Abstract
Anaplastic large-cell lymphoma (ALCL) accounts for 15% of all peripheral T-cell lymphomas globally and can be further divided into subcategories, of which patients with ALK-negative ALCL have dismal prognosis and overall survival. We established a patient-derived xenograft (PDX) and in vitro model (designated PTCL-S1) of TP63-rearranged ALK-negative ALCL from the primary tumour site of a 55-year old Chinese woman. Whole genome sequencing of the patient’s tumour identified various mutations including AKT1 and NOTCH1, as well as the TP63-TBL1XR1 gene fusion. RNA sequencing followed by Sanger sequencing confirmed the gene rearrangement in original tumour, PDX and PTCL-S1 cell line. Immunohistochemistry profiling of the PDX model and cell-line were consistent with the patient’s primary tumour sample (CD3 + /CD30 + /CD79a-). Cytotoxic agents (doxorubicin, etoposide and gemcitabine) commonly used in ALCL treatment exhibited potent anti-proliferative activity in the cell-line. In conclusion, the established PTCL-S1 cell line can be a useful tool for further investigation of the understanding of TP63-rearranged ALK-negative ALCL.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13577-025-01264-1.
Keywords: Anaplastic large cell lymphoma (ALCL), TP63 rearrangement, TP63-TBL1XR1 fusion, Tumour models, Patient-derived xenograft
Introduction
Systemic anaplastic large-cell lymphoma (ALCL) belongs to a family of nodal peripheral T-cell lymphomas (PTCL) that are rare globally [1]. Conventionally, ALCL is divided into ALK (anaplastic lymphoma kinase)-positive and ALK-negative subtypes with divergent pathobiology and clinical phenotype [2]. While ALK-positive ALCL is generally defined by good response to conventional chemotherapy and favourable prognosis, ALK-negative ALCL belongs to a heterogeneous subgroup that is poorly characterized, with widely disparate clinical outcomes [3, 4]. Notably, ALK-negative ALCL may be further stratified into distinct molecular subtypes based on genomic rearrangements of DUSP22 and TP63. These molecular alterations are mutually exclusive and make up a minority of ALK-negative ALCL, accounting for between 13 and 30% and 2–8% cases, respectively [3, 5–7]. While DUSP22 rearrangement has been associated with survival outcomes of up to 90% at five years, TP63 rearrangement has been shown to portend a dismal prognosis [3, 5].
Contemporary anthracycline-based “CHOP” or “CHOP-like” regimens are typically used in the first-line treatment of ALCL, regardless of known molecular alterations. The incorporation of brentuximab vedotin, a CD30-targeted antibody–drug conjugate, is also approved in this setting, following the pivotal ECHELON-2 study [8], though outcomes remain poor for ALK-negative/TP63-rearranged cases. The urgent need for better understanding of the disease pathobiology and for improved therapeutic strategies in TP63-rearranged ALCL is however, hampered by the lack of ex vivo model systems for mechanistic studies and evaluation of drug candidates.
In this study, we established a novel patient-derived murine xenograft and cell-line model of ALK-negative ALCL with a TP63 rearrangement (designated PTCL-S1). We characterized the genomic profile of this model via whole genome sequencing and RNA sequencing, and examined ex vivo responses to a panel of conventional chemotherapeutic agents.
Materials and methods
Clinical biospecimen and data acquisition
Clinical information was obtained from hospital electronic medical records. Basic demographic data including sex, age and ethnicity of the index patient was checked against her National Registry Identification Card. All histological parameters were reviewed by expert haemato-pathologists. Written informed consent from the patient for use of clinical data and biospecimens was obtained in accordance with the Declaration of Helsinki. Ethics approval from the SingHealth Centralized Institution Review Board (CIRB 2018/3084) was obtained for tissue collection and patient consent protocols.
Clinical and pathological description of the patient
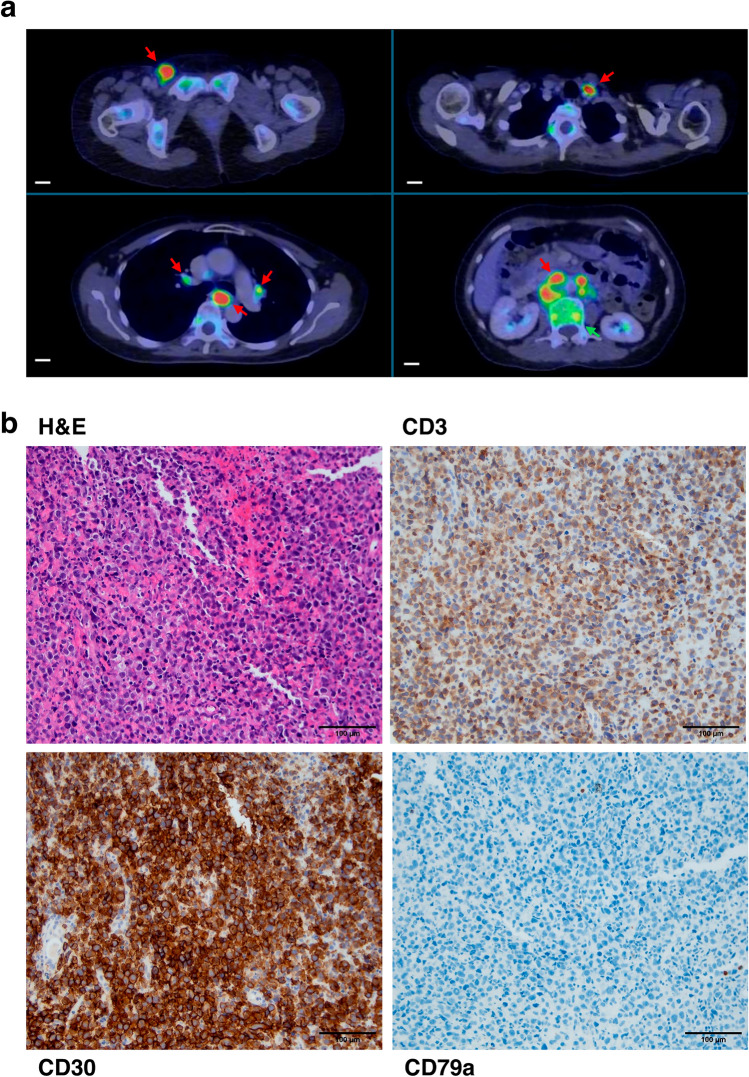
A 55 year old Chinese woman was diagnosed with stage 2 anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma (ALCL) of the posterior nasopharynx. She had presented with intermittent nasal discharge and epistaxis over a 3 month duration, associated with right-sided hearing impairment, blocked nose and unintentional weight loss of 5 kg. 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG-PET/CT) imaging showed a FDG-avid mass in the posterior nasopharynx extending into the skull base and sphenoid sinus, as well as FDG-avid cervical lymph nodes bilaterally. Biopsy of the posterior nasopharyngeal mass showed negativity for ALK staining on immunohistochemistry. In addition, fluorescence in situ hybridization for ALK and DUSP22 rearrangements were both negative. Large tumour cells with nucleolated, rounded and irregular hyperchromatic nuclei, enclosed in ample, pale cytoplasm were observed, staining strongly positive for CD30, CD3 and CD2. EBV-encoded small RNA (EBER), ALK, CD20, CD79A, CD10, CD56, CD123 and TCL-1 were all negative. Ki-67 was positive in 70–80% of tumour cells. She achieved complete remission following six cycles of induction ICE (ifosfamide, carboplatin, etoposide) chemotherapy without consolidation with autologous stem cell transplantation (ASCT). However, the patient eventually relapsed 5-years later, presenting with involvement of lymph nodes above and below diaphragm, bone marrow, and cerebrospinal fluid. Excision of the right inguinal node and bone marrow biopsy confirmed relapse of ALK-negative ALCL (Fig. 1). She was treated with four cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) and two cycles of high-dose methotrexate, achieving complete metabolic remission, followed by consolidation ASCT. Unfortunately, she relapsed approximately one month post-engraftment and passed away shortly.
Fig. 1.
Clinical and pathological features of patient with ALK-negative ALCL. a 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG-PET/CT) imaging taken at time of relapse showed hypermetabolic supradiaphragmatic and infradiaphragmatic lymphadenopathy (red arrows), including the right inguinal, left supraclavicular, subcarinal, peri-hilar and para-aortic regions, as well as FDG-avid bony lesions indicative of marrow infiltration (green arrow) (scale bar: 2 cm). Transverse diameter of dominant nodes: (Top Left to Right) 2–1.2 cm (Bottom Left to Right) 1.7–5 cm. b IHC of patient tumour tissue revealed to be strongly and uniformly positive for CD30 and CD3, while being negative for CD79a (scale bar: 100 µm).
Establishment of patient-derived xenograft (PDX)
Primary tumour tissue before chemotherapy from the patient was dissected and implanted into the right flank of NSG mice subcutaneously. Measurements were taken twice a week and passaged when tumour volume hit > 1000 cm3, but not exceeding 2000 cm3. The PDX was considered established after it had been successfully passaged for 4 generations. Xenograft studies were conducted in compliance with animal protocols approved by the SingHealth Institutional Animal Care and Use Committee (IACUC).
Establishment of patient-derived cell line
Tissue from the established PDX was digested and homogenized using human tissue dissociation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer’s protocol. PTCL-S1 was maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% foetal bovine serum (FBS), 10% horse serum (HS), 2 mM L-glutamine and 100 units/ml of Penicillin/Streptomycin (Thermo Fisher Scientific, Waltham, MA, US) in a humidified incubator at 37 ℃, 5% CO2. Culture medium was changed every 3–4 days and mouse cell depletion was done twice, at passage 5 and passage 10, using Mouse Cell Depletion Kit (Miltenyi Biotec, Singapore). Cells were maintained as described for more than 60 passages.
Short tandem repeat (STR) analysis
PTCL-S1 cells were collected and sent for standard STR genotyping analysis (Axil Scientific, Singapore) to establish a unique genomic fingerprint of the established cell line as well as to check for any cross-contamination.
Cell proliferation assay
Cell proliferation was measured by cell counting with the automated cell counter Countess II (Thermo Fisher Scientific, Waltham, MA, US). PTCL-S1 cells were seeded at 3 × 105 cells/well in 24-well plate in triplicate and cultured for 0, 24, 48 and 72 h. Cells were counted at each time point and doubling time was calculated using the formula of where T = time in hours, e = final cell number and b = initial cell number. Characterization of drug response in PTCL-S1 was performed using cytotoxic drugs that are commonly used in clinical practice as part of standard treatment regimens, including etoposide, gemcitabine and doxorubicin (Selleck Chemicals, USA). Drug cytotoxicity was tested by seeding 2 × 104 PTCL-S1 cells per ml media in 24-well plates and treated with various concentrations of each drug. Cells were incubated for 72 h and then resuspended and 100 µl of cells were plated in 96-well plate in triplicates. Cell viability was assessed using Promega CellTiter-glo following manufacturer’s instructions (Promega, Madison, WI, US) and measuring the absorbance at 480 nm using Tecan M200 Infinite 96-well plate reader (Tecan, Männedorf, Switzerland). Cell viability was calculated as a percentage of control absorbance and IC50 values were estimated using GraphPad Prism 9 (GraphPad Software, CA, USA).
Immunohistochemistry
Histological investigation was performed on 4 µm thick sections of a representative formalin-fixed paraffin-embedded (FFPE) tumour and cell line samples. Tumour from the PDX was fixed in 10% neutral-buffered formalin and made into FFPE blocks for validation using immunohistochemistry. The sections were deparaffinized and stained with haematoxylin and eosin (H&E) as well as antibodies against CD3, CD30 and CD79a following standard protocols in the clinical laboratory of the Singapore General Hospital. Immunohistochemistry profiles of the xenograft and cell line were compared with the original tumour.
RNA extraction, RNA sequencing and RT-PCR for fusion detection
Cells were collected and RNA were isolated from PTCL-S1 using Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA, USA). RNA was quantified using NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, MA, USA) and the integrity of RNA was determined by electrophoresis using the 2100 Bioanalyzer (Agilent Technologies, USA). Reverse transcription polymerase chain reaction (RT-PCR) was performed using BioRad iScript cDNA synthesis Kit according to manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA, USA). Whole transcriptome sequencing was performed on the MGI G400 platform (MGI Tech, China) using standard manufacturer’s protocol. The reads were aligned to the human genome hg19 RefSeq reference transcriptome by STAR [9] and fusion detection was performed using CICERO v1.9.6 [10].
DNA extraction and whole genome sequencing
Genomic DNA was extracted using Qiagen DNeasy Blood & Tissue kit (Qiagen, Valencia, CA, USA) following manufacturer’s instructions and quantified using NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, MA, USA). Whole genome sequencing was performed on the Illumina HiSeq X Ten platform as paired-end 150-base pair reads (Macrogen, Singapore).
Mutational variant-calling on DNA sequencing reads
DNA sequencing adaptors, trailing low-quality bases and polyG sequences were removed by fastp (v0.19.6) [11]. The pre-processed reads were aligned using BWA-MEM (v0.7.17) [12] to the hs37d5 human reference genome. Polymerase chain reaction (PCR) duplicates were subsequently marked by Sambamba (v0.6.5) [13]. The alignment coverage statistics were computed by Qualimap [14] (v2.2.1). Short-variants were called using Strelka2 (v2.9.4). wAnnovar (November 2020) [15] was used to annotate the short-variants. The filtering criteria on the short-variants were further filtered against low complexity regions, dbSNP(v132) positions without COSMIC IDs, simple repeats and homopolymers as described in JQ Lim et al., 2022 [16]. Structural rearrangements (SR) were called using Manta (v1.6.0) [17] and annotated by annotSV (v1.2). [18]. Each candidate SR was subjected to the following filtering criteria: (1) SR is supported by at least 3 discordant read-pairs and at least 3 soft-clipped reads, and the sum of all supporting reads is at least 10; (2) at least 10 × coverage in both tumour and matching-normal data; and (3) SR is at least 1000 bp in size.
Validation of somatic mutations and gene fusion by Sanger sequencing
Specific flanking primer pairs for the selected mutations and fusion product to be validated were designed using Primer-BLAST and the primer sequences are as per specified.
NOTCH1-Fwd: 5’-GAGTAGCTGTGCTGCGAGG-3’.
NOTCH1-Rev: 5’-AACCAATACAACCCTCTGCG-3’.
AKT1-Fwd: 5’-GAGGCCAAGGGGATACTTACG-3’.
AKT1-Rev: 5’-TTCTGTCGCTGGCCCTAAGA-3’.
TP63-TBL1XR1-Fwd: 5’-CCCCAGCTCATTTCTCTTGGAA-3’.
TP63-TBL1XR1-Rev: 5’-GGCAAACCCATCATAGGAACC-3'.
TBL1XR1-TP63-Fwd: 5’-CTGTGCCTGGAACCCTGTT-3’.
TBL1XR1-TP63-Rev: 5’-CGCGTGGTCTGTGTTATAGG-3’.
Two primer pairs were used to detect the TP63-TBL1XR1 gene fusion products. PCR was performed by using 1 st Base REDiant II PCR master mix (Axil Scientific, Singapore) following manufacturer’s instructions. The resulting amplicons were sent out for Sanger sequencing by 1 st Base (Axil Scientific, Singapore) and analysed.
Data availability
All data, xenograft and cell lines that support the findings of this study are available from the corresponding authors upon reasonable request.
Results
Establishment and characterization of patient-derived xenograft (PDX) model
Patient tumour was dissected and subcutaneously injected into NSG mice, with solid lymphoma tissue developing after 8 days of transplantation (Fig. 2a). IHC of the harvested tumour after 4 generations revealed similar characteristics to the original patient tumour, i.e. positive for CD3, CD30 and negative for CD79a (Fig. 2b) This indicates that the xenograft can propagate and retain the characteristics of the original tumour.
Fig. 2.
PDX establishment from patient tissue. a Solid lymphoma developed 8 days following subcutaneous implantation of tumour tissue into NSG mice. b IHC of established PDX revealed similar characteristics to the original patient tumour (scale bar: 100 µm)
Establishment and morphological characterization of patient-derived cell-line PTCL-S1
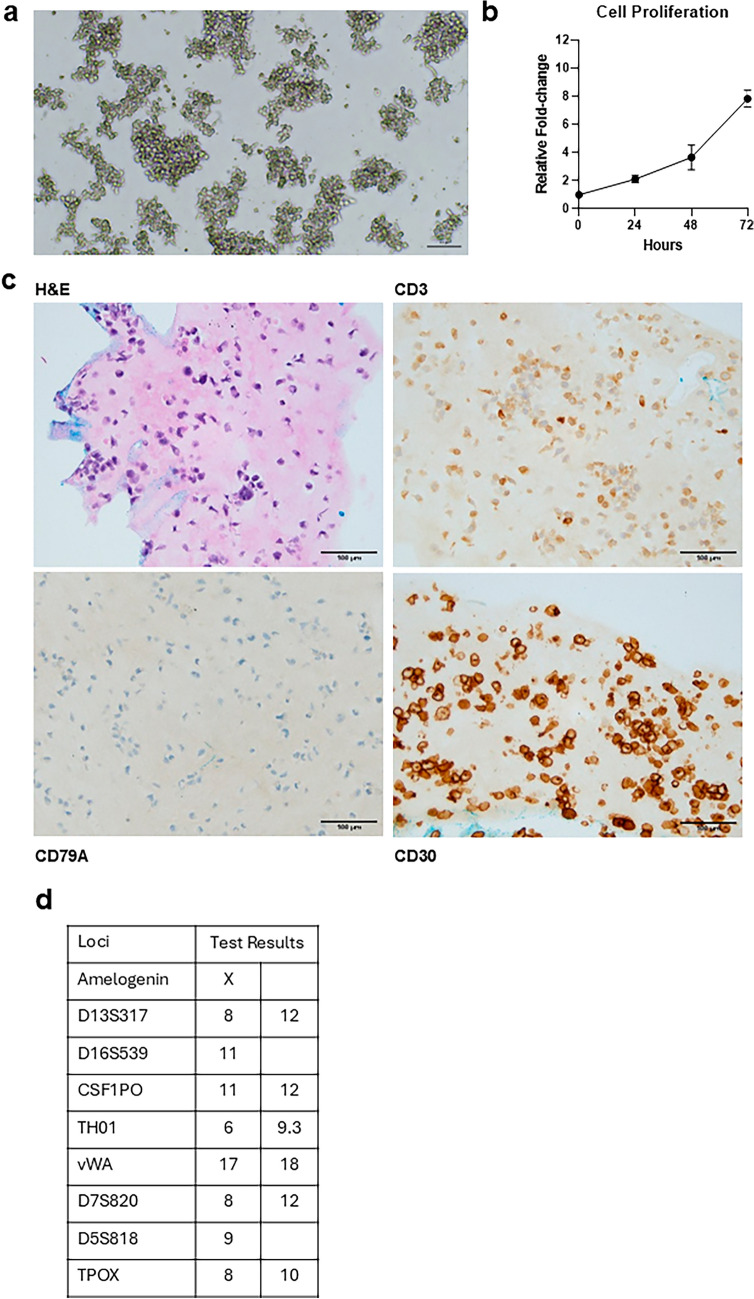
The cell line PTCL-S1 was derived from the established PDX tissue and the cells grew in suspension for more than 60 passages. PTCL-S1 are mostly spherical, with some irregularities in shape where some cells appear to be elongated, and they tend to grow in clumps (Fig. 3a). They have a doubling time of 22.26 ± 0.82 h (Fig. 3b) and are interleukin-2 (IL-2) independent. Further IHC investigations were conducted and compared with both original patient tissue and PDX tissue. The results revealed that PTCL-S1 highly expresses CD30 and CD3, and is negative for CD79a (Fig. 3c), which is concordant with the patient tumour and PDX tissue. STR profiling demonstrated PTCL-S1’s unique DNA fingerprint, with no matches to any cell-line from known databases (Fig. 3d).
Fig. 3.
Characterization of PTCL-S1 cell-line. a Brightfield image of PTCL-S1 at 10 × magnification (scale bar: 100 µm) at passage 31. PTCL-S1 cells are mostly spherical with some irregularities in their shape, appearing to be elongated, and they tend to grow in clumps. b PTCL-S1 cells grow readily in RPMI-1640 supplemented with 10% FBS, 10% HS and has an estimated doubling time of 22.26 ± 0.82 h. c IHC of established cell line at passage 10. IHC revealed that PTCL-S1 highly expresses CD30 and CD3 and is negative for CD79a (scale bar: 100 µm). d STR profiling of PTCL-S1 demonstrated a unique DNA fingerprint of the cell line, with no evidence of cross-contamination with cell-lines from known databases
Genomic characterization of PTCL-S1
The patient-matched bulk tumoural tissue and non-tumoural blood are whole-genome sequenced and generated a total of 224.7 Gbp (Q30, read 1: 91.5%, read 2: 68.2%) and 110.7 Gbp (Q30, read 1: 85.2%, read 2: 70.9%) of data, respectively. After alignment to the human reference genome, the libraries achieved an effective PCR duplicated coverage of 61.5 × and 31.2x, respectively (Supplementary Table 1). Genomic investigation of patient tumour using Whole Genome Sequencing (WGS) detected a series of heterozygous mutations with variant allele frequencies (VAF) between 0.1 and 0.27, of which 5 nonsynonymous mutations FAT3 (c.C12623T; VAF 0.27), NRG1 (c.C929T; VAF 0.21), SLFN5 (c.C1064T; VAF 0.21), CLRN1 (c.T275A; VAF 0.17), AKT1 (c.G49A; VAF 0.13), 1 frameshift deletion TNRC6C (c.1837delA; VAF 0.26) and 1 stop-gain mutation NOTCH1 (c.C7216T; VAF 0.10) were observed. We then validated the mutations in 2 genes, namely NOTCH1 (c.C7216T) and AKT1 (c.G49A) (Fig. 4a), in the PDX and PTCL-S1 cell line (Fig. 4b) using Sanger sequencing. These mutations were concordant across all samples. WGS of the patient tumour also detected the fusion of TP63-TBL1XR1, showing a balanced inversion of TP63 and TBL1XR1 (Fig. 4c.) Sanger sequencing of the cDNA from PDX and the cell line verified that the fusion product was present (Fig. 4d).
Fig. 4.
Genomic characterization of PTCL-S1. Validation of genomic characteristics in PDX and cell line was performed by sanger sequencing at passage 4 and 58 respectively. a NOTCH1 and AKT1 somatic mutations were detected by Whole Genome Sequencing (WGS). b Both somatic mutations were verified and validated in the PTCL-S1 PDX and cell-line by Sanger sequencing. c TP63-TBL1XR1 fusion was identified following WGS of the primary tumour and RNA-Seq of PTCL-S1 cell-line passage 40. d Verification and validation of the TP63-TBL1XR1 fusion in the PTCL-S1 PDX and cell-line by sanger sequencing
In vitro characterization of responses to conventional cytotoxic agents
Following morphological and genomic characterization of PTCL-S1, we investigated the drug response of PTCL-S1 to conventional PTCL treatment regimen (Fig. 5), such as Etoposide, Gemcitabine and Doxorubicin. The cells were incubated with different concentrations of the respective drugs for 72 h before performing ATP assay to assess their drug sensitivity and establishing the IC50. PTCL-S1 demonstrated a reduction in cell viability following drug treatment in a dose-dependent manner and showed potent sensitivity to all selected drugs with an IC50 of 7.48 nM, 0.349 nM and 2.12 nM to etoposide, gemcitabine and doxorubicin respectively.
Fig. 5.
Drug response characterization of PTCL-S1 to standard PTCL treatment regimens. PTCL-S1 treated with the various drugs showed reduction of viability in a dose-dependent manner with reasonable sensitivity to each drug. All drug response experiments were performed using cells between passage 30–40
Discussion
ALCL is a subset of PTCL derived from Th17 cells with the characteristic expression of IL-17A and IL-17F, and is uniformly CD30-positive in nature [19, 20]. ALCL can be further characterized into ALK-negative and ALK-positive which can affect disease prognosis. ALK-negative patients typically perform worse [20] and are more prone to relapses when compared with ALK-positive patients, at 49% and 43% for 5 year overall survival (OS) and progression-free survival (PFS) respectively [21]. There is a need to understand the molecular and genetic features of ALK-negative ALCL to help improve patient outcome and understanding of the disease.
Chromosomal rearrangements seen exclusively in ALK-negative ALCL patients include TP63 rearrangement, which results from a TP63-TBL1XR1 intrachromosomal inversion [5, 22] and DUSP22 rearrangement. Patients harbouring TP63 rearrangements perform worse overall where DUSP22-rearranged patients in the study by Pedersen et al. had an 80% 5 year OS but the two patients with TP63 rearrangements both died within 2 years of diagnosis [5]. Another report by Parrilla Castellar et al. reported 90% 5 year OS for DUSP22-rearranged patients when compared with only 17% OS in TP63-rearranged patients [3]. Meanwhile, the sole case of TP63-rearranged patient in the study by Hapgood died within 6 months of diagnosis [23].
ALK-negative ALCLs are difficult to study due to their genetic and biological heterogeneity, and there are unique subtypes within this classification. Cases with abnormal immunophenotypes can result in confusion with other neoplasms [24]. In one case, fluorescence in situ hybridization (FISH) was unable to identify the TP63-rearrangement and chromosomal rearrangements were only picked up by RNA-seq [25].
According to our knowledge, other than the 21 ALCL cell models described in the 2004 report by Drexler and MacLeod [26], there are a total of 29 published ALCL cell models now. However, many are lost or do not have sufficient information on the genetic and molecular features as shown in Table 1. Of which, only 10 are known to be ALK-negative, with only 7 available from a commercial cell bank. There is currently only 1 cell line, DL-40, which was first reported in 1990 [27], and then subsequently found to harbour the TP63-TBL1XR1 gene fusion using RNA-seq [28]. DL-40 was originally derived from the peripheral blood of a case in keeping with aggressive ALCL in a terminal leukemic phase, whereas our current PTCL-S1 model is derived from a primary tumour at an earlier disease state upon diagnosis and before chemotherapy. The clinical implications of TP63-rearrangements have been widely discussed in previous studies as mentioned and the addition and establishment of new in vitro models, particularly those that harbour gene rearrangements, are important to further our study of the disease.
Table 1.
ALCL cell lines in publications
| Cell line | Published Year | Patient age and sex | Sample Site | ALK status | Translocation | Cellosaurus accession number | Availability | References |
|---|---|---|---|---|---|---|---|---|
| DL-40 | 1990 | 64F | Peripheral Blood | – | TP63-TBL1XR1 | CVCL_2889 | JCRB | [27, 28] |
| TK-ALCL1 | 2024 | 59 M | Lymph Node | + | NPM-ALK | CVCL_E2T4 | Authors | [31] |
| AMS3 | 1994 | 23 M | Tumour | + | NPM-ALK | CVCL_H629 | ? | [32] |
| CHIC | 2011 | 32 M | Cerebrospinal fluid | + | NPM-ALK | CVCL_E2T5 | Authors | [33] |
| COST | 2004 | 4 M | Peripheral Blood | + | NPM-ALK | CVCL_9491 | ? | [34] |
| DEL | 1990 | 12 M | Pleural Effusion | + | NPM-ALK | CVCL_1170 | DSMZ | [35] |
| HSC-M1 | 2001 | 5F | Bone Marrow | + | NPM-ALK | CVCL_H630 | Lost | [36] |
| JB6 | 1990 | 12 M | Peripheral Blood | + | NPM-ALK | CVCL_H633 | ? | [37] |
| Karpas-299 | 1988 | 25 M | Peripheral Blood | + | NPM-ALK | CVCL_1324 | DSMZ | [38] |
| Ki-JK | 1993 | 15 M | Pleural Effusion | + | NPM-ALK | CVCL_2093 | DSMZ/JCRB | [39] |
| L-82 | 2002 | 24F | Pleural Effusion | + | NPM-ALK | CVCL_2098 | DSMZ | [40] |
| SU-DHL-1 | 1974 | 10 M | Pleural Effusion | + | NPM-ALK | CVCL_0538 | ATCC/DSMZ | [41] |
| SR/SR786 | 1988 | 11 M | Pleural Effusion | + | NPM-ALK | CVCL_1711 | ATCC/DSMZ/NCI-DTP | [42] |
| SUP-M2 | 1989 | 5F | Cerebrospinal fluid | + | NPM-ALK | CVCL_2209 | DSMZ | [43] |
| UCONN-L2 | 1995 | ? | Lymph Node | + | NPM-ALK | CVCL_A693 | ? | [44] |
| FE-PD | 1994 | 46F | Peripheral Blood | – | MKLN1-AS1-DUSP22 | CVCL_H614 | Authors | [28, 45] |
| MAC-2A | 1989 | 47 M | ? | – | PCM1-JAK2 | CVCL_H637 | DSMZ |
[28], [46] |
| TLBR-1 | 2010 | 42F | Tumour | – | – | CVCL_L177 | DSMZ | [47] |
| TLBR-2 | 2012 | 43F | Tumour | – | – | CVCL_A1EY | DSMZ | [48] |
| TLBR-3 | 2012 | 45F | Tumour | – | – | CVCL_A1EZ | DSMZ | [48] |
| TLBR-4 | 2017 | ? | Tumour | – | – | CVCL_A1FA | DSMZ | [49] |
| DL-95 | 1998 | 40 M | ? | ? | ? | CVCL_IR15 | ? | [50] |
| JK | 1999 | 58 M | Tumour | – | ? | CVCL_H635 | ? | [51] |
| Mac-2 | 1988 | 47 M | ? | – | ? | CVCL_H632 | ? | [52] |
| MAC-2B | 1989 | 47 M | ? | – | ? | CVCL_H638 | DSMZ | [46] |
| MH-1 | 1984 | 61 M | Bone Marrow | ? | ? | CVCL_H639 | ? | [53] |
| SU-DHL-3 | 1974 | 35 M | Pleural Effusion | ? | ? | CVCL_W769 | ATCC/DSMZ | [41] |
| SU-LL-1 | 1993 | ?M | ? | ? | ? | CVCL_B5D7 | ? | [54] |
| USP-91 | 1993 | 14 M | Pleural Effusion | ? | ? | CVCL_H634 | ? | [55] |
*MAC-2A and MAC-2B are sublines of MAC-2
In this study, we established and characterized an ALCL cell line, PTCL-S1, which has been derived from patient tumour. This cell line has been shown to retain genomic and immunohistochemical profiles of the original tissue, i.e. TP63-TBL1XR1 gene fusion, NOTCH1 and AKT1 mutations, CD30 and CD3 positive, CD79a negative. In keeping with previous studies, NOTCH1 mutations have also been described in ALCL. In addition, NOTCH1 mutations have been reported in other clinical cases. Larose et al. previously reported NOTCH1 T349P variant in 12% of their ALCL samples, and this variant was found to confer growth advantage in their study [29]. In another report by Zhong et al., they found that NOTCH1 was the second most common mutation (22.7%) among ALK-negative patients in their cohort [30]. It has also shown to be responsive to contemporary ALCL treatment agents, including doxorubicin, etoposide and gemcitabine. Taken together, this indicates that PTCL-S1 can be useful as a TP63-rearranged ALK-negative ALCL in vitro model to investigate new treatment candidates as well as to better understand the genetic and molecular features of ALK-negative TP63-rearranged ALCL.
In conclusion, we established an ALK-negative ALCL PDX and cell line model harbouring TP63 rearrangement that can not only recapitulate the original characteristics of the patient tumour, but also propagate in vivo. This model shows promise to be used in the understanding of disease mechanism of TP63-rearrangement associated ALCL as well as developing new treatments to better patient outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
K.X.Y.C. analyzed the data and prepared the first draft the manuscript; S.T.L. and J.Y.C. provided clinical information and samples; J.Q.L. performed the bioinformatic analyses; K.X.Y.C., E.C.Y.L., Z.L., N.A.B.M.T., D.H. processed tissue and performed experiments; J.Y.C. contributed to data interpretation; J.Y.C. and C.K.O. conceptualized the study, interpreted the results, had unrestricted access to all data, and revised the manuscript. All authors agreed to submit the manuscript, read and approved the final draft, and take full responsibility for its content.
Funding
This work was supported by the Tanoto Foundation Professorship in Medical Oncology, New Century Foundation Limited, Ling Foundation, Singapore Ministry of Health’s National Medical Research Council Research Transition Award (TA21jun-0005), Large Collaborative Grant (OFLCG18May-0028 and OFLCG23May-0039), TETRAD II Collaborative Centre Grant (CG21APR2002), the SingHealth Duke-NUS AM/ACP-Designated Philanthropic Fund Grant Award (08/FY2023/EX/27-A65), as well as the Khoo Bridge Funding Award (Duke-NUS-KBrFA/2025/0090) provided by Duke-NUS Medical School and the “Estate of Tan Sri Khoo Teck Puat”.
Declarations
Conflict of interests
The authors declare no competing interests.
Ethical approval
Ethics approval from the SingHealth Centralized Institution Review Board was obtained for tissue collection and consent protocols (CIRB 2018/3084). Xenograft studies were conducted in compliance with animal protocols approved by the SingHealth Institutional Animal Care and Use Committee (IACUC) (2018/SHS/1371).
Informed consent
Written informed consent from the patient for use of clinical data and biospecimens was obtained in accordance with the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jason Yongsheng Chan, Email: jason.chan.y.s@nccs.com.sg.
Choon Kiat Ong, Email: gmsock@nus.edu.sg.
References
- 1.O’Connor OA, Ma H, Chan JYS, Kim SJ, Yoon SE, Kim WS. Peripheral T-cell lymphoma: from biology to practice to the future. Cancer Treat Rev. 2024;129:102793. 10.1016/j.ctrv.2024.102793. [DOI] [PubMed] [Google Scholar]
- 2.Yap DRY, Lim JQ, Huang D, Ong CK, Chan JY. Emerging predictive biomarkers for novel therapeutics in peripheral T-cell and natural killer/T-cell lymphoma. Front Immunol. 2023;26(14):1068662. 10.3389/fimmu.2023.1068662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124:1473–80. 10.1182/blood-2014-04-571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang EWY, Tan YH, Chan JY. Novel clinical risk stratification and treatment strategies in relapsed/refractory peripheral T-cell lymphoma. J Hematol Oncol. 2024;17(1):38. 10.1186/s13045-024-01560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen MB, Hamilton-Dutoit SJ, Bendix K, et al. DUSP22 and TP63 rearrangements predict outcome of ALK-negative anaplastic large cell lymphoma: a Danish cohort study. Blood. 2017;130(4):554–7. 10.1182/blood-2016-12-755496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkhi M, Bal A, Das A, et al. ALK-negative anaplastic large cell lymphoma (ALCL): prognostic implications of molecular subtyping and JAK-STAT pathway. Appl Immunohistochem Mol Morphol. 2021;29(9):648–56. 10.1097/PAI.0000000000000936. (PMID: 33901030). [DOI] [PubMed] [Google Scholar]
- 7.Sibon D, Bisig B, Bonnet C, et al. ALK-negative anaplastic large cell lymphoma with DUSP22 rearrangement has distinctive disease characteristics with better progression-free survival: a LYSA study. Haematologica. 2023;108(6):1590–603. 10.3324/haematol.2022.281442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz S, O’Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229–40. 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian L, Li Y, Edmonson MN, et al. CICERO: a versatile method for detecting complex and diverse driver fusions using cancer RNA sequencing data. Genome Biol. 2020;21(1):126. 10.1186/s13059-020-02043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. q-bioGN. 2013:arXiv:1303.3997v1301.
- 13.Tarasov A, Vilella AJ, Cuppen E, Nijman IJ, Prins P. Sambamba: fast processing of NGS alignment formats. Bioinformatics. 2015;31(12):2032–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okonechnikov K, Conesa A, Garcia-Alcalde F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 2016;32(2):292–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang X, Wang K. wANNOVAR: annotating genetic variants for personal genomes via the web. J Med Genet. 2012;49(7):433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim JQ, Huang D, Chan JY, et al. A genomic-augmented multivariate prognostic model for the survival of natural-killer/T-cell lymphoma patients from an international cohort. Am J Hematol. 2022;97(9):1159–69. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Schulz-Trieglaff O, Shaw R, et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32(8):1220–2. [DOI] [PubMed] [Google Scholar]
- 18.Geoffroy V, Herenger Y, Kress A, et al. AnnotSV: an integrated tool for structural variations annotation. Bioinformatics. 2018;34(20):3572–4. [DOI] [PubMed] [Google Scholar]
- 19.Iqbal J, Weisenburger DD, Greiner TC, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 2010;115:1026–36. 10.1182/blood-2009-06-227579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shustov A, Soma L. Anaplastic Large cell lymphoma: contemporary concepts and optimal management. T-Cell NK-Cell Lymphomas. 2019;176:127–44. 10.1007/978-3-319-99716-2_6. [DOI] [PubMed] [Google Scholar]
- 21.Shustov A, Soma L. ALK-negative anaplastic large cell lymphoma: features and outcomes of 235 patients from the international T-Cell Project. Blood Adv. 2021. 10.1182/bloodadvances.2020001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasmatzis G, Johnson SH, Knudson RA, et al. Genome-wide analysis reveals recurrent structural abnormalities of TP63 and other p53-related genes in peripheral T-cell lymphomas. Blood. 2012;120:2280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hapgood G, Ben-Neriah S, Mottok A, et al. Identification of high-risk DUSP22-rearranged ALK-negative anaplastic large cell lymphoma. Br J Haematol. 2019;186(3):e28–31. 10.1111/bjh.15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie W, Medeiros LJ, Fan G, Li S, Xu J. Systemic ALK-negative anaplastic large cell lymphoma: insights into morphologic, immunophenotypic, genetic and molecular characteristics. Human Pathol. 2025;105671:156. 10.1016/j.humpath.2024.105671. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed N, Ketterling RP, Nowakowski GS, Dasari S, Feldman AL. RNAseq identification of FISH-cryptic BCL6::TP63 rearrangement in ALK-negative anaplastic large-cell lymphoma. Histopathology. 2022;81(2):275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drexler HG, MacLeod RA. Malignant hematopoietic cell lines: in vitro models for the study of anaplastic large-cell lymphoma. Leukemia. 2004;18(10):1569–71. 10.1038/sj.leu.2403465. (PMID: 15356658). [DOI] [PubMed] [Google Scholar]
- 27.Kubonishi I, Sonobe H, Miyagi T, et al. A Ki-1 (CD30)-positive T (E+, CD4+, Ia+)-cell line, DL-40, established from aggressive large cell lymphoma. Cancer Res. 1990;50(23):7682–5 (PMID: 1979248). [PubMed] [Google Scholar]
- 28.Ng SY, Yoshida N, Christie AL, et al. Targetable vulnerabilities in T- and NK-cell lymphomas identified through preclinical models. Nat Commun. 2018;9:2024. 10.1038/s41467-018-04356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larose H, Prokoph N, Matthews JD, et al. Whole Exome Sequencing reveals NOTCH1 mutations in anaplastic large cell lymphoma and points to Notch both as a key pathway and a potential therapeutic target. Haematologica. 2021;106(6):1693–704. 10.3324/haematol.2019.238766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong LH, Wu ZD, Wang JC, et al. Molecular profiling of Chinese systemic anaplastic large cell lymphoma patients: novel evidence of genetic heterogeneity. Ann Transl Med. 2021;9(2):128. 10.21037/atm-20-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sungwan P, Panaampon J, Kariya R, et al. Establishment and characterization of TK-ALCL1: a novel NPM-ALK-positive anaplastic large-cell lymphoma cell line. Hum Cell. 2024;37(4):1215–25. 10.1007/s13577-024-01077-8. (Epub 2024 May 16 PMID: 38755432). [DOI] [PubMed] [Google Scholar]
- 32.Shiota M, Fujimoto J, Takenaga M, et al. Diagnosis of t(2;5)(p23;q35)-associated Ki-1 lymphoma with immunohistochemistry. Blood. 1994;84(11):3648–52. 10.1182/blood.V84.11.3648.bloodjournal84113648. [PubMed] [Google Scholar]
- 33.Thielen C, Bisig B, Gofflot, et al. CHIC cells: a novel ALK+ cell line derived from a relapsed anaplastic large cell lymphoma. Br J Haematol. 2011;152(3):356–60. 10.1111/j.1365-2141.2010.08414.x. [DOI] [PubMed] [Google Scholar]
- 34.Lamant L, Espinos E, Duplantier M, et al. Establishment of a novel anaplastic large-cell lymphoma-cell line (COST) from a “small-cell variant” of ALCL. Leukemia. 2004;18(10):1693–8. 10.1038/sj.leu.2403464. (PMID: 15356659). [DOI] [PubMed] [Google Scholar]
- 35.Barbey S, Gogusev J, Mouly H, et al. DEL cell line: a “malignant histiocytosis” CD30+ t(5;6)(q35;p21) cell line. Int J Cancer. 1990;45(3):546–53. 10.1002/ijc.2910450329. (PMID: 2307542). [DOI] [PubMed] [Google Scholar]
- 36.Al-Hashmi I, Decoteau J, Gruss HJ, et al. Establishment of a cytokine-producing anaplastic large-cell lymphoma cell line containing the t(2;5) translocation: potential role of cytokines in clinical manifestations. Leuk Lymphoma. 2001;40(5–6):599–611. 10.3109/10428190109097658. (PMID: 11426532). [DOI] [PubMed] [Google Scholar]
- 37.Kadin ME, Cavaille-Col MW, Sioutos N, et al. Childhood Ki-1+ anaplastic large cell lymphoma: establishment and characterization of a new tumor cell line transplantable to SCID mice. Blood. 1990;76(Suppl. 1):354a–354a.2114934 [Google Scholar]
- 38.Fischer P, Nacheva E, Mason DY, et al. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin’s lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor beta-chain gene. Blood. 1988;72(1):234–40 (PMID: 3260522). [PubMed] [Google Scholar]
- 39.Shimakage M, Dezawa T, Tamura S, et al. A Ki-1-positive cell line expressing Epstein-Barr virus antigens, established from a child with Ki-1-positive lymphoma. Intervirology. 1993;36(4):215–24. 10.1159/000150340. (PMID: 8169113). [DOI] [PubMed] [Google Scholar]
- 40.Merz H, Lange K, Gaiser T, et al. Characterization of a novel human anaplastic large cell lymphoma cell line tumorigenic in SCID mice. Leuk Lymphoma. 2002;43(1):165–72. 10.1080/10428190210193. (PMID: 11908723). [DOI] [PubMed] [Google Scholar]
- 41.Epstein AL, Kaplan HS. Biology of the human malignant lymphomas. I. Establishment in continuous cell culture and heterotransplantation of diffuse histiocytic lymphomas. Cancer. 1974;34(6):1851–72. [DOI] [PubMed] [Google Scholar]
- 42.Su IJ, Balk SP, Kadin ME. Molecular basis for the aberrant expression of T cell antigens in postthymic T cell malignancies. Am J Pathol. 1988;132(2):192–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan R, Smith SD, Hecht BK, et al. Lack of involvement of the c-fms and N-myc genes by chromosomal translocation t(2;5)(p23;q35) common to malignancies with features of so-called malignant histiocytosis. Blood. 1989;73(8):2155–64 (PMID: 2525056). [PubMed] [Google Scholar]
- 44.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1995;267(5196):316–7. 10.1126/science.267.5196.316-b. [DOI] [PubMed] [Google Scholar]
- 45.del Mistro A, Leszl A, Bertorelle R, et al. A CD30-positive T cell line established from an aggressive anaplastic large cell lymphoma, originally diagnosed as Hodgkin’s disease. Leukemia. 1994;8(7):1214–9 (PMID: 8035614). [PubMed] [Google Scholar]
- 46.Su IJ, Kadin ME. Expression of growth factor/receptor genes in postthymic T cell malignancies. Am J Pathol. 1989;135(3):439–45. [PMC free article] [PubMed] [Google Scholar]
- 47.Lechner MG, Lade S, Liebertz DJ, et al. Breast implant-associated, ALK-negative, T-cell, anaplastic, large-cell lymphoma: establishment and characterization of a model cell line (TLBR-1) for this newly emerging clinical entity. Cancer. 2011;117(7):1478–89. 10.1002/cncr.25654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lechner MG, Megiel C, Church CH, et al. Survival signals and targets for therapy in breast implant-associated ALK–anaplastic large cell lymphoma. Clin Cancer Res. 2012;18(17):4549–59. 10.1158/1078-0432.CCR-12-0101. (Epub 2012 Jul 12 PMID: 22791880). [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Zhang Y, Petrus MN, et al. Cytokine receptor signaling is required for the survival of ALK- anaplastic large cell lymphoma, even in the presence of JAK1/STAT3 mutations. Proc Natl Acad Sci U S A. 2017;114(15):3975–80. 10.1073/pnas.1700682114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kubonishi I, Machida H, Miyoshi I. Ki-lymphoma and interleukin-6. Ann Intern Med. 1998;128(6):506–7. 10.7326/0003-4819-128-6-199803150-00021. [DOI] [PubMed] [Google Scholar]
- 51.Schiemann WP, Pfeifer WM, Levi E, Kadin ME, Lodish HF. A deletion in the gene for transforming growth factor beta type I receptor abolishes growth regulation by transforming growth factor beta in a cutaneous T-cell lymphoma. Blood. 1999;94(8):2854–61 (PMID: 10515889). [PubMed] [Google Scholar]
- 52.Kadin ME, Sako D, Morton C, Newcom SR, Su I-J. Characterization of a neoplastic T cell line from a patient with cutaneous T cell lymphoma and regressing T cell skin lesions. Lab Invest. 1988;58:45a–45a. [Google Scholar]
- 53.Kadin ME, Holt L, Nasu K, Najfeld V. Malignant histiocytosis: establishment and characterization of a neoplastic cell line, MH1. Lab Invest. 1984;50:29a–29a. [Google Scholar]
- 54.Saltman D, Morgan R, Cleary ML, de Lange T. Telomeric structure in cells with chromosome end associations. Chromosoma. 1993;102(2):121–8. 10.1007/BF00356029. (PMID: 8432193). [DOI] [PubMed] [Google Scholar]
- 55.Umiel T, Rettenmier CW, Siegel S, et al. Establishment and characterization of a human mixed-lineage, T-lymphoid/myeloid cell line (USP-91). Blood. 1993;82(6):1829–37 (PMID: 8400235). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, xenograft and cell lines that support the findings of this study are available from the corresponding authors upon reasonable request.