Abstract
In Tetrahymena cells, constitutive phosphorylation of histone H1 phenocopies the loss of H1 from chromatin. Regulation of transcription by H1 phosphorylation is achieved by altering the overall charges of a small domain. Here, we further explore the electrostatic properties of this domain and the mechanism by which it regulates transcription. We demonstrate that the regulatory effect of the clustered charges does not require any long-range interaction and is position independent. However, when the same number of charges was dispersed throughout the H1 molecule, the effect became undetectable. The results are explained by a nucleation-propagation model and provide in vivo evidence that the synergy of the clustered positive charges plays a role in histone function and gene regulation.
Posttranslational modifications of nonglobular histone tails result in histone heterogeneity that is associated with different structural and functional states of chromatin. In particular, reversible acetylation, methylation, and phosphorylation are involved in important processes such as histone deposition, transcriptional regulation, chromatin remodeling, and chromosome condensation (1–4). These histone covalent modifications, alone or in combination, may generate patterns, referred to as a “histone code,” that can interact differentially with regulatory protein factors (5). The specificity of such interactions between histones and other chromatin-associated proteins is best illustrated by recent studies showing that methylation of lysine 9 of histone H3 creates a binding site for the chromo-domains of HP1 and Swi6 proteins (6, 7). In addition, x-ray crystallographic analyses have shown that the conserved bromodomain of several transcription coactivators, such as p300/CBP-associated factor (P/CAF) and TBP-associated factor TAF (II)250, can specifically bind to acetylated lysine residues on histone N-terminal tails (8, 9).
In addition to marking specific residues, modifications such as acetylation and phosphorylation also reduce the positive electrostatic charge carried by histones. As a result, these modifications also are likely to affect the interaction between the negatively charged DNA and the positively charged histone tails, which is coulombic in nature. Therefore, it would not be surprising if modifications such as the multiple acetylations recognized by TAF(II)250 (10) also weaken histone–DNA electrostatic interactions to facilitate chromatin remodeling that enables transcription factors to gain access to key DNA elements (11). However, there is surprisingly little evidence that changes in histone tails significantly affect nucleosome structure or stability, probably because of the overwhelmingly large binding energy contributed by the core histone globular domains.
Interactions between linker histones and DNA depend even more on coulombic forces than core histone–DNA interactions, as revealed by the fact that H1 dissociates from chromatin at lower salt concentrations than core histones. Recent studies have shown that modulation of the coulombic interactions between H1 and DNA by posttranslational modification is physiologically important (12, 13). We showed that the expression of the CyP1 gene in starved Tetrahymena cells requires dephosphorylation of the macronuclear linker histone. CyP1 expression was shown to be regulated by the net charge of a 19-residue region (residues 35–54) of H1 containing the five phosphorylation sites. If the total number of charges of that region was the same as that of fully phosphorylated H1, CyP1 expression was inhibited. If the total charges of the region were the same as those of fully dephosphorylated H1, CyP1 expression was strongly induced. The charge altering mutations need only to be in this region and not at the actual phosphorylation sites; and these effects are independent of the hydrophobicity of the region. These studies demonstrated that phosphorylation of H1 in Tetrahymena regulates the expression of specific genes by changing the overall charge of a small domain (referred to as a “charge patch”) of the H1 molecule, and not by phosphate recognition or by creating a site-specific charge.
Regulation of a charge patch by posttranslational modification of a core histone has also been shown to have an important (essential) function (14). In Tetrahymena, mutating all six acetylation sites in the N-terminal tail of the conserved histone variant H2A.Z to arginines, which cannot be acetylated, was lethal. This lethality can be rescued by retaining a single acetylatable lysine or by mutations that reduce the positive charge at any of the acetylation sites or at other (non-acetylation) sites in the N-terminal tail. Such coulombic interactions also could explain many of the effects of mutating acetylation sites of the histone H4 tail in yeast (15). These results argue strongly that posttranslational modifications are likely to affect transcription by regulating nonspecific coulombic interactions between DNA and core histones as well as linker histones.
To further explore the electrostatic properties of a charge patch and the mechanism by which it regulates transcription, we set up three experiments to analyze the phosphorylation of H1 in greater detail. Here, we demonstrate that the regulatory effect of the clustered charges does not require any long-range interaction and is position independent. However, when the same number of charges was dispersed throughout the H1 molecule, the effect became undetectable.
Materials and Methods
Cell and Culture Conditions.
Wild-type (WT) Tetrahymena thermophila strain CU428 mpr1-1/mpr1-1 (MPR1, mp-s, VII) was kindly provided by P. J. Bruns (Cornell University). Cells were grown in medium (16) containing 1% proteose peptone (SPP).
Construct Information.
In the nine circular permutation constructs of the charge patch, WT and mutant (A, E) charge patches, from residues 35 to 54, were moved to different regions of H1 in a circularly permutated way (Fig. 1A) by overlapping PCR (17). In each of the nine mutations, the first three amino acids of the H1 protein were retained for proper translation.
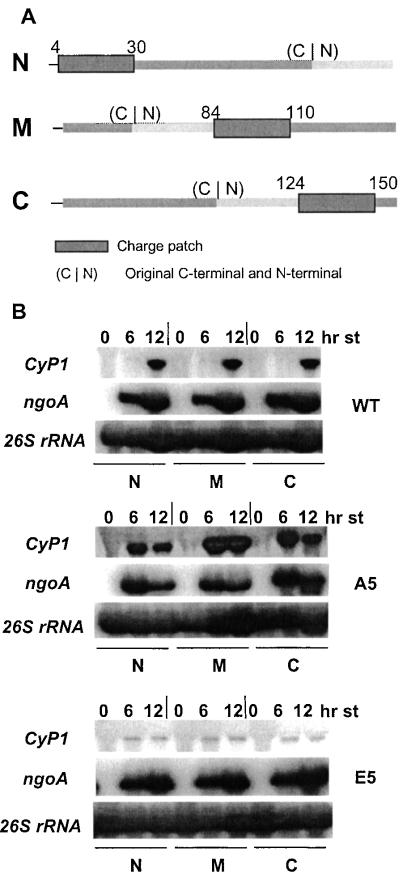
Figure 1.
(A) Circular permutation of the charge patch. Wild-type (WT) and mutant (A and E) charge patches, from residues 35 to 54, were moved to different regions of H1 in a circularly permutated way. In N, the patch was moved to the extreme N terminus of the protein, from position 4 to 30. In M, the patch was moved near the center of H1, from residues 84 to 110. In C, the patch was moved to the C terminus, to positions 124–150. (C|N) is the junction of the C and N terminus if the ends of H1 were ligated. (B) The Charge patch functions at different sites in H1. Whole-cell RNAs (10 μg) were isolated from WT-N, M, and C; A-N, M, and C; and E-N, M, and C strains at 0, 6, and 12 h of starvation. They were analyzed by Northern blots probed with CyP1 and ngoA genes. 26s rRNA was used as loading control.
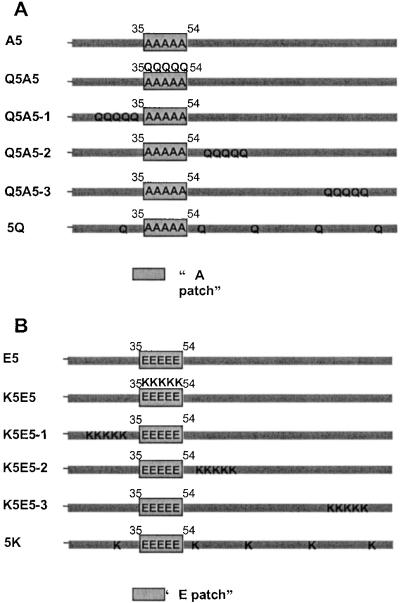
In “patch” and “dispersed” K to Q mutations, Q5A5-1 is like Q5A5 except that five lysines (K19, -23, -24, -28, and -29) on the N-terminal side of the phosphorylation region were changed to glutamines. In Q5A5-2, five lysines (K61, -62, -63, -66, and -68) at the immediate C-terminal side of the phosphorylation region were changed to glutamines. In Q5A5-3, five lysines (K135, -138, -139, -142, and -149) in the C-terminal region of H1 were changed to glutamines. In 5Q, five lysines (K29, K60, K87, K117, and K150) were changed to glutamines. The net charges of the Q5A5, Q5A5-1, -2, and -3, and 5Q versions of H1 were the same, similar to that of E5.
In patch and dispersed neutral to K mutations, K5E5-1 is like K5E5 except that five neutral residues (H17, P21, A25, I26, and A27) at the N-terminal side of the phosphorylation region were changed to lysines. In K5E5-2, five neutral residues (T60, I64, H65, T67, and T69) at the immediate C-terminal side of the phosphorylation region were changed to lysines. In K5E5-3, five neutral residues (P136, A137, A141, T145, and A148) in the C-terminal region of H1 were changed to lysines. In 5K, residues A27, T59, S90, V120, and A149 were changed to lysines. The net charges of the K5E5, K5E5-1, -2, and -3, and 5K versions of H1 were the same, similar to that of A5.
All these constructs contained a neo2 cassette inserted into 3′ flanking region of the HHO1 gene (13).
Gene Replacement by Biolistic Transformation.
Mutation constructs containing a flanking neo2 gene conferring paromomycin (pm) resistance in Tetrahymena were digested with KpnI and SacII, biolistically transformed into CU428 cells as described (13), and selected in paromomycin sulfate to a final concentration of 2.5 mg/ml.
Northern Blot Analysis.
RNA was isolated from starved cells with Trizol (Life Technologies), electrophoresed in 2.2 M formaldehyde-1.2% agarose gels, blotted and hybridized (18). [α-32P]dATP-labeled random primed probes were obtained as follows: The probe for rRNA was a 2 kb HindIII fragment from pBS26S encoding the Tetrahymena 26S rRNA (19). The CyP1 probe was synthesized from two PstI fragments (0.5 kb, 0.7 kb) from pCyP1 (20). The ngoA probe was a 1.1 kb PstI fragment from pC5.5 (21). Hybridizations were done as described in ref. 12.
Results
Because the five phosphorylation sites of H1 are clustered in a small, specific region of Tetrahymena H1, the first question we addressed was whether the regulatory effects of phosphorylation required that the charge patch be in its normal location. Three mutations were constructed for each of the wild-type, A5 (replacing all of the phosphorylation sites with alanines, mimicking fully dephosphorylated H1), and E5 (replacing the sites with glutamates, mimicking fully phosphorylated H1) versions of the HHO1 gene (13). These molecules were changed in a circularly permutated way so that the phosphorylation patch was at the N-terminal location (N), in the third quarter (M), or at the C-terminal part (C) of H1, respectively (Fig. 1A). In these mutated H1s, the immediate context of the patch is preserved as much as possible, but the location of the patch within the protein is dramatically changed and any long-range intramolecular interactions are likely to be disrupted.
The nine constructs were introduced into T. thermophila by biolistic transformation, and CyP1 was used as the reporter gene to examine the effects of the mutations. The results were clear and surprising, and can be easily summarized: CyP1 expression depends only on the original modification status of the phosphorylation sites and is independent of the location of the patch. In cells with the WT patch (W-N, W-M, and W-C strains) at any of the three locations, CyP1 was induced, as occurs with WT H1, after 12 h of starvation, when the majority of the WT H1 normally becomes dephosphorylated (13). In contrast, in the three A5 strains (A-N, A-M, and A-C), CyP1 was highly induced after only 6 h of starvation as shown previously for A5 cells (13). In the three E5 (E-N, E-M, and E-C) cells, CyP1 expression was markedly inhibited even after 24-hour starvation as shown earlier (13) for the E5 strain (Fig. 1B). These results demonstrate that the phosphorylation domain of H1 functions independent of its position in the molecule, even in a molecule whose overall sequence arrangement has been markedly rearranged. These results are consistent with the previous studies demonstrating that linker histone tail domains (equivalent to the entire Tetrahymena H1, which has no globular domain) have little structure in solution (22).
A trivial explanation of these results is that the mutated H1s were changed so much that they did not bind to chromatin at all. However, this is not likely to be the case. If circular permutation altered H1 structure so that it could not bind to chromatin, all three mutations should have behaved like the H1 null mutation, in which CyP1 induction is inhibited (23). However, the WT and A5 mutations exhibit similar levels and timing of CyP1 induction as H1s with the same residues located at the original position of the phosphorylation patch. Therefore, only the E5 mutation series could be explained by arguing that the mutant H1s fail to bind to chromatin. However, the original E5 mutation of H1 shows only a slight reduction in its binding affinity to chromatin compared with A5 when assayed by salt elution (unpublished observation). Because the WT and A5 permutation mutations didn't behave as H1 null mutations, it is highly unlikely that the E5 permutation leads to complete dissociation of H1 from chromatin.
To examine the effects of the position of the charge patch under conditions that minimized long range perturbations of H1 structure, a series of mutations were made by changing five lysines (Ks) to glutamines (Qs) in regions spanning ≈15 residues at various positions of the H1 molecule containing mutated phosphorylation sites (A5). These mutations are equivalent to creating a region at randomly chosen positions in the molecule with reduced positive charges that is similar to the original, fully phosphorylated patch. Two of these “Q-patches” were introduced immediately flanking the charge patch on either side, whereas the third one is very close to the carboxyl terminus (Fig. 2A). These constructs resemble the previously studied Q5A5 construct (12), in which five glutamines were introduced within an A5 patch at the normal position of the wild-type phosphorylation region. However, an important difference between these three new mutations and the original Q5A5 mutation is that the original charge patch remains in its unphosphorylated state, while completely new regions elsewhere in the molecule take on the charge properties of a fully phosphorylated patch. If all three patches fail to mimic phosphorylated H1, we can conclude that the patch needs to be specifically located within its normal local context. If the two closely flanking Q-patches behave like E5, just as Q5A5 did, but the distal one does not, it would suggest that the charge patch has boundaries that define it as an independent functional unit. If all three mutations have the same phenotype as that of Q5A5, then we can conclude that the charge patch can be placed in any context, anywhere in the molecule.
Figure 2.
(A) Patch and dispersed locations of K to Q mutations. In A5, the five phosphorylation sites were changed to alanine, producing a constitutively unphosphorylated H1. Starting with A5, in Q5A5, five lysines in the region of the phosphorylation sites were changed to glutamines, reducing the positive charge without introducing any negatively charged molecules that resemble phosphates. Q5A5-1, -2, and -3 are like Q5A5 except that five lysines on the N-terminal side of the phosphorylation region, at the immediate C-terminal side of the phosphorylation region and in the C-terminal region of H1, were changed to glutamines. In 5Q, five lysines (≈30 aa apart) were changed to glutamines. The net charges of the Q5A5, Q5A5-1, -2, and –3, and 5Q versions of H1 were the same, similar to that of E5. (B) Patch and dispersed locations of neutral to K mutations. In E5, the five phosphorylation sites were changed to glutamic acids, mimicking a constitutively phosphorylated H1. Starting with E5, in K5E5 five neutral residues in the region of the phosphorylation sites were changed to lysines, introducing positive charges. K5E5-1, -2, and -3 are like K5E5 except that five neutral residues at the N-terminal side of the phosphorylation region, at the immediate C-terminal side of the phosphorylation region, and in the C-terminal region of H1 were changed to lysines. In 5K, five neutral residues (≈30 aa apart) were changed to lysines. The net charges of the K5E5, K5E5-1, -2, and –3, and 5K versions of H1 were the same, similar to that of A5.
We also introduced “K-patches” into the E5 version of the HHO1 gene. In these mutants, five clustered neutral residues were changed to lysines (Ks) at varying distance on either side of the phosphorylation sites, at approximately the same regions where the Q-patches were introduced (Fig. 2B). These mutations combined with the E5 mutations at the phosphorylation sites will mimic A5 H1 in overall charge while retaining a charge patch that mimics the fully phosphorylated state. These three constructs, K5E5-1, -2, and -3 serve as controls for the Q-patch constructs, and vice versa.
All six mutations were transformed into Tetrahymena, and CyP1 expression was analyzed. Q5A5-1, -2, and -3 mutants showed phenotypes similar to those of the E5 and Q5A5 strains. CyP1 induction was inhibited in all three strains even after long starvation. K5E5-1, -2 and -3 mutants showed phenotypes similar to those of A5 and K5E5 strains. CyP1 was induced early during starvation in all three strains. The expression of another starvation-inducible gene ngoA, whose induced expression is independent of H1, was not affected in either of the two experiments (Fig. 3).
Figure 3.
Total RNA (10 μg) isolated from cells after 0, 6, and 12 h of starvation, was analyzed by Northern blotting using CyP1 and ngoA specific probes. 26S rRNA was used as loading control. (A) Q5A5-1, Q5A5-2, Q5A5-3, and 5Q strains. (B) K5E5-1, K5E5-2, K5E5-3, and 5K strains.
The circular permutation experiment and the Q-patch and the K-patch experiments suggest that the function of H1 phosphorylation in regulating CyP1 transcription depends either on the net charge of the protein or on the existence of a small region of reduced positive charge, but not on the particular location of the charge patch. To determine whether the charges need to be clustered to affect transcription, we examined the effects of the same number of charge-altering mutations spaced evenly throughout the molecule compared with the clustered changes described above. We made five K to Q replacements spread over the whole length of the A5 version of H1 and five neutral residues to K mutations throughout the E5 version of H1. In the 5Q construct, K29, K60, K87, K117, and K150, were changed to glutamine. The total charge of this H1 is similar to that of the E5 mutation and to that of the Q5A5 series of mutations (Fig. 2A). In the 5K construct, we started with the E5 mutation and changed A27, T59, S90, V120, and A149 to Ks. This mutation has an overall charge similar to A5 and to the K5E5 series of mutations (Fig. 2B).
The results clearly showed that, when the changes of charge were dispersed, they could no longer regulate transcription of CyP1. In the 5Q mutant, CyP1 was induced early during starvation, just as would be expected from the A5 and K5E5 strains. In the 5K mutant, the CyP1 activation is inhibited after 12-hour starvation, just as in the E5 and Q5A5 strains. Again, the expression of the starvation-inducible gene ngoA was not affected in these experiments (Fig. 3). These results are strikingly different from the results observed when charge changes were clustered, inasmuch as these mutations produced phenotypes dictated by the sequence of the original charge patch, and not by the net charge on the molecule. Thus, when the same charge-altering mutations were dispersed throughout the H1 molecule instead of being clustered, their effect was undetectable.
Discussion
Examination of the charge density across WT and mutated Tetrahymena H1 indicated that phosphorylation of the WT sequence, the E5 mutations, and the clustered mutations in the Q5A5 series served to convert regions from being positively charged to the most negatively charged segment of the molecule. In addition, in those cases where they have been mapped (24–27), phosphorylation sites in other linker histones are clustered. These observations, and our previous findings that phosphorylation of H1 and deletion of H1 have indistinguishable effects on the transcription of two genes (13), led us to propose a nucleation-propagation model for the interaction of DNA and H1 (Fig. 4). In this scenario, the first step of both H1 association and disassociation from DNA/chromatin is rate limiting. The nucleation center for H1 disassociation is the part of H1 with the highest negative charge density (or lowest positive charge density), and the disassociation rate is positively correlated with the negative charge density. When clusters of either positive or negative charges are introduced into H1, new nucleation centers for association and disassociation are created, and the dynamics of H1 binding to DNA/chromatin are dramatically changed. Dispersed charges have no impact on H1 function because they do not create new nucleation centers, so single charges introduced outside of the nucleation center have very little effect on H1 binding.
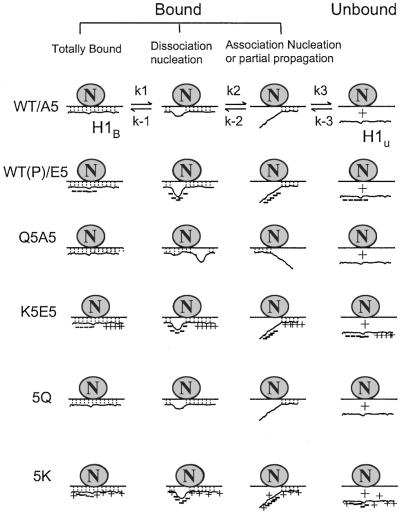
Figure 4.
The nucleation-propagation model of H1-DNA interactions. H1 is proposed to be constantly exchanging, with bound and unbound fractions in equilibrium (30, 31). For all forms of H1, dissociation is nucleated from the region with the lowest positive charge and then propagated to other parts of the molecule. Similarly, association is nucleated from the region with the highest positive charges. In WT cells with fully dephosphorylated H1 or in A5 cells, nucleation of dissociation is relatively low, whereas nucleation of reassociation is high. When WT H1 is phosphorylated or the phosphorylation sites are mutated to glutamic acid (E5), the phosphorylation region is negatively charged and becomes the nucleation center for dissociation. In the Q5A5 series, reducing positive charges creates new nucleation centers in other parts of H1, again shifting the equilibrium toward dissociation. In the K5E5 series, although a nucleation site is produced by the five Es at the phosphorylation sites, we hypothesize that the newly introduced positive charge patches could produce a nucleation center that increases the association rate or that the newly introduced positive charges could produce a kinetic barrier to propagation. In 5Q and 5K cells, the introduced charges are spread out throughout the whole molecule. Because no new nucleation center was created, they behave like A5 or E5 cells, respectively.
In vitro physical chemistry studies testing the binding affinities of polycation oligopeptides to double-stranded DNA indicate that nonspecific binding is driven primarily by coulombic interactions (28). Interestingly, the binding constants for oligopeptides with dispersed positive charges are 1–2 orders of magnitude less than the values for oligopeptides with the same number of charges in a clustered form (29). Our results provide in vivo evidence that such synergy of clustered positive charges can play a role in regulating histone function and gene regulation.
In summary, we have demonstrated that the linker histone H1 in Tetrahymena functions like a charge patch-based switch that is regulated by phosphorylation. Presumably, the H1 kinase recognition motif is the target of upstream regulatory signals. The only feature of the molecule that regulates the downstream effects on transcription seems to be its highly localized charge density. Mechanistically, these properties are similar to the prominent effects of clustered charges observed in simple model systems of polycation oligo-peptide binding to DNA. Considering that clustered, charge-reducing, covalent modifications also occur on the highly basic tails of core histones, this mechanism is likely to be a general feature of histone-mediated gene regulation. However, it should be emphasized that the charge patch mechanism, although stressing the importance of coulombic DNA–histone interactions, does not exclude the participation of protein–histone interactions in gene regulation as proposed in the “histone code” hypothesis. We believe it is likely that there also are pathways that can recognize more subtle differences in histones with different site-specific patterns of covalent modifications to add additional layers of complexity to the function of histones in gene regulation.
Acknowledgments
We are grateful to Josephine Bowen for critically reading the manuscript. This work was supported by Grant GM21793 from the National Institutes of Health.
Abbreviation
- WT
wild type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cheung P, Allis C D, Sassone-Corsi P. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 2.Roth S Y, Allis C D. Cell. 1996;87:5–8. doi: 10.1016/s0092-8674(00)81316-1. [DOI] [PubMed] [Google Scholar]
- 3.Turner B M. Bioessays. 1995;17:1013–1015. doi: 10.1002/bies.950171204. [DOI] [PubMed] [Google Scholar]
- 4.Ura K, Kurumizaka H, Dimitrov S, Almouzni G, Wolffe A P. EMBO J. 1997;16:2096–2107. doi: 10.1093/emboj/16.8.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strahl B D, Allis C D. Nature (London) 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 6.Lachner M, O'Carroll N, Rea S, Mechtler K, Jenuwein T. Nature (London) 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama J, Rice J C, Strahl B D, Allis C D, Grewal SI. Science. 2001;292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 8.Bannister A J, Zegerman P, Partridge J F, Miska E A, Thomas J O, Allshire R C, Kouzarides T. Nature (London) 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 9.Dhalluin C, Carlson J E, Zeng L, He C, Aggarwal A K, Zhou M M. Nature (London) 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson R H, Ladurner A G, King D S, Tjian R. Science. 2000;288:1422–1425. doi: 10.1126/science.288.5470.1422. [DOI] [PubMed] [Google Scholar]
- 11.Tse C, Sera T, Wolffe A P, Hansen J C. Mol Cell Biol. 1998;18:4629–4638. doi: 10.1128/mcb.18.8.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dou Y L, Gorovsky M A. Mol Cell. 2000;6:225–231. doi: 10.1016/s1097-2765(00)00024-1. [DOI] [PubMed] [Google Scholar]
- 13.Dou Y L, Mizzen C A, Abrams M, Allis C D, Gorovsky M A. Mol Cell. 1999;4:641–647. doi: 10.1016/s1097-2765(00)80215-4. [DOI] [PubMed] [Google Scholar]
- 14.Ren Q, Gorovsky M A. Mol Cell. 2001;7:1329–1335. doi: 10.1016/s1097-2765(01)00269-6. [DOI] [PubMed] [Google Scholar]
- 15.Megee P C, Morgan B A, Smith M M. Genes Dev. 1995;9:1716–1727. doi: 10.1101/gad.9.14.1716. [DOI] [PubMed] [Google Scholar]
- 16.Gorovsky M A, Yao M-C, Keevert J B, Pleger G L. Methods Cell Biol. 1975;9:311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Russell D W. Molecular Cloning. Plainview, NY: Cold Spring Harbor Lab. Press; 2001. [Google Scholar]
- 18.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley Interscience; 1988. [Google Scholar]
- 19.Engberg J, Nielsen H. Nucleic Acids Res. 1990;18:6915–6919. doi: 10.1093/nar/18.23.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karrer K M, Stein-Gavens S. J Protozool. 1990;37:409–414. doi: 10.1111/j.1550-7408.1990.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 21.Martindale D W, Bruns P J. Mol Cell Biol. 1983;3:1857–1865. doi: 10.1128/mcb.3.10.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He X, Rines D R, Espelin C W, Sorger P K. Cell. 2001;106:195–206. doi: 10.1016/s0092-8674(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 23.Shen X T, Gorovsky M A. Cell. 1996;86:475–483. doi: 10.1016/s0092-8674(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Hayashi H, Iwai K. J Biochem. 1987;102:369–376. doi: 10.1093/oxfordjournals.jbchem.a122063. [DOI] [PubMed] [Google Scholar]
- 25.Hill C S, Rimmer J M, Green B N, Finch J T, Thomas J O. EMBO J. 1991;10:1939–1948. doi: 10.1002/j.1460-2075.1991.tb07720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hohmann P, Tobey R A, Gurley L R. J Biol Chem. 1976;251:3685–3692. [PubMed] [Google Scholar]
- 27.Sung M T, Freedlender E F. Biochemistry. 1978;17:1884–1190. doi: 10.1021/bi00603a013. [DOI] [PubMed] [Google Scholar]
- 28.Anderson C F, Record M T., Jr J Phys Chem. 1993;97:7116–7126. [Google Scholar]
- 29.Padmanabhan S, Zhang W, Capp M W, Anderson C F, Record M T J. Biochemistry. 1997;36:5193–5206. doi: 10.1021/bi962927a. [DOI] [PubMed] [Google Scholar]
- 30.Lever M A, Th'ng J P H, Sun X J, Hendzel M J. Nature (London) 2000;408:873–876. doi: 10.1038/35048603. [DOI] [PubMed] [Google Scholar]
- 31.Misteli T, Gunjan A, Hock R, Bustin M, Brown D T. Nature (London) 2000;408:877–881. doi: 10.1038/35048610. [DOI] [PubMed] [Google Scholar]