Abstract
Background
Hepatocellular carcinoma (HCC), a common and lethal form of liver cancer, includes mitochondrial dysfunction in its pathogenesis.
Objectives
This study investigated the relationship between mitochondrial function-related genes and HCC progression.
Methods
We systematically retrieved mitochondrial genetic data from the MitoCarta database and identified differentially expressed genes through Gene Expression Omnibus analysis. Weighted gene co-expression network analysis was subsequently employed to construct co-expression networks and identify key modules associated with HCC progression. We evaluated 113 machine learning algorithms to develop mitochondrial gene-based prognostic models. Gene set enrichment analysis further delineated the pathways and biological processes enriched in the module hub genes, offering mechanistic insights into HCC. Immune infiltration analysis using CIBERSORT highlighted the pivotal roles of M1 and M2 macrophages in HCC. Finally, therapeutic candidates targeting critical genes were explored using computational drug prediction, molecular docking, and molecular dynamic simulations, providing novel strategies for HCC-targeted therapy.
Results
Stepwise Logistic Regression with Gradient Boosting Machine was chosen as the optimal model (area under the curve [AUC] = 0.977). Moreover, 15 potential HCC biomarkers were identified, including PSMD4 (AUC = 0.888), TBCE (AUC = 0.879), and CKS1B (AUC = 0.860). Additionally, fluoxetine and paroxetine were predicted as potential HCC drugs and validated through molecular docking and dynamic simulations.
Conclusions
This study highlights the prognostic significance of mitochondrial function-related genes in HCC and establishes a framework for developing innovative diagnostic and therapeutic interventions. Future research should prioritize clinical validation of these findings and evaluate the translational potential of the identified drug candidates in HCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-03216-5.
Keywords: Mitochondria, Hepatocellular carcinoma, Machine learning, Prognostic modelling, Drug prediction, Molecular docking
Introduction
Hepatocellular carcinoma (HCC) is a significant global health concern, ranking sixth among common malignancies and third in terms of tumor mortality [1]. Globally, the incidence of HCC attributed to metabolic risks has continued to increase since 1990. The highest mortality burden and disability-adjusted life years were consistently observed in high sociodemographic index (SDI) countries, whereas sustained increases in these metrics were evident in low-to-middle SDI nations; this trend imposes a growing clinical burden on global public health systems [2]. Current treatment paradigms for HCC primarily comprise early stage surgical resection [3], targeted therapy [4], and immunotherapy [5]. However, clinical management of HCC remains challenging due to drug resistance, high metastatic potential, and elevated recurrence rates, which collectively contribute to compromised patient survival outcomes. These limitations underscore the critical need to develop innovative strategies to improve early detection, implement preventive measures, and optimize therapeutic interventions at disease onset [6].
The application of high-throughput sequencing technology in HCC has facilitated the discovery of the underlying mechanisms that contribute to HCC development [7, 8]. Owing to discrepancies in molecular characteristics, patients at the same stage of the disease may exhibit comparable tumor morphology and clinical presentation, yet demonstrate different responses to the same treatment strategies. Therefore,the utilization of advanced methodologies is imperative for identifying prognostic markers in HCC, with the objective of enhancing the predictive accuracy of patient outcomes.
An increasing number of studies have employed histological techniques to gain a deeper understanding of tumor biology. The mitochondria are vital organelles that regulate cellular energy production, metabolism, proliferation, and apoptosis. The altered function of mitochondria is a hallmark of cancer and plays a pivotal role in cell proliferation and death [9, 10]. In addition to the key bioenergetic genes encoded by the mitochondria, the mitochondria-associated genome comprises more than 1000 genes, and there are certain genetic predispositions that may lead to mitochondrial dysfunction [11]. Emerging evidence indicates that mitochondrial dysfunction plays a crucial role in the pathogenesis and progression of HCC by influencing cellular metabolism, oxidative stress, and cell fate. In addition, dysregulation of mitochondrial dynamics, impaired mitophagy, and altered mitochondrial metabolism are frequently observed in HCC and contribute to tumor initiation, growth, and therapy-resistance [12].
Given the role of mitochondrial dysfunction in HCC progression, we hypothesize that mitochondria-related genes influence tumorigenesis through both intrinsic cellular pathways and tumor immune microenvironment interactions. Towards this end, gene co-expression analysis, machine learning-based prognostic modeling, and immune infiltration profiling were integrated to construct and validate a comprehensive HCC prognosis model and explore its clinical application value. The research approach is illustrated in Fig. 1.
Fig. 1.
Flow chart of the study approach
Materials and methods
Data sources for genes associated with mitochondrial function in hepatocellular carcinoma
In this study, HCC datasets were obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/gds/), and three datasets were selected for further analysis, GSE54236, GSE76427, and GSE84402. The probes in each dataset were annotated and converted into standard gene names according to the corresponding platform annotation files. Expression profiles from the GSE54236 and GSE76427 datasets were merged to create a training set that was adjusted for batch effects using the ‘sva’ package. This training set was subsequently analyzed using weighted gene co-expression network analysis (WGCNA) and machine learning. In contrast, GSE84402 was used as a standalone validation dataset. MitoCarta is a comprehensive and authoritative database of human mitochondrial genes that offers researchers the ability to download data for analysis. The mitochondrial-related genes set was downloaded from the MitoCarta database (https://personal.broadinstitute.org/scalvo/MitoCarta3.0/human.mitocarta3.0.html), and 1136 mitochondrial-related genes were obtained after eliminating duplicates.
Analysis of differentially expressed genes
Following standardization and normalization of the dataset, the differential genes between HCC and control samples were analyzed using the'limma' R package. The absolute value of LogFC was greater than 1.5 times the screening criteria, which was set at a corrected P-value of less than 0.05. Volcano and heat maps were generated using the R software packages'ggplot2' and'pheatmap,’ respectively, to illustrate the significant differentially expressed genes (DEGs).
Weighted gene co-expression network analysis
The ‘WGCNA’ package was employed in the current study, as it offers a suite of R functions for the analysis of weighted correlation networks, including the examination of co-expression networks derived from gene expression data [13]. Co-expression networks were constructed using the merged dataset, retaining genes with standard deviations greater than 0.5. The minimum number of genes in each module was set to 60. Subsequently, the adjacency matrix was transformed into a topological overlap matrix, which was used to construct multiple gene modules and hierarchical clustering trees. Subsequently, the correlation between each module and clinical features was evaluated. Module gene significance (GS) and module membership (MM) were calculated to quantify the relevance and significance of the genes to the biological modules and clinical information. Significant modules and genes were then extracted for subsequent analyses.
Enrichment analysis of DEGs in HCC
To accurately identify important genes, the intersection of modular genes in DEGs was assessed to identify the set of HCC hub genes, and then, the intersection with mitochondria-related genes was assessed to obtain the final hub genes. Subsequently, the hub genes were subjected to GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses using the ‘Hs.eg.db’ and ‘clusterProfiler’ packages in R to identify the related signaling pathways. A P-value of less than 0.05 was used to identify the biological processes (BP), cellular components (CC), molecular functions (MF), and pathways implicated in the DEGs. The results were visualized using ‘ComplexHeatmap,’ ‘RColorBrewer,’ ‘ggplot2,’ and ‘enrichplot’ packages.
Exploring the construction of prognostic models using 12 machine learning algorithms for mitochondrial gene-related HCC
This study integrated 12 machine learning algorithms, including stepwise logistic regression (StepGLM), random forest (RF), gradient boosting machine (GBM), least absolute shrinkage and selection operator (Lasso), generalized linear model boosting (glmBoost), naive Bayes (NaiveBayes), support vector machine (SVM), elastic net (Enet), linear discriminant analysis (LDA), partial least squares regression generalized linear model (plsRglm), ridge regression (Ridge), and extreme gradient boosting (XGBoost) [14]. The 113 combinatorial algorithms derive from pairwise integrations of distinct base models, with classification of the secondary algorithm in each ensemble determined by α-value thresholds.These algorithms, with their unique capabilities, were combined to construct 113 prognostic models in order to enhance the predictive capacity for HCC prognosis. We applied these algorithm combinations to input genes derived from DEGs intersected with key module genes identified using WGCNA and mitochondria-related genes for training, and independent validation was performed using the external dataset GSE84402.
During model training and evaluation processes, the performance of the models was assessed using the receiver operating characteristic (ROC) curve, with the area under the curve (AUC) serving as an indicator of classification ability. Throughout the analysis, various algorithm combinations were thoroughly compared, and the model with the highest average AUC was selected as the optimal model for prognostic feature selection and classification prediction of mitochondria-related genes in HCC.
The StepGLM method uses stepwise regression with the step function, with the core parameter direction specifying variable selection trajectories. Rooted in generalized linear regression, this approach employs the Akaike information criterion (AIC) as the primary metric for feature selection. For the GBM component, key hyperparameters were configured as follows: loss function with the Bernoulli distribution, initial tree count of 10,000, learning rate of 0.001, tree depth of 3, and minimum node sample size of 10.
Model optimization was conducted using tenfold cross-validation to identify the iteration number, yielding a minimal validation error. The final model was trained using this optimal tree configuration to ensure robust generalization performance.
Evaluation of the diagnostic utility of pivotal biomarkers for HCC
The diagnostic value of the identified biomarkers was evaluated by plotting a ROC curve using the ‘pROC package’ in R. The expression levels and diagnostic efficacy of the candidate markers were further validated using the validation dataset (GSE84402). A confusion matrix was used to evaluate the performance of the prediction model. The genes obtained from the key module were displayed using volcano plots, created with the ‘ggplot’ package. The differences between the genes in the key modules of the HCC and control groups were shown as box plots. Additionally, ROC curves were plotted for each gene in the key module to assess the diagnostic value of individual genes.
Gene set enrichment analysis
Gene set enrichment analysis (GSEA) was used to examine the functional enrichment of the gene sets between the two groups. GSEA identifies a set of genes that exhibit a common biological function or regulatory signature and demonstrates coordinated expression changes within a given dataset, even when these changes are relatively subtle. This approach allows for the inclusion and analysis of genes that may exhibit only minor differences in expression but have a common impact. The R package'clusterProfiler' was used for the purpose of performing GSEA on differential genes. A P-value of less than 0.05 was set, and the gene set'c2.cp.kegg.Hs.symbols.gmt' was selected as a reference gene set.
Evaluation of the abundance and differential expression of immune cell subtypes
To investigate the functional relevance of the hub genes in the tumor immune microenvironment, we used the CIBERSORT algorithm. This analysis directly investigates the hypothesis that mitochondrial dysfunction-associated genes may regulate immune cell recruitment or polarization, thereby influencing HCC prognosis. Abundance and correlation of immune-infiltrating cells were analyzed using the CIBERSORT deconvolution algorithm. The CIBERSORT method depends on the LM22 immune cell subtype expression matrix, which was analyzed qualitatively and quantitatively using the CIBERSORT R package. The results of immune infiltration were filtered to preserve immune infiltration with a P-value of less than 0.05, and box plots of the DEGs were generated to demonstrate the relationship between immune cells and pivotal genes. Spearman’s correlation analysis was subsequently employed to further investigate the cell/gene relationship [15].
Hub gene potential drug prediction and molecular docking
The small molecule structures of potential drugs were obtained from PubChem, and hydrogenation of these small molecules was performed using AutoDock software (version 4.2.6, https://autodock.scripps.edu/downloads/). The Protein Data Bank (PDB) format files of key proteins were acquired from the AlphaFold protein structure database (https://alphafold.ebi.ac.uk/), imported into AutoDock, and a suitable Grid Box was set for molecular docking. Finally, the conformation with the lowest docking binding energy was selected as the final docking result and saved in ‘pdbqt’ format. The docking results were visualized using PyMOL software (Version 2.6.0, https://pymol.org/2/). Additionally, the results were validated using CB-dock2 (https://cadd.labshare.cn/cb-dock2/index.php) [16, 17].
Molecular dynamic simulations
Molecular dynamic (MD) simulations of the ligand-receptor complex were conducted using GROMACS (version 2022.2). The initial structure of the simulation was derived from an optimal docking model generated using molecular docking. The protein topology file was created using the AMBER99SB-ILDN force field, and the ligand topology file was generated using the ACPYPE script with the AMBER force field. The system was placed in a triclinic box solvated with TIP3P water molecules, and periodic boundary conditions were applied. To neutralize the system, counterions (Na + or Cl −) were added. Energy minimization was performed using the steepest descent and conjugate gradient methods for approximately 1000 steps to relax all the atoms. Subsequently, the system was equilibrated using a canonical ensemble (NVT) at 300 K for 100 ps, followed by an isothermal-isobaric ensemble (NPT) at 300 K for 100 ps. Finally, a production MD simulation was carried out for 100 ns to analyze the dynamic binding mode and stability of the ligand-receptor complex. The results were visualized and analyzed using the Origin software.
Results
Identification and validation of DEGs in HCC
Following batch correction using the ‘sva’ package, principal component analysis (PCA) plots confirmed decreased batch effects between the merged datasets (GSE54236 and GSE76427). A total of 1776 DEGs were identified, as shown in the volcano plot (Fig. 2).
Fig. 2.
Identification of DEGs: a represents the boxplot before batch correction; b illustrates the boxplot after batch correction; c displays the principal component analysis (PCA) plot before batch correction; d shows the PCA plot following batch correction; e presents the volcano plot of DEGs after batch correction; (f) illustrates the heatmap of DEGs post-batch correction
WGCNA identifies key modules associated with HCC progression
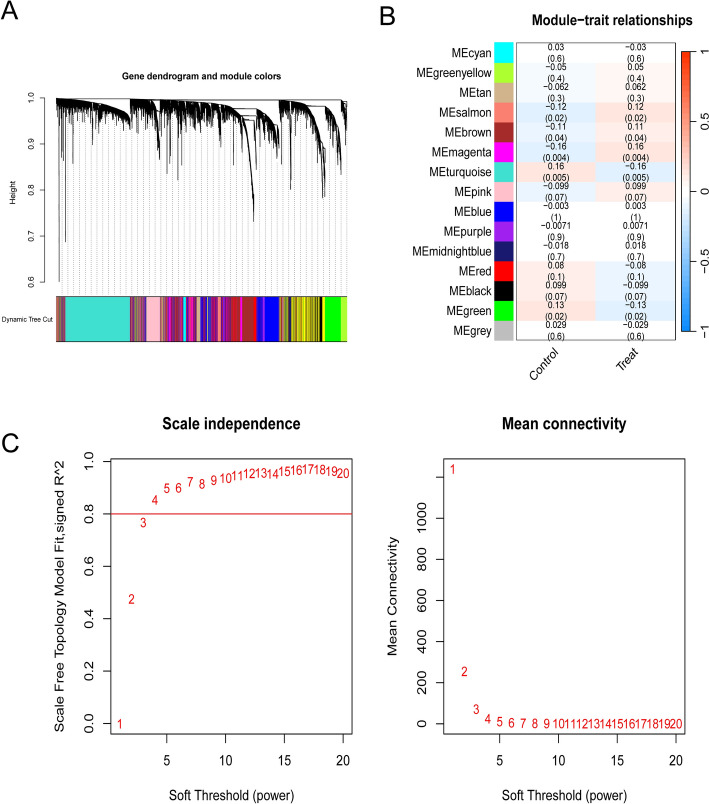
WGCNA was constructed for GSE54236 and GSE76427, which were merged into a single de-batched dataset to identify the co-expression modules and genes associated with HCC. As illustrated in Fig. 3, the highest correlation with HCC was observed in the MEmagenta module (r = -0.16, P = 0.004), which yielded 450 module genes.
Fig. 3.
Results of weighted gene co-expression analysis: a scale-free and average connectivity plots; b module correlation heatmap; c gene co-expression network mapping
GO and KEGG enrichment analyses
GO and KEGG analyses facilitated a comprehensive understanding of the biological functions, pathways, and interactions of similarly expressed proteins. In total, 185 GO and 2 KEGG items were obtained, and the top 10 results for GO items were presented according to the default sorting. The proteins identified were found to be involved in a number of pathways, including those pertaining to mitochondrial translation, mitochondrial gene expression, positive regulation of the lipid catabolic process, mitochondrial ribosomes, structural constituents of the ribosome, bile acid binding, and ribosomes (Fig. 4). These pathways are critical for the proliferation and metabolic reprogramming of HCC cells. See Supplementary material 1 for details.
Fig. 4.
Gene enrichment analysis and PPI interaction network analysis: a Gene Ontology (GO) enrichment analysis histogram; b GO enrichment analysis loop diagram; c Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment pathway
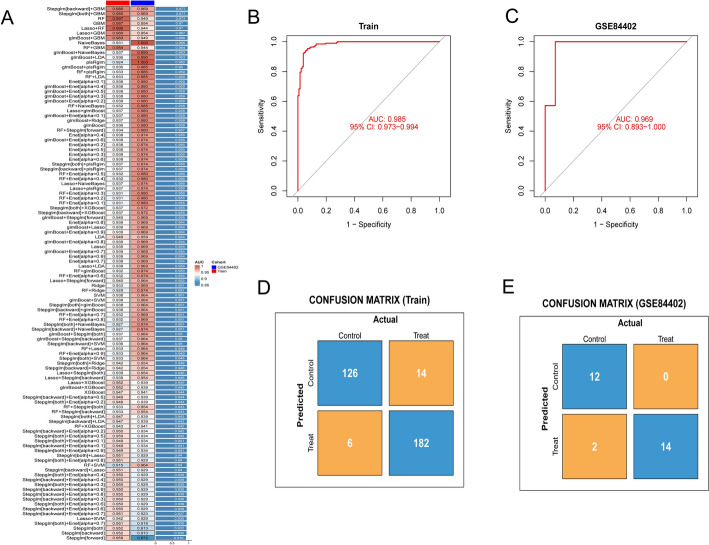
Machine learning screening of key genes with diagnostic values
Among the 113 combinations of machine learning evaluated, the StepGLM + GBM model demonstrated the highest predictive performance (Fig. 5a). This model incorporated 15 hub genes and showed strong diagnostic accuracy with an AUC of 0.985 (95% confidence interval [CI] 0.973–0.994) in the training cohort (Fig. 5b) and 0.969 (95% CI 0.893–1.000) in the validation cohort (GSE84402, Fig. 5c). The confusion matrices further validated the reliability of the model, showing high sensitivity and specificity for distinguishing patients with HCC from controls. In the training cohort, the sensitivity was 96.8% (182/[182 + 6]), and the specificity was 90.0% (126/[126 + 14]) (Fig. 5d). In the validation cohort, the sensitivity was 87.5% (14/[14 + 2]), and the specificity was 100% (12/[12 + 0]) (Fig. 5e). These results collectively demonstrated the robust predictive performance of the StepGLM + GBM model. The 15 potential biomarkers predicted by the optimal machine learning model StepGLM + GBM are detailed in Supplementary material 2. The key genes were PSMD4 (AUC = 0.888), TBCE (AUC = 0.879), and CKS1B (AUC = 0.860).
Fig. 5.
Machine learning was used to construct prognostic models for mitochondria-associated HCC genes.[] indicate categorization based on α-value thresholds, while the + symbol denotes algorithmic integration pairs: a the model constructed using the StepGLM + GBM algorithms was the most optimal; b efficacy of the StepGLM + GBM model was assessed using ROC curves in both the training and c validation cohorts; efficacy of the model biomarkers was assessed using the confusion matrix in both the training and validation cohorts; d confusion matrix for the training set; e confusion matrix for the validation sets
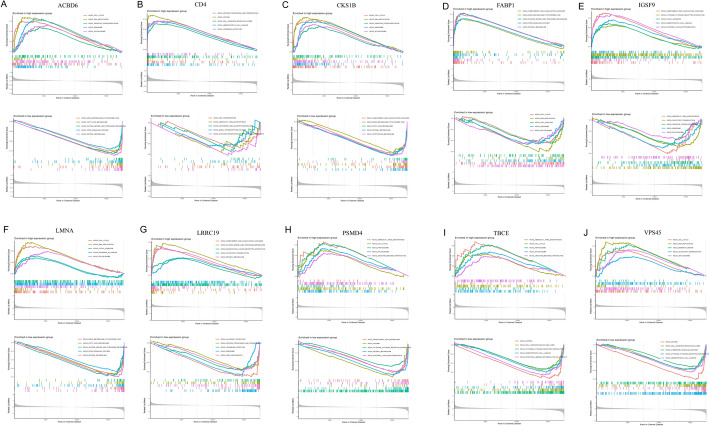
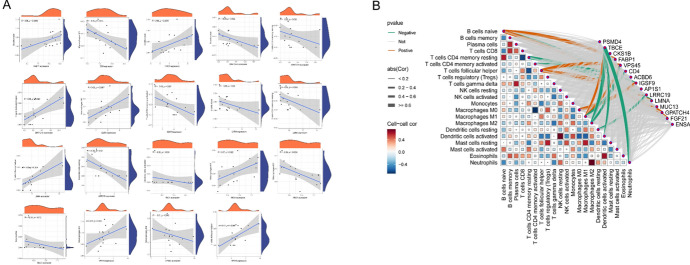
GSEA enrichment results
GSEA identified significant enrichment of mitochondria-related KEGG pathways in the high- and low-expression gene groups within the key modules. Specifically, the high-expression group showed pronounced enrichment in pathways associated with oxidative phosphorylation (ACBDA, NDUFB7, and COX7C) and mitochondrial fatty acid metabolism (CPT1A and ACADM), whereas the low-expression group was enriched for pathways linked to apoptosis regulation (CASP3 and BAX) and reactive oxygen species (ROS) detoxification (SOD2, GPX4) (Fig. 6). Notably, genes such as CD4 and FARP1, which have been implicated in immune modulation and mitochondrial membrane dynamics, were prominently represented in these pathways. These findings highlight the dual role of mitochondrial dysfunction in energy metabolism and redox homeostasis in HCC progression, suggesting potential therapeutic targets for mitochondrial pathway modulation.
Fig. 6.
Results of GSEA enrichment analysis of hub genes: a ACBD gene, b CD4 gene, c CKS1B gene, d FABP1 gene, e IGSF9 gene, f LMNA gene, g LRRC19 gene, h PSMD4 gene, i TBCE gene, j VPS45 gene
Immune infiltration analysis
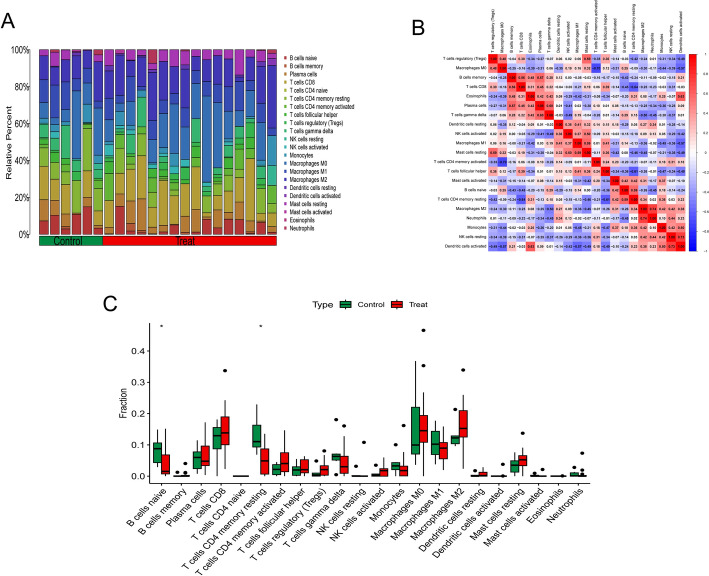
Building on the identification of the 15 mitochondria-related hub genes (Sect. 3.4), we evaluated their association with immune cell infiltration. The CIBERSORT algorithm facilitated a more detailed examination of the interrelationship between the pivotal modular genes of mitochondria-associated HCC and immune system function. Figure 7a illustrates the proportions of the 22 immune cells present in each HCC and control sample. A strong negative correlation was observed between the CD4 memory T cells and M0 macrophages (r = -0.70), whereas dendritic cells (DCs) exhibited a positive correlation with resting natural killer cells (NK cells resting) (r = 0.73, Fig. 7b). Figure 7c illustrates the different expression of the 22 cell types genes. Significant changes were observed in the activation of CD8 and CD4 memory T cells, M0 and M1 macrophages, and DCs compared to those in the control group.
Fig. 7.
Analysis of immune cell infiltration of key genes: a stacked histogram showing the proportion of immune cells in the experimental and control groups; b heatmap showing the correlation of model hub genes with 22 immune cell infiltrations; c bar graph comparing 22 immune cells in the experimental and control groups
Hub gene and immune cell correlation results
Correlation analyses were performed for model genes and immune cells, resulting in 19 significant correlations. In the correlation analysis, TBCE was negatively correlated with DCs, M1 and M2 macrophages, and neutrophils, whereas FABP1 was significantly positively correlated with B cells, as shown in Fig. 8.
Fig. 8.
Immuno-correlation results are shown: a Immune cells associated with each hub gene and their correlation coefficients; b Positive and negative regulatory relationships between genes and immune cells, where brown represents positive correlations and green represents negative correlations
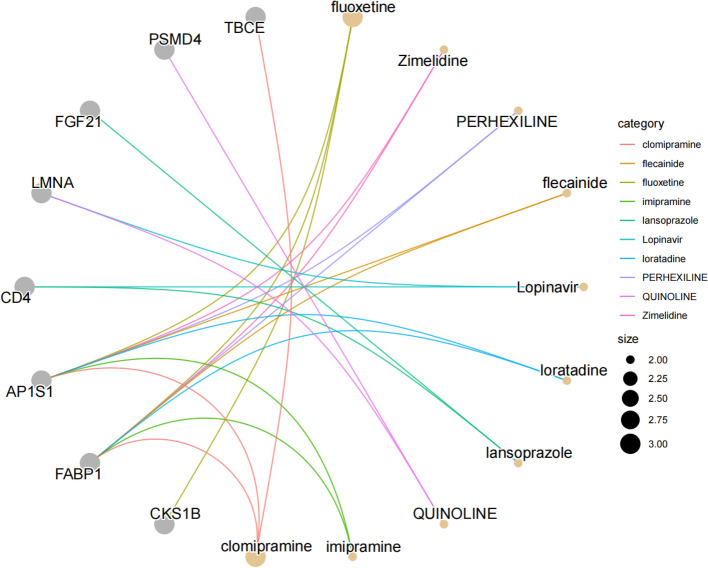
Analysis of drug projections
Given that the majority of drugs exert therapeutic effects by targeting proteins, we systematically explored drug-gene interactions and drug repurposing potential. Based on this analysis, fluoxetine and clomipramine were identified as promising candidates for HCC therapy. Specifically, molecular investigations revealed that both compounds targeted FABP1 and AP1S1. Furthermore, FABP1 emerged as a central hub, demonstrating interactions with multiple pharmacological agents, highlighting its potential as a therapeutic target. These findings provide a foundation for the development of targeted therapies for HCC (Fig. 9).
Fig. 9.
Target drug enrichment results. The grey nodes represent genes, orange nodes represent drugs, and connecting lines represent drug prediction relationships
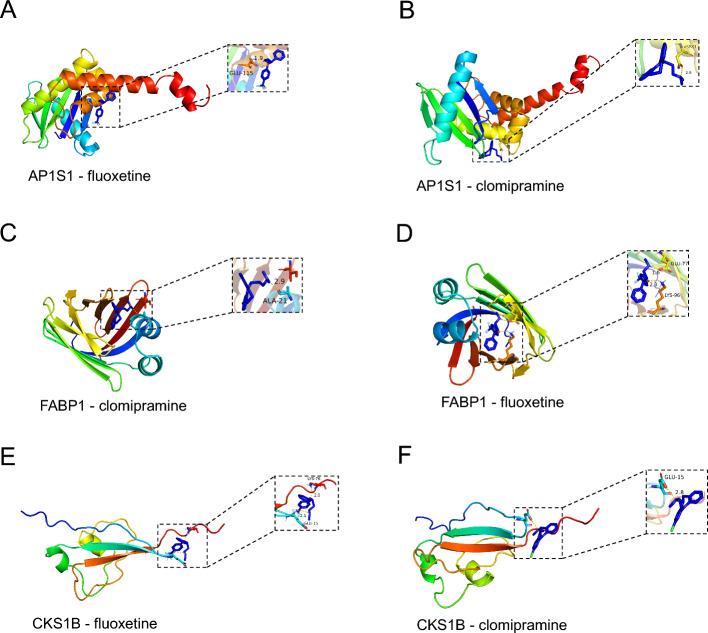
Molecular docking
A molecular docking approach was used to predict the binding interactions between key drugs and hub genes. The binding energies of both the primary active ingredient and the primary target were -5.0 kcal/mol. As the binding energy decreases, the binding activity and ease of binding to the target increase. The results of the molecular docking analysis indicated that the genes and predicted drugs could form stable conformations, as shown in Table 1. A visual representation of the complete set of molecular docking results is presented in Fig. 10. Supplementary material 3 contains results from validation with CB-Dock2.
Table 1.
Molecular docking results of partial drugs and targets
| Predicted drugs | Protein | Alphafold ID | binding energy | Amino acid residues with hydrogen bonding interactions |
|---|---|---|---|---|
| fluoxetine | AP1S1 | AF-P61966-F1-v4 | − 6.2 | GLU-115 |
| clomipramine | AP1S1 | AF-P61966-F1-v4 | − 5.66 | ASP-91 |
| clomipramine | FABP1 | AF-P07148-F1-v4 | − 7.77 | ALA-21 |
| fluoxetine | FABP1 | AF-P07148-F1-v4 | − 6.78 | GLU-77, LYS-96 |
| clomipramine | CKS1B | AF-P61024-F1-v4 | − 6.15 | GLU-115 |
| fluoxetine | CKS1B | AF-P61024-F1-v4 | − 5.62 | GLU-115, LYS-76 |
Fig. 10.
Molecular docking demonstration of partial drugs and targets: Binding pattern of a fluoxetine with AP1S1; b clomipramine with AP1S1; c clomipramine with FABP1; d fluoxetine with FABP1 e fluoxetine with CKS1B; and f clomipramine with CKS1B
MD simulation results
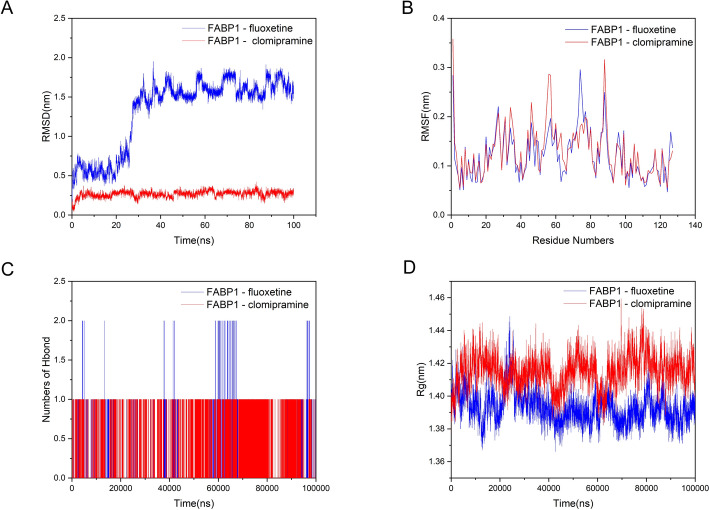
To validate the molecular docking and visualization results, we conducted further analyses using MD simulations. The root mean square deviation (RMSD) reflects the fluctuation in protein conformation and can be used to assess the stability of ligand binding to the target protein, with lower values indicating more stable binding. The RMSD values of the FABP1-fluoxetine and FABP1-clomipramine complexes remained relatively stable during the middle and late stages (Fig. 11a). The root-mean-square fluctuation (RMSF) reflects the fluctuation in individual amino acid residues within the protein. Figure 11b illustrates that the overall structures of the FABP1-fluoxetine and FABP1-clomipramine complexes were relatively stable, although some regions exhibited fluctuations. Hydrogen bonds are among the strongest noncovalent interactions, and the number of hydrogen bonds formed during the simulation reflects the binding strength. Figure 11c shows the changes in hydrogen bond numbers during the simulation, with FABP1-clomipramine maintaining one to two hydrogen bonds, indicating good binding strength. The radius of gyration (Rg) is commonly used to assess the overall compactness of a protein and can also be used to measure molecular affinity. Indeed, the Rg values of the proteins remained relatively stable during the simulation (Fig. 11d). These results demonstrated stable interactions among the two well-performing docking complexes, providing potential therapeutic agents for the treatment of HCC.
Fig. 11.
Results of the molecular dynamic simulation: a Temporal evolution of the root mean square deviation (RMSD); b residue-specific root mean square fluctuation (RMSF) profile; c dynamic variation of hydrogen bond (Hbond) numbers; d distribution characteristics of radius of gyration (Rg)
Discussion
HCC is the most prevalent liver cancer. Despite recent advances in diagnostic techniques and therapeutic modalities, the prognosis of HCC remains unfavorable due to delays in diagnosis [18, 19]. Therefore, identifying reliable biomarkers for improving patient prognosis and facilitating the development of effective therapeutic strategies is crucial. This study aimed to develop reliable prognostic models based on mitochondria-related genetic markers by integrating diverse machine learning techniques to facilitate the advancement of personalized targeted therapeutic regimens for HCC in clinical practice. In this study, we constructed and validated a prognostic model for HCC by integrating 12 machine learning methods with the objective of identifying markers of HCC prognosis and mitochondria-related genes. WGCNA is a method for examining gene expression profiles that enables the grouping or organization of genes that exhibit similar expression patterns [20]. This approach permits investigation of the correlation between gene modules and phenotypes [21]. To enhance the reliability of the results, we employed a combination of WGCNA and machine learning approaches to investigate the potential of mitochondria-related genes to develop a prognostic model for HCC. Additionally, we explored the significance of prognostic genes in immune infiltration, which revealed that mitochondrial dysfunction in HCC drives tumor progression not only through metabolic reprogramming, but also via immune microenvironment modulation. We built upon these findings and identified target drugs for the treatment of HCC based on mitochondrial function; this was achieved by predicting the potential of hub genes as therapeutic drug candidates and assessing their interactions using molecular docking studies.
GO and KEGG enrichment analyses indicated that mitochondrial translation and ribosomes play significant roles in the development of HCC. Hub genes are closely associated with mitochondrial translation. In a study by Han et al., circMTO1 (a mitochondrial translation optimization 1 homologue; hsa_circRNA_0007874/hsa_circRNA_104135) was found as a potential target for HCC therapy, as it was significantly down-regulated in HCC tissues [22].Mitochondrial topoisomerase IB (TOP1MT), a nuclear-encoded topoisomerase exclusively localized in the mitochondria, alleviates the topological stress generated during mitochondrial DNA (mtDNA) replication and transcription. As reported by Baechler et al., TOP1MT expression is upregulated in cancer tissues, and its genetic deletion suppresses tumor growth in both human and murine models of colorectal and hepatocellular carcinomas. Due to mitochondrial dysfunction, TOP1MT-knockout (TOP1MT-KO) cells exhibit glycolytic dependency, which restricts the supply of anabolic metabolites and energy required for cancer cell proliferation in nutrient-deprived tumor microenvironments. Mechanistically, Baechler et al. demonstrated that TOP1MT interacts with mitochondrial ribosomal subunits to ensure mitochondrial translation and assembly of OXPHOS complexes, which are essential for sustaining tumorigenic growth [23].Antibiotics targeting mitochondrial translation (e.g., erythromycin, chloramphenicol, tetracycline-class) can eradicate cancer stem cells within diverse cancer cell populations [24].Furthermore, modulating genes associated with mitochondrial translation (such as EF-Tu knockout) induces mitochondrial dysfunction and suppresses tumor growth [25, 26].Additionally, approximately 80% of ribosomal proteins in eukaryotes possess non-canonical functions; these proteins participate in cancer progression by regulating tumorigenesis and therapeutic resistance [27].This further suggests the potential role of ribosomal dysregulation in HCC.Ji et al. demonstrated through integrated analyses of public databases combined with experimental validation that mitochondrial ribosomal protein L12 (MRPL12) drives HCC progression by orchestrating mitochondrial metabolic reprogramming [28].Ribosomal proteins, which are the major components of ribosomes, are involved not only in protein synthesis, but also in the development and progression of various cancers, including HCC. Mitochondria possess their own ribosomes (designated mitochondrial ribosomes), which facilitate the synthesis of limited quantities of proteins, and they are indispensable for the biosynthesis of oxidative phosphorylation systems [29]. Xie et al. discovered that the upregulation of MRPL9 markedly enhanced tumor proliferation and metastasis and inhibited the cell cycle by facilitating the G1/S phase transition. Additionally, MRPL9 accelerates epithelial-mesenchymal transition (EMT), which is pivotal during the initial stages of HCC metastasis [30]. The upregulation of ribosomal proteins in HCC may contribute to disease progression through a complex mechanism. This suggests that ribosomal proteins may serve as novel biomarkers for the early diagnosis of HCC [31].In summary, the mitochondrial translation and ribosomes are associated with HCC progression. We expect to further explore the mechanisms of key targets based on these findings, and thus plan to use systematic machine learning models for the analysis of key genes.
In this study, the screening process with machine learning yielded key modular genes closely related to the mitochondria and HCC. The StepGLM + GBM framework was selected because of its superior performance in handling complex datasets characterized by high-dimensional features and missing values. This model demonstrated exceptional classification accuracy and achieved the highest AUC in comparative testing. PSMD4, TBCE, CKS1B, and FABP1 represent key genes identified through machine learning approaches. As a core component of the immunoproteasome complex, PSMD4 plays a pivotal role in tumor antigen presentation, further supporting its significance in cancer immunology. Ubiquitin-dependent protein degradation pathways play a pivotal role in cellular regulation, including control of the cell cycle, differentiation, and apoptosis. The 26S proteasome subunit ubiquitin receptor non-ATPase 4 (PSMD4), a member of the ubiquitin protease family, is highly expressed in HCC and plays a pivotal role in disease progression through the Akt/COX2 pathway and p53 inhibition [32]. Li et al. discovered that the elevated expression of microtubule protein folding cofactor E (TBCE) is linked to resistance to platinum-based chemotherapy. Furthermore, high TBCE expression was associated with poorer prognosis and earlier recurrence in patients with HCC. Their findings also revealed that at the mechanistic level, silencing of TBCE significantly affected cytoskeletal rearrangement, which in turn increased cisplatin-induced cell cycle arrest and apoptosis [33]. Huang et al. observed that in HCC, overexpression of the CKS1B protein was associated with clinical aggressiveness, but not with p27 (Kip1) turnover [34]. In vitro experiments conducted by Liu et al. demonstrated that CKS1B, an oncogene, promoted the proliferation and metastasis of HCC cells by activating the JAK/STAT3 signaling pathway. These findings suggest that CKS1B may serve as a new therapeutic target for HCC [35].Liver fatty acid-binding protein (L-FABP) functions as an intracellular lipid chaperone that selectively binds unsaturated free fatty acids. This protein facilitates ligand trafficking to mitochondria and peroxisomes, playing an essential role in modulating lipid metabolic pathways and signal transduction cascades [36, 37].Research by Barbara P. Atshaves et al. demonstrated that FABP1 facilitates the transport of long-chain fatty acids (LCFAs) to mitochondria, maintaining fatty acid oxidation homeostasis and thereby preventing metabolic dysregulation [38]. The tumorigenesis and progression are intricately linked to immune regulatory mechanisms. To delve into the interactive relationship between key genes and immune cells, we subsequently performed immune infiltration analysis to explore the immune microenvironment of mitochondria-related HCC genes at the functional level.
Immune infiltration analysis demonstrated that the key genes were closely associated with a multitude of immune cells. Notably, the interactions between mitochondrial dysfunction-related hub genes (FABP1 and TBCE) and macrophage polarization (M1/M2) may underlie their prognostic significance in HCC. Our findings indicate that a greater focus on M1 and M2 macrophages may lead to the identification of novel prospects and therapeutic avenues for HCC treatment. In oncology research, David Dum et al. employed tissue microarray analysis to profile FABP1 protein expression across 150 tumor types. The highest FABP1 positivity rates were observed in colorectal adenoma (86%), colorectal adenocarcinoma (71.1%), and hepatocellular carcinoma (65.3%), followed by ovarian mucinous carcinoma (34.6%), cholangiocarcinoma (21.6%), and various adenocarcinomas of digestive origin (10–23%). These findings collectively suggest FABP1's potential as a promising diagnostic biomarker, demonstrating consistency with the current study's results [39].For instance, FABP1, a lipid-binding protein that was highly expressed in our model, has been implicated in the regulation of fatty acid metabolism, which is known to influence macrophage polarization. Low-dose FABP1 appears to reprogram HCC-associated tumor-associated macrophages (TAMs) by reversing their polarization from the immunosuppressive M2 phenotype to the tumor-suppressive M1 phenotype, consequently inhibiting HCC cell proliferation [40].
Weiwei Tang et al. [40]demonstrated that TAMs in stage III HCC exhibit significant upregulation of FABP1 compared with stage II HCC tissues. Meanwhile, the study revealed that loss-of-function of FABP1 in TAMs could inhibit HCC progression in vitro. Specifically, FABP1 interacts with peroxisome proliferator-activated receptor γ (PPARG)/CD36 within TAMs, thereby enhancing mitochondria-related fatty acid oxidation (FAO) in HCC.
Both FABP1 and vascular endothelial growth factor receptor (VEGFR) demonstrate co-upregulation in HCC, exhibiting significant positive correlation. Mechanistically, FABP1 promotes HCC cell migration via activating VEGFR2/SRC proto-oncogene tyrosine-protein kinase signaling and engaging the focal adhesion kinase (FAK)/cell division cycle 42 (CDC42) pathway [41].
Yeung et al. [42] demonstrated that M2 macrophages lead to a poor prognosis in HCC and promote tumor invasion through CCL22-induced EMT [14]. In their initial study, Sharen et al. showed that M1-like tumor-associated macrophages may promote the proliferation of HCC cells and enhance their anti-apoptotic ability through the activation of the NF-κB signaling pathway [43]. Although this study has revealed that these targets play an important role in the progression mechanism of HCC, we further expect to build on these core genes to provide new breakthrough directions for subsequent HCC treatment. Therefore, carrying out drug prediction research is of great practical significance.
Based on drug predictive analytics, Fluoxetine and clomipramine may be effective drugs for the treatment of HCC. Lee et al. demonstrated that fluoxetine might exert apoptotic effects in ovarian cancer cell lines by inducing alterations in mitochondrial membrane permeability, resulting in cytochrome C release and subsequent caspase-3 activation via ROS-dependent NF-κB activation [44]. In vivo experiments conducted by Hsu et al. demonstrated that fluoxetine exhibited anticancer properties against HCC and non-small cell lung cancer (NSCLC). The findings indicated that fluoxetine could impede tumor growth by reducing the expression of proliferative and anti-apoptotic proteins and by inhibiting the AKT/NF-κB and ERK/NF-κB signaling pathways [45]. Moreover, fluoxetine induces apoptotic signaling by activating caspase-3, -8, and -9, suggesting that it may be a promising adjuvant therapy for patients with HCC or NSCLC. Previous studies report that sertraline and fluoxetine suppress tumor growth in gastric cancer, melanoma, and non-small cell lung cancer by inhibiting mammalian target of rapamycin (mTOR) activity, with fluoxetine demonstrating particular therapeutic potential against HCC. Research by Huan Zhang et al. reveals these antidepressants block the protein kinase B (AKT)/mTOR signaling pathway, consequently inhibiting HCC cell proliferation across in vitro models, xenografts, and diethylnitrosamine/carbon tetrachloride (DEN/CCl₄)-induced primary liver cancer murine models. Furthermore, sertraline and fluoxetine exert synergistic effects with sorafenib—the first-line standard agent for advanced HCC—co-suppressing tumor cell viability both in vitro and in vivo [46].Hsiang-Lin Chan et al. conducted a population-based cohort study to examine differential effects of individual selective serotonin reuptake inhibitors (SSRIs) on HCC risk. Their findings revealed that SSRI exposure—specifically including fluoxetine—was associated with significantly reduced HCC incidence in a dose-dependent manner, indicating potential chemopreventive properties against hepatocarcinogenesis [47].
In vitro studies by A-Reum Mun et al. demonstrate that fluoxetine induces apoptosis in human HCC Hep3B cells through mitochondrial membrane potential collapse, dysregulated reactive oxygen species (ROS) generation, and modulation of mitogen-activated protein kinase (MAPK) signaling activity [48].Complementary in vitro research by Wei-Ting Chen et al. demonstrates that fluoxetine induces dual apoptotic pathways (extrinsic and intrinsic) while simultaneously impairing both anti-apoptotic and invasive capacities of HCC cells through ERK/NF-κB signaling downregulation. These collective findings substantiate fluoxetine's therapeutic potential as a promising candidate for HCC treatment [49].
This study establishes clomipramine as a viable therapeutic candidate for HCC. Previous research similarly indicates its substantial anticancer potential, demonstrating significant efficacy against diverse malignancies including prostate cancer, lung cancer, and gliomas, with emerging evidence supporting its therapeutic utility in HCC. Fu-Chia Shih et al. further identified that desmethylclomipramine—a bioactive metabolite of clomipramine—retains antidepressant properties while exhibiting potent cytostatic effects against lung cancer cells. Mechanistically, desmethylclomipramine exerts antitumor activity by inactivating the Akt/GSK-3β/Mcl-1 survival axis and triggering cathepsin B/caspase-8-mediated mitochondrial apoptosis, suggesting therapeutic promise for mesenchymal-type carcinomas [50].Prior research demonstrates synergistic therapeutic effects from clomipramine-gemcitabine cotreatment in HT-1376 bladder carcinoma cells, inducing substantial cancer cell death [51]. Similarly, the clomipramine derivative desmethylclomipramine potentiates cytotoxicity when paired with mitomycin C in RT-4112 transitional cell carcinoma lines [51]. In enzalutamide-resistant prostate cancer models, combination regimens incorporating clomipramine produce marked reductions in tumor burden both in vitro and in vivo [52].Beyond demonstrating potential inhibitory effects against prostate cancer, Belén Congregado Ruiz et al.'s narrative review indicates that clomipramine and related antidepressants exhibit the capacity to overcome acquired resistance to anti-androgen agents in castration-resistant prostate cancer (CRPC), effectively resensitizing tumor cells to previously administered androgen receptor signaling inhibitors [53].In lung cancer therapeutics, Yuan Wang et al. demonstrated through cellular and murine models that combinatorial targeting of AIP4 with the clinical antidepressant clomipramine synergistically enhances PD-1/PD-L1 immune checkpoint blockade (ICB) efficacy. This dual therapeutic strategy effectively suppresses ICB-resistant tumor progression in both immunocompetent and humanized mouse models [54].BBB-penetrant antidepressants demonstrate significant cytotoxicity against neoplastic cells across multiple studies. Autophagy has been specifically identified as a critical mediator underlying the antitumor effects of these agents. Edgar Petrosyan et al. comprehensively reviewed FDA-approved antidepressants exhibiting cytotoxic activity in diverse tumor models, wherein autophagic dysregulation was established as playing a predominant mechanistic role. Notably, fluoxetine paradoxically induces autophagic flux while clomipramine functions as a potent autophagy inhibitor. Collectively, these pharmacologic agents correlate with favorable therapeutic outcomes in various neoplastic models, including glioblastoma [55].The apoptosis regulator B-cell lymphoma 2 (Bcl-2) represents a critical therapeutic target in anticancer drug development, with its overexpression conferring chemoresistance in malignant cells—a mechanism potentially linked to clomipramine's anticancer activity. Employing computational drug repurposing strategies, Noor Rahman et al. systematically screened FDA-approved compounds for Bcl-2 inhibitory potential. Through integrated in vitro validation, biophysical analyses, and in silico simulations, their comprehensive assessment identified clomipramine hydrochloride as exhibiting marked anti-proliferative potency and functioning as a potent Bcl-2 inhibitor, revealing significant therapeutic promise in oncology [56].In HCC therapeutic assessment, Xinyang Hu et al. demonstrated through integrated in vitro and in vivo investigations that GP73 mediates vimentin polymerization processes. Their work further establishes clomipramine as a promising vimentin-targeted therapeutic agent for metastatic HCC patients exhibiting elevated sGP73 levels, thereby providing preclinical evidence supporting its translational relevance [57].Overall, these evidence validate our computational prediction of clomipramine as a repurposed potential therapeutic for HCC.
To further validate the binding potential and complex stability between predicted target genes and investigational drug candidates, this study implemented rigorous computational validation through molecular docking simulations and molecular dynamics analyses.Molecular docking demonstrated that small-molecule drugs exhibited robust binding to the hub genes, and the formation of hydrogen bonds at the binding site resulted in a stable conformation. These observations suggest that the combination of the predicted drugs may prove to be a valuable therapeutic strategy for the treatment of HCC.
The novelty of this study lies in the integration of association mechanisms between mitochondria-related genes, immune cell infiltration and the occurrence of HCC. Previous studies predominantly employed reductionist approaches—focusing singularly on mitochondrial dynamics or ribosomal proteins through isolated methodologies—with emphasis on individual genes such as EZH2, GRPEL2, and NDRG1. Crucially, the relationships between mitochondrial functional genes and HCC prognosis remain scarcely investigated. Our work integrates mitochondrial functional genomics data to construct prognostic signatures using multi-algorithmic bioinformatics frameworks and machine learning ensembles, thereby identifying 15 core biomarkers (including PSMD4) that interconnect mitochondrial translation, immune infiltration, and tumor microenvironment remodeling. This systems-level analytical paradigm prioritizes holistic genomic integration and model architecture over single-gene analysis, with computational validation through drug prediction, molecular docking, and molecular dynamics simulations. We constructed disease prediction models by combining 12 machine learning models, including StepGLMv, RF, and GBM, into 113 algorithms. This approach identified key biomarkers, such as PSMD4, TBCE, and CKS1B. Subsequently, based on the DGIdb, we conducted potential drug evaluation analyses and predicted that two FDA-approved antidepressant drugs, clomipramine and fluoxetine, would have high binding affinities for the target proteins. These findings have been partially reported in experimental and clinical studies. Molecular docking demonstrated satisfactory binding interactions between the targets and drugs, which were further validated by 100 ns MD simulations to confirm binding stability.
In summary, this study reveals that mitochondria-related genes play a significant role in the progression of HCC. For instance, FABP1 facilitates the transport of long-chain fatty acids to mitochondria to maintain oxidative homeostasis and influences tumor cells through immune pathways. It exerts anti-cancer effects by regulating the polarization of macrophages from immunosuppressive M2 phenotype to anti-tumor M1 phenotype. Subsequenty, drug prediction based on these findings indicates that fluoxetine and clomipramine are potential candidates for HCC treatment.
However, the current study was limited by the lack of functional experiments and validation of clinical data. While fluoxetine and clomipramine emerged as top candidates computationally, their biological relevance remains purely speculative without empirical evidence. Bioinformatics-based predictive models inherently possess intrinsic limitations, notably the constrained capacity to fully recapitulate complex in vivo pharmacodynamic processes. Consequently, subsequent research necessitates systematic in vitro and in vivo pharmacological validation to bridge computational predictions with mechanistic verification, thereby generating preliminary leads for translational development.
Conclusions
By employing bioinformatics and machine-based methodologies, we identified differential genes associated with HCC pathogenesis and elucidated the underlying molecular pathways of HCC. Subsequently, the DEGs were integrated with the GEO database, and 1136 mitochondrial genes were integrated to develop a prognostic model for mitochondria-associated HCC using 113 combinations of machine learning algorithms. We identified 15 pivotal genes and conducted a comprehensive investigation into HCC diagnosis and treatment using immune infiltration analysis, drug prediction, and molecular docking. Our findings offer novel insights into the molecular diagnosis and treatment of HCC based on mitochondrial mechanisms. However, our study had some limitations as it was based on data from the GEO database, which offers limited pathological characteristics of patients. Consequently, identifying more practical and valuable factors is needed to predict treatment efficacy. The interplay between HCC and mitochondria remains to be explored further. Finally, this study lacked experimental evidence for potential prognostic gene expression, necessitating further investigation.
Supplementary Information
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- HCC
Hepatocellular carcinoma
- DEGs
Differentially expressed genes
- WGCNA
Weighted gene co-expression network analysis
- GSEA
Gene set enrichment analysis
- GEO
Gene Expression Omnibus
- IRGs
Immune-related genes
- TOM
Topological overlap matrix
- GS
Gene significance
- MM
Module membership
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- BP
Biological processes
- CC
Cellular components
- MF
Molecular functions
- SuperPC
Supervised principal component
- StepGLM
Stepwise logistic regression
- RF
Random forest
- GBM
Gradient boosting machine
- Lasso
Least absolute shrinkage and selection operator
- glmBoost
Generalized linear model boosting
- NaiveBayes
Naive Bayes
- SVM
Support vector machine
- Enet
Elastic net
- LDA
Linear discriminant analysis
- plsRglm
Partial least squares regression generalized linear model
- Ridge
Ridge regression
- XGBoost
Extreme gradient boosting
- plsRcox
Cox partial least squares regression
- RMSD
Root mean square deviation
- RMSF
Root mean square fluctuation
- Hbond
Hydrogen bond
- Rg
Radius of gyration
- ROC
Receiver operating characteristic
- EMT
Epithelial-mesenchymal transition
- NSCLC
Non-small cell lung cancer
Author contributions
Fei Gao: Conceptualization; methodology; data curation; formal analysis. Fei Teng: Writing-original draft; methodology; software. Yuxiang Wan: Validation; visualization; writing—reviewing and editing. Qiaoli Zhang: Writing–review and editing;funding acquisition; supervision. Jinchang Huang: Supervision; verifying the cited references;writing– review & editing;funding acquisition. All authors read and approved the final article. The work reported in the article has been performed by the authors. All authors read and approved the final article. The work reported in the article has been performed by the authors.
Funding
This study was funded by the National Natural Science Foundation of China (No. 82074545), Project of Beijing University of Chinese Medicine (2022-JYB-JBZR-042), and the Jiebangguashuai Fund Project of the Beijing University of Chinese Medicine (No.2023-JYB-JBZD-038).
Data availability
The data generated in this study are readily accessible from the corresponding author, and we encourage other researchers to extend the work presented herein in future investigations.
Declarations
Ethics approval and consent to participate
This study utilized exclusively publicly available genomic data from the Gene Expression Omnibus (GEO) and MitoCarta databases.No additional ethical approval was required for this secondary computational analysis. All authors were involved in the research and made substantive contributions to this work.
Consent for publication
All authors approved the submitted version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Gao and Fei Teng contributed equally to the article.
Contributor Information
Qiaoli Zhang, Email: zhangqiaoli1009@126.com.
Jinchang Huang, Email: zryhhuang@163.com.
References
- 1.Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. [DOI] [PubMed]
- 2.Wu C, Targher G, Byrne CD, et al. Global, regional, and national burden of primary liver cancer attributable to metabolic risks: an analysis of the global burden of disease study 1990–2021. Am J Gastroenterol. 2025; 10.14309/ajg.0000000000003288. [DOI] [PubMed]
- 3.Wang X, Cao J, Li J. Anatomic liver resection based on portal territory with margin priority for hepatocellular carcinoma. JAMA Surg. 2024;159(6):710–1. 10.1001/jamasurg.2023.5904. [DOI] [PubMed] [Google Scholar]
- 4.Vogel A, Grant RC, Meyer T, et al. Adjuvant and neoadjuvant therapies for hepatocellular carcinoma. Hepatology. 2023. 10.1097/HEP.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 5.Sun R, Moraleda JM, Wei LJ. Quantification of treatment effect of Tislelizumab vs sorafenib for hepatocellular carcinoma. JAMA Oncol. 2024;10(5):674. 10.1001/jamaoncol.2024.0116. [DOI] [PubMed] [Google Scholar]
- 6.Arvind A, Redmon K, Singal AG. Persisting challenges in the early detection of hepatocellular carcinoma. Expert Rev Anticancer Ther. 2025. 10.1080/14737140.2025.2467184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao S, Yin X, Chen T, et al. ALDH2 is a prognostic biomarker and related with immune infiltrates in HCC[J]. Am J Cancer Res. 2021;11(11):5319–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Jing Q, Yuan C, Zhou C, et al. Comprehensive analysis identifies CLEC1B as a potential prognostic biomarker in hepatocellular carcinoma. Cancer Cell Int. 2023;23(1):113. 10.1186/s12935-023-02939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao X, Zhang J, Huang G, et al. The crosstalk between HIFs and mitochondrial dysfunctions in cancer development. Cell Death Dis. 2021;12(2):215. 10.1038/s41419-021-03505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth KG, Mambetsariev I, Kulkarni P, et al. The mitochondrion as an emerging therapeutic target in cancer. Trends Mol Med. 2020;26(1):119–34. 10.1016/j.molmed.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rath S, Sharma R, Gupta R, et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021;49(D1):D1541–7. 10.1093/nar/gkaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen TH, Lin SH, Lee MY, et al. Mitochondrial alterations and signatures in hepatocellular carcinoma. Cancer Metastasis Rev. 2025;44(1):34. 10.1007/s10555-025-10251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xun D, Li X, Huang L, et al. Machine learning-based analysis identifies a 13-gene prognostic signature to improve the clinical outcomes of colorectal cancer. J Gastrointest Oncol. 2024;15(5):2100–16. 10.21037/jgo-24-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–7. 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Yang X, Gan J, et al. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022;50(W1):W159–64. 10.1093/nar/gkac394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannon M, Stevenson J, Stahl K, et al. DGIdb 5.0: rebuilding the drug-gene interaction database for precision medicine and drug discovery platforms. Nucleic Acids Res. 2024;52(D1):D1227–35. 10.1093/nar/gkad1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Wei C. Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis. 2020;7(3):308–19. 10.1016/j.gendis.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34(2):153–9. 10.1053/j.semdp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Tang J, Kong D, Cui Q, et al. Prognostic genes of breast cancer identified by gene co-expression network analysis. Front Oncol. 2018;8:374. 10.3389/fonc.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiaojing S, Li M. Construction of novel 10 signatures in diabetic retinopathy construction of model based on WGCNA: Mechanism of action of RPL3 and MRPL16 protein. Int J Biol Macromol. 2025;286: 138235. 10.1016/j.ijbiomac.2024.138235. [DOI] [PubMed] [Google Scholar]
- 22.Han D, Li J, Wang H, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66(4):1151–64. 10.1002/hep.29270. [DOI] [PubMed] [Google Scholar]
- 23.The mitochondrial type IB topoisomerase drives mitochondrial translation and carcinogenesis| Nature Communication. [2025–06–14]. https://www.nature.com/articles/s41467-018-07922-3. [DOI] [PMC free article] [PubMed]
- 24.McKee EE, Ferguson M, Bentley AT, et al. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob Agents Chemother. 2006;50(6):2042–9. 10.1128/AAC.01411-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong R, Chen P, Polireddy K, et al. An RNA-binding protein, Hu-antigen R, in pancreatic cancer epithelial to mesenchymal transition, metastasis, and cancer stem cells. Mol Cancer Ther. 2020;19(11):2267–77. 10.1158/1535-7163.MCT-19-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skrtić M, Sriskanthadevan S, Jhas B, et al. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2011;20(5):674–88. 10.1016/j.ccr.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie J, Zhang W, Liang X, et al. RPL32 promotes lung cancer progression by facilitating p53 degradation. Mol Ther Nucleic Acids. 2020;21:75–85. 10.1016/j.omtn.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji X, Yang Z, Li C, et al. Mitochondrial ribosomal protein L12 potentiates hepatocellular carcinoma by regulating mitochondrial biogenesis and metabolic reprogramming. Metabolism. 2024;152:155761. 10.1016/j.metabol.2023.155761. [DOI] [PubMed] [Google Scholar]
- 29.de Silva D, Tu YT, Amunts A, et al. Mitochondrial ribosome assembly in health and disease. Cell Cycle. 2015;14(14):2226–50. 10.1080/15384101.2015.1053672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie C, Hu J, Hu Q, et al. Classification of the mitochondrial ribosomal protein-associated molecular subtypes and identified a serological diagnostic biomarker in hepatocellular carcinoma. Frontiers in Surgery. 2022;9:1062659. 10.3389/fsurg.2022.1062659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su Q, Sun H, Mei L, et al. Ribosomal proteins in hepatocellular carcinoma: mysterious but promising. Cell Biosci. 2024;14(1):133. 10.1186/s13578-024-01316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Fang S, Rong F, et al. PSMD4 drives progression of hepatocellular carcinoma via Akt/COX2 pathway and p53 inhibition. Hum Cell. 2023;36(5):1755–72. 10.1007/s13577-023-00935-1. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Chen S, Dong Z, et al. Concurrent silencing of TBCE and drug delivery to overcome platinum-based resistance in liver cancer. Acta Pharmaceutica Sinica B. 2023;13(3):967–81. 10.1016/j.apsb.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang CW, Lin CY, Huang HY, et al. CKS1B overexpression implicates clinical aggressiveness of hepatocellular carcinomas but not p27(Kip1) protein turnover: an independent prognosticator with potential p27 (Kip1)-independent oncogenic attributes? Ann Surg Oncol. 2010;17(3):907–22. 10.1245/s10434-009-0779-8. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Zhao D. CKS1B promotes the progression of hepatocellular carcinoma by activating JAK/STAT3 signal pathway. Anim Cells Syst. 2021;25(4):227–34. 10.1080/19768354.2021.1953142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang G, Bonkovsky HL, de Lemos A, et al. Recent insights into the biological functions of liver fatty acid binding protein 1. J Lipid Res. 2015;56(12):2238–47. 10.1194/jlr.R056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu L, Li L, Li J, et al. Chemiluminescence immunoassay method of urinary liver fatty-acid-binding protein as a promising candidate for kidney disease. J Fluoresc. 2023;33(3):1191–200. 10.1007/s10895-022-03120-z. [DOI] [PubMed] [Google Scholar]
- 38.Atshaves BP, Martin GG, Hostetler HA, et al. Liver fatty acid-binding protein and obesity. J Nutr Biochem. 2010;21(11):1015–32. 10.1016/j.jnutbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dum D, Ocokoljic A, Lennartz M, et al. FABP1 expression in human tumors: a tissue microarray study on 17,071 tumors. Virchows Archiv. 2022;481(6):945–61. 10.1007/s00428-022-03394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang W, Sun G, Ji GW, et al. Single-cell RNA-sequencing atlas reveals an FABP1-dependent immunosuppressive environment in hepatocellular carcinoma. J Immunother Cancer. 2023;11(11): e007030. 10.1136/jitc-2023-007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ku CY, Liu YH, Lin HY, et al. Liver fatty acid-binding protein (L-FABP) promotes cellular angiogenesis and migration in hepatocellular carcinoma. Oncotarget. 2016;7(14):18229–46. 10.18632/oncotarget.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeung OWH, Lo CM, Ling CC, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62(3):607–16. 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 43.Sharen G, Cheng H, Hu X, et al. M1-like tumor-associated macrophages enhance proliferation and anti-apoptotic ability of liver cancer cells via activating the NF-κB signaling pathway. Mol Med Rep. 2022;26(5):331. 10.3892/mmr.2022.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CS, Kim YJ, Jang ER, et al. Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-kappaB. Basic Clin Pharmacol Toxicol. 2010;106(6):446–53. 10.1111/j.1742-7843.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- 45.Hsu LC, Tu HF, Hsu FT, et al. Beneficial effect of fluoxetine on anti-tumor progression on hepatocellular carcinoma and non-small cell lung cancer bearing animal model. Biomed Pharmacother. 2020;126:110054. 10.1016/j.biopha.2020.110054. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Xu H, Tang Q, et al. The selective serotonin reuptake inhibitors enhance the cytotoxicity of sorafenib in hepatocellular carcinoma cells. Anticancer Drugs. 2021;32(8):793–801. 10.1097/CAD.0000000000001067. [DOI] [PubMed] [Google Scholar]
- 47.Chan HL, Chiu WC, Chen VCH, et al. SSRIs associated with decreased risk of hepatocellular carcinoma: a population-based case-control study. Psychooncology. 2018;27(1):187–92. 10.1002/pon.4493. [DOI] [PubMed] [Google Scholar]
- 48.Mun AR, Lee SJ, Kim GB, et al. Fluoxetine-induced apoptosis in hepatocellular carcinoma cells. Anticancer Res. 2013;33(9):3691–7. [PubMed] [Google Scholar]
- 49.Chen WT, Hsu FT, Liu YC, et al. Fluoxetine induces apoptosis through extrinsic/intrinsic pathways and inhibits ERK/NF-κB-modulated anti-apoptotic and invasive potential in hepatocellular carcinoma cells in vitro. Int J Mol Sci. 2019;20(3):757. 10.3390/ijms20030757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shih FC, Lin CF, Wu YC, et al. Desmethylclomipramine triggers mitochondrial damage and death in TGF-β-induced mesenchymal type of A549 cells. Life Sci. 2024;351: 122817. 10.1016/j.lfs.2024.122817. [DOI] [PubMed] [Google Scholar]
- 51.Rossi M, Rotblat B, Ansell K, et al. High throughput screening for inhibitors of the HECT ubiquitin E3 ligase ITCH identifies antidepressant drugs as regulators of autophagy. Cell Death Dis. 2014;5(5): e1203. 10.1038/cddis.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen HG, Yang JC, Kung HJ, et al. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene. 2014;33(36):4521–30. 10.1038/onc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strategies to re-sensitize castration-resistant prostate cancer to antiandrogen therapy[EB/OL]. [2025-06-14]. https://www.mdpi.com/2227-9059/11/4/1105. [DOI] [PMC free article] [PubMed]
- 54.MTSS1 curtails lung adenocarcinoma immune evasion by promoting AIP4-mediated PD-L1 monoubiquitination and lysosomal degradation| cell discovery[EB/OL]. [2025–06–14]. https://www.nature.com/articles/s41421-022-00507-x. [DOI] [PMC free article] [PubMed]
- 55.Petrosyan E, Fares J, Cordero A, et al. Repurposing autophagy regulators in brain tumors. Int J Cancer. 2022;151(2):167–80. 10.1002/ijc.33965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahman N, Zafar H, Atia-Tul-wahab, et al. Drug repurposing for the identification of new Bcl-2 inhibitors: In vitro, STD-NMR, molecular docking, and dynamic simulation studies. Life Sci. 2023;334:122181. 10.1016/j.lfs.2023.122181. [DOI] [PubMed] [Google Scholar]
- 57.Hu X, Yuan S, Zhou S, et al. Golgi-protein 73 facilitates vimentin polymerization in hepatocellular carcinoma. Int J Biol Sci. 2023;19(12):3694–708. 10.7150/ijbs.85431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are readily accessible from the corresponding author, and we encourage other researchers to extend the work presented herein in future investigations.