Abstract
We have used gene targeting to generate mice with a homozygous deficiency in trp2, a cation channel expressed in the vomeronasal organ (VNO). Trp2 mutant animals reveal a striking reduction in the electrophysiological response to pheromones in the VNO, suggesting that trp2 plays a central role in mediating the pheromone response. These mutants therefore afford the opportunity to examine the role of the VNO in the generation of innate sexual and social behaviors in mice. Trp2 mutant males and nursing females are docile and fail to initiate aggressive attacks on intruder males. Male–female sexual behavior appears normal, but trp2 mutant males also vigorously mount other males. These results suggest that the cation channel trp2 is required in the VNO to detect male-specific pheromones that elicit aggressive behaviors and dictate the choice of sexual partners.
Animals exhibit behavioral repertoires that are often innate and result in stereotyped sexual and social responses to their environment. Innate behaviors do not require learning or experience and are likely to reflect the activation of developmentally programmed neural circuits. The appropriate expression of an innate behavioral array frequently requires signals from the outside world. Mice rely heavily on olfactory information to sense their environment. In mice, odorants are recognized by two anatomically and functionally distinct sensory organs, the main olfactory epithelium (MOE) and the vomeronasal organ (VNO) (1, 2). The main olfactory epithelium is thought to recognize odors that provide information about the world at large and can result in measured behavioral responses. In contrast, the VNO has traditionally been implicated in the recognition of pheromones, odorants that provide information about the social and sexual status of other individuals within the species (3, 4). Activation of the VNO is thought to result in innate neuroendocrine and behavioral responses.
In mammals, the chemical nature of the pheromones that activate the VNO to elicit innate behavioral responses has not been elucidated (5). Moreover, it has been difficult to sort out the relative roles of the MOE and the VNO in mediating specific behaviors. In male hamsters, removal of the olfactory bulb, which receives input from both the MOE and the VNO, abolishes the sexual response (6). Removal of the VNO alone diminishes the robustness of the male mating response, but does not eliminate sexual behavior (7). The consequence of VNO removal is most apparent in sexually naive animals, suggesting that with experience the main olfactory system assumes an increasingly important role in the sexual response (8, 9). Other innate behaviors, including lordosis in female pigs (10) in response to the male hormone, androstenone, or suckling behavior in newborn rabbits (11) in response to mammary secretions, are unaffected by removal of the VNO. These innate behavioral responses are likely to be elicited by pheromones that activate the main olfactory system. Thus, mammals have evolved innate behavioral arrays mediated by pheromones that activate both the main and vomeronasal olfactory system.
It is possible to genetically distinguish the behavioral contributions of the two olfactory systems by mutating essential signaling components of the “two noses” independently. The signal transduction cascades that translate odor binding into alterations in membrane potential differ in the MOE and VNO. In the MOE, odorant binding to one of a large family of seven transmembrane receptors (12) initiates a G-protein cascade that ultimately activates a cyclic nucleotide-gated cation channel (13). In the VNO, receptor activation is thought to regulate a distinct cation channel, trp2. Antibodies to trp2 detect this channel solely in VNO neurons but low levels of mRNA are detected in testes as well (14, 15). Trp2 is homologous to osm-9, a trp-like channel that mediates olfactory responses in the nematode Caenorhabditis elegans (16), suggesting that trp2 may be the odorant-activated transduction channel in the VNO.
We have used gene targeting to generate mice with a homozygous deficiency in trp2. Trp2 mutant animals reveal a striking reduction in the electrophysiological response to pheromone mixtures in the VNO, suggesting that trp2 plays a central role in mediating the pheromone response. These mutants therefore afford the opportunity to define the role of the VNO in the generation of innate sexual and social behaviors in mice.
Materials and Methods
Generation of trp2−/− Mice.
A 21-kb genomic clone containing the trp2 gene was isolated from a phage library derived from a 129SvJ mouse (Stratagene). Restriction enzyme mapping and sequence analysis revealed a 6.1-kb KpnI fragment that contains the putative transmembrane domains 3–6, as well as a portion of the carboxyl-terminal coding sequence (see Fig. 5A, which is published as supporting information on the PNAS web site, www.pnas.org). This fragment was deleted in the targeting construct, being replaced by a neor gene (NEB pGT-N29, New England Biolabs). Flanking the neor gene in the targeting construct is a 5′ 5.1-kb KpnI fragment and a 3′ 2.4-kb KpnI/HindIII fragment. This construct was electroporated into 129SvEv ES cells that were cultured and G418-selected as described (17). Southern blots were used to identify a positive clone that was expanded and injected into C57BL6/J blastocysts. The resultant male germline chimeras were then crossed to C57BL6/J females to produce trp2+/− mice.
Electrophysiology.
Local field potentials [the electro-vomeronasogram (EVG)] were recorded from the microvillous layer of intact VNO sensory epithelia as described (18). Urine was collected from the outer urethra of 20 female and 15 male adult control mice (age range: 7–14 weeks). Samples were pooled according to sex, frozen as aliquots, and stored at −20°C. Artificial urine (19) consisted of (in mM): 120 NaCl, 40 KCl, 20 NH4OH, 4 CaCl2, 2.5 MgCl2, 15 NaH2PO4, 20 NaHSO4, and 333 urea, adjusted to pH 7.4 (NaOH).
Animals.
Trp2+/− breeding pairs of mixed 129SvEv × C57BL6/J background were used to generate trp2−/− mutant animals and +/− or +/+ littermate controls. Mice were weaned at 21 days of age, at which time they were segregated four to five per cage in single-sex groups with food and water available ad libitum. Animals were maintained in a temperature-controlled barrier facility on a 14:10 light-dark cycle. All behavioral testing, with the exception of the marking assays, was conducted at night. Testing was initiated 1 h after the onset of the dark cycle and was completed within 4 h. Testing sessions were recorded on videotape under infrared illumination (Sony TRV-88) and were analyzed after the completion of the experiment by an observer blind to genotype.
Resident–Intruder Aggression Assay.
Male mutant and control mice were isolated at 9–11 weeks of age for a period of 4–5 weeks before testing. Testing lasted 15 min, and began when a group-housed, sexually inexperienced adult 129SvEv strain “intruder” male was placed in the home cage of the test mouse, whose bedding had not been changed for at least 4 days. Aggressive behavior was defined as biting, chasing, or wrestling/tumbling. Recorded parameters include latency to first attack, cumulative attack duration, and number of bites, as well as latency to mount, cumulative mount duration, and number of mounts. Some mice were later retested after receiving mating experience, which consisted of 10 days with B6D2F1/J females (all of which eventually produced litters). This second resident–intruder assay began 30 min after the removal of the female from resident's home cage.
Mating Assay.
One week after the completion of the resident–intruder aggression assay, male mutant and control mice, still sexually inexperienced, were used in a mating assay. The testing period lasted 30 min, and began when a wild-type estrous female of the B6D2F1/J strain was placed in the home cage of the test mouse. Latency to first mount and intromission, as well as the total number of mounts and intromissions, were scored.
Mating Choice Assay.
Male mice were tested before and after mating experience for 10 days. The testing period lasted 15 min, and began when a 129SvEv strain male and a nonestrous B6D2F1/J female were simultaneously placed in the home cage of the test mouse. Nonestrous females were used to minimize the effect of female receptivity in this assay. Previously mentioned parameters of mating and aggressive behavior were scored.
Maternal Aggression Assay.
trp2 and control females were paired with C57BL6/J stud males for 10 days and then isolated in separate cages where they produced litters. On postpartum days 5, 7, and 9, female mice were tested for maternal aggression in a manner similar to males—i.e., by the introduction of a 129SvEv intruder male for 15 min. The females were tested on multiple days to maximize the chance that aggressive behaviors would be observed. The pups were removed from the cage 3 min before the onset of testing to avoid the possibility of injury to the pups, which does not alter the aggression of the mother (20).
Marking Assays.
Nonlittermate trp2 and control males, which had been singly housed and had not received sexual experience, were placed on opposite sides of a wire mesh barrier in a two-chamber cage lined with filter paper for 30 min. At the end of the test session, urine marking patterns were visualized with UV transillumination (21) and the number of marks was scored. Nonlittermates were then randomly assigned to pairs and given daily experience with each other (21), in which they were placed together in a neutral cage lined with filter paper. There were 11 control–mutant pairs, six mutant–mutant pairs, and five control–control pairs. Pairing sessions lasted 30 min for the first 3 days, and 15 min on the last 3 days. After each session the mice were returned to their home cages. On day 7, marking behavior was assayed for each pair of mice as described above. Those pairs in which a clear dominance relationship was not established as indicated by marking pattern were repaired for 3 h and marking behavior was reassayed the following day.
Results
Gene Targeting at the Trp2 Locus.
Mice with a homozygous deletion in the trp2 gene were generated to permit us to examine the role of trp2 in VNO function, as well as the role of the VNO in mediating innate behaviors. The trp2 gene consists of 13 exons and encodes a cation channel comprised of six transmembrane domains and a pore region. A 6.1-kb segment encoding transmembrane domains 3 through 6, including the pore (exons 6–11), was substituted with a PGK-neo cassette by homologous recombination in ES cells (see Fig. 5A and Supporting Materials and Methods, which are published as supporting information on the PNAS web site). Recombinants bearing a deletion at the trp2 locus were identified by Southern blot analysis and injection of these ES cells into blastocysts yielded chimeric mice that transmitted the mutation through their germ line. Heterozygous and homozygous animals were obtained and the absence of trp2 in homozygous animals was confirmed by in situ hybridization and by immunohistochemistry, using an anti-trp2 antibody (see Fig. 5B). The electrophysiological responses to pheromone mixtures and sexual and social behaviors were then examined in trp2 mutant mice.
Electrophysiological Responses in the VNO of Trp2 Mutant Mice.
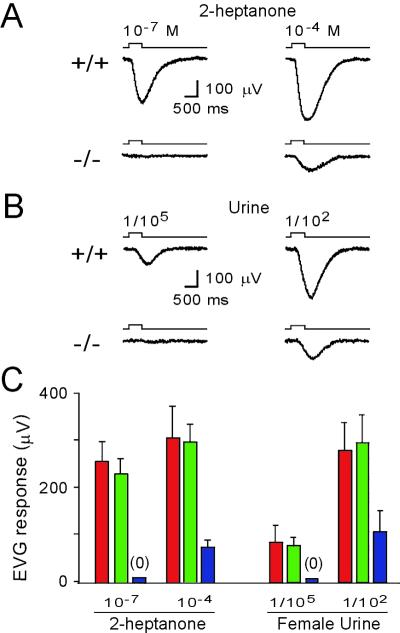
Sensory neurons in mouse VNO can detect pheromonal ligands isolated from urine with exquisite sensitivity and specificity (18, 22). To define the role of trp2 in pheromone transduction, we used an intact VNO preparation to record local field potentials (EVG) from the surface of the sensory epithelium (18). Pulses (500-ms) of 2-heptanone, a molecule previously shown to activate the VNO (18), elicited negative field potentials in a concentration-dependent manner (Fig. 1 A and C). Wild-type (+/+) and trp2 heterozygotes (+/−) showed a robust response to 10−7 M 2-heptanone. An increase in the stimulus strength to 10−4 M further increased the EVG amplitude. In contrast, trp2 mutants (−/−) did not respond to 2-heptanone at 10−7 M, but did exhibit a small response to 2-heptanone at 10−4 M (Fig. 1 A and C). On average, the size of the EVG response to 2-heptanone at 10−4 M in trp2 mutants is reduced to about 25% of control mice.
Figure 1.
Strongly diminished responses in trp2−/− vomeronasal sensory neurons to synthetic and natural stimuli. (A) Representative EVG responses from wild-type (Upper) and trp2−/− mice (Lower). Responses were induced by 500-ms pulses of 2-heptanone at 10−7 M or 10−4 M, respectively. (B) Representative EVG responses from wild-type (Upper) and trp2−/− mice (Lower) induced by 500-ms pulses of urine diluted 1/105 or 1/102, respectively. (C) Histograms showing collected results (mean ± SD) from +/+ (red), +/− (green), and −/− (blue) mice. Peak EVG responses from male and female animals were pooled. For stimulation with 10−7 M 2-heptanone, no responses are observed in trp2 mutants (n = 11). With 10−4 M 2-heptanone, responses are reduced to about 25% of control mice [+/+: 305 ± 66 μV (n = 7); trp2−/−: 77 ± 15 μV (n = 4); P < 0.0001]. For stimulation with urine diluted 1/105, no responses are observed in trp2 mutants (n = 4). With urine diluted 1/102, responses are reduced to about 37% of control mice [+/+: 277 ± 61 μV (n = 6); trp2−/−: 103 ± 45 μV (n = 10); P < 0.0001]. Responses from +/+ and +/− mice were essentially identical (P = 0.21–0.80).
We observed a similar reduction in sensitivity of the trp2 mutant vomeronasal neurons to pheromone mixtures in urine (Fig. 1 B and C). In wild-type and heterozygous mice, a urine-evoked field potential was observed at dilutions of urine as low as 1/105. No response is observed with trp2 mutants at this concentration. Peak EVG responses to female urine, at dilutions of 1/102, are reduced to approximately 37% in the trp2 mutants when compared with wild-type mice (Fig. 1 B and C). This reduction in the response was observed with either male or female urine as stimulus, independent of whether recordings were obtained from male or female VNO (data not shown). Artificial urine at dilutions of 1/102 gave no measurable EVG response in either wild-type or mutant mice. Thus, trp2 mutant mice reveal a significant reduction in the electrophysiological response to single ligands, as well as pheromone mixtures, indicating that trp2 plays a central role in mediating pheromone responsivity.
Sexual Behavior in Trp2 Mice.
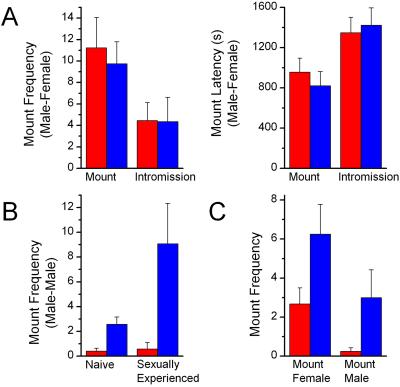
We next examined male sexual behavior in trp2 mutants. The mating sequence in male rodents consists of an initial phase of olfactory exploration, followed by multiple episodes of mounting and intromission, and ending with ejaculation (23). Four-month-old trp2 mutant (−/−, n = 11) and control (+/−, n = 12) male mice, never exposed to females after weaning and therefore sexually naive, were observed in mating assays. An estrous female was added to the male's home cage and the latency and frequency of mounting, intromission, and ejaculation were recorded over 30 min. All animals exhibited mounting and intromission during this period. Only a few of the sexually inexperienced males in this genetic background mated to ejaculation. The latency and frequency of mounting and intromission were not statistically different in mutant and control mice (Fig. 2A). Controls exhibited an average of 11.2 ± 2.8 mounts during the mating assay, compared with 9.7 ± 2.0 for trp2 mutants. Similar results were obtained with mutant and control mice that were sexually experienced rather than naive (data not shown).
Figure 2.
Trp2−/− males mate normally with females but display increased mounting toward other males. (A) Equivalent sexual behavior toward estrous females is exhibited by sexually naive trp2 (n = 11, blue bars) and control (n = 12, red bars) male mice in a 30-min mating test. (B) Increased intermale mounting is observed in trp2−/− mice (n = 23) in comparison to controls (n = 25) upon the addition of a male to their home cage in a 15-min test. Both before and after mating experience with females, trp2 males exhibited a significantly greater number of intermale mounts than controls (mount frequency, P < 0.001; Mann–Whitney U test). In fact, trp2−/− mice displayed a significant increase in intermale mounting after sexual experience with females (mount frequency, P < 0.05, Wilcoxon signed rank test). (C) trp2−/− males continue to mount males at a high rate even when a female is present. Sexually experienced trp2−/− (n = 12) and control (n = 12) male mice were observed for behaviors exhibited upon the simultaneous addition of a male and nonestrous female mouse into their home cage in a 15-min test. Trp2 males exhibit a 2-fold preference for mounting females, whereas in the control group there is a 10-fold preference for mounting females.
Although we observed no apparent effect of the trp2 deletion on male–female sexual behaviors, we observed dramatic increase in male–male mounting in trp2 mutants. Sexually inexperienced, singly housed males were exposed to wild-type intruder males for 15 min. Under these social circumstances, 4 of 25 control mice exhibited a low frequency of male–male mounting. Trp2 mutants (n = 23), however, reveal a dramatic increase in intermale mounting behavior with 61% of mutant mice exhibiting a high frequency of mounting the intruder male (Fig. 2B). Although intromission is not achieved, the intermale behavior resembles mouse male–female mounting behavior in that the male approaches the intruder from behind, grabs with its forepaws, and exhibits rapid pelvic thrusting motions. Both the number of mounts and mount duration were significantly greater in trp2 mutants as compared with controls (P < 0.001). The number of intermale mounts exhibited by trp2 mutants in a 15-min test period (2.57 ± 0.59) was more than six times higher than controls (0.4 ± 0.22).
Males used in the experiments were separated from females at weaning. We next asked whether intermale mounting behavior persists after mating experience with females. Trp2 mutant and control males were therefore paired with breeder females for 10 days. Females from pairings with both control and mutant mice produced litters, indicating that all males had engaged in sexual behavior to ejaculation with females. Prior sexual encounters with females results in a greater than 3-fold increase in subsequent male–male mounting frequency and mount duration in trp2 mutant mice (Fig. 2B). These differences in intermale mounting between naive and sexually experienced animals were not observed for control mice. Thus, the male–male mounting behavior observed in naive trp2 mice persists in a more vigorous form following sexual experience with females.
We next examined mating preference by presenting trp2 mutant and control male mice with a male and female simultaneously. A docile intruder male and a nonestrous female were introduced together into the home cage of sexually experienced trp2 mutant and control mice for 15 min. Nonestrous females were used to minimize the differential receptivity of males and females in this assay. Control males exhibited a relative decrease in mounting behavior in this assay, perhaps because they often attacked the intruder male. Nevertheless, control males mounted females ten times more frequently than males (Fig. 2C), whereas trp2 mutants exhibit only a 2-fold preference for females. Thus, mutant males reveal a high frequency of intermale mounting that persists even when males are confronted with a male–female choice of sexual partner.
Trp2 Mutant Mice Are Not Aggressive.
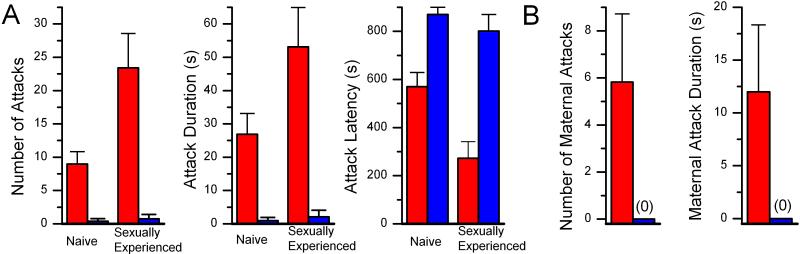
The observation that male trp2 mice exhibit mounting behaviors in response to intruder males contrasts with the behavior of control mice that aggressively attack intruders. We therefore examined aggression in trp2 animals more extensively in a resident–intruder assay. In this assay, a male intruder is added to the home cage of a singly housed resident test mouse. After a period of vigorous olfactory exploration, wild-type resident mice will initiate vigorous attacks against the intruder. Trp2 males exhibit a striking diminution in all parameters of aggressivity. In experiments with sexually inexperienced control mice, sniffing was followed by biting attacks, chasing, violent tumbling, and fighting. Trp2 mice exhibited the same olfactory exploratory behavior as wild-type mice, but rarely initiated biting attacks. Only 1 of 23 mutants (4.3%) exhibited aggressive attack behavior compared with 64% of controls (n = 25). All measures of aggressive behavior, including latency to first attack, attack frequency, and total attack duration, were significantly different between mutant and control mice (P < 0.0001; Fig. 3A). The number of bites, as well as the duration of attacks, was greater than 25-fold higher for control versus trp2 mutant animals.
Figure 3.
Lack of aggressive behavior in trp2−/− males and lactating females. (A) trp2−/− males are not aggressive in a resident–intruder assay. Trp2 (n = 23, blue bars) and control (n = 25, red bars) male mice were observed for behaviors elicited by the addition of a male to their home cage for 15 min prior to and after mating experience with females. Trp2 males were significantly different from control males by all measures of aggressive behavior (latency to attack, attack frequency and attack duration, P < 0.0001, Mann–Whitney U test). There was also a significant increase in all parameters of aggressive behavior after sexual experience in the control group (P < 0.05, Wilcoxon signed rank test), but sexual experience did not stimulate aggression in the trp2−/− group. (B) trp2−/− lactating females are not aggressive in a maternal aggression assay. Lactating trp2 (n = 11) and control (n = 11) mothers were tested for aggression toward intruder males in a 15-min test. Trp2 females were significantly different from control females by all measures of aggressive behavior (P < 0.05). Forty-five percent of control females responded aggressively toward intruder males on at least one of the test sessions, whereas trp2 females never initiated attacks.
The males used in this initial resident intruder assay were separated from female littermates at weaning and were therefore sexually inexperienced. Because exposure to females often increases male aggression (24), we next asked whether the absence of aggressive behavior in trp2 mutant mice persists after mating experience. Mutant and control mice were paired with breeder females for 10 days under conditions where verified ejaculation occurred. Control males exhibited a 3-fold (P < 0.001) increase in attack frequency, a 2-fold increase in attack duration, and a 2-fold decrease in latency after mating experience (Fig. 3A). Sexual experience, however, did not increase aggressive behavior in trp2 mutant mice (Fig. 3A). We also asked whether the docile trp2 males respond more aggressively to intruders after prior fighting experience. Trp2 and control males were paired with other males under conditions where all mice engaged in fighting (see marking and dominance assays below). At the end of the fighting regimen, resident intruder assays were performed. No enhancement in any parameter of aggressivity was observed in either control or mutant mice (data not shown). Thus, intermale aggression is virtually absent in trp2 mutants and cannot be enhanced by prior mating or fighting experience.
The Absence of Maternal Aggression in Trp2 Females.
Female mice are usually not aggressive toward intruders, but lactating females vigorously attack intruder males (25). We therefore examined maternal aggression in control (n = 11) and trp2 mutant (n = 11) females. Pregnant females were isolated in separate cages and allowed to produce litters. On postpartum days 5, 7, and 9, females were tested for maternal aggression by first removing the pups and then introducing an intruder male. Five of 11 control females exhibited intense aggressive attacks that, at the extreme, involved 33 separate bites in a 15-min session. In contrast, none of the 11 trp2 lactating females attacked intruder males over the course of three trials (Fig. 3B). These data indicate that both male and female trp2 mutant mice fail to exhibit aggressive responses to intruder males.
Defects in Territorial Marking in Trp2 Mutant Males.
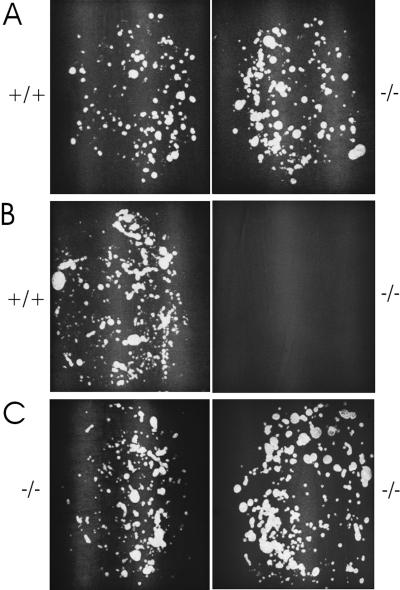
Mammals often employ marking behavior to communicate information about territorial boundaries or social status (26). In laboratory mice, marking is a male-specific, testosterone-dependent behavior in which dominant males will scatter the floor of the cage broadly with small marks of urine (21, 27). Subordinate males suppress marking and void their urine in large pools in the periphery (21). It is therefore possible to distinguish dominant from subordinate males by examining their marking patterns. In initial experiments, we asked whether marking behavior differs between mutant and control male mice. Before fighting or mating experiences, singly housed trp2 (n = 11) and control (n = 11) males were placed on opposite sides of a wire mesh barrier in a two-chambered cage lined with filter paper for 30 min. At the end of the test session, the urine marking patterns were visualized by UV transillumination. The marking rates of both trp2 and control males, before fighting experience, were similar and both revealed dominant patterns (Fig. 4A).
Figure 4.
trp2−/− males become subordinate to control males and exhibit altered territorial behavior when paired with other trp2 males. (A) trp2−/− and control males mark in a dominant fashion before fighting experience with each other. trp2−/− (n = 11) and control (n = 11) males, which had been singly housed, were placed on opposite sides of a wire mesh barrier in a two-chamber cage lined with filter paper for 30 min. The total number of marks by controls (89.8 ± 20.7) and mutants (91.1 ± 24.0) did not significantly differ. (B) trp2−/− males become subordinated and suppress marking after repetitive pairing with controls (n = 11 pairs). In 10 of 11 of cases the control was dominant over the trp2 mutant, as assessed by marking. (C) trp2−/− males do not form normal dominant–subordinate relationships. After repetitive pairing as in B, analysis of the marking patterns of mutant pairs (n = 6 pairs) reveals that in all cases, neither member of the pair suppressed marking behavior in the presence of the other. In contrast, analysis of marking patterns of control pairs (n = 5 pairs, not shown) demonstrated that in all cases one of the males became dominant over the other.
When the wire mesh separating a pair of control mice is removed, vigorous fighting ensues. After repetitive encounters, a victor emerges and a clear dominance relationship is established. The dominant member of the pair continues to mark with a dominant pattern, whereas the subordinate mouse suppresses marking in the victor's presence. When trp2 mutant males confront one another in this assay, fighting is rarely observed and instead male–male mounting occurs. These encounters between trp2 mice do not alter the initial marking behavior and both members of the pair continue to mark in a dominant pattern (Fig. 4C). Finally, when trp2 mutant and control mice were paired, fighting ensues with the control mouse emerging as victor in 10 of 11 encounters. This results in the suppression of dominant marking patterns in the mutant and persistent dominant territorial marking in the victorious control mice (Fig. 4B). Thus, trp2 mutant males fail to exhibit aggression and as a consequence fail to establish territorial dominance relationships.
Discussion
All animals have evolved a repertoire of innate behaviors. The innate behavioral arrays associated with mating and aggression, for example, are exhibited by all individuals within a species but often vary between species (28,29). The observation that stereotyped aggression or mounting occurs in naive animals suggests that the nervous system is wired to effect these social and reproductive behaviors without a major requirement for learning or experience. The existence of programmed neural circuits that govern innate behavioral repertoires implies that these behaviors must be tightly regulated to assure that they occur in an appropriate social context. The developmental and temporal control of behaviors can be mediated by internal regulators such as hormones and by external cues recognized by sensory systems.
One set of signals in the environment, the pheromones, is recognized by sensory neurons in the vomeronasal organ (18, 22). Pheromones are molecules that provide information about the social, sexual, and reproductive status of other individuals within a species and elicit neuroendocrine and behavioral responses. We have generated mice with a homozygous deficiency in trp2, a cation channel expressed predominantly in the VNO (14, 15). In these mutants, the VNO exhibits a dramatic reduction in the electrophysiological response to pheromone mixtures, allowing us to examine the regulatory role of the VNO in eliciting innate behavioral responses. Surprisingly, male–female mating behavior is unaltered in trp2 mutants but male–male mounting is now observed with far greater frequencies than in wild-type mice. Moreover, these mutants fail to exhibit aggressive behaviors in a variety of different assays. As a consequence, trp2 males assume the submissive role after encounters with control males. Similar behavioral consequences of a trp2 mutation were recently reported (30). We cannot at present determine whether the trp2 mutation results in a total loss of VNO function or whether a partial loss of function might elicit neomorphic phenotypes. Nonetheless, these data suggest that one important role of the VNO is the recognition of male-specific cues that regulate the expression of different behavioral repertoires.
Aggressive Behavior.
Aggressive behavior results from a complex interplay between innate hormonal regulators and environmental cues. In males, aggressive behaviors require androgens during a critical period of development, presumably to establish the requisite neural circuitry (31, 32). Circulating androgens are also required during adulthood to facilitate the aggressive response (32). In adult male red deer, for example, aggression is cyclical during the year and the aggressive cycle is causally related to changing androgen levels (33). In female mice, attack behaviors are largely restricted to periods of nursing, again revealing a hormonal dependence of aggressivity (25).
Superimposed on innate endocrine regulators are environmental cues that assure that aggressive behaviors are elicited only in appropriate situations. Our studies, along with previous ablation experiments (34–36), demonstrate that aggression in both sexes requires a functional VNO. Trp2 mutant mice do not exhibit characteristic aggressive responses to an intruder male and this docile behavior is not altered by fighting or mating experience. The simplest interpretation of these data are that males express a pheromone recognized by the VNO that elicits attack behavior in other mice. This behavioral response is innate but is regulated internally by hormonal status and externally by pheromones in the environment.
Sexual Behavior.
The repertoire of sexually dimorphic mating behaviors in mice is also innate; it is observed in naive animals without prior learning or experience. Similar to aggression, sexual behaviors are dependent both on hormonal status and environmental cues. In male mice, castration at birth or mutations in the androgen receptor (37) abolish the sexual response to females. Olfactory cues are also essential to elicit the male sexual response to assure that mating occurs in the appropriate social context. Efforts to discern the relative contribution of the two olfactory systems to mate recognition and mating behavior reveal a complex interplay between the “two noses.” Olfactory bulbectomy, which destroys input from both the main olfactory epithelium and the VNO, abolishes male mating behavior (6). However, disruption of the MOE alone or the surgical ablation of the VNO does not eliminate sexual behavior (38). Thus, the recognition of olfactory cues is obligatory for the expression of male sexual behaviors and these cues can activate both the main and vomeronasal olfactory systems. In accord with these conclusions, our studies demonstrate that genetic disruption of VNO function in trp2 mutants does not affect the male sexual response to female mice.
One striking behavior exhibited by trp2 mutants is frequent intermale mounting. In sexually experienced trp2 mutants, the frequency of male–male mounting is sixteen times greater than in control animals. Moreover, when trp2 mutants are simultaneously exposed to male and female partners, trp2 males continue to mount other males at a frequency far greater than is observed in control animals. One possible explanation for the frequency of intermale mounting in trp2 mutants is that it reflects a “displacement” behavior (39). The diminished aggression observed in trp2 mutants may reveal an increase in other behaviors, such as intermale mounting. This is unlikely, however, because mutants in other genes that show decreased aggression (40) do not exhibit this unusual sexual response.
One model consistent with our data argues that mounting is an innate behavior in males that is enhanced by female pheromones that activate the main olfactory systems and inhibited by male pheromones by VNO signaling. Trp2 males, therefore, show diminished ability to discriminate between the sexes and persist in mounting both males and females. Our data suggest that the VNO may be essential for the recognition of male pheromones that elicit aggressive behaviors and suppress mounting behaviors.
Supplementary Material
Acknowledgments
We thank Monica Mendelsohn, Adriana Nemes, and Xiao-Hong Li for advice and expert technical assistance; Nirao Shah for discussion and for help with the marking assay; Rene Hen and Sylvie Ramboz for help with the behavioral assays; Catherine Dulac for sharing data before publication; members of the Axel lab for much discussion and advice; Tom Jessell for critically reading the manuscript; and Phyllis Kisloff for preparation of the manuscript. This work was supported by the Howard Hughes Medical Institute and by a grant from the National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant NS29832 (to R.A.), National Institutes of Health/National Institute on Deafness and Other Communication Disorders Grants DC05249 (to F.Z.) and DC00347 (to T.L.-Z.), and National Institute of Mental Health Grant MH01888 (to C.R.Y.).
Abbreviations
- VNO
vomeronasal organ
- MOE
main olfactory epithelium
- EVG
electro-vomeronasogram
References
- 1.McCotter R E. Anat Rec. 1912;6:299–318. [Google Scholar]
- 2.Winans S S, Scalia F. Science. 1970;170:330–332. doi: 10.1126/science.170.3955.330. [DOI] [PubMed] [Google Scholar]
- 3.Halpern M. Annu Rev Neurosci. 1987;10:325–362. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- 4.Wysocki C J. Neurosci Biobehav Rev. 1979;3:301–341. doi: 10.1016/0149-7634(79)90015-0. [DOI] [PubMed] [Google Scholar]
- 5.Johnston R E. Ann NY Acad Sci. 1998;855:333–348. doi: 10.1111/j.1749-6632.1998.tb10592.x. [DOI] [PubMed] [Google Scholar]
- 6.Winans S S, Powers J B. Behav Biol. 1974;10:461–471. doi: 10.1016/s0091-6773(74)92043-4. [DOI] [PubMed] [Google Scholar]
- 7.Powers J B, Winans S S. Science. 1975;187:961–963. doi: 10.1126/science.1145182. [DOI] [PubMed] [Google Scholar]
- 8.Meredith M. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffer C A, Johnston R E. Physiol Behav. 1994;55:129–138. doi: 10.1016/0031-9384(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 10.Dorries K M, Adkins-Regan E, Halpern B P. Brain Behav Evol. 1997;49:53–62. doi: 10.1159/000112981. [DOI] [PubMed] [Google Scholar]
- 11.Hudson R, Distel H. Physiol Behav. 1986;37:123–128. doi: 10.1016/0031-9384(86)90394-x. [DOI] [PubMed] [Google Scholar]
- 12.Buck L, Axel R. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Gold G H. Nature (London) 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- 14.Liman E R, Corey D P, Dulac C. Proc Natl Acad Sci USA. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann T, Schaefer M, Schultz G, Gudermann T. Biochem J. 2000;351:115–122. doi: 10.1042/0264-6021:3510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colbert H A, Smith T L, Bargmann C I. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mombaerts P, Wang F, Dulac C, Chao S K, Nemes A, Mendelsohn M, Edmondson J, Axel R. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 18.Leinders-Zufall T, Lane A P, Puche A C, Ma W, Novotny M V, Shipley M T, Zufall F. Nature (London) 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- 19.McClinic J R. The Physiology of the Human Body. New York: Wiley; 1978. [Google Scholar]
- 20.Svare B, Betteridge C, Katz D, Samuels O. Physiol Behav. 1981;26:253–258. doi: 10.1016/0031-9384(81)90020-2. [DOI] [PubMed] [Google Scholar]
- 21.Desjardins C, Maruniak J A, Bronson F H. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 22.Holy T E, Dulac C, Meister M. Science. 2000;289:1569–1572. doi: 10.1126/science.289.5484.1569. [DOI] [PubMed] [Google Scholar]
- 23.Stone C P. J Comp Psychol. 1922;2:95–153. [Google Scholar]
- 24.Goyens J, Noirot E. Dev Psychobiol. 1975;8:79–84. doi: 10.1002/dev.420080111. [DOI] [PubMed] [Google Scholar]
- 25.Gandelman R. Horm Behav. 1972;3:23–28. doi: 10.1016/0018-506x(72)90003-7. [DOI] [PubMed] [Google Scholar]
- 26.Ralls K. Science. 1971;171:443–449. doi: 10.1126/science.171.3970.443. [DOI] [PubMed] [Google Scholar]
- 27.Maruniak J A, Desjardins C, Bronson F H. Am J Physiol. 1977;233:E495–E499. doi: 10.1152/ajpendo.1977.233.6.E495. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz K. On Aggression. New York: Harcourt Brace Jovanovich; 1966. [Google Scholar]
- 29.Beach F A. Hormones and Behavior. New York: Paul B. Hoeber; 1948. [Google Scholar]
- 30.Stowers L, Holy T E, Meister M, Dulac C, Koentges G. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 31.Bronson F H, Desjardins C. Endocrinology. 1969;85:971–974. doi: 10.1210/endo-85-5-971. [DOI] [PubMed] [Google Scholar]
- 32.Edwards D A. Physiol Behav. 1969;4:333–338. [Google Scholar]
- 33.Lincoln G A, Guiness F, Short R V. Horm Behav. 1972;3:375–396. [Google Scholar]
- 34.Clancy A N, Coquelin A, Macrides F, Gorski R A, Noble E P. J Neurosci. 1984;4:2222–2229. doi: 10.1523/JNEUROSCI.04-09-02222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bean N J. Physiol Behav. 1982;29:433–437. doi: 10.1016/0031-9384(82)90262-1. [DOI] [PubMed] [Google Scholar]
- 36.Maruniak J A, Wysocki C J, Taylor J A. Physiol Behav. 1986;37:655–657. doi: 10.1016/0031-9384(86)90300-8. [DOI] [PubMed] [Google Scholar]
- 37.Ohno S, Geller L N, Lai EVY. Cell. 1974;4:235–242. doi: 10.1016/0092-8674(74)90137-8. [DOI] [PubMed] [Google Scholar]
- 38.Winans S S, Powers J B. Brain Res. 1977;126:325–344. doi: 10.1016/0006-8993(77)90729-6. [DOI] [PubMed] [Google Scholar]
- 39.Bastock M, Morris D, Moynihan M. Behavior. 1953;6:66–84. [Google Scholar]
- 40.Miczek K A, Maxson S C, Fish E W, Faccidomo S. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.