Abstract
Background
Pancreatic exocrine insufficiency (PEI) results from impaired exocrine function of the pancreas, causing distressing symptoms like steatorrhea, diarrhea, abdominal distention, bloating, and pain. Treatment involves pancreatic enzyme replacement therapy (PERT), yet compliance with clinical guidelines and its real-world effectiveness are unclear. This systematic review aimed to assess the concordance between real-world PERT doses and clinical guidelines and to evaluate the effectiveness of PERT doses, considering their average administration.
Methods
A systematic search of MEDLINE and EMBASE up to June 2023 identified observational studies reporting PERT doses and their effects on diarrhea and nutrition in PEI patients. Studies were classified based on adherence to current clinical guidelines for PERT dosing.
Results
Twenty-five observational studies involving 3818 patients met the inclusion criteria. In 40% of the studies, average PERT doses were lower than the recommended 40,000–50,000 lipase units (LU) per meal. Significant alleviation of diarrhea was observed in nearly all studies with lower-than-recommended doses, but none showed benefits in nutritional status. PERT doses compliant with guidelines helped reduce diarrhea in most studies and improved or maintained nutritional status.
Conclusions
This review revealed that real-world PERT doses were lower than European guidelines in 40% of studies. While lower doses alleviated gastrointestinal symptoms, they were insufficient for maintaining normal nutritional status. Therefore, PERT dosing should aim for nutritional improvement by adhering to guideline-recommended doses. Individualized dosing, considering both symptom management and nutritional status, is essential due to varying responses to treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-025-09011-0.
Keywords: Pancreatic enzyme replacement therapy, Pancreatic enzyme insufficiency, Clinical practice guidelines, Real-world data, Systematic review
Introduction
Pancreatic exocrine insufficiency (PEI) is a medical complication of pancreatic diseases, which results in maldigestion and malabsorption of nutrients due to reduced quantity or activity of secreted pancreatic enzymes [1]. Several conditions may be the primary cause of PEI, and the most probable are cystic fibrosis, acute/chronic pancreatitis (AP/CP), pancreatic cancer (PC), pancreatic duct obstruction, and pancreatic resection or surgery [2–4]. Less common etiologies of PEI include intestinal diseases (celiac or inflammatory bowel disease), states after gastrointestinal operations, genetic syndromes, and metabolic disorders (type 1 and 2 diabetes mellitus) [5]. The estimated prevalence of predisposition to PEI varies between conditions, from about 5% in patients with symptoms suggesting irritable bowel syndrome to nearly 100% in patients with inoperable pancreatic cancer [6, 7]. The prevalence of PEI appears to increase with the severity of the primary disease—PEI is observed in 30% of patients with mild changes of CP and 85% with severe changes of CP [3, 5, 8].
Due to nonspecific and varied symptoms, often confused with other gastrointestinal conditions, PEI remains underdiagnosed, especially in patients with mild manifestations or early stages of the disease [5]. In order to diagnose exocrine insufficiency in chronic pancreatitis, it is recommended that fecal elastase-1 (FE-1) activity be measured. The determination is performed on a single stool sample, with normal concentrations of the enzyme exceeding 200 μg/g of stool. An elastase-1 activity of less than 200 μg/g is indicative of mild exocrine pancreatic insufficiency, whereas a value of less than 100 μg/g, especially less than 50 μg/g, is indicative of severe exocrine pancreatic insufficiency [9, 10]. However, this test has limited specificity and sensitivity, particularly in cases of mild to moderate disease [11].
Diarrhea, steatorrhea, and weight loss are the hallmarks of PEI. Other common gastrointestinal symptoms of PEI include abdominal pain, flatulence, and distention [1, 6, 12]. Qualitative assessment of PEI symptoms can be performed using patient-reported outcome instruments [13].
In addition to clinical symptoms, several nutritional deficiencies have been described in PEI patients, including fat-soluble vitamins (A, D, E, K), minerals and trace elements (zinc, selenium, magnesium), and serum proteins (lipoproteins, prealbumin, retinol-binding protein) [14–16]. As a result of persistent malnutrition, PEI may lead to rare but severe complications, including osteoporosis, sarcopenia, impaired night vision, infections due to decreased immunity, coagulation problems, anemia, peripheral neuropathy, and ataxia [1, 6]. These malnutrition-related manifestations have been demonstrated to negatively impact a patient’s quality of life [12, 15]. Furthermore, malnourished PEI patients are believed to suffer more pain episodes and require more hospitalization than those with normal nutritional status [17]. Finally, severe malnutrition, including apolipoprotein A1 and lipoprotein A or C alterations, is a well-known risk factor for cardiovascular events [18, 19]. Therefore, PEI therapy should be focused not only on avoiding clinically relevant gastrointestinal symptoms but also on preventing weight loss and malnutrition.
The only known treatment for PEI is pancreatic enzyme replacement therapy (PERT), which is prandial, oral supplementation of exogenous pancreatic enzymes. The primary goal of PERT is to provide exocrine enzymes at a level considered sufficient to relieve clinical symptoms and improve the nutritional status of patients [2]. Modern formulations of PERT consist of pancreatic enzymes encapsulated in mini-microspheres or micro-pellets with an acid-protective coating designed to prevent enzymes from releasing in the stomach’s acid environment [10]. Until now, several medicinal products have been approved for marketing in PEI conditions (specific brand names differ in region/countries). Formulations differ in the composition of amylase, protease, and lipase amounts [20]. As lipase levels are believed to be the most crucial for normal nutritional status maintenance, the issue of proper PERT administration mainly focuses on lipase intake [2]. In the last decades, there has been no consensus on appropriate PERT dosing—doses recommended by clinical practice guidelines range from 10,000 lipase units (LU) for snacks up to 80,000 LU per main meal [21, 22]. In accordance with the European guidelines on chronic pancreatitis, the recommended enzyme dose is a minimum of 40,000–50,000 LU [23, 24]. Conversely, the Polish Society of Gastroenterology permits the commencement of enzyme therapy with 30,000–40,000 LU, which can be safely augmented up to 50,000 or even 75,000–80,000 LU per meal, contingent on the clinical response [10]. It is unexplored whether PERT administration in actual clinical routines has complied with these recommendations, as only a few studies have been published on this topic [25–27]. Secondarily, whether the recommended doses are clinically effective in resolving gastrointestinal and nutritional problems in PEI patients remains unknown.
To answer these questions, we performed a systematic review of real-world data to assess the compliance of real-world PERT dosing with the guidelines and evaluate its effects on improving gastrointestinal symptoms and nutritional status in PEI patients.
Methods
Search Strategy
The systematic review followed the PRISMA guidelines [28], and the review protocol was registered in PROSPERO (CRD42023434776). A systematic search of major medical databases, including MEDLINE (via Pubmed) and EMBASE, was conducted to identify real-world evidence published until June 2023. Keywords for the MEDLINE and EMBASE search included queries for PEI and related diseases, PERT, and observational studies combined with appropriate Boolean operators. Detailed search strategies are presented in the supplementary appendix. Additionally, references of the included studies were also screened.
Inclusion and Exclusion Criteria
Observational studies published in English were eligible if they reported data on PEI patients treated with PERT in a real-world setting, provided dosage information expressed in LU per meal or day (regardless of the type of pharmacopeia, as 1 FIP/PhEur = 1 USP for lipase [29]), and provided data on patients’ nutritional status, diarrhea, or other clinical outcomes. Studies performed exclusively in children were not included. Similarly, studies focusing on patients with cystic fibrosis were also excluded, as they primarily involve pediatric patients and require a separate analysis due to disease-specific guidelines and significantly different PERT dosing recommendations. Additionally, studies published only as conference proceedings were not included.
Study Selection and Quality Assessment
Two independent analysts screened database records and full-text articles at each stage of the selection process. Two reviewers also independently extracted information about PERT doses and treatment effects from included studies. Any discrepancies between reviewers on the selection and extraction process were resolved by consensus. The quality of the included studies was assessed according to the criteria proposed by NICE for case series or by the Newcastle–Ottawa Scale (NOS) for cohort and cross-sectional studies [30, 31].
Outcomes of Interest and Statistical Analysis
The dosage of PERT expressed in LU was the primary endpoint of this analysis. An average dose of PERT was defined as the mean or the median dose reported by authors or calculated from the number of PERT capsules received. If PERT doses were reported for several subgroups, an average dose for the entire study population was calculated by aggregating data from these subgroups, if possible.
For the assessment of concordance between real-world lipase intake and clinical practice guidelines, the United European Gastroenterology (HaPanEU/UEG) guidelines were used [24]. Although this document was specifically developed for the diagnosis and management of chronic pancreatitis, its recommendations were extrapolated by the authors of this review to all analyzed PEI indications. This approach is justified for two reasons: firstly, no other relevant European clinical guidelines for all PEI conditions had been published in recent years, and secondly, these recommendations were based on several previous world clinical guidelines and clinical trials dedicated to a broad spectrum of PEI disorders. After the completion of the systematic review and manuscript preparation, updated UEG guidelines for PEI were published (December 2024) [32]. While these recent guidelines did not specify recommended dosing range stating that the initial doses of PERT vary mainly depending on the patient’s age [32], the doses we adopted (as outlined below) were confirmed by the guidelines as effective in adult patients, further supporting our approach.
The HaPanEU/UEG guideline recommends a minimum PERT dose of 40,000–50,000 LU for main meals, and these doses may be doubled or even tripled in case of unsatisfactory clinical response [24]. As a result, it has been proposed that recommended PERT dosing ranges between 40,000 and 150,000 LU/meal, corresponding with 120,000–450,000 LU/day, assuming that PERT is supplemented three times daily with main meals. Compliance of average PERT doses with HaPanEU/UEG recommendations was analyzed as a dichotomous variable (yes/no) [24]. A four-grade scale was developed to assess the compatibility between real-world and recommended lipase intake (Table 1).
Table 1.
PERT dose compatibility scale
| Compliance | Available measurements in studies | |
|---|---|---|
| Mean or median | Range | |
| YES | ||
| Complete | The mean/median dose of PERT in a study is within the ranges of recommended doses by clinical guidelines | Ranges of doses used in a study are within ranges recommended by guidelines |
| Partial | NA | Ranges for real-world doses and recommended doses overlap |
| NO | ||
| Lower | The mean/median dose of PERT in a study is below the minimum recommended by guidelines | Ranges for real-world doses in a study are lower and do not overlap with ranges for recommended doses |
| Higher | The mean/median dose of PERT in a study is above the maximum recommended by guidelines | Ranges for real-world doses are higher and do not overlap with ranges for recommended doses |
NA not applicable, PERT pancreatic enzyme replacement therapy
Diarrhea and nutritional status were analyzed as secondary endpoints. Other outcomes, including body weight, various gastrointestinal symptoms, quality of life, and overall survival, were also extracted, if available. Unless otherwise specified, PERT doses and continuous effect measurements were presented as mean and range. Dichotomous endpoints were analyzed as a percentage of patients with an event. A meta-analysis of clinical outcomes was initially planned; however, due to the limited number of studies for each outcome and their heterogeneity, it was not feasible to conduct. The summary of PERT effectiveness was based on p values reported in studies and the authors’ conclusions.
Results
Study Selection and Characteristics
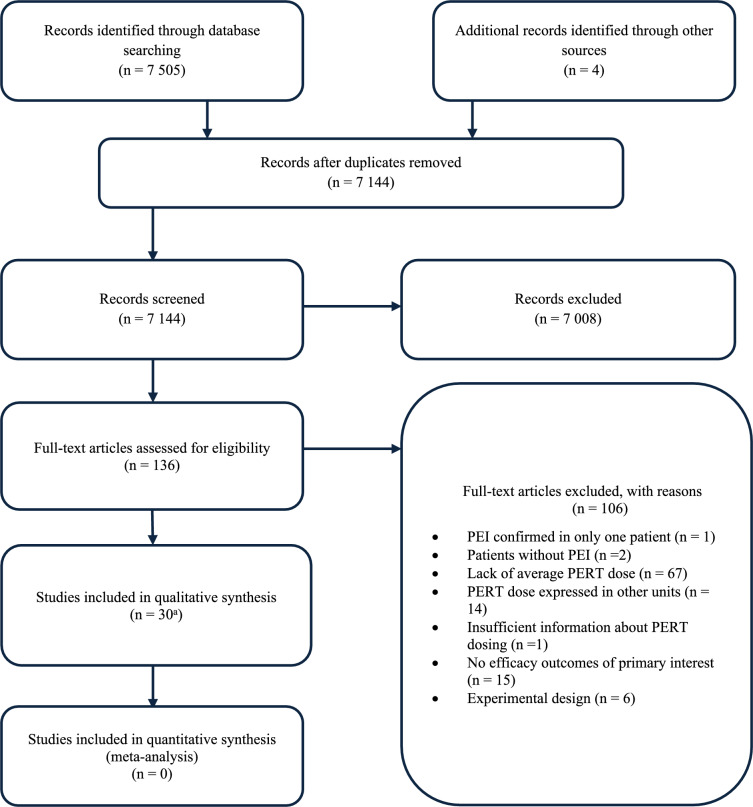
The search of MEDLINE and EMBASE retrieved 7509 records, and after removing duplicates, 7144 records were qualified for further assessment. Based on titles and abstracts, 7008 papers were discarded because they did not match the predefined inclusion criteria, and 136 full-text articles were assessed for eligibility. Finally, 30 studies were considered relevant for this review [33–62]. The main reasons for exclusion are presented in the PRISMA flow diagram (Fig. 1), while the list of excluded studies is shown in the supplementary appendix.
Fig. 1.
PRISMA flow diagram
A total number of 3818 patients with PEI from 30 studies (30 publications; one publication described two original studies, and one study was described in two papers) were included in this analysis (Table 2). Eight studies were performed in chronic pancreatitis patients, three in pancreatic cancer, and eight in patients after pancreatectomy. The remaining nine studies were conducted in patients with various underlying causes of PEI, including HIV infection, hepatocellular carcinoma, irritable bowel syndrome, celiac disease, and states after esophagectomy, among others. Most of the included studies were prospective (20 studies), single-center (24 studies), and conducted in Europe (19 studies). The remaining studies were performed in the United States (n = 4), Japan (n = 2), India (n = 3), Argentina (n = 1), and South Korea (n = 1). Sample sizes were relatively small, and only nine studies reported data on more than 100 patients. Most studies did not provide the trade name and type of PERT formulation. Detailed characteristics of included studies are described in the supplementary appendix.
Table 2.
| Indication | No. of studies | P/R/CS | S/M | No. of patients | Study size [pts] |
|---|---|---|---|---|---|
| Pancreatic exocrine insufficiency (PEI) | 11 | 7/4/0 | 11/0 | 693 | 20–210 |
| Chronic pancreatitis (CP) | 8 | 6/0/2 | 6/2 | 1889 | 29–987 |
| Pancreatectomy (PY) | 8 | 6/1/1 | 5/3 | 875 | 25–355 |
| Pancreatic cancer (PC) | 3 | 1/2/0 | 2/1 | 361 | 91–160 |
| All | 30 | 20/7/3 | 24/6 | 3818 | 20–987 |
CS cross-sectional, M multi-center, P prospective, R retrospective, S single center
The methodological quality of the included studies was highly heterogeneous. Scores for case series ranged from 4 to 7 points, according to the NICE questionnaire. The most common reasons for lower quality scores included retrospective design, single-center origin, and lack of consecutiveness. The quality of the cohort and cross-sectional studies assessed by NOS was good. None of the studies was excluded from this analysis due to their low quality.
Average Doses of PERT
Average PERT doses varied broadly between individual studies, ranging from 15,000 to 96,000 LU/meal. An average dose of PERT in chronic pancreatitis patients was between 20,000 and 96,000 LU/meal. In patients with pancreatectomy, an average PERT dose was 25,000–66,000 LU/meal; however, in some post-pancreatectomy individuals, lipase intake was escalated even to 216 000 LU/meal [43]. Patients with pancreatic cancer and various PEI conditions received an average of about 25,000–75,000 LU/meal (Table 3).
Table 3.
Average doses of PERT—compliance with HaPanEU/UEG
| Study | Indication | N | PERT doses | Compliance with HaPanEU/UEG | References | |
|---|---|---|---|---|---|---|
| Mean or median* | Range or IQR** | |||||
| LU/meal | ||||||
| Naffouje 2018 | PEI | 9 | NDA | 20,000–40,000 | YES (partial) | [33] |
| Yilmaz 2018 | PEI | 12 | 50,000 | NDA | YES (complete) | [34] |
| Huddy 2013 | PEI | 22 | 25,000 | NDA | NO (lower) | [36] |
| Kiefer 2018 | PEI | 23 | NDA | 25,000–50,000 | YES (partial) | [37] |
| Rinzivillo 2020 | PEI | 7 | NDA | 40,000–50,000 | YES (complete) | [38] |
| Saif 2020 | PEI | 19 | 72,000 | 72,000–72,000 | YES (complete) | [39] |
| Shandro 2020 | PEI | 103 | 50,000* | 10,000–100,000 | YES (complete) | [40] |
| Diaz-Gonzalez 2021 | PEI | 6 | 25,000 | 25 000–25,000 | NO (lower) | [56] |
| Desai 2023 | PEI | 50 | 15,000 | NDA | NO (lower) | [58] |
| Olmos 2022 | PEI | 7 | 50,000 | NA | YES (complete) | [59] |
| Struyvenberg 2017 | PY | 39 | 66,000* | 9000–216,000 | YES (complete) | [43] |
| Saluja 2019 | PY | 16 | 25,000 | NDA | NO (lower) | [47] |
| Kim 2018 | PY | 47 | NDA | 40,000–80,000 | YES (complete) | [46] |
| Latenstein 2021 | PY | 62 | NDA | 25,000–56,250 | YES (partial) | [60] |
| Dominguez-Munoz 2010 | CP | 21 | 20,000* | 20,000–60,000 | NO (lower) | [49] |
| Dominguez-Munoz 2007 | CP | 29 | 40,000 | NDA | YES (complete) | [50] |
| Min 2018 | CP | 77 | 96,000 | NDA | YES (complete) | [51] |
| Dieguez-Castillo 2020 | CP | 28 | 50,000 | 50,000–50,000 | YES (complete) | [52] |
| Arutla 2021 | CP | 201 | 24,694a | 10,000–40,000 | NO (lower) | [57] |
| Dominguez-Munoz 2007 | CP | |||||
| 1st year | 30 | 20,000* | 0–40,000 | NO (lower) | [50] | |
| 2nd year | 20 | 40,000* | 40,000–60,000 | YES (complete) | [50] | |
| Saito 2017 | PC | 46 | 48,000 | NDA | YES (complete) | [54] |
| Dominguez-Munoz 2018 | PC | 49 | 75,000* | NDA | YES (complete) | [55] |
| LU/day | ||||||
| Yilmaz 2018 | PEI | 12 | 150,000 | NDA | YES (complete) | [34] |
| Evans 2010 | PEI | 20 | 45,000 | 10,000–120,000 | NO (lower) | [35] |
| Diaz-Gonzalez 2021 | PEI | 6 | 75,000 | 75,000–75,000 | NO (lower) | [56] |
| Desai 2023 | PEI | 50 | 45,000 | NDA | NO (lower) | [58] |
| Barbier 2013 | PY | 25 | 150,000* | 75,000–450,000 | YES (complete) | [42] |
| Crippa 2011 | PY | 65 | 80,000* | 30,000–160,000 | NO (lower) | [44] |
| Suzuki 2016 | PY | 41 | 125,145 | NDA | YES (complete) | [45] |
| Latenstein 2021 | PY | 62 | NDA | 75,000–181,250 | YES (partial) | [60] |
| Kroon 2022 | PY | 25 | 150,000* | NDA | YES (partial) | [61] |
| 21 | 112,500* | NDA | ||||
| D’Haese 2014 | CP | 294 | 94,630a | NDA | NO (lower) | [48] |
| Dominguez-Munoz 2007 | CP | 29 | 120,000 | NDA | YES (complete) | [50] |
| Kempeneers 2020 | CP | 266 | 100,000* | 75,000–175,000** | NO (lower) | [53] |
| Dominguez-Munoz 2018 | PC | 49 | 325,000* | 200,000–400,000 | YES (complete) | [55] |
| Trestini 2021 | PC | 55 | 80,000* | NDA | NO (lower) | [62] |
CP chronic pancreatitis, IQR Interquartile Range, NDA no data available, PEI pancreatic enzyme insufficiency, PY pancreatectomy
aOwn calculations
*Median, **IQR
Daily Doses of PERT
In most PEI studies, patients received between 80,000 and 150,000 LU/day, which translates to 26,000–50,000 LU/meal, assuming daily doses correspond with lipase supplementation for three main meals per day. A significant deviation from the pattern was observed in three studies, in which the median PERT doses were 45 000 or 325,000 LU/day [23, 35, 58]. In some individuals, daily lipase supplementation did not exceed 10,000 LU, while in others, even 450,000 LU needed to be administered (Table 3) [35, 42].
Compliance of Average Doses of PERT with Clinical Practice Guidelines
Compliance of average PERT doses with HaPanEU/UEG guidelines is presented in Table 3. Complete and partial compliance were confirmed in thirteen and three studies in which lipase intake was expressed in LU/meal. Average PERT doses in seven studies were distinctly lower than recommended. A similar distribution of compliance was observed in studies where PERT doses were expressed in LU/day; however, this conclusion needs to be interpreted with caution due to methodological simplification. Average PERT doses did not exceed those recommended by guidelines in any study.
Clinical Efficacy of Average Doses of PERT
Doses Lower Than Recommended
Diarrhea was evaluated in 6 out of 12 studies with average PERT doses lower than recommended by European guidelines. In five studies, lower PERT doses reduced diarrhea [35, 36, 44, 48, 56]. In the Arutla 2021 study, no differences were observed in the diarrhea rates between chronic pancreatitis patients who were compliant with their prescribed PERT dose, those who were non-compliant, and those who were not receiving PERT [57]. However, as the study was designed as cross-sectional, it is unclear if prescribed PERT doses had no therapeutic effect or if other factors influenced these results.
The benefits of PERT use were also reported on other gastrointestinal symptoms, including lowered levels of FE1 and reduced steatorrhea [35, 36, 44, 47, 48, 53, 58, 62]. Lower doses of PERT could stabilize weight or lead to weight gain in patients from 6 of 10 studies.
Weight results were mixed in the remaining four studies, with about half of the patients experiencing weight gain or stabilization and the other half experiencing weight loss or no significant difference in weight between PERT and no enzyme supplementation [44, 47, 53, 57]. In one study, lower doses of PERT improved patients’ quality of life, which can be explained by reduced abdominal pain [48]. However, no difference was observed in the quality of life in studies comparing lower-than-recommended doses of PERT with no-PERT [53, 57].
Regardless of the positive impact on gastrointestinal symptoms, lower doses of PERT were insufficient to improve patients’ nutritional status (Table 4) [48–50].
Table 4.
Summary of PERT average doses and their efficacy
| Study | PERT | Compl. | N | Diarrhea | Nutrition | General | Weight | Steatorr. | Other GI | QoL | Survival | Conclusions | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D’Haese 2014 | 94 630 LU/day | Lower | 294 | ✓ | X | NR | ✓ | ✓ |
✓ (pain) |
✓ | NR | PERT was effective in reducing GI symptoms and weight loss as well as in improving QoL; however, it was not enough in reducing malabsorption | [48] |
| Evans 2010 | 45 000 LU/day | Lower | 20 | ✓ | NR | ✓ | ✓ | NR |
✓ (FE1) |
NR | NR | PERT was effective in weight stabilization, normalizing FE1, reducing stool frequency as well as in resolving all symptoms in some patients | [35] |
| Huddy 2013 | 25 000 LU/meal | Lower | 10 | ✓ | NR | NR | ✓ | ✓ | NR | NR | NR | PERT was effective in increasing weight and providing symptomatic benefits in diarrhea/steatorrhea | [36] |
| Dominguez-Munoz 2007 | 20 000 LU/meal | Lower | 30 | NR | X | NR | NR | NR | NR | NR | NR | PERT was too low to improve nutritional status measured by a breath test | [50] |
| Dominguez-Munoz 2010 | 20 000 LU/meal | Lower | 21 | NR | X | NR | NR | NR | NR | NR | NR | PERT was not enough to prevent malnutrition in about half of the patients | [49] |
| Crippa 2011 | 80 000 LU/day | Lower | 65 | ✓ | NR | NR | = | ✓ |
= (pain) |
NR | NR | PERT was effective in weight gain/stabilization in about half of the patients and in reducing steatorrhea and diarrhea in most of the patients; abdominal pain was still present in 22% of the patients | [44] |
| Saluja 2019 | 25 000 LU/meal | Lower | 16 | NR | NR | NR | = | ✓ | NR | NR | NR | PERT was effective in weight gain/stabilization in about 44% of the patients and reducing steatorrhea in all of them | [47] |
| Kempeneers 2020 | 100 000 LU/day | Lower | 266 | NR | NR | NR | = | NR | ✓ | = | NR | PERT was effective in reducing GI symptoms compared to lack of PERT, but groups were similar in terms of weight and QoL | [53] |
| Diaz-Gonzalez 2021 | 25 000 LU/meal | Lower | 6 | ✓ | NR | NR | ✓ | NR |
✓ (FE1) |
NR | NR | PERT resolved diarrhea/weight loss in all of the patients and restored FE1 levels in 83% of them | [56] |
| Arutla 2021 | 24 694 LU/meal | Lower | 201 | = | = | NR | = | = |
= (pain) |
= a | NR | No differences between PERT and no-PERT groups were observed, as well as between compliant and non-compliant PERT groups | [57] |
| Desai 2023 | 15 000 LU/meal | Lower | 50 | NR | = | NR | ✓ | NR | ✓ | NR | NR | PERT was effective in stabilizing weight and improving GI symptoms in some of the patients. Mean values of albumin and hemoglobin also increased; however, they are below population norms | [58] |
| Trestini 2021 | 80,000 LU/day | Lower | 55 | NR | NR | NR | ✓ | NR | ✓ | NR | ✓ | PERT was effective in improving GI symptoms in PEI patients; survival of patients with PERT also improved compared with lack of PERT | [62] |
| Naffouje 2018 | 20–40,000 LU/meal | Partial | 9 | ✓ | NR | NR | NR | NR | NR | NR | NR | PERT was effective in resolving diarrhea in nearly all of the patients | [33] |
| Kiefer 2018 | 25–50,000 LU/meal | Partial | 23 | NR | NR | NR | ✓ | ✓ | NR | NR | NR | PERT was effective in resolving steatorrhea as well as helped to gain weight in some patients | [37] |
| Latenstein 2021 | 25–56 250 LU/meal | Partial | 62 | NR | NR | ✓ | NR | NR | NR | NR | NR | PERT resolved or decreased PEI complaints in 81% of patients | [60] |
| Kroon 2022 | 112,5–150 000 LU/day | Partial | 46 | NR | NR | ✓ | NR | NR | NR | NR | NR | PERT was effective in resolving symptoms in 44–70% of patients | [61] |
| Barbier 2013 | 150 000 LU/day | Complete | 25 | = | NR | NR | X | NR | NR | X | NR | PERT did not prevent weight loss in 60% of the patients and diarrhea in 20%; QoL was decreased due to fatigue and diarrhea | [42] |
| Suzuki 2016 | 125 145 LU/day | Complete | 41 | ✓ | ✓ | NR | ✓ | NR | NR | NR | NR | PERT was effective in stabilizing BMI, reducing diarrhea, and normalizing albumin level | [45] |
| Saito 2017 | 48 000 LU/meal | Complete | 46 | NR | ✓ | NR | ✓ | NR | NR | NR | ✓ | PERT was effective in stabilizing body weight, maintaining nutritional status, and improving overall survival compared to a lack of PERT | [54] |
| Yilmaz 2018 | 50 000 LU/meal | Complete | 12 | ✓ | NR | ✓ | NR | NR | NR | NR | NR | Nearly all of the patients receiving PERT became asymptomatic or experienced a reduction of diarrheas | [34] |
| Dominguez-Munoz 2007 | 40,000 LU/meal | Complete | 29 | NR | ✓ | NR | NR | NR | NR | NR | NR | PERT was able to improve nutritional status (expressed as an increase in CFA and breath test results) as well as to normalize these outcomes at a numerically high rate in the patients | [50] |
| Dominguez-Munoz 2007 | 40 000 LU/meal | Complete | 20 | NR | ✓ | NR | ✓ | NR | NR | NR | NR | PERT was effective in gaining weight and improving nutritional status in all of the patients | [50] |
| Min 2018 | 96 000 LU/meal | Complete | 77 | NR | NR | NR | NR | ✓ | NR | NR | NR | PERT was effective in resolving steatorrhea in 90% of CP patients | [51] |
| Kim 2018 | 40–80 000 LU/meal | Complete | 47 | NR | ✓ | NR | NR | NR | NR | NR | NR | PERT was effective in improving or maintaining nutritional status in 74% of patients | [46] |
| Dieguez-Castillo 2020 | 50 000 LU/meal | Complete | 28 | NR | = | NR | = | NR |
= (FE1) |
NR | NR | No differences were observed between the PERT and no-PERT groups in terms of weight, vitamin deficiency, or FE-1 levels | [52] |
| Rinzivillo 2020 | 40–50 000 LU/meal | Complete | 4 | NR | NR | NR | NR | NR | ✓ | NR | NR | PERT was effective in resolving abdominal symptoms in all of the patients | [38] |
| Dominguez-Munoz 2018 |
325 000 LU/day |
Complete | 40 | NR | NR | NR | NR | NR | NR | NR | ✓ | PERT was effective in improving overall survival in patients with baseline weight loss compared to no-PERT | [55] |
| Saif 2020 | 72 000 LU/meal | Complete | 19 | ✓ | X | NR | ✓ | NR | ✓ | NR | NR | PERT was effective in improving GI symptoms and reversing weight loss, but vitamin/mineral deficiencies were still observed in individuals | [39] |
| Shandro 2020 | 50 000 LU/meal | Complete | 81 | NR | NR | ✓ | NR | NR | ✓ | NR | NR | PERT was effective in improving GI symptoms, and 65% responded to treatment | 40] |
| Olmos 2022 | 50 000 LU/meal | Complete | 7 | NR | NR | NR | NR | NR | ✓ | NR | NR | PERT was effective in improving GI symptoms, including stool scores, distention, and pain | [59] |
✓ PERT dose enough to improve or stabilize symptoms, X PERT dose not enough to improve symptoms; = mixed results, NR not reported.
Not shown above: results from Struyvenberg 2017 study—clinical efficacy of PERT was dose-dependent.
aAccording to the authors, there is no difference in the score between patients on PERT and those without (no numerical data was presented).
Doses Compliant with Guidelines
Ten of eighteen studies assessed gastrointestinal symptoms with complete or partial compliance of PERT doses with the recommended guidelines. In four studies, average PERT doses effectively improved diarrhea, and in two studies, they improved steatorrhea in PEI patients. The recommended 150,000 LU/day was insufficient to relieve gastrointestinal symptoms, including diarrhea, and to prevent weight loss in two studies [42, 52]. Nutritional status was evaluated in seven studies, where doses equal to or higher than 40,000 LU/meal led to maintenance or improvement of nutritional status, expressed as albumin level or retinol-binding protein level. Improvements were also observed in fat digestion measured by CFA and breath tests [39, 45, 46, 49, 50, 52, 54].
In five studies, PERT doses recommended by the HaPanEU/UEG were also effective in preventing weight loss and in four studies in improving general PEI symptoms [34, 37, 39, 41, 45, 50, 54, 58, 62]. In two studies, PERT therapy improved patients’ overall survival compared to a lack of enzyme supplementation [54, 55]. Detailed results are presented in the supplementary appendix.
Another study (Struyvenberg 2017) showed that PERT efficacy increases with higher doses [43]. Rates of diarrhea, steatorrhea, abdominal distention, gas, and bloating were much lower in patients with doses > 96,000 LU/meal compared with those who received doses ≤ 36,000 LU/meal (data shown in the supplementary appendix) [43].
Discussion
This study is the first systematic review investigating PERT dosing and its real-world effectiveness in resolving gastrointestinal symptoms and improving nutritional status. Adequate lipase supplementation is necessary to improve the quality of a patient’s life by reducing gastrointestinal symptoms. Confirmation of response to PERT is mainly obtained by reducing PEI-specific symptoms like diarrhea, steatorrhea, abdominal pain, and flatulence and avoiding weight loss. However, according to the extensive literature, optimal therapy of PEI should not only relieve gastrointestinal symptoms but also normalize digestion and ensure normal nutritional status on a biochemical level to prevent secondary complications of malnutrition [49, 50, 63–65].
There is no consensus on the appropriate dose of PERT for patients with PEI. Some gastrointestinal societies recommend administering 25,000 LU/meal, while others consider 72,000 LU/meal the minimum [4, 10, 21, 66]. For example, Australasian guidelines suggest initiating treatment with the lowest recommended dose of 25,000–40,000 LU per meal for patients with PEI [66]. In contrast, British guidelines recommend starting with at least 50,000 LU per meal and 25,000 LU per snack with patients encouraged to adjust their dose if ineffective [67]. The most recent UEG guidelines state that initial PERT dosing depends primarily on patient age (adult or child), PEI severity, and meal fat content. The most recent UEG guidelines state that initial PERT dosing depends primarily on patient age (adult or child), PEI severity, and meal fat content. However, doses of 40,000–50,000 LU per meal have been recognized as clinically effective [32]. The guidelines used in our analysis (HaPanEU/UEG 2018) recommended a wide range of lipase intake, 40,000–150,000 LU/meal, which translates to about 120,000–450,000 LU/day [23]. Average lipase intake was lower than the recommended 40,000 LU/meal in 40% of the studies included in this analysis. This observation is supported by recent analyses of PERT administration patterns in the US, showing that about 31% of chronic pancreatitis and 27.5% of pancreatic cancer patients who initiated PERT were prescribed doses ≥ 40,000 LU/meal [27, 68]. Another analysis performed by Barkin et al. revealed that 36% of members of the Inspire Pancreatitis or Pancreatic Cancer Support communities were prescribed suboptimal doses of PERT < 40,000 LU per meal [69].
Adherence to PERT treatment seems to be higher in Europe compared to the USA. Recent studies showed that 85.6% of Swedish patients were given PERT according to the HaPanEU/UEG guidelines, while in the Netherlands, 76.3% of patients with PEI initiated PERT [25, 26]. However, there is still an issue with adjusting the PERT dose—available data showed that the dose is not increased even in cases of enzyme ineffectiveness in 37.9% of patients [25].
Several reasons may explain the observed non-compliance with guidelines in real-world practice. Firstly, previous practice guidelines, particularly local and regionally limited, recommended slightly lower lipase doses than European guidelines [21, 22]. For example, the guidelines of the Polish Society of Gastroenterology and the Polish Pancreatic Club, developed by the co-authors of this systematic review, recommended 30,000–40,000 LU per meal in the treatment of chronic pancreatitis [10], which, in light of the results of this systematic review, may be insufficient. Therefore, in the case of many local guidelines, it may be necessary to revise the current recommendations toward increasing the recommended initial PERT dose. Secondly, physicians might initiate lower doses and gradually increase them if there is no improvement in gastrointestinal symptoms. A third potential explanation may be the cost of PERT. According to the analysis by Prescott et al., in 2016, 37% of prescriptions for PERT in the USA were never filled [70]. Physicians may prescribe lower doses of PERT to reduce costs and not discourage patients from taking therapy for financial reasons.
Notably, the latest data indicate that adequate PERT doses were more commonly prescribed by pancreas specialists (82.8%) compared with primary care physicians (29.2%) and gastroenterologists (55.0%) [68]. Better dosage adjustment by more qualified specialists may be associated with greater knowledge and consideration of gastrointestinal symptoms and nutritional status in PEI patients.
This systematic review confirmed that various PERT doses have been used in the last two decades. Nonetheless, prandial doses of PERT rarely exceeded 50,000 LU. Average enzyme supplementation in included studies ranged between 20,000 and 96,000 LU/meal and 45,000–325,000 LU/day. In most of the included studies, lower-than-recommended doses (< 40 000 and < 120 000 LU/day) were sufficient to improve PEI symptoms, including defecation urgency and consistency and weight loss, but could not prevent malabsorption and malnutrition.
However, it is uncertain if deficient lipase intake helps resolve gastrointestinal symptoms. In the Evans 2010 study, 45,000 LU/day was sufficient to reduce diarrhea in 90% of celiac patients, almost three times less than the recommended dose [35]. It has to be noted that PEI diagnosis in these patients was obtained solely by lowered FE1 concentration, which often gives a false-positive result [71]. According to the authors, low concentrations of FE1 in celiac patients may be an artifact resulting from stool dilution characteristic of this condition [35]. Then, it is possible that part of the study population was misdiagnosed with PEI, and their symptoms improved not due to lipase supplementation but because of diet modification leading to stool concentration. Additionally, adherence to a gluten-free diet promotes villous recovery [72], which may further contribute to symptom resolution. Regarding the uncertainties mentioned above, a minimal dose of PERT effective in resolving gastrointestinal symptoms seems to be 25,000 LU/meal. Unfortunately, this dose appears insufficient to improve patients’ nutritional status.
The utilization of PERT plays a crucial role in maintaining optimal nutritional status in PEI patients. The latest research showed that patients with chronic pancreatitis who did not receive PERT had a significantly higher prevalence of vitamin E deficiency (75 vs. 0%, p = 0,012) and osteoporosis (43 vs. 5.6%, p = 0,013) than those receiving PERT. Notably, 75% of osteoporosis cases in the PERT group occurred in patients with inadequate dosing [73]. Our systematic review showed that despite the gastrointestinal symptom benefits obtained by lower-than-recommended doses of PERT, abnormally low nutritional parameters are detected in up to 70% of patients. Doses recommended by the European guidelines, 40,000–50,000 LU/meal, were generally effective in symptomatic benefit and improving or maintaining nutritional status.
For example, in the study by Yilmaz et al., 75% of patients treated with 50,000 LU/meal observed an improvement in diarrhea, with 25% becoming asymptomatic. Unfortunately, nutritional status was not evaluated in this study [34]. Results from other studies showed that a lipase intake of 40,000 LU/meal led to CFA normalization in 66% of patients, while a median dose 20 000 LU/meal was linked with persistent malnutrition in 67% of patients, which was reversed in all patients by doubling the PERT dose [49, 50].
It was necessary to increase the PERT dose during the study course from the initial dose in other included studies (e.g., [42, 45]). However, in one study, even doses recommended by guidelines (150 000 LU/day) were suboptimal, as 68–74% of patients complained about steatorrhea, and weight loss and fat restriction were present in 39–60% and 40–58% of patients, respectively [42, 45]. These findings confirmed that the initial recommended doses are insufficient in some PEI patients. Therefore, the European guidelines allow for an increase of up to 150,000 LU/meal [23]. Nonetheless, according to the results of this systematic review, this does not seem to be a common practice.
In only four of the included studies, an average lipase intake exceeded the minimum recommended doses (40,000–50,000 LU/meal). In the remaining studies, despite suboptimal treatment response, the average lipase intake did not exceed the minimum recommended doses. As we know, enzyme doses should be individually adjusted based on the severity of symptoms, residual pancreatic function, enteric physiology, patient’s age, primary condition, and dietary fat intake [43, 74].
In the included studies, there was a tendency for higher doses of PERT to provide better control of PEI symptoms than lower doses. These outcomes are supported by results from Struyvenberg 2017 study, in which steatorrhea, bloating, abdominal distention, and gas decreased with high doses of pancreatic enzymes (> 36 000, > 96 000 LU/meal) compared with low doses (≤ 36 000 LU/meal), and in which low lipase intake was significantly related to unintended weight loss [43].
Many reasons other than suboptimal dose may also be responsible for the insufficient response to PERT, including those associated with patient compliance, such as an inadequate diet with high-fat content, alcohol abuse, sporadic lipase intake, or incorrect timing of administration [48]. Other factors include acidic intestinal pH, other intestinal diseases, and using acid-sensitive or macrosphere preparations that do not mix effectively with chyme [74]. According to recent guidelines, recommended enzyme formulations should contain mini-microspheres and micro-pellets with dimensions less than 2 mm [23]. It has been shown that formulations with microspheres or pellets with a diameter of 1.0–1.2 have 25% higher therapeutic efficacy than those with a diameter of 1.8–2.0 mm [10]. Also, enteric-coated formulations of PERT are generally preferred to avoid acid-mediated inactivation lipase in the stomach [10, 23]. Hence, patients with inadequate PERT benefits should first ensure that none of the above factors apply to the observed lack of efficacy and then consider increasing the lipase dose. Some authors also conclude that the efficacy of PERT is higher when it is self-dosed flexibly rather than fixed by physicians [75]. It is possible that patients with suboptimal responses were not instructed to adjust their lipase intake.
This systematic review has some limitations, mainly due to the heterogeneity of studies and sample size. No meta-analysis could be performed due to patient characteristics, study design, and follow-up discrepancies. Many of the included studies were not primarily designed to evaluate or determine the efficacy of the lipase dosing. Notably, the trade name of the enzyme formulation was not mentioned in most studies, so it is also unknown if macrosphere or microsphere formulations were used. Moreover, values of average PERT doses were obtained mainly from medical records, physicians, and investigators, not directly from patients. How many patients self-modified their prescribed dose to achieve the desired response is uncertain.
The limitations of this systematic review also extend to the parameters used for assessment and the lack of comprehensive information in the included studies regarding patients’ dietary habits. Nutritional status assessment presents challenges, particularly due to the use of albumin as a marker, which is not a reliable indicator in conditions with chronic inflammation, as its levels may be influenced by disease-related factors rather than actual malnutrition [76].
Moreover, the included studies often lacked detailed data on key factors influencing nutritional status, such as inadequate caloric intake, uncontrolled diabetes, and micronutrient deficiencies. Variations in dietary intake—such as frequent small meals, skipped meals, or reliance on fortified foods and nutritional supplements—can significantly impact enzyme requirements, making standardized dosing approaches less applicable to all patients. However, despite these limitations, this systematic review provided essential insight into real-world PERT dosing and its effectiveness.
To conclude, our systematic review revealed that the real-world doses of PERT were lower than recommended by European guidelines in 40% of identified studies. While lower doses (< 40,000 LU/meal) were found to alleviate gastrointestinal symptoms in most studies, they were insufficient to ensure the normal nutritional status of PEI patients. These findings suggest that relying solely on gastrointestinal symptoms may not be optimal when determining PERT dosing. Instead, the goal should be to achieve nutritional improvement, which requires adhering to guideline-recommended doses. Due to various factors affecting response to treatment, appropriate PERT doses should be established individually, considering symptom management and nutritional status.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The study was sponsored by Viatris, which provided funding for the study implementation and publication fee. The company had no influence on the study methodology, results, or conclusions.
Abbreviations
- AP
Acute pancreatitis
- CP
Chronic pancreatitis
- CFA
Coefficient of fat absorption
- FE1
Fecal elastase-1
- LU
Lipase units
- NOS
Newcastle–Ottawa Scale
- PEI
Pancreatic enzyme insufficiency
- PERT
Pancreatic enzyme replacement therapy
Author Contributions
Conceptualization: Roland Kadaj-Lipka, Grażyna Rydzewska, Magdalena Monica, Anita Stożek-Tutro, and Przemysław Ryś; Methodology: Magdalena Monica, Anita Stożek-Tutro, and Przemysław Ryś; Database searches and evidence selection: Magdalena Monica and Anita Stożek-Tutro; Formal analysis and investigation: Magdalena Monica, Anita Stożek-Tutro, and Przemysław Ryś; Writing—original draft preparation: Magdalena Monica, Anita Stożek-Tutro, and Przemysław Ryś; Writing—review and editing: Roland Kadaj-Lipka and Grażyna Rydzewska; Funding acquisition: Przemysław Ryś; Resources: Magdalena Monica, Anita Stożek-Tutro, and Przemysław Ryś; Supervision: Roland Kadaj-Lipka, Grażyna Rydzewska, and Przemysław Ryś. All authors read and approved the final manuscript.
Funding
The study was sponsored by Viatris.
Data Availability
Data Sharing Statement: All data that support the findings of this study have been included in the article and its supplementary materials.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stevens T, Darwin L. Exocrine pancreatic insufficiency. 2018 [cited 2021 Feb 9]. https://www.uptodate.com/contents/exocrine-pancreatic-insufficiency
- 2.Forsmark CE. Diagnosis and management of exocrine pancreatic insufficiency. Current Treatment Options in Gastroenterology. 2018;16:306–315. [DOI] [PubMed] [Google Scholar]
- 3.Lévy P, Domínguez-Muñoz E, Imrie C, Löhr M, Maisonneuve P. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterology Journal. 2014;2:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabater L, Ausania F, Bakker OJ, Boadas J, Domínguez-Muñoz JE, Falconi M et al. Evidence-based guidelines for the management of exocrine pancreatic insufficiency after pancreatic surgery. Annals of Surgery. 2016;264:949–958. [DOI] [PubMed] [Google Scholar]
- 5.Singh VK, Haupt ME, Geller DE, Hall JA, Diez PMQ. Less common etiologies of exocrine pancreatic insufficiency. World Journal of Gastroenterology. 2017;23:7059–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Othman MO, Harb D, Barkin JA. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. International Journal of Clinical Practice. 2018;72:e13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel MJ, Asbun H, Stauffer J, Raimondo M. Pancreatic exocrine insufficiency in pancreatic cancer: A review of the literature. Digestive and Liver Disease. 2015;47:1013–1020. [DOI] [PubMed] [Google Scholar]
- 8.Hart PA, Conwell DL. Diagnosis of exocrine pancreatic insufficiency. Current Treatment Options in Gastroenterology. 2015;13:347–353. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez-Muñoz JE, Hardt PD, Lerch MM, Löhr MJ. Potential for screening for pancreatic exocrine insufficiency using the fecal elastase-1 test. Digestive Diseases and Sciences. 2017;62:1119–1130. [DOI] [PubMed] [Google Scholar]
- 10.Kadaj-Lipka R, Lipiński M, Adrych K, Durlik M, Gąsiorowska A, Jarosz M et al. Diagnostic and therapeutic recommendations for chronic pancreatitis: Recommendations of the Working Group of the Polish Society of Gastroenterology and the Polish Pancreas Club. Gastroenterology Review. 2018;13:167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parihar V, Ballester R, Ridgway PF, Conlon KC, Gibney J, Ryan BM. Screening for undiagnosed pancreatic exocrine insufficiency (PEI) in a cohort of diabetic patients using faecal elastase testing and PEI scoring system. Acta Diabetologica. 2024;61:1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Löhr M. Clinical and laboratory diagnosis of chronic pancreatitis. The University of Michigan Library [cited 2021 Aug 13]. http://pancreapedia.org/?q=node/9463
- 13.Johnson CD, Arbuckle R, Bonner N, Connett G, Dominguez-Munoz E, Levy P et al. Qualitative assessment of the symptoms and impact of Pancreatic Exocrine Insufficiency (PEI) to inform the development of a Patient-Reported Outcome (PRO) instrument. Patient. 2017;10:615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindkvist B, Domínguez-Muñoz JE, Luaces-Regueira M, Castiñeiras-Alvariño M, Nieto-Garcia L, Iglesias-Garcia J. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology. 2012;12:305–310. [DOI] [PubMed] [Google Scholar]
- 15.Afghani E, Sinha A, Singh VK. An overview of the diagnosis and management of nutrition in chronic pancreatitis. Nutrition in Clinical Practice. 2014;29:295–311. [DOI] [PubMed] [Google Scholar]
- 16.Vujasinovic M, Hedström A, Maisonneuve P, Valente R, von Horn H, Löhr J-M et al. Zinc deficiency in patients with chronic pancreatitis. WJG. 2019;25:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindkvist B, Phillips ME, Domínguez-Muñoz JE. Clinical, anthropometric and laboratory nutritional markers of pancreatic exocrine insufficiency: Prevalence and diagnostic use. Pancreatology. 2015;15:589–597. [DOI] [PubMed] [Google Scholar]
- 18.Domínguez-Muñoz JE. Pancreatic exocrine insufficiency: Diagnosis and treatment. Journal of Gastroenterology and Hepatology. 2011;26:12–16. [DOI] [PubMed] [Google Scholar]
- 19.Montalto G, Sores M, Carroccio A, Scafidi E, Barbagallo CM, Ippolito S et al. Lipoproteins and chronic pancreatitis. Pancreas. 1994;9:137–138. [DOI] [PubMed] [Google Scholar]
- 20.Löhr J-M, Hummel FM, Pirilis KT, Steinkamp G, Körner A, Henniges F. Properties of different pancreatin preparations used in pancreatic exocrine insufficiency. European Journal of Gastroenterology & Hepatology. 2009;21:1024–1031. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmeister A, Mayerle J, Beglinger C, Büchler M, Bufler P, Dathe K et al. English language version of the S3-consensus guidelines on chronic pancreatitis: Definition, aetiology, diagnostic examinations, medical, endoscopic and surgical management of chronic pancreatitis. Zeitschrift für Gastroenterologie. 2015;53:1447–1495. [DOI] [PubMed] [Google Scholar]
- 22.Toouli J, Biankin A, Oliver M. Management of pancreatic exocrine insufficiency: Australasian Pancreatic Club recommendations. The Medical Journal of Australia. 2010;193:461–467. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez-Munoz JE, Drewes AM, Lindkvist B, Ewald N, Czakó L, Rosendahl J et al. Recommendations from the United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. Pancreatology. 2018;18:847–854. [DOI] [PubMed] [Google Scholar]
- 24.Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterology Journal. 2017;5:153–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan M, Rutkowski W, Vujasinovic M, Löhr JM. Adherence to European guidelines for treatment and management of pancreatic exocrine insufficiency in chronic pancreatitis patients. JCM. 2021;10:2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rijk FE, Kempeneers MA, Bruno MJ, Besselink MG, Goor H, Boermeester MA et al. Suboptimal care for chronic pancreatitis patients revealed by moderate to low adherence to the United European Gastroenterology evidence-based guidelines (HaPanEU): A Netherlands nationwide analysis. United European Gastroenterology Journal. 2020;8:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsmark CE, Tang G, Xu H, Tuft M, Hughes SJ, Yadav D. The use of pancreatic enzyme replacement therapy in patients with a diagnosis of chronic pancreatitis and pancreatic cancer in the US is infrequent and inconsistent. Aliment Pharmacology & Therapeutics. 2020;51:958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn RJ, Eyting S, Henniges F, Potthoff A. In vitro comparison of physical parameters, enzyme activity, acid resistance, and pH dissolution characteristics of enteric-coated pancreatic enzyme preparations: Implications for clinical variability and pharmacy substitution. The Journal of Pediatric Pharmacology and Therapeutics. 2007;12:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quality assessment for case series. [cited 2021 Feb 9]. https://www.nice.org.uk/guidance/cg3/documents/appendix-4-quality-of-case-series-form2
- 31.Wells G, Shea B, O’Connell D. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. 2000 [cited 2018 Nov 8]. http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf
- 32.Dominguez-Muñoz JE, Vujasinovic M, De La Iglesia D, Cahen D, Capurso G, Gubergrits N et al. European guidelines for the diagnosis and treatment of pancreatic exocrine insufficiency: UEG, EPC, EDS, ESPEN, ESPGHAN, ESDO, and ESPCG evidence-based recommendations. UEG Journal. 2024;13:125–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naffouje S, Salti G. Transient exocrine pancreatic insufficiency: First report of an unrecognized complication of cytoreductive surgery and HIPEC. Anticancer Research. 2018;38:2353–2358. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz A, Hagberg L. Exocrine pancreatic insufficiency is common in people living with HIV on effective antiretroviral therapy. Infectious Diseases. 2018;50:193–199. [DOI] [PubMed] [Google Scholar]
- 35.Evans KE, Leeds JS, Morley S, Sanders DS. Pancreatic insufficiency in adult celiac disease: Do patients require long-term enzyme supplementation? Digestive Diseases and Sciences. 2010;55:2999–3004. [DOI] [PubMed] [Google Scholar]
- 36.Huddy JR, Macharg FMS, Lawn AM, Preston SR. Exocrine pancreatic insufficiency following esophagectomy: Pancreatic insufficiency post-esophagectomy. Diseases of the Esophagus. 2013;26:594–597. [DOI] [PubMed] [Google Scholar]
- 37.Kiefer T, Krahl D, Osthoff K, Thuss-Patience P, Bunse J, Adam U et al. Importance of pancreatic enzyme replacement therapy after surgery of cancer of the esophagus or the esophagogastric junction. Nutrition and Cancer. 2018;70:69–72. [DOI] [PubMed] [Google Scholar]
- 38.Rinzivillo M, De Felice I, Magi L, Annibale B, Panzuto F. Occurrence of exocrine pancreatic insufficiency in patients with advanced neuroendocrine tumors treated with somatostatin analogs. Pancreatology. 2020;20:875–879. [DOI] [PubMed] [Google Scholar]
- 39.Saif MW, Romano A, Smith MH, Patel R, Relias V. Chronic use of long-acting somatostatin analogues (SSAs) and exocrine pancreatic insufficiency (EPI) in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs): An under-recognized adverse effect. Cancer Medicine Journal 2020;3:75–84. [PMC free article] [PubMed] [Google Scholar]
- 40.Shandro BM, Ritehnia J, Chen J, Nagarajah R, Poullis A. The investigation and management of pancreatic exocrine insufficiency: A retrospective cohort study. Clinical Medicine. 2020;20:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shandro BM, Chen J, Ritehnia J, Poullis A. Associations with pancreatic exocrine insufficiency: An United Kingdom single-centre study. World Journal of Clinical Cases. 2021;9:9469–9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbier L, Jamal W, Dokmak S, Aussilhou B, Corcos O, Ruszniewski P et al. Impact of total pancreatectomy: short- and long-term assessment. HPB. 2013;15:882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Struyvenberg MR, Fong ZV, Martin CR, Tseng JF, Clancy TE, Fernández-del Castillo C et al. Impact of treatments on diabetic control and gastrointestinal symptoms after total pancreatectomy. Pancreas. 2017;46:1188–1195. [DOI] [PubMed] [Google Scholar]
- 44.Crippa S, Tamburrino D, Partelli S, Salvia R, Germenia S, Bassi C et al. Total pancreatectomy: Indications, different timing, and perioperative and long-term outcomes. Surgery. 2011;149:79–86. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki S, Miura J, Shimizu K, Tokushige K, Uchigata Y, Yamamoto M. Clinicophysiological outcomes after total pancreatectomy. Scandinavian Journal of Gastroenterology. 2016;51:1526–1531. [DOI] [PubMed] [Google Scholar]
- 46.Kim E, Kang JS, Han Y, Kim H, Kwon W, Kim JR et al. Influence of preoperative nutritional status on clinical outcomes after pancreatoduodenectomy. HPB. 2018;20:1051–1061. [DOI] [PubMed] [Google Scholar]
- 47.Saluja SS, Kiran S, Mishra PK, Ramaswamy D, Varshney VK, Godhi S et al. Long-term functional outcome after pancreatoduodenectomy for periampullary carcinoma with morphological correlation. Pancreas. 2019;48:1182–1187. [DOI] [PubMed] [Google Scholar]
- 48.D’Haese JG, Ceyhan GO, Demir IE, Layer P, Uhl W, Löhr M et al. Pancreatic enzyme replacement therapy in patients with exocrine pancreatic insufficiency due to chronic pancreatitis: a 1-year disease management study on symptom control and quality of life. Pancreas. 2014;43:834–841. [DOI] [PubMed] [Google Scholar]
- 49.Domínguez-Muñoz JE, Iglesias-García J. Oral pancreatic enzyme substitution therapy in chronic pancreatitis: is clinical response an appropriate marker for evaluation of therapeutic efficacy? JOP. 2010;11:158–162. [PubMed] [Google Scholar]
- 50.Domínguez-Muñoz JE, Iglesias-García J, Vilariño-Insua M, Iglesias-Rey M. 13C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clinical Gastroenterology and Hepatology. 2007;5:484–488. [DOI] [PubMed] [Google Scholar]
- 51.Min M, Patel B, Han S, Bocelli L, Kheder J, Vaze A et al. Exocrine pancreatic insufficiency and malnutrition in chronic pancreatitis: identification, treatment, and consequences. Pancreas. 2018;47:1015–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diéguez-Castillo C, Jiménez-Luna C, Martín-Ruiz JL, Martínez-Galán J, Prados J, Torres C et al. Role of exocrine and endocrine insufficiency in the management of patients with chronic pancreatitis. JCM. 2020;9:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kempeneers MA, Ahmed Ali U, Issa Y, van Goor H, Drenth JPH, van Dullemen HM et al. Natural course and treatment of pancreatic exocrine insufficiency in a nationwide cohort of chronic pancreatitis. Pancreas. 2020;49:242–248. [DOI] [PubMed] [Google Scholar]
- 54.Saito T, Hirano K, Isayama H, Nakai Y, Saito K, Umefune G et al. The role of pancreatic enzyme replacement therapy in unresectable pancreatic cancer: A prospective cohort study. Pancreas. 2017;46:341–346. [DOI] [PubMed] [Google Scholar]
- 55.Domínguez-Muñoz JE, Nieto-Garcia L, López-Díaz J, Lariño-Noia J, Abdulkader I, Iglesias-Garcia J. Impact of the treatment of pancreatic exocrine insufficiency on survival of patients with unresectable pancreatic cancer: A retrospective analysis. BMC Cancer. 2018;18:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Díaz-González Á, Belmonte E, Sapena V, Sanduzzi-Zamparelli M, Darnell A, Díaz A et al. Pancreatic insufficiency in patients under sorafenib treatment for hepatocellular carcinoma. Journal of Clinical Gastroenterology. 2021;55:263–270. [DOI] [PubMed] [Google Scholar]
- 57.Arutla M, Sarkar S, Unnisa M, Sarkar P, Raj MA, Mrudula MR et al. Malnutrition after pancreatic enzyme replacement therapy in chronic pancreatitis: Risk factors in real world practice. Pancreatology. 2021;21:34–41. [DOI] [PubMed] [Google Scholar]
- 58.Desai H, Patel A. Role of pancretic enzyme supplementation in pancreatic exocrine deficiency. Asian Journal of Pharmaceutical and Clinical Research. 2022;16:23–25. [Google Scholar]
- 59.Olmos JI, Piskorz MM, Litwin N, Schaab S, Tevez A, Bravo-Velez G et al. Exocrine pancreatic insufficiency is undiagnosed in some patients with diarrhea-predominant irritable bowel syndrome using the Rome IV Criteria. Dig Dis Sci. 2022;67:5666–5675. [DOI] [PubMed] [Google Scholar]
- 60.Latenstein AEJ, Blonk L, Tjahjadi NS, de Jong N, Busch OR, de Hingh IHJT et al. Long-term quality of life and exocrine and endocrine insufficiency after pancreatic surgery: A multicenter, cross-sectional study. HPB. 2021;23:1722–1731. [DOI] [PubMed] [Google Scholar]
- 61.Kroon VJ, Daamen LA, Tseng DSJ, de Vreugd AR, Brada LJH, Busch OR et al. Pancreatic exocrine insufficiency following pancreatoduodenectomy: A prospective bi-center study. Pancreatology. 2022;22:1020–1027. [DOI] [PubMed] [Google Scholar]
- 62.Trestini I, Carbognin L, Peretti U, Sperduti I, Caldart A, Tregnago D et al. Pancreatic enzyme replacement therapy in patients undergoing first-line gemcitabine plus nab-paclitaxel for advanced pancreatic adenocarcinoma. Frontiers in Oncology. 2021;11:688889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ockenga J. Importance of nutritional management in diseases with exocrine pancreatic insufficiency. HPB. 2009;11:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vantini I, Benini L, Caliari S, Fioretta A. What Kind of Pancreatic Extracts To Use? In: Pederzoli P, Cavallini G, Bassi C, Falconi M, editors. Facing the pancreatic dilemma. Berlin, Heidelberg: Springer Berlin Heidelberg; 1994 [cited 2018 Nov 9], pp. 154–78. http://www.springerlink.com/index/10.1007/978-3-642-79167-3_21
- 65.Laterza L, Scaldaferri F, Bruno G. Pancreatic function assessment. European Review for Medical and Pharmacological Sciences. 2013;17:65–71. [PubMed] [Google Scholar]
- 66.Smith RC, Smith SF, Wilson J, Pearce C, Wray N, Vo R et al. Summary and recommendations from the Australasian guidelines for the management of pancreatic exocrine insufficiency. Pancreatology. 2016;16:164–180. [DOI] [PubMed] [Google Scholar]
- 67.Phillips ME, Hopper AD, Leeds JS, Roberts KJ, McGeeney L, Duggan SN et al. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol. 2021;8:e000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivoleti P, Dolan RD, Yang AL, Jin DX, Banks PA, McNabb-Baltar J. Provider differences in monitoring and management of exocrine pancreatic insufficiency in chronic pancreatitis. Pancreas. 2022;51:25–27. [DOI] [PubMed] [Google Scholar]
- 69.Barkin JA, Harb D, Kort J, Barkin JS. Real-world patient experience with pancreatic enzyme replacement therapy in the treatment of exocrine pancreatic insufficiency. Pancreas. 2023 [cited 2024 Sep 11]. https://journals.lww.com/10.1097/MPA.0000000000002273 [DOI] [PMC free article] [PubMed]
- 70.Prescott J. Pancreatic enzyme replacement therapy: A view from behind the counter [cited 2020 Feb 9]. https://www.pharmacytimes.com/publications/issue/2016/october2016/r773_october2016
- 71.Vanga RR, Tansel A, Sidiq S, El-Serag HB, Othman MO. Diagnostic performance of measurement of fecal elastase-1 in detection of exocrine pancreatic insufficiency: systematic review and meta-analysis. Clinical Gastroenterology and Hepatology. 2018;16:1220-1228.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galli G, Esposito G, Lahner E, Pilozzi E, Corleto VD, Di Giulio E et al. Histological recovery and gluten-free diet adherence: a prospective 1-year follow-up study of adult patients with coeliac disease. Aliment Pharmacol Ther. 2014;40:639–647. [DOI] [PubMed] [Google Scholar]
- 73.Parhiala M, Ukkonen M, Sand J, Laukkarinen J. Osteoporosis and sarcopenia are common and insufficiently diagnosed among chronic pancreatitis patients. BMC Gastroenterology. 2023;23:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sikkens ECM, Cahen DL, van Eijck C, Kuipers EJ, Bruno MJ. The daily practice of pancreatic enzyme replacement therapy after pancreatic surgery: a Northern European Survey: Enzyme replacement after surgery. Journal of Gastrointestinal Surgery. 2012;16:1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sikkens ECM, Cahen DL, van Eijck C, Kuipers EJ, Bruno MJ. Patients with exocrine insufficiency due to chronic pancreatitis are undertreated: A Dutch national survey. Pancreatology. 2012;12:71–73. [DOI] [PubMed] [Google Scholar]
- 76.Don BR, Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Seminars in Dialysis. 2004;17:432–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data Sharing Statement: All data that support the findings of this study have been included in the article and its supplementary materials.