Abstract
Chronic exposure to cocaine induces long-term adaptations that are likely to involve changes in transcription factor expression. This possibility has not been examined in the cocaine-exposed human brain. The transcription factor nurr1 is highly expressed in rodent midbrain dopamine neurons and is essential for their proper phenotypic development. Here we show that human NURR1 gene expression is robust within control subjects and reduced markedly within the dopamine neurons of human cocaine abusers. NURR1 is known to regulate transcription of the gene encoding the cocaine-sensitive dopamine transporter (DAT). We show here that DAT gene expression also is reduced markedly in the dopamine neurons of NURR1-deficient cocaine abusers, suggesting that NURR1 plays a critical role in vivo in controlling human DAT gene expression and adaptation to repeated exposure to cocaine.
Chronic cocaine exposure induces long-term adaptations that are incompletely understood at the molecular level but most likely involve changes in transcription factor gene expression (1). The transcription factor nurr1 is an orphan nuclear receptor of the steroid/thyroid hormone receptor superfamily (2, 3) that plays a pivotal role in the proper development of dopamine neurons (4–6). It is conceivable that nurr1 also plays a role in maintaining dopamine neuron phenotype within the mature nervous system. Consistent with this possibility, recent data (7, 8) suggest that nurr1 regulates transcription of the gene encoding the cocaine-sensitive dopamine transporter (DAT), a plasma membrane transport protein that regulates extracellular dopamine concentrations (9). To date, expression of the NURR1 gene (the human homolog of rodent nurr1) has not been examined in human brain. In the present experiments, we examine NURR1 gene expression within cocaine-exposed adult human midbrain.
Materials and Methods
Tissue Acquisition and Subject Characterization.
Postmortem brain specimens were obtained from Miami, Florida (study 1) and Wayne County, Michigan (study 2) during routine autopsy and analyzed as described (10, 11). Medicolegal investigations were conducted by forensic pathologists. The cause and manner of death were determined after evaluating the circumstances of death, toxicology data, and autopsy results. All cases were evaluated for common drugs of abuse (including alcohol), and positive urine screens were confirmed by quantitative analysis of blood. Blood (and, in study 1, brain) levels of cocaine and metabolites were determined as described (12). The cocaine overdose cases used in the present study tested negative for other drugs of abuse. Control subjects were drug-free and died as a result of cardiovascular disease (n = 8), gunshot wounds (n = 6), or multiple trauma (n = 3). Cocaine abuse and control subject groups were matched closely in terms of age, sex/race, postmortem interval (PMI), and tissue pH: study 1 controls (29 ± 2 yr, 43% black male, 16 ± 2 h PMI) compared with cocaine subjects (34 ± 2 yr, 50% black male, 11 ± 1 h PMI); study 2 controls (39 ± 2 yr, 70% black male, <20 h PMI, brain pH 6.5 ± 0.5) compared with cocaine subjects (42 ± 3 yr, 50% black male, <20 h PMI, brain pH 6.5 ± 0.5).
In Situ Hybridization Histochemistry and Quantitative Analysis.
In situ hybridization was carried out as described (11, 13). Midbrain sections (≈12 μm thick) from different subjects were matched with regard to rostrocaudal plane and processed in parallel by using 35S-labeled antisense riboprobes for hNURR1 (nucleotides 7,822–8,199 from GenBank accession no. AB017586), hDAT (nucleotides 509–848; ref. 14), and human vesicular monoamine transporter 2 (VMAT2, nucleotides 682–883 from GenBank accession no. NM_003054). Signal intensity (grain density) within 50 dopamine neurons, spanning the entire mediolateral extent of the substantia nigra ventral tier, was quantified under dark field illumination (11, 13) by an investigator blind to any information regarding the subjects.
Immunohistochemistry.
Fresh-frozen thaw-mounted tissue sections were fixed in 3% paraformaldehyde and then incubated for 48 h with anti-NURR1 polyclonal antibody (1:100; Santa Cruz Biotechnology). NURR1-immunoreactivity was visualized by using a standard avidin-biotin-based procedure (Vector Laboratories) with nickel enhancement of 3,3′-diaminobenzidine as chromagen.
Results
NURR1 Gene Expression in the Human Midbrain.
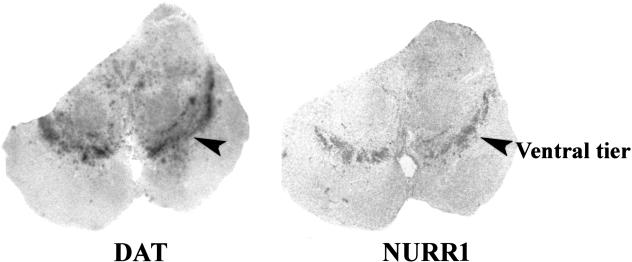
Because the distribution of NURR1 gene expression in human brain has not been reported previously, we first examined NURR1 expression using coronal slices of human midbrain. The NURR1 gene was found to be expressed abundantly in the ventral midbrain with a pattern precisely paralleling that of the DAT, a highly specific phenotypic marker of dopamine neurons (Fig. 1). At a microscopic level, NURR1 mRNA was visualized within nearly every midbrain dopamine cell (Fig. 2A). No NURR1 probe binding was seen in the presence of 100-fold excess of unlabeled probe (data not shown).
Figure 1.
Distribution of DAT and NURR1 gene expression in human midbrain. Autoradiographic experiments revealed a parallel distribution of DAT mRNA and NURR1 mRNA in adjacent coronal sections of adult human midbrain. The region of highest signal intensity, the ventral tier of the substantia nigra (indicated by the arrowheads), was subjected to further analyses.
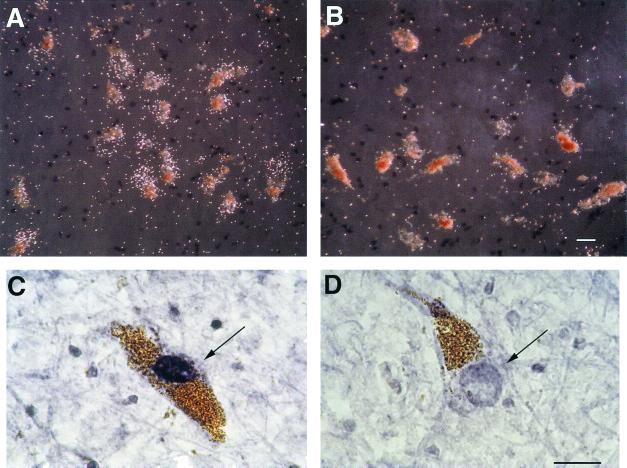
Figure 2.
Decreased NURR1 mRNA and protein abundances within the dopamine neurons of cocaine abusers. (A and B) NURR1 mRNA, visualized as grains overlying neuromelanin-containing dopamine neurons, in a representative drug-free subject (A) and cocaine abuser (B). (C and D) NURR1 immunoreactivity (indicated by the arrows) within the nucleus of a dopamine neuron from a drug-free control subject (C) and a cocaine abuser (D). (Scale bars, 25 μm.)
Quantification of NURR1 Gene Expression in Cocaine Abusers and Control Subjects.
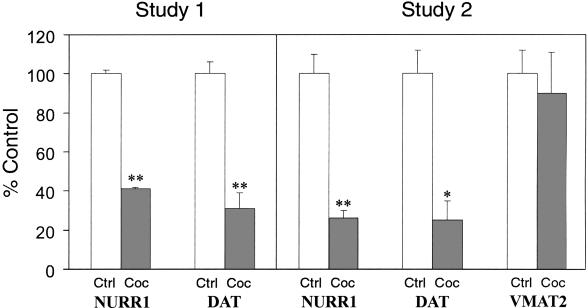
To determine the effects of chronic cocaine abuse on NURR1 gene expression in these neurons, the dopamine cell-rich ventral tier of the substantia nigra of cocaine abusers and matched, drug-free control subjects were analyzed by an experimenter blind to subject identity. In contrast to drug-free controls, we found that dopamine neurons of cocaine abusers expressed very low levels of NURR1 mRNA (compare Fig. 2 A with B). Quantitative analysis confirmed a significant reduction in the abundance of NURR1 mRNA in cocaine abusers (Fig. 4). To assess the robustness of this finding, we analyzed a second, independent set of subjects from a second study site. We found nearly identical cocaine-related decreases in NURR1 mRNA abundance in these subjects (Fig. 4). In keeping with the diminished abundance of NURR1 mRNA, NURR1-immunoreactivity also was decreased significantly within the nuclei of dopamine cells of cocaine abusers compared with matched controls (compare Fig. 2 D with C).
Figure 4.
Summary of changes in gene expression within the dopamine neurons of cocaine abusers. Bars represent the means ± SE (n = 5–7 for study 1; n = 6–9 for study 2). *, P < 0.001; **, P < 0.0001. Ctrl, control subjects; Coc, cocaine abusers.
DAT and VMAT2 Gene Expression in Cocaine Abusers and Control Subjects.
Because the transcription factor NURR1 regulates DAT gene transcription in vitro (7, 8), DAT gene expression also was examined in these subjects. The normally high abundance of DAT mRNA within ventral tier dopamine cells of control subjects (Fig. 1) was reduced by 70–75% in both study groups of cocaine abusers studied compared with drug-free controls (individual cases shown in Fig. 3 A and B; data summarized in Fig. 4). There was a significant correlation (r2 = 0.7067, P < 0.0005, n = 20) between DAT and NURR1 mRNA abundances among the subjects.
Figure 3.
Decreased DAT mRNA but unchanged VMAT2 mRNA levels within dopamine neurons of cocaine abusers. (A and B) DAT mRNA in a control subject (A) and cocaine abuser (B). (C and D) VMAT2 mRNA in a control subject (C) and cocaine abuser (D). (Scale bar, 25 μm.)
The VMAT2 represents a distinct transporter protein expressed in high abundance within dopamine neurons but not known to be regulated by NURR1. VMAT2 mRNA abundance was unaltered in the dopamine neurons of the cocaine abusers who exhibited striking losses of NURR1 and DAT gene expression (compare Fig. 3 C with D; Fig. 4).
Discussion
We show here that NURR1, a transcription factor critical for the development of the midbrain dopamine cell phenotype, continues to be expressed abundantly in adult human dopamine neurons. These data are consistent with findings in the adult rodent brain (15) and suggest that NURR1 may play a role in the maintenance, as well as the development, of the dopamine phenotype. We have demonstrated previously that NURR1 activates transcription of the dopamine cell-specific DAT gene in cell culture (7, 8). The corresponding cocaine-related decreases in NURR1 and DAT gene expression seen in the present study are consistent with the possibility that NURR1 exerts a significant influence over the level of human DAT gene expression in vivo as well.
The cocaine-related reduction in DAT gene expression that we find in the present study is consistent with recent reports associating cocaine abuse or cocaine-induced excited delirium with decreased DAT mRNA (16, 17) and DAT protein (18). Inconsistent changes in DAT ligand binding (17–19) may reflect modulation of the binding sites, phosphorylation state or subcellular distribution of the DAT in the brains of cocaine abusers. In any case, because the DAT is a major site of action for cocaine in the brain, cocaine-induced decreases on DAT gene transcription could represent an important component of the compensatory mechanisms that occur as a result of chronic drug exposure.
We found that although DAT mRNA levels were decreased in cocaine abusers, there was no change in the level of VMAT2 mRNA. VMAT2 mediates the intracellular storage of dopamine (and other monoamine neurotransmitters) and serves as another phenotypic marker for midbrain dopamine neurons (20). A number of conditions that change DAT expression do not alter VMAT2, which is thought to provide an excellent index of dopamine cell integrity (10, 21). Therefore the data suggest that changes in NURR1 and DAT expression seen in cocaine abusers are gene-specific and not indicative of some generalized pathological process within midbrain dopamine neurons. Further studies will be required to elucidate the cellular and molecular mechanisms linking chronic cocaine abuse to decreased NURR1 gene expression.
Profiling drug-related changes in transcription factor expression in human brain may provide a forensic assay for chronic cocaine abuse. Such studies may extend our knowledge of the molecular basis of addiction and ultimately lead to the development of novel therapeutic strategies.
Acknowledgments
We thank Drs. Gregory Kapatos and Jonathan Pollock for their helpful suggestions and encouragement. We also thank Dr. Robert Edwards for the human VMAT2 clone. Finally, we acknowledge Ms. Lisa Brownschidle and Ms. Margaret Basile for their excellent technical assistance. These studies were supported by National Institutes of Health Grant DA06470.
Abbreviations
- DAT
dopamine transporter
- VMAT2
vesicular monoamine transporter 2
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hyman S E. Nat Neurosci. 1999;2:855–856. doi: 10.1038/13147. [DOI] [PubMed] [Google Scholar]
- 2.Mages H W, Rilke O, Bravo R, Senger G, Kroczek R A. Mol Endocrinol. 1994;8:1583–1591. doi: 10.1210/mend.8.11.7877627. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zetterstrom R H, Solomin L, Jansson L, Hoffer B J, Olson L, Perlmann T. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 5.Saucedo-Cardenas O, Quintana-Hau J D, Le W D, Smidt M P, Cox J J, De Mayo F, Burbach J P, Conneely O M. Proc Natl Acad Sci USA. 1998;95:4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castillo S O, Baffi J S, Palkovits M, Goldstein D S, Kopin I J, Witta J, Magnuson M A, Nikodem V M. Mol Cell Neurosci. 1998;11:36–46. doi: 10.1006/mcne.1998.0673. [DOI] [PubMed] [Google Scholar]
- 7.Sacchetti P, Brownschidle L A, Granneman J G, Bannon M J. Mol Brain Res. 1999;74:167–174. doi: 10.1016/s0169-328x(99)00275-2. [DOI] [PubMed] [Google Scholar]
- 8.Sacchetti P, Mitchell T, Granneman J G, Bannon M J. J Neurochem. 2001;76:1565–1572. doi: 10.1046/j.1471-4159.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- 9.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature (London) 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 10.Staley J K, Talbot J Z, Ciliax B J, Miller G W, Levey A I, Kung M P, Kung H F, Mash D C. Brain Res. 1997;747:219–229. doi: 10.1016/s0006-8993(96)01196-1. [DOI] [PubMed] [Google Scholar]
- 11.Bannon M J, Whitty C J. Neurology. 1997;48:969–977. doi: 10.1212/wnl.48.4.969. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez A, Andollo W, Hearn W L. Forensic Sci Int. 1994;65:149–156. doi: 10.1016/0379-0738(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 13.Whitty C J, Paul M A, Bannon M J. J Comp Neurol. 1997;382:394–400. [PubMed] [Google Scholar]
- 14.Bannon M J, Poosch M S, Xia Y, Goebel D J, Cassin B, Kapatos G. Proc Natl Acad Sci USA. 1992;89:7095–7099. doi: 10.1073/pnas.89.15.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Q, Castillo S O, Nikodem V M. Neuroscience. 1996;75:221–230. doi: 10.1016/0306-4522(96)00159-5. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Segal D M, Moraes C T, Mash D C. Mol Brain Res. 1999;73:181–185. doi: 10.1016/s0169-328x(99)00233-8. [DOI] [PubMed] [Google Scholar]
- 17.Little K Y, McLaughlin D P, Zhang L, McFinton P R, Dalack G W, Cook E H, Jr, Cassin B J, Watson S J. Arch Gen Psychiatry. 1998;55:793–799. doi: 10.1001/archpsyc.55.9.793. [DOI] [PubMed] [Google Scholar]
- 18.Wilson J M, Levey A I, Bergeron C, Kalasinsky K, Ang L, Peretti F, Adams V I, Smialek J, Anderson W R, Shannak K, et al. Ann Neurol. 1996;40:428–439. doi: 10.1002/ana.410400312. [DOI] [PubMed] [Google Scholar]
- 19.Hurd Y L, Herkenham M. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- 20.Peter D, Finn J P, Klisak I, Liu Y, Kojis T, Heinzmann C, Roghani A, Sparkes R S, Edwards R H. Genomics. 1993;18:720–723. doi: 10.1016/s0888-7543(05)80383-0. [DOI] [PubMed] [Google Scholar]
- 21.Bannon M J, Sacchetti P, Granneman J G. In: Psychopharmacology: The Fourth Generation of Progress. Watson S J, editor. Williams & Wilkins, Philadelphia: Lippincott; 2000. http://www.acnp.org/G4 , http://www.acnp.org/G4. . [Google Scholar]