Abstract
Transient stimulation of secretion in calf chromaffin cells is invariably followed by rapid endocytosis (RE), a clathrin- and K+-independent process with a half time of several seconds. Here we show that when exocytosis is triggered in a more sustained manner, a much slower form of endocytosis (SE) replaces RE. SE is complete within 10 min and is abolished when anticlathrin antibodies are introduced into the cell or when intracellular K+ is removed. RE, but not SE, is blocked by intracellular administration of antidynamin-1 antibodies; the inverse specificity was found for antidynamin-2 antibodies. Replacement of extracellular Ca2+ by Ba2+ or Sr2+ completely blocked RE but had little effect on SE. Thus chromaffin cells exhibit two kinetically and mechanistically distinct forms of endocytosis that are coupled to different extents of exocytosis and are mediated by different isoforms of dynamin. We surmise that RE is associated with the transient fusion (“kiss-and-run”) mechanism of transmitter release and is the prevalent means of vesicle recapture and recycling under normal physiological conditions, whereas the clathrin-based SE mechanism comes into play only at higher levels of stimulation and may be associated with complete fusion of vesicles with the plasma membrane.
The mechanisms of endocytosis that contribute to the recycling of synaptic vesicles at nerve terminals and dense-core vesicles in neuroendocrine cells are still actively debated (1). The predominant model suggests that vesicle membrane merges with the plasma membrane and that recovery is primarily accomplished by clathrin-coated vesicles, which bud either from the plasma membrane or from cisternae located at some distance from the active zone or sites of exocytosis (2–4). By contrast, the “kiss-and-run” or transient fusion hypothesis proposes that vesicles are recovered intact directly at the active zone and may then be directly refilled with transmitter without the necessity of the sorting processes thought to be integral to the coated vesicle mechanism (1, 5). Recent experiments showing kinetically distinct types of endocytosis at nerve terminals (6–8) suggest that models of synaptic vesicle recycling that consider only clathrin-based mechanisms (3, 4) are too simplistic.
We have postulated that the rapid (complete within several seconds) endocytosis (RE) processes seen in calf chromaffin cells (9–11), other types of neurosecretory cells (e.g., 12–14), as well as synaptic terminals (8, 15), comprise the endocytotic arm of the transient fusion mechanism (1). Capacitance measurements of membrane retrieval in calf chromaffin cells reveal that RE is a process that ensues after very mild stimulation (release of ≈250–600 vesicles). It is kinetically complex and has three time constants (ultrafast, ≈0.3 sec; fast-1, ≈3 sec; and fast-2, ≈13 sec); similar kinetics were subsequently reported in adult bovine chromaffin cells (16, 17). RE may manifest as “excess retrieval” in the first round of stimulation, but subsequent rounds (using identical stimulation with identical Ca2+ influx) usually show “compensatory retrieval” where cell membrane capacitance (Cm) values return directly to near baseline (9, 14). As we have found no mechanistic differences between these processes (e.g., both are blocked by antidynamin IgGs, substitution of extracellular Ca2+ or anticalmodulin peptides but are resistant to anticlathrin IgGs), we consider them to be alternate modes of the same basic RE process.
RE is a strictly Ca2+-dependent process that uses the fission protein dynamin to recover presumptive vesicular membrane after secretion (9–11). Strikingly, all forms of RE are independent of clathrin (9) and are much faster than conventional receptor-mediated endocytosis, as reported in a variety of studies (18). Here we show that a slower form of endocytosis (complete within several minutes) takes over when chromaffin cells are subjected to more sustained stimulation. We demonstrate that this form differs mechanistically from RE and corresponds to a clathrin-coated vesicle process likely akin to receptor-mediated endocytosis. In particular, RE is mediated by dynamin-1, whereas the slower form is mediated by dynamin-2, both of which are present in chromaffin cells.
Methods
Cell Culture and Electrophysiology.
Calf chromaffin cells were obtained by collagenase digestion of adrenal medullae from 10- to 12-week-old bovine calves followed by gradient centrifugation to purify chromaffin cells (9). Culture conditions were as described previously (9); cells were used 2–6 d after preparation. The standard patch–pipette solution contained (in mM): Hepes (30), K-glutamate (90), Cs-glutamate (20), K-EGTA (0.1), NaCl (12), MgCl2 (5), ATP (2), GTP (0.35), pH 7.2, with KOH (final [K+] 120 mM; nucleotides added fresh from stocks just before experiment). The bath solution consisted of (in mM): Hepes (10), tetraethylammonium Cl (150), CaCl2 (2), glucose (10), and 1 μM tetrodotoxin, pH 7.3. Other modifications were as noted in figure legends. In some experiments, the perforated patch technique was used. The pipette tip was first dipped in amphotericin-free internal pipette solution, then backfilled with amphotericin-B (Calbiochem; final concentration 240 μg/ml in internal solution, diluted fresh from 60 mg/ml of DMSO stock). After obtaining a cell-attached gigaseal, ≈5 min elapsed before the series resistance dropped to 10–15 MΩ, when recording was begun.
Antibody Treatment.
Cells were loaded with antibodies (purified IgGs) via the patch pipette, as described (9, 10). Anticlathrin monoclonal IgG (X22) was obtained from Affinity Bioreagents (Golden, CO) and used as described (9). Polyclonal antidynamin-1 IgG (Affinity Bioreagents) and monoclonal antidynamin-2 IgG (BD-Transduction Laboratories, Lexington, KY) were loaded into cells at 1 mg/ml. Prior to experiment, all antibodies were centrifugally dialyzed (Centricon-30; Millipore) into internal pipette solution. After whole-cell break-in, 10 min was allowed for the IgG to reach its target before recording.
Immunochemistry.
Immunoblots for dynamin-1 and -2 were performed on tissue extracts as previously described (9). Specificity of the isoform-specific antibodies was confirmed by immunoblot against total extracts of tissues known to express only dynamin-1 (synaptosomes) and only dynamin-2 (fibroblasts). Dynamin-1 IgG did not react with fibroblast proteins, and dynamin-2 IgG did not react with synaptosome proteins (data not shown). Chromaffin cells grown on coverslips were prepared for double-label immunocytochemistry by fixation in 4% paraformaldehyde/PBS. Samples were then reacted with antidynamin-2 IgG (1:1,000) followed by antidynamin-1 IgG (1:1,000). Secondary antibody detection was with fluorescein anti-mouse IgG and Texas red anti-rabbit IgG, respectively. Samples were viewed in an Olympus (New Hyde Park, NY) FluoView confocal microscope, digitized by using fluoview software and analyzed by using image j (NIH).
Results
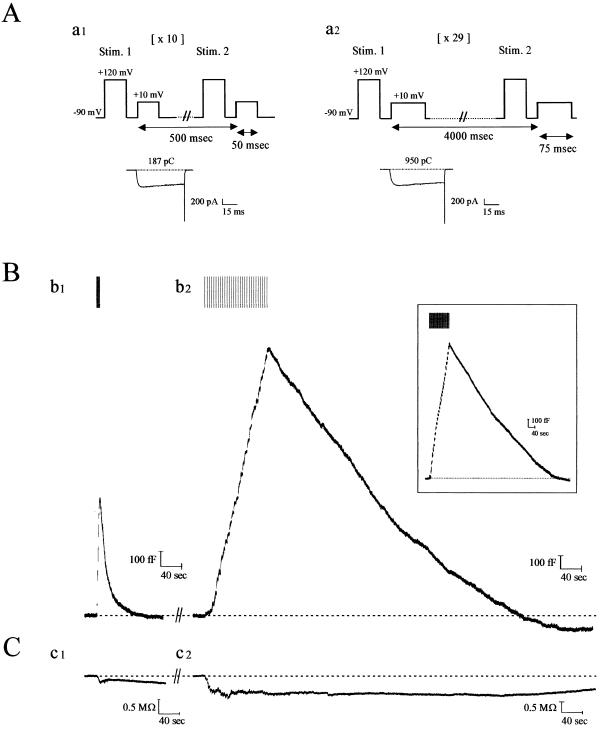
Cm recordings reveal that RE invariably follows exocytosis in calf chromaffin cells when these cells are mildly stimulated (9–11). To determine whether alternate membrane recovery processes exist in response to various secretory stimuli, we subjected isolated cells to depolarizing pulse trains of different duration or frequency and recorded the accompanying Cm changes (Fig. 1A). As shown in Fig. 1Bb1, when cells are subjected to 10 depolarizations of 50 msec, Cm rises with exocytosis but then rapidly declines to baseline within ≈40 sec (20 sec on average), typical of RE as previously characterized by us (refs. 9–11; Table 1). However, when stimulation is increased several-fold (29 × 75 msec depolarizations), RE disappears by the end of the stimulation period. Instead, a slower form of membrane retrieval ensues, which gradually returns Cm to baseline within ≈10 min at 23°C (Fig. 1Bb2; Table 1). Unlike RE, slow endocytosis (SE) is well described by a single kinetic component with a constant rate (≈140 fF/min). SE was also found when cells were immediately stimulated with the sustained protocol [i.e., without prior RE; Fig. 1Bb2 (Inset)]. Moreover, very similar parameters for RE and SE were obtained when cells were stimulated in perforated patch mode (Table 1). Together with our previous data showing that RE remains intact even during long-term whole-cell recording in calf chromaffin cells (9–11), these results suggest that SE is not a kinetically modified form of RE arising from “washout” of some critical endocytotic component.
Figure 1.
Kinetics of endocytosis are altered with degree of stimulation of calf chromaffin cells. (A) Patch-clamped calf chromaffin cells were stimulated with depolarizing pulse trains of 10 × 50 msec (with 0.5 sec between each stimulus; a1) or 29 × 75 msec (with 4 sec between each stimulus; a2). Before each depolarization, a prepulse was administered to recruit facilitation L-type Ca2+ channels in this preparation (9). Typical Ca2+ currents recorded from the first depolarizations in a1 and a2 are shown below the protocols (numbers indicate cumulative Ca2+ entry for each protocol; note this is 5.5-fold larger for the sustained versus the transient paradigm). (B) Continuous Cm recordings after formation of whole-cell configuration from a single calf chromaffin cell, sequentially stimulated with the two protocols illustrated in A. (b1) With transient stimulation, exocytosis (rising phase of Cm trace) was immediately followed by RE (falling phase of Cm trace). (b2) After a 12-min recovery period, during which baseline Cm was attained and remained stable, a second round of secretion was elicited with the sustained protocol. Under these conditions, endocytosis was slow, and Cm returned to baseline in ≈9 min (see Table 1 for average values). (Inset) SE was evoked during the first round of stimulation, 3 min after establishing whole-cell configuration. Results from a typical experiment are shown [mean values (n = 12) were peak current (676 ± 44 pA), current integral (767 ± 36 pC), total Cm increase (1,750 ± 125 fF), rate of endocytosis (134 ± 20 fF/min) and endocytosis duration (10 ± 0.6 min), respectively]. (C) Conductance traces accompanying the records in B show that this parameter does not change in parallel and thus does not significantly contaminate the Cm records. In total, >90% of the cells tested expressed both forms of endocytosis.
Table 1.
Statistical analysis of the molecular requirements for SE versus RE in chromaffin cells
| Conditions | Peak current, pA | Cumulative current integral, pC | Total Cm increase, fF | Rate of endocytosis, fF/min | Endocytosis duration, min |
|---|---|---|---|---|---|
| SE | |||||
| 120 mM K+ (n = 46) | 684 ± 25 | 807 ± 18 | 1,888 ± 123 | 138 ± 9 | 10.1 ± 0.33 |
| 0 mM K+ (n = 24) | 686 ± 22 | 794 ± 28 | 1,945 ± 166 | — | — |
| Anticlathrin IgG (n = 18) | 690 ± 24 | 809 ± 33 | 1,911 ± 190 | — | — |
| Antidynamin-1 IgG (n = 21) | 700 ± 37 | 808 ± 28 | 2,024 ± 169 | 140 ± 10 | 10.5 ± 0.43 |
| Antidynamin-2 IgG (n = 18) | 701 ± 29 | 802 ± 40 | 1,790 ± 128 | — | — |
| Strontium (n = 16) | 761 ± 34 | 1,047 ± 63 | 1,877 ± 210 | 98 ± 13.5 | 15 ± 1 |
| Perforated patch (n = 18) | 680 ± 32 | 787 ± 40 | 2,010 ± 142 | 150 ± 12 | 11 ± 0.7 |
| RE | |||||
| 120 mM K+ (n = 89) | 741 ± 24 | 190.2 ± 4.5 | 605 ± 34.7 | 6,055 ± 137.2 | 0.328 ± 0.013 |
| Antidynamin-1 IgG (n = 73) | 683 ± 22 | 189.8 ± 4.9 | 717 ± 40.7 | — | — |
| Antidynamin-2 IgG (n = 30) | 740 ± 39 | 194.2 ± 7.5 | 613 ± 49.3 | 5,771 ± 143.8 | 0.313 ± 0.024 |
| Perforated patch (n = 34) | 699 ± 38 | 188.7 ± 6.9 | 608 ± 52.1 | 6,009 ± 189 | 0.314 ± 0.019 |
Explanation of measurements: Peak current corresponds to the maximum Ca2+ current amplitude evoked by the first pulse in the train of stimulation. Cumulative current integral is calculated from the total number of Ca2+ ions entering the cell during the entire stimulation period (10 depolarizations of 50 ms for RE and 29 of 75 ms for SE). Total Cm increase is the maximum increase of membrane capacitance at the end of the train of stimulation. Rate of endocytosis for SE was obtained by measuring the slope of the declining Cm trace. Rate of endocytosis for RE was obtained by measuring the steepest downward slope of Cm trace. The endocytosis duration is the time required for Cm to return to baseline from its maximum level after stimulation. Perforated patch values (120 mM K+) were not significantly different from whole-cell controls. Measurements are given as means ± SEM. Dashes indicate not significantly different from zero.
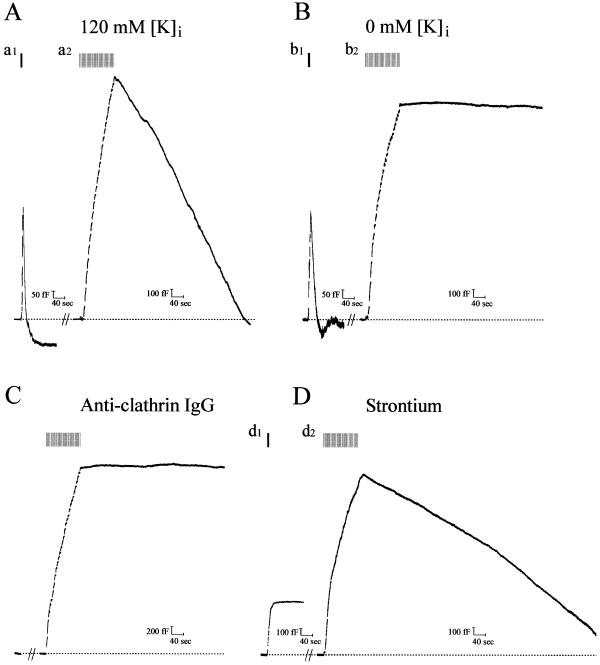
The duration of SE is within the range previously reported for recovery of dense-core vesicle markers deposited on the chromaffin cell surface after a long depolarizing stimulus (19, 20), or for horseradish peroxidase uptake after secretion in chromaffin cells (21). Given that the average rate of SE is about 45-fold slower than the fast-1 form of RE (Table 1; see also refs. 9, 10), we tested whether the two processes were mechanistically different. Clathrin-coated vesicle-based endocytosis (including receptor-mediated endocytosis) depends on intracellular K+ (22). Previously, we showed that removal of K+ from the patch–pipette solution (promoting diffusional K+ loss from the cytoplasm) had no effect on RE (ref. 9; Fig. 2Bb1). By contrast, as shown in Fig. 2Bb2, this strategy abolished SE (compare with 120 mM K control; Fig. 2Aa2; Table 1). To examine whether SE depends on the assembly of clathrin cages, we introduced into the cell affinity-purified monoclonal anticlathrin antibody (23), which is known to inhibit receptor-mediated endocytosis in chromaffin and other cells but does not influence RE (both “excess retrieval” and “compensatory endocytosis;” ref. 9 and refs. therein). Anticlathrin IgG effectively blocked SE, but irrelevant IgGs were without effect (Fig. 2C and data not shown). Thus, SE is most likely mediated by clathrin, suggesting that coated vesicle endocytosis can be directly measured electrophysiologically in these cells. Because RE is strictly Ca2+-dependent in calf chromaffin cells, replacement of extracellular Ca2+ with certain alien divalent cations ablates the process while allowing exocytosis to continue (9, 10). To examine the Ca2+ dependence of SE, we replaced extracellular Ca2+ with either Ba2+ or Sr2+, neither of which support RE. As shown in Fig. 2Dd1, RE is abolished under these conditions, but SE still occurs (Fig. 2Dd2), albeit with slower kinetics (Table 1; results with Ba2+ were comparable, including the slowed SE time course; data not shown). These results suggest that SE is independent of, or less influenced by, Ca2+ or has a divalent cation specificity different to RE.
control; Fig. 2Aa2; Table 1). To examine whether SE depends on the assembly of clathrin cages, we introduced into the cell affinity-purified monoclonal anticlathrin antibody (23), which is known to inhibit receptor-mediated endocytosis in chromaffin and other cells but does not influence RE (both “excess retrieval” and “compensatory endocytosis;” ref. 9 and refs. therein). Anticlathrin IgG effectively blocked SE, but irrelevant IgGs were without effect (Fig. 2C and data not shown). Thus, SE is most likely mediated by clathrin, suggesting that coated vesicle endocytosis can be directly measured electrophysiologically in these cells. Because RE is strictly Ca2+-dependent in calf chromaffin cells, replacement of extracellular Ca2+ with certain alien divalent cations ablates the process while allowing exocytosis to continue (9, 10). To examine the Ca2+ dependence of SE, we replaced extracellular Ca2+ with either Ba2+ or Sr2+, neither of which support RE. As shown in Fig. 2Dd1, RE is abolished under these conditions, but SE still occurs (Fig. 2Dd2), albeit with slower kinetics (Table 1; results with Ba2+ were comparable, including the slowed SE time course; data not shown). These results suggest that SE is independent of, or less influenced by, Ca2+ or has a divalent cation specificity different to RE.
Figure 2.
Slow endocytosis is mediated by a clathrin-coated vesicle pathway and is independent of Ca entry. Cm records from cells in which secretion was evoked as in Fig. 1 for both RE and SE. (A) Pipette solution contained 120 mM K+; SE ensues and is complete within ≈8 min. (B) Pipette solution contained 0 mM K+ (replaced by Cs-glutamate); SE is absent (b2), but RE is unaffected (b1). No significant difference in Cm rise between the sustained protocols in A and B was found (see Table 1 for average values of this and other parameters from these experiments). (C) Anticlathrin IgG (1 mg/ml) diffused into the cell from the patch pipette in the presence of 120 mM K+. Monoclonal anticlathrin IgG [×22] was dialyzed against internal pipette solution; 10 min was allowed to elapse between attaining a whole-cell patch and recording an exo/endocytotic cycle. Note that no SE takes place in the presence of the IgG (n = 18). RE (not shown) was unaffected by this maneuver, as previously described (9). Cm increase was similar to control. (D) Ca2+ dependence of RE but not SE. Bath Ca2+ was replaced by equimolar Sr2+, and stimulation was conducted to elicit either RE (d1) or SE (d2). RE was blocked, whereas SE continued and was complete within ≈15 min. No significant difference in Cm increase was found in Sr2+ versus Ca2+.
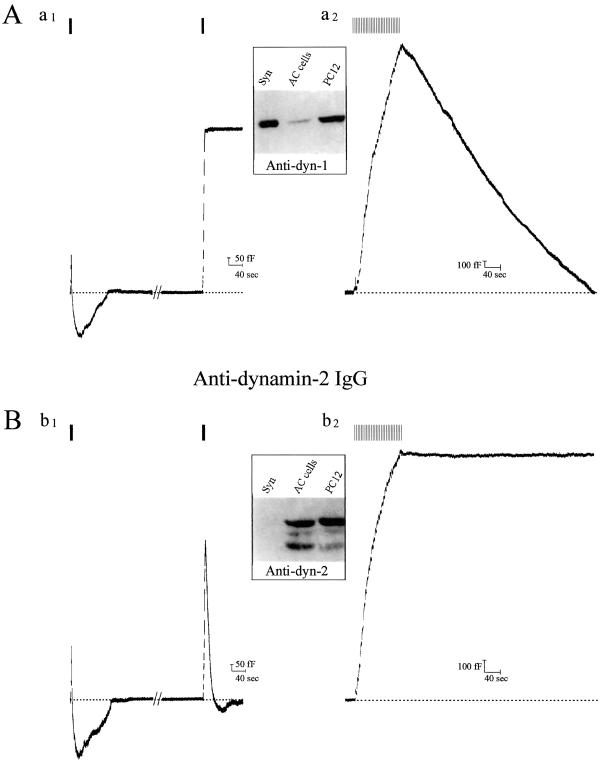
Dynamins are a family of proteins that are involved in vesicular fission reactions at various membranes and have been implicated in both RE and receptor-mediated endocytosis (for reviews, see refs. 24, 25). Although dynamin-2 has a ubiquitous distribution, dynamin-1 is found primarily in neurons and some neuroendocrine cells. Chromaffin cells express both dynamins-1 and -2, and we previously hypothesized that these isoforms might regulate different types of endocytosis (11). Using isolated pleckstrin-homology domains as dominant negatives, we showed that interference with dynamin-1 but not -2 function specifically blocks RE (11). To establish whether RE and SE depend on the function of different dynamin isoforms, we diffused antidynamin-1- and -2-specific antibodies into calf chromaffin cells before stimulation. As expected (11), antidynamin-1 but not -2 IgG was able to block RE (Fig. 3 Aa1 and Bb1). By contrast, antidynamin-2 IgG interfered with SE, whereas antidynamin-1 IgG was inactive (Fig. 3 Aa2 and Bb2). Neither antibody affected the kinetics of exocytosis or the magnitude of Ca2+ currents, indicating the absence of nonspecific effects (mean data summarized in Table 1). Immunoblotting confirmed that calf chromaffin cells express both isoforms of dynamin (Fig. 3; see ref. 11). These results demonstrate that both RE and SE are mediated by dynamin, but that different isoforms are predominantly linked to the two mechanisms. Given this finding, it seemed possible that dynamin-1 and -2 distribution in calf chromaffin cells might be distinct, which was confirmed by localizing the two isoforms by immunofluorescence microscopy (Fig. 4). Each isoform displayed a punctate distribution, but little or no overlap was found by careful analysis of merged images. Similar results were obtained in other cell types that express both isoforms of dynamin (data not shown).
Figure 3.
Dynamin-1 mediates RE, whereas dynamin-2 mediates SE. Cm records from cells in which either affinity-purified (A) antidynamin-1-specific IgG or (B) antidynamin-2-specific IgG, both at 1 mg/ml, were introduced into calf chromaffin cells followed by transient or sustained stimulation. In A, note that antidynamin-1 IgG inhibits RE (a1) but has no effect on SE (a2). In B, antidynamin-2 IgG has no effect on RE (b1) but blocks SE (b2). Note in Bb1 that two rounds of exocytosis/RE occur, whereas in Aa1 the second round of RE is blocked after the antibody has diffused into the cell (in the first round, RE is normal because insufficient antibody has diffused into the cell; the extent of the first exocytosis in Aa1 and Bb1 appears smaller because of simultaneous endocytosis that is largely absent in the second round). (Insets) Reactivity of antidynamin-1 (c1)- and -2 (c2)-specific antibodies with lysates from rat brain synaptosomes (Syn; 10 μg of protein); calf chromaffin (AC) cells (100 μg), and PC12 cells (100 μg); immunoblots were performed as described (9) and developed by using enhanced chemiluminescence.
Figure 4.
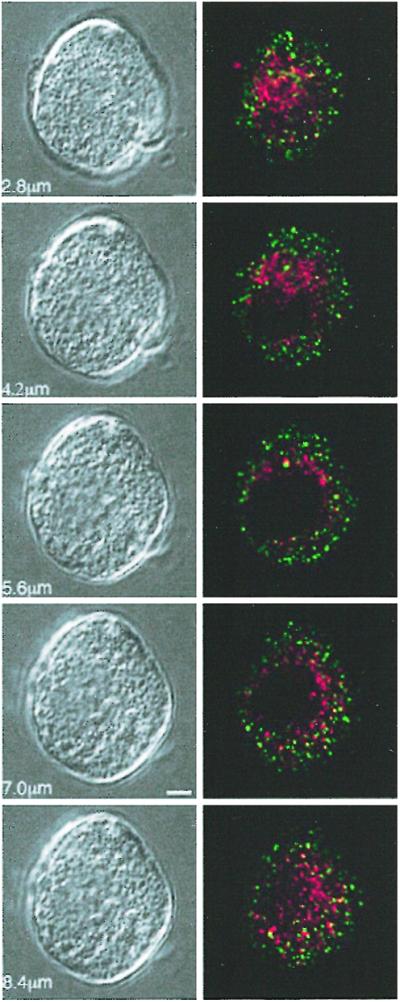
Dynamin-1 and -2 are differentially distributed in calf chromaffin cells. Fixed chromaffin cells were double-labeled with antidynamin-1 (green)- and -2 (red)-specific IgGs with appropriate fluorescent secondary IgGs and viewed by confocal microscopy (Olympus FluoView). Series of single 0.5 μm sections through one cell (bottom of cell, Top) shown at Right with corresponding differential interference contrast microscopy images at Left (Bar = 2 μm). Note the minimal overlap (pixels registering for both signals) indicating the distinct distribution of the two proteins. Image representative of >1,000 sections from >50 different cells.
Discussion
The present data may contribute to a longstanding controversy in neurobiology with respect to the transient and complete fusion models of exo/endocytosis, which may be coupled to entirely separate pathways of vesicle recycling (1). Ceccarelli et al. were the first to hypothesize that synaptic vesicles regenerate by two different pathways, depending on the degree of stimulation (5). However, morphological markers for the “kiss-and-run” pathway, claimed to be the major mechanism of recycling under physiological conditions, were lacking. As a consequence, the pathway that is prevalent under tetanic stimulation conditions, where the decimated synaptic vesicle population is slowly regenerated in part by easily recognizable coated vesicles (2), has become the accepted paradigm in the field. Although recent data from various synaptic preparations have uncovered different rates of membrane retrieval in response to variations in stimulation (6–8), the mechanistic details remain unknown. We show here that chromaffin cells resemble synaptic terminals with respect to the existence of different endocytotic processes and their dependence on stimulation intensity. We verify, to our knowledge for the first time, that these modes of membrane recovery are mechanistically divergent, as summarized in Table 2. RE, elicited by transient stimulation, is a clathrin-independent process that may be the last step in kiss-and-run exocytosis. SE, on the other hand, is activated with sustained stimulation and involves clathrin and, most likely, coated vesicles.
Table 2.
Comparison of the properties of RE and SE in chromaffin cells
| Property | RE | SE |
|---|---|---|
| Activation | Low–moderate physiological stimulation | Moderate–high physiological stimulation |
| Rate | ∼6,000 fF/min | ∼140 fF/min |
| Clathrin dependence | None | Required |
| K+ dependence | None | Required |
| Ca2+ dependence | Required | None or minimal |
| Dynamin dependence | Dynamin-1 mediates | Dynamin-2 mediates |
Other distinctions are apparent from present and previous work: e.g., whereas RE exhibits a specific requirement for Ca2+, SE does not (refs. 9 and 10; Fig. 2D). Furthermore, different isoforms of dynamin are preferentially used by the two processes: dynamin-1 mediates RE (11), whereas dynamin-2 mediates SE, which we show here with specific antibodies (Fig. 3). Early speculation that the function of different dynamins might be largely redundant has generally given way to the view that these proteins are biochemically and functionally distinct (11, 24–27). Dynamin-2 most likely controls receptor-mediated endocytosis in virtually all somatic cells, as it is ubiquitously distributed and this form of endocytosis is universal. By contrast, dynamin-1 has a much more restricted expression and is especially concentrated at nerve terminals, where it may participate in RE-like events governing rapid synaptic vesicle recycling. As clathrin-based endocytosis indubitably takes place at synaptic terminals (2–4), the question arises whether dynamin-1 could participate in both an RE-like event and parallel clathrin-based endocytosis in the same cell. Although this issue remains unresolved, one possibility is that the very high concentration of dynamin-1 at synapses could enable this protein to mediate both processes. In support of this contention, mutant dynamin-1 expression can inhibit receptor-mediated endocytosis in a number of cell types, probably by interfering with the normal function of endogenous dynamin-2 (28, 29). Notably, mutant dynamin-2 overexpression is much more efficient in this regard (27). Another possibility is that participation in either process might depend on the phosphorylation state of the protein. Dynamin-1 is dephosphorylated during nerve terminal depolarization (30), which affects its ability to associate with other proteins, including those purportedly involved in coated vesicle assembly (31). Dephosphorylated dynamin-1 might mediate RE, whereas the phosphorylated form mediates SE, or vice versa.
Several other questions related to the control of endocytotic processes and the mechanism of vesicle recycling are raised by the present work. Can RE be proven to form the endocytotic arm of kiss-and-run secretion in these cells? Patch amperometry combined with the functional tools described here might answer this question. In addition, morphological analysis after different degrees of stimulation may be useful: preliminary studies have recently shown diverse endosomal vesicular populations in stimulated chromaffin cells that likely originate in mechanistically distinct endocytotic events (32). What factor(s) control the switch from RE to SE? It would seem that either some scarce component (possibly dynamin-1 itself) becomes rate-limiting for RE or that some other component reaches a threshold that inhibits RE and perhaps simultaneously activates SE. It remains to be determined whether the SE pathway described here results in dense-core vesicle recycling, as has been proposed for the clathrin-coated vesicle pathway in nerve terminals containing small synaptic vesicles (2–4). Earlier results in adult bovine chromaffin cells showed that vesicular membrane components retrieved after a strong stimulus to secretion by clathrin-based processes are ultimately recycled back to chromaffin granules in a sorting process taking several hours (for review, see ref. 33). We recently showed, by using amperometric methods in calf chromaffin cells, that intracellular dynamin antagonism causes a rapid decline in secretion (34), suggesting that a recycling pool contributes significantly to ongoing secretory competence, just as in neurons. Thus, as in other aspects of the secretory process, chromaffin cells may provide a useful model for the mechanism of vesicle recycling.
Acknowledgments
We thank M. E. Brown for technical assistance. This work was supported by Public Health Service Grants IBN-9985874, DK58921, MH47181 (C.R.A.), and GM-56396 (H.C.P.).
Abbreviations
- RE
rapid endocytosis
- SE
slow endocytosis
- Cm
cell membrane capacitance
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Palfrey H C, Artalejo C R. Neuroscience. 1998;83:969–989. doi: 10.1016/s0306-4522(97)00453-3. [DOI] [PubMed] [Google Scholar]
- 2.Heuser J E, Reese T E. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heuser J E. Cell Biol Int Rep. 1989;13:1063–1076. doi: 10.1016/0309-1651(89)90020-9. [DOI] [PubMed] [Google Scholar]
- 4.De Camilli P, Slepnev V I, Shupliakov O, Brodin L. In: Synapses. Cowan W M, Sudhof T C, Stevens C F, editors. Baltimore, MD: Johns Hopkins Univ. Press; 2000. pp. 217–274. [Google Scholar]
- 5.Ceccarelli B, Hurlbut W P, Mauro A. J Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards D A, Guatimosim C, Betz W J. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- 7.Koenig J H, Ikeda K. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens C F, Williams J H. Proc Natl Acad Sci USA. 2000;97:12828–12833. doi: 10.1073/pnas.230438697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artalejo C R, Henley J R, McNiven M A, Palfrey H C. Proc Natl Acad Sci USA. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artalejo C R, Elhamdani A, Palfrey H C. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- 11.Artalejo C R, Lemmon M A, Schlessinger J, Palfrey H C. EMBO J. 1997;16:1565–1574. doi: 10.1093/emboj/16.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas P, Lee A K, Wong J G, Almers W. J Cell Biol. 1994;124:667–676. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansvelder H D, Kits K S. J Neurosci. 1998;18:81–92. doi: 10.1523/JNEUROSCI.18-01-00081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhamdani A, Brown M E, Artalejo C R, Palfrey H C. J Neurosci. 2000;20:2495–2503. doi: 10.1523/JNEUROSCI.20-07-02495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavalali E T, Klingauf J, Tsien R W. Philos Trans R Soc London B. 1999;354:337–346. doi: 10.1098/rstb.1999.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith C, Neher E. J Cell Biol. 1997;139:885–894. doi: 10.1083/jcb.139.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engisch K, Nowycky M. J Physiol. 1998;506:591–608. doi: 10.1111/j.1469-7793.1998.591bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henkel A W, Almers W. Curr Opin Neurobiol. 1996;6:350–357. doi: 10.1016/s0959-4388(96)80119-x. [DOI] [PubMed] [Google Scholar]
- 19.Phillips J H, Burridge K, Wilson S P, Kirschner N. J Cell Biol. 1983;97:1906–1917. doi: 10.1083/jcb.97.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patzak A, Winkler H. J Cell Biol. 1986;102:510–515. doi: 10.1083/jcb.102.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Grafenstein H, Roberts C S, Baker P F. J Cell Biol. 1986;103:2343–2352. doi: 10.1083/jcb.103.6.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkin J M, Brown M S, Goldstein J L, Anderson R G W. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 23.Doxsey S J, Brodsky F M, Blank G S, Helenius A. Cell. 1987;50:453–463. doi: 10.1016/0092-8674(87)90499-5. [DOI] [PubMed] [Google Scholar]
- 24.McNiven M A, Cao H, Pitts K R, Yoon Y. Trends Biochem Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- 25.van der Bliek A M. Trends Cell Biol. 1999;9:96–102. doi: 10.1016/s0962-8924(98)01490-1. [DOI] [PubMed] [Google Scholar]
- 26.Warnock D E, Baba T, Schmid S L. Mol Biol Cell. 1997;8:2553–2562. doi: 10.1091/mbc.8.12.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschuler Y, Barbas S M, Terlecky L J, Tang K, Hardy S, Mostov K E, Schmid S L. J Cell Biol. 1998;143:1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herskovits J S, Burgess C C, Obar R A, Vallee R B. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Bliek A M, Redelmeier T E, Damke H, Tisdale E, Meyerowitz E M, Schmid S L. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J P, Sim A T, Robinson P J. Science. 1994;265:970–974. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- 31.Slepnev V I, Ochoa G C, Butler M H, Grabs D, DeCamilli P. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- 32.Henkel A W, Horstmann H, Henkel M K. FEBS Lett. 2001;505:414–418. doi: 10.1016/s0014-5793(01)02861-7. [DOI] [PubMed] [Google Scholar]
- 33.Winkler H. Handbk Exp Pharmacol. 1988;90/I:43–117. [Google Scholar]
- 34.Elhamdani A, Palfrey H C, Artalejo C R. Neuron. 2001;31:819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]