Abstract
The limbic localization of the arginine vasopressin V1b receptor has prompted speculation as to a potential role of this receptor in the control of emotional processes. To investigate this possibility, we have studied the behavioral effects of SSR149415, the first selective and orally active non-peptide antagonist of vasopressin V1b receptors, in a variety of classical (punished drinking, elevated plus-maze, and light/dark tests) and atypical (fear/anxiety defense test battery and social defeat-induced anxiety) rodent models of anxiety, and in two models of depression [forced swimming and chronic mild stress (CMS)]. When tested in classical tests of anxiety, SSR149415 produced anxiolytic-like activity at doses that ranged from 1 to 30 mg/kg (i.p. or p.o.), but the magnitude of these effects was overall less than that of the benzodiazepine anxiolytic diazepam, which was used as a positive control. In contrast, SSR149415 produced clear-cut anxiolytic-like activity in models involving traumatic stress exposure, such as the social defeat paradigm and the defense test battery (1–30 mg/kg, p.o.). In the forced swimming test, SSR149415 (10–30 mg/kg, p.o.) produced antidepressant-like effects in both normal and hypophysectomized rats. Moreover, in the CMS model in mice, repeated administration of SSR149415 (10 and 30 mg/kg, i.p.) for 39 days improved the degradation of the physical state, anxiety, despair, and the loss of coping behavior produced by stress. These findings point to a role for vasopressin in the modulation of emotional processes via the V1b receptor, and suggest that its blockade may represent a novel avenue for the treatment of affective disorders.
Arginine vasopressin (AVP) is a cyclic nonapeptide that is synthesized centrally in the hypothalamus. Although it participates in the hypothalamic-pituitary-adrenal axis, regulating pituitary ACTH (corticotropin) secretion by potentiating the stimulatory effects of corticotropin releasing factor (CRF), extrahypothalamic AVP-containing neurons have been characterized in the rat, notably in the medial amygdala, that innervate limbic structures such as the lateral septum and the ventral hippocampus (1). In these latter structures, AVP was suggested to act as a neurotransmitter, exerting its action by binding to specific G protein-coupled receptors, i.e., V1a and V1b (2–4), which are widely distributed in the central nervous system, including the septum, cortex, and hippocampus (2, 5).
Because the pioneering studies of David De Wied and colleagues (6, 7), it has been widely accepted that AVP is involved in various types of behavioral processes (8). Early work paid attention to the possible role of this peptide in learning and memory, in particular with regard to avoidance behavior (for review, see ref. 9), but also in antypiresis, scent marking, and social communication (for reviews, see refs. 10 and 11). For instance, several studies showed that centrally administered AVP in rats reverses drug-induced memory loss and affects long-term memory processes, improving both the consolidation and retrieval phases (12, 13). The neuroanatomical distribution of AVP and its receptors has also prompted speculation about their functional role in emotional processes leading to studies that investigated the behavioral action of central infusion of peptide V1 receptor antagonists in animal models of anxiety. For example, the intra-septal application of the mixed V1a/b receptor antagonist d(CH2)5Tyr(Et)VAVP was found to produce anxiolytic-like effects in the elevated plus-maze (EPM) test in rats (14). Moreover, infusion of an antisense oligodeoxynucleotide to the V1a subtype mRNA into the septum of rats reduced anxiety in the EPM (15). Moreover, chronic immobilization stress has been shown to increase V1b receptor mRNA levels in the rat brain (16). Whereas this latter finding suggests that the V1b receptor subtype may be predominantly involved in the modulatory action of AVP on emotional processes, no selective V1b receptor antagonist was available to directly verify this concept.
Recently, the first non-peptide antagonist at the V1b receptor, SSR149415, has been described in our laboratory, thus opening new avenues for exploring the pathophysiology of stress-related illnesses such as anxiety and depressive disorders. This compound displays high affinities for both native and recombinant human and rat V1b receptors (human, Ki = 4.2 and 1.5 nM, respectively; rat, Ki = 3.7 and 1.3 nM, respectively), 60- and 800-fold selectivity for human and rat V1b as compared with V1a receptor, and was inactive in more than 90 binding assays for neurotransmitters and peptides (including corticotropin releasing factor and enkephalins; ref. 17). SSR149415 did not modify the V1a-mediated vascular response in rats after AVP administration up to 10 and 30 mg/kg, i.p. and per os (p.o.), respectively. It is a potent antagonist at the V1b receptor, as shown by its ability to inhibit AVP-induced Ca2+ increase in Chinese hamster ovary cells expressing the human or rat V1b receptor (Ki = 1.26 and 0.73 nM, respectively), and AVP-induced ACTH secretion in corticotroph cells in rats (17). In addition, the drug inhibited restraint-stress-induced ACTH secretion in rats (17). Here, we examined the effects of SSR149415 by using a variety of classical and atypical rodent models of anxiety, and two models of depression.
Materials and Methods
Animals.
Male Sprague–Dawley and Wistar rats (Iffa Credo, Charles River Breeding Laboratories), weighing 180–290 g at the time of testing, were used. They were housed in groups of three to eight. Male CD1, OF1, and BALB/c mice weighing 17–32 g (Charles River Breeding Laboratories, Iffa Credo or Janvier) were used. CD1 mice were housed in groups of twenty; those used in the mouse defense test battery (MDTB) and in the chronic mild stress (CMS) were housed singly; and BALB/c mice used in the light/dark test were housed in groups of six. Male Long Evans rats (400–500 g; Iffa Credo) were used in the MDTB. All animals were maintained under standard laboratory conditions and kept on a 12-h light/dark cycle with light onset at 6 a.m.
Drugs.
Compounds were prepared as solutions or suspensions in physiological saline containing Tween 80 (0.1%) or DMSO (5%) and Cremophor EL (5%). The drugs used were diazepam, flumazenil, fluoxetine, imipramine, and SSR149415 ((2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxyphenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidinecarboxamide; Sanofi-Synthelabo). Drugs were given in a constant volume of 2 (rats) or 20 (mice) ml/kg.
Punished Drinking Test in Rats.
The procedure was a modification of that described by Vogel et al. (18). Rats, deprived of water for 48 h before testing, were placed in cages with a stainless steel grid floor. Each cage contained a drinking tube connected to a 50-ml buret filled with tap water. Trials were started only when the animal's tongue had entered in contact with the drinking tube for the first time. An electric shock (0.6 mA/500 ms) was delivered to the tongue after every twenty licks. The number of shocks was recorded during a 5-min period. Tests were performed 30 min after i.p. injection of SSR149415 or diazepam. In a second experiment, SSR149415 (10 mg/kg i.p.) and the benzodiazepine (BZ) receptor antagonist flumazenil (10 mg/kg i.p.) were tested alone or in combination.
Elevated Plus-Maze Test in Rats.
The apparatus is based on that described by Pellow et al. (19). All parts were made of poly(vinyl chloride). It was elevated to a height of 50 cm with two open (50 × 10 cm) and two enclosed arms (50 × 10 × 50 cm), arranged so that the arms of the same type were opposite to each other. The apparatus was equipped with infrared beams and sensors capable of measuring arm activity for 4 min. In addition, rats were observed via video link by an observer located in an adjacent room. This arrangement allowed the recording of attempts at entry into open arms followed by avoidance responses, including stretched attend posture (the rat stretches forward and retracts to original position). Tests were performed 60 min after p.o. administration of the drugs. In a second experiment, the duration of the anxiolytic-like action of 10 mg/kg (p.o.) of SSR149415 was investigated. Rats were tested 1 h, 1 h 30 min, 3 h, or 6 h postadministration.
Light/Dark Test in Mice.
The test apparatus is based on that described by Misslin et al. (20). It consisted of two poly(vinyl chloride) boxes (20 × 20 × 14 cm). One of these boxes was darkened. A desk lamp placed 20 cm above the lit box provided the room illumination. An opaque plastic tunnel (5 × 7 × 10 cm) separated the dark box from the illuminated one. The apparatus was equipped with infrared beams capable of recording during a 4-min period: (i) time spent by mice in the lit box, and (ii) number of tunnel crossings. Tests were performed 30 min after i.p. administration of the drugs.
Mouse Defense Test Battery.
The test was conducted in an oval runway as described previously (21). (i) Pretest, 3 min: 30 or 120 min after i.p. or p.o. administration of diazepam or SSR149415, respectively, subjects were placed into the runway for a 3-min period, in which line crossings were recorded. (ii) The rat avoidance test: Immediately after this period, the experimenter introduced a hand-held dead rat at one end of the runway and brought it up to the subject. If the subject fled, avoidance distance was recorded. (iii) Chase/flight test: The rat was again brought up to the subject. The following parameters were recorded: number of stops and chase speed. (iv) Straight alley: By closing the two doors, the runway was converted to a straight alley. The rat was introduced in one end of the straight alley. During 30 s, the number of approaches/withdrawals was recorded. (v) Forced contact: The experimenter brought the rat up to contact the subject in the straight alley. For each such contact, vocalizations, upright postures, and bites by the subjects were noted.
Social Defeat Stress-Induced Anxiety in the EPM in Mice.
The procedure was a modification of that described by Miczek (22). A naive mouse was placed in the cage of a resident male aggressor, which was selected for high levels of aggression (23). The experimenter interrupted social agonistic behaviors, and the intruder was removed from the area when it displayed a submissive posture after being attacked. Thereafter, the intruder was returned to the resident cage for 60 min and placed in a cylindrical wire mesh enclosure to avoid physical contact or injury. At the end of the interaction period, the intruder mouse was placed onto the central platform of the EPM during a 5-min period. Time spent in open arms was recorded. The drugs were given p.o. 30 (diazepam) or 60 (SSR149415) min before social defeat.
Forced Swimming Test in Rats.
The procedure was a modification of that described by Porsolt et al. (24). Animals were placed in individual glass cylinders containing water. Two swimming sessions were conducted (an initial 15-min pretest followed 24 h later by a 6-min test). The duration of immobility was measured for a 6-min period. The drugs were given p.o. twice (15 min after the first session on day 1, and 60 min before session 2 on the second day).
Chronic Mild Stress.
This test is based on the procedure originally designed by Willner et al. (25) for rats. The CMS protocol consists of the sequential application of a variety of mild stressors, including restraint, forced swimming, water deprivation, and pairing with another stressed animal, in a schedule that lasts for 3 weeks, and is repeated thereafter. Parallels between human depression and chronically stressed animals have been drawn from the reduction of the efficiency with which even the smallest tasks are accomplished in depressed patients, leading to the inability to maintain minimal personal hygiene, and the decrease in grooming behavior seen in stressed animals. In this latter case, there is a degradation of the physical state of the coat, consisting of a loss of fur and dirty fur. Thus, we measured physical state about once a week over the entire CMS period, which lasted 7 weeks. At the end of the CMS procedure, mice were tested in the following models: (i) the EPM, to assess the impact of CMS on anxiety levels (anxiety was evaluated because individuals with major depressive episode frequently present with anxiety and phobias); (ii) the forced swimming test, to measure despair and coping in an aversive situation; (iii) the MDTB, to assess anxiety levels and stress coping in a threatening situation (i.e., forced contact with a Long Evans rat). The administration of SSR149415 (10 and 30 mg/kg, i.p., once a day) or fluoxetine (10 mg/kg, i.p., once a day) started 4 weeks after the beginning of the CMS and lasted until all tests were completed (week 9; in total, 39 days of treatment). The forced swimming test, EPM, and MDTB were performed 30, 31, and 32 to 39 days after the beginning of the treatments, respectively.
Miscellaneous.
Possible side effects of SSR149415 were assessed in the rotarod in mice for ataxia, in the traction test in mice for myorelaxation, by measuring electroencephalographic (EEG) activity in rats for sedation, and in the Morris water maze task in rats for learning and memory.
Results
Punished Drinking Test in Rats.
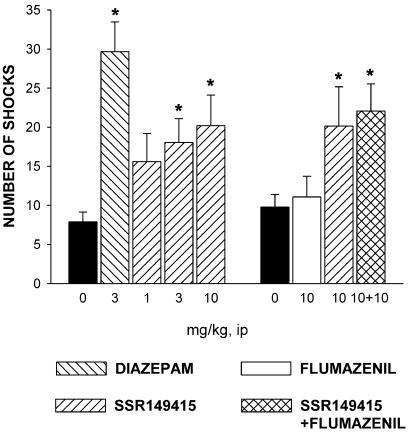
SSR149415 significantly increased punished responding at 3 and 10 mg/kg, whereas diazepam produced similar effects at 3 mg/kg i.p. (χ2 = 21.7, P < 0.001; Fig. 1). When coadministered with SSR149415 at 10 mg/kg, the BZ antagonist flumazenil did not modify the anxiolytic-like effects of the V1b antagonist (ANOVA: F = 3.5, P = 0.02; Fig. 1).
Figure 1.
Effects of diazepam and SSR149415 alone or in combination with the BZ receptor antagonist flumazenil in the punished drinking conflict test in rats. Data represent mean ± SEM; *, P < 0.05 (Kruskal–Wallis or Dunnett). n = 13–20.
Elevated Plus-Maze in Rats.
SSR149415 and diazepam increased significantly the percentage of entries into open arms at 10 mg/kg p.o. (F = 3.2, P = 0.03) and reduced attempts (F = 3.4, P = 0.02) at 10 and 30 mg/kg (SSR149415), and at 10 mg/kg (diazepam) (Table 1). Neither drug significantly modified the closed arm entries. The oral time course of the anxiolytic-like action of SSR149415 performed at 10 mg/kg indicated that effects lasted up to 3 h (data not shown).
Table 1.
Effects of SSR149415 and diazepam in the elevated plus-maze in rats
| Compound | Dose, mg/kg, p.o. | % entries open arms | Attempts | Closed arm entries |
|---|---|---|---|---|
| 0 | 22.1 ± 5.9 | 7.1 ± 0.5 | 8.6 ± 0.6 | |
| Diazepam | 10 | 52.4 ± 8* | 3.3 ± 0.9* | 6.3 ± 1 |
| SSR149415 | 3 | 35.4 ± 5.8 | 5.4 ± 0.8 | 7 ± 0.7 |
| 10 | 49.3 ± 8* | 4.6 ± 0.8* | 6.3 ± 0.4 | |
| 30 | 36 ± 6.1 | 4.1 ± 0.7* | 7.1 ± 1.1 |
Data represent mean ± SEM;
, P < 0.05 (Dunnett's t test). n = 7.
Light/Dark Test in Mice.
SSR149415 and diazepam modified the time spent in the lit box (SSR149415: χ2 = 12.2, P = 0.02; diazepam: χ2 = 13.1, P = 0.01) and the number of tunnel crossings (SSR149415: χ2 = 9.7, P = 0.05; diazepam: χ2 = 29.9, P = 0.0001) (Table 2). SSR149415 increased significantly the former measure at 1, 10, and 30 mg/kg, whereas diazepam produced similar effects from 1 mg/kg. Tunnel crossings were increased by SSR149415 at 1 and 10 mg/kg, and by diazepam between 0.3 and 3 mg/kg.
Table 2.
Effects of SSR149415 and diazepam in the light/dark test in mice
| Compound | Dose, mg/kg | Time lit box, s | Tunnel crossings |
|---|---|---|---|
| Diazepam | 0 | 32.3 ± 12.2 | 4.5 ± 1 |
| (i.p.) | 0.3 | 60.1 ± 12.1 | 16.6 ± 3.4* |
| 1 | 74.56 ± 14.2* | 13.5 ± 1.2* | |
| 3 | 96.3 ± 10.5* | 18.1 ± 1.7* | |
| 10 | 104.2 ± 23.1* | 6.6 ± 2 | |
| SSR149415 | 0 | 12.06 ± 5.6 | 5.5 ± 1.8 |
| (i.p.) | 1 | 53.1 ± 10.7* | 13.9 ± 2.9* |
| 3 | 39.7 ± 10.1 | 15.9 ± 2.3 | |
| 10 | 65.7 ± 13.5* | 15.9 ± 3* | |
| 30 | 47.9 ± 10.4* | 11.4 ± 2.1 |
Data represent mean ± SEM;
, P < 0.05 (Kruskal-Wallis test). n = 13–16.
Mouse Defense Test Battery.
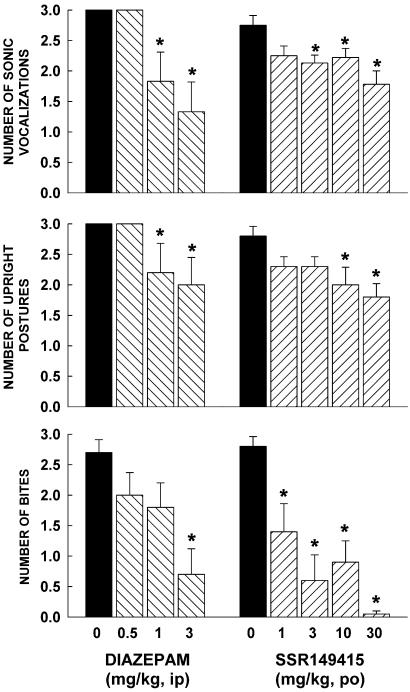
SSR149415, but not diazepam significantly decreased avoidance distance at 30 mg/kg (F = 2.7, P = 0.05; Table 3). When mice were chased by the rat or constrained in a straight alley, only diazepam significantly modified risk assessment behavior (stops: F = 8.5, P = 0.0008; approach/withdrawal: F = 3, P = 0.05). The drug decreased stops from 0.5 mg/kg, and increased approaches followed by withdrawals from 1 mg/kg (Table 3). SSR149415 and diazepam significantly decreased defensive aggression (SSR149415: vocalizations, F = 4.2, P = 0.007; upright postures, F = 2.9, P = 0.03; bites, F = 10.2, P = 0.0001; diazepam: vocalizations, F = 6, P = 0.004; upright postures, F = 2.7, P = 0.05; bites, F = 5.4, P = 0.007; Fig. 2). They decreased vocalizations from 1 and 3 mg/kg, respectively. Upright postures were reduced by SSR149415 and diazepam from 10 and 1 mg/kg, respectively. Defensive biting was attenuated by the V1b receptor antagonist at all doses, and by diazepam at 3 mg/kg. Finally, neither drug treatment significantly altered activity before (line crossings) and during (chase speed) exposure to the rat (Table 3).
Table 3.
Effects of SSR149415 and diazepam on several behavioral responses displayed by mice before (line crossings) and during (chase speed, avoidance, and risk assessment) exposure to a Long Evans rat in the Mouse Defense Test battery
| Compound | Dose, mg/kg | Activity
|
Flight: avoidance distance, cm | Risk assessment
|
||
|---|---|---|---|---|---|---|
| Line crossings | Chase speed, m/s | Stops | Approaches-withdrawals | |||
| Diazepam | 0 | 114.8 ± 12.1 | 0.8 ± 0.1 | 165.8 ± 16.6 | 13.8 ± 1 | 0.2 ± 0.2 |
| (i.p.) | 0.5 | 153.8 ± 12.7 | 0.7 ± 0.1 | 131.7 ± 21.6 | 7.3 ± 1.6* | 0.5 ± 0.2 |
| 1 | 135.8 ± 9.7 | 0.8 ± 0.1 | 122.7 ± 23.1 | 7.5 ± 1.4* | 1.5 ± 0.4* | |
| 3 | 122.2 ± 21 | 0.6 ± 0.1 | 106.5 ± 20 | 4 ± 1.5* | 1.2 ± 0.5* | |
| SSR149415 | 0 | 101.3 ± 9.7 | 0.9 ± 0.1 | 176.8 ± 12.6 | 11.1 ± 0.7 | 0 ± 0 |
| (p.o.) | 1 | 127.8 ± 8.2 | 0.8 ± 0.1 | 137.5 ± 11.7 | 8.8 ± 1 | 0 ± 0 |
| 3 | 122.1 ± 13.7 | 0.8 ± 0.1 | 151 ± 4.3 | 8.1 ± 1.1 | 0 ± 0 | |
| 10 | 119.7 ± 5.4 | 0.8 ± 0.1 | 152.4 ± 14.5 | 9.6 ± 0.5 | 0.1 ± 0.1 | |
| 30 | 122.7 ± 10.5 | 0.8 ± 0.1 | 121.6 ± 11.9* | 8.3 ± 1 | 0 ± 0 | |
Data represent mean ± SEM.
, P < 0.05 (Dunnett's t test). n = 6–9.
Figure 2.
Effects of SSR149415 and diazepam on defensive aggression on forced contact with a Long Evans rat in the mouse defense test battery. Data represent mean ± SEM; *, P < 0.05 (Dunnett). n = 6–9.
Social Defeat Stress-Induced Anxiety in the EPM in Mice.
There was a significant effect in both drug-treatment groups for the percentage of time in open arms: SSR149415, F = 32.9, P < 0.01; diazepam, F = 4.9, P < 0.05. Social defeat had a significant impact on the performance of mice exposed to the EPM as was shown by the dramatic decrease in the percentage of time spent in open arms in stressed animals as compared with control mice (Table 4). This anxiogenic-like effect of stress was significantly antagonized by SSR149415 at 3 mg/kg and by diazepam at 4 mg/kg (Table 4).
Table 4.
Effects of SSR149415 and diazepam on social defeat-induced anxiogenic-like behavior in the elevated plus-maze in mice
| Compounds | Condition | Dose, mg/kg | % time open arms |
|---|---|---|---|
| Diazepam | Non-stressed | 0 | 42.7 ± 4.5 |
| (p.o.) | Stressed | 0 | 20.3 ± 6.3* |
| Stressed | 4 | 42.9 ± 7† | |
| SSR149415 | Non-stressed | 0 | 36 ± 2.9 |
| (p.o.) | Stressed | 0 | 6.3 ± 2.1* |
| Stressed | 3 | 21.4 ± 2.7† |
Data represent mean ± SEM;
, P < 0.05 (vs. non-stressed animals); †, P < 0.05 (vs. vehicle-treated stressed mice, Mann-Whitney test). n = 9–10.
Forced Swimming Test in Rats.
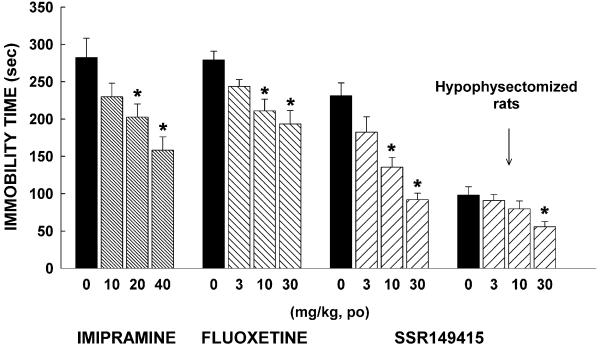
In normal rats, there was a significant effect on immobility time with SSR149415 (F = 14.8, P = 0.0001), fluoxetine (F = 7.3, P = 0.001), and imipramine (F = 6.7, P = 0.002). SSR149415 and fluoxetine significantly decreased this measure from 10 mg/kg, whereas imipramine produced such effects from 20 mg/kg (Fig. 3). In hypophysectomized rats, immobility time was diminished when compared with normal rats, but SSR149415 was still able to decrease it, although this effect reached statistical significance only at 30 mg/kg (Fig. 3).
Figure 3.
Effects of SSR149415, imipramine, and fluoxetine in the forced swimming test in rats. Data represent mean ± SEM; *, P < 0.05 (Dunnett). n = 7–13.
Chronic Mild Stress.
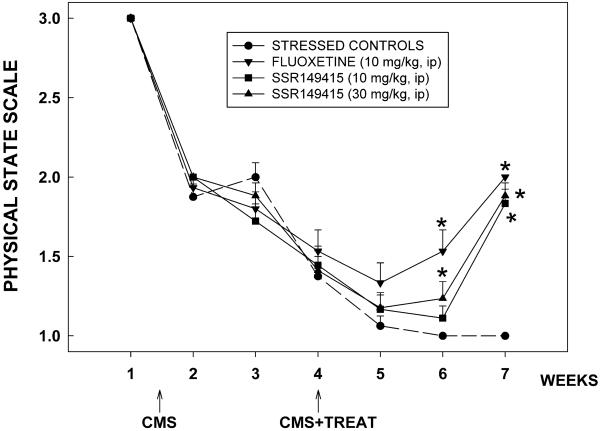
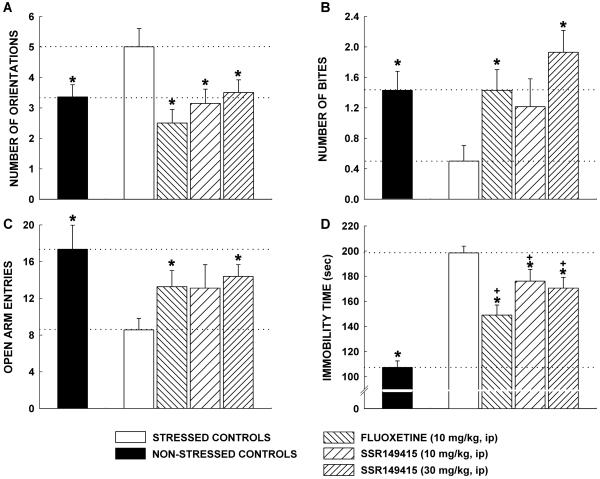
There was a significant degradation in the physical state of the coat of mice because of stress (F = 5.1, P < 0.0001; Fig. 4). In the vehicle-treated group, the effect lasted till the end of the 7-week CMS. In contrast, the degradation of the physical state of the animal's coat was significantly improved by SSR149415 (30 mg/kg) and fluoxetine (10 mg/kg) after 2 weeks of treatment (CMS week 6), an effect that lasted until the CMS was over. The 10 mg/kg dose of SSR149415 produced similar effects 3 weeks after the beginning of treatment. Moreover, chronically stressed mice made fewer entries into the open arms of the EPM (T = 4.5, P = 0.05; Fig. 5C) and displayed significantly more orientations when chased by a rat in the MDTB (F = 3.8, P = 0.007; Fig. 5A) as compared with nonstressed controls. SSR149415 and fluoxetine reversed these anxiogenic-like effects of stress and their performances were no longer significantly different from nonstressed controls. Furthermore, mice chronically stressed were unable to show a normal coping response as they displayed significantly fewer bites on forced contact with the rat in the MDTB than nonstressed control animals (F = 3.4, P = 0.01). The coping behavior was fully restored by SSR149415 (30 mg/kg) and fluoxetine (10 mg/kg) (Fig. 5B) as levels of biting reached those of nonstressed controls. Finally, chronically stressed control mice showed greater immobility in the forced swimming test than nonstressed control animals (F = 28.7, P < 0.0001), which may indicate increased levels of despair in the former. Although SSR149415 and fluoxetine significantly reversed the increase in immobility, their performances were still different from nonstressed controls (Fig. 5D).
Figure 4.
Effects of repeated administration of SSR149415 and fluoxetine on the degradation of the physical state of the coat of animals in chronically stressed mice. Data represent mean ± SEM; *, P < 0.05 (vs. vehicle-treated stressed mice, Dunnett). n = 15–18.
Figure 5.
Effects of repeated administration of SSR149415 and fluoxetine for 30–39 days on chronic mild stress-induced (A and C) anxiogenic-like behavior when mice are chased by a rat in the MDTB and elevated plus-maze test, respectively; (B) loss of coping behavior on forced contact with a rat in the MDTB; (D) despair behavior in the forced swimming test. Data represent mean ± SEM; *, P < 0.05 (vs. stressed mice); +, P < 0.05 (vs. nonstressed mice, Student or Dunnett). n = 15–18.
Miscellaneous.
SSR149415 administered up to 100 mg/kg did not significantly modify performance of mice in the rotarod and traction tests. Neither did the drug modify sleep patterns after electroencephalographic analysis or impair learning in the Morris water maze up to 30 mg/kg in rats.
Discussion
The results of the present series of experiments show that the selective non-peptide V1b receptor antagonist SSR149415 displays a behavioral profile that is consistent with an anxiolytic- and antidepressant-like action. When tested in classical animal models of anxiety, such as the light/dark, the EPM, and the punished drinking tests, SSR149415 produced anxiolytic-like effects at doses that ranged from 1 to 30 mg/kg, depending on the model used. The absence of significant modifications in the number of closed arm entries [a reliable measure of locomotor activity (26)] in the EPM indicates that the anxiolytic-like activity was observed at doses that did not impair motor activity. Moreover, it is unlikely that the positive effects of SSR149415 in the punished drinking test are due to decreased sensitivity to electric shocks because compounds that are endowed with analgesic properties, such as morphine, are inactive in conflict tests (27). The finding that the BZ receptor antagonist flumazenil did not block the anxiolytic-like activity of SSR149415 in the punished drinking test provides undisputed evidence that these effects are at least not mediated by an action at γ-aminobutyric acid type A (GABAA)/BZ receptors. It is important to note that the magnitude of the anxiolytic-like action of SSR149415 in these models was overall less than that of the BZ anxiolytic diazepam, which was used as a positive control. Whether this result may indicate a less efficacious anxiolytic-like potential of V1b receptor antagonists compared with BZs, or suggests that these compounds may have a different spectrum of therapeutic activity in anxiety disorders than BZs, remains to be determined. Results obtained with SSR149415 in the MDTB may, however, be relevant to this issue. The earlier MDTB studies have suggested that this procedure provides a model capable of responding to, and differentiating anxiolytic drugs of different classes through specific profiles of effect on different measures (28). Here, SSR149415 failed to modify significantly risk assessment, a behavior that has been shown to be particularly sensitive to BZs, i.e., drugs used against generalized anxiety disorder (GAD), but it produced clear-cut effects on defensive aggression, a behavior that is claimed to be associated with certain aspects of stress disorders after traumatic events (29), thereby suggesting that SSR149415 may be useful in these conditions rather than in GAD. In agreement with this idea are results from the social defeat stress-induced anxiety paradigm, where SSR149415 completely antagonized the heightened emotionality in the EPM produced by prior (stressful) exposure to an aggressive resident.
To investigate potential antidepressant-like effects of SSR149415, we used the forced swimming and CMS tests, two classical acute and chronic models of depression, respectively (24, 25). Results from the forced swimming test showed that SSR149415 produced dose-dependent antidepressant-like activity. These effects were comparable to those observed with the reference antidepressants, fluoxetine and imipramine. Importantly, the finding that the antidepressant-like effects of SSR149415 were still present, albeit at a higher dose, in hypophysectomized rats indicates that this action does not necessarily involve pituitary-adrenal axis blockade, thereby suggesting that extrahypothalamic V1b receptors may play a role in these effects. The antidepressant potential of SSR149415 was confirmed in the CMS procedure where the drug was given repeatedly for 30 to 39 days. SSR149415 improved the degradation of the physical state of the coat of stressed animals. This finding suggests that SSR149415 normalized grooming, an activity impaired by repeated stress. CMS caused the appearance of an “anxious” profile as was evidenced by the findings from the EPM and the MDTB. These behavioral changes were not seen in animals treated with SSR149415, indicating that the drug was able to prevent the stress-induced increase in anxiety levels. In addition, stress coping was impaired by CMS. In the forced swimming test, stressed mice displayed a greater tendency toward despair behavior than nonstressed animals. In the MDTB, stressed mice were unable to show normal defensive attack reactions when confronted with a natural threat. Whereas SSR149415 only partially improved despair, it completely normalized defensive attack. These findings thus indicate that SSR149415 is able to restore a normal coping response when animals are exposed to inescapable aversive stimuli. Overall, the effects of SSR149415 in the CMS paralleled those of repeated treatment with fluoxetine. Based on the well accepted predictive validity of the CMS (25), the present findings indicate that SSR149415 has antidepressant-like properties that are comparable in terms of efficacy of the effects to those of a classical antidepressant.
The site at which the anxiolytic- and antidepressant-like actions of SSR149415 are exerted remains to be determined, but it likely involves V1b receptors located on neurons in the amygdala, septum, hippocampus, or hypothalamus, regions associated with the integration and transduction of stressful stimuli (30). Altogether, the present findings suggest that SSR149415 will serve as a useful tool for further probing the functional importance of brain AVP system in emotional processes, and suggest that blockade of V1b receptors may represent a novel strategy for the treatment of major depressive and some forms of anxiety disorders.
Acknowledgments
The expert technical assistance of C. Aliaga, G. Gout, M. Lhermitte, and N. Moindrot is greatly appreciated. The automation of most procedures used here was carried out by B. Kleinberg, to whom we are thankful. The authors would like to thank Drs. J. Alexander and G. Le Fur for critically reading the manuscript.
Abbreviations
- AVP
arginine vasopressin
- BZ
benzodiazepine
- EPM
elevated plus-maze
- CMS
chronic mild stress
- MDTB
mouse defense test battery
- p.o.
per os
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.De Vries G J, Buijs R M. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 2.Lolait S J, O'Carroll A M, Mahan L C, Felder C C, Button D C, Young W S, III, Mezey E, Brownstein M J. Proc Natl Acad Sci USA. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young L J, Toloczko D, Insel T R. J Neuroendocrinol. 1999;11:291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- 4.Vaccari C, Lolait S J, Ostrowski N L. Endocrinology. 1998;139:5015–5033. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- 5.Morel A, O'Carroll A M, Brownstein M J, Lolait S J. Nature (London) 1992;356:523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- 6.De Wied D. Int J Neuropharmacol. 1965;4:157–167. doi: 10.1016/0028-3908(65)90005-5. [DOI] [PubMed] [Google Scholar]
- 7.De Wied D. J Endocrinol. 1970;48:xlv–xlvi. [PubMed] [Google Scholar]
- 8.Koob G F, Bloom F E. Annu Rev Physiol. 1982;44:571–582. doi: 10.1146/annurev.ph.44.030182.003035. [DOI] [PubMed] [Google Scholar]
- 9.Engelmann M, Wotjak C T, Neumann I, Ludwig M, Landgraf R. Neurosci Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 10.Alescio-Lautier B, Metzger D, Soumireu-Mourat B. Rev Neurosci. 1993;4:239–266. doi: 10.1515/revneuro.1993.4.3.239. [DOI] [PubMed] [Google Scholar]
- 11.Dantzer R, Bluthe R M. Crit Rev Neurobiol. 1992;6:243–255. [PubMed] [Google Scholar]
- 12.Bohus B, Kovacs G L, De Wied D. Brain Res. 1978;157:414–417. doi: 10.1016/0006-8993(78)90052-5. [DOI] [PubMed] [Google Scholar]
- 13.Bohus B, Borrell J, Koolhaas J M, Nyakas C, Buwalda B, Compaan J C, Roozendaal B. Ann N Y Acad Sci. 1993;689:285–299. doi: 10.1111/j.1749-6632.1993.tb55554.x. [DOI] [PubMed] [Google Scholar]
- 14.Liebsch G, Wotjak C T, Landgraf R, Engelmann M. Neurosci Lett. 1996;217:101–104. [PubMed] [Google Scholar]
- 15.Landgraf R, Gerstberger R, Montkowski A, Probst J C, Wotjak C T, Holsboer F, Engelmann M. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilera G, Rabadan-Diehl C. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 17.Serradeil-Le Gal C, Wagnon J, Simiand J, Griebel G, Lacour C, Guillon G, Barberis C, Brossard G, Soubrié P, Nisato D, et al. J Pharmacol Exp Ther. 2002;300:1122–1130. doi: 10.1124/jpet.300.3.1122. [DOI] [PubMed] [Google Scholar]
- 18.Vogel J R, Beer B, Clody D E. Psychopharmacologia. 1971;21:1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- 19.Pellow S, Chopin P, File S E, Briley M. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 20.Misslin R, Belzung C, Vogel E. Behav Process. 1989;8:119–132. doi: 10.1016/S0376-6357(89)80010-5. [DOI] [PubMed] [Google Scholar]
- 21.Griebel G, Sanger D J, Perrault G. Aggress Behav. 1997;23:19–31. [Google Scholar]
- 22.Miczek K A. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 23.Simiand J, Keane P E, Barnouin M C, Keane M, Soubrié P, Le Fur G. Fundam Clin Pharmacol. 1993;7:413–427. [PubMed] [Google Scholar]
- 24.Porsolt R D, Le Pichon M, Jalfre M. Nature (London) 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 25.Willner P, Muscat R, Papp M. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 26.Cruz A P M, Frei F, Graeff F G. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 27.Treit D. Neurosci Biobehav Rev. 1985;9:203–222. doi: 10.1016/0149-7634(85)90046-6. [DOI] [PubMed] [Google Scholar]
- 28.Griebel G, Sanger D J. In: Animal Models of Human Emotion and Cognition. Haug M, Whalen R E, editors. Washington, DC: Am. Psychol. Assoc.; 1999. pp. 75–85. [Google Scholar]
- 29.Blanchard R J, Griebel G, Henrie J A, Blanchard D C. Neurosci Biobehav Rev. 1997;21:783–789. doi: 10.1016/s0149-7634(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 30.Herman J P, Cullinan W E. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]