Abstract
This retrospective cohort study investigated the effects of statin use on 5-year clinical outcomes, particularly all-cause mortality, in patients with breast cancer. Clinical data of 971,808 patients who received a diagnosis of breast cancer between 2010 and 2020 were collected from the TriNetX platform. Eligible patients were classified as statin users (98,761) or nonusers (691,644). Statin use was defined by a prescription of statins being given within 3 years after breast cancer diagnosis. All-cause mortality and cardiovascular incidence were evaluated from Aalen–Johansen cumulative incidence curves. After 1:1 propensity score matching, all-cause mortality outcomes were analyzed in terms of hazard ratios and risk ratios. Our studies revealed that the risk of all-cause mortality was lower in statin users than in nonusers (hazard ratio: 0.798; risk ratio: 0.721; p < 0.001). Subgroup analysis revealed that the protective effect of statins against all-cause mortality was more pronounced in older patients; those with a higher body mass index; and those with higher cholesterol, triglyceride, or low-density lipoprotein levels. The effects were prominent also in patients with estrogen receptor-negative or progesterone receptor-negative tumors. Statin use was associated with improved survival in patients with breast cancer, particularly older patients, those with hormone receptor-negative tumors, and those with metabolic dysregulation. Our findings indicate a possible link between statin use and reduced mortality in breast cancer patients, warranting further investigation in prospective controlled studies.
Keywords: all-cause mortality, breast cancer, survival, statin
1. Introduction
Breast cancer is one of the most common malignancies worldwide; its progression is influenced by genetic, metabolic, and environmental factors [1,2]. It is a heterogeneous disease classified into various molecular subtypes, such as luminal A breast cancer, luminal B breast cancer, HER2-enriched breast cancer, and triple-negative breast cancer (TNBC) [3]. These subtypes differ in their biological behaviors and therapeutic responses [4]. Post-treatment disease progression varies markedly across the subtypes [5]. The most aggressive subtype of breast cancer is TNBC, characterized by the lack of estrogen receptor (ER), progesterone receptor (PR), and HER2 expression. TNBC carries a high risk of early progression and has limited options for targeted therapy, resulting in the worst prognosis [6]. Therefore, developing new therapeutic strategies for different breast cancer subtypes would benefit patients.
Statins, widely known for their cholesterol-lowering effects, have recently gained attention in breast cancer research for their potential to regulate key oncogenic pathways [7,8,9]. These drugs are generally classified into two types on the basis of solubility: lipophilic statins (e.g., simvastatin, fluvastatin, atorvastatin, and pitavastatin) and hydrophilic statins (e.g., pravastatin and rosuvastatin) [10]. Statins exert their anticancer effects primarily by inhibiting 3-hydroxy-3-methylglutaryl-CoA reductase, thereby disrupting cholesterol biosynthesis and altering key cellular processes such as proliferation, apoptosis, and metastasis [11,12,13,14]. Notably, the overexpression of 3-hydroxy-3-methylglutaryl-CoA reductase is strongly associated with increased tumor aggression and poor prognosis [15]. Statins suppress tumor progression mainly by inhibiting cell survival-related signaling cascades, such as the phosphoinositide 3-kinase/protein kinase B (AKT) and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways, thus inducing cell cycle arrest and reducing tumor cell proliferation [10,16,17,18]. In addition, statins suppress the prenylation of RHO and RAC proteins, regulating cytoskeletal reorganization, cell motility, and adhesion and thereby reducing metastatic potential [13,19]. Emerging research suggests that statins modulate the tumor microenvironment by alleviating inflammation and enhancing immune surveillance, thus improving cancer outcomes [20,21]. In summary, statins potentially improve breast cancer outcomes by modulating cancer cell growth, metastasis, and the tumor microenvironment.
The effects of statins on breast cancer progression remain inconclusive and depend on the breast cancer subtype. In luminal A and luminal B subtypes, which rely on ER signaling, statins may affect ER activity by altering the composition of lipid rafts [22,23,24]. Previous studies revealed that the use of statins to inhibit cholesterol biosynthesis enhanced the efficacy of AKT inhibitors in breast cancer [16,25,26]. However, a study demonstrated that neither ER-positive breast cancer cells nor ER-positive organoids are sensitive to the combination of AKT inhibitors and pitavastatin [16]. In HER2-enriched breast cancer, statins may disrupt lipid raft structures that support the localization of HER2 receptor, inhibiting HER2-related signaling, potentiating HER2-targeted therapy (e.g., trastuzumab), and delaying resistance development [22]. Guo et al. reported that the effects of statins were relatively pronounced in hormone receptor-positive/HER2-negative breast cancer [27]. Collectively, these findings indicate that the efficacy of statins in treating breast cancer remains questionable, necessitating further research. Although epidemiological and preclinical data suggest that statins exert anticancer effects, clinical trial results remain inconclusive [28]. Further validation through real-world data is necessary, particularly to clarify the efficacy of statins in treating various breast cancer subtypes and the therapeutic benefits of these drugs for high-risk populations, such as patients with obesity or metabolic syndrome. To address these research gaps, the present retrospective study investigated the effect of statins on breast cancer progression and explored the potential applications of these drugs in personalized therapy.
2. Methods
2.1. Data Source
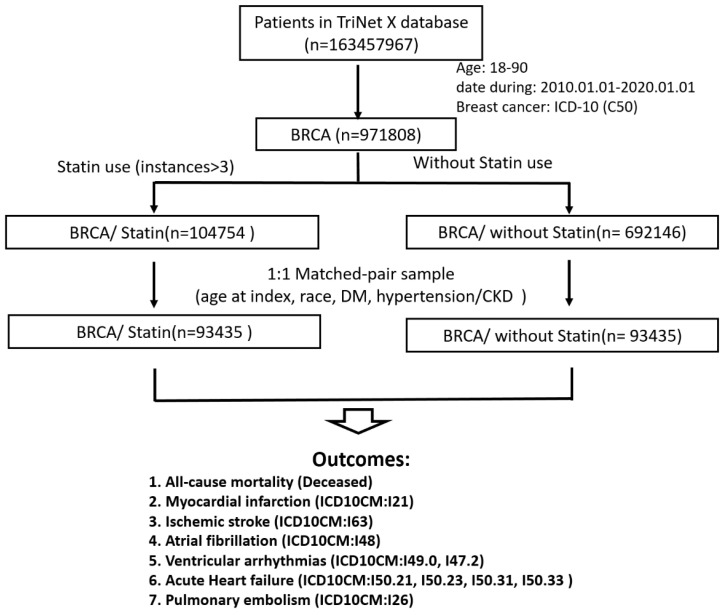
The study protocol was approved by the institutional review board of our hospital. Patient data were extracted from the TriNetX Global Collaborative Network, which contains the data of 163,457,967 patients across 140 health-care centers worldwide. TriNetX analyses anonymized patient data in compliance with the Health Insurance Portability and Accountability Act and were therefore exempt from institutional Human Research Ethics review processes. This study included 971,808 patients (age: 18–90 years) who received a diagnosis of breast cancer (International Classification of Diseases, Tenth Revision, Clinical Modification code: C50) between 1 January 2010 and 1 January 2020. They were stratified into statin users and nonusers.
2.2. Data Analysis
Statin use was defined by the presence of a prescription of any statin (atorvastatin, rosuvastatin, simvastatin, pravastatin, lovastatin, pitavastatin, or fluvastatin) being given within 3 years after the first recorded diagnosis of breast cancer. Patients were only included in the statin use group if they had at least three prescriptions of a statin. Patients in the statin nonusers group had never been prescribed any of the above statins.
To minimize confounding effects, 1:1 propensity score matching was performed, with adjustments for age at initial breast cancer diagnosis, sex, race, diabetes mellitus (DM), kidney disease, and hypertension. Thus, two well-matched cohorts of 93,435 patients each were formed. The following 5-year clinical outcomes were assessed: all-cause mortality and cardiovascular events (International Classification of Diseases, Tenth Revision, Clinical Modification codes: I21, I26, I47.2, I48, I49.0, I50.21, I50.23, I50.31, I50.33, and I63). Patients having any of these outcomes before receiving a breast cancer diagnosis were excluded from the risk analysis for that specific outcome. In the Kaplan–Meier survival analysis, a time window was applied to include patients who made ≥1 outpatient visit after 5 years from the first diagnosis of breast cancer. This ensured that data from both groups were not censored due to having their last clinical fact recorded during the outcome period. Data collection was completed on 7 March 2025.
2.3. Statistical Analysis
All statistical analyses were conducted using the analytical tools available within the TriNetX platform. Aalen–Johansen cumulative incidence curves were used to assess all-cause mortality and the incidence of cardiovascular events. Kaplan–Meier survival analysis was applied to estimate survival probabilities over a 5-year follow-up period, while Cox proportional hazards regression was used to calculate hazard ratios (HRs) for clinical outcomes between groups during this timeframe. The Risk Ratio (RR) compares the probability of all-cause mortality in statin users to that in nonusers, indicating how much the risk changes with statin use. The Odds Ratio (OR) compares the odds of all-cause mortality between statin users and nonusers, and is commonly applied in case–control studies and logistic regression models.
3. Results
3.1. Clinical Characteristics of Patients Stratified by Statin Use
From the TriNetX data, we identified 971,808 patients (age: 18–90 years) who received a breast cancer diagnosis during the study period. The statin user and nonuser groups had 104,754 and 692,146 patients, respectively. Among statin users, the cumulative incidence of cardiovascular events was significantly higher than the rate of mortality (37.446% vs. 5.083%; Figure 1). Among nonusers, the incidence of cardiovascular events was similar to the rate of mortality (9.768% vs. 9.805%). These results highlight a difference in outcomes between the two groups. Compared with nonusers, statin users tended to be older at the index date (67.8 ± 10.6 vs. 58.6 ± 14.1 years) and have a higher body mass index (30.3 ± 7.2 vs. 27.7 ± 6.8 kg/m2). The following comorbidities were more prevalent among statin users than among nonusers (Table 1): DM (22.3% vs. 3.1%), kidney disease (7.3% vs. 1.5%), and hypertension (46.6% vs. 10.5%). Furthermore, blood levels of cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein were significantly lower in statin users than in nonusers, whereas those of triglycerides were significantly higher in statin users than in nonusers (Table 1).
Figure 1.
Cumulative incidence of all-cause mortality and cardiovascular events in patients with breast cancer. The figures depict 5-year cumulative incidence in (A) statin users and (B) nonusers.
Table 1.
Patients’ baseline characteristics before and after propensity score matching.
| Characteristic Name | Before Propensity Score Matching | After Propensity Score Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients with Statins (n = 104,754) | Patients without Statins (n = 692,146) | p Value | Std diff | Patients with Statins (n = 93,435) | Patients without Statins (n = 93,435) | p Value | Std diff | |
| Basic demographics (%) | ||||||||
| Age at Index, mean (mean+/−SD) | 67.8 +/− 10.6 | 58.6 +/− 14.1 | <0.001 | 0.74 | 67.6 +/− 10.7 | 68.0 +/− 10.9 | <0.001 | 0.038 |
| White (%) | 70.80% | 53.60% | <0.001 | 0.36 | 71.20% | 71.60% | 0.045 | 0.009 |
| Black or African American (%) | 14.80% | 7.00% | <0.001 | 0.25 | 13.70% | 13.10% | <0.001 | 0.019 |
| Asian (%) | 3.90% | 5.00% | <0.001 | 0.05 | 3.90% | 3.90% | 0.559 | 0.003 |
| Laboratory, Mean ± SD | ||||||||
| Glucose [Mass/volume] in Serum, Plasma, or Blood | 118.1 +/− 47.1 | 105.4 +/− 34.3 | <0.001 | 0.31 | 116.0 +/− 45.3 | 113.3 +/− 43.4 | <0.001 | 0.06 |
| Alanine aminotransferase [Enzymatic activity/volume] in Serum, Plasma, or Blood | 23.8 +/− 27.2 | 26.8 +/− 57.4 | <0.001 | 0.07 | 23.9 +/− 26.7 | 27.3 +/− 63.1 | <0.001 | 0.071 |
| Aspartate aminotransferase [Enzymatic activity/volume] in Serum or Plasma | 25.3 +/− 32.3 | 31.6 +/− 94.5 | <0.001 | 0.09 | 25.3 +/− 30.8 | 33.3 +/− 95.8 | <0.001 | 0.112 |
| Alkaline phosphatase [Enzymatic activity/volume] in Serum, Plasma, or Blood | 84.3 +/− 47.7 | 94.2 +/− 97.8 | <0.001 | 0.13 | 84.1 +/− 48.1 | 97.5 +/− 92.6 | <0.001 | 0.182 |
| Lactate dehydrogenase [Enzymatic activity/volume] in Serum or Plasma | 248.1 +/− 243.6 | 270.7 +/− 360.5 | <0.001 | 0.074 | 247.6 +/− 247.8 | 292.2 +/− 380.9 | <0.001 | 0.139 |
| Bilirubin.Total [Mass/volume] in Serum, Plasma, or Blood | 0.5 +/− 0.7 | 0.6 +/− 1.1 | <0.001 | 0.084 | 0.5 +/− 0.6 | 0.6 +/− 1.2 | <0.001 | 0.096 |
| Albumin [Mass/volume] in Serum, Plasma, or Blood | 4.0 +/− 0.5 | 4.0 +/− 0.6 | <0.001 | 0.025 | 4.0 +/− 0.5 | 3.9 +/− 0.6 | <0.001 | 0.278 |
| Cholesterol [Mass/volume] in Serum or Plasma | 183.5 +/− 47.2 | 196.3 +/− 41.3 | <0.001 | 0.287 | 185.1 +/− 47.0 | 192.6 +/− 43.7 | <0.001 | 0.166 |
| Cholesterol in LDL [Mass/volume] in Serum or Plasma | 101.3 +/− 39.3 | 111.7 +/− 33.3 | <0.001 | 0.287 | 102.5 +/− 39.3 | 109.6 +/− 34.7 | <0.001 | 0.192 |
| Cholesterol in HDL [Mass/volume] in Serum or Plasma | 54.1 +/− 18.7 | 60.0 +/− 21.1 | <0.001 | 0.296 | 54.6 +/− 18.8 | 57.6 +/− 21.7 | <0.001 | 0.15 |
| Triglyceride [Mass/volume] in Serum, Plasma, or Blood | 138.4 +/− 83.0 | 114.6 +/− 70.5 | <0.001 | 0.308 | 137.8 +/− 83.1 | 122.9 +/− 74.3 | <0.001 | 0.189 |
| Hemoglobin A1c/Hemoglobin.total in Blood | 6.8 +/− 1.5 | 6.1 +/− 1.3 | <0.001 | 0.494 | 6.7 +/− 1.5 | 6.4 +/− 1.5 | <0.001 | 0.194 |
| BMI | 30.3 +/− 7.2 | 27.7 +/− 6.8 | <0.001 | 0.364 | 30.1 +/− 7.1 | 28.8 +/− 7.2 | <0.001 | 0.175 |
| Diagnosis (%) | ||||||||
| Diabetes Mellitus | 22.30% | 3.10% | <0.001 | 0.602 | 17.60% | 16.60% | <0.001 | 0.027 |
| Acute Kidney Failure and Chronic Kidney Disease | 7.30% | 1.50% | <0.001 | 0.288 | 6.40% | 6.30% | 0.276 | 0.005 |
| Hypertensive Diseases | 46.60% | 10.50% | <0.001 | 0.871 | 43.40% | 43.60% | 0.253 | 0.005 |
3.2. Effects of Statin Use on Survival
After 1:1 propensity score matching for age, race, DM, hypertension, and kidney disease, each of the two matched groups comprised 93,435 patients (Figure 2). The following clinicodemographic characteristics were well balanced between statin users and nonusers after propensity score matching (Table 1): age on the index date (67.6 ± 10.7 vs. 68.0 ± 10.9 years), race, DM prevalence (17.6% vs. 16.6%), kidney disease prevalence (6.4% vs. 6.3%), and hypertension prevalence (43.4% vs. 43.6%).
Figure 2.
Flowchart depicting the study pipeline. A cohort of patients with breast cancer was selected from the TriNetX platform. The patients were stratified by statin use into two groups (statin users and nonusers) and subjected to 1:1 propensity score matching.
Statin use was significantly associated with a reduced risk of all-cause mortality in patients with breast cancer (risk ratio [RR]: 0.721; 95% confidence interval [CI]: 0.705–0.738; p < 0.001; Table 2). Moreover, Kaplan–Meier survival analysis revealed that statin use was associated with a relatively long survival (HR: 0.798; 95% CI: 0.729–0.873; p < 0.001; Figure 3). These findings suggest that statins confer their protective effects by reducing the risk of all-cause mortality. However, statin use was significantly associated with elevated risks of cardiovascular events such as myocardial infarction (RR: 4.390; 95% CI: 4.105–4.694), ischemic stroke (RR: 4.250; 95% CI: 4.009–4.505), atrial fibrillation (RR: 2.272; 95% CI: 2.190–2.358), ventricular arrhythmias (RR: 2.644; 95% CI: 2.560–2.731), acute heart failure (RR: 3.502; 95% CI: 3.305–3.711), and pulmonary embolism (RR: 1.864; 95% CI: 1.757–1.978).
Table 2.
Associations of statin use with various outcomes in patients with breast cancer.
| Outcomes | Groups | Cases Followed |
Incident Cases | Risk (%) | Risk Ratio | Odds Ratio | Risk Difference (p Value) |
|---|---|---|---|---|---|---|---|
| All-cause Mortality | with statins | 93,236 | 10,412 | 11.2 | 0.721 (0.705, 0.739) | 0.686 (0.668, 0.705) | <0.001 |
| without statins | 92,578 | 14,332 | 15.5 | ||||
| Myocardial Infarction | with statins | 90,661 | 4454 | 4.9 | 4.390 (4.105, 4.694) | 4.565 (4.264, 4.887) | <0.001 |
| without statins | 92,569 | 1036 | 1.1 | ||||
| Ischemic Stroke | with statins | 89,437 | 5653 | 6.3 | 4.250 (4.009, 4.505) | 4.469 (4.210, 4.744) | <0.001 |
| without statins | 92,048 | 1369 | 1.5 | ||||
| Atrial Fibrillation | with statins | 86,617 | 8473 | 9.8 | 2.272 (2.190, 2.358) | 2.410 (2.317, 2.507) | <0.001 |
| without statins | 88,226 | 3798 | 4.3 | ||||
| Ventricular Arrhythmias | with statins | 85,840 | 11,919 | 13.9 | 2.644 (2.560, 2.731) | 2.909 (2.808, 3.013) | <0.001 |
| without statins | 89,702 | 4711 | 5.3 | ||||
| Acute Heart Failure | with statins | 91,903 | 4999 | 5.4 | 3.502 (3.305, 3.711) | 3.646 (3.436, 3.869) | <0.001 |
| without statins | 92,775 | 1441 | 1.6 | ||||
| Pulmonary Embolism | with statins | 92,007 | 3043 | 3.3 | 1.864 (1.757, 1.978) | 1.894 (1.782, 2.013) | <0.001 |
| without statins | 92,281 | 1637 | 1.8 |
Figure 3.
Kaplan–Meier curves depicting 5-year all-cause mortality rates in patients with breast cancer (BRCA) stratified by statin use.
3.3. Subgroup Analysis of All-Cause Mortality
A detailed subgroup analysis of 5-year all-cause mortality in patients with breast cancer revealed that statins significantly reduced the risk of all-cause mortality across all subgroups (Figure 4). However, statins exerted no significant effect on this risk in younger patients (odds ratio [OR]: 0.937; 95% CI: 0.757–1.160; p = 0.549). The protective effects of these drugs were more pronounced in older patients (OR: 0.699; 95% CI: 0.680–0.719; p < 0.001) and those with a higher body mass index (>30 kg/m2; (OR: 0.720; 95% CI: 0.681–0.762; p < 0.001). The statin-induced reduction in the risk of all-cause mortality was larger in patients with ER-negative breast cancer (OR: 0.696; 95% CI: 0.634–0.764; p < 0.001) and patients with PR-negative breast cancer (OR: 0.771; 95% CI: 0.664–0.895; p < 0.001) than in their counterparts with the corresponding conditions. Furthermore, the protective benefits of statins were greater in patients with higher blood levels of cholesterol (>220 mg/dL; OR: 0.643; 95% CI: 0.571–0.724; p < 0.001), triglycerides (>200 mg/dL; OR: 0.540; 95% CI: 0.479–0.680; p < 0.001), and LDL (>160 mg/dL; OR: 0.451; 95% CI: 0.393–0.518; p < 0.001). These findings highlight the efficacy of statin use in improving survival in patients with breast cancer, particularly older patients and those with high triglyceride, cholesterol, or LDL levels.
Figure 4.
Subgroup analysis of 5-year mortality rate in patients with breast cancer stratified by statin use.
4. Discussion
Our findings revealed that statin use in patients with breast cancer was associated with a significant reduction in the risk of all-cause mortality (HR: 0.798; 95% CI: 0.729–0.873; p < 0.001). This finding aligns with those of epidemiological studies highlighting an association between statin use and improved survival in patients with breast cancer [29,30,31]. Statins exert antitumor effects by inhibiting the mevalonate pathway, thereby disrupting key oncogenic signaling cascades such as the phosphoinositide 3-kinase/AKT and RAS/mitogen-activated protein kinase pathways, ultimately suppressing tumor proliferation and metastasis [13,17,18,19,32]. In addition to suppressing oncogenic pathways, statins may decelerate cancer progression by altering the tumor microenvironment. Li et al. demonstrated that lovastatin markedly downregulated the paclitaxel-induced expression of programmed death-ligand 1 and increased the activity of CD8+ T cells, thus supporting paclitaxel-mediated inhibition of tumor growth [33]. Previous review papers indicated that statins may improve breast cancer outcomes by promoting tumor cell apoptosis and reducing metastasis [34,35,36]. However, their long-term effects and optimal use remain uncertain. Consequently, the efficacy of statins may vary depending on the patient’s physical condition, breast cancer subtype, the specific statin used, and the timing of administration [34].
Guo et al. reported that statins significantly reduced the risk of cancer-specific mortality, particularly in patients with hormone receptor-positive and HER2-negative breast cancer [27]. Similarly, Murto et al. indicated that statins reduced the risk of mortality in patients with ER-positive breast cancer [37]. Corroborating these findings, Scott et al. reported a significant association between statin use and reduced mortality risk in patients with breast cancer, particularly those with ER-positive tumors, postmenopausal women, and those with advanced-stage disease [38]. Statins may disrupt ER signaling by inhibiting the mevalonate pathway, thereby enhancing the efficacy of endocrine therapy in hormone receptor-positive breast cancer [22,39]. However, the present study revealed that both patients with ER-negative or PR-negative tumors benefited more from statin use than did their counterparts, implying that statins exert direct antitumor effects independent of hormone signaling. A study reported that inhibiting cholesterol biosynthesis with statins enhanced the efficacy of AKT inhibitors in treating TNBC xenografts and patient-derived ER-negative breast cancer organoids [16]. However, ER-positive breast cancer cells and organoids did not respond to the combination of an AKT inhibitor and pitavastatin. Statins suppress the HER2-induced AKT and extracellular signal-regulated kinase pathway by altering the localization of RAC1, thereby improving prognosis in patients with HER2-positive breast cancer [40]. We found that patients with HER2-positive tumors benefited more from statin use than did those with HER2-negative tumors.
Our results indicated that statin users were older and had a higher BMI compared to nonusers, which likely reflects a correlative observation rather than a causal relationship. It is likely that this pattern arises due to confounding by indication, as individuals who are older or have higher BMI are at greater risk for cardiovascular diseases and are thus more likely to be prescribed statins. Therefore, the difference in age and BMI between statin users and nonusers probably reflects underlying clinical risk profiles rather than an effect of statin use itself. In addition, the cumulative incidence of cardiovascular events was higher than the rate of mortality among statin users. However, among nonusers, the cumulative incidence of cardiovascular events was similar to the rate of mortality. Therefore, patients with breast cancer who were prescribed statin therapy might have had an elevated cardiovascular risk at baseline, likely attributable to comorbidities such as hypertension, DM, and obesity, which were more prevalent among statin users than among nonusers. Evidence suggests that the presence of metabolic disorders often confounds the benefits of statin use, making it challenging to identify the direct effects of statins on breast cancer outcomes [41]. Although statin use is associated with a reduced risk of all-cause mortality, it is also associated with elevated risks of cardiovascular events, such as myocardial infarction, ischemic stroke, and acute heart failure. This association may be attributable to the higher baseline cardiovascular risk in statin users than in nonusers. Thus, clinicians must carefully assess cardiovascular risk in patients with breast cancer receiving statin therapy.
This study yielded several key findings regarding statin use in patients with breast cancer. Unlike other studies suggesting broad protective effects across all age groups, our study revealed no significant survival benefits of statins in younger patients (OR: 0.937; 95% CI: 0.757–1.160; p = 0.549). The protective effects of statins were relatively pronounced in patients with a high risk of metabolic syndrome, particularly those with high blood levels of cholesterol (>220 mg/dL), triglycerides (>200 mg/dL), and LDL (>160 mg/dL). These findings suggest that statins are particularly beneficial for older patients and those with severe lipid metabolism disorders, supporting the notion that cholesterol biosynthesis plays a key role in the progression of breast cancer.
Our study has several limitations. First, statin users had a higher baseline cardiovascular risk than did nonusers, making it challenging for us to determine whether the observed reduction in the risk of all-cause mortality was due to statin use itself or indirect factors such as improved overall medical care. Second, we did not differentiate between lipophilic statins and hydrophilic statins. Although we did not perform subgroup analyses based on statin lipophilicity, prior studies have suggested that lipophilic statins may exert stronger anticancer effects than hydrophilic statins due to their enhanced ability to penetrate extrahepatic tissues [25,42,43,44]. Third, precise data on the dose and duration of statin use were unavailable. Finally, the possibility of a selection bias cannot be ignored, given that the prevalence of metabolic comorbidities and the likelihood of receiving long-term medical care were relatively high among statin users; this might have led to the early detection of cancer progression in statin users and enabled them to have access to advanced treatment options, partially explaining the observed survival benefits. In addition, a review paper reported that statins reduce breast cancer recurrence rates when administered after diagnosis [34]. Although recurrence-related survival outcomes could offer further insights, the lack of recurrence-specific data within the TriNetX platform limits our ability to conduct such analyses. Future studies with access to detailed recurrence and progression data are warranted.
5. Conclusions
This study adds to the growing body of evidence suggesting that statins offer survival benefits for patients with breast cancer, particularly older patients and those with metabolic dysregulation. However, the increased cardiovascular risk in statin users highlights the need for personalized therapeutic strategies that balance the benefits of improved survival with the management of cardiovascular risk.
Author Contributions
C.-H.L. and J.W. were responsible for project administration, methodology, and manuscript preparation. J.W. contributed to manuscript editing and TriNetX data analysis. K.-W.T., C.-F.C., and K.-C.L. supervised the study and were involved in manuscript preparation and revision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol was approved by the Institutional Review Board of Taipei Tzu Chi Hospital (approval number: 14-IRB015) on 18 February 2025.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taiwan (grant numbers: TCRD-TPE-111-36 and TCMF-CM3-112-03).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Karthikayan A., Sureshkumar S., Kadambari D., Vijayakumar C. Low serum 25-hydroxy vitamin D levels are associated with aggressive breast cancer variants and poor prognostic factors in patients with breast carcinoma. Arch. Endocrinol. Metab. 2018;62:452–459. doi: 10.20945/2359-3997000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sotiriou C., Pusztai L. Gene-expression signatures in breast cancer. N. Engl. J. Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perou C.M., Sorlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Sorlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 7.Murtola T.J., Visvanathan K., Artama M., Vainio H., Pukkala E. Statin use and breast cancer survival: A nationwide cohort study from Finland. PLoS ONE. 2014;9:e110231. doi: 10.1371/journal.pone.0110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckwitt C.H., Clark A.M., Ma B., Whaley D., Oltvai Z.N., Wells A. Statins attenuate outgrowth of breast cancer metastases. Br. J. Cancer. 2018;119:1094–1105. doi: 10.1038/s41416-018-0267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjarnadottir O., Romero Q., Bendahl P.O., Jirstrom K., Ryden L., Loman N., Uhlen M., Johannesson H., Rose C., Grabau D., et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res. Treat. 2013;138:499–508. doi: 10.1007/s10549-013-2473-6. [DOI] [PubMed] [Google Scholar]
- 10.Hosseini F.S., Ahmadi A., Kesharwani P., Hosseini H., Sahebkar A. Regulatory effects of statins on Akt signaling for prevention of cancers. Cell Signal. 2024;120:111213. doi: 10.1016/j.cellsig.2024.111213. [DOI] [PubMed] [Google Scholar]
- 11.Campbell M.J., Esserman L.J., Zhou Y., Shoemaker M., Lobo M., Borman E., Baehner F., Kumar A.S., Adduci K., Marx C., et al. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–8714. doi: 10.1158/0008-5472.CAN-05-4061. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh-Choudhury N., Mandal C.C., Ghosh-Choudhury N., Ghosh Choudhury G. Simvastatin induces derepression of PTEN expression via NFkappaB to inhibit breast cancer cell growth. Cell Signal. 2010;22:749–758. doi: 10.1016/j.cellsig.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gopalan A., Yu W., Sanders B.G., Kline K. Simvastatin inhibition of mevalonate pathway induces apoptosis in human breast cancer cells via activation of JNK/CHOP/DR5 signaling pathway. Cancer Lett. 2013;329:9–16. doi: 10.1016/j.canlet.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Denoyelle C., Vasse M., Korner M., Mishal Z., Ganne F., Vannier J.P., Soria J., Soria C. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: An in vitro study. Carcinogenesis. 2001;22:1139–1148. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 15.Schointuch M.N., Gilliam T.P., Stine J.E., Han X., Zhou C., Gehrig P.A., Kim K., Bae-Jump V.L. Simvastatin, an HMG-CoA reductase inhibitor, exhibits anti-metastatic and anti-tumorigenic effects in endometrial cancer. Gynecol. Oncol. 2014;134:346–355. doi: 10.1016/j.ygyno.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillis A.L., Martin T.D., Manchester H.E., Hogstrom J., Zhang N., Lecky E., Kozlova N., Lee J., Persky N.S., Root D.E., et al. Targeting Cholesterol Biosynthesis with Statins Synergizes with AKT Inhibitors in Triple-Negative Breast Cancer. Cancer Res. 2024;84:3250–3266. doi: 10.1158/0008-5472.CAN-24-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Shafei N.H., Zaafan M.A., Kandil E.A., Sayed R.H. Simvastatin ameliorates testosterone-induced prostatic hyperplasia in rats via modulating IGF-1/PI3K/AKT/FOXO signaling. Eur. J. Pharmacol. 2023;950:175762. doi: 10.1016/j.ejphar.2023.175762. [DOI] [PubMed] [Google Scholar]
- 18.Hu T., Shen H., Huang H., Yang Z., Zhou Y., Zhao G. Cholesterol-lowering drug pitavastatin targets lung cancer and angiogenesis via suppressing prenylation-dependent Ras/Raf/MEK and PI3K/Akt/mTOR signaling. Anti-Cancer Drugs. 2020;31:377–384. doi: 10.1097/CAD.0000000000000885. [DOI] [PubMed] [Google Scholar]
- 19.Afshordel S., Kern B., Clasohm J., Konig H., Priester M., Weissenberger J., Kogel D., Eckert G.P. Lovastatin and perillyl alcohol inhibit glioma cell invasion, migration, and proliferation--impact of Ras-/Rho-prenylation. Pharmacol. Res. 2015;91:69–77. doi: 10.1016/j.phrs.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Mao W., Cai Y., Chen D., Jiang G., Xu Y., Chen R., Wang F., Wang X., Zheng M., Zhao X., et al. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint blockade in non-small cell lung cancer. JCI Insight. 2022;7:e161940. doi: 10.1172/jci.insight.161940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W., Hu J.W., He X.R., Jin W.L., He X.Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021;40:241. doi: 10.1186/s13046-021-02041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyder T., Marti J.L.G., Nasrazadani A., Brufsky A.M. Statins and endocrine resistance in breast cancer. Cancer Drug Resist. 2021;4:356–364. doi: 10.20517/cdr.2020.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centonze G., Natalini D., Piccolantonio A., Salemme V., Morellato A., Arina P., Riganti C., Defilippi P. Cholesterol and Its Derivatives: Multifaceted Players in Breast Cancer Progression. Front. Oncol. 2022;12:906670. doi: 10.3389/fonc.2022.906670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centonze G., Natalini D., Grasso S., Morellato A., Salemme V., Piccolantonio A., D’Attanasio G., Savino A., Bianciotto O.T., Fragomeni M., et al. p140Cap modulates the mevalonate pathway decreasing cell migration and enhancing drug sensitivity in breast cancer cells. Cell Death Dis. 2023;14:849. doi: 10.1038/s41419-023-06357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckwitt C.H., Shiraha K., Wells A. Lipophilic statins limit cancer cell growth and survival, via involvement of Akt signaling. PLoS ONE. 2018;13:e0197422. doi: 10.1371/journal.pone.0197422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park Y.H., Jung H.H., Ahn J.S., Im Y.H. Statin induces inhibition of triple negative breast cancer (TNBC) cells via PI3K pathway. Biochem. Biophys. Res. Commun. 2013;439:275–279. doi: 10.1016/j.bbrc.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 27.Guo H., Malone K.E., Heckbert S.R., Li C.I. Statin use and risks of breast cancer recurrence and mortality. Cancer. 2024;130:3106–3114. doi: 10.1002/cncr.35362. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin D.J., Haslam A., Prasad V. Cardiovascular/anti-inflammatory drugs repurposed for treating or preventing cancer: A systematic review and meta-analysis of randomized trials. Cancer Med. 2024;13:e7049. doi: 10.1002/cam4.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giorello M.B., Marks M.P., Osinalde T.M., Padin M.D.R., Wernicke A., Calvo J.C., Chasseing N.A., Vellon L. Post-surgery statin use contributes to favorable outcomes in patients with early breast cancer. Cancer Epidemiol. 2024;90:102573. doi: 10.1016/j.canep.2024.102573. [DOI] [PubMed] [Google Scholar]
- 30.McKechnie T., Brown Z., Lovrics O., Yang S., Kazi T., Eskicioglu C., Parvez E. Concurrent Use of Statins in Patients Undergoing Curative Intent Treatment for Triple Negative Breast Cancer: A Systematic Review and Meta-Analysis. Clin. Breast Cancer. 2024;24:e103–e115. doi: 10.1016/j.clbc.2023.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Jia X., Lu Y., Xu Z., Mu Q. Impact of statin use on breast cancer recurrence and mortality before and after diagnosis: A systematic review and meta-analysis. Front. Oncol. 2023;13:1256747. doi: 10.3389/fonc.2023.1256747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamal A., Boerner J., Assad H., Chen W., Simon M.S. The Effect of Statins on Markers of Breast Cancer Proliferation and Apoptosis in Women with In Situ or Early-Stage Invasive Breast Cancer. Int. J. Mol. Sci. 2024;25:9587. doi: 10.3390/ijms25179587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L., Wang H., Zhang S., Gao S., Lu X., Pan Y., Tang W., Huang R., Qiao K., Ning S. Statins inhibit paclitaxel-induced PD-L1 expression and increase CD8+ T cytotoxicity for better prognosis in breast cancer. Int. J. Surg. 2024;110:4716–4726. doi: 10.1097/JS9.0000000000001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Wyhe R.D., Rahal O.M., Woodward W.A. Effect of statins on breast cancer recurrence and mortality: A review. Breast Cancer. 2017;9:559–565. doi: 10.2147/BCTT.S148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atale N., Wells A. Statins as Secondary Preventive Agent to Limit Breast Cancer Metastatic Outgrowth. Int. J. Mol. Sci. 2025;26:1300. doi: 10.3390/ijms26031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marti J.L.G., Wells J.Z., Wells A. Statins as a Secondary Preventive Agent for Metastatic Cancer. J. Thorac. Oncol. 2023;18:e125–e126. doi: 10.1016/j.jtho.2023.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Murto M.O., Simolin N., Arponen O., Siltari A., Artama M., Visvanathan K., Jukkola A., Murtola T.J. Statin Use, Cholesterol Level, and Mortality Among Females With Breast Cancer. JAMA Netw. Open. 2023;6:e2343861. doi: 10.1001/jamanetworkopen.2023.43861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott O.W., TinTin S., Harborg S., Kuper-Hommel M.J.J., Lawrenson R., Elwood J.M. Post-diagnostic statin use and breast cancer-specific mortality: A population-based cohort study. Breast Cancer Res. Treat. 2023;199:195–206. doi: 10.1007/s10549-022-06815-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee K., Noh E., Moon S.J., Joo Y.Y., Kang E.J., Seo J.H., Park I.H. Statin use in patients with hormone receptor-positive metastatic breast cancer treated with everolimus and exemestane. Cancer Med. 2023;12:5461–5470. doi: 10.1002/cam4.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato C., Iizuka-Ohashi M., Honda M., Konishi E., Yokota I., Boku S., Mizuta N., Morita M., Sakaguchi K., Taguchi T., et al. Additional statin treatment enhances the efficacy of HER2 blockade and improves prognosis in Rac1-high/HER2-positive breast cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2024;1870:167458. doi: 10.1016/j.bbadis.2024.167458. [DOI] [PubMed] [Google Scholar]
- 41.Jiralerspong S., Goodwin P.J. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J. Clin. Oncol. 2016;34:4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 42.Manthravadi S., Shrestha A., Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis. Int. J. Cancer. 2016;139:1281–1288. doi: 10.1002/ijc.30185. [DOI] [PubMed] [Google Scholar]
- 43.Liu B., Yi Z., Guan X., Zeng Y.X., Ma F. The relationship between statins and breast cancer prognosis varies by statin type and exposure time: A meta-analysis. Breast Cancer Res. Treat. 2017;164:1–11. doi: 10.1007/s10549-017-4246-0. [DOI] [PubMed] [Google Scholar]
- 44.Koohestanimobarhan S., Salami S., Imeni V., Mohammadi Z., Bayat O. Lipophilic statins antagonistically alter the major epithelial-to-mesenchymal transition signaling pathways in breast cancer stem-like cells via inhibition of the mevalonate pathway. J. Cell Biochem. 2019;120:2515–2531. doi: 10.1002/jcb.27544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained in the article.