Abstract
Central nervous system (CNS) tumor with BCL6 corepressor gene BCOR/BCORL1 fusion is an extremely rare tumor entity, with fewer than 40 cases reported. These tumors are distinct from the WHO 2021-defined CNS tumor with BCOR internal tandem duplication. Even rarer are CNS tumors that match to the methylation class of CNS tumors with BCOR/BCORL1 fusion, but lack fusions and instead harbor truncating small nucleotide variants in BCOR. To our knowledge, only two other cases of this scenario have been previously reported. Due to their scarcity and morphological features that mimic oligodendrogliomas and ependymomas, the diagnosis of CNS tumor with BCOR/BCORL1 fusion can be challenging, and misdiagnoses are not uncommon. Histologic findings of Olig2 positivity with focal to absent GFAP warrant further evaluation for this tumor entity. Moreover, no standard of care therapy exists for these tumors, making treatment selection difficult. We present a case of a 37-year-old woman with a midline CNS tumor with BCOR/BCORL1 fusion, harboring a pathogenic BCOR c.626del (p.S209Cfs*7) (Exon 4) variant, who was successfully treated with definitive radiation therapy and adjuvant temozolomide. Notably, EMA showed focal strong dot-like perinuclear immunoreactivity, which has not been previously reported in these tumors. This case adds to the limited but growing body of evidence supporting the use of radiation and temozolomide in treating tumors matching the methylation class of CNS tumors with BCOR/BCORL1 fusion without a detectable fusion.
Keywords: PNET, CNS tumor with BCOR/BCOR(L1) fusion, CNS tumor with BCOR internal tandem duplication, ependymoma, temozolomide
1. Introduction
The World Health Organization (WHO)’s classification of central nervous system (CNS) tumors continues to undergo revisions to improve the diagnostic accuracy for CNS-primitive neuroectodermal tumors (PNETs). Each new iteration refines and expands upon the existing diagnostic categories. The 2021 WHO classification of CNS tumors defined a new CNS-PNET tumor entity: CNS tumor with BCL6 corepressor gene (BCOR) internal tandem duplication (ITD) [1,2,3]. The BCOR gene, located on Xq26.1, is a tumor suppressor gene and an epigenetic regulator that mediates cell differentiation and structural development, while contributing to polycomb repressive complexes (PRCs) [4,5,6,7,8,9,10]. PRC1 is a main regulator of cellular outcome and stem cell existence [10,11]. PRC1.1 alterations are associated with BCOR and BCOR(L1) [10]. Accordingly, a loss of function mutation in the BCOR gene interrupts tumor suppression functioning and thereby leads to tumorigenesis. Additionally, ITDs and oncogenic gene fusions are alternate drivers of tumorigenesis [3,12,13,14].
The updated WHO classification schemes have increasingly incorporated DNA methylation profiling as an ancillary diagnostic tool. A diagnosis of CNS tumor with BCOR-ITD can be designated by histologic criteria coupled with an ITD in Exon 15 of BCOR or DNA methylation profiling in unresolved cases [2]. CNS tumor with a BCOR or BCOR Like 1 (L1) fusion entity has a unique DNA methylation signature that is molecularly distinct from CNS tumor with BCOR-ITD and is not yet recognized in the WHO classification of CNS tumors [15]. BCOR-L1 is a BCOR homolog and is an intense transcriptional corepressor when tethered to a heterologous promoter [9,16]. Limited information exists for this exceptionally rare tumor that is characterized by the presence of a BCOR/BCOR(L1) fusion, copy number variations within chromosomes Xp11.4 and 22q12.31, or truncating small nucleotide variants [14,17]. Moreover, little is known about the relationship between BCOR-fusion tumors and BCOR-ITDs. However, biological differences have been reported between these tumors. In stark contrast to BCOR-ITD tumors, BCOR-fusion tumors more commonly occur in adults [14,18,19]. Evidence is conflicting for any sex differences [14,20]. The median age at presentation for patients with BCOR-fusion tumors is 27–30 years of age, but broadly presents between the ages of 5 and 72 years [14,20]. The median progression-free survival for these tumors is 16 months, but ranges from 1 to 86 months [14,18,19]. The median overall survival is 27 months and data suggest that patients with BCOR-fusion tumors portend better prognosis compared to BCOR-ITD [18,19,20].
On diagnostic imaging, CNS tumors with BCOR/BCOR(L1) fusion commonly arise within or adjacent to the ventricles and are characteristically T1-hypointense and T2-hyperintense [17,19]. These tumors can be well-demarcated or diffusely infiltrating and with or without enhancement [17,18].
CNS tumors with BCOR/BCOR(L1) fusion are mimickers of other diseases. Histology demonstrates intra- and inter-tumor heterogeneity, including oligodendroglioma-like, ependymoma-like, and embryonal features, frequently with focal calcifications and/or myxoid change [14,17]. Tumor cells show diverse cytology ranging from round to ovoid, and occasionally angulated nuclei with delicate to hyperchromatic chromatin and clear to eosinophilic cytoplasm, occasionally with intracytoplasmic vacuolation [14,17]. Malignant features include necrosis and elevated mitotic activity, and less commonly, microvascular proliferation [14,19]. Ependymoma-like perivascular pseudorosettes and/or oligodendroglioma-like chicken-wire vasculature are consistently encountered [14,17,19,21]. Olig2 is commonly expressed, and GFAP may be focally present or absent [14,17,19]. Accordingly, these tumors can be misdiagnosed as supratentorial ependymoma, oligodendroglioma, astrocytoma, glioblastoma, or ganglioglioma [14]. However, molecular advances have improved upon the classification of CNS tumors. The presence of MYCN amplification or fusions in ZFTA or YAP1 is suggestive of ependymoma [1]. The presence of mutations in IDH1, IDH2, 1p/19q, TERT promoter, CIC, FUBP1, and NOTCH1 is suggestive of oligodendroglioma [1].
Positivity for glial markers in the presence of a BCOR fusion justifies a provisional designation as glioma with BCOR fusion [14]. BCOR fusion or a matching methylation class distinguishes these CNS tumors from BCOR-ITD [14]. BCOR-fusion methylation class tumors are characterized by EP300 or CREBBP as BCOR/BCOR(L1)-fusion partners, an MEAF6::CXXC5 fusion, a BCOR stop mutation, or CNV breakpoints at the EP300 and BCOR loci on chromosomes X or 22 [14,17].
There are fewer than 40 reported cases, warranting further investigations into the clinical behavior and radiographic, pathologic, and genomic features of this rare tumor entity [17]. No standard of care therapy exists, further highlighting the need to deepen our understanding of CNS tumors with BCOR/BCOR(L1) fusion. Herein, we present a case of a 37-year-old female with a CNS tumor harboring a pathologic BCOR c.626del (p.S209Cfs*7) (Exon 4) variant that matched the same methylation class as CNS tumors with BCOR/BCOR(L1) fusion, but without a detectable fusion, who was successfully treated with radiation followed by adjuvant temozolomide.
2. Case Presentation
A 37-year-old female presented to an outside institution with syncope, altered mental status, and visual disturbance (Figure 1). Magnetic resonance imaging (MRI) and computed tomography (CT) demonstrated a 4.3 cm enhancing, diffusion restricting, partially calcified, partially cystic mass involving the tectum, right thalamus, third ventricle, and right lateral ventricle. Severe locoregional mass effect caused brainstem compression and obstructive hydrocephalus (Figure 2). A biopsy was performed with an initial diagnosis of craniopharyngioma. She responded with significant neurologic recovery following shunt placement and was discharged with steroids. The patient subsequently presented to our institution with bilateral lower extremity weakness and gait instability. Neurological examination revealed moon facies and bilateral hip flexor weakness. A brain MRI demonstrated interval tumor progression (Figure 3A,B) and she underwent a craniotomy for tumor debulking of the right thalamic component (Figure 3C,D).
Figure 1.
Patient timeline (months).
Figure 2.
Initial imaging demonstrating an enhancing (T1 and T1+), diffusion-restricting (DWI and ADC), partially calcified (CT), partially cystic (CT), T2-intermediate (T2), tectal-intraventricular mass causing obstructive hydrocephalus (GRE and FLAIR).
Figure 3.
Serial post-contrast imaging demonstrating tumor growth and ventricular shunting (A,B), tumor debulking (C,D), continued tumor growth (E,F), and response to radiation treatment (G,H).
Pathological examination demonstrated a densely cellular neoplasm with elevated mitotic activity and extensive perivascular pseudorosettes (Figure 4). Angulated hyperchromatic nuclei, focal calcifications, and focal myxoid change were present. Necrosis, microvascular proliferation, and oligodendroglioma-like features were not identified within the tissue sections available for histological examination. Olig2 showed strong to moderate immunopositivity in the majority of tumor nuclei (Figure 5B). GFAP was patchy positive with a subset of fragments demonstrating more prominent expression in perivascular regions (Figure 5A). EMA was patchy positive in a strong perinuclear dot-like pattern (Figure 5C). SOX10, synaptophysin, and CAM5.2 were negative. The estimated Ki-67 proliferative index was high (10–30%; Figure 5D). The morphology and intraventricular location bore similarities to supratentorial ependymoma. However, Olig2 positivity in the majority of tumor nuclei raised concern for an alternate diagnosis.
Figure 4.
Hematoxylin and eosin staining. (A) Ependymoma-like tumor histomorphology with extensive perivascular pseudorosettes. (B) Elevated mitotic activity with three mitoses (arrows) in 10 high power fields. (C) Focal microcalcifications. (D) Microcysts and myxoid change.
Figure 5.
(A) Patchy positive GFAP with a subset of fragments demonstrating more prominent expression in perivascular regions. (B) Strong to moderate OLIG2 immunopositivity in the majority of tumor nuclei. (C) Patchy positive EMA with a strong perinuclear dot-like pattern. (D) High Ki-67 (10–30%).
Further investigation with the Mayo Clinic Neuro-Oncology Expanded Gene Panel with Rearrangement revealed EGFR c.1088C>T (p.T363I) (Exon 9) and c.1793G>T (p.G598V) (Exon 15), clinically relevant variants, as well as a BCOR c.626del (p.S209Cfs*7) (Exon 4) variant. Variants of unknown significance were uncovered, including NOTCH1 c.3076G>A (Exon 19), FUBP1 c.263A>T (Exon 4), and MET c.3272C>T (Exon 15). The fusion assay did not detect a BCOR/BCOR(L1) fusion, prompting further analysis with DNA methylation profiling. The composite methylation profile on the Heidelberg classifier versions 11b6 and 12b6 and the NCI/Bethesda classifier versions 2.0 and 3.0 indicated a consensus match to CNS tumor with BCOR/BCOR(L1) fusion. Dimensionality reduction with UMAP (uniform manifold approximation and projection) and t-SNE (t-distributed stochastic neighbor embedding) also placed the tumor into the same class (Figure 6).
Figure 6.
Methylation-based t-SNE distribution. Reference DNA methylation classes (Bethesda CNS tumor classifier v3.0): CNS_BCOR_FUS: CNS tumor with BCOR/BCORL1 fusion; CNS_BCOR_ITD: CNS tumor with BCOR internal tandem duplication; CNS_PLAG_AMP: CNS embryonal tumor with PLAG-family amplification; EPN_ST_SE: subependymoma and ependymoma, supratentorial; EPN_ST_ZFTA_FUS: supratentorial ependymoma, ZFTA fusion-positive; EPN_SPINE_SE: subependymoma and ependymoma, spinal; and SP_EPN_MYCN_LIKE: spinal ependymoma, MYCN-amplified-like.
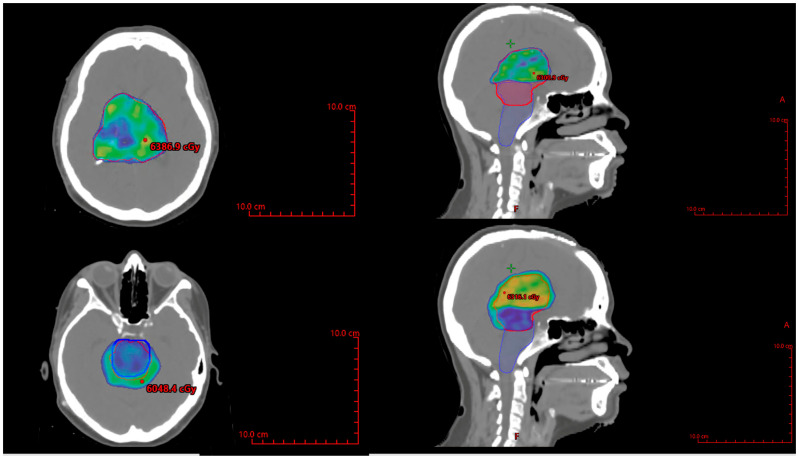
She subsequently developed a bilateral upgaze palsy. Her radiation planning brain MRI showed continued tumor progression (Figure 3E,F). Following a multi-disciplinary discussion coupled with lack of any actionable targets on expression profiling, the decision was made to proceed with external beam radiation therapy (59.4 Gy in 33 fractions) followed by six cycles of adjuvant temozolomide 150–200 mg/m2. The planning target volume overlapping the brainstem was significant and was prescribed to receive 54 Gy through dose painting to respect brainstem tolerance (Figure 7). The patient tolerated the treatment well, with improvement in her bilateral upwards gaze palsy and headaches within 2 weeks of starting radiation therapy. She completed radiation treatment with Grade 1 fatigue.
Figure 7.
Axial and sagittal views of the radiation treatment plan with PTV shown in red, and brainstem shown in blue, with color wash set to 5940 cGy in the top panels, and 5400 cGy in the bottom panels. PTV = planning treatment volume.
Following three cycles of temozolomide, her MRI brain showed response to treatment with significant tumor reduction (Figure 3G,H). Her symptoms resolved, and she remains alive at 11-month follow-up. Ethical guidelines set out by the Declaration of Helsinki were followed in the preparation of this report, and the patient provided written consent.
3. Discussion
The updated WHO classification of CNS tumors improves upon the prior classification schemas by incorporating histologic, molecular, and epigenetic characteristics into an integrated diagnosis [22]. DNA methylation profiling has emerged as an effective means to identify new CNS tumor entities with distinct genetic and epigenetic features within the CNS-PNET group. BCOR-ITD is a newly identified tumor among CNS-PNETs, wherein a match to this methylation class on DNA methylation profiling is an essential criterion for unresolved cases.
BCOR alterations occur in a variety of tumors, including CNS tumors with BCOR/BCOR(L1) fusion, CNS tumors with BCOR-ITD, gliomas, medulloblastomas, and specific sarcomas [3,14,17,23]. CNS tumors with BCOR/BCOR(L1) fusion are characterized by the presence of a BCOR fusion, often paired with EP300 as the typical fusion partner, with or without hallmark CNV within chromosomes 22q12.31 and Xp11.4 [17]. However, CNS tumors without BCOR/BCOR(L1) fusion that instead harbor copy number variations within chromosome Xp11.4 and 22q12.31 or truncating small nucleotide variants can match to the same methylation class as those with BCOR/BCOR(L1) fusion [14,17]. To our knowledge, this is the third reported case of a CNS tumor with BCOR/BCOR(L1) fusion matching the methylation class with a BCOR small nucleotide variant (BCOR c.626del (p.S209Cfs*7) (Exon 4)) instead of a fusion. These cases are exceptionally rare, but share similar molecular characteristics [14]. BCOR deletions hinder PRC1.1 tumor suppressor functioning and incomplete PRC1.1 dysfunction may be sufficiently adequate to promote tumorigenesis [10,24].
CNS tumors with BCOR/BCOR(L1) fusion predominantly occur in adults [14,18,19], consistent with the age of our patient at diagnosis of 37 years. In keeping with the reported radiographic features of CNS tumors with BCOR/BCOR(L1) fusion and predilection for the ventricular region, our patient’s tumor involved the lateral ventricle and demonstrated T1 hypointensity and T2 hyperintensity [17]. These tumors can appear well-demarcated or diffusely infiltrating and with or without enhancement [17,20]. Our patient’s tumor was well-demarcated, enhancing, diffusion-restricting, partially calcified, and partially cystic.
Collectively, the histomorphologic examination of our patient’s tumor corroborates other reported cases of CNS tumors with BCOR/BCOR(L1) fusion, which exhibit diverse histologic findings including oligodendroglial-like chicken-wire vasculature and/or ependymomal-like perivascular pseudorosettes [17,19]. With the exception of supratentorial ZFTA fusion-positive ependymomas which can show patchy Olig2 positivity, extensive Olig2 and patchy GFAP positivity are otherwise atypical in ependymomas [19] and should prompt further consideration for an alternative diagnosis such as CNS tumors with BCOR/BCOR(L1) fusion, as substantiated in our case. Malignant features, including necrosis and elevated mitotic activity are frequently observed in these tumors [19]. Microvascular proliferation is less commonly demonstrated [19]. Our patient’s tumor was highly proliferative with frequent mitotic activity and an estimated Ki-67 ranging from 10–30%. Notably, EMA showed focal strong dot-like perinuclear immunoreactivity, which has not been previously reported in these tumors.
Our patient’s tumor also harbored EGFR p.G598V and p.T363I pathologic variants. In glioblastoma, EGFR p.T363I has been reported in cases of glioblastoma in a single tumor sector [25]. In contrast, EGFR p.G598V is one of the most common extracellular domain missense mutations [26]. EGFR p.G598V is not known to be associated with survival [26]. However, preclinical data suggest that glioblastoma with EGFR missense mutants may be responsive to EGFR kinase inhibitors [27,28,29]. The presence of these EGFR missense mutations in our patient’s tumor may be therapeutically relevant for second-line therapy in the setting of recurrence [26,28]. However, treatment resistance [29] has also been demonstrated in certain cases [28].
In view of the limited evidence on therapies for this extremely rare tumor that matched the methylation class of CNS tumors with BCOR/BCORL1 fusion without a detectable fusion, we reasoned that radiation therapy was warranted given the high proliferative activity. Temozolomide was also selected for its efficacy in CNS tumors with BCOR/BCORL1 fusion and its favorable toxicity profile [16,30,31]. Bevacizumab, carboplatin, ifosfamide, cisplatin, and etoposide also have proven efficacy, but carry greater risks of toxicities [16,19,30] (Table 1).
Table 1.
CNS Tumor with BCOR fusion treatments.
| PMID | Age | Sex | Location | Fusion | Initial Diagnosis | Treatment | PFS (Months) |
OS (Months) |
|---|---|---|---|---|---|---|---|---|
| Pisapia et al. [30] | 15 | M | right frontal, left temporal, left occipital | BCOR:CREBBP | diffuse astrocytic glioma, with molecular features of glioblastoma | RT with concomitant TMZ | 18 | 27 |
| Baressi et al. [18] | 45 | M | right frontal | CREBBP:BCORL1 | - | RT and TMZ | 1 | 1 |
| Yamazaki et al. [16] | 17 | F | right frontal | CREBBP:BCORL1 | - | resection | 18 | - |
| Tauziède-Espariat et al. [20] | 13 | M | right temporal | EP300:BCOR | - | GTR and chemotherapy | 16 | 16 |
| Tauziède-Espariat et al. [20] | 27 | M | left frontal | EP300:BCOR | - | GTR, chemotherapy and focal RT | 27 | 27 |
| Tauziède-Espariat et al. [19] | 64 | M | left frontal | EP300:BCOR | high-grade glioneuronal tumor | GTR | 12 | 12 |
| Tauziède-Espariat et al. [19] | 40 | M | within the right lateral ventricle | EP300:BCOR | high-grade glioneuronal tumor | STR, carboplatin and VP16 | 12 | 12 |
| Torre et al. [21] | 12 | M | right frontal | EP300:BCOR | oligodendroglioma | extraventricular drainage, STR | 4.5 | 6 |
| Torre et al. [21] | 10 | F | left basal ganglia and thalamus | EP300:BCOR | glioblastoma | STR | 1 | 7 |
| Torre et al. [21] | 18 | M | right medial occipital | EP300:BCOR | dysembryoplastic neuroepithelial tumor (DNET) versus glioneuronal tumor | STR | 15 | 42 |
| Fukuoka et al. [32] | 72 | M | occipital | EP300:BCORL1 | anaplastic ependymoma G3 | resection, RT (40 Gy), chemotherapy | 24 | 33 |
| Xu et al. [31] | 43 | F | right frontotemporal | EP300:BCOR | - | NTR, RT (5200 cGy), adjuvant chemotherapy | 8 | 8 |
| Xu et al. [31] | 54 | M | right frontal | EP300:BCOR; BCOR-L3MBTL2 | - | GTR, RT (6000 cGy) and adjuvant TMZ | 16 | 16 |
| Yamazaki et al. [16] | 18 | F | right frontal | - | - | resection | 33 | - |
| Yamazaki et al. [16] | 21 | F | right frontal | - | - | resection, RT (54 Gy), TMZ | 89 | - |
| Yamazaki et al. [16] | 25 | F | right frontal | - | - | resection, SRS, adjuvant chemotherapy with bevacizumab, surgery, later addition of ifosfamide, cisplatin, and etoposide | - | 115 |
RT = radiation therapy; TMZ = temozolomide; GTR = gross total resection; STR = subtotal resection; and SRS = stereotactic radiosurgery.
We acknowledge that several limitations exist in this study. The nature of a single case may not reflect the broader population of CNS tumors with BCOR/BCORL1 fusions in their entirety or those tumors matching to the same methylation class. The short follow-up in our patient limits the understanding of the durability of this treatment regimen and the sustainability of the response to therapy.
Future investigations should be directed at deepening our understanding of the clinical and biological behavior of these tumors. Evidence suggests that these tumors are unique and distinguishable from CNS tumors with BCOR-ITD. Therapeutics represent an open area of investigation, and forthcoming studies should be directed at identifying effective therapies for these tumors. As we cultivate our understanding of CNS tumors with BCOR/BCORL1 fusions, the management for these patients will also continue evolve.
Our case adds to the limited but growing body of evidence for a new CNS tumor type or subtype that may be incorporated into the future WHO classification of CNS tumors. Substantial evidence, including therapeutic options, remains to be uncovered for CNS tumors with BCOR(L1) fusions.
4. Conclusions
A CNS tumor with BCOR/BCOR(L1) fusion is a rare tumor entity, not yet defined in the 2021 WHO classification of CNS tumors. Our case adds to the growing body of evidence for definitive radiation and temozolomide for the treatment of this rare tumor entity.
Abbreviations
The following abbreviations are used in this manuscript:
| CNS | central nervous system tumor |
| BCOR | BCL6 corepressor gene |
| CT | computed tomography |
| GTR | gross total resection |
| ITD | internal tandem duplication |
| MRI | magnetic resonance imaging |
| RT | radiation therapy |
| SRS | stereotactic radiosurgery |
| STR | subtotal resection |
| t-SNE | t-distributed stochastic neighbor embedding |
| TMZ | temozolomide |
| UMAP | uniform manifold approximation and projection |
| WHO | World Health Organization |
Author Contributions
J.L.—data collection, analysis, interpretation, manuscript drafting, and final approval. W.Y.—data analysis, editing, and final approval. K.A.—data analysis, interpretation, editing, and final approval. E.C.—data collection, interpretation, editing, and final approval. C.H.—data collection, interpretation, editing, and final approval. F.P.K.H.—editing and final approval. M.Z.—data collection, drafting, and final approval. A.R.—data collection and final approval. G.F.—data collection and final approval. D.N.—data collection and final approval. C.A.Y.—study concept, data collection, analysis, interpretation, manuscript drafting, editing, revision, and final approval. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the University of California, Irvine Institutional Review Board (#7459 on 18 June 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis D.N., Wesseling P., Aldape K., Brat D.J., Capper D., Cree I.A., Eberhart C., Figarella-Branger D., Fouladi M., Fuller G.N., et al. cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020;30:844–856. doi: 10.1111/bpa.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturm D., Orr B.A., Toprak U.H., Hovestadt V., Jones D.T.W., Capper D., Sill M., Buchhalter I., Northcott P.A., Leis I., et al. New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. Cell. 2016;164:1060–1072. doi: 10.1016/j.cell.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astolfi A., Fiore M., Melchionda F., Indio V., Bertuccio S.N., Pession A. BCOR involvement in cancer. Epigenomics. 2019;11:835–855. doi: 10.2217/epi-2018-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huynh K.D., Fischle W., Verdin E., Bardwell V.J. BCoR, a novel corepressor involved in BCL-6 repression. Genes. Dev. 2000;14:1810–1823. doi: 10.1101/gad.14.14.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gearhart M.D., Corcoran C.M., Wamstad J.A., Bardwell V.J. Polycomb group and SCF ubiquitin ligases are found in a novel BCOR complex that is recruited to BCL6 targets. Mol. Cell Biol. 2006;26:6880–6889. doi: 10.1128/MCB.00630-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Junco S.E., Wang R., Gaipa J.C., Taylor A.B., Schirf V., Gearhart M.D., Bardwell V.J., Demeler B., Hart P.J., Kim C.A. Structure of the polycomb group protein PCGF1 in complex with BCOR reveals basis for binding selectivity of PCGF homologs. Structure. 2013;21:665–671. doi: 10.1016/j.str.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi W.I., Jeon B.N., Yoon J.H., Koh D.I., Kim M.H., Yu M.Y., Lee K.M., Kim Y., Kim K., Hur S.S., et al. The proto-oncoprotein FBI-1 interacts with MBD3 to recruit the Mi-2/NuRD-HDAC complex and BCoR and to silence p21WAF/CDKN1A by DNA methylation. Nucleic Acids Res. 2013;41:6403–6420. doi: 10.1093/nar/gkt359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagan J.K., Arnold J., Hanchard K.J., Kumar R., Bruno T., Jones M.J., Richard D.J., Forrest A., Spurdle A., Verdin E., et al. A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J. Biol. Chem. 2007;282:15248–15257. doi: 10.1074/jbc.M700246200. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer E.J., Wang H.C., Karp H.Q., Meyer C.A., Cejas P., Gearhart M.D., Adelman E.R., Fares I., Apffel A., Lim K., et al. BCOR and BCORL1 Mutations Drive Epigenetic Reprogramming and Oncogenic Signaling by Unlinking PRC1.1 from Target Genes. Blood Cancer Discov. 2022;3:116–135. doi: 10.1158/2643-3230.BCD-21-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackledge N.P., Rose N.R., Klose R.J. Targeting Polycomb systems to regulate gene expression: Modifications to a complex story. Nat. Rev. Mol. Cell Biol. 2015;16:643–649. doi: 10.1038/nrm4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossmann V., Tiacci E., Holmes A.B., Kohlmann A., Martelli M.P., Kern W., Spanhol-Rosseto A., Klein H.U., Dugas M., Schindela S., et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118:6153–6163. doi: 10.1182/blood-2011-07-365320. [DOI] [PubMed] [Google Scholar]
- 13.Damm F., Chesnais V., Nagata Y., Yoshida K., Scourzic L., Okuno Y., Itzykson R., Sanada M., Shiraishi Y., Gelsi-Boyer V., et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood. 2013;122:3169–3177. doi: 10.1182/blood-2012-11-469619. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z., Rajan S., Chung H.J., Raffeld M., Panneer Selvam P., Schweizer L., Perry A., Samuel D., Giannini C., Ragunathan A., et al. Molecular and clinicopathologic characteristics of gliomas with EP300::BCOR fusions. Acta Neuropathol. 2022;144:1175–1178. doi: 10.1007/s00401-022-02508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gojo J., Kjaersgaard M., Zezschwitz B.V., Capper D., Tietze A., Kool M., Haberler C., Pizer B., Hoff K.V. Rare embryonal and sarcomatous central nervous system tumours: State-of-the art and future directions. Eur. J. Med. Genet. 2023;66:104660. doi: 10.1016/j.ejmg.2022.104660. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki A., Arai Y., Fukuoka K., Nakano Y., Hama N., Nakata S., Makino K., Kuroda J.I., Shinojima N., Mukasa A., et al. Diffusely infiltrating glioma with CREBBP-BCORL1 fusion showing overexpression of not only BCORL1 but BCOR: A case report. Brain Tumor Pathol. 2022;39:171–178. doi: 10.1007/s10014-022-00435-4. [DOI] [PubMed] [Google Scholar]
- 17.Ebrahimi A., Waha A., Schittenhelm J., Gohla G., Schuhmann M.U., Pietsch T. BCOR::CREBBP fusion in malignant neuroepithelial tumor of CNS expands the spectrum of methylation class CNS tumor with BCOR/BCOR(L1)-fusion. Acta Neuropathol. Commun. 2024;12:60. doi: 10.1186/s40478-024-01780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barresi V., Cardoni A., Miele E., Pedace L., Masotto B., Nardini C., Barresi S., Rossi S. CNS tumor with CREBBP::BCORL1 Fusion and pathogenic mutations in BCOR and CREBBP: Expanding the spectrum of BCOR-altered tumors. Acta Neuropathol. Commun. 2024;12:8. doi: 10.1186/s40478-024-01726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tauziede-Espariat A., Uro-Coste E., Sievers P., Nicaise Y., Mariet C., Siegfried A., Pierron G., Guillemot D., Benzakoun J., Pallud J., et al. CNS tumor with EP300::BCOR fusion: Discussing its prevalence in adult population. Acta Neuropathol. Commun. 2023;11:26. doi: 10.1186/s40478-023-01523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tauziede-Espariat A., Pierron G., Siegfried A., Guillemot D., Uro-Coste E., Nicaise Y., Castel D., Catalaa I., Larrieu-Ciron D., Chaynes P., et al. The EP300:BCOR fusion extends the genetic alteration spectrum defining the new tumoral entity of “CNS tumors with BCOR internal tandem duplication”. Acta Neuropathol. Commun. 2020;8:178. doi: 10.1186/s40478-020-01064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torre M., Meredith D.M., Dubuc A., Solomon D.A., Perry A., Vasudevaraja V., Serrano J., Snuderl M., Ligon K.L., Alexandrescu S. Recurrent EP300-BCOR Fusions in Pediatric Gliomas with Distinct Clinicopathologic Features. J. Neuropathol. Exp. Neurol. 2019;78:305–314. doi: 10.1093/jnen/nlz011. [DOI] [PubMed] [Google Scholar]
- 22.Jaunmuktane Z., Capper D., Jones D.T.W., Schrimpf D., Sill M., Dutt M., Suraweera N., Pfister S.M., von Deimling A., Brandner S. Methylation array profiling of adult brain tumours: Diagnostic outcomes in a large, single centre. Acta Neuropathol. Commun. 2019;7:24. doi: 10.1186/s40478-019-0668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips J.J., Gong H., Chen K., Joseph N.M., van Ziffle J., Bastian B.C., Grenert J.P., Kline C.N., Mueller S., Banerjee A., et al. The genetic landscape of anaplastic pleomorphic xanthoastrocytoma. Brain Pathol. 2019;29:85–96. doi: 10.1111/bpa.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremer J., Kottke R., Johann P.D., von Hoff K., Brazzola P., Grotzer M.A., Kool M., Rushing E., Gerber N.U. A single supratentorial high-grade neuroepithelial tumor with two distinct BCOR mutations, exceptionally long complete remission and survival. Pediatr. Blood Cancer. 2020;67:e28384. doi: 10.1002/pbc.28384. [DOI] [PubMed] [Google Scholar]
- 25.Mahlokozera T., Vellimana A.K., Li T., Mao D.D., Zohny Z.S., Kim D.H., Tran D.D., Marcus D.S., Fouke S.J., Campian J.L., et al. Biological and therapeutic implications of multisector sequencing in newly diagnosed glioblastoma. Neuro Oncol. 2018;20:472–483. doi: 10.1093/neuonc/nox232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binder Z.A., Thorne A.H., Bakas S., Wileyto E.P., Bilello M., Akbari H., Rathore S., Ha S.M., Zhang L., Ferguson C.J., et al. Epidermal Growth Factor Receptor Extracellular Domain Mutations in Glioblastoma Present Opportunities for Clinical Imaging and Therapeutic Development. Cancer Cell. 2018;34:163–177.e167. doi: 10.1016/j.ccell.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J.C., Vivanco I., Beroukhim R., Huang J.H., Feng W.L., DeBiasi R.M., Yoshimoto K., King J.C., Nghiemphu P., Yuza Y., et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006;3:e485. doi: 10.1371/journal.pmed.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes T.K., Aquilanti E., Persky N.S., Yang X., Kim E.E., Brenan L., Goodale A.B., Alan D., Sharpe T., Shue R.E., et al. Comprehensive mutational scanning of EGFR reveals TKI sensitivities of extracellular domain mutants. Nat. Commun. 2024;15:2742. doi: 10.1038/s41467-024-45594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivanco I., Robins H.I., Rohle D., Campos C., Grommes C., Nghiemphu P.L., Kubek S., Oldrini B., Chheda M.G., Yannuzzi N., et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2012;2:458–471. doi: 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisapia D.J., Ohara K., Bareja R., Wilkes D.C., Hissong E., Croyle J.A., Kim J.H., Saab J., MacDonald T.Y., Beg S., et al. Fusions involving BCOR and CREBBP are rare events in infiltrating glioma. Acta Neuropathol. Commun. 2020;8:80. doi: 10.1186/s40478-020-00951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y., Hou Y., Gao X., Li J., Jiang D., Li Z., Hu P., Wang Y., Wen Y., Yao X., et al. Report two adult cases of high-grade neuroepithelial neoplasm harbouring EP300::BCOR fusions with comprehensive molecular detection. Brain Pathol. 2023;33:e13177. doi: 10.1111/bpa.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuoka K., Kanemura Y., Shofuda T., Fukushima S., Yamashita S., Narushima D., Kato M., Honda-Kitahara M., Ichikawa H., Kohno T., et al. Significance of molecular classification of ependymomas: C11orf95-RELA fusion-negative supratentorial ependymomas are a heterogeneous group of tumors. Acta Neuropathol. Commun. 2018;6:134. doi: 10.1186/s40478-018-0630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical restrictions.