Abstract
We analyzed for the first time the expression of chemokines in subpopulations of the murine immune system at the single-cell level. We demonstrate in vitro and in a model of murine listeriosis that macrophage inflammatory protein (MIP)-1α, MIP-1β, regulated on activation normal T cell expressed and secreted (RANTES), and activation-induced, T cell-derived, and chemokine-related cytokine (ATAC)/lymphotactin are cosecreted to a high degree with IFN-γ by activated individual natural killer (NK), CD8+ T, and CD4+ T helper 1 (Th1) cells. Functionally, ATAC and the CC chemokines cooperate with IFN-γ in the up-regulation of CD40, IL-12, and tumor necrosis factor-α, molecules playing a central role in the effector phase of macrophages. Our data indicate that (i) MIP-1α, MIP-1β, RANTES, and ATAC are not only chemoattractants but also coactivators of macrophages, (ii) MIP-1α, MIP-1β, RANTES, and ATAC constitute together with IFN-γ a group of “type 1 cytokines,” and (iii) these cytokines act together as a functional unit that is used by NK cells in the innate phase and then “handed over” to CD8+ T cells in the antigen-specific phase of the immune defense, thus bridging the two components of a Th1 immune reaction.
During the innate and adaptive phases of an immune response, intercellular communication occurs through cell–cell contact and via soluble mediators, among them chemokines. Structurally, chemokines can be grouped into the CX3C, CXC, CC, and C families (1). Some of the chemokines are expressed constitutively and are involved primarily in the organization of lymphoid tissue (“homeostatic chemokines”). “Inflammatory chemokines” such as macrophage inflammatory protein (MIP)-1α (CCL3), MIP-1β (CCL4), and regulated on activation normal T cell expressed and secreted (RANTES, CCL5) typically are induced de novo in response to infection and recruit effector cells to the site of pathogen entry (2). MIP-1α, MIP-1β, and RANTES are secreted by a variety of cells, among them macrophages, activated natural killer (NK) cells, and T cells (3). All three CC chemokines use CCR5 as their common receptor, whereas RANTES and MIP-1α also bind to CCR1 (4). CCR5 is expressed on macrophages, dendritic cells, and activated T helper (Th)1 cells. Through this receptor, the three CC chemokines potently attract monocytes/macrophages, NK cells, and distinct subpopulations of T cells (4–6).
Activation-induced, T cell-derived, and chemokine-related cytokine (ATAC), the only member of the C family of chemokines, was cloned by us (7) and independently as lymphotactin (8) and SCM-1 (9) by others. ATAC, in contrast to most chemokines, has a rather restricted expression pattern, being secreted mainly by activated CD8+ T (7) and NK (ref. 10; unpublished results) cells. The function of ATAC/lymphotactin is less well defined at present, because early reports on T cell chemotaxis in response to lymphotactin have not been substantiated by others (11–13). GPR5 (XCR1; ref. 14), only recently recognized as the ATAC-specific receptor (13), has been identified in T and NK cells by reverse transcription (RT)-PCR (15).
We studied the expression and function of ATAC, MIP-1α, MIP-1β, and RANTES in murine listeriosis. In the early phase of this disease, NK cells abundantly secrete IFN-γ, the primary promoter of the Th1 immune response. IFN-γ increases the capacity of macrophages to present antigen, generate microbicidal levels of oxygen and nitrogen intermediates, and release IL-12 (16, 17). Furthermore, the early IFN-γ release contributes to the differentiation of T cells to Th1/Tc1 cells (18). The key role of IFN-γ in Th1-based immunity is underlined by the observation that mice deficient for IFN-γ or its receptor have a defective resistance to Listeria monocytogenes, Mycobacterium bovis, and vaccinia virus (19–21).
In the present report we show that MIP-1α, MIP-1β, RANTES, and ATAC are not only cosecreted with IFN-γ at the single-cell level in vitro and in vivo but also act synergistically on macrophages as common target cells. Furthermore, our data indicate that both NK and CD8+ T cells use IFN-γ, MIP-1α, MIP-1β, RANTES, and ATAC as a “functional unit” to drive the Th1 response to certain pathogens in vivo.
Materials and Methods
Mice.
C57BL/6 and BALB/c mice were bred and maintained under specific pathogen-free conditions and used at 8–12 weeks of age. Mice transgenic for the ovalbumin (OVA)323–339 peptide-specific DO11.10 α/β T cell antigen receptor (22), a gift from D. Loh (Washington University, St. Louis), were maintained on the BALB/c background.
Antibodies and Flow Cytometry.
Murine ATAC-specific mAb MTAC-2 was obtained by immunizing Lewis rats with His-tagged murine ATAC (Val-22–Gly-114) and fusing the spleen cells to myeloma P3x63Ag8.653 (American Type Culture Collection; unpublished data). Affinity-purified goat antisera directed to the chemokines MIP-1α, MIP-1β, RANTES, and MIP-2 were obtained from R & D Systems, and anti-IFN-γ-inducible protein 10 sera were from DPC Biermann (Bad Nauheim, Germany). R-phycoerythrin-conjugated antibodies against murine granulocyte/macrophage colony-stimulating factor (MP1–22E9), IL-2 (JES6–5H4), and tumor necrosis factor-α (TNF-α, MP6-XT22) were obtained from PharMingen. For staining of MCP-1 we used mAb 2H5 (PharMingen). Staining of IFN-γ was performed with mAb AN18.17.24 (23) or mAb XMG1.2 (PharMingen). The following mAb directed to cell surface antigens were used: GK1.5 (CD4), 53-6.72 (CD8), 5C6 (CD11b), M5/114.15.2 (MHC class II), GL1 (CD86, all from ATCC), DX5 (pan-NK cells and NK T cells), 16–10A1 (CD80, both from PharMingen), and FGK (CD40, a kind gift of Ton Rolink, Basel). ICOS-L was detected by using a murine ICOS–human Ig fusion protein (24). The mAbs used were coupled to digoxigenin-N-hydroxysuccinimidester (Roche, Mannheim, Germany), fluorescein-N-hydroxysuccinimidester (Molecular Probes), or phycoerythrin (Cyanotech, Kailua-Kona, Hawaii) according to standard procedures. To block unspecific binding of antibodies to Fc receptors, all antibodies directed to surface antigens were diluted in 100 μg/ml mAb 2.4G2 (Fcγ II/III receptor, ATCC) and 50 μg/ml purified rat Ig (Nordic, Tilberg, The Netherlands). The intracellular staining procedure including specific blocking controls and detection of digoxigenin-labeled antibodies was performed as described (25). Samples were analyzed on a FACScalibur by using CELLQUEST software (Becton Dickinson).
Statistical Analysis of Intracellular Cytokine Coexpression.
Correlation of cytokine coexpression was calculated from dot plots by using the test for φ correlation coefficients (26) according to the equation φ = (ad − bc)/[(a + b)(c + d)(a + c)(b + d)0.5], with a = percentage of cells in the lower left, b = percentage of cells in the lower right, c = percentage of cells in the upper left, and d = percentage of cells in the upper right quadrant. A φ value ≥0.1 was considered as significant (26).
L. monocytogenes Infection.
For primary infection, mice were injected i.v. with a sublethal dose of strain EGD [BALB/c, 4 × 103 colony-forming units (cfu); C57BL/6, 6 × 103 cfu]. For secondary infection, mice were injected i.v. with 105 cfu on day 35.
Splenocyte Cultures.
Splenocytes (2 × 106 per ml) from healthy mice were stimulated with phorbol 12-myristate 13-acetate (20 ng/ml) and ionomycin (1 μg/ml) for 6 h in the presence of 5 μg/ml brefeldin A for the last 3 h (all from Sigma). Splenocytes from Listeria-infected mice were either incubated in 5 μg/ml brefeldin A only for 5 h or restimulated with Listeria-specific peptides. To this end, BALB/c splenocytes were incubated with the immunodominant H2-Kd-restricted peptide LLO91–99 and C57BL/6 splenocytes were incubated with the H2-M3-restricted peptide fMIGWII (both at 10−6 M) for 6 h, with brefeldin A added for the last 3 h.
Th1 and Th2 Cultures.
Naive CD4+ CD62L+ OVA-TCRtg/tg T cells were magnetically sorted and differentiated into Th1 and Th2 subsets as described (25).
Bone Marrow-Derived Macrophages (BMMs).
BMMs were prepared from the femora of C57BL/6 mice as described (27). Briefly, resting BMMs were obtained after culture in Iscove's modified Dulbecco's medium (IMDM, Biochrom, Berlin) containing 1% FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 30 μg/ml holotransferrin (Sigma), and 30 ng/ml recombinant murine macrophage colony-stimulating factor (R & D Systems) in hydrophobic 5-cm PetriPerm dishes (Sartorius). For functional experiments, the medium was replaced on day 10 of culture by IMDM containing 1% FCS. After infection of BMM with live Listeria (108 cfu per dish), extracellular Listeria were killed 1 h postinfection (p.i.) with medium containing 10 μg/ml gentamycin (Sigma) and 1% FCS. Infected BMMs were incubated further in the presence of various combinations of murine IFN-γ (0.1 ng/ml), MIP-1α, MIP-1β, RANTES, and ATAC/lymphotactin (all at 50 ng/ml, all from R & D Systems; endotoxin level < 0.1 ng/μg protein as determined by the limulus amoebacyte lysate method). After 24 h of culture, the levels of TNF-α, IL-12 p70, and NO were determined in the supernatants, and the BMMs were analyzed for expression of MHC class II, CD40, CD80, CD86, and ICOS-L by flow cytometry. For chemokine-receptor expression analysis by RT-PCR, resting BMMs (1 × 106) were stimulated with IFN-γ (50 ng/ml), lipopolysaccharide (500 ng/ml, Sigma), or IL-12 (10 ng/ml, PharMingen) for 20 h in IMDM containing 1% FCS or infected with live Listeria (108 cfu per dish) as described above.
RT-PCR.
Total RNA from resting and stimulated BMMs was prepared with the RNeasy Midi kit (Qiagen, Chatsworth, CA) and reverse-transcribed into cDNA by using oligo(dT) primers and SuperScript II (GIBCO/BRL). PCR amplification was performed with primers specific for the murine ATAC receptor and β-actin (15) or for murine CCR5 (5′-CAGGATGGATTTTCAAGGG-3′ and 5′-AAGAGCAGGTCAGAGATGGC-3′).
Detection of IL-12 and TNF-α in Culture Supernatants.
IL-12 p70 was measured by ELISA using mAb 9A5 and biotinylated mAb C17.8. TNF-α was detected by ELISA using mAb G281-2626 and biotinylated mAb MP6-XT3 (all from PharMingen).
Results
Establishment of Intracellular Flow Cytometry Detection of Murine Chemokines.
Our earlier work has demonstrated that the ATAC mRNA is expressed strongly in activated CD8+ T cells but only weakly in activated CD4+ T cells (7). To further analyze this strongly biased expression pattern at the protein level, we generated the mAb MTAC-2 suitable for intracellular flow cytometry detection of murine ATAC. For comparative experiments, a panel of antisera specific for murine inflammatory chemokines (MIP-1α, MIP-1β, RANTES, MIP-2, MCP-1, and IFN-γ-inducible protein 10) also were labeled with digoxigenin. C57BL/6 or BALB/c splenocytes were activated with phorbol 12-myristate 13-acetate, ionomycin, and brefeldin A, and the secretion of chemokines was determined in NK cells and subsets of T cells and compared with the secretion of IFN-γ. Interestingly, the overall frequency of cells positive for the analyzed chemokine group and also IFN-γ was similar in all lymphocyte subsets analyzed (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org); 30–70% of activated DX5+ cells and up to 40% of CD8+ splenic T cells secreted ATAC, MIP-1α, MIP-1β, RANTES, or IFN-γ. In clear contrast, not only ATAC but also MIP-1α, MIP-1β, and RANTES were detected in only ≈2% of activated CD4+ T cells (Fig. 6). In contrast to these findings, MIP-2 could be detected in up to 4% of activated CD4+ T cells but was absent in activated CD8+ T cells and NK cells, and no signal was obtained for MCP-1 and IFN-γ-inducible protein 10 (data not shown).
ATAC, MIP-1α, MIP-1β, and RANTES are Cosecreted with IFN-γ in Polyclonally Activated NK and T Cells.
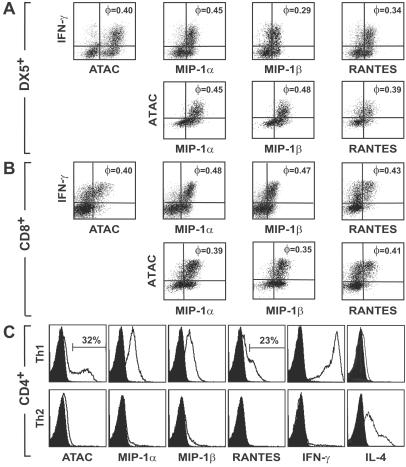
The strongly biased expression of ATAC, MIP-1α, MIP-1β, and RANTES suggested that these molecules are cosecreted by a functional subset of NK and T cells. In preliminary experiments IFN-γ but not IL-2, TNF-α, and granulocyte/macrophage colony-stimulating factor showed a consistent cosecretion pattern with ATAC and the CC chemokines both in activated NK cells and T cells (Fig. 7, which is published as supporting information on the PNAS web site) and therefore was chosen for further studies. When DX5+ cells were polyclonally activated, virtually all IFN-γ-producing cells simultaneously released ATAC, MIP-1α, MIP-1β, or RANTES (Fig. 1A), giving high values of φ, a parameter introduced to validate the statistical significance of cosecretion frequencies (a random cosecretion would give a value of 0, a 100% coincidence a value of 1; see Materials and Methods). Furthermore, all ATAC-secreting DX5+ cells also generated MIP-1α and MIP-1β and were highly correlated with RANTES (Fig. 1A), thus indicating that all five molecules had a high probability of being secreted by the same DX5+ cells. A similar level of cosecretion was observed also with activated CD8+ T cells, with correlation coefficients ranging from 0.35 to 0.48 (Fig. 1B). The high degree of cosecretion of ATAC and the CC chemokines with IFN-γ in NK and CD8+ T cells suggested that the small population of CD4+ T cells releasing these cytokines may have a Th1 phenotype. To examine this question we analyzed in vitro polarized Th1 or Th2 cells derived from OVA-TCRtg/tg mice. MIP-1α, MIP-1β, and IFN-γ were secreted by virtually all Th1 cells, and ATAC and RANTES were released by ≈20–30% of Th1 cells (Fig. 1C). In clear contrast, no significant signals were obtained for ATAC, MIP-1α, MIP-1β, RANTES, and IFN-γ in Th2 cells (Fig. 1C). This biased expression of chemokines in Th1 versus Th2 cells was not caused by different expression kinetics (Fig. 8, which is published as supporting information on the PNAS web site). Of all chemokines analyzed, only MIP-2 was selectively present in Th2 cells, albeit at low levels (3%; data not shown).
Figure 1.
ATAC, MIP-1α, MIP-1β, RANTES, and IFN-γ are cosecreted in polyclonally activated NK cells, CD8+ T cells, and Th1-differentiated CD4+ T cells. C57BL/6 splenocytes were stimulated with phorbol 12-myristate 13-acetate, ionomycin, and brefeldin A for 6 h, stained for DX5 or CD8, fixed, and counterstained with the respective cytokine/chemokine reagents. (A) Gate on DX5+ cells. (B) Gate on CD8+ cells. The correlation coefficient φ is indicated in the right upper quadrants. Shown is representative of four experiments. (C) OVA-specific Th1 or Th2 cells were restimulated after 2 weeks of differentiation with phorbol 12-myristate 13-acetate, ionomycin, and brefeldin A for 6 h and analyzed for the expression of ATAC, the CC chemokines, IFN-γ, and IL-4. Black profiles indicate isotype controls. The data given were obtained in one representative experiment out of two.
Cosecretion of ATAC, MIP-1α, MIP-1β, and RANTES with IFN-γ by NK Cells in the Innate Phase of L. monocytogenes Infection.
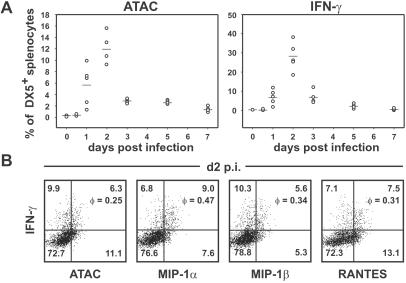
The biased expression in CD4+ versus CD8+ T cells, the exclusive induction in Th1 cells, and the close association with IFN-γ in T and NK cells suggested that ATAC, MIP-1α, MIP-1β, and RANTES constitute a group of “type 1 chemokines.” On the basis of these expression data we hypothesized that these type 1 chemokines, together with IFN-γ, represent a functional group of “type 1 cytokines.” We tested our hypothesis in vivo in the L. monocytogenes model, a classical Th1 model (17, 28, 29). BALB/c mice were infected with a sublethal dose of Listeria, and DX5+ splenocytes were analyzed for ATAC and IFN-γ secretion on days 1–7 p.i. without further restimulation in vitro. No signal could be observed at the 0- and 12-h time points, but intracellular ATAC and IFN-γ became detectable in a significant proportion of DX5+ cells on day 1, and the maximum of positive cells was seen on day 2 p.i., when ≈12% of DX5+ cells secreted ATAC and 28% secreted IFN-γ (Fig. 2A). Similar data were obtained with C57BL/6 mice (data not shown). Day 2 p.i. therefore was chosen for a detailed cosecretion analysis of the type 1 cytokines by NK cells. Interestingly, the synthesis of IFN-γ by DX5+ cells activated by Listeria in vivo again was correlated statistically with the secretion of the chemokine group. Approximately half of the IFN-γ producers could be found in the 10–20% fraction of cells secreting ATAC, MIP-1α, MIP-1β, or RANTES, giving φ values of 0.25–0.47, thus indicating quite a substantial nonrandom cosecretion (Fig. 2B). One should note that these numbers represent rather conservative estimates; IFN-γ producers activated in vivo exhibit low levels of chemokines per cell and, although clearly shifted when compared with the IFN-γ nonproducers (Fig. 2B), do not quantitatively “cross” the quadrant threshold used for statistical analysis. In noninfected controls, none of the cytokines analyzed were detectable in DX5+ cells (data not shown). Overall, secretion of IFN-γ by NK cells in vivo clearly was correlated positively with the synthesis of ATAC, MIP-1α, MIP-1β, and RANTES.
Figure 2.
Correlated secretion of IFN-γ with ATAC, MIP-1α, MIP-1β, and RANTES by NK cells in the innate phase of L. monocytogenes infection. (A) Time course of ATAC and IFN-γ secretion in splenic DX5+ cells. BALB/c mice were infected with L. monocytogenes (five mice per group), and the spleens were removed at various time points. Splenocytes were incubated with brefeldin A for 5 h without restimulation and analyzed for the expression of ATAC and IFN-γ by flow cytometry. Shown is the percentage of DX5+ cells secreting ATAC or IFN-γ in the course of listeriosis. (B) On day 2 (d2) p.i. splenocytes from individual Listeria-infected BALB/c mice were stained for DX5 and counterstained for IFN-γ versus ATAC, MIP-1α, MIP-1β, or RANTES. The percentage of cells in each quadrant and the correlation coefficient φ for each staining pair are indicated. Analysis of one representative animal out of eight is shown.
Cosecretion of ATAC, MIP-1α, MIP-1β, RANTES, and IFN-γ by Antigen-Specific CD8+ T Cells in the Adaptive Phase of L. monocytogenes Infection.
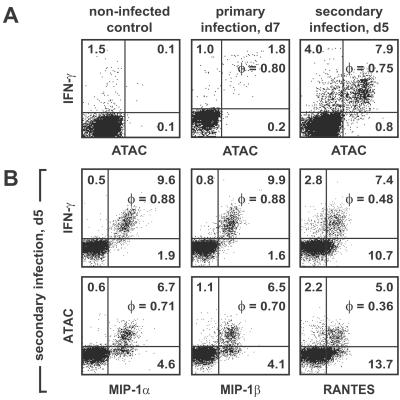
To assess the involvement of the type 1 chemokines also in the adaptive phase of the immune response to Listeria, we analyzed CD4+ and CD8+ splenic T cells after incubation with brefeldin A alone. No signals were obtained between days 1 and 14 p.i. (data not shown). Therefore, splenocytes of the infected BALB/c mice were restimulated on days 3, 5, 7, and 14 p.i. with MHC class I-restricted Listeria-specific peptides LLO91–99 (Fig. 3) or p60 (data not shown) for 6 h, with brefeldin A present for the last 3 h. On day 7 we observed 2.8% of cells positive for IFN-γ and ≈1.8% of cells positive for ATAC and IFN-γ, giving a very high φ value of 0.8 (Fig. 3A). Similar expression levels and correlations were found for MIP-1α, MIP-1β, and RANTES with IFN-γ (data not shown). In the noninfected control, a background synthesis of IFN-γ by 1.5% of cells but no synthesis of ATAC or the CC chemokines was observed (Fig. 3A).
Figure 3.
Cosecretion of ATAC, MIP-1α, MIP-1β, RANTES, and IFN-γ by antigen-specific CD8+ T cells during the adaptive phase of L. monocytogenes infection. BALB/c mice were infected with a sublethal dose of L. monocytogenes. On day 7 (d7) p.i. of primary infection or day 5 (d5) p.i. of a secondary infection, splenocytes of individual mice were restimulated with the Listeria-specific peptide LLO91–99 for 6 h in the presence of brefeldin A. Cells were stained for CD8, fixed, and counterstained for IFN-γ, ATAC, MIP-1α, MIP-1β, or RANTES. (A) Cosecretion of IFN-γ with ATAC by Listeria-specific CD8+ T cells in primary and secondary infection. (B) Cosecretion of IFN-γ and ATAC with MIP-1α, MIP-1β, and RANTES by Listeria-specific CD8+ T cells in secondary infection. Analysis of one representative animal out of six is shown.
To analyze the cytokine/chemokine coexpression in a recall response, BALB/c mice were reinfected with Listeria 35 days after primary infection. On day 5 of reinfection the percentages of CD8+ splenic T cells synthesizing the type 1 chemokines and IFN-γ rose dramatically, with 11.9% of cells secreting IFN-γ on peptide restimulation and 7.9% of cells secreting both IFN-γ and ATAC, giving a φ value of 0.75 (Fig. 3A). As in the primary infection, IFN-γ synthesis was highly correlated with the secretion of MIP-1α, MIP-1β, and RANTES, and the same was true when ATAC was correlated with the CC chemokines (Fig. 3B). Of note, the percentages of peptide-responsive CD8+ T cells in our studies, both in primary and secondary infection, were in good accordance with the frequencies of Listeria-specific CD8+ T cells reported by Busch et al. (30) using the tetramer technique. We did not detect any specific signals for the type 1 chemokines or IFN-γ in CD4+ T cells in any of the experiments performed (data not shown). Similar data were obtained also with C57BL/6 mice in the primary and secondary infection by using the Listeria-specific peptide fMIGWII (data not shown). Taken together, our data demonstrated a very high degree of cosecretion of the type 1 chemokines with IFN-γ by Listeria-specific CD8+ T cells both during primary and secondary infection.
BMMs Respond Synergistically to the type 1 Cytokines by up-regulation of CD40 and Synthesis of IL-12 and TNF-α.
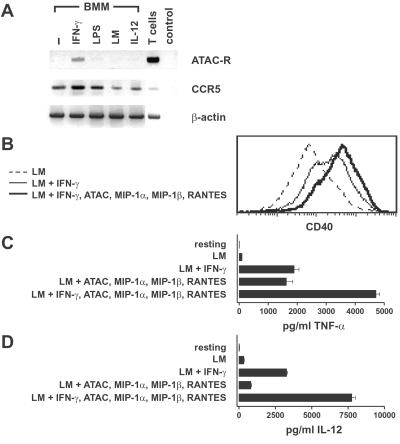
Macrophages communicate with NK and T cells in the innate and adaptive phases of an immune response and have a central role as effector cells in the defense against Th1-inducing pathogens after IFN-γ activation (28, 31). We therefore tested the possibility that macrophages represent a common physiological target for the combined group of type 1 chemokines and IFN-γ. In a first step, BMMs were analyzed by RT-PCR for the expression of the ATAC receptor and CCR5, the common receptor for MIP-1α, MIP-1β, and RANTES. ATAC receptor mRNA was not present in resting BMMs but became detectable after exposure to IFN-γ, whereas stimulation with lipopolysaccharide, infection by Listeria, or incubation with IL-12 was without effect (Fig. 4A). In contrast, CCR5 mRNA was present in resting BMMs, as reported earlier (32), and was up-regulated further by IFN-γ and lipopolysaccharide (Fig. 4A).
Figure 4.
Functional effects of the type 1 chemokines and IFN-γ on macrophages. (A) BMMs were exposed to IFN-γ, lipopolysaccharide (LPS), or IL-12 or were infected with live L. monocytogenes as described in Materials and Methods. After 24 h of culture, total RNA was analyzed by RT-PCR for the ATAC receptor, CCR5, and β-actin. RNA from purified (99%) CD8+ T cells (T cells) was used as a positive control, and samples without mRNA template were used as a negative control (control). (B) BMMs were infected with Listeria and were either left untreated (LM, dashed line) or treated additionally with IFN-γ alone (thin line) or a combination of IFN-γ and ATAC, MIP-1α, MIP-1β, and RANTES (thick line). After 24 h, BMMs were collected and analyzed for CD40 cell surface expression by using flow cytometry. One representative experiment out of five is shown. (C and D) BMMs were either left untreated (resting) or infected with L. monocytogenes for 1 h. Infected BMMs were either cultured in medium or stimulated with cytokines as indicated. After 24 h, supernatants were harvested and tested for the presence of TNF-α (C) and IL-12 (D). The values represent means of duplicates (TNF-α and IL-12) ± SD. One representative out of four experiments is shown.
In terms of function, preliminary experiments showed that noninfected BMMs do not respond functionally to any of the type 1 cytokines or combinations thereof. IFN-γ at 0.1 ng/ml induced a suboptimal expression of MHC class II, and was used at this concentration to detect costimulatory effects of MIP-1α, MIP-1β, RANTES, and ATAC. IFN-γ alone markedly increased CD40 cell surface expression on Listeria-infected BMMs, in particular on a subpopulation of cells (mean fluorescence intensity = 286; Fig. 4B and Table 1, which is published as supporting information on the PNAS web site). The combined effect of IFN-γ and ATAC, MIP-1α, MIP-1β, and RANTES lead to a further up-regulation of CD40 on the entire BMM population (mean fluorescence intensity = 497; Fig. 4B). A detailed analysis determined that all cytokines of the type 1 group contributed to the observed CD40 up-regulation, with IFN-γ being the most important factor (Table 1). Interestingly, expression of MHC class II, CD80, CD86, and ICOS-L was not affected beyond the up-regulation achieved with IFN-γ alone (data not shown), suggesting a specific synergistic action of the type 1 cytokines on CD40 induction.
When the supernatants of the same BMM culture were analyzed for the presence of inflammatory mediators, we observed substantial production of TNF-α in Listeria-infected cultures exposed to IFN-γ or to ATAC, MIP-1α, MIP-1β, and RANTES (Fig. 4C). In the combined presence of the type 1 chemokines plus IFN-γ, the TNF-α production was increased further (Fig. 4C). Regarding IL-12, we observed a 10-fold induction after addition of IFN-γ to Listeria-infected BMMs (Fig. 4D). Here, the chemokine group alone had at best modest effects but clearly was synergistic with IFN-γ (Fig. 4D).
Discussion
Our establishment of intracellular flow cytometry detection for MIP-1α, MIP-1β, RANTES, MIP-2, and ATAC allowed for the first time to correlate chemokines with cytokines at the single-cell level in the murine immune system. Interestingly, the secretion of MIP-1α, MIP-1β, RANTES, and ATAC in polyclonally activated NK and T cells was highly associated with the secretion of IFN-γ, the prototypic Th1 cytokine (Fig. 1). In T cells differentiated into Th1 and Th2 cells in vitro, MIP-1α, MIP-1β, RANTES, and ATAC, similar to IFN-γ, were present exclusively in Th1 cultures (Fig. 1). These central findings were the basis for our hypothesis that MIP-1α, MIP-1β, RANTES, and ATAC are type 1 chemokines. We tested our hypothesis in murine listeriosis, a prototypic Th1 model (28, 29). Here, secretion of IFN-γ by NK cells on days 1 and 2 p.i. clearly was correlated with the secretion of MIP-1α, MIP-1β, RANTES, and ATAC at the single-cell level (Fig. 2). This phenomenon was not restricted to NK cells, because during the adaptive phases of primary and secondary infections we observed a clear cosecretion of IFN-γ, MIP-1α, MIP-1β, RANTES, and ATAC by individual Listeria-specific CD8+ T cells (Fig. 3). The cosecretion of the type 1 chemokines with IFN-γ in NK and CD8+ T cells at various stages of listeriosis suggested that all these mediators may act on the same cells. We tested this assumption on macrophages, a central target population for IFN-γ. Interestingly, with Listeria-infected macrophages we observed a synergistic effect of the type 1 chemokines with IFN-γ on the up-regulation of CD40, a receptor mediating the proinflammatory action of CD40 ligand expressed on activated T and NK cells (33). Furthermore, synergistic effects of the five type 1 cytokines were seen on the production of TNF-α and IL-12, the central inducer of the Th1 immune response. Despite a mild cooperative effect of the chemokine group with IFN-γ on the release of NO, no increased intracellular killing of bacteria was detectable (data not shown), suggesting that the combined action of the type 1 chemokines with IFN-γ has regulatory rather than microbicidal effects. Together, these data demonstrate that MIP-1α, MIP-1β, RANTES, and ATAC are not only to a high degree cosecreted with IFN-γ at the single-cell level but also synergize functionally with IFN-γ on a common target population.
Our results extend the understanding of the biological role of a number of chemokines. MIP-1α, MIP-1β, and RANTES are being regarded principally as chemoattractants for monocytes/macrophages and distinct populations of lymphocytes (5, 6, 34) despite an early report by Fahey et al. (35) on the activation of thioglycollate-elicited macrophages by MIP-1α. Only recently it became apparent that MIP-1α, MIP-1β, and RANTES have additional functions in the immune system, because they were found to augment the cytolytic capacity of T and NK cells and to costimulate T cell proliferation and IL-2 synthesis (36, 37). Our finding of a coordinated secretion and function of MIP-1α, MIP-1β, RANTES, and ATAC with IFN-γ in a number of settings in vitro and in vivo makes it attractive to view all biological effects of these chemokines in the context of the Th1/Th2 dichotomy of the immune response. Schrum et al. (38) were the first to notice that human peripheral blood mononuclear cells respond to extracts of the Th1-inducing pathogen Yersinia enterocolitica by a cytokine pattern including IFN-γ, MIP-1α, and RANTES, but they did not characterize the responsible cell populations. Bradley et al. (39) observed the expression of ATAC/lymphotactin, MIP-1α, and MIP-1β in in vitro Th1-polarized but not Th2-polarized T cell populations by using RNase protection assays. In two very recent gene-expression profiling experiments of in vitro Th1- and Th2-polarized human T cells, IFN-γ, MIP-1α, MIP-1β, RANTES, and ATAC/lymphotactin were among the seven transcripts (of 22,096 and 6,000 examined, respectively) with the strongest bias in expression toward Th1 cells (40, 41). When these in vitro data are taken together with our in vivo expression analyses of MIP-1α, MIP-1β, RANTES, ATAC, and IFN-γ, little doubt remains about the central role of this group of cytokines in the Th1 immune response of T cells.
Our results indicate that the association of MIP-1α, MIP-1β, RANTES, and ATAC with IFN-γ is not limited to T cells but is found also in NK cells. It has been shown earlier that human NK cells are able to release IFN-γ, MIP-1α, MIP-1β, and RANTES upon activation in vitro (42, 43). These analyses were complemented recently by microarray profiling of murine NK cells crosslinked via Ly-49D showing the highest up-regulation indices for ATAC/lymphotactin, MIP-1α, and MIP-1β (44). These results, obtained exclusively in vitro, are substantiated by our data on the cosecretion of MIP-1α, MIP-1β, RANTES, and ATAC with IFN-γ by NK cells in vivo. Collectively, these data suggest also that NK cells may be intimately involved in the development of Th1 immunity. Because the cosecretion of the analyzed molecules is not restricted to T(h) cells but is similarly relevant for NK cells, one should consider MIP-1α, MIP-1β, RANTES, and ATAC together with IFN-γ as type 1 cytokines rather than Th1 cytokines.
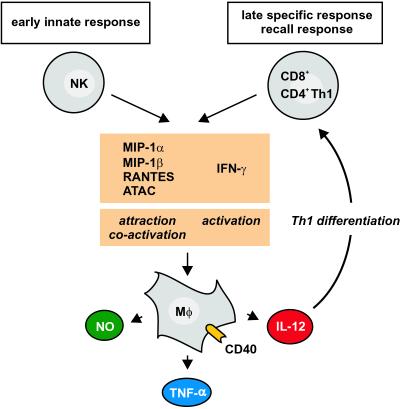
It is important to consider the temporal involvement of the type 1 chemokines in vivo in the context of their functional role. In listeriosis, the type 1 chemokines, released by NK cells on days 1 and 2 of infection, are involved in the early attraction of macrophages, which play a major role in the first line of defense against Listeria (34, 39, 45). Our functional results suggest that macrophages, once attracted to the site of pathogen entry, will release under the synergistic influence of the type 1 chemokines and IFN-γ the inflammatory cytokine TNF-α and also IL-12, the leading inducer of Th1 T cell differentiation (46). Interestingly, from day 3 of listeriosis onward, the secretion of NK cell-derived type 1 chemokines and IFN-γ rapidly declines almost to resting levels and does not reappear. In the second phase of infection, T cell-derived type 1 chemokines and IFN-γ come into play, becoming detectable around day 7 (day 5 of reinfection). Our data thus suggest that the type 1 chemokines plus IFN-γ, as a functional unit, are “handed over” from NK cells in the innate phase to CD8+ T cells in the antigen-specific phase of the immune response (Fig. 5), thus bridging the innate and adaptive components of the immune system (47, 48). From the biological point of view, this handing over of the type 1 chemokines and IFN-γ from NK cells to T cells may ensure the specificity of the continued immune response after the initial innate reaction. Independent of the secreting cell type and the time point of release, the type 1 chemokines together with IFN-γ will attract and activate macrophages (Fig. 5).
Figure 5.
Model for the role of the type 1 chemokines and IFN-γ as a functional unit in the course of a Th1-inducing infection. MIP-1α, MIP-1β, RANTES, ATAC, and IFN-γ are cosecreted early by NK cells and later by CD8+ T/CD4+ Th1 cells. In both phases, the type 1 chemokines attract and, together with IFN-γ, coactivate macrophages to release NO, TNF-α, and IL-12. The chemokine group MIP-1α, MIP-1β, RANTES, and ATAC thus constitute together with the cytokine IFN-γ a functional unit that is used both by cells of the innate and adaptive immunity to drive the type 1 immune reaction in vivo.
In consequence of all available data, it is attractive to view the type 1 chemokines MIP-1α, MIP-1β, RANTES, and ATAC on equal terms with the key Th1 cytokines IFN-γ, IL-12, and IL-18 as communicators between NK cells, macrophages, and Th1 T cells, the effectors of cellular immunity. This concept is supported by the observation that the deletion of the IFN-γ or the CCR5 genes results in an increased susceptibility of mice to a variety of Th1-inducing pathogens including L. monocytogenes (19, 20, 49).
Supplementary Material
Acknowledgments
The expert technical assistance of K. Ranke and P. Jahn is gratefully acknowledged. We thank H. W. Mittrücker for support in the Listeria model and H. W. Mages and A. Hutloff for critical reading of the manuscript. This work was supported by Deutsche Forschungsgemeinschaft Grant SFB421.
Abbreviations
- MIP
macrophage inflammatory protein
- RANTES
regulated on activation normal T cell expressed and secreted
- NK
natural killer
- Th
T helper
- ATAC
activation-induced, T cell-derived, and chemokine-related cytokine
- RT
reverse transcription
- OVA
ovalbumin
- TNF-α
tumor necrosis factor-α
- cfu
colony-forming unit(s)
- BMM
bone marrow-derived macrophage
- p.i.
postinfection
References
- 1.Zlotnik A, Yoshie O. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 2.Moser B, Loetscher P. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 4.Murphy P M, Baggiolini M, Charo I F, Hebert C A, Horuk R, Matsushima K, Miller L H, Oppenheim J J, Power C A. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 5.Uguccioni M, D'Apuzzo M, Loetscher M, Dewald B, Baggiolini M. Eur J Immunol. 1995;25:64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 6.Taub D D, Conlon K, Lloyd A R, Oppenheim J J, Kelvin D J. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 7.Müller S, Dorner B, Korthäuer U, Mages H W, D'Apuzzo M, Senger G, Kroczek R A. Eur J Immunol. 1995;25:1744–1748. doi: 10.1002/eji.1830250638. [DOI] [PubMed] [Google Scholar]
- 8.Kelner G S, Kennedy J, Bacon K B, Kleyensteuber S, Largaespada D A, Jenkins N A, Copeland N G, Bazan J F, Moore K W, Schall T J. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Imai T, Kakizaki M, Nishimura M, Yoshie O. FEBS Lett. 1995;360:155–159. doi: 10.1016/0014-5793(95)00093-o. [DOI] [PubMed] [Google Scholar]
- 10.Hedrick J A, Saylor V, Figueroa D, Mizoue L, Xu Y, Menon S, Abrams J, Handel T, Zlotnik A. J Immunol. 1997;158:1533–1540. [PubMed] [Google Scholar]
- 11.Dorner B, Müller S, Entschladen F, Schröder J M, Franke P, Kraft R, Friedl P, Clark-Lewis I, Kroczek R A. J Biol Chem. 1997;272:8817–8823. doi: 10.1074/jbc.272.13.8817. [DOI] [PubMed] [Google Scholar]
- 12.Bleul C C, Fuhlbrigge R C, Casasnovas J M, Aiuti A, Springer T A. J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida T, Imai T, Kakizaki M, Nishimura M, Takagi S, Yoshie O. J Biol Chem. 1998;273:16551–16554. doi: 10.1074/jbc.273.26.16551. [DOI] [PubMed] [Google Scholar]
- 14.Heiber M, Docherty J M, Shah G, Nguyen T, Cheng R, Heng H H, Marchese A, Tsui L C, Shi X, George S R. DNA Cell Biol. 1995;14:25–35. doi: 10.1089/dna.1995.14.25. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida T, Izawa D, Nakayama T, Nakahara K, Kakizaki M, Imai T, Suzuki R, Miyasaka M, Yoshie O. FEBS Lett. 1999;458:37–40. doi: 10.1016/s0014-5793(99)01114-x. [DOI] [PubMed] [Google Scholar]
- 16.Boehm U, Klamp T, Groot M, Howard J C. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann S H E. In: Fundamental Immunology. 4th Ed. Paul W E, editor. Philadelphia: Lippincott–Raven; 1999. pp. 1335–1371. [Google Scholar]
- 18.Mosmann T R, Sad S. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 19.Harty J T, Bevan M J. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 21.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 22.Murphy K M, Heimberger A B, Loh D Y. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 23.Prat M, Gribaudo G, Comoglio P M, Cavallo G, Landolfo S. Proc Natl Acad Sci USA. 1984;81:4515–4519. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mages H W, Hutloff A, Heuck C, Büchner K, Himmelbauer H, Oliveri F, Kroczek R A. Eur J Immunol. 2000;30:1040–1047. doi: 10.1002/(SICI)1521-4141(200004)30:4<1040::AID-IMMU1040>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Richter A, Löhning M, Radbruch A. J Exp Med. 1999;190:1439–1450. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Löhning M, Grogan J L, Coyle A J, Yazdanbakhsh M, Meisel C, Gutierrez-Ramos J C, Radbruch A, Kamradt T. J Immunol. 1999;162:3882–3889. [PubMed] [Google Scholar]
- 27.Flesch I, Kaufmann S H. J Immunol. 1987;138:4408–4413. [PubMed] [Google Scholar]
- 28.Unanue E R. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 29.North R J, Dunn P L, Conlan J W. Immunol Rev. 1997;158:27–36. doi: 10.1111/j.1600-065x.1997.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 30.Busch D H, Pilip I M, Vijh S, Pamer E G. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 31.Bancroft G J, Schreiber R D, Unanue E R. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 32.Jarmin D I, Nibbs R J, Jamieson T, de Bono J S, Graham G J. Exp Hematol (Charlottesville, Va) 1999;27:1735–1745. doi: 10.1016/s0301-472x(99)00115-0. [DOI] [PubMed] [Google Scholar]
- 33.Stout R D, Suttles J. Immunol Today. 1996;17:487–492. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 34.Wolpe S D, Davatelis G, Sherry B, Beutler B, Hesse D G, Nguyen H T, Moldawer L L, Nathan C F, Lowry S F, Cerami A. J Exp Med. 1988;167:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahey T J, 3rd, Tracey K J, Tekamp-Olson P, Cousens L S, Jones W G, Shires G T, Cerami A, Sherry B. J Immunol. 1992;148:2764–2769. [PubMed] [Google Scholar]
- 36.Taub D D, Sayers T J, Carter C R, Ortaldo J R. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 37.Taub D D, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 38.Schrum S, Probst P, Fleischer B, Zipfel P F. J Immunol. 1996;157:3598–3604. [PubMed] [Google Scholar]
- 39.Bradley L M, Asensio V C, Schioetz L K, Harbertson J, Krahl T, Patstone G, Woolf N, Campbell I L, Sarvetnick N. J Immunol. 1999;162:2511–2520. [PubMed] [Google Scholar]
- 40.Nagai S, Hashimoto S, Yamashita T, Toyoda N, Satoh T, Suzuki T, Matsushima K. Int Immunol. 2001;13:367–376. doi: 10.1093/intimm/13.3.367. [DOI] [PubMed] [Google Scholar]
- 41.Rogge L, Bianchi E, Biffi M, Bono E, Chang S Y, Alexander H, Santini C, Ferrari G, Sinigaglia L, Seiler M, et al. Nat Genet. 2000;25:96–101. doi: 10.1038/75671. [DOI] [PubMed] [Google Scholar]
- 42.Perussia B. Curr Opin Immunol. 1991;3:49–55. doi: 10.1016/0952-7915(91)90076-d. [DOI] [PubMed] [Google Scholar]
- 43.Fehniger T A, Shah M H, Turner M J, VanDeusen J B, Whitman S P, Cooper M A, Suzuki K, Wechser M, Goodsaid F, Caligiuri M A. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 44.Ortaldo J R, Bere E W, Hodge D, Young H A. J Immunol. 2001;166:4994–4999. doi: 10.4049/jimmunol.166.8.4994. [DOI] [PubMed] [Google Scholar]
- 45.Didier P J, Paradis T J, Gladue R P. Inflammation. 1999;23:75–86. doi: 10.1023/a:1020243701890. [DOI] [PubMed] [Google Scholar]
- 46.Trinchieri G. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 47.Medzhitov R, Janeway C A J. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 48.Fearon D T, Locksley R M. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y, Kurihara T, Ryseck R P, Yang Y, Ryan C, Loy J, Warr G, Bravo R. J Immunol. 1998;160:4018–4025. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.