Abstract
Plant HKT proteins comprise a family of cation transporters together with prokaryotic KtrB, TrkH, and KdpA transporter subunits and fungal Trk proteins. These transporters contain four loop domains in one polypeptide with a proposed distant homology to K+ channel selectivity filters. Functional expression in yeast and Xenopus oocytes revealed that wheat HKT1 mediates Na+-coupled K+ transport. Arabidopsis AtHKT1, however, transports only Na+ in eukaryotic expression systems. To understand the molecular basis of this difference we constructed a series of AtHKT1/HKT1 chimeras and introduced point mutations to AtHKT1 and wheat HKT1 at positions predicted to be critical for K+ selectivity. A single-point mutation, Ser-68 to glycine, was sufficient to restore K+ permeability to AtHKT1. The reverse mutation in HKT1, Gly-91 to serine, abrogated K+ permeability. This glycine in P-loop A of AtHKT1 and HKT1 can be modeled as the first glycine of the K+ channel selectivity filter GYG motif. The importance of such filter glycines for K+ selectivity was confirmed by interconversion of Ser-88 and Gly-88 in the rice paralogues OsHKT1 and OsHKT2. Surprisingly, all HKT homologues known from dicots have a serine at the filter position in P-loop A, suggesting that these proteins function mainly as Na+ transporters in plants and that Na+/K+ symport in HKT proteins is associated with a glycine in the filter residue. These data provide experimental evidence that the glycine residues in selectivity filters of HKT proteins are structurally related to those of K+ channels.
Transport proteins bind their substrates with high specificity but low energy barriers. The structural basis of this phenomenon is unknown for most transporters, with the exception of K+ channels. Functional mutagenesis of the Drosophila Shaker channel, computer modeling, and mapping of its substrate-binding site with channel-blocking toxins identified loops at the outer surface of the protein as critical for K+ selectivity (1–3). These P-loops are highly conserved in sequence and include a glycine-tyrosine-glycine (GYG) motif, the K+ channel signature (4). Mutation of either glycine of the GYG motif resulted in loss of K+ selectivity (4). Each of the channel's four α-subunits carries one P-loop between two transmembrane domains. The solution of the structure of the bacterial KcsA homotetramer revealed that the four P-loops indeed constitute the outer pore of the channel, the narrowest point being formed by the backbone carbonyl groups of the first glycines of the GYG motif (5). The selectivity of the channel for K+ is mediated by those filter glycines and by electrostatic interactions inside the pore (6–10).
HKT proteins from plants, TrkH subunits from bacteria, Trk1 from fungi, and cation-translocating subunits of bacterial K+- and Na+-ATPases have been hypothesized to harbor four P-loop-like domains in a single polypeptide chain and to resemble tetrameric K+ channels in pore architecture (11–13). This model is supported by genetically selected, model-independent mutations in HKT1 that increase K+ selectivity and reduce Na+ permeation, because mutations lie in the proposed P-loop domains (14, 15). In addition, site-directed mutations in a predicted P-loop altered the Na+ specificity of HKT/Trk/KtrB transporters (16, 17). The four-P-loop hypothesis has been further strengthened by the recent elucidation of the membrane topology of Arabidopsis AtHKT1 (18).
Wheat HKT1 was cloned by functional complementation of a K+-uptake-deficient yeast trk1,2 deletion mutant (19). HKT1 homologs were identified from Arabidopsis (20), eucalyptus (21), rice (22), and ice plant (H. Su and H. J. Bohnert, GenBank accession no. AF367366). Wheat HKT1 functions as a K+/Na+-symporter (14), and eucalyptus HKT-mediated K+ currents were stimulated by Na+ (21, 23). K+/Na+-symport has also been confirmed for the related bacterial transporters KtrB (17) and NtpJ (24). Arabidopsis AtHKT1 neither complements the yeast trk1,2 deletion nor mediates K+ currents in Xenopus oocytes (20). However, AtHKT1 caused inward Na+ currents in oocytes and Na+ hypersensitivity when expressed in yeast, showing that it functions as a Na+ and not a K+ transporter in eukaryotic expression systems. HKT1-type transporters have been suggested to contribute to Na+ transport and to affect salinity stress (14, 19, 20). K+/Na+ selectivity is of particular importance in plant nutrition. K+ fulfills a variety of physiological functions and is the most abundant cation in plants, whereas Na+ is toxic to glycophytes, including most crop plants (for reviews, see refs. 25–28).
To understand the molecular basis of K+/Na+ selectivity in HKT transporters, we constructed chimeras of wheat HKT1 and Arabidopsis AtHKT1 and introduced point mutations in the predicted first P-loop of either protein. We identified a glycine residue in the first P-loop as crucial for K+ selectivity and transport. The presented results provide strong evidence for the model that HKT/Trk-type transporters have K+ channel-related P-loops.
Materials and Methods
HKT Plasmid Construction.
HKT1-AtHKT1 and AtHKT1-HKT1 chimera were constructed by PCR followed by substitution of the corresponding regions in the plasmids pYES2:HKT1 and pYES2:AtHKT1 (14, 20). Mutagenesis of the plasmids pYES2:HKT1, pYES2:AtHKT1 and pYES2:OsHKT1 (14, 20, 22) was performed with the U.S.E. Mutagenesis kit (Amersham Pharmacia) and two-step PCR. All constructs were verified by sequencing.
Expression of HKTs in Yeast.
All transporter constructs were expressed under the control of the Gal1 promoter in the plasmid pYES2 (Invitrogen). Saccharomyces cerevisiae strain CY162 [MATa,Δtrk1, trk2∷pCK64, his3, leu2, ura3, trp1, ade2 (29)] and strain G19 [MATa, his3, leu2, ura3, trp1, ade2, ena1∷HIS3∷ena4 (30)] were grown in 8 mM H3PO4/12 mM arginine/2 mM MgSO4/0.2 mM CaCl2/0.8 g/liter CSM-ura (Bio101), supplemented with trace elements, vitamins, 2% galactose, 0.3% sucrose, and 1% agarose for plate assays. K+ concentration was adjusted with KCl. For complementation of the CY162 S. cerevisiae strain with chimeric HKTs, uracil-selective medium contained 6.7 g/liter yeast nitrogen base (Difco), 2% galactose, 2% sucrose, and 2% agar.
Recording of Xenopus Oocytes.
Capped complementary RNA (cRNA) of HKT1 and AtHKT1 was prepared with the mMESSAGE mMACHINE kit (Ambion, Austin, TX). Xenopus laevis oocytes were prepared as described (31). Oocytes were kept at 16°C in Barth's solution [88 mM NaCl/1 mM KCl/2.4 mM NaHCO3/0.33 mM Ca(NO3)2/0.41 mM CaCl2/0.82 mM MgSO4/10 mM Hepes-NaOH, pH 7.4]. Ionic currents were recorded 1 day after injection of cRNA (10 ng per oocyte) by two-electrode voltage clamping, in a bath solution containing 6 mM MgCl2, 1.8 mM CaCl2, the indicated concentrations of K+ and Na+ (as glutamate salts), 10 mM Mes-1,3-bis(tris[hydroxymethyl]methylamino)propane, pH 5.5, and osmolality of 240 mosmol/kg with D-mannitol. Voltage clamping and data acquisition were performed with voltage-clamp amplifiers (Cornerstone model TEV-200, Dagan Instruments, Minneapolis, or AxoClamp 2B, Axon Instruments, Foster City CA).
Collection and Analysis of Arabidopsis thaliana Ecotypes.
Seeds were obtained from the Arabidopsis Biological Resource Center (ABRC, Ohio State University, Columbus), kindly provided by J. Borevitz and J. Chory (Salk Institute). A 1,138-bp fragment of AtHKT1, comprising the codon for Ser-68 at nucleotide 286, was amplified from genomic DNA by PCR with the primers AtHKT1-start (5′-GGCTGACAATTTCCATGTACGTGTA-3′) and AtHKT1-antisense3 (5′-CCAAGATAGCTGGGGAAAGTGTA-3′). PCR products were digested with EarI (New England Biolabs).
Phylogenetic Analyses.
Sequence alignments, calculation of phylogenetic trees, and bootstrapping were performed with CLUSTALX (32). Dendrograms were displayed with TREEVIEW (33). For Fig. 7b, the following segments were used as P-loop-like domains A, B, C, and D, respectively: HKT1 77–93, 232–248, 356–372, 459–475; AtHKT1 54–70, 199–215, 323–339, 428–444; EcHKT1 81–97, 248–264, 372–388, 477–493; EcHKT2 80–96, 247–263, 371–387, 476–492; OsHKT1 and OsHKT2 74–90, 230–246, 354–370, 455–471; McHKT1 80–96, 202–218, 326–342, 431–447; PbHKT1 (D) LNITIEVVSAYGNVGFS.
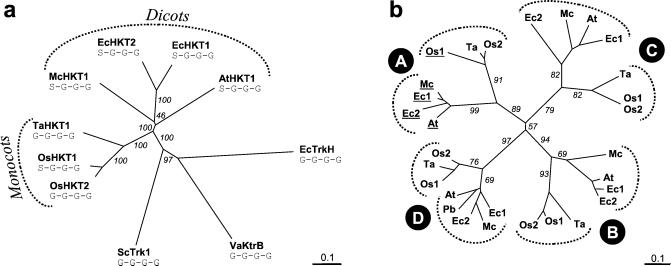
Figure 7.
Phylogenetic analysis of HKT proteins. (a) Dendrogram of the plant HKT family: Full-length sequences of HKT proteins were aligned with ClustalX (32). Escherichia coli EcTrkH (GenBank accession no. P21166), V. alginolyticus KtrB (BAA32063), and S. cerevisiae Trk1 (M21328) were included for comparison. Predicted filter residues of each of the four P-loops are indicated for all transporters. Numbers are the percentage of positives in 1,000 runs of bootstrapping. (b) Dendrogram of all P-loops from plant HKTs: Segments of 17 aa constituting the predicted P-loops A–D were extracted from the seven known plant HKTs and from a translated expressed sequence tag from poplar (PbHKT1). P-loops with a serine are underlined. Numbers are the percentage of positives in 1,000 runs of bootstrapping. Ta, HKT1 (GenBank accession no. AAA52749); At, AtHKT1 (AAF68393); Ec1, EcHKT1 (AAF97728); Ec2, EcHKT2 (AAD53890); Mc, McHKT1 (AAK52962); Os1, OsHKT1 (BAB61790); Os2, OsHKT2 (BAB61791); Pb, PbHKT1 (AI167044).
Results
The N Terminus of HKT1 Is Crucial for Rescue of K+ Deficiency in Yeast.
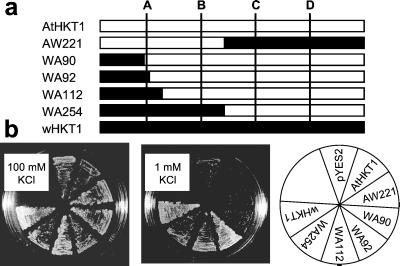
HKT1 (533 aa) and AtHKT1 (506 aa) share about 40% overall identity and exhibit almost identical hydropathy profiles (19, 20). To narrow down the region(s) required for K+ transport we constructed a series of wheat (“w”) HKT1-Arabidopsis AtHKT1 chimeras at aligned residues: AtHKT1 (1–221)-HKT1 (255–533) (AW221), HKT1 (1–90)-AtHKT1 (68–506) (WA90), HKT1 (1–92)-AtHKT1 (70–506) (WA92), HKT1 (1–112)-AtHKT1 (90–506) (WA112), and HKT1 (1–254)-AtHKT1 (222–506) (WA254; Fig. 1a). The chimeras and their parent proteins were expressed in the K+-uptake-deficient yeast mutant CY162 (34), deleted in the two K+ transporters Trk1 and Trk2. Expression of HKT1 permitted CY162 cells to grow on 1 mM K+, but AtHKT1 did not (Fig. 1b). Replacement of the N-terminal half of AtHKT1 with the corresponding part of HKT1 (WA254) rescued CY162, whereas the reciprocal construct (AW221) did not suppress the trk1,2 phenotype. The first 92 aa of HKT1 were sufficient to rescue CY162 cells when substituted into AtHKT1 (WA92); the first 90 (WA90), however, were not (Fig. 1b). All chimeras rendered CY162 cells more sensitive to Na+ (data not shown) as shown for HKT1 and AtHKT1 (14, 20). These data suggest that functionality of the chimeras was preserved. The fact that only constructs containing the N terminus of HKT1 rescued CY162 cells indicated the importance of that region for K+ transport.
Figure 1.
Expression of AtHKT1-HKT1 chimeras in yeast. (a) Schematic representation of chimera constructs. Parts from AtHKT1 are depicted in white and designated by an “A”, those from wheat HKT1 are in black and designated by a “W”. The predicted P-loop-like domains (A, B, C, D) of the transporters are indicated by lines. (b) Suppression of the K+-uptake-deficient phenotype by the chimeric proteins at 1 mM K+ (Middle). Expression of HKT1, WA254, WA112, and WA92 allowed growth at 1 mM K+. AtHKT1, AW221, and WA90, in contrast, did not suppress the trk1,2 null phenotype.
A Putative Filter Glycine for K+ Selectivity Is Absent in AtHKT1.
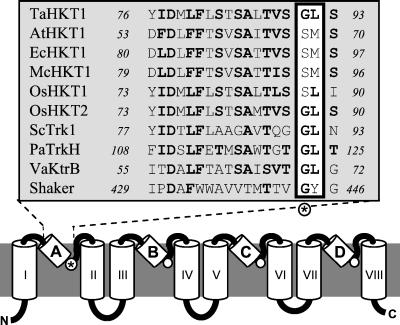
The presumed P-loops are highly conserved within HKT/Trk/KtrB proteins but have only about three to four amino acid residues homologous to the complete K+ channel P-loops (Fig. 2). Alignment of the presumed P-loops of HKT/Trk/KtrB proteins with known P-loops from K+ channels reveals a glycine residue in HKT/Trk/KtrB transporters at the position modeled as corresponding to the first glycine of the GYG motif (Fig. 2). HKT, Trk, KtrB each have four putative P-loops (11, 13, 18). The four glycines, one at the end of each of four P-loops, are invariably present throughout bacterial TrkH and KtrB proteins as well as fungal Trk transporters. The same holds true for HKT1, the four glycines being Gly-91, Gly-246, Gly-370, and Gly-477. In P-loop A of AtHKT1, however, a serine (Ser-68) is at the expected filter position (Fig. 2), whereas the following three glycines are preserved (Gly-213, Gly-337, Gly-446). Furthermore, whereas in P-loop A of other transporters the following residue is predominantly a leucine (i.e., Leu-92 in HKT1), in AtHKT1 it is methionine (Met-69, Fig. 2). Apparent replacement of the predicted filter glycine in P-loop A by serine also occurs in HKT proteins identified from eucalyptus, rice, and ice plant (Fig. 2).
Figure 2.
Structural model of HKT proteins. Model of HKT/Trk/KtrB transporters as in refs. 13 and 18, based on the known structure of K+ channels (5). Predicted P-loops are labeled A–D, transmembrane domains are labeled I–VIII. The alignment shows P-loop A of various plant HKTs compared with Trk1 from S. cerevisiae (M21328), TrkH from Pseudomonas aeruginosa (AAG06598), KtrB from Vibrio alginolyticus (BAA32063), and to the P-loop of the Drosophila Shaker channel (S00479). The residue corresponding to the first glycine of the K+ channel GYG motif is marked with an asterisk.
Site-Directed Mutagenesis of Predicted Filter Glycines.
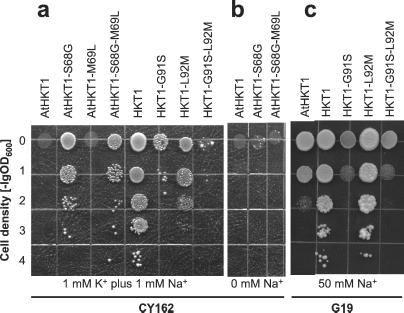
To test the hypothesis that the terminal glycine residues in the P-loop-like domains of HKT/Trk/KtrB transporters are related to K+ selectivity P-loop filters in K+ channels, we mutated Ser-68 of AtHKT1 to glycine (AtHKT1-S68G). Mutant and parent transporters were expressed in the yeast mutant CY162. Expression of AtHKT1-S68G permitted growth at low K+ concentrations but AtHKT1 had no such effect (Fig. 3a). The converse mutation in HKT1, Gly-91 to serine (HKT1-G91S), reduced complementation of the Δtrk1,2 phenotype (Fig. 3a), whereas HKT1 allowed growth at 1 mM K+. Thus, substitution of a single amino acid, Ser-68 to Gly, is sufficient to restore K+ transport to AtHKT1 as measured by rescue of CY162 cells. However, AtHKT1-S68G was not as effective in suppressing the Δtrk1,2 phenotype as was HKT1, and HKT1-G91S still promoted growth of CY162 cells better than did AtHKT1 (Fig. 3a), indicating further determinants of K+ transport in HKT1 proteins. Four additional constructs were made to assess the role of the leucine residue after the predicted filter glycine in P-loop A: AtHKT1-M69L, AtHKT1-S68G-M69L, HKT1-L92M, and HKT1-G91S-L92M. Only AtHKT1-S68G-M69L and HKT1-L92M allowed growth at 1 mM K+, the constructs with a glycine at the predicted filter position. Whether the following residue was leucine or methionine had no effect on growth of CY162 cells (Fig. 3a). Complementation of K+ uptake by AtHKT1-S68G required the presence of Na+ in the external medium. AtHKT1-S68G and AtHKT1-S68G-M69L did not support growth of CY162 cells at low [K+] in the absence of Na+ (Fig. 3b). These data correlate with the Na+/K+ symport function of this transporter family (14, 15, 17, 24).
Figure 3.
Expression of AtHKT1 and HKT1 constructs in S. cerevisiae CY162. (a) Complementation of K+-uptake deficiency by the various HKT1 and AtHKT1 constructs. CY162 cells expressing AtHKT1 and HKT1 constructs were plated on 1 mM K+ in a dilution series from OD600 = 1 to OD600 = 10−4. Only constructs with a glycine at the predicted filter position in P-loop A (68 in AtHKT1, 91 in HKT1) suppressed the trk1,2 phenotype. The media were supplemented with 1 mM Na+. (b) Dependence of K+-uptake complementation on external Na+. The same experiment as shown in a was performed in the absence of Na+. (c) Expression of AtHKT1 and HKT1 constructs in the Na+ hypersensitive yeast strain G19. Cells were plated on 50 mM Na+, 1 mM K+ in a dilution series from OD600 = 1 to OD600 = 10−4.
Salt Sensitivity in Yeast Mediated by HKT Constructs.
Na+ sensitivity by HKT1 mutants was analyzed by expressing them in the yeast strain G19 (Δena1–4), which is hypersensitive to salt stress because it is disrupted in four tandemly repeated P-type Na+-ATPase genes (30). Expression of HKT1 constructs increased the salt sensitivity of G19 cells (Fig. 3c). Although this negative effect was moderate for HKT1, it was more pronounced for AtHKT1. Mutation of Gly-91 to serine (HKT1-G91S) markedly increased the salt sensitivity to about the same extent as AtHKT1. HKT1-L92M rendered G19 cells as Na+ sensitive as HKT1, and HKT1-G91S-L92M rendered them as Na+ sensitive as HKT1-G91S.
Na+/K+ Selectivity of HKT Constructs Expressed in Xenopus Oocytes.
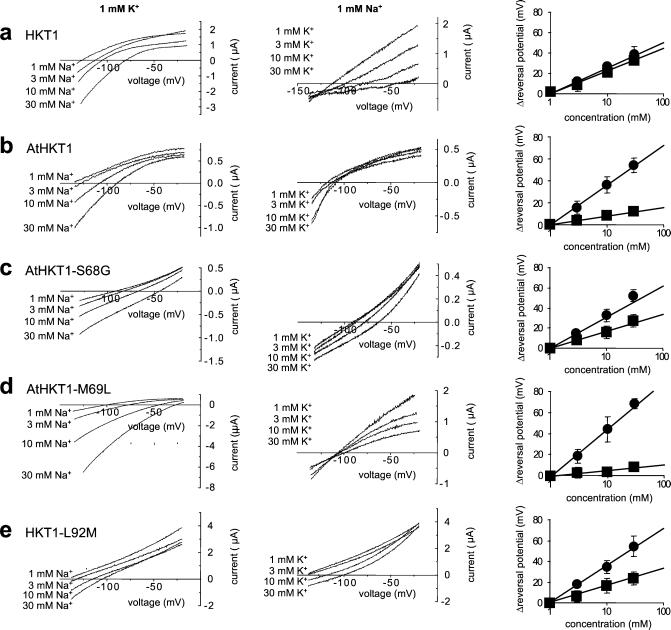
To address the substrate specificity of the HKT1 constructs directly, parent and mutated transporters were expressed in Xenopus oocytes. AtHKT1 and HKT1 both showed positive shifts in the reversal potential upon increasing [Na+] confirming Na+ permeability in oocytes (Fig. 4 a and b Left). The same was observed for the other tested constructs (Fig. 4 Left). Note that absolute reversal potentials varied, which may have been due to both differences in HKT isoforms and variation in oocyte properties, thus enabling semiquantitative comparisons (Fig. 4 Right). The addition of external K+ produced a positive shift in the reversal potential of the HKT1-mediated steady-state currents, showing K+ permeability (Fig. 4a Center) (14, 19, 35). The reversal potential of AtHKT1-injected oocytes was not significantly affected by the external K+ concentration (Fig. 4b Center), consistent with earlier findings (20) and with the lack of suppression of K+ deficiency by AtHKT1 in yeast (Figs. 1 and 3a). AtHKT1-S68G, however, caused positive shifts in the reversal potential of oocytes with increasing [K+], demonstrating that this point mutation confers K+ permeability to AtHKT1 (Fig. 4c, middle panel). Calculation of K+ to Na+ permeability ratios (PK+/PNa+) (15) showed values of 40.8 for HKT1, 0.07 for AtHKT1, 4.7 for AtHKT1-S68G, and 0.033 for AtHKT1-M69L. HKT1-G91S and HKT1-G91S-L92M did not elicit any Na+ or K+ currents, indicating that these constructs may not be properly expressed in Xenopus oocytes (data not shown). The mutation AtHKT1-M69L did not cause potassium-dependent shifts in the reversal potential, consistent with lack of complementation of K+ deficiency by AtHKT1-M69L (Figs. 4d Center and 3a). However, AtHKT1-M69L-mediated outward currents were sensitive to K+. AtHKT1-M69L-mediated outward currents decreased with increasing [K+] (Fig. 4d Center), an effect which was also observed for HKT1 (Fig. 4a Center). The reverse mutation in HKT1-L92M abolished this dampening effect of K+ on outward currents (Fig. 4e Center). Current reversal results are summarized in Fig. 4 Right, which shows the measured reversal potential shifts as a function of Na+ and K+ concentrations.
Figure 4.
Expression of AtHKT1 and HKT1 constructs in Xenopus oocytes. Xenopus oocytes were injected with cRNA of HKT1, AtHKT1, and the indicated mutated transporters. (Left) Ionic currents were recorded at 1 mM K+, while varying Na+ concentrations from 1 to 30 mM. (Middle) Ionic currents were recorded at 1 mM Na+, while varying [K+] from 1 to 30 mM. (Right) Reversal potential shifts as a function of ion concentration (circles, Na+; squares, K+). Only transporters with a glycine at the filter position in P-loop A (HKT1, AtHKT1-S68G, HKT1-L92M) were more permeable to K+ as indicated by large positive shifts in the reversal potential with increasing external [K+].
AtHKT1 Ser-68 Is Conserved Throughout A. thaliana Ecotypes.
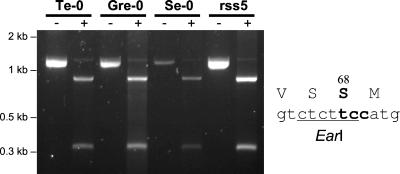
The findings above show that a serine in place of glycine in the first P-loop of AtHKT1 renders this transporter more Na+ selective. We examined whether this serine is conserved throughout A. thaliana ecotypes from different areas of the Northern hemisphere (36). The codon TCC for Ser-68 in AtHKT1 is part of a recognition site for the restriction endonuclease EarI (Fig. 5). We used PCR amplification of an AtHKT1 fragment from genomic DNA followed by EarI digestion to screen for AtHKT1 variants with a residue other than serine at the first P-loop. One hundred six different A. thaliana ecotypes were tested as well as two A. thaliana Col-0 lines with reduced salt sensitivity (rss3–3B and rss5–5A, deposited in ABRC by J. Werner and R. Finkelstein, University of California, Santa Barbara). The amplified AtHKT1 fragments of all 106 ecotypes were cut by EarI (Fig. 5). Thus Ser-68 is conserved throughout A. thaliana ecotypes.
Figure 5.
RFLP analysis of AtHKT1 from different Arabidopsis ecotypes. A 1,138-bp portion of AtHKT1 that comprises the codon for Ser-68 was amplified from genomic DNA of 106 A. thaliana ecotypes by PCR (−, no enzyme added). That codon is part of a EarI restriction site (Right). Digestion of the PCR product with EarI yields two fragments of 850 and 288 bp, provided the codon for Ser-68 is not mutated (+, treated with EarI). Representative data for four Arabidopsis ecotypes are illustrated and ecotype names are given on top of lanes. All 106 ecotypes tested exhibited the same pattern, indicating that Ser-68 is conserved throughout AtHKT1 proteins.
Glycine or Serine in P-Loop A Determines K+ Selectivity Also in Rice HKTs.
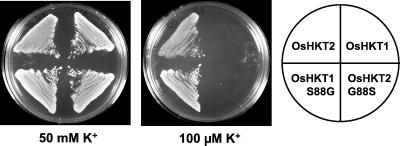
Two HKT genes have recently been identified in rice. Although OsHKT1 was a singleton in the Nipponbare cultivar, the cultivar Pokkali contained two closely related genes OsHKT1 and OsHKT2 (22). The predicted OsHKT1 from Pokkali and Nipponbare are identical and, like AtHKT1, have a serine at the predicted filter position in P-loop A (Ser-88, Fig. 2). OsHKT2, however, has a glycine at that position (Gly-88), like HKT1. Only OsHKT2 suppressed the trk1,2 phenotype of strain CY162; OsHKT1 did not allow growth on low K+ concentrations (22). To test further the hypothesis that the presence of glycine in P-loop A determines K+ permeability, we mutated Ser-88 of OsHKT1 to glycine (OsHKT1-S88G) and Gly-88 of OsHKT2 to serine (OsHKT2-G88S). CY162 cells expressing OsHKT1 did not grow on 100 μM K+, whereas OsHKT1-S88G expressing cells were able to grow on 100 μM K+ (Fig. 6). OsHKT2-G88S, in contrast to OsHKT2, did not permit growth at low [K+] (Fig. 6). Thus, as with AtHKT1 and HKT1, switch from serine to glycine in P-loop A was sufficient to confer K+ complementation and the reverse switch abolished K+ complementation.
Figure 6.
Site-directed mutagenesis of OsHKT1 and OsHKT2. Expression of OsHKT2 permits K+-uptake-deficient cells to grow on 100 μM K+, but OsHKT1 does not. Mutation of Ser-88 to glycine in OsHKT1 (OsHKT1-S88G) restores K+-uptake complementation, whereas mutation of Gly-88 to serine in OsHKT2 (OsHKT2-G88S) abrogates K+-uptake complementation.
Phylogenetic Analysis of Plant HKT Proteins.
Having identified the predicted filter residue in P-loop A as a determinant of K+ selectivity, we superimposed the information on that particular amino acid, serine or glycine, on a phylogenetic tree of plant HKT proteins. Monocot and dicot HKTs are clearly separated (Fig. 7a). All known dicot HKTs have a serine at the expected filter position in P-loop A (S-G-G-G type), whereas the transporters from monocots are of either the S-G-G-G or the G-G-G-G type (Fig. 7a). Plant HKTs are thought to share a common ancestor with bacterial TrkH, KtrB, and fungal Trk proteins (11) which are of the G-G-G-G type. To investigate the origin of the glycine to serine mutation in P-loop A we compared the individual P-loop sequences of all known HKT proteins (Fig. 7b). The P-loops segregate first according to their position (A, B, C, or D), and within each position according to their phylogenetic origin (dicot or monocot; Fig. 7b). These data demonstrate that monocot and dicot HKTs share a common ancestor with four P-loop-like domains.
Discussion
Structural models of HKT/Trk proteins suggested that these transporters contain P-loop-like domains and are related to K+ channels (11, 13). Here we provide functional evidence that the P-loop-like domains indeed determine the substrate specificity of HKT transporters. A glycine at the predicted filter position in P-loop A is necessary and sufficient for K+ permeation in HKT1 and AtHKT1. However, other residues also contribute to cation selectivity. AtHKT1-S68G was less permeable to K+ than HKT1 in oocytes and less effective in suppressing K+ uptake deficiency in yeast (Figs. 3a and 4). A leucine adjacent to the filter glycine conferred K+ sensitivity to outward currents mediated by HKT1 and AtHKT1, whereas a methionine at that position relieved this K+ effect (Fig. 4). Surprisingly, EcHKT1 and EcHKT2 from eucalyptus transported K+ when expressed in Xenopus oocytes, although both proteins have a serine at the predicted filter position in P-loop A (21, 23). However, mutations in rice OsHKTs (Fig. 6) and mutation of the predicted filter residues in P-loops A and C of the bacterial transporter KtrB reduced K+ permeability, confirming the importance of those residues for K+ selectivity (N. Tholema, N.U., P.M., J.I.S., and E.P.B., unpublished data). Our findings provide experimental support for the hypothesis that plant HKTs are structurally related to K+ channels. These data further support the hypothesis that the Na+-K+ cotransporter HKT1 has channel-like transport modes (35).
Null mutations in AtHKT1 have recently been found to suppress the salt-overly-sensitive phenotype of the Arabidopsis sos3 mutant (37, 38), confirming a proposed contribution of HKT proteins to salinity stress (14, 19, 20). The rice cultivar Pokkali with two HKT paralogues, one of which has a filter glycine in P-loop A, is more salt-resistant than the cultivar Nipponbare which only has the Ser-88 variant OsHKT1 (22; see Fig. 2). Furthermore, salt tolerance in Pokkali correlated with a decrease in expression of OsHKTs and reduced uptake of Na+ during salt stress (39). Our findings support the notion that those HKTs with a serine in P-loop A function mainly as Na+ transporters, possibly contributing to Na+ accumulation and salt toxicity (14, 20, 35, 40, §§).
The HKT proteins known from dicots all have a serine at the predicted filter position in P-loop A. Monocot and dicot HKTs clearly separate on a phylogenetic tree (Fig. 7a), but comparison of individual P-loops shows that they share a common ancestor transporter with 4-loops (Fig. 7b). To elucidate the evolutionary history of plant HKTs more sequences from dicots and from monocots other than grasses are required. Of particular interest would be the HKT1 homologs from ginkgo or magnoliids, because these plants are considered directly related to common ancestors of monocots and dicots (41). Identification of such HKT genes will show whether the archetypal plant HKT transporter has a G-G-G-G or S-G-G-G signature.
Structure–function analyses of animal K+ channels have shown that the four glycines form a highly selective K+ filter in the K+ channel pore. The highly conserved P-loops of K+ channels all show the characteristic GYG signature sequence. On the other hand the presented results suggest that the P-loops of HKT/Trk/KtrB transporters show identity only in one of the glycine residues that mediates K+ selectivity. The selectivity filters of HKT/Trk/KtrB transporters may have evolved independently due to a requirement for K+ selectivity or may have a common ancestor with K+ channels in an archetypal K+-binding site. The conserved glycine residues in HKT/Trk/KtrB transporters reinforce the importance of four glycines as a structural solution for creating a K+-binding site.
Acknowledgments
We are thankful to Y. Murata for help with Xenopus oocytes and to J. Borevitz and J. Chory for providing A. thaliana ecotypes. This work was supported by U.S. Department of Agriculture Grant 2001-35304-09988 and National Institutes of Health Grant 1P42ES10337 (to J.I.S.), a grant-in-aid for scientific research and a Center of Excellence (COE) grant from the Ministry of Education, Science, Sports and Culture of Japan (to N.U.), a grant-in-aid for Research for the Future Program (JSPS-RFTF00L01604) from the Japan Society for the Promotion of Science (to K.Y.), and Human Frontiers Science Program and Swiss National Science Foundation fellowships (to P.M.).
Footnotes
Laurie, S., Feeney, K., Maathuis, F., Heard, P. & Leigh, R. (2001) in 12th International Workshop on Plant Membrane Biology, Madison, WI, p. 120 (abstr.).
References
- 1.Durell S R, Guy H R. Biophys J. 1992;62:238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacKinnon R, Yellen G. Science. 1990;250:276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo P, MacKinnon R. Science. 1995;268:307–310. doi: 10.1126/science.7716527. [DOI] [PubMed] [Google Scholar]
- 4.Heginbotham L, Lu Z, Abramson T, MacKinnon R. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle D A, Morais Cabral J, Pfuetzner R A, Kuo A, Gulbis J M, Cohen S L, Chait B T, MacKinnon R. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura R L, Anderson J A, Gaber R F. J Biol Chem. 1997;272:1011–1018. doi: 10.1074/jbc.272.2.1011. [DOI] [PubMed] [Google Scholar]
- 7.Roux B, MacKinnon R. Science. 1999;285:100–102. doi: 10.1126/science.285.5424.100. [DOI] [PubMed] [Google Scholar]
- 8.Morais-Cabral J H, Zhou Y, MacKinnon R. Nature (London) 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Morais-Cabral J H, Kaufman A, MacKinnon R. Nature (London) 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 10.Berneche S, Roux B. Nature (London) 2001;414:73–77. doi: 10.1038/35102067. [DOI] [PubMed] [Google Scholar]
- 11.Durell S R, Hao Y, Nakamura T, Bakker E P, Guy H R. Biophys J. 1999;77:775–788. doi: 10.1016/S0006-3495(99)76931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durell S R, Bakker E P, Guy H R. Biophys J. 2000;78:188–199. doi: 10.1016/S0006-3495(00)76584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durell S R, Guy H R. Biophys J. 1999;77:789–807. doi: 10.1016/S0006-3495(99)76932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubio F, Gassmann W, Schroeder J I. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 15.Rubio F, Schwarz M, Gassmann W, Schroeder J I. J Biol Chem. 1999;274:6839–6847. doi: 10.1074/jbc.274.11.6839. [DOI] [PubMed] [Google Scholar]
- 16.Diatloff E, Kumar R, Schachtman D P. FEBS Lett. 1998;432:31–36. doi: 10.1016/s0014-5793(98)00833-3. [DOI] [PubMed] [Google Scholar]
- 17.Tholema N, Bakker E P, Suzuki A, Nakamura T. FEBS Lett. 1999;450:217–220. doi: 10.1016/s0014-5793(99)00504-9. [DOI] [PubMed] [Google Scholar]
- 18.Kato Y, Sakaguchi M, Mori Y, Saito K, Nakamura T, Bakker E P, Sato Y, Goshima S, Uozumi N. Proc Natl Acad Sci USA. 2001;98:6488–6493. doi: 10.1073/pnas.101556598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schachtman D P, Schroeder J I. Nature (London) 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- 20.Uozumi N, Kim E J, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker E P, Nakamura T, Schroeder J I. Plant Physiol. 2000;122:1249–1260. doi: 10.1104/pp.122.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairbairn D J, Liu W, Schachtman D P, Gomez-Gallego S, Day S R, Teasdale R D. Plant Mol Biol. 2000;43:515–525. doi: 10.1023/a:1006496402463. [DOI] [PubMed] [Google Scholar]
- 22.Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A. Plant J. 2001;27:129–138. doi: 10.1046/j.1365-313x.2001.01077.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Fairbairn D J, Reid R J, Schachtman D P. Plant Physiol. 2001;127:283–294. doi: 10.1104/pp.127.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawano M, Abuki R, Igarashi K, Kakinuma Y. J Bacteriol. 2000;182:2507–2512. doi: 10.1128/jb.182.9.2507-2512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder J I, Ward J M, Gassmann W. Annu Rev Biophys Biomol Struct. 1994;23:441–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- 26.Tyerman S D, Skerrett I M. Sci Hortic (Amst) 1999;78:175–235. [Google Scholar]
- 27.Blumwald E, Aharon G S, Apse M P. Biochim Biophys Acta. 2000;1465:140–151. doi: 10.1016/s0005-2736(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 28.Serrano R, Rodriguez-Navarro A. Curr Opin Cell Biol. 2001;13:399–404. doi: 10.1016/s0955-0674(00)00227-1. [DOI] [PubMed] [Google Scholar]
- 29.Anderson J A, Huprikar S S, Kochian L V, Lucas W J, Gaber R F. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quintero F J, Garciadeblas B, Rodriguez-Navarro A. Plant Cell. 1996;8:529–537. doi: 10.1105/tpc.8.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schachtman D P, Schroeder J I, Lucas W J, Anderson J A, Gaber R F. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- 32.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page R D. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 34.Gaber R F, Styles C A, Fink G R. Mol Cell Biol. 1988;8:2848–2859. doi: 10.1128/mcb.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gassmann W, Rubio F, Schroeder J I. Plant J. 1996;10:869–882. doi: 10.1046/j.1365-313x.1996.10050869.x. [DOI] [PubMed] [Google Scholar]
- 36.Alonso-Blanco C, Koornneef M. Trends Plant Sci. 2000;5:22–29. doi: 10.1016/s1360-1385(99)01510-1. [DOI] [PubMed] [Google Scholar]
- 37.Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee B H, Matsumoto T K, Koiwa H, Zhu J K, Bressan R A, Hasegawa P M. Proc Natl Acad Sci USA. 2001;98:14150–14155. doi: 10.1073/pnas.241501798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J P, Zhu J K. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- 39.Golldack D, Kamasani U R, Quigley F, Bennett J, Bohnert H J. Plant Physiol. 1997;114:S529. [Google Scholar]
- 40.Wang T B, Gassmann W, Rubio F, Schroeder J I, Glass A D. Plant Physiol. 1998;118:651–659. doi: 10.1104/pp.118.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raven P H, Evert R F, Eichhorn S E. Biology of Plants. New York: Freeman; 1999. pp. 517–553. [Google Scholar]