Abstract
This study investigated the natural bioactive compounds in Polygonum cuspidatum Sieb. et Zucc. (P. cuspidatum) by fractionating a 70% ethanol extract using n-hexane, chloroform, ethyl acetate, n-butanol, and water. The total polyphenol and flavonoid contents of each fraction were determined, and their antioxidant activities were evaluated using DPPH, ABTS, and FRAP assays. Additionally, the anti-diabetic potential was assessed via α-glucosidase inhibitory activity, while anti-obesity activity was evaluated using lipase inhibitory activity. The fractions were also tested for tyrosinase and elastase inhibitory activities to assess their skin-whitening and anti-wrinkle potential, and their antibacterial activity against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa was determined using the agar diffusion method. Finally, bioactive compounds were identified and quantified using HPLC and GC–MSD. The results showed that the ethyl acetate fraction possessed the highest total polyphenol content (0.53 ± 0.01 g GAE/g) and total flavonoid content (0.19 ± 0.02 g QE/g). It also exhibited strong antioxidant activity, with the lowest DPPH radical scavenging IC50 (0.01 ± 0.00 mg/mL), ABTS radical scavenging IC50 (0.06 ± 0.00 mg/mL), and the highest FRAP value (6.02 ± 0.30 mM Fe2+/mg). Moreover, it demonstrated potent enzyme inhibitory activities, including tyrosinase inhibitory activity (67.78 ± 2.50%), elastase inhibitory activity (83.84 ± 1.64%), α-glucosidase inhibitory activity (65.14 ± 10.29%), and lipase inhibitory activity (85.79 ± 1.04%). In the antibacterial activity, the ethyl acetate fraction produced a clear inhibitory zone of 19.50 mm against Staphylococcus aureus, indicating notable antibacterial activity. HPLC-PDA and GC–MSD analyses identified tannic acid and emodin as the major bioactive constituents. These findings suggest that the ethyl acetate fraction of P. cuspidatum extract, rich in polyphenol and flavonoid compounds, is a promising natural source of bioactive ingredients for applications in the food, pharmaceutical, and cosmetic industries. Further research is needed to explore its mechanisms and therapeutic applications.
Keywords: Polygonum cuspidatum Sieb. et Zucc., antioxidant activity, enzyme inhibitory activity, chromatographic analyses
1. Introduction
Polygonum cuspidatum Sieb. et Zucc. (P. cuspidatum), a perennial herb of the Polygonaceae family, is widely distributed across East Asia, including China, Japan, and Korea [1]. The dried rhizomes of this plant, known as P. cuspidatum, have been traditionally used in Chinese medicine for their effects in clearing heat and detoxifying, promoting diuresis, removing jaundice, and activating blood circulation to remove blood stasis. Clinically, it is commonly employed to treat liver and gallbladder diseases, rheumatic pain, and menstrual disorders [2]. With increasing research into natural medicines and plant resources, the active ingredients of P. cuspidatum and their multitarget pharmacological mechanisms have become a research focus. Current studies have shown that P. cuspidatum is rich in anthraquinones, such as emodin, chrysophanol, and rhein; stilbenes like resveratrol; flavonoids such as quercetin, kaempferol, and isoquercitrin; terpenoids like β-sitosterol and campesterol; as well as tannins and polyphenols [3,4,5,6]. These components have demonstrated significant antioxidant, anti-inflammatory, antibacterial, anticancer, metabolic-regulating, and skin-whitening activities in both in vitro and in vivo experiments [7,8]. Among these, antioxidant activity is considered the core mechanism underlying the diverse pharmacological effects of P. cuspidatum. Its primary mechanisms include the clearance of excessive reactive oxygen species (ROS), enhancement of superoxide dismutase (SOD) and glutathione (GSH) activities, and inhibition of lipid peroxidation, which significantly alleviates oxidative damage in HepG2 cells [9]. Moreover, resveratrol, an active compound in P. cuspidatum, induces autophagy and inhibits α-MSH induced melanogenesis, thereby achieving a whitening effect [10]. Furthermore, P. cuspidatum shows significant potential in natural medicine research. Its major active compounds, including resveratrol, emodin, and polydatin, inhibit xanthine oxidase activity, reduce blood uric acid levels, and exhibit antihyperuricemic effects while regulating the onset and pathological progression of chronic metabolic diseases through multiple mechanisms [11]. The highly diverse chemical composition of P. cuspidatum leads to the presence of numerous inactive impurities in its crude extracts, potentially affecting the accuracy of biological activity studies. Over the past few decades, research on the extraction of phenolic compounds from plants has primarily focused on advanced purification techniques such as solid-phase extraction (SPE), countercurrent chromatography (CCC), and centrifugal partition chromatography (CPC). However, these methods, while efficient in compound separation, are often complicated and associated with high operational costs [12,13]. In contrast, solvent fractionation has been widely applied in natural product research due to its efficiency, ease of operation, and low cost, particularly in polarity classification and preliminary purification [14]. Therefore, this study employed solvent fractionation, sequentially using n-hexane, chloroform, ethyl acetate, n-butanol, and water, to achieve the preliminary separation of components with different polarities. This method reduces interference from inactive impurities and improves the purity of the target compounds, providing a more reliable foundation for subsequent biological activity evaluation and component analysis.
2. Results
2.1. Total Polyphenol and Flavonoid Contents of Solvent Fractions from P. cuspidatum
Phenolic compounds are important secondary metabolites widely present in plants, commonly found in fruits, vegetables, grains, and medicinal herbs. The phenolic hydroxyl groups (–OH) in their structures enable them to scavenge reactive oxygen species (ROS), thereby mitigating oxidative damage, delaying aging, and promoting overall health [15]. This class includes flavonoids, anthocyanins, tannins, catechins, isoflavones, lignans, and resveratrol, all of which exhibit diverse biological activities such as antioxidant, antibacterial, anticancer, hepatoprotective, and lipid-lowering effects. Among them, flavonoids, characterized by a flavone backbone, are key pigments in leaves, flowers, fruits, and stems and play multiple roles in plants by absorbing ultraviolet radiation, defending against pathogens and herbivores, regulating growth and development, and modulating enzyme activity [16]. In this study, we measured the total polyphenol content in the solvent fractions of P. cuspidatum using a gallic acid standard curve, and the results are presented in Table 1. The ethyl acetate fraction exhibited the highest total polyphenol content (0.53 ± 0.01 g GAE/g), followed by the chloroform fraction (0.37 ± 0.01 g GAE/g), the n-butanol fraction (0.36 ± 0.02 g GAE/g), the n-hexane fraction (0.12 ± 0.00 g GAE/g), and the aqueous fraction (0.10 ± 0.01 g GAE/g), showing a statistically significant decreasing trend (p < 0.05). Additionally, we measured the total flavonoid content in the solvent fractions using a quercetin standard curve, and these results are also presented in Table 1. The ethyl acetate fraction exhibited the highest total flavonoid content (0.19 ± 0.02 g QE/g), followed by the n-butanol fraction (0.14 ± 0.01 g QE/g), the chloroform fraction (0.09 ± 0.00 g QE/g), the n-hexane fraction (0.08 ± 0.00 g QE/g), and the aqueous fraction (0.04 ± 0.00 g QE/g), also showing a statistically significant decreasing trend (p < 0.05). The significantly higher total polyphenol and flavonoid contents in the ethyl acetate fraction can be primarily attributed to its intermediate polarity, which offers optimal solubility and strong affinity for moderately polar phenolic compounds containing both hydrophilic hydroxyl groups and hydrophobic aromatic rings [17]. These findings indicate that ethyl acetate is the most effective solvent for extracting polyphenolic compounds, including flavonoids, from P. cuspidatum, making it particularly suitable for the isolating of bioactive phenolic constituents. Moreover, previous studies on medicinal plants such as Curcuma longa (turmeric) have also demonstrated the high efficiency of ethyl acetate in extracting phenolic and flavonoid compounds, further supporting the present findings [18]. This reinforces the role of ethyl acetate as a medium-polarity solvent capable of selectively enriching valuable phytochemicals and underscores the importance of solvent selection based on compound polarity in the development of natural products and strategies for functional ingredient extraction.
Table 1.
Total polyphenol and flavonoid contents of solvent fractions from P. cuspidatum.
| Solvent | Total Polyphenol Content (g GAE/g) 1 |
Total Flavonoid Content (g QE/g) 2 |
|---|---|---|
| n-hexane fraction | 0.12 ± 0.00 c34 | 0.08 ± 0.00 c34 |
| chloroform fraction | 0.37 ± 0.01 b | 0.09 ± 0.00 c |
| ethyl acetate fraction | 0.53 ± 0.01 a | 0.19 ± 0.02 a |
| n-butanol fraction | 0.36 ± 0.02 b | 0.14 ± 0.01 b |
| aqueous fraction | 0.10 ± 0.01 d | 0.04 ± 0.00 d |
1 GAE: gallic acid equivalent mg/g. 2 QE: quercetin equivalent mg/g. 3 Mean ± SD (n = 3). 4 a–d Means Duncan’s different letters within a column differ significantly (p < 0.05).
2.2. Antioxidant Activities (DPPH, ABTS Radical Scavenging Activity, and Ferric Reducing Antioxidant Power (FRAP) Assay) of Solvent Fractions from P. cuspidatum
The DPPH radical scavenging assay is a widely used in vitro method to evaluate the antioxidant capacity of compounds as it reflects the ability of antioxidants to donate hydrogen atoms or electrons to neutralize free radicals [19]. This method is based on the ability of antioxidants to scavenge stable DPPH radicals. The ABTS radical scavenging assay assesses antioxidant capacity by measuring the neutralization of ABTS+ radicals generated by potassium persulfate [20]. The FRAP assay quantifies the total reducing power of a sample by evaluating the ability of antioxidants to reduce ferric-TPTZ (Fe3+-TPTZ) to ferrous-TPTZ (Fe2+-TPTZ) [21]. The DPPH radical scavenging activity of solvent fractions from P. cuspidatum, expressed by the IC50 values, is presented in Table 2. The ethyl acetate fraction and L-ascorbic acid exhibited the highest antioxidant activities (0.01 ± 0.00 mg/mL), followed by the n-butanol fraction (0.02 ± 0.00 mg/mL), the chloroform fraction (0.07 ± 0.00 mg/mL), the aqueous fraction (0.10 ± 0.01 mg/mL), and the n-hexane fraction (0.25 ± 0.20 mg/mL), showing a statistically significant decreasing trend (p < 0.05). The ABTS radical scavenging activity of solvent fractions from P. cuspidatum, expressed by the IC50 values, is presented in Table 2. The ethyl acetate fraction and L-ascorbic acid exhibited the highest antioxidant activities (0.06 ± 0.00 mg/mL), followed by the n-butanol fraction (0.13 ± 0.00 mg/mL), the chloroform fraction (0.14 ± 0.00 mg/mL), the aqueous fraction (0.51 ± 0.00 mg/mL), and the n-hexane fraction (0.64 ± 0.19 mg/mL), showing a statistically significant decreasing trend (p < 0.05). The ferric reducing antioxidant power (FRAP) assay of solvent fractions from P. cuspidatum is presented in Table 2. The ethyl acetate fraction exhibited the highest antioxidant activity (6.02 ± 0.30 mM Fe2+/mg), followed by the n-butanol fraction (2.95 ± 0.57 mM Fe2+/mg), the chloroform fraction (1.84 ± 0.14 mM Fe2+/mg), the aqueous fraction (0.32 ± 0.02 mM Fe2+/mg), and the n-hexane fraction (0.22 ± 0.05 mM Fe2+/mg), showing a statistically significant decreasing trend (p < 0.05). The differences in antioxidant activity among the solvent fractions of P. cuspidatum can primarily be attributed to variations in the electron or hydrogen donation capabilities of phenolic compounds, which play a crucial role in scavenging free radicals and reducing oxidants [22]. Polyphenolic compounds are well known for their redox properties, effectively neutralizing reactive species by donating electrons or hydrogen atoms, thereby interrupting oxidative chain reactions and protecting biological macromolecules from oxidative damage [23]. Fractions extracted using medium-polarity solvents, such as ethyl acetate and n-butanol, exhibited higher antioxidant activities, indicating that these solvents can selectively enrich compounds with strong redox activity. Moreover, similar solvent polarity-dependent extraction profiles have been reported in other medicinal plants, such as Leucaena leucocephala, where ethyl acetate demonstrated high efficiency in extracting polyphenolic and flavonoid compounds [24]. These bioactive compounds typically contain hydroxyl groups attached to aromatic rings, which confer superior free radical scavenging abilities. Collectively, these findings underscore the importance of rational solvent selection based on polarity to enhance the extraction efficiency and quality of natural antioxidants from plant materials.
Table 2.
DPPH and ABTS radical scavenging activities and ferric reducing antioxidant power (FRAP) values of solvent fractions from P. cuspidatum.
| Solvent | DPPH Radical Scavenging Activity IC50 (mg/mL) 1 |
ABTS Radical Scavenging Activity IC50 (mg/mL) 1 |
FRAP Value (mM Fe2+/mg) |
|---|---|---|---|
| n-hexane fraction | 0.25 ± 0.20 a23 | 0.64 ± 0.19 a23 | 0.22 ± 0.05 d23 |
| chloroform fraction | 0.07 ± 0.00 c | 0.14 ± 0.00 c | 1.84 ± 0.14 c |
| ethyl acetate fraction | 0.01 ± 0.00 d | 0.06 ± 0.00 d | 6.02 ± 0.30 a |
| n-butanol fraction | 0.02 ± 0.00 d | 0.13 ± 0.00 c | 2.95 ± 0.57 b |
| aqueous fraction | 0.10 ± 0.01 b | 0.51 ± 0.00 b | 0.32 ± 0.02 d |
| L-ascorbic acid | 0.01 ± 0.00 d | 0.06 ± 0.00 d | - |
1 Inhibitory activity was expressed as the mean of the 50% inhibitory concentration from triplicate determinations, obtained by interpolation of the concentration-inhibition curve. 2 Mean ± SD (n = 3). 3 a–d Means Duncan’s different letters within a column differ significantly (p < 0.05).
2.3. Tyrosinase and Elastase Inhibitory Activities of Solvent Fractions from P. cuspidatum
Tyrosinase plays a crucial role in regulating skin pigmentation by mediating melanin synthesis and is a key target for improving skin health [25]. This study evaluated the inhibitory effects of various solvent fractions from P. cuspidatum on tyrosinase activity. As shown in Table 3, at a concentration of 0.1 mg/mL, the positive control, Kojic acid, exhibited an inhibitory activity of 69.26 ± 0.10%. Among the solvent fractions, the ethyl acetate fraction exhibited the highest inhibitory activity, with a rate of 67.78 ± 2.50%, followed by the n-butanol fraction (61.64 ± 0.65%), chloroform fraction (59.93 ± 1.95%), aqueous fraction (56.31 ± 0.86%), and n-hexane fraction (53.60 ± 1.61%), showing a statistically significant decreasing trend (p < 0.05). Ethyl acetate, as a medium-polar solvent, effectively extracts medium-polar compounds from P. cuspidatum, such as resveratrol, emodin, and piceid. These compounds are closely associated to tyrosinase inhibitory activity [26]. The polarity of ethyl acetate allows it to interact effectively with the structures of these compounds, thereby enhancing their bioactivity and resulting in the strongest enzyme inhibitory activity [27]. Consequently, the ethyl acetate fraction demonstrates significant potential for skin whitening, particularly in applications aimed at inhibiting melanin production.
Table 3.
Tyrosinase and elastase inhibitory activities of solvent fractions from P. cuspidatum.
| Solvent | Tyrosinase Inhibitory Activity (%) |
Elastase Inhibitory Activity (%) |
|---|---|---|
| n-hexane fraction | 59.93 ± 1.95 c14 | 68.69 ± 1.31 bc14 |
| chloroform fraction | 53.60 ± 1.61 d | 64.65 ± 1.50 c |
| ethyl acetate fraction | 67.78 ± 2.50 a | 83.84 ± 1.64 a |
| n-butanol fraction | 61.64 ± 0.65 b | 74.75 ± 1.74 b |
| aqueous fraction | 56.31 ± 0.86 cd | 72.73 ± 0.60 bc |
| standard | 69.26 ± 0.10 e2 | 63.64 ± 0.59 c3 |
1 Mean ± SD (n = 3). 2 Kojic acid. 3 L-Ascorbic acid. 4 a–e Means Duncan’s different letters within a column differ significantly (p < 0.05).
Elastase is an enzyme responsible for the degradation of elastin, which plays a critical role in maintaining skin elasticity. This study evaluated the inhibitory effects of different solvent fractions from P. cuspidatum on elastase activity. As shown in Table 3, at a concentration of 0.1 mg/mL, the positive control, L-ascorbic acid, exhibited an inhibitory activity of 63.64 ± 0.59%. The ethyl acetate fraction exhibited the strongest elastase inhibitory activity, reaching 83.84 ± 1.64%, followed by the n-butanol fraction (74.75 ± 1.74%), aqueous fraction (72.2 ± 0.60%), n-hexane fraction (68.69 ± 1.31%), and chloroform fraction (64.65 ± 1.50%), with a statistically significant decreasing trend (p < 0.05). The potent elastase inhibitory activity of the ethyl acetate fraction may be attributed to its content of medium-polar compounds, such as resveratrol, emodin, and piceid. High-performance liquid chromatography coupled with mass spectrometry (HPLC-Q-TOF-MS/MS) [28] has shown that these compounds are closely associated with elastase inhibitory activity. Therefore, the ethyl acetate fraction not only holds potential for skin whitening but also plays a significant role in anti-aging and maintaining skin elasticity.
2.4. α-Glucosidase and Lipase Inhibitory Activities of Solvent Fractions from P. cuspidatum
α-Glucosidase and lipase are involved in the digestion and absorption of carbohydrates and lipids, respectively, and their enzymatic activities are closely associated with metabolic disorders such as obesity and type 2 diabetes. The inhibitory activity of α-glucosidase can delay glucose absorption and reduce postprandial blood glucose levels, while lipase inhibitory activity helps decrease dietary fat absorption. Both mechanisms are considered effective strategies for regulating metabolic health [29,30]. The α-glucosidase inhibitory activity of solvent fractions from P. cuspidatum is presented in Table 4. At the test concentration of 0.1 mg/mL, the positive control acarbose showed 34.83 ± 0.31% inhibitory activity. Among the fractions, the ethyl acetate fraction exhibited the highest inhibitory activity (65.14 ± 0.29%), followed by the n-butanol fraction (35.87 ± 0.56%), the chloroform fraction (12.87 ± 0.15%), the aqueous fraction (6.46 ± 0.40%), and the n-hexane fraction (5.56 ± 0.59%), showing a statistically significant decreasing trend (p < 0.05). The lipase inhibitory activity of solvent fractions from P. cuspidatum is presented in Table 4. At the test concentration of 0.1 mg/mL, the positive control orlistat showed 98.18 ± 0.18% inhibitory activity. Among the fractions, the n-hexane fraction exhibited the highest inhibitory activity (85.93 ± 1.00%), followed by the ethyl acetate fraction (85.79 ± 0.61%), the n-butanol fraction (84.35 ± 0.77%), the aqueous fraction (79.99 ± 0.70%), and the chloroform fraction (78.78 ± 2.16%), showing a statistically significant decreasing trend (p < 0.05). The ethyl acetate fraction exhibited the highest α-glucosidase inhibitory activity, significantly surpassing the other fractions (p < 0.05). This inhibitory effect is likely attributed to moderately polar phenolic and flavonoid compounds, such as polydatin and resveratrol, which can bind to key amino acid residues within the enzyme’s active site. This binding either blocks substrate access or induces conformational changes, resulting in competitive or non-competitive inhibitory activity [31]. Molecular docking studies further corroborate these interactions, demonstrating strong binding affinities between these compounds and the catalytic residues of tyrosinase and elastase, thereby elucidating the potential molecular mechanisms underlying their enzyme inhibitory activities [32]. Regarding lipase inhibitory activity, the n-hexane, ethyl acetate, and n-butanol fractions exhibited comparable inhibitory activity rates of 85.93%, 85.79%, and 84.35%, respectively (p > 0.05). These fractions are believed to contain non-polar terpenoids, anthraquinone derivatives such as emodin-8-O-glucoside, and esterified stilbenoids like polydatin, which likely interact with the enzyme’s catalytic site, interfering with substrate hydrolysis [33]. Overall, the varying polarity of chemical constituents in P. cuspidatum suggests multiple mechanisms of enzyme inhibitory activity, highlighting its potential as a natural multi-target metabolic modulator.
Table 4.
α-Glucosidase and lipase inhibitory activities of solvent fractions from P. cuspidatum.
| Solvent | α-Glucosidase Inhibitory Activity (%) |
Lipase Inhibitory Activity (%) |
|---|---|---|
| n-hexane fraction | 5.560 ± 0.59 d14 | 85.93 ± 1.00 b14 |
| chloroform fraction | 12.87 ± 0.15 c | 78.78 ± 2.16 c |
| ethyl acetate fraction | 65.14 ± 0.29 a | 85.79 ± 0.61 b |
| n-butanol fraction | 35.87 ± 0.56 b | 84.35 ± 0.77 b |
| aqueous fraction | 6.460 ± 0.40 d | 79.99 ± 0.70 c |
| standard | 34.83 ± 0.31 b2 | 98.18 ± 0.18 a3 |
1 Mean ± SD (n = 3). 2 Acarbose. 3 Orlistat. 4 a–d Means Duncan’s different letters within a column differ significantly (p < 0.05).
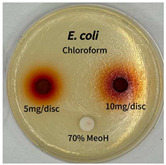
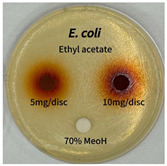
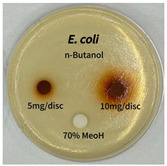
2.5. Antibacterial Activity of Solvent Fractions from P. cuspidatum Broth Against Selected Microbial Strains
Plant-derived antibacterial agents are mainly categorized as phenolic acids, polyphenols, quinones, flavonoids, flavonols, tannins, terpenoids, and lectins [34]. Although the precise antibacterial mechanisms of these natural products have not been fully elucidated, studies have shown that phenolics and flavonoids inhibit microbial growth by binding essential nutrients such as water and inorganic salts [35]. Additionally, quinones, particularly anthraquinone derivatives, exert antibacterial effects by generating reactive oxygen species (ROS) through redox cycling, which leads to damage to microbial DNA, proteins, and cell membranes [36]. Microorganisms are generally classified into bacteria and fungi. Bacteria are classified as Gram-positive or Gram-negative based on differences in their cell wall structure. Gram-positive bacteria possess a thicker peptidoglycan layer, whereas Gram-negative bacteria have a thinner one. These structural differences result in varying susceptibilities to antibacterial agents [37,38]. Such structural variations may contribute to the differential antibacterial effects observed for P. cuspidatum extracts against various microbial strains. The antibacterial activity of solvent fractions from P. cuspidatum is presented in Table 5 and Table 6. At a concentration of 5 mg/disc, the ethyl acetate, n-hexane, and aqueous fractions exhibited antibacterial activity against Staphylococcus aureus. At 10 mg/disc, the ethyl acetate fraction showed antibacterial activity against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. In addition, the ethyl acetate, aqueous, n-hexane, and n-butanol fractions consistently exhibited antibacterial activity against Staphylococcus aureus. HPLC-PDA analysis in this study revealed that although the chloroform fraction contained the highest concentration of emodin and relatively low levels of tannic acid, the ethyl acetate fraction exhibited the strongest antibacterial activity. This observation suggests that antibacterial efficacy is not solely dependent on the concentration of individual compounds but may also be influenced by potential synergistic interactions among multiple bioactive constituents. The hydrophobic components in the chloroform fraction may inhibit the release and diffusion of emodin, whereas the matrix of the ethyl acetate fraction appears to facilitate the dissolution and dispersion of various active compounds, including polyphenols and flavonoids, thereby enhancing the overall antibacterial effect. Previous studies have shown that polyphenol compounds such as resveratrol and tannic acid exert antibacterial effects by disrupting bacterial membranes, inhibiting key enzymatic activities, or inducing oxidative stress responses [39]. Moreover, ethyl acetate, a medium-polarity solvent, has polarity characteristics that facilitate the extraction and stabilization of amphiphilic bioactive components. This increases their solubility in aqueous environments and enhances their permeability across cell membranes, thereby improving their bioavailability and antibacterial potency. In summary, these findings emphasize the importance of the polarity distribution of chemical constituents in plant extracts, as well as their compatibility with the polarity of the extraction solvent, in determining antibacterial efficacy. This provides valuable theoretical support for the functional development of natural products.
Table 5.
Antibacterial activity date of solvent fractions from P. cuspidatum.
| Microorganism | Solvent | 5 mg/disc | 10 mg/disc |
|---|---|---|---|
| Staphylococcus aureus | n-hexane fraction | 10.0 1 | 12.0 |
| chloroform fraction | - 2 | - | |
| ethyl acetate fraction | 17.0 | 19.5 | |
| n-butanol fraction | - | 10.0 | |
| aqueous fraction | 9.50 | 12.5 | |
| Escherichia coli | n-hexane fraction | - | - |
| chloroform fraction | - | - | |
| ethyl acetate fraction | - | 10.0 | |
| n-butanol fraction | - | - | |
| aqueous fraction | - | - | |
| Pseudomonas aeruginosa | n-hexane fraction | - | - |
| chloroform fraction | - | - | |
| ethyl acetate fraction | - | 10.0 | |
| n-butanol fraction | - | - | |
| aqueous fraction | - | - |
1 Size of clear zone (mm). 2 Not detected.
Table 6.
Graphical representation of the antibacterial activities of solvent fractions from P. cuspidatum.
| Microorganism | n-Hexane Fraction | Chloroform Fraction | Ethyl Acetate Fraction | n-Butanol Fraction | Aqueous Fraction |
|---|---|---|---|---|---|
| Staphylococcus aureus |

|

|

|

|

|
| Escherichia coli |

|
|
|
|

|
| Pseudomonas aeruginosa |

|

|

|

|

|
2.6. High-Performance Liquid Chromatography with Photodiode Array Detection (HPLC-PDA) Quantification of Tannic Acid and Emodin
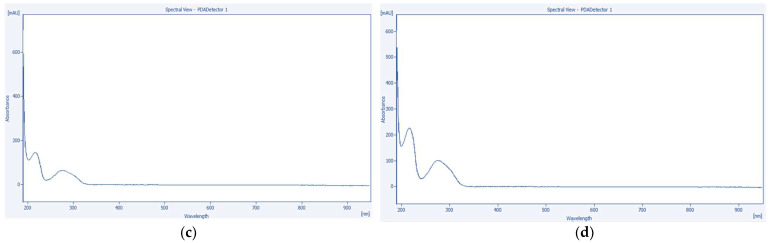
Quantitative analysis of tannic acid and emodin in the solvent fractions of P. cuspidatum was performed using HPLC-PDA. The retention times of tannic acid and emodin were 1.71 min and 8.01 min, respectively. Both analytes exhibited baseline separation with symmetrical peak shapes and no tailing, indicating excellent chromatographic resolution and robust method performance. Representative chromatograms and quantification results are shown in Figure 1 and Figure 2.
Figure 1.
Chromatographic and photodiode array (PDA) spectral data of tannic acid: (a) HPLC chromatogram of the tannic acid standard solution showing 3 peaks; (b) chromatogram of the P. cuspidatum solvent fractions showing 13 peaks; (c) PDA spectrum of the tannic acid standard; and (d) PDA spectrum of the corresponding sample peak. The identical spectral profiles confirm the presence of tannic acid in the sample matrix.
Figure 2.
Chromatographic and photodiode array (PDA) spectral data of emodin: (a) HPLC chromatogram of the emodin standard solution showing 6 peaks; (b) chromatogram of the P. cuspidatum solvent fractions showing 8 peaks; (c) PDA spectrum of the emodin standard; and (d) PDA spectrum of the corresponding sample peak. The identical spectral profiles confirm the presence of emodin in the sample matrix.
2.6.1. Evaluation of Linearity, Sensitivity, and Precision
Systematic validation of the developed HPLC-PDA method was performed for linearity, sensitivity, and precision. As summarized in Table 7, tannic acid and emodin exhibited excellent linearity over the concentration range of 25 to 500 ppm, with correlation coefficients (R2) of 0.9999 and 0.9995, respectively. The limits of detection (LOD) were 9.24 ppm for tannic acid and 1.82 ppm for emodin, while the limits of quantification (LOQs) were 27.94 ppm and 5.81 ppm, respectively. The relative standard deviations (RSDs) for replicate measurements were below 0.01%, indicating excellent repeatability. Chromatographic analysis showed retention times of 1.72 min for tannic acid (detected at 270 nm) and 7.96 min for emodin (detected at 254 nm), both achieving baseline separation with symmetrical peak shapes and no significant tailing. These findings confirm that the method can reliably quantify the target compounds in P. cuspidatum solvent fractions accurately.
Table 7.
Retention times, linear range, calibration curves, limits of detection (LODs), and limits of quantitation (LOQs) of tannic acid and emodin compounds.
| Parameter | Tannic Acid | Emodin |
|---|---|---|
| λ 1 (nm) | 270 | 254 |
| tR 2 (min) | 1.72 | 7.96 |
| RSD 3 (%) | 0.01 | 0.01 |
| Linear range (ppm) | 25–500 | 25–500 |
| Regression equation 4 | Y = 30.43845X − 550.88152 | Y = 3.94432X + 33.84235 |
| r 2 5 | 0.9999 | 0.9995 |
| LOD (ppm) | 9.24 | 1.82 |
| LOQ (ppm) | 27.94 | 5.81 |
1 Detection wavelength. 2 Retention times. 3 Relative standard deviation. 4 Y: peak area, X: concentration (ppm). 5 Coefficient of determination.
2.6.2. Evaluation of Method Precision and Accuracy
Systematic validation of precision and accuracy was conducted for the HPLC-PDA analytical method. As shown in Table 8, tannic acid exhibited excellent repeatability, with intra-day and inter-day relative standard deviations (RSDs) not exceeding 0.03% and 0.01%, respectively. Emodin also demonstrated strong repeatability across all tested concentrations, with intra-day RSD ≤ 0.38% and inter-day RSD ≤ 0.11%. In both cases, inter-day precision was slightly superior to intra-day values, indicating stable method performance over time. Regarding accuracy, tannic acid showed recovery values ranging from 81.78% to 91.82% at 25 ppm, which increased to a range of 94.59% to 103.5% at 50 and 75 ppm. Emodin maintained consistent accuracy across all concentration levels, with recoveries ranging from 99.19% to 109.8%. These results confirm that the developed HPLC-PDA method possesses satisfactory reproducibility and reliability and is suitable for the quantitative determination of target compounds in P. cuspidatum solvent fractions.
Table 8.
Precision and accuracy of the HPLC-PDA method for tannic acid and emodin compounds.
| Compound | Conc. 1 (ppm) |
Analysis type | Measured Conc. 2 (ppm) |
RSD 3 (%) |
Accuracy 4 (%) |
RSD (%) |
|---|---|---|---|---|---|---|
| Tannic acid | 25 | Intra-day (n = 3) |
27.53 | 0.03 | 81.78 | 0.01 |
| 50 | 45.45 | 0.02 | 97.60 | 0.03 | ||
| 75 | 73.80 | 0.03 | 103.5 | 0.04 | ||
| 25 | Inter-day (n = 3) |
29.14 | 0.01 | 91.82 | 0.02 | |
| 50 | 47.96 | 0.01 | 94.59 | 0.01 | ||
| 75 | 72.30 | 0.00 | 102.2 | 0.00 | ||
| Emodin | 25 | Intra-day (n = 3) |
23.23 | 0.38 | 99.19 | 0.57 |
| 50 | 49.80 | 0.29 | 106.3 | 0.23 | ||
| 75 | 78.14 | 0.16 | 101.0 | 0.18 | ||
| 25 | Inter-day (n = 3) |
24.17 | 0.05 | 102.0 | 0.09 | |
| 50 | 50.49 | 0.05 | 109.8 | 0.06 | ||
| 75 | 79.88 | 0.09 | 107.4 | 0.11 |
1 Theoretical concentration of the compound. 2 Measured concentration of the compound. 3 Relative standard deviation. 4 Percentage recovery of the compound.
2.6.3. Quantitative Analysis of Tannic Acid and Emodin by HPLC-PDA
In this study, tannic acid and emodin were quantified in different solvent fractions of P. cuspidatum using HPLC-PDA; the results are summarized in Table 9. Tannic acid was found at the highest concentration in the ethyl acetate fraction (0.272 ± 0.004 mg/g), followed by the chloroform fraction (0.105 ± 0.005 mg/g). Lower concentrations were observed in the n-hexane (0.028 ± 0.002 mg/g) and n-butanol (0.044 ± 0.002 mg/g) fractions, while no tannic acid was detected in the aqueous fraction. Emodin was most abundant in the chloroform fraction (0.270 ± 0.019 mg/g), with moderate levels in the n-hexane fraction (0.103 ± 0.003 mg/g). Only trace amounts were found in the ethyl acetate (0.012 ± 0.002 mg/g) and n-butanol (0.011 ± 0.003 mg/g) fractions, and it was not detected in the aqueous fraction. The extraction efficiency of tannic acid and emodin varied significantly across different solvent fractions, primarily due to the interplay between solvent polarity and the structural characteristics of each compound. Tannic acid (Figure 3), a hydrolyzable tannin rich in phenolic hydroxyl groups and aromatic rings, exhibits amphiphilic properties. Ethyl acetate, a medium-polarity solvent, yielded the highest extraction (0.272 ± 0.004 mg/g), likely due to its ability to interact with both hydrophilic and hydrophobic regions through hydrogen bonding and π–π interactions [40]. In contrast, the non-polar solvent n-hexane exhibited limited solubility, whereas overly polar solvents such as n-butanol and water may excessively stabilize hydrophilic moieties, thus impairing overall solubility. Emodin (Figure 4), a hydrophobic anthraquinone with a rigid tricyclic aromatic structure and weak polarity, showed the highest extraction efficiency in the chloroform fraction (0.270 ± 0.019 mg/g). Chloroform’s moderate polarity effectively solubilized the aromatic skeleton of emodin, whereas n-hexane, despite its low polarity, tended to extract lipids rather than rigid aromatic compounds. Ethyl acetate and n-butanol, being relatively more polar, were not suitable for efficiently dissolving emodin [41]. Both compounds were undetectable in the aqueous fraction, indicating extremely low water solubility. In summary, the optimal extraction of phenolic and anthraquinone-type compounds depends on the precise alignment of solvent polarity with the functional group composition of the target molecules. This understanding provides a theoretical basis for developing efficient extraction protocols for antioxidant, pharmaceutical, and cosmeceutical applications.
Table 9.
HPLC-PDA analysis of tannic acid and emodin compounds in solvent fractions from P. cuspidatum.
| Solvent | Tannic Acid (mg/g) | Emodin (mg/g) |
|---|---|---|
| n-hexane fraction | 0.028 ± 0.002 d12 | 0.028 ± 0.002 d |
| chloroform fraction | 0.105 ± 0.005 b | 0.105 ± 0.005 b |
| ethyl acetate fraction | 0.272 ± 0.004 a | 0.272 ± 0.004 a |
| n-butanol fraction | 0.044 ± 0.002 c | 0.044 ± 0.002 c |
| aqueous fraction | - 3 | - |
1 Mean ± SD (n = 3). 2 a–d Means Duncan’s different letters within a column differ significantly (p < 0.05). 3 Not detected.
Figure 3.
Chemical structure of tannic acid, a representative hydrolyzable tannin characterized by multiple phenolic hydroxyl groups and a polyaromatic framework.
Figure 4.
Chemical structure of emodin, a hydrophobic anthraquinone with a rigid tricyclic aromatic structure and weak polarity.
2.7. Comprehensive Gas Chromatography–Mass Spectrometry Detection GC–MSD Analysis of Solvent Fractions from P. cuspidatum
To systematically characterize the chemical constituents of P. cuspidatum, sequential solvent partitioning was performed, followed by analysis using GC–MSD. Five solvent fractions of differing polarity n-hexane, chloroform, ethyl acetate, n-butanol, and aqueous were analyzed to reveal the polarity-dependent distribution of secondary metabolites. The n-hexane fraction exhibited the highest chemical diversity and was predominantly composed of hydrophobic, low-polarity compounds. Notable constituents included silicon-based compounds such as methyltris(trimethylsiloxy)silane (11.94%) and arsenous acid tris(trimethylsilyl) ester (9.57%), both of which have been associated with antibacterial and antidiabetic activities. In addition, minor amounts of cyclic trisiloxanes (3.91%) and flavonoid derivatives such as 1-benzopyrylium, 3,7-dihydroxy-2-(4-hydroxyphenyl) (2.52%), contributed antioxidant and antibacterial properties (Table 10, Figure 5). The chloroform fraction was enriched in potent antioxidant and antibacterial agents, notably 7,9-di-tert-butyl-1-oxaspiro [4,5] deca-6,9-diene-2,8-dione (14.17%), phenol, 2,4-bis(1,1-dimethylethyl) (3.72%), and histamine dihydrochloride (5.54%). These compounds exhibited synergistic antioxidant, anti-inflammatory, and antibacterial activities (Table 10, Figure 5). In the ethyl acetate fraction, histamine dihydrochloride was the predominant constituent (20.76%), suggesting strong antioxidant potential. Additionally, the detection of AICAR (1H-imidazole-4-carboxamide, 5-amino; 3.49%) and 1-benzopyrylium derivatives (7.79%) suggests potential anti-inflammatory and antibacterial properties (Table 10, Figure 1). The n-butanol fraction was characterized by elevated levels of 2,5-di-tert-butyl-1,4-benzoquinone (19.10%) and dibutyl phthalate (19.49%), both compounds recognized for their antioxidant and antibacterial activities. Moderate concentration of AICAR (4.30%) and histamine dihydrochloride (7.81%) further contributed the strong bioactivity profile of this fraction (Table 10, Figure 5). The aqueous fraction, as the most polar extract, primarily contained hydrophilic antioxidant and antibacterial constituents, such as 1-benzopyrylium, 3,7-dihydroxy-2-(4-hydroxyphenyl) (17.68%), histamine dihydrochloride (13.96%), and 1,10-phenanthrolinium chloride (13.70%) (Table 10, Figure 5). Overall, the n-hexane fraction exhibited the highest diversity of volatile compounds, attributed to its ability to solubilize lipophilic and low-polarity constituents, including waxes, siloxanes, and other lipid-soluble metabolites. In contrast, more polar solvents such as ethyl acetate, n-butanol, and water primarily extracted non-volatile or high-boiling-point polar compounds, leading to reduced chemical diversity in GC–MSD analysis. This polarity-driven partitioning behavior underscores the pivotal role of solvent polarity in determining phytochemical extraction efficiency. To comprehensively evaluate both quantitative and qualitative attributes, this study integrated HPLC-PDA quantification of key phenolic and flavonoid markers with in vitro antioxidant, anti-inflammatory, and enzyme inhibitory activity. GC–MSD analysis was subsequently conducted on the most bioactive fractions, enabling a robust correlation between chemical composition and biological activity. This multi-platform analytical strategy provides a scientifically robust foundation for the potential development of P. cuspidatum in pharmaceutical, nutraceutical, and cosmeceutical applications.
Table 10.
Chemical constituents identified in the solvent fractions derived from P. cuspidatum by GC–MSD analysis.
| Compound | tR
1 (min) |
Chemical Class | Major Compounds (Relative Abundance, %) |
Reported Biological Activity |
|---|---|---|---|---|
|
n-hexane fraction |
8.001 | Flavonoids | 1-Benzopyrylium, 3,7-dihydroxy-2-(4-hydroxyphenyl) (2.52%) | Antibacterial |
| 10.33 | Biogenic amines | Histamine dihydrochloride (1.39%) | Antioxidant | |
| 12.07 | Nucleotide intermediates | 1H-Imidazole-4-carboxamide, 5-amino(AICAR) (1.60%) | Anti-inflammatory, Antioxidant |
|
| 12.20 | Nitrogen heterocycles | 1,10-phenanthrolinium chloride (1.45%) | Antioxidant, Antibacterial |
|
| 13.85 | Phthalate esters | 1,2-benzene-dicarboxylic acid, dimethyl ester (3.71%) | Antioxidant | |
| 19.10 | Spirocyclic diketones | 7,9-Di-tert-butyl-1-oxaspiro (4,5) deca-6,9-diene-2,8-dione (3.40%) | Antioxidant | |
| 27.31 | Organoarsenic compounds | Arsenous acid, tris(trimethylsilyl) ester (9.57%) | Antidiabetic | |
| 27.68 | Organosilicon compounds | methyltris(trimethylsiloxy)silane (11.94%) | Antibacterial | |
| 27.71 | Cyclic siloxanes | Cyclotrisiloxane, hexamethy (3.91%) | Antioxidant, Antibacterial | |
| chloroform fraction | 7.906 | Biogenic amines | Histamine dihydrochloride (5.54%) | Antioxidant |
| 14.51 | Phenolic antioxidants | 2,4-Di-tert-butylphenol (3.72%) | Antibacterial, Anti-inflammatory |
|
| 19.10 | Spirocyclic diketones | 7,9-Di-tert-butyl-1-oxaspiro (4,5) deca-6,9-diene-2,8-dione (14.17%) | Antioxidant | |
| ethyl acetate fraction | 5.032 | Flavonoids | 1-Benzopyrylium, 3,7-dihydroxy-2-(4-hydroxyphenyl) (7.79%) | Antibacterial |
| 13.85 | Biogenic amines | Histamine dihydrochloride (20.76%) | Antioxidant | |
| 17.88 | Nucleotide intermediates | 1H-Imidazole-4-carboxamide, 5-amino(AICAR) (3.49%) | Anti-inflammatory, Antioxidant | |
| 19.37 | Nitrogen heterocycles | 1,10-phenanthrolinium chloride (3.61%) | Antioxidant, Antibacterial | |
| 29.22 | Phthalate esters | 1,2-benzene-dicarboxylic acid, dimethyl ester (2.99%) | Antioxidant | |
|
n-butanol fraction |
7.906 | Biogenic amines | Histamine dihydrochloride (7.81%) | Antioxidant |
| 14.85 | Nucleotide intermediates | 1H-Imidazole-4-carboxamide, 5-amino(AICAR) (4.30%) | Anti-inflammatory, Antioxidant |
|
| 19.10 | Quinones | 2,5-di-tert-butyl-1,4-benzoquinone (DTBBQ) (19.10%) | Antioxidant, Antibacterial | |
| 19.49 | Phthalate esters | Dibutyl phthalate (19.49%) | Antibacterial | |
| aqueous fraction |
4.545 | Nitrogen heterocycles | 1,10-phenanthrolinium chloride (13.70%) | Antioxidant, Antibacterial |
| 12.07 | Biogenic amines | Histamine dihydrochloride (13.96%) | Antioxidant | |
| 19.10 | Flavonoids | 1-Benzopyrylium, 3,7-dihydroxy-2-(4-hydroxyphenyl) (17.68%) | Antibacterial |
1 Retention times.
Figure 5.
GC–MSD chromatograms profiles of P. cuspidatum solvent fractions (a) n-hexane fraction, (b) chloroform fraction, (c) ethyl acetate fraction, (d) n-butanol fraction, and (e) aqueous fraction.
3. Discussion
The ethyl acetate fraction of P. cuspidatum exhibited significant antioxidant, anti-diabetic, anti-obesity, skin-whitening, anti-aging, and antibacterial activities in multiple in vitro assays. These biological effects are primarily attributed to the synergistic actions of abundant natural bioactive compounds, particularly phenolics and flavonoids. Qualitative and quantitative analyses using HPLC–PDA and GC–MSD identified key compounds, including tannic acid, emodin, and several benzopyran derivatives, thereby providing strong evidence for the molecular basis of its bioactivities. At the molecular level, the identified compounds exhibited potent free radical scavenging activity and the ability to inhibit reactive oxygen species through electron donation and metal ion chelation, thereby protecting against oxidative stress and preserving cellular integrity. The moderate polarity of the ethyl acetate fraction facilitated the co-extraction of both lipophilic and hydrophilic compounds, enabling effective interactions with multiple target enzymes such as α-glucosidase, lipase, tyrosinase, and elastase. Such interactions, likely mediated through hydrogen bonding and hydrophobic forces, modulate enzymatic activity and contribute to the observed hypoglycemic, anti-obesity, depigmenting, and anti-wrinkle effects. Moreover, the antibacterial effects may result from multiple mechanisms, including disruption of microbial membranes, inhibitory activity of metabolic enzymes, and interference with DNA synthesis. These multifaceted pharmacological activities underscore the potential of the ethyl acetate fraction as a valuable natural source for the development of multifunctional therapeutic agents. Collectively, the findings support the hypothesis that the ethyl acetate fraction exerts its effects via a multi-target, multi-pathway synergistic mechanism. However, this study did not assess the toxicity or safety profile of the ethyl acetate fraction or its isolated compounds. Comprehensive in vitro and in vivo toxicity evaluations are essential to ensure safety before any clinical or commercial application. Future research should prioritize these assessments to fully validate the therapeutic potential of P. cuspidatum extracts. Additionally, further investigations employing molecular docking, cellular assays, and in vivo models are warranted to elucidate the precise targets and mechanisms of action of the bioactive constituents.
4. Materials and Methods
4.1. Chemicals and Plant Material
The P. cuspidatum material used in this study was purchased from a local herbal market in Yeongcheon, Gyeongsangbuk-do, Republic of Korea, in September 2024. The dried roots were ground to a particle size of 25–40 mesh using a mechanical grinder and stored in a desiccator until further use.
Gallic acid (Sigma-Aldrich, St. Louis, MO, USA), 1,1-diphenyl-2-picrylhydrazyl (DPPH) (Sigma-Aldrich, St. Louis, MO, USA), 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ) (Sigma-Aldrich, St. Louis, MO, USA), ABTS diammonium salt (Sigma-Aldrich, St. Louis, MO, USA), L-3,4-dihydroxyphenylalanine (L-DOPA) (Sigma-Aldrich, St. Louis, MO, USA), mushroom tyrosinase (Sigma-Aldrich, St. Louis, MO, USA), N-succinyl-(Ala)3-p-nitroanilide (Sigma-Aldrich, St. Louis, MO, USA), porcine pancreatic lipase (Sigma-Aldrich, St. Louis, MO, USA), and 3-(N-morpholino)propanesulfonic acid (MOPS) (Sigma-Aldrich, St. Louis, MO, USA) were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Ferric chloride hexahydrate (FeCl3·6H2O) (Samchun Pure Chemical Co., Ltd. Seoul, Republic of Korea) and iron(II) sulfate hexahydrate (FeSO4·6H2O) (Samchun Pure Chemical Co., Ltd. Seoul, Republic of Korea) were obtained from Samchun Pure Chemical Co., Ltd. (Seoul, Republic of Korea). All solvents used for extraction, high-performance liquid chromatography-photodiode array (HPLC-PDA)(YOUNG IN Chromass, Anyang-si, Gyeonggi-do, Republic of Korea), and analytical procedures were of guaranteed reagent (GR) grade and were purchased from Duksan Pure Chemicals Co., Ltd. (Ansan-si, Gyeonggi-do, Republic of Korea).
4.2. Extraction and Solvent Fractionation
A total of 200 g of P. cuspidatum powder was extracted three times at room temperature with 70% ethanol (v/v) at a solvent-to-material ratio of 10:1 (v/w), with each extraction lasting 24 h. After each round, the mixture was filtered through Whatman No. 2 filter paper (Cytiva Ltd., Maidstone, UK). The combined filtrates were subsequently concentrated by freeze-drying using a lyophilizer (Model HFD-1, Huachen Instrument Co., Ltd., Zhengzhou, China) to remove ethanol and obtain the crude ethanolic extract. The dried extract was reconstituted in 200 mL of distilled water and sequentially extracted with various solvents, as illustrated in Figure 6. First, the aqueous solution was mixed with n-hexane in a 1:1 (v/v) ratio and fractionated using a separatory funnel. The organic phase was collected and concentrated under reduced pressure using a rotary evaporator (Model WEV-1001 L, Daihan Scientific Co., Ltd., Seoul, Republic of Korea) at 40 °C. The extraction yielded 3.03% of the n-hexane fraction. The remaining aqueous layer was sequentially partitioned with chloroform, ethyl acetate, and n-butanol using the same procedure. The extraction yielded 9.61%, 24.48%, and 8.59% of the chloroform, ethyl acetate, and-butanol fractions, respectively. The final aqueous fraction had a yield of 32.78%, as detailed in Table 11. All fractions were stored at temperatures below 4 °C and were dissolved in 70% methanol prior to subsequent bioactivity and chemical analyses.
Figure 6.
Stepwise solvent fractionation scheme of the 70% ethanolic extract of P. cuspidatum. The dried extract was successively partitioned with n-hexane, chloroform, ethyl acetate, and n-butanol, resulting in the corresponding solvent fractions.
Table 11.
Extraction yields of solvent fractions from P. cuspidatum.
| Solvent | Yield (%, w/w 1) |
|---|---|
| n-hexane fraction | 3.03 |
| chloroform fraction | 9.61 |
| ethyl acetate fraction | 24.5 |
| n-butanol fraction | 8.59 |
| aqueous fraction | 32.8 |
1 yield (%) was calculated against the dried P. cuspidatum.
4.3. Total Polyphenol and Flavonoid Contents of Solvent Fractions from P. cuspidatum
The total polyphenol content was measured using the Folin–Denis method [42]. A 200 μL aliquot of the sample was mixed with 200 μL of Folin–Ciocalteu’s phenol reagent (Sigma-Aldrich, St. Louis, MO, USA) and allowed to react for 3 min at room temperature. Then, 3 mL of 10% sodium carbonate was added, and the mixture was incubated in the dark for 60 min. The absorbance was measured at 765 nm using a UV–Vis spectrophotometer (Optizen Pop-s, KLab, Daejeon, Republic of Korea). The total polyphenol content was expressed as mg gallic acid equivalents (GAE) per gram of sample, calculated using a standard curve obtained with gallic acid (Sigma-Aldrich, St. Louis, MO, USA). The total flavonoid content was measured following the method of Jia et al. [43]. A 250 μL aliquot of the sample, diluted with distilled water, was mixed with 1 mL of distilled water and 75 μL of 5% sodium nitrite (w/v, Samchun Pure Chemical Co., Ltd., Seoul, Republic of Korea). After 5 min, 150 μL of 10% aluminum chloride (w/v, Junsei Chemical Co., Ltd.) was added, and the mixture was allowed to stand for 6 min. Then, 500 μL of 1 M sodium hydroxide (Junsei Chemical Co., Ltd., Tokyo, Japan) was added, and, after 11 min, the absorbance was measured at 510 nm. The total flavonoid content was expressed as mg quercetin equivalents (QEs) per gram of sample, calculated using a standard curve obtained with quercetin (Sigma-Aldrich, St. Louis, MO, USA).
4.4. Antioxidant Activities (DPPH, ABTS Radical Scavenging Activity and Ferric Reducing Antioxidant Power (FRAP) Assay) of Solvent Fractions from P. cuspidatum
The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity was evaluated according to the methods of Blois [44] and others [45]. Briefly, 500 μL of 0.2 mM DPPH solution was mixed with 500 μL of the sample and allowed to react in the dark for 30 min at room temperature. The absorbance was measured at 517 nm using a UV–Vis spectrophotometer (Optizen Pop-s, KLab Inc., Seoul, Republic of Korea). The reduction in absorbance caused by DPPH radical scavenging was assessed. Blank samples were prepared by substituting the sample with the extraction solvent. The DPPH scavenging activity (%) was calculated using the formula below, and the 50% inhibitory concentration (IC50) was determined. L-ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA) was used as a reference control for comparison and analysis. The DPPH radical scavenging activity was calculated using the following formula:
| DPPH radical scavenging activity (%) = (1 − (A/B)) × 100 |
A stands for “The absorbance of the sample”, and B stands for “The absorbance of the control”.
The ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity was measured following the methods of Pellegrini et al. [46] and others [47]. To prepare the ABTS radical solution, 88 μL of 140 mM potassium persulfate solution was added to 5 mL of distilled water, followed by the addition of two tablets of ABTS diammonium salt (Sigma-Aldrich, St. Louis, MO, USA) to achieve a final concentration of 7 mM ABTS. This solution was left in the dark for 14–16 h, and the resulting ABTS solution was diluted with absolute ethanol at a ratio of 1:88 (v/v) to adjust the absorbance to 0.70 ± 0.02 at 734 nm. Blank samples were prepared by substituting the sample with the extraction solvent. The ABTS scavenging activity (%) was calculated using the formula below, and the 50% inhibitory concentration (ABTS IC50) was determined. L-ascorbic acid was used as positive control for comparison and analysis. The ABTS radical scavenging activity was calculated using the following formula:
| ABTS radical scavenging activity (%) = (1 − (A/B)) × 100 |
A stands for “The absorbance of the sample”, and B stands for “The absorbance of the control”.
The ferric reducing antioxidant power (FRAP) assay was measured according to the method of Benzie et al. [48]. The FRAP reagent was prepared by mixing pre-prepared acetate buffer (0.3 M, pH 3.6; Sigma-Aldrich, St. Louis, MO, USA) with 10 mM 2,4,6-tripyridyl-S-triazine (TPTZ; Sigma-Aldrich, St. Louis, MO, USA) and 20 mM ferric chloride solution (Samchun Pure Chemical Co., Ltd., Seoul, Republic of Korea) at a ratio of 10:1:1 (v/v/v). The sample (30 μL), diluted in 70% methanol, was mixed with 900 μL of the FRAP reagent and 90 μL of distilled water. After vortexing, the mixture was left to stand at 37 °C for 10 min, and the absorbance was measured at 593 nm. The FRAP value was expressed as mM of iron sulfate hexahydrate per gram of sample, calculated using a standard curve obtained with iron sulfate hexahydrate (Samchun Pure Chemical Co., Ltd., Seoul, Republic of Korea).
4.5. Tyrosinase and Elastase Inhibitory Activities of Solvent Fractions from P. cuspidatum
Tyrosinase inhibitory activity was measured according to the method outlined by Choi et al. [49]. The reaction mixture contained 500 μL of 0.1 M potassium phosphate buffer (pH 6.8), 200 μL of sample, and 200 μL of 10 mM L-DOPA (dihydroxy-phenylalanine, Sigma-Aldrich, St. Louis, MO, USA). Then, 100 μL of enzyme solution (mushroom tyrosinase, Sigma-Aldrich Co.) was added. The mixture was incubated at 37 °C for 15 min, and the change in absorbance at 475 nm was measured. The inhibitory activity was calculated based on the change in dopachrome absorbance. Kojic acid (Sigma-Aldrich, St. Louis, MO, USA) was used as the control. The tyrosinase inhibitory activity was calculated using the following formula:
| Tyrosinase inhibitory activity (%) = (1 − (A − B)/C) × 100 |
A stands for “The absorbance at 475 nm determined with sample”, B stands for “The absorbance at 475 nm determined with buffer instead of enzyme”, and C stands for “The absorbance at 475 nm determined with buffer instead of sample”.
Elastase inhibitory activity was measured following the method described by Choi et al. [49]. A mixture of 0.2 M Tris-HCl buffer (pH 8.0) 350 μL and n-succinyl-(Ala)3-p-nitroanilide (Sigma-Aldrich, St. Louis, MO, USA) 125 μL was prepared. Subsequently, 20 μL of the sample was added and 5 μL of elastase (pancreatic from porcine pancreas, PPE, 1.4 units/mg, Sigma-Aldrich Co.) at a concentration of 50 μL/mL was added. The reaction was carried out at 37 °C in an incubator for 20 min. The absorbance was then measured at 410 nm using a UV–Vis spectrophotometer (Optizen Pop-s, KLab Inc., Seoul, Republic of Korea). Elastase inhibitory activity was calculated using the formula below, and ascorbic acid, known for its benefits in aging skin, was used as a control for comparison and analysis. The elastase inhibitory activity was calculated using the following formula:
| Elastase inhibitory activity (%) = (1 − (A − B)/C) × 100 |
A stands for “absorbance at 410 nm determined with sample”, B stands for “absorbance at 410 nm determined with buffer instead of enzyme”, and C stands for “absorbance at 410 nm determined with buffer instead of sample”.
4.6. α-Glucosidase and Lipase Inhibitory Activities of Solvent Fractions from P. cuspidatum
The α-glucosidase inhibitory activity was evaluated using a modified spectrophotometric method based on the enzyme–substrate reaction, as described by Eom et al. [50]. Briefly, 450 μL of α-glucosidase solution (0.1 U/mL) was mixed with 50 μL of either the sample solution or 0.1 M sodium phosphate buffer (pH 6.8). The mixture was incubated at 37 °C for 15 min. After incubation, 500 μL of 1 mM p-NPG (p-nitrophenyl-α-D-glucopyranoside, Sigma-Aldrich, St. Louis, MO, USA) was added as the substrate and further incubated for 5 min. The amount of p-nitrophenol released by enzymatic hydrolysis was measured at 405 nm using a UV–Vis spectrophotometer (Optizen Pop-s, KLab Inc., Seoul, Republic of Korea). The α-glucosidase inhibitory activity was calculated using the formula below. Acarbose, a well-known α-glucosidase inhibitor, was used as a positive control for comparative analysis. The α-glucosidase inhibitory activity was calculated using the following formula:
| α-Glucosidase inhibitory activity (%) = (1 − (A/B)) × 100 |
A stands for “The absorbance of the sample”, and B stands for “The absorbance of the control”.
The lipase inhibitory activity was determined using the method described by Kim et al. [51] with slight modifications. For the preparation of the enzyme buffer, 0.3 mg of porcine pancreatic lipase (triacylglycerol acyl-hydrolase, EC 3.1.1.3; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 30 μL of 10 mM MOPS (3-[N-morpholino] propanesulfonic acid; Sigma-Aldrich, St. Louis, MO, USA) and 30 μL of 1 mM EDTA (pH 6.8), followed by the addition of 850 μL of Tris buffer (100 mM Tris-HCl, 5 mM CaCl2, pH 7.0). After preparation, 100 μL of the sample solution was added to the enzyme buffer and incubated at 37 °C for 15 min. Subsequently, 20 μL of 10 mM p-nitrophenyl palmitate (p-NPP; Sigma-Aldrich, St. Louis, MO, USA) was added to initiate the enzymatic reaction, which was further incubated at 37 °C for an additional 15 min. The degree of hydrolysis of p-NPP to p-nitrophenol was quantified by measuring absorbance at 400 nm using a UV–Vis spectrophotometer (Optizen Pop-s, KLab Inc., Seoul, Republic of Korea). The lipase inhibitory activity was calculated using the formula below. Orlistat, a clinically approved lipase inhibitor, was used as a positive control for comparative analysis. The lipase inhibitory activity was calculated using the following formula:
| Lipase inhibitory activity (%) = (1 − (B − b)/A) × 100 | (1) |
A stands for “absorbance at 400 nm determined with buffer instead of sample”, B stands for “absorbance at 400 nm determined with sample”, and b stands for “absorbance at 400 nm determined with buffer instead of enzyme”.
4.7. Antibacterial Activities of Solvent Fractions from P. cuspidatum Broth Against Selected Microbial Strains
The antibacterial activities of solvent fractions derived from P. cuspidatum broth was evaluated using the disc diffusion assay, as previously described by Blois [52]. Seven microbial strains were selected for the antibacterial activity testing, including Gram-positive bacteria: Staphylococcus aureus KCTC 1621 and Gram-negative bacteria: Escherichia coli KCTC 1112 and Pseudomonas aeruginosa KCTC 2450. These strains were obtained from the Korean Collection for type culture (KCTC). The bacterial strains used for antibacterial activity were cultured under the conditions specified in Table 12. Each strain was inoculated into nutrient broth and cultured at 30 °C and 37 °C for 24–30 h through three subcultures. The absorbance at 600 nm was measured, and strains with absorbance values falling within the range of 0.2 to 0.4 (corresponding to 1 × 105 CFU/mL) were selected for antibacterial activity testing. For antibacterial activity testing, the activated strains were evenly spread on nutrient agar to prepare the test plates. Test solutions were prepared for each sample to achieve concentrations of 5 and 10 mg per disc. Paper discs (8 mm) were impregnated with each sample solution, dried to evaporate the solvent, and then placed in close contact with the agar surface of the test plates. After incubating at 30 °C or 37 °C for 24–30 h, the diameter (mm) of the clear zone around each disc, indicating inhibitory activity against microbial growth, was measured to compare antibacterial efficacy.
Table 12.
List of strains used for antibacterial experiments.
| Microorganism | Media 1 | Temperature (°C) | |
|---|---|---|---|
| Staphylococcus aureus | NA/NB | 30 | |
| Escherichia coli | NA/NB | 30 | |
| Pseudomonas aeruginosa | NA/NB | 37 | |
1 NA: nutrient agar, NB: nutrient broth.
4.8. HPLC-PDA Quantification of Tannic Acid, and Emodin
Quantitative analysis of gallic acid, tannic acid, and emodin was performed using a YOUNG IN Chromass ChroZen HPLC system (YOUNG IN Chromass, Anyang-si, Gyeonggi-do, Republic of Korea) equipped with a quaternary pump, autosampler, and photodiode array (PDA) detector. Separation was performed using a Poroshell 120 EC-C18 column (4.6 × 150 mm, 4 μm; Agilent Technologies, Santa Clara, CA, USA). For tannic acid, isocratic elution was performed using a mobile phase consisting of 50% solvent A (0.5% (v/v) acetic acid in water) and 50% solvent B (methanol) over 0–20 min. Detection was conducted at 270 nm, with a flow rate of 1.0 mL/min, column temperature set at 30 °C, and injection volume of 10 μL. For emodin, isocratic elution was carried out using 75% solvent A (0.1% (v/v) phosphoric acid in water) and 25% solvent B (methanol) over 30 min. Detection was performed at 254 nm, with the same flow rate, column temperature, and injection volume as described above.
4.9. HPLC-PDA Method Validation
The analytical method was validated in accordance with the guidelines of European Commission Decision 2002/657/EC. The validation parameters included linear range, calibration curves, sensitivity, precision, and accuracy.
4.9.1. Linearity, Sensitivity, and Precision
Linearity was assessed by analyzing a series of standard working solutions at various concentrations using the established HPLC-PDA method. Calibration curves were generated by plotting peak area (Y) against analyte concentration (X), with the correlation coefficient (R2) indicating reliable quantification within the validated range. Sensitivity was evaluated by determining the limits of detection (LODs) and quantification (LOQs), based on signal-to-noise ratios of 3 and 10, respectively. Low-concentration spiked samples were analyzed to estimate these limits. The LOQ values met the acceptance criteria for recovery (>70%) and precision (RSD ≤ 20%), confirming the method’s reliability at low concentrations. Precision was evaluated by calculating the relative standard deviation (RSD) for both intra-day and inter-day repeatability. Intra-day precision was assessed by analyzing three replicate samples at different concentrations within a single day, while inter-day precision was determined by repeating the same procedure over three consecutive days. The method was considered acceptably precise if the RSD was less than 15%.
4.9.2. Accuracy and Specificity
Accuracy was determined through recovery experiments by spiking known amounts of analytes into blank matrices at three concentration levels (25–500 ppm). Each level was analyzed in six replicates following the same sample preparation procedure. Recovery rates (%) were calculated by comparing the measured concentrations to the theoretical spiked values, ensuring the method’s trueness and reliability. Specificity was confirmed by analyzing blank matrices, standard solutions, and matrix-spiked samples. Chromatographic results demonstrated that the target analytes were well resolved under the optimized conditions, with no interference from endogenous matrix components, indicating a high selectivity.
4.10. GC–MSD Quantification of Solvent Fractions from P. cuspidatum
GC–MSD analysis was conducted using an Agilent 5975 GC–MSD system equipped with an HP-5ms capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). Helium was used as the carrier gas at a constant flow rate of 1.0 mL/min. The GC oven temperature was initially held at 60 °C for 3 min, then ramped to 240 °C at 5 °C/min, and further increased to 300 °C, where it was held for 5 min. The injector temperature was set to 230 °C. Mass spectra were acquired in electron ionization (EI) mode at 70 eV.
4.11. Statistical Analysis
All experiments were conducted with measurements repeated at least three times, and the results were expressed as mean ± standard error. Statistical analysis was performed using the SPSS Statistics 22.0 software system (ANOVA, SPSS Inc., Chicago, IL, USA). Analysis of variance (ANOVA) was carried out to validate the results. For significant findings, Duncan’s multiple range test was employed for post hoc analysis at a significance level of p < 0.05.
5. Conclusions
In conclusion, the ethyl acetate fraction of P. cuspidatum demonstrated the most diverse and potent bioactivities among all solvent fractions, including antioxidant, anti-inflammatory, enzyme inhibitory, and antibacterial effects. These bioactivities are closely associated with the presence of phenolic and flavonoid compounds identified through HPLC–PDA and GC–MSD analyses. These findings provide a scientific basis for developing this fraction as a potential active ingredient in functional foods, pharmaceuticals, and cosmetic formulations. Further studies are warranted to elucidate the in vivo efficacy and precise molecular targets of these bioactive constituents.
Acknowledgments
We sincerely thank Woonjung Kim and Yuri Kang for their valuable assistance and support during this study. All individuals mentioned in the acknowledgments have given their consent to be acknowledged publicly.
Author Contributions
Conceptualization, Y.C. and W.K.; methodology, Y.C. and W.K.; software, Y.C. and Y.K.; validation, Y.C., W.K. and Y.K.; formal analysis, Y.C.; investigation, Y.C.; resources, W.K. and Y.K.; data curation, Y.C.; writing—original draft preparation, Y.C. and W.K.; writing—review and editing, Y.C. and W.K.; visuali-zation, Y.C.; supervision, Y.K. and W.K.; project administration, Y.K. and W.K.; funding acquisition, W.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated during this study are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the Korea Institute for Advancement of Technology (KIAT) grant funded by the Korean government (MOTIE) (RS-2024-00409639, the HRD Program for Industrial Innovation) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2023-00281517).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hsu C.Y., Chan Y.P. Antioxidant activity of extract from Polygonum. Biol. Res. 2007;40:13–21. doi: 10.4067/S0716-97602007000100002. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y., Zhang T., Wang L., Liu X., Li H. Research progress on chemical constituents in roots and rhizomes of Polygonum cuspidatum and their pharmacological activities. Chin. Tradit. Herb. Drugs. 2016;47:3055–3063. [Google Scholar]
- 3.Kirino A., Nakahara K., Matsuura M. Analysis and functionality of major polyphenolic components of Polygonum cuspidatum. J. Food Sci. Technol. 2012;49:99–105. doi: 10.3177/jnsv.58.278. [DOI] [PubMed] [Google Scholar]
- 4.Peng W., Qin R., Li X., Zhou H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb. et Zucc.: A review. J. Ethnopharmacol. 2013;148:729–745. doi: 10.1016/j.jep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Quinty V., Morel S., Vallet C., Couteau C., Coiffard L. Screening and evaluation of dermo-cosmetic activities of the invasive plant species Polygonum cuspidatum. Cosmetics. 2022;9:83. doi: 10.3390/plants12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fazary A.E., Ju Y.H. Feruloyl esterases as biotechnological tools: Current and future perspectives. Acta Biochim. Biophys. Sin. 2007;39:811–828. doi: 10.1111/j.1745-7270.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., Zhao L., Cheng X., Ma Y. Tissue-specific transcriptome analyses reveal candidate genes for stilbene, flavonoid and anthraquinone biosynthesis in the medicinal plant Polygonum cuspidatum. BMC Genom. 2021;22:353. doi: 10.1186/s12864-021-07658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia Y., Liu L., Wu X., Zhao W. Advances for pharmacological activities of Polygonum cuspidatum: A review. Phytother. Res. 2023;37:285–303. doi: 10.1080/13880209.2022.2158349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarma A.D., Mallick A.R., Ghosh A.K. Free radicals and their role in different clinical conditions: An overview. Int. J. Pharma Sci. Res. 2010;1:185–192. [Google Scholar]
- 10.Kim Y.J., Uyama H., Kobayashi S. Autophagy induced by resveratrol suppresses α-MSH-induced melanogenesis. Biochem. Biophys. Res. Commun. 2014;452:161–167. doi: 10.1111/exd.12337. [DOI] [Google Scholar]
- 11.Li H., Zhang Q., Wang C. Separation and purification of the main components from Polygonum cuspidatum and their anti-hyperuricemia effect. Chin. J. Nat. Med. 2021;19:641–649. [Google Scholar]
- 12.Ito Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 13.Exarchou V., Godejohann M., van Beek T.A., Gerothanassis I.P., Vervoort J. LC–UV–solid-phase extraction–NMR–MS combined with a cryogenic flow probe and its application to the identification of compounds present in Greek oregano. Anal. Chem. 2003;75:6288–6294. doi: 10.1021/ac0347819. [DOI] [PubMed] [Google Scholar]
- 14.Chua L.S., Lau C.H., Chew C.Y., Ismail N.I.M. Solvent fractionation and acetone precipitation for crude saponins from Eurycoma longifolia extract. J. Appl. Res. Med. Aromat. Plants. 2019;12:1416. doi: 10.3390/molecules24071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandoval-Acuña C., Ferreira J., Speisky H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014;559:75–90. doi: 10.1016/j.abb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Agati G., Stefano G., Biricolti S., Tattini M. Mesophyll distribution of antioxidant flavonoids in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 2009;104:853–861. doi: 10.1093/aob/mcp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gębalski J., Małkowska M., Wnorowska S., Gawenda-Kempczyńska D., Strzemski M., Wójciak M., Słomka A., Styczyński J., Załuski D. Ethyl Acetate fraction from Eleutherococcus divaricatus root extract as a promising source of compounds with anti-hyaluronidase, anti-tyrosinase, and antioxidant activity but not anti-melanoma activity. Molecules. 2024;29:3640. doi: 10.3390/molecules29153640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh D.Y., Kim H.S. Total flavonoid content and antioxidant activities of turmeric (Curcuma longa L.) extracts in Jindo, Korea. J. Environ. Sci. Int. 2019;28:393–401. doi: 10.5322/JESI.2019.28.4.393. [DOI] [Google Scholar]
- 19.Brand-Williams W., Cuvelier M.E., Berset C. Use of a Free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 20.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 21.Choi J.S., Kim H.Y., Seo W.T., Lee J.H., Cho K.M. Roasting enhances antioxidant effect of bitter melon (Momordica charantia L.) increasing in flavan-3-ol and phenolic acid contents. Food Sci. Biotechnol. 2012;21:19–26. doi: 10.1007/s10068-012-0003-7. [DOI] [Google Scholar]
- 22.Aydin S., Kadioğlu G. Total phenolic content and antioxidant activity of Thymus vulgaris, Curcuma longa, propolis and their mixtures. J. Biol. Sci. 2022;13:13 [Google Scholar]
- 23.Simunkova M., Barbierikova Z., Jomova K., Hudecova L., Lauro P., Alwasel S.H., Alhazza I., Rhodes C.J., Valko M. Antioxidant vs. Prooxidant properties of the flavonoid, kaempferol, in the presence of Cu (II) Ions: A ROS-scavenging activity, fenton reaction and DNA damage study. Int. J. Mol. Sci. 2021;22:1619. doi: 10.3390/ijms22041619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarma P., Kashyap B., Gurumayum S., Sarma S., Baruah P., Swargiary D., Saikia A., Deka R.C., Borah J.C. Antihyperglycemic potential of quercetin-3-glucoside isolated from Leucaena leucocephala seedpods via the SIRT1/AMPK/GLUT4 signaling cascade. ACS Omega. 2024;9:32429–32443. doi: 10.1021/acsomega.3c09672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Im D.Y., Lee K.I. Tyrosinase inhibitory activity and melanin production inhibitory activity of the extract and fractions from Paeoniae Radix. Korean J. Pharmacogn. 2011;42:323–328. [Google Scholar]
- 26.Wang H., Dong Y., Xiu Z.L. Microwave-assisted aqueous two-phase extraction of piceid, resveratrol and emodin from Polygonum cuspidatum by ethanol/ammonium sulphate systems. Biotechnol. Lett. 2008;30:2079–2084. doi: 10.1007/s10529-008-9815-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.S., Lee E.B., Yu Y.J., Kim G.W., Kim W.J., Choi D.K. Ethyl acetate fraction from a Catalpa ovata G. Don extract inhibits α-MSH-induced melanogenesis through the cAMP/CREB pathway. Int. J. Mol. Sci. 2023;25:151. doi: 10.3390/ijms25010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du R., Ye L., Chen X., Meng Y., Zhou L., Chen Q., Zheng G., Hu J., Shi Z. Screening of key components for melanogenesis inhibition of Polygonum cuspidatum extract based on the spectrum–effect relationship and molecular docking. Molecules. 2024;29:857. doi: 10.3390/molecules29040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tundis R., Loizzo M.R., Menichini F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010;10:315–331. doi: 10.2174/138955710791331007. [DOI] [PubMed] [Google Scholar]
- 30.Birari R.B., Bhutani K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today. 2007;12:879–889. doi: 10.1016/j.drudis.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Kim H.J., Ha S.C., Park S.W. Inhibition of tyrosinase and lipoxygenase activities by resveratrol and its derivatives from seeds of Paeonia lactiflora. Prev. Nutr. Food Sci. 2002;7:447–450. doi: 10.3746/jfn.2002.7.4.447. [DOI] [Google Scholar]
- 32.Wang R., Fan R., Meng T., Wang L. Exploration of the inhibitory mechanisms of trans-polydatin/resveratrol on α-glucosidase by multi-spectroscopic analysis, in silico docking and molecular dynamics simulation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023;299:122866. doi: 10.1016/j.saa.2023.122866. [DOI] [PubMed] [Google Scholar]
- 33.Chang Y.X., Ge A.H., Jiang Y., Azietaku J.T., Li J., Gao X.-M. A bioactivity-based method for screening, identification of lipase inhibitors, and clarifying the effects of processing time on lipase inhibitory activity of Polygonum multiflorum. Evid. Based Complement. Altern. Med. 2016;2016:5965067. doi: 10.1155/2016/5965067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hintz T., Matthews K.K., Di R. The use of plant antimicrobial compounds for food preservation. Biomed. Res. Int. 2015;2015:246264. doi: 10.1155/2015/246264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y.T., Labbe R.G., Shetty K. Inhibition of Listeria monocytogenes in fish and meat systems by use of oregano and cranberry phytochemical synergies. Appl. Environ. Microbiol. 2004;70:5672–5678. doi: 10.1128/AEM.70.9.5672-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qun T., Zhou T., Hao J., Wang C., Zhang K., Xu J., Wang X., Zhou W. Antibacterial activities of anthraquinones: Structure–activity relationships and action mechanisms. RSC Med. Chem. 2023;14:1446–1471. doi: 10.1039/D3MD00116D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alhumaid S., Al Mutair A., Alawi Z.H., Al-Omari A. Antimicrobial susceptibility of gram-positive and gram-negative bacteria: A 5-year retrospective analysis at a multi-hospital healthcare system in Saudi Arabia. Antimicrob. Resist. Infect. Control. 2021;10:36. doi: 10.1186/s12941-021-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima S.L., Colombo A.L., de Almeida Júnior J.N. Fungal cell wall: Emerging antifungals and drug resistance. Front. Microbiol. 2019;10:2573. doi: 10.3389/fmicb.2019.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramanian M., Goswami M., Chakraborty S., Jawali N. Resveratrol induced inhibition of Escherichia coli proceeds via membrane oxidation and independent of diffusible reactive oxygen species generation. Redox Biol. 2014;2:865–872. doi: 10.1016/j.redox.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deogratias G., Shadrack D.M., Munissi J.J.E., Kinunda G.A., Jacob F., Mtei R.P., Masalu R.J., Mwakyula I., Kiruri L.W., Nyandoro S.S. Hydrophobic π-π stacking interactions and hydrogen bonds drive self-aggregation of luteolin in water. J. Mol. Graph. Model. 2022;116:108243. doi: 10.1016/j.jmgm.2022.108243. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y.C., Wu P., Li Y.B., Liu T.C., Zhang L., Zhou Y.H. Natural deep eutectic solvents as new green solvents to extract anthraquinones from Rheum palmatum L. RSC Adv. 2018;8:15069–15077. doi: 10.1039/C7RA13581E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folin O., Denis W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912;12:239–243. doi: 10.1016/S0021-9258(18)88697-5. [DOI] [Google Scholar]
- 43.Jia Z.S., Tang M.C., Wu J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/s0308-8146(98)00102-2. [DOI] [Google Scholar]
- 44.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 45.Jeong S.J., Kim K.H., Yook H.S. Whitening and antioxidant activities of solvent extracts from hot-air dried Allium hookeri. J. Korean Soc. Food Sci. Nutr. 2015;44:832–839. doi: 10.3746/jkfn.2015.44.6.832. [DOI] [Google Scholar]
- 46.Pellegrini N., Re R., Yang M., Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) radical cation decolorization assay. Methods Enzymol. 1999;299:379–389. [Google Scholar]
- 47.Kim K.H., Kim H.J., Byun M.W., Yook H.S. Antioxidant and antimicrobial activities of ethanol extract from six vegetables containing different sulfur compounds. J. Korean Soc. Food Sci. Nutr. 2012;41:577–583. doi: 10.3746/jkfn.2012.41.5.577. [DOI] [Google Scholar]
- 48.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 49.Choi M.H., Kim K.H., Yook H.S. Antioxidant activity of fermented Kaempferia parviflora and inhibitory action against tyrosinase and elastase. J. Korean Soc. Food Sci. Nutr. 2018;47:1076–1084. doi: 10.3746/jkfn.2018.47.11.1076. [DOI] [Google Scholar]
- 50.Eom S.H., Lee S.H., Yoon N.Y., Jung W.K., Jeon Y.J., Kim S.K., Lee M.S., Kim Y.M. α-Glucosidase and α-amylase inhibitory activities of phlorotannins from Eisenia bicyclis. J. Sci. Food Agric. 2012;92:2084–2090. doi: 10.1002/jsfa.5585. [DOI] [PubMed] [Google Scholar]
- 51.Kim J.H., Kim H.J., Park H.W., Youn S.H., Choi D.Y., Shin C.S. Development of inhibitors against lipase and α-glucosidase from derivatives of Monascus pigment. FEMS Microbiol. Lett. 2007;276:93–98. doi: 10.1111/j.1574-6968.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 52.Joshi S.C., Verma A.R., Mathela C.S. Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food Chem Toxicol. 2010;48:37–40. doi: 10.1016/j.fct.2009.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are included in this article.