Abstract
Objectives
This study aims to elucidate the complex molecular and cellular landscape of pancreatic ductal adenocarcinoma (PDAC) by identifying key regulatory non-coding RNAs (ncRNAs), hub protein-coding genes, and Intercellular communication pathways that may serve as prognostic biomarkers and therapeutic targets.
Background
Pancreatic cancer remains one of the deadliest malignancies worldwide, characterized by late diagnosis, limited treatment response, and poor prognosis. Among its histological subtypes, PDAC accounts for over 80% of cases and is defined by a highly fibrotic and immunosuppressive tumor microenvironment (TME).
Methods
We performed a comprehensive bioinformatics analysis integrating multiple transcriptomic datasets from the NCBI Gene Expression Omnibus (GEO), including mRNA, miRNA, lncRNA, and circRNA profiles from PDAC and adjacent normal tissues. Differential expression analysis was conducted using GEO2R, followed by functional enrichment via DAVID. Hub genes were identified from protein–protein interaction (PPI) networks constructed using STRING and validated using GEPIA2. A competing endogenous RNA (ceRNA) network was developed to investigate regulatory ncRNA–mRNA axes. To refine these findings, single-cell RNA-seq (scRNA-seq) data were analyzed to resolve the cellular origin of hub genes and ncRNAs, and CellChat was employed to model intercellular communication within the TME.
Results
We identified several dysregulated genes and ncRNAs implicated in key oncogenic pathways, including ECM remodeling, inflammation, and immune evasion. The ceRNA network highlighted functional interactions between circRNAs, lncRNAs, and miRNAs regulating key hub genes. Single-cell analysis revealed cell-type-specific expression of hub genes—e.g., FN1 and COL11A1 in fibroblasts, CXCL8 in macrophages, and ITGA3 in ductal cells—and uncovered a macrophage–endothelial CXCL8–ACKR1 signaling axis potentially driving tumor-associated angiogenesis. Moreover, correlations with immune cell infiltration and drug sensitivity further underscored the translational relevance of the identified molecular targets.
Conclusion
Our analysis combining bulk and single-cell transcriptomics provides a multi-scale view of PDAC pathogenesis. The findings highlight the interplay between ncRNAs, hub genes, and cellular crosstalk in shaping the tumor ecosystem and suggest novel targets for precision therapeutic intervention and biomarker development.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-025-01815-8.
Keywords: Bioinformatics analysis, Pancreatic Cancer, Non-coding RNA, RNA-seq, circRNA-miRNA-mRNA, Single-cell, CellChat, scRNA-seq
Introduction
Pancreatic cancer is one of the deadliest forms of malignancies worldwide, characterized by an exceptionally high mortality rate and extremely poor prognosis. Among the histological subtypes of Pancreatic cancer, pancreatic ductal adenocarcinoma (PDAC) is the most common, accounting for approximately 80–90% of cases [1, 2]. PDAC is notable for its aggressive clinical behavior, rapid progression, and late-stage diagnosis, which severely limit therapeutic options and result in dismal five-year survival rates of less than 10% [3].
The risk of developing PDAC increases substantially with advancing age, especially between 60 and 80 years, and is influenced by a combination of genetic and environmental risk factors, including familial syndromes, chronic pancreatitis, diabetes mellitus, smoking, heavy alcohol consumption, and obesity [2–4]. Despite significant advances in surgical techniques, adjuvant chemotherapy, and molecular research, outcomes for PDAC patients have remained largely unchanged, with the majority of diagnoses occurring at advanced, often metastatic stages [1, 3].
Early diagnosis remains a major clinical challenge in PDAC due to its asymptomatic nature in initial stages and the lack of reliable early biomarkers. Conventional tumor markers such as carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) suffer from limited sensitivity and specificity, particularly in detecting early lesions [4, 5]. Moreover, imaging modalities, while critical for staging and monitoring, often fail to detect early or small PDAC lesions [4]. Consequently, there is a pressing need for novel molecular biomarkers and targeted therapeutic strategies.
In recent years, non-coding RNAs (ncRNAs), including circular RNAs (circRNAs), microRNAs (miRNAs), and long non-coding RNAs (lncRNAs), have emerged as critical regulators of PDAC pathogenesis and progression. CircRNAs, with their covalently closed loop structures, exhibit remarkable stability and tissue-specific expression, functioning predominantly as competing endogenous RNAs (ceRNAs) that modulate miRNA activity and influence gene regulatory networks [1, 5]. Similarly, miRNAs play crucial roles by binding to the 3' untranslated regions (UTRs) of target mRNAs, mediating post-transcriptional repression or degradation [5]. Aberrant expression of specific miRNAs can drive tumorigenesis, metastasis, and therapy resistance in PDAC.
Technological advances in high-throughput sequencing and integrative bioinformatics analyses have accelerated the discovery of novel ncRNA-mediated regulatory axes involved in PDAC. For example, alterations in the circRNA_102049/miR-455-3p/CD80 axis have been implicated in immune modulation and tumor progression [6]. In addition, understanding the interplay between tumor cells and the immunosuppressive tumor microenvironment (TME)—including cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs)—has shed light on mechanisms of immune evasion and metastasis in PDAC [3].
Rather than introducing a novel analytical pipeline, our study strategically combines bulk and single-cell transcriptomic data to uncover cell-type-specific mechanisms underlying pancreatic ductal adenocarcinoma (PDAC). Bulk RNA-seq was used to identify candidate genes and regulatory ncRNAs, and scRNA-seq provided cellular resolution to localize these findings within the tumor microenvironment.
Materials and methods
Data preparation and differential expression analysis
To achieve a multi-layered understanding of PDAC, we adopted a two-tiered strategy: (1) identification of differentially expressed genes and regulatory ncRNAs using bulk transcriptomic datasets; (2) mapping of these targets onto specific cell types using single-cell RNA-seq analysis, including clustering, annotation, and cell–cell communication modeling.
The following criteria guided the selection of datasets: (1) the presence of both pancreatic cancer and corresponding normal tissue samples, (2) the application of either high-throughput sequencing or microarray technologies, (3) the inclusion of metadata detailing patient demographics, and (4) prior usage and validation in peer-reviewed publications. These criteria were established to ensure the reliability and biological relevance of the findings. Accordingly, we retrieved six datasets (GSE119794, GSE130688, GSE171485, GSE196009, GSE69362, and GSE79634) from the NCBI Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) to obtain comprehensive gene expression profiles [7].
Datasets GSE119794, GSE130688, GSE171485, and GSE196009 were employed to obtain expression profiles of protein-coding genes in both pancreatic cancer and normal tissue samples. Additionally, we extracted miRNA sequencing data from tumor and normal pancreatic tissues of PAAD patients, along with clinical information and demographic data, from the TCGA database using the GDCRNATools package. To analyze circRNA expression, we utilized the GSE69362 and GSE79634 datasets, which provide detailed microarray data for PDAC and normal controls.
Furthermore, to gain insight into the tumor microenvironment at single-cell resolution, we included the GSE197177 dataset in our analysis. This single-cell RNA-seq dataset comprises transcriptomic profiles of thousands of individual cells derived from PDAC tissues and normal pancreatic tissue samples. GSE197177 met all predefined criteria and provided the basis for cell clustering, cell-type annotation, and inference of intercellular communication networks using the Seurat and CellChat packages. A summary of all datasets included in this study is presented in Table 1.
Table 1.
Detailed information of all datasets
| Profile | RNA type | Platform | Experiment type | Sample size (PDAC/control) | Region | Year |
|---|---|---|---|---|---|---|
| GSE119794 | mRNA | GPL11154 | Illumina HiSeq 2000 | 10/10 | China | 2019 |
| GSE130688 | mRNA | GPL16791 | Illumina HiSeq 2500 | 15/15 | Brazil | 2022 |
| GSE171485 | mRNA | GPL11154 | Illumina HiSeq 2000 | 6/6 | China | 2022 |
| GSE196009 | mRNA | GPL17303 | Ion Torrent Proton | 13/6 | Japan | 2022 |
| GSE69362 | circRNA | GPL19978 | Agilent-069978 Arraystar | 6/6 | China | 2021 |
| GSE79634 | circRNA | GPL19978 | Agilent-069978 Arraystar | 20/20 | China | 2019 |
| GSE197177 | mRNA | GPL18573 | Illumina NextSeq 500 | 3/1 | China | 2023 |
Differentially Expressed Genes (DEGs) were identified between cancerous and normal samples using the NCBI's GEO2R online tool (http://www.ncbi.nlm.nih.gov/geo/geo2r/). We analyzed significantly different miRNA expression profiles of PAAD and determined the significantly different genes of the cancerous tissues patients vs. normal samples with the limma package.
Functional enrichment analysis
To investigate the functional significance of differentially expressed mRNAs (DEMs), we performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov) [8]. Enriched pathways and functional categories were identified using a significance threshold of P < 0.05. Based on the statistical ranking of P-values, the top ten most enriched pathways were selected for subsequent analysis.
Protein–protein interaction (PPI) networks construction and Hub Gene identification
To construct the protein–protein interaction (PPI) network of DEMs, we employed the widely recognized online platform STRING (Search Tool for the Retrieval of Interacting Genes) (https://string-db.org/) [9]. STRING predicts potential functional associations among DEMs. The resulting interaction network was further analyzed using the CytoHubba plug-in in Cytoscape, a tool commonly applied to identify key hub genes within complex biological networks.
Validation of the hub genes expression and survival analysis
GEPIA2 is a comprehensive web-based platform that analyzes RNA sequencing expression data from more than 9000 tumors and 8000 normal tissue samples, integrating datasets from the TCGA and GTEx projects through a unified analytical pipeline [10]. We utilized GEPIA2 to validate the mRNA expression levels of hub genes identified through the CytoHubba analysis of the PPI network in pancreatic adenocarcinoma (PAAD). We extracted the top 500 differentially expressed genes associated with Overall Survival (OS) and Disease-Free Survival (DFS) to explore potential prognostic biomarkers from GEPIA2. These genes were then intersected with our DEMs to identify overlapping candidates with prognostic significance.
Analyzing immune cell infiltration and predicting drug sensitivity
In this study, we utilized the Gene Set Cancer Analysis (GSCA) platform (http://bioinfo.life.hust.edu.cn/GSCA/#/), a freely available resource for comprehensive genomic, pharmacogenomic, and immunogenomic analyses in cancer research. GSCA integrates diverse genomic datasets from TCGA, GDSC, and CTRP databases [11]. It integrates multidimensional genomic data from the TCGA, GDSC, and CTRP databases. We specifically employed this tool to examine the associations between the expression levels of hub genes, immune cell infiltration patterns, and drug sensitivity profiles.
CeRNA network construction
CircMine (http://www.biomedical-web.com/circmine/home) is a robust and comprehensive platform developed for exploring circRNA datasets and assessing their clinical and biological relevance in human diseases [12]. By integrating, normalizing, and analyzing circRNA transcriptomic data, CircMine facilitates the identification of potential disease associations. Our study used CircMine to obtain general information on differentially expressed circRNAs (DECs) and predict their potential miRNA targets.
Additionally, we employed StarBase (v2.0, https://rnasysu.com/encori/index.php), a widely used database, to predict interactions between the identified miRNAs and hub genes [13]. Based on these analyses, we successfully constructed a functional competing endogenous RNA (ceRNA) network encompassing circRNAs, miRNAs, and mRNAs.
Preprocessing, quality control, and data integration of scRNA-seq data
Single-cell RNA-seq data from PDAC tumors and adjacent normal tissues were processed using Seurat package [14]. Raw gene–barcode count matrices were first filtered to ensure high data quality. Cells with fewer than 300 or more than 6,000 detected genes or with greater than 20% mitochondrial gene content were excluded to eliminate potential doublets, dead cells, or low-complexity droplets. Doublets were further identified using the scDblFinder package and excluded from downstream analyses [15]. To normalize the data while accounting for sequencing depth and technical noise, we applied the SCTransform method. In addition, cell cycle phase scores (S and G2/M) were calculated based on canonical human gene sets and regressed out to minimize clustering bias caused by proliferative states. Samples from different individuals and conditions were integrated using Seurat’s SCT-based anchor integration framework, which also includes a correction for batch effects by aligning shared biological variation across datasets. This approach enabled the harmonization of cellular states across tumor and normal groups, facilitating direct comparative analysis.
Dimensionality reduction, clustering, and visualization
Dimensionality reduction and clustering were performed on the integrated dataset using the Seurat v5 framework. Variable features were identified from the integrated assay to capture the most informative genes across all cells. Principal component analysis (PCA) was then applied to reduce the dataset's dimensionality.
The first 20 principal components were used to construct a shared nearest neighbor (SNN) graph, followed by clustering using a graph-based Louvain algorithm with a resolution parameter of 1.0 to define transcriptionally distinct cell populations. Low-dimensional embeddings were generated using t-distributed Stochastic Neighbor Embedding (t-SNE) to visualize cell populations and inter-group relationships.
Cell-type annotation
Cell-type identities were assigned using a combination of automated reference-based annotation and manual marker-based curation. Automated annotation was conducted using SingleR with the Human Primary Cell Atlas as a reference [16]. Manual refinement was based on established canonical markers and cluster-specific gene expression (e.g., CD3D for T cells, CD68 for macrophages, and EPCAM for epithelial cells) from the Azimuth database [17].
Differential gene expression and enrichment analysis
To identify transcriptional alterations between tumor and normal conditions within each shared cell type, differential expression analysis was conducted using the MAST statistical framework [18]. Genes were considered significantly differentially expressed if they satisfied both an adjusted p-value < 0.05 (Benjamini–Hochberg correction) and an absolute log2 fold change greater than 0.25.
Cell–Cell communication analysis
To investigate intercellular signaling interactions, the CellChat package was applied to tumor samples [19]. Using cell-type annotations and normalized expression data, overexpressed ligand-receptor pairs were identified and used to compute signaling probabilities between cell types. Pathway-level interactions were inferred, and CXCL and FN1 signaling networks were selected for in-depth analysis.
Data visualization
To effectively visualize our analytical results, we employed SRplot (https://bioinformatics.com.cn/srplot), a user-friendly online platform designed for creating a variety of plots, including volcano plots, bubble charts, and alluvial diagrams [20]. These visualizations facilitated a clearer interpretation of the data.
We used Cytoscape (https://cytoscape.org/), a widely adopted open-source software tool for visualizing complex molecular interaction networks for network construction and analysis [21]. Cytoscape was pivotal in identifying key regulatory subnetworks and hub genes and constructing the circRNA–miRNA–mRNA interaction network by integrating molecular relationships with gene expression data. Furthermore, dataset overlaps were determined using Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/index.html), an online tool for generating Venn diagrams [22].
Results
Identification of DEMs, DEMIs, and DECs
The primary objective of this study was to identify key ncRNAs (circRNAs and miRNAs) and protein-coding genes that may regulate the progression of pancreatic cancer. Using the GEO database, our investigation began by analyzing gene expression patterns in pancreatic cancer tumors and healthy pancreatic tissues.
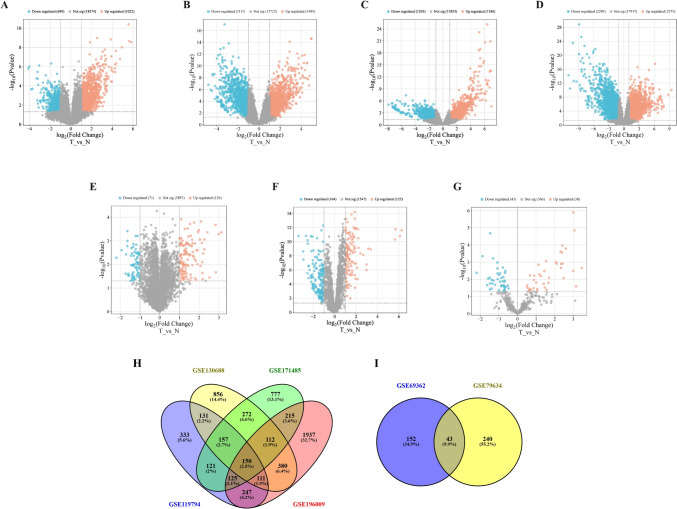
We applied a cutoff threshold of P-value < 0.05 and |log2FC|≥ 1 to identify DEGs. The datasets GSE119794, GSE130688, GSE171485, and GSE196009 contained 1721 (1022 up-regulated and 669 down-regulated), 2169 (1485 up-regulated and 1111 down-regulated), 2389 (1203 up-regulated and 1186 down-regulated), and (2290 up-regulated and 2373 down-regulated) DEMs, respectively. To identify differentially expressed miRNAs (DEMIs), we analyzed the TCGA database miRNA-Seq data, identifying 81 DEMIs (38 up-regulated and 43 down-regulated) with screening criteria P-value < 0.05. Moreover, our study uncovered 43 DECs that were shared between the GSE69362 and GSE79634 datasets, based on P-value < 0.05 and |log2FC|≥ 1 (Table 2). Volcano plots visually depict the distribution of DEGs between pancreatic cancer and non-tumor tissues across all datasets (Fig. 1A–G). Venn diagram analysis revealed that 150 DEMs and 43 DECs were shared among datasets (Fig. 1H and I).
Table 2.
The expression details of top 10 hub genes in pancreatic cancer
| GeneID | Symbol | Description | EnsemblGeneID | log2FoldChange | padj | pvalue |
|---|---|---|---|---|---|---|
| 1301 | COL11A1 | collagen type XI alpha 1 chain | ENSG00000060718 | 2.569493 | 0.019577 | 0.000444 |
| 1308 | COL17A1 | collagen type XVII alpha 1 chain | ENSG00000065618 | 3.443897 | 0.014026 | 0.000247 |
| 1294 | COL7A1 | collagen type VII alpha 1 chain | ENSG00000114270 | 1.506222 | 0.154001 | 0.0234 |
| 3576 | CXCL8 | C-X-C motif chemokine ligand 8 | ENSG00000169429 | 1.930308 | 0.090181 | 0.00786 |
| 2335 | FN1 | fibronectin 1 | ENSG00000115414 | 1.829018 | 0.066648 | 0.00403 |
| 3673 | ITGA2 | integrin subunit alpha 2 | ENSG00000164171 | 2.038208 | 0.001526 | 5.7E-06 |
| 3675 | ITGA3 | integrin subunit alpha 3 | ENSG00000005884 | 1.623104 | 0.02984 | 0.000949 |
| 3914 | LAMB3 | laminin subunit beta 3 | ENSG00000196878 | 2.964776 | 0.000127 | 8.87E-08 |
| 3918 | LAMC2 | laminin subunit gamma 2 | ENSG00000058085 | 3.033904 | 0.000503 | 8.01E-07 |
| 4312 | MMP1 | matrix metallopeptidase 1 | ENSG00000196611 | 3.31104 | 0.000258 | 2.44E-07 |
Fig. 1.
(A–G) Volcano plots illustrating differentially expressed genes (DEGs) between tumor (T) and normal (N) pancreatic tissues across seven GEO datasets. (A) GSE119794, (B) GSE130688, (C) GSE171485, (D) GSE196009, (E) GSE69362, (F) GSE79634, and (G) miRNA-TCGA. Up-regulated genes are shown in red, down-regulated in blue, and non-significant genes in gray. (H) Venn diagram showing the overlap of DEGs among four GEO datasets (GSE130688, GSE171485, GSE196009, and GSE119794), identifying 150 genes shared across all datasets. (I) Venn diagram indicating the intersection of DECs between GSE69362 and GSE79634, identifying 43 shared genes
GO and KEGG pathway analyses of the DEMs
To explore the biological relevance of DEMs in pancreatic cancer, we conducted GO and KEGG pathway enrichment analyses (Supplementary File 1). GO analysis further classified the DEMs into three principal categories: Biological Process (BP), Cellular Component (CC), and Molecular Function (MF).
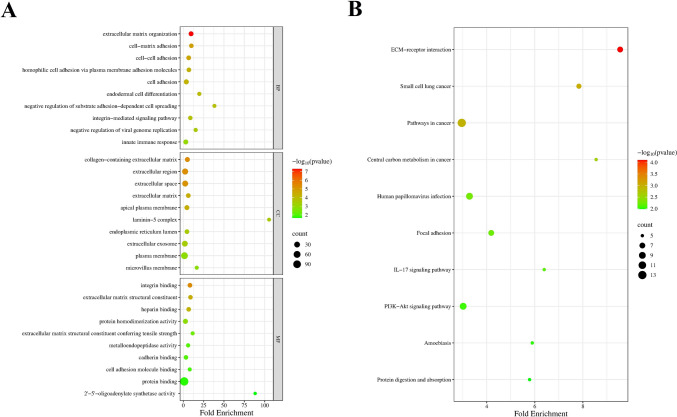
In the BP category, genes were predominantly associated with extracellular matrix organization, various forms of cell adhesion, including cell–matrix and cell–cell adhesion, and integrin-mediated signaling pathways. Processes involved in immune responses and the regulation of viral genome replication were also enriched, suggesting potential roles in both structural and immunological contexts. In the CC category, enriched terms mainly included the extracellular matrix, extracellular region, and space, as well as membrane-associated structures such as the plasma membrane and apical surfaces. Notably, components like the laminin complex and extracellular vesicles were also significantly represented, indicating cell communication and structural support involvement. For the MF category, enriched terms were primarily related to integrin binding, structural constituents of the extracellular matrix, and protein binding. Several terms also pointed to enzymatic activities and molecular interactions critical for extracellular signaling and matrix remodeling (Fig. 2A).
Fig. 2.
GO and KEGG analysis of DEMs in PDAC. (A) GO terms associated with DEMs in PDAC. (B) KEGG pathway related to the DEMs
To gain deeper insights into the molecular pathways involved, a KEGG pathway enrichment analysis was conducted. The analysis revealed significant enrichment in pathways related to ECM receptor interactions and focal adhesion, emphasizing the importance of cell–matrix communication and adhesion in the context of the studied genes. Several cancer-related pathways were also enriched, including those involved in small-cell lung cancer, central carbon metabolism in cancer, and general cancer signaling, suggesting potential roles in tumorigenesis and metabolic reprogramming. Additionally, pathways associated with immune signaling, such as the IL-17 signaling pathway and PI3K-Akt signaling, were identified, highlighting the involvement of inflammatory and survival-related processes. Other enriched pathways included those related to viral infection, such as human papillomavirus infection, protein digestion, and amoebiasis, indicating broader biological implications of the target genes (Fig. 2B).
Construction of the PPI network and identification of the Hub genes
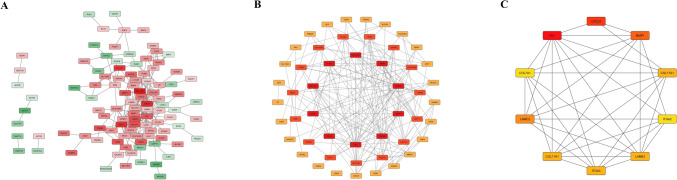
To investigate the molecular interactions among DEMs in pancreatic cancer, we constructed a PPI network using the STRING database, based on 150 common DEMs. The resulting network consisted of 101 nodes and 236 edges, revealing a complex web of gene interactions (Fig. 3A). To identify central regulatory genes within this network, we applied the cytoHubba plug-in in Cytoscape, a tool specifically designed for hub gene detection. By employing the degree ranking method, we identified the top 50 hub genes with the highest connectivity within the network (Fig. 3B). Several key genes were identified as potentially crucial contributors to the progression of pancreatic cancer, particularly in the context of extracellular matrix remodeling, cell adhesion, and invasion (Fig. 3C). Table 2 details the expression patterns of these hub genes in pancreatic cancer and non-tumor tissues.
Fig. 3.
Identification of the hub genes from the PPI network. (A) Initial PPI network of differentially expressed genes (DEGs) in PDAC, constructed using the STRING database. Nodes represent genes, with red indicating upregulation and green indicating downregulation. The intensity of the color reflects the magnitude of the expression change. (B and C) Genes ranked within the top 100 and top 10 by the Degree method were defined as hub genes. The ranking of these hub genes is indicated by a gradient that shifts from red to yellow, representing higher rankings. Nodes with higher centrality and connectivity are shown in deeper red, representing potential hub genes
Confirming hub gene expression with GEPIA2 analysis
We employed the GEPIA2 database, which integrates RNA sequencing data from the TCGA and GTEx projects, to validate the expression patterns of the identified hub genes. Specifically, the expression levels of COL11A1, COL17A1, COL7A1, CXCL8, FN1, ITGA2, ITGA3, LAMB3, LAMC2, and MMP1 were assessed in pancreatic cancer samples and compared to those in normal pancreatic tissues (Fig. 4).
Fig. 4.
Hub genes expression in PDAC Tumor vs. Normal tissues
The analysis revealed that all ten hub genes exhibited expression trends consistent with our differential expression results from the GEO datasets, reinforcing their potential roles in pancreatic cancer development. This validation not only strengthens the reliability of our findings but also underscores the importance of these genes as promising therapeutic targets in pancreatic cancer.
Survival analysis for prognostic biomarker identification
To identify potential prognostic biomarkers for pancreatic cancer, we performed an integrated survival analysis by extracting and categorizing differentially expressed genes (DEGs) from our dataset. We obtained the top 500 genes associated with overall survival (OS) and disease-free survival (DFS) from the GEPIA2 database. By cross-referencing these survival-related genes with our DEGs, we identified a subset of genes consistently altered across both datasets, enabling the identification of robust prognostic candidates. Genes with significant prognostic value (log-rank p < 0.05 and consistent trends in both OS and DFS) were prioritized. As a result, seven genes (ANLN, CDH3, COL17A1, ITGA3, KRT7, LAMA3, and SAMD9) were recognized as key prognostic markers (Supplementary Fig. 1).
Correlation between hub gene expression and drug sensitivity and immune cells in pancreatic cancer
To explore the potential therapeutic implications of the identified genes, we investigated their correlation with drug sensitivity using GDSC pharmacogenomic data. Several genes, including FN1, ITGA3, CXCL8, MMP1, and ITGA2, showed a consistent positive correlation with resistance to a wide range of chemotherapeutic agents, such as methotrexate, PHA-793887, vorinostat, and 5-fluorouracil. Conversely, a significant negative correlation was observed between the expression of some genes, particularly COL11A1, FN1, and ITGA3, and sensitivity to targeted agents such as bortezomib, dasatinib, and docetaxel, indicating that these genes might serve as predictive biomarkers for drug responsiveness (Supplementary Fig. 2A).
Furthermore, to investigate the immunological relevance of the identified key genes, we analyzed their correlation with immune cell infiltration levels in PAAD. Most of the genes, including FN1, COL11A1, COL17A1, ITGA2, and LAMB3, showed a strong positive correlation with infiltration scores and with innate immune cells such as dendritic cells (DCs), monocytes, and macrophages. Interestingly, several genes, including LAMC2, COL17A1, and ITGA3, were negatively correlated with subsets of adaptive immune cells, particularly cytotoxic T cells, Th1, and NK cells (Supplementary Fig. 2B).
Construction of the ceRNA network
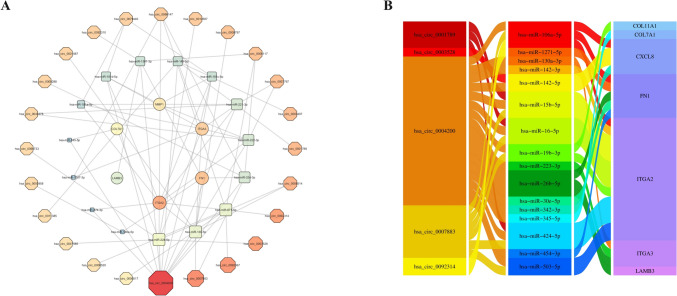
Recent evidence suggests that circRNAs can regulate gene expression through various mechanisms, notably by functioning as molecular sponges for miRNAs. We utilized the CircMine and StarBase databases to investigate this regulatory axis and predict potential miRNA targets for each circRNA. Subsequently, StarBase was employed to identify miRNAs interacting with the hub genes identified in our study. Based on these analyses, we constructed interaction networks linking circRNAs to miRNAs and hub genes (Fig. 5A). Next, we determined the overlap between the predicted and the DEMIs identified in our dataset. By integrating these results, we assembled a comprehensive ceRNA network connecting regulatory ncRNAs with mRNAs. To further refine the network, we focused on interactions characterized by negative regulation among circRNA–miRNA, lncRNA–miRNA, and miRNA–mRNA pairs. To enhance the clarity and interpretability of the results, we prioritized and visualized the top five circRNAs with the highest centrality scores within the ceRNA network (Fig. 5B).
Fig. 5.
Comprehensive ceRNA Interaction Networks and Regulatory Axes in PDAC. (A) circRNA/lncRNA–miRNA–mRNA interaction network, illustrating the competitive binding relationships between ncRNAs and mRNAs. The size and color of the nodes represent the strength of interaction and their centrality within the network, respectively. (B) Regulatory axis network of circRNAs, illustrating the routes of circRNA-mediated control, from circRNAs to miRNAs and on to their mRNA targets
Cellular heterogeneity and hub gene expression landscape in PDAC revealed by single-cell analysis
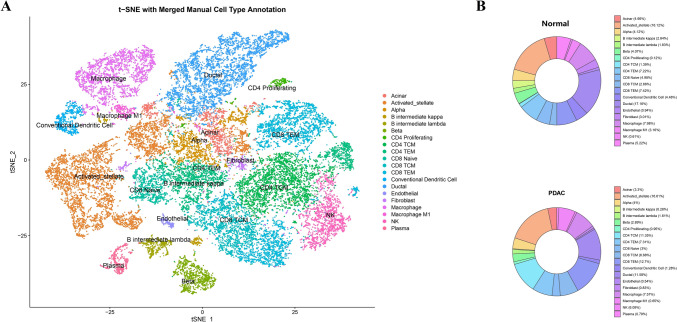
To characterize the cellular architecture of PDAC, we performed dimensionality reduction and unsupervised clustering of single-cell RNA-seq data from tumor and adjacent normal tissues. A total of 20 transcriptionally distinct cell populations were identified, including epithelial cells (ductal, acinar), stromal compartments (fibroblasts, endothelial cells), and diverse immune subsets such as macrophages, CD4/CD8 T cells, dendritic cells, and B cells (Fig. 6A). we quantified the proportion of each cell type within the tumor versus normal samples. Tumor tissues exhibited a higher relative abundance of fibroblasts, CD8 TEM cells, and activated stellate cells, indicative of a reprogrammed and immunomodulatory tumor microenvironment (Fig. 6B).
Fig. 6.
Single-cell transcriptomic landscape and cell-type composition in PDAC and normal tissues. (A) t-SNE visualization of single-cell transcriptomes from PDAC and adjacent normal tissues, showing manual cell-type annotation based on canonical marker genes. A total of 20 major cell populations were identified, including ductal, acinar, fibroblast, immune, and endothelial cells. (B) Donut charts display the relative proportions of each cell type in normal versus PDAC samples, highlighting major shifts in cellular composition
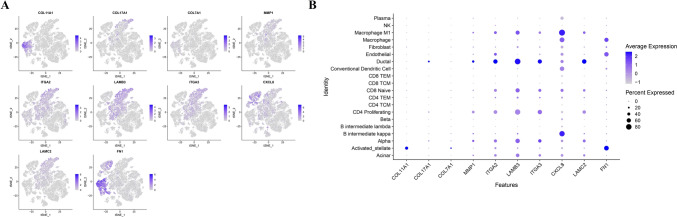
Next, we analyzed the expression of ten hub genes with known roles in tumor progression (COL11A1, COL17A1, COL7A1, MMP1, FN1, ITGA2, ITGA3, LAMB3, LAMC2, and CXCL8). These genes displayed distinct cell-type-specific expression patterns, with prominent enrichment in fibroblasts, macrophages, and endothelial cells (Fig. 7A).
Fig. 7.
Single-cell expression patterns of candidate prognostic genes across annotated cell populations. (A) t-SNE feature plots showing the expression distribution of ten hub genes (COL11A1, COL17A1, COL7A1, MMP1, ITGA2, LAMB3, ITGA3, CXCL8, LAMC2, FN1) in the integrated scRNA-seq dataset. Expression intensity is represented by a color gradient. (B) Dot plot visualizing the average expression and percentage of cells expressing each gene across major cell types. Notably, MMP1 and CXCL8 were enriched in endothelial and macrophage populations, while FN1 and LAMC2 showed high expression in activated stellate and ductal cells, respectively
To further quantify expression specificity, we generated a dot plot (Fig. 6B) showing both average expression levels and the percentage of expressing cells across clusters. Notably, COL11A1 and FN1 were strongly expressed in activated stellate cells and fibroblasts, while CXCL8 showed enrichment in macrophage populations, supporting their roles in extracellular matrix remodeling and inflammatory signaling. These findings highlight the cell-type–specific expression landscape of candidate genes implicated in tumor progression and microenvironmental remodeling in PDAC (Fig. 7B).
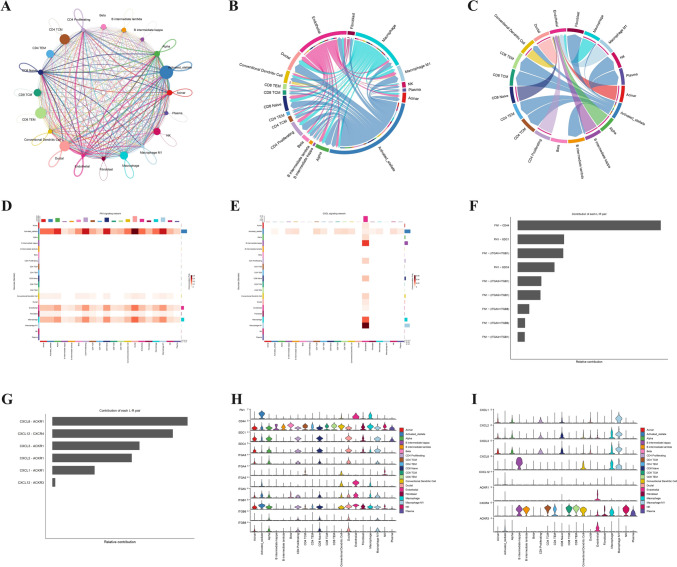
Global cell–cell communication landscape highlights FN1 and CXCL signaling as key intercellular pathways
To explore how different cell populations interact in the PDAC microenvironment, we used the CellChat framework to infer intercellular communication networks. The global interaction map revealed extensive crosstalk between fibroblasts, macrophages, endothelial cells, and T cell subsets, with stromal and myeloid compartments acting as central hubs (Fig. 8A–C). Among the predicted pathways, FN1 and CXCL signaling emerged as dominant axes of intercellular communication. FN1 signaling was driven largely by fibroblasts and endothelial cells, engaging receptors such as CD44, ITGA5, and ITGB1 on recipient cell types (Fig. 8D–F). Simultaneously, CXCL ligands, particularly CXCL8, were identified as top contributors in ligand-receptor analysis, with the CXCL8–ACKR1 axis ranking highest in relative signaling strength (Fig. 8G). These findings prompted a deeper analysis of CXCL-driven interactions.
Fig. 8.
Inference of Cell–Cell Communication Networks in PDAC Using CellChat Analysis. (A) Global intercellular communication network showing the intensity and diversity of signaling interactions among 20 identified cell types. Edge width represents the strength of signaling; node size reflects the total number of interactions per cell type. (B) Circle plot highlighting the inferred communication network of the FN1 signaling pathway, revealing major sending and receiving cell populations. (C) Circle plot showing the communication network of the CXCL signaling pathway, particularly active between immune cells and stromal/epithelial compartments. (D) Heatmap of signaling roles (outgoing signals) by cell type for the FN1 pathway. (E) Heatmap of signaling roles (incoming signals) for the CXCL pathway, showing endothelial cells as dominant receivers. (F) Bar plot showing relative contribution of ligand–receptor pairs to the FN1 signaling pathway. (G) Bar plot of the top contributing ligand–receptor pairs in the CXCL signaling pathway, with CXCL8–ACKR1 ranking highest. (H) Violin plots showing cell-type–specific expression of FN1 pathway-related genes (FN1, CD44, ITGA5, ITGB1, etc.). (I) Violin plots of CXCL pathway genes across cell types, including CXCL1–3, CXCL8, ACKR1, CXCR1/2, and adhesion molecules
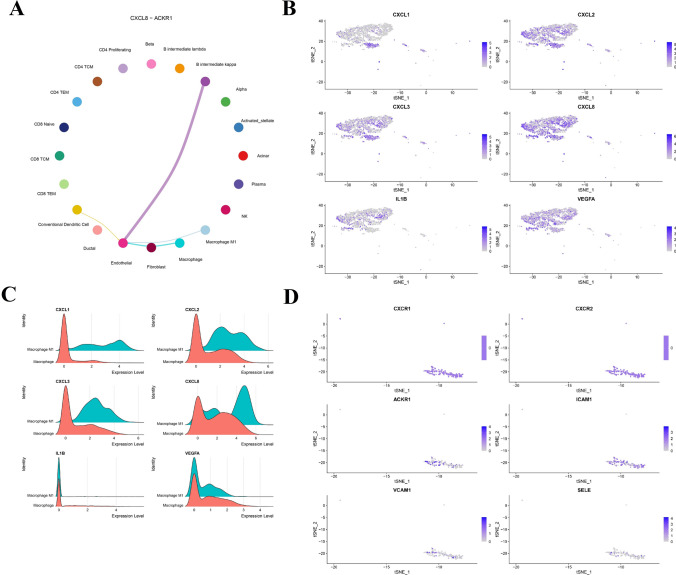
Macrophage-to-endothelium CXCL8–ACKR1 signaling axis orchestrates inflammatory crosstalk in PDAC
A focused analysis of the CXCL signaling pathway uncovered a macrophage-centered inflammatory circuit. The CXCL8–ACKR1 interaction was identified as a dominant communication route from M1-like macrophages to endothelial cells (Fig. 9A), suggesting its critical role in modulating vascular inflammation and leukocyte recruitment. tSNE and density plots confirmed strong expression of CXCL1, CXCL2, CXCL3, CXCL8, IL1B, and VEGFA in macrophages, while ACKR1 was exclusively enriched in endothelial cells (Fig. 9B–C). Classical CXCL receptors such as CXCR1 and CXCR2 showed minimal expression, emphasizing the importance of ACKR1 as a noncanonical chemokine receptor in PDAC. Furthermore, adhesion molecules ICAM1, VCAM1, and SELE were highly expressed in endothelial cells (Fig. 9D), reinforcing the presence of an inflamed, immune-permissive vasculature. These findings collectively suggest that the CXCL8–ACKR1 axis not only mediates inflammatory crosstalk but may also contribute to tumor-associated angiogenesis, potentially through the synergistic actions of CXCL8 and VEGFA secreted by macrophages and the activation of endothelial adhesion and chemokine signaling pathways.
Fig. 9.
Characterization of the CXCL8–ACKR1 Signaling Axis Between Macrophages and Endothelial Cells in PDAC. (A) CellChat circle plot depicting the CXCL8–ACKR1 interaction, with M1-like macrophages as primary senders and endothelial cells as main receivers, suggesting a macrophage-driven inflammatory signaling axis. (B) tSNE plots showing expression of CXCL1, CXCL2, CXCL3, CXCL8, IL1B, and VEGFA across all single cells, with strong enrichment observed in macrophage clusters. (C) Ridge plots comparing expression levels of key inflammatory ligands between macrophages and M1 macrophages, confirming upregulation of CXCL chemokines, IL1B, and VEGFA in M1-like subtypes. (D) tSNE expression plots of CXCR1, CXCR2, ACKR1, and adhesion molecules (ICAM1, VCAM1, SELE) showing specific expression of ACKR1 and adhesion molecules in endothelial cells, supporting their role as targets of macrophage-derived chemokine signaling
Functional reprogramming of tumor-associated macrophages: DEG and network analysis
To elucidate the transcriptional landscape of tumor-associated macrophages, we performed DEG analysis between macrophage populations in tumor versus normal tissues. GO enrichment analysis indicated strong activation of antiviral defense, cytokine signaling, and inflammatory response pathways (Supplementary Fig. 3A). Cellular component analysis highlighted genes associated with endosomes, plasma membrane, and cytoplasmic complexes, while molecular functions were enriched for RNA binding, pattern recognition receptor activity, and ATP/GTP binding.
KEGG pathway enrichment further implicated immune-related pathways such as NOD-like receptor signaling, HTLV-I infection, and COVID-19/influenza response, along with lipid metabolism and atherosclerosis, reflecting the dual inflammatory–metabolic reprogramming of PDAC macrophages (Supplementary Fig. 3B) (Supplementary File 2). The PPI network analysis identified central hub genes such as ISG15, IFIT3, DDX60, IRF7, and OAS2, which likely act as upstream regulators of innate immunity and type I interferon signaling (Supplementary Fig. 3C). These findings reveal a highly active, interferon-enriched macrophage population that may drive immune remodeling and interface with the CXCL8–ACKR1 axis described earlier.
Discussion
One of the major contributors to PDAC’s aggressive nature is the complex network of molecular alterations, including the dysregulation of ncRNAs such as lncRNAs, circRNAs, and miRNAs. Extensive research documents that the expression of ncRNAs is dysregulated in pancreatic cancer. Their dysregulation can be attributed to a variety of underlying mechanisms, including chromosomal abnormalities, alterations in transcriptional control, epigenetic regulation, RNA modifications, and disruptions in the machinery responsible for ncRNAs biogenesis [23].
Our use of single-cell transcriptomics was designed not as a separate analysis, but as a refinement and contextualization of the bulk-derived hub genes. For example, FN1 and COL11A1—initially identified through PPI network analysis in bulk datasets—were found to be specifically enriched in fibroblasts and activated stellate cells in the scRNA-seq data, suggesting a functional role in ECM remodeling within the PDAC stroma. This cell-specific mapping provided an added layer of biological insight beyond conventional bulk analysis.
The volcano plot and heatmap presented the significantly DEGs between PDAC tissues and normal samples. The distribution indicates a substantial number of both upregulated and downregulated transcripts, suggesting strong transcriptional dysregulation in PDAC. The clustering seen in the heatmap implies consistent expression patterns across biological replicates, highlighting the robustness of the identified DEGs. Specifically, the expression levels of COL11A1, COL17A1, COL7A1, CXCL8, FN1, ITGA2, ITGA3, LAMB3, LAMC2, and MMP1 were assessed in pancreatic cancer samples and compared to those in normal pancreatic tissues. These include COL11A1, COL17A1, and COL7A1, which are involved in collagen formation and structural integrity of the extracellular matrix. Elevated expression of COL11A1 in both the epithelial and stromal components of pancreatic ductal adenocarcinoma (PDAC) tissue has been shown to correlate with key clinicopathological features of the disease [24]. Similarly, COL17A1 is significantly overexpressed in pancreatic cancer tissues relative to normal pancreatic tissue. This gene plays a crucial role in promoting the proliferation, migration, and invasion of pancreatic cancer cells by regulating epithelial-mesenchymal transition (EMT) pathways. Due to its oncogenic activity and clinical significance, COL17A1 has emerged as both a potential prognostic biomarker and a promising therapeutic target in PDAC management [25]. Additionally, genes such as FN1 (fibronectin 1), ITGA2, and ITGA3 are central to integrin-mediated cell adhesion and intracellular signaling, which are critical processes in tumor progression. The involvement of integrin alpha (ITGA) family members in PDAC progression has been previously documented [26]. Specifically, Liquan et al. reported that upregulation of ITGA2 is associated with poor clinical outcomes and may influence immune modulation in pancreatic cancer [27]. Furthermore, the study by Idichi et al. demonstrated that the oncogenic receptors ITGA3 and ITGB1 are directly regulated by miR-124-3p in PDAC cells. Overexpression of these receptors was strongly linked to poor prognosis in PDAC patients [28]. In addition, research by Lei et al. revealed a significant association between FN1 expression and key cellular processes, including cell viability, apoptosis, and cell cycle regulation in pancreatic cancer cells. [29].
Additionally, LAMB3 and LAMC2, encoding laminin subunits, are central to basement membrane organization and tumor cell migration. Wang et al. reported that LAMC2 contributes to the formation of an acidic extracellular microenvironment in pancreatic cancer, which facilitates tumor cell invasion through the activation of the Akt/NHE1 signaling pathway [30]. Similarly, another study showed that LAMB3 influences key cellular processes in PDAC, including cell cycle arrest and apoptosis, and modulates tumor cell proliferation, invasion, and metastasis by regulating the PI3K/Akt signaling axis [31]. Additionally, MMP1, a member of the matrix metalloproteinase family, is well-recognized for its role in degrading the extracellular matrix (ECM) and promoting invasive behavior in pancreatic cancer cells [32, 33]. Supporting this, Xu et al. found that MMP-1 is significantly overexpressed in pancreatic carcinoma and serves as an independent prognostic marker with strong diagnostic value for tumor staging and lymph node metastasis [34].
Notably, CXCL8 (also known as IL-8) is a pro-inflammatory chemokine that contribute to tumor-promoting inflammation and angiogenesis. The study by Matsuo et al. demonstrated that CXCL8, secreted by pancreatic cancer cells, and CXCL12, produced by fibroblasts, act synergistically to promote angiogenesis in vitro. This cooperative effect enhances the proliferation, invasion, and tube formation capabilities of human umbilical vein endothelial cells (HUVECs), highlighting a critical mechanism by which the tumor microenvironment facilitates neovascularization [35].
Together, these genes may represent critical molecular drivers of pancreatic cancer aggressiveness and serve as potential targets for therapeutic intervention. Many of the upregulated transcripts are associated with key oncogenic pathways influenced by ncRNAs. According to recent studies, dysregulation of miRNAs and lncRNAs alters post-transcriptional gene regulation, contributing to PDAC progression by modulating apoptosis, angiogenesis, and immune evasion [36, 37].
Functional enrichment and KEGG pathway analysis identify several biological processes and signaling cascades enriched in DEGs. Notably, pathways involved in extracellular matrix interaction, PI3K-AKT signaling, and immune regulation are prominently featured. These pathways are known to contribute to PDAC tumorigenesis, stromal remodeling, and resistance to therapy [38–40].
Our Kaplan–Meier survival analysis revealed that the selected hub genes (ANLN, CDH3, COL17A1, ITGA3, KRT7, LAMA3, and SAMD9) have significant prognostic implications for patients with PDAC. Patients categorized based on differential expression levels of these hub genes exhibited distinct survival outcomes, highlighting their potential as prognostic biomarkers. This suggests that alterations in the expression patterns of these genes may reflect underlying biological differences that influence disease progression and clinical prognosis. Consequently, the identified hub genes can facilitate patient stratification into distinct risk groups, potentially improving individualized patient management and treatment decisions. These findings underscore the necessity for further experimental validation and clinical studies to confirm their applicability as reliable prognostic indicators in pancreatic cancer management.
The immune infiltration analysis reveals correlations between expression of key genes and the abundance of various immune cell populations. This analysis suggests an immunosuppressive microenvironment in PDAC and highlights the role of specific genes in modulating tumor–immune interactions. including FN1, COL11A1, COL17A1, ITGA2, and LAMB3, showed a strong positive correlation with infiltration scores and with innate immune cells such as dendritic cells (DCs), monocytes, and macrophages. This suggests a possible link between ECM remodeling and the recruitment of innate immune components in the tumor microenvironment [40]. Interestingly, several genes, including LAMC2, COL17A1, and ITGA3, were negatively correlated with subsets of adaptive immune cells, particularly cytotoxic T cells, Th1, and NK cells (Fig. 7B). These findings may reflect an immunosuppressive microenvironment shaped by the overexpression of ECM-related genes, potentially contributing to immune evasion in pancreatic cancer [40, 41].
Drug Sensitivity analysis illustrates the relationship between hub gene expression and sensitivity to several chemotherapeutic agents. FN1, ITGA3, CXCL8, MMP1, and ITGA2, showed a consistent positive correlation with resistance to a wide range of chemotherapeutic agents, such as methotrexate, PHA-793887, vorinostat, and 5-fluorouracil. These findings suggest that overexpression of these genes may contribute to drug resistance mechanisms in pancreatic cancer cells, potentially through enhanced adhesion, extracellular matrix remodeling, or inflammatory signaling [39, 40]. Conversely, a significant negative correlation was observed between the expression of some genes, particularly COL11A1, FN1, and ITGA3, and sensitivity to targeted agents such as bortezomib, dasatinib, and docetaxel, indicating that these genes might serve as predictive biomarkers for drug responsiveness. These results underscore the importance of incorporating gene expression profiles into personalized treatment strategies and suggest that targeting these pathways may improve therapeutic efficacy in pancreatic cancer The inverse correlations observed imply that certain genes may serve as predictors of drug resistance, offering a basis for personalizing therapy in PDAC.
The ceRNA network presented in Fig. 8 integrates lncRNAs, miRNAs, and mRNAs, illustrating potential regulatory axes that influence PDAC biology. In this ceRNA network has_circ_0004200, has_circ_0007883, has-mir-15b-5p, has-mir-424-5p, and ITGA2 show a strong interaction with other RNAs. Such networks underscore the complex post-transcriptional regulatory landscape and point to novel interactions that may be therapeutically targetable.
In our study, several circRNAs, including hsa_circ_0004200, hsa_circ_0007883 (circANKRD17), hsa_circ_0003528 (circSEC24A), hsa_circ_0092314 (circRANBP1), and hsa_circ_0001789 (circRAB11FIP1), were identified as potential regulatory molecules contributing to PDAC pathogenesis. Accumulating evidence has revealed the diverse functional roles of these circRNAs in cancer biology, primarily through their modulation of miRNA-mediated pathways.
For example, hsa_circ_0007883 (circANKRD17) has been previously associated with promoting glycolysis and tumor progression by sponging miR-143 in breast cancer, thereby increasing hexokinase 2 expression, which drives the Warburg effect and an aggressive tumor phenotype [42]. In ovarian cancer, circANKRD17 has been identified as a transcriptional target of the EMT-related transcription factor ZEB1, which significantly enhances proliferation, migration, and EMT processes, suggesting a broader role in promoting malignancy across various cancers [43].
Similarly, hsa_circ_0003528 (circSEC24A) has been shown to have prominent oncogenic roles across multiple cancer types. In triple-negative breast cancer (TNBC), circSEC24A promotes malignant behaviors via sponging miR-215, thereby facilitating tumor cell proliferation, migration, and metastasis [44]. Additionally, in non-small cell lung cancer (NSCLC), circSEC24A contributes to malignant transformation and immune evasion by modulating the miR-511-3p/PDL1 axis, highlighting its critical function in cancer immune escape mechanisms [45].
Furthermore, hsa_circ_0001789 (circRAB11FIP1) has recently emerged as a potential diagnostic biomarker in gastric carcinoma, with its dysregulation correlating significantly with tumor aggressiveness, invasion depth, and metastatic potential. Although less characterized, hsa_circ_0092314 (circRANBP1) and hsa_circ_0004200 (circ_chr15_00053) may similarly play essential roles in cancer progression and warrant further exploration, given their predicted regulatory interactions with miRNAs.
Consistent with these findings, the identified circRNAs in our PDAC study represent promising candidates for further investigation. They likely exert their effects through ceRNA networks involving crucial miRNAs and downstream target genes, potentially contributing to critical oncogenic pathways such as metabolic reprogramming, EMT, cell adhesion, and immune modulation. Consequently, these circRNAs could serve as novel biomarkers for prognosis and therapeutic targets, potentially advancing personalized treatment strategies in PDAC.
In our study, miR-424-5p was found to be significantly downregulated in pancreatic ductal adenocarcinoma (PDAC). Previous research has shown that miR-424-5p exerts tumor-suppressive effects in various cancer types. For instance, in laryngeal squamous cell carcinoma, overexpression of miR-424-5p inhibited proliferation, migration, and invasion by targeting the oncogene CADM1 [46]. In our ceRNA network, FN1, a key gene involved in extracellular matrix organization and tumor invasiveness, was identified as a predicted target of miR-424-5p and was concurrently upregulated in PDAC. This inverse correlation supports a potential regulatory relationship, where downregulation of miR-424-5p may contribute to FN1 overexpression and tumor progression.
Similarly, miR-15b-5p was also found to be downregulated in PDAC and has been described in previous studies as a tumor suppressor involved in regulating genes associated with cell survival and adhesion. Notably, ITGA2, another upregulated hub gene in our analysis, was predicted as a direct target of miR-15b-5p. The observed downregulation of miR-15b-5p may relieve its inhibitory effect on ITGA2, thereby enhancing integrin-mediated signaling and cell–matrix interactions that promote tumor invasion and metastasis in PDAC.
One of the key strengths of this study lies in the integration of bulk and single-cell transcriptomic analyses, which collectively illuminate the molecular and cellular complexity of pancreatic ductal adenocarcinoma (PDAC). Bulk RNA-seq revealed a panel of upregulated hub genes—including FN1, COL11A1, ITGA2, ITGA3, and CXCL8—that are implicated in extracellular matrix remodeling, immune cell recruitment, and invasive tumor behavior. However, the spatial and cellular origins of these transcriptional signatures remained elusive until further dissected by scRNA-seq.
Our scRNA-seq analysis identified 20 distinct cell populations, capturing the heterogeneous nature of the PDAC tumor microenvironment (TME). This approach enabled precise attribution of hub gene expression to specific cellular subsets. FN1 and COL11A1 were highly expressed in fibroblasts and activated stellate cells, consistent with their roles in desmoplastic stroma formation. In contrast, ITGA2, ITGA3, and LAMC2 were upregulated in malignant ductal epithelial cells, highlighting their involvement in epithelial-mesenchymal transition (EMT) and cell adhesion. Importantly, CXCL8 showed strong expression in macrophages and ductal cells, suggesting dual roles in inflammation and tumor progression.
A major highlight of our study is the use of CellChat to explore intercellular communication within the TME. Through this analysis, we identified CXCL8–ACKR1 as a dominant signaling axis from M1-like macrophages to endothelial cells, forming a macrophage-centered inflammatory loop. This atypical chemokine receptor, ACKR1, lacks classical G-protein signaling but plays a pivotal role in shaping chemokine gradients, leukocyte recruitment, and endothelial activation [47]. Notably, ACKR1 was exclusively expressed on endothelial cells in our dataset, and co-expression of adhesion molecules (e.g., ICAM1, VCAM1, SELE) reinforces the notion of a pro-inflammatory vasculature conducive to immune infiltration.
This finding is supported by prior studies suggesting that ACKR1/DARC not only acts as a scavenger of pro-inflammatory chemokines like CXCL8, CXCL1, and CXCL2 but also regulates vascular integrity and immune cell trafficking [47, 48]. In the context of PDAC, where immune exclusion is a hallmark, the enrichment of ACKR1 + endothelial cells may paradoxically promote leukocyte entry, suggesting a dynamic balance between immune activation and evasion. Furthermore, the involvement of macrophage-derived VEGFA and inflammatory cytokines (e.g., IL1B) in this axis supports a role in tumor-associated angiogenesis beyond conventional pro-angiogenic pathways.
In addition to the CXCL axis, our data revealed that FN1-integrin interactions (particularly via ITGA3, ITGA2, ITGB1, and ITGAV) mediate strong crosstalk between fibroblasts, ductal cells, and endothelial compartments. This matrix-mediated communication likely contributes to tissue stiffness, stromal expansion, and therapeutic resistance—hallmarks of aggressive PDAC. These dual signaling axes—CXCL8–ACKR1 and FN1–integrin—offer a mechanistic framework linking immune–stromal–vascular interactions in the PDAC TME.
While this study presents an integrative and multi-dimensional bioinformatics framework that uncovers key molecular players and intercellular communication pathways in PDAC, several limitations must be acknowledged. First, the findings are based entirely on computational predictions derived from publicly available bulk and single-cell transcriptomic datasets. Although the integration of these datasets improves biological resolution and enhances analytical depth, the inherent heterogeneity across studies, such as differences in sample preparation, sequencing platforms, and clinical variables, may introduce batch effects and potential biases. Moreover, the identification of pivotal signaling axes, such as the CXCL8–ACKR1-mediated macrophage–endothelial interaction and FN1–integrin–driven stromal–epithelial crosstalk, while biologically plausible and supported by expression patterns, remains inferred rather than demonstrated. Without functional validation, it is difficult to confirm whether these signaling pathways actively contribute to immune infiltration, angiogenesis, or therapeutic resistance in PDAC. Furthermore, while central non-coding RNAs and hub genes were prioritized using network centrality metrics, these rankings do not substitute for mechanistic insight.
Looking ahead, future research could benefit from the integration of artificial intelligence (AI) and functional genomic screening to strengthen the translational relevance of our findings. For instance, Tang et al. developed SpaRx, a deep learning–based framework capable of capturing single-cell spatial heterogeneity in drug response using multi-omics data, enabling more precise patient stratification and therapeutic prediction in PDAC [49]. Incorporating such AI-driven models into our ceRNA and intercellular communication networks could reveal more nuanced subtypes and drug sensitivities. Additionally, Wang et al. constructed CRISPRoffT, a comprehensive database for CRISPR/Cas9 off-target prediction, which facilitates more accurate and safe gene editing strategies for experimental validation [50]. By leveraging CRISPR-based functional screens, the oncogenic relevance of key genes and RNAs identified in our study—such as FN1, CXCL8, or circANKRD17—could be directly assessed in PDAC models, potentially accelerating the identification of therapeutic targets.
In addition, co-culture systems or 3D tumor organoids incorporating macrophages, fibroblasts, and endothelial cells would provide a more physiologically relevant context to assess the proposed cell–cell communication pathways. Lastly, the CXCL8–ACKR1 axis, though intriguing as a noncanonical inflammatory and angiogenic driver, requires in vivo validation, such as blocking or enhancing ACKR1 signaling in murine PDAC models or patient-derived xenografts (PDXs), to determine its translational significance. Addressing these limitations through rigorous experimental studies and stratified analyses will strengthen the translational potential of bioinformatics-driven discoveries, paving the way for their application in clinical settings.
Conclusion
In summary, our integrative multi-omics and single-cell transcriptomic analyses provide a comprehensive landscape of the molecular and cellular mechanisms driving pancreatic ductal adenocarcinoma (PDAC). The combination of bulk and single-cell transcriptomic data allowed us to (1) identify key hub genes with cell-type-specific expression patterns, (2) elucidate dynamic communication pathways shaping the PDAC ecosystem, and (3) propose cell-specific therapeutic vulnerabilities. By identifying a panel of robust prognostic biomarkers and mapping their expression across diverse cell types, we highlight the pivotal roles of extracellular matrix remodeling, immune modulation, and angiogenesis in PDAC progression. Notably, the CXCL8–ACKR1 signaling axis emerged as a key macrophage-to-endothelium communication pathway, potentially mediating tumor-associated inflammation and vascular activation. Furthermore, our drug sensitivity analysis suggests that specific hub genes may serve as predictive markers of chemotherapeutic responsiveness. Collectively, these findings not only deepen our understanding of PDAC pathobiology but also offer promising targets for precision diagnosis and therapeutic intervention.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure 1. Survival analysis was conducted to assess the impact of gene expression on PDAC patient outcomes.
Supplementary file1 (JPG 3691 kb)
Supplementary Figure 2. Analysis of Gene Expression Correlations with Drug Sensitivity and Immune Infiltration in PDAC. (A) Analysis of the relationship between mRNA expression levels and drug sensitivity using data from the GDSC database, showing how specific genes correlate with the sensitivity to various drugs. (B) Examination of the correlation between gene expression and immune cell infiltration in PDAC.
Supplementary file2 (JPG 1152 kb)
Supplementary Figure 3. Functional Enrichment and Network Analysis of Macrophage-Specific Differentially Expressed Genes in PDAC. (A) Gene Ontology (GO) enrichment analysis of macrophage-associated DEGs across three categories: Biological Processes, Cellular Components, and Molecular Functions. Key enriched terms include defense response to virus, inflammatory response, and cytokine production, highlighting the immunologically active role of macrophages in the tumor microenvironment. (B) KEGG pathway enrichment of the same DEGs, revealing associations with NOD-like receptor signaling, viral infection pathways, HTLV-I signaling, and lipid and atherosclerosis-related signaling, implicating macrophages in both inflammatory and metabolic regulation in PDAC. (C) Protein–protein interaction (PPI) network constructed using the STRING database, with nodes colored by degree centrality.
Supplementary file3 (JPG 2515 kb)
Supplementary File 1. GO and KEGG analysis data
Supplementary file4 (XLSX 29 kb)
Supplementary File 2. Differentially expressed Genses for Macrophages
Supplementary file5 (XLSX 30 kb)
Acknowledgements
Not applicable.
Author’s contribution
AG and MS conceived and designed the study. AG, MZ, SG and HK conducted the data acquisition and analysis and drafted and wrote the manuscript. MS supervised, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript and accept personal responsibility for the author’s contribution.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The sequence data supporting this study's findings are derived from publicly available datasets. These datasets are accessible through the Gene Expression Omnibus (GEO) repository: https://www.ncbi.nlm.nih.gov/geo/.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sharma GG, Okada Y, Von Hoff D, Goel A. Non-coding RNA biomarkers in pancreatic ductal adenocarcinoma. Semin Cancer Biol. 2021;75:153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Z, Liu W. Pancreatic cancer: a review of risk factors, diagnosis, and treatment. Technol Cancer Res Treat. 2020;19:1533033820962117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, et al. The molecular biology of pancreatic adenocarcinoma: translational challenges and clinical perspectives. Signal Transduct Target Ther. 2021;6(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morani AC, et al. Hereditary and sporadic pancreatic ductal adenocarcinoma: current update on genetics and imaging. Radiol Imaging Cancer. 2020;2(2): e190020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortoglou M, et al. Non-coding RNAs in pancreatic ductal adenocarcinoma: new approaches for better diagnosis and therapy. Transl Oncol. 2021;14(7): 101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, et al. circRNA circ_102049 Implicates in Pancreatic Ductal Adenocarcinoma Progression through Activating CD80 by Targeting miR-455-3p. Mediators Inflamm. 2021;2021:8819990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherman BT, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022;50(W1):W216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szklarczyk D, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Z, et al. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CJ, et al. GSCA: an integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief Bioinform. 2023. 10.1093/bib/bbac558. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, et al. Circmine: a comprehensive database to integrate, analyze and visualize human disease-related circRNA transcriptome. Nucleic Acids Res. 2022;50(D1):D83-d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J-H, et al. StarBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2013;42(D1):D92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Y, et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat Biotechnol. 2024;42(2):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Germain PL, et al. Doublet identification in single-cell sequencing data using scDblFinder. F1000Res. 2021;10:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aran D, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20(2):163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao Y, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573-3587.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finak G, et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015;16(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin S, Plikus MV, Nie Q. CellChat for systematic analysis of cell-cell communication from single-cell and spatially resolved transcriptomics. bioRxiv, 2023: p. 2023.11.05.565674. [DOI] [PubMed]
- 20.Tang D, et al. SRplot: a free online platform for data visualization and graphing. PLoS ONE. 2023;18(11): e0294236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveros JC. Venny. An interactive tool for comparing lists with Venn's diagrams. (2007–2015); Available from: https://bioinfogp.cnb.csic.es/tools/venny/index.html.
- 23.Li Z, Zhang T, Yang X, Peng Y. Role of noncoding RNA and protein interaction in pancreatic cancer. Chin Med J. 2025;138(09):1019–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, et al. The COL11A1/Akt/CREB signaling axis enables mitochondrial-mediated apoptotic evasion to promote chemoresistance in pancreatic cancer cells through modulating BAX/BCL-2 function. J Cancer. 2021;12(5):1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, et al. COL17A1 facilitates tumor growth and predicts poor prognosis in pancreatic cancer. Biochem Biophys Res Commun. 2022;632:1–9. [DOI] [PubMed] [Google Scholar]
- 26.Yang R-R et al. Comprehensive Analysis on the Expression and Prognostic value of ITGA Family in Human Pancreatic Adenocarcinoma. 2022.
- 27.Jin L, et al. High expression ITGA2 affects the expression of MET, PD-L1, CD4 and CD8 with the immune microenvironment in pancreatic cancer patients. Front Immunol. 2023. 10.3389/fimmu.2023.1209367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idichi T, et al. Involvement of anti-tumor miR-124-3p and its targets in the pathogenesis of pancreatic ductal adenocarcinoma: direct regulation of ITGA3 and ITGB1 by miR-124-3p. Oncotarget. 2018;9(48):28849–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei X, et al. Comprehensive analysis of abnormal expression, prognostic value and oncogenic role of the hub gene FN1 in pancreatic ductal adenocarcinoma via bioinformatic analysis and in vitro experiments. PeerJ. 2021;9: e12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, et al. LAMC2 modulates the acidity of microenvironments to promote invasion and migration of pancreatic cancer cells via regulating AKT-dependent NHE1 activity. Exp Cell Res. 2020;391(1): 111984. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, et al. LAMB3 mediates apoptotic, proliferative, invasive, and metastatic behaviors in pancreatic cancer by regulating the PI3K/Akt signaling pathway. Cell Death Dis. 2019;10(3):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knapinska AM, Estrada C-A, Fields GB. The roles of matrix metalloproteinases in pancreatic cancer. Prog Mol Biol Transl Sci. 2017;148:339–54. [DOI] [PubMed] [Google Scholar]
- 33.Kurnia I, et al. Molecular patho-mechanisms of cervical cancer (MMP1). Ann Med Surg. 2022;77: 103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X, Ji H, Guo Z, Lu X. Elevated serum MMP-1 associated with advanced disease stage and lymph node metastasis in patients with pancreatic carcinoma. Am J Cancer Res. 2023;13(11):5405–17. [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuo Y, et al. CXCL8/IL-8 and CXCL12/SDF-1α co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer. 2009;124(4):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, et al. Long non-coding RNAs in pancreatic cancer: biologic functions, mechanisms, and clinical significance. Cancers (Basel). 2022;14(9):2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong Y, et al. Deciphering the role of NcRNAs in Pancreatic Cancer immune evasion and drug resistance: a new perspective for targeted therapy. Front Immunol. 2024;15:2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Javadrashid D, et al. Pancreatic cancer signaling pathways, genetic alterations, and tumor microenvironment: the barriers affecting the method of treatment. Biomedicines. 2021;9(4):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, et al. The extracellular matrix: a key accomplice of cancer stem cell migration, metastasis formation, and drug resistance in PDAC. Cancers (Basel). 2022;14(16):3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Z, et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. 2023;22(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad RS, Eubank TD, Lukomski S, Boone BA. Immune cell modulation of the extracellular matrix contributes to the pathogenesis of pancreatic cancer. Biomolecules. 2021. 10.3390/biom11060901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H, et al. Circankrd17 promotes glycolysis by inhibiting miR-143 in breast cancer cells. J Cell Physiol. 2023;238(12):2765–77. [DOI] [PubMed] [Google Scholar]
- 43.Cai W, Zhang Q. The transcription factor ZEB1 mediates the progression of epithelial ovarian cancer by promoting the transcription of CircANKRD17. J Biochem Mol Toxicol. 2022;36(8): e23086. [DOI] [PubMed] [Google Scholar]
- 44.Chang R, Yu H, Li S, Pan J. CircRNA hsa_circ_0003528/miR-215 is considered a potential target for predictive prognosis and therapy for triple-negative breast cancer. Mol Biol Rep. 2024;51(1):901. [DOI] [PubMed] [Google Scholar]
- 45.Wang G, et al. Hsa_circ_0003528 promotes cell malignant transformation and immune escape via increasing oncogene PDL1 through sponging miR-511-3p in non-small cell lung cancer. Environ Toxicol. 2023;38(6):1347–60. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, et al. MiR-424-5p promotes proliferation, migration and invasion of laryngeal squamous cell carcinoma. Onco Targets Ther. 2019;12:10441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crawford KS, Volkman BF. Prospects for targeting ACKR1 in cancer and other diseases. Front Immunol. 2023;14:2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horuk R. The duffy antigen receptor for chemokines DARC/ACKR1. Front Immunol. 2015;6:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Z, et al. SpaRx: elucidate single-cell spatial heterogeneity of drug responses for personalized treatment. Brief Bioinform. 2023. 10.1093/bib/bbad338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang G, et al. CRISPRoffT: comprehensive database of CRISPR/Cas off-targets. Nucleic Acids Res. 2025;53(D1):D914-d924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Survival analysis was conducted to assess the impact of gene expression on PDAC patient outcomes.
Supplementary file1 (JPG 3691 kb)
Supplementary Figure 2. Analysis of Gene Expression Correlations with Drug Sensitivity and Immune Infiltration in PDAC. (A) Analysis of the relationship between mRNA expression levels and drug sensitivity using data from the GDSC database, showing how specific genes correlate with the sensitivity to various drugs. (B) Examination of the correlation between gene expression and immune cell infiltration in PDAC.
Supplementary file2 (JPG 1152 kb)
Supplementary Figure 3. Functional Enrichment and Network Analysis of Macrophage-Specific Differentially Expressed Genes in PDAC. (A) Gene Ontology (GO) enrichment analysis of macrophage-associated DEGs across three categories: Biological Processes, Cellular Components, and Molecular Functions. Key enriched terms include defense response to virus, inflammatory response, and cytokine production, highlighting the immunologically active role of macrophages in the tumor microenvironment. (B) KEGG pathway enrichment of the same DEGs, revealing associations with NOD-like receptor signaling, viral infection pathways, HTLV-I signaling, and lipid and atherosclerosis-related signaling, implicating macrophages in both inflammatory and metabolic regulation in PDAC. (C) Protein–protein interaction (PPI) network constructed using the STRING database, with nodes colored by degree centrality.
Supplementary file3 (JPG 2515 kb)
Supplementary File 1. GO and KEGG analysis data
Supplementary file4 (XLSX 29 kb)
Supplementary File 2. Differentially expressed Genses for Macrophages
Supplementary file5 (XLSX 30 kb)
Data Availability Statement
The sequence data supporting this study's findings are derived from publicly available datasets. These datasets are accessible through the Gene Expression Omnibus (GEO) repository: https://www.ncbi.nlm.nih.gov/geo/.