Abstract
The Tallis-Leyton model is a simple model of parasite acquisition where parasites accumulate in the host without affecting the host’s mortality, or eliciting any immune reaction from the host. Furthermore, the parasites do not reproduce in the host. We examine how the variability in parasite loads among hosts is affected by the rate of infectious contacts, the distribution of parasite entering the host during infectious contacts, the host’s age, and the distribution of parasite lifetimes. Motivated by empirical studies in parasitology, variability is examined in the sense of the Lorenz order and related metrics. Perhaps counterintuitively, increased variability in the distribution of parasite lifetimes is seen to decrease variability in the parasite loads among hosts.
Keywords: Aggregation, Convex order, Gini index, Lorenz order, Negative binomial distribution, Pietra index
Introduction
The distribution of parasites among their host population typically displays a high degree of variation; some hosts are infected with many parasites while many hosts have comparatively few. This phenomenon is almost universally observed in wild populations (Shaw and Dobson 1995; Poulin 2007). Following the usual practice in the parasitology literature, we call this phenomenon aggregation (Pielou 1977; Wilson et al. 2001; Poulin 2011).
Unfortunately, there is no universally accepted measure of aggregation. Instead, different authors use different metrics of aggregation to summarise the parasite’s distribution (Pielou 1977; McVinish and Lester 2020; Morrill et al. 2023). Despite claims that these measures have identical interpretations and more-or-less predict each other (Reiczigel et al. 2014), different methods can give opposing answers (McVinish and Lester 2020, Figure 1). The most commonly used measures of aggregation in theoretical models are the variance-to-mean ratio (Isham 1995; Barbour and Pugliese 2000; Herbert and Isham 2000; Peacock et al. 2018) and the k parameter of the negative binomial distribution (Anderson and May 1978a, b; Rosà and Pugliese 2002; Schreiber 2006; McPherson et al. 2012), where the negative binomial distribution has mean m and variance 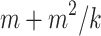 . Both of these measures can be interpreted as quantifying how over dispersed the distribution of parasite load is relative to a Poisson distribution.
. Both of these measures can be interpreted as quantifying how over dispersed the distribution of parasite load is relative to a Poisson distribution.
An alternative view of aggregation was put forward by Poulin (1993), arguing that a measure of the discrepancy between the observed distribution of parasites in the hosts and the ideal distribution where all hosts are infected with the same number of parasites would be the best measure of aggregation. This view puts the Lorenz ordering of distributions (Lorenz 1905; Arnold and Sarabia 2018) central in the study of aggregation. While the coefficient of variation is perhaps the most widely known measure having a direct relationship to the Lorenz order (Arnold and Sarabia 2018, Sections 5.2.1 & 5.4), Poulin (1993) proposed using a different index, D, as a measure of this discrepancy. It can be shown that, up to a factor that goes to one as the sample size increases, Poulin’s D is Gini’s concentration ratio (Gini 1914, 2005), also known as the estimator of the Gini index (Arnold and Sarabia 2018, Equation 5.85). Poulin’s D has since become one of the standard measures of aggregation used in studies of wild parasite populations (Herrero-Cófreces et al. 2021; Rodríguez-Hernández et al. 2021; Schrock et al. 2025).
The aim of this paper is to characterize how different processes in the Tallis-Leyton model (Tallis and Leyton 1969) shape parasite aggregation in the sense of the Lorenz ordering and related indices. Only population values of these indices are considered, rather than their estimators. Despite the growing importance of the Lorenz order in empirical studies of parasite distributions following Poulin’s proposal, we are unaware of any other theoretical study of parasite acquisition using the Lorenz order. In Sect. 2 we review some background on the Lorenz ordering and the closely related convex ordering of distributions. The Tallis-Leyton model is analysed in Sect. 3. We first show show how the host’s parasite load can be represented as a compound Poisson distribution. This representation is then applied to determine how each of the model parameters affect the Lorenz ordering of the distribution of parasites in the host. The final part of the analysis shows that the host’s parasite load is asymptotically normally distributed in the limit as the rate of infectious contacts goes to infinity. This allows the indices to be approximated in terms of the mean and variance. The paper concludes with a discussion of future challenges in analysing models of parasite aggregation.
Background
Tallis-Leyton model
Tallis and Leyton (1969) proposed the following model for the parasite load M(a) of a definitive host at age a, conditional on survival of the host to age a. The host is parasite free at birth so  . During its lifetime, the host makes infectious contacts following a Poisson process with constant rate
. During its lifetime, the host makes infectious contacts following a Poisson process with constant rate 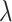 . At each infectious contact, a random number of parasites N enter the host. Once a parasite enters the host, it survives for a random period of time T. The lifetimes of parasites, numbers of parasites entering the host at infectious contacts, and the Poisson process of infectious contacts are all independent. The parasites are assumed to have no effect on the host mortality so the host’s parasite load at age a is independent of the host surviving to age a. Henceforth, we will simply refer to M(a) as the parasite load of a host age a. Although we won’t make use of this fact, we note that this process also describes an infinite server queue with bulk arrivals and general independent service times (Holman et al. 1983).
. At each infectious contact, a random number of parasites N enter the host. Once a parasite enters the host, it survives for a random period of time T. The lifetimes of parasites, numbers of parasites entering the host at infectious contacts, and the Poisson process of infectious contacts are all independent. The parasites are assumed to have no effect on the host mortality so the host’s parasite load at age a is independent of the host surviving to age a. Henceforth, we will simply refer to M(a) as the parasite load of a host age a. Although we won’t make use of this fact, we note that this process also describes an infinite server queue with bulk arrivals and general independent service times (Holman et al. 1983).
Let 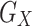 ,
, 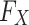 and
and 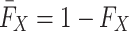 denote the probability generating function (PGF), distribution function, and survival function of a random variable X. We write
denote the probability generating function (PGF), distribution function, and survival function of a random variable X. We write 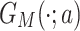 for the PGF of M(a). Tallis and Leyton (1969) showed
for the PGF of M(a). Tallis and Leyton (1969) showed
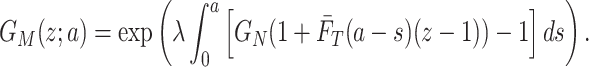 |
1 |
Using the well known relationship between the moments of a random variable and derivatives of its PGF at zero, we see that the mean and variance of M(a) are
 |
2 |
 |
3 |
Hence, the variance-to-mean ratio is
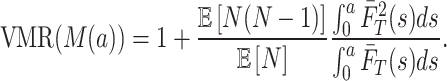 |
4 |
Assuming 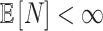 and
and 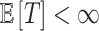 , the limiting distribution of parasite load as
, the limiting distribution of parasite load as 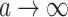 exists and has PGF
exists and has PGF
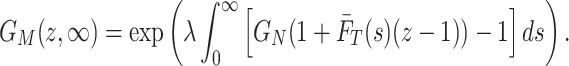 |
5 |
An appropriate rescaling of the host age, rate of infectious contacts, and parasite lifetimes leaves the distribution of the host’s parasite load unchanged. Specifically, for any  let
let  represent the parasite load of host age a in the Tallis-Leyton model with parameters
represent the parasite load of host age a in the Tallis-Leyton model with parameters  ,
,  and
and 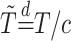 . Then
. Then 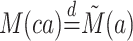 . To see this, apply the change of variable
. To see this, apply the change of variable 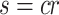 in the integral in (1) for the PGF of M(ca):
in the integral in (1) for the PGF of M(ca):
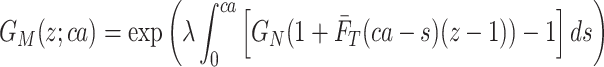 |
6 |
 |
7 |
Upon noting  , it follows that
, it follows that  .
.
Convex order and Lorenz order
Lorenz (1905) proposed the Lorenz curve as a graphical measure of inequality. The following general definition of the Lorenz curve was given by Gastwirth (1971).
Definition
The Lorenz curve 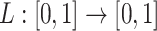 for the distribution F with finite mean
for the distribution F with finite mean 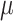 is given by
is given by
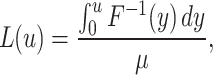 |
8 |
where 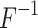 is the quantile function
is the quantile function
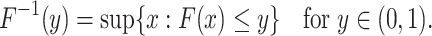 |
9 |
Adapting the description in Arnold and Sarabia (2018, Section 3.1) to a parasitology context, the Lorenz curve L(u) represents the proportion of the parasite population infecting the least infected u proportion of the host population. When all hosts are infected with the same number of parasites, the Lorenz curve is given by 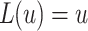 and is called the egalitarian line. The Lorenz curve never rises above the egalitarian line, that is
and is called the egalitarian line. The Lorenz curve never rises above the egalitarian line, that is 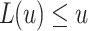 for all
for all 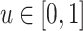 .
.
The Lorenz curve defines a partial order on the class of all distributions on 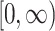 with finite mean (Arnold and Sarabia 2018, Definition 3.2.1).
with finite mean (Arnold and Sarabia 2018, Definition 3.2.1).
Definition
Let X and Y be random variables with the respective Lorenz curves denoted 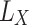 and
and 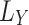 . We say X is smaller in the Lorenz order, denoted
. We say X is smaller in the Lorenz order, denoted 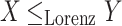 if
if 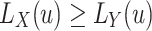 for every
for every 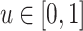 .
.
The negative binomial distribution, which is extensively used in parasitology, can be compared in the Lorenz order (McVinish and Lester 2024). Specifically, let 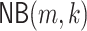 denote the negative binomial distribution with mean m and variance
denote the negative binomial distribution with mean m and variance 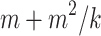 . Then
. Then
-
(i)
for any
 and
and 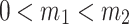 ,
, 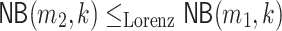 , and
, and -
(ii)
for any
 and
and  ,
,  .
.
Closely related to the Lorenz order is the convex order of distributions.
Definition
Let X and Y be two random variables such that  . We say X is smaller than Y in the convex order, denoted
. We say X is smaller than Y in the convex order, denoted  , if
, if 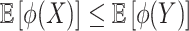 for all convex functions
for all convex functions 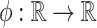 , provided the expectations exist.
, provided the expectations exist.
These two orderings are related since  if and only if
if and only if
 |
10 |
for every continuous convex function  (Arnold and Sarabia 2018, Corollary 3.2.1). In other words,
(Arnold and Sarabia 2018, Corollary 3.2.1). In other words,
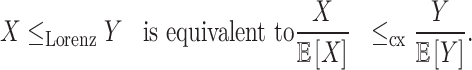 |
11 |
Shaked and Shanthikumar (2007, Section 3.A) provide an extensive review of results on the convex order. We briefly mention some of the important results that are used in our analysis.
The convex order is closed under weak limits provided the expectations also converge (Shaked and Shanthikumar 2007, Theorem 3.A.12 (c)).
The convex order is closed under mixtures (Shaked and Shanthikumar 2007, Theorem 3.A.12 (b)). Let X, Y, and
 be random variables and write
be random variables and write  and
and  for the conditional distributions of X and Y given
for the conditional distributions of X and Y given 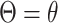 . If
. If  for all
for all 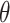 in the support of
in the support of  , then
, then 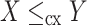 . As an application of this property we can say that if
. As an application of this property we can say that if  and Z is an independent non-negative random variable, then
and Z is an independent non-negative random variable, then  .
.- The convex order is closed under convolutions (Shaked and Shanthikumar 2007, Theorem 3.A.12 (d)). Let
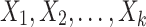 and
and 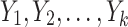 be two sets of independent random variables. If
be two sets of independent random variables. If  for
for 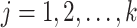 , then
, then 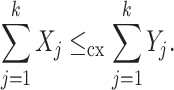
12 - Combining the properties of closure under mixtures and closure under convolutions, we see the convex order is closed under random sums so
for any non-negative integer random variable K. As an application of the closure under random sums property of the convex order, consider two random variables K and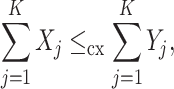
13 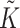 that related by binomial thinning. That is,
that related by binomial thinning. That is, 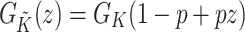 for some
for some  . Then
. Then 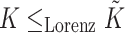 (McVinish and Lester 2020, Section 3)
(McVinish and Lester 2020, Section 3) - The closure under random sums property can be adapted to the case where the
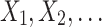 and
and 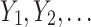 are two iid sequences with
are two iid sequences with 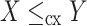 , and
, and 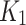 and
and  are non-negative integer random variables such that
are non-negative integer random variables such that  . In this case, (Shaked and Shanthikumar 2007, Theorem 3.A.13) implies
. In this case, (Shaked and Shanthikumar 2007, Theorem 3.A.13) implies 
14 The survival function can be used to establish if two random variables can be compared in the convex order. If X and Y are two random variables with the same mean and
 has a single sign change from positive to negative, then
has a single sign change from positive to negative, then  (Shaked and Shanthikumar 2007, Theorem 3.A.44(b)). This property can also be used to characterise the convex order (Shaked and Shanthikumar 2007, Theorem 3.A.45).
(Shaked and Shanthikumar 2007, Theorem 3.A.44(b)). This property can also be used to characterise the convex order (Shaked and Shanthikumar 2007, Theorem 3.A.45).
Measures of aggregation
In practice, levels of aggregation are compared with numerical summaries rather than using the entire Lorenz curve. If we accept the Lorenz order as the way to compare aggregation in parasite-host systems (Poulin 1993; McVinish and Lester 2020), then our measures of aggregation should respect the Lorenz order. That is, if 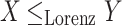 , then the measure of aggregation
, then the measure of aggregation 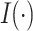 should satisfy
should satisfy  . Arnold and Sarabia (2018, Chapter 5) review several inequality measures and these can be applied as measures of aggregation. We restrict our attention in this paper to the following four measures respecting the Lorenz order; the coefficient of variation, the Gini index, the Pietra index (also known as the Hoover index, or the Robin-Hood index) and
. Arnold and Sarabia (2018, Chapter 5) review several inequality measures and these can be applied as measures of aggregation. We restrict our attention in this paper to the following four measures respecting the Lorenz order; the coefficient of variation, the Gini index, the Pietra index (also known as the Hoover index, or the Robin-Hood index) and  .
.
The coefficient of variation is given by
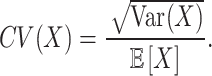 |
15 |
This measure is rarely used in parasitology, though it is mentioned in some reviews on parasite aggregation such as Wilson et al. (2001) and McVinish and Lester (2020). As means and variances are commonly reported in empirical studies and are often easily calculated for theoretical models, it may be useful in some contexts. For example, from Eqs. (2) and (3), the squared coefficient of variation for the Tallis-Leyton model is
 |
16 |
The Gini index (Gini 1914, 2005) is given by twice the area between the egalitarian line and the Lorenz curve. For a random variable X, the Gini index can be expressed as
 |
17 |
where 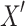 is an independent random variable with
is an independent random variable with  (Arnold and Sarabia 2018, Page 47). The Pietra index is given by the maximum vertical distance between the egalitarian line and the Lorenz curve (Pietra 1915, 2014). McVinish and Lester (2020) argue that this index could be useful due to its simple interpretation as the proportion of the parasite population that would need to be redistributed among the hosts in order for all hosts to have the same parasite load. The Pietra index can be expressed as
(Arnold and Sarabia 2018, Page 47). The Pietra index is given by the maximum vertical distance between the egalitarian line and the Lorenz curve (Pietra 1915, 2014). McVinish and Lester (2020) argue that this index could be useful due to its simple interpretation as the proportion of the parasite population that would need to be redistributed among the hosts in order for all hosts to have the same parasite load. The Pietra index can be expressed as
 |
18 |
(Arnold and Sarabia 2018, Lemma 5.3.1). In general, the dependence of the Pietra index on the mean is not smooth. For example, the Pietra index for the Poisson distribution with mean 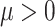 is
is
 |
19 |
where m is the smallest integer greater than or equal to 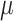 (Ramasubban 1958). While the Pietra index in this instance is continuous in
(Ramasubban 1958). While the Pietra index in this instance is continuous in 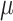 , it is not differentiable with respect to
, it is not differentiable with respect to 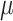 at integer values of
at integer values of 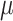 . Similar behaviour will be observed in the numerical results reported in Sect. 3.
. Similar behaviour will be observed in the numerical results reported in Sect. 3.
Prevalence, the probability that a host is infected by at least one parasite, is an important quantity in parasitology (Jovani and Tella 2006; Kura et al. 2022). Although prevalence is not usually thought of as a measure of aggregation, we may express  in terms of the Lorenz curve. From the definition of the Lorenz curve,
in terms of the Lorenz curve. From the definition of the Lorenz curve, 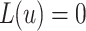 if
if 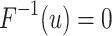 . From the definition of the quantile function,
. From the definition of the quantile function, 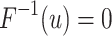 for
for  . As the Lorenz curve is continuous and
. As the Lorenz curve is continuous and 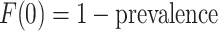 , we see
, we see
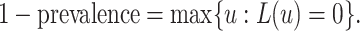 |
20 |
Prevalence for the Tallis-Leyton model can be evaluated directly from the PGF as
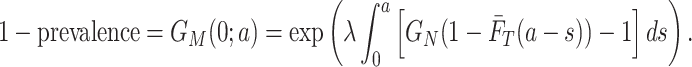 |
21 |
There is a close connection between the Pietra index and prevalence. If  , then
, then
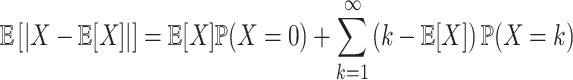 |
22 |
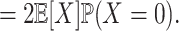 |
23 |
Hence,
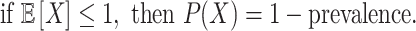 |
24 |
More generally, the four indices are constrained by the following inequality
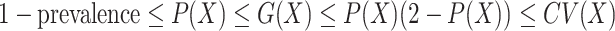 |
25 |
(Taguchi 1968; McVinish and Lester 2020).
The Gini index and Pietra index can be further related to the coefficient of variation when the distribution of parasites is approximately normal. Suppose  is a sequence of random variables such that
is a sequence of random variables such that
 |
26 |
where  . As
. As 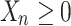 with probability one, the above limit is only possible if
with probability one, the above limit is only possible if 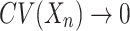 . Nevertheless, the ratio of the Gini index to the coefficient of variation still has a well defined limit. The Gini index of
. Nevertheless, the ratio of the Gini index to the coefficient of variation still has a well defined limit. The Gini index of  can be expressed as
can be expressed as
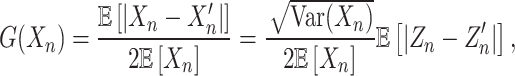 |
27 |
where  is an independent random variable with
is an independent random variable with 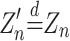 . Since
. Since  , the collection of random variables
, the collection of random variables  is uniformly integrable and
is uniformly integrable and  . Applying the asymptotic normality and uniform integrability of the
. Applying the asymptotic normality and uniform integrability of the  ,
,
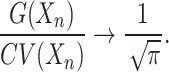 |
28 |
Similarly, the Pietra index of 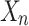 can be expressed as
can be expressed as
 |
29 |
Applying the asymptotic normality and uniform integrability of the 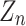 ,
,
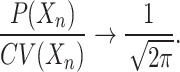 |
30 |
Numerical evaluation of aggregation measures from the PGF
From Eq. (16), the coefficient of variation can be relatively easily evaluated for the Tallis-Leyton model. Numerical integration of  and
and 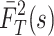 may be required, but the dependence on age and
may be required, but the dependence on age and 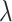 is explicit. Similarly,
is explicit. Similarly,  could be evaluated with a single numerical integration using (21). On the other hand, evaluation of the Gini and Pietra indices require evaluation of the probability mass function. In the examples of the next section, we numerically evaluate the probability mass function of M(a) by inverting
could be evaluated with a single numerical integration using (21). On the other hand, evaluation of the Gini and Pietra indices require evaluation of the probability mass function. In the examples of the next section, we numerically evaluate the probability mass function of M(a) by inverting  using the Abate-Whitte algorithm (Abate and Whitt 1992). The algorithm was implemented in MATLAB (The MathWorks Inc. 2022a) using the vpa function in the Symbolic Math Toolbox (The MathWorks Inc. 2022b) for high precision arithmetic. The code used to evaluate the indices is available from McVinish (2025).
using the Abate-Whitte algorithm (Abate and Whitt 1992). The algorithm was implemented in MATLAB (The MathWorks Inc. 2022a) using the vpa function in the Symbolic Math Toolbox (The MathWorks Inc. 2022b) for high precision arithmetic. The code used to evaluate the indices is available from McVinish (2025).
Analysis of the Tallis-Leyton model
In this section we characterize how the different processes in the Tallis-Leyton model shape parasite aggregation in the sense of the Lorenz ordering and the related indices discussed in Sect. 2.3. The analysis begins with a representation of the host’s parasite load, M(a), as a random variable having a compound Poisson distribution. This representation is used extensively to understand how the rate of infectious contacts ( ), the distribution of the number of parasites (N) that enter the host during an infectious contact, the age of the host (a), and lifetime distribution of the parasites (T) all affect the distribution of a host’s parasite load in terms of the Lorenz order. When comparing the host’s parasite load in two systems, the parameters of the second parasite-host system is distinguished by a tilde.
), the distribution of the number of parasites (N) that enter the host during an infectious contact, the age of the host (a), and lifetime distribution of the parasites (T) all affect the distribution of a host’s parasite load in terms of the Lorenz order. When comparing the host’s parasite load in two systems, the parameters of the second parasite-host system is distinguished by a tilde.
Compound Poisson representation
Let n be a non-negative integer, 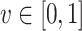 , and let X(n, v) denote a random variable from a
, and let X(n, v) denote a random variable from a  distribution, with
distribution, with 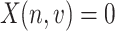 with probability 1 when
with probability 1 when 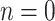 . Our first result will represent a host’s parasite load M(a) as a random sum of independent and identically distributed random variables.
. Our first result will represent a host’s parasite load M(a) as a random sum of independent and identically distributed random variables.
Theorem 1
Assume N has a distribution on the non-negative integers and T has a continuous distribution on  . For
. For 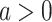 , define V to be a random variable on
, define V to be a random variable on  with distribution function
with distribution function
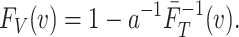 |
31 |
Let 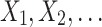 be a sequence of independent random variables with the same distribution as X(N, V), where N and V are independent. Let
be a sequence of independent random variables with the same distribution as X(N, V), where N and V are independent. Let  be a Poisson process with rate
be a Poisson process with rate 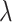 that is independent of the sequence
that is independent of the sequence  Then
Then
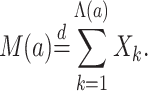 |
32 |
Proof
We first determine the PGF of X(N, V). The PGF of X(n, v) is 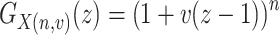 . By conditioning on N, the PGF of X(N, v) is seen to be
. By conditioning on N, the PGF of X(N, v) is seen to be
 |
33 |
By conditioning on V and then applying the distribution function of V (31), we can write the PGF of X(N, V) as
 |
34 |
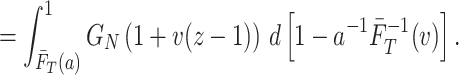 |
35 |
We now determine the PGF of the right-hand side of Eq. (32). Conditioning on 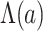 and noting that
and noting that 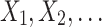 is a sequence of independent random variables with the same distribution as X(N, V), the PGF of
is a sequence of independent random variables with the same distribution as X(N, V), the PGF of 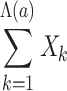 is seen to be
is seen to be
 |
36 |
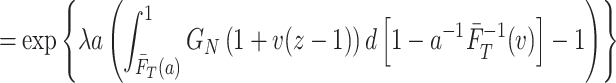 |
37 |
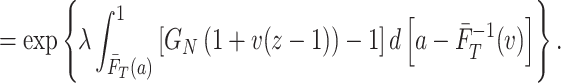 |
38 |
Upon making the substitution 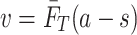 so
so 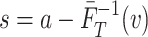 , the PGF of
, the PGF of  can be expressed as
can be expressed as
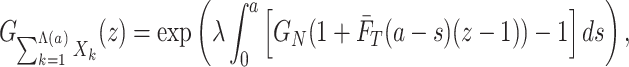 |
39 |
which by Eq. (1) is  .
. 
Rate of infectious contacts
In this section we examine the effect of the rate of infectious contacts (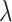 ) on the parasite aggregation. The rate of infectious contacts has no effect on the variance-to-mean ratio (4), whereas the coefficient of variation is strictly decreasing as the rate of infectious contacts increases (16). The following result shows an increase in the rate of infectious contacts decreases parasite aggregation in the sense of the Lorenz order.
) on the parasite aggregation. The rate of infectious contacts has no effect on the variance-to-mean ratio (4), whereas the coefficient of variation is strictly decreasing as the rate of infectious contacts increases (16). The following result shows an increase in the rate of infectious contacts decreases parasite aggregation in the sense of the Lorenz order.
Theorem 2
If 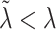 and all other model parameters are equal, then
and all other model parameters are equal, then  .
.
Proof
Set 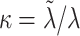 . Let
. Let  be a sequence of independent random variables having the same distribution as X(N, V) and let
be a sequence of independent random variables having the same distribution as X(N, V) and let 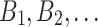 be a sequence of independent
be a sequence of independent  random variables that are also independent of the
random variables that are also independent of the  . As
. As  and the convex order is closed under mixtures,
and the convex order is closed under mixtures, 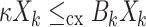 . The PGF of
. The PGF of 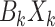 is
is 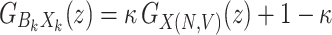 . Let
. Let 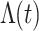 be a Poisson process with rate
be a Poisson process with rate  . As the convex order is closed under random sums,
. As the convex order is closed under random sums,
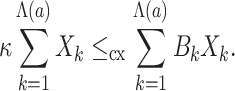 |
40 |
By Theorem 1, 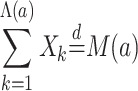 . To determine the distribution of
. To determine the distribution of 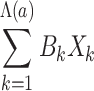 , we evaluate its PGF
, we evaluate its PGF
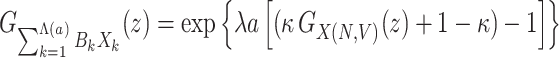 |
41 |
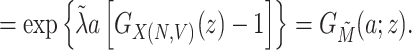 |
42 |
Hence, 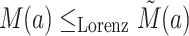 .
. 
Figure 1 shows the four indices (coefficient of variation, Gini, Pietra, and 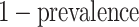 ) for a host aged 3 with rate of infectious contacts (
) for a host aged 3 with rate of infectious contacts ( ) in [0.25, 128], the number of parasites (N) entering the host at infectious contacts having a
) in [0.25, 128], the number of parasites (N) entering the host at infectious contacts having a  distribution, and the parasite lifetimes (T) having a
distribution, and the parasite lifetimes (T) having a 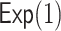 distribution. All four indices are strictly decreasing as the rate of infectious contacts increases. The coefficient of variation (16) is not displayed for small values of
distribution. All four indices are strictly decreasing as the rate of infectious contacts increases. The coefficient of variation (16) is not displayed for small values of 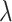 as it is proportional to
as it is proportional to 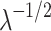 . For
. For  , the expected parasite load is less than one so the Pietra index and
, the expected parasite load is less than one so the Pietra index and 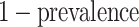 are equal for
are equal for 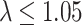 following (24). As expected from the discussion in Sect. 2.3, the Pietra index appears to display some discontinuity in the first derivative at points where the expected parasite load is integer valued. This behaviour is less apparent at larger values of
following (24). As expected from the discussion in Sect. 2.3, the Pietra index appears to display some discontinuity in the first derivative at points where the expected parasite load is integer valued. This behaviour is less apparent at larger values of 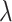 .
.
Fig. 1.
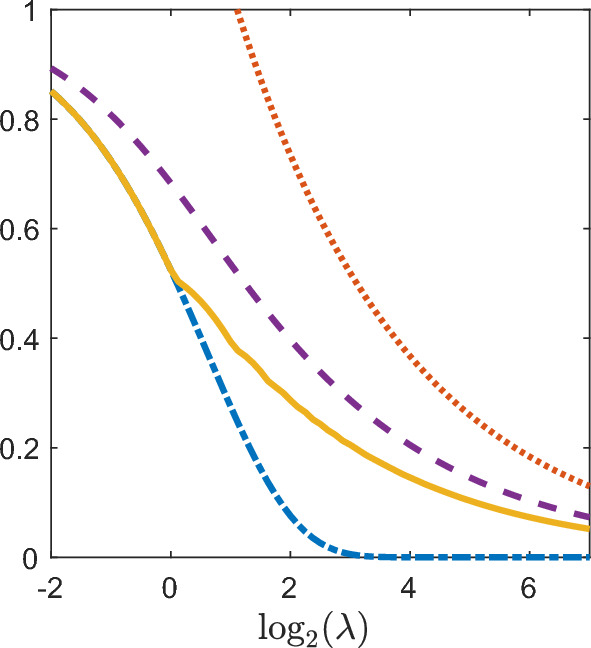
Plot of the coefficient of variation (orange dotted line), Gini index (purple dashed line), Pietra index (yellow solid line), and  (blue dot-dashed line) for a host aged 3 in the Tallis-Leyton model with
(blue dot-dashed line) for a host aged 3 in the Tallis-Leyton model with  , and
, and 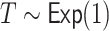 . Since
. Since 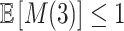 for
for  , the Pietra index and
, the Pietra index and 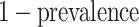 coincide on that interval of
coincide on that interval of 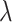 as expected (24)
as expected (24)
Distribution of N
We now consider the role of the distribution of the number of parasites (N) that enter the host during an infectious contact. As a concrete example, suppose 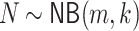 , where m is the mean and the variance is
, where m is the mean and the variance is 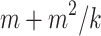 . From (4), the variance-to-mean ratio of the parasite load M(a) is
. From (4), the variance-to-mean ratio of the parasite load M(a) is
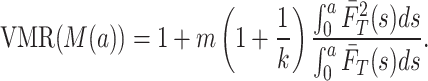 |
43 |
We see that the variance-to-mean ratio is increasing in m but decreasing in k. In contrast, the coefficient of variation of M(a) is decreasing in both m and k.
The next two results show increased variability in the number of parasites entering the host during an infectious contact leads to increased parasite aggregation in the sense of the Lorenz order. The first of these results uses the convex order, which requires the distributions being compared to have the same expectation.
Theorem 3
Suppose that N and  are non-negative integer valued random variables such that
are non-negative integer valued random variables such that 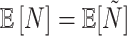 and
and 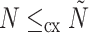 . Assume that all other model parameters are equal. Then
. Assume that all other model parameters are equal. Then 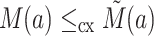 .
.
Proof
Using an extension of the closure under random sums property of the convex order Shaked and Shanthikumar (2007, Theorem 3.A.13),
 |
44 |
As the convex order is closed under mixtures, 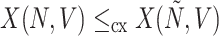 . Let
. Let 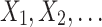 be a sequence of independent random variables having the same distribution as X(N, V) and let
be a sequence of independent random variables having the same distribution as X(N, V) and let  be a sequence of independent random variables having the same distribution as
be a sequence of independent random variables having the same distribution as 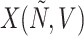 . As the convex order is closed under random sums,
. As the convex order is closed under random sums,
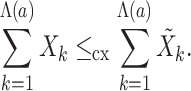 |
45 |
Theorem 1 shows 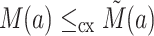 .
. 
For distributions with different means, we consider only the case where N and 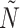 are related by binomial thinning. Recall that if
are related by binomial thinning. Recall that if 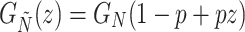 for some
for some  , then
, then 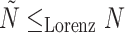 .
.
Theorem 4
Suppose that  for some
for some  and all other model parameters are equal. Then
and all other model parameters are equal. Then 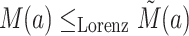 .
.
Proof
Let  and
and  be independent standard uniform random variables. Then standard conditioning arguments show
be independent standard uniform random variables. Then standard conditioning arguments show
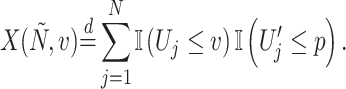 |
46 |
As the convex order is closed under mixtures,
 |
47 |
As the convex order is closed under random sums,  . Following the same arguments as in the proof of Theorem 3, we see
. Following the same arguments as in the proof of Theorem 3, we see  . Hence,
. Hence, 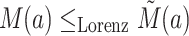 .
. 
When the distribution of the number of parasite has a 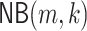 distribution, Theorems 3 and 4 together show that an increase in m or k will decrease parasite aggregation in the sense of the Lorenz order.
distribution, Theorems 3 and 4 together show that an increase in m or k will decrease parasite aggregation in the sense of the Lorenz order.
Corollary 5
Suppose  and
and  with
with 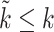 and
and  . Assume that all other model parameters are equal. Then
. Assume that all other model parameters are equal. Then 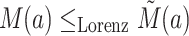 .
.
Proof
Let  be the parasite load for a host of age a in the Tallis-Leyton model with
be the parasite load for a host of age a in the Tallis-Leyton model with 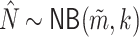 and all other model parameters equal. The PGF of the
and all other model parameters equal. The PGF of the 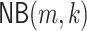 distribution is
distribution is
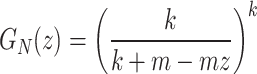 |
48 |
and 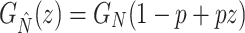 with
with  . Theorem 4 implies
. Theorem 4 implies 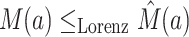 . Since
. Since 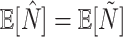 and
and 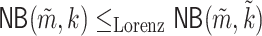 ,
,  . Theorem 3 implies
. Theorem 3 implies  . As the Lorenz ordering is transitive,
. As the Lorenz ordering is transitive,  .
. 
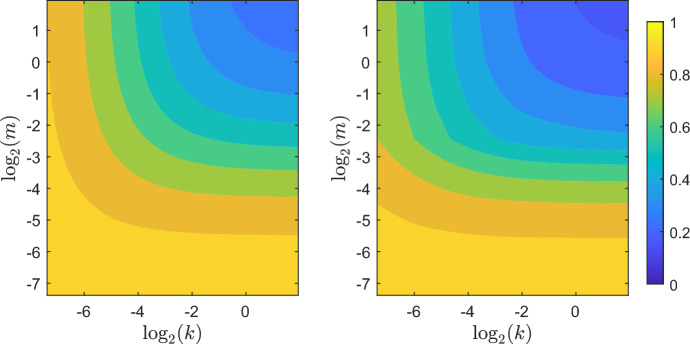
Figure 2 shows the Gini and Pietra indices for a parasite host system with host aged 10, rate of infectious contacts 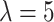 , the distribution of the number of parasites (N) that enter the host during an infectious contact following a
, the distribution of the number of parasites (N) that enter the host during an infectious contact following a 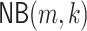 distribution, and parasite lifetimes (T) having an
distribution, and parasite lifetimes (T) having an 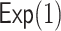 distribution. Both indices are decreasing in both m and k as we expect from the above results. The contours of both the Gini and Pietra indices tend to become parallel to the respective axes as
distribution. Both indices are decreasing in both m and k as we expect from the above results. The contours of both the Gini and Pietra indices tend to become parallel to the respective axes as 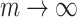 and
and 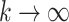 . This is a consequence of the limiting behaviour of the negative binomial distribution (Adell and Cal 1994). The contours of the Pietra index display some discontinuity in the first derivative for
. This is a consequence of the limiting behaviour of the negative binomial distribution (Adell and Cal 1994). The contours of the Pietra index display some discontinuity in the first derivative for 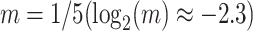 , which corresponds to a host’s expected parasite load being 1.
, which corresponds to a host’s expected parasite load being 1.
Fig. 2.
Contour plots showing Gini index (Left) and Pietra index (Right) for a host aged 10 in the Tallis-Leyton model with 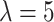 ,
, 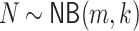 and
and 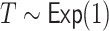
It is natural to consider which distribution for N results in the least aggregated distribution for the host’s parasite load. This requires determining the smallest distribution in the convex ordering. The distributions being compared must have the same expected value. Let n be a non-negative real number. Define the random variable N such that
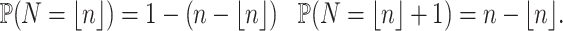 |
49 |
In the supplementary material of McVinish and Lester (2020) it was shown for any random variable 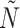 on the non-negative integers with
on the non-negative integers with  is larger than N in the convex order. That is,
is larger than N in the convex order. That is, 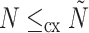 and we can say N has the smallest distribution in convex order with expectation n. When
and we can say N has the smallest distribution in convex order with expectation n. When 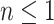 , the smallest distribution in convex order for N leads to M(a) having a Poisson distribution. There is no largest distribution in the convex order.
, the smallest distribution in convex order for N leads to M(a) having a Poisson distribution. There is no largest distribution in the convex order.
Host age
We now examine the effect of the host’s age (a) on parasite aggregation. Differentiating (4) with respect to a shows the variance-to-mean ratio is a decreasing function of the host’s age. Since the expected parasite load is increasing in age, the coefficient of variation is also decreasing in the host’s age. The following result shows that parasite aggregation in the sense of Lorenz order decreases as the host age increases.
Theorem 6
If 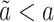 , then
, then 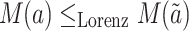 .
.
The proof is built from the following lemmas.
Lemma 7
Let V have the distribution (31) and let  have the distribution (31) with a replaced by
have the distribution (31) with a replaced by  . Let
. Let  independent of V, and let
independent of V, and let  independent of
independent of  . Then
. Then 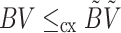 .
.
Proof
Note that
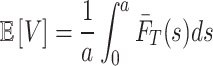 |
50 |
so  . We show that
. We show that 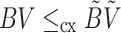 by examining the sign changes of
by examining the sign changes of 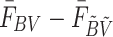 . The survival functions of BV and
. The survival functions of BV and  are
are
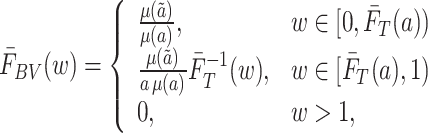 |
51 |
and
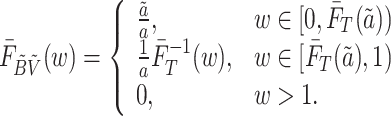 |
52 |
Since 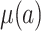 is increasing in a and
is increasing in a and 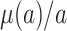 is decreasing in a,
is decreasing in a,
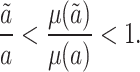 |
53 |
Hence, 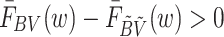 for all
for all 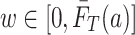 . On
. On 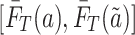 ,
, 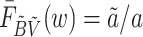 whereas
whereas  decreases from
decreases from 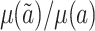 to
to 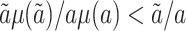 . For all
. For all  ,
, 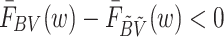 . Hence,
. Hence, 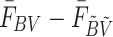 has a single sign change from positive to negative. Hence,
has a single sign change from positive to negative. Hence, 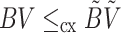 (Shaked and Shanthikumar 2007, Theorem 3.A.44).
(Shaked and Shanthikumar 2007, Theorem 3.A.44). 
Lemma 8
For any convex function 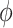 and any non-negative integer valued random variable N that is independent of
and any non-negative integer valued random variable N that is independent of 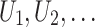 ,
,  is a convex function in v.
is a convex function in v.
Proof
As the binomial distribution 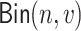 is a regular exponential family of distribution with expectation linear in v, Schweder (1982, Proposition 2) implies
is a regular exponential family of distribution with expectation linear in v, Schweder (1982, Proposition 2) implies  is convex in v for any positive integer n. As non-negative weighted sums of convex functions are also convex, it follows that
is convex in v for any positive integer n. As non-negative weighted sums of convex functions are also convex, it follows that  is a convex function in v.
is a convex function in v. 
Proof of Theorem 6
As  , if b takes values in
, if b takes values in 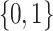 , then
, then  . Applying Shaked and Shanthikumar (2007, Theorem 3.A.21) with Lemmas 7 and 8,
. Applying Shaked and Shanthikumar (2007, Theorem 3.A.21) with Lemmas 7 and 8,
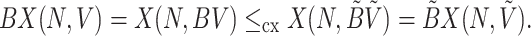 |
54 |
Since the convex order is transitive and closed under mixtures,
 |
55 |
In the notation of Theorem 1,  , where
, where 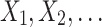 is a sequence of independent random variables with
is a sequence of independent random variables with 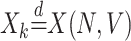 . From the thinning property of the Poisson process and Theorem 1, we can write
. From the thinning property of the Poisson process and Theorem 1, we can write 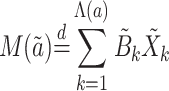 , where
, where  is a sequence of independent random variables with
is a sequence of independent random variables with  and
and 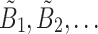 is a sequence of independent
is a sequence of independent  random variables that are also independent of the
random variables that are also independent of the  . As the convex order is closed under random sums,
. As the convex order is closed under random sums,
 |
56 |

Figure 3 shows the four indices (coefficient of variation, Gini, Pietra, and  ) for the parasite load M(a) of a host aged a in the Tallis-Leyton model with rate of infectious contacts
) for the parasite load M(a) of a host aged a in the Tallis-Leyton model with rate of infectious contacts 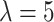 , the number of parasites (N) entering the host during an infectious contact following a
, the number of parasites (N) entering the host during an infectious contact following a 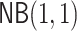 distribution, and parasite lifetimes (T) following an
distribution, and parasite lifetimes (T) following an  distribution. All four indices are strictly decreasing in host age. The Pietra index appears to be crudely interpolated, however all indices were evaluated on the same grid with a step size of 0.01. The ages where the Pietra index appears non-differentiable are those ages where the expected parasite load of the host is integer valued. Specifically, the expected parasite load of the host is
distribution. All four indices are strictly decreasing in host age. The Pietra index appears to be crudely interpolated, however all indices were evaluated on the same grid with a step size of 0.01. The ages where the Pietra index appears non-differentiable are those ages where the expected parasite load of the host is integer valued. Specifically, the expected parasite load of the host is  so the host has integer valued expected parasite load at ages 0.22, 0.51, 0.92 and 1.61. As in Fig. 1, the Pietra index coincides with
so the host has integer valued expected parasite load at ages 0.22, 0.51, 0.92 and 1.61. As in Fig. 1, the Pietra index coincides with 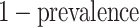 for
for 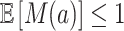 , that is for
, that is for  .
.
Fig. 3.

Plot of the coefficient of variation (orange dotted line), Gini index (purple dashed line), Pietra index (yellow solid line), and  (blue dot-dashed line) for a host in the Tallis-Leyton model with
(blue dot-dashed line) for a host in the Tallis-Leyton model with  ,
, 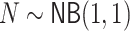 , and
, and  . Since
. Since  for
for  , the Pietra index and
, the Pietra index and 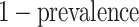 coincide for
coincide for 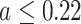 as expected (24)
as expected (24)
Parasite lifetime distribution
We now assess the effect of variability in the parasite lifetime distribution (T) on parasite aggregation. Rather than assuming  , we will assume that
, we will assume that 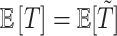 and
and  has a single sign change from positive to negative. As noted in the last bullet point of Sect. 2.2, these conditions imply
has a single sign change from positive to negative. As noted in the last bullet point of Sect. 2.2, these conditions imply  . The below result shows that increased variability in the parasite lifetimes decreases parasite aggregation in the sense of the Lorenz order. In particular, the result implies that the host’s parasite load is most aggregated when parasites have constant lifetimes.
. The below result shows that increased variability in the parasite lifetimes decreases parasite aggregation in the sense of the Lorenz order. In particular, the result implies that the host’s parasite load is most aggregated when parasites have constant lifetimes.
Theorem 9
Suppose 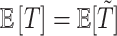 and
and 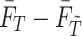 has a single sign change from positive to negative. Assume all other model parameters are equal. Then
has a single sign change from positive to negative. Assume all other model parameters are equal. Then  .
.
Proof
We first show that for all  ,
,
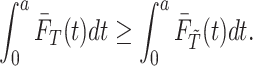 |
57 |
Define the function 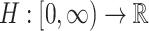 as
as
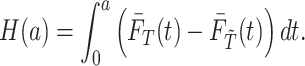 |
58 |
By definition  . As
. As 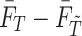 has a single sign change from positive to negative, H first increases and then decreases on
has a single sign change from positive to negative, H first increases and then decreases on 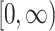 . Since
. Since 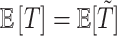 ,
, 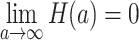 . Hence,
. Hence, 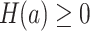 for all
for all 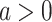 and (57) is established. For any
and (57) is established. For any 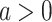 , set
, set  such that
such that
 |
59 |
It follows from (57) that 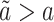 . Let
. Let 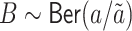 . Let V have distribution (31) and let
. Let V have distribution (31) and let 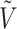 have the distribution (31) with a replaced by
have the distribution (31) with a replaced by  and T replaced by
and T replaced by  . The survival functions of
. The survival functions of 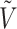 and BV are
and BV are
 |
60 |
and
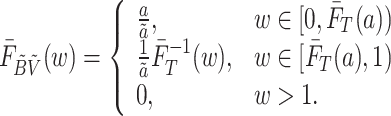 |
61 |
Since 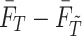 has a single sign change from positive to negative, it follows that
has a single sign change from positive to negative, it follows that  also has a single sign change from positive to negative. Hence,
also has a single sign change from positive to negative. Hence,  (Shaked and Shanthikumar 2007, Theorem 3.A.44). Applying Lemma 8 and Shaked and Shanthikumar (2007, Theorem 3.A.21) together shows
(Shaked and Shanthikumar 2007, Theorem 3.A.44). Applying Lemma 8 and Shaked and Shanthikumar (2007, Theorem 3.A.21) together shows 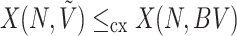 . From Theorem 1,
. From Theorem 1,  and
and 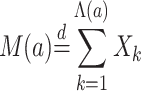 , where
, where 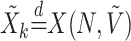 and
and 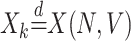 . Let
. Let 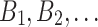 be a sequence of independent
be a sequence of independent 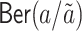 random variables that are also independent of
random variables that are also independent of  By construction
By construction 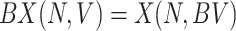 . From the thinning property of the Poisson process,
. From the thinning property of the Poisson process, 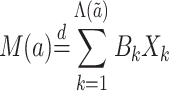 . As the convex order is closed under random sums, we see
. As the convex order is closed under random sums, we see  . Letting
. Letting  and noting that the convex order is closed under weak limits, we see
and noting that the convex order is closed under weak limits, we see 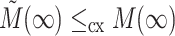 .
. 
That increasing variability in the parasite lifetimes decreases parasite aggregation seems counter-intuitive. However, if we consider the extreme case where the parasite lifetimes are constant, then we see that at any given age the host will either have all or none of the hosts from a previous infectious contact. Therefore, it is natural to expect this to lead to the greatest parasite aggregation. On the other hand, greater variability in the parasite lifetimes effectively spreads out when parasites die, leading to less parasite aggregation.
Asymptotic normality
As noted previously, when the host’s parasite load to converges to a normal distribution, the Gini and Petra indices can each be approximate by a constant multiple of the coefficient of variation as indicated by the limits (28) and (30). The final result shows that when the rate of infectious contacts in the Tallis-Leyton model tends to infinity, the distribution of the host’s parasite load converges to a normal distribution.
Theorem 10
Suppose there exists positive constants 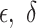 and C such that
and C such that
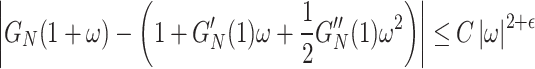 |
62 |
for all  such that
such that 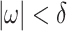 . Then
. Then
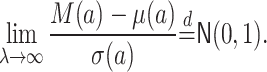 |
63 |
Proof
The characteristic function of M(a) is 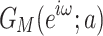 . We aim to show that
. We aim to show that
 |
64 |
The result then follows by Lévy’s convergence theorem. Define
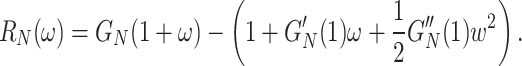 |
65 |
For non-negative integers n and real x define
 |
66 |
Then 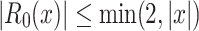 and
and
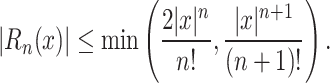 |
67 |
(Williams 1991, pg 183). Note that
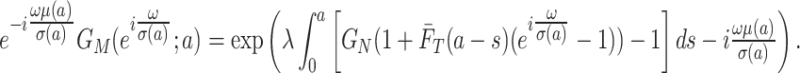 |
68 |
From the expressions for  and
and  ,
,
 |
69 |
From the expression for 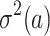 and the fact that
and the fact that
 |
70 |
we obtain
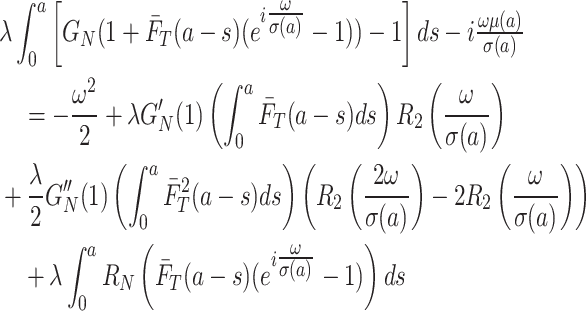 |
71 |
Using the bound (67) and the fact that  , we see
, we see
 |
72 |
and
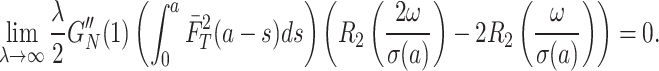 |
73 |
Finally, using 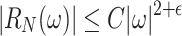 together with the bound (67) and the fact that
together with the bound (67) and the fact that 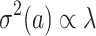 , we see
, we see
 |
74 |
Hence, the limit (64) holds. 
Figure 4 compares the probability mass function of the host’s parasite load, M(a), in the Tallis-Leyton model with the probability density function of the approximating normal distribution. The Tallis-Leyton model used a host aged 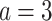 , number of parasites (N) entering the host during an infectious having a
, number of parasites (N) entering the host during an infectious having a 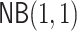 distribution, and parasite lifetimes (T) having an
distribution, and parasite lifetimes (T) having an 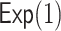 distribution. When the rate of infectious contact
distribution. When the rate of infectious contact 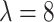 , the probability mass function still shows some right skewness. The normal approximation in this instance places a non-negligible probability on values less than zero. When
, the probability mass function still shows some right skewness. The normal approximation in this instance places a non-negligible probability on values less than zero. When  , the probability mass function is very close to symmetric and the normal distribution provides a good approximation. Figure 5 shows the Gini and Pietra indices together with the approximations based on the limits (28) and (30). In this instance the approximations of the Gini and Pietra indices appear reasonably accurate even for
, the probability mass function is very close to symmetric and the normal distribution provides a good approximation. Figure 5 shows the Gini and Pietra indices together with the approximations based on the limits (28) and (30). In this instance the approximations of the Gini and Pietra indices appear reasonably accurate even for 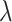 as small as 2 where the normal approximation is poor.
as small as 2 where the normal approximation is poor.
Fig. 4.

Probability mass function (blue bars) and approximating normal probability density function (red line) for a host aged 3 in the Tallis-Leyton model with  ,
,  , and
, and 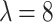 (left) and
(left) and 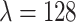 (right)
(right)
Fig. 5.
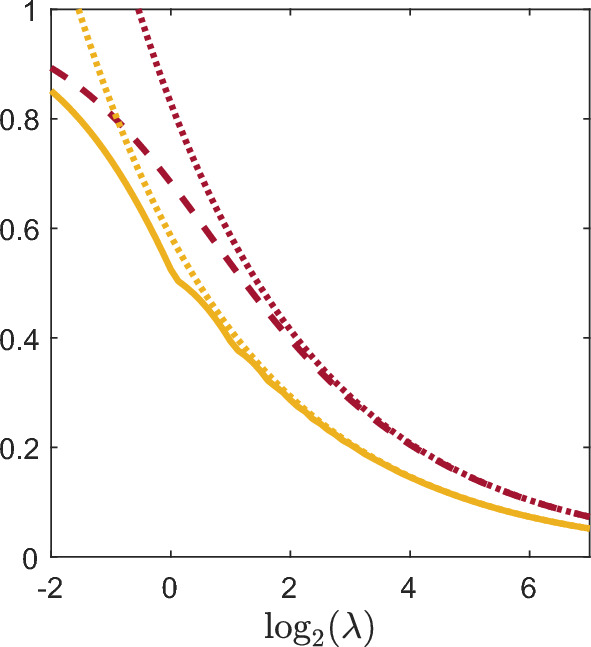
Gini index (purple dashed line) and Pietra index (yellow line) together with the asymptotic normal approximations (dotted lines) for a host aged 3 in the Tallis-Leyton model with 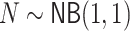 and
and 
Discussion
This study examined how variation in M(a), the parasite load of age a hosts, in the Tallis-Leyton model is affected by the host age, the rate of infectious contacts (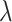 ), the distribution of the number of parasites (N) entering the host during an infectious contact, and the distribution of parasite lifetimes (T). Variation in the parasite load was quantified by several aggregation metrics. While there are many aggregation measures used in the parasitology literature, this study focused on measures related to the Lorenz ordering of distributions, specifically the coefficient of variation, the Gini index, Pietra index, and
), the distribution of the number of parasites (N) entering the host during an infectious contact, and the distribution of parasite lifetimes (T). Variation in the parasite load was quantified by several aggregation metrics. While there are many aggregation measures used in the parasitology literature, this study focused on measures related to the Lorenz ordering of distributions, specifically the coefficient of variation, the Gini index, Pietra index, and 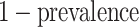 . The Lorenz based measures of aggregation all decrease together if variation in the distribution decreases in the sense of the Lorenz order and are constrained by equality (24) and inequality (25). Furthermore, when the parasite load has approximately a normal distribution the Gini and Pietra indices can each be approximate by a constant multiple of the coefficient of variation.
. The Lorenz based measures of aggregation all decrease together if variation in the distribution decreases in the sense of the Lorenz order and are constrained by equality (24) and inequality (25). Furthermore, when the parasite load has approximately a normal distribution the Gini and Pietra indices can each be approximate by a constant multiple of the coefficient of variation.
The analysis showed that an increase in the rate of infectious contacts or an increase in the host age results in a decrease in the aggregation of parasite load using the Lorenz based measures. These results are perhaps not surprising in light of the behaviour of the Poisson distribution, which decreases in the Lorenz order as the mean increases. It might also be expected that increased variability in the the number of parasites entering the host during an infectious contact results in increased aggregation of parasite load using the Lorenz based measures. However, that increased variation in the parasite lifetimes decreases parasite aggregation in the limit as host age tends to infinity seems counter-intuitive. This result can be understood as variability in parasite lifetimes spreads out when parasites die and hence results in less variable parasite loads.
Although only four measure of aggregation based on the Lorenz order were explicitly mentioned in this study, these results extend to any other index respecting the Lorenz order. On the other hand, measures of aggregation not based on the Lorenz order may behave differently. For example, the variance-to-mean ratio is not affected by changes to the rate of infectious contacts. Also, if the number of parasites entering the host during an infectious contact has a 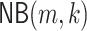 distribution, then an increase in m results in an increase in the variance-to-mean ratio, but the Lorenz based measures decrease.
distribution, then an increase in m results in an increase in the variance-to-mean ratio, but the Lorenz based measures decrease.
Unfortunately, the population dynamics of parasites are often more complicated than what is represented in the Tallis-Leyton model. Some parasites need multiple hosts to complete its life cycle. Once a parasite finds a host it may be subject to intraspecific and interspecific competition for resources. Furthermore, parasites often interact with the host either by stimulating an immune response from the host or by increasing the host’s mortality rate.
Isham (1995) proposed a simple stochastic model that incorporates parasite induced host mortality. In Isham’s model, the host acquires parasites following the same dynamics as the Tallis-Leyton model and parasite lifetimes are assumed exponentially distributed. The important difference in Isham’s model is that each parasite present in the host increases the host’s death rate by a fixed amount  . A complete analysis of Isham’s model in terms of the Lorenz order is beyond the scope of this paper. In a special case, however, we see that parasite induced host mortality increases aggregation of the parasite distribution in the sense of the Lorenz order. When the number of parasites that enter the host at an infectious contact follows a geometric distribution, an explicit expression for the limiting distribution is possible. Specifically, if
. A complete analysis of Isham’s model in terms of the Lorenz order is beyond the scope of this paper. In a special case, however, we see that parasite induced host mortality increases aggregation of the parasite distribution in the sense of the Lorenz order. When the number of parasites that enter the host at an infectious contact follows a geometric distribution, an explicit expression for the limiting distribution is possible. Specifically, if 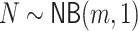 , then
, then
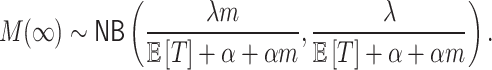 |
75 |
As the negative binomial distribution is decreasing in Lorenz order in both mean and k, it follows that indices respecting the Lorenz order are increasing in the parasite induced host mortality rate. In contrast, the variance-to-mean ratio is  so it is not affected by the parasite induced mortality.
so it is not affected by the parasite induced mortality.
A complete examination Isham’s model in terms of the Lorenz order may prove challenging. Even computing the Gini and Pietra indices may present difficulties since they require absolute moments, which are often not easily evaluated. In that case, the coefficient of variation may prove useful since it respects the Lorenz order, is easily evaluated, and can be used to approximate the Gini and Pietra indices when the distribution is approximately normal.
Acknowledgements
The author express his thanks to the associate editor and two referees for their detailed and thoughtful comments on the original version of the paper.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Data availability
The code used to generate the figures in this paper are publicly available on Zenodo (McVinish 2025).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abate J, Whitt W (1992) Numerical inversion of probability generating functions. Oper Res Lett 12:245–251. 10.1016/0167-6377(92)90050-D [Google Scholar]
- Adell JA, De La Cal J (1994) Approximating gamma distributions by normalized negative binomial distributions. J Appl Probab 31:391–400. 10.2307/3215032 [Google Scholar]
- Anderson RM, May RM (1978a) Regulation and stability of host-parasite population interactions: I. Regulatory processes. J Anim Ecol 47:219–247. 10.2307/3933 [Google Scholar]
- Anderson RM, May RM (1978b) Regulation and stability of host-parasite population interactions: II. Destabilizing processes. J Anim Ecol 47:249–267. 10.2307/3934 [Google Scholar]
- Arnold BC, Sarabia JM (2018) Majorization and the Lorenz order with applications in applied mathematics and economics. Springer, New York. 10.1007/978-3-319-93773-1 [Google Scholar]
- Barbour AD, Pugliese A (2000) On the variance-to-mean ratio in models of parasite distributions. Adv Appl Probab 32:701–719. 10.1239/aap/1013540240 [Google Scholar]
- Gastwirth JL (1971) A general definition of the Lorenz curve. Econometrica 39:1037–1039. 10.2307/1909675 [Google Scholar]
- Gini C (1914) Sulla misura della concentrazione e della variabilità dei caratteri. Atti Ist Ven 73:1203–1248 [Google Scholar]
- Gini C (2005) On the measurement of concentration and variability of characters. METRON Int J Stat 63:3–38 [Google Scholar]
- Herbert J, Isham V (2000) Stochastic host-parasite interaction models. J Math Biol 40:343–371. 10.1007/s002850050184 [DOI] [PubMed] [Google Scholar]
- Herrero-Cófreces S, Flechoso MF, Rodríguez-Pastor R, Luque-Larena JJ, Mougeot F (2021) Patterns of flea infestation in rodents and insectivores from intensified agro-ecosystems. Parasites Vectors 14:16. 10.1186/s13071-020-04492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Holman DF, Chaudhry ML, Kashyap BRK (1983) On the service system
 . Eur J Oper Res 13:142–145. 10.1016/0377-2217(83)90075-9 [Google Scholar]
. Eur J Oper Res 13:142–145. 10.1016/0377-2217(83)90075-9 [Google Scholar] - Isham V (1995) Stochastic models of host-macroparasite interaction. Ann Appl Probab 5:720–740. 10.1214/aoap/1177004702 [Google Scholar]
- Jovani R, Tella JL (2006) Parasite prevalence and sample size: misconceptions and solutions. Trends Parasitol 22:214–218. 10.1016/j.pt.2006.02.011 [DOI] [PubMed] [Google Scholar]
- Kura K, Truscott JE, Collyer BS, Phillips A, Garba A, Anderson RM (2022) The observed relationship between the degree of parasite aggregation and the prevalence of infection within human host populations for soil-transmitted helminth and schistosome infections. Trans R Soc Trop Med Hyg 116:1226–1229. 10.1093/trstmh/trac033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MO (1905) Methods of measuring the concentration of wealth. Publ Am Stat Assoc 9:209–219. 10.2307/2276207 [Google Scholar]
- McPherson NJ, Norman RA, Hoyle AS, Bron JE, Taylor NGH (2012) Stocking methods and parasite-induced reductions in capture: modelling Argulus foliaceus in trout fisheries. J Theor Biol 312:22–33. 10.1016/j.jtbi.2012.07.017 [DOI] [PubMed] [Google Scholar]
- McVinish R (2025) Numerical evaluation of aggregation indices for the Tallis-Leyton model. Zenodo. 10.5281/zenodo.15293018 [Google Scholar]
- McVinish R, Lester RJG (2020) Measuring aggregation in parasite populations. J R Soc Interface 17:20190886. 10.1098/rsif.2019.0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVinish R, Lester RJG (2024) A graphical exploration of the relationship between parasite aggregation indices. PLoS ONE 19:e0315756. 10.1371/journal.pone.0315756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill A, Poulin R, Forbes MR (2023) Interrelationships and properties of parasite aggregation measures: a user’s guide. Int J Parasitol 53:763–776. 10.1016/j.ijpara.2023.06.004 [DOI] [PubMed] [Google Scholar]
- Peacock SJ, Bouhours J, Lewis MA, Molnár PK (2018) Macroparasite dynamics of migratory host populations. Theor Popul Biol 120:29–41. 10.1016/j.tpb.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Pielou EC (1977) Mathematical ecology, Chapter 8. Wiley, New York [Google Scholar]
- Pietra G (1915) Delle relazioni tra gli indici di variabilità (nota I). Atti Ist Ven 74:775–792 [Google Scholar]
- Pietra G (2014) On the relationships between variability indices (note I). METRON 72:5–16. 10.1007/s40300-014-0034-3 [Google Scholar]
- Poulin R (1993) The disparity between observed and uniform distributions: a new look at parasite aggregation. Int J Parasitol 23:931–944. 10.1016/0020-7519(93)90060-C [DOI] [PubMed] [Google Scholar]
- Poulin R (2007) Are there general laws in parasite ecology? Parasitology 134:763–776. 10.1017/S0031182006002150 [DOI] [PubMed] [Google Scholar]
- Poulin R (2011) Evolutionary ecology of parasites, Chapter 6, 2nd edn. Princeton University Press, Princeton [Google Scholar]
- Ramasubban TA (1958) The mean difference and the mean deviation of some discontinuous distributions. Biometrika 45:549–556. 10.1093/biomet/45.3-4.549 [Google Scholar]
- Reiczigel J, Marozzi M, Fábián I, Rózsa L (2019) Biostatistics for parasitologists—a primer to quantitative parasitology. Trends Parasitol 35:277–281. 10.1016/j.pt.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Hernández K, Álvarez Mendizábal P, Chapa-Vargas L, Escobar F, González-García F, Santiago-Alarcon D (2021) Haemosporidian prevalence, parasitaemia and aggregation in relation to avian assemblage life history traits at different elevations. Int J Parasitol 51:365–378. 10.1016/j.ijpara.2020.10.006 [DOI] [PubMed] [Google Scholar]
- Rosà R, Pugliese A (2002) Aggregation, stability, and oscillations in different models for host-macroparasite interactions. Theor Popul Biol 61(3):319–334. 10.1006/tpbi.2002.1575 [DOI] [PubMed] [Google Scholar]
- Schreiber SJ (2006) Host-parasitoid dynamics of a generalized Thompson model. J Math Biol 52:719–732. 10.1007/s00285-005-0346-2 [DOI] [PubMed] [Google Scholar]
- Schrock SAR, Walsman JC, DeMarchi J, LeSage EH, Ohmer MEB, Rollins-Smith LA, Briggs CJ, Richards-Zawacki CL, Woodhams DC, Knapp RA, Smith TC, Haddad CFB, Becker CG, Johnson PTJ, Wilber MQ (2025) Do fungi look like macroparasites? Quantifying the patterns and mechanisms of aggregation for host-fungal parasite relationships. Proc R Soc B 292:20242013. 10.1098/rspb.2024.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweder T (1982) On the dispersion of mixtures. Scand J Stat 9:165–169 [Google Scholar]
- Shaked M, Shanthikumar JG (2007) Stochastic orders. Springer, New York. 10.1007/978-0-387-34675-5 [Google Scholar]
- Shaw DJ, Dobson AP (1995) Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology 111:S111–S133. 10.1017/s0031182000075855 [DOI] [PubMed] [Google Scholar]
- Taguchi T (1968) Concentration-curve methods and structures of skew populations. Ann Inst Stat Math 20:107–141. 10.1007/BF02911628 [Google Scholar]
- Tallis GM, Leyton MK (1969) Stochastic models of populations of helminthic parasites in the definitive host. I. Math Biosci 4:39–48. 10.1016/0025-5564(69)90006-6 [Google Scholar]
- The MathWorks Inc. (2022a) Matlab version: 9.13.0.2080170 (r2022b) update 1
- The MathWorks Inc. (2022b) Symbolic math toolbox (r2022b)
- Williams D (1991) Probability with martingales. Cambridge University Press, Cambridge. 10.1017/CBO9780511813658 [Google Scholar]
- Wilson K, Bjørnstad O, Dobson A, Merler S, Poglayen G, Randolph S, Read A, Skorping A (2001) Heterogeneities in macroparasite infections: patterns and processes. In: Hudson P, Rizzoli A, Grenfell B, Heesterbeek H, Dobson A (eds) The ecology of wildlife diseases. Oxford University Press, Oxford, pp 6–44. 10.1093/oso/9780198506201.003.0002 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The code used to generate the figures in this paper are publicly available on Zenodo (McVinish 2025).