Abstract
Senile plaques and neurofibrillary tangles, the two hallmark lesions of Alzheimer's disease, are the results of the pathological deposition of proteins normally present throughout the brain. Senile plaques are extracellular deposits of fibrillar β-amyloid peptide (Aβ); neurofibrillary tangles represent intracellular bundles of self-assembled hyperphosphorylated tau proteins. Although these two lesions are often present in the same brain areas, a mechanistic link between them has yet to be established. In the present study, we analyzed whether tau plays a key role in fibrillar Aβ-induced neurite degeneration in central neurons. Cultured hippocampal neurons obtained from wild-type, tau knockout, and human tau transgenic mice were treated with fibrillar Aβ. Morphological analysis indicated that neurons expressing either mouse or human tau proteins degenerated in the presence of Aβ. On the other hand, tau-depleted neurons showed no signs of degeneration in the presence of Aβ. These results provide direct evidence supporting a key role for tau in the mechanisms leading to Aβ-induced neurodegeneration in the central nervous system. In addition, the analysis of the composition of the cytoskeleton of tau-depleted neurons suggested that the formation of more dynamic microtubules might confer resistance to Aβ-mediated neurodegeneration.
Recent research on the field of Alzheimer's disease (AD) has been focused on the mechanisms leading to the formation of senile plaques and neurofibrillary tangles, the two hallmark lesions of this neurodegenerative disease. Both lesions are the result of the pathological deposition of proteins normally distributed throughout the brain. Senile plaques are extracellular deposits of fibrillar β-amyloid peptide (Aβ), a cleavage product of the amyloid precursor protein (see refs. 1–5 and refs. therein). Neurofibrillary tangles, on the other hand, are intracellular bundles of self-assembled hyperphosphorylated tau proteins (6–10). Although these two lesions are often present in the same brain areas, a mechanistic link between them has yet to be established. Indirect evidence from several studies using cultured neurons suggests a role for tau in the generation of dystrophic neurites in the presence of fibrillar Aβ. The common picture emerging from these studies indicates that deposition of fibrillar Aβ induces phosphorylation of tau followed by progressive degeneration of neuronal processes (11–16). In addition, several groups have reported that kinases known to phosphorylate tau in vitro were activated in response to fibrillar Aβ deposition. When young neurons were incubated in the presence of fibrillar Aβ, both GSK3β and CDK5 were activated (11, 17). In mature hippocampal neurons, on the other hand, fibrillar Aβ-induced neurotoxicity was accompanied by the sustained activation of mitogen-activated protein kinase (MAPK) (14–16, 18). Taken collectively, these results suggest a key role for tau in the generation of dystrophic neurites in response to fibrillar Aβ treatments. To obtain direct evidence of the participation of tau in this process, we analyzed the effect of fibrillar Aβ on neurite degeneration in tau-depleted hippocampal neurons. The results presented here indicate that hippocampal neurons depleted of tau by homologous recombination techniques do not degenerate in the presence of fibrillar Aβ. In addition, analysis of the composition of the cytoskeleton of tau-depleted neurons suggests that the presence of an increased pool of unstable microtubules might render these cells resistant to degeneration caused by fibrillar Aβ deposits.
Materials and Methods
Preparation of Hippocampal Cultures.
Embryonic day (E)16 embryos obtained from wild-type, homozygous tau knockout, and human tau transgenic mice on a murine tau null background were used to prepare primary hippocampal cultures as described (19, 20). Briefly, hippocampi were dissected and freed of meninges. The cells were dissociated by trypsinization (0.25% for 15 min at 37°C) followed by trituration with a fire-polished Pasteur pipette. The cell suspension was then plated onto poly-l-lysine-coated coverslips in MEM with 10% horse serum. After 4 h, the coverslips were transferred to dishes containing an astroglial monolayer and maintained in MEM containing N2 supplements (21) plus ovalbumin (0.1%) and sodium pyruvate (0.1 mM). For biochemical experiments, hippocampal neurons were plated at high density (500,000 cells/60-mm dish) in MEM with 10% horse serum. After 4 h, the medium was changed to glia-conditioned MEM containing N2 supplements (21) plus ovalbumin (0.1%) and sodium pyruvate (0.1 mM).
Aβ Aggregation and Treatment.
Synthetic Aβ (1–40), obtained from Sigma, was dissolved in N2 medium at 0.5 mg/ml and incubated for 4 days at 37°C to preaggregate the peptide (14). Fibrillar Aβ was added to the culture medium at a final concentration of 20 μM, and the cells were grown in its presence for 1–4 days as described (14, 18). Soluble Aβ (1–40) was used as additional control.
Immunocytochemical Procedures.
Hippocampal neurons cultured for 4 weeks were fixed for 20 min with 4% paraformaldehyde in PBS containing 0.12 M sucrose. They were then permeabilized in 0.3% Triton X-100 in PBS for 5 min and rinsed twice in PBS. The cells were preincubated in 10% BSA in PBS for 1 h at 37°C and exposed to the primary antibodies (diluted in 1% BSA in PBS) overnight at 4°C. Finally, the cultures were rinsed in PBS and incubated with secondary antibodies for 1 h at 37°C. The following primary antibodies were used: anti-α-tubulin (clone DM1A) and polyclonal antitubulin from Sigma, and anti-tau (clone tau-5, ref. 22). The following secondary antibodies were used: anti-mouse IgG fluorescein-conjugated and anti-rabbit IgG rhodamine-conjugated (Boehringer Mannheim).
To quantify neurite degeneration, control and Aβ-treated cultures were stained with a tubulin antibody as described above. Ninety fields were analyzed for each experimental condition and the number of processes showing signs of degeneration (tortuous course of the neurites, varicosities, and/or fragmentation of processes) was counted. In addition, cell viability was assessed by counting the number of live/dead neurons using the ethidium homodimer/calcein a.m. (Molecular Probes) combination of vital dyes as previously described (18). Briefly, 30 days in culture, hippocampal neurons were incubated for 30 min at 37°C using the Live/Dead Viability/Cytotoxicity Kit. Fifty nonoverlapping microscopic fields from three independent experiments were analyzed for each experimental condition.
Protein Determination, Electrophoresis, and Immunoblotting.
Cultures were rinsed twice in warmed PBS, scraped into Laemmli buffer, and homogenized in a boiling water bath for 5 min. The protein concentration was determined by the method of Lowry et al. (23) as modified by Bensadoun and Weinstein (24). SDS–polyacrylamide gels were run according to Laemmli (25). Transfer of protein to Immobilon membranes (Millipore, Bedford, MA) and immunodetection were performed according to Towbin et al. (26) as modified by Ferreira et al. (27, 28). The following antibodies were used: anti-α-tubulin (clone DM1A, 1:3,000; Sigma); anti-tau [clone 5E2, 1:20 (8); tau-5, 1:100 (22); anti-MAP2 (clone AP14, 1:500); antiacetylated tubulin (clone 6–11-B1, 1:1,000), antityrosinated tubulin (clone Tub-1A2, 1:1,000), anti-MAP1A (clone HM-1, 1:100), anti-MAP1B (clone AA6, 1:100) all from Sigma, anti-Class III β-tubulin (clone Tuj1, 1:1,000, Chemicon), antidetyrosinated tubulin (a generous gift from Gary Borisy, Northwestern University, 1:5,000), anti-ERK2 (Santa Cruz, Biotechnology, 1:2,000); and antiactive MAPK (Biosource International, Camarillo, CA, 1:100). Secondary antibodies conjugated to horseradish peroxidase (1:1,000, Promega) followed by enhanced chemiluminescence reagents (Amersham Pharmacia Biotech) were used for the detection of proteins. Densitometry was performed by using a Bio-Rad 700 flatbed scanner (Bio-Rad) and molecular analyst software (Bio-Rad). Films and membranes were scanned at 600 dots per inch by using light transmittance, and pixel volume analysis was performed on the appropriate bands. Densitometric values were normalized by using α-tubulin or total MAPK as internal controls. Scanning of the Western blots demonstrated the curve to be linear in the range used for each antibody.
Treatment with Cytoskeletal Disrupting Drugs.
Hippocampal neurons obtained from wild-type, tau knockout, and human tau transgenic mice were kept in culture for 4 weeks and then treated with either nocodazole or Taxol (both from Sigma). Nocodazole was added, at a final concentration of 10 μg/ml, for 1 h, and cytoskeletal fractions were prepared as described (27, 28). Briefly, cultures were rinsed in a microtubule stabilizing buffer (MTSB, 0.13 M Hepes/2 mM MgCl2/10 mM EGTA; pH 6.9) for 2 min and then extracted in MTSB plus 0.2% triton X-100 for 4 min and scraped into Laemmli buffer as previously described. In another set of experiments, cultures were pretreated with Taxol (1 or 10 μM) for 6 h (27) and then incubated with or without fibrillar Aβ for 24 h. Neurons were then fixed and immunostained or used to prepare cytoskeletal fractions as described above.
Results
Tau-Depleted Hippocampal Neurons Did Not Degenerate in the Presence of Fibrillar Aβ.
Primary cultures of hippocampal neurons were prepared from wild-type or homozygous tau knockout E16 mouse embryos and were cultured for 4 weeks. These mature hippocampal neurons were cultured in the presence of fibrillar Aβ at a final concentration of 20 μM for 24–96 h, at which point the cultures were fixed and analyzed. Severe degeneration was apparent in fibrillar Aβ-treated wild-type cells when compared with nontreated control cells. Comparable to those described in rat cultures, the signs of degeneration observed in these cells included the formation of tortuous processes, the presence of varicosities along neurites and retraction of neuritic processes (see refs. 14, 18). These signs of neurite degeneration were evident as early as 24 h after the addition of fibrillar Aβ. After 96 h, most of the neuritic processes extended by wild-type neurons (90 ± 5%) had completely degenerated (Fig. 1), and most of the neurons were dead (87 ± 7%). In contrast, no signs of neurite degeneration were observed when tau-depleted neurons were grown in the presence of fibrillar Aβ for 24 h. Even 96 h after the addition of fibrillar Aβ, most of the processes extended by tau-depleted neurons (85 ± 7%) displayed normal morphological characteristics (Fig. 2), and only a few of them died (20 ± 5%).
Figure 1.
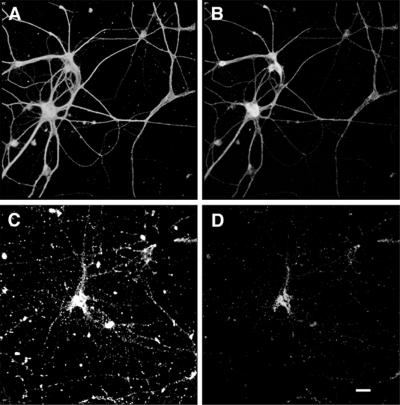
Fibrillar Aβ induced neurite degeneration in mature hippocampal neurons. Hippocampal neurons obtained from wild-type E16 embryos were kept in culture for 4 weeks (A and B) and then incubated in the presence of fibrillar Aβ for 4 days (C and D). Cells were fixed and double stained with tubulin (polyclonal antitubulin, Sigma) (A and C) and tau (clone tau-5) (B and D) antibodies. Note the massive neurite degeneration induced by fibrillar Aβ (C and D). (Bar = 20 μm.)
Figure 2.
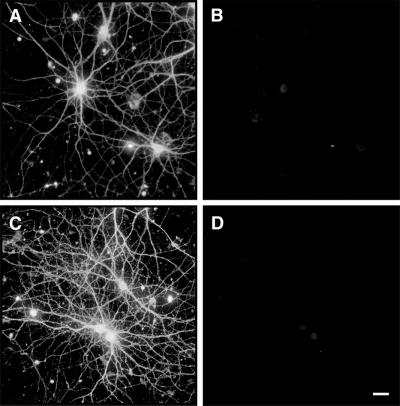
Tau-depleted hippocampal neurons did not degenerate in the presence of fibrillar Aβ. Hippocampal neurons obtained from tau knockout E16 embryos were kept in culture for 4 weeks (A and B) and then incubated in the presence of fibrillar Aβ for 4 days (C and D). Cells were fixed and double stained with tubulin (polyclonal antitubulin, Sigma) (A and C) and tau (clone tau-5) (B and D) antibodies. Most of the processes extended by tau knockout neurons showed normal morphological characteristic in the presence of fibrillar Aβ (C). (Bar = 20 μm.)
Neurons Expressing Human Tau Degenerated in the Presence of Fibrillar Aβ.
The experiments described above suggested that tau might play a key role in the mechanisms leading to the degeneration of neurites in the presence of fibrillar Aβ. For this to be the case, the re-expression of tau in cultured hippocampal neurons should restore their susceptibility to degeneration in the presence of fibrillar Aβ. To test this hypothesis, we repeated these experiments with cultures prepared from human tau transgenic mice generated on a murine tau knockout background. These mice expressed only the human tau protein (20). Hippocampal neurons obtained from these mice were cultured for 4 weeks, and the level of tau expression was quantified by Western blot analysis where anti-human tau antibodies recognized one main band. The level of tau expression in human tau transgenic cultures was approximately 63% of those observed in wild-type mouse controls at 4 weeks after plating (see also Fig. 4 and Table 2). However, the level of expression of the only human isoform expressed in these neurons was significantly higher than the corresponding murine isoform (63 ± 5% vs. 42 ± 4%, respectively). When these human tau cultures were exposed to fibrillar Aβ, they degenerated in a manner similar to the Aβ-treated wild-type cultures (Fig. 3). Four days after the addition of fibrillar Aβ, the majority (87 ± 6%) of the processes on hippocampal neurons expressing human tau had degenerated (Fig. 3) and most of the cells died (79 ± 9%). These results provide direct evidence that tau is a major downstream target for Aβ-induced neurodegenerative processes.
Figure 4.
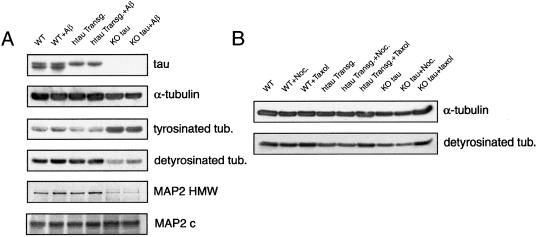
(A) Western blot analysis of the content of microtubular proteins in wild-type (wt), human tau transgenic (htau transg), and knockout (KO) tau hippocampal neurons kept in culture for 4 weeks and incubated in the presence or absence of fibrillar Aβ for 4 days. Equal amounts of total protein (40 μg) were loaded in each lane. (B) Western blot analysis of the content of α-tubulin and detyrosinated tubulin in cytoskeletal fractions prepared from wild-type (wt), human tau transgenic (htau transg), and knockout (KO) tau hippocampal neurons kept in culture for 4 weeks and incubated in the presence or absence of nocodazole or Taxol. Nocodazole-resistant fractions were normalized using the content of α-tubulin as internal controls.
Table 2.
Content of microtubular proteins in whole-cell extracts (upper part of table) and cytoskeletal fractions (lower part of table) obtained from wild-type, human tau (htau) transgenic, and tau knockout hippocampal neurons cultured in the presence of fibrillar Aβ, nocodazole (nocod), or Taxol
| Protein | Phenotype
|
|||||
|---|---|---|---|---|---|---|
| Wild type
|
htau transgenic
|
Tau knockout
|
||||
| No treatment | Aβ* | No treatment | Aβ | No treatment | Aβ | |
| Tyr-tubulin | 100 | 97 ± 12 | 99 ± 9 | 111 ± 10 | 160 ± 15† | 154 ± 12† |
| Detyrosinated tubulin | 100 | 112 ± 8 | 109 ± 9 | 117 ± 12 | 72 ± 10† | 68 ± 10† |
| Acetylated tubulin | 100 | 110 ± 15 | 118 ± 17 | 102 ± 14 | 111 ± 12 | 120 ± 15 |
| Class III β-tubulin | 100 | 102 ± 7 | 88 ± 10 | 77 ± 18 | 79 ± 16 | 87 ± 18 |
| MAP1B | 100 | 95 ± 7 | 92 ± 9 | 88 ± 15 | 90 ± 12 | 95 ± 15 |
| MAP2a + b | 100 | 120 ± 10 | 110 ± 13 | 125 ± 11 | 56 ± 7† | 54 ± 6† |
| MAP2c | 100 | 103 ± 5 | 110 ± 11 | 115 ± 9 | 167 ± 12† | 157 ± 10† |
| Tau | 100 | 101 ± 6 | 63 ± 5 | 58 ± 8 | ND | ND |
| No treatment | Nocod‡ | Taxol§ | No treatment | Nocod | Taxol | No treatment | Nocod | Taxol | |
|---|---|---|---|---|---|---|---|---|---|
| α-tubulin | 100 | 89 ± 9 | 115 ± 10† | 98 ± 7 | 91 ± 7 | 118 ± 8† | 88 ± 4† | 86 ± 5† | 115 ± 10† |
| Detyr. tubulin | 100 | 99 ± 5 | 122 ± 7† | 94 ± 8 | 84 ± 5 | 110 ± 5† | 72 ± 8† | 47 ± 8† | 118 ± 6† |
Protein amounts are expressed as % of wild-type untreated controls. Each number represents the mean ± SEM. Samples were prepared from three independent cultures for each experimental condition. ND, not detected.
Aβ fibrils were added at a final concentration of 20 μM.
Differs from control cultures, P < 0.001.
Nocodazole was added at a final concentration of 10 μg/ml. Nocodazole-resistant fractions were normalized by using the content of α-tubulin as internal controls.
Taxol was added at a final concentration of 10 μM.
Figure 3.
Cultured hippocampal neurons expressing human tau degenerated in the presence of fibrillar Aβ. Hippocampal neurons obtained from human tau transgenic mice were kept in culture for 4 weeks and then incubated in the presence (C and D) or absence (A and B) of fibrillar Aβ for 4 days. Cells were fixed and double stained with tubulin (polyclonal antitubulin, Sigma) (A and C) and tau (clone tau-5) (B and D) antibodies. (Bar = 20 μm.)
Fibrillar Aβ Activated MAPK in Hippocampal Neurons Obtained from Tau Knockout and Human Tau Transgenic Mice.
Aβ neurotoxicity has been reported to result in tau hyperphosphorylation (11–18). In agreement with these findings, we have previously shown that the addition of fibrillar Aβ to mature hippocampal neurons kept in culture for more than 28 days resulted in the sustained activation of MAPK (14, 18). Because no degeneration was observed when tau-depleted neurons were cultured in the presence of fibrillar Aβ, we analyzed whether the MAPK signal transduction was activated as we previously observed in wild-type controls treated with Aβ. The content of active MAPK was determined in hippocampal cultures obtained from wild-type, tau knockout, and human tau transgenic mice, all treated with fibrillar Aβ and compared with untreated controls. Whole cell extracts were prepared from 30-day in vitro cultures treated with or without fibrillar Aβ for 24 h. Immunoblots were reacted with an antibody that recognizes phospho-specific MAPK (ERK2 active forms) and normalized by using an antibody that recognizes total MAPK (phosphorylated and dephosphorylated ERK2). We have previously shown that this method of detection of MAPK activation gives results comparable to those obtained by means of in vitro kinase assays (14, 18). A significant increase (>80%) in active MAPK levels was detected in wild-type Aβ-treated cells as compared with nontreated controls (Table 1). Similar results were obtained from cultures prepared from knockout and human tau transgenic mouse embryos (Table 1). In these cultures, the addition of fibrillar Aβ resulted in increased MAPK activity (≈75 and 89%, respectively) when compared with nontreated controls (see also Table 1).
Table 1.
Activation of MAPK by fibrillar Aβ in mature hippocampal cultures prepared from wild-type, tau knockout, and human tau transgenic mice
| Genotype | Treatment | Active MAPK (% of untreated control) |
|---|---|---|
| Wild type | None | 100 |
| Wild type | Aβ fibrils* | 180 ± 15† |
| Tau knockout | None | 98 ± 17 |
| Tau knockout | Aβ fibrils | 175 ± 10† |
| htau transgenic | None | 115 ± 19 |
| htau transgenic | Aβ fibrils | 189 ± 12† |
Cultures were prepared and Western blots run as described. Enhanced chemiluminescence was used to detect proteins. The numbers represent the means ± SEM obtained from three experiments.
Aβ fibrils were added at a final concentration of 20 μM.
Differs from untreated control; P < 0.001.
Analysis of the Complement of Microtubular Proteins in Tau Knockout Hippocampal Neurons.
Because the results described above indicated that tau-depleted neurons were not susceptible to the neurodegeneration typically observed in the presence of fibrillar Aβ, we analyzed the composition of their microtubular system. Microtubules play an important role in neurite elongation and maintenance in cultured neurons. Changes in the levels of tubulin subunits and/or in the complement of microtubule-associated proteins result in altered rates of growth of axonal and dendritic processes in cultured hippocampal neurons (28). We initially analyzed the complement of other non-tau MAPs in these cells. No changes in the total levels of MAP1B, MAP1A, or MAP2 were detected in tau-depleted neurons when compared with wild-type or human tau transgenic controls. However, we did detect changes in the expression of MAP2 isoforms. In mature wild-type and human tau transgenic hippocampal neurons, high molecular weight MAP2 (predominantly MAP2b) isoforms were more abundant than the lower molecular weight isoform (MAP2c). Conversely, there was a significant increase in MAP2c and a decrease in MAP2b levels in tau-depleted neurons as compared with those expressing wild-type murine tau and those expressing the human tau transgene (Fig. 4 and Table 2).
We also analyzed the complement of tubulin in these cells. In addition to total tubulin and neuron-specific class III β-tubulin, we analyzed the levels of acetylated and detyrosinated tubulin as markers of stable microtubules and tyrosinated tubulin as a marker of unstable microtubules (29, 30). No significant changes were detected in the content of total tubulin, acetylated tubulin, or class III neuron-specific tubulin in human tau transgenic or tau knockout mice when compared with wild-type untreated cultures (Table 2). On the other hand, our results demonstrated a significant increase in tyrosinated tubulin and a concomitant decrease in detyrosinated tubulin in tau-depleted neurons when compared with either their wild-type or human tau transgenic counterparts (Fig. 4 and Table 2). These results suggested the presence of a more dynamic population of microtubules in tau-deficient neurons as compared with wild-type or human tau transgenic controls. To further test the stability of the microtubular system, wild-type, human tau transgenic, and tau knockout cultures were treated with nocodazole for 1 h at a final concentration of 10 μg/ml to induce the depolymerization of unstable microtubules (31). Nocodazole-resistant microtubule fractions were then prepared, and the content of detyrosinated tubulin was determined. Significantly less protein was recovered in nocodazole-resistant fractions obtained from tau-depleted neurons when compared with those obtained from tau-expressing neurons. In addition, a significant decrease in detyrosinated tubulin (stable microtubules) was detected in nocodazole-treated tau-deficient neurons when compared with neurons expressing either murine or human tau (Fig. 4, Table 2).
Finally, we determined whether the more dynamic microtubular system observed in tau knockout neurons was responsible, at least in part, for preventing Aβ-induced neurodegeneration. To test this hypothesis, tau-depleted neurons were pretreated with Taxol (a microtubule-stabilizing factor) for 6 h (1 and 10 μM, final concentration) and then incubated in the presence of fibrillar Aβ for 24 h. The addition of Taxol (both doses tested) induced a significant increase in stable microtubules in tau knockout cultures, and detyrosinated tubulin reached levels comparable to those observed in neurons expressing either murine or human tau (Figs. 4 and 5, Table 2). Severe neurodegeneration was detected when tau-depleted neurons, in which the microtubules had been stabilized by Taxol, were incubated with fibrillar Aβ (Fig. 5). No differences were detected when these cultures were compared with wild-type or human tau transgenic controls treated with Taxol and incubated with fibrillar Aβ (Fig. 5).
Figure 5.
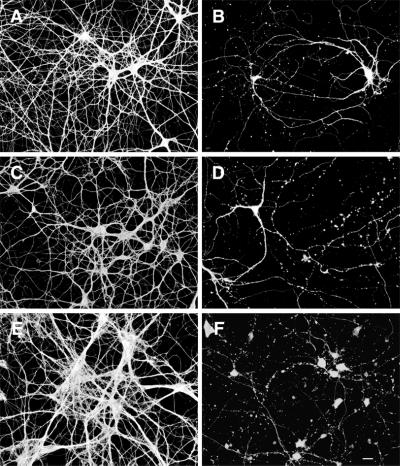
Fibrillar Aβ induced neurite degeneration in Taxol-treated tau-depleted hippocampal neurons. Wild-type (A and B), tau knockout (C and D), and human tau transgenic (E and F) hippocampal neurons kept in culture for 4 weeks were pretreated with Taxol for 6 h and then cultured for 24 h in the presence (B, D, and F) or absence (A, C, and E) of fibrillar Aβ. Cells were then fixed and stained by using a tubulin antibody (clone DMIA, SIGMA). Severe neurodegeneration was detected in Taxol-treated tau knockout hippocampal neurons exposed to fibrillar Aβ (D) as well as in neurons expressing either murine (B) or human (F) tau isoforms. (Bar = 20 μm.)
Discussion
The results presented herein provide direct evidence supporting a key role for tau in the mechanisms leading to fibrillar Aβ-induced neurodegeneration in hippocampal neurons. In addition, they suggest that changes in the composition of the cytoskeleton that result in the formation of more dynamic microtubules might confer resistance to degeneration in the presence of this peptide.
The lack of neurite degeneration in tau knockout hippocampal neurons and the restoration of the neurotoxic effect of fibrillar Aβ by the re-expression of tau provide direct evidence for a key role of tau in this neurodegenerative process. However, the mechanism by which tau mediates neurite degeneration in the context of the AD process is not completely understood. Recently, more than 15 different pathogenic mutations in the tau gene were shown to associate with human autosomal dominant frontotemporal dementias (reviewed in ref. 32). However, tau mutations do not associate with any known form of familial AD, suggesting that tau filament formation is a more distal event in the disease process. AD then would begin when fibrillar Aβ triggers posttranslational modifications of tau (i.e., phosphorylation and/or truncation) that could favor its aggregation as cytoplasmic filamentous inclusions (reviewed in ref. 33). Whether these aggregates are toxic or interfere in essential cell processes is unknown. Perhaps pathological aggregates of hyperphosphorylated tau alter the transport of essential materials to the distal ends of the neuronal processes. As a consequence of this altered transport, a progressive degeneration initiated at the distal end of the processes and moving retrogradely toward the cell body takes place. Experimental data supporting this hypothesis indicated that the overexpression of tau in CHO or differentiated neuroblastoma cells (N2a cell line) resulted in a differential impairment of plus-end-directed transport mediated by kinesin-like motors (34, 35). In the absence of tau (i.e., knockout mice), no alteration of axonal transport should occur in the presence of fibrillar Aβ permitting essential elements to reach distal portions of the neuritic processes and prevent neurodegeneration.
It has been suggested that Aβ-induced hyperphosphorylation of tau could also lead to neurite degeneration by decreasing microtubule stabilization (36). Under this premise, tau-depleted neurons should have less stable microtubules and therefore an increased susceptibility to degeneration. Our results suggested that only the subpopulation of stable microtubules containing detyrosinated but not the one containing acetylated tubulin was decreased in the absence of tau. These results suggest that tau might be involved (and/or associated) with the stabilization of only one subset of microtubules in central neurons, the one containing detyrosinated tubulin. These data are in agreement with previous observations indicating that in neurons, unlike in other cell types, acetylation and detyrosination define two different subsets of stable microtubules (37). The decrease in detyrosinated microtubules is accompanied by a significant increase in tyrosinated tubulin in tau-depleted neurons. This increase in unstable microtubules could be driven by the absence of tau and/or by the increase in the level of MAP2c. This juvenile MAP has been linked with the polymerization of a more plastic cytoskeleton during the initial phases of development (38–40). In addition, the continued expression of MAP2c in brain areas undergoing active growth during adulthood (i.e., the olfactory bulb) suggests its role in the formation of dynamic microtubules characteristic of rapid neurite elongation (40). These results suggest that the presence of a more dynamic microtubular system might allow neurons to compensate for degenerative cues. Our results showing that tau-depleted neurons in which their microtubules had been stabilized by Taxol became susceptible to degeneration in the presence of fibrillar Aβ seem to support this view. However, we could not rule out the possibility that Aβ induced neurite degeneration in Taxol-treated cells through mechanisms different from the ones activated in the absence of Taxol. Taxol could induce neurite degeneration by impairing anterograde axonal transport (41). Alternatively, Taxol could induce apoptosis in central neurons by activating a pool of c-jun protein kinase in the nucleus of central neurons (42). However, the lack of signs of neurite degeneration in control Taxol-treated wild-type, tau knockout, and human tau transgenic neurons argues against the possibility that these mechanisms are primarily responsible for Aβ-induced neurodegeneration in these cells.
Regardless of the mechanisms involved, the present results strongly suggest a key role for tau in neurodegeneration associated with fibrillar Aβ deposition in the central nervous system. The elucidation of the precise mechanisms underlying fibrillar Aβ-induced neurite degeneration awaits further investigation.
Acknowledgments
This work was supported by National Institutes of Health Grants NS39080 (A.F.) and AG15383 (M.P.V.) and Alzheimer's Association Grant Zen-98-012 (M.P.V.).
Abbreviations
- MAPK
mitogen-activated protein kinase
- AD
Alzheimer's disease
- Aβ
β-amyloid peptide
- En
embryonic day n
References
- 1.Glenner G G, Wong C W. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 2.Vitek M P, Rasool C G, de Sauvage F, Vitek S M, Bartus R T, Beer B, Ashton R A, Macq A F, Maloteaux J M, Blume A J. Brain Res. 1988;464:121–131. doi: 10.1016/0169-328x(88)90004-6. [DOI] [PubMed] [Google Scholar]
- 3.Yankner B A, Mesulam M M. N Engl J Med. 1991;325:1849–1857. doi: 10.1056/NEJM199112263252605. [DOI] [PubMed] [Google Scholar]
- 4.Haass C, Selkoe D J. Cell. 1993;75:1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe D J. Annu Rev Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- 6.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung Y-C, Zaidi M S, Wisniewski H M. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 7.Kondo J, Honda T, Mori H, Hamada Y, Miura R, Ogawara M, Ihara Y. Neuron. 1988;1:827–834. doi: 10.1016/0896-6273(88)90130-4. [DOI] [PubMed] [Google Scholar]
- 8.Kosik K S, Joachim C L, Selkoe D J. Proc Natl Acad Sci USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nukina N, Ihara Y. J Biochem. 1986;99:1541–1544. doi: 10.1093/oxfordjournals.jbchem.a135625. [DOI] [PubMed] [Google Scholar]
- 10.Wood J G, Mirra S S, Pollock N L, Binder L I. Proc Natl Acad Sci USA. 1986;508:320–324. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takashima A, Noguchi K, Sato K, Hoshimo T, Imahori K. Proc Natl Acad Sci USA. 1993;90:7789–7793. doi: 10.1073/pnas.90.16.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzo A, Yankner B A. Proc Natl Acad Sci USA. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busciglio J, Lorenzo A, Yeh J, Yankner B A. Neuron. 1995;14:879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira A, Lu Q, Orecchio L, Kosik K S. Mol Cell Neurosci. 1997;9:220–234. doi: 10.1006/mcne.1997.0615. [DOI] [PubMed] [Google Scholar]
- 15.Shea T B, Deargay A N, Ekinci F J. Neurosci Res Comm. 1998;22:45–49. [Google Scholar]
- 16.Ekinci F J, Malik K, Shea T B. J Biol Chem. 1999;274:30322–30327. doi: 10.1074/jbc.274.42.30322. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez A, Toro R, Caceres A, Maccioni R B. FEBS Lett. 1999;459:421–426. doi: 10.1016/s0014-5793(99)01279-x. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport M, Ferreira A. J Neurochem. 2000;74:125–133. doi: 10.1046/j.1471-4159.2000.0740125.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira A, Li L, Chin L S, Greengard P, Kosik K S. Mol Cell Neurosci. 1996;8:286–299. doi: 10.1006/mcne.1996.0064. [DOI] [PubMed] [Google Scholar]
- 20.Dawson H N, Ferreira A, Eyster M V, Binder L I, Vitek M P. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- 21.Bottenstein J E, Sato G. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LoPresti P, Szuchet S, Papasozomenos S C, Zinkowski R P, Binder L I. Proc Natl Acad Sci USA. 1995;92:10369–10373. doi: 10.1073/pnas.92.22.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry O H, Reserbrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Bensadoun A, Weinstein R. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Towbin H, Staehelein T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4354–4356. [Google Scholar]
- 27.Ferreira A, Caceres A. J Neurosci Res. 1992;32:516–529. doi: 10.1002/jnr.490320407. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira A, Busciglio J, Caceres A. Dev Brain Res. 1989;49:215–228. doi: 10.1016/0165-3806(89)90023-0. [DOI] [PubMed] [Google Scholar]
- 29.Piperno G, LeDizet M, Chang X. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulze E, Asai D J, Bulinski J C, Kirschner M. J Cell Biol. 1987;105:2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira A, Kincaid R, Kosik K S. Mol Biol Cell. 1993;4:1225–1238. doi: 10.1091/mbc.4.12.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee V M, Trojanowski J Q. Neuron. 1999;24:507–510. doi: 10.1016/s0896-6273(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 33.Garcia M L, Cleveland D W. Curr Opin Cell Biol. 2001;13:41–48. doi: 10.1016/s0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 34.Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E-M, Mandelkow E. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinczek B, Ebneth A, Mandelkow E-M, Mandelkow E. J Cell Sci. 1999;112:2355–2367. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- 36.Dreschel D N, Hyman A A, Cobb M H, Kirschner M. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robson S J, Burgoyne R D. Cell Motil Cytoskel. 1989;12:273–282. doi: 10.1002/cm.970120408. [DOI] [PubMed] [Google Scholar]
- 38.Matus A. Curr Opin Cell Biol. 1990;2:10–14. doi: 10.1016/s0955-0674(05)80024-9. [DOI] [PubMed] [Google Scholar]
- 39.Matus A. J Cell Sci Suppl. 1991;15:61–67. doi: 10.1242/jcs.1991.supplement_15.9. [DOI] [PubMed] [Google Scholar]
- 40.Weisshaar B, Doll T, Matus A. Development (Cambridge, UK) 1992;116:1151–1161. doi: 10.1242/dev.116.4.1151. [DOI] [PubMed] [Google Scholar]
- 41.Theiss C, Meller K. Cell Tissue Res. 2000;299:213–224. doi: 10.1007/s004419900120. [DOI] [PubMed] [Google Scholar]
- 42.Figueroa-Masot X A, Hetman M, Higgins M J, Kobot N, Xia Z. J Neurosci. 2001;21:4657–4667. doi: 10.1523/JNEUROSCI.21-13-04657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]