Abstract
In contrast to the high degree of experience-dependent plasticity usually exhibited by cortical representational maps, a number of experiments performed in visual cortex suggest that the basic layout of orientation preference maps is only barely susceptible to activity-dependent modifications. In fact, most of what we know about activity-dependent plasticity in adults comes from experiments in somatosensory, auditory, or motor cortex. Applying a stimulation protocol that has been proven highly effective in other cortical areas, we demonstrate here that enforced synchronous cortical activity induces major changes of orientation preference maps (OPMs) in adult cats. Combining optical imaging of intrinsic signals and electrophysiological single-cell recordings, we show that a few hours of intracortical microstimulation (ICMS) lead to an enlargement of the cortical representational zone at the ICMS site and an extensive restructuring of the entire OPM layout up to several millimeters away, paralleled by dramatic changes of pinwheel numbers and locations. At the single-cell level, we found that the preferred orientation was shifted toward the orientation of the ICMS site over a region of up to 4 mm. Our results show that manipulating the synchronicity of cortical activity locally without invoking training, attention, or reinforcement, OPMs undergo large-scale reorganization reminiscent of plastic changes observed for nonvisual cortical maps. However, changes were much more widespread and enduring. Such large-scale restructuring of the visual cortical networks indicates a substantial capability for activity-dependent plasticity of adult visual cortex and may provide the basis for cognitive learning processes.
Training and learning induce powerful reorganization of the adult brain in parallel to an improvement of perceptual and behavioral abilities (1). Cortically, enhancement of sensorimotor abilities are assumed to be mediated by network adaptations based on alterations of synaptic efficacy. Although all cortical areas share a substantial degree of similarities (2), most of what we know about activity-dependent plasticity in adults comes from experiments in somatosensory, auditory, or motor cortex (3–6), leaving open whether visual cortex differs in terms of capacities and ease for modifiability (7–9). In fact, a number of experiments performed in visual cortex suggest that the basic layout of orientation preference maps (OPMs) is only barely susceptible to activity-dependent modifications (10–16). This apparent stability has even raised the suspicion that OPMs may be prespecified by genetic instructions (13, 17–20). On the other hand, in vitro studies using slice preparations from adult visual cortex demonstrated very clearly the existence of the entire cellular repertoire required to mediate synaptic plasticity (21).
We approached the puzzle about the potential reorganizational capacities of adult visual cortex by combining optical imaging of intrinsic signals and single-cell electrophysiology to record signatures of plastic changes evoked by a stimulation protocol that has been proven highly effective in other cortical areas (5, 6, 9). Instead of manipulating environmental constraints, we directly enforced local, intracortical synchronous activity by electrical intracortical microstimulation (ICMS; refs. 22 and 23) or “focal electrical stimulation,” as called by others (24, 25). ICMS consists of repetitively applied electrical pulses delivered by a microelectrode. The resulting temporally synchronized neuronal discharges are highly effective for the induction of reorganizational processes. ICMS has been used successfully to study rapid, i.e., plastic changes inducible within a few hours in adult motor, somatosensory, and auditory cortex and thalamus; these changes were fully reversible (23–32). The region of tissue that is stimulated by direct activation during ICMS is very small (50–100 μm; refs. 22–23). Because of this locality, and because ICMS can be applied at any desired location within a map, ICMS works largely independently of the peripheral and subcortical pathways and of the constraints provided by particularities of a sensory pathway and its preprocessing. Based on experimental and theoretical work, it has been suggested that the detailed topography of the OPMs arise from a meticulous balance of both local and global mechanisms based on feed-forward and recurrent inputs (33–38). Particularly, the extended network of horizontal connections is assumed to play a crucial role in mediating and stabilizing a mature pattern of OPMs (8, 15, 39–43). Thus, ICMS is optimally suited for addressing the role of local mechanisms and global interactions for the modifiability of the topography of cortical maps, and more generally, for performing a comparative analysis of reorganizational capacities across areas and modalities. Our results show that in adult visual cortex plastic processes modify in a systematic way large portions of the OPMs over several millimeters, including dramatic changes in the layout of pinwheel pattern. Accordingly, widespread restructuring in the functional topography of adult visual cortex can be induced by locally imposing synchronous activity that emphasizes the crucial role of intracortical mechanisms through large and distributed networks.
Materials and Methods
General Animal Preparation.
Twenty-four cats (3–12 months old, average of 7.5 months) were anesthetized for surgery with 25 mg/kg ketamine hydrochloride in combination with 1.5 mg/kg xylacine. During experiments, the animals were continuously infused with 3–9 mg/h of pentobarbital (Nembutal) together with dextrose and electrolytes. Animals were paralyzed by continuous infusions of gallamine triethiodide (2 mg/kg/h). Treatment conformed to National Institutes of Health guidelines; all experiments were approved by the German Animal Care and Use Committee. Respiration was adjusted for an end-tidal CO2 between 3.5 and 4.0%. Pupils were dilated by atropine, and nictitating membranes were retracted by neosynephrine. Contact lenses were used to cover the eyes and to focus them on the monitor. For optical imaging, the skull was opened between Horsley–Clarke coordinates A0 and A10 and L0 and L5 above area 18 of the left hemisphere. A stainless-steel chamber was cemented onto the skull, the dura was removed, and the chamber was filled with silicone oil and sealed with a coverglass.
Optical Recording and Data Acquisition.
For optical measurements, we used a Lightstar II imaging system (LaVision, Göttingen, Germany) with a 2 MHz A/D converter and a slow scan 12-bit charge-coupled device camera. Single images were obtained with acquisition times of 80-ms duration. Averaging was performed within and across trials. Reflectance changes were accumulated between 800 and 2800 ms after stimulus onset. Each trial was separated by a pause of about 20 s. The cortex was illuminated with a 610 nm light source. Visual stimuli were moving square-wave gratings of high-contrast presented at four orientations moving in eight directions at a spatial frequency of 0.15 cycles per degree and a drift velocity of 16° per s. The stimuli were presented to the contralateral eye on a 21-inch monitor (120 Hz, noninterlaced) and covered 50 × 50° visual angle. Nonstimulus control (blank) images were recorded before each trial presentation. Image acquisition was triggered by respiration. During each presentation, the grating was moved in one direction and moved in the opposite during another presentation. Data acquired for opposite directions were averaged. Each stimulus was repeated between 20 and 60 times.
Image Processing.
Images were computed by dividing a stimulus condition by a cocktail-blank (single-condition map, SCM). For quantitative analysis, each SCM was median filtered (width = 0.09 mm) and smoothed with a Gaussian filter (width = 0.05–0.1 mm). To correct for unequal illumination within single frames, we subtracted a widely smoothed (smoothing width = 0.8–1.5 mm) image from its original one, a procedure equivalent to a highpass-filter but conserving spatial structures in the crucial range of orientation domains (cf. 41). For contrast enhancement, SCMs of orthogonal orientations were subtracted. The SCMs were used to calculate OPMs by vectorially summing for each pixel the responses to all orientations. The angle of the resulting preferred stimulus orientation at each pixel was color coded.
To analyze the relative representational areas of the orientation domains, we considered three orientation classes relative to the preferred orientation at the ICMS site: the preferred orientation at the ICMS site ± 22.5° (preferred orientation), orientations orthogonal to the preferred orientation (± 67.5 to 90°, orthogonal orientation) and orientations between ± 22.5 to 67.5° (oblique orientations). The two classes containing oblique orientations were pooled. Based on pixel counts for each class, we computed its relative occurrence.
Two OPMs were compared by computing the cross correlation between these maps. To correct for shifts of the recording frame, we calculated the correlation coefficient between two maps at x,y-offsets between −40 to + 40 pixels (each pixel corresponds to 13 μm of cortical surface) resulting in a two-dimensional array of correlation coefficients. The maximum correlation coefficient was used for further analysis (cf. 12).
Electrophysiology.
By using linear arrays of seven quartz glass-coated platinum/tungsten microelectrodes (3–4 MΩ, evenly spaced by 750 μm), single cells were recorded in the central representation of area 18. Penetration depth was 0.7–1.0 mm. One of the electrodes was used to apply ICMS. For each cell, orientation tuning curves were derived before and after ICMS from poststimulus time histograms (PSTHs) after presentation of eight oriented stimuli (spaced by 22.5°) in the receptive field of each neuron. For quantitative analysis, orientation tuning curves were computed by using the spike count within 30 to 80 ms after stimulus onset, which corresponds to the peak area in the PSTH. Activity was normalized to the maximal activity of each cell. Preferred orientation was computed by vectorial averaging of the responses to each presented orientation.
ICMS.
ICMS was applied through a microelectrode at a so-called ICMS site. The stimulation consisted of a burst of capacitance-coupled and charged-balanced pulses of 6 μA and 200 μs duration applied at 1 Hz (bursts at 300 Hz for 40 ms; for details see refs. 22, 23, 26, and 27).
The significance of all effects was tested by univariate analysis of variance (ANOVA) and posthoc t tests.
Results
Plasticity of Orientation Preference Maps.
The experimental schedule of the optical imaging experiments consisted of first establishing a baseline of the layout and stability of OPMs under control conditions by repeated measurements. Subsequently, ICMS was applied for 3 to 4 h at a selected location (ICMS site) within the OPM. During this period, no visual stimuli were presented. After termination of ICMS, assessment of OPMs was repeated several times up to 18 h after termination of ICMS.
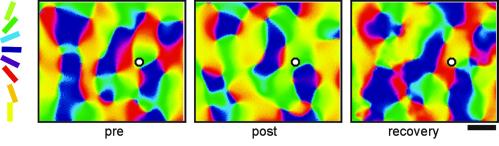
Under control conditions, SCMs and OPMs showed the well known pattern of orientation domains (Fig. 1). In all ICMS-stimulated animals (n = 10), ICMS induced a profound reorganization of the orientation maps. A typical ICMS effect consisted of an enlargement of the cortical representational zone at and around the ICMS site. Consequently, as illustrated for the SCMs in Fig. 1 a–d, the representation of the preferred orientation at the ICMS site (left oblique) increased in size. On average, the relative area of the orientation domains representing the orientation of the ICMS-site was enlarged from 21.4% to 35.5%. The domains representing orientations oblique to the preferred orientation of the ICMS-site were reduced from 25.8% pre-ICMS to 20.8% post-ICMS, and the domains representing orthogonal orientations from 25.2% to 19.7% (Fig. 2a).
Figure 1.
ICMS-induced plasticity of SCMs for different stimulus orientations (a–d) and OPMs (e) recorded in right visual cortex (area 18) of an adult cat. For both SCMs and OPMs, two control conditions are shown (pre1 and pre2) recorded within 2 h before ICMS, after 4 h of ICMS (post), and 4 h after termination of ICMS (recovery). In the SCMs, dark indicates increased activation. Stimulus orientations are indicated by the gratings. Color code of orientation preference used for OPMs is indicated. Image of the cortical surface obtained at 546 nm is shown at the bottom. (Bar = 1 mm.) ICMS sites are marked (white circles). Comparison of the two control conditions recorded within 2 h (pre1, pre2) indicates high reproducibility of SCMs and OPMs. In contrast, note the considerable expansion of cortical territory (after ICMS–post) representing the orientation preference of the ICMS site (d, left oblique, orientation coded in orange to red in OPMs). There is substantial reorganization (major changes marked by arrowheads) of the OPM within the entire observation window (3.5 mm × 2.9 mm). Note that there is no complete recovery of the entire layout after termination of ICMS. Some portions within the map appear to recover well (white triangles in the recovery map), whereas others do not (black triangles). Correlation coefficients (see Fig. 2) were pre1-pre2, 0.53; pre1-post, 0.27; pre2-post, 0.27; pre1-rec, 0.48; and pre2-rec, 0.48. The spatial extent of the cortical territory representing different orientations recovered to normal (see Fig. 2a), although the exact spatial distributions and locations of activity within the OPM remained dissimilar from the preconditions (see Fig. 2b).
Figure 2.
Summary diagram of ICMS-induced changes in OPMs. (a) Changes in the relative sizes of iso-orientation domains. Mean values and SDs are shown for different stimulus orientations relative to the orientation preference of the ICMS site (red squares, preferred orientation; green circles, oblique orientations; blue diamonds, orthogonal orientations) at different recording sessions (pre-ICMS, post-ICMS, after recovery). Note the significant enlargement of domains representing the orientation at the ICMS site at the cost of domains representing neighboring and orthogonal orientations (ANOVA, F4,103 = 8.75, P < 0.0001; posthoc t tests, P < 0.001 for the preferred orientation and P < 0.05 for the other orientations). Representational areas after recovery were not significantly different from the precondition (P > 0.6). In this and all following figures, significance is indicated with: **, P < 0.01; *, P < 0.05; and n.s., not significant. (b) Average two-dimensional cross-correlation coefficients describing map similarity between two pre-ICMS maps (pre-pre, n = 10), pre- and post-ICMS maps (pre-post, n = 10), preICMS maps and maps after the recovery period (pre-rec, n = 6), and pre- and post-sham stimulation (pre-sham, n = 2). Means, black dots; SEs, boxes; and SDs, whiskers. Univariate ANOVA (F3,24 = 8.27, P < 0.0006) and posthoc t tests revealed that the correlation coefficient for pre-post comparison (0.24) was significantly lower than that for comparison of two pre maps (0.48, P < 0.005). After several hours of termination of ICMS, the correlation coefficient had an intermediate value (0.34), which was not significantly different from the pre-pre condition (P = 0.18). Sham stimulation had no effect (0.50, P = 0.99).
Generally, these changes were widespread leading to extensive restructuring of the entire OPM layout. As shown in Fig. 1e and Fig. 3, the enlargement of the domain representing the orientation at the ICMS site (Fig. 1e, left-oblique, coded in red; Fig. 3 vertical, coded in yellow) consisted of a broadening and a shift of orientation domains. In addition, new domains representing the orientation at the ICMS site emerged at some distances away from the ICMS site. Furthermore, domains oblique or orthogonal to that orientation contracted or even disappeared. As a consequence, the regular pinwheel pattern found under control conditions underwent substantial changes up to 3 mm around the ICMS site. Existing pinwheels disappeared and new pinwheels emerged. Within this observation frame, we found no evidence for a distance-dependency of these effects.
Figure 3.
Example of an OPM exhibiting progressive changes of the OPM layout after termination of ICMS. Conventions here are the same as in Fig. 1. Maps were recorded before (Left), after 4 h of ICMS (Center), and 15 h after termination of ICMS (Right). Color code is indicated. (Bar = 1 mm.) ICMS sites are marked (white circles). Note the considerable expansion of cortical territory representing the orientation preference of the ICMS site (vertical orientation coded in yellow) resulting in widespread changes within the entire observation window. Note that there is no complete recovery of the layout even after 15 h. A partial recovery is restricted to the caudal (Right) portions of the map. In the middle and rostral (Left) portions of the map, there are progressive changes, particularly of the horizontal orientation (coded in blue) in the middle and the left side of the map. Correlation coefficients were 0.21 for pre-post and 0.32 for pre-rec.
To quantitatively assess the ICMS-induced changes of map layout, OPMs were compared pairwise by computing a two-dimensional cross correlation for pre-pre, pre-post, and pre-recovery conditions. Under control conditions, which consisted of repeated measurements within several hours, the spatial patterns of activity were rather stable, indicated by a mean cross-correlation coefficient of 0.48 (pre-pre, Fig. 2b). After ICMS, the described changes of the OPM layout were reflected by a strong reduction of the correlation coefficient to 0.24. Accordingly, our data suggest that after ICMS, the maps are not completely shuffled, but differ substantially from the initial state.
To assess whether the maps recovered from the ICMS effects, OPMs were recorded up to 18 h after termination of ICMS (right column in Fig. 1). In general, we found no evidence for a complete recovery from the ICMS effects within our observation period. Depending on the location within an individual OPM, orientation domains seemed to continue to change, although others tended to come back to the pattern observed pre-ICMS. Across animals, we also found a considerable variation in the degree of recovery. In some animals (see Fig. 1), a high degree of similarity between pre and post maps was reestablished only 2 h after ICMS. In others, even after 15 h, the OPM patterns remained fairly different from the pre-ICMS conditions (see Fig. 3). This variable behavior was expressed in an intermediate pre-recovery correlation coefficient of 0.34 (pre-rec, Fig. 2b), which is in between the values found for pre-pre and pre-post comparison. The large variance observed for the pre-recovery condition reflects the large interindividual variability. Thus, although the representational areas on average returned to control values (24.6%, 24.5%, and 24.3%; preferred, oblique, and orthogonal orientations), the exact spatial distributions and locations of activity within the OPMs often remained dissimilar from the preconditions.
Plasticity of Single-Cell Tuning.
To confirm the optical imaging data, and to provide additional information about plastic changes of single-cell tuning properties, we performed electrophysiological experiments in nine additional animals. We found that ICMS induced major changes of the orientation tuning. Neurons most susceptible to plastic changes were those that, under control conditions, differed most from the orientation at the ICMS site, whereas cells with similar orientation preference showed only slight or no changes (Fig. 5b). Effects consisted of a shift of the preferred orientation toward the orientation present at the ICMS site. This behavior was observed equally for both narrowly and broadly tuned neurons (Fig. 4). Occasionally, we found a slight broadening of the tuning curves, so that orientations at or close to the orientation preference of the ICMS site were included in the post-ICMS tuning curves (Fig. 4). Accordingly, a subpopulation of cells that was characterized pre-ICMS by different orientation preference adopted the tuning of the ICMS site. This behavior resembled that found for the changes of orientation domains in the optical imaging (OI) data (see Fig. 5a): cortical territories showed substantial changes in orientation preference whereas others remained unaffected. In contrast, the width of the orientation-tuning curves was not affected by ICMS in a consistent manner (Fig. 4). We also found no distance-dependence of the described effects from the ICMS site within the tested range of up to 4 mm. When combined, the electrophysiological data confirmed the existence of clear changes in orientation tuning after ICMS for a subpopulation of neurons.
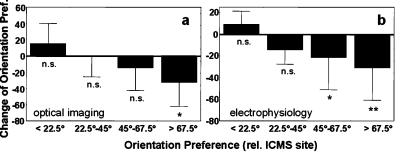
Figure 5.
(a) Changes in orientation preference in OPMs (means and SD). Maps from all optical imaging experiments were binned (10 × 10 pixels). All binned pixels were grouped with respect to the orientation preference at the ICMS sites (<22.5°, 22.5–45°, 45–67.5°, >67.5°). Changes in orientation preference were obtained after subtraction of the scatter in orientation preference derived from repeated prepre measurements. Note a significant change in orientation preference only in those pixels with a relative orientation preference difference of >67.5° in comparison to pre-ICMS orientation preference (ANOVA, F(3.13302) = 414.68; P < 0.00001; posthoc t test, P < 0.0001 for relative orientations of >67.5°). Pixels with a smaller orientation difference (22.5–67.5°) or pixels with similar or identical orientation preferences as the ICMS sites (<22.5°) showed no significant effect. (b) Changes in orientation preference computed for the single-cell orientation tuning measured before and after ICMS. Data from all cells (n = 33) are grouped with respect to the cells' difference in orientation preference before ICMS relative to the preferred orientation at the ICMS sites (means and SD). Note a significant change in orientation preference only in the groups of cells with a relative orientation preference of >45° pre-ICMS (ANOVA, F3,29 = 8.02, P < 0.001; posthoc t test, P < 0.05 and P < 0.001 for relative orientations of 45–67.5° and >67.5°, respectively).
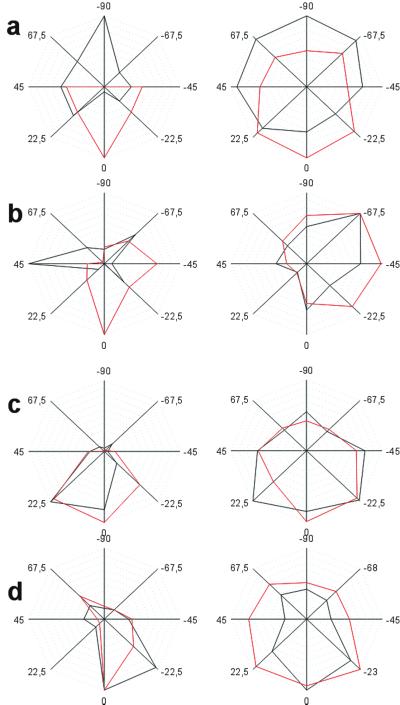
Figure 4.
Analysis of ICMS-induced changes of orientation tuning as obtained from electrophysiological experiments. (a–d) Normalized orientation-tuning curves are plotted pre- (black) and post-ICMS (red). In each case, two representative neurons are illustrated that either had a preferred orientation difference relative to the ICMS site of >67.5° (a), 45–67.5° (b), <45° (c), or were located at the ICMS site (d). Orientation preference is always expressed relative to the orientation preference of the ICMS site (0°). Note a significant change in orientation preference only in the groups of cells with a relative orientation preference of >45° pre-ICMS. See also Fig. 5 a and b. Neurons with a smaller orientation difference (22.5–45°) or neurons with similar or identical orientation preferences as the ICMS sites (<22.5°) showed no significant effect (both were P > 0.05; see c and d). Accordingly, ICMS-induced changes of orientation tuning consisted of a shift of the preferred orientation toward the orientation present at the ICMS site. This behavior was observed for narrow and broadly tuned neurons (a and b). Generally, tuning width was not systematically affected by ICMS. ANOVA revealed neither a significant main effect tuning width pre- and post-ICMS (F1,29 = 2.30, P = 0.14) nor an interaction with the preICMS orientation preference group (F3,29 = 0.80, P = 0.50).
We performed a number of controls to rule out that the observed changes were caused by unspecific side effects of ICMS or to intrinsic variability of the maps. In addition to the pre-pre controls described above, we performed sham experiments, in which the entire ICMS procedure was executed, except that no current was applied through the ICMS electrode. These experiments revealed no changes of the OPMs. The lack of map changes under sham-conditions was corroborated by the correlation analysis (pre-sham) that showed normal pre-pre values (0.50; Fig. 2b).
Discussion
Our results demonstrate that OPMs in visual cortical area 18 of adult cats are consistently and specifically modifiable. By using a protocol of ICMS that has frequently been used for the induction of plastic reorganization in nonvisual cortical and subcortical areas (23–32), we were able to induce substantial changes in the layout of OPMs of adult cat area 18. We confirmed this reorganization by single-cell recordings that demonstrated significant changes of orientation preference for a subpopulation of neurons. Accordingly, visual cortex plasticity follows rules similar to those described for other modalities, namely an activity-dependent enlargement of that representational territory that was synchronously stimulated. However, changes were not restricted to the cortical territory surrounding the ICMS site but included a restructuring of the OPMs over several millimeters with major changes of the pinwheel pattern.
ICMS Protocol.
Correlated spiking of pre and postsynaptic neurons results in strengthening or weakening of synapses, depending on the temporal order (44). The ICMS protocol differs from conventional methods of low- or high-frequency stimulation to induce synaptic plasticity such as long-term potentiation (LTP) or long-term depression (LTD). When we used a slice preparation of adult rat somatosensory cortex, we found that ICMS close to the stimulation site evoked a depression of local field potentials, implying a distinct form of synaptic depression of excitatory mechanisms (32).
Immunohistochemical techniques used to characterize in vivo ICMS in somatosensory cortex revealed a down-regulation of parvalbumin around the ICMS site, but an up-regulation of glutamate more remote to it.¶ In contrast, receptive field size increased in both zones (6, 27), indicating that different mechanisms might yield the same net effects (31). It has yet to be investigated whether similar results hold for visual cortex of cats. However, independent of how synaptic plasticity is induced, there is controversy how in vivo reorganization can be explained in terms of LTP or LTD (9, 45).
Stability of OPMs.
As shown by the pre-pre recordings, OPMs display a considerable degree of stability, although there is also evidence for some variability occurring over time. In the context of the present study, it was important to show that spontaneous fluctuations of the OPMs were clearly smaller than the changes evoked by ICMS. Although by eye the pre1-pre2 maps look rather similar (see Fig. 1), correlation coefficients were in the range of 0.5 only. However, these values are in close relation to a so-called “similarity index” used in studies of developmental plasticity where map similarity was compared over weeks. A similarity index of 0.75 used to indicate map similarity (11, 12) is equivalent to a correlation coefficient of 0.5. Recently, correlation coefficients in the range of 0.5 observed within a few hours were taken as an indication that the general map structure was not changed (17). When combined, the degree of stability of OPMs we observed under control or sham conditions is within the range reported.
Local Stimulation Induces Widespread Reorganization.
Our study also revealed profound differences of the ICMS-induced plastic changes compared with other sensory or motor cortices (23, 26, 27, 31): OPM changes were widespread, covering several millimeters around the ICMS site. By using optical imaging and single-cell recordings, we found no evidence for a distance-dependency over several millimeters around the ICMS site, indicating that the efficacy of ICMS must drop off beyond that zone; the investigation of its exact range is the subject of further experiments. Because ICMS activates directly only a local group of neurons (22, 23), the occurrence of widespread changes implies that nonlocal interactions among populations of visual cortical neurons are critically involved in the reorganization of OPMs. Conceivably, the system of long-range horizontal connections characterized by patchy axonal projections of up to 8 mm (39–43) seems capable of mediating the observed widespread changes. Recently, local adaptation was shown to induce nonlocal effects; after short-term adaptation to one orientation, systematic repulsive shifts in orientation preference were observed that included changes in orientation tuning away from the adapting stimulus indicative for network interactions (8, 38). Generally, restructuring of networks has been shown to affect the degree of cooperativity between neurons (46). In studies exploring neural synchrony before and after ICMS-induced reorganization in somatosensory and auditory cortex, it has been shown that interaction between neuron pairs was increased after ICMS, as indicated by an enhancement of correlated firing (27, 30). This effect was restricted to that cortical territory that underwent map changes, which supports the view that interactions might play an important role in mediating map changes (27).
Partial Recovery of Reorganizational Changes.
Furthermore, we demonstrate a partial recovery within a time period, which in somatosensory, auditory, or motor cortex leads to a largely complete recovery from ICMS-induced reorganization (23, 26, 27, 31). The observation that the pattern of orientation domains does not recover to its initial configuration but may evolve into a new pattern requires further analysis. One interesting explanation of this behavior is suggested by a computational model describing the structure of OPMs by the attractors of a nonlinear dynamics of cortical reorganization (37). Because such a dynamics has multiple attractors, a perturbation may drive the OPM into a different basin of attraction, resulting in a global rearrangement of the pattern as it converges to the new attractor.
Is Adult Visual Cortex Plasticity Different?
We clearly demonstrated the plastic nature of adult visual cortex, but also showed that the extent and the reversibility of reorganizational changes were unusual compared with other areas and modalities. Nearly three decades ago, adult cats exposed to a visual environment consisting of only vertical stripes were reported to show clear signs of plastic changes (47). In the adaptation study described (8, 38), major changes of orientation preference and response magnitude were evoked, indicating a remarkable capacity for rapid stimulus-dependent changes of OPMs, particularly at and around pinwheel centers. That adult human visual cortex is capable of long-term changes by simple training procedures was corroborated in partially blind subjects who obtained some restitution of their visual field (48).
Besides the question whether visual cortex plasticity might be different from plastic capacities observed in other sensory areas, there is controversy about the modifiability of orientation maps, even in developing visual cortex (13, 14), although visual cortical maps are believed to emerge by activity-dependent mechanisms (20, 49, 50). It was suggested that sensory experience and long-range cortical processing may not be essential for the expression of a basic pattern of orientation selectivity but may be indispensable for the full maturation of tuning and topographical layout (15). By using a pairing protocol similar to that introduced by Fregnac et al. (51), it was reported that the general structure of OPMs was preserved, despite strong shifts in orientation preference (17). This observation is clearly different from our data, which showed that ICMS altered the general map structure. Recent studies in monkey visual cortex combining perceptual learning with electrophysiological recordings lend further support to the hypothesis that visual cortex might differ from other primary sensory areas in terms of stability and modifiability (7, 52).
When combined, our data show activity-dependent reorganizational changes of large portions of OPMs beyond the locally affected orientation, implying a restructuring of the entire underlying cortical network. At the single-cell level, changes consisted of significant shifts of the preferred orientation toward the orientation of the ICMS site. It remains to be seen how behavioral improvement associated with perceptual learning codes into a network with such adaptive properties.
Acknowledgments
We thank Dr. Fred Wolf for insightful discussion and critical suggestions on earlier versions of this manuscript. The work was supported by the Institute for Neuroinformatics at the Ruhr-University Bochum, the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 509 (to H.R.D.), and the Volkswagen-Stiftung I 73035 (to B.G.).
Abbreviations
- OPM
orientation preference map
- ICMS
intracortical microstimulation
- SCM
single-condition map
- OI
optical imaging
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Benali, A., Eysel, U. T. & Dinse, H. R. (1999) Soc. Neurosci. Abstr. 25, 691.
References
- 1.Recanzone G H. In: The New Cognitive Neurosciences. Gazzaniga M S, editor. Cambridge, MA: MIT Press; 2000. pp. 237–250. [Google Scholar]
- 2.Schüz A, Miller R. Cortical Areas, Unity and Diversity: Conceptual Advances in Brain Research. London: Harwood; 2002. [Google Scholar]
- 3.Weinberger N M. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert C D. Curr Opin Neurobiol. 1996;6:269–274. doi: 10.1016/s0959-4388(96)80083-3. [DOI] [PubMed] [Google Scholar]
- 5.Buonomano D V, Merzenich M M. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 6.Dinse H R, Merzenich M M. In: Perceptual Learning. Fahle M, Poggio T, editors. Cambridge, MA: MIT Press; 2002. pp. 19–42. [Google Scholar]
- 7.Crist R E, Li W, Gilbert C D. Nat Neurosci. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- 8.Dragoi V, Rivadulla C, Sur M. Nature (London) 2001;411:80–86. doi: 10.1038/35075070. [DOI] [PubMed] [Google Scholar]
- 9.Dinse H R, Böhmer G. In: Cortical Areas, Unity and Diversity: Conceptual Advances in Brain Research. Schüz A, Miller R, editors. London: Harwood; 2002. pp. 311–348. [Google Scholar]
- 10.Das A, Gilbert C D. Nature (London) 1995;375:780–784. doi: 10.1038/375780a0. [DOI] [PubMed] [Google Scholar]
- 11.Godecke I, Bonhoeffer T. Nature (London) 1996;379:251–254. doi: 10.1038/379251a0. [DOI] [PubMed] [Google Scholar]
- 12.Chapman B, Stryker M P, Bonhoeffer T. J Neurosci. 1996;16:6443–6453. doi: 10.1523/JNEUROSCI.16-20-06443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengpiel F, Goedecke I, Stawinski P, Hubener M, Löwel S, Bonhoeffer T. Neuropharmacology. 1998;37:607–621. doi: 10.1016/s0028-3908(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 14.Sengpiel F, Stawinski P, Bonhoeffer T. Nat Neurosci. 1999;2:727–732. doi: 10.1038/11192. [DOI] [PubMed] [Google Scholar]
- 15.White L E, Coppola D M, Fitzpatrick D. Nature (London) 2001;411:1049–1052. doi: 10.1038/35082568. [DOI] [PubMed] [Google Scholar]
- 16.Blakemore C. Philos Trans R Soc London B. 1977;278:425–434. doi: 10.1098/rstb.1977.0052. [DOI] [PubMed] [Google Scholar]
- 17.Schuett S, Bonhoeffer T, Hubener M. Neuron. 2001;32:325–337. doi: 10.1016/s0896-6273(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 18.Singer W, Tretter F, Yinon U. J Physiol. 1982;324:239–248. doi: 10.1113/jphysiol.1982.sp014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crair M C, Gillespie D C, Stryker M P. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller K D, Erwin E, Kayser A. J Neurobiol. 1999;41:44–57. doi: 10.1002/(sici)1097-4695(199910)41:1<44::aid-neu7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Kirkwood A, Rioult M C, Bear M F. Nature (London) 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 22.Stoney S D, Thompson W D, Asanuma H. J Neurophysiol. 1968;31:659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]
- 23.Nudo R J, Jenkins W M, Merzenich M M. Somatosens Motor Res. 1990;7:463–483. doi: 10.3109/08990229009144720. [DOI] [PubMed] [Google Scholar]
- 24.Sakai M, Suga N. Proc Natl Acad Sci USA. 2001;98:3507–3512. doi: 10.1073/pnas.061021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X, Suga N. J Neurophysiol. 2001;85:1078–1087. doi: 10.1152/jn.2001.85.3.1078. [DOI] [PubMed] [Google Scholar]
- 26.Recanzone G H, Merzenich M M, Dinse H R. Cereb Cortex. 1992;2:181–196. doi: 10.1093/cercor/2.3.181. [DOI] [PubMed] [Google Scholar]
- 27.Dinse H R, Recanzone G H, Merzenich M M. NeuroReport. 1993;5:173–176. doi: 10.1097/00001756-199311180-00020. [DOI] [PubMed] [Google Scholar]
- 28.Sil'kis I G, Rapoport S S. Neurosci Behav Physiol. 1995;25:322–339. doi: 10.1007/BF02360045. [DOI] [PubMed] [Google Scholar]
- 29.Joublin F, Spengler F, Wacquant S, Dinse H R. Biol Cybern. 1996;74:275–286. doi: 10.1007/BF00652228. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado P E, Gerstein G L. Exp Brain Res. 1996;112:431–441. doi: 10.1007/BF00227949. [DOI] [PubMed] [Google Scholar]
- 31.Dinse H R, Godde B, Zepka R, Shuishi-Haupt S, Spengler F, Hilger T. Adv Neurol. 1997;73:195–178. [PubMed] [Google Scholar]
- 32.Heusler P, Cebulla B, Boehmer G, Dinse H R. Exp Brain Res. 2000;135:300–310. doi: 10.1007/s002210000530. [DOI] [PubMed] [Google Scholar]
- 33.von der Malsburg C. Kybernetik. 1973;14:85–100. doi: 10.1007/BF00288907. [DOI] [PubMed] [Google Scholar]
- 34.Somers D C, Nelson S B, Sur M. J Neurosci. 1995;15:5448–5465. doi: 10.1523/JNEUROSCI.15-08-05448.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sompolinsky H, Shapley R. Curr Opin Neurobiol. 1997;7:514–522. doi: 10.1016/s0959-4388(97)80031-1. [DOI] [PubMed] [Google Scholar]
- 36.Somers D C, Todorov E V, Siapas A G, Toth L J, Kim D S, Sur M. Cereb Cortex. 1998;8:204–217. doi: 10.1093/cercor/8.3.204. [DOI] [PubMed] [Google Scholar]
- 37.Wolf F, Geisel T. Nature (London) 1998;395:73–78. doi: 10.1038/25736. [DOI] [PubMed] [Google Scholar]
- 38.Dragoi V, Sharma J, Sur M. Neuron. 2000;28:287–298. doi: 10.1016/s0896-6273(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 39.Rockland K S, Lund J S. Science. 1982;215:1532–1534. doi: 10.1126/science.7063863. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert C D, Wiesel T N. J Neurosci. 1989;9:2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kisvarday Z F, Toth E, Rausch M, Eysel U T. Cereb Cortex. 1997;7:605–618. doi: 10.1093/cercor/7.7.605. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt K E, Kim D S, Singer W, Bonhoeffer T, Lowel S. J Neurosci. 1997;17:5480–5492. doi: 10.1523/JNEUROSCI.17-14-05480.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Löwel S, Schmidt K E, Kim D S, Wolf F, Hoffsummer F, Singer W, Bonhoeffer T. Eur J Neurosci. 1998;10:2629–2643. doi: 10.1046/j.1460-9568.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- 44.Bi G, Poo M. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 45.Rioult-Pedotti M S, Friedman D, Hess G, Donoghue J P. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 46.Krupa D J, Nicolelis M A. Prog Brain Res. 2000;128:161–172. doi: 10.1016/S0079-6123(00)28014-X. [DOI] [PubMed] [Google Scholar]
- 47.Creutzfeldt O D, Heggelund P. Science. 1975;188:1025–1027. doi: 10.1126/science.1145187. [DOI] [PubMed] [Google Scholar]
- 48.Kasten E, Wust S, Behrens-Baumann W, Sabel B A. Nat Med. 1998;4:1083–1087. doi: 10.1038/2079. [DOI] [PubMed] [Google Scholar]
- 49.Stryker M P. In: Development of the Visual System. Lam D M, Shatz C J, editors. Cambridge, MA: MIT Press; 1991. pp. 267–287. [Google Scholar]
- 50.Weliky M, Katz L C. Nature (London) 1997;386:680–685. doi: 10.1038/386680a0. [DOI] [PubMed] [Google Scholar]
- 51.Fregnac Y, Shulz D, Thorpe S, Bienenstock E. Nature (London) 1988;333:367–370. doi: 10.1038/333367a0. [DOI] [PubMed] [Google Scholar]
- 52.Schoups A, Vogels R, Qian N, Orban G. Nature (London) 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]