Abstract
Mycobacterium avium subsp. hominissuis (M. avium) is a pathogen that causes pulmonary and systemic infection in humans. Mycobacterial infection activates both pro-inflammatory and anti-inflammatory pathways modulating these routes to escape killing. eDNA has a role for environmental survival and biofilm formation of M. avium. We hypothesized that M. avium eDNA might play a role in macrophages survival. To investigate the macrophage response to M. avium eDNA, we utilized two virulent strains of M. avium, eDNA-deficient mutants, and nonvirulent Mycobacterium smegmatis. eDNA-deficient mutants were attenuated at macrophage survival and yielded significantly higher IL-1β than wildtype bacterium, while M. avium, but not M. smegmatis, suppresses IL-1β production and NLRP3 expression by host macrophages. We also observed that M. avium triggered IFN-β production in a DNA-dependent manner but did not have an effect on cGAS expression. These data indicate that M. avium strains modulate macrophage responses in an eDNA dependent manner.
Keywords: Mycobacterium avium, Inflammasome, NLRP3, Cytokines, Macrophages, STING, cGAS, AIM2, eDNA export, Survival, Intracytoplasmic
Introduction
In recent years, the number of M. avium infections have been increasing worldwide (Prevots and Marras 2015). Treatment of pulmonary infections requires multiple antibiotics for a minimum of 12 months and is only effective in 50–60% of patients (Griffith et al. 2007; Ingen et al. 2013). Mycobacterial biofilms are ubiquitous in household plumbing, natural water sources, and the soil and have been associated with infection in immunocompetent individuals (Griffith et al. 2007; Vega-Dominguez et al. 2020). To sustain a chronic infection, mycobacteria must evade the host immune system and find supportive niches to foster bacterial growth and spreading. A key component of innate immunity is the ability for cells to sense stress or foreign material from viruses, bacteria, and damage-associated molecular patterns (DAMPs). And one way to accomplish this is through the inflammasome pathways.
Manipulation of the host inflammasome response is a vital part of bacterial pathogenesis. M. tuberculosis, for example, differentially modulates the NLRP3 inflammasome depending on the stage of infection, implicating its necessity in both bacterial clearance and dissemination during M. tuberculosis infection (Rastogi et al. 2021; Xu et al. 2020). Early in the infection M. tuberculosis inhibits NLRP3 inflammasome activation in host macrophages via PknF and Hip1 (Rastogi et al. 2021; Madan-Lala et al. 2011). Once chronic infection is established, M. tuberculosis then promotes NLRP3 activation to trigger secretion of pro-inflammatory IL-1β, leading to pyroptosis and bacterial cell-to-cell spread (Guo et al. 2015). In patients with M. avium lung disease, NLRP3 activation and IL-1β secretion are attenuated in PBMCs (Wu et al. 2019). NLRP3 activation is a tightly regulated process involving phosphorylation of specific sites, ubiquitination control, and the interaction of specific effectors with NLRP3 (Swanson et al. 2019). The ASC adaptor protein is regulated by phosphorylation activation at Tyr146 by Spleen Tyrosine Kinase (SYK) (Hara et al. 2013). SYK has been implicated in NLRP3 activation during M. tuberculosis infection (Lee et al. 2012). BRCA1/BRCA2-containing complex E3 ubiquitin ligase (BRCC3) deubiquitinates NLRP3, which promotes NLRP3 protein complex formation and activation (Py et al. 2013). During M. tuberculosis infection of macrophages, lysosomal release of activated Cathepsin B is required for NLRP3 activation (Amaral et al. 2018). Both M. avium and M. tuberculosis infection increase the expression of Cathepsin B in macrophages (Amaral et al. 2018; Gutierrez et al. 2008).
Mycobacteria species produce robust biofilms leading to increased antimicrobial resistance. Composition of biofilm extracellular matrix within mycobacterial species can differ. For example, M. avium strain A5 is a high-biofilm producing strain of M. avium that exports large amounts of extracellular DNA (eDNA) in the biofilm compared to M. avium strain 104 (Rose and Bermudez 2016; Chakraborty et al. 2021). Proteins for eDNA export in M. avium strain A5 were identified within a unique 50-kbp genomic island which and is present in other mycobacterial species including, Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium intracellulare, M. avium subsp. hominissuis strain 3388, which are all associated to human infections (Rose and Bermudez 2016). Previous work has shown that M. avium strain A5 is able to invade epithelial cells more efficiently than M. avium strain 104, forming aggregates on the cells surface, a precursor to biofilm formation; suggesting that biofilm-related genes may play a role during infection (Yamazaki et al. 2006). eDNA is a critical structural component of the extracellular matrix in M. avium strain A5 biofilms, and forms a protective barrier against antimicrobial peptides and antibiotics (Rose et al. 2015). We thought that if the bacterium has a complex system to export DNA, it may use it to survive within phagocytic cells.
To piece together the role of eDNA during host macrophage infection we looked at a pro-inflammatory DNA sensor (AIM2) and primarily anti-inflammatory DNA sensor (cGAS/STING). The AIM2 inflammasome is a cytosolic dsDNA sensor, which induces cleavage of pro-IL-1β by caspase 1 upon activation, similar to NLRP3 (Guo et al. 2015). M. smegmatis, Mycobacterium fortuitum, Mycobacterium kansasii induce AIM2 activation whereas M. tuberculosis inhibits AIM2 activation in vitro via the ESX-1 system (Shah et al. 2013). To demonstrate AIM2 importance, knockout mice given M. tuberculosis are highly susceptible to infection compared to wildtype, which suggests that AIM2 plays a vital role in host defense for specific mycobacterial strains (Saiga et al. 2012). Additionally, both NLRP3 and AIM2 require SYK phosphorylation for IL-18 secretion, another pro-inflammatory cytokine (Hara et al. 2013). Though AIM2 is an important DNA sensor It’s not the only one within a cell.
The STING pathway is a cytosolic surveillance pathway typically involved in the antiviral response and is characterized by the production of type I interferons (IFN-α and IFN-β) and subsequent activation of autophagy (Schoggins et al. 2014; Watson et al. 2015). Briefly, Cytosolic dsDNA binds to cyclic GMP-AMP synthase (cGAS), dimerizing stimulator of interferon genes (STING) to induce IFN regulatory factor 3 (IRF3), upregulating Type I interferons (Burdette and Vance 2013). During M. tuberculosis infection cytosolic bacterial DNA is detected by the cGAS-STING pathway, leading to the production of type I interferons which have a suppressive effect on NLRP3 activation and IL-1β secretion (Watson et al. 2015; Ma et al. 2020; Wassermann et al. 2015; Boxx and Cheng 2016; Guarda et al. 2011). In this study we examine the interaction of eDNA producing M. avium strain A5 with host inflammasomes as well as modulation of DNA sensing mechanisms. We utilize three eDNA-deficient mutants from our previous work (Rose and Bermudez 2016). A knocked down metal-dependent hydrolase (7d3) found within the virulent 50-kb island. A suppressed serine/threonine kinase, PknB (9e11) located within an efflux pump operon. Also, a Ftsk/SpoIIIE-DNA pore ATPase (11e7) known to export eDNA. We demonstrate that M. avium strain A5 modulates inflammatory pathways during early infection. Additionally, we demonstrated the important role of bacterial eDNA in anti-inflammatory signaling during the intra-macrophage phase of infection. Taken together, the exportation of eDNA is used as a mechanism for M. avium A5 to promote residency and dissemination strategies evading host killing mechanisms.
Methods
Bacteria maintenance and propagation
Mycobacterium avium subsp. hominissuis strains 104 (M. avium 104) and A5 (M. avium A5), were originally isolated from the blood of AIDS patients (Aronson et al. 1999). M. avium 104 is a reference strain and M. avium A5 exports eDNA (Rose and Bermudez 2016). M. smegmatis mc2155 (M. smegmatis) was obtained from American Type Culture Collection (ATCC). M. smegmatis is a non-pathenogenic mycobacteria that produces eDNA. M. avium 104, M. avium A5, and Mycobacterium smegmatis were grown on Middlebrook 7H10 agar supplemented with 10% w/v oleic acid, albumin, dextrose, and catalase (OADC, Hardy Diagnostics) for 7–10 days at 37 °C. M. avium A5 transposon mutants were grown on 7H10 agar supplemented with 10% w/v OADC and 400 µg/ml Kanamycin for 7–10 days at 37 °C.
Macrophage tissue culture
THP-1 (TIB-202) human monocytes were obtained from the ATCC and maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gemini Bio-Products) at 37 °C with 5% CO2. THP-1 cells were seeded in 48-well plates at a density of 3.5 × 105 cells per well, 24-well plates at 1 × 106 cells/well, and 6-well plates at 2 × 106 cells/well. THP-1 cells were differentiated with 50 ng/ml of Phorbol 12-myristate 13-acetate (PMA; Sigma Aldrich) for 24 h, followed by 24 h in media without PMA, prior to use in experiments. The number of THP-1 cells in the monolayer was monitor as described (Danelishvili et al. 2004).
eDNA-deficient M. avium A5 isolates
M. avium A5 MycomarT7 phagemid-based transposon mutants were screened for deficiency in eDNA export during biofilm formation (Rose and Bermudez 2016). Briefly, the Mmt7 phagemid was transduced into M. avium A5, and the resulting transposon mutant library was screened. The library was screened for deficiency in eDNA export in biofilms via eDNA/optical density (OD) value for each mutant was calculated by normalizing the fluorescence reading for day 7 RFU of eDNA bound to propidium iodide to the starting OD of the respective sample. The eDNA/O.D. for wildtype A5 was 30,000. The location of transposon insertion selected eDNA-deficient mutants was determined using ligation-mediated PCR (LMPCR) as previously described.
M. avium survival assays in macrophages
M. avium 104 and A5, M. smegmatis, and eDNA-deficient mutants were added to THP-1 cells at a MOI of 10, synchronized by centrifugation for 10 min at 150 ×g, and allowed to infect for 1 h. Bacteria inoculums for these assays were formed in 1X HBSS supplemented with 0.05% Tween-20 (Sigma-Aldrich). After infection, extracellular bacteria were removed by two wash steps with 1X HBBS followed by treatment with gentamicin sulfate (Sigma-Aldrich) for 1 h (100 µg/ml) and an additional wash step with 1X HBSS to remove dead bacteria and antibiotic. Cells were lysed at appropriate timepoints with 0.1% Triton-X for 10 min and resulting lysates were serially diluted and plated. Colony forming units (CFUs) were enumerated at 1, 24, 48, and 72 h post infection (h.p.i.). LPS activated macrophages were generated by treating cells two hours before infection with 10 ng/mL LPS.
Chemical inhibition of NLRP3 during M. avium infections
Infection with M. avium 104 and A5 was carried out as described above. THP-1 cells were treated with 1 µM MCC950 (Millipore, Sigma) for 24 h after infection. Cells were lysed and CFUs enumerated at 1, 24, and 48 h.p.i. Growth curves of both M. avium 104 and M. avium A5 were cultured in 7H9 broth supplemented with 10% w/v OADC at 37˚ C in a shaking incubator. MCC950 (1 µM) was added at day 0. Control tubes were left untreated. The absorbance at 595 nm was measured at day 0, 2, 4 and 6.
Gene expression during M. avium infections
THP-1 cells were infected with M. avium 104, M. avium A5, M. smegmatis, and M. avium A5 mutants as described above. RNA was isolated using the RNeasy mini kit (Qiagen). Genomic DNA was removed by treatment with DNase I recombinant (Roche Diagnostics) for 1 h at 37 °C. The DNase was inactivated with Turbo DNase-inactivation reagent (Turbo DNA-free kit, ThermoFisher Scientific) for 2 min at 37 °C. Inactivation reagent was removed via centrifugation for 1 min at 10,000 ×g, and RNA transferred to new collection tubes. RNA samples were stored at −4 °C until cDNA synthesis. cDNA was transcribed from host RNA using the iScript cDNA synthesis kit (Bio-Rad). The quality of cDNA was tested by PCR with Gold 360 master mix using the manufacturer’s specifications (ThermoFisher Scientific). The qPCR reaction was performed using iQ SYBR Green Supermix (Bio-Rad) and an iCycler (CFX Connect Real-Time Systems, Bio-Rad) as previously described (Anes et al. 2006). Primers were designed in NCBI Blast using sequences from GenBank (National Center for Biotechnology Information) and listed in Table 1.
Table 1.
Primers for qPCR
| Primer name | Sequence (5′-3′) | Designer |
|---|---|---|
| NLRP3 forward | CTTCTCTGATGAGGCCCAAG | J. Joseph |
| NLRP3 reverse | GCAGCAAACTGGAAAGGAAG | J. Joseph |
| AIM2 forward | CAACGTGCTGCACCAAAAGT | J. Joseph |
| AIM2 reverse | GCTTGCCTTCTTGGGTCTCA | J. Joseph |
| Cathepsin B forward | TGTGGGGACGGCTGTAAT | J. Joseph |
| Cathepsin B reverse | GCTATTGGAGACGCTGTAGG | J. Joseph |
| cGAS forward | GGAGCCCTGCTGTAACACTT | J. Joseph |
| cGAS reverse | GTGAGAGAAGGATAGCCGCC | J. Joseph |
| STING forward | CAGCCTTGGTTCTGCTGAGT | J. Joseph |
| STING reverse | ACCCCGTTTAACAGCAGTCC | J. Joseph |
| Beta-actin forward | CATGTACGTTGCTATCCAGGC | J. Joseph |
| Beta-actin reverse | CTCCTTAATGTCACGCACGAT | Wang, 2013 |
IL-1β and IFN-β protein quantification via ELISA
Supernatants from infections carried out as described above were collected at 1, 6, 24, and 48 h.p.i. Supernatants were immediately spun down to remove cell debris for 10 min at 150 × g and stored at −20 °C until analyzed. IL-1β concentration was quantified with the Human IL-1β ELISA kit according to manufacturer’s specifications (ThermoFisher Scientific). IFN-β was quantified using the Human IFN-β ELISA kit according to manufacturer’s specifications (ThermoFisher Scientific).
Statistical analysis
All described experiments were repeated at least three times and data shown are representative of the biological replicates. Comparisons between two groups were analyzed in graphpad Prism using the two-tailed Student t test and ANOVA with multiple comparisons. Results with p-values below 0.05 were considered significant.
Results
eDNA-deficient mutants are attenuated in THP-1 macrophages
eDNA has been previously shown to be important for M. avium A5 biofilm formation. A library of M. avium strain A5 Mmt7 transposon mutants was previously screened for eDNA-deficiency during biofilm formation (Rose and Bermudez 2016). We selected three mutants with varying eDNA deficiencies summarized in Table 2. We tested the three mutants for their capacity to infect and survive in THP-1 macrophages, 7d3 (metal-dependent hydrolase), 9e11 (PknB-serine/threonine kinase) and 11e7 (Ftsk/SpoIIIE-DNA pore ATPase) compared to M. avium A5. All three mutants infected macrophages similarly to WT (Fig. 1A). After infection, mutants were extracted from THP-1 macrophages at 24 h.p.i and CFUs enumerated. All three mutants showed significantly reduced survival compared to M. avium A5 whether in naïve or LPS activated macrophages (Fig. 1B). Next, the growth curve of each mutant was compared to M. avium A5 (Fig. 1C). Replication of mutants 7d3, 9e11, and 11e7 bacteria was similar to M. avium A5 by day 2, however mutant 9e11 was significantly lower than M. avium A5 by day 4 (Fig. 1C). The reduced replication rate exhibited by mutant 9e11 by day 4 demonstrates inhibition of important pathways for PknB but has not affected the intracellular survival at 24 h.p.i. Taken together these data demonstrate the importance of eDNA for survival of M. avium A5 in macrophages. Since we have eDNA deficient mutants that are attenuated in macrophages, we investigated immune evasion through inflammasome modulation.
Table 2.
eDNA-deficient M. avium A5 mutants
| Location of transposon | Mutant | eDNAa (d7/O.D.) | G-C conent | Conserved domain | Neighboring genesc |
|---|---|---|---|---|---|
| MAVA5_03380 | 11e7 | 6177 | 61.6 | FtsK/SpoIIIE (DNA pore ATPase) | Hypothetical, plasmid replication, integration, and excision activator. Integrase, tRNA-Phe, tRNA-Asp, tRNA-glu, tRNA-Lys |
| MAVA5_10275b | 7d3 | 9918 | 57.8 | Metal-dependent hydrolase | TetR family transcriptional regulator. acetyl-CoA carboxylase, acetyl-CoA carboxylase subunit alpha, acyl-CoA dehydrogenase, long-chain fatty acid-CoA ligase, TetR family transcriptional regulator |
| MAVA5_13430 | 9e11 | 19,286 | 71.8 | PknB (Serine/threonine kinase) | Hypothetical, hypothetical, hypothetical, exinulease ABC subunit UvrB/DNA Polymerase III, EmrB efflux pump/Major facilitator superfamily |
aWT A5 eDNA (d7/O.D) was ~ 30,000
bGene is in 50 kb genomic region that is associated with DNA export
cItalics: downstream, Normal: upstream
Fig. 1.
eDNA-deficient mutants are attenuated in THP-1 macrophages. eDNA-deficient mutant characteristics were compared to wildtype M. avium A5 during invasion and survival in THP-1 macrophages. A Uptake of eDNA-deficient mutants compared to wildtype M. avium A5 at 1 h.p.i. B Survival of eDNA-deficient A5 mutants compared to wildtype M. avium A5 in THP-1 macrophages at 24 h.p.i. C Growth curve of wildtype M. avium A5 and eDNA mutants in 7H9 broth (not inside macrophages). Absorbance at 595 nm was read at day 0, 2, 4, and 6. Statistical comparisons are between WT and mutants. Data are representative of three independent experiments. Statistical comparisons: *P < 0.05; **P < 0.005, ***P < 0.0005, ****P < 0.0001
eDNA-deficient mutants alter IL-1β secretion
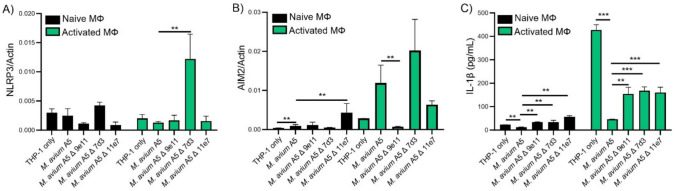
Here we compared eDNA-deficient mutants to M. avium A5 and uninfected macrophages (naïve or LPS activated). In naïve macrophages, eDNA deficient mutants as the WT bacterium did not cause upregulation of NLRP3 expression at 24 h.p.i. (Fig. 2A). Interestingly, mutant 7d3 significantly upregulated NLRP3 with LPS activated macrophages (Fig. 2A). When investigating AIM2, mutant 7d3 had upregulated expression similar to M avium A5 in LPS activated macrophages while mutant 9e11 had similar gene expression to uninfected macrophages (Fig. 2B). Uninfected macrophages gene expression determined background levels. Since NLRP3 and AIM2 stimulate IL-1β production, we determined cytokine protein levels. In naïve macrophages all mutants increased IL-1β levels compared to WT and uninfected macrophages. LPS-treated macrophages demonstrated that mutants still suppressed IL-1β but not as significantly as M. avium A5 (Fig. 2C). Even though gene expression for both NLRP3 and AIM2 is increased in mutant 7d3, there is no corresponding IL-1β increase. This could be due to the protein being actively degraded or it’s post translational processing is impaired. These mutants can help narrow down specific interactions that affect pro-inflammatory cytokine levels in macrophages. The observation that NLRP3 expression did not correlate with the increased IL-1β during mutant infection, indicates that M. avium is affecting NLRP3 activation somewhere between gene expression and IL-1β production.
Fig. 2.
eDNA-deficient mutants alter IL-1β secretion through inflammasome pathways. eDNA-deficient mutants compared to wildtype M. avium A5 infecting either naïve or LPS activated macrophages after 24 h.p.i. NLRP3 (A) and AIM2 (B) gene expression were quantified by qPCR and IL-1β protein levels (C) were quantified by ELISA. Data are representative of three independent experiments. Statistical comparisons: *P < 0.05; **P < 0.005, ***P < 0.0005
eDNA exporting M. avium strain A5 modulates NLRP3 expression differently than M. avium strain 104
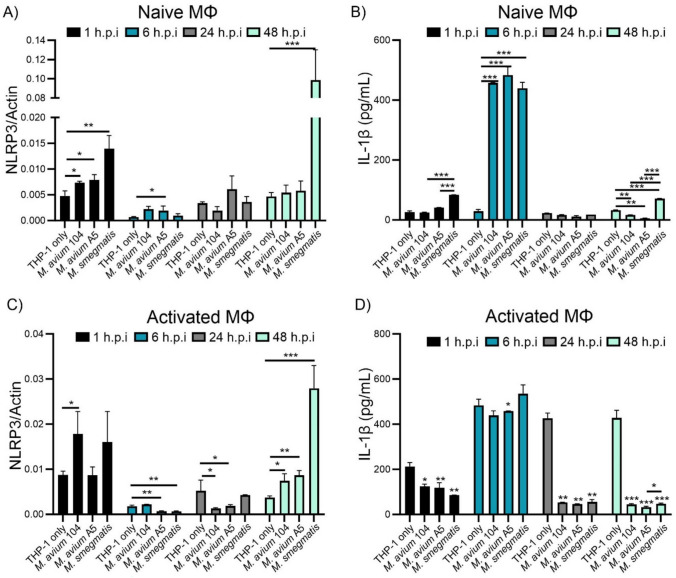
To determine if eDNA export has an effect on host cell stress sensors, we examined the gene expression of NLRP3. We compared M. avium strain A5, a high eDNA exporter, with M. avium strain 104, a lower eDNA exporter. Since regulation of the inflammasome response needs to occur early on during infection, time points were chosen between 1–48 h.p.i. M. smegmatis was a non-pathogenic strain of mycobacteria that can be killed by macrophages by 48 h.p.i but produces eDNA. Uninfected macrophages determine normal cellular expression of genes. NLRP3 expression was upregulated significantly at 1 h.p.i in all strains tested (Fig. 3A) compared to uninfected macrophages. The most upregulation was observed in M. smegmatis. Over time, NLRP3 expression in all treatments beings to reduce and at 48 h.p.i. M. avium 104 and M. avium A5 have similar levels to uninfected macrophages. M. smegmatis, however, greatly increases expression of NLRP3 at 48 h.p.i (Fig. 3A). This over expression is in response to intracellular killing of M. smegmatis and the release of PAMPs into the cytosol (Anes et al. 2006). This is not seen with M. avium strains, as both strains used to survive in macrophages.
Fig. 3.
M. avium species suppresses NLRP3 expression and IL-1β secretion by THP-1 macrophages. Naive and LPS activated macrophages were infected with either M. avium 104, M. avium A5, or M. smegmatis to determine NLRP3 gene expression and IL-1β protein section over 48 h.p.i. NLRP3 gene expression in A naïve and C LPS activated THP-1macrophages quantified by qPCR. IL-1β protein levels in supernatants from B naive and D LPS activated THP-1 macrophages quantified by ELISA. Statistical comparisons represent significant differences from uninfected cells at each timepoint. Data are representative of three independent experiments. Statistical comparisons: *P < 0.05; **P < 0.005, ***P < 0.0005
NLRP3 leads to the activation of IL-1β, a pro-inflammatory cytokine, and ultimately pyroptosis in macrophages. We examined if the upregulation seen in NLRP3 expression affects the quantity of IL-1β protein secreted by macrophages. When naïve macrophages are infected with M. avium 104, M. avium A5 or M. smegmatis, IL-1β protein concentration significantly (~ 500 pg/ml) increases by 6 h.p.i. (Fig. 3B). After 6 h.p.i. IL-1β protein concentration drops down to background levels. At 48 h.p.i. M. avium strains reduce protein levels while M. smegmatis increases protein concentration compared to the concentration of protein seen in uninfected macrophages.
Naïve macrophages are not the only type of cell mycobacteria will encounter in the host. Macrophages can be activated into M1 or M2 phenotypes depending on cell signaling (Yunna et al. 2020). A stimulator of M1 macrophages, LPS, also induces upregulation of NLRP3 (Gritsenko et al. 2020; Zheng et al. 2013). Macrophage activation via LPS stimulation and measurable IL-1β production takes 2–4 h (Gritsenko et al. 2020). However, LPS-induced NLRP3 inflammasome activation occurs within 15 min (Song et al. 2017). We compared M. avium 104 to M. avium 5 and M. smegmatis after LPS stimulation to determine what effects they have on inflammasome regulation in activated macrophages.
NLRP3 expression is upregulated by LPS activated macrophages in all treatment groups at 1 h.p.i. except M. avium A5 (Fig. 3C) compared to no LPS activated cells (Fig. 3A). M. avium 104 and M. smegmatis infected macrophages had a significant upregulation of NLRP3 after 1 h.p.i compared to uninfected cells. Interestingly, M. avium A5 does not increase NLRP3 expression beyond the effects of LPS activated macrophages, similar to uninfected macrophages after 1 h.p.i. Again, after early stimulation, NLRP3 expression decreases over time, until 48 h.p.i, where all strains of mycobacteria increase NLRP3 expression (Fig. 3C). In addition to NLRP3 upregulation due to TLR4 signaling, the LPS can lead to increased bacteria killing in macrophages which may lead to the observable NLRP3 increase. Activated macrophages infected with mycobacteria initially reduce IL-1β protein levels at 1 h.p.i, but after 6 h.p.i, IL-1β levels increase to uninfected amounts. Interestingly, after 6 h.p.i. IL-1β protein levels in all mycobacterial strains tested are suppressed, diminishing this pro-inflammatory pathway (Fig. 3D) while uninfected LPS activated macrophages remains high. Interestingly, the level of IL-1β of M. smegmatis infected naïve macrophages was significantly more than control THP-1 cells compared to the same infection in LPS treated macrophages. LPS treated macrophages have been shown to reduce survival of M. smegmatis by 60% within 24 h (Garg et al. 2006). This should lead to stimulation of NLRP3 and its downstream effects but was not observed. Perhaps an unknown variable of gene regulation or protein processing is affected by mycobacterial species but seen more prominently with non-pathogenic mycobacteria. We wanted to investigate how M. avium A5 might be mitigating the NLRP3 pathway via regulation of NLRP3 checkpoints.
Chemical inhibition of NLRP3 activation does not affect early survival of M. avium in macrophages
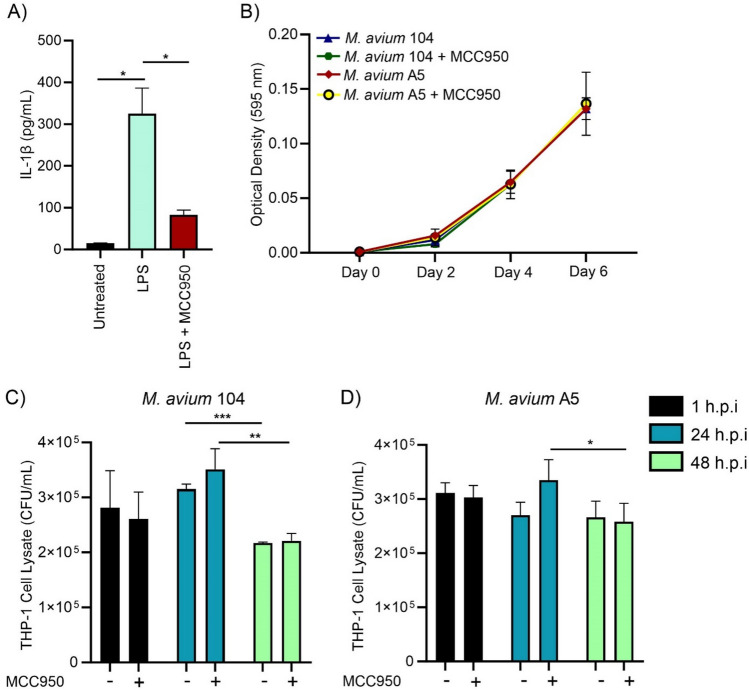
We investigated whether inhibition of NLRP3 with compound MCC950 affected M. avium A5 and M. avium 104 invasion and survival in macrophages. To confirm the inactivation of NLRP3, IL-1β protein levels were determined for LPS stimulated macrophages, LPS and MCC 950 treated macrophages, and untreated macrophages. LPS again increased IL-1β (~ 350 pg/ml), while MCC950 significantly reduced IL-1β levels to ~ 100 pg/ml (Fig. 4A). M. avium strains 104 and A5 were grown in 7H9 broth with or without NLRP3 inhibition to determine if this compound affects bacterial replication directly (Fig. 4B). Over 6 days of culture, there was no observable difference in growth capacity of either strain. After confirming reduction of NLRP3 activation by MCC950 and its effects on bacterial growth, we investigated the survival of M. avium strains in THP-1 macrophages. Treatment with MCC950 had no effect on M. avium 104 (Fig. 4C) survival compared to no inhibition of NLRP3. This observation was also seen in M. avium A5 (Fig. 4D). These data indicate that NLRP3 inflammasome activation does not affect invasion or early survival in macrophages. However, since M. avium possesses the ability to actively reduce NLRP3 activation early in infection, the effects of external NLRP3 inhibition may be indistinguishable from bacteria-induced suppression.
Fig. 4.
Chemical inhibition of NLRP3 activation does not affect M. avium survival in macrophages. NLRP3 activation was inhibited by MCC950 (1 µM) in macrophages at the time of invasion with either M. avium 104 or M. avium A5. A Confirmation of NLRP3 reduction through IL-1β protein production. B Bacterial growth with MCC950 (1 µM) compound for 1 h.p.i. Absorbance was measured at 595 nm over 6 days. M. avium 104 (C) and M. avium (D) invasion and survival in LPS activated macrophages. Statistical comparisons: *P < 0.05; **P < 0.005, ***P < 0.0005
Expression of eDNA sensors AIM2 and cGAS/STING after M. avium strain A5 infection
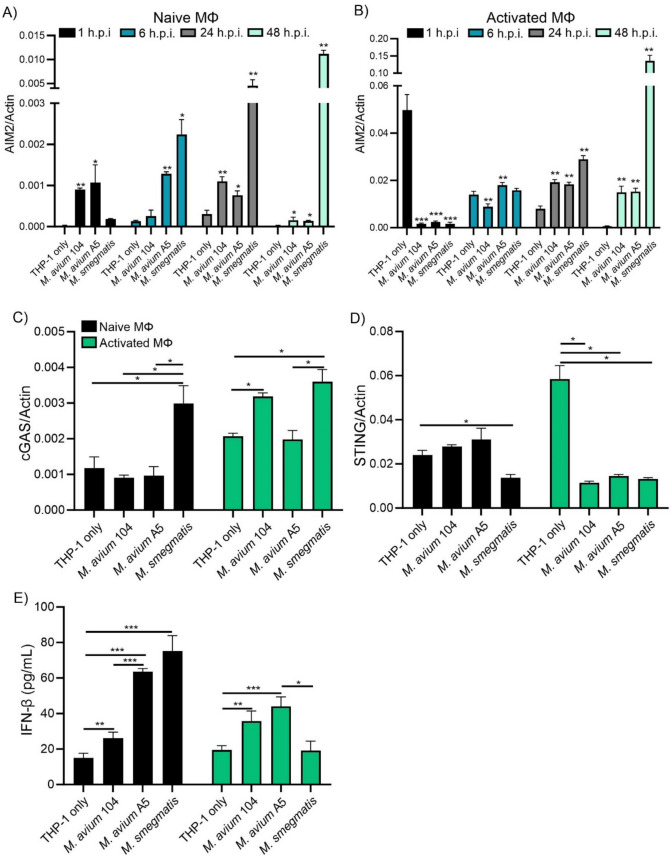
The NLRP3 inflammasome is only one pathway that produces IL-1β. The mechanism by which M. avium A5 reduces IL-1β production may be due to AIM2 inflammasome activation. The AIM2 inflammasome senses foreign and host dsDNA in the cytosol. To determine if eDNA produced from mycobacteria effects the AIM2 pathway, we measured gene expression by qPCR. Early infection of naïve THP-1 with M. smegmatis showed an increasing amount of AIM2 over 48 h.p.i (Fig. 5A). M. avium A5 initially increased AIM2 over 6 h.p.i but then gradually declined after 48 h.p.i (Fig. 5A). M. avium104 significantly upregulated AIM2 up to 24 h.p.i but less than high eDNA producing strain M. avium A5 and after 48 h.p.i upregulation was reduced (Fig. 5A). M. avium A5 exports higher quantities of extracellular DNA in the biofilm, which could explain why the initial spike in AIM2 expression differs from 104. When THP-1 macrophages are activated with LPS, uninfected cells upregulated AIM2 at 1 h, then expression remained relatively low (Fig. 5B). Like NLRP3, AIM2 inflammasome expression was initially upregulated by LPS stimulation, but was reduced over 48 h.p.i. M. smegmatis upregulation of AIM2 increases over 48 h.p.i and is much more than uninfected macrophages (Fig. 5B). Both M. avium strains have significantly elevated AIM2 over 48 h.p.i but there was no difference between eDNA exporting strains (Fig. 5B). With high expression of AIM2 we would expect increased levels of IL-1β, but as observed previously, IL-1β is suppressed especially in M. smegmatis.
Fig. 5.
M. avium species suppresses the expression of DNA sensors AIM2, cGAS, and STING, and triggers IFN-β secretion. Naïve and LPS activated macrophages were infected with either M. avium 104, M. avium A5, or M. smegmatis to determine DNA sensor gene expression and IFN-β protein production. AIM2 gene expression in naïve macrophages (A) and LPS activated macrophages (B) over 48 h.p.i. determined by qPCR. cGAS (C) and STING (D) gene expression after 24 h.p.i. quantified by qPCR. IFN-β protein levels (E) after 24 h.p.i. quantified by ELISA. Data are representative of at least two independent experiments. Statistical comparisons: *P < 0.05; **P < 0.005, ***P < 0.0005
dsDNA can also be detected by the cGAS/STING pathway. Activation of this pathway leads to the production of IFN-β, a pleiotropic cytokine, and has been shown to act in a host detrimental manner during chronic bacterial infections (Manca et al. 2001; Manzanillo et al. 2012). IFN-β may counteract the effects of pro-inflammatory cytokine signaling by regulating gene expression (Boxel-Dezaire et al. 2006). Since cGAS/STING is a multistep pathway, we investigated cGAS and STING gene expression separately. cGAS is the cytosolic sensor that binds to dsDNA and produces a secondary messenger (cGAMP) that leads to STING activation downstream. THP-1 macrophages infected with M. avium strains showed no increase in cGAS expression while non-pathogenic M. smegmatis led to a significant increase (Fig. 5C). Activated macrophages showed an increase of cGAS overall but a significant increase in M. avium 104 and M. smegmatis at 24 h.p.i. (Fig. 5C). M. avium A5 did not increase cGAS expression after 24 h.p.i. Different strains of mycobacteria can utilize various pathways for survival including expression of cGAS which can be modulated in various ways including Beclin-1 controlling autophagy or ubiquitination and phosphorylation mediated signaling (Yu and Liu 2021). STING activation leads to the IFN-β transcription. STING expression in macrophages is not upregulated in M. avium infections. Activated macrophages demonstrated the upregulation of STING gene expression while all mycobacterial strains tested actively reduced STING expression (Fig. 5D). IFN-β protein levels were determined for mycobacteria. Macrophages infected with eDNA generating mycobacteria produce high levels of IFN-β, M. avium A5 (~ 65 pg/ml) and M. smegmatis (~ 77 pg/ml) while M. avium 104 induces much lower levels (~ 30 pg/ml) (Fig. 5E). LPS activation reduces IFN-β levels for eDNA producing mycobacteria (M. avium A5 and M. smegmatis) compared to naïve macrophages but increased IFN-β for M. avium 104 (Fig. 5E). M. smegmatis was reduced to normal uninfected macrophages. Since LPS stimulates pro-inflammatory cytokines, a reduction in anti-inflammatory IFN-β would allow for host killing mechanism to prevail. Mycobacteria may use IFN-β to reduce the pro-inflammatory state of the macrophage especially when these bacteria actively reduce levels of IL-1β as seen previously. eDNA has been demonstrated to effectively modulate the inflammatory state of macrophages so to understand these effects more in depth we utilized mutants deficient in eDNA export.
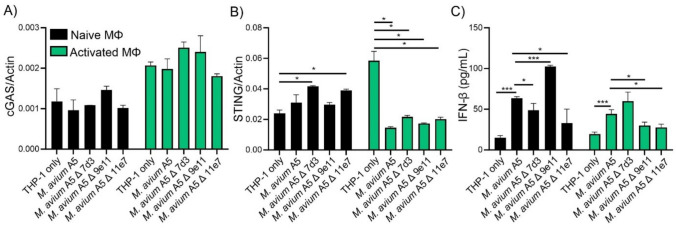
M. avium A5 eDNA-deficient mutants downregulate STING expression in activated macrophages and modulate IFN-β production
eDNA-deficient mutants affect the secretion of IFN-β through the cGAS/STING pathway. M. avium A5 previously showed no effect on cGAS upregulation compared to uninfected THP-1 macrophages. Whether or not these cells were activated by LPS addition. Attenuated mutants did not stimulate the upregulation of cGAS in naïve macrophages similar to M. avium A5 (Fig. 6A). This pattern was also seen in activated macrophages. eDNA export did not affect the gene expression of cGAS in THP-1 macrophages. In naïve macrophages, mutants 7d3 and 11e7 upregulated STING gene expression (Fig. 6B). STING gene expression was greatly reduced only after infection of activated macrophages similar to M. avium A5 (Fig. 6B). cGAS/STING stimulates IFN-β production. eDNA-deficient mutants 7d3 and 11e7 had significantly reduced levels of IFN-β compared to M. avium A5 in naïve macrophages (Fig. 6C). Mutant 9e11, the serine/threonine kinase, greatly stimulated anti-inflammatory activation and IFN-β production. In activated macrophages, IFN-β levels were reduced compared to M. avium A5, likely due to the heightened inflammatory environment generated by LPS (Fig. 6C). However, these levels of IFN-β were still higher than uninfected macrophages, demonstrating that M. avium is still able to induce anti-inflammatory signaling. These data indicate an importance for eDNA export to promote anti-inflammatory signal expression during infection of macrophages to evade host killing mechanisms. Additional work is needed to dissect the mechanism what is truly affecting the modulation of inflammatory responses in host macrophages.
Fig. 6.
eDNA-deficient mutants modulates STING expression and IFN-β production in macrophages. Naïve and LPS activated macrophages were infected with either e DNA-deficient mutants (7d3, 9e11, 11e7) or wildtype M. avium A5. DNA sensor gene expression of cGAS (A) and STING (B) at 24 h.p.i. determined by qPCR. IFN-β protein levels (C) at 24 h.p.i quantified by ELISA. Data are representative of three independent experiments. Statistical comparisons: *P < 0.05; **P < 0.005, ***P < 0.0005
Discussion
Intracellular pathogens, like mycobacteria, modulate cytosolic sensing pathways to avoid macrophage killing mechanisms and halt premature cell death. We found that M. avium 104 and M. avium A5 modulate NLRP3 inflammasome expression and activation early in infection. The NLRP3, AIM2 inflammasomes and cGAS/STING are cytosolic sensors are important during mycobacterial infection. IL-1β secretion spiked early in the infectious process but dropped to background levels up to 48 h.p.i. It appears to differ from M. smegmatis which triggers high levels of NLRP3 activation, but the subsequent IL-1β secretion is countered by cGAS-STING dependent IFN-β production (Kim et al. 2020). In LPS activated macrophages infected with M. avium, IL-1β protein levels increased at 6 h.p.i compared to uninfected macrophages, which could explain the increased bacterial killing after LPS stimulation. LPS is a potent macrophage activator and induces autophagy, pro-inflammatory cytokine secretion, nitric oxide, and enhance bacterial killing (Meng and Lowell 1997). LPS also triggers NLRP3 activation through a non-canonical pathway (Guo et al. 2015). It has also been shown to induce AIM2 and STING expression in macrophages (Lugrin and Martinon 2018; Ning et al. 2020), explaining the upregulation of NLRP3, AIM2, and STING expression and IL-1β production in uninfected cells stimulated with LPS.
Nonvirulent M. smegmatis induced significantly greater expression of NLRP3 and IL-1β production at 48 h.p.i than M. avium. IL-1β production by M. smegmatis-infected macrophages at 48 h.p.i, however, it was not increased by LPS-stimulation. M. smegmatis is not adapted to survival in human macrophages, and phagocytes clear M. smegmatis infection by 24–48 h following phagocytosis. M. smegmatis does not inhibit phagosome acidification during early infection, and the process of phagosome maturation is not altered (Kuehnel et al. 2001). Thus, phagosome maturation and subsequent intracellular killing of M. smegmatis could lead to increased cytosolic PAMPS which could explain the upregulation of NLRP3 and AIM2 at 48 h.p.i.
The activation of the NLRP3 inflammasome may play different roles at different stages of infection. A previous study demonstrated, the early macrophage response to M. avium infection is pro-inflammatory, but is reversed once the bacteria are internalized (Greenwell-Wild et al. 2002). M. tuberculosis induces NLRP3 activation during chronic infection to facilitate cell-to-cell spread (Xu et al. 2020). We observed no effect on intracellular M. avium survival in the presence of an NLRP3 inhibitor. This gives credence to the idea that M. avium possesses mechanisms by which to evade early inflammasome activation and modulate the pro-inflammatory response to further intracellular replication. Perhaps similar strategy is employed by M. tuberculosis to inhibit NLRP3 activation during early stages of infection (Be et al. 2012), by expressing Phosphokinase F (PknF) (Rastogi et al. 2021). M. avium A5 has a homolog for PknF, and is the gene disrupted in mutant 9e11 (MAVA5_13430; PknB). MAVA5_13430 is an operon that contains several genes encoding for hypothetical proteins, efflux pump, and transporters. Mutant 9e11 induces significantly higher IL-1β production than M. avium A5, which could be due to the lack of functional PknB. The same increase of IL-1β, however, was observed for other eDNA-deficient mutants, raising the possibility that increased IL-1β may be the consequence of bacterial killing.
We also examined the modulation of DNA-sensing pathways AIM2 and cGAS/STING in the macrophage during M. avium and M. smegmatis infection. Activation of the AIM2 inflammasome leads to pro-inflammatory IL-1β production, which increases anti-bacterial activity. Activation of the cGAS/STING pathway leads to the production of type I interferon IFN-β. The role of type I interferons in NTM infection is inconclusive. Prolonged expression contributes to persistence during chronic viral and bacterial infections. IFN-β promotes chronic mycobacterial infections by inducing anti-inflammatory IL-10 production and suppressing pro-inflammatory IL-12 and IFN-γ (Boxx and Cheng 2016; Teles et al. 2013). This limits inflammation-related tissue damage but slows bacterial clearance.
While the role of STING in M. tuberculosis is well-described, the importance of STING in NTM infection is still unclear. Recent research demonstrated that M. abscessus, a rapidly growing NTM, induces high levels of cGAS/STING-derived IFN-β, which counteracts NLRP3-derived IL-1β, and facilitates bacterial survival (Kim et al. 2020). During M. tuberculosis infection, the cGAS/STING pathway is activated by cytosolic mycobacterial DNA, and mycobacterial c-di-AMP (Watson et al. 2015; Liu et al. 2022). This activation induces autophagy, which has a negative effect on inflammasome activation and bacterial survival (Watson et al. 2015). However, M. tuberculosis is able to survive by inhibiting autophagy and reducing the availability of STING by secreting the protein MmsA (Maphasa et al. 2021; Sun et al. 2020). M. avium has a homolog of the MmsA gene and may be able to utilize a similar mechanism. Further work could determine if MmsA is involved in the modulation of STING during M. avium pathogenesis.
We found that high eDNA-producing M. avium strain A5 induced significantly higher AIM2 expression and IFN-β production than low eDNA-producing M. avium 104 in naïve macrophages. However, LPS stimulation reduced these differences, indicating crosstalk between inflammasomes and cGAS/STING. Interestingly, M. smegmatis was unable to induce IFN-β production after 24 h like the M. avium strains. This could be due to LPS treatment inducing more bacteria killing in the macrophages and the other strains ability to modulate their environment by affecting signaling pathways. We found that M. avium A5 eDNA-deficient mutants induced varied responses in human macrophages. Infections with three mutants lack the LPS-induced upregulation of STING expression similar to WT M. avium A5. This observation suggests that bacterial eDNA may not be involved in the modulation of STING expression. While there is no evidence of intracellular eDNA export by M. avium, intracellular DNA export has been established during M. tuberculosis infection, so it is feasible for mycobacteria to accomplish this in the host. The observation, however, of reduced IFN-β during mutants 7d3 and 11e7 infection, compared to the increased IFN-β induced by WT A5, supports the hypothesis that eDNA is being secreted in the host macrophage. Future work would examine cGAS/STING activation and DNA export at later time points to further the knowledge in this question.
All the three M. avium A5 mutants were attenuated at intracellular survival in human macrophages. Mutant 11e7 (MAVA5_03380; DNA pore ATPase) is the most deficient at DNA export, which explains the reduction in AIM2 expression and IFN-β production compared to the wildtype bacterium. These differences are likely due to lower amounts of cytosolic mycobacterial DNA. MAVA5_03380 is homologous to M. tuberculosis gene Rv3871, which encodes an ESX-1 Type VII secretion system protein EccB, that contains a FtsK/SpoIIIE domain, similar to MAVA5_03380, and is involved in protein secretion by the type VII secretion system, interaction with the host, and evasion of the host immune response (Brodin et al. 2006; Stanley et al. 2003). EccB interacts with EspL (Rv3876), which is the only ESX-1 protein found in M. avium (MAVA5_16875) (Jeffrey et al. 2017). EspL is essential for M. tuberculosis virulence and mediates ESX-1 function. Given the similarity of the ESX secretion systems, EspL may interact with an alternate ESX secretion system that is present in the M. avium genome. This could explain the attenuation of macrophage survival observed by mutant 11e7 (Fig. 1).
Mutant 9e11 is the least eDNA-deficient mutant used in this study, which could mean that there is sufficient cytosolic DNA to trigger the cGAS/STING pathway. This could explain the unexpected increase in IFN-β production observed following 9e11 infection of macrophages. Additionally, intracellular killing could release increased DNA into the cytosol. The gene disrupted in mutant 9e11, MAVA5_13430, has a serine/threonine protein kinase B domain (PknB), which is essential for growth, stress response and metabolic regulation in M. tb (Grundner et al. 2005). Additionally, MAVA5_13430 has three homologs in the M. tuberculosis genome: Rv2914c (PknI), Rv2088 (PknJ), and Rv1746 (PknF). All three genes encode serine/threonine protein kinases, which carry out a range of functions in the host. PknI regulates bacterial growth in the host and specifically slows bacterial growth in the macrophage (Gopalaswamy et al. 2009). PknJ phosphorylates several substrates in the host, including pyruvate kinase, and macrophage-entry associated protein GroEL2 (Arora et al. 2010; Vinod et al. 2021). PknF interacts with an ABC transporter encoded by Rv1747, to inhibit the NLRP3 inflammasome during M. tuberculosis infection (Rastogi et al. 2021). The lack of any of these functions could explain the significant attenuation of extracellular and intracellular growth displayed by the 9e11 mutant (Fig. 4). This gene would be an ideal candidate for further study since it appears to be central to survival in the host and environment.
Mutant 7d3 (MAVA5_10275; hydrolase) is not as DNA deficient as 11e7, so there may be sufficient cytosolic DNA to induce AIM2 expression, cGAS/STING activation, and IFN-β production. Unexpectedly, 7d3 induced significant upregulation of NLRP3 and AIM2 in LPS activated macrophages, but IL-1β production was unaffected. Mutant 7d3 induced significantly lower IFN-β than WT A5 in naïve macrophages and significantly higher levels in activated macrophages. The neighboring genes upstream and downstream of MAVA5_10275 are involved in glycolysis and cellular metabolism. Additionally, the homolog of MAVA5_10275 in M. abscessus, MAB_2069, is a metal-dependent hydrolase involved in fatty acid metabolism. MAB_2069 interacts with AraC transcriptional regulator appY, which regulates the response to oxidative stress, acid stress and antibiotics in E. coli (Dale et al. 2022). AraC transcriptional regulators are known to be important for its role on virulence and response to stress (Gallegos et al. 1997). Disruption of MAVA5_10275 and surrounding genes in mutant 7d3 could interfere with the utilization of host lipids, and adaptation to intracellular conditions, leading to the observed attenuation of this mutant in the macrophage, but not extracellular growth (Fig. 1).
Exploring the ability of mycobacteria biofilms to not only allow for survival in an intracellular or extracellular environment but to utilize these pathways for pathogenicity is beginning to be understood. M. tuberculosis has been shown to manipulate inflammasomes allowing pathogenesis. In this study, our focus to determine the effects of eDNA exporting mycobacteria in the context of survival in host macrophages occurred through modulation of inflammasome pathways. eDNA-deficient mutants being attenuated in macrophages demonstrated its importance for survival. eDNA producing M. avium A5 modulates inflammasome processes differently than M. tuberculosis. With the complex nature of inflammasome pathways continued work is need to better understand how eDNA producing M. avium survive in host macrophages leading to new targets for therapies and better patient outcomes.
Author contributions
JJ: Participated in the design of experiments, performed the experiments, wrote the manuscript; ALP: performed experiments, participated in the design of experiments, wrote the manuscript; LEB, idealized the studies, design the experiments, edited the manuscript, funded the work. All the authors participated in the analyze of the data.
Funding
This work was supported by the Microbiology Foundation of San Francisco. Grant 1001.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jayanthi J. Joseph and Amy Leestemaker-Palmer contributed equally to the report.
References
- Amaral EP, Riteau N, Moayeri M, Maier N, Mayer-Barber KD, Pereira RM, Lage SL, Kubler A, Bishai WR, D’Império-Lima MR, Sher A, Andrade BB (2018) Lysosomal cathepsin release is required for NLRP3-inflammasome activation by Mycobacterium tuberculosis in infected macrophages. Front Immunol 9:1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anes E, Peyron P, Staali L, Jordao L, Gutierrez MG, Kress H, Hagedorn M, Maridonneau-Parini I, Skinner MA, Wildeman AG, Kalamidas SA, Kuehnel M, Griffiths G (2006) Dynamic life and death interactions between Mycobacterium smegmatis and J774 macrophages. Cell Microbiol 8:939–960 [DOI] [PubMed] [Google Scholar]
- Aronson T, Holtzman A, Glover N, Boian M, Froman S, Berlin OGW, Hill H, Stelma G (1999) Comparison of large restriction fragments of Mycobacterium avium isolates recovered from AIDS and non-AIDS patients with those of isolates from potable water. J Clin Microbiol 37:1008–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora G, Sajid A, Gupta M, Bhaduri A, Kumar P, Basu-Modak S, Singh Y (2010) Understanding the role of PknJ in Mycobacterium tuberculosis: biochemical characterization and identification of novel substrate pyruvate kinase A. PLoS ONE 5:e10772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Be NA, Bishai WR, Jain SK (2012) Role of Mycobacterium tuberculosis pknD in the pathogenesis of central nervous system tuberculosis. BMC Microbiol 12:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxx GM, Cheng G (2016) The roles of type I interferon in bacterial infection. Cell Host Microbe 19:760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P, Majlessi L, Marsollier L, de Jonge MI, Bottai D, Demangel C, Hinds J, Neyrolles O, Butcher PD, Leclerc C, Cole ST, Brosch R (2006) Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun 74:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Vance RE (2013) STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol 14:19–26 [DOI] [PubMed] [Google Scholar]
- Chakraborty P, Bajeli S, Kaushal D, Radotra BD, Kumar A (2021) Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis. Nat Commun 12:1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AG, Porcu A, Mann J, Neidle S (2022) The mechanism of resistance in Escherichia coli to ridinilazole and other antibacterial head-to-head bis-benzimidazole compounds. Med Chem Res 31:1176–1191 [Google Scholar]
- Danelishvili L, Poort MJ, Bermudez LE (2004) Identification of Mycobacterium avium genes up-regulated in cultured macrophages and in mice. FEMS Microbiol Lett 239:41–49 [DOI] [PubMed] [Google Scholar]
- Gallegos MT, Schleif R, Bairoch A, Hofmann K, Ramos JL (1997) Arac/XylS family of transcriptional regulators. Microbiol Mol Biol Rev 61:393–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Vitvitsky V, Gendelman HE, Banerjee R (2006) Monocyte differentiation, activation, and mycobacterial killing are linked to transsulfuration-dependent redox metabolism. J Biol Chem 281:38712–38720 [DOI] [PubMed] [Google Scholar]
- Gopalaswamy R, Narayanan S, Chen B, Jacobs WR, Av-Gay Y (2009) The serine/threonine protein kinase PknI controls the growth of Mycobacterium tuberculosis upon infection. FEMS Microbiol Lett 295:23–29 [DOI] [PubMed] [Google Scholar]
- Greenwell-Wild T, Vázquez N, Sim D, Schito M, Chatterjee D, Orenstein JM, Wahl SM (2002) Mycobacterium avium Infection and modulation of human macrophage gene expression. J Immunol 169:6286–6297 [DOI] [PubMed] [Google Scholar]
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K (2007) An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416 [DOI] [PubMed] [Google Scholar]
- Gritsenko A, Yu S, Martin-Sanchez F, Diaz-del-Olmo I, Nichols E-M, Davis DM, Brough D, Lopez-Castejon G (2020) Priming is dispensable for NLRP3 inflammasome activation in human monocytes in vitro. Front Immunol. 10.3389/fimmu.2020.565924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundner C, Gay LM, Alber T (2005) Mycobacterium tuberculosis serine/threonine kinases PknB, PknD, PknE, and PknF phosphorylate multiple FHA domains. Protein Sci Publ Protein Soc 14:1918–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J (2011) Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34:213–223 [DOI] [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting JP-Y (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Mishra BB, Jordao L, Elliott E, Anes E, Griffiths G (2008) NF-κB activation controls phagolysosome fusion-mediated killing of mycobacteria by macrophages. J Immunol 181:2651–2663 [DOI] [PubMed] [Google Scholar]
- Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E, Tybulewicz V, Mitsuyama M (2013) Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. 12. Nat Immunol 14:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey B, Rose SJ, Gilbert K, Lewis M, Bermudez LE (2017) Comparative analysis of the genomes of clinical isolates of Mycobacterium avium subsp. hominissuis regarding virulence-related genes. J Med Microbiol 66:1063–1075 [DOI] [PubMed] [Google Scholar]
- Kim B-R, Kim B-J, Kook Y-H, Kim B-J (2020) Mycobacterium abscessus infection leads to enhanced production of type 1 interferon and NLRP3 inflammasome activation in murine macrophages via mitochondrial oxidative stress. PLOS Pathog 16:e1008294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehnel MP, Goethe R, Habermann A, Mueller E, Rohde M, Griffiths G, Valentin-Weigand P (2001) Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cell Microbiol 3:551–566 [DOI] [PubMed] [Google Scholar]
- Lee H-M, Yuk J-M, Kim K-H, Jang J, Kang G, Park JB, Son J-W, Jo E-K (2012) Mycobacterium abscessus activates the NLRP3 inflammasome via Dectin-1–Syk and p62/SQSTM1. Immunol Cell Biol 90:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Pang X, Zhang H, Ji P (2022) The cGAS-STING pathway in bacterial infection and bacterial immunity. Front Immunol. 10.3389/fimmu.2021.814709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugrin J, Martinon F (2018) The AIM2 inflammasome: sensor of pathogens and cellular perturbations. Immunol Rev 281:99–114 [DOI] [PubMed] [Google Scholar]
- Ma R, Ortiz Serrano TP, Davis J, Prigge AD, Ridge KM (2020) The cGAS-STING pathway: The role of self-DNA sensing in inflammatory lung disease. FASEB J off Publ Fed Am Soc Exp Biol 34:13156–13170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan-Lala R, Peixoto KV, Re F, Rengarajan J (2011) Mycobacterium tuberculosis hip1 dampens macrophage proinflammatory responses by limiting toll-like receptor 2 activation. Infect Immun 79:4828–4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, Freedman VH, Kaplan G (2001) Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci U S A 98:5752–5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS (2012) Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11:469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maphasa RE, Meyer M, Dube A (2021) The macrophage response to Mycobacterium tuberculosis and opportunities for autophagy inducing nanomedicines for tuberculosis therapy. Front Cell Infect Microbiol. 10.3389/fcimb.2020.618414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Lowell CA (1997) Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med 185:1661–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning L, Wei W, Wenyang J, Rui X, Qing G (2020) Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide. Clin Transl Med 10:e228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevots DR, Marras TK (2015) Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 36:13–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py BF, Kim M-S, Vakifahmetoglu-Norberg H, Yuan J (2013) Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49:331–338 [DOI] [PubMed] [Google Scholar]
- Rastogi S, Ellinwood S, Augenstreich J, Mayer-Barber KD, Briken V (2021) Mycobacterium tuberculosis inhibits the NLRP3 inflammasome activation via its phosphokinase PknF. PLOS Pathog 17:e1009712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SJ, Bermudez LE (2016) Identification of bicarbonate as a trigger and genes involved with extracellular DNA export in mycobacterial biofilms. mBio. 10.1128/mBio.01597-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SJ, Babrak LM, Bermudez LE (2015) Mycobacterium avium possesses extracellular DNA that contributes to biofilm formation, structural integrity, and tolerance to antibiotics. PLoS ONE 10:e0128772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, Yamamoto M, Takeda K (2012) Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol 24:637–644 [DOI] [PubMed] [Google Scholar]
- Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V, Dabelic R, Manicassamy B, Aitchison JD, Aderem A, Elliott RM, García-Sastre A, Racaniello V, Snijder EJ, Yokoyama WM, Diamond MS, Virgin HW, Rice CM (2014) Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505:691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Bohsali A, Ahlbrand SE, Srinivasan L, Rathinam VAK, Vogel SN, Fitzgerald KA, Sutterwala FS, Briken V (2013) Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-β and AIM2 inflammasome-dependent IL-1β production via its ESX-1 secretion system. J Immunol 191:3514–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Liu Z-S, Xue W, Bai Z-F, Wang Q-Y, Dai J, Liu X, Huang Y-J, Cai H, Zhan X-Y, Han Q-Y, Wang H, Chen Y, Li H-Y, Li A-L, Zhang X-M, Zhou T, Li T (2017) NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell 68:185-197.e6 [DOI] [PubMed] [Google Scholar]
- Stanley SA, Raghavan S, Hwang WW, Cox JS (2003) Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci U S A 100:13001–13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang W, Dong C, Xiong S (2020) Mycobacterium tuberculosis MmsA (Rv0753c) interacts with STING and blunts the type I interferon response. mBio 11:e03254-e3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KV, Deng M, Ting JP-Y (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19:477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles RMB, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, Komisopoulou E, Kelly-Scumpia K, Chun R, Iyer SS, Sarno EN, Rea TH, Hewison M, Adams JS, Popper SJ, Relman DA, Stenger S, Bloom BR, Cheng G, Modlin RL (2013) Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science 339:1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxel-Dezaire AHH, Rani MRS, Stark GR (2006) Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25:361–372 [DOI] [PubMed] [Google Scholar]
- van Ingen J, Ferro BE, Hoefsloot W, Boeree MJ, van Soolingen D (2013) Drug treatment of pulmonary nontuberculous mycobacterial disease in HIV-negative patients: the evidence. Expert Rev Anti Infect Ther 11:1065–1077 [DOI] [PubMed] [Google Scholar]
- Vega-Dominguez P, Peterson E, Pan M, Di Maio A, Singh S, Umapathy S, Saini DK, Baliga N, Bhatt A (2020) Biofilms of the non-tuberculous Mycobacterium chelonae form an extracellular matrix and display distinct expression patterns. Cell Surf 6:100043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod V, Pushkaran AC, Kumar A, Mohan CG, Biswas R (2021) Interaction mechanism of Mycobacterium tuberculosis GroEL2 protein with macrophage Lectin-like, oxidized low-density lipoprotein receptor-1: an integrated computational and experimental study. Biochim Biophys Acta BBA Gen Subj 1865:129758 [DOI] [PubMed] [Google Scholar]
- Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, Schmid-Burgk JL, Schmidt T, Hornung V, Cole ST, Ablasser A (2015) Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe 17:799–810 [DOI] [PubMed] [Google Scholar]
- Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS (2015) The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17:811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M-F, Shu C-C, Wang J-Y, Yan B-S, Lai H-C, Chiang B-L, Wu LS-H, Yu C-J (2019) NLRP3 inflammasome is attenuated in patients with Mycobacterium avium complex lung disease and correlated with decreased interleukin-1β response and host susceptibility. Sci Rep 9:12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Qi H, Li J, Sun L, Gong J, Chen Y, Shen A, Li W (2020) Mycobacterium tuberculosis infection up-regulates MFN2 expression to promote NLRP3 inflammasome formation. J Biol Chem 295:17684–17697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Danelishvili L, Wu M, Hidaka E, Katsuyama T, Stang B, Petrofsky M, Bildfell R, Bermudez LE (2006) The ability to form biofilm influences Mycobacterium avium invasion and translocation of bronchial epithelial cells. Cell Microbiol 8:806–814 [DOI] [PubMed] [Google Scholar]
- Yu L, Liu P (2021) Cytosolic DNA sensing by cGAS: regulation, function, and human diseases. Signal Transduct Target Ther 6:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunna C, Mengru H, Lei W, Weidong C (2020) Macrophage M1/M2 polarization. Eur J Pharmacol 877:173090 [DOI] [PubMed] [Google Scholar]
- Zheng X-F, Hong Y-X, Feng G-J, Zhang G-F, Rogers H, Lewis MAO, Williams DW, Xia Z-F, Song B, Wei X-Q (2013) Lipopolysaccharide-induced M2 to M1 macrophage transformation for IL-12p70 production is blocked by candida albicans mediated up-regulation of EBI3 expression. PLoS ONE 8:e63967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.