Abstract
The endoplasmic reticulum (ER) Ca2+ sensor STIM1, best-known for its essential role in triggering influx of extracellular Ca2+ via Ca2+-release-activated channels when ER stores become depleted, unexpectedly also regulates Ca2+ entry through voltage-gated Ca2+ channels. In response to a drop in ER luminal Ca2+ level, this ER membrane-spanning sensor can contact voltage-gated Ca2+ channels in the plasma membrane and thereby inhibit Ca2+ influx through them. This previously unappreciated, interaction between ER Ca2+ level and magnitude of Ca2+ influx via voltage-gated Ca2+ channels may turn out to powerfully impact Ca2+ signaling in excitable cells, including neurotransmitter release, structural and functional postsynaptic plasticity, and transcription factor translocation.
Keywords: voltage-gated calcium channel, L-type calcium channel, STIM1, endoplasmic reticulum, endoplasmic reticulum calcium
Introduction
Ca2+ is the lingua franca of intracellular communication. In neurons, transduction of electrical activity into important cytoplasmic Ca2+ signals is accomplished in large part through the activity of voltage-gated calcium channels (CaVs). In keeping with such broad capability, the activity of CaVs is made highly adjustable by assembling these channels within macromolecular signaling modules replete with regulatory machinery. Among the many forms of such regulation are several involving Ca2+ and Ca2+ signaling proteins, such as Ca2+-dependent inactivation of CaVs, CaV-initiated Ca2+-induced Ca2+ release (CICR) from intracellular stores, and retrograde control of CaVs by Ca2+-release channels (1).
In 2010, two groups reported the surprising and transformative finding that CaVs are subject to strong regulation by the ER Ca2+ sensor, stromal interaction molecule-1 (STIM1) (2, 3). Previous work had shown that STIM1 is expressed in a wide range of tissues, including prominently and most famously in T lymphocytes and in other non-excitable tissues of lung, liver, kidney, as well as in excitable tissues of heart, skeletal muscle, smooth muscle, glandular tissue, and throughout the brain. This work had also shown that STIM1 is a single-pass ER membrane protein bearing a Ca2+ binding EF-hand in its N-terminal intraluminal region which allows STIM1 to report ER Ca2+ concentration ([Ca2+]ER) (4, 5). Following release of Ca2+ from ER stores, for example in response to Gq-coupled receptor/inositol 1,4,5-trisphosphate receptor activity, the resulting depletion of Ca2+ from the ER results in unloading of Ca2+ from STIM1’s EF hand, activation and oligomerization of STIM1, and extension of the cytosolic domains of this complex across the gap between the ER and plasma membrane (PM). In breakthrough work that revealed the fundamental mechanism of stores-operated Ca2+ entry (SOCE) (Box1), the C-terminal domain of the extended STIM1 complex contacts and activates Ca2+ release-activated channels (CRAC) embedded in the plasma membrane (PM) (6–8), which allows influx of Ca2+ that both acts as a cytosolic signal and replenishes ER stores. It now appears that this same mechanism, involving STIM1 or its relative STIM2, is largely responsible for SOCE and regulation of [Ca2+]ER in neurons (9–11) and other excitable cells (12). The 2010 reports from the Dolmetsch and Gill research groups that STIM1 regulates CaVs as well as CRAC channels were wholly unexpected not only because CaVs, in addition to CRAC channels, were discovered to be regulated by STIM1, but also because Ca2+ influx via CaVs was found to be attenuated, rather than activated, by interaction with STIM1.
The ability of STIM1 to physically engage its PM signaling partners is supported by regions of close apposition of cortical ER with the PM. The intermembrane gap of such ER-PM junctions has been found to range from ~10 to 17 nm (13–15). This gap is narrow enough that the extended conformation of the cytosolic C-terminal domain of STIM1 can reach across it to the PM. In considering the dynamics, localization and restriction of subcellular Ca2+ signals it should be noted that ER-PM junctions are tiny, thin spaces—only ~0.1% the diameter of neuronal somata, ~1% the diameter of a dendrite, ~2% the diameter of a dendritic spine, and a little narrower than the neuronal synaptic cleft (~20 nm).
Reciprocal control of voltage-gated Ca2+ channels by STIM1
Neuronal CaVs are composed of three subunits, one α1, one α2δ and one β subunit. These channels are functionally dominated by their single α1 subunit, which forms the permeation pathway for Ca2+, detects membrane depolarization arising from action potentials or excitatory postsynaptic potentials, and undergoes conformational changes from closed to open to inactivated states. Neuronal CaVs are variously specialized for neurotransmitter release (CaV2 subfamily, corresponding to P/Q-, N- and R-type channels), regulation of gene expression (CaV1, corresponding to L-type channels) and postsynaptic/somatic Ca2+ signaling (CaV1 and CaV2), or repetitive firing (CaV1.3 and the T-type CaV3 channels) (Table 1) (16). While depolarization is the only natural stimulus that directly causes CaVs to open, their probability of opening is modulated by many factors including interaction with their own accessory α2δ and β channel subunits (α2δ1–4, β1–4), channel phosphorylation by multiple types of kinases, or binding of regulatory factors such as calmodulin or the βγ subunits of Gi/o proteins.
Table 1.
Neuronal voltage-gated Ca2+ channel subunits.
| Subfamily | Functional type | Systematic name | α1 subunit |
|---|---|---|---|
|
| |||
| cav1 | L-type | cav1.2 | α11.2 |
| L-type | cav1.3 | α11.3 | |
| cav2 | P/Q-type | cav2.1 | α12.1 |
| N-type | cav2.2 | α12.2 | |
| R-type | cav2.3 | α12.3 | |
| Cav3 | T-type | cav3.1 | α13.1 |
| T-type | cav3.2 | α13.2 | |
| T-type | cav3.3 | α13.3 | |
Dolmetsch and colleagues (2) and Gill and colleagues (3) added to the already extensive list of CaV regulatory processes when they discovered that depletion of [Ca2+]ER and consequent activation of STIM1 can attenuate the activity of voltage-gated Ca2+ channels. Using rat cultured cortical neurons (endogenous CaV1-,3 channels), the A7r5 vascular smooth muscle cell line (endogenous CaV1 channels), and HEK293 cells which lack endogenous CaVs but were transfected with CaV1.2 L-type Ca2+ channels, these pioneering groups found that diverse pharmacological interventions resulting in depletion of ER Ca2+ stores attenuated depolarization-evoked intracellular Ca2+ ([Ca2+]i) elevation and also reduced the amplitude of voltage-clamped Ca2+ current. Linking stores depletion-mediated inhibition of CaV activity to STIM1 were two pieces of evidence: first, overexpression of STIM1 in cortical neurons led to reduction in depolarization-driven rises in [Ca2+]i, and reduced or eliminated CaV current in CaV1.2-transfected HEK293 cells. Second, STIM1 defective in Ca2+-binding (STIM1-D76A mutation) and hence chronically active, when overexpressed in A7r5 cells or CaV1.2-transfected HEK293 cells, caused constitutive reduction in CaV1.2 activity (3).
In cells with a well-characterized mechanism of STIM1 action, albeit with CRAC channels, would STIM1 also suppress CaV1.2 activity? Park et al. 2010 (2) tested this by transfecting Jurkat T lymphocytes with CaV1.2 subunits (α11.2, α2δ1 and β1b), and found no depolarization-evoked rise in [Ca2+]i, as though endogenous STIM1 had chronically shut off the exogenous CaVs. In contrast, Jurkat T lymphocyte mutants that lack functional STIM1 displayed, when transfected with CaV1.2 subunits, depolarization-driven elevation in [Ca2+]i. Overexpression of STIM1 in these mutants restored wild-type functionality, eliminating CaV1.2-dependent [Ca2+]i rises. Finally, knockdown of STIM1 in wild-type Jurkat T lymphocytes allowed CaV1.2-dependent [Ca2+]i rises, confirming that STIM1 suppresses CaV1.2 activity in these cells, as it appeared to do in cortical neurons and other cells
The Gill and Dolmetsch groups also showed, using HEK293 cells transfected with CaV1.2 and STIM1, that immunoprecipitation of STIM1 coimmunoprecipitated CaV1.2, and vice versa, suggesting that these two proteins can interact directly. Using neuroblastoma cells that express both of these partners natively, STIM1 was shown to be associated with immunoprecipitated CaV1.2 as well. Molecular dissection of this interaction revealed that STIM1’s cytosolic CRAC activation domain (CAD; residues 342–448), which binds and activates Orai-based CRAC channels, also interacts with CaV1.2 channels, though in this case inhibiting channel activity. Thus deletion of CAD from STIM1 prevented STIM1 suppression of [Ca2+]i rises, whereas expression of the CAD peptide alone strongly suppressed CaV1.2 activity (2, 3). Moreover, immunoprecipitation of CAD coimmunoprecipitated CaV1.2, indicating direct interaction (2).
Within STIM1’s CAD domain, substitution of alanine at two adjacent positions, L347 and Q348, prevented STIM1 activation of CRAC channels (17), and this double substitution mutant also failed to inhibit CaV1.2 channels (3). In contrast, STIM1Δ441–448 is incapable of activating CRAC channels, but this mutation retains the ability to inhibit CaV1.2. Thus the site on STIM1 that inhibits CaV1.2 overlaps but is not identical to that which activates Orai-based CRAC channels (3).
Which parts of CaV1.2 are contacted by STIM1? In HEK293 cells expressing the CaV1.2 α1 subunit alone, stores depletion was still able to suppress Ca2+ influx, showing that the channel accessory α2δ and β subunits are not needed for depletion-driven inhibition of CaV1.2 activity (3). Functional requirement for the CaV1.2 C-terminal domain was demonstrated by the finding that CaV1.2 lacking its C-terminal domain supported depolarization-dependent rises in [Ca2+]i that were unaffected by the presence of STIM1 (2). Coimmunoprecipitation experiments with HEK293 cells overexpressing STIM1, or its CAD domain, along with individual intracellular domains of CaV1.2 revealed that STIM1 binds to a region in the cytoplasmic distal C-terminal of CaV1.2, encompassed by residues 1809–1908 (2). This specific region of the CaV1.2 C terminal region is distinct from other C-terminal sites, such as those that bind calmodulin, calmodulin kinase II, and A-kinase anchoring proteins; sites involved in protein kinase A-dependent enhancement of channel activity, including sites of Ser/Thr phosphorylation; or domains involved in Ca2+-dependent inactivation.
Finally, in cultured hippocampal neurons, overexpression of STIM1 greatly reduced the fraction of CaV1.2 channels on the cell surface (2) via a dynamic-dependent mechanism. Thus STIM1 can acutely inhibit CaV1.2 activity via stores depletion and it can also chronically reduce CaV1.2 activity by promoting cellular internalization of these channels.
STIM1 inhibition of L-type CaVs in neurons
In addition to its key role in non-excitable cells, STIM1 is clearly involved in neuronal ER Ca2+ signaling and Ca2+ homeostasis. For example, in cerebellar Purkinje neurons, STIM1 has been shown to mediate mGluR1 activation of TRPC3 channels and thereby support slow excitatory synaptic transmission that is involved in cerebellar motor control (10). STIM1 is present throughout neurons, as ER pervades dendritic shafts, projects into large dendritic spines in a labile fashion, and also helps form a synaptopondin-bearing structure in the base of spines known as the spine apparatus (18–21). And it turns out, STIM1 exerts strong modulatory control over CaV activity in neurons in order to regulate diverse cellular functions.
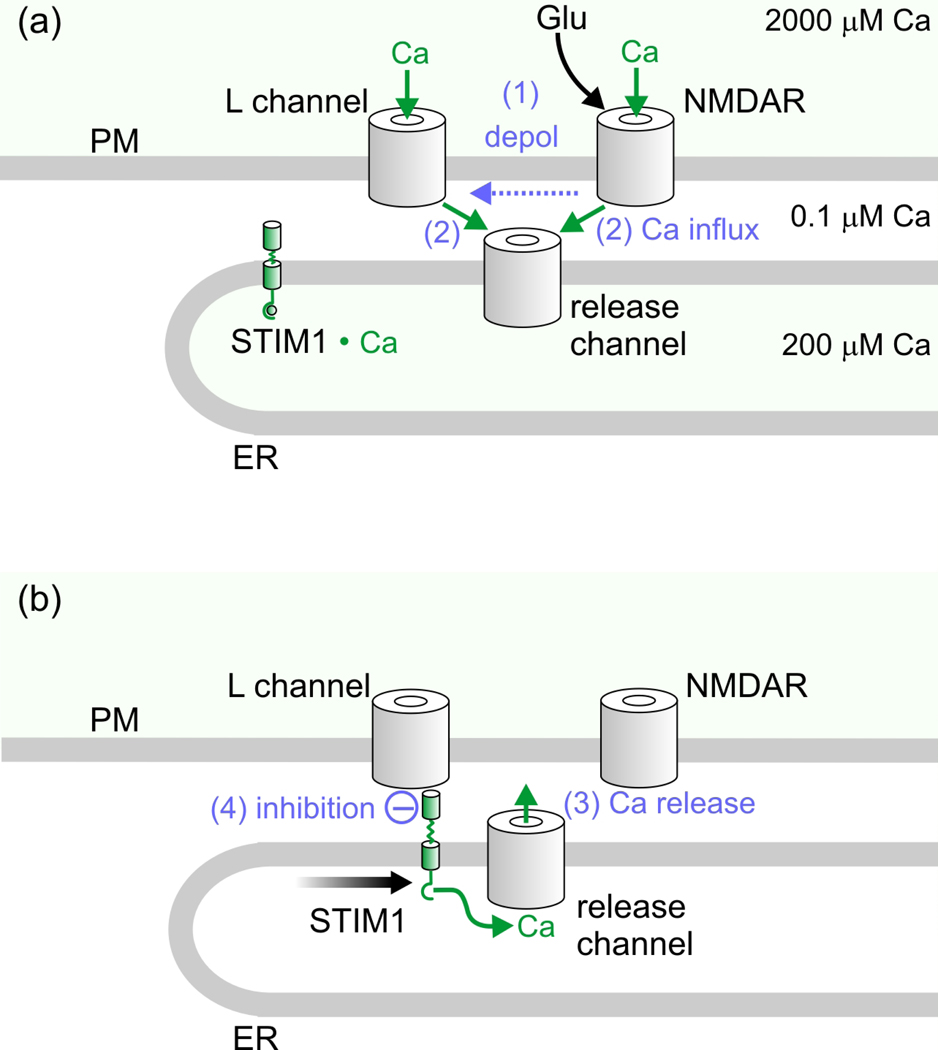
Using cultured hippocampal neurons, Dittmer et al. discovered that the neurotransmitter glutamate, acting through N-methyl-D-aspartate (NMDA) receptor-dependent depletion of ER Ca2+ stores, could initiate STIM1 inhibition of L-type Ca2+ current (22) (Figure 1). In their experiments, responses of voltage-clamped L-type current to bath applied glutamate (15 s), or of simultaneously-imaged cytoplasmic and ER Ca2+ to uncaging of glutamate (60 laser flashes in 1 min) near a single dendritic spine, were measured. In either case, NMDA receptors initiated CICR and neuronal depolarization, the latter in turn activating L-type Ca2+ channels and hence additional CICR. Completing the negative feedback loop, stores depletion-activated STIM1 inhibited subsequent Ca2+ entry through L-type Ca2+ channels. RNAi knockdown of STIM1 prevented inhibition, and inhibition was restored by co-expression of an RNAi-insensitive STIM1.This group also used Förster resonance energy transfer (FRET) to gauge interaction between overexpressed, fluorescent protein-labeled CaV1.2 and STIM1, which showed that the time course of interaction between these partners reflected the time course of inhibition of the channel. Previous work had shown that Ca2+ influx through NMDA receptors can trigger CICR from the ER, including in dendritic spines (23–26). It seems possible, or even likely however, that other pathways that release Ca2+ from the ER may, in other experimental conditions or neuronal cell types, also initiate STIM1 inhibition of L-type channels.
Figure 1.
Activation by glutamate of STIM1 feedback inhibition of L-type CaVs in neurons. (a) Activation of NMDA receptors (NMDAR) by glutamate (plus glycine). NMDAR activity (step 1) depolarizes the plasma membrane, activating CaV1 channels (L channels) and thereby (step 2) generating Ca2+ influx. NMDAR activity also directly provides (step 2) influx of Ca2+. Listed on the right of the panel are approximate concentrations of Ca2+ in the extracellular space, and prior to influx and release of Ca2+, in the ER-PM junctional space and in the ER luminal space. (b) Combined influx of Ca2+ via NMDARs and CaV1 channels (step 2) triggers a large release of Ca2+ from stores (step 3, CICR process), resulting in loss of Ca2+ from its binding site within STIM1’s ER lumen-localized EF hand and thus activation of STIM1. Activated STIM1 translocates along the ER membrane, clusters, binds to CaV1 channels, and (step 4) reduces the probability of CaV1 channel opening in response to subsequent depolarizing stimuli.
Singh and colleagues studied STIM1 regulation of CaV1.3-dependent rhythmic firing in dopaminergic neurons of the substantia nigra (27). In these neurons, stores depletion reduced Ca2+ current and neuronal firing rate, and also activated TRPC1 channels, which are known to be able to function as stores-operated channels (12). In TRPC1 knock-out neurons, firing rate was elevated above wild-type level, and it could not be reduced by stores depletion. In addition, SOCE was largely eliminated and stores depletion could no longer inhibit Ca2+ current. Anti-STIM1 was found to coimmunoprecipitate CaV1.3, and stores depletion increased anti-STIM1 pull-down of CaV1.3.
Using SH-SY5Y neuroblastoma cells, these workers showed that knockdown of TRPC1 strongly attenuated Ca2+ influx following stores depletion, as in dopamine neurons, consistent with the idea that TRPC1 channels function in both of these kinds of cells as a SOC (27). TRPC1 knockdown also increased Ca2+ current amplitude, but in cells in which CaV1.3 was also knocked down, TRPC1 knockdown had no effect on Ca2+ current amplitude, indicating that TRPC1 inhibited CaV1.3 activity. Like TRPC1 knockdown, STIM1 knockdown strongly attenuated TRPC1-mediated Ca2+ influx following stores depletion, and increased Ca2+ current density. Store depletion promoted increased interaction among all three of STIM1, TRPC1 and CaV1.3, as measured in reciprocal coimmunoprecipitation experiments. The results suggest that store depletion drives STIM1 binding and activation ofTRPC1, and TRPC1 additionally facilitates STIM1 interaction with, and inhibition of, CaV1.3. This is reminiscent of the observation by Gill and colleagues that Orai-based CRAC channels facilitated STIM1 interaction with CaV1.2 (3). In the case of TRPC1, however, it is curious that block of Ca2+ flux through TRPC1 channels also blocked depletion-mediated, STIM1-dependent inhibition of CaV1.3, as though Ca2+ itself is involved. In any event, STIM1- and TRPC1-dependent inhibition of CaV1.3 channels is proposed to be neuroprotective, by reducing the firing frequency and thus Ca2+ load in dopaminergic neurons (27).
Roles for STIM1 regulation of CaVs in neuronal physiology
Beyond limiting Ca2+ load and thereby minimizing the risk of cell death in dopaminergic neurons (27), in what other physiological tasks is STIM1 control of CaVs involved? Dittmer and colleagues (22) found that CaV1.2-dependent nuclear translocation of the NFATc3 transcription factor was attenuated by STIM1 inhibition of L-type channels, suggesting that this negative feedback pathway may be involved in control of neuronal gene expression. They also found, in spines stimulated via glutamate uncaging at a frequency that initiates spine enlargement and long-term potentiation of post-synaptic responses (28), that spine ER volume was increased in a STIM1- and L channel-dependent manner. This response was initiated by activation of NMDA receptors, in agreement with previous findings (25).This aligns with findings that STIM1 knockout impairs long-term potentiation (29), and that STIM1 overexpression impairs long-term depression and improves contextual learning (30).
The evidence to date indicates that STIM1 directly regulates (inhibits) only L-type CaVs in neurons, but will that prove true? Ryan and colleagues have reported that STIM1, activated by stores depletion, reduces action potential-driven presynaptic, CaV2-dependent Ca2+ influx and synaptic vesicle exocytosis (31). How activated STIM1 is coupled to CaV2-dependent Ca2+ influx, and hence exocytosis, is unexplored, but direct interaction with CaV2 channels is an attractive hypothesis advanced by these authors. In this proposed mechanism, as CaV2 channels circulate between release zones and other regions of the presynaptic terminal, activated STIM1 could bind and trap those traveling outside of release zones, thereby reducing the number of channels available to occupy release zones, reducing action potential-driven Ca2+ influx, and reducing synaptic vesicle exocytosis.
Future directions
Specifics of the mechanism of interaction of STIM proteins with CaVs remain to be elucidated: How does STIM1 binding to a floppy C-terminal tail inhibit opening of CaVs, and might other binding domains be involved? Are there ER-PM junctions specialized to support STIM1 inhibition of CaVs? Other questions in need of answers include: what kinds of CaVs can be inhibited (directly) by STIM1 other than the known CaV1.2 and CaV1.3 L-type channels? In neurons, how might gene expression controlled by nuclear translocation of the NFAT transcription factors be coordinately, and reciprocally, regulated by STIM1-Orai versus STIM1-CaV1.2? How do CaV1.2 and STIM1 work together to control ER content in spines undergoing synaptic plasticity? By what mechanism(s) might STIM1 regulate presynaptic CaV2 function? The answers to these and other stimulating questions await.
Box 1.
Molecular components that support stores-operated Ca2+ entry (SOCE)
| IP3R1, IP3R2, IP3R3 | inositol trisphosphate receptors, release ER Ca2+ |
| RYR1, RyR2, RyR3 | ryanodine receptors, also release ER Ca2+ |
| STIM1, STIM2 | high, and low, affinity sensors of [Ca2+]ER; activate stores-operated channels (SOCs) |
| Orai1, Orai2, Orai3 | Ca2+-release activated Ca2+ (CRAC) channels, a class of SOCs |
| TRPC1, other TRPCs can function as STIMI -activated SOCs | |
Highlights.
Key in stores-operated Ca2+ entry, STIM1 also controls voltage-gated Ca2+ channels
STIM1, sensing a drop in ER Ca2+, binds and inhibits voltage-gated Ca2+ channels
STIM1 and voltage-gated Ca2+ channels together control dendritic spine ER content
STIM1 tunes voltage-gated Ca2+ channel-dependent transcription factor translocation
STIM1 inhibits transmitter release, perhaps by voltage-gated Ca2+ channel inhibition
Acknowledgements
This work was supported by NIH grant R01MH102338.
Footnotes
Conflict of interest statement
Nothing declared.
Bibliography
• of special interest – References (31)
•• of outstanding interest – References (2, 3, 22, 27)
- 1.Nakai J, et al. (1996) Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 380(6569):72–75. [DOI] [PubMed] [Google Scholar]
- 2. Park CY, Shcheglovitov A, & Dolmetsch R (2010) The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science 330(6000):101–105. ••The authors’ paradigm-changing work describes the discovery that Ca2+ influx via voltage-gated Ca2+ channels is sensitive to [Ca2+]ER, via channel interaction with the ER Ca2+ sensor STIM1.
- 3. Wang Y, et al. (2010) The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330(6000):105–109. ••The authors’ paradigm-changing work describes the discovery that Ca2+ influx via voltage-gated Ca2+ channels is sensitive to [Ca2+]ER, via channel interaction with the ER Ca2+ sensor STIM1.
- 4.Liou J, et al. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol 15(13):1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roos J, et al. (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol 169(3):435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feske S, et al. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441(7090):179–185. [DOI] [PubMed] [Google Scholar]
- 7.Vig M, et al. (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312(5777):1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang SL, et al. (2006) Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. U. S. A 103(24):9357–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruszczynska-Biegala J, Pomorski P, Wisniewska MB, & Kuznicki J (2011) Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons. PLoS One 6(4):e19285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann J, et al. (2014) STIM1 controls neuronal Ca2+ signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior. Neuron 82(3):635–644. [DOI] [PubMed] [Google Scholar]
- 11.Sun S, et al. (2014) Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 82(1):79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakriya M & Lewis RS (2015) Store-operated calcium channels. Physiol. Rev 95(4):1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu MM, Buchanan J, Luik RM, & Lewis RS (2006) Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol 174(6):803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lur G, et al. (2009) Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP3 receptors. Curr. Biol 19(19):1648–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orci L, et al. (2009) STIM1-induced precortical and cortical subdomains of the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A 106(46):19358–19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catterall WA (2011) Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3(8):a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan JP, et al. (2009) SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nature cell biology 11(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spacek J & Harris KM (1997) Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci 17(1):190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holbro N, Grunditz A, & Oertner TG (2009) Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc. Natl. Acad. Sci. U. S. A 106(35):15055–15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlachos A, et al. (2009) Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. J. Neurosci 29(4):1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korkotian E, Frotscher M, & Segal M (2014) Synaptopodin regulates spine plasticity: mediation by calcium stores. J. Neurosci 34(35):11641–11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dittmer PJ, Wild AR, Dell’Acqua ML, & Sather WA (2017) STIM1 Ca2+ sensor control of L-type Ca2+-channel-dependent dendritic spine structural plasticity and nuclear signaling. Cell Rep. 19:321–334. ••This study demonstrates that STIM1 regulation of L-type Ca2+ channels is engaged in dendrites by physiologically-relevant stimuli such as glutamate uncaging, and that this process controls dendritic spine ER content as well as nuclear translocation of the NFATc3 transcription factor.
- 23.Emptage N, Bliss TV, & Fine A (1999) Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron 22(1):115–124. [DOI] [PubMed] [Google Scholar]
- 24.Korkotian E & Segal M (1998) Fast confocal imaging of calcium released from stores in dendritic spines. Eur. J. Neurosci 10(6):2076–2084. [DOI] [PubMed] [Google Scholar]
- 25.Ng AN, Doherty AJ, Lombroso PJ, Emptage NJ, & Collingridge GL (2014) Rapid regulation of endoplasmic reticulum dynamics in dendritic spines by NMDA receptor activation. Mol. Brain 7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose CR & Konnerth A (2001) Stores not just for storage: intracellular calcium release and synaptic plasticity. Neuron 31(4):519–522. [DOI] [PubMed] [Google Scholar]
- 27. Sun Y, et al. (2017) Inhibition of L-type Ca2+ channels by TRPC1-STIM1 complex is essential for the protection of dopaminergic neurons. J. Neurosci 37(12):3364–3377. ••Dopaminergic neurons of the substantia nigra rely in part upon CaV1.3 L-type Ca2+ channels for their rhythmic firing, but the resulting Ca2+ influx is thought to contribute to death of these neurons in Parkinson’s disease. The authors show that the stores-operated channel TRPC1 acts as a scaffold for STIM1 to interact with and inhibit the activity of CaV1.3 L-type Ca2+ channels, thereby serving a neuroprotective role.
- 28.Matsuzaki M, Honkura N, Ellis-Davies GC, & Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429(6993):761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Alvarez G, et al. (2015) Impaired spatial memory and enhanced long-term potentiation in mice with forebrain-specific ablation of the Stim genes. Front. Behav. Neurosci 9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majewski L, et al. (2017) Overexpression of STIM1 in neurons in mouse brain improves contextual learning and impairs long-term depression. Biochim. Biophys. Acta 1864(6):1071–1087. [DOI] [PubMed] [Google Scholar]
- 31. de Juan-Sanz J, et al. (2017) Axonal endoplasmic reticulum Ca2+ content controls release probability in CNS nerve terminals. Neuron 93(4):867–881 e866. •These authors report that STIM1, activated in presynaptic terminals by depletion of ER Ca2+ stores, inhibits action potential-triggered influx of presynaptic Ca2+ and thus reduces exocytosis of neurotransmitter. Though the mechanism of inhibition is currently unknown, the non-L-type CaV2 channels that support transmitter release are an intriguing target.