Abstract
Carbonic anhydrases (CAs) are crucial for cancer cells to survive in hypoxia. Here we show that our newly synthesised 1,8-naphthalimide-piperazine-amidobenzenesulfonamide derivative, namely compound Q, specifically targets CA IX and causes cell death in colorectal cancer. Compound Q stably binds to the zinc atom in the active pocket of CA IX and selectively inhibits the activity of this enzyme. It kills SW480 cells under normoxic and hypoxic conditions, with an IC50 of 17.03 ± 1.09 μM and 10.90 ± 0.46 μM, respectively. The inhibitory effect of compound Q against CA IX activity is better under hypoxic conditions and it has low toxicity on normal colon with an IC50 of 38.83 ± 1.98 μM. Compound Q also inhibits tumour growth in the colorectal cancer SW480 xenograft model and it shows no adverse effects on nude mice body weight. Our analyses also demonstrate that compound Q induces ferroptosis, apoptosis and autophagy in colorectal cancer and we believe that these are the main mechanisms by which it promotes cell death in this cancer. Taken together, our data indicate that compound Q is a potent and selective CA IX inhibitor that is promising for the treatment of colorectal cancer.
Compound Q targets the transmembrane CA IX protein and inhibits its activity, leading to cell death in colorectal cancer by inducing ferroptosis, apoptosis and autophagy.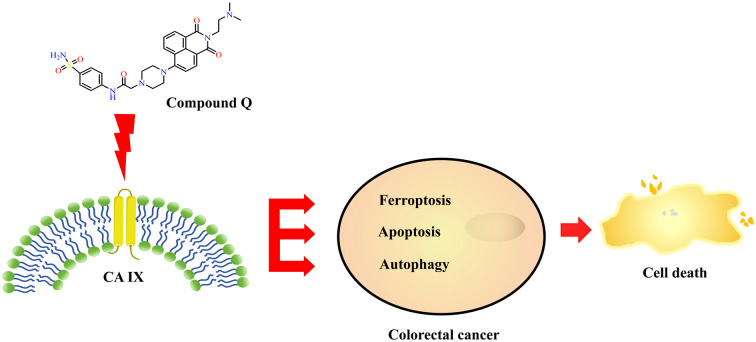
Introduction
Approximately 10% of global cancer cases in 2024 are caused by colorectal cancer (CRC), putting it as the third most common cancer in the world and the second leading cause of cancer-related deaths worldwide.1 Various treatment options are available to treat and manage this disease including surgery, radiotherapy, targeted chemotherapy and immunotherapy.2 However, the toxicity and resistance that are developed in CRC are concerning and therefore new therapeutic strategies are demanded to improve the prognosis of CRC patients.
Our group found that carbonic anhydrases (CAs) have a high potential to be a molecular target for CRC. CAs regulate multiple physiological processes and are crucial to maintain the homeostasis of the internal environment.3,4 CA IX is one of the isoenzymes of human CAs, which is expressed in many solid tumours to help adapt to hypoxic environments.5–8 The distribution of CA IX in cancer cells is related to the occurrence and development of multiple tumours. Therefore, we believe that CA IX can become a drug target for the treatment efficacy, drug resistance, and prognosis of CRC.9,10
CA IX is linked to ferroptosis in cancer cells. Ferroptosis is a novel programmed cell death mechanism that differs from apoptosis and autophagy, and it has a great potential for the treatment of cancer, neurodegenerative diseases, and ischemia–reperfusion injury.11–16 Studies found that inhibiting CA IX affects autophagy activity, suggesting that this enzyme can promote tumour growth and drug resistance by regulating autophagy.17–19 We also notice that iron-induced death and autophagy can regulate each other.20–22 In this case, we believe that inducing ferroptosis and autophagy may bypass apoptosis resistance and become a new treatment strategy for cancer.
Sulfonamide inhibitors are an early studied class of CA inhibitors.23 The CA IX inhibitor, namely SLC-0111, has been used successfully in phase I clinical trials for the treatment of advanced metastatic solid tumours.24–26 Studies have also shown that 1,8-naphthalimide is promising for cancer treatment as introducing different substituents at its 4-position can result in efficient and low-toxicity derivatives.27,28 Considering the potential of sulfonamides and 1,8-naphthalimide derivatives, we synthesised 1,8-naphthalimide piperazine amidobenzenesulfonamide derivatives based on 1,8-naphthalimide and sulfonamide structures. These derivatives target CA IX and kill breast cancer cells by inducing ferroptosis.29
Therefore, from here, we continue synthesising a new 4-(N-dithiobenzylpiperazine)-1,8-naphthylamide derivative with the substituent N,N′-dimethylethylenediamine, namely compound Q. This compound has good selectivity and inhibitory activity against CA IX in SW480 cells. Since CA IX is induced by hypoxia and mainly expressed in hypoxic tumour areas,30,31 we decided to focus on this study under hypoxic conditions. So far, we found that compound Q has great cytotoxicity towards CRC cells in the presence of low oxygen. This may improve the therapeutic effect on solid tumours, which we believe is promising for the development of chemotherapy drugs targeting hypoxic CRC cells.
Results and discussion
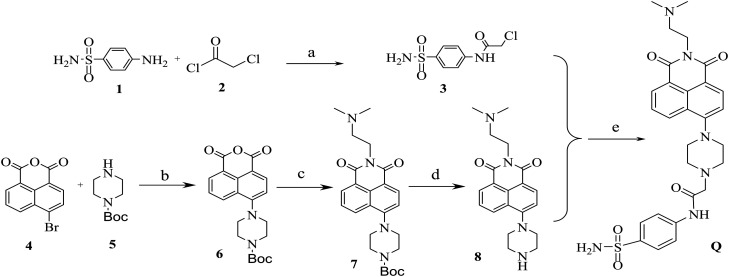
The synthesis of compound Q
Compound Q was synthesised using p-aminobenzenesulfonamide and 4-bromo-1,8-naphthalic anhydride as outlined in Scheme 1. The reaction of p-aminobenzenesulfonamide 1 with chloroacetyl chloride 2 in acetone and potassium carbonate (K2CO3) at 25 °C gave the intermediate compound 3. The reaction of 4-bromo-1,8-naphthalic anhydride 4 with N-Boc-piperazine 5 in anhydrous ethylene glycol methyl ether at 125 °C produced the intermediate 6. The intermediate compound 7 was obtained by treating the intermediate compound 6 with N,N′-dimethylethylenediamine in ethanol at 80 °C. Then, the intermediate compound 7 was deprotected with trifluoroacetic acid to afford the intermediate compound 8. The reaction of the intermediate compound 3 and compound 8 in N,N-dimethylformamide at 130 °C finally produced compound Q. We evaluated the structure of compound Q using 1H NMR, 13C NMR and HR-MS spectroscopy, and the data are provided in the ESI.†
Scheme 1. Synthetic route through which compound Q was produced. Reagents and reaction conditions: (a) acetone, K2CO3, 25 °C, 4 h; (b) ethylene glycol methyl ether, 125 °C, 3 h; (c) ethanol, 80, 3 h; (d) trifluoroacetic acid, triethylamine, 25 °C, 3 h; (e) N,N-dimethylformamide, 130 °C, 4 h.
Compound Q inhibited CA IX at its active site
Molecular docking showed that the sulfonamide group of compound Q and SLC-0111 penetrated deep into the active pocket of CA IX protein (PDB 5FL4) and ionised as well as chelated the zinc ions at the bottom of the active cavity (Fig. 1). The amino acid residues that were involved in the interaction between compound Q and CA IX protein were Gln92, His94, Val121, Arg129, Val130, Asp131, Leu134, Leu140, Val142, Leu199, Thr200 and Pro203, many of which were consistent with the literature reports on Gln92, Val130, Leu134, Leu199, Thr201, and Pro203.32 The amino acid residues that were involved in the interaction between SLC-0111 and CA IX protein also overlapped with compound Q and the original ligand (Fig. 1). We also indicate the hydrogen bonds formed between compound Q and SLC-0111 with CA IX protein due to the important roles of these bonds in maintaining the conformation of secondary, tertiary, and quaternary protein structures (Fig. 1). Compared to the positive control SLC-0111, compound Q formed more hydrogen bonds with CA IX protein, hence creating a more stable conformation between compound Q and CA IX. In agreement with a previous report,32 Zn2+ was connected to the amino acids His94, His96, and His119 at the bottom of the CA IX pocket (Fig. 1A). The ionisation of the amino group on the sulfonyl group of compound Q allowed interaction with the Zn2+ coordination. The two oxygen atoms on the sulfonyl group interacted with the amino acid residues Thr200 (1.9 Å, 135.2°), Leu199 (2.6 Å, 152.3°), and His94 (2.6 Å, 113.5°) to form hydrogen bonds. The hydrogen atom of the amino group on the sulfonamide group also formed hydrogen bonds with the amino acid residue Thr200 (2.1 Å, 111.9°). On another end of compound Q, one carbonyl oxygen atom on the 1,8-naphthalimide skeleton and two hydrogen atoms on the methyl group formed hydrogen bonds with the amino acid residues Arg129 (2.4 Å, 130.8°) and Asp131 (1.9 Å, 131.2°) (2.9 Å, 95.3°), respectively.
Fig. 1. 2D and 3D diagrams of the predicted binding of CA IX (PDB 5FL4) with (A) compound Q and (B) SLC-0111. In the 3D diagrams, the green colour indicates compound Q or SLC-0111, the yellow straight line represents hydrogen bond interaction, the yellow dotted line represents hydrogen bond angle, and the grey ball represents zinc atom.
After examining the binding of compound Q on the CA IX enzyme, we determined its inhibition against CA IX using p-nitrophenol acetate (4-PNA) as a substrate. For this, we compared the efficacy of compound Q in inhibiting CA IX with CA II and we used SLC-0111, the first-in-class of selective CA IX inhibitors, as a positive control. Like SLC-0111, our results indicated that compound Q decreased the activity of both CA IX and CA II with an inhibitory IC50 value of 0.08 μM and 0.52 μM, respectively (Table 1). Compound Q also showed a selectivity index (SI) of 6.54 towards CA IX over CA II, which is close to the SI of 10.49 of SLC-0111 towards CA IX over CA II. Altogether, this demonstrates that compound Q is a selective CA IX inhibitor, and it has better potency and selectivity to CA IX over CA II.
Table 1. In vitro inhibition of compound Q against CA II and CA IX.
| Compound | CA IX | CA II | SIa (CAII/CA IX) |
|---|---|---|---|
| IC50 (μM) ± SD | IC50 (μM) ± SD | ||
| Q | 0.08 ± 0.00 | 0.52 ± 0.18 | 6.54 |
| SLC-0111 | 0.05 ± 0.01 | 0.57 ± 0.16 | 10.49 |
Selectivity index (SI) = CA II IC50 value/CA IX IC50 value.
Compound Q inhibited the proliferation of CRC cells
We hypothesise that compound Q has a strong inhibitory activity under hypoxic conditions33 as CA IX is highly expressed in the hypoxic area of solid tumours.3,19 This would be good for cancer treatment strategies, and therefore we selected colorectal cancer HCT116 and SW480 cell lines to determine its cytotoxicity using the MTT assay. The results indicated that compound Q moderately inhibited the proliferation of HCT116 cells with an IC50 of 23.17 μM and 14.79 μM under normoxic and hypoxic condition, respectively (Table 2). Compared to HCT116 cells, compound Q exhibited a better inhibitory activity against SW480 cells with an IC50 of 17.03 μM and 10.90 μM under normoxic and hypoxic condition, respectively. These IC50s were higher than that of mitonafide, which is a positive control to compound Q, and lower than that of SLC-0111 (Table 2). Indeed, the IC50 of compound Q was lower under hypoxia than normoxia, suggesting that SW480 cells were more sensitive to compound Q in the absence of oxygen. These results confirmed our hypothesis that compound Q is more potent under hypoxic conditions.
Table 2. Antiproliferative activity of compound Q on CRC and normal colon cell lines.
| Compound | IC50a (μM) | ||||
|---|---|---|---|---|---|
| SW480 (normoxia) | SW480 (hypoxia) | HCT116 (normoxia) | HCT116 (hypoxia) | NCM460 (normoxia) | |
| Q | 17.03 ± 1.09 | 10.90 ± 0.46 | 23.17 ± 4.50 | 14.79 ± 1.68 | 38.83 ± 1.98 |
| SLC-0111 | >40 | >40 | >40 | >40 | >40 |
| Mitonafide | 12.61 ± 1.57 | 9.14 ± 2.78 | 8.34 ± 1.60 | 6.58 ± 0.82 | 15.46 ± 0.77 |
The IC50 values are expressed as mean ± SD of three separate experiments.
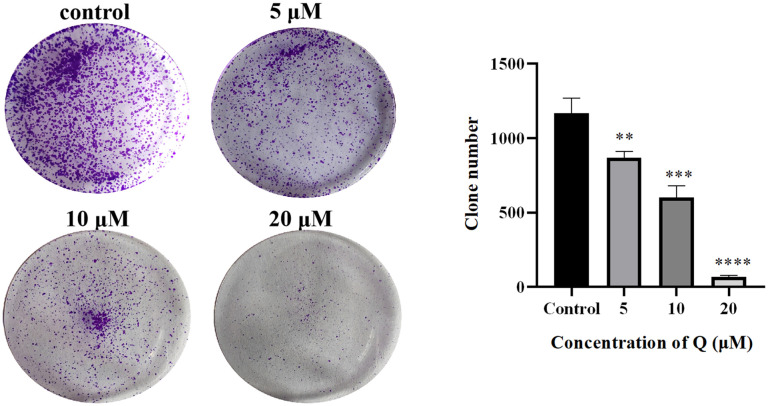
While this is the case in hypoxic conditions, compound Q was safe to normal colon NCM460 cells as it had high tolerance with an IC50 of more than 40 μM (Table 2). Based on these results, we chose 5, 10 and 20 μM compound Q for the treatment under hypoxia. We also determined the effects of compound Q on the growth of SW480 cells using colony formation assays. This was done to gain more confidence with our decision with these concentrations under hypoxic conditions. Our results indicated that the formation of SW480 colony decreased significantly in a dose-dependent manner after 24 h of exposure to compound Q (Fig. 2). This is like other sulfonamide compounds that also have inhibitory effects on CRC cells.34 Based on these results, we believe that compound Q could improve the prognosis of CRC patients, as hypoxia is the main cause of drug resistance and poor prognosis in this disease.35
Fig. 2. Compound Q suppressed the colony formation of CRC cells. Colony formation assays were performed after 24 h of treatment with compound Q under hypoxic (0, 5, 10, 20 μM) conditions. DMSO (0.5% v/v) was used as a vehicle control. **p < 0.01, ***p < 0.001, ****p < 0.0001 versus control.
Compound Q decreased the migration capacity of CRC cells
Previously, CA IX has been reported to enhance the motility and invasion capacity of cancer cells.36 Therefore, we evaluated the effect of compound Q on the migration of CRC cells to determine if it could prevent the progression of this disease. Our results showed that in contrast to the control group, compound Q decreased the migration of SW480 cells in a dose-dependent manner (Fig. 3), demonstrating the therapeutic potential of this compound in inhibiting CRC cells from metastasising.
Fig. 3. The inhibition of cell migration by compound Q. Compound Q (0, 5, 10, 20 μM) blocked the migration of CRC cells under hypoxia as illustrated in the statistical analysis of SW480 cell migration. DMSO (0.5% v/v) was used as a vehicle control. Scale bar: 200 μm. ****p ≤ 0.0001 compared to the control group.
Compound Q caused cell cycle arrest at the G2/M phase
Since compound Q was implicated in the growth of SW480 cells, we then performed cell cycle analysis to determine if cell proliferation was inhibited due to cell cycle arrest. The phase distribution of SW480 cells in the cell cycle was determined using a flow cytometer after propidium iodide (PI) staining. We found that compound Q increased SW480 cell ratio at the G2/M phase in a concentration-dependent manner (Fig. 4). Specifically, the percentage of cells at the G2/M phase increased gradually from 17.81% in the control group to 19.73%, 23.53% and 24.41% in response to 5, 10 and 20 μM compound Q. This is in line with previous research by Abbas et al., who found that sulfonamide compounds could arrest cell cycle in the G2/M phase in various lines of cancer including breast and liver cancer.37 These data indeed further supported the ability of compound Q in inhibiting the activity of CRC cells.
Fig. 4. SW480 cells were treated with compound Q under hypoxia at the concentrations of 5, 10 and 20 μM. After 24 h, the cells were harvested and fixed with ethanol overnight before they were stained with PI and analysed by flow cytometry. Data are expressed as mean ± SD from three independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 compared to control.
Compound Q induced apoptosis in CRC cells
Apoptosis failure contributes to the development and progression of CRC, apart from poor response to chemotherapy and radiotherapy. The activation of apoptosis is one of the treatment approaches for CRC patients. Therefore, we determined whether compound Q led to apoptosis in SW480 cells as part of its mechanism of inducing cell death. We found that this compound dose-dependently increased the proportion of apoptotic CRC cells (Fig. 5). Specifically, the apoptotic cells increased from 10.73 ± 1.67% in the control group to 22.14 ± 2.20%, 27.41 ± 3.50%, and 41.92 ± 4.73% at 5, 10, and 20 μM compound Q. These results were consistent with the findings by Tuluce et al.,38 and therefore we further analysed the expression of apoptosis-related proteins such as caspase-3, Bcl-2 and Bax. The activation of caspase-3 is a hallmark of apoptosis, and the cleaved caspase-3 protein serves as a significant biomarker of apoptosis.39
Fig. 5. Compound Q induced apoptosis in SW480 cells. (A) SW480 cells were treated with compound Q under hypoxia (0, 5, 10, 20 μM) for 24 h. The cells were stained with Annexin V-FITC/PI and analysed by flow cytometry. Data are expressed as mean ± SD from three independent experiments. ****p < 0.0001 compared to control. (B) Total lysates treated with compound Q were subjected to Western blotting to detect the apoptotic signature proteins caspase-3/cleaved-caspase-3, Bcl-2 and Bax. (C) SW480 cells were treated with compound Q under hypoxia (0, 5, 10, 20 μM) for 24 h and the cells were stained with JC-1 dye. Scale bar: 100 μm.
When the caspase apoptotic pathway is activated, caspase-3 cleaves specific substrates, thereby mediating the apoptosis process. When apoptosis signals appear in cells, the expression of Bax and Bcl-2 is also affected. Our analyses indicated that the expression of caspase-3/cleaved-caspase-3 and Bax was upregulated after 24 h of exposure to compound Q, whilst the expression of Bcl-2 was downregulated (Fig. 5B). This agrees with the fact that in CRC, Bcl-2 and Bax are negatively correlated, and the overexpression of the anti-apoptotic Bcl-2 protein correlates with the recurrence, poor prognosis and chemoresistance of this cancer.40
To further understand the implications of compound Q on SW480 cells, we tracked changes that occurred upon its treatment in the mitochondria. This is because a decrease in the mitochondrial membrane potential (MMP) is an important event in apoptosis.41 For this, we conducted JC-1 assays to detect apoptosis after treating SW480 cells for 24 h with compound Q at 0, 5, 10, 20 μM under hypoxia. JC-1 is a mitochondria selective aggregate dye that will show a change in emission colour from green to red if the MMP becomes more polarised.42 Basically, JC-1 forms “J-aggregates” at high membrane potential, hence displaying red fluorescence. However, it is green in colour if the MMP decreases. In our case, the fluorescence variations of JC-1 in the compound Q-treated cells implied that the drug decreased the MMP in a concentration-dependent manner, suggesting that compound Q evoked apoptosis in CRC cells (Fig. 5C).
Compound Q induced ferroptosis in CRC cells
The inhibition of CA IX activity was reported previously to promote ferroptosis in cancer cells.43 Therefore, to determine if compound Q might have caused ferroptosis in SW480 cells, we performed cell viability assays. We used the ferroptosis inhibitor, ferrostatin-1 (Fer-1), to verify if it could reverse the inhibition of cell proliferation by compound Q. Interestingly, the viability of SW480 cells improved in the presence of 5 μM Fer-1 compared to compound Q alone, and this effect was observed after 48 h of treatment, suggesting that ferroptosis might be a unique mechanism through which compound Q induced cell death in CRC cells (Fig. 6A).
Fig. 6. Compound Q induced ferroptosis in SW480 cells. (A) MTT assay was performed on SW480 cells exposed to compound Q with and without the presence of 5 μM Fer-1 under hypoxic conditions. (B) SW480 cells were treated with compound Q for 24 h under hypoxia (5, 10, 20 μM) and subjected to C11 BODIPY 581/591 staining for lipid peroxide formation. Cells were visualised under a fluorescence microscope using a 20× magnification objective with a scale of 100 μm. Red fluorescence denotes the non-oxidised state, and green fluorescence denotes the oxidised state. (C) SW480 cells were treated with compound Q for 24 h under hypoxia (5, 10, 20 μM), and the cells were harvested and stained with DCFH-DA and analysed by flow cytometry. Data are expressed as mean ± SD from three independent experiments. **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to control. (D) SW480 cells were treated with compound Q under hypoxia (5, 10, 20 μM) for 24 h and total cell lysates were extracted to detect the ferroptosis signature proteins such as FACL4, GPX4, and SLC7A11.
Since ferroptosis is characterised by reduced cell defence against oxidative stress, altered iron homeostasis, and abnormal lipid peroxidation in mammalian cells,44 we measured the levels of lipid peroxide and reactive oxygen species (ROS) to support our findings that the death of SW480 cells was due to ferroptosis. For this, we detected the intracellular lipid peroxide by a C11 BODIPY 581/591 probe and evaluated the formation of intracellular ROS using a DCFH-DA fluorescent probe. We found that the increment of lipid peroxide occurred in a dose-dependent manner in the presence of compound Q (Fig. 6B). Indeed, the fluorescence intensity was low when Fer-1 was co-incubated with compound Q compared to compound Q alone, indicating that the ferroptosis inhibitor Fer-1 decreased the production of lipid peroxide in SW480 cells. We also found that the increase in the cellular ROS content occurred parallel with lipid peroxidation in SW480 cells with compound Q increased the mean fluorescence of the DCFH-DA probe in a dose-dependent manner (Fig. 6C).
Additionally, compound Q downregulated the expression of intracellular ferroptosis-related proteins, GPX4 and SLC7A11 (Fig. 6D), whilst it upregulated the expression of FACL4 (Fig. 6D). These suggest that compound Q might trigger ferroptosis via the GPX4 and FACL4 pathways. This is because the inactivation of GPX4 is a critical event in the process of ferroptosis.45 GPX4 is a key regulator of ferroptosis, and it inhibits this process by reducing lipid peroxides. SLC7A11 is a key component of the cystine–glutamate antiporter system (Xc-), which is responsible for transporting extracellular cystine into cells while exporting glutamate out of cells. SLC7A11-mediated cystine uptake is the rate-limiting step in GSH synthesis, and SLC7A11 indirectly supports GPX4 activity by maintaining GSH levels. GPX4 and SLC7A11 constitute the system Xc-GSH-GPX4 ferroptosis regulatory pathway.46 FACL4 is a key positive regulator of ferroptosis, as it converts the long-chain polyunsaturated fatty acids (PUFAs) into acyl-CoA, which is then integrated into cell membrane phospholipids. PUFAs are the primary substrates for lipid peroxidation, and thus FACL4 promotes ferroptosis by elevating the synthesis of PUFAs.47
Compound Q changed the morphology of mitochondria in CRC cells
Ferroptosis can be distinguished morphologically from apoptosis, autophagy and necrosis. In this case, if cells are undergoing ferroptosis, mitochondria will undergo ultrastructural changes such as decreased volume, decreased mitochondrial cristae, increased density of mitochondrial membrane, and rupture of the outer mitochondrial membrane.48 Therefore, we further verified ferroptosis induced by compound Q by observing the mitochondria morphological changes using transmission electron microscopy (TEM). From this analysis, we found that the mitochondria in the untreated cells were morphologically normal with well-distributed cristae (Fig. 7). However, after exposing the cells to compound Q, their mitochondria changed in a dose-dependent manner, with ruptured and small-sized mitochondria, reduced mitochondrial cristae and increased membrane density. Unexpectedly, autophagic vesicles were also observed in the cells treated with compound Q, indicating that ferroptosis occurred together with autophagy (Fig. 7).
Fig. 7. Changes in the mitochondria and cellular substructure due to ferroptosis. SW480 cells were treated with compound Q for 24 h at the concentrations of 0, 5, and 15 μM under hypoxia. The cells were fixed with 2.5% glutaraldehyde, and morphological changes were viewed by TEM. Green arrows indicate ferroptosis whereby the mitochondria are wrinkled with the internal crest disappearing and colour deepening. Red arrows indicate autophagy. Scale bars: 2 μm for the top figures and 500 nm for the bottom figures.
Compound Q induced autophagy in CRC cells
Since we found autophagic vesicles in the cytoplasm of SW480 cells after 24 h of treatment with compound Q (Fig. 7), we conducted a cell viability assay to determine if the antitumour activity of compound Q was due to autophagy. For this, we treated the cells with the autophagy inhibitor, namely 3-methyladenine (3-MA), to determine if it triggered cell proliferation inhibited by compound Q. We found that the viability of SW480 cells was improved after 48 h of treatment with compound Q in the presence of 3-MA (50 μM), indicating that autophagy was a part of the mechanisms by which compound Q induced cell death in CRC (Fig. 8B). We confirmed this result with monodansylcadaverine (MDC) staining, as it could label autophagosomes through ion capture and had specific binding with membrane lipid. Relative to control, we observed a stronger fluorescence intensity upon the treatment with compound Q and this occurred dose-dependently, both of which indicating that more autophagosomes were generated in the presence of compound Q (Fig. 8A).
Fig. 8. Compound Q induced autophagy in SW480 cells. (A) The viability of SW480 cells in the presence of compound Q and 50 μM of 3-MA. (B) SW480 cells were treated for 24 h with compound Q under hypoxia (5, 10, 20 μM). The cells were stained with MDC and visualised under a fluorescence microscope using a 20× magnification objective at a scale of 100 μm. (C) Western blotting was performed on SW480 cells after 24 h of treatment with compound Q under hypoxia (5, 10, 20 μM). Total cell lysates were used to detect the autophagy signature proteins p62 and LC3B.
We also examined the expression of autophagy-related proteins LC3B and p62 (SQSTM1) to further validate the occurrence of autophagy. LC3B is an autophagy marker, which is a subfamily of microtubule-associated protein 1 light chain 3 (LC3). Under external stimuli such as starvation, the LC3B precursor (LC3B-I) undergoes ubiquitin-like modification to be converted into LC3B-II, which binds to autophagosome membranes to promote the formation and expansion of autophagosomes. In this case, there is a strong correlation between the amount of LC3B-II and the number of autophagosomes.49 p62 is a scaffold protein that is highly expressed in tumour cells and is responsible for recognising and transporting ubiquitinated proteins to autophagosomes. When autophagy is activated, p62 levels decrease, whereas they will accumulate when autophagy is inhibited.50 After treating SW480 cells with compound Q, changes in the expression of LC3B-I, LC3B-II, and p62 were consistent with our expectations (Fig. 8C). This indicated that the autophagy level was upregulated in SW480 cells following compound Q treatment. This confirmed our results in the morphological studies and in vitro antitumour activity assays.
Compound Q inhibited colorectal tumour growth in vivo
To further evaluate the antitumour efficacy of compound Q, we established a xenograft model by implanting SW480 cells in female BALB/c nude mice. The mice were randomly assigned to three groups after the tumour volume reached approximately 100 mm3, and they were treated with compound Q (20 or 40 mg kg−1) by intraperitoneal injection for 21 consecutive days. We found that compound Q suppressed tumour growth after 21 days of treatment compared to the vehicle group, as shown in Fig. 9A and C. Similarly, compound Q inhibited SW480 tumour growth in a dose-dependent manner with tumour growth inhibition (TGI) of 44.8% and 63.1% at a dose of 20 and 40 mg kg−1, respectively (Fig. 9B). These nude mice did not have any obvious body weight loss or adverse effect over the course of treatment, indicating that compound Q is safe for use (Fig. 9D).
Fig. 9. The effects of compound Q on an SW480 xenograft model. Nude mice were injected intraperitoneally with compound Q (20 or 40 mg kg−1) every 3 days for 21 consecutive days. (A) Pictures of the isolated tumour tissues after 21 days of administration with compound Q. (B) The weight of the tumours excised at the end of compound Q treatment. (C) Changes in tumour volume in different treatment groups measured every 3 days. (D) The effect of compound Q on the body weight of the SW480 xenograft model.
Conclusions
Compound Q was synthesised and characterised as the inhibitor of CA IX for the treatment of CRC. It has a comparable enzyme inhibitory activity and selectivity against CA IX to SLC-0111. Molecular docking revealed that the benzenesulfonamide structure of this compound penetrated deeply into the CA IX binding pocket, while the 1,8-naphthalimide structure interacted with the peripheral region of the active site. In vitro antiproliferative assays showed that compound Q exhibited a superior antitumour activity compared to SLC-0111. In particular, it showed an enhanced inhibitory effect under hypoxic conditions. Additionally, this compound demonstrated a concentration-dependent activity against nude mice xenograft models, and it had no significant side effects on the mice body weight. Mechanistic investigations indicated that compound Q induced cell cycle arrest at the G2/M phase and promoted apoptosis. It also induced ferroptosis and autophagy in CRC cells. Altogether, these findings suggest that compound Q represents a promising novel therapeutic agent for the intervention of CRC.
Experimental
Reagents and instruments
All reagents were purchased and used without further purification unless otherwise stated. NMR spectra were measured on a Bruker Avance AV400 instrument, and mass spectra were examined on a Bruker spectrometer with tetramethylsilane (TMS) as an internal standard in DMSO-d6. High-resolution ESI-MS spectra were recorded on an Agilent 1290-6545 UHPLC-QTOF mass spectrometer and high-performance liquid chromatography (HPLC) was conducted using a 1260-II HPLC system (Agilent Corporation). The purity of compound Q was determined by HPLC.
Synthesis of compound Q
Compound Q was synthesised as illustrated in Scheme 1. Firstly, the reaction of p-aminobenzenesulfonamide 1 (2.15 g, 12.5 mmol) with chloroacetyl chloride 2 (2.00 mL, 25 mmol) in 25 mL acetone and potassium carbonate (K2CO3, 3.45 g, 25 mmol) at 25 °C gave the intermediate compound 3. To the reaction solution water was added to end the reaction and the solution was extracted with ethyl acetate. The organic layer was retained and dried by spin evaporation. The intermediate 6 was obtained by reacting 4-bromo-1,8-naphthalic anhydride 4 (2.77 g, 10 mmol) with N-Boc-piperazine 5 (5.59 g, 30 mmol) in 50 mL anhydrous ethylene glycol methyl ether at 125 °C for 3 h. The reaction was monitored by thin-layer chromatography (TLC). At the end of the reaction, compound 6 was obtained by filtration and washed with anhydrous ethanol. The intermediate compound 7 was obtained by treating the intermediate compound 6 (0.38 g, 1 mmol) with N,N′-dimethylethylenediamine (0.131 mL, 1.20 mmol) in 50 mL ethanol at 80 °C for 3 h. At the end of the reaction, compound 7 was purified using a silicone chromatography column with a dichloromethane : methanol (20 : 1 v/v) system as the mobile phase. The intermediate 7 was deprotected with 10 mL dichloromethane and 5 mL trifluoroacetic acid at 25 °C for 3 h to afford the intermediate compound 8. After the reaction was completed, the intermediate compound 8 was spun-dried by spin distillation. Finally, the reaction of the intermediate 3, compound 8 and NaCO3 in a molar ratio of 2 : 1 : 2 in 20 ml N,N-dimethylformamide (DMF) at 130 °C for 4 h produced a benzenesulphonamide–naphthamide derivative, namely compound Q. After solvent removal, compound Q was purified using a silicone chromatography column with a dichloromethane : methanol (20 : 1 v/v) system as the mobile phase. The structures for the intermediate compounds were confirmed by 1H NMR, 13C NMR and HR-MS spectroscopy.29 Compound Q was dissolved in absolute DMSO to a concentration of 100 mM of mother liquor and diluted with culture medium or physiological saline solution as needed for subsequent experiments.
Compound Q
2-(4-(2-(2-(Dimethylamino)ethyl)-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)piperazin-1-yl)-N-(4-aminosulfonylphenyl)acetamide
HR-MS (m/z): calculated for C28H32N6O5S [M + H]+: 565.2155; found: 565.2228. 1H NMR (400 MHz, DMSO-d6) δ 10.18 (s, 1H), 8.48–8.44 (t, 2H), 8.42–8.40 (d, 1H), 7.86–7.82 (t, 3H), 7.79–7.76 (d, 2H), 7.38–7.36 (d, 1H), 7.28 (s, 2H), 4.16–4.13 (t, 2H), 3.35 (s, 8H), 2.89 (s, 4H), 2.22 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 169.35, 164.10, 163.56, 156.16, 142.03, 139.01, 132.72, 131.18, 131.07, 129.63, 127.10, 126.54, 125.75, 123.02, 119.48, 115.99, 115.55, 61.95, 56.80, 53.08, 52.99, 45.50, 37.53. HPLC purity: 97.39%.
Molecular docking of compound Q and CA IX
The binding of compound Q, SLC-0111 and the original ligand (9EK)32 to CA IX (PDB 5FL4) was examined by molecular docking using Discovery Studio version 2019 software, carried out on the operating platform Windows 11. First, we utilised the prepare ligands module of the Discovery Studio to pre-process small molecules, including original ligand molecules, using the default parameters, followed by energy minimisation. Next, we downloaded CA IX protein from the RCSB protein database and analysed it and used the prepare protein module of the software to process the crystal structure of the protein using the default parameters. For proteins that had undergone certain processes, we selected their eutectic original ligands to generate docking sites, deleted the original ligands, and used the dock ligands (CDOCKER) module of the software to dock the proteins with the prepared small molecules using the default parameters. We selected the model with high stabilisation energy and the binding pattern was generated by PyMOL (version 1.7.X). The redocking of CA IX (PDB 5FL4) with the original ligand compound (9EK) showed a root-mean-square deviation (RMSD) of 1.17 Å, demonstrating the effectiveness of the docking protocol.
CA inhibitory activity assay
The inhibition of compound Q against CA II and CA IX was tested by esterase hydrolysis method. We used p-nitrophenol acetate (4-PNA) in this assay as CAs catalysed the hydrolysis of a colourless p-nitrophenol acetate (4-PNA) to produce yellow 4-p-nitrophenol. Therefore, the activity of CAs was measured by determining the absorbance of 4-p-nitrophenol at 405 nm.51 Additionally, we used the commercially available CA IX inhibitor, namely SLC-0111, as a positive control.
Cell culture
Human CRC HCT116 and SW480 cell lines, and normal colon cell line, NCM460 cells, were purchased from the National Collection of Authenticated Cell Cultures (China). The cells were cultured in Dulbecco's modified Eagle medium (DMEM, Gibco) containing 10% (v/v) foetal bovine serum (FBS, Gibco), 100 U mL−1 penicillin (Sigma) and 100 U mL−1 streptomycin (Sigma). The cells were incubated in a humidified atmosphere containing 5% CO2. The cells were cultured with cobalt(ii) chloride (CoCl2) in a concentration of 100 μM to generate a hypoxic condition.52,53
Cell viability assay
We performed MTT assays to determine cell viability under normoxia and hypoxia and the results were compared with positive control drugs, namely mitonafide and SLC-0111. In this assay, 5.0 × 103 cells were seeded per well in 96-well plates and incubated for 24 h at 37 °C under normoxia and hypoxia. After 48 h of treatment with compound Q, cells were incubated with 5 mg mL−1 MTT solution for 4 h at 37 °C. The formazan crystal formed was dissolved in 150 μL of absolute dimethyl sulfoxide (DMSO) and the plates were read at 490 nm with a microplate reader (BioTek, USA).
Colony formation assay
The long-term inhibitory effects of compound Q on SW480 cells were determined by colony formation assays. Cells were seeded at a density of 1.0 × 103 cells per well in 6-well plates and treated for 24 h with compound Q at 0, 5, 10 and 20 μM under hypoxia. The cells were then cultured with fresh enriched DMEM for another seven days until the number of cells in most of individual clones exceeded 50 cells. Colonies formed were fixed with 4% paraformaldehyde (PFA) and stained with 0.5% crystal violet followed by imaging.
Wound healing assay
SW480 cells were seeded in 6-well plates (3.0 × 105 per well) and incubated under hypoxic conditions. After reaching confluency, the monolayer cells were scratched with a sterile 200 μL pipette tip. The cells were then incubated for 24 h with compound Q at 0, 5, 10, 20 μM under hypoxia. After that, the cells were grown in fresh medium for 48 h under hypoxic conditions. The wounded area at the centre was measured at 0, 24, 48 and 72 h. The wound healing rate was determined by measuring the wound ratio between the closure and the original area54 using Adobe Photoshop 2020 software.
Cell cycle analysis
SW480 cells (1.0 × 105 per well) were seeded in 6-well plates under hypoxia and incubated for 24 h at 37 °C. The cells were treated with compound Q at 0, 5, 10, 20 μM under hypoxia for 24 h. Then, the cells were harvested, washed twice with cold PBS, and fixed in cold 75% ethanol at −20 °C overnight. The cells were washed twice with cold PBS and stained with 100 μg mL−1 RNase A (Sigma, USA) for 30 min at 37 °C. The cells were washed and resuspended with PBS containing 50 μg mL−1 propidium iodide (PI, Sigma, USA) for 30 min at room temperature in the dark. Cell cycle phase distributions were analysed by flow cytometry (BD FACSAria III, USA).
Annexin V-FITC/PI staining
Apoptosis was analysed using Annexin V-FITC/PI staining. In this assay, SW480 cells (1.0 × 105 per well) were seeded in 6-well plates under hypoxia and incubated for 24 h with various concentrations of compound Q (0, 5, 10, 20 μM). The cells were harvested, washed twice with 2 mL cold PBS, and stained with Annexin V FITC/PI (BD) according to the manufacturer's protocol. The cells were analysed by flow cytometry (BD FACSAria III, USA).
Detection of mitochondrial membrane potential
SW480 cells (1.0 × 105 per well) were seeded in 6-well plates and incubated for 24 h. The cells were treated with 0, 5, 10, 20 μM compound Q under hypoxia. After 24 h, the cells were washed twice with 2 mL cold PBS and stained with JC-1 dye (Beyotime, China) according to the manufacturer's protocol, followed by imaging using a fluorescence microscope (BioTek, USA).
Measurement of ROS
SW480 cells (1.0 × 105 per well) were treated with compound Q at 0, 5, 10, 20 μM under hypoxia for 24 h. The cells were harvested and washed with cold PBS and stained with diacetyldichlorofluorescein (DCFH-DA) probe (Beyotime, China) according to the manufacturer's protocol. The formation of ROS was measured by flow cytometry (BD FACSAria III, USA).
Detection of intracellular lipid peroxidation
SW480 cells (1.0 × 105 per well) were treated with compound Q at 0, 5, 10, 20 μM under hypoxia. After 24 h, the cells were washed with 2 mL cold PBS prior to staining with C11-BODIPY (Thermo Fisher, Cat# D3861) according to the manufacturer's protocol. The samples were imaged using a fluorescence microscope (BioTek, USA).
Morphological analysis by TEM
SW480 cells (1.0 × 105 per well) were treated with 5 and 15 μM compound Q under hypoxia. After 24 h, the cells were fixed with 3% glutaraldehyde and subjected to electron microscopy using a JEM-1400Flash microscope (JEOL; Tokyo, Japan).
MDC staining
We detected autophagic vesicles in autophagic cells by MDC staining. Basically, MDC was used as a tracer of autophagic vesicles whereby all acidic vacuoles in the cells were visualised by this staining. For this, SW480 cells (1.0 × 105 per well) were treated with 5, 10 and 20 μM compound Q under hypoxia. After 24 h, the cells were stained with MDC (Beyotime, C3018S, Nanjing, China) for 30 min at 37 °C in the dark, washed with the assay buffer three times, and visualised under a fluorescence microscope (BioTek, USA).
Western blotting
SW480 cells (1.0 × 105 per well) were treated for 24 h with 5, 10 and 20 μM compound Q under hypoxia. The cells were lysed on ice for 15 min in RIPA buffer. The homogenates were centrifuged at 12 000 rpm for 15 min and the supernatant was used for the western blotting. Prior to this, protein content was determined by the bicinchoninic acid (BCA) assay (Beyotime Biotechnology, China) and the lysates were made up to 20 μg. The lysates were mixed with SDS-PAGE sample buffer and boiled for 10 min at 100 °C. Samples were loaded into the well of either 12%, 10% or 8% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad, USA). Membranes were blocked for 1.5 h in 5% (w/v) non-fat milk and incubated with a primary antibody at 4 °C overnight. The membranes were washed three times in Tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated with horseradish peroxidase (HRP) conjugated with rabbit or mouse IgG for 1.5 h at room temperature. After that, the membranes were washed three times with TBST, and protein bands were detected using an enhanced chemiluminescence system (BeyoECL Plus, Beyotime). β-Actin was used as a loading control. The primary antibodies used were purchased from Abcam (Cambridge, UK), with the dilutions as follows: GPX4 (ab125066; 1 : 2000), FACL4 (ab155282; 1 : 5000), SLC7A11 (ab307601; 1 : 1000), Bcl-2 (EPR17509; 1 : 2000), Bax (ab32503; 1 : 5000), caspase3/cleaved-caspase-3 (ab32351; 1 : 5000), p62 (ab280086; 1 : 1000), LC3B (ab192890; 1 : 2000), β-actin (66009-1; 1 : 10 000), HRP-conjugated anti-mouse/rabbit IgG secondary antibodies (Proteintech, Wuhan, China; 1 : 4000).
Antitumour efficacy test in nude mice
The in vivo antitumour activity of compound Q was evaluated in BALB/c nude mice implanted with SW480 cells. The nude mice were randomly divided into three groups: control and two experimental groups (low-dose and high-dose groups), with five nude mice in each group. When the tumours reached a volume of 80 to 120 mm3, the first group was intraperitoneally injected with 100 μL of physiological saline solution as the vehicle control. The second and third groups were treated with compound Q at the dose of 20 mg kg−1 and 40 mg kg−1, respectively. The drug was also administered intraperitoneally in a volume of 100 μL. The nude mice were injected every 3 days for 21 consecutive days. Tumour volume and body weight were recorded every day after the treatment. We measured the tumour volume using an electronic digital caliper by measuring the tumour length (A) and width (B) to calculate the volume (V = AB2/2). All nude mice were euthanised via cervical dislocation after 3 weeks of treatment, and tumour samples were collected for further analysis.
Animal ethics
All animal procedures were performed in accordance with the Administration of Affairs Concerning Experimental Animals, the National Guideline for Animal Experiments, and the Institutional Animal Care and Use Committee (IACUC) of Guilin Medical University. The study met the requirements of animal welfare ethics and passed the investigation by the Animal Ethics Committee of the university with the approval code GLMC-IACUC-20251001.
Statistical analysis
Statistical analyses were performed using the GraphPad PRISM software version 8.0. Data are presented as mean ± standard deviation (SD) of at least three independent experiments. Data were analysed using one-way ANOVA and p values were indicated appropriately in the figures. Mean differences were considered significant at the 0.05 levels, unless otherwise stated in the figure legends.
Author contributions
NSA, XLM: study design and supervision of the project. XQZ, YZ, RZH: experimental design. XQZ, YXH, QLL, HRL: performing experiments. XQZ, NSA, XLM: data analysis, manuscript preparation and revision. All authors have read and agreed with the manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the Project Program of Guangxi Key Laboratory of Drug Discovery and Optimisation, Guilin Medical University (No. GKLPMDDO-2023-B03 and GKLPMDDO-2022-Z01), and the Guilin Scientific Research and Technology Development Plan Project (No. 20220129-2). It was also supported by the Ministry of Higher Education, Malaysia, via the Fundamental Research Grant Scheme (FRGS/1/2023/SKK06/UITM/02/1). The authors acknowledge the Scientific Experiment Centre from Guilin Medical University for their staff support and technical advice.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5md00348b
Data availability
The data supporting this article have been included as part of the ESI.†
References
- Rastin F. Javid H. Oryani M. A. Int. Immunopharmacol. 2024;126:111055. doi: 10.1016/j.intimp.2023.111055. [DOI] [PubMed] [Google Scholar]
- Kiran N. S. Yashaswini C. Maheshwari R. Bhattacharya S. Prajapati B. G. ACS Pharmacol. Transl. Sci. 2024;7(4):967–990. doi: 10.1021/acsptsci.4c00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. Q. Ma X. L. Ariffin N. S. Eur. J. Pharmacol. 2024;976:176677. doi: 10.1016/j.ejphar.2024.176677. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Scozzafava A. Expert Opin. Ther. Pat. 2000;10(5):575–600. doi: 10.1517/13543776.10.5.575. [DOI] [PubMed] [Google Scholar]
- Mboge M. Y. Mahon B. P. McKenna R. Frost S. C. Metabolites. 2018;8(1):19. doi: 10.3390/metabo8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P. C. Swayampakula M. Dedhar S. Metabolites. 2018;8(1):1–11. doi: 10.3390/metabo8010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran C. T. Biochem. J. 2016;473(14):2023–2032. doi: 10.1042/BCJ20160115. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Capasso C. Pathogens. 2017;6(3):1–13. doi: 10.3390/pathogens6030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran C. T. J. Enzyme Inhib. Med. Chem. 2016;31(3):345–360. doi: 10.3109/14756366.2015.1122001. [DOI] [PubMed] [Google Scholar]
- Swietach P. Hulikova A. Vaughan-Jones R. D. Harris A. L. Oncogene. 2010;29(50):6509–6521. doi: 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- Stockwell B. R. Friedmann Angeli J. P. Bayir H. Bush A. I. Conrad M. Dixon S. J. Fulda S. Gascón S. Hatzios S. K. Kagan V. E. Noel K. Jiang X. Linkermann A. Murphy M. E. Overholtzer M. Oyagi A. Pagnussat G. C. Park J. Ran Q. Rosenfeld C. S. Salnikow K. Tang D. Torti F. M. Torti S. V. Toyokuni S. Woerpel K. A. Zhang D. D. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X. He Z. Zhang Y. Fang Y. Liu D. Wu H. Ji J. Xi Y. Ye L. Yang X. Zhai G. Chem. Eng. J. 2023;468:143729. doi: 10.1016/j.cej.2023.143729. [DOI] [Google Scholar]
- Hassannia B. Vandenabeele P. Vanden-Berghe T. Cancer Cell. 2019;35(6):830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Luo M. Y. Su J. H. Gong S. X. Liang N. Huang W. Q. Chen W. Wang A. P. Tian Y. Front. Cell Dev. Biol. 2021;9:1–18. doi: 10.3389/fcell.2021.733908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafe S. C. Vizeacoumar F. S. Venkateswaran G. Nemirovsky O. Awrey S. Brown W. S. McDonald P. C. Carta F. Metcalfe A. Karasinska J. M. Huang L. Muthuswamy S. K. Schaeffer D. F. Renouf D. J. Supuran C. T. Vizeacoumar F. J. Dedhar S. Sci. Adv. 2021;7(35):0364. doi: 10.1126/sciadv.abj0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N. Lin Q. Zuo W. B. Chen W. B. Huang S. Han Y. S. Liang X. J. Zhu X. Huo S. D. Nanoscale Horiz. 2023;8:783–793. doi: 10.1039/d2nh00494a. [DOI] [PubMed] [Google Scholar]
- Levy J. Towers C. Thorburn A. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Jiang L. Chew S. H. Hirayama T. Sekido Y. Toyokuni S. Redox Biol. 2019;26:101297. doi: 10.1016/j.redox.2019.101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temiz E. Koyuncu I. Durgun M. Caglayan M. Gonel A. Güler E. M. Kocyigit A. Supuran C. T. Int. J. Mol. Sci. 2021;22(11):6098. doi: 10.3390/ijms22116098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Wang Y. Liu J. Kang R. Tang D. Cancer Gene Ther. 2021;28(1–2):55–63. doi: 10.1038/s41417-020-0182-y. [DOI] [PubMed] [Google Scholar]
- Gao M. Monian P. Pan Q. Zhang W. Xiang J. Jiang X. Cell Res. 2016;26(9):1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai E. Han L. Liu J. Xie Y. Kroemer G. Klionsky D. J. Zeh H. J. Kang R. Wang J. Tang D. Autophagy. 2020;16(11):2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran C. T. Metabolites. 2017;7(3):48. doi: 10.3390/metabo7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran C. T. Expert Opin. Invest. Drugs. 2021;30(12):1197–1208. doi: 10.1080/13543784.2021.2014813. [DOI] [PubMed] [Google Scholar]
- McDonald P. C. Chia S. Bedard P. L. Chu Q. Lyle M. Tang L. Singh M. Zhang Z. Supuran C. T. Renouf D. J. Dedhar S. Am. J. Clin. Oncol. 2020;43(7):484–490. doi: 10.1097/COC.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreucci E. Ruzzolini J. Peppicelli S. Bianchini F. Laurenzana A. Carta F. Supuran C. T. Calorini L. J. Enzyme Inhib. Med. Chem. 2019;34(1):117–123. doi: 10.1080/14756366.2018.1532419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S. M. Liang G. B. Wang H. L. Jiang H. Ma X. L. Wei J. H. Huang R. Z. Zhang Y. Eur. J. Med. Chem. 2024;263:115937. doi: 10.1016/j.ejmech.2023.115937. [DOI] [PubMed] [Google Scholar]
- Chen X. M. Zhou J. Y. Liu S. Q. Song L. H. Wang H. L. Wang Q. Liang S. M. Lu L. Wei J. H. Huang R. Z. Bioorg. Med. Chem. Lett. 2023;85:129218. doi: 10.1016/j.bmcl.2023.129218. [DOI] [PubMed] [Google Scholar]
- Liang Q. L. Zhang S. Liu J. J. Zhou X. Q. Ariffin N. S. Wei J. H. Shi C. Y. Ma X. L. Zhang Y. Huang R. Z. Bioorg. Chem. 2024;150:107596. doi: 10.1016/j.bioorg.2024.107596. [DOI] [PubMed] [Google Scholar]
- Pastorekova S. Gillies R. J. Cancer Metastasis Rev. 2019;38(1):65. doi: 10.1007/s10555-019-09799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkeila E. Talvinen K. Jaakkola P. M. Minn H. Syrjanen K. Sundström J. Pyrhönen S. Br. J. Cancer. 2009;100(6):874–880. doi: 10.1038/sj.bjc.6604949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitans J. Kazaks A. Balode A. Ivanova J. Zalubovskis R. Supuran C. T. Tars K. J. Med. Chem. 2015;58(22):9004–9009. doi: 10.1021/acs.jmedchem.5b01343. [DOI] [PubMed] [Google Scholar]
- Tafreshi N. K. Lloyd M. C. Proemsey J. B. Bui M. M. Kim J. Gillies R. J. Morseet D. L. Mol. Imaging Biol. 2016;18:219–231. doi: 10.1007/s11307-015-0885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi F. Hasan P. Queen A. Hussain A. Khan P. Zeya B. King H. M. Rana S. Garrison J. Alajmi M. F. Rizvi M. M. A. Zahid M. Imtaiyaz Hassan M. Abid M. Bioorg. Chem. 2020;98:103754. doi: 10.1016/j.bioorg.2020.103754. [DOI] [PubMed] [Google Scholar]
- Xu K. Zhan Y. Yuan Z. Qiu Y. Wang H. Fan G. Wang J. Li W. Cao Y. Shen X. Zhang J. Liang X. Yin P. Mol. Ther. 2019;27(10):1810. doi: 10.1016/j.ymthe.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloranta K. Pihlajoki M. Liljestrom E. Nousiainen R. Soini T. Lohi J. Cairo S. Wilson D. B. Parkkila S. Heikinheimo M. Front. Oncol. 2023;13:1118268. doi: 10.3389/fonc.2023.1118268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas H. Nossier E. S. El-Manawaty M. A. El-Bayaa M. N. Sci. Rep. 2024;14(1):1–16. doi: 10.1038/s41598-024-62864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluce Y. Ahmed B. A. Koyuncu I. Durgun M. J. Bioenerg. Biomembr. 2018;50:107–116. doi: 10.1007/s10863-018-9749-9. [DOI] [PubMed] [Google Scholar]
- Myeza N. Slabber C. Munro O. Q. Sookai S. Zacharias S. C. Martins-Furness C. Harmse L. Eur. J. Pharmacol. 2024;978:176764. doi: 10.1016/j.ejphar.2024.176764. [DOI] [PubMed] [Google Scholar]
- Abraha A. M. Ketema E. B. World J. Gastrointest. Oncol. 2016;8(8):583–591. doi: 10.4251/wjgo.v8.i8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly J. D. Grubb D. R. Lawen A. Apoptosis. 2003;8:115–128. doi: 10.1023/A:1022945107762. [DOI] [PubMed] [Google Scholar]
- Sivandzade F. Bhalerao A. Cucullo L. Bio-Protoc. 2019;9(1):3128. doi: 10.21769/BioProtoc.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P. C. Dedhar S. Front. Mol. Biosci. 2023;10:1327310. doi: 10.3389/fmolb.2023.1327310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. Shen J. Jiang J. Wang F. Min J. Signal Transduction Targeted Ther. 2013;8(1):1–30. doi: 10.1038/s41392-023-01606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L. Liu P. Jin Y. Ning Z. Yang Y. Gao H. Eur. J. Med. Chem. 2022;244:114861. doi: 10.1016/j.ejmech.2022.114861. [DOI] [PubMed] [Google Scholar]
- Yan H. Talty R. Aladelokun O. Bosenberg M. Johnson C. H. Br. J. Cancer. 2023;128(8):1439. doi: 10.1038/s41416-023-02149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S. Proneth B. Tyurina Y. Y. Panzilius E. Kobayashi S. Ingold I. Irmler M. Beckers J. Aichler M. Walch A. Prokisch H. Trumbach D. Mao G. Qu F. Bayir H. Füllekrug J. Scheel C. H. Wurst W. Schick J. A. Kagan V. E. Angeli J. P. Conrad M. Nat. Chem. Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. F. Zou T. Tuo Q. Z. Xu S. Li H. Belaidi A. A. Lei P. Signal Transduction Targeted Ther. 2021;6(1):49. doi: 10.1038/s41392-020-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y. Mizushima N. Yamamoto A. Oshitani-Okamoto S. Ohsumi Y. Yoshimori T. J. Cell Sci. 2004;117:2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- Islam M. A. Sooro M. A. Zhang P. Int. J. Mol. Sci. 2018;19(5):1405. doi: 10.3390/ijms19051405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerzada M. N. Khan P. Ahmad K. Hassan M. I. Azam A. Eur. J. Med. Chem. 2018;155:13–23. doi: 10.1016/j.ejmech.2018.05.034. [DOI] [PubMed] [Google Scholar]
- Law P. C. Auyeung K. K. Chan L. Y. Ko J. K. BMC Complementary Altern. Med. 2012;12:160. doi: 10.1186/1472-6882-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. Sun X. Wang W. Lu S. Oncol. Rep. 2010;24(1):97–104. doi: 10.3892/or_00000833. [DOI] [PubMed] [Google Scholar]
- Ariffin N. S. Acta Histochem. 2023;125(7):152074. doi: 10.1016/j.acthis.2023.152074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.†