Abstract
BEC (CPILE) is a virulence factor of the pathogen, Clostridium perfringens, which has caused foodborne outbreaks in Japan. BEC is a binary toxin that comprises the enzymatic A-component (BECa) and the B-component (BECb); the latter forms a membrane pore to translocate the A-component into target cells. Although BEC differs from other binary toxins in that the B-component alone shows enterotoxic activity, the reason for this remains unclear. We focus on the narrowest region of BECb-pore formed by not phenylalanine residues conserved in other binary toxins including iota toxin B-component (Ib) but serine residues. Comparisons between BECb and BECb (S405F) where the serine residue forming the narrowest region is substituted to the phenylalanine residue reveal that the serine residue is responsible for both cytotoxicity and enterotoxic activity. Though attempts to prepare the BECb-pore were unsuccessful, we reveal the cryo-EM structure of Ib (F454S) where the phenylalanine residue forming the narrowest region is substituted to the serine residue as a surrogate of BECb. Furthermore, Ib (F454S) increases current conductance to nine times that of Ib due to the larger pore diameter and the hydrophilic nature. These results suggest that BECb functions as a pore-forming toxin and as a translocation channel for BECa.
Subject terms: Pathogens, Permeation and transport, Cryoelectron microscopy
Via structural and mutational-functional analyses, the BEC toxin from C. perfringens is shown to use a unique serine-lined pore structure critical for toxicity, distinguishing it from other binary toxins that rely on conserved phenylalanine residues.
Introduction
Clostridium perfringens is one of the most common animal and human pathogens and is responsible for causing diseases such as gas gangrene and necrotic enteritis. The virulence of this pathogen is mediated by at least 17 different toxins, including four major toxins (α, β, ε, and ι), an enterotoxin called C. perfringens enterotoxin (CPE), and other minor toxins. Each strain produces only a subset of these toxins, and there is considerable variety in the toxins produced in the different strains. C. perfringens strains were historically classified into five types, A–E, according to their production of the four major toxins1–3. This classification was recently expanded into seven types, A–G4.
C. perfringens is a frequent cause of human foodborne illness: It was estimated to have caused 4.0 million foodborne illnesses worldwide in 20105 and was reported as the second most common foodborne illness-causing pathogenic bacterium in the United States6,7. C. perfringens type F strains containing the cpe gene (formerly called cpe-positive strains of type A) are associated with food poisoning in humans, and epidemiological and experimental studies showed that CPE plays critical roles in the pathogenesis of food poisoning8.
In four foodborne outbreaks that occurred in Japan from 1997 to 2010, the patients’ symptoms included mainly diarrhea and abdominal pain and appeared after median incubation periods of 9.5–15.5 h9. Although the clinical and epidemiological characteristics indicated that the outbreaks were caused by C. perfringens, the C. perfringens isolates obtained from patients of the four outbreaks did not harbor the cpe gene or secrete CPE protein to culture supernatants. Irikura et al. and Yonogi et al. identified a new enteropathogenic factor that caused the four foodborne outbreaks and named it C. perfringens iota-like enterotoxin (CPILE) and binary enterotoxin of C. perfringens (BEC), respectively10,11. BEC (CPILE) is a binary toxin consisting of BECa (CPILEa) and BECb (CPILEb).
A binary toxin generally consists of an enzymatic A-component that is responsible for attacking a target protein in the target cell and a B-component that is responsible for forming a membrane pore on the target cell to transport the A-component into the cell. In addition to BEC, the reported binary toxins include C. perfringens iota toxin (Ia and Ib), C. spiroforme toxin (CSTa and CSTb), C. botulinum C2 toxin (C2I and C2II), and Clostridioides difficile transferases (CDTa and CDTb)12. The A- and B-components are both secreted from bacteria as soluble forms. After the B-component binds to a specific receptor on a target cell, the N-terminal pro sequence is proteolytically cleaved to induce the B-component to undergo oligomerization and soluble prepore formation. The A-component binds to the prepore, and the A-component-prepore complex enters the target cell by endocytosis. The low pH found in the endosome induces a conformational change from the prepore to the transmembrane pore form. The A-component translocates through the pore, enters the cytosol, and attacks the target protein. The A-components, Ia, CSTa, C2I, CDTa, and BECa, function as ADP-ribosyltransferases to mono-ADP-ribosylate G-actin and induce actin depolymerization13.
We recently revealed the Ib-pore, Ia-bound Ib-pore, and CDTa-bound CDTb-pore structures by cryo-EM analysis14,15. Ib and CDTb form a heptameric transmembrane pore that exhibits a narrow region called the ϕ-clamp, which has a diameter of 6 Å and is formed by the phenylalanine residues of each protomer, and the stem region, which is 90 Å in length and 15 Å in diameter. One molecule of the A-component binds to the heptameric pore. The A-component is thought to unfold during translocation in order to pass through the narrow ϕ-clamp and stem regions14,15. The amino acid sequences of the B-components are similar except for those encoding the receptor binding domain, and the ϕ-clamp is also conserved in Ib, CDTb, C2II, and CSTb. The ϕ-clamp is thought to be essential for translocation of A-components16. However, the amino acid sequence alignment suggests that BECb employs serine residue for this purpose (Fig. 1a).
Fig. 1. Cryo-EM structures of Ib- and Ib (F454S)-pores and predicted structures of BECb- and BECb (S405F)-pores.
a Amino acid sequence alignment among the B-components of binary toxins. b Cryo-EM density map of Ib (F454S)-pore refined with C7 symmetry. Local resolution is displayed. c Ib (F454S)-pore structure. d Surface electrostatic potential at pH 7.0 of Ib (F454S) (red, negative; blue, positive). e Close-up view of the s-clamp of Ib (F454S)-pore. Side chains of amino acid residues forming the clamp are shown in stick model. Transparent surface electrostatic potential at pH 7.0 is also shown. f Close-up view of the ϕ-clamp of Ib (WT)-pore (PDB ID: 6klw). g BECb (WT)-pore structure predicted by AF3. h Surface electrostatic potential at pH 7.0 of BECb (WT)-pore. i Close-up view of the s-clamp of BECb (WT)-pore. j Close-up view of the ϕ-clamp of BECb (S405F)-pore predicted by AF3. Electrostatic potentials were calculated using PDB2PQR49 and APBS programs50.
Binary toxins are considered to exhibit in vivo toxic activity only when both their A- and B-components are present. The combined components of iota toxin result in lethality in mice and induce dermonecrotic lesions, whereas the individual components show minimal biological activity17,18. Similarly, the combined components of C2 toxin are lethal to mice, while the separate components exhibit little biological activity19. Furthermore, it has been reported that the combined components induce fluid accumulation (FA) in the rabbit small intestine, even though the effect of the individual components was not demonstrated20–22. In contrast, BEC does not require the A-component for FA, despite the amino acid sequence similarity in both the A- and B-components11. FA is induced by BECb alone and is enhanced by the co-inoculation of BECa. In C. perfringens, the type E strain containing the iota gene is not associated with food poisoning in humans, and BEC has been identified as the enteropathogenic factor responsible for food poisoning in humans. It remains unclear why BECb alone shows FA activity. In this study, we compare the structures, cytotoxicity, enterotoxic activity, and conductance between BECb (WT) and BECb (S405F) in which the serine residue forming the narrowest region of the BECb (WT) pore is substituted to the phenylalanine residue. Furthermore, we also compare them between Ib (WT) and Ib (F454S) in which the phenylalanine residue forming the narrowest region of the Ib (WT) pore is substituted to the serine residue. These comparisons reveal that the serine residues that form the narrowest region of the BECb pore are responsible for its FA activity and cytotoxicity.
Results
The clamp structure formed by serine residues
The amino acid sequences other than the receptor binding domain are well conserved among the B-components of binary toxins, including BECb. However, the phenylalanine residue forming the ϕ-clamp is replaced with serine residue in BECb (Fig. 1a). Because the structure of the BECb-pore has not yet been shown experimentally, we sought to determine the BECb-pore structure. Unfortunately, despite the addition of 10% ethanol following the Ib-pore preparation method which we established previously14, the oligomerization of BECb did not succeed (Supplementary Fig. 1). Therefore, to experimentally clarify the structure of the B-component with a clamp formed by serine residues, we determined the cryo-EM structure of Ib (F454S)-pore in which the phenylalanine residue forming the ϕ-clamp is substituted to the serine residue as a surrogate of BECb-pore. The cryo-EM structure of Ib (F454S)-pore was determined at 2.78 Å and 2.36 Å resolution with C1 and C7 symmetry, respectively (Fig. 1b and Supplementary Figs. 2–4), which are higher than that of wild type Ib-pore structure (2.9 Å resolution, EMD-0721). When comparing the maps generated with C1 and C7 symmetry, the receptor-binding domain was partially missing in the C1 symmetry map (Supplementary Fig. 3d). However, no other notable differences were observed. No differences were observed in the s-clamp region either (Supplementary Fig. 3e). Therefore, the higher-resolution map generated with C7 symmetry was used for model building and analysis. The overall structure of Ib (F454S)-pore was nearly identical to that of Ib-pore (Fig. 1c), and the root mean square deviation (rmsd) value between their overall structures was 0.41 Å (Supplementary Fig. 5). Although the interior of Ib (F454S)-pore showed a negative electrostatic potential similar to that of Ib-pore (Fig. 1d), the diameter of the clamp of Ib (F454S)-pore was 10.4 Å, larger than that of the ϕ-clamp of Ib-pore (6.0 Å) (Fig. 1e, f). We designate the clamp formed by serine residues as the s-clamp. To further examine the flexibility of the overall structure and the s-clamp region, 3D variability analysis was conducted. Among the ten classes, differences were observed only in the length of the barrel (Supplementary Fig. 6a). No differences were observed in the s-clamp region (Supplementary Fig. 6b). Accordingly, three classes with sufficient barrel length and seven classes with insufficient barrel length were grouped and subjected to reconstruction and refinement. However, differences were observed among these maps only in the length of the barrel. These results indicate that the analyzed Ib (F454S) does not exhibit significant structural flexibility in the s-clamp region.
On the other hand, the BECb-pore structure was predicted by AlphaFold 3 (AF3)23, which generated a BECb heptameric pore structure similar to the experimentally determined Ib- and Ib (F454S)-pore structures (Fig. 1g). In the predicted pore structure, the pore interior showed a negative electrostatic potential (Fig. 1h). The diameter of the s-clamp was 12.9 Å (Fig. 1i), which was more than twice the 6 Å diameter of the ϕ-clamp of the Ib-pore. To investigate the influences of the s-clamp of the BECb-pore to cytotoxicity, enterotoxic activity, and conductance, we decided to compare BECb and BECb (S405F) in which the serine residue forming the serine-clamp is substituted to the phenylalanine residue, and also compare Ib and Ib (F454S). Therefore, we also predicted the structure of the BECb (S405F)-pore using AF3. The overall structure of BECb (S405F)-pore was nearly identical to that of BECb-pore, and the rmsd value was 0.53 Å (Supplementary Fig. 5). In the predicted structure of BECb (S405F)-pore, the diameter of the ϕ-clamp was 10.9 Å (Fig. 1j), which was smaller than the s-clamp of BECb but larger than the ϕ-clamp of Ib. The larger diameter of BECb (S405F) ϕ-clamp, predicted by AF3, is likely due to the proline residue adjacent to the serine residue in BECb s-clamp, which constrains the loop’s structure containing the s-clamp (Fig. 1a).
BECb alone induces and BECa enhances cytotoxicity toward MDCK cells
Although it has been reported that BECb alone exhibits and BECa enhances cytotoxicity toward Vero and L929 cells10, cytotoxicity toward MDCK cells was unknown. Therefore, we investigated whether BEC toxin exhibits cytotoxicity toward MDCK cells. BECb alone induced dose-dependent cytotoxicity (Fig. 2a), cell detachment (Fig. 2b), and cell shrinkage (Supplementary Fig. 7). Furthermore, the ability of BECa to enhance the cytotoxicity caused by BECb was confirmed by a viability test (Fig. 2a). When MDCK cells were treated with BECb plus BECa, cells in the process of becoming round with protrusions were observed (Supplementary Fig. 7). The rounded cells resembled those induced by both A- and B-components of the other binary toxins, which can together induce microtubule-based protrusions24. Our data therefore suggest that the cell rounding was caused not by BECb, but rather by BECa upon its translocation into the cytosol via the BECb-pore.
Fig. 2. Cell viability and detachment from culture plates after a 24 h incubation with the indicated toxins in MDCK, A431, and L929 cells.
a Cell viability with the indicated toxins at the noted concentrations. BECa and Ia were applied at 1 µg/ml. Small and big circles show the individual values of three independent experiments and the means ± standard deviations, respectively. The values are normalized to the untreated control. b Toxin-induced detachment from culture plates. The attached cells were stained with crystal violet. The B-components were applied at 1 µg/ml. Ia and CPILEa were applied at 1 µg/ml with 1 µg/ml Ib and CPILEb, respectively. AMBnTβCD and HSβCD were applied at 10 µM.
Replacement of the s-clamp with a ϕ-clamp reduces the cytotoxicity of BECb against MDCK cells
Previous work showed that substitution of the ϕ-clamp influences ion permeability and conductance16,25, prompting us to speculate that the s-clamp of BECb may influence its cytotoxicity. To test this possibility, we compared the cytotoxicity of wild-type (WT) BECb and a mutant version harboring a ϕ-clamp instead of the s-clamp (S405F) in MDCK cells. Viability was higher (Fig. 2a) and detachment was decreased (Fig. 2b) among cells treated with BECb (S405F) compared to those treated with BECb although BECb (S405F) induced morphological changes similar to those caused by BECb (Supplementary Fig. 7). These results indicate that substitution of the s-clamp with a ϕ-clamp reduces the cytotoxicity of BECb against MDCK cells.
Replacement of the Ib ϕ-clamp with an s-clamp renders Ib cytotoxic toward MDCK cells
To further investigate the difference in the cytotoxicity of the s- and ϕ-clamps, we compared the cytotoxicity of WT Ib and a mutant Ib carrying an s-clamp instead of the ϕ-clamp (F454S) against MDCK cells. First, we confirmed that Ib alone did not cause cytotoxicity or morphological changes at even 10 µg/ml (Fig. 2a), and that treatment with Ib plus Ia caused the well-characterized cell rounding with protrusions and cytotoxicity (Supplementary Fig. 7). In contrast, treatment with Ib (F454S) led to morphological changes (Supplementary Fig. 7). Cell viability assay showed that Ib (F454S) dose-dependently induced cytotoxicity (Fig. 2a). Cell detachment was also observed (Fig. 2b). These results show that replacement of the Ib ϕ-clamp with the s-clamp generates cytotoxicity against MDCK cells.
Replacement of the s- and ϕ-clamps does not affect oligomer formation on MDCK cells
To test whether clamp replacement affects the oligomer and/or pore formations of CDTb and Ib, we used western blotting to detect the formation of their oligomers on cells. Both mutants formed oligomers in amounts comparable to their respective WT versions (Supplementary Fig. 8). It is unclear whether the detected oligomeric bands represented the prepore or pore forms. Regardless, this result indicates that replacement of the BECb s-clamp with a ϕ-clamp and the Ib ϕ-clamp with an s-clamp does not inhibit their oligomer formation on cells.
A pore blocker protects MDCK cells from BECb and Ib (F454S)
To investigate why BECb alone induces cytotoxicity against MDCK cells, we used a seven-fold symmetric β-cyclodextrin carrying seven positively charged groups: per-6-S-(3-amino-methyl)benzylthio-β-cyclodextrin (AMBnTβCD). Previous studies showed that AMBnTβCD blocks the ion current through binary toxin B-component pores reconstituted into planar lipid bilayers26 and protects cultured cells27,28 and animal models29 from intoxication with binary toxins. Because BECb-pore has a negative electrostatic potential similar to those of other binary toxins (Fig. 1h, i), we expected that AMBnTβCD would also protect MDCK cells from BECb. Indeed, when MDCK cells were treated with 1 µg/ml BECb and 10 µM AMBnTβCD for 24 h, there was almost no evidence of the cell detachment seen under treatment with BECb alone (Fig. 2b). In addition, no morphological changes were observed. To confirm that AMBnTβCD functions as a pore blocker, we also used another β-cyclodextrin carrying seven negatively charged groups: each of the primary hydroxy group is substituted with a sulfate group (HSβCD). HSβCD did not protect MDCK cells from BECb (Fig. 2b). These results suggest that the pores formed in cytoplasmic membranes by BECb contribute to its cytotoxicity. AMBnTβCD also protected and HSβCD did not protect MDCK cells from BECb (S405F) and Ib (F454S) (Fig. 2b), indicating that the pores formed in cytoplasmic membranes by BECb (S405F) and Ib (F454S) also contribute to the cytotoxicities of these versions.
Clamp replacements of BECb and Ib have the same effects in A431 and L929 cells as seen in MDCK cells
To assess whether our findings were specific to MDCK cells, we performed the same experiments using A431 and L929 cells. The cytotoxicity of BECb in A431 cells had not previously been reported. The following results were obtained for both A431 and L929 cells. BECb alone induced dose-dependent cytotoxicity (Fig. 2a), cell detachment (Fig. 2b), and morphological change similar to those observed in MDCK cells (Supplementary Fig. 7). BECa enhanced the cytotoxicity caused by BECb (Fig. 2a). When cells were treated with BECb plus BECa, cells in the process of becoming round with protrusions were observed (Supplementary Fig. 7), suggesting that the cell rounding was caused by BECa upon its translocation into the cytosol via the BECb-pore. S405F mutation of BECb slightly reduced cytotoxicity (Fig. 2a) although morphological changes caused by BECb (S405F) were similar to those caused by BECb (Supplementary Fig. 7). The co-application of AMBnTβCD protected cells from BECb and BECb (S405F), suggesting that BECb and BECb (S405F)-pore on the cytoplasmic membrane contribute to the cytotoxicity (Fig. 2b).
L929 cells are insensitive to iota toxin, likely because they lack the Ib receptor, lipolysis-stimulated lipoprotein receptor (LSR)10. In contrast, A431 cells were sensitive to Ib alone, as reported previously (Fig. 2a). Cells treated with Ib alone exhibited cell swelling (Supplementary Fig. 7). However, treatment with Ib (F454S) induced stronger cytotoxicity (Fig. 2a) and cell detachment than Ib alone (Fig. 2b). Replacements of the BECb s-clamp with a ϕ-clamp and the Ib ϕ-clamp with an s-clamp did not inhibit their ability to form oligomers on the cells (Supplementary Fig. 8). The co-application of AMBnTβCD protected A431 cells from Ib although the reason why AMBnTβCD could not protect cells from Ib (F454S) remains unclear. Taken together, these results obtained in A431 and L929 are similar to those obtained in MDCK cells.
The effects of BECb on MDCK, L929, and A431 cells were similar; however, the concentrations of BECb required to induce cytotoxicity varied significantly among the cell types. This difference between cell lines is likely attributable to variations in the number of receptors present on each cell line and the duration of its retention on the cell membrane. Further research including identification of BECb receptor is needed to fully understand the difference.
Clamp replacements of BECb and Ib lead to the same effects on enterotoxic activity as observed for cytotoxicity
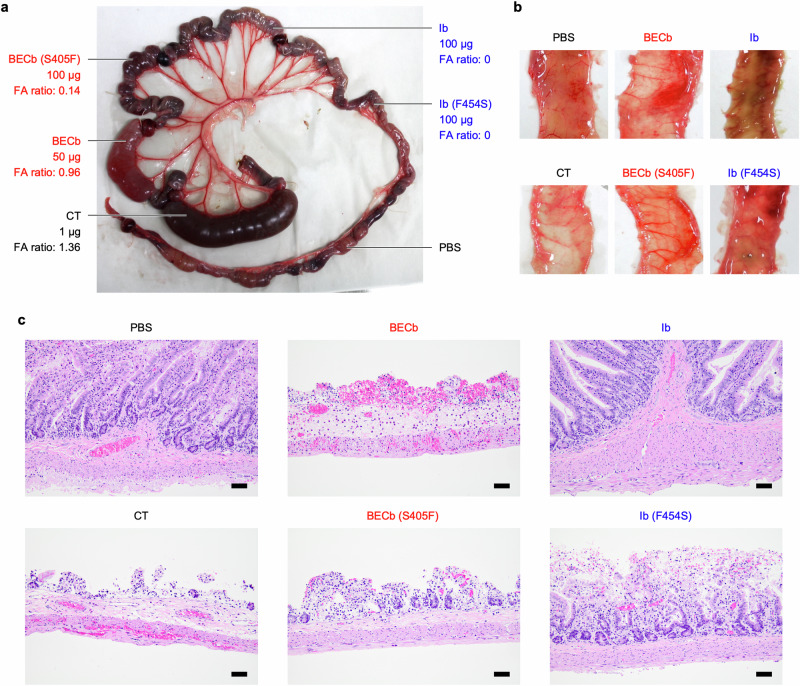
It has been reported that BECb alone shows fluid-accumulating (FA) activity in the suckling mouse assay11 and a rabbit ileal loop assay (unpublished data; under submission). To investigate how clamp replacement of BECb affected FA activity, rabbit ileal loop tests were performed. The loop injected with 50 µg of BECb showed FA activity (FA ratio = 0.96): It turned red with hemorrhagic fluid (Fig. 3a) and the intestinal wall became thin, lost elasticity, and underwent hemorrhage (Fig. 3b). Histologically, severe villous blunting, edema, hemorrhage, and dilated blood vessels were observed (Fig. 3c). In contrast, the loop injected with 100 µg of BECb (S405F) showed less FA activity (FA ratio = 0.14) (Fig. 3a): The fluid was not hemorrhagic, and the intestinal wall was not as thin as that of the BECb (Fig. 3b). At the histologic level, moderate villous blunting and fusion and edema were observed (Fig. 3c). These results indicate that replacement of the BECb s-clamp with a ϕ-clamp reduces not only cytotoxicity, but also enterotoxic activity and histological lesions.
Fig. 3. Rabbit ileal loop test.
a Ileal loops were injected with purified B-component, cholera toxin (CT, positive control), or PBS (negative control). The injected toxin mass and the fluid accumulation (FA) ratio are shown. b Lumen sides of the ileal loops. The presented photos were taken after the lumenal sides were rinsed with saline. c Histological sections of the ileal loops. Hematoxylin and eosin staining. Scale bar: 50 µm.
We also performed ileal loop tests with Ib and Ib (F454S). In this test, the loop injected with 100 µg of Ib did not show FA activity (FA ratio = 0) (Fig. 3a): The fluid was not hemorrhagic, and the intestinal wall appeared similar to that of the negative control (Fig. 3b). The histologic damage was not observed (Fig. 3c). The loop injected with 100 µg of Ib (F454S) did not also show FA activity (FA ratio = 0) (Fig. 3a). Although the fluid was not hemorrhagic, the lumen of the intestinal wall was covered with thick mucus (Fig. 3b). This mucus was not observed in loops injected with PBS, CT, BECb, BECb (S405F), or Ib. At the histologic level, moderate villous blunting and hemorrhage were observed in loops treated with Ib (F454S) (Fig. 3c). These results indicate that replacement of the Ib ϕ-clamp with an s-clamp generates not only cytotoxicity but also histological lesions.
Electrophysiological observation of the difference between the s-clamp and ϕ-clamp
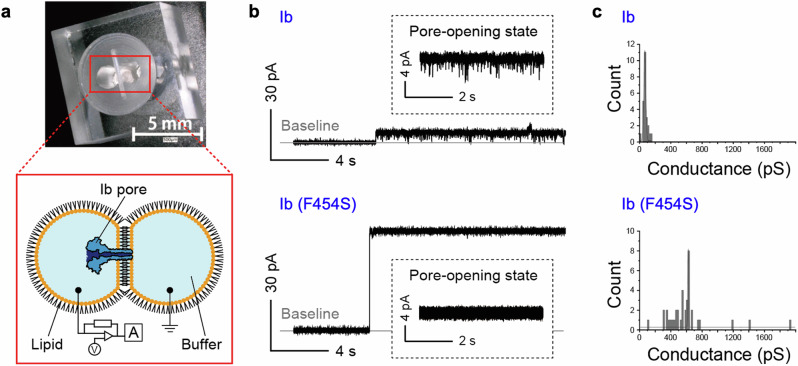
To further investigate the differences in cytotoxicity between B-components harboring the s- versus ϕ-clamp, we used electrophysiological single-channel measurement to compare the ion flux between ϕ-clamp-containing Ib and s-clamp-containing Ib (F454S) (Fig. 4a). Figure 4b shows typical current-time traces of single-channel recordings obtained from Ib and Ib (F454S). Transient current blocking in the pore-opening state was observed only for Ib, probably owing to the hydrophobic gating of the ϕ-clamp30. Regarding the pore-opening current conductance, the histogram showed a Gaussian distribution with a peak at 67 pS for Ib and at 620 pS for Ib (F454S) (Fig. 4c). The size of the pore can be theoretically calculated using the peak value of the current conductance and Hille’s equation as follows:
where r is the pore radius, l is the pore length between the clamp and the tip of the stem region (11.7 nm for the Ib), ρ is the solvent resistivity, and R is the resistance of the pore. R is calculated as V/I, where I is the current through the pore and V is the applied voltage between two chambers. Using the experimentally obtained R values, the pore diameters were calculated to be 0.30 nm for Ib and 0.94 nm for Ib (F454S). The pore diameters were also computationally investigated in the trajectory (Supplementary Fig. 9), which average values were approximately to the experimental results (Supplementary Table 1).
Fig. 4. Electrophysiological measurements.
a Image showing the microdevice used for lipid bilayer preparation and a schematic illustration of the electrophysiological measurements. b Typical current-time traces from single-channel recordings of Ib and Ib (F454S). c Histogram of the current conductances of Ib and Ib (F454S) under a step-like increase from 0 A (baseline).
Discussion
BEC is the enteropathogenic factor responsible for outbreaks of C. perfringens foodborne illness that occurred in Japan within the past 30 years. BEC is the first binary toxin found to serve as a major virulence factor in foodborne illness among humans. It differs from other binary toxins in that it does not require the A-component to exert FA activity. We found that BECb has an s-clamp instead of the ϕ-clamp that is conserved in other binary toxins, and investigated how clamp exchange (between serine and phenylalanine) in BECb and Ib could affect cytotoxicity. In all of the tested cell lines (MDCK, A431, and L929 cells), the following results were obtained: (1) BECb alone caused cytotoxicity. (2) BECa enhanced the cytotoxicity induced by BECb alone and induced rounding with protrusions. (3) Replacement of the s-clamp with a ϕ-clamp in BECb reduced its cytotoxicity. (4) Replacement of the ϕ-clamp with an s-clamp in Ib generated or increased cytotoxicity. We also investigated how clamp exchange in BECb and Ib affected enterotoxicity, and obtained results were consistent with those obtained for cytotoxicity: Replacement of the s-clamp with a ϕ-clamp in BECb reduced FA and histological lesions, and replacement of the ϕ-clamp with an s-clamp in Ib resulted in histological lesions without FA. Therefore, our study shows that the cytotoxic and enterotoxic activities of BECb depend on the amino acids that form the clamp of the oligomeric transmembrane pore.
Unfortunately, we were not able to prepare BECb-prepore or -pore, and thus could not perform cryo-EM analysis or electrophysiological observation of BECb-pore. The predicted structure of BECb-pore was overall similar to that of Ib-pore, but the predicted diameter of the s-clamp was more than twice that of the ϕ-clamp of Ib-pore. Electrophysiological observations of Ib and Ib (F454S) showed that replacement of the ϕ-clamp with an s-clamp increased the pore-opening current conductance by a factor of about 9. This significant increase in conductance is probably due to the increased diameter of the s-clamp and the hydrophilic nature of serine residues. The pore diameter of Ib (F454S) calculated from pore conductance (0.94 nm) was similar to those of the pore-forming toxins (PFTs), hemolysin (1.4 nm), and antimicrobial peptides (1.1 nm)31. PFTs primarily act to alter the concentrations of K+, Ca2+, and ATP in the target cell cytosol, and thereby trigger secondary effects32. Therefore, the conductance increase resulting from replacement of the ϕ-clamp with an s-clamp suggests that Ib (F454S) and also BECb function as pore-forming toxins on the cytoplasmic membrane. This proposal is supported by our observation that the pore-blocker, AMBnTβCD, protected cells from BECb.
Binary toxins were historically thought to cause cytotoxicity only when the A- and B-components were both present. However, recent studies showed that in some cases cytotoxicity can be induced by the B-component alone. Ib alone causes cell swelling, ATP depletion, and finally necrosis in A431 and A549 cells, whereas no such effect is seen in MDCK, Vero, CHO, Caco-2, HT-29, and DLD-1 cells33. CDTb alone causes cell rounding in Vero and Caco-2 cells34,35. C2II does not show cytotoxicity but induces morphological changes and decreases chemotaxis in primary human polymorphonuclear leukocytes (PMN) but not human epithelial cells (HeLa), human endothelial cells (HUVEC), and a murine macrophage line (J774A.1)36. These reports collectively suggest that Ib, CDTb, and C2II function as PFTs on the cytoplasmic membrane. It is generally believed that the acidic environment within endosomes promotes the conformational transition from the prepore to the pore state37. However, considering these previous reports that Ib and CDTb induce cytotoxicity, as well as our findings that BECb exhibits toxicity as a PFT, it is likely that pore formation of the B-component does not exclusively occur within endosomes but can also take place on the cell membrane.
Although both Ib (WT) and BECb (S405F) possess a ϕ-clamp, Ib (WT) showed no cytotoxicity toward MDCK cells and only weak cytotoxicity toward A431 cells, whereas BECb (S405F) showed strong cytotoxicity toward these cells. Although both Ib (F454S) and BECb (WT) have an s-clamp, the cytotoxicity of Ib (F454S) was weaker than that of BECb (WT) in all tested cells. These differences may be attributed to differences in the efficiencies of prepore formation, prepore-to-pore transformation, endocytosis-mediated internalization, and/or residence time at the cytoplasmic membrane, all of which may arise because Ib and BECb use different receptors.
BEC is the first binary toxin identified as a major virulence factor in human foodborne illness caused by C. perfringens, and it may be included in a future expansion of the C. perfringens classification scheme4. Here, we report that the s-clamp of BEC is responsible for its cytotoxic and enterotoxic activity and that BECb functions as both a pore-forming toxin and a translocation channel for BECa (Supplementary Fig. 10). The structure of BECb could not be determined, leaving it unclear whether BECb truly adopts the predicted heptameric structure. However, similar differences observed between Ib (WT) and Ib (F454S) in cytotoxicity and rabbit ileal loop experiments were also seen between BECb (WT) and BECb (S405F). These findings support the conclusion that differences in the clamp are responsible for the observed variations in toxicity. Furthermore, AMBnTβCD has been reported to be effective not only against Ib and BECb but also against C2II and PA. Therefore, AMBnTβCD is an attractive drug candidate for targeting binary PFTs.
Methods
Expression and purification of enzymatic components of the iota and BEC toxins
Ia (UniProt ID: Q46220) lacking its signal peptide (residues 42–454) was cloned into a pET21a vector with a C-terminal hexa-histidine. The full-length BECa (UniProt ID: X5I2D7) was cloned into a pET23a vector with a C-terminal TEV protease cleavage site followed by a hexa-histidine. Both proteins were overexpressed in the Escherichia coli strain, SoluBL21. Transformants were cultivated in LB medium containing 50 µg/ml ampicillin at 37 °C until the absorbance at 600 nm reached 0.6–0.8. After induction with 0.5 mM isopropyl β-D-1-thiogalactopyranoside, the culture was incubated for 20 h at 25 °C. The harvested cells were resuspended in lysis buffer containing 20 mM Tris pH 8.0, 300 mM NaCl, and 20 mM imidazole, and disrupted by sonication. The sample was centrifuged, and the supernatant was loaded onto a Ni-NTA agarose resin. The column was washed first with lysis buffer and then with lysis buffer lacking NaCl, and elution was performed with an elution buffer comprising 20 mM Tris pH 8.0 and 400 mM imidazole. The eluted fractions were loaded onto a HiTrap-Q HP 5 ml column (GE Healthcare) equilibrated with 20 mM Tris pH 8.0 and eluted with a linear gradient from 0 to 500 mM NaCl. The Ia or BECa fractions were finally loaded onto a Superdex 75 column (GE Healthcare) with 10 mM Tris pH 8.0 and 100 mM NaCl. The concentrated Ia or BECa fractions were rapidly frozen in liquid nitrogen and stored at −80 °C.
Expression and purification of binding components of the iota and BEC toxins
Ib (UniProt ID: Q46221) lacking its signal peptide (residues 40–875), Ib (F454S), BECb (UniProt ID: X5HZK7) (residues 1–799), and BECb (S405F) were individually cloned into a pGEX4T-1 vector. Their proteins were overexpressed in the Escherichia coli strain, BL21 Star (DE3). Transformants were cultivated in super broth medium containing 50 µg/ml ampicillin at 37 °C until the absorbance at 600 nm reached 0.6–0.8. After induction with 1 mM isopropyl β-D-1-thiogalactopyranoside, the culture was incubated for 16 h at 23 °C. The harvested cells were resuspended in lysis buffer containing 20 mM Tris pH 8.0, 150 mM NaCl, 2 mM CaCl2, and 5 mM dithiothreitol, and disrupted by sonication. The sample was centrifuged, and the supernatant was loaded onto glutathione sepharose 4B resin (GE Healthcare). The column was washed with lysis buffer, and elution was performed with an elution buffer containing 20 mM Tris pH 8.0, 150 mM NaCl, and 10 mM reduced glutathione. The eluted fractions were concentrated, and the buffer was exchanged to 20 mM Tris pH 8.0, 50 mM NaCl, and 2.5 mM CaCl2.
Cleavage of the N-terminal pro sequences of the binding components of the iota and BEC toxins
Purified Ib and BECb were diluted to 1 mg/ml with 20 mM Tris pH 8.0, 50 mM NaCl, and 2 mM CaCl2. To cleave the N-terminal pro sequences, 1 ml of 1 mg/ml Ib or BECb was treated with 1 µl of 1 mg/ml chymotrypsin or trypsin dissolved in 1 mM HCl and 2 mM CaCl2 at room temperature for 1 h, respectively. The reactions were quenched by adding 10 µl of 100 mM phenylmethylsulfonyl fluoride (PMSF) dissolved in ethanol. The samples were rapidly frozen in liquid nitrogen and stored at -80 °C.
Cell cultivation
A431 (RCB0202), L929 (RCB1422), and MDCK (RCB0995) cells were obtained from RIKEN BRC through the National Bio-Resource Project of MEXT/AMED, Japan. All cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin. Incubation was carried out at 37 °C with 5% CO2 under humidified conditions.
Morphological observation and crystal-violet staining
Twenty-four-well plates were seeded with 600 µl/well of cells suspended at 1 × 105 cells/ml (A431) or 0.5 × 105 cells/ml (L929 and MDCK). After being incubated for 24 h, the cells were washed twice with medium and treated with 200 µl of medium containing 1 µg/ml of the toxin B (binding)-component with or without 1 µg/ml of the toxin A (enzymatic)-component, 10 µM AMBnTβCD, or 10 µM HSβCD. Cell morphologies were observed after 1, 3, and 24 h using an inverted optical microscope. After observation at 24 h, the medium was removed and the cells were stained with 0.2% crystal violet at room temperature for 30 min. The stained cells were washed with tap water and air-dried at room temperature.
Cell viability assay
Ninety-six-well plates were seeded with 100 µl/well of cells suspended at 0.5 × 105 cells/ml, and incubated for 24 h. The cells were then treated with 200 µl of medium containing 0.001–10 µg/ml of B-component with or without 1 µg/ml of the A-component. The plates were incubated for another 24 h, loaded with 10 µl/well of assay solution from a Cell Counting Kit-8 (Dojindo) and incubated for 3 h, and absorbance was measured at 450 nm.
Detection of Ib and BECb oligomers formed on cells
Twenty-four-well plates were seeded with 600 µl/well of cells suspended at 8 × 105 cells/ml (A431) or 0.75 × 105 cells/ml (MDCK). The plates were incubated for 24 h, washed twice with chilled medium, and treated at 4 °C for 1.5 h with 200 µl/well of medium containing 2.5 µg/ml of Ib or BECb, to allow cell surface binding. The cells were washed twice with chilled medium to remove the unbound toxins and treated with 200 µl/well of toxin-free medium at 37 °C for 1 h to promote oligomerization on the cell surface. The detached cells were pelleted by centrifugation, and the detached and attached cells in each well were lysed with SDS-PAGE sample buffer containing a protease inhibitor cocktail. The samples and markers (ECL DualVue Western Blotting Markers, Cytiva) were resolved by SDS-PAGE and blotted to a polyvinylidene difluoride membrane. The membrane was incubated in phosphate-buffered saline with Tween-20 (PBS-T) containing 5% instant nonfat dry milk for 30 min at room temperature. The membrane was incubated overnight with specific antibody (1:5000 anti-Ib antibody or 1:10,000 anti-BECb antibody) in PBS-T. The membrane was subsequently incubated with an anti-rabbit antibody diluted in PBS-T for 30 min at r.t. For the visualization of the markers, we added S-protein-HRP conjugate and incubated for another 30 min at r.t. The proteins were detected with the Pierce ECL Western blotting substrate (ThermoFisher) using a LAS 4000 mini (Cytiva).
Rabbit intestinal loop test
A Japanese White male rabbit (9 weeks old) was provided by Japan SLC, Inc. The rabbit was housed in a cage (one rabbit per cage) at 25 °C until the loop test. Six intestinal loops were made under anesthesia with isoflurane and medetomidine hydrochloride. Each sample or mixture was injected into an intestinal loop at 0.5 ml. Cholera toxin (1 µg; FUJIFILM Wako) was injected as a positive control, and PBS was injected as a negative control. The loops were examined approximately 15 h after injection. The fluid accumulation (FA) ratio was calculated based on the volume of fluid in the loop (ml)/length of the loop (cm). After the FA ratio was determined, each loop was examined for macropathological and histopathological findings: The loops were dissected and observed macropathologically and then fixed with 10% buffered formalin. Histological specimens stained with hematoxylin-eosin were prepared by Biopathology Institute Co., Ltd. This test was repeated more than two times for each toxin, and the toxins were prepared freshly for each test. Representative image is shown in Fig. 3. The Animal Experiment Committee at Tokyo Metropolitan Institute of Public Health approved the animal experiments performed in this study. The experiments were humanely conducted under the regulation and permission of the committee. We have complied with all relevant ethical regulations for animal use.
Lipid bilayer preparation and reconstitution of Ib-pores
Lipid bilayers were prepared in a microdevice using the droplet-contact method, as previously described38,39 (Fig. 4a). Briefly, the wells of the device were filled with n-decane (0.8 μl) containing 10 mg/ml DPhPC. Buffered solution (4.7 μl) containing Ib-pore (final concentration 900 ng/μl) was loaded to the chamber connected to the recording terminal, while buffered solution (4.7 µl) alone was loaded to the chamber connected to the ground terminal. In this study, the buffered solution consisted of 1 M KCl and 10 mM MOPS, pH 7.0. Within a few minutes, a lipid bilayer formed on the parylene C film that separated the two chambers, and Ib formed nanopores by reconstitution in the lipid bilayer. If the lipid bilayer ruptured during this process, it was reassembled by tracing with a hydrophobic stick at the droplet interface. The channel current was recorded using a Pico patch-clamp amplifier (Tecella) connected to each chamber. The signals were detected using an 8 kHz low-pass Bessel filter at a sampling rate of 40 kHz. A constant voltage of +100 mV was applied from the recording side, and the ground side was grounded. Data were analyzed using Clampfit 11.2 (Molecular Devices), Excel (Microsoft), and Origin pro 8.5 J (Light Stone).
Molecular dynamics simulation
All-atom molecular dynamics (MD) simulations were performed to confirm the ϕ-clamp and s-clamp motion of the Ib-pore. The Ib structure (PDB ID: 6KLX)14 was embedded in a DPPC membrane (200 lipids per leaflet) with 1 M KCl at 298 K using the CHARMM-GUI membrane builder40. The F454S (s-clamp) mutant was generated using the mutagenesis tool in PyMOL. Following the CHARMM-GUI protocol, the systems were initially equilibrated (with additional equilibration for 500 ps). The simulation was subsequently conducted with a total simulation time of 100 ns. The HOLE software41 was used to evaluate the pore diameters of the ϕ-clamp and s-clamp. All simulations were performed using GROMACS-2021.242 and the CHARMM36m force field43. The trajectory files were analyzed using the analysis tools installed in GROMACS.
Ib (F454S)-pore preparation
Two point 1 mg of Ib (F454S) were treated with 2.1 µg α-chymotrypsin (SIGMA) at 25 °C for 1 h, and proteolysis was terminated by adding PMSF (final 1 mM). Next, Ib (F454S) was incubated with ethanol (final 10%) and LMNG (final 0.03% (w/v)) at 37 °C for 1 h. It was loaded onto a density gradient bed containing 10–30% glycerol, 50 mM HEPES pH 7.5, 100 mM NaCl, 1 mM CaCl2, and 0.03% (w/v) LMNG and ultracentrifuged at 230,139 × g for 16 h, and five drops were collected by punching a hole at the bottom of the centrifuge tube. After measuring the absorbance of each fraction at 280 nm, the peak fractions were collected and replaced with 10 mM HEPES pH 7.5, 1 mM CaCl2, and 0.003% (w/v) LMNG using PD10. Finally, purified Ib (F454S)-pore was concentrated to 0.669 mg/ml.
Cryo-EM data acquisition of Ib (F454S)-pore
The Ib (F454S)-pore was applied to a glow-discharged Quantifoil grid (R1.2/1.3, Cu 300 mesh). The grid was automatically blotted at 4 °C for 3.5 s in 100% humidity and plunged into liquid ethane using a Vitrobot Mark IV (Thermo Fisher Scientific). Cryo-EM imaging was performed at an acceleration voltage of 300 kV using a Titan Krios (Thermo Fisher Scientific) equipped with Cs corrector (CEOS, GmbH). The images were recorded with a K3 direct electron detector (Gatan) in counting mode and data were automatically collected using SerialEM at a physical pixel size of 0.675 Å/pixel. The defocus range was −0.6 to −1.8 µm, and the total electron dose was 50 e/Å2. Details of data acquisition are described in Supplementary Table 2.
Image processing of cryo-EM data with C1 symmetry
The entire image processing was carried out with cryoSPARC v3.3.244. All 7080 movies were subjected to Full-Frame Motion Correction and Patch CTF Estimation. Next, a template based on wild-type Ib map (unpublished) was generated by Create Templates. Using the template, a total of 1,379,432 particles were picked from the 7080 micrographs by Template Picker, and the picks were extracted with a box size of 450 pixels. After the first 2D classification was carried out, 897,298 particles were retained. Several rounds of Heterogeneous refinement with Ab-Initio reconstruction map and wild-type Ib map were performed to clean up the dataset, resulting in a total of 354,215 particles retained. Homogeneous refinement generated a map with a resolution of 2.95 Å. Then, Homogeneous refinement optimizing per-particle defocus and per-group CTF params, followed by Non-Uniform refinement optimizing per-particle defocus and per-group CTF params, improved the resolution to 2.78 Å. The resolutions were determined using the 0.143 criterion of the FSC. The processing strategy is presented in Supplementary Fig. 2.
Image processing of cryo-EM data with C7 symmetry
Using 897,298 particles after the first 2D classification in image processing with C1 symmetry, several rounds of Heterogeneous refinement with Ab-Initio reconstruction map and wild-type Ib map were performed to clean up the dataset, resulting in a total of 401,021 particles retained. The second 2D classification, Local Motion Correction, and two rounds of Homogeneous refinement were performed, resulting in a map with the resolution of 2.42 Å. The particles were divided into ten classes using 3D classification, and then several Homogeneous refinements and Non-Uniform refinement optimizing per-particle defocus were carried out using 190,663 particles combined the class 3, 8, and 9, yielding a map with the resolution of 2.38 Å. Finally, Homogeneous refinement was performed to improve the resolution to 2.36 Å. The resolutions were determined using the 0.143 criterion of the FSC. The processing strategy is presented in Supplementary Fig. 3. Furthermore, 3D variability analysis was performed using 355,144 particles from all classes in the 3D classification. After Homogeneous refinements were carried out for the particles of each class, Ab-initio reconstructions were performed for two groups: 156,562 particles from classes 6, 7, and 8 which show sufficient β-barrel, and 198,582 particles from classes 0–5 and 9 which show insufficient β-barrel. Using the reconstructed maps, Homogeneous refinement was carried out, yielding maps with resolutions of 2.82 Å and 3.12 Å, respectively. The processing strategy is presented in Supplementary Fig. 6.
Model building and refinement of Ib (F454S)-pore
The map refined with C7 symmetry was used for model building. The initial rigid-body fit of the wild-type Ib structure (unpublished) was applied the map using USCF ChimeraX45. All Phe454 (F454) of the model were mutated to Ser and fitted to the map using COOT46. The model was refined by real-space refinement and manually modified using PHENIX47 and COOT. The flip state of the side chains of Asn, Gln, and His residues of the final model was corrected and validated by MolProbity48. The refinement and validation statistics are described in Supplementary Table 2.
Statistics and reproducibility
All data in this study, except for structural analysis show at least three independent experiments unless otherwise stated.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Masahiro Nagahama and Masaya Takehara (Tokushima Bunri University) for technical support of cell culture procedures, Daisuke Irikura (HORIBA, Ltd.) for providing an anti-BECb antibody, and Noriko Konishi (Tokyo Metropolitan Institute of Public Health) for assisting with rabbit ileal loop test. This work was supported by JSPS KAKENHI Grant Number 18K06170, 21H02452, and 24K01993. This research was supported by Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number JP25ama121001.
Author contributions
T. Yoshida and H. Tsuge participated in research design. T. Yoshida, Y. Ninomiya, Y. Uchida, N. Sakoda, and T. Yamada prepared proteins. Y. Ninomiya and J. Kishikawa performed cryo-EM data acquisition. T. Yoshida, Y. Ninomiya, and T. Yamada performed cryo-EM data image processing and model building. T. Yoshida, Y. Uchida, N. Sakoda, and VA. Karginov performed experiments using MDCK, A431, and L929 cells. C. Monma performed rabbit intestinal loop test. S. Takiguchi, S. Fujita, and R. Kawano performed electrophysiological observation and MD simulation. All authors contributed to writing the manuscript.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Janesh Kumar and Tobias Goris. A peer review file is available.
Data availability
The Cryo-EM dataset was deposited to the Electron Microscopy Public Image Archive (https://www.ebi.ac.uk/pdbe/emdb/empiar/) under accession code of EMPIAR-12300. The Cryo-EM maps and coordinate were deposited to the Electron Microscopy Data Bank (EMDB) and Protein Data Bank (PDB) with the accession codes EMD-39544 (C7 symmetry), EMD-64005 (C1 symmetry), and PDB 8YRM, respectively. The source data of cell viability assay in Fig. 2a and rabbit ileal loop test in Fig. 3 are also provided as Supplementary Data 1 and 2, respectively. All other data are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Toru Yoshida, Chie Monma, Yuki Ninomiya.
Contributor Information
Toru Yoshida, Email: yoshidat@fc.jwu.ac.jp.
Hideaki Tsuge, Email: tsuge@cc.kyoto-su.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-025-08519-5.
References
- 1.Rood, J. I. & Cole, S. T. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol. Rev.55, 621–648 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Songer, J. G. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev.9, 216–234 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petit, L., Gibert, M. & Popoff, M. R. Clostridium perfringens: toxinotype and genotype. Trends Microbiol.7, 104–110 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Rood, J. I. et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe53, 5–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirk, M. D. et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med.12, e1001921 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scallan, E. et al. Foodborne illness acquired in the United States–major pathogens. Emerg. Infect. Dis.17, 7–15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grass, J. E., Gould, L. H. & Mahon, B. E. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998-2010. Foodborne Pathog. Dis.10, 131–136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarker, M. R., Carman, R. J. & McClane, B. A. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol.33, 946–958 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Monma, C. et al. Four foodborne disease outbreaks caused by a new type of enterotoxin-producing Clostridium perfringens. J. Clin. Microbiol.53, 859–867 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irikura, D. et al. Identification and characterization of a new enterotoxin produced by Clostridium perfringens isolated from food poisoning outbreaks. PLoS One10, e0138183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonogi, S. et al. BEC, a novel enterotoxin of Clostridium perfringens found in human clinical isolates from acute gastroenteritis outbreaks. Infect. Immun.82, 2390–2399 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stiles, B. G., Wigelsworth, D. J., Popoff, M. R. & Barth, H. Clostridial binary toxins: iota and C2 family portraits. Front. Cell Infect. Microbiol.1, 11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margarit, S. M., Davidson, W., Frego, L. & Stebbins, C. E. A steric antagonism of actin polymerization by a Salmonella virulence protein. Structure14, 1219–1229 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Yamada, T. et al. Cryo-EM structures reveal translocational unfolding in the clostridial binary iota toxin complex. Nat. Struct. Mol. Biol.27, 288–296 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Kawamoto, A. et al. Cryo-EM structures of the translocational binary toxin complex CDTa-bound CDTb-pore from Clostridioides difficile. Nat. Commun.13, 6119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krantz, B. A. et al. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science309, 777–781 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiles, B. G. & Wilkins, T. D. Clostridium perfringens iota toxin: synergism between two proteins. Toxicon24, 767–773 (1986). [DOI] [PubMed] [Google Scholar]
- 18.Stiles, B. G. & Wilkins, T. D. Purification and characterization of Clostridium perfringens iota toxin: dependence on two nonlinked proteins for biological activity. Infect. Immun.54, 683–688 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohishi, I., Iwasaki, M. & Sakaguchi, G. Purification and characterization of two components of botulinum C2 toxin. Infect. Immun.30, 668–673 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurazono, H., Hosokawa, M., Matsuda, H. & Sakaguchi, G. Fluid accumulation in the ligated intestinal loop and histopathological changes of the intestinal mucosa caused by Clostridium botulinum C2 toxin in the pheasant and chicken. Res Vet. Sci.42, 349–353 (1987). [PubMed] [Google Scholar]
- 21.Geric, B. et al. Binary toxin-producing, large clostridial toxin-negative Clostridium difficile strains are enterotoxic but do not cause disease in hamsters. J. Infect. Dis.193, 1143–1150 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Redondo, L. M. et al. Effects of Clostridium perfringens iota toxin in the small intestine of mice. Anaerobe48, 83–88 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature630, 493–500 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwan, C. et al. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog.5, e1000626 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun, J., Lang, A. E., Aktories, K. & Collier, R. J. Phenylalanine-427 of anthrax protective antigen functions in both pore formation and protein translocation. Proc. Natl. Acad. Sci. USA105, 4346–4351 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezrukov, S. M. et al. Interactions of high-affinity cationic blockers with the translocation pores of B. anthracis, C. botulinum, and C. perfringens binary toxins. Biophys. J.103, 1208–1217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nestorovich, E. M., Karginov, V. A., Popoff, M. R., Bezrukov, S. M. & Barth, H. Tailored ß-cyclodextrin blocks the translocation pores of binary exotoxins from C. botulinum and C. perfringens and protects cells from intoxication. PLoS One6, e23927 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roeder, M. et al. Tailored cyclodextrin pore blocker protects mammalian cells from Clostridium difficile binary toxin CDT. Toxins6, 2097–2114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moayeri, M., Robinson, T. M., Leppla, S. H. & Karginov, V. A. In vivo efficacy of beta-cyclodextrin derivatives against anthrax lethal toxin. Antimicrob. Agents Chemother.52, 2239–2241 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamini, G. et al. Hydrophobic gating and 1/f noise of the anthrax toxin channel. J. Phys. Chem. B125, 5466–5478 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Watanabe, H. et al. Analysis of pore formation and protein translocation using large biological nanopores. Anal. Chem.89, 11269–11277 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Bischofberger, M., Iacovache, I. & van der Goot, F. G. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe12, 266–275 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Nagahama, M. et al. Clostridium perfringens iota-toxin b induces rapid cell necrosis. Infect. Immun.79, 4353–4360 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landenberger, M. et al. The cytotoxic effect of Clostridioides difficile pore-forming toxin CDTb. Biochim. Biophys. Acta Biomembr.1863, 183603 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Ernst, K. et al. Characterization and Pharmacological Inhibition of the Pore-Forming Clostridioides difficile CDTb Toxin. Toxins13, 390 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisele, J. et al. The pore-forming subunit C2IIa of the binary Clostridium botulinum C2 toxin reduces the chemotactic translocation of human polymorphonuclear leukocytes. Front. Pharm.13, 810611 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerding, D. N., Johnson, S., Rupnik, M. & Aktories, K. Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes5, 15–27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu, K. et al. De novo design of a nanopore for single-molecule detection that incorporates a β-hairpin peptide. Nat. Nanotechnol.17, 67–75 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi, N., Hiratani, M. & Kawano, R. Pattern recognition of microRNA expression in body fluids using nanopore decoding at subfemtomolar concentrations. JACS Au2, 1829–1838 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem.35, 1997–2004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph14, 376 (1996). 354–360. [DOI] [PubMed] [Google Scholar]
- 42.Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem.26, 1701–1718 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods14, 71–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci.32, e4792 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr.60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol.74, 531–544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr.66, 12–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dolinsky, T. J., Nielsen, J. E., McCammon, J. A. & Baker, N. A. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res.32, W665–W667 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jurrus, E. et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci.27, 112–128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The Cryo-EM dataset was deposited to the Electron Microscopy Public Image Archive (https://www.ebi.ac.uk/pdbe/emdb/empiar/) under accession code of EMPIAR-12300. The Cryo-EM maps and coordinate were deposited to the Electron Microscopy Data Bank (EMDB) and Protein Data Bank (PDB) with the accession codes EMD-39544 (C7 symmetry), EMD-64005 (C1 symmetry), and PDB 8YRM, respectively. The source data of cell viability assay in Fig. 2a and rabbit ileal loop test in Fig. 3 are also provided as Supplementary Data 1 and 2, respectively. All other data are available from the corresponding author on reasonable request.