Abstract
We have investigated the role of cAMP in NO- and prostanoid-independent relaxations that are widely attributed to an endothelium-derived hyperpolarizing factor (EDHF). Under control conditions EDHF-type relaxations evoked by acetylcholine (ACh) in rabbit iliac arteries were transient, but in the presence of the cAMP phosphodiesterase inhibitor isobutylmethylxanthine (IBMX) or the cell permeant cAMP analog 8-bromo-cAMP, relaxations became sustained with their maxima potentiated ≈2-fold. Relaxation was associated with transient ≈1.5-fold elevations in smooth muscle cAMP levels with both mechanical and nucleotide responses being abolished by interrupting gap junctional communication with the connexin-mimetic peptide Gap 27 and by endothelial denudation. However, IBMX induced a sustained endothelium-independent ≈2-fold rise in cAMP levels, which was not further amplified by ACh, suggesting that the contribution of cAMP to the EDHF phenomenon is permissive. After selective loading of the endothelium with calcein AM, direct transfer of dye from the endothelium to the media was enhanced by IBMX or 8-bromo-cAMP, but not by 8-bromo-cGMP, whereas Gap 27 promoted sequestration within the intima. ACh-induced hyperpolarizations of subintimal smooth muscle in arterial strips with intact endothelium were abolished by Gap 27 and the adenylyl cyclase inhibitor 2′,5′-dideoxyadenosine but were unaffected by IBMX. By contrast, in strips partially denuded of endothelium, IBMX enhanced the transmission of hyperpolarization from the endothelium to remote smooth muscle cells. These findings support the hypothesis that endothelial hyperpolarization underpins the EDHF phenomenon, with cAMP governing subsequent electrotonic signaling via both myoendothelial and homocellular smooth muscle gap junctions.
Keywords: connexin‖acetylcholine‖IBMX‖8-bromo-cAMP‖cGMP
It is widely recognized that agonists such as acetylcholine (ACh) evoke endothelial hyperpolarization and smooth muscle relaxations that are independent of the synthesis of nitric oxide (NO) and vasodilator prostanoids (1). In guinea pig and hamster arterioles, hyperpolarizations induced either by ACh or the injection of electrical current into the endothelium may be transmitted to smooth muscle cells through a passive electrotonic mechanism, and in the converse sense, action potentials originating in smooth muscle cells can be detected in the endothelium (2–4). Such direct intercellular communication depends on the coupling of apposing cells by gap junctions, which are constructed from connexin (Cx) proteins and possess an aqueous central pore (5). In arterioles, myoendothelial gap junctions behave as simple ohmic resistors without rectification, so that complex electrical events are reduced in amplitude by 10–20% when conducted between the endothelium and media in either direction, but are preserved in temporal form (4). By contrast, in thick-walled arteries, it has been argued that the endothelium cannot act as a significant source of hyperpolarizing current because the large bulk of the media will result in current dissipation (6). An alternative hypothesis, therefore, is that an endothelium-derived hyperpolarizing factor (EDHF) is released into the extracellular space by the endothelium on stimulation with ACh to activate smooth muscle K+ channels and thereby mediate relaxation (7, 8). However, bioassay experiments with sandwich preparations of rabbit conduit arteries, constructed from closely apposed strips of endothelium-intact and -denuded vessel, fail to demonstrate the release of a freely diffusible EDHF after administration of ACh (9–11). Because there is no gap junctional communication between the “donor” endothelium and “detector” smooth muscle in such vessel composites, direct endothelial-smooth muscle coupling thus appears central to the EDHF-type response evoked by endothelium-dependent agonists.
Previous studies have nevertheless provided evidence that EDHF-type responses evoked by ACh in rabbit conduit arteries may not be exclusively passive because relaxation is associated with endothelium-dependent elevations in smooth muscle cAMP levels, and therefore attenuated by inhibition of adenylyl cyclase with P-site agonists such as 2′,5′-dideoxyadenosine (2′,5′-DDA) and inhibition of protein kinase A (12). Furthermore, EDHF-type relaxations are potentiated by inhibition of cAMP hydrolysis with the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX), while remaining susceptible to the combination of apamin plus charybdotoxin (12). Sensitivity to these peptide toxins is a hallmark of the EDHF phenomenon and thought to reflect the opening of apamin-sensitive small conductance K+ channels (SKCa) and charybdotoxin-sensitive large and intermediate conductance K+ channels (BKCa and IKCa) located on endothelial cells (1). Consistent with a role for gap junctions, EDHF-type relaxations and cAMP accumulation evoked by ACh are inhibited by synthetic connexin-mimetic peptides that interrupt intercellular communication in a connexin-specific fashion and by 18α-glycyrrhetinic acid, an aglycone that disrupts gap junction plaques at points of cell–cell contact (11–13).
Because there is evidence that elevations in cAMP levels may rapidly enhance gap junctional communication (14–18), in the present study we have tested the hypothesis that this nucleotide underpins EDHF-type relaxations in conduit arteries by facilitating the electrotonic spread of hyperpolarizing current from the endothelium into and through the media. Mechanical responses were correlated with measurements of smooth muscle cAMP levels and membrane potential, and modulation of gap junctional permeability by cAMP was assessed by a dye transfer technique whereby the entire endothelial monolayer was loaded with the cell permeant tracer calcein AM. In addition to providing electrical continuity, gap junctions allow the transfer of molecules <1,000 Da in size between coupled cells (5), so that after intracellular cleavage of the acetoxymethyl moiety responsible for endothelial uptake, a reduction in molecular mass (from 1,000 to 623 Da) allows diffusion of calcein into the media. Connexins may be classified on the basis of molecular mass (in kDa) with three main subtypes (Cxs 37, 40, and 43) being found in endothelial and vascular smooth muscle cells in rabbit arteries (19). In the present study, a peptide homologous to the Gap 27 domain of the second extracellular loop of Cxs 37 and 43 was used to interrupt gap junctional communication, and has previously been shown to inhibit EDHF-type responses through a receptor-independent mechanism that does not involve nonspecific effects on contraction, relaxation, or hyperpolarization (20–22).
Materials and Methods
Iliac and femoral arteries were removed from male New Zealand White rabbits (2–2.5 kg) killed with sodium pentobarbitone (120 mg/kg; i.v.), transferred to oxygenated (95% O2/5% CO2, pH 7.4) Holmans buffer (composition in mM: 120 NaCl, 5 KCl, 2.5 CaCl2, 1.3 NaH2PO4, 25 NaHCO3, 11 glucose, and 10 sucrose) at room temperature, and stripped of adherent tissue.
Mechanical Responses.
Iliac artery rings 2–3 mm wide were suspended in organ chambers containing oxygenated Holmans buffer at 37°C with resting tension set at ≈0.25 g. During an initial equilibrium period of 1 h, the tissues were repeatedly washed with fresh buffer, and tension was readjusted after stress relaxation, followed by incubation for 40 min with NG-nitro-l-arginine methyl ester (l-NAME, 300 μM) and 10 μM indomethacin. Rings were constricted with phenylephrine (PE, 1 μM) and cumulative concentration-relaxation curves to ACh constructed. Some preparations also were incubated with Gap 27 (amino acid sequence SRPTEKTIFII, 600 μM; Sigma Genosys, UK) or with 20 μM IBMX for 40 min before addition of ACh. As IBMX depressed contraction, the concentration of PE used in experiments with this agent was increased from 1 to 3 μM. In separate experiments, rings constricted by 1 μM PE were partially relaxed by 1 mM 8-bromo-cAMP, and tension was restored to control level with 3 μM PE before concentration-relaxation curves for ACh were constructed. The endothelium-dependent nature of responses to ACh was confirmed in rings from which the endothelium had been removed by gentle abrasion.
RIA.
Unmounted rings of iliac artery were incubated in oxygenated Holmans solution (37°C) containing l-NAME and indomethacin in the presence or absence of Gap 27 or IBMX as described above. Subsequently, 1 μM PE was introduced into the buffer followed by 3 μM ACh after 3 min. The rings were then frozen in liquid N2 at time points up to 180 s and stored at −70°C before extraction in 6% trichloroacetic acid, neutralization with water saturated diethyl ether, and RIA of cAMP and cGMP content (Amersham Biosciences). Nucleotide levels were expressed relative to protein content determined by a dye-binding assay (Bio-Rad). Previous studies have shown that nucleotide measurements in iliac artery rings reflect their smooth muscle content as the mass of the endothelium is negligible compared to the media (11, 12).
Dye Transfer.
Femoral arteries were mounted in a Living Systems perfusion myograph (Living Systems Instrumentation, Burlington, VT) and perfused with oxygenated Holmans buffer (35°C) at a flow rate of 0.1 ml/min at a constant pressure of 25 mmHg. The vessels were allowed to equilibrate for 30 min then perfused with 300 μM l-NAME and 10 μM indomethacin for 60 min followed, additionally, by either 600 μM Gap 27, 20 μM IBMX, 500 μM 8-bromo-cAMP, or 500 μM 8-bromo-cGMP for 30 min. The preparations were allowed to return to room temperature, and 10 μM calcein AM (Molecular Probes) was perfused through the lumen for 30 min before washout with dye-free buffer at 35°C for 30 min. In control experiments arteries were perfused with 10 μM calcein, which is membrane impermeable. The vessels were subsequently removed from the myograph and fixed in 0.1 M PBS containing 0.2% glutaraldehyde and 2% formalin for 90 min before cryopreservation in OCT compound (Agar Scientific, Stanstead, UK), cooled by liquid N2. Cryosections of transverse rings (10 μm thick) were prepared and mounted onto poly-l-lysine-coated slides under Fluorsave (Calbiochem) and imaged with a TCS four-dimensional confocal laser scanning system (Leica) with the filters set for 490 nm excitation and 525 nm emission. A gallery of several images was collected at 0.5-μm steps for each sample followed by image processing by using Leica scanware software to obtain a maximum projection image. A pixel intensity profile across the vessel wall was then obtained with matlab software and fitted to a monoexponential to derive a space constant describing the decay of medial fluorescence as a function of distance from the intima, i.e., the distance over which fluorescence decremented by 1/e or ≈63%.
Microelectrode Studies.
Iliac artery strips were held adventitia down in an oxygenated (95% O2/5% CO2) organ chamber by a Harp slice grid (ALA Scientific Instruments, Westbury, NY) superfused (2 ml/min at 37°C) with Holmans solution containing 300 μM l-NAME and 10 μM indomethacin. Transmembrane potential was recorded with glass capillary microelectrodes (tip resistance of 60–110 MΩ) filled with 3 M KCl and connected to the headstage of a SEC-10LX amplifier (NPI Electronic, Tamm, Germany). Successful impalements were achieved after a sudden negative drop in potential from baseline and a stable signal for at least 2 min. To ensure recordings were made from smooth muscle cells, the microelectrode was advanced into the subintima by using a PCS-5000 micromanipulator (Burleigh Instruments, Fishers, NY) until there had been 2–3 such negative deflections. After first obtaining control responses to ACh by direct administration into the organ chamber under conditions of no flow, the strips were washed for 1 h with fresh buffer before incubation for 40 min with either 600 μM Gap 27, 20 μM IBMX, or 200 μM 2′,5′-DDA and then repeat exposure to ACh. In a separate series of experiments, the anatomically downstream one-half of the iliac artery strip was denuded of endothelium by abrasion (subsequently confirmed by staining with 1% Evans blue). The vessel was then exposed to ACh with the microelectrode impaled first in smooth muscle cells just within (≈0.2 mm) the endothelium-intact region and then downstream at a distance of 1.5 mm from the residual endothelium. This protocol was subsequently repeated after incubation with IBMX for 40 min. In other experiments the entire vessel was denuded of endothelium before the effects of ACh on membrane potential were recorded in the absence and presence of IBMX.
Statistical Analysis.
All data are given as mean ± SE, where n denotes the number of animals studied for each data point, and were compared by the Student's t test for paired or unpaired data as appropriate. P < 0.05 was considered as significant. Concentration-response curves and the time course of nucleotide accumulation were assessed by ANOVA followed by the Bonferroni multiple comparisons test.
Results
Mechanical Responses.
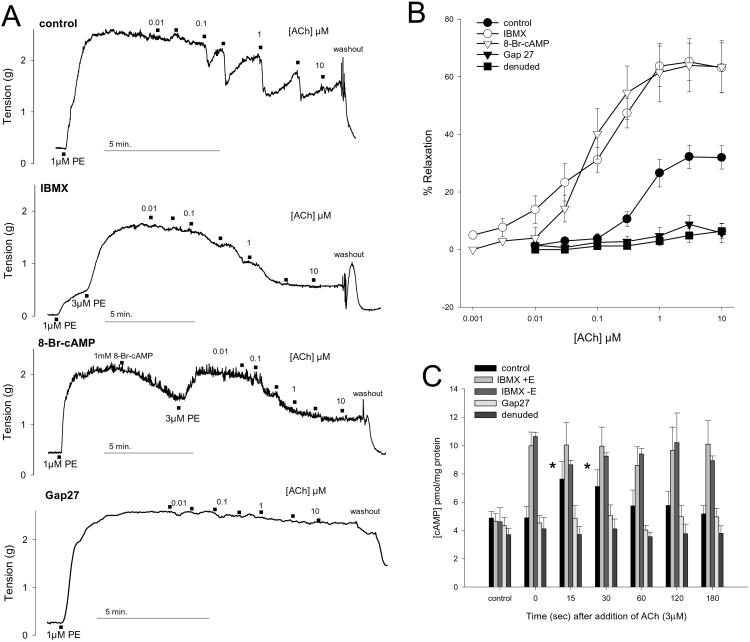
EDHF-type relaxations evoked by ACh reached a maximum of 32.2 ± 4.1% of PE-induced tone at a concentration of 3 μM with an EC50 value of 561 ± 100 nM (n = 14, Fig. 1). After incubation with 20 μM IBMX or 1 mM 8-bromo-cAMP, maximal relaxation was potentiated to 65.2 ± 6.5% and 64.0 ± 9.2% with leftward shifts in EC50 values to 99.5 ± 30.9 nM and 79.0 ± 10.0 nM, respectively (P < 0.05 in each case, n = 6 and 4; Fig. 1). Reductions in tone after administration of ACh were generally transient, but in the presence of IBMX or 8-bromo-cAMP they were sustained (Fig. 1). Preparations incubated with 600 μM Gap 27 or denuded of their endothelium were essentially devoid of vasorelaxant activity (n = 4 and 5; Fig. 1). Constrictions evoked by 1 μM PE were 1.86 ± 0.14 g (n = 14, data pooled from all experiments with intact endothelium) and were unaffected by incubation with Gap 27 or endothelial denudation. In experiments involving IBMX or 8-bromo-cAMP, initial tension was restored by 3 μM PE to levels that did not differ significantly from control at 1.90 ± 0.32 g and 1.95 ± 0.26 g (n = 6 and 4, respectively).
Figure 1.
Mechanical relaxations and cAMP accumulation in rabbit iliac artery rings incubated with 300 μM l-NAME and 10 μM indomethacin and constricted by 1 μM or 3 μM phenylephrine. (A) Representative traces demonstrating that EDHF-type relaxations to ACh were transient but sustained in the presence of 20 μM IBMX or 1 mM 8-bromo-cAMP. Responses were abolished by 600 μM Gap 27. (B) Concentration-relaxation curves confirming potentiation by IBMX and 8-bromo-cAMP and loss of response after endothelial denudation or incubation with Gap 27. Data shown for four to six animals in each case. (C) Under control conditions, 3 μM ACh induced a transient peak in cAMP levels in rings with intact endothelium but not in rings incubated with Gap 27 or from which the endothelium had been removed. IBMX (20 μM) caused equivalent sustained increases in cAMP levels in both endothelium-intact and -denuded preparations that were unaffected by subsequent addition of ACh. Histograms show results pooled from 4 to 10 experiments. *, P < 0.05 compared with time 0.
Nucleotide Accumulation.
Basal cAMP levels were ≈4 pmol/mg protein in both endothelium-intact and -denuded rings and not significantly altered by incubation with 300 μM l-NAME and 10 μM indomethacin and administration of 1 μM PE (n = 5 and 10, respectively). In preparations with endothelium 3 μM ACh induced a transient rise in cAMP levels that peaked at ≈7.5 pmol/mg protein after 15–30 s before falling toward baseline (n = 5, Fig. 1). This nucleotide response was abolished by endothelial removal and by incubation with 600 μM Gap 27 (n = 10 and 8, respectively; Fig. 1). cAMP levels were significantly increased to ≈10 pmol/mg protein in both endothelium-intact and -denuded rings incubated with 20 μM IBMX, but were not subsequently altered by administration of ACh (n = 7 and 4, respectively; Fig. 1). In rings with intact endothelium basal cGMP levels were 1.05 ± 0.23 pmol/mg protein (n = 13) and were reduced to 0.34 ± 0.08 pmol/mg protein on incubation with l-NAME, indomethacin, and PE (P < 0.05, n = 6) and to 0.47 ± 0.05 pmol/mg protein in the additional presence of 20 μM IBMX (P < 0.05, n = 7). In neither experimental group were cGMP levels significantly altered by administration of ACh (data not shown).
Dye Transfer.
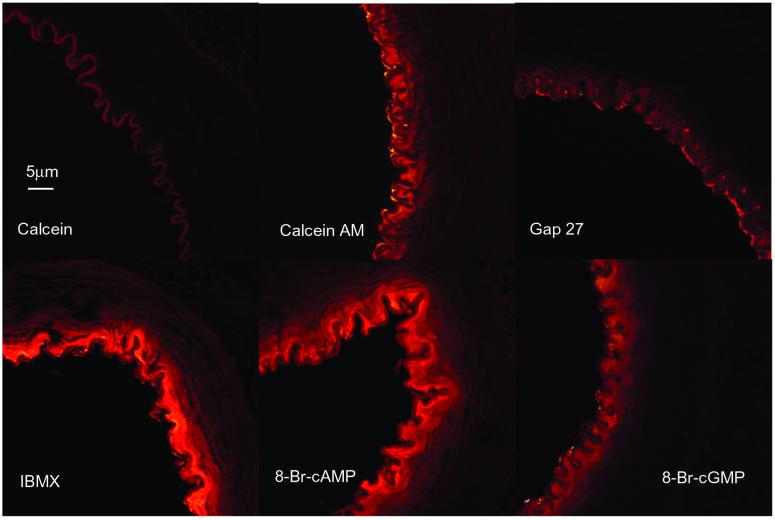
Dye was detected within the vessel wall after intraluminal perfusion with calcein AM but not calcein (Fig. 2). Under control conditions medial fluorescence decayed with a space constant of 8.54 ± 0.39 μm (n = 3), whereas dye was localized almost exclusively within the endothelium in arteries perfused with 600 μM Gap 27. Perfusion with 20 μM IBMX or 1 mM 8-bromo-cAMP significantly increased the space constant to 11.50 ± 0.80 and 12.50 ± 0.95 μm, respectively (n = 6 and 5, P < 0.05), whereas its value in arteries perfused with 1 mM 8-bromo-cGMP was 8.39 ± 0.44 μm (n = 4) and did not differ significantly from control.
Figure 2.
Dye transfer in isolated rabbit femoral arteries. After intraluminal perfusion with calcein, only autofluorescence of the internal elastic lamina was evident, whereas the cell permeant calcein AM allowed penetration of dye into subjacent smooth muscle cells. Diffusion of dye was prevented by 600 μM Gap 27, enhanced by 20 μM IBMX and 1 mM 8-bromo-cAMP, but unaffected by 1 mM 8-bromo-cGMP. All images shown at the same magnification.
Smooth Muscle Membrane Potential.
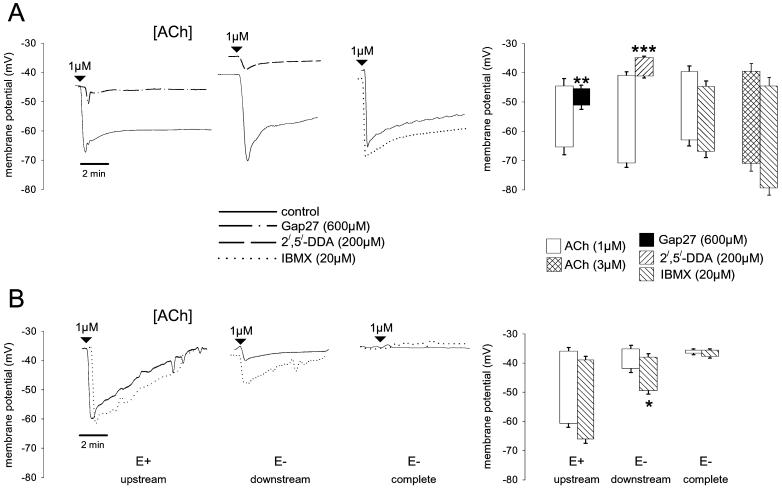
In arteries with intact endothelium, 600 μM Gap 27 did not affect the resting membrane potential of subintimal smooth muscle cells which was −45.3 ± 3.8 mV compared to −46.7 ± 3.0 mV in the presence of the peptide (n = 3). However, Gap 27 attenuated hyperpolarizations evoked by 1 μM ACh with maximal responses reduced from −19.4 ± 2.3 mV to −5.0 ± 1.0 mV (P < 0.01, n = 3, Fig. 3) and sustained hyperpolarization reduced from −11.2 ± 2.1 mV to −1.3 ± 0.8 mV (P < 0.05, n = 3, Fig. 3). Smooth muscle cells were slightly depolarized by 200 μM 2′,5′-DDA from −41.7 ± 3.2 mV to −36.0 ± 1.7 mV (n = 3). In this series of experiments, maximal and sustained hyperpolarizations to 1 μM ACh were −27.0 ± 1.5 mV and −16.8 ± 2.8 mV and were reduced to −5.7 ± 0.4 mV and −1.5 ± 0.6 mV, respectively, in the presence of 2′,5′-DDA (P < 0.005 and n = 3 in each case, Fig. 3). Under resting conditions 20 μM IBMX slightly hyperpolarized smooth muscle cells from −40.6 ± 2.2 mV to −44.9 ± 2.1 mV (n = 9). In this series of experiments, maximal and sustained hyperpolarizations evoked by 1 μM ACh were −21.6 ± 2.1 mV and −14.8 ± 3.4 mV in the absence and −20.9 ± 2.6 mV and −13.4 ± 3.8 mV in the presence of IBMX and did not differ significantly (n = 9, Fig. 3). Maximal and sustained hyperpolarizations evoked by 3 μM ACh were −29.3 ± 4.2 mV and −15.2 ± 5.2 mV in the absence and −32.4 ± 2.9 mV and −17.4 ± 5.6 mV in the presence of IBMX, and were similarly unaffected by phophodiesterase inhibition (n = 5, Fig. 3).
Figure 3.
Effects of ACh on subintimal smooth muscle membrane potential in strips of rabbit iliac artery. (A) Representative traces from endothelium-intact preparations showing rapid initial hyperpolarizations to 1 μM ACh that were sustained 10–15 mV below resting membrane potential. Changes in membrane potential were almost abolished by 600 μM Gap 27 and 200 μM 2′,5′-DDA but unaffected by 20 μM IBMX. Note that 2′,5′-DDA caused a small depolarization and IBMX a small hyperpolarization, whereas Gap 27 was without effect. Histograms show results pooled from three to nine such experiments. (B) Representative traces from strips in which the endothelium had been either partially (50%) or completely removed. IBMX did not affect hyperpolarizations evoked by 1 μM ACh in regions where the endothelium was still intact (E+ upstream). By contrast, small conducted hyperpolarizations detected 1.5 mm from the edge of the residual endothelium were amplified by IBMX (E− downstream). In strips entirely denuded of endothelium, there was no electrical response to ACh either in the presence or absence of IBMX (E− complete). Histograms show maximal potential changes pooled from three such experiments in each case. *, P < 0 05; **, P < 0.01, and ***, P < 0.001 compared with control.
In arterial strips from which the endothelium had been partly removed by abrasion, maximal hyperpolarizations evoked by 1 μM ACh were −22.5 ± 0.7 mV in the absence and −24.8 ± 1.1 mV in the presence of 20 μM IBMX when impalement of subintimal smooth muscle was just within the anatomically upstream region of the vessel that still possessed endothelium. When impalement was made 1.5 mm in the downstream denuded region, control maximal hyperpolarizations to 1 μM ACh were reduced to −5.5 ± 0.6 mV but were increased to −13.4 ± 2.7 mV by the presence of IBMX (P < 0.05, n = 3, Fig. 3). In strips completely denuded of endothelium, no hyperpolarizing response could be detected either in the presence or absence of IBMX (Fig. 3).
Discussion
The present study has identified a permissive role for cAMP in the genesis of NO- and prostanoid-independent EDHF-type arterial relaxations evoked by ACh. The salient finding is that the signaling pathways that underlie such responses in isolated ilio-femoral rabbit arteries depend on the electrotonic spread of hyperpolarization from the endothelium into the media via myoendothelial and homocellular smooth muscle gap junctions whose permeability and electrical conductance are regulated by cAMP.
In ring preparations constricted by phenylephrine in the presence of l-NAME and indomethacin, ACh generally evoked transient relaxations in which an initial rapid decrease in tone over seconds returned toward control level within ≈2 min. Concentrations of ACh that caused maximal relaxation (≈35% of developed tone) also evoked transient ≈1.5-fold increases in smooth muscle cAMP levels, as previously reported (11, 12). New insights into the role of cAMP in the EDHF phenomenon were provided by the potentiation of EDHF-type relaxations to ACh in rings incubated with IBMX, when responses became sustained rather than transient in nature. This amplifying effect of IBMX was not dependent on dynamic increases in cAMP accumulation as this phophodiesterase inhibitor induced similar sustained ≈2-fold elevations in the cAMP content of rings both with and without intact endothelium, and 8-bromo-cAMP, a cell permeant analog of cAMP, also potentiated and prolonged mechanical responses to ACh. Direct measurement of cGMP levels confirmed that blockade of NO synthesis was essentially complete under the experimental conditions used and that IBMX, which inhibits hydrolysis of both cGMP and cAMP (23), did not potentiate the EDHF-type relaxant response to ACh by amplifying the biochemical consequences of residual NO activity (24).
The central involvement of endothelial-smooth muscle coupling via gap junctions was confirmed by observations that relaxations and cAMP accumulation were both abolished by endothelial denudation and incubation with the connexin-mimetic peptide Gap 27. Because there is evidence that cAMP can enhance the molecular permeability and electrical conductance of gap junctions (14–18), we therefore investigated its ability to modulate dye transfer in the vessel wall. Attempts to demonstrate communication via myoendothelial gap junctions by injecting tracer dyes into individual endothelial cells are usually unsuccessful (25–27), presumably because of preferential diffusion of dye between cells in the endothelial monolayer, which are highly coupled by large gap junction plaques (19, 28). To overcome this “sink-source” problem, we therefore selectively loaded the entire endothelium of femoral artery segments by intraluminal perfusion with the cell permeant dye calcein AM (29), so that fluorescence could be assessed in the media by confocal microscopy after hydrolysis to calcein. Transfer of calcein from the endothelium to subjacent smooth muscle cells was enhanced to an equivalent extent by IBMX and 8-bromo-cAMP, with the space constant for diffusion derived by quantitative image analysis increased from ≈8 to ≈12 μm in each case. Enhancement of dye transfer appeared specific for cAMP/8-bromo-cAMP because no analogous effect was observed after incubation with 8-bromo-cGMP. Although the role of myoendothelial gap junctions was confirmed by experiments with Gap 27, which markedly restricted penetration of calcein into the media, cAMP also may modulate the subsequent diffusion of calcein via gap junctions coupling smooth muscle cells because there was substantially greater smooth muscle fluorescence in preparations incubated with IBMX or 8-bromo-cAMP.
To further delineate the roles of myoendothelial and smooth muscle gap junctions in the EDHF phenomenon, we investigated the effects of ACh on smooth muscle membrane potential in arterial strips impaled via their intimal surface. In the presence of intact endothelium, 1 and 3 μM ACh evoked rapid subintimal hyperpolarizations of ≈20 and ≈30 mV, respectively, followed by a rise to a plateau that was generally sustained 10–20 mV below baseline for at least 10 min, thus contrasting with the transience of the associated mechanical responses. Such ACh-evoked smooth muscle hyperpolarizations correspond closely in magnitude and time course to those reported for the endothelium of the rabbit aortic valve (30) and are therefore likely to reflect electrotonic spread of hyperpolarization via myoendothelial gap junctions. Indeed, Gap 27 effectively abolished subintimal smooth muscle hyperpolarizations evoked by ACh, and no changes in membrane potential were observed in strips devoid of endothelium. In partially denuded strips, remote conducted smooth muscle hyperpolarizations, monitored ≈1.5 mm from the edge of the unabraded endothelium, were markedly attenuated and abbreviated relative to control, but were prolonged and amplified ≈3-fold in magnitude after inhibition of cAMP hydrolysis with IBMX. These findings suggest that the relatively brief elevation in smooth muscle cAMP levels evoked by ACh, which persisted for just 15–30 s, is unable to support sustained conduction of endothelial hyperpolarization into the outer layers of the media, whereas hyperpolarization and relaxation are prolonged by the stable elevations in smooth muscle cAMP levels that result from inhibition of cAMP hydrolysis by IBMX. The observation that subintimal hyperpolarizations evoked by ACh when the overlying endothelium was intact were sustained and not amplified by IBMX suggests that myoendothelial gap junctions are selectively maintained in a high conductance state by the continuous 2-fold increase in endothelial cAMP synthesis that results from administration of ACh (12). Indeed, the adenylyl cyclase inhibitor 2′,5′-DDA, which has previously been shown to abolish EDHF-type relaxations and cAMP accumulation in rabbit iliac arteries (11, 12), abolished subintimal smooth muscle potential changes evoked by ACh. Although cAMP could theoretically hyperpolarize endothelial and smooth muscle cells by activating K+ channels directly (31, 32), this action is unlikely to contribute significantly to the EDHF phenomenon in rabbit arteries as IBMX hyperpolarized, and 2′,5′-DDA depolarized, subintimal muscle cells by <5 mV in strips with intact endothelium. Furthermore, IBMX did not affect smooth muscle membrane potential in endothelium-denuded preparations, despite causing sustained 2-fold increases in cAMP levels.
The KCa channel-mediated endothelial hyperpolarization that is critical to the EDHF phenomenon is sustained principally by capacitative Ca2+ influx triggered by depletion of the endoplasmic reticulum Ca2+ store by agonists such as ACh (33, 34). Indeed, cyclopiazonic acid, which also depletes this internal store by blocking Ca2+ uptake directly, stimulates receptor-independent EDHF-type relaxations in a range of artery types (20, 33–35). Because this agent also stimulates endothelial cAMP synthesis (36), capacitative Ca2+ influx may not simply regulate endothelial hyperpolarization but also its conduction into the media via myoendothelial gap junctions. Indeed, EDHF-type relaxations evoked by cyclopiazonic acid, like those evoked by ACh, are attenuated by 2′,5′-DDA and by Gap 27 in rabbit arteries (12). The mechanism responsible for elevations in smooth muscle cAMP levels, which may additionally enhance electrotonic conduction through the media, is less clear. Possibilities include: (i) diffusion of endothelium-derived cAMP into subjacent smooth muscle cells via gap junctions (13), (ii) hyperpolarization-induced reductions in smooth muscle [Ca2+]i, which could in theory activate the Ca2+-inhibited type V and VI adenylyl cyclase isoforms and/or suppress the Ca2+-stimulated type I phosphodiesterase (23, 37–39), and (iii) transfer via gap junctions of a novel mediator produced within the endothelium that stimulates adenylyl cyclase or inhibits cAMP hydrolysis within smooth muscle (10, 11). Each of these possibilities would be consistent with the observation that Gap 27 was able to prevent cAMP accumulation in iliac artery rings with intact endothelium.
In conclusion, we have demonstrated that EDHF-type relaxations evoked by ACh in rabbit arteries have an essential requirement for coexistent cAMP synthesis. The cellular actions of this nucleotide encompass specific characteristics of the EDHF phenomenon reported in the literature such as activation of hyperpolarizing K+ channels. However, the present findings suggest that its principal mode of action is to enhance electrotonic spread of endothelial hyperpolarization through the vessel wall. Although gap junction-dependent relaxations are most pronounced in small resistance arteries (19), consistent with an electrotonic aetiology, an associated synthesis of cAMP may be essential for EDHF-type relaxations in conduit arteries.
Acknowledgments
We thank Dr. D. Parthimos for help with image analysis software. The work was supported by the Medical Research Council.
Abbreviations
- ACh
acetylcholine
- 2′,5′-DDA
2′,5′-dideoxyadenosine
- IBMX
isobutylmethylxanthine
- EDHF
endothelium-derived hyperpolarizing factor
- l-NAME
NG-nitro-l-arginine methyl ester
- PE
phenylephrine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Edwards G, Weston A H. J Physiol (London) 2001;531:299. doi: 10.1111/j.1469-7793.2001.0299i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerson G G, Segal S S. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 3.Coleman H A, Tare M, Parkington H C. J Physiol (London) 2001;531:359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Klemm M F, Edwards F R, Suzuki H. J Physiol (London) 2001;535:181–195. doi: 10.1111/j.1469-7793.2001.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brink P R. Acta Physiol Scand. 1998;164:349–356. doi: 10.1046/j.1365-201X.1998.00439.x. [DOI] [PubMed] [Google Scholar]
- 6.Beny J-L. News Physiol Sci. 1999;14:68–73. doi: 10.1152/physiologyonline.1999.14.2.68. [DOI] [PubMed] [Google Scholar]
- 7.Campbell W B, Harder D R. Circ Res. 1999;84:484–488. doi: 10.1161/01.res.84.4.484. [DOI] [PubMed] [Google Scholar]
- 8.Popp R, Bauersachs J, Hecker M, Fleming I, Busse R. J Physiol (London) 1996;497:699–709. doi: 10.1113/jphysiol.1996.sp021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plane F, Pearson T, Garland C J. Br J Pharmacol. 1995;115:31–38. doi: 10.1111/j.1476-5381.1995.tb16316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutcheson I R, Chaytor A T, Evans W H, Griffith T M. Circ Res. 1999;84:53–63. doi: 10.1161/01.res.84.1.53. [DOI] [PubMed] [Google Scholar]
- 11.Chaytor A T, Taylor H J, Griffith T M. Am J Physiol. 2002;282:H1548–H1555. doi: 10.1152/ajpheart.00903.2001. [DOI] [PubMed] [Google Scholar]
- 12.Taylor H J, Chaytor A T, Edwards D H, Griffith T M. Biochem Biophys Res Commun. 2001;283:583–589. doi: 10.1006/bbrc.2001.4791. [DOI] [PubMed] [Google Scholar]
- 13.Griffith T M, Taylor H J. Biochem Biophys Res Commun. 1999;263:52–57. doi: 10.1006/bbrc.1999.1313. [DOI] [PubMed] [Google Scholar]
- 14.Burghardt R C, Barhoumi R, Sewall T C, Bowen J A. J Membr Biol. 1995;148:243–253. doi: 10.1007/BF00235042. [DOI] [PubMed] [Google Scholar]
- 15.Chanson M, White M M, Garber S S. Am J Physiol. 1996;271:C533–C539. doi: 10.1152/ajpcell.1996.271.2.C533. [DOI] [PubMed] [Google Scholar]
- 16.Abudara V, Eyzaguirre C, Saez J C. Adv Exp Med Biol. 2000;475:359–369. doi: 10.1007/0-306-46825-5_33. [DOI] [PubMed] [Google Scholar]
- 17.Gladwell S J, Jefferys J G. Neurosci Lett. 2001;300:1–4. doi: 10.1016/s0304-3940(01)01530-0. [DOI] [PubMed] [Google Scholar]
- 18.Grazul-Bilska A T, Reynolds L P, Bilski J J, Redmer D A. Biol Reprod. 2001;65:777–783. doi: 10.1095/biolreprod65.3.777. [DOI] [PubMed] [Google Scholar]
- 19.Berman R S, Martin P E M, Evans W H, Griffith T M. Microvasc Res. 2002;63:115–128. doi: 10.1006/mvre.2001.2352. [DOI] [PubMed] [Google Scholar]
- 20.Chaytor A T, Evans W H, Griffith T M. J Physiol (London) 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dora K A, Martin P E M, Chaytor A T, Evans W H, Garland C J, Griffith T M. Biochem Biophys Res Commun. 1999;254:27–31. doi: 10.1006/bbrc.1998.9877. [DOI] [PubMed] [Google Scholar]
- 22.Richards G R, Weston A H, Burnham M P, Feletou M, Vanhoutte P M, Edwards G. Br J Pharmacol. 2001;134:1–5. doi: 10.1038/sj.bjp.0704256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugnier C, Komas N. Eur Heart J. 1993;14, Suppl. 1:141–148. [PubMed] [Google Scholar]
- 24.Cohen R A, Plane F, Najibi S, Huk I, Malinski T, Garland C J. Proc Natl Acad Sci USA. 1997;94:4193–4198. doi: 10.1073/pnas.94.8.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segal S S, Beny J-L. Am J Physiol. 1992;263:H1–H7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- 26.Beny J-L, Pacicca C. Am J Physiol. 1994;266:H1465–H1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- 27.Little T L, Beyer E C, Duling B R. Am J Physiol. 1995;268:H729–H739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- 28.Chaytor A T, Martin P E, Edwards D H, Griffith T M. Am J Physiol. 2001;280:H2441–H2450. doi: 10.1152/ajpheart.2001.280.6.H2441. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg G S, Bechberger J F, Naus C C. BioTechniques. 1995;18:490–497. [PubMed] [Google Scholar]
- 30.Ohashi M, Satoh K, Itoh T. Br J Pharmacol. 1999;126:19–26. doi: 10.1038/sj.bjp.0702262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graier W F, Kukovetz W R, Groschner K. Biochem J. 1993;291:263–267. doi: 10.1042/bj2910263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wigham C G, Turner H C, Swan J, Hodson S A. Pflügers Arch. 2000;440:866–870. doi: 10.1007/s004240000357. [DOI] [PubMed] [Google Scholar]
- 33.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Br J Pharmacol. 1997;120:1328–1334. doi: 10.1038/sj.bjp.0701027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagaud G J, Skarsgard P L, Laher I, van Breemen C. J Pharmacol Exp Ther. 1999;290:832–839. [PubMed] [Google Scholar]
- 35.Taylor H J, Chaytor A T, Evans W H, Griffith T M. Br J Pharmacol. 1998;125:1–4. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamata K, Umeda F, Kasuya Y. J Cardiovasc Pharmacol. 1996;27:601–606. doi: 10.1097/00005344-199604000-00022. [DOI] [PubMed] [Google Scholar]
- 37.Bolz S S, De Wit C, Pohl U. Br J Pharmacol. 1999;128:124–134. doi: 10.1038/sj.bjp.0702775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oishi H, Budel S, Schuster A, Stergiopulos N, Meister J, Beny J-L. Cell Calcium. 2001;30:261–267. doi: 10.1054/ceca.2001.0233. [DOI] [PubMed] [Google Scholar]
- 39.Murthy K S, Makhlouf G M. Mol Pharmacol. 1998;54:122–128. doi: 10.1124/mol.54.1.122. [DOI] [PubMed] [Google Scholar]