Abstract
Background
The administration of preoperative oral carbohydrate (POC) has been shown to enhance patient well-being and expedite postoperative recovery. Nevertheless, evidence regarding its efficacy in orthopedic patients remains insufficient and warrants further investigation. This study aimed to address gaps by evaluating the effect of preoperative oral dextrose (POD) intake on perioperative well-being parameters (including thirst, hunger, anxiety, pain, nausea, and vomiting) in patients undergoing hip and lower extremity surgeries with spinal anesthesia.
Methods
In this randomized controlled trial, 70 adult patients scheduled for orthopedic surgery were assigned to either the dextrose (POD intake) group or the fasting group (routine fasting periods of 8–10 h). Perioperative subjective well-being, including measures of thirst, hunger, anxiety, pain, nausea, and vomiting, was evaluated using the Visual Analogue Scale (VAS) both preoperatively and at multiple postoperative time points (T0, T6h, and T24h). Adverse postoperative events were monitored for 24 h following surgery.
Results
Significant improvements in perioperative thirst and pain scores were observed in the dextrose group compared to the fasting group (p = 0.041 and p = 0.003, respectively). The dextrose group consistently reported lower VAS scores for thirst, hunger, pain, and anxiety across all time points (T0, T6h, T24h) (p < 0.001). No significant differences were found between groups for nausea or vomiting (p > 0.05).
Conclusion
The administration of preoperative oral carbohydrate (POC) showed no clinically significant impact on perioperative nausea or vomiting in orthopedic patients. However, it significantly improved other perioperative well-being parameters such as thirst, hunger, anxiety, and pain, highlighting its potential to enhance patient comfort and recovery outcomes.
Trial registration
Iranian Registry of Clinical Trials, IRCT20191017045139N2. Registered on 28 January 2024.
Keywords: Fasting, Nausea, Orthopedic surgery, Preoperative oral carbohydrate, Thirst, Vomiting, Well-being
Introduction
Surgical interventions trigger a cascade of catabolic responses characterized by the release of stress hormones, such as cortisol, along with the activation of pro-inflammatory pathways. These responses contribute to the development of insulin resistance (IR) and metabolic disturbances [1–4] (Fig. 1).
Fig. 1.
Preoperative stressors plus hormonal response to surgical stress [1, 2, 4, 12, 47, 59, 65, 66]. HPA: hypothalamic–pituitary–adrenal axis; SAM: sympathetic-adrenomedullary system; CRH: Corticotropin-Releasing Hormone; ACTH: Adrenocorticotropic Hormone; IL1β: Interleukin 1 beta; IL6: Interleukin 6; IL8: Interleukin 8; TNFα: Tumor Necrosis Factor α; CRP: C-Reactive Protein; PI3K: Phosphoinositide 3-kinase; GLUT4: Glucose Transporter Type 4
Prolonged preoperative fasting heightens metabolic stress [5, 6], by raising glucocorticoid and glucagon levels and lowering insulin sensitivity [1, 2, 7]. It also disrupts glucose metabolism, leading to complications such as confusion, discomfort, headache, and electrolyte imbalance [5, 8].
Addressing these metabolic disruptions and perioperative discomforts, including dehydration, anxiety, thirst, hunger, dry mouth, postoperative nausea and vomiting (PONV) [9–11] are essential for enhancing postoperative recovery and overall quality of life [8].
Orthopedic patients face unique perioperative challenges, including the physiological stress associated with surgery, pain, and the risks inherent to spinal anesthesia (SA) [5, 8]. Malnutrition in orthopedic patients, especially those with hip fractures, exacerbates the catabolic effects of surgical stress, leading to insulin resistance, muscle loss, and impaired recovery. Given the negative impact of prolonged fasting on the metabolic state [3, 12], revised fasting guidelines have endorsed the consumption of clear liquids up to 2–3 h before surgery [13, 14].
The Enhanced Recovery After Surgery (ERAS) program is a strategic approach designed to mitigate metabolic disturbances and improve patient comfort during the perioperative period [4, 12]. ERAS protocols recommend preoperative oral carbohydrate (POC) intake to mitigate surgical stress and counteract the adverse fasting effects [3]. POC intake enhances insulin sensitivity, immune response, and reduces postoperative metabolic complications [3, 6, 10, 15] (Fig. 2). Additionally, it alleviates perioperative discomforts, such as thirst, hunger, dry mouth, fatigue, anxiety, nausea, and vomiting, thereby enhancing sleep quality, energy and patient satisfaction [16–20].
Fig. 2.
Mechanisms behind the effects of Preoperative Oral Carbohydrate (POC) intake as part of the ERAS protocol in enhancing patient comfort or well-being [3, 5, 19, 26, 67]
Despite a significant body of evidence supporting POC intake, there remain notable inconsistencies and gaps in the implementation and adherence to this strategy across different healthcare settings [3, 5, 19]. While systematic reviews and meta-analyses report reductions in IR, its effect on overall patient well-being remains inconclusive [21]. For example, Doo et al. found no improvements in patient satisfaction or well-being following thyroidectomy [6]. Similarly, studies involving laparoscopic cholecystectomy and lumbar disc surgery reported no significant differences in perioperative pain, nausea, vomiting, or fatigue between fasting and carbohydrate groups [22, 23]. Sada et al. observed no significant effect on overall well-being, despite reductions in postoperative thirst in colorectal surgery patients [18].
While POC intake is well-supported across multiple surgical specialties [24], its specific role in orthopedic procedures, particularly hip and lower extremity surgeries, remains unclear and underexplored [25].
The ERAS guidelines currently refrain from recommending POC intake for these surgeries due to limited data on both immediate and long-term postoperative outcomes [25]. For instance, While Ertural et al. demonstrated reductions in anxiety, pain, thirst, hunger, and nausea in hip arthroplasty (HA) patients under SA [26], other studies have indicated no significant improvement in patient well-being following POC intake in those undergoing total knee arthroplasty (TKA) or hip procedures under epidural or SA [27–29].
Additionally, Kweon et al. [10] reported that POC intake in patients undergoing cephalomedullary nailing for proximal femur fractures under SA failed to relieve anxiety, nausea, or vomiting.
These inconsistencies in POC intake highlight the need for further investigation in hip and lower extremity orthopedic procedures using SA, given that orthopedic patients face unique perioperative challenges, including the significant physiological stress of surgery [29], and that ERAS protocols advocate for SA to reduce opioid use, provide reliable analgesia, cause little disturbance to hemodynamics [30, 31] and have a positive postoperative outcome over general anesthesia [32].
Despite these challenges, optimizing postoperative recovery and patient well-being remains a key priority in orthopedic surgery [8]. Nonetheless, the lack of robust evidence supporting POC intake underscores the need for further research in this area [25].
This study aims to address gaps by evaluating the effect of preoperative oral dextrose (POD) intake on perioperative well-being parameters (including thirst, hunger, anxiety, pain, nausea, and vomiting) in patients undergoing hip and lower extremity surgeries with spinal anesthesia. We hypothesize that POD intake will significantly improve subjective well-being parameters compared to standard fasting in this population. Through this investigation, the study aims to optimize perioperative well-being and contribute to the advancement of orthopedic surgical care.
Methods
Study design
This single-blind, randomized controlled clinical trial was conducted between January and August 2024 at two hospitals in Kashan, Iran, involving eligible participants.
Participants
The sample size was calculated based on mean VAS pain scores (6.1 ± 2.1 and 4.8 ± 1.8) from a previous study [5]. With 80% power and a 95% confidence level, a minimum of 35 participants per group was required (Formula 1). The online-generated block randomization (1:1 ratio) with a fixed block size of four was performed. Allocation concealment was ensured using sealed, opaque envelopes prepared by an independent epidemiologist and opened by the investigator (first author) only at the time of intervention. Data analysis was performed by a statistician who was blinded to group allocation, with assignments coded as A (dextrose group) and B (fasting group). However, the investigator responsible for recording VAS scores was not blinded. Moreover, due to the nature of the intervention, patients knew their group assignment (dextrose or fasting).
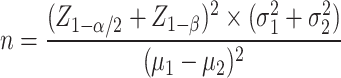 |
1 |
The inclusion criteria included: adult patients (≥ 18 years), consent to participate in this study, scheduled for elective orthopedic surgeries of the hip or lower extremities under SA, surgery duration of at least two hours, effective comprehension and communication skills, and classification as American Society of Anesthesiologists (ASA) physical status class I or II.
Exclusion criteria included participant withdrawal, conversion to general anesthesia due to inadequate SA, a delay of over three hours between dextrose intake and transfer to the OR, or intake less than two hours before anesthesia (Patients who consumed dextrose less than 120 min before anesthesia were excluded due to aspiration risk, in line with ERAS guidelines on 90-minute gastric emptying for clear fluids). Additional exclusions were intraoperative blood transfusion, surgery cancellation, perioperative mortality, and a history of conditions such as uncontrolled hypertension, motion sickness, gastroparesis, gastroesophageal reflux disease(GERD), esophageal disorders (strictures, achalasia), severe systemic diseases (brain, heart, lungs, liver, kidneys), morbid obesity (BMI > 40 kg/m²), metabolic or endocrine disorders, pathological fractures, or suspected musculoskeletal issues.
Intervention
In the afternoon of the day before surgery, patients who had been referred to the surgical units for hip or lower extremities surgery provided their consent, and the first author randomly allocated them into either the fasting group (n = 35) or the dextrose group (n = 35). All participants adhered to the study protocol (Fig. 3).
Fig. 3.
CONSORT flow diagram
All patients in both groups received 1,000 mL of a 0.3% NaCl in 3.33% dextrose solution after the NPO period commenced at 10:00 p.m. This routine care was undertaken to maintain an adequate effective circulating volume, preserve normal electrolyte balance, and meet the metabolic demands during fasting. The fluid volume was calculated according to the Holliday and Segar formula [33], which allocates 100 mL for the first 10 kg of body mass, a method applied to both adult and pediatric patients. Moreover, as part of routine care, all patients received intravenous Ringer’s solution was started at 10 mL/kg/h 30 min before spinal anesthesia and continued at 2 mL/kg/h during surgery [34].
Patients in the fasting group adhered to standard preoperative fasting guidelines, refraining from solids and liquids after 10:00 p.m. The dextrose group also fasted from solids after 10:00 p.m. These patients received 200 mL of 10% oral dextrose solution (10 g glucose/100 mL, osmolality: 555.2 mOsm/L, delivering 34 kcal/100 mL) 2–3 h before surgery (approximately 6:00–6:30 a.m.). Patients were instructed to consume the entire dextrose solution gradually over 30–60 min. No additional clear fluids were permitted.
Adherence to the dextrose intervention was monitored by the investigator (first author) who directly observed patients consuming the solution and recorded the volume ingested.
While ERAS protocols often recommend complex carbohydrates like 12.5% maltodextrin, we selected 10% dextrose due to its availability, lower cost, and institutional policy limitations [35].
Perioperative management
On arrival in the OR, all patients underwent monitoring with noninvasive blood pressure (NIBP), electrocardiogram (ECG), pulse oximeter for assessing O2 saturation (SaO2), heart rate (HR), and axillary body temperature measurements. Body temperature was maintained between 36 °C and 37 °C, while the OR temperature was kept between 22 °C and 25 °C. No prophylactic antiemetics (e.g., ondansetron) were administered. However, if ondansetron was required postoperatively based on the anesthesiologist’s clinical judgment, all administered doses were recorded during data collection.
An experienced anesthesiologist performed spinal anesthesia (SA) in the sitting position using a 25–27-gauge spinal Quincke-tip needle (EXEL INT International Company) at the L3-L4 or L4-L5 interspace. After confirming cerebrospinal fluid flow, a standard dose of 12–20 mg of hyperbaric bupivacaine 0.5% was injected into the subarachnoid space based on the required level of anesthesia, and patients were positioned supine [34].
Subsequently, 50 to 100 micrograms of fentanyl and 1 to 2 milligrams of midazolam were administered to all patients, with dosages determined based on weight, time of surgery, and clinical judgment [34, 36]. The surgeon, based on the clinical diagnosis or fracture location, selected the surgical approach and type of implant or prosthesis.
Intraoperatively, patients received supplemental oxygen (5 L/min via facial mask). Postoperatively, Ringer’s infusion rate was maintained at 2 mL/kg/h to account for intraoperative losses such as blood loss and fluid redistribution [34, 36].
All patients received routine care in the post-anesthesia care unit (PACU), including temperature management with blankets. NIBP, HR, SaO2, and ECG of the patients were regularly monitored and recorded every five minutes, and temperature was measured every 10 min. The PACU temperature ranged from 25 °C to 27 °C during the study.
Measurement
-
(A)
Demographic and Clinical Information Questionnaire that included age, gender, education, marital status, type of surgery, type of implant used, pathological site (unilateral or bilateral), Body Mass Index (BMI), duration of anesthesia, duration of surgery and blood loss during surgery.
-
(B)
Subjective well-being was assessed using numerical Visual Analogue Scale (VAS) scores [9, 16, 26, 28] for six parameters: thirst, hunger, anxiety, pain, nausea, and vomiting. Each was rated on a scale from 0 (none) to 10 (severe). Specifically, thirst, hunger, and anxiety were rated from 0 (no thirst/hunger/anxiety) to 10 (extreme thirst/hunger/anxiety), while pain, nausea, and vomiting followed the same 0–10 scale for severity. Assessments were performed at three time points: 30 min before spinal anesthesia (T0), six hours postoperatively (T6h), and 24 h postoperatively (T24h).
Data analysis
Data analysis was performed using IBM SPSS Statistics, version 27.0 (IBM Corp., Chicago, IL). Descriptive statistics were presented as means and standard deviations for quantitative variables and as frequencies and percentages for categorical variables. The normality of quantitative data was assessed using skewness and kurtosis indices. Skewness values within ± 2 and kurtosis values within ± 5 were considered indicative of a normal distribution. Chi-square or Fisher’s exact test, and two-tailed Student’s t-tests were used to compare the patients’ categorical and quantitative demographic and clinical characteristics, respectively. Repeated measures analysis of variance (ANOVA) assessed changes in mean VAS scores within and between groups across the three time points. Mauchly’s test was applied to assess sphericity (P > 0.05). If sphericity was violated (P < 0.05), the Greenhouse–Geisser correction was employed. In addition, the Bonferroni post hoc test was used for pairwise comparisons. P < 0.05 was considered statistically significant.
Ethical considerations
Ethical approval was obtained from the Kashan University of Medical Sciences Ethics Committee under the code of IR.KAUMS.NUHEPM.REC.1402.071. This study was registered in the Iranian clinical trial registration system under the number IRCT20191017045139N2. The information and objectives of the study were provided to all participants, and they were assured that their information would remain confidential. Furthermore, all participants signed an informed consent form. The study also adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines [37].
Results
Demographic and clinical information
The mean age of all patients was 63 ± 19.45 years, and their age range was 19–91 years. Moreover, the mean BMI of all patients was 26.89 ± 4.95 (kg/m2), and the BMI range was between 17.30 and 47.75 (kg/m2). In addition, the mean duration of surgery for all patients was 127.64 ± 25.94 min, with a range of 120 to 190 min. The mean duration of anesthesia for all patients was 156.64 ± 26.13 min with a range of 135 to 210 min. All patients had unilateral pathologies or injuries. Furthermore, no patients experienced severe nausea or vomiting during the initial 24-hour postoperative period, thereby eliminating the necessity for antiemetic therapy.
As shown in Table 1, the results of the Chi-square test and Fisher’s exact test did not show a significant difference between the two groups in terms of qualitative demographic and clinical characteristics (P ≥ 0.05). Independent t-test also showed that there was no statistically significant difference between the two groups in terms of quantitative variables (P ≥ 0.05).
Table 1.
Baseline demographic and clinical characteristics
| Characteristics | Groups | |||
|---|---|---|---|---|
| Fasting (N = 35) |
Dextrose (N = 35) |
P value | ||
| Gender, N (%) | Female | 22 (62.9%) | 14 (40%) | 0.56a |
| Male | 13 (37.1%) | 21 (60%) | ||
| Marital status, N (%) | Single | 6 (42.9%) | 8 (57.1%) | 0.76b |
| Married | 43 (50.6%) | 42 (49.4%) | ||
| Widowed | 2 (66.7%) | 1 (33.3%) | ||
| Divorced | 1 (2.9%) | 0 (0.0%) | ||
| Education level, N (%) | Illiterate | 10 (28.6%) | 13 (37.1%) | 0.45b |
| Junior school | 16 (45.7%) | 14 (40.0%) | ||
| High school | 9 (25.7%) | 6 (17.1%) | ||
| University | 0 (0.0%) | 2 (5.7%) | ||
| Clinical diagnosis | Hip Fracture | 3 (8.6%) | 2 (5.7%) | 0.17b |
| Hip Osteoarthritis | 5 (14.3%) | 1 (2.9%) | ||
| Femoral Neck Fracture | 8 (22.9%) | 8 (22.9%) | ||
| Subtrochanteric Fracture | 4 (11.4) | 6 (17.1) | ||
| Femoral Shaft Fracture | 7 (20%) | 14 (40%) | ||
| Knee Osteoarthritis | 5 (14.3%) | 7 (20.0%) | ||
| Tibia Fracture | 4 (11.4%) | 1 (2.9%) | ||
| Type of surgery, N (%) | ORIF | 24 (68.6%) | 24 (68.6%) | 0.29b |
| THA | 4 (11.4%) | 3 (8.6%) | ||
| TBA | 2 (5.7%) | 7 (20%) | ||
| TKA | 5 (14.3%) | 1 (2.9%) | ||
| Type of implant, N (%) | Hip Prosthesis | 4 (11.4%) | 3 (8.6%) | 0.22b |
| Bipolar Prosthesis | 2 (5.7%) | 7 (20%) | ||
| Dynamic Hip Screw | 6 (17.1%) | 1 (2.9%) | ||
| Gamma Nail | 4 (11.4%) | 6 (17.1%) | ||
| Intramedullary Nail | 2 (5.7%) | 1 (2.9%) | ||
| Knee Prosthesis | 5 (14.3%) | 1 (2.9%) | ||
| Screw and Plate | 12 (34.3%) | 16 (45.7%) | ||
| Age (years), mean ± SD | 64.74 ± 18.36 | 61.26 ± 20.6 | 0.45c | |
| Body Mass Index (kg/m²), mean ± SD | 27.11 ± 5.27 | 26.67 ± 4.66 | 0.70c | |
| Duration of Anesthesia (minutes), mean ± SD | 163.31 ± 22.00 | 157.57 ± 20.77 | 0.26c | |
| Duration of Surgery (minutes), mean ± SD | 136.29 ± 19.86 | 132.46 ± 17.17 | 0.39c | |
| Blood Loss During Surgery (milliliters), mean ± SD | 725.43 ± 188.88 | 658.29 ± 184.41 | 0.13c | |
TKA Total Hip Arthroplasty, TBA Hip Bipolar Arthroplasty, TKA Total Knee Arthroplasty, ORIF Open Reduction and Internal Fixation
aChi-Square test; bFisher’s Exact Test; cIndependent T-test
Perioperative patient well-being
Thirst Visual Analogue Scale Score (TVASS)
A significant time-by-group interaction observed for TVASS (F = 3.38, p = 0.041), with greater improvements in the dextrose group at all time points (p < 0.001). Both groups showed time-related reductions (p < 0.001), though in the fasting group, no significant difference was noted between T0 and T24h (p = 1.00) (Table 2; Fig. 4).
Table 2.
Within- and between-group comparisons of the mean TVASSs and HVASSs at three time points
| Outcomes | Groups | Between-group comparisonsb | ||||
|---|---|---|---|---|---|---|
| Fasting (n = 35) mean ± SD |
Dextrose (n = 35) mean ± SD |
|||||
| TVASS (0–10) | T0 | 6.0 ± 2.67 | 3.23 ± 2.56 | ˂0.001 | ||
| T6h | 8.37 ± 1.89 | 4.31 ± 2.32 | ˂0.001 | |||
| T24h | 5.89 ± 2.41 | 1.77 ± 1.98 | ˂0.001 | |||
|
Within-group comparisons (Effect of time) |
F = 31.75 ES = 0.478 p ˂ 0.001 |
F = 26.13 ES = 0.438 p ˂ 0.001 |
Time-group interactiona | |||
| Pairwise comparisons c | Difference T0 & T6h | p ˂ 0.001 | P = 0.03 | Mauchly’s Test | Greenhouse ‒ Geisser | |
| Difference T0 & T24h | P = 1.00 | P = 0.007 |
χ 2 = 6.65 P = 0.03 |
F = 3.38 ES = 0.041 P ˂ 0.04 |
||
| Difference T6h & T24h | p ˂ 0.001 | p ˂ 0.001 | ||||
| HVASS (0–10) | T0 | 3.71 ± 2.35 | 1.74 ± 2.21 | ˂0.001 | ||
| T6h | 4.63 ± 2.65 | 2.0 ± 2.27 | ˂0.001 | |||
| T24h | 2.40 ± 2.39 | 0.57 ± 1.35 | ˂0.001 | |||
|
Within-group comparisons (Effect of time) |
F = 21.09 ES = 0.38 p˂0.001 |
F = 9.72 ES = 0.22 p˂0.001 |
Time-group interactiona | |||
| Pairwise comparisonsc | Difference T0 & T6h | P = 0.07 | P = 1.00 | Mauchly’s Test | Sphericity Assumed | |
| Difference T0 & T24h | P = 0.002 | P = 0.008 |
χ 2 = 1.65 P = 0.43 |
F = 1.31 ES = 0.019 P = 0.27 |
||
| Difference T6h & T24h | p ˂ 0.001 | p ˂ 0.001 | ||||
TVASS Thirst Visual Analogue Scale Score, HVASS Hunger Visual Analogue Scale Score, T0 30 min before anesthesia induction, T6h 6 h after surgery, T24h 24 h after surgery, SD standard deviation, ES Effect Size
a Repeated Measures ANOVA; b Independent T-test; c Bonferroni Statistics
Fig. 4.
The mean and 95% Confidence Interval (CI) of thirst visual analogue scale score (A: TVASS), hunger visual analogue scale score (B: HVASS), nausea visual analogue scale score (C: NVASS), vomiting visual analogue scale score (D: VVASS), pain visual analogue scale score (E: PVASS), and anxiety visual analogue scale score (F: AVASS) in the dextrose and fasting groups at three time points (T0, T6h, and T24h)
Hunger Visual Analogue Scale Score (HVASS)
For HVASS, although no interaction effect was detected (F = 1.31, p = 0.27), both groups improved over time (p < 0.001). The dextrose group exhibited significantly lower scores than the fasting group across all assessments (p < 0.001), with notable improvements from T0 to T24h and T6h to T24h (Table 2; Fig. 4).
Nausea Visual Analogue Scale Score (NVASS)
NVASS showed no significant between-group differences (F = 2.37, p = 0.10). In the fasting group, nausea rose postoperatively (T0–T6h) and later declined (T6h–T24h) (both p < 0.001), while in the dextrose group, changes were not significant. Mean NVASS scores remained within the 1–2.5 range, indicating mild clinical intensity (Table 3; Fig. 4).
Table 3.
Within- and between-group comparisons of the mean NVASSs and VVASSs at three time points
| Outcomes | Groups | Between-group comparisonsb | ||||
|---|---|---|---|---|---|---|
| Fasting (n = 35) mean ± SD |
Dextrose (n = 35) mean ± SD |
|||||
| NVASS (0–10) | T0 | 1.03 ± 1.22 | 1.43 ± 1.78 | p = 0.27 | ||
| T6h | 2.40 ± 2.42 | 2.0 ± 2.0 | p = 0.45 | |||
| T24h | 1.34 ± 1.64 | 1.17 ± 1.15 | P = 0.61 | |||
|
Within-group comparisons (Effect of time) |
F = 13.60 ES = 0.28 p = 0.001 |
F = 7.19 ES = 0.17 p˂0.001 |
Time-group interactiona | |||
| Pairwise comparisons c | Difference T0 & T6h | p˂0.001 | P = 0.216 | Mauchly’s Test | Greenhouse ‒ Geisser | |
| Difference T0 & T24h | P = 0.70 | P = 0.99 |
χ 2 = 12.24 p = 0.002 |
F = 2.37 ES = 0.03 p = 0.10 |
||
| Difference T6h & T24h | p˂0.001 | p < 0.001 | ||||
| VVASS (0–10) | T0 | 1.06 ± 1.23 | 1.09 ± 0.88 | p = 0.91 | ||
| T6h | 2.83 ± 2.26 | 3.00 ± 1.91 | p = 0.69 | |||
| T24h | 2.34 ± 2.05 | 2.09 ± 1.56 | P = 0.55 | |||
|
Within-group comparisons (Effect of time) |
F = 17.39 ES = 0.34 p˂0.001 |
F = 20.37 ES = 0.37 p˂0.001 |
Time-group interactiona | |||
| Pairwise comparisonsc | Difference T0 & T6h | p˂0.001 | p˂0.001 | Mauchly’s Test | Sphericity Assumed | |
| Difference T0 & T24h | p˂0.001 | P = 0.008 |
χ 2 = 0.47 p = 0.79 |
F = 0.56 ES = 0.008 p = 0.57 |
||
| Difference T6h & T24h | P = 0.32 | P = 0.007 | ||||
NVASS Nausea Visual Analogue Scale Score, VVASS Vomiting Visual Analogue Scale Score, T0 30 min before anesthesia induction, T6h 6 h after surgery, T24h 24 h after surgery, SD standard deviation, ES Effect Size
a Repeated Measures ANOVA; b Independent T-test; c Bonferroni Statistics
Vomiting Visual Analogue Scale Score (VVASS)
VVASS also revealed no group-level effect (F = 0.56, p = 0.57). In both groups, vomiting increased early postoperatively and declined by T24h (p < 0.001). Mean scores remained within mild ranges [1–3], without statistically significant differences between groups (Table 3; Fig. 4).
Anxiety Visual Analogue Scale Score (AVASS)
Although AVASS did not show a significant interaction (F = 3.12, p = 0.80), both groups improved over time (p < 0.001). The dextrose group had significantly lower anxiety levels at T6h (p = 0.008) and T24h (p = 0.02), suggesting a postoperative anxiolytic effect (Table 4; Fig. 4).
Table 4.
Within- and between-group comparisons of the mean AVASSs and PVASSs at three time points
| Outcomes | Groups | Between-group comparisonsb | ||||
|---|---|---|---|---|---|---|
| Fasting (n = 35) mean ± SD |
Dextrose (n = 35) mean ± SD |
|||||
| AVASS (0–10) | T0 | 4.86 ± 1.78 | 4.0 ± 2.21 | p = 0.08 | ||
| T6h | 0.94 ± 1.34 | 0.26 ± 0.61 | p = 0.008 | |||
| T24h | 1.0 ± 1.87 | 0.23 ± 069 | P = 0.02 | |||
|
Within-group comparisons (Effect of time) |
F = 48.69 ES = 0.59 p ˂ 0.001 |
F = 52.42 ES = 0.61 p ˂ 0.001 |
Time-group interactiona | |||
| Pairwise comparisons c | Difference T0 & T6h | p ˂ 0.001 | p ˂ 0.001 | Mauchly’s Test | Greenhouse ‒ Geisser | |
| Difference T0 & T24h | p ˂ 0.001 | p ˂ 0.001 |
χ 2 = 51.21 p ˂ 0.001 |
F = 3.12 ES = 0.01 p = 0.8 |
||
| Difference T6h & T24h | P = 1.00 | P = 1.00 | ||||
| PVASS (0–10) | T0 | 7.63 ± 2.07 | 6.06 ± 1.55 | p ˂ 0.001 | ||
| T6h | 8.54 ± 1.86 | 6.26 ± 2.16 | p ˂ 0.001 | |||
| T24h | 7.66 ± 2.16 | 4.40 ± 1.92 | p ˂ 0.001 | |||
|
Within-group comparisons (Effect of time) |
F = 8.59 ES = 0.204 p ˂ 0.001 |
F = 30.37 ES = 0.476 p ˂ 0.001 |
Time-group interactiona | |||
| Pairwise comparisonsc | Difference T0 & T6h | P = 0.03 | P = 1.00 | Mauchly’s Test | Greenhouse ‒ Geisser | |
| Difference T0 & T24h | P = 1.00 | p ˂ 0.001 |
χ 2 = 16.66 p ˂ 0.001 |
F = 6.86 ES = 0.09 P = 0.003 |
||
| Difference T6h & T24h | p = 0.001 | p ˂ 0.001 | ||||
AVASS Anxiety Visual Analogue Scale Score, PVASS Pain Visual Analogue Scale Score, T0 30 min before anesthesia induction, T6h 6 h after surgery, T24h 24 h after surgery, SD standard deviation, ES Effect Size
aRepeated Measures ANOVA; b Independent T-test; c Bonferroni Statistics
Pain Visual Analogue Scale Score (PVASS)
A significant interaction was found for PVASS (F = 8.86, p = 0.003), with consistently lower pain scores in the dextrose group (p < 0.001). While both groups improved over time (p < 0.001), the dextrose group showed significant reductions from T6h onward, whereas fasting group improvements were less consistent (Table 4; Fig. 4).
In this study, the assumption of sphericity, equal variances of differences between time points, was tested using Mauchly’s test. When violated, Greenhouse-Geisser corrections were applied.
Discussion
Effect of POD intake on perioperative thirst
Our findings indicated that POD intake effectively reduces perioperative thirst, in patients undergoing orthopedic surgery under SA. Several studies support this result, demonstrating that POD intake mitigates thirst across various surgical contexts, including both orthopedic and non-orthopedic surgeries [1, 10, 18, 38, 39]. For instance, Kweon et al. and Akbuğa & Başer reported significant thirst reductions with POC intake [1, 10]. Similarly, patients with Type 2 Diabetes undergoing TKA experienced improved thirst sensations following POC intake, though this improvement was not statistically significant compared to warm water. These findings suggest that both POC and warm water were equally effective in alleviating preoperative thirst [40].
These findings are supported by physiological mechanisms, wherein prolonged fasting depletes glycogen stores, induces a stress response, disrupts plasma osmolarity, and alters glucose metabolism, leading to increased blood glucose and thirst, and exacerbating xerostomia due to preoperative anxiety and medications [1, 11, 41].
Several plausible mechanisms may explain the reduction in thirst following oral carbohydrate or dextrose intake. For example, evidence suggests that POC intake can mitigate psychological factors such as fear and anxiety [26], which in turn reduces sympathetic nervous system activation. This decrease in sympathetic drive improves salivary secretion and helps alleviate dry mouth, thereby diminishing the sensation of thirst [11].
Moreover, antidiuretic hormone (ADH), a key regulator of water-electrolyte and carbohydrate metabolism that is influenced by both plasma osmolarity and non-osmotic factors like blood volume and stress, may be reduced by POC/POD intake [42]. This reduction in ADH levels optimizes plasma osmolarity and attenuates the central neural drive responsible for thirst. Collectively, these mechanisms offer a comprehensive explanation for the improved thirst response observed in patients receiving oral carbohydrates or dextrose compared to those maintained in a fasting state.
In orthopedic patients, diminished gastrointestinal and salivary secretions further compound dehydration, xerostomia, and fatigue, highlighting the importance of POD intake in managing perioperative thirst [1, 11, 41].
However, despite these positive effects, some studies involving diverse surgical populations found no significant differences in postoperative thirst scores between POD intake, placebo, and fasting [21, 43]. These discrepancies may stem from confounding variables such as intraoperative fluid loss, hemorrhage, diuretic use, PACU stay duration, surgery type, and anesthetic agents like opioids and anticholinergics [1, 11, 41]. Further research is necessary to clarify the effect of POD intake on perioperative thirst.
Effect of POD intake on perioperative hunger
While the time-group interaction was insignificant, the results of the t-test indicated that POD intake significantly alleviates perioperative hunger in patients undergoing orthopedic procedures under SA. Over time, the mean hunger score decreased more markedly in the dextrose group compared to the fasting group. Although both groups demonstrated statistically significant reductions in hunger, the clinical impact was more pronounced in the dextrose group, indicating an additional therapeutic benefit of the intervention.
Our findings align with several studies demonstrating the efficacy of POC intake in reducing hunger in different surgical patients [17, 19, 43]. Harsten et al. found that 400 mL of a 12.5% POC solution before total hip arthroplasty (THA) significantly reduced postoperative hunger [44]. Ertural et al. observed that 1200 mL of POC intake significantly lowered hunger scores from day one to five postoperatively [26]. Additional studies further support these findings, highlighting significant reductions in hunger across various orthopedic procedures [10, 40, 45, 46]. POC intake up to two hours before surgery activates anabolic pathways, replenishes glycogen, enhances glucose uptake, and elevates insulin levels, thereby mitigating the catabolic effects of fasting [47, 48].
The observed reduction in hunger sensation following POD administration (2–3 h before surgery) is likely mediated by the coordinated activation of interconnected metabolic and neuroendocrine pathways. Specifically, dextrose ingestion may rapidly elevate blood glucose levels, stimulating insulin secretion from pancreatic β-cells. This insulin surge exerts dual effects: [1] it potentiates leptin release from adipose tissue, which acts via hypothalamic receptors to amplify central satiety signaling [49], and [2] it suppresses ghrelin (hunger hormone) secretion from gastric cells, thereby attenuating peripheral hunger signals [50]. Concurrently, insulin binding within the hypothalamus activates the PI3K/PKB signaling cascade, which may modulate appetite-regulatory neurons through two distinct mechanisms: inhibition of orexigenic neuropeptides (e.g., neuropeptide Y) and potentiation of anorexigenic pathways [48]. These mechanisms may explain the diminished hunger observed in patients receiving dextrose compared to those who were fasting.
Conversely, a meta-analysis of six non-orthopedic studies found no significant reduction in hunger, indicating potential variability due to surgical type, study design, or intervention methods [21]. Despite conflicting evidence, our study highlights the benefits of POD intake in reducing perioperative hunger, necessitating further research to resolve inconsistencies.
Effect of POD intake on perioperative pain
The results of the present study revealed that POD intake significantly mitigates perioperative pain. The findings of some other studies reported substantial reductions exclusively in postoperative pain levels, following POC intake in orthopedic patients [5, 26, 44, 46].
Contrarily, Yap et al. found no notable reduction in postoperative pain among patients undergoing hip fracture surgery [28]. Prolonged fasting raises C-reactive protein (CRP) levels and driving oxidative stress and pain [8]. The POC intake was associated with significantly lower levels of interleukin-6 (IL-6) and CRP, two markers of inflammation contributing to pain. Given that surgical wound pain triggers specific inflammatory and metabolic responses, POC intake might serve as an adjunctive pain-reducing method [5, 17, 26, 44].
In conclusion, the administration of POD significantly improves perioperative pain, potentially enhancing patient satisfaction and expediting recovery [3, 9, 19, 26, 51]. The findings support incorporating POD into preoperative protocols and highlight the need for robust trials to confirm its clinical effectiveness.
Effect of POD intake on perioperative nausea and vomiting
While POD intake showed benefits in reducing thirst, hunger, and pain, its effects on nausea and vomiting are less clear. However, POD intake resulted in clinically significant improvements in nausea scores at both 6 and 24 h post-surgery, as well as better vomiting scores at the 24-hour mark.
Interpreting these outcomes remains complex. In our study, VAS scores for them were low across both groups, with minimal differences between fasting and dextrose groups. This absence of significant perioperative nausea and vomiting complaints suggests that POD intake may not confer additional antiemetic benefits beyond standard fasting protocols. Studies across orthopedic (THA, TKA, hip fracture [HF], proximal femur, osteoporotic fractures) [10, 24, 27, 28, 52–55] and non-orthopedic procedures [6, 17, 23, 39] reported no significant differences in perioperative nausea and vomiting between patients receiving POC (400–1200 mL), plain water, or IV dextrose.
However, some evidence contradicts these findings. Ertural et al. demonstrated that 1200 mL of POC intake significantly reduced postoperative nausea in HA patients [26]. In addition, Harsten et al. reported reduced vomiting scores following 400 mL of POC before THA surgery [44]. The inconsistency in results may reflect variables such as history of PONV, and anesthetic protocols [56–58]. Notably, SA is preferred over general anesthesia for lower limb orthopedic surgery, as SA reduces PONV and opioid use [12, 58–60]. Moreover, preoperative anxiety, a significant predictor of PONV, can activate central dopaminergic pathways, exacerbating nausea and vomiting [56, 61]. Some Studies showed that POC intake reduces anxiety, potentially contributing to lower PONV rates [19, 26, 62]. The present study supports this correlation, with significant anxiety reduction observed in the dextrose group at 6 and 24 h postoperatively, aligning with minimal perioperative nausea and vomiting incidence.
Overall, it can be said that despite these promising findings, ERAS protocols do not explicitly address POC’s role in PONV prevention [47, 56], emphasizing the need for additional research to optimize POD intake strategies for PONV reduction.
Effect of POD intake on perioperative anxiety
The findings revealed that POD intake significantly reduced anxiety at T6h and T24h but showed no statistically significant differences at T0 point. Anxiety reductions at T6h and T24h suggest a postoperative anxiolytic effect, possibly due to attenuated stress responses (e.g., lower cortisol) or faster psychological recovery from reduced discomfort [12, 26, 63]. For instance, POD intake may serve as a metabolic primer by enhancing glucagon-like peptide-1 (GLP-1) secretion via gut-mediated pathways, thereby sustaining postoperative glucose homeostasis and mitigating stress hormones like cortisol, which is directly associated with a reduction in anxiety [64].
Additionally, over time, anxiety scores decreased more in the dextrose group than in the fasting group. Although both groups showed statistically significant reductions, the clinical impact was greater with dextrose, suggesting an added therapeutic benefit.
This aligns with studies demonstrating POC’s capacity to alleviate preoperative anxiety [16, 19, 26]. For instance, Ertural et al. reported sustained anxiety reductions for up to five days post-surgery [26]. In addition, Hausel et al. found similar effects within 40 to 90 min post-intake [16]. Moreover, Shi et al. found that POC intake significantly reduced anxiety scores at 40 and 90 min post-intake in patients [15].
Nonetheless, several studies have reported no significant reduction in anxiety, indicating that POC intake alone may not be sufficient to alleviate the complex psychological stressors experienced during the perioperative period [6, 18].
Preoperative anxiety often stems from prolonged fasting, diagnosis uncertainty, and surgical anticipation, factors that may not be entirely mitigated by carbohydrate intake alone. This combination can lead to silent suffering and difficulty managing symptoms [17, 26, 39, 65]. Given the contradictory results in this area, further investigation is warranted to explore the broader psychological benefits of POC intake in the perioperative stage.
Limitations & future directions
This study has important limitations. It was conducted in only two hospitals, one public and one private, limiting generalizability to more diverse healthcare settings. Future studies should replicate the intervention across varied geographic and clinical settings to strengthen external validity.
Perioperative IR, a key metabolic indicator, was not assessed. Including IR metrics in future research could provide deeper insights into the physiological effects of POC intake.
The absence of a placebo group and patient blinding restrict causal inference. To distinguish true metabolic effects from placebo responses, future trials should use three-arm designs (fasting, water placebo, and dextrose).
Participant inclusion was limited to ASA I–II, excluding higher-risk patients. Research involving ASA III or IV populations would improve applicability to broader clinical practice.
Finally, while dextrose was used, its comparative effectiveness against other carbohydrates, such as maltodextrin, remains unclear. Trials within orthopedic ERAS protocols, assessing outcomes like mobility and hospital stay, are strongly recommended to refine perioperative strategies.
Conclusion
In patients undergoing orthopedic surgery, POD intake showed no clinically significant improvement in perioperative nausea and vomiting. However, it significantly improved several perioperative parameters, such as reductions in thirst, hunger, anxiety, and pain. Further randomized controlled trials with larger sample sizes are required to validate and expand upon these findings.
Acknowledgements
The authors express their profound gratitude to all patients who willingly participated in this study, whose cooperation was pivotal to its success. The authors are equally indebted to Kashan University of Medical Sciences for providing financial support with Registration Number 402180 (dated January 14, 2024), which made this research possible. This research is derived from a Master of Science in Nursing thesis of the first author.
Abbreviations
- POC
Preoperative Oral Carbohydrate
- POD
Preoperative Oral Dextrose
- SA
Spinal Anesthesia
- VAS
Visual Analogue Scale
- PONV
Postoperative Nausea and Vomiting
- ASA
American Society of Anesthesiologists
- ERAS
Enhanced Recovery After Surgery
- IR
Insulin Resistance
- PACU
Post-Anesthesia Care Unit
- ORIF
Open Reduction and Internal Fixation
- TKA
Total Knee Arthroplasty
- THA
Total Hip Arthroplasty
- TBA
Hip Bipolar Arthroplasty
- HF
Hip fracture
- TVASS
Thirst Visual Analogue Scale Score
- HVASS
Hunger Visual Analogue Scale Score
- NVASS
Nausea Visual Analogue Scale Score
- VVASS
Vomiting Visual Analogue Scale Score
- AVASS
Anxiety Visual Analogue Scale Score
- PVASS
Pain Visual Analogue Scale Score
- HPA
Hypothalamic–Pituitary–Adrenal Axis
- SAM
Sympathetic-Adrenomedullary System
- CRH
Corticotropin-releasing hormone
- ACTH
Adrenocorticotropic Hormone
- IL1β
Interleukin 1 Beta
- IL6
Interleukin 6
- IL8
Interleukin 8
- TNFα
Tumor Necrosis Factorα
- CRP
C-Reactive Protein
- PI3K
Phosphoinositide 3-Kinase
- GLUT4
Glucose Transporter Type 4
- SaO2
O2 saturation
- NIBP
noninvasive blood pressure
- ECG
electrocardiogram
- BMI
Body Mass Index
- ES
effect size
- GLP-1
Glucagon-Like Peptide-1
Author contributions
M.R.Z., M.GH., and M.R. collaborated on the conceptualization and design of this study. M.GH. assumed primary responsibility for data collection, while M.R.Z., MGH, M.R., M.A.S., and A.Y.collectively analyzed and interpreted the dataset. M.R.Z. and M.R. played key roles in drafting and refining the manuscript. All authors thoroughly reviewed and approved the final version of the manuscript for publication.
Funding
This research was financially supported by the Vice Chancellor for Research and Technology at Kashan University of Medical Sciences, as part of a registered project (Registration Number: 402180, dated January 14, 2024).
Data availability
The datasets generated and analyzed during this study are accessible upon reasonable request by contacting the corresponding author.
Declarations
Ethics approval and consent to participate
This study adhered to the ethical standards outlined in the Declaration of Helsinki. Ethical approval was obtained from the ethics committee of Kashan University of Medical Sciences with code IR.KAUMS.NUHEPM.REC.1402.071. Informed written consent was obtained from all participants, who voluntarily enrolled in the study. Participants were informed of their right to withdraw at any stage, and the confidentiality of their personal information was strictly maintained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akbuğa GA, Başer M (2021) Effect of preoperative oral liquid carbohydrate intake on blood glucose, fasting-thirst, and fatigue levels: a randomized controlled study. Braz J Anes 71(3):247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimenta GP, de Aguilar-Nascimento JE (2014) Prolonged preoperative fasting in elective surgical patients. Nutr Clin Pract 29(1):22–28 [DOI] [PubMed] [Google Scholar]
- 3.Tall J, Nygren J (2020) Preoperative fasting and carbohydrate treatment. In: Ljungqvist O, Francis NK, Urman RD (eds) Enhanced recovery after surgery: A complete guide to optimizing outcomes. Springer International Publishing, Cham, pp 31–36 [Google Scholar]
- 4.Schricker T, Lattermann R, Carli F (2020) Physiology and pathophysiology of ERAS. In: Ljungqvist O, Francis NK, Urman RD (eds) Enhanced recovery after surgery: A complete guide to optimizing outcomes. Springer International Publishing, Cham, pp 11–22 [Google Scholar]
- 5.Chaudhary NK, Sunuwar DR, Sharma R, Karki M, Timilsena MN, Gurung A et al (2022) The effect of pre-operative carbohydrate loading in femur fracture: a randomized controlled trial. BMC Musculoskelet Disord 23(1):819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doo AR, Hwang H, Ki M-J, Lee J-R, Kim D-C (2018) Effects of preoperative oral carbohydrate administration on patient well-being and satisfaction in thyroid surgery. Kja 71(5):394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakob SM, Stanga Z (2010) Perioperative metabolic changes in patients undergoing cardiac surgery. Nutrition 26(4):349–353 [DOI] [PubMed] [Google Scholar]
- 8.Hu Z-C, He L-J, Chen D, Li X-B, Feng Z-H, Fu C-W et al (2019) An enhanced recovery after surgery program in orthopedic surgery: a systematic review and meta-analysis. J Orthop Surg Res 14(1):77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Çakar E, Yilmaz E, Çakar E, Baydur H (2017) The effect of preoperative oral carbohydrate solution intake on patient comfort: A randomized controlled study. J Perianesth Nurs 32(6):589–599 [DOI] [PubMed] [Google Scholar]
- 10.Kweon S-h, Js P (2020) Oral carbohydrate administration in patients undergoing cephalomedullary nailing for proximal femur fractures: an analysis of clinical outcomes and patient satisfaction. Geriatric Orthop Surg Rehabilitation 11:2151459320958609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seyhan Ak E, Türkmen A, Sinmaz T, Biçer ÖS (2023) Evaluation of thirst in the early postoperative period in patients undergoing orthopedic surgery. J Perianesth Nurs 38(3):448–453 [DOI] [PubMed] [Google Scholar]
- 12.Carli F (2015) Physiologic considerations of enhanced recovery after surgery (ERAS) programs: implications of the stress response. Can J Anesthesia/Journal Canadien D’anesthésie 62(2):110–119 [DOI] [PubMed] [Google Scholar]
- 13.Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration (2017) Application to healthy patients undergoing elective procedures: an updated report by the American society of anesthesiologists task force on preoperative fasting and the use of Pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology 126(3):376–393 [DOI] [PubMed] [Google Scholar]
- 14.Smith I, Kranke P, Murat I, Smith A, O’Sullivan G, Søreide E et al (2011) Perioperative fasting in adults and children: guidelines from the European society of anaesthesiology. Eur J Anaesthesiol| EJA 28(8):556–569 [DOI] [PubMed]
- 15.Shi Y, Dong B, Dong Q, Zhao Z, Yu Y (2021) Effect of preoperative oral carbohydrate administration on patients undergoing Cesarean section with epidural anesthesia: A pilot study. J Perianesth Nurs 36(1):30–35 [DOI] [PubMed] [Google Scholar]
- 16.Hausel J, Nygren J, Lagerkranser M, Hellström PM, Hammarqvist F, Almström C et al (2001) A Carbohydrate-Rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg 93(5):1344–1350 [DOI] [PubMed]
- 17.Zhang Y, Min J (2020) Preoperative carbohydrate loading in gynecological patients undergoing combined spinal and epidural anesthesia. J Invest Surg 33(7):587–595 [DOI] [PubMed] [Google Scholar]
- 18.Sada F, Krasniqi A, Hamza A, Gecaj-Gashi A, Bicaj B, Kavaja F (2014) A randomized trial of preoperative oral carbohydrates in abdominal surgery. BMC Anesthesiol 14(1):93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang W, Meng G, Yang C, Sun Y, Zhong W, Lu Y (2024) Effect of preoperative oral carbohydrate on the postoperative recovery quality of patients undergoing daytime oral surgery: a randomized controlled trial. Perioper Med 13(1):102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu W, Yan Y, Sun Y, Fan Z, Fang N, Zhang Y et al (2021) Implementation of enhanced recovery after surgery (ERAS) protocol for elderly patients receiving surgery for intertrochanteric fracture: a propensity score-matched analysis. J Orthop Surg Res 16(1):469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong E, Chen Y, Ren Y, Zhou Y, Di C, Zhou Y et al (2022) Effects of preoperative carbohydrate loading on recovery after elective surgery: A systematic review and bayesian network meta-analysis of randomized controlled trials. Front Nutr 9:951676 [DOI] [PMC free article] [PubMed]
- 22.Bisgaard T, Kristiansen VB, Hjortsø NC, Jacobsen LS, Rosenberg J, Kehlet H (2004) Randomized clinical trial comparing an oral carbohydrate beverage with placebo before laparoscopic cholecystectomy. Br J Surg 91(2):151–158 [DOI] [PubMed] [Google Scholar]
- 23.Dilmen OK, Yentur E, Tunali Y, Balci H, Bahar M (2017) Does preoperative oral carbohydrate treatment reduce the postoperative surgical stress response in lumbar disc surgery? Clin Neurol Neurosurg 153:82–86 [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Jin X-q, Li X-y, Sun L, Peng Y (2023) The association between oral carbohydrate intake before orthopedic surgery for osteoporotic fractures and outcomes in elderly patients. J Orthop Surg Res 18(1):966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pradhan A, Srinivasan A, Raj S, Howard D, Aujla RS (2025) A systematic review and meta-analysis of preoperative carbohydrate drink loading prior to elective hip and knee arthroplasty. J Clin Orthop Trauma 63:102928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ertural F, Küçükakça Çelik G, Özçelik H (2023) Effect of oral carbohydrate solution administered before hip arthroplasty on preoperative anxiety and postoperative patient comfort: A randomized controlled trial. J Perianesth Nurs 38(3):461–468 [DOI] [PubMed] [Google Scholar]
- 27.Kadado A, Shaw JH, Ayoola AS, Akioyamen NO, North WT, Charters MA (2022) Effects of preoperative Carbohydrate-rich drinks on immediate postoperative outcomes in total knee arthroplasty: A randomized controlled trial. JAAOS - J Am Acad Orthop Surg 30(11):e833–e41 [DOI] [PubMed] [Google Scholar]
- 28.Yap KS, Loh PS, Foong YX, Mok CZ, Ong T, Khor HM (2024) A feasibility study on preoperative carbohydrate loading in older patients undergoing hip fracture surgery. BMC Geriatr 24(1):401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljunggren S, Hahn RG (2012) Oral nutrition or water loading before hip replacement surgery; a randomized clinical trial. Trials 13:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soffin EM (2020) Enhanced recovery after surgery (ERAS) protocols in orthopedic patients. In: MacKenzie CR, Cornell CN, Memtsoudis SG (eds) Perioperative care of the orthopedic patient. Springer International Publishing, Cham, pp 143–150 [Google Scholar]
- 31.Li J, Zhu H, Liao R (2019) Enhanced recovery after surgery (ERAS) pathway for primary hip and knee arthroplasty: study protocol for a randomized controlled trial. Trials 20(1):599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Memtsoudis SG, Cozowicz C, Bekeris J, Bekere D, Liu J, Soffin EM et al (2019) Anaesthetic care of patients undergoing primary hip and knee arthroplasty: consensus recommendations from the international consensus on Anaesthesia-Related outcomes after surgery group (ICAROS) based on a systematic review and meta-analysis. Br J Anaesth 123(3):269–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holliday MA, Segar WE (1957) The maintenance need for water in parenteral fluid therapy. Pediatrics 19(5):823–832 [PubMed] [Google Scholar]
- 34.Moheb M, Rezaei M, Azizi-Fini I, Atoof F, Saadati MA (2022) Comparison of the effect of Forced-air warming and warmed intravenous fluid on the comfort and prevention of shivering after spinal anesthesia in patients undergoing orthopedic surgery. J Perianesth Nurs 37(6):865–871 [DOI] [PubMed] [Google Scholar]
- 35.Mousavie SH, Negahi A, Hosseinpour P, Mohseni M, Movassaghi S (2021) The effect of preoperative oral versus parenteral dextrose supplementation on pain, nausea, and quality of recovery after laparoscopic cholecystectomy. J Perianesth Nurs 36(2):153–156 [DOI] [PubMed] [Google Scholar]
- 36.Ghafarzadegan R, Zarei M, Norouzi N, Ajorpaz NM, Lotfi SM, Rasooli Manesh SM et al (2025) Efficacy of an Iranian herbal medicine formula for postoperative constipation in trauma patients with hip and lower limb fractures: A triple-blind, placebo-controlled randomized clinical trial. Int J Orthop Trauma Nurs 57:101163 [DOI] [PubMed] [Google Scholar]
- 37.Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacotherapeutics 1(2):100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng P-L, Loh E-W, Chen J-T, Tam K-W (2021) Effects of preoperative oral carbohydrate on postoperative discomfort in patients undergoing elective surgery: a meta-analysis of randomized controlled trials. Langenbecks Arch Surg 406(4):993–1005 [DOI] [PubMed] [Google Scholar]
- 39.Tran S, Wolever TMS, Errett LE, Ahn H, Mazer CD, Keith M (2013) Preoperative carbohydrate loading in patients undergoing coronary artery bypass or spinal surgery. Anesth Analg 117(2):305–313 [DOI] [PubMed]
- 40.Lai Y, Cai Y, Ding Z, Huang C, Luo Z, Zhou Z Effect of preoperative carbohydrate loading on postoperative recovery of individuals who have type 2 diabetes after total knee arthroplasty: A randomized controlled trial. J Arthroplasty 40(3):665–671 [DOI] [PubMed]
- 41.Aroni P, Fonseca LF, Ciol MA, Margatho AS, Galvão CM (2020) The use of mentholated popsicle to reduce thirst during preoperative fasting: A randomised controlled trial. J Clin Nurs 29(5–6):840–851 [DOI] [PubMed] [Google Scholar]
- 42.Vantyghem M-C, Balavoine A-S, Wémeau J-L, Douillard C (2011) Hyponatremia and antidiuresis syndrome. Ann Endocrinol 72(6):500–512 [DOI] [PubMed] [Google Scholar]
- 43.Wang X-H, Wang Z-Y, Shan Z-R, Wang R, Wang Z-P (2025) Effects of preoperative oral carbohydrates on recovery after lapar oscopic cholecystectomy: A Meta-analysis of randomized controlled trials. J Perianesth Nurs 40(1):169–180 [DOI] [PubMed]
- 44.Harsten A, Hjartarson H, Toksvig-Larsen S (2012) Total hip arthroplasty and perioperative oral carbohydrate treatment: a randomised, double-blind, controlled trial. Eur J Anaesthesiol 29(6):271–274 [DOI] [PubMed]
- 45.Deng Y, Fang Y, Li H, Chen J, An J, Qiao S et al (2020) A preoperative Whey protein and glucose drink before hip fracture surgery in the aged improves symptomatic and metabolic recovery. Asia Pac J Clin Nutr 29(2):234–238 [DOI] [PubMed] [Google Scholar]
- 46.Chada R, Maryada V, Mulpur P, Reddy A, Maska A (2019) Does preoperative carbohydrate loading help outcomes in total knee replacement surgery. J Nutr Med Diet Care 5(1):1–7 [Google Scholar]
- 47.Ackerman RS, Tufts CW, DePinto DG, Chen J, Altshuler JR, Serdiuk A et al (2020) How sweet is this?? A review and evaluation of preoperative carbohydrate loading in the enhanced recovery after surgery model. Nutr Clin Pract 35(2):246–253 [DOI] [PubMed] [Google Scholar]
- 48.Wang ZG, Wang Q, Wang WJ, Qin HL (2010) Randomized clinical trial to compare the effects of preoperative oral carbohydrate versus placebo on insulin resistance after colorectal surgery. Br J Surg 97(3):317–327 [DOI] [PubMed] [Google Scholar]
- 49.Guo L, Liu P, Jiang X, Shan Z, Wang R, Wang Z (2024) Effects of oral carbohydrate loading in patients scheduled for painless bidirectional endoscopy: a prospective randomized controlled trial. Langenbecks Arch Surg 409(1):275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh M, Chaudhary M, Vashistha A, Kaur G (2015) Evaluation of effects of a preoperative 2-hour fast with glutamine and carbohydrate rich drink on insulin resistance in maxillofacial surgery. J Oral Biology Craniofac Res 5(1):34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G (2014) Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl 96(1):15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ljunggren S, Hahn RG (2012) Oral nutrition or water loading before hip replacement surgery; a randomized clinical trial. Trials 13(1):97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blum CL, Akerman M, Callari M, Jordan E, Capozzi JD (2019) Association of nausea and length of stay with carbohydrate loading prior to total joint arthroplasty. J Clin Outcomes Manag 26(4):175–179 [Google Scholar]
- 54.Choi YS, Cho BW, Kim HJ, Lee YS, Park KK, Lee B (2022) Effect of preoperative oral carbohydrates on insulin resistance in older adults who underwent total hip or knee arthroplasty: A prospective randomized trial. J Am Acad Orthop Surg 30(20):971–978 [DOI] [PubMed] [Google Scholar]
- 55.Shin S, Choi YS, Shin H, Yang IH, Park KK, Kwon HM et al (2021) Preoperative carbohydrate drinks do not decrease postoperative nausea and vomiting in type 2 diabetic patients undergoing total knee Arthroplasty-A randomized controlled trial. J Am Acad Orthop Surg 29(1):35–43 [DOI] [PubMed] [Google Scholar]
- 56.Stoops S, Kovac A (2020) New insights into the pathophysiology and risk factors for PONV. Best Pract Res Clin Anaesthesiol 34(4):667–679 [DOI] [PubMed] [Google Scholar]
- 57.Kranke P, Wilhelm W, Eberhart L (2020) Management of postoperative nausea and vomiting (PONV). In: Ljungqvist O, Francis NK, Urman RD (eds) Enhanced recovery after surgery: A complete guide to optimizing outcomes. Springer International Publishing, Cham, pp 195–202 [Google Scholar]
- 58.Choi YS, Kim TW, Chang MJ, Kang SB, Chang CB (2022) Enhanced recovery after surgery for major orthopedic surgery: a narrative review. Knee Surg Relat Res 34(1):8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Helander EM, Webb MP, Menard B, Prabhakar A, Helmstetter J, Cornett EM et al (2019) Metabolic and the surgical stress response considerations to improve postoperative recovery. Curr Pain Headache Rep 23(5):33 [DOI] [PubMed] [Google Scholar]
- 60.De Cassai A, Geraldini F, Boscolo A, Pasin L, Pettenuzzo T, Persona P et al (2021) General anesthesia compared to spinal anesthesia for patients undergoing lumbar vertebral surgery: A Meta-Analysis of randomized controlled trials. J Clin Med 10(1):102 [DOI] [PMC free article] [PubMed]
- 61.Yu L, Chen S, Huang M, Chen B, Fang X (2013) 278– The effects of preoperative psychological status on the incidence of postoperative nausea and vomiting following gynecological laparoscopic surgery. Eur Psychiatry 28:121920709 [Google Scholar]
- 62.Yilmaz N, Cekmen N, Bilgin F, Erten E, Ozhan M, Coşar A (2013) Preoperative carbohydrate nutrition reduces postoperative nausea and vomiting compared to preoperative fasting. J Res Med Sci 18(10):827–832 [PMC free article] [PubMed] [Google Scholar]
- 63.Rizvanović N, Nesek Adam V, Čaušević S, Dervišević S, Delibegović S (2019) A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int J Colorectal Dis 34:1551–1561 [DOI] [PubMed] [Google Scholar]
- 64.Cheng X, Guo J ( 2025) Preoperative oral carbohydrate for lower extremity arthroplasty: A systematic review and Meta-Analysis. J Arthroplasty. 10.1016/j.arth.2025.01.045 [DOI] [PubMed]
- 65.Gillis C, Ljungqvist O, Carli F (2022) Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br J Anaesth 128(3):434–448 [DOI] [PubMed] [Google Scholar]
- 66.Hanalis-Miller T, Nudelman G, Ben-Eliyahu S, Jacoby R (2022) The effect of Pre-operative psychological interventions on psychological, physiological, and immunological indices in oncology patients: A scoping review. Front Psychol 13:839065 [DOI] [PMC free article] [PubMed]
- 67.Ljungqvist O (2009) Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract Res Clin Anaesthesiol 23(4):401–409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during this study are accessible upon reasonable request by contacting the corresponding author.






