Abstract
The St. Césaire 1 Neanderthal skeleton of a young adult individual is unique in its association with Châtelperronian artifacts from a level dated to ca. 36,000 years ago. Computer-tomographic imaging and computer-assisted reconstruction of the skull revealed a healed fracture in the cranial vault. When paleopathological and forensic diagnostic standards are applied, the bony scar bears direct evidence for the impact of a sharp implement, which was presumably directed toward the individual during an act of interpersonal violence. These findings add to the evidence that Neanderthals used implements not only for hunting and food processing, but also in other behavioral contexts. It is hypothesized that the high intra-group damage potential inherent to weapons might have represented a major factor during the evolution of hominid social behavior.
Keywords: Châtelperronian‖paleopathology‖tool use‖trauma‖computer tomography
The St. Césaire 1 Neanderthal partial skeleton was discovered in 1979 at the site of La Roche à Pierrot (near the village of St. Césaire, Charente Maritime, France), a collapsed rock shelter comprising a sequence of Mousterian, Châtelperronian, and Aurignacian deposits (1). The skeleton was recovered from level EJ0P (1), which contains a Châtelperronian assemblage thermoluminescence-dated to ≈36,000 years ago (2, 3). This ensemble represented the first direct evidence for the association of Neanderthals with Châtelperronian implements. Together with a similar association from the site of Arcy-sur-Cure (4), these finds spurred an intense and ongoing debate over the evolutionary, paleodemographic, and cultural relationships between local Neanderthal populations and the early modern human (EMH) newcomers during the early Upper Paleolithic in Europe.
The St. Césaire 1 skeleton is fragmented and partially eroded, but the reconstructed craniomandibular and long bone diaphyseal morphology permitted significant inferences regarding the phyletic status and behavioral specializations of this individual. The craniomandibular morphology of the specimen largely corresponds to the “classical” Neanderthal type (5). Tooth microwear analysis suggests a meat-rich diet comparable to that of earlier Neanderthals and modern hunting societies (6). The morphology of the well-preserved right femoral shaft indicates Neanderthal-type hyperarctic body proportions, but cross-sectional biomechanical analysis suggests locomotor patterns closer to those of EMH than of classic Neanderthals (7).
Here we report on recently discovered paleopathological aspects of the morphology of the fossil. During computer-assisted reconstruction of the skull, we detected a healed fracture in the cranial vault. When paleopathological diagnostic standards (8, 9) are applied, this bony scar bears direct evidence for the impact of a sharp implement, which may have been directed toward the individual during an act of interpersonal violence. We discuss the possible behavioral context of this evidence and its implications for hominid behavior during the Middle-to-Upper Paleolithic transition in Europe.
Materials and Methods
The St. Césaire skeletal remains belong to a young adult, possibly male, individual. All preserved cranial and postcranial elements derive from a spatially confined area with a diameter of no more than 70 cm (1). The excavation yielded no signs of a burial pit; yet the local inhomogeneity in the distribution of rocks and implements at the spot where the skeleton was found, as well as its association with Dentalium shells (10), may be indicative of an intentional burial (11). The postcranium is represented by fragments of the axial skeleton and the limb bones, some of which were found in anatomical connection. The skull was lying on its right side, with the upper and lower jaws in anatomical association. Most of the preserved cranial structures come from the right side and comprise the mandible and maxillae (up to the left lateral incisors), the face, and the right anterolateral region of the braincase. The internal lamina of the cranial vault bones is partially eroded, and with the exception of several isolated teeth, the left cranial half is missing (Fig. 1). Deterioration and loss of these elements is probably because of temporary exposure and weathering of the upper layers of the sediment in which the fossil was embedded (1).
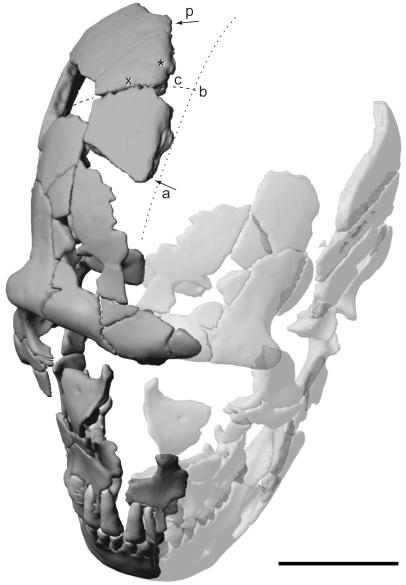
Figure 1.
Computerized reconstruction of the St. Césaire 1 Neanderthal skull (mirror-imaged completed parts are transparent) showing the bony scar in the right apical cranial vault. The skull is seen from the direction in which the hypothetical blow was exerted. Arrows indicate the anteroposterior (a and p) extent of the preserved lateral border of the injury (c, coronal suture; b, bregma; *, location of the coronal cross section through the injury (see Fig. 2b); ×, location of the parasagittal cross section through the coronal suture (see Fig. 3c); the dotted line indicates the reconstructed position of the midsagittal plane of the cranial vault). Note oblique, off-midline position of the scar. (Scale bar = 5 cm.)
The physical reconstruction of the fragmented right hemimandible and skull was performed by one of us (B.V.) and revealed taphonomic deformation of the face relative to the braincase, resulting in an everted position of the cranial vault relative to the midplane of the skull. As an effect of the general flattening of the cranial morphology, the anatomical connections between larger reconstructed pieces remained inconclusive. Among these was an apical vault fragment representing substantial portions of the right frontal and parietal bones joined along the coronal suture. This fragment is delimited by postmortem fractures on its anterior, posterior, and lateral sides (Fig. 1). The medial margin, however, exhibits a smooth border, which extends in an anteroposterior direction across the coronal suture and was initially interpreted as representing the partially fused sagittal (interparietal) and metopic (interfrontal) sutures.
To correct the taphonomic deformation of the skull and re-assess the anatomical position of this piece, we performed a computerized reconstruction of the skull. Virtual reconstruction was indispensable because the brittleness of the original fossil material prevented physical disassembly and manipulation of the specimen. Following three-dimensional data acquisition with computer tomography (CT), all fragments were isolated electronically from filling material and then recomposed on the computer screen, according to procedures and criteria described in ref. 12 (a detailed account of the new reconstruction will be given elsewhere). One result was that the medial border of the isolated cranial vault piece clearly does not correspond to the midsagittal plane of the skull (Fig. 1), nor does it represent normal cranial anatomy at its true position lateral of the midsagittal plane.
We established a differential diagnostic scheme, according to which this unusual morphology, its underlying causes, and behavioral context could be studied at increasing levels of detail (Table 1). For the comparative analysis of the external and internal structure of the bone, we used a sample of adult specimens comprising (i) an archeological specimen exhibiting a healed slash resulting from sharp trauma to the cranial vault (13), (ii) three specimens with healed trepanations [one neolithic (14) and two modern skulls], and (iii) four specimens exhibiting metopic sutures and/or persisting fontanelles.
Table 1.
Differential diagnostic scheme for trauma analysis in fossil hominids
| Criterion* | Range of possible alternatives | ||||||
|---|---|---|---|---|---|---|---|
| Paleopathology, proximate causes | |||||||
| Incident | In vivo | • | Postmortem | ||||
| Etiology | Trauma | • | Pathology | ||||
| Epigenetic variant | |||||||
| Type of trauma | Direct | • | Indirect | ||||
| Type of lesion | Penetrating, sharp | • | Blunt | ||||
| Severity of trauma | Mild | • | Fatal | ||||
| Post-traumatic | Complete healing | • | Chronic impairment | ||||
| Object causing lesion | Implement | • | Natural object | ||||
| Implement type | Stone | ? | Wood | ||||
| Forensics, ultimate causes | |||||||
| Behavioral context | Intentional | • | Accidental | ||||
| Motivation | Aggression | • | Ritual | ||||
| Medical | |||||||
| Action | Casual (short-term) | • | Premeditated (long-term) | ||||
| Actor | Opponent | • | Self-infliction | ||||
| Social context | Intragroup | • | Intergroup (intra-/interspecific) | ||||
| Recovery | Autonomous | • | Supported (nursing) | ||||
Paleopathology
The medial border of the cranial vault fragment of the St. Césaire Neanderthal deviates in its external and internal structure from both postmortem fractures and interosseous sutures. The external lamina of the bone is rounded off toward the medial margin, and the diploic region is covered with cortical bone (Fig. 2). This morphology is characteristic of in vivo apposition of bone matrix and excludes postmortem abrasion and erosion as potential mechanisms. Bone apposition at structural boundaries may occur under two different circumstances: normal periosteal growth in sutural tissue and bone regeneration following an injury. The off-axis position and oblique orientation of the margin (Fig. 1) exclude that it represents parts of the interparietal and a supposed metopic suture. On the other hand, a presumed suture in parasagittal position would imply the existence of a large persisting fontanelle or a bregmatic ossicle (15). However, the fairly straight margin does not correspond to a border around a fontanelle or a suture around a bregmatic ossicle. Furthermore, the cross-sectional structure of the bone clearly differs from that of an interosseous suture, which exhibits a corrugated border with less dense cortical bone than the border of a healed wound (Fig. 3). The most probable cause of the observed morphology is therefore bone regeneration following a lesion, a scenario that is corroborated by the close match of the cross-sectional morphology of the St. Césaire fragment (Fig. 3a) with that of comparative specimens exhibiting healed trepanations and scars (Fig. 3 b, e, and f).
Figure 2.
Anatomy of a blow. (a) Mediolateral view of the right border of the St. Césaire 1 cranial vault injury (same symbols as in Fig. 1: a and p, anterior and posterior delimitations of the scar, c, position of the coronal suture, and *, location of the cross section in b. The drawing indicates areas of bone remodeling (gray) and areas affected by postmortem fracturing (hatched). (b and c) Comparative morphology of the injured vault bones in St. Césaire 1 (b) and a specimen from a medieval graveyard (13) exhibiting a healed scar resulting from sharp trauma on the left frontal bone (c) (both specimens in anteroposterior view, coronal cross sections); the CT sections show bone remodeling at the margin of the injured external lamina (*) and a space (▵) between the dislocated internal lamina and the diploë, probably filled by connective tissue. (Scale bar = 5 cm.)
Figure 3.
Comparative cross-sectional morphology of bone injuries and sutures. (a and b) Healed scars of St. Césaire 1 and a medieval specimen (same coronal sections as in Fig. 2 b and c). (c) St. Césaire 1 coronal suture (parasagittal section through × in Fig. 1). (d) Metopic suture in a recent specimen (coronal section). (e) Large occipital trepanation during early phases of healing [Ensisheim skull (14), coronal section]. (f) Healed occipital trepanation in a recent specimen (coronal section). Note apposition of dense cortical bone at the injured borders, resulting in a smooth contour, as opposed to the less dense and corrugated aspect of the sutural borders. (Scale bar = 5 cm.)
In a next step, we analyzed the morphology of the injury with the aim to infer the proximate mechanical causes of the lesion and to reconstruct its posttraumatic history. As evidenced by the nearly straight border of the scar, the individual most probably suffered a lesion from a blade-shaped object. The resulting slash in the cranial vault is preserved on its right (lateral) side over a length of 68 mm; it was probably slightly longer in vivo. The left (medial) side of the groove was lost during fossilization. Judging from the degree of bone remodeling along the margin, the injury reached its greatest depth near the coronal suture, corresponding to the presumed primary site of impact. Toward the anterior and posterior ends, the groove tapers off. This morphology most closely matches the pattern of direct, sharp trauma. Under these biomechanical conditions, the high but localized stresses caused by the impacting object lead to puncturing/cutting of the scalp and the external lamina of the bone, comminution of the diploë, and separation/displacement of bone fragments from the internal lamina (9). The St. Césaire specimen in fact bears evidence that at the primary site of the impact the internal lamina was fractured and parts of it were dislocated whereas, toward the periphery of the slash, it was only partially severed (Figs. 1 and 2). The interpretation of the slash as a linear fracture resulting from blunt trauma is a less likely scenario because the compressive forces induced by blunt objects lead to nonlocal deformation of the cranial vault, typically resulting in multiple radial and tangential fractures around the center of impact (9, 16).
Comparison with an archeological specimen exhibiting a healed slash on the frontal bone permits further inferences regarding the severity of the injury and the subsequent healing process (Fig. 2). Given the moderate depth of the St. Césaire scar, it appears that the lesion was relatively mild. Cross-sectional CT images (Figs. 2 and 3) show that the injured region was extensively remodeled during lifetime; the severed external lamina was smoothed out by bone resorption and deposition, and the dislocated parts of the internal lamina were fixated, probably through formation of connective tissue in the diploic region. The external and cross-sectional morphology of the injured region does not show any signs of post-traumatic infection (e.g., periostitis or osteomyelitis), and the degree of healing is relatively advanced. Considering that bone healing is visible only 2–3 weeks after a traumatic event (9), it can be concluded that the individual survived the injury for at least some months, such that a direct causal connection between the trauma and the individual's death is unlikely.
Behavioral Context
By using forensic criteria originally developed for trauma analysis in extant and archeological populations (9), it is possible to conceive of various scenarios under which the St. Césaire injury could have occurred, assess their relative likelihood, and discuss their behavioral and motivational implications. The anteroposterior orientation of the slash as well as its apical position are indicative of a blow or a thrust exerted against the individual from the front or from behind, assuming that the individual was in an upright position. These spatial relationships indicate an intentional action, effected with an implement rather than a natural object. Accidental injury, such as falling onto a sharp edge, a rockfall, or an unintentional blow, such as resulting from a hunting incident, are less likely explanations; comparative forensic evidence suggests that accidental trauma typically affects the sides of the cranial vault, as opposed to the apical location of intentional injuries (16).
Inferences regarding the nature of the implement, the agents causing the lesion, and the behavioral context must remain tentative. From a mechanical point of view, the severity of sharp trauma depends on the mass, impact velocity, and incisiveness of the weapon. The wide range of possible combinations of these parameters encompasses a correspondingly wide spectrum of implements that might have been used to inflict the wound. For example, the Châtelperronian stone tools recovered from the site of St. Césaire are relatively small and exhibit retouched, blunt edges. To attain the kinetic energy necessary to penetrate bone, considerable acceleration, probably through hafting (17), would have been essential. Implications regarding the nature of the weaponry are complicated by the fact that the function of Châtelperronian implements is poorly understood and the preserved stone industry probably represents a small fraction of the actual in vivo spectrum of available tools.
Comparative evidence from historical populations suggests that interpersonal conflict behavior resulting in cranial vault fractures is relatively frequent (18, 19). Given the characteristic apical location of the injury in St. Césaire, direct interaction with another individual is the most parsimonious scenario. Theoretically, the injury may have resulted from intragroup, intergroup, or even interspecific conflict behavior. The first scenario is the most likely one because in socially organized species the vast majority of interpersonal interactions occur at the within-group level (20). Population densities were low during the Late Pleistocene, such that mutual avoidance during the rare encounters between different groups (both intra- and interspecific) might have represented the optimum strategy for the resolution of potential conflict (21). Nevertheless, it must be considered that patchy resource distribution might have induced temporary between-group competition.
Another aspect that requires consideration is the motivational background of the conflict in which the St. Césaire Neanderthal was involved. Motivations may range from a premeditated assault to a brief argument emerging from a temporary conflict between individuals, such as over social status, access to potential mates, or intragroup resources. Overall, a likely scenario for the interpersonal violence in St. Césaire is intragroup tempers with available “weaponry.” The immediate effects of the trauma were probably serious, implying heavy bleeding, cerebral commotion, and temporary impairment. Although it is possible that the individual sustained these adverse effects autonomously, it can be assumed that it had benefited at least to some extent from initial intragroup assistance.
Discussion
The St. Césaire cranial injury adds to the extremely small sample of specimens bearing direct evidence that Neanderthals used implements during acts of interpersonal violence. The only other clearly documented example, probably older than 50,000 years, comes from the Shanidar 3 Neanderthal. This specimen exhibits a slash in the superior margin of the ninth left rib, resulting from a penetrating implement, which remained stuck between neighboring ribs until the individual's death, but which was lost postmortem (22, 23). These rare cases of tool-mediated wounds must be interpreted in an evolutionary, behavioral, and cultural context, using comparative data on trauma in Neanderthals and fossil EMH, as well as modern evidence on the relationship among trauma, interpersonal violence, and tool use in human and nonhuman primates.
Neanderthals were shown to differ from fossil and extant EMH populations in both the frequency and pattern of skeletal injury: The overall incidence of trauma was comparatively high and concentrated to the head and neck (21). Differences in pattern of trauma between Neanderthals and the EMH from the Upper Paleolithic have not yet been investigated systematically, but it appears that the overall frequency of trauma was lower in the EMH populations, whereas the proportion of head and neck injuries was probably as elevated as in the Neanderthals (E. Trinkaus, personal communication). Taking into account that injuries tend to accumulate over an individual's lifetime, such that older individuals typically exhibit a greater number of lesions (21, 24), it is worth noting that immature specimens of Neanderthals [Devil's Tower and Le Moustier (25, 26) and EMH (Qafzeh 11)] also bear evidence for craniofacial trauma. The elevated frequency of trauma might therefore reflect not only a high risk of injury during close-quarter hunting of medium to large-sized game (21), but a physically demanding and stressful life from early ontogenetic stages (27, 28).
Against this comparative background, the dearth of direct evidence for tool involvement in fossil hominid trauma may be interpreted in two different ways. On one hand, it may reflect a low in vivo frequency of intentional or accidental tool-inflicted injuries relative to other types of trauma. It also may reflect the limits of paleopathological diagnosis; in a fossil, a lesion induced by an implement can be recognized only if evidence of healing is still present postmortem and the morphology of the scar permits inferences regarding the responsible weapon. The probability of detecting such injuries is further reduced through the effects of diagenesis, which tends to degrade the paleopathological evidence (9). Following this line of argument, a relevant proportion of the nondiagnostic injuries may represent tool-induced trauma and/or may be seen in the context of interpersonal violence rather than accident.
Data from nonhuman primates indicate that interpersonal violence within social networks represents a major cause of trauma. For example, in the Gombe chimpanzees more than one-half of the observed skeletal lesions are fractures and bite wounds resulting from intragroup violence (24), and several cases of fatal outcome of intragroup clashes have been reported in both wild and captive chimpanzee groups (29). A question arises whether there is evidence of tool use in interpersonal conflict situations in nonhuman primates. The cultural variability and contextual diversity of tool production and use have been studied extensively in various primate communities (30–32), revealing a general behavioral basis to aim objects, but not tools, toward conspecifics in the context of conflict behavior. However, although object-throwing to intimidate group members is fairly common (30, 33), the purposeful and directed use of tools designed for a distinct function during acts of interpersonal violence has not been reported. On the basis of the currently available evidence, it appears therefore that hominids differ from nonhuman primates in their ability to produce and use tools in an expanded, multifunctional context, including conflict behavior. Accordingly, the St. Césaire 1 and Shanidar 3 wounds indicate that Neanderthals transformed a “tool” into a “weapon”; in other words, they used an implement in a functional context which differed from that for which it was originally designed.
The cognitive ability to use tools multifunctionally was most probably acquired earlier during hominid evolution. Direct evidence for this hypothesis is scant, but it appears that the cognitive capacities and technical skills of early hominids are typically underestimated because relatively little is known about the in vivo level of complexity of tool production and utilization. Nevertheless, evidence for hafting of Mousterian stone implements with heat-processed bitumen (17, 34) and for the use of aerodynamically designed wooden hunting spears (35) suggest that the variety, effectiveness, and material sophistication of Middle and Late Pleistocene implements, and therefore of their utilization, was considerable. The intentional use of implements in the context of intragroup conflict must have had a major impact during hominid evolution because the availability of highly effective hunting and/or food-processing tools in interpersonal conflict created a new and considerable potential for intragroup damage, a potential that required specific behavioral adjustments with which to cope. Intragroup aggression in primate societies must be understood as one specific behavioral option in a complex network of social interactions, which is typically balanced by active reconciliatory behavior (20) and/or the minimization of social interactions under crowding conditions (36).
If we adhere to the hypothesis that the St. Césaire individual was injured in an act of intragroup violence and was later assisted to some extent during healing, this fossil lesion sheds light on both the disruptive/deleterious and integrative/supportive aspects of Neanderthal behavior. This fits well into the picture that Neanderthals were capable of sustaining severely impaired individuals over extended periods of time (21), and that this behavioral competence was already present in the Middle Pleistocene (37). We must therefore conceive that Neanderthals, depending on the context, inflicted wounds to conspecifics and nursed the injured, using aggressive and integrative behavioral elements as tools in a network of social interactions. Within this basic hominid pattern of behavior, implements probably played a crucial role because of their high effectiveness in interpersonal violence and because they represented an additional level of complexity of social interactions.
The difficulty to infer an indisputable behavioral context from the observed skeletal traumatic alterations is therefore not only because of the limitations of paleodiagnosis but also may be indicative of the behavioral polyvalence of tool use in Neanderthals. Accordingly, the links among form, purpose, and effective function of a tool might have been relatively loose. The most prominent behavioral context of Neanderthal tool use directed toward conspecifics is cannibalism, for which evidence has been advanced since the early days of Neanderthal research (38). However, even the clearest evidence for butchering of conspecifics from the site of Moula-Guercy (39, 40) ultimately remains unresolved in terms of its behavioral and motivational context. Implications that Neanderthal tool use was far more sophisticated than generally assumed also comes from other sources, notably the evidence of burial and a hypothetical case of cranial vault deformation (41).
Conclusions
To reconstruct the causes and consequences of the traumatic event that affected the St. Césaire individual, we followed a maximum-likelihood approach based on comparative fossil and actualistic data. We come to the conclusion that the cranial injury was inflicted with a tool during an act of intragroup interpersonal violence. In a wider context, we suggest that the basic behavioral and cognitive abilities to use implements during interpersonal conflict were probably already present early during hominid evolution and may represent a significant aspect of the evolution of social tool use. Accordingly, it can be assumed that in this specific respect, no major “transition” from Neanderthal-specific to EMH-specific behavioral patterns during the early Upper Paleolithic took place. This process most likely went across species and was eminently patterned, both spatially and temporally. Although genetic, developmental, and morphological data suggest that Neanderthals and EMH are separated at the species level (42–44), their ways to balance between aggressive and cooperative tool-mediated behavioral patterns were largely similar.
Acknowledgments
This study has greatly benefited from discussions with E. Trinkaus, T. Böhni, R. Majcen, C. van Schaik, J. Zilhão, H. Thieme, and M. Schultz, as well as from the comments and suggestions of three anonymous reviewers. The constant support of P. Stucki is gratefully acknowledged. We thank K. Alt, U. Bochsler, and Ch. Lanz for kindly providing the archeological specimens. We also thank Gea Bijl for technical assistance during CT scanning of the comparative sample. This work was supported by the Swiss National Science Foundation.
Abbreviations
- EMH
early modern human(s)
- CT
computer tomography
References
- 1.Lévêque F, Vandermeersch B. C R Acad Sci. 1980;291:187–189. [Google Scholar]
- 2.Valladas H, Geneste J-M, Joron J-L, Chadelle J-P. Nature (London) 1986;322:335–344. [Google Scholar]
- 3.Mercier N, Valladas H, Joron J L, Reyss J L, Lévêque F, Vandermeersch B. Nature (London) 1991;351:737–739. doi: 10.1038/351737a0. [DOI] [PubMed] [Google Scholar]
- 4.Hublin J J, Spoor F, Braun M, Zonneveld F, Condemi S. Nature (London) 1996;381:224–226. doi: 10.1038/381224a0. [DOI] [PubMed] [Google Scholar]
- 5.Vandermeersch B. Bull Mém Soc Anthropol Paris. 1984;1:191–196. [Google Scholar]
- 6.Lalueza C, Pérez-Pérez A, Turbón D. Am J Phys Anthropol. 1996;100:367–387. doi: 10.1002/(SICI)1096-8644(199607)100:3<367::AID-AJPA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Trinkaus E, Ruff C B, Churchill S E, Vandermeersch B. Proc Natl Acad Sci USA. 1998;95:5836–5840. doi: 10.1073/pnas.95.10.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courville C B. In: Diseases in Antiquity. Brothwell D, Sandison A T, Dawson W R, editors. Springfield, IL: Thomas; 1967. pp. 606–622. [Google Scholar]
- 9.Lovell N C. Yearbook Phys Anthropol. 1997;40:139–170. [Google Scholar]
- 10.Lévêque F, Backer A M, Guilbaud M, editors. Context of a Late Neandertal. Madison, WI: Prehistory Press; 1993. [Google Scholar]
- 11.Vandermeersch B. In: Context of a Late Neandertal. Lévêque F, Backer A M, Guilbaud M, editors. Madison, WI: Prehistory Press; 1993. [Google Scholar]
- 12.Zollikofer C P E, Ponce de León M S, Martin R D. Evol Anthropol. 1998;6:41–54. [Google Scholar]
- 13.Lanz C. Schweiz Ärzteztg. 1998;79:935–938. [Google Scholar]
- 14.Alt K W, Jeunesse C, Buitrago-Tellez C H, Wächter R, Böes E, Pichler S. Nature (London) 1997;387:360. doi: 10.1038/387360a0. (lett.). [DOI] [PubMed] [Google Scholar]
- 15.Hauser G, De Stefano G F. Epigenetic Variants of the Human Skull. Stuttgart: E. Schweizerbart'sche Verlagsbuchhandlung; 1989. [Google Scholar]
- 16.Fischer H. In: Handbuch der Speziellen Pathologischen Anatomie und Histologie. Uehlinger E, editor. Vol. 9. Berlin: Springer; 1972. pp. 353–424. [Google Scholar]
- 17.Boëda E, Connan J, Dessort D, Muhesen S, Mercier N, Valladas H, Tisnérat N. Nature (London) 1996;380:336–338. [Google Scholar]
- 18.Walker P L. Am J Phys Anthropol. 1989;80:313–324. doi: 10.1002/ajpa.1330800305. [DOI] [PubMed] [Google Scholar]
- 19.Weber J, Czarnetzki A. Am J Phys Anthropol. 2001;114:352–356. doi: 10.1002/ajpa.1047. [DOI] [PubMed] [Google Scholar]
- 20.de Waal F B. Science. 2000;289:586–590. doi: 10.1126/science.289.5479.586. [DOI] [PubMed] [Google Scholar]
- 21.Berger T D, Trinkaus E. J Archaeol Sci. 1995;22:841–852. [Google Scholar]
- 22.Trinkaus E, Zimmerman M R. Am J Phys Anthropol. 1982;57:61–67. doi: 10.1002/ajpa.1330570108. [DOI] [PubMed] [Google Scholar]
- 23.Trinkaus E. The Shanidar Neanderthals. New York: Academic; 1983. [Google Scholar]
- 24.Jurmain R. Am J Phys Anthropol. 1989;80:229–237. doi: 10.1002/ajpa.1330800211. [DOI] [PubMed] [Google Scholar]
- 25.Zollikofer C P E, Ponce de León M S, Martin R D, Stucki P. Nature (London) 1995;375:283–285. doi: 10.1038/375283b0. [DOI] [PubMed] [Google Scholar]
- 26.Ponce de León M S, Zollikofer C P E. Anat Rec. 1999;254:474–489. doi: 10.1002/(sici)1097-0185(19990401)254:4<474::aid-ar3>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Ogilvie M D, Curran B K, Trinkaus E. Am J Phys Anthropol. 1989;79:25–41. doi: 10.1002/ajpa.1330790104. [DOI] [PubMed] [Google Scholar]
- 28.Trinkaus E. J Archaeol Sci. 1995;22:121–142. [Google Scholar]
- 29.Fawcett K, Muhumuza G. Am J Primatol. 2000;51:243–247. doi: 10.1002/1098-2345(200008)51:4<243::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 30.McGrew W C. Chimpanzee Material Culture: Implications for Human Evolution. Cambridge, U.K.: Cambridge Univ. Press; 1992. [Google Scholar]
- 31.Whiten A, Goodall J, McGrew W C, Nishida T, Reynolds V, Sugiyama Y, Tutin C E, Wrangham R W, Boesch C. Nature (London) 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 32.van Schaik C P, Knott C D. Am J Phys Anthropol. 2001;114:331–342. doi: 10.1002/ajpa.1045. [DOI] [PubMed] [Google Scholar]
- 33.van Schaik C P, Deaner R O, Merrill M Y. J Hum Evol. 1999;36:719–741. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- 34.Mania D, Toepfer V. Königsaue: Gliederung, Oekologie und Mittelpaläolithische Funde der letzten Eiszeit. Berlin: Deutscher Verlag der Wissenschaften VEB; 1973. [Google Scholar]
- 35.Thieme H. Nature (London) 1997;385:807–810. doi: 10.1038/385807a0. [DOI] [PubMed] [Google Scholar]
- 36.Aureli F, de Waal F B. Am J Primatol. 1997;41:213–228. doi: 10.1002/(SICI)1098-2345(1997)41:3<213::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Lebel S, Trinkaus E, Faure M, Fernandez P, Guerin C, Richter D, Mercier N, Valladas H, Wagner G A. Proc Natl Acad Sci USA. 2001;98:11097–11102. doi: 10.1073/pnas.181353998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorjanovič-Kramberger D. Korr Deutsch Ges Anthropol Ethnol Urgesch. 1908;39:108–112. [Google Scholar]
- 39.Defleur A, Dutour O, Valladas H, Vandermeersch B. Nature (London) 1993;362:214. doi: 10.1038/362214a0. (lett.). [DOI] [PubMed] [Google Scholar]
- 40.Defleur A, White T, Valensi P, Slimak L, Crégut-Bonnoure E. Science. 1999;286:128–131. doi: 10.1126/science.286.5437.128. [DOI] [PubMed] [Google Scholar]
- 41.Pap I, Tillier A-M, Arensburg B, Chech M. Ann Hist-Nat Hung Nat Mus. 1996;88:233–270. [Google Scholar]
- 42.Krings M, Capelli C, Tschentscher F, Geisert H, Meyer S, von Haeseler A, Grossschmidt K, Possnert G, Paunovič M, Pääbo S. Nat Genet. 2000;26:144–146. doi: 10.1038/79855. [DOI] [PubMed] [Google Scholar]
- 43.Ponce de León M S, Zollikofer C P E. Nature (London) 2001;412:534–538. doi: 10.1038/35087573. [DOI] [PubMed] [Google Scholar]
- 44.Stringer C B, Gamble C. In Search of the Neanderthals: Solving the Puzzle of Human Origins. London: Thames and Hudson; 1993. [Google Scholar]