Abstract
Generation of CD8+ memory T cells requires antigenic stimulation through T cell receptor (TCR); however, maintenance of CD8+ memory T cells seems to be mediated by cytokines, such as IL-15, in a TCR-independent manner. Compared with the TCR-induced activation, less is known about the mechanisms of IL-15 action. We report here a comparative and kinetic analysis of the responses of memory phenotype CD8+ T cells to IL-15 or TCR (anti-CD3) stimulation in vitro. These two stimuli induce highly similar responses in memory phenotype CD8+ T cells as measured by cellular proliferation, gene expression changes, synthesis of effector molecules (IFNγ, tumor necrosis factor β, granzyme B, and perforin), and induction of cytotoxicity. From 189 genes/expressed sequence tags (ESTs) whose expression changed in CD8+ memory T cells after IL-15 and anti-CD3 stimulation identified by cDNA microarray analysis, 77% of the genes/ESTs exhibit a highly similar pattern of expression between IL-15 and anti-CD3-treated cells, and only 16% and 7% of the genes/ESTs are differentially expressed in response to IL-15 and anti-CD3 treatments, respectively. These results show that IL-15 and anti-CD3 stimulation induced remarkably similar gene expression and effector function. Thus, IL-15 acts not only as a crucial growth factor but also as an antigen-independent activator of effector functions for CD8+ memory T cells.
Upon antigenic challenge, naïve T lymphocytes differentiate into effector T cells, and subsequently some of them become memory T cells. Memory T lymphocytes are long-lived and are capable of further differentiation and proliferation to become effector cells in the subsequent antigenic encounter (1). These unique features of memory lymphocytes provide the cellular basis for immunological memory, a hallmark of the adaptive immunity.
Generation of memory lymphocytes depends on antigenic stimulation, but the survival of memory lymphocytes seems to be antigen independent and requires cytokines (2–4). Among various implicated cytokines (5–8), strong evidence shows that IL-15 can promote proliferation and long-term survival of memory phenotype CD8+ T cells in an antigen-independent fashion (4, 7, 9). IL-15 is secreted by many types of cells, but not by T cells (10). The IL-15 receptor consists of a private α-chain, and shared IL-2 receptor β- and γ-chains that are expressed individually or together to form various functional receptors with different affinities and signaling capabilities (10). The interaction of IL-15 with its receptors leads to potent and selective proliferation of CD8+ memory T cells (7, 11–13). However, the molecular mechanisms underlying IL-15-mediated proliferation and maintenance of CD8+ memory T cells are not fully understood.
In contrast to the antigen-independent proliferation induced by IL-15, the engagement of T cell receptor (TCR) with MHC class I-peptide complex of the target cells induces antigen-specific memory CD8+ T cells to proliferate and differentiate into effector cells (14). The differentiation process results in several physiological changes in memory CD8+ T cells including expression of surface-activation markers, production of cytokines, and synthesis of effector molecules. Despite apparent differences in the initial ligand/receptor interaction, the fact that both IL-15 and TCR engagement are capable of inducing CD8+ memory T cell proliferation suggests that these two pathways share some common downstream events.
To determine molecular and cellular changes induced by IL-15, and to compare IL-15- and TCR-mediated stimulation, we conducted a parallel analysis of genome-scale gene expression, proliferation, effector molecule production, and cytotoxicity in memory phenotype CD8+ T cells stimulated in vitro with IL-15 or anti-CD3 monoclonal antibodies (mAbs). We report here a remarkable resemblance in all these parameters in memory phenotype CD8+ T cells treated with either IL-15 or anti-CD3. Furthermore, we have identified 189 cDNA clones whose expression levels were significantly changed in memory phenotype CD8+ T cells after IL-15 and/or anti-CD3 stimulation. Approximately 77% of those cDNA clones exhibited a similar pattern of changes with either IL-15 or anti-CD3 treatment. In addition to these similarities, we have also identified differences between these two stimulations in gene expression and in surface-activation marker expression (CD2 and CD53). These results show that the distinct stimulation by IL-15 and anti-CD3 share overall downstream events, and suggest that IL-15 not only promotes survival but also activates the effector function of memory phenotype CD8+ T cells.
Materials and Methods
Isolation of Memory Phenotype CD8+ T Cells from Peripheral Blood.
We applied the immunomagnetic separation method to isolate memory phenotype CD8+ T cells from peripheral blood based on previously characterized phenotype (15). In brief, blood was obtained from normal donors of the National Institutes of Health Blood Bank and National Institute on Aging Clinical Core Laboratory, and mononuclear cells were isolated by Ficoll gradient centrifugation (Organon Teknika–Cappel). Memory phenotype CD8+ T cells were then isolated by removing other types of cells through incubating with a panel of mouse mAbs, against CD4, CD19, CD11b, CD14, CD16, MHC class II, erythrocytes, platelets, and CD45RA. Antibody-bound cells were subsequently removed by incubation with anti-mouse IgG-conjugated magnetic beads (Qiagen, Chatsworth, CA). The purified memory phenotype CD8+ T cells consist of approximately 90–95% CD8+ CD45R0+ T cells and 0.1% CD45RA+CD27− cells by fluorescence-activated cell sorter analysis.
Stimulation of Memory Phenotype CD8+ T Cells in Vitro.
The procedures for in vitro stimulation of purified memory phenotype CD8+ T cells were essentially as described (16). Anti-CD3 (OKT3) mAb conjugated magnetic beads (Dynal, Great Neck, NY) were gifts from C. June (University of Pennsylvania). IL-15 was obtained from PeproTech (Boston). Cells were resuspended at 2 × 106 cells per ml in RPMI1640 medium (Life Technologies, Rockville, MD) supplemented with 10% FBS (Gemini Biological Products, Calabasas, CA) and 1× penicillin-streptomycin (Life Technologies), mixed with anti-CD3-conjugated beads (used for cellular changes and gene expression analysis) or immobilized on plate (used for the cytotoxicity assay), or IL-15, and incubated for defined time points before harvest. The proliferation measurement was previously described (17).
cDNA Microarray Filters, Procedure, and Analysis.
The custom made filters consist of 4,604 cDNA clones that were selected from an initial screening over 40,000 unique cDNA clones obtained from Research Genetics (Huntsville, AL), Incyte Genomics (St. Louis), and cloned in this laboratory as described (17). The procedures for mRNA isolation, cDNA probe synthesis, and labeling were performed as described (17). Image files were collected from the PhosphorImager (Amersham Pharmacia), and were processed by using the P-SCAN analysis software as described (17). In brief, the hybridization spots on the image of the microarray were located, and the average image intensity was then determined. These numerical intensities of each spot were normalized filterwide, and the relatively over- and underexpressed genes between two conditions were determined by a 2-fold mean ratio change. The identity of the selected clones was confirmed by sequencing. The relative changes of the expression were presented in color (red = increase, green = decrease, black = no change) by using cluster and treeview (M. Eisen, Stanford University).
Western Blot Analysis.
Western blot analysis was carried out as described (18). The membranes were probed sequentially with mouse anti-human proliferating cell nuclear antigen (Santa Cruz Biotechnology), Granzyme B (PharMingen), and rabbit anti-human perforin (Santa Cruz Biotechnology). Signals were detected by using the ECL+Plus detection system (Amersham Pharmacia) according to the manufacturer's instructions. The membranes were stripped and probed again with anti-ZAP70 antibody (gift from R. Wange, National Institute on Aging).
Cytokine Assays.
Supernatants were collected from in vitro stimulated memory phenotype CD8+ T cells. IFN-γ and tumor necrosis factor (TNF) β concentrations were determined by using ELISA Immunoassay Kit [BioSource International (Camarillo, CA) and R & D Systems] according to the manufacturer's instructions.
Redirected Cytotoxicity Assay.
The cytotoxic activity of memory phenotype CD8+ T cells after stimulation was determined by a redirected cytotoxicity assay. Five million Fas− L1210 target cells were labeled with 200 μCi of 51Cr in 250 μl of FCS for 1h at 37°C. After washing in Hepes-saline (0.1 M Hepes/0.15 M NaCl, pH 7.6), the cells were biotinylated with 0.2 mM NHS-LC-biotin (Calbiochem) in Hepes-saline for 30 min at 4°C, washed, and streptavidin-coated by incubation with 20 μg/ml of streptavidin (Sigma) in Hepes-saline with 1% BSA for 30 min at room temperature. Effector T cells, freshly isolated and stimulated memory phenotype CD8+ T cells, were mixed with the target cells in culture medium at different ratios in the presence or absence of 2.5 μg/ml of biotinylated anti-CD3. After 4 h of incubation the supernatants were harvested and counted in a gamma counter. The corrected percent lysis was calculated from the released 51Cr, with the spontaneous release subtracted.
Results
IL-15 and Anti-CD3 Stimulation Induce Similar Cellular Proliferation in Memory Phenotype CD8+ T Cells in Vitro.
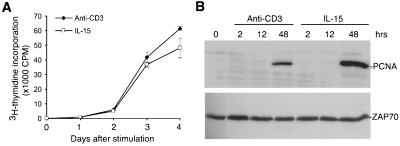
To compare stimulation of memory phenotype CD8+ T cells by IL-15 and anti-CD3 mAb, we determined responses to titrated concentrations of both stimuli. IL-15 at 100 ng/ml and anti-CD3-conjugated magnetic beads at a 1:1 bead:cell ratio induced proliferation at plateau level (data not shown). These conditions were then applied to assess the proliferation kinetics during a 4-day period of memory phenotype CD8+ T cells isolated from peripheral blood of normal donors. It was found that memory phenotype CD8+ T cells proliferate at a similar rate in response to either IL-15 or anti-CD3 treatment (Fig. 1A). In good agreement with the cellular proliferation, proliferating cell nuclear antigen, a biochemical marker of proliferating cells, was detected in memory phenotype CD8+ T cells at 2 days after either IL-15 or anti-CD3 stimulation (Fig. 1B).
Figure 1.
Cellular proliferation of memory phenotype CD8+ T cells after IL-15 and anti-CD3 treatment in vitro. (A) Profiles of [3H]thymidine incorporation of memory phenotype CD8+ T cells after in vitro stimulation. Isolated memory phenotype CD8+ T cells were cultured in the presence of 100 ng/ml IL-15 or anti-CD3 mAb-conjugated magnetic beads, and assayed for [3H]thymidine incorporation at days 1, 2, 3, and 4. (B) Western blot analysis of proliferating cell nuclear antigen protein levels in memory phenotype CD8+ T cells after stimulation with IL-15 or anti-CD3 for 2, 12, and 48 h. One representative of two independent blots was shown. ZAP-70 is used as loading standard.
Genome-Scale Gene Expression in Memory Phenotype CD8+ T Cells after IL-15 and Anti-CD3 Stimulation.
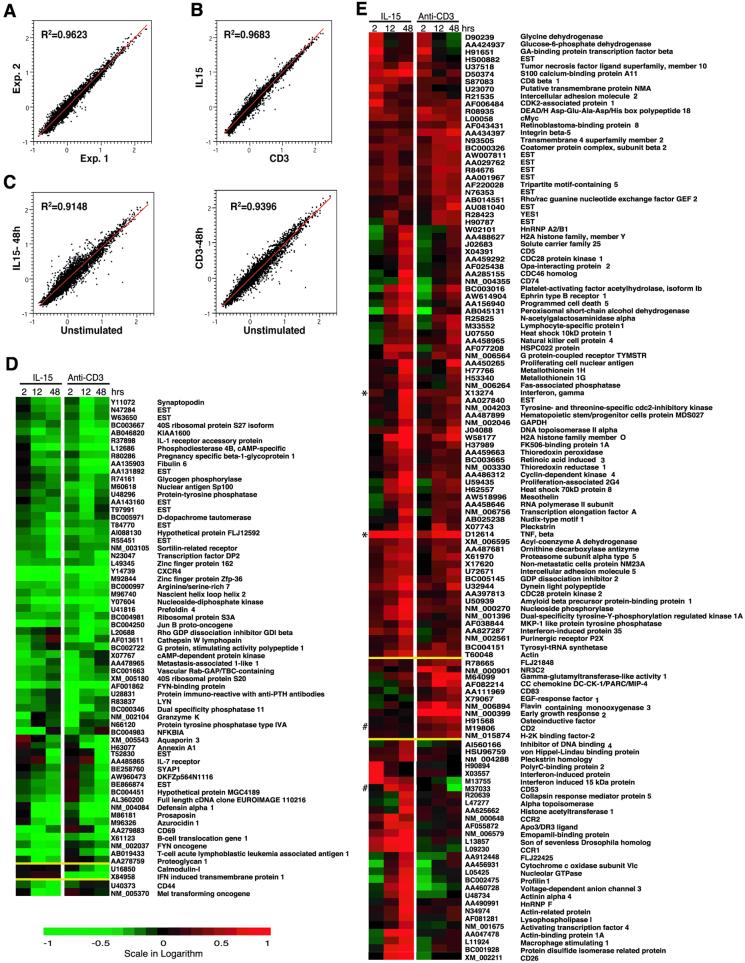
To assess and compare general gene expression changes in response to these stimuli, memory phenotype CD8+ T cells stimulated with IL-15 or anti-CD3 for 2, 12, and 48 h were collected for gene expression analysis by using cDNA microarray. Our custom-made cDNA microarray filters consist of 4,608 cDNA clones that were selected from the initial screening of expression of more than 40,000 unique human clones by CD4+ and CD8+ T cells. Two independent experiments with a total of six independent measurements of each cDNA clone under every experimental condition were analyzed (http://www.grc.nia.nih.gov/branches/li/weng/arraydata2.htm). The reproducibility between two experiments was excellent, because the average R2 of duplicate linear regressions for all seven conditions was 0.9527 ± 0.0188 (Fig. 2A). The gene expression changes were quite similar between IL-15 and anti-CD3 treatments, because the average R2 of duplicate linear regressions for all three time points was 0.9609 ± 0.0135 (Fig. 2B). The stimulation-induced general changes are shown in Fig. 2C.
Figure 2.
Gene expression profiles in memory phenotype CD8+ T cells after IL-15 or anti-CD3 treatment in vitro. (A) Example of gene expression comparison in duplicate experiments (1 and 2 of unstimulated cells): 4,608 clones present in the duplicates were compared. The average R2 of duplicate linear regressions for all time points was 0.9527 ± 0.0188. (B) Example of the similarity in gene expression between IL-15 and anti-CD3 treatment. (The data at the 2-h time point are shown.) The average R2 of duplicate linear regressions for all three time points between two stimulation conditions was 0.9609 ± 0.0135. (C) The difference between stimulated and unstimulated memory phenotype CD8+ T cells: 4,608 clones were compared. IL-15 on the right and anti-CD3 on the left after treatment at 48 h were shown. (D and E) Genes that were down- (D) and up- (E) regulated after in vitro stimulation with IL-15 or anti-CD3 for 2, 12, and 48 h are shown. The clones were clustered in the order of common between two conditions, differentially expressed in IL-15 and anti-CD3 separated by yellow lines. Each colored square represents the mean ratio of stimulated over resting cells of one cDNA clone. The mean value was derived from two independent hybridization experiments with a total of six independent replicate measurements for each cDNA clone. The scale of the intensity ratio is −1 to 1 in logarithm value (10-fold down- to 10-fold up-regulation), with green indicating a decrease and red indicating an increase. The GenBank accession number and gene names are indicated at the right. The genes were clustered by using CLUSTER and TREEVIEW software.
A total of 189 clones whose expression was changed at least 2 fold at one or more time points after stimulation compared with those of freshly isolated memory phenotype CD8+ T cells were identified (Fig. 2 D and E). Overall, approximately 77% of cDNA clones (145 of 189 clones) exhibited a similar pattern of changes after either IL-15 or anti-CD3 stimulation. The differential expression of clones was arbitrarily defined by an intensity change of 2-fold or greater in expression under one stimulation condition but an intensity change of 1.3-fold or less under the other condition at the same time point. Based on this criterion, only 16% of the identified clones were differentially expressed in IL-15 (31 of 189) and 7% clones were differentially expressed in anti-CD3 (13 of 189 clones) stimulated memory phenotype CD8+ T cells.
Sixty-five cDNA clones [56 genes and 9 expressed sequence tags (ESTs)] were down-regulated by at least 2-fold at one of three time points during a 2-day period (Fig. 2D). The majority of those down-regulated clones (approximately 94% of the clones) exhibited similar changes in response to IL-15 and anti-CD3 treatments. Two genes each were down-regulated uniquely in response to IL-15 stimulation and anti-CD3 treatment in memory phenotype CD8+ T cells (Fig. 2D).
One hundred and twenty-four cDNA clones (115 genes and 9 ESTs) were up-regulated significantly in at least one of three time points over a 2-day period after IL-15 or anti-CD3 stimulation (Fig. 2E). The gene expression patterns of the majority of up-regulated cDNA clones (84 cDNA clones) were similar between IL-15 and anti-CD3 stimulated memory phenotype CD8+ T cells (Fig. 2E). Only 29 genes were differentially up-regulated in IL-15, and 11 genes in anti-CD3 stimulated cells (Fig. 2E).
Functional Characteristics of Commonly Down- and Up-Regulated Genes in Memory Phenotype CD8+ T Cells after IL-15 and Anti-CD3 Stimulation.
Recent studies show that the status of resting CD4+ T lymphocytes is maintained through expression of a large number of genes that function in preventing cellular proliferation, activation, and apoptosis (17, 19–21). Antigenic stimulation in vivo or crosslinking of TCR in vitro results in down-regulated expression of those genes in lymphocytes. Among 65 down-regulated cDNA clones in memory phenotype CD8+ T cells after IL-15 and anti-CD3 stimulation, we found two clones, B cell translocation gene I and GDP-dissociation inhibitor, whose functions include inhibiting cellular proliferation and activation (Fig. 2D). In addition, several signaling related molecules such as cAMP-dependent protein kinase, cAMP-specific phosphodiesterase 4B, nucleoside-diphosphate kinase, FYN-binding protein, and protein tyrosine phosphatase type IVA were also down-regulated (Fig. 2D). Their precise roles in resting and in activated memory phenotype CD8+ T cells are not yet understood.
Activation of T cells also induces enhanced expression of numerous genes (17, 21, 22). Previous studies have categorized these activation-induced genes into several functional groups including transcriptional regulation, signal transduction, cell cycle regulation, apoptosis, activation, and effector function. Some up-regulated genes identified here in memory phenotype CD8+ T cells after IL-15 or anti-CD3 stimulation function in those known categories, but the roles of many other genes/ESTs in memory phenotype CD8+ T cell activation and proliferation remain to be defined.
Both IL-15 and Anti-CD3 Stimulation Induces IFNγ and TNFβ Production in Memory Phenotype CD8+ T Cells.
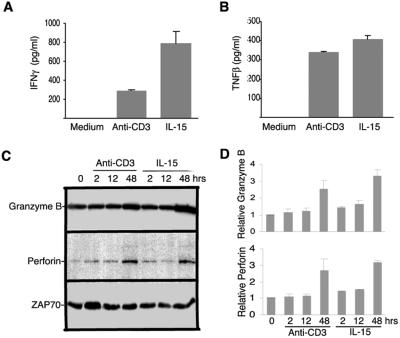
One of the characterized features of CD8+ T cell activation is synthesis of certain cytokines (23). IFNγ and TNFβ were two key cytokines whose mRNA was up-regulated as early as 2 h in memory phenotype CD8+ T cells after IL-15 and anti-CD3 stimulation from cDNA array analysis (Fig. 2E*), which was subsequently confirmed by RNase protection assay (data not shown). To determine protein expression of these two cytokines, we measured IFNγ and TNFβ by ELISA and detected both proteins in the supernatant of memory phenotype CD8+ T cells after 2 days stimulation with either IL-15 or anti-CD3 (Fig. 3 A and B) but not in the supernatant of unstimulated cells.
Figure 3.
Effector molecule synthesis in memory phenotype CD8+ T cells after IL-15 or anti-CD3 treatment in vitro. IFNγ (A) and TNFβ (B) production in memory phenotype CD8+ T cells. Supernatants were collected 2 days after culture, and measured for the IFNγ and TNFβ by ELISA. Three independent donors were measured, and the results from a representative donor are shown. (C) Granzyme B and perforin protein synthesis in memory phenotype CD8+ T cells. Freshly isolated and stimulated memory phenotype CD8+ T cells were lysed and analyzed by Western blotting for granzyme B and perforin protein. The stimulation conditions are indicated at the top. ZAP70 is used as a loading standard. (D) The relative levels of Granzyme B and perforin protein were quantified based on the intensity of ZAP70 and average from two independent experiments by densitometry and IMAGEQUANT software.
Both IL-15 and Anti-CD3 Stimulation Induce Granzyme B and Perforin Production in Memory Phenotype CD8+ T Cells.
Granzymes and perforin are two key effector molecules in CD8+ T cell-mediated cytolysis (23, 24). The mRNA of both granzyme B and perforin were detected in freshly isolated cells and were up-regulated in in vitro stimulated memory phenotype CD8+ T cells from cDNA array analysis and RNase protection assay, but neither of them reached 2-fold increase after stimulation (data not shown). To examine granzymes and perforin protein expression in stimulated memory phenotype CD8+ T cells, we measured the protein levels of granzyme B and perforin by Western blot. Granzyme B protein was detected whereas perforin protein levels were very low in freshly isolated memory phenotype CD8+ T cells (Fig. 3 C and D). After IL-15 and anti-CD3 stimulation, both granzyme B and perforin were not increased at 2 and 12 h, but were significantly increased at 48 h after stimulation (Fig. 3 C and D).
IL-15-Induced Cytotoxic Activity in Memory Phenotype CD8+ T Cells.
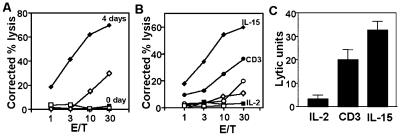
To determine the functional consequences of induction of cytotoxic effector molecules in memory phenotype CD8+ T cells after IL-15 treatment, we used a redirected cytotoxicity assay to measure the granule exocytosis cytotoxicity pathway of these cells. When freshly isolated memory phenotype CD8+ T cells were assayed, no detectable cytotoxicity existed, but after 4 days of culture with IL-15 (100 ng/ml), significant cytotoxic activity was detected (Fig. 4A). IL-15 induced equal or greater levels of cytotoxicity than anti-CD3 in memory phenotype CD8+ T cells in eight of nine independent donors, with a typical result shown in Fig. 4B. In contrast, IL-2 alone (10 units/ml) did not induce significant cytotoxicity in most donors (e.g., Fig. 4B), whereas minimal cytotoxicity was seen in three of nine donors. Mean cytotoxicity induced in all nine donors, expressed as lytic units, is shown in Fig. 4C. Because the redirected cytotoxicity assay was mediated by means of an anti-CD3 antibody, we compared CD3 expression in IL-15- and anti-CD3-treated memory phenotype CD8+ T cells by flow cytometry and found no significant difference in CD3 expression between these two different treatments (data not shown).
Figure 4.
Cytotoxic function of IL-15- and anti-CD3-stimulated memory phenotype CD8+ T cells. (A) Freshly isolated (labeled as control, squares) and IL-15 (diamonds) stimulated (4 days in culture) memory phenotype CD8+ T cells were tested by redirected cytotoxicity assay. The target cells (Fas− L1210) were streptavidin-coated and mixed with the memory phenotype CD8+ T cells in the presence (closed symbols) or absence (open symbols) of biotinylated anti-CD3 mAb. (B) One representative donor of nine independent donors analyzed is shown. Purified memory phenotype CD8+ T cells were cultured with IL-15 or -2 for 4 days, or on plate-bound anti-CD3 for 1 day and transferred to fresh wells and cultured without exogenous cytokine for another 3 days. Cytotoxicity was then measured on Fas− L1210 target cells by redirected cytotoxicity assay as above. Stimulation: IL-15 (diamonds), anti-CD3 mAb (circles), and IL-2 (squares). (C) Mean cytotoxicity, expressed in lytic units (100× reciprocal of the number of cells required to produce 50% lysis) of memory phenotype CD8+ T cells from nine independent donors stimulated with IL-2, CD3, or IL-15. (Error bars show SEM.)
Differentially Down- and Up-Regulated Genes in Memory Phenotype CD8+ T Cells After IL-15 and Anti-CD3 Stimulation.
Among the cDNA clones that are differentially regulated after IL-15 stimulation but not after anti-CD3 stimulation, two (CD44 and Mel transforming oncogene) were down-regulated (Fig. 2D), and 29 were up-regulated (Fig. 2E). Six of the up-regulated genes are known for their involvement in the immune response: two IFN-induced proteins (15- and 56-kDa proteins), two chemokine receptors (CCR1 and CCR2), CD26, and CD53# (Fig. 2E). Other genes include transcriptional regulators (activating transcription factor 4, heterogeneous ribonucleoprotein F, and inhibitor of DNA binding 4) and cytoskeleton proteins (actinin alpha 4, profilin-1, actin-binding protein 1A, and actin-related protein).
Among the cDNA clones that are differentially regulated after anti-CD3 stimulation, two were down-regulated, IFN-induced transmembrane protein I and calmodulin-1 (Fig. 2D), and 11 were up-regulated including CD2#, CD83, epidermal growth factor-response factor 1, and CC-chemokine (Fig. 2E).
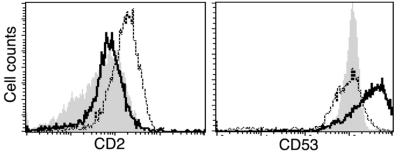
To determine whether the differential quantity of mRNA translates to different expression at the protein level, we measured CD53 and CD2 expression by fluorescence-activated cell sorter analysis. A significant increase in CD53 expression was detected only in IL-15-treated CD8+ memory T cells, whereas a significant increase of CD2 expression was found only in anti-CD3 treated in memory phenotype CD8+ T cells (Fig. 5).
Figure 5.
Profiles of cell surface marker expression on memory phenotype CD8+ T cells after IL-15 or anti-CD3 treatment in vitro. Fluorescent intensity (FI) of CD2- or CD53-expressing memory phenotype CD8+ T cells before and after stimulation with IL-15 or anti-CD3. The FI overlays of CD2 (Left) and CD53 (Right) on the surface of memory phenotype CD8+ T cells of unstimulated (gray filled) and stimulated with IL-15 (dotted line) or anti-CD3 (solid line) for 48 h are shown. All experiments were performed with cells from three donors, and the representative results from one donor are shown.
Discussion
The molecular and cellular events of IL-15 action on memory phenotype CD8+ T cells have been assessed in parallel with TCR-mediated activation. Our analysis points to a remarkable resemblance between IL-15- and anti-CD3-induced changes. First, memory phenotype CD8+ T cells proliferate at a similar rate in response to both stimulation conditions during a course of 4 days. Second, a gene expression analysis by cDNA microarray showed that these cells display a striking similarity in the overall changes in gene expression profile (77% of total changed clones) induced by IL-15 and anti-CD3 stimulation. Third, protein analysis of functional effector molecules including IFNγ, TNFβ, granzyme B, and perforin exhibited similar expression in memory phenotype CD8+ T cells after either stimulation. Fourth, cytotoxic activity in these cells was induced by both stimuli. Finally, differentially induced genes/ESTs after IL-15 or anti-CD3 stimulation were identified. These results identify the components of IL-15 action and reveal similarities between IL-15 stimulation and antigen-mediated activation in memory phenotype CD8+ T cells. Thus, the survival and effector functions of CD8+ memory T cells could be maintained by IL-15 in the absence of antigen.
Activation of memory CD8+ T cells through TCR engagement results in a series of changes leading to proliferation and production of effector molecules including IL-2. Thus, some of the observed gene expression after anti-CD3 stimulation could be caused by the IL-2R, which shares common β- and γ-chains with the IL-15R. Although we were unable to detect IL-2 protein in anti-CD3-stimulated memory phenotype CD8+ T cells (data not shown), we cannot rule out a contribution of IL-2R-induced genes. Their influence would be expected to increase with time after stimulation, and would be predicted to lead to more similarity between anti-CD3- and IL-2-induced genes at later times. However, we observed that the difference in gene expression between IL-15- and anti-CD3-stimulated memory CD8+ T cells increases with time, i.e., greater differences occur in gene expression between anti-CD3 and IL-15 stimulation at 48 h than at 2 or 12 h (Fig. 2E). Therefore, the contribution of IL-2 to the extensive similarities of memory CD8+ T cell response to anti-CD3 or IL-15 is likely to be minimal.
The physiological importance of IL-15 in the homeostasis of CD8+ memory T cells has been demonstrated from both in vivo (9, 25–27) and in vitro studies (7, 28). IL-15 is capable of selectively stimulating memory phenotype CD8+ T cells through initial interaction with IL-2Rβ chain (7) and leads to a series of signaling events that include activation of JAK/STAT pathways (10, 12, 29). However, the molecular nature of downstream events of IL-15 action is less well characterized. In this report, we analyzed the gene expression changes in memory phenotype CD8+ T cells for 2 days after IL-15 treatment and identified 176 cDNA clones (63 down- and 113 up-regulated) whose expression was significantly changed at one or more times during the course. One general picture emerging from such kinetic assessments of gene expression reveals that the down-regulation occurs early and is stable over time, whereas the up-regulation occurs at all time points with more genes increased at the later points (10, 12, 29). This kinetic pattern of expression suggests that the down-regulated clones serve to maintain the “resting” status of the cells, that they may not play active roles in stimulated cells, and that genes related to cell division and effector function are not significantly induced until the later time points.
Indeed, recent studies of gene expression in mouse and human T cells revealed that a large group of genes were down-regulated after stimulation (17, 21, 22), and that some of those down-regulated genes prevent cells from entering the cell cycle and apoptosis (30, 31). Our results show that IL-15-induced down-regulation of genes with diverse functions relating to cell surface receptors (IL-7 receptor, CD44, CD69, and CXCR4), and many signaling molecules (LYN, FYN, FYN-binding protein, Jun B proto-oncogene, IL-1 receptor-binding protein, cAMP-dependent protein kinase, and NFKBIA, etc.). It is conceivable that these highly expressed genes may play roles in yet to be characterized functions in resting memory phenotype CD8+ T cells. Further characterization of their function will shed new light on the mechanisms underlying the maintenance of the “resting” status, as well as the survival of CD8+ memory T cells.
IL-15 induced numerous up-regulated genes/ESTs in memory phenotype CD8+ T cells. Many of those genes are involved in signal transduction, transcription regulation, T cell activation, cell cycle, apoptosis, and effector functions, which are characteristic changes shared with TCR-mediated antigen-specific activation. The enhanced expression of those DNA-replication- and cell-cycle-related genes correlates well with cellular proliferation, suggesting that IL-15 stimulation, like TCR crosslinking, effectively turns on the cell cycle machinery in memory phenotype CD8+ T cells. The IL-15-induced up-regulation of cytotoxic effector proteins also correlates with measurements of cytotoxic effector function of these cells. In contrast to findings in another system (32), fresh memory phenotype CD8+ T cells did not show cytotoxic activity, but a 4-day culture in IL-15 induced at least as much cytolytic function as anti-CD3 stimulation. Such TCR-independent activation may explain examples of bystander killing in vivo.
Despite a remarkable similarity in gene expression, a few differentially expressed clones were identified under IL-15 or anti-CD3 stimulation in memory phenotype CD8+ T cells. Among them, we have identified two surface molecules, CD53 and CD2, that are differentially expressed after these two forms of stimulation. CD53, a glycoprotein involving signal transduction (33), is selectively enhanced after IL-15 treatment. CD2, another glycoprotein involving T cell signaling and cell adhesion (34), is significantly increased after anti-CD3 stimulation. The differential expression of these cell surface markers indicates the differences of memory phenotype CD8+ T cells under those two stimulation conditions. These markers are valuable for further determining the significance of the subtle differences of IL-15 and TCR crosslinking-induced changes in CD8+ memory T cells.
Acknowledgments
We thank Richard Hodes and Dan Longo for reading the manuscript and insightful comments. We also thank Kevin Becker for making custom array filters, Peter Munson for the assistance of using P-SCAN, Stephen Shaw and Carl June for providing valuable antibodies, and the National Institutes of Health blood bank and National Institute on Aging Clinical Core Lab for assistance in obtaining blood samples.
Abbreviations
- TNF
tumor necrosis factor
- TCR
T cell receptor
- mAb
monoclonal antibody
- EST
expressed sequence tag
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sprent J, Surh C D. Curr Opin Immunol. 2001;13:248–254. doi: 10.1016/s0952-7915(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 2.Unutmaz D, Pileri P, Abrignani S. J Exp Med. 1994;180:1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tough D F, Sun S, Zhang X, Sprent J. Vaccine. 2000;18:1642–1648. doi: 10.1016/s0264-410x(99)00500-9. [DOI] [PubMed] [Google Scholar]
- 4.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann T A, Dubois S, Tagaya Y. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 6.Schluns K S, Kieper W C, Jameson S C, Lefrancois L. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 8.Huang L R, Chen F L, Chen Y T, Lin Y M, Kung J T. Proc Natl Acad Sci USA. 2000;97:3406–3411. doi: 10.1073/pnas.060026497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X C, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek T R, Strom T B. Nat Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 10.Tagaya Y, Bamford R N, DeFilippis A P, Waldmann T A. Immunity. 1996;4:329–336. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 11.Russell S M, Johnston J A, Noguchi M, Kawamura M, Bacon C M, Friedmann M, Berg M, McVicar D W, Witthuhn B A, Silvennoinen O, et al. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 12.Beadling C, Guschin D, Witthuhn B A, Ziemiecki A, Ihle J N, Kerr I M, Cantrell D A. EMBO J. 1994;13:5605–5615. doi: 10.1002/j.1460-2075.1994.tb06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanegane H, Tosato G. Blood. 1996;88:230–235. [PubMed] [Google Scholar]
- 14.Doherty P C, Christensen J P. Annu Rev Immunol. 2000;18:561–592. doi: 10.1146/annurev.immunol.18.1.561. [DOI] [PubMed] [Google Scholar]
- 15.Hamann D, Baars P A, Rep M H, Hooibrink B, Kerkhof-Garde S R, Klein M R, Van Lier R A. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weng N, Levine B L, June C H, Hodes R J. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K, Li Y, Prabhu V, Young L, Becker K G, Munson P J, Weng N N. J Immunol. 2001;166:7335–7344. doi: 10.4049/jimmunol.166.12.7335. [DOI] [PubMed] [Google Scholar]
- 18.Liu K, Hodes R J, Weng N. J Immunol. 2001;166:4826–4830. doi: 10.4049/jimmunol.166.8.4826. [DOI] [PubMed] [Google Scholar]
- 19.Kuo C T, Veselits M L, Leiden J M. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 20.Marrack P, Mitchell T, Bender J, Hildeman D, Kedl R, Teague K, Kappler J. Immunol Rev. 1998;165:279–285. doi: 10.1111/j.1600-065x.1998.tb01245.x. [DOI] [PubMed] [Google Scholar]
- 21.Teague T K, Hildeman D, Kedl R M, Mitchell T, Rees W, Schaefer B C, Bender J, Kappler J, Marrack P. Proc Natl Acad Sci USA. 1999;96:12691–12696. doi: 10.1073/pnas.96.22.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogge L, Bianchi E, Biffi M, Bono E, Chang S Y, Alexander H, Santini C, Ferrari G, Sinigaglia L, Seiler M, et al. Nat Genet. 2000;25:96–101. doi: 10.1038/75671. [DOI] [PubMed] [Google Scholar]
- 23.Harty J T, Tvinnereim A R, White D W. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 24.Henkart P A. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 25.Lodolce J P, Boone D L, Chai S, Swain R E, Dassopoulos T, Trettin S, Ma A. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 26.Marks-Konczalik J, Dubois S, Losi J M, Sabzevari H, Yamada N, Feigenbaum L, Waldmann T A, Tagaya Y. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy M K, Glaccum M, Brown S N, Butz E A, Viney J L, Embers M, Matsuki N, Charrier K, Sedger L, Willis C R, et al. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanchot C, Lemonnier F A, Perarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 29.Ihle J N, Kerr I M. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 30.Marrack P, Mitchell T, Hildeman D, Kedl R, Teague T K, Bender J, Rees W, Schaefer B C, Kappler J. Curr Opin Immunol. 2000;12:206–209. doi: 10.1016/s0952-7915(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 31.Kuo C T, Leiden J M. Annu Rev Immunol. 1999;17:149–187. doi: 10.1146/annurev.immunol.17.1.149. [DOI] [PubMed] [Google Scholar]
- 32.Opferman J T, Ober B T, Ashton-Rickardt P G. Science. 1999;283:1745–1748. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 33.Olweus J, Lund-Johansen F, Horejsi V. J Immunol. 1993;151:707–716. [PubMed] [Google Scholar]
- 34.Hahn W C, Burakoff S J, Bierer B E. J Immunol. 1993;150:2607–2619. [PubMed] [Google Scholar]