Abstract
To determine the influence of Bcl-2 on the developmental biology of myocytes, we analyzed the population dynamics of this cell type in the heart of transgenic (TG) mice overexpressing Bcl-2 under the control of the α-myosin heavy chain promoter. TG mice and non-TG (wild type, WT) mice were studied at 24 days, 2 months, and 4 months after birth. Bcl-2 overexpression produced a significant increase in the percentage of cycling myocytes and their mitotic index. These effects were strictly connected to the expression of the transgene, as demonstrated in isolated myocytes. The formation of mitotic spindle and contractile ring was identified in replicating cells. These typical aspects of mitosis were complemented with the demonstration of karyokinesis and cytokinesis to provide structural evidence of cell division. Apoptosis was low at all ages and was not affected by Bcl-2. The higher cell replication rate in TG was conditioned by a decrease in the expression of the cell-cycle inhibitors, p21WAF1 and p16INK4a, and by an increase in Mdm2-p53 complexes. In comparison with WT, TG had 0.4 × 106, 0.74 × 106, and 1.2 × 106 more myocytes in the left ventricle at 24 days, 2 months, and 4 months, respectively. Binucleated myocytes were 12% and 25% larger in WT than in TG mice at 2 and 4 months of age. Taken together, these observations reveal a previously uncharacterized replication-enhancing function of Bcl-2 in myocytes in vivo in the absence of stressful conditions.
In vivo and in vitro studies have strengthened the notion that one of the main functions of Bcl-2 is to inhibit cell death (1–3). However, the antiapoptotic function of Bcl-2 is stimulus dependent, as in erythroid cells (4), or cell specific, as in the central nervous system (5). These results suggest that the impact of Bcl-2 varies in different cells, tissues, and physiologic states. Bcl-2 has positive consequences on the viability of the ischemic brain and myocardium (2, 3, 6) or exerts minimal or no detectable changes in these organs (2, 7). Importantly, myocyte survival was increased by Bcl-2 exclusively under stressful conditions, such as hypoxia. The developmental influence of this protein on physiologic postnatal cardiac growth, if any, remains to be identified.
Myocyte size and number are regulated by the changes in cardiac hemodynamics that reflect the increases in body weight and circulating blood volume with postnatal and adult life (8). Myocytes constitute only 30% of cardiac cells but occupy 85% of the myocardium; they are the major determinants of the anatomy of the heart. For these reasons, we tested two alternative but not exclusive hypotheses on the role of Bcl-2 in cardiac myocytes during maturation and adulthood.
It is widely believed that myocyte division ceases at birth and myocyte number does not increase postnatally (8). Thus, any change in the rate of cell death would be of paramount importance in preserving functioning myocytes. In this regard, we have shown that a subpopulation of myocytes maintains its replicative potential throughout life (8–10), and primitive cells with stem cell characteristics are present in the human myocardium (11). Bcl-2 may protect these myocytes and critically enhance the size of this replicating pool by attenuating the expression of cell-cycle inhibitors such as p53, p21WAF1, and p16INK4a, or by increasing gene products, such as Mdm2, that favor cell multiplication by interfering with p53 (12). Therefore, to test these hypotheses, transgenic mice were generated overexpressing the human bcl-2 cDNA under the control of the α-myosin heavy chain promoter. The population kinetics of myocytes was analyzed at 24 days, 2 months, and 4 months of age. These intervals were chosen because they represent the stages at which the early postnatal pattern of cell multiplication is believed to have ceased, the transgene has reached full level of expression, and myocytes have acquired the differentiated phenotype.
Materials and Methods
Transgenic Mice.
Bcl-2 transgenic (TG) mice were generated in the FVB/N strain by inserting the full-length coding sequence of the human bcl-2 cDNA linked to the FLAG tag into an expression plasmid driven by the full-length murine myosin heavy chain promoter (13). Transgenic mice were identified by Southern blot. Genomic DNA was extracted from the tail tissue of 3-week-old mice, digested with HindIII, and hybridized with the α32P-dCTP-labeled cDNA probe obtained from pBS2SK+-bcl-2. Transgene expression was determined by Western blot and immunocytochemistry with antihuman Bcl-2 and anti-FLAG. The transgene was confirmed to be limited to the cardiac tissue with a minor extension to part of the pulmonary vasculature. One heterozygous TG line with a high level of human Bcl-2 expression was studied. By confocal microscopy, antihuman Bcl-2 in TG was detected in 68 ± 17% myocytes (age, 2–4 months; n = 7). Wild type (WT) served as controls and were negative. Myocyte cytoplasm was identified by α-sarcomeric actin (8–11).
Echocardiography and Hemodynamics.
Echocardiography was performed in conscious mice at 2 and 4 months. From M-mode tracings, left ventricular (LV) end-systolic and -diastolic diameters, anterior and posterior wall thickness were obtained. The ejection fraction was estimated (14). At the time of killing, mice were anesthetized, and LV pressures and + and − dP/dt were measured (14, 15). Hearts were arrested in diastole and fixed by perfusion or used for myocyte isolation (12). Protocols were approved by New York Medical College.
Cardiac Anatomy.
In mice at 24 days (TG = 10; WT = 11), 2 months (TG = 10; WT = 9), and 4 months (TG = 9; WT = 11), the heart was arrested in diastole and fixed with formalin. LV longitudinal axis, wall thickness, chamber diameter, and volume were evaluated. Wall thickness in diastole, chamber radius, and end-diastolic pressure were used to compute mid-wall diastolic stress (14) as well as diastolic myocyte stress from the endocardium to the epicardium (16).
Myocyte Isolation.
Myocytes were dissociated from 8 TG and 8 WT mice each at 24 days and 2 months, and from 21 TG and 18 WT mice at 4 months (12). The degree of contamination from nonmyocytes was 2–3%. The average yield of LV myocytes was 2–3 × 106.
Myocyte Volume and Number.
In each LV, 500 myocytes, stained by propidium iodide (PI), were examined to determine the fraction of mononucleated, binucleated, and trinucleated cells. Cell volume was obtained in 200 binucleated, 50 mononucleated, and 50 trinucleated myocytes in each LV. The number of each cell type was calculated from the quotient of their LV aggregate volume and the corresponding myocyte volume (14). Sampling: 8 TG and 8 WT each at 24 days, 2 months, and 4 months.
Bromodeoxyuridine (BrdUrd), Ki67, and Mitotic Index.
Sections were incubated with BrdUrd or Ki67 antibodies and stained with PI and cardiac myosin antibody. The percentages of myocyte nuclei labeled by BrdUrd or Ki67 and the fraction of myocyte nuclei in mitosis were obtained by confocal microscopy (10, 14). Sampling for BrdUrd and Ki67: 24 days (TG = 6; WT = 7), 2 months (TG = 7; WT = 7), and 4 (TG = 6; WT = 7) months; mitotic index: 24 days (TG = 6; WT = 8), 2 months (TG = 7; WT = 7), and 4 months (TG = 6; WT = 7). Numbers of myocyte nuclei examined for BrdUrd were: TG, 24 days = 99,296; 2 months = 149,666; 4 months = 128,024; WT, 24 days = 146,610; 2 months = 152,817; 4 months = 128,002. Numbers of myocyte nuclei examined for Ki67 were: TG, 24 days = 94,019, 2 months = 189,318, 4 months = 158,530; WT, 24 days = 114,300, 2 months = 206,066, 4 months = 162,402. The percentage of myocyte nuclei undergoing mitosis was obtained by sampling a large number of myocyte nuclei: TG, 24 days = 221,265, 2 months = 311,519, 4 months = 266,595; WT, 24 days = 324,057, 2 months = 295,140; 4 months = 281,566.
Terminal Deoxynucleotidyl Transferase (TdT).
Sections were incubated with TdT and biotin-16-dUTP (15). Sampling: 6 TG and 7 WT each at 24 days, 2 months, and 4 months. Number of myocyte nuclei examined: TG, 24 days = 226,994, 2 months = 271,440, 4 months = 274,441; WT, 24 days = 255,895, 2 months = 306,711, 4 months = 288,744.
BrdUrd, Ki67, Human Bcl-2, and Mitotic Index in Isolated Myocytes.
These analyses were restricted to TG (n = 6) and WT (n = 6) at 4 months. In some cases, myocytes were labeled with rabbit polyclonal connexin 43 antibody (Sigma). Numbers of myocytes examined for BrdUrd were: TG = 107,753 and WT = 118,440. Numbers of myocytes examined for Ki67 were: TG = 111,552 and WT = 102,858. Numbers of myocytes examined for mitosis were: TG = 1,021,690 and WT = 645,020.
Western Blot and Immunoprecipitation.
The quantities of p53, p16INK4a, p21WAF1, and the fraction of Mdm2 bound to p53 were measured in 7 TG and 4 WT mice (12).
Statistics.
Results: X ± SD. Significance: Student's t test and Bonferroni method.
Results
Myocyte Division.
Myocyte replication in TG and WT mice was measured after six injections of BrdUrd at 12 h intervals and one injection at 50–60 min before killing. BrdUrd labeling identifies cells in S-phase. The number of labeled myocyte nuclei was 58%, 46%, and 45% higher in TG than in WT mice at 24 days, 2 months, and 4 months, respectively (see Fig. 5 a–j, which is published as supporting information on the PNAS web site, www.pnas.org). The number of Ki67+ cells was lower than BrdUrd-labeled cells. Ki67 recognizes cycling myocytes (10) at the time of observation. Ki67 was 90%, 53%, and 58% higher in TG than in WT mice at 24 days, 2 months, and 4 months.
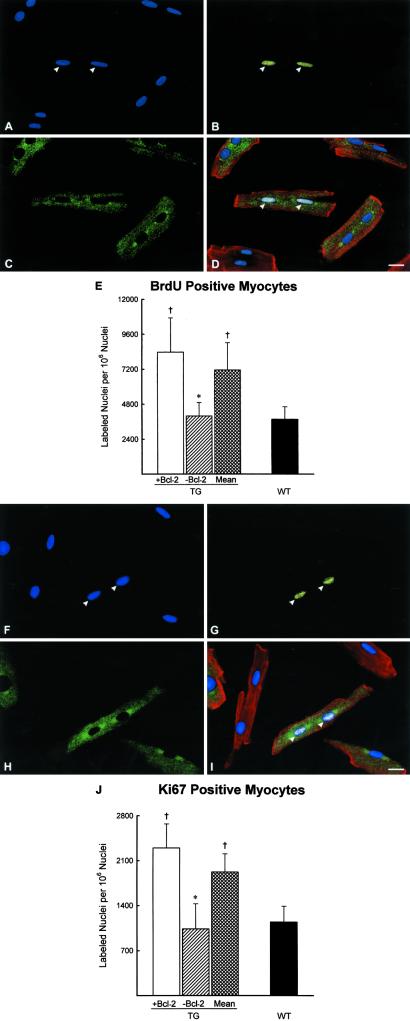
Because only 68% of myocytes in TG hearts scored positive for human Bcl-2, the relationship between Bcl-2 expression and BrdUrd labeling was determined at 4 months in isolated myocytes. Mice were injected with BrdUrd every 8 h for 7 days, and the distribution of BrdUrd-labeled cells in Bcl-2+ and Bcl-2− cells was evaluated. More than a 2-fold higher BrdUrd labeling was coupled with the expression of the transgene. Ki67 expression behaved in a similar manner (Fig. 1 a–j).
Figure 1.
(A–J) Identification of cycling myocytes in isolated cells. (A and F) PI labeling of nuclei (blue). (B and G) BrdUrd (B, yellow) and Ki67 (G, yellow) labeling. (C and H) Human Bcl-2 (green). (D and I) PI and BrdUrd (D, bright) and PI and Ki67 (I, bright) labeling of nuclei (arrowheads), human Bcl-2 (green), and α-sarcomeric actin of myocyte cytoplasm (red). (A–D, F–I) TG mice at 4 months. (Bar = 10 μm.) (E and J) BrdUrd- (E) and Ki67-labeled (J) nuclei in myocytes from TG expressing (+) and nonexpressing (−) Bcl-2 and in myocytes collected from WT mice. Results are means ± SD. * and †, P < 0.05 vs. Bcl-2+ and mean in TG and WT mice, respectively.
Mitotic figures in myocytes were identified in tissue sections from all groups of mice (see Fig. 6 a–k, which is published as supporting information on the PNAS web site). Moreover, the formation of the mitotic spindle by tubulin and the generation of the contractile ring by actin accumulation in the region of cytoplasmic separation were detected. Karyokinesis and cytokinesis also were found. As expected, mitotic indices decreased with age and were higher in TG than in WT mice at all intervals.
With the exception of anaphase and telophase, Ki67 is detected throughout the cell cycle. These two phases of mitosis, however, are very short and do not significantly contribute to the duration of the cell cycle. Mitosis lasts nearly 1 h, and this time is relatively constant in most proliferating cells (10). Thus, the ratio of Ki67-to-mitotic index represents a reasonable estimate of the duration of the myocyte cell cycle in vivo (values in Fig. 5j divided by values in Fig. 6k). In WT and TG, this parameter averaged ≈23 h at all ages. Therefore, the increase in the number of cycling myocytes in TG is most likely caused by an expansion in the replicating cell population and not by a prolonged cell cycle.
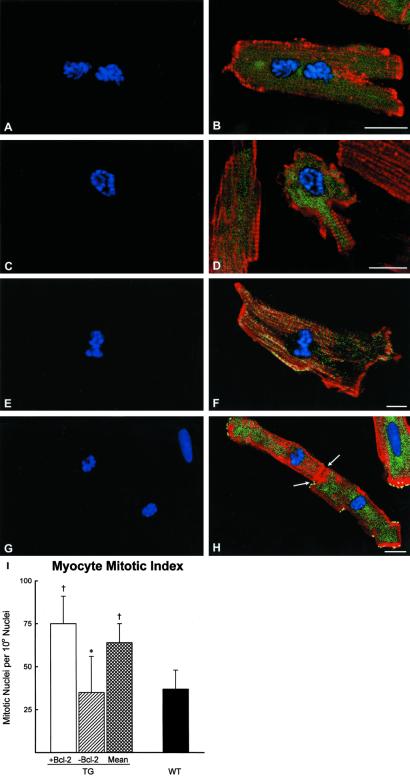
To dissect further the role of Bcl-2 in myocyte proliferation, mitotic indices were obtained in myocytes positive and negative for human Bcl-2, isolated from TG and WT mice at 4 months. Consistent with the distribution of BrdUrd- and Ki67-positive cells in these two myocyte populations, mitosis was 2-fold higher in myocytes expressing the transgene (Fig. 2 a–i). Interestingly, Bcl-2− myocytes from TG had a mitotic index similar to WT. Myocyte cytokinesis was identified for the first time in dissociated myocytes (Fig. 2 g and h). It is noteworthy that connexin 43 was absent in the region of cell separation where actin was accumulated, constituting the contractile ring.
Figure 2.
(A–I) Mitotic figures in isolated myocytes from TG mice at 4 months. (A, C, E, and G) Metaphase chromosomes (PI; blue). (B, D, F, and H) Metaphase chromosomes (PI; blue), Bcl-2 (green), myocyte cytoplasm (α-sarcomeric actin; red). (H) Yellow represents connexin 43 at the periphery of the dividing cells; arrows point to the contractile ring forming during cytokinesis. (Bar = 10 μm.) (I) Mitotic nuclei in myocytes from TG expressing (+) and nonexpressing (−) Bcl-2 and in myocytes collected from WT mice. Results are means ± SD. * and †, P < 0.05 vs. Bcl-2+ and mean in TG and WT mice, respectively.
Myocyte Volume and Number.
The parameters listed above indicate that myocyte replication occurred from weaning to adulthood in WT, and Bcl-2 overexpression enhanced this phenomenon. However, none of these measurements provided a quantitative evaluation of cell multiplication. The postnatal increase of cardiac mass involves myocyte hypertrophy and myocyte proliferation. Myocardial growth, however, is complicated by myocyte death (8). Additionally, myocytes are mononucleated, binucleated, and trinucleated.
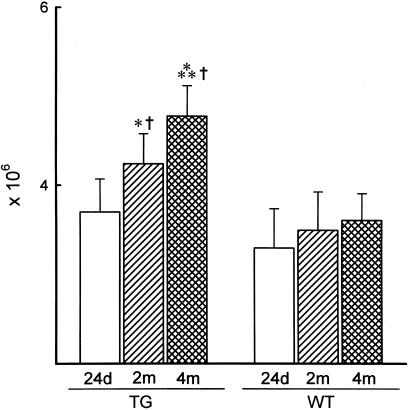
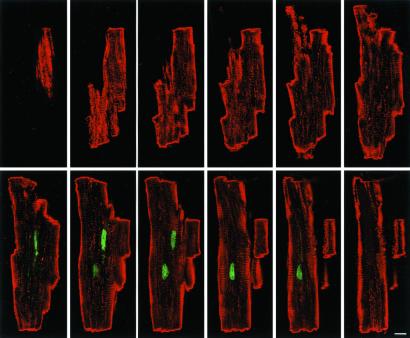
In TG and WT cells at all intervals, mononucleated myocytes comprised an average of 4.8%, binucleated was 94%, and trinucleated was 1.2%. Myocyte volume of each cell class was similar in TG and WT at 24 days, but it increased in both groups at 2 months. At 4 months, cell volume increased again in WT. Myocyte volume was determined by confocal microscopy (Fig. 3). These determinations demonstrated that binucleated myocytes were 12 and 25% larger in WT than in TG at 2 and 4 months, respectively (Fig. 7 a–f, which is published as supporting information on the PNAS web site). In TG, the total number of mononucleated and trinucleated myocytes did not vary up to 4 months, whereas binucleated cells increased progressively with age. From 24 days to 4 months, a 28% increase in binucleated myocytes was found. In contrast, in WT, the number of each myocyte class was essentially established at 24 days, because no further increase was noted at the two subsequent intervals. Conversely, total myocyte number in TG increased 15 and 29% from 24 days to 2 and 4 months, respectively (Fig. 4). When TG and WT were compared, myocyte number at 2 and 4 months was 21 and 32% higher in TG than in WT.
Figure 4.
Total number of myocytes in TG and WT mice. Results are means ± SD. *, **, and †, P < 0.05 vs. values at 24 days and at 2 months in each group of mice and values in WT mice, respectively.
The lack of increase in myocyte number with maturation in WT is at variance with the levels of BrdUrd incorporation, Ki67 expression, and mitotic index measured in these cells. Therefore, myocyte apoptosis was evaluated and found to be 144 ± 14 × 106, 102 ± 10 × 106, and 107 ± 19 × 106 in WT at 24 days, 2 and 4 months, respectively. Corresponding values in TG were 152 ± 18 × 106, 111 ± 12 × 106 and 101 ± 26 × 106. The extent of apoptosis was similar in TG and WT, indicating that Bcl-2 had no effect on cell viability in early postnatal development. Failure to detect an increase in myocyte number in growing WT hearts despite cell replication was because of a rate of apoptosis similar to the rate of cell growth. Because the rate of apoptosis in TG was similar to WT, the increase in myocyte number in TG constituted an underestimation of the degree of cell replication with age.
Cycling Myocytes.
The relative values of BrdUrd+ and Ki67+ myocytes were used in combination with the total number of ventricular muscle cells to assess the actual number of replicating myocytes in TG and WT at each age interval. An identical approach was used to compute the number of myocytes in mitosis. In this manner, the number of BrdUrd+ myocytes varied from a minimum of 6,570 per ventricle of 4 months WT to a maximum of 26,159 per ventricle of 24 days TG (values in Fig. 5e and × values in Fig. 4). Similarly, the expression of Ki67 ranged from a minimum of 3,720 per ventricle of 4 months WT to a maximum of 14,800 per ventricle of 24 days TG. Mitotic myocytes were at minimum 154 per ventricle of 2 months WT and at maximum 777 per ventricle of 24 days TG.
Heart Size and Function.
Heart (H) and LV weights (mg) were similar in TG and WT mice at 24 days (TG: H = 73 ± 8, LV = 55 ± 6, n = 11; WT: H = 66 ± 9, LV = 51 ± 6, n = 11), 2 months (TG: H = 124 ± 12, LV = 98 ± 10, n = 11; WT: H = 117 ± 14, LV = 92 ± 13, n = 11), and 4 months (TG: H = 143 ± 12, LV = 110 ± 9, n = 11; WT: H = 134 ± 12, LV = 104 ± 10, n = 11). The similarity in these gross anatomical parameters between TG and WT mice was consistent with a larger number of myocytes of smaller size in TG mice. LV chamber volume and wall thickness increased postnatally in TG and WT mice. Body weight increased in both groups of mice with time, and heart weight-to-body weight ratios did not vary.
Echocardiograms were obtained in conscious mice (Fig. 8 a–f, which is published as supporting information on the PNAS web site). Systolic and diastolic dimensions and ejection fraction remained constant from 2 to 4 months in TG and WT mice (not shown). Similarly, ventricular hemodynamics at killing showed that LV end-diastolic and systolic pressures and + and − dP/dt were comparable in both groups. Echocardiography and functional data could not be collected at 24 days because of the small size of the animals. Chamber radius and wall thickness, in combination with the in vivo evaluation of LV end-diastolic pressure, allowed the computation of diastolic wall stress. The knowledge of the number of myocytes across the wall (16) permitted the translation of mural stress at the cellular level from the endocardium to the epicardium. Loading was higher in myocytes distributed in the endocardium and decreased progressively toward the epicardium. Diastolic myocyte stress was consistently lower in Bcl-2 than in WT mice, and age enhanced this difference. The increased number of myocytes across the wall strongly suggests that new myocytes were added mostly in parallel rather than in series.
Cell-Cycle Regulators.
To identify the basis of enhanced myocyte replication with Bcl-2 overexpression, the protein levels of p53, Mdm2 bound to p53, p21WAF1 and p16INK4a were measured in myocytes isolated from WT and TG mice at 4 months. These gene products were selected because p53 arrests cells in the cell cycle by activating p21WAF1 (17), which is a p53-dependent gene. Mdm2 is a p53-dependent gene that forms Mdm2-p53 inactive complexes opposing p53 function (12, 18). Mdm2 reduces the stability of p53, thus further affecting its transcriptional activity (18). p16INK4a is a marker of cellular senescence (19) that blocks the entry of cells into the cell cycle. p16INK4a interacts with CDK4 and CDK6, preventing the phosphorylation of G1-cyclins (17). The quantity of p53 was comparable in myocytes from WT and TG. The fraction of Mdm2, p90, and p57–58 bound to p53 was higher in TG than in WT cells. Thus, the relatively higher level of free p53 (not bound to Mdm2) in myocytes from WT was accompanied by a 3-fold greater accumulation of p21WAF1. Conversely, attenuation of p53 function in TG was coupled with a markedly reduced amount of p21WAF1. p16INK4a was apparent in myocytes from WT and barely detectable in TG. Biochemical data can be found in Fig. 9, which is published as supporting information on the PNAS web site.
Discussion
Bcl-2 and Myocyte Proliferation.
The current results expand on the notion that Bcl-2 overexpression leads to an increase in the number of cells in organs by interfering with apoptotic cell death (1–3). Bcl-2 TG mice possess an increased number of stem cells and progenitor cells in the bone marrow, and these adaptations have been linked to the antiapoptotic property of this protein (3). Similarly, targeted expression of Bcl-2 in oocytes confers resistance to naturally developing and chemotherapy-induced apoptosis, increasing the number of female germ cells (20). Moreover, the number of neurons in the brain, Purkinje cells in the cerebellum, and cells in the liver increases in Bcl-2 transgenic mice (4, 5). In apparent contrast with the present findings, another relevant aspect of Bcl-2 has been connected with its ability to negatively regulate cell-cycle progression in vitro (21). Bcl-2 delays the entry of cells in G1 by increasing the formation of the pocket protein p130. Cell-cycle arrest also can occur at the G1-S transition, retarding BrdUrd incorporation (21), or later in the cell cycle, resulting in the accumulation of cells in G2-M (22).
The antiapoptotic and antiproliferative functions of Bcl-2 seem at variance with our observations in which forced expression of Bcl-2 was restricted to cardiac myocytes. Because the transgene was driven by the cardiac α-myosin heavy chain promoter, its expression did not start until late in the prenatal period and reached its maximal expression around puberty. Thus, it is fair to assume that most of the effects of the transgene were limited to postnatal life, as suggested by the fact that the total number of myocytes was not different in TG and WT at 24 days.
Bcl-2 TG hearts were characterized by a higher number of myocytes in the adult, a larger fraction of cycling myocytes, and a higher mitotic index. Myocytes were smaller in TG than in WT mice. Apoptosis was essentially identical in TG and WT mice. Conversely, inhibition of apoptosis occurs in vitro in myocytes infected with an adenoviral vector carrying human Bcl-2 (23), pointing to differences in the behavior of Bcl-2 in myocyte viability in vivo and in vitro. In the latter case, however, cells were exposed to hypoxia and reoxygenation (23). The difference between our data and published work may reflect the targeted expression of the transgene in myocytes and its activation during postnatal life only. The increased Bcl-2 level in adult myocytes may promote cell survival under severe conditions of stress (3, 6), which were not tested here.
The effect of Bcl-2 overexpression on p53 and p53-dependent genes is consistent with its promotion of myocyte replication. Bcl-2 overexpression down-regulates p53 function through the formation of Mdm2-p53 inactive complexes and markedly attenuates the level of p21WAF1 in myocytes. Additionally, p16INK4a becomes barely detectable in TG myocytes. The combination of these factors suggests that the myocyte replicative potential is enhanced by the ability of Bcl-2 to modulate positively the entry of nonterminally differentiated myocytes into the cell cycle. However, the effector pathway implicated in Bcl-2-mediated control of cell-cycle inhibitors remains to be identified. Within the limitations of in vivo data, our results indicate that Bcl-2 does not increase the number of replicating cells by prolonging the duration of the cell cycle but by truly expanding the number of dividing cells. This result is accomplished by increasing both the subpopulation of myocytes able to cycle and the probability of cells to enter the cell cycle.
Myocyte regeneration is a phenomenon that accompanies the normal development of the heart (8). Bcl-2 does not initiate this process but significantly amplifies its magnitude, facilitating its recognition and unequivocal documentation because it produces a net increase in myocyte number after puberty. The need for using several techniques to document myocyte proliferation in the adult heart is particularly apparent when the developmental changes of the heart in WT mice are considered. This fact might help to explain the resistance to the concept of myocyte regeneration, which is still common among researchers in the cardiovascular field. The number of myocytes does not increase with age in these animals, despite the fact that a relevant number of cells are cycling and in mitosis. Cell death counteracted cell replication, resulting in a relatively constant aggregate number of myocytes in the ventricle. The excess of 3 × 105 newly formed myocytes from 24 days to 4 months in WT mice did not increase significantly the number of ventricular myocytes. This cell replication vs. cell death phenomenon is not uncommon because similar results have been found in the failing human heart and in animal models of human disease (8, 10, 16). It could be argued that although BrdUrd labeling was consistent with myocyte multiplication, it also could be caused by DNA repair or polyploidization. Neither of these two processes can be confused with cell regeneration. However, this is not the case for Ki67 protein that is expressed only in dividing cells and is not implicated in DNA repair (24). The recognition of mitosis further weakens the argument against myocyte replication. The preservation of cell number in the normal mouse heart at 2 and 4 months of age with ongoing cell death can be accomplished only by myocyte mitotic division. Contrasting results, challenging the notion of cell regeneration, have been published (25). Unfortunately, observations in the mouse heart did not use multiple injections of BrdUrd to favor the identification of cells duplicating DNA, nor did they measure the extent of cell death taking place in the normal and overloaded heart (25). More convincingly, the degree of cell death detected in pathologic states in humans and animals would lead to the disappearance of the heart in the absence of cell multiplication (26, 27); a 1.2% loss of myocytes by apoptosis and necrosis would abolish the cardiac muscle mass in less than 3 months.
Overexpression of Bcl-2 allowed a direct demonstration of myocyte proliferation. From ≈1 to 4 months of age, 1.1 × 106 new myocytes were formed in the left ventricle of TG mice, despite the chronic loss of cells by apoptosis. Myocyte growth was characterized by high levels of BrdUrd and Ki67 labeling and mitotic images in muscle cells. The fraction of myocytes in mitosis was greater than in the normal human heart (9, 10), reaching values found in animals and patients with severe failure (8, 9). Furthermore, cell regeneration limited myocyte hypertrophy in the adult Bcl-2 mouse. From 2 to 4 months, cell size remained essentially constant. This outcome did not occur in WT mice, in which the imbalance between cell death and repair resulted in myocyte hypertrophy. Myocyte multiplication and the absence of cellular hypertrophy have been observed in mice overexpressing IGF-1 (28), c-myc (13), or large T antigen (29). It might be relevant to indicate that our findings in the left ventricle may not be comparable to the growth response of the right ventricle, which is exposed to a much lower pressure during postnatal development and adulthood.
Bcl-2 and Ventricular Function.
Bcl-2 TG mice and non-TG mice were characterized by hearts of comparable weight and cavitary size. However, TG mice had more myocytes, smaller in volume. The thickness of the ventricular wall was comparable in the two groups of animals but, as a consequence of the cellular differences, TG contained more myocytes within the wall than WT mice. This condition was more apparent in the fully matured adult mouse. The increase in the parallel addition of newly formed myocytes within the wall of TG mice provided another unequivocal demonstration of myocyte proliferation in these animals with maturation (8). The combination of mural myocyte number, chamber radius, and end-diastolic pressure resulted in a significant attenuation in diastolic wall stress at the cellular level in TG mice. This positive effect was apparent in most of the cells distributed across the ventricular wall. Such a phenomenon increased in older animals, emphasizing the critical role of cell proliferation in the definition of myocyte loading (8, 16); the same magnitude of stress is distributed among a larger number of cells across the wall. Similar positive and negative adaptations have been shown in pathologic states of the heart associated with myocyte regeneration or severe restructuring of the wall with decreases in the mural number of cells (30).
Myocyte mechanics is influenced by factors that include cell size, phosphorylation of regulatory proteins, Ca2+ transients, myofilament Ca2+ sensitivity, and Ca2+ release and uptake from the sarcoplasmic reticulum (31, 32). Bcl-2 regulates Ca2+ metabolism, increases the Ca2+ permeability of the endoplasmic reticulum membrane, modulates mitochondrial Ca2+ homeostasis, and protects cells from accumulation of Ca2+ in the mitochondria (1). These properties suggest that the Bcl-2 transgenic heart possessed a greater reserve in response to sudden changes in physiologic and pathologic loads. In conclusion, targeted expression of bcl-2 in cardiac myocytes has no effect on baseline apoptosis and does not interfere with the reentry of cells into the cell cycle. This finding does not exclude that the antiapoptotic and antiproliferative function of Bcl-2 manifests itself in cardiac diseases of ischemic and nonischemic origin. Importantly, during late postnatal development and maturation, Bcl-2 promotes myocyte replication, attenuates hypertrophy, and improves the performance of the young adult heart.
Supplementary Material
Figure 3.
Optical sectioning by confocal microscopy of an isolated myocyte from a WT mouse at 4 months. Cell was labeled with PI (green) and α-sarcomeric actin antibody (red). The optical sections were taken at 1.0-μm intervals. (Bar = 10 μm.)
Acknowledgments
We thank Dr. A. Haunstetter for providing a founding pair of Bcl-2 transgenic mice. This work was supported by National Institutes of Health Grants HL-38132, HL-39902, AG-15756, HL-65577, HL-66923, AG-17042, and HL-65573.
Abbreviations
- TG
transgenic
- WT
wild type
- LV
left ventricular
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Green D R, Reed J C. Science. 1998;281:130–134. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 2.Merry D E, Korsmeyer S J. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- 3.Brocheriou V, Hagège A A, Oubenaïssa A, Lambert M, Mallet V O, Duriez M, Wassef M, Kahn A, Menassche P, Gilgenkrantz H. J Gene Med. 2000;2:326–333. doi: 10.1002/1521-2254(200009/10)2:5<326::AID-JGM133>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Lacronique V, Varlet P, Mayeux P, Porteu A, Gisselbrecht S, Kahn A, Lacombe C. Blood. 1997;90:3050–3056. [PubMed] [Google Scholar]
- 5.Wang H-D, Fukuda T, Suzuki T, Hashimoto K, Liou S-Y, Momoi T, Kosaka T, Yamamoto K, Nakanishi H. Neuroscience. 1999;57:1–12. doi: 10.1002/(SICI)1097-4547(19990701)57:1<1::AID-JNR1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Chua C C, Ho Y S, Hamdy R C, Chua B H. Am J Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 7.Akli S, Taffett G, Pocius J, Pham T, Entman M L, Michael L H, Schneider M D. Circulation. 1999;100, Suppl. 1:774. [Google Scholar]
- 8.Anversa P, Kajstura J. Circ Res. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami C A, Anversa P. Proc Natl Acad Sci USA. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beltrami A P, Urbanek K, Kajstura J, Yan S-M, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami C A, Anversa P. New Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 11.Quaini F, Urbanek K, Beltrami A P, Finato N, Beltrami C A, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 12.Leri A, Liu Y, Wang X, Kajstura J, Malhotra A, Meggs L G, Anversa P. Circ Res. 1999;84:752–756. doi: 10.1161/01.res.84.7.752. [DOI] [PubMed] [Google Scholar]
- 13.Robbins R J, Swain J L. Am J Physiol. 1992;262:H590–H597. doi: 10.1152/ajpheart.1992.262.2.H590. [DOI] [PubMed] [Google Scholar]
- 14.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine D M, Leri A, Anversa P. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, Setoguchi M, Wang X, Andreoli A M, Leri A, Malhotra A, Kajstura J, Anversa P. Circ Res. 1999;84:1007–1019. doi: 10.1161/01.res.84.9.1007. [DOI] [PubMed] [Google Scholar]
- 16.Anversa P, Olivetti G. In: Handbook of Physiology, Section 2: The Cardiovascular System, The Heart. Page E, Fozzard H A, Solaro R J, editors. Oxford: Oxford Univ. Press; 2002. pp. 75–144. [Google Scholar]
- 17.Sherr C J, Roberts J M. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 18.Momand J, Zambetti G P. J Cell Biochem. 1997;64:343–352. [PubMed] [Google Scholar]
- 19.Hara E, Smith R, Parry D, Hidetoshi T, Stone S, Peters G. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita Y, Perez G I, Maravei D V, Tilly K I, Tilly J L. Mol Endocrinol. 1999;13:841–850. doi: 10.1210/mend.13.6.0306. [DOI] [PubMed] [Google Scholar]
- 21.Vairo G, Soos T J, Upton T M, Zalvide J, DeCaprio J S, Ewen M E, Koff A, Adams J M. Mol Cell Biol. 2000;20:4745–4753. doi: 10.1128/mcb.20.13.4745-4753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto K, Ichijo H, Korsmeyer S J. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang P M, Haunstetter A, Aoki H, Usheva A, Izumo S. Circ Res. 2000;87:118–125. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]
- 24.Scholzen T, Gerdes J. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Soonpaa M H, Field L J. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 26.Narula J, Haider N, Virmani R, DiSalvo T G, Kolodgie F D, Hajjar R J, Schmidt U, Semigran M J, Dec G W, Khaw B A. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 27.Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami C A, Kajstura J, Anversa P. Circ Res. 1999;85:856–866. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]
- 28.Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy C J, Baserga R, Anversa P. Proc Natl Acad Sci USA. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daud A I, Lanson N A, Claycomb W C, Field L J. Am J Physiol. 1993;264:H1693–H1700. doi: 10.1152/ajpheart.1993.264.5.H1693. [DOI] [PubMed] [Google Scholar]
- 30.Anversa P, Zhang X, Li P, Capasso J M. J Clin Invest. 1992;89:618–629. doi: 10.1172/JCI115628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redaelli G, Malhotra A, Li B, Li P, Sonnenblick E H, Hofmann P A, Anversa P. Circ Res. 1998;82:594–603. doi: 10.1161/01.res.82.5.594. [DOI] [PubMed] [Google Scholar]
- 32.Marks A R. Circ Res. 2000;87:8–11. doi: 10.1161/01.res.87.1.8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.