Abstract
Mammalian genomes are subdivided into euchromatic A compartments that contain mostly active chromatin, and inactive, heterochromatic B compartments. However, it is not well understood how A and B genome compartments are established and maintained. Here we study SMCHD1, an SMC-like protein best known for its role in X chromosome inactivation, in human male myoblasts. SMCHD1 colocalizes with Lamin B1 and the heterochromatin mark H3K9me3. Loss of SMCHD1 leads to extensive heterochromatin and Lamin B1 depletion at the nuclear lamina, acquisition of active chromatin states and increased DNA methylation along chromosomes. In absence of SMCHD1, long range intra-chromosomal contacts between B compartments are lost while many new TADs and loops are formed. Inactivation of SMCHD1 promotes numerous B to A compartment transitions accompanied by activation of silenced genes. The data suggests that SMCHD1 functions as an anchor for heterochromatin domains at the nuclear lamina ensuring that these domains are poorly accessible to DNA methyltransferases and to epigenome modification enzymes that typically operate in active chromatin. Thus, the properties of SMCHD1 in heterochromatin maintenance extend well beyond its role in X chromosome inactivation.
Subject terms: Gene silencing, Epigenomics, Experimental models of disease
The authors characterize SMCHD1 as a nuclear lamina-associated protein in human myoblasts where it serves as an anchor for heterochromatin. Loss of SMCHD1 leads to B-to-A compartment transitions and numerous changes in 3D chromatin organization.
Introduction
The genome of eukaryotes consists of segments of euchromatin and heterochromatin. Euchromatin contains transcriptionally active genomic regions whereas heterochromatin is largely depleted of genes and contains transcriptionally silent sequences and repetitive DNA1–6. Histone H3 lysine 9 (H3K9) methylation is a hallmark of heterochromatin3. Almost half of the human genome consists of heterochromatin, but the structural and functional role of heterochromatin is only partially understood7–9. At the three-dimensional level, mammalian chromosomes are subdivided into A compartments that contain mostly active chromatin and B compartments that consist chiefly of heterochromatin10–13. However, it is not clear how these compartments are established and maintained.
Structural maintenance of chromosomes flexible hinge domain containing 1 (SMCHD1) was initially identified in a genetic screen as a repressor of mouse metastable epialleles14. SMCHD1, which exists as a homodimer15, may be functionally related to the microrchidia (MORC) proteins, a family of conserved GHKL-type ATPases involved in chromatin compaction and gene silencing16. The SMCHD1 protein is involved in X chromosome inactivation by facilitating the hypermethylation of CpG islands and by other chromatin-based repression mechanisms14,17,18. SMCHD1 is mutated in two unrelated human genetic diseases, facioscapulohumeral muscular dystrophy type 2 (FSHD2)19,20 and Bosma arrhinia microphtalmia syndrome (BAMS)21,22, a rare developmental defect. The alterations of SMCHD1 in FSHD2 are generally considered loss of function mutations whereas this has been less clear for the BAMS mutations18. Although the function of SMCHD1 in X chromosome inactivation is quite well characterized23–29, we know relatively little about the role of this protein in the control of chromatin structure on autosomes. Previous work has shown that SMCHD1 is a regulator of HOX gene clusters, protocadherin clusters and of imprinted gene expression17,30–34, but its role is thought to be restricted to a limited number of autosomal gene regions.
However, given the ubiquitous and cell type independent expression of SMCHD1 in male and female cells, we reasoned that SMCHD1 may have roles that could go well beyond regulating the X chromosome and these specific gene loci. Because of potential disease relevance, we focused here on human myoblast cells. We characterized the genomic properties of SMCHD1 as a nuclear lamina-associated protein. Using gene inactivation in combination with detailed genome-wide DNA methylation and chromatin structure analysis including HiC mapping, we show that loss of SMCHD1 in these somatic human cells leads to a genome-wide perturbation of heterochromatin structure, whereby many heterochromatic B compartment regions lose contact to each other and may undergo transition to euchromatic A compartments accompanied by major changes in 3D chromatin structure and epigenome landscape.
Results
SMCHD1 colocalizes with Lamin B1 and the heterochromatin mark H3K9me3
The male immortalized human myoblast cell line LHCN-M2 was grown under standard conditions without differentiation. Using these cells, we performed a comprehensive characterization of SMCHD1 and its relationship to DNA methylation, chromatin modifications, gene expression, and three-dimensional genome structure (Fig. 1A). To map the genomic distribution of SMCHD1, we used Dam-ID, a method in which a small adenine methyltransferase domain is attached to the N-terminus of the SMCHD1 protein25. The methylated adenines, which are established on DNA in the vicinity of the tagged protein, are then mapped using cleavage with the 6-methyladenine-dependent restriction enzyme DpnI and high-throughput sequencing (Fig. 1B). Conceptually, this technique indicates where a protein ‘has been’ (and has introduced DNA adenine methylation), which is different from direct in situ mapping by ChIP-sequencing which captures a protein at a moment in time. Inspection of the Dam-ID SMCHD1 signals along all chromosomes showed a generally broad distribution of the signal in large megabase-size blocks that were interrupted by areas of much weaker SMCHD1 Dam-ID signal (Fig. 1B; Supplementary Data 1). The Dam-only sequencing controls showed only a low and non-specific signal along the genome and the SMCHD1 signal was normalized relative to the Dam only signal (Fig. 1B). Known SMCHD1-bound genes such as the protocadherin gene cluster on chromosome 5 and several imprinted genes showed more restricted but clearly positive areas of SMCHD1 Dam-ID signal (Supplementary Fig. 1). We did not detect SMCHD1 at HOX gene loci in this cell type. The large SMCHD1 blocks tended to be localized in gene-poor areas of the chromosomes. Because gene-depleted areas of the genome are known to be associated with the nuclear lamina and with heterochromatin and transcriptional repression9,35–37, we mapped in parallel the distribution of Lamin B1 by Dam-ID sequencing and of the characteristic heterochromatin mark histone H3 lysine 9 trimethylation (H3K9me3) by standard ChIP sequencing (Fig. 1B–E; and Supplementary Fig. 2). SMCHD1, Lamin B1, and H3K9me3 were extensively colocalized (Fig. 1B–H; and Supplementary Fig. 2A, 2C). Heat maps show the clear colocalization of the three mapped features over lamina-associated domain (LAD) regions (Fig. 1C–E). Venn diagrams (Fig. 1F) and scatter plots (Fig. 1H) further reveal the strongly correlated localization of SMCHD1 and Lamin B1. Polycomb complexes have been linked with SMCHD1 function, for example during X chromosome inactivation and other silencing events26,27,34,38–40. The Polycomb marks H3K27me3 and H2AK119ub1 were only weakly associated with SMCHD1 in human myoblasts (Fig. 1H). Colocalization of SMCHD1 and Lamin B1 was found along all chromosomes (Supplementary Fig. 2A). Only chromosomes 19 and 22, two gene-rich chromosomes, did not contain many SMCHD1 and Lamin B1 blocks (Supplementary Fig. 2A).
Fig. 1. Colocalization of SMCHD1 with Lamin B1 and H3K9me3.
A Outline of the experimental approaches used in this study. B Dam-ID sequencing shows the localization of SMCHD1 and Lamin B1 signals along chromosome 4. Two replicates each are shown. The signals were normalized relative to Dam-only signal. H3K9me3 was mapped by ChIP-sequencing. The density of Refseq genes (bottom) shows that the SMCHD1 blocks preferentially lie within gene-poor regions. C Heat map of SMCHD1-DamID signal over LAD regions. D Heat map of Lamin B1-DamID signal over LAD regions. E Heat map of H3K9me3 ChIP-seq signal over LAD regions. F More than 80% of SMCHD1 and Lamin B1 regions coincide. G tSNE plots of SMCHD1-bound and SMCHD1-unbound bins (500 kb) at the whole genome level. All bins were plotted based on the combinatorial mean of H3K9me3, H3K4me1, H3K4me3, H3K27ac, H3K36me2, H3K36me3 and Lamin B1 levels across all bins in two dimensions, using t-Distributed Stochastic Neighbor Embedding (t-SNE). H Scatter plots show a strong positive correlation between SMCHD1 and Lamin B1 DamID signals (r = 0.99, p < 2.2e − 16) and between SMCHD1 and H3K9me3 (r = 0.78, p < 2.2e − 16) but marginal or negative correlation with other chromatin marks (H3K4me1: r = −0.03, p = 0.025. H3K4me3: r = −0.38, p < 2.2e − 16. H3K27ac: r = −0.18, p < 2.2e − 16. H3K36me2: r = 0.18, p < 2.2e − 16. H3K36me3: r = −0. 018, p = 0.16. H3K27me3: r = 0.1, p = 3.2e − 15. H2AK119ub1: r = 0.033, p = 0.012). A two-sided correlation significance test was calculated by Pearson’s correlation coefficient to determine the strength and direction of the relationship between the two signals. I Cell fractionation experiments and Western blots showing the enrichment of SMCHD1, Lamin B1, G9a (EHMT2), SETDB1, H3K9me3, tubulin, and total histone H3 in cytoplasmic (S1), soluble chromatin (S2), tightly chromatin-associated (S3) and insoluble cellular fractions (S4) of LHCN-M2 cells. Source data are provided as a Source Data file.
Because of the unexpected and extensive colocalization of SMCHD1 and Lamin B1, we verified our Dam-ID sequencing method by monitoring the genomic signals produced by heterochromatin protein CBX1 (HP1-beta) Dam-ID, and by MBD3 Dam-ID (Supplementary Fig. 3). CBX1 signal was found at its known preferred binding regions, which included LADs, where it partially overlapped with SMCHD1 and Lamin B1, and at several other sites that were only weakly positive or lacked SMCHD1/LaminB1 signal. On the other hand, MBD3, a protein known to localize to promoters, gene bodies and enhancers of active genes41, was found mostly in gene-dense areas in a pattern that was completely different from that of SMCHD1 and Lamin B1 (Supplementary Fig. 3). Furthermore, we examined published Dam-ID sequencing data for SMCHD1, Lamin B1 and CBX1 from mouse embryo fibroblasts. This published data also showed a high degree of overlap between SMCHD1 and Lamin B1 (Supplementary Fig. 4). The overlap existed even though the data were obtained by two different laboratories25,42. We also aligned CBX1 from mouse fibroblasts showing partial overlap with SMCHD1 and Lamin B1 (Supplementary Fig. 4).
To analyze SMCHD1 cellular localization, we tried several commercial antibodies, but none produced a reliable signal that was absent in the knockout controls. We then transfected the various Dam-ID constructs, which carry a V5-tag and stained the cells with anti-V5 antibody (Supplementary Fig. 5A). Lamin B1 and SMCHD1 showed predominantly a localization near the nuclear periphery and MBD3 was found throughout the nucleus, consistent with the Dam-ID mapping data. CBX1 was seen both near the lamina and within the nucleus using the V5 antibody. Two CBX1 antibodies for direct staining of this protein detected it mostly in the interior of the nucleus (Supplementary Fig. 5B).
The distributions of SMCHD1, Lamin B1, and H3K9me3 over LAD borders showed a sharp rise for SMCHD1 and Lamin B1 and a more gradual increase of H3K9me3 from the borders into the LADs themselves (Supplementary Fig. 2B). As expected, the euchromatic (active) marks H3K4me1, H3K4me3, H3K27 acetylation, H3K36me2, H3K36me3, and RNA polymerase II, which we also mapped in the LHCN-M2 cells, were depleted within the LAD regions (Supplementary Fig. 2B). The facultative heterochromatin (Polycomb) marks H3K27me3 and H2AK119ub1 were reduced within the SMCHD1 and Lamin B1 associated regions but tended to accumulate at LAD boundaries and within gene-rich regions (Supplementary Fig. 2B, and Supplementary Fig. 2C). Principal component analysis (PCA) showed the distinct differences between the SMCHD1-bound and non-bound regions based on the mapped histone modification patterns and Lamin B1 binding pattern (Fig. 1G). Supplementary Fig. 2C shows an example of the differential chromatin modifications present at SMCHD1-positive and SMCHD1-negative regions along the same chromosomal segment and Supplementary Fig. 2D summarizes the differential distributions of these marks.
To obtain further support for the association of SMCHD1 with the nuclear lamina, we performed cell fractionation experiments. We tested the presence of SMCHD1, Lamin B1, the H3K9 methyltransferases G9A (EHMT2) and SETDB1 and of H3K9me3 in cytoplasmic (S1), soluble chromatin (S2), tightly chromatin-associated (S3), and insoluble (lamina-containing) (S4) cellular fractions by Western blotting (Fig. 1I). While the histones were extracted into the S2 and S3 fractions, SMCHD1, Lamin B1 and the H3K9 methylation enzymes remained in the insoluble S4 fraction consistent with our genomic mapping data (Fig. 1I).
SMCDH1 maintains heterochromatin architecture at the nuclear lamina
To study the function of SMCHD1 in muscle cells, we used CRISPR/Cas9 technology to inactivate this gene. Figure 2A shows the complete loss of SMCHD1 protein in three independent knockout cell clones relative to three independent wildtype cell clones, which were subsequently used throughout the study. Supplementary Fig. 6A shows the genotypes of the knockout clones. Removal of SMCHD1 did not have a major effect on cell growth rates or cell cycle phase distribution of the cells.
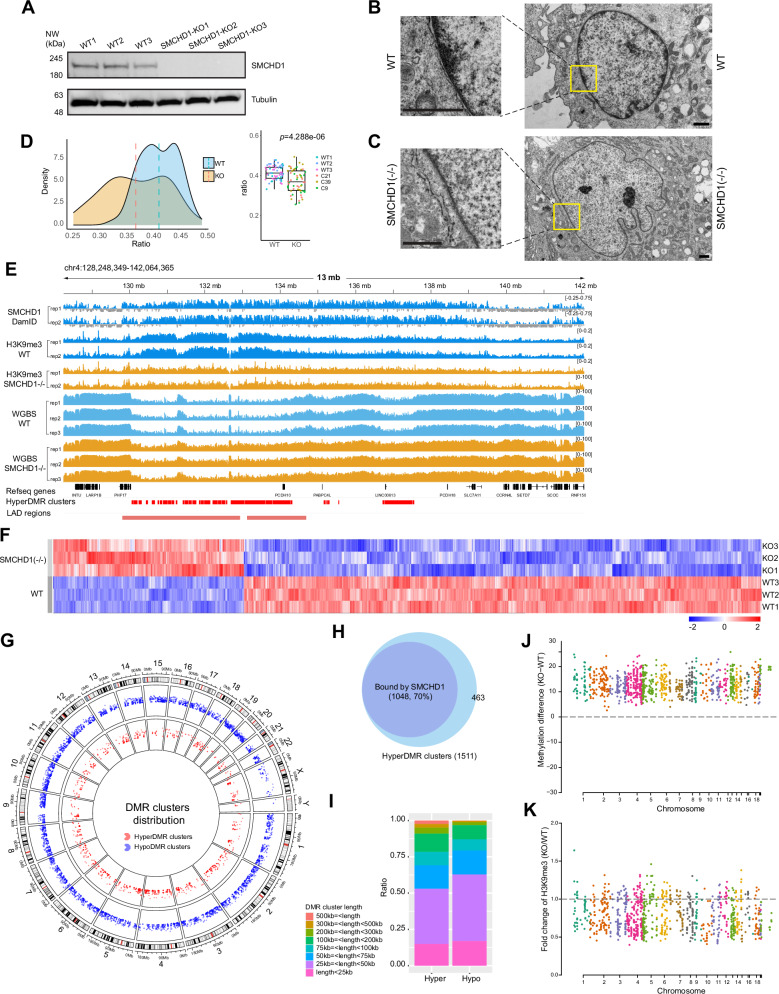
Fig. 2. Inactivation of SMCHD1 leads to changes in heterochromatin, H3K9me3 and DNA methylation patterns.
A CRISPR/Cas9-mediated inactivation of SMCHD1 in LHCN-M2 myoblasts. Three wildtype and three SMCHD1-deficient clones were analyzed by Western blot. Source data are provided as a Source Data file. B Transmission electron microscopy (TEM) shows dense heterochromatin staining at the nuclear periphery in wildtype (WT) cells. The magnified squares show the nuclear envelope regions in more detail. Bars = 1 μm. C TEM shows much weaker staining of heterochromatin at the nuclear periphery in SMCHD1 knockout cells. Bars = 1 μm. D Densitometric quantitation of nuclear lamina-associated heterochromatin regions in WT and SMCHD1 KO cells. Regions under the nuclear envelope were quantitated by densitometry of WT (n = 59) and KO (n = 58) cells. The heterochromatin staining intensities were normalized to the total area of the nucleus. The midline is the median of the data, with the upper and lower limits of the box being the third and first quartile (75th and 25th percentile) respectively. Whiskers extend to the most extreme data points within 1.5 × inter-quartile range (IQR) from the box bounds, and minima and maxima within the whiskers indicate the range of non-outlier data. Statistical significance was assessed using a two-sided t-test (p = 4.288e-06). Source data are provided as a Source Data file. E Analysis of H3K9me3 by ChIP-seq and of DNA methylation by whole genome bisulfite sequencing (WGBS) in wildtype and SMCHD1-deficient myoblasts. Genome browser views of a region of chr4 are shown. The localization of SMCHD1 is shown in the top two tracks. Refseq genes, hypermethylation DNA clusters, and LAD regions are shown at the bottom. Tracks for wildtype cells (blue) and SMCHD1−/− cells (orange) are shown. F Heat map of differentially methylated regions (DMRs) between SMCHD1 knockout (KO) and wildtype (WT) cells. G Circos plot showing hypomethylation DMR (hypoDMR, blue) and hypermethylation DMR (hyperDMR, red) clusters along all chromosomes. H Overlap between SMCHD1-bound regions and DNA hypermethylation clusters. I Stacked bar plot showing the different length profiles of hypermethylation (Hyper) and hypomethylation (Hypo) DMR clusters. Source data are provided as a Source Data file. J Manhattan plot showing differences in methylation (%) at hypermethylation DMR clusters in SMCHD1-bound regions between KO and WT cells for all chromosomes. Each dot represents a hypermethylation DMR cluster. K Manhattan plot showing differences in H3K9me3 at the same hypermethylation DMR clusters as shown in panel J.
We used transmission electron microscopy (TEM) to monitor structural changes of the nucleus in SMCHD1-deficient cells. In wildtype myoblasts (Fig. 2B), we observed electron-dense clusters of heterochromatin at the nuclear periphery, as expected. However, in cells lacking SMCHD1, a much thinner layer of heterochromatin at the outer boundaries of the nucleus was observed (Fig. 2C). Densitometric quantification of the heterochromatin layer near the nuclear surface shows a significant reduction of staining intensity in the knockout cells (p = 4.288e-06, t-test) (Fig. 2D). Next, we analyzed myoblast cells obtained from FSHD2 patients carrying heterozygous loss of function mutations in SMCHD1 (Supplementary Fig. 6B–D). Like in the immortalized myoblasts with inactive SMCHD1, we found a reduction of heterochromatin density at nuclear lamina attached regions in myoblasts from FSHD2 patients (p = 2.184e-10, t-test; Supplementary Fig. 6B–D).
SMCHD1 has previously been linked to H3K9 trimethylation15,43 suggesting a connection between SMCHD1, likely via the bridging proteins LRIF1/HBIX1 and HP1, and the constitutive heterochromatin histone mark H3K9me3. The colocalization of SMCHD1 and H3K9me3 we observed in human muscle cells (Fig. 1B–H) supports a tight connection between SMCHD1 and constitutive heterochromatin. Inspection of genome browser data and formal analysis show that many regions with megabase-size SMCHD1 blocks in wildtype cells undergo a loss of H3K9me3 in the SMCHD1 knockout cells (Fig. 2E; and Supplementary Fig. 7A, 7B, 7D, 7E) supporting a role of SMCHD1 in heterochromatin integrity. This reduction of H3K9me3 was further monitored by immunofluorescence staining with an anti-H3K9me3 antibody in wildtype and SMCHD1 knockout cells (Supplementary Fig. 8A, 8B). The pronounced H3K9me3 staining near the nuclear periphery was significantly reduced in the knockout cells (Supplementary Fig. 8C, 8D).
Re-organization of DNA methylation in SMCHD1-deficient muscle cells
Since SMCHD1 has previously been implicated in the control of DNA methylation14,30,32,44, we performed whole genome bisulfite sequencing of the three wildtype myoblast cell clones and three SMCHD1 knockout clones (Fig. 2E–J; and Supplementary Fig. 7A). In total, there were 115,400 hypomethylated differentially methylated regions (DMRs) and 48,500 hypermethylated DMRs in the SMCHD1-/- cells. These DMRs occurred along all chromosomes (Fig. 2G). Upon closer inspection, we found that the hypomethylation DMRs were shorter and more scattered along the genome than the hypermethylation DMRs, which tended to occur in tightly linked longer clusters (Fig. 2E, I; Supplementary Fig. 7A). A chromosome analysis shows the more scattered nature of the hypomethylation clusters compared to the hypermethylation clusters. The length of hypermethylation clusters is increased relative to length of hypomethylation clusters (Fig. 2I; and Supplementary Fig. 9). The overall preponderance of hypomethylation DMRs over hypermethylation DMRs in terms of numbers is consistent with a role of SMCHD1 as a negative regulator of 5-methylcytosine oxidase enzymes, which catalyze DNA demethylation. These enzymes should be more active in the absence of SMCHD1 as previously reported32.
The hypermethylated regions displayed very unusual and interesting features. First, most hyper-DMRs occurred in dense clusters (n = 1511; Supplementary Data 2) and as such covered not only genes but larger genomic regions of several megabases. DMR clusters were defined as having five or more DMRs within 10 kb, and the DMR clusters were joined when the maximum distance between the DMR clusters was less than 10 kb. Examples are shown in Fig. 2E, and Supplementary Fig. 7A, and in several subsequent Figures. Second, the hypermethylation DMR clusters (70%) were preferentially associated with SMCHD1-bound regions (Fig. 2H). Third, hypermethylation DMR regions show a concomitant loss of H3K9me3 (Figs. 2E, J and Supplementary Fig. 7A, 7D, 7E). The latter inverse correlation between DNA methylation and the heterochromatin modification H3K9me3 is unusual because the two inactive gene marks usually coexist, for example for repression of repetitive DNA sequences8,45. In this context, it is of note that DNA CpG methylation, although commonly associated with gene repression when it occurs at promoters or enhancers, should also be viewed as a euchromatin mark. DNA methylation levels are much higher in gene-dense regions relative to gene-poor regions of chromosomes. DNA methylation in gene bodies has an activating role in gene regulation and is positively correlated with gene expression states46,47. In our system, DNA hypermethylation in the knockouts occurred along LAD regions bound by SMCHD1 in wildtype cells. LAD regions generally tend to be only partially methylated in most cell types and have been referred to as partially methylated domains (PMDs)48. In essence, loss of SMCHD1 converts these partially methylated domains into a state of higher methylation, with concomitant loss of H3K9me3.
We determined if the observed DNA methylation changes in absence of SMCHD1 occurred in specific genome compartments (Supplementary Fig. 10). We observed only slightly less hypermethylation than hypomethylation at genic regions but over 4-times more hypomethylation at intergenic genome compartments (Supplementary Fig. 10A, 10B). Hypermethylation at promoter regions was more pronounced at downregulated than at upregulated genes, as expected (see below for data on gene expression analysis) (Supplementary Fig. 10C, 10D).
Acquisition of active chromatin marks in SMCHD1 bound regions after loss of SMCHD1
We mapped several active chromatin marks in wildtype and in SMCHD1-depleted cells. All ChIP-seq samples of the same genotype showed high levels of correlation (Supplementary Fig. 11A). The major differences were again found in SMCHD1-marked regions, most notably in those that acquired long stretches of DNA hypermethylation (Supplementary Fig. 7). Regions that acquired clustered DNA hypermethylation and lost H3K9me3 simultaneously gained extensive signals for H3K36me2, H3K36me3, numerous peaks of H3K27 acetylation, H3K4me1 and H3K4me3 (Supplementary Fig. 7A). This observation was made genome-wide for hypermethylation DMR clusters (Supplementary Fig. 7B). PCA separated the hypermethylated DMR clusters between wildtype cells and SMCHD1-deficient cells when all mapped histone modifications were considered (Supplementary Fig. 7C). For the hypomethylation DMR clusters, the reverse phenomenon was observed, i.e., loss of active marks, but this occurred to a much lesser extent (Supplementary Fig. 7B). These regions showed a limited gain of H3K9me3 (Supplementary Fig. 7B, 7D). Hypermethylation DMR clusters in SMCHD1-marked regions with a concomitant loss of H3K9me3 occurred along all chromosomes (Figs. 2J, K; and Supplementary Fig. 7E).
We also mapped the structural proteins CTCF and the cohesin subunit RAD21 in wildtype and SMCHD1 knockout cells. Also here, within the SMCHD1-associated regions and long-range hypermethylation DMR clusters, we observed a strong gain of many coinciding CTCF and RAD21 peaks in the knockout cells. Examples are shown in the bottom panels of Supplementary Fig. 7A, and genome-wide analysis is presented in Supplementary Fig. 7B.
Control of genome compartmentalization by SMCHD1
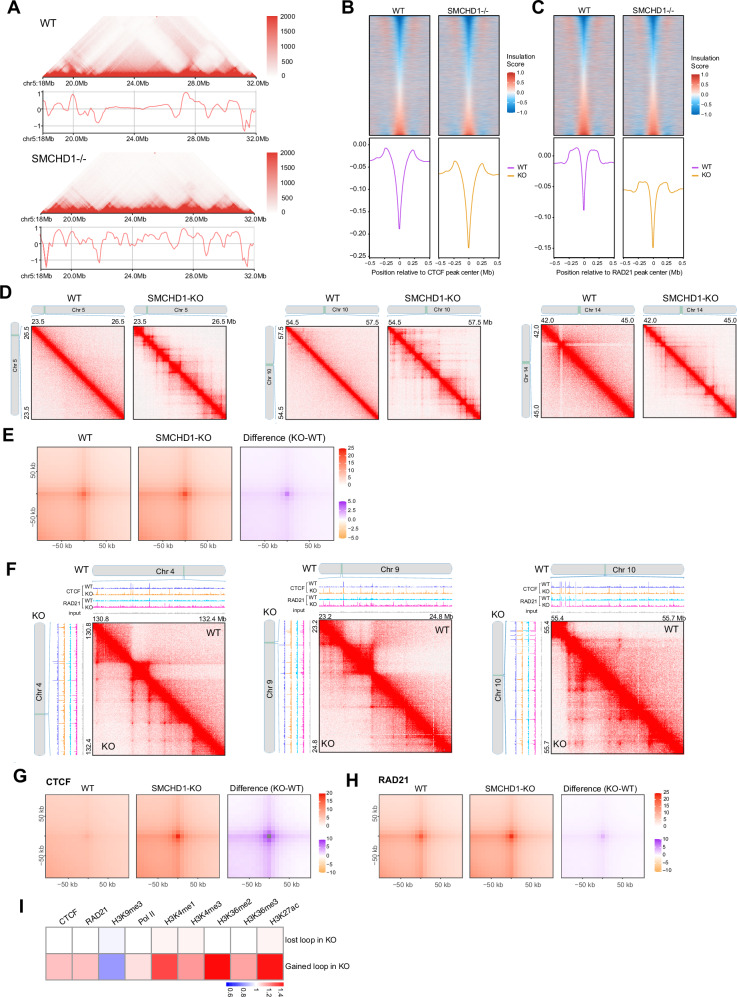
The changes in CTCF and cohesin (RAD21 subunit) localization suggested to us that the three-dimensional genome architecture may undergo substantial changes when SMCHD1 is inactivated. To obtain a detailed picture of 3D genome structure in wildtype and SMCHD1-depleted muscle cells, we used the HiC 3.0 technique49,50 on two biological replicates each of wildtype and SMCHD1 knockout LHCN-M2 myoblasts. We obtained between 2.1 and 2.5 billion aligned contact reads for each sample providing detailed resolution down to a level of a few kilobases (Supplementary Fig. 11B). We pooled the data from the biological replicates, which were well correlated (Supplementary Fig. 11C), to achieve high read density. Figure 3A shows contact maps along the entire chromosome 4 as an example. We observed the loss of many long-range (larger than 1 Mb) contacts in the SMCHD1−/− cells (Fig. 3A, B). However, when examining the chromosomes at higher magnification, we revealed a gain of shorter-range contacts within windows of single to a few megabases (Fig. 3B). As shown for the different contacts on chr4 as an example (Fig. 3C), the average probability to gain contacts in the SMCHD1 knockout relative to wildtype cells is increased for shorter contact ranges and then declines for the longer-range contacts.
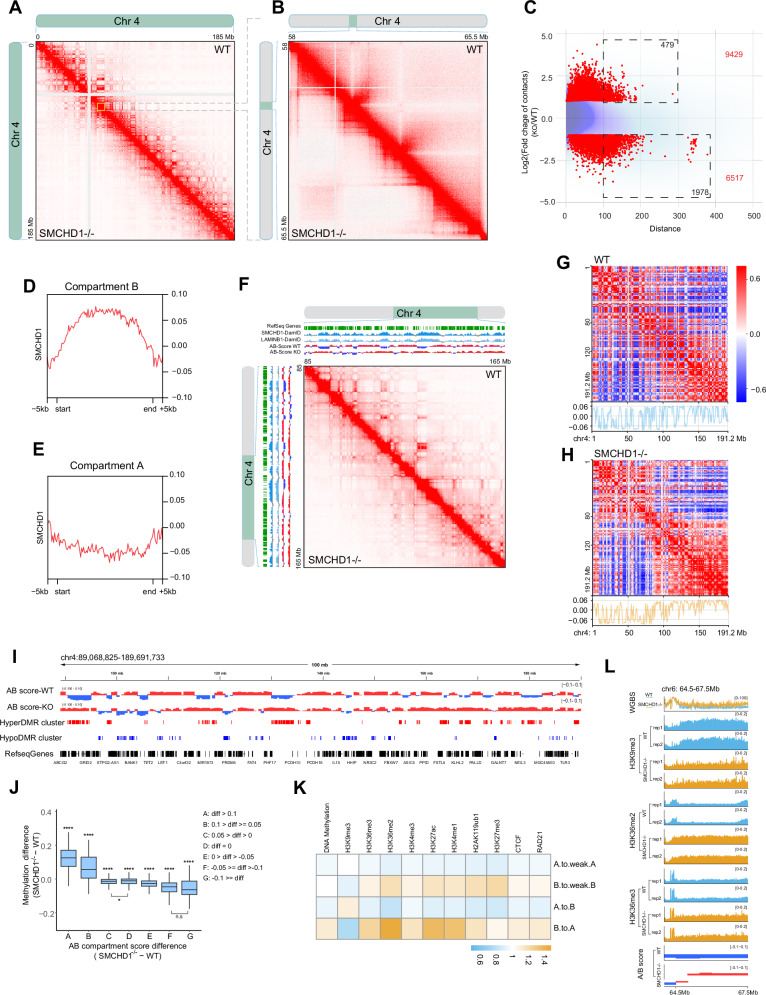
Fig. 3. SMCHD1 controls genome compartmentalization.
A In situ Hi-C contact maps showing contacts along the entire chromosome 4 in wildtype (WT) and SMCHD1−/− myoblasts. Many long-range contacts are diminished or lost, along with gain of short-range contacts in the SMCHD1-deficient cells. B Enlargement of an area on chromosome 4 from panel A shows gain of numerous short-range contacts in the SMCHD1−/− cells. C MD plot showing the distribution density of log-fold change in interaction strength of contacts (Y axis) relative to interaction distance (X axis) on all B-to-A transitioned regions at chr4. X axis is defined as the distance between two interacting regions, expressed in unit-length of the 10 kb resolution Hi-C data. Darker purple areas indicate regions with a higher concentration of interactions. Red points: Significant differential interactions detected by HiCcompare (Fold change > 2 and p < 0.05). The total number of differential interaction regions is labeled in red in the figure, and the number of differential interaction regions at long distance ( > 100, equals >1 Mb) is labeled in black in the dashed rectangle. D Distribution of SMCHD1 Dam-ID signal over B compartments derived from HiC analysis. E Distribution of SMCHD1 Dam-ID signal over A compartments derived from HiC analysis. F HiC contacts and Hi-C eigenvector (PC1) analysis in WT and SMCHD1−/− cells across an 80 Mb region of chromosome 4. The SMCHD1 and Lamin B1 signals are shown along with B compartments (blue) and A compartments (red). B-to-A transitions can be seen in the knockout cells compared to wildtype. G Heat maps showing the PC1 for chromosome 4 at 500-kb resolution in wildtype cells. Bottom: Line plot showing corresponding PC1 for chromosome 4. H Heat maps showing the PC1 for chromosome 4 at 500-kb resolution in SMCHD1−/− cells. Bottom: Line plot showing corresponding PC1 for chromosome 4. I Detailed display of A and B compartments in WT and SMCHD1−/− cells along with their positional relationship to hyperDMR clusters, hypoDMR clusters and Refseq genes. J Boxplot showing association between compartment score (Hi-C Eigenvector) change and DNA methylation change. The midline is the median of the data, with the upper and lower limits of the box being the third and first quartile (75th and 25th percentile). Whiskers extend to the most extreme data points within 1.5 × inter-quartile range (IQR) from the box bounds, and minima and maxima within the whiskers indicate the range of non-outlier data. A two-sided Wilcoxon signed-rank test was performed for statistical analysis (*p ≤ 0.05, **** p ≤ 0.0001; the group labeled with ‘****’ indicates the difference is highly significant relative to any other group, except difference between groups C and D (*p ≤ 0.05), and difference between groups F and G (F, G) (n.s indicates not significant). The numbers of each group: n(A) = 111, n(B) = 197, n(C) = 2735, n(D) = 2135, n(E) = 2431, n(F) = 260, n(G) = 53). Exact p values and source data are provided as a Source Data file. K. Heatmap showing changes of chromatin marks, DNA methylation, CTCF and RAD21 in relation to compartment transitions in SMCHD1−/− cells. L. Changes in DNA methylation (WGBS) and chromatin marks over a B-to-A compartment transition for a representative region on chromosome 6.
We next assigned chromatin compartments (A versus B) using Eigenvector principal component analysis49. Most B (inactive chromatin) but not A (active) compartments were associated with SMCHD1 signal blocks (Figs. 3D, E). We observed that loss of SMCHD1 promoted many B-to-A compartment transitions (Fig. 3F–I; shift of blue to red color of the compartment scores). These transitions occurred specifically in SMCHD1-associated regions (Fig. 3F). Furthermore, B to A compartment transitions were confirmed when comparing the heatmaps of PC1 over the whole chromosome in WT and SMCHD1−/− cells (Fig. 3G, H).
Almost half (n = 317) of all B compartments transitioned to A compartments whereas a lower number of A compartments changed into B (Supplementary Fig. 12A) but over much smaller regions (Figs. 3F–I; Supplementary Fig. 12B). Of the remaining “non-transitioning” B compartments (N = 328), many of them (n = 146) became converted into weak B compartments (Supplementary Fig. 12A). This means that over 70% of all B compartments are converting to weak B or to A compartments. Most B-to-A transitioning regions contained DNA hypermethylation clusters (Fig. 3I; and Supplementary Fig. 12B). The greatest DNA methylation differences were observed at the highest compartment score differences, reflecting B-to-A transitions (Fig. 3J). Globally, B-to-A compartment transitions were associated with a loss of H3K9me3 and with gains of DNA methylation, H3K36me2, H3K36me3, H3K4me1, H3K4me3, H3K27Ac, CTCF and RAD21 (Fig. 3K; and Supplementary Fig. 12C). Figure 3L shows an example from chromosome 6, where a B-to-A compartment transition is accompanied by changes in several of these chromatin marks.
Loss of intrachromosomal B compartment contacts
Importantly, there were many long-range contacts between different SMCHD1-marked B compartments (B-to-B contacts) along the same chromosome that were lost in SMCHD1 knockout cells (Fig. 4A, Fig. 4C, D; Supplementary Fig. 13A and 13B). This data supports the proposal that SMCHD1 is an important anchor point for heterochromatin regions that are distant from each other on the linear chromosomes. A more high-resolution analysis shows that there were many contact points (dots) between two individual B compartments (B-to-B contacts) as indicated by a cloud of dots in shapes of distinct broader stripes (Fig. 4A; and Supplementary Fig. 13A, 13B). However, these dots and stripes disappear in the SMCHD1 knockout cells.
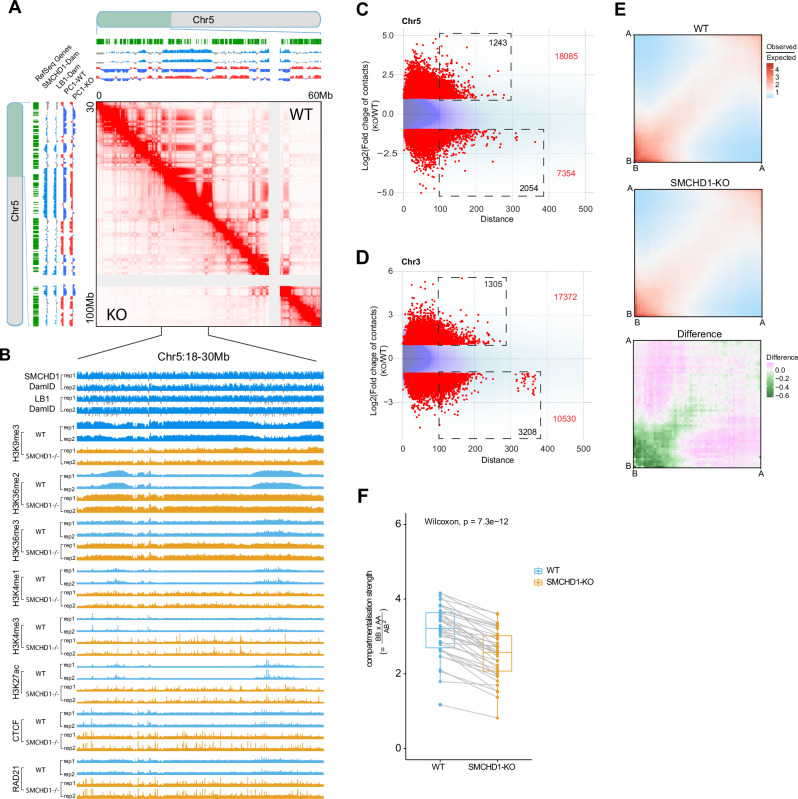
Fig. 4. Loss of contacts between B compartments in SMCHD1-depleted cells.
A In situ Hi-C contact map showing contacts between individual B compartments (broad stripes) across a 60-Mb region of chromosome 5 and their loss in SMCHD1-/- cells. The SMCHD1 and Lamin B1 (LB1) signals are shown along with B compartments (blue) and A compartments (red) on top and left. B SMCHD1, Lamin B1 (LB1) and histone modification patterns in WT and SMCHD1-/- cells in a magnified representative region from (A). C, D MD plots showing the distribution density of log-fold change in interaction strength of contacts (Y axis) relative to interaction distance (X axis) on all B loss regions at chr5 (C) and Chr3 (D). Darker purple areas indicate regions with a higher concentration of interactions. Lighter blue areas represent regions with fewer data points. Red points: Significant differential interactions detected by HiCcompare (Fold change >2 and p < 0.05). The total number of differential interaction regions is labeled in red in the figure, and the number of differential interaction regions at long distance ( > 100, equals > 1 Mb) is labeled in black in the dashed rectangle. E Saddle plots showing the genome-wide compartmentalization and differences between WT and SMCHD1−/− cells. Differential saddles with green colors denoting fewer B-to-B inter-compartment interactions in SMCHD1−/− cells compared to wildtype cells as shown on the right. F Boxplot of the compartmentalization strength per each chromosome arm (dots). This data corresponds to the proportion of intra-compartment (B-to-B and A-to-A) contacts versus inter-compartment (A-to-B and B-to-A) contacts and is calculated for each chromosome arm separately. Each pair of connected points represents a matched chromosome arm, with lines highlighting paired measurements (n = 39). The midline is the median of the data, with the upper and lower limits of the box being the third and first quartile (75th and 25th percentile) respectively. Whiskers extend to the most extreme data points within 1.5 × inter-quartile range (IQR) from the box bounds, and minima and maxima within the whiskers indicate the range of non-outlier data. Statistical significance was assessed using a two-sided Wilcoxon signed-rank test (p = 7.3 × 10−12), Source data are provided as a Source Data file.
The B-to-A compartment transitions and loss of inter-B compartment contacts were associated with loss of the repressive histone mark H3K9me3 and gain of many active marks, including H3K36me2, H3K36me3, H3K4me1, H3K4me3, H3K27Ac, as well as gain of CTCF and RAD21 binding peaks (Fig. 4B; and Supplementary Fig. 13C, 13D, 13E). Figure 4E shows the loss of B-to-B compartment contacts in SMCHD1−/− cells using genome-wide analysis. Compartmentalization strength (inter-B and inter-A) is decreased along all chromosome arms except for chr 19q, a very gene-rich chromosome arm, which shows a minor reduction (Fig. 4F).
SMCHD1 organizes LADs and 3D genome structure
B to A compartment transitions were targeted to SMCHD1-associated regions (Fig. 3). We observed that these same regions partially lose their association with Lamin-B1 as shown by diminishing Lamin B1 signal over B-to-A transitioning compartments (Fig. 5A, B). This reduction was not caused by poor performance of the Dam-ID reactions in the knockout cells because we observed increased Lamin B1 signals at several genomic regions, for example at the left side of Fig. 5B (indicated by a green rectangle) and closer to the telomeres/subtelomeres of several chromosomes (Fig. 5C). A quantitative analysis of Lamin B1 signal reduction in absence of SMCHD1 at Lamin B1-associated regions is shown in Fig. 5D.
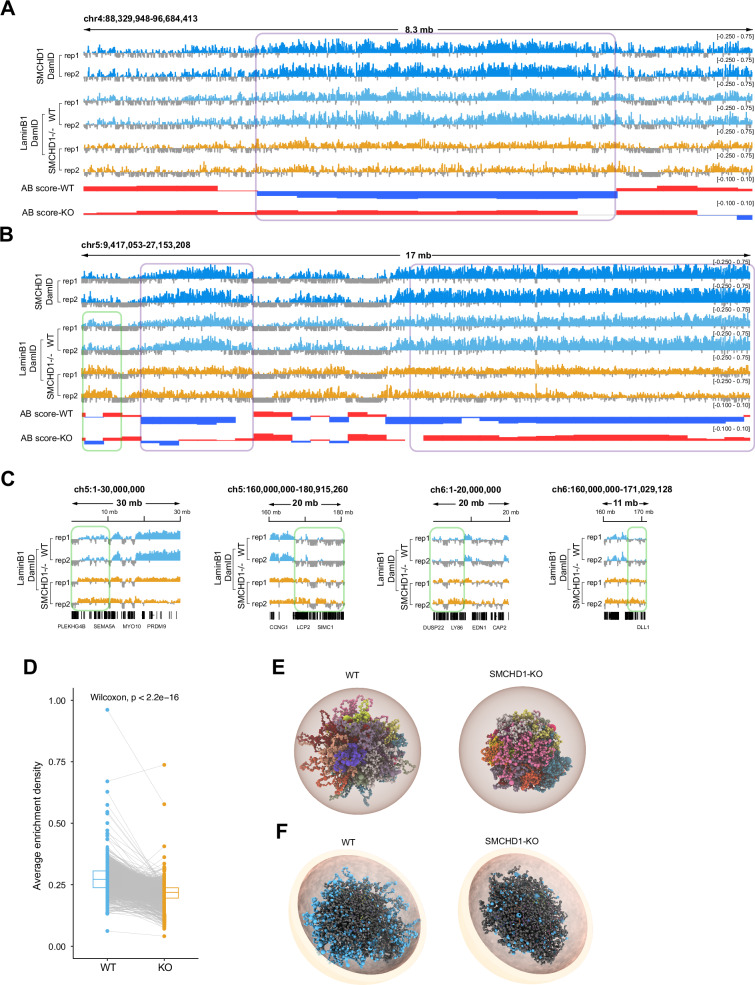
Fig. 5. Loss of LADs and alterations of 3D chromatin structure in SMCHD1-deficent cells.
A Plots showing changes in Lamin B1 over a B-to-A compartment transitions for a region on chr4 in WT and SMCHD1−/− cells. Two replicates each of Dam-ID sequencing are shown along with B compartments (blue) and A compartments (red). The signals were normalized relative to Dam-only signal. B Genome browser views of an area of chr5 showing the distribution of SMCHD1 and of Lamin B1 in wildtype and in SMCHD1-KO cells (see panel A for details). C Genome browser views showing the increased enrichment of LaminB1 in SMCHD1−/− cells by Dam-ID sequencing at representative areas of chr5 and chr6 (indicated by green rectangles). D Plot showing the average enrichment density of Lamin B1 at Lamin-associated genomic regions in wildtype (WT) and in SMCHD1 KO cells. Each pair of connected points represents a LaminB1-associated genomic region (n = 1242), with lines highlighting paired measurements. The midline is the median of the data, with the upper and lower limits of the box being the third and first quartile (75th and 25th percentile) respectively. Statistical significance was assessed using a two-sided Wilcoxon signed-rank test (p < 2.2e − 16). Source data are provided as a Source Data file. E Structural 3D genome modeling reveals changes of the spatial distribution of individual chromosomes for the whole genome in WT and SMCHD1-KO cells. Chrom3D models of the LHCN-M2 genome were built from Hi-C and Lamin B1-DamID sequencing data used as positional constraints for TADs and LADs. Tomographic views show individual chromosomes (differentially colored) modeled as beads on a string, each bead representing a TAD identified in the Hi-C data. Radius was set to 5 μm as a default parameter. F Tomographic view of 3D structure showing spatial distribution of individual chromosomes for the whole genome in WT and SMCHD1-KO cells. The LAD-associated genomic regions are indicated as blue spheres. Grey spheres represent TADs without LAD association. Radius was set to 5 μm as a default parameter.
To further analyze this phenomenon at the global level, we performed Chrom3D, a method that uses 3D TAD information obtained from the HiC data along with data on Lamin-B1-associated regions to construct a 3D model of nuclear and chromatin structure51,52. Analyzing the distribution of the chromosomes inside the nucleus, we observed a striking shrinkage of the territorial volume that the chromosomes occupy in the SMCHD1-deficient cells relative to wildtype cells (Fig. 5E; and Movies S1 and S2). Chrom3D shows the peripheral localization of LADs in the nuclear shell in wildtype myoblasts (Fig. 5F; and see Movies S3 and S5). However, in SMCHD1-KO cells, much of the Lamin B1 signal has disappeared from the nuclear periphery and shows a more random organization (Fig. 5F; Movies S4 and S6). At the level of single chromosomes, we noted an outside localization of the Lamin B1 associated regions on chromosomes 2, 3, and 6 (and most other chromosomes) in wildtype cells but a more compact organization of the chromosomes in the SMCHD1-deficient cells (Supplementary Fig. 14A–C). The exceptions were smaller, gene-rich chromosomes, which have few LADs and few SMCHD1-bound sequences. An example is chromosome 19, where substantial Lamin B1 signals are only found near the centromere (Supplementary Fig. 14D). This chromosome shows no major structural differences between wildtype and knockout cells (Supplementary Fig. 14D) suggesting that the structural changes we observe on the other chromosomes are linked to SMCHD1/Lamin B1-associated genomic features. Combined, the data shows that SMCHD1 is an important regulator that maintains genome compartments and nuclear structure.
Topologically associated domains and loops are remodeled by inactivation of SMCHD1
When zooming in to reveal further details, in parallel with the B-to-A compartment transitions and the loss of the long-range contacts between different B compartments, we observe a gain of distinct sets of shorter-range contacts suggesting the emergence of new topologically associated domains (TADs) (Figs. 4C, D; and Fig. 6A; Supplementary Fig. 15A, 15B).
Fig. 6. Gains of TADs and loops in SMCHD1-deficient cells are linked to CTCF and Cohesin/RAD21 binding.
A Hi-C contact map and insulation profiles at a representative region on chromosome 5 in wildtype cells (upper panel) and SMCHD1−/− cells (lower panel). B Insulation heatmap and histogram profile of insulation scores at 10 kb resolution spanning a 500 kb window at CTCF peak centers between WT and SMCHD1−/− cells. The color maps show strong insulation in blue and weak insulation in red. C Insulation heatmap and histogram profile of insulation scores at 10 kb resolutions spanning a 500 kb window at RAD21 peak centers between WT and SMCHD1−/− cells. Color maps show strong in blue and weak insulation in red. D Hi-C contact maps showing more loops at representative regions on chromosomes 5 (left panel), 10 (middle panel), and 14 (right panel) after loss of SMCHD1. E Aggregate peak analysis (APA) of all chromatin loops shows increased interaction intensity in SMCHD1−/− cells versus wild-type cells. The differential APA analysis plot with purple denotes more interactions in SMCHD1−/− cells compared to the wildtype cells. F Gains of loops at representative regions on chromosomes 4 (left panel), 9 (middle panel), and 10 (right panel), linked to newly arising RAD21 and CTCF peaks in SMCHD1 KO cells as shown in the ChIP-seq tracks. G APA analysis of chromatin loops located at CTCF binding sites detected in wildtype and SMCHD1−/− cells, which shows increased interaction intensity in SMCHD1−/− cells versus wild-type cells. Differential APA analysis plot with purple denotes more interactions in SMCHD1−/− cells compared to wildtype cells. H APA analysis of chromatin loops located RAD21 binding sites detected in wildtype and SMCHD1−/− cells, which shows increased interaction intensity in SMCHD1−/− cells versus wild-type cells. A differential APA analysis plot with purple denotes more interactions in SMCHD1−/− cells compared to the wildtype cells. I. Heatmap showing differences in CTCF, RAD21, Pol II, and chromatin marks over gained or lost loop intervals in SMCHD1−/−- cells.
We called differential TADs at different levels of resolution, ranging from 10 kb to 250 kb (Supplementary Fig. 16). This data shows no major differences depending on resolution. Alignment of the HiC data with ChIP-seq peaks of CTCF and RAD21 showed that the new TAD boundaries in the knockout are marked by new CTCF and RAD21 binding (Fig. 6B, C; and Supplementary Fig. 15C). Heatmap and histogram profiles of insulation scores showed strong insulation at the CTCF and RAD21 binding sites in SMCHD1−/− cells at a global level, compared WT cells (Fig. 6B, C). Aggregate TAD analysis is depicted in Supplementary Fig. 15D and shows an increase in TAD formation upon loss of SMCHD1, both within TADs and with neighboring TADs (Supplementary Fig. 15E). Furthermore, cumulative distribution plots for insulation scores comparing WT versus SMCHD1−/− cells also showed a statistically significant difference for the whole genome (p < 2.2e-16), with the slope of the SMCHD1−/− curve being shallower before zero and steeper after zero, indicative of greater fluctuation of insulation scores and more TADs (Supplementary Fig. 15F). A similar pattern was observed on presentative chromosomes 4, 10 and 13 in WT and SMCHD1−/− cells (Supplementary Fig. 15G).
Our dataset provided sufficient resolution to examine loop formation well below the 5 kb resolution. First, we determined if differential loop calling was dependent on the level of HiC resolution. We found that loop calling was similar at levels of resolution of 5 kb, 10 kb, and 20 kb (Supplementary Fig. 17). We found that the loss of SMCHD1 leads to the formation of many new loops (e.g., dots in Figs. 6D, F; and Supplementary Fig. 15C). At the whole genome level, we saw an increase of contacts at the loops in the absence of SMCHD1 (Fig. 6E). The new loops were anchored at newly arising CTCF and RAD21 peaks in the SMCHD1 knockout cells, in some cases in combination with CTCF peaks that preexisted in the wildtype cells (Fig. 6F). Global analysis of chromatin loops located at CTCF or RAD21 binding sites detected in wildtype and SMCHD1−/− cells showed increased interaction intensity in SMCHD1-deficient cells (Fig. 6G, H). The new loop formation was associated with the gain of active chromatin components, most strongly with gains of H3K4me1, H3K36me2, and H3K27 Ac and with a loss of H3K9me3 (Fig. 6I).
SMCHD1 regulation of transcription
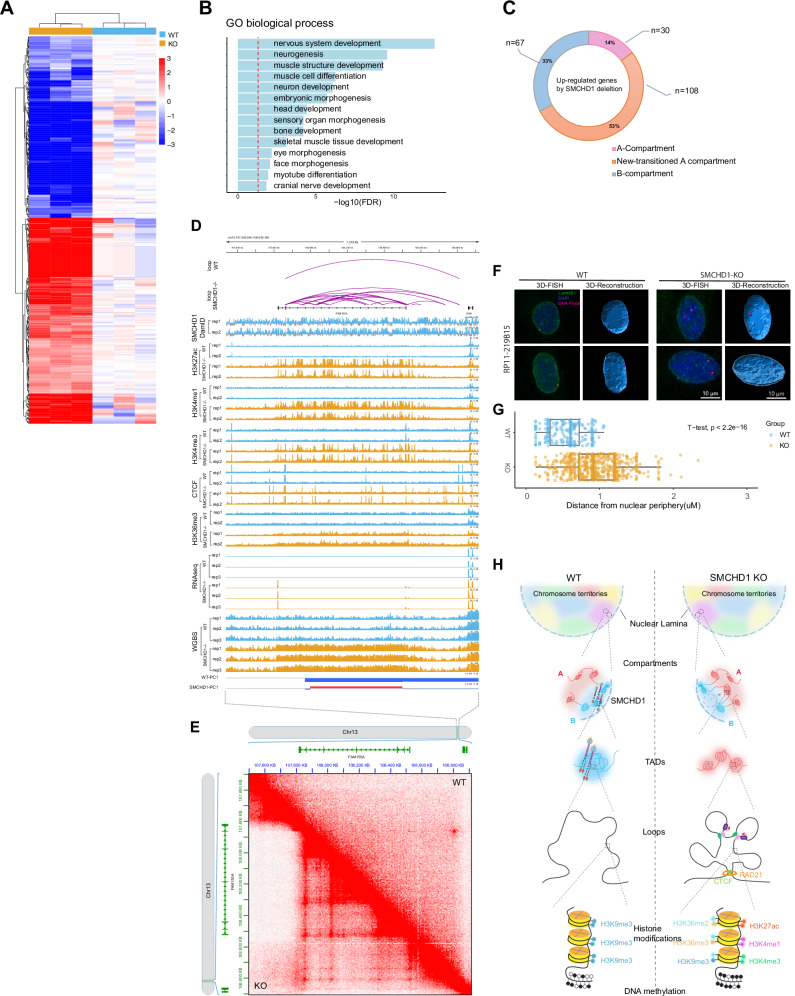
Changes in gene expression in the knockout cells were relatively limited, likely reflecting the fact that SMCHD1 chiefly is associated with gene-poor regions of chromosomes. Using RNA-seq, we found 208 upregulated genes and 180 downregulated genes (fold change > 2, FDR < 0.05) (Fig. 7A; and Supplementary Data 3). Gene ontology analysis showed enrichment of terms related to muscle cell development and neurogenesis (Fig. 7B; Supplementary Fig. 18A). Of particular interest were those upregulated genes that we found in SMCHD1-bound regions which underwent B-to-A compartment transitions, usually in parallel with acquisition of active chromatin marks. More than half of all upregulated genes were localized within B-to-A transitioning compartments (Fig. 7C).
Fig. 7. Changes in gene expression and 3D interactions after loss of SMCHD1.
A Heat map of gene expression changes. B GO analysis of differentially expressed genes. C The relationship between differential gene activation and compartment transitions. D The FAM155A gene is upregulated in parallel with acquisition of active marks and extensive new loop formation in SMCHD1−/− cells. This panel shows browser tracks for RNA-seq data, distribution of active histone marks and mapped loops in WT and SMCHD1 KO myoblasts. E Raw HiC data showing the gain of loops at the FAM155A locus. F 3D-DNA FISH analysis of FAM155A locus positioning (red dots) in the nucleus of WT (left) and SMCHD1 KO cells (right). Representative FISH images are shown, with probe distance from the nuclear periphery. Bars = 10 μm; 3D reconstructed models of FAM155A locus positioning were generated by DAPI staining and FISH positioning using Imaris (see “Methods”). G Quantification of the distance of each FISH focus to the nuclear periphery in WT and SMCHD1 KO cells (0 = periphery defined as the border of DAPI staining). WT nuclei (n = 117) and SMCHD1 KO nuclei (n = 310) were analyzed; bars = 10 μm. The midline is the median of the data, with the upper and lower limits of the box being the third and first quartile (75th and 25th percentile), respectively. Whiskers extend to the most extreme data points within 1.5 × inter-quartile range (IQR) from the box bounds, and minima and maxima within the whiskers indicate the range of non-outlier data. Statistical significance was assessed using a two-sided t-test (p < 2.2e − 16). Source data are provided as a Source Data file. H. Model of SMCHD1 function in 3D epigenome landscape maintenance.
The likely disease-causing gene activated in muscle cells of FSHD2 patients that carry SMCHD1 mutations and/or a contraction of the D4Z4 repeat units at chromosome 4q35 (FSHD1) is DUX420 encoding an embryonic transcription factor53–55. Using our RNA-seq data and RT-PCR, we did not observe an upregulation of DUX4 expression, nor did we observe upregulation of the well-characterized DUX4 target genes ZSCAN4, MBD3L2, and TRIM43 in SMCHD1 knockout cells (Supplementary Fig. 18C). In addition, DNA methylation patterns at the telomeric DUX4 repeat unit did not change after SMCHD1 loss. This finding is consistent with previous results in which inactivation of SMCHD1 in human somatic cells did not reactivate this locus44. We were only able to activate DUX4 and demethylate the locus by combined inactivation of SMCHD1 and treatment of the cells with the DNA methylation inhibitor 5-azadeoxycytidine (5-azaC-dR) but not with 5-azadeoxycytidine alone.
We next determined if formation of new 3D contacts may globally underlie gene activation events in SMCHD1-deficient cells. Examples are shown in Supplementary Fig. 18B for the genes CCL2 and NAP1L3. These de novo loop formations were associated with gain of active chromatin marks (Supplementary Fig. 18B). The promoters of upregulated genes were associated with gain of active marks whereas downregulated genes lost active marks, as expected (Supplementary Fig. 18D).
One of the most highly upregulated genes, FAM155A, is shown in Fig. 7D. This gene encodes a component of hetero-tetrameric NALCN sodium channels56–58. FAM155A was highly upregulated after SMCHD1 knockout (Fig. 7D). At the FAM155A locus, we observed de novo formation of CTCF and RAD21 binding sites and extensive de novo loop formation in absence of SMCHD1 along with gain of many active chromatin modifications (Fig. 7D). The de novo loop formation in absence of SMCHD1 is easily visible from the raw HiC data (Fig. 7E). Consistent with the reorganization of the epigenomic state and B to A compartment transition of these sequences, we observed a movement of the average FAM155A locus-specific FISH signal from a mostly peripheral location in the nucleus to positions more internal and away for the nuclear lamina (Fig. 7F, G).
The model in Fig. 7H summarizes our findings and highlights the role of SMCHD1 in maintaining the integrity of B compartments and the proper segregation of inactive and active chromatin domains.
Discussion
Here we present a genome-wide analysis of SMCHD1 function in human muscle cells. We show that SMCHD1 colocalizes and copurifies with Lamin B1 and is colocalized extensively with the LAD-associated heterochromatin mark H3K9me3. Conceptually, DamID marks DNA where SMCHD1 (and Lamin B1) have been, rather than where these proteins are at a moment in time. Rather than being fixed at the LADs, the DamID data therefore may suggest that SMCHD1 moves around throughout the genome, particularly at the LADs, but perhaps spends more time at regions that have previously been reported with SMCHD1 ChIP-seq. We also tried to localize SMCHD1 using antibody-based immunofluorescence staining. Out of five commercially available antibodies, only two showed a loss of signal in the knockout cells. These two antibodies were directed against the same epitope localized in the N-terminal ATPase domain of the protein and showed a speckled nuclear distribution with little enhancement at the nuclear lamina region. C-terminal SMCHD1-GFP fusion proteins also did not show lamina association in MEFs or neural stem cells from mice27. The apparent discrepancy of these results with the SMCHD1-Dam-ID patterns at the nuclear lamina, and at the protein level, is currently unexplained but it is possible that the endogenous SMCHD1 protein is poorly accessible when present within the LAD regions or that the GFP tag interferes with LAD association. Another study has analyzed SMCHD1-Dam-ID patterns in female mouse embryo fibroblast cells using the data of Wang et al.25 who focused on the role of SMCHD1 in X inactivation. The authors reported a strong colocalization of SMCHD1 and lamin-associated protein beta (LAP2β) on autosomes in this cell type59. We confirmed co-localization of SMCHD1 and Lamin B1 in murine fibroblasts using publicly available data (Supplementary Fig. 4). In a proteomics study, SMCHD1 was found as an interactor of LAP2β, along with the proteins HP1β, LRIF1, and EHMT2, a H3K9 methyltransferase59. These findings support our data on LAD association of this protein and suggest that SMCHD1 localization on autosomes may not be entirely cell type dependent. We also emphasize that all major changes in epigenetic marks and in 3D genome organization observed in our study when SMCHD1 was inactivated do occur at regions bound by SMCHD1 and Lamin B1, further supporting the genomic localization patterns we observed.
Depletion of SMCHD1 leads to a reduction of H3K9me3 along many SMCHD1-bound regions, a visible loss of heterochromatin at the nuclear periphery as determined by transmission electron microscopy, and to a partial loss of Lamin B1 at these regions (Figs. 2, 5). Unexpectedly, the loss of H3K9me3 occurs in parallel with a gain of DNA methylation which increases over megabase size regions that lose H3K9me3. The loss of SMCHD1 leads to an uncoupling of the two marks at larger genomic scales.
Along with the loss of H3K9me3, we observe a gain of many active chromatin marks, including promoter and enhancer marks (H3K4me3, H3K4me1, H3K27Ac), active gene body marks (H3K36me3, RNA polymerase), the more broadly distributed euchromatin modification H3K36me2, as well as the chromosomal structural proteins, CTCF and RAD21. The changes observed reflect the conversion of heterochromatin to euchromatin (B-to-A compartment transitions) at SMCHD1-bound regions as determined by 3D compartment analysis.
The gene expression changes we observed in myoblasts lacking functional SMCHD1 may contribute to FSHD2. One of the most strongly upregulated genes was FAM155A, a component of sodium channels. Alterations in the NALCN sodium leak channels, which FAM155A is a component of, have been linked to hypotonia, a form of muscle weakness60. It is conceivable that an imbalance of NALCN subunits due to strong overexpression of FAM155A may contribute to a muscle cell phenotype. It remains to be determined if and how changes to the nuclear structure as we observed in SMCHD1-deficient cells may also contribute to FSHD2. Future in-depth analysis of patient-derived samples will be needed to address this point. Myocytes utilize nuclear mechanosignaling mechanisms to respond to changes in the environment and transmit them through the nuclear envelope and lamina to the nucleus to alter transcription61. Intriguingly, mutations in several nuclear envelope and lamina-associated proteins, Lamin A/C, Emerin, Nesprin1/2, and LAP1, are all linked to different forms of muscular dystrophy61,62.
The fact that SMCHD1 plays an important part in organizing stretches of constitutive heterochromatin on autosomes in human somatic cells has previously not been appreciated. Prior studies have focused predominantly on the function of SMCHD1 in X chromosome inactivation14,23–26 or in the control of a few autosomal gene clusters, such as protocadherin and HOX genes24,33. Although the structure of the inactive X chromosome is unique, there are similarities in SMCHD1 function in X inactivation and in autosomal heterochromatin maintenance. SMCHD1 antagonizes TADs and compartmentalization and merges the S1 and S2 compartments on the inactive X chromosome in mouse embryo fibroblasts or mouse neural progenitor cells (Xi)23–25. However, the role of H3K9me3 in X chromosome inactivation is less defined although a link to SMCHD1 has been observed28. SMCHD1 was shown to be recruited to the Xi by a pathway involving XIST, HNRNPK and the PRC1 Polycomb complex via histone H2AK119 ubiquitylation27. Polycomb components play an important role in X chromosome inactivation63,64 but their contribution to constitutive heterochromatin is not clear since this mark occurs across both A and B compartments with perhaps less involvement in B compartments. Furthermore, SMCHD1 is critical for XIST RNA spreading on the Xi26. The exact roles of SMCHD1 on the inactive X and at autosomal heterochromatin regions are expected to be different. Prior Hi-C studies of SMCHD1 knockouts have not observed substantial compartment switching or changes in TADs on autosomes23–25. However, Wang et al. did find that SMCHD1, although highly concentrated on the Xi, was also found at autosomal gene-poor regions of mouse embryo fibroblast cells25. The differences between our data and some published work with regards to 3D genome organization raise the important questions as to whether our findings are unique to muscle cells, occur only in human and not in mouse cells (unlikely, given the inter-species conservation of SMCHD1), or are a feature of only male cells, which lack an inactive X chromosome. Future work should address all these possibilities but would require generation of different genetic models in different species and in male and female cell types.
Removal of SMCHD1 leads to the loss of many inter-B compartment contacts, a weakening of B compartments and switches from B into A compartments. Thus, the SMCHD1 protein functions as an important global tether of heterochromatic B compartments, likely by tying a substantial fraction of heterochromatin to the nuclear lamina (see model in Fig. 7H).
Our data suggests that the B compartment (heterochromatin) is physically inaccessible in wildtype cells to a group of euchromatic histone and DNA modification enzymes and to at least some structural proteins. The model is consistent with a study in which promoters from numerous silent LAD-associated genes show strong activity when moved onto episomal plasmids36. Previous work has also shown that SMCHD1 limits access of the Polycomb H3K27 methylation activity to chromatin24. One particularly interesting finding concerns DNA methylation changes which occurred as long stretches of hypermethylation within the B-to-A switched regions in absence of SMCHD1. The hypermethylation is likely carried out by the de novo DNA methyltransferases DNMT3A and/or DNMT3B. The activities of these enzymes are stimulated by the euchromatic histone marks H3K36me2 for DNMT3A65, and by H3K36me3 for DNMT3A and DNMT3B46,66. These two histone modifications also substantially increased within the B-to-A converted regions (Fig. 4B, and Supplementary Fig. 12C, 13E). It is a general misconception that heterochromatin is enriched in DNA methylation. The partial methylation of LADs has typically been explained by late replication or by a DNMT sequence preference for these domains67,68. However, here we offer another explanation, namely that there is much-reduced accessibility of heterochromatin to the de novo DNA methyltransferases and the H3K36 histone methyltransferases which create improved substrates for the DNA methylation enzymes. These regions easily become H3K36-methylated, and de novo DNA-methylated upon a compartment switch (B-to-A).
We report here that regions of gene-poor heterochromatin will flip to become euchromatin by removal of a single chromosomal structural protein. Our data suggests that the spatial segregation of chromatin drives its transcriptional state rather than resulting from it although the order of events cannot easily be determined for genes that switch their expression state. The 3D genome architecture at the sub-megabase scale is regulated by the CTCF/Cohesin system, in which loop extrusion by Cohesin and the arrest of this process at CTCF-bound sites leads to the formation of loops and TADs. Notably, depletion of CTCF causes the disappearance of most TADs while leaving the higher order organization of chromatin into A and B compartments largely unchanged69. The cellular determinants of compartment organization have remained elusive. Here we report that the protein SMCHD1 is one critical component of the molecular machinery that maintains genome compartments, 3D-chromatin architecture, and epigenome landscape. The structurally related MORC family proteins use ATP-binding to control their dimerization and entrap DNA, forming chromatin loops by compaction70. Future studies will determine if SMCHD1 uses a similar or different mechanism to stabilize B compartments.
Methods
Cell lines
LHCN-M2, a human male myoblast cell line immortalized with hTERT and CDK4, was obtained from Evercyte (Vienna, Austria). LHCN-M2 cells were cultured on 0.1% gelatin-coated tissue culture plates in DMEM / medium 199 (4:1) supplemented with 15% fetal bovine serum, 0.02 M HEPES, pH 7.1, 0.03 μg/mL zinc sulfate, 1.4 μg/mL vitamin B12, 0.055 μg/mL dexamethasone, 2.5 ng/mL hepatocyte growth factor, 10 ng/mL basic FGF, 60 units/mL penicillin and 60 μg/mL streptomycin.
Myoblasts from healthy individuals (n = 3) or from patients with FSHD2 (n = 3) were obtained from the FSHD Registry at the University of Rochester. The genotype of 19MB015 was SMCHD1 + : c.610 A > G, that of 19MB016 was SMCHD1 + : c.1647+3 A > G, and 19MB017 had a 1.2 megabase deletion encompassing the SMCHD1 gene. Normal and patient myoblasts were cultured in F-10 Nutrient Media supplemented with 20% FBS, 1% penicillin/streptomycin, 10 ng/ml bFGF and 1 µM dexamethasone.
Generation of knockout LHCN-M2 myoblast lines using CRISPR-Cas9
SMCHD1−/− LHCN-M2 cell lines were generated by pLentiCRISPR-E with the blasticidin resistance gene generated from pLentiCRISPR-E-puromycin (a gift from Phillip Abbosh; Addgene plasmid # 78852). The pLentiCRISPR-E-Blast vector carrying the appropriate SMCHD1 sgRNAs was co-transfected into HEK293FT cells with lentiviral packing plasmid psPAX2 and envelope plasmid pMD2.GVG. For transfection of a 10 cm dish, FuGENE HD reagent (Promega, E2311) was diluted into 2 ml Opti-MEM and then the following DNA was added: 9.9 μg plentiCRISPR-E-Blast, 7.5 μg psPAX2 and 5 μg pMD2.GVG. Viral particles were harvested at 48 h after transfection and frozen at −80 °C. LHCN-M2 cells were transduced with plentiCRISPR-E-Blast-SMCHD1 virus, and single-cell clones were selected in 10 μg/mL blasticidin (10 μg/mL). Protein expression levels in the knockout cells were confirmed by Western blot. All SMCHD1 knockout clones were further confirmed by the presence of frameshift mutations detected by Sanger sequencing of the CRISPR-targeted region, which was PCR-amplified from genomic DNA and cloned into Topo-TA cloning vector (Thermo, 450030).
Western blot
Western blots were performed as previously described32, with minor modifications. Cells were lysed in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA and proteinase inhibitor cocktail (Roche,11873580001) on ice for 1 h, then the cell lysate was centrifuged at 12,000 × g for 15 min at 4 °C. The centrifuged cell lysates were then separated on 4%–15% SDS-polyacrylamide gels and transferred onto PVDF membranes (Bio-Rad) by wet transfer at 4 °C. The membranes were incubated with blocking buffer (5% non-fat milk, 0.1% Tween-20 in PBS) for 1 h at room temperature, and the membranes were then incubated with the indicated primary antibody at 4 °C overnight. We washed the PVDF membranes with PBS-Tween (0.1%), followed by incubation with peroxidase-conjugated secondary antibodies for 1 h at room temperature. Blotting signals were detected using ECL Prime detection reagent (GE Healthcare). Antibodies used for Western blots were anti-SMCHD1 (1:2,500, Bethyl Laboratories, A302-871A), anti-alpha-tubulin (1:10,000, Abcam, ab7291), HRP goat anti-rabbit IgG (Active Motif, 1:10,000, 15015), HRP goat anti-mouse IgG (1:10,000, Active Motif, 15014). Lamin B1 (Abcam, ab16048), G9a/EHMT2 (R&D Systems, PP-A8620A-00), SETDB1 (Proteintech, 11231-1-AP), H3K9me3 (Abcam, ab8898) and histone H3 (Abcam, ab1791).
Chromatin fractionation
Chromatin fractionation was performed as previously described71, with minor modifications. LHCN-M2 cells were washed in PBS and extracted in cytoskeleton (CSK) buffer (10 mM PIPES, pH 6.8, 100 mM NaCl, 1 mM EGTA, 300 mM sucrose, 3 mM MgCl2, protease inhibitors, and 1 mM phenylmethylsulfonyl fluoride (Thermo Fisher Scientific, 36978) supplemented with 1 mM dithiothreitol and 0.5% Triton X-100). We incubated the reaction on ice for 5 min, and then separated the cytoskeletal fraction from soluble proteins by centrifugation at 800 × g for 3 min. The supernatant was designated as the S1 fraction. The pellets were washed with an additional volume of CSK buffer. We solubilized chromatin by adding 25 U of ribonuclease-free DNase (Invitrogen) in CSK buffer at 37 °C for 30 min, followed by adding ammonium sulfate in CSK buffer to a final concentration of 250 mM, then incubated the reaction on ice for 5 min and pelleted the samples at 2500 × g for 3 min at 4 °C. The supernatant was designated as the S2 fraction. We washed the pellet with CSK buffer, then treated the pellet with CSK buffer including 2 M NaCl for 5 min at 4 °C and centrifuged at 2500 × g for 3 min at 4 °C. The supernatant after this step was designated as the S3 fraction. We washed the remaining insoluble pellet with CSK buffer supplemented with 2 M NaCl twice. The insoluble pellets were then treated with 8 M urea buffer and the solubilized sample was considered as the nuclear matrix–containing fraction (S4). Supernatants from each extraction step were quantified and analyzed by SDS-PAGE and immunoblotting.
RNA preparation and RNA sequencing (RNA-seq)
Total RNA was extracted from LHCN-M2 cells using the PureLinkTM RNA Mini kit (Ambion, 12183020), according to the manufacturer’s protocol. Total RNA integrity was verified by Agilent 2100 Bioanalyzer (Agilent Technologies) and quantified with a NanoDrop 8000 instrument (Thermo Fisher). RNA-seq libraries were prepared from total RNA with the Standard Kapa stranded mRNA library prep Kit (KAPA Biosystems, KR0960) according to the manufacturer’s protocols. RNA-seq was performed with three biological replicates (for both WT and SMCHD1 KO). Library size distributions were validated on the Bioanalyzer (Agilent Technologies). Sequencing was performed with an Illumina NextSeq500 system. Library de-multiplexing was performed following Illumina standards.
Whole-genome bisulfite library preparation and sequencing
Total genomic DNA was isolated using the Quick-DNA Miniprep Plus kit (Zymo Research, D4070). WGBS libraries were prepared according to the manufacturer’s instructions using the Swift, Accel-NGS Methyl-Seq DNA Library Kit (Swift Biosciences, 30024) and Zymo’s EZ DNA Methylation-Lightning kit (Zymo Research, D5030). Sequencing was performed with an Illumina Novaseq 6000 instrument with 150-bp paired-end read runs.
ChIP-seq
Chromatin state maps (H3K27ac, H3K4me1, H3K4me3, H3K36me2, H3K36me3, H3K9me3, H3K27me3 and H2AK119ub1) and binding profiles for RNA polymerase II, CTCF insulator protein and the RAD21 cohesin protein subunit were generated by ChIP-seq. Briefly, cells were cross-linked with 1% formaldehyde for 10 min at room temperature and the reaction was quenched with 125 mM glycine. Crosslinked cells were lysed in lysis buffer (50 mM HEPES-KOH, pH 7.9, 140 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% NP40, 0.25% Triton X-100, supplemented with cOmplete protease inhibitors (Roche)) and incubated on ice for 10 min. Following centrifugation at 4500 × g for 5 min at 4 °C in a benchtop centrifuge, the cell pellets were then washed with wash buffer (10 mM Tris-Cl, pH 8.1, 200 mM NaCl, 1 mM EDTA, pH 8.0, 0.5 mM EGTA, pH 8.0, supplemented with cOmplete proteinase inhibitors) and shearing buffer (0.1% SDS, 1 mM EDTA, 10 mM Tris-Cl, pH 8.1, supplemented with cOmplete proteinase inhibitors). Then, the cell pellets were resuspended in shearing buffer and sonicated with a Covaris E220 Evo sonicator to shear the DNA to 300 to 500 bp size fragments. The resulting lysate was supplied with NaCl and Triton X-100 to reach a final concentration of 150 mM NaCl and 1% Triton X-100, then cleared by centrifugation for 10 min at 20,000 × g, and then incubated with washed Dynabeads Protein G (Invitrogen, 10004D) and antibody overnight on a rotator at 4 °C. Antibodies were as follows: CTCF (Active Motif, 61311), H3K27ac (Abcam, ab4729), H3K9me3 (Abcam, ab8898), H3K36me2 (CST, 2901S), H3K36me3 (Abcam, ab9050), H3K4me1 (Abcam, ab8895), H3K4me3 (Millipore, 07-473), H3K27me3 (CST, 9733), H2AK119ub1 (CST, 8240), RNA Pol2 (Millipore, 05-623) and RAD21 (Abcam, ab992).
Beads were then collected and washed with low salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM HEPES-KOH, pH 7.9, 150 mM NaCl supplemented with cOmplete proteinase inhibitors), high salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM HEPES-KOH, pH 7.9, 500 mM NaCl supplemented with cOmplete proteinase inhibitors) and LiCl buffer (100 mM Tris-Cl, pH 7.5, 500 mM LiCl, 1% NP40, 1% sodium deoxycholate) twice and with TE buffer (10 M Tris-Cl, pH 8.1, 1 mM EDTA) once. ChIP DNA samples were eluted with Proteinase K digestion buffer (20 mM HEPES, pH 7.9, 1 mM EDTA, 0.5% SDS) and 1 µl of Proteinase K (20 mg/mL). Purified DNA was quantified for library preparation with Qubit sensitivity dsDNA HS Kit. Libraries were then prepared using the TruSeq ChIP Sample Preparation Kit (Illumina, IP-202–1012, IP-202–1024) according to the manufacturer’s instruction. Briefly, 5 ng of ChIP-DNA was used for input and IP samples. Libraries were amplified using 14 cycles on a thermocycler. Libraries then were quantified and validated using the Agilent High Sensitivity DNA Kit and bioanalyzer. Sequencing was performed with an Illumina HiSeq 2500 instrument with 150-bp paired-end read runs. All ChIP-Seq experiments were processed in parallel with whole cell extract input controls.
DamID-sequencing and library preparation for SMCHD1 and Lamin B1
To map the enrichment patterns of SMCHD1 and Lamin B1, we generated DamID-seq libraries following a protocol described previously25,72, with minor modifications outlined below. Briefly, full length SMCHD1 and Lamin B1 sequences were PCR amplified from oligo-dT-primed cDNA and cloned into pENTR-vector (Thermo Fisher Scientific). The SMCHD1 and Lamin B1 cDNAs were subsequently cloned into pLgw EcoDam-V5-RFC173 (a gift from Bas van Steensel, Addgene plasmid # 59209), to construct pLgw EcoDam-V5-SMCHD1 and EcoDam-V5-Lamin B1 by gateway cloning. The expression of EcoDam-V5-SMCHD1 fusion protein (Dam-SMCHD1) and EcoDam-V5-Lamin B1 fusion protein (Dam-LaminB1) were confirmed by Western blot in LHCN-M2 cells transiently transfected with pLgw-EcoDam-V5-SMCHD1 or pLgw-EcoDam-V5-Lamin B1. To generate stable cell lines with low expression of Dam, Dam-SMCHD1 and Dam-Lamin B1, respectively, the strong CMV promoter was removed by using the Q5 Site-Directed Mutagenesis Kit (NEB, E0554S), to ensure that the fragments harboring the Dam fusion protein were driven by the HSP70 promoter. The puromycin resistance gene was then cloned into the vector for screening single clones. Stably transfected cells were selected in 1 µg/mL puromycin (Thermo Fisher Scientific, A1113803) for 10 days, then single clones of the transfected cells were selected and maintained with 1 µg/mL puromycin. To further validate the Dam-ID sequencing method, we performed Dam-ID mapping with two additional proteins, CBX1 and MBD3 as controls. pLgw EcoDam-V5-CBX1 (a gift from Bas van Steensel, Addgene plasmid # 59211) and pLgw EcoDam-V5-MBD3 (gift from Paul Wade, Addgene plasmid # 55605) were packaged into lentivirus, which was used to infect LHCN-M2 cells for 72 h to obtain low expression of Dam-fusion proteins driven by the HSP70 promoter. Genomic DNA of stable cell lines or lentivirus-infected cells was purified using the Quick-DNA Miniprep Plus kit (Zymo Research, D4070) with the RNase A digestion step included. The RNase-treated genomic DNAs were then digested with DpnI overnight (at least 12 h) at 37 °C. We cleaned up the DpnI-digested DNA with the Qiagen PCR Purification Kit according to the manufacturer’s instructions. We ligated the DamID adaptors to 750 ng (up to 15 µl) DpnI-digested DNA for 2 h at 16 °C (15 μL DpnI-digested DNA, 2 μL 10×T4 DNA ligase buffer, 0.8 μL dsAdR, 1.2 μL H2O), followed by 10 min incubation at 65 °C to inactivate the ligase. We added 19 μL of premade TaDa DpnII digestion buffer (4 μL 10× DpnII buffer, 15 μL H2O) and 1 μL of DpnII enzyme into the mixture and digested the mixture at 37 °C for 3 h. We added 118 μL premade DamID PCR buffer (16 μL 10×cDNA buffer, 2.5 μL DamID_PCR primer (50 μM), 3.2 μL 10 mM dNTPs, 96.3 μL water) and 2 μL of Advantage2 cDNA polymerase enzyme to the DpnII-digested DNA, split the PCR reaction into 4 × 40 μL reactions, then perform PCR with the programs as followed: 68 °C for 10 min, 1 cycle of 94 °C for 30 sec, 65 °C for 5 min, 68 °C for 15 min. Then we added 3 cycles of 94 °C for 30 sec, 65 °C for 1 min, 68 °C for 10 min, and 17 cycles of 94 °C for 30 sec, 65 °C for 1 min, 68 °C for 2 min, followed by a final extension step with 68 °C for 5 mins. We purified PCR products with Qiagen PCR Purification Kit according to the manufacturer’s instructions. We sonicated 2 μL of purified DNA with a Covaris E220 Evo sonicator to shear the DNA to 300 to 500 bp size fragments. We incubated 1 μL of AlwI enzyme (NEB, R0513S) and sheared mixtures at 37 °C for overnight to remove the DamID adaptors. We cleaned up the mixtures with AMPure Beads according to the manufacturer’s instructions. Then, we incubated 500 ng of cleaned DNA in 20 μL with 2.5 μL end-repair enzyme mix (1.14 μL T4 DNA polymerase (3 U/μL), 0.23 μL Klenow fragment (5 U/μl), 1.14 μL T4 polynucleotide kinase) and 7.5 μL of end-repair buffer (3 μL 10× T4 DNA ligase buffer, 1.2 μL 10 mM dNTPs, 3.3 μL H2O) at 30 °C for 30 mins. Adenylation of 3′ ends was then performed by incubating the mixture with 0.75 μl of Klenow 3′–5′ exo- enzyme at 37 °C for 30 min followed by incubating with 2.5 μL of sequencing adaptor and 2.5 μL of NEB quick ligase enzyme at 30 °C for 30 min. Two rounds of AMPure beads cleanup were then performed according to the manufacturer’s instructions. The purified DNA was then amplified by PCR using the following conditions: 98 °C for 30 sec, 6 cycles of 98 °C for 10 sec, 60 °C for 30 sec, 72 °C for 30 sec, then a final extension at 72 °C for 5 mins. Sequencing was performed with an Illumina HiSeq 2500 instrument with 150-bp paired-end read runs.
Dam-V5-tagged subcellular protein localization and Immunofluorescent staining
Constructs were transiently transfected into LHCN-M2 cells growing in 8-well chamber slides (MatTek) for 72 h before imaging. The target proteins were then tracked by immunofluorescent staining following a protocol described previously74, with minor modifications outlined below. Briefly, the cells were fixed with freshly prepared 4% paraformaldehyde for 15 min at room temperature. Cells were washed with PBS three times and were then permeabilized with 0.2% Triton X-100 in PBS for 15 min at room temperature, followed by incubation with blocking buffer containing 1% BSA/0.05% Tween-20 in PBS at room temperature for 1 h. The cells were incubated with primary antibodies in a blocking buffer overnight at 4 °C. The cells were washed with PBS three times and incubated with secondary antibodies in the blocking buffer at room temperature for 1 h. Cells were then covered with antifade mountant (with DAPI) for further imaging. Antibody information: anti-V5 (1:500, Thermo, 46-0705), anti-mouse Alexa Fluor 488 (1:1000, Invitrogen A11001). For detecting H3K9me3 and CBX1 in LHCN-M2 cells, we followed the same IF protocol, with the H3K9me3 antibody (1:500, Abcam, ab8898), CBX1 antibodies (1:500, Abcam, ab10811 and ab10478), anti-Rabbit Alexa Fluor 488 (1:1000, Invitrogen A11034), anti-chicken-Alexa Fluor 488 (1:1000, Invitrogen A11039). The cell images were collected using a Zeiss LSM 880 equipped with an Axio Observer 7 inverted microscope body and acquired with Zen Black (version 2.3) software.
In situ Hi-C library preparation and sequencing
In situ Hi-C was performed as described previously50,75. Briefly, 5 × 106 cells were cross-linked with 1% formaldehyde for exactly 10 min at room temperature. We added 1.25 mL of 2.5 M glycine to quench the cross-linking reaction for 5 min at room temperature, and then placed the cells on ice for at least 15 min. We pelleted the cells by centrifugation at 1000 × g for 10 min. We washed the pellet with DPBS (Thermo Fisher Scientific,14190-144), then cross-linked with disuccinimidyl glutarate (Thermo Fisher Scientific, 20593, final concentration 3 mM) for 40 min at room temperature. Cross-linked cells were lysed in ice-cold lysis buffer (10 mM Tris-Cl, pH 8.0, 10 mM NaCl, 0.2% Igepal CA-630, MP Biomedicals, 198596) containing 10 μL protease inhibitor cocktail. The nuclei were digested with 400 U DdeI and 400 U DpnII (NEB) at 37 °C overnight with interval shaking (900 rpm, 30 sec on, 4 min off). We incubated the Hi-C samples with biotinylation buffer (7 μL 10x NEBuffer 3.1, 1.5 μL 10 mM dCTP, 1.5 μL 10 mM dGTP, 1.5 μL 10 mM dTTP, 37.5 μL 0.4 mM biotin-14-dATP, 11 μL H2O, 10 µL Klenow DNA polymerase) for 4 h at 23 °C in a thermomixer (900 rpm, 30 sec on, 4 min off). We incubated the ligation mix (240 μL 5x ligation buffer, 120 μL 10% Triton X-100, 12 μL 10 mg/ml BSA, 243 μL H2O, 50 μL 1 U/ μL T4 DNA ligase) with the biotinylation mix for 4 h at 16 °C in a thermomixer with interval shaking (900 rpm, 30 sec on, 4 min off). To each Hi-C sample, we added 50 μL of 10 mg/ml proteinase K and incubated for 2 h at 65 °C with interval shaking (900 rpm, 30 sec on, 4 min off), then added another 50 μL of 10 mg/ml proteinase K to each Hi-C sample and continued incubating overnight at 65 °C. We prepared biotin removal reactions in PCR tubes as follows: 15 μg of Hi-C DNA sample, 13 μL 10x NEB buffer 2.1, 3.25 μL 1 mM dATP, 3.25 μL 1 mM dGTP, 13 μL 1 mM 3000 U/ml T4 DNA polymerase and filled up to 130 μL with water. We transferred two 65 μL aliquots from each 130 μl reaction to a thermocycler with the following cycling parameters: 20 °C for 4 h, 75 °C for 20 min, hold at 4 °C. We sonicated the samples on a Covaris E220 Evo sonicator to shear the DNA to a fragment size distribution of 300 to 500 bp. We performed double size selection on the sheared DNA using 0.8X-1.1X Agencourt AMPure XP beads (Beckman Coulter, A63881) according to the manufacturer’s instructions. We incubated end-repair mix (7 μL 10× ligation buffer, 7 μL 2.5 mM dNTP mix, 2.5 μL 3 U/μL T4 DNA polymerase, 2.5 μL 10 U/μl T4 polynucleotide kinase, 0.5 μL 5 U/μl Klenow DNA polymerase, 4.5 μL H2O) and size-selected HiC libraries in a thermocycler with the following cycling parameters: 20 °C for 30 min, 75 °C for 20 min, hold at 4 °C. The products were then purified with MyOne Streptavidin C1 beads (Thermo Fisher Scientific, 65001), dA-tailed, and ligated to 5 μL 15 μM adaptor at 37 °C for 2 h. Hi-C libraries were then amplified by PCR using the following conditions: 98 °C for 30 sec, 4 cycles of 98 °C for 10 sec, 65 °C for 30 sec, 72 °C for 30 sec, then a final extension at 72 °C for 2 mins. In situ Hi-C on WT and SMCHD1 knockout LHCN-M2 cells were performed in two biological replicates, which were sequenced on an Illumina Novaseq 6000 machine with 150 bp paired-end read runs.
Electron microscopy
Our present study employed a comprehensive methodology to prepare and visualize cellular ultrastructure using transmission electron microscopy (TEM). To ensure precise cellular preservation, cells were initially fixed using a solution composed of 2% paraformaldehyde and 2.5% glutaraldehyde in a 0.1 M cacodylate buffer at room temperature for 2 h. Following the fixation step, the cells underwent a series of washing steps with 0.1 M cacodylate buffer (3 washes of 20 minutes each) to remove any residual fixative. Subsequently, post-fixation was carried out by immersing the cells in a solution containing 1% osmium tetroxide and 1.25% potassium ferrocyanide in a 0.1 M cacodylate buffer for 1 h at room temperature. After several buffer washes, the cells were immersed in a mixture of 50% ethanol and 1% uranyl acetate for 30 minutes as part of the en-bloc staining process. Dehydration was then performed by exposing the cells to ascending ethanol concentrations (50%, 70%, 90%, and 95%), ultimately reaching 100% absolute ethanol. The cells were treated with propylene oxide for 20 minutes. Infiltration of the cells was achieved by immersing them in a mixture of LX-112 resin (Ladd Research, USA, 21210) and propylene oxide, with varying ratios (1:3, 1:1, 3:1) for 2 h each and overnight in 100% resin. Samples were placed in a 60 °C oven for polymerization for 3 days. Ultrathin sections ( ~70 nm) were obtained using a Leica Ultracut UCT ultramicrotome and mounted on copper-rhodium grids. Post-staining was carried out with aqueous uranyl acetate and Reynolds’ lead citrate. The high-resolution imaging of the prepared samples was conducted using a Tecnai Spirit G2 BioTWIN transmission electron microscope operating at 120 kV, facilitated by a digital capture system utilizing an Orius 832 CCD Camera and Gatan software.
Quantification and Statistical Analysis
RNA-seq data analysis
For RNA-seq analysis, we followed our standard procedures32,76. Seventy-five base pair reads were trimmed with Trim Galore (v0.4.0), trimmed reads were then aligned to the human genome hg19 with STAR (version 2.5.1), and gene count was performed with STAR. Differential gene expression was called by the Limma (v3.38.2) statistical package19. P values for differential expression were adjusted for multiple testing correction using the Benjamini-Hochberg method in the stats package. Statistical significance for differentially expressed genes was fold change > 2 with q < 0.05. Pheatmap package was used to generate heat maps. The GO function annotation was performed with PANTHER (v18.0). The motif analysis for the promoter of the differentially expressed genes in this study was performed with ‘findMotifsGenome.pl’ function of Homer (v4.11.1).
WGBS data analysis
For WGBS data analysis, following our standard procedures32,76, 150 bp paired-end whole-genome bisulfite reads were trimmed using TrimGalore (v0.5.0) with the following parameters to remove library preparation artifacts and low quality bases: --length 50, --clip_R1 10, --clip_R2 18, --three_prime_clip_R1 10, and --three_prime_clip_R2 10. Trimmed reads were aligned to the reference genome (hg19) using Bismark77 (v0.19.0) and Bowtie278 (v2.3.3.1) with the following parameters: -X 1000, --nucleotide_coverage, and --bowtie2. Duplicates were marked and removed using the deduplicate_bismark script provided with Bismark. CpG methylation values were extracted using the bismark_methylation_extractor script provided with Bismark and the following parameters: --no_overlap, --comprehensive, --merge_non_CpG, and --cytosine_report. We used DMRseq79 version 0.99.0 to identify DMRs. Briefly, CpG loci with fewer than three reads were not considered for DMR calling, and a single CpG coefficient cutoff of 0.05 was used for candidate regions. Significant DMRs were identified using a q value < 0.05. Genomic coordinates of Hypermethylation-DMR clusters (Hyper-DMR clusters) or Hypomethylation-DMR clusters (Hypo-DMR clusters) were then defined based on Hyper-DMRs (Hypo-DMRs) called from the last step. Consecutive Hyper- (or Hypo-) DMRs smaller than 10 kb bins were merged. At least five merged Hyper-DMRs (or Hypo-DMRs) were considered as a Hyper-DMR cluster (or Hypo-DMR cluster) for further analysis. These DMR clusters are listed in Supplementary Data 2.
ChIPseq data analysis
The adapter and low-quality sequences were trimmed from 3′ and 5′ ends by Trimgalore (v0.5.0). Subsequently, the trimmed reads were aligned to hg19 with Bowtie2 (versionv2.5.1). The aligned reads were then deduplicated using PicardTools (http://broadinstitute.github.io/picard). ChIP-peaks were then analyzed with MACS280. All ChIP data was normalized against the corresponding input controls using the ‘-c’ option of MACS2. For CTCF, RAD21, H3K4me3 and H3K27ac, ChIP-Seq peaks were called using the ‘callpeak’ function of MACS2 with --nomodel -f BAMPE -g hs -q 0.05 -B. For H3K9me3, H3K36me2, H3K36me3, H3K4me1, H3K27me3, H2AK119ub1, and Pol II, the ‘–broad’ option of MACS2 was used to identify broad peaks with --nomodel --broad -f BAMPE -g hs --broad-cutoff 0.1 -B. Bigwig tracks of ChIP-seq were generated using bamCoverage of deeptools81 (v3.5.5) to compute coverage across the entire genome. Heatmaps and enrichment profiles of average Counts Per Million mapped reads (CPM) were performed using deeptools. Overlap of genomic regions and peaks contained within them was determined with the bedtools (v2.30.0). Enrichment patterns of different ChIPseq data was generated by genomation82 (v1.34.0) and deeptools.
Hi-C data analysis
The in situ Hi-C data were processed with a standard pipeline of Juicer83, using a pipeline code available at (https://github.com/theaidenlab). Within sample normalization was performed using the Knight-Ruiz (KR) method84. We generated Hi-C data comprised of two sets of biological replicates for each WT and SMCHD1 knockouts. Each map contained an average of 2.4 billion contacts with mapping quality (MAPQ) score > 30. The contacts correlation analysis for biological replicates was performed at 500 kb resolution with R package multiHiCcompare (v 1.20.0)85, then the two biological replicates for each genotype were merged to get higher resolution (average of 4.8 billion contacts) for further analysis. For each chromosome in each sample, compartments were called using the ‘eigenvector’ option of Juicer tools (https://github.com/theaidenlab). The eigenvector was calculated to delineate compartments, which was the first principal component of the Pearson’s correlation matrix of the Observed/Expected, and the sign of the first eigenvector was used to assign compartment labels. We used H3K9me3 and H3K27ac data to flip the sign of the eigenvector such that the value of the first eigenvector was larger than 0 indicating an active A compartment and values smaller than 0 indicating an inactive B compartment. The heatmap and line plot of the AB correlation matrix eigenvector was generated by FAN-C86 (v 0.9.1) based on the PC1 information generated by Juicer.
HiCCUPS (https://github.com/theaidenlab) was used to identify the chromatin loops with default parameters for high resolution maps in HiCCUPS described with all the available flags (-m 512 -c (all chromosomes) -r 5000,10000 -k KR -f .1,.1 -p 4,2 -i 7,5, -t 0.02,1.5,1.75,2 -d 20000,20000,50000), as described previously87. All the code used in the above steps is publicly available at (https://github.com/theaidenlab). Juicebox83 was used for visualization of the Hi-C data.
To show the average contact count distribution of loops and their surroundings between WT and SMCHD1 knockouts, we performed an aggregate peak analysis (APA) of loop calls using the R package GENOVA88 (v1.0.0.9.98) at 10 kb resolution. To show the average contact count distribution of differential TADs and their surroundings in WT and SMCHD1 knockouts, we performed an aggregated TAD analysis (ATA) using the ATA-tool from the R package GENOVA (v1.0.0.9.98), including the up and downstream 500 kb regions, from Hi-C contact maps at 10 kb resolution. R package HicCompare (1.30.0)89 was applied to determine the differential interaction regions between WT and SMCHD1 knockouts in HiC datasets at specific chromosomes.
To show that the general conclusions are not affected by the levels of resolution, five types of differential TAD boundaries were identified by the R package TADcompare (v1.18.0)90 at different resolution. In addition, the insulation score of TADs in WT and SMCHD1 knockout cells at different resolutions was also determined with FAN-C86 (v 0.9.1). The differential loops between WT and SMCHD1 knockouts were confirmed at different resolution with the APA analysis using the R package GENOVA 87 (v1.0.0.9.98).
Analysis of SMCHD1 DamID-seq datasets
DamID-seq 150 bp paired end reads were trimmed with Trim Galore (v0.5.0). The trimmed reads were then aligned to hg19 with Bowtie2 (v2.5.1). To get the normalization of signals generated by Dam-fusion proteins over background, the aligned reads were binned into GATC fragments according to GATC sites, then were normalized to Dam-only control, using damidseq_pipeline91. The log2 (Dam-fusion/Dam alone) values of each GATC fragment was then calculated. To identify all Dam-SMCHD1 and Dam-Lamin B1 binding sites, all Dam-fusion data was normalized against the corresponding Dam-only control using the ‘-c’ option of MACS2 with major parameters –broad, -min-length 50000, --max-gap 50000 and -broad-cutoff 0.0001.
Electron microscopy analysis
Quantifications of TEM images were performed using Fiji version (2.9.0) by thresholding the 32-bit TEM images. The outer border of each nucleus was traced using the freehand tool, here a stylus pen on a touch screen for precise selection. The heterochromatin was then identified by invert and binary functions, and the measurements > threshold limit was set to measure the area of the heterochromatin near the nuclear periphery and the total nuclear threshold. The percentage of lamina-adjacent heterochromatin was calculated by dividing the lamina-adjacent heterochromatin by the total nuclear area. Finally, the relationship between normal and FSHD2 samples, as well as between wildtype and SMCHD1-depleted LHCN-M2 cells was plotted based on the percentage of heterochromatin. Independent data from three samples with N = 30, 20, and 20 nuclei for normal myoblasts and N = 20, 20, and 20 nuclei for FSHD2 myoblasts were obtained, as well as three different clones with N = 19, 20, and 20 nuclei for LHCN-M2 WT cells and N = 19, 19, and 20 nuclei for the SMCHD1 mutant LHCN-M2 cells. Visualization of the data and statistical testing were performed using R (version 4.1.2).
3D genome modeling
3D genome models for WT and SMCHD1-KO LHCN-M2 cells were generated with Chrom3D (v1.0.2)51, following a protocol described previously, with minor modifications52. Briefly, the TADs were generated using the Arrowhead algorithm87, which was used in Chrom3D simulations as chains of contiguous beads for modeling chromosomes. Centromeres and assembly gaps were filtered from the TAD data which were subsequently used for the next step. LADs (LaminB1-DamID data generated in this study) were matched to corresponding TADs and used as peripheral sub-nuclear constraints in the simulations. The significant intra-chromosomal and inter-chromosomal interactions were identified with the non-central hypergeometric distribution algorithm, part of Chrom3D. Gtrack files then were generated from significantly interacting TADs and LADs using the Python scripts makeGtrack.py, which were used as inputs for the next 3D simulation step with Chrom3D. Chrom3D simulations were performed for each condition with a nuclear occupancy of 0.05, with 2 million simulation steps and parameter -- nucleus, which was set to push the beads towards the nuclear interior. The nuclear boundary was modeled as a sphere with a radius of 5 μm. The simulated Chrom3D models then were visualized with ChimeraX92.
IF, 3D-FISH and imaging
Cells were fixed in 2% PFA/1 x PBS (pH 7.4) for 10 min at room temperature. After three rinses in 1 x PBS, cells were permeabilized in iced-cold 0.4% Triton- X-100/1 x PBS for 5 min on ice followed by three rinses in 1 x PBS. Cells are then incubated in the blocking solution (2.5% BSA, 10% normal goat serum, 0.1% Tween 20 in 1 x PBS) for 30 min at room temperature. Cells were incubated with the Lamin B1 antibody (Abcam, ab16048) diluted in blocking solution at 4 °C overnight. Cells were then washed in 0.2% BSA, 0.1% Tween-20, 1 x PBS for three times at room temperature with shaking, followed by incubation with the Alexa Fluor™ 488 secondary antibody (Thermo Fisher, A-11008) diluted in blocking solution, for 1 h at room temperature. After three washes in 0.1% Tween-20, 1 x PBS at room temperature with shaking in the dark, cells were then post-fixed in 2% PFA, 1 x PBS for 10 min at room temperature in the dark. Following three rinses in 1 x PBS, cells were incubated with 0.1 µg/μl RNase A in 1 x PBS for one h at 37 °C. After three rinses in 1 x PBS, cells were permeabilized in ice-cold 0.7% Triton-X-100, 0.1 M HCl for 10 min on ice. After three rinses in 1 x PBS, cells were denatured in 50% formamide, 2 x SSC for 30 min at 80 °C. Cells were hybridized with the probes overnight at 37 °C in a dark and humid chamber. Cells were washed in 2x SSC for 30 min at 37 °C in the dark with shaking, followed by washing in in 2x SSC for 30 min at room temperature, then in 1x SSC for 30 min at room temperature. After dehydration, cells were mounted in mounting medium containing DAPI.
Confocal Z-stacks were collected using a Zeiss LSM 880 equipped with an Axio Observer 7 inverted microscope body and acquired with Zen Black (version 2.3) software using 405 nm diode, 488 nm argon ion, 561 nm DPSS, and 633 nm HeNe laser lines. Emitted light was detected through a Zeiss Plan-apochromat 63x/1.4 NA oil immersion objective, using an Airyscan Fast GaAsP detector. Eight-bit images were collected sequentially at 1024 × 1024 pixel resolution, using 0.3 µm z-steps, a pixel dwell of 0.51 µs and an optical zoom of 1.0.
FISH spatial and quantification analysis
Optical Z-stacks of FISH-stained samples were imported into Imaris v10.1.0 (Oxford Instruments) for analysis. The Cell module’s cell and vesicle option was used to quantify the distances of the FISH probe to the edge of the nucleus. The DAPI signal was used as the “cell” object and detected in the cell portion of the Cell module with the following settings: automatic threshold (no background subtraction), smoothing filter = 0.132 µm, distance from image border in XY < 1 µm (to remove any incomplete cells), and volume of cell < 737 µm3 (to remove possibly dividing cells). The FISH probes were then detected in the vesicle portion of the Cell module with the following settings: automatic threshold with background subtraction filter = 0.527 µm, estimated diameter = 0.7 µm, and quality filter with automatic settings. A final object filter was used to exclude any cells containing more than two detected FISH probes to minimize potentially dividing cells included in the analysis. The volume and diameter of the nuclei, and distance of the FISH probe to the edge of the nucleus were exported for further statistical analysis with R (version 4.1.2).
Quantification of H3K9me3 immunostaining
Multi-scene czi files were first split into individual scenes using Zen’s Split Scenes (Write Files) module before importing into CellProfiler (v4.2.6)93 for generating masks of nuclei. The DAPI signal intensity was rescaled to full range of the image before applying a gaussian filter sigma=1. The IdentifyPrimaryObjects module was used on the gaussian blurred image to segment nuclei (diameter 90–200 pixels, Ostu three class—foreground, and threshold correction 0.8). The resulting objects were exported as a binary 8-bit tiff for further processing in ImageJ/Fiji (v1.54p)94. A custom ImageJ macro script was written to run particle analyzer on the mask images to generate an ROI for each nucleus, apply a gaussian filter sigma=1 on the GFP channel, and then draw a straight line across the maximum diameter of each nucleus and measure the pixel intensity values along the line on the gaussian smoothed image (for easier interpretation when plotting). Both the CellProfiler pipeline and custom ImageJ macro script, are available here: (https://github.com/vaioic/Published_Scripts/tree/main/Pfeifer_Lab/Plot_Profile). The profile of H3k9me3 IF density across the cell was then plotted with R package ggplot2 (v3.5.1) and statistical testing (t-test) was performed using R (v4.1.2).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We thank the members of the Van Andel Institute Core Facilities (Genomics, Bioinformatics and Biostatistics, Optical Imaging, Cryo-electron Microscopy) for their support and technical assistance. We are grateful to Yinghui Song and Jidong Shan from the Molecular Cytogenetics Core at Albert Einstein College of Medicine for fluorescence in situ hybridization. This work was supported by NIH grant R01AR079174 to GPP.
Author contributions
Conceptualization, G.P.P., Z.H.; experimental design, Z.H., W.C., and G.P.P.; investigation, Z.H., W.C., I.R., L.C., R.T.; data analysis, Z.H., W.C., K.G., and G.P.P.; writing, Z.H. and G.P.P.; visualization, Z.H., I.R., L.C.; supervision, G.P.P.; funding acquisition, G.P.P.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data are available in the main text or the Supplementary Material. Source Data are provided with this paper. Raw sequencing files and processed data files generated in this study are deposited and freely available from Gene Expression Omnibus (GEO: GSE251747- GSE251751). All data is provided at GEO GSE251747 (ChIP-seq), GSE251748 (Dam-ID-seq), GSE251749 (HiC), GSE251750 (RNA-seq), and GSE251751 (WGBS). Source data are provided with this paper.
Code availability
Both the CellProfiler pipeline and custom ImageJ macro script, are available here: (https://github.com/vaioic/Published_Scripts/tree/main/Pfeifer_Lab/Plot_Profile).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-62211-0.
References
- 1.Allshire, R. C. & Madhani, H. D. Ten principles of heterochromatin formation and function. Nat. Rev. Mol. Cell Biol.19, 229–244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen, A., Colmenares, S. U. & Karpen, G. H. Heterochromatin: guardian of the genome. Annu Rev. Cell Dev. Biol.34, 265–288 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Montavon, T. et al. Complete loss of H3K9 methylation dissolves mouse heterochromatin organization. Nat. Commun.12, 4359 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padeken, J., Methot, S. P. & Gasser, S. M. Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance. Nat. Rev. Mol. Cell Biol.23, 623–640 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, O., Burton, A., Dean, C., Gasser, S. M. & Torres-Padilla, M. E. Heterochromatin definition and function. Nat. Rev. Mol. Cell Biol.24, 691–694 (2023). [DOI] [PubMed] [Google Scholar]
- 6.McCarthy, R. L., Zhang, J. & Zaret, K. S. Diverse heterochromatin states restricting cell identity and reprogramming. Trends Biochem Sci.48, 513–526 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haws, S. A., Simandi, Z., Barnett, R. J. & Phillips-Cremins, J. E. 3D genome, on repeat: Higher-order folding principles of the heterochromatinized repetitive genome. Cell185, 2690–2707 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grewal, S. I. S. The molecular basis of heterochromatin assembly and epigenetic inheritance. Mol. Cell83, 1767–1785 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Steensel, B. & Belmont, A. S. Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell169, 780–791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn, E. H. & Misteli, T. Molecular basis and biological function of variability in spatial genome organization. Science365 (2019). [DOI] [PMC free article] [PubMed]
- 11.Gibcus, J. H. & Dekker, J. The hierarchy of the 3D genome. Mol. Cell49, 773–782 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowley, M. J. & Corces, V. G. Organizational principles of 3D genome architecture. Nat. Rev. Genet.19, 789–800 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belmont, A. S. Nuclear compartments: an incomplete primer to nuclear compartments, bodies, and genome organization relative to nuclear architecture. Cold Spring Harb. Perspect. Biol.14, a041268 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blewitt, M. E. et al. SmcHD1, containing a structural-maintenance-of-chromosomes hinge domain, has a critical role in X inactivation. Nat. Genet.40, 663–669 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Brideau, N. J. et al. Independent mechanisms target SMCHD1 to trimethylated histone H3 lysine 9-modified chromatin and the inactive X chromosome. Mol. Cell Biol.35, 4053–4068 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moissiard, G. et al. MORC family ATPases required for heterochromatin condensation and gene silencing. Science336, 1448–1451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gendrel, A. V. et al. Epigenetic functions of SMCHD1 repress gene clusters on the inactive X chromosome and on autosomes. Mol. Cell Biol.33, 3150–3165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurzau, A. D., Blewitt, M. E., Czabotar, P. E., Murphy, J. M. & Birkinshaw, R. W. Relating SMCHD1 structure to its function in epigenetic silencing. Biochem Soc. Trans.48, 1751–1763 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daxinger, L., Tapscott, S. J. & van der Maarel, S. M. Genetic and epigenetic contributors to FSHD. Curr. Opin. Genet Dev.33, 56–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemmers, R. J. et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat. Genet.44, 1370–1374 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon, C. T. et al. De novo mutations in SMCHD1 cause Bosma arhinia microphthalmia syndrome and abrogate nasal development. Nat. Genet.49, 249–255 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Jansz, N., Chen, K., Murphy, J. M. & Blewitt, M. E. The epigenetic regulator SMCHD1 in development and disease. Trends Genet.33, 233–243 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Gdula, M. R. et al. The non-canonical SMC protein SmcHD1 antagonises TAD formation and compartmentalisation on the inactive X chromosome. Nat. Commun.10, 30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansz, N. et al. Smchd1 regulates long-range chromatin interactions on the inactive X chromosome and at Hox clusters. Nat. Struct. Mol. Biol.25, 766–777 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Wang, C. Y., Jegu, T., Chu, H. P., Oh, H. J. & Lee, J. T. SMCHD1 Merges Chromosome Compartments and Assists Formation of Super-Structures on the Inactive X. Cell174, 406–421.e25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, C. Y., Colognori, D., Sunwoo, H., Wang, D. & Lee, J. T. PRC1 collaborates with SMCHD1 to fold the X-chromosome and spread Xist RNA between chromosome compartments. Nat. Commun.10, 2950 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansz, N. et al. Smchd1 targeting to the inactive X Is dependent on the Xist-HnrnpK-PRC1 pathway. Cell Rep.25, 1912–1923.e9 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Ichihara, S., Nagao, K., Sakaguchi, T., Obuse, C. & Sado, T. SmcHD1 underlies the formation of H3K9me3 blocks on the inactive X chromosome in mice. Development149 (2022). [DOI] [PubMed]
- 29.Sakakibara, Y. et al. Role of SmcHD1 in establishment of epigenetic states required for the maintenance of the X-inactivated state in mice. Development145 (2018). [DOI] [PubMed]
- 30.Gendrel, A. V. et al. Smchd1-dependent and -independent pathways determine developmental dynamics of CpG island methylation on the inactive X chromosome. Dev. Cell23, 265–279 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, K. et al. Genome-wide binding and mechanistic analyses of Smchd1-mediated epigenetic regulation. Proc. Natl. Acad. Sci. USA112, E3535–E3544 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang, Z. et al. The chromosomal protein SMCHD1 regulates DNA methylation and the 2c-like state of embryonic stem cells by antagonizing TET proteins. Sci Adv7 (2021). [DOI] [PMC free article] [PubMed]
- 33.Tapia Del Fierro, A. et al. SMCHD1 has separable roles in chromatin architecture and gene silencing that could be targeted in disease. Nat. Commun.14, 5466 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benetti, N. et al. Maternal SMCHD1 regulates Hox gene expression and patterning in the mouse embryo. Nat. Commun.13, 4295 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towbin, B. D. et al. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell150, 934–947 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Leemans, C. et al. Promoter-intrinsic and local chromatin features determine gene repression in LADs. Cell177, 852–864.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Sandoval, A. & Gasser, S. M. On TADs and LADs: spatial control over gene expression. Trends Genet.32, 485–495 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Bowness, J. S. et al. Xist-mediated silencing requires additive functions of SPEN and Polycomb together with differentiation-dependent recruitment of SmcHD1. Cell Rep.39, 110830 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goossens, R. et al. A proteomics study identifying interactors of the FSHD2 gene product SMCHD1 reveals RUVBL1-dependent DUX4 repression. Sci. Rep.11, 23642 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanigasuriya, I. et al. Smchd1 is a maternal effect gene required for genomic imprinting. Elife9 (2020). [DOI] [PMC free article] [PubMed]
- 41.Shimbo, T. et al. MBD3 localizes at promoters, gene bodies and enhancers of active genes. PLoS Genet.9, e1004028 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong, X. et al. Lamin C is required to establish genome organization after mitosis. Genome Biol.22, 305 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nozawa, R. S. et al. Human inactive X chromosome is compacted through a PRC2-independent SMCHD1-HBiX1 pathway. Nat. Struct. Mol. Biol.20, 566–573 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Dion, C. et al. SMCHD1 is involved in de novo methylation of the DUX4-encoding D4Z4 macrosatellite. Nucleic Acids Res.47, 2822–2839 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du, J., Johnson, L. M., Jacobsen, S. E. & Patel, D. J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol.16, 519–532 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neri, F. et al. Intragenic DNA methylation prevents spurious transcription initiation. Nature543, 72–77 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Rauch, T. A., Wu, X., Zhong, X., Riggs, A. D. & Pfeifer, G. P. A human B cell methylome at 100-base pair resolution. Proc. Natl. Acad. Sci. USA106, 671–678 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lister, R. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature462, 315–322 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science326, 289–293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lafontaine, D. L., Yang, L., Dekker, J. & Gibcus, J. H. Hi-C 3.0: Improved Protocol for Genome-Wide Chromosome Conformation Capture. Curr. Protoc.1, e198 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulsen, J. et al. Chrom3D: three-dimensional genome modeling from Hi-C and nuclear lamin-genome contacts. Genome Biol.18, 21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paulsen, J., Liyakat Ali, T. M. & Collas, P. Computational 3D genome modeling using Chrom3D. Nat. Protoc.13, 1137–1152 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Hendrickson, P. G. et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat. Genet.49, 925–934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whiddon, J. L., Langford, A. T., Wong, C. J., Zhong, J. W. & Tapscott, S. J. Conservation and innovation in the DUX4-family gene network. Nat. Genet.49, 935–940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Iaco, A. et al. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet.49, 941–945 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang, Y. & Chen, L. Structure and mechanism of NALCN-FAM155A-UNC79-UNC80 channel complex. Nat. Commun.13, 2639 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou, L., Liu, H., Zhao, Q., Wu, J. & Yan, Z. Architecture of the human NALCN channelosome. Cell Discov.8, 33 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kschonsak, M. et al. Structural architecture of the human NALCN channelosome. Nature603, 180–186 (2022). [DOI] [PubMed] [Google Scholar]
- 59.Wong, X. et al. Mapping the micro-proteome of the nuclear lamina and lamina-associated domains. Life Sci Alliance4 (2021). [DOI] [PMC free article] [PubMed]
- 60.Al-Sayed, M. D. et al. Mutations in NALCN cause an autosomal-recessive syndrome with severe hypotonia, speech impairment, and cognitive delay. Am. J. Hum. Genet.93, 721–726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, B., Powers, J. D., McCulloch, A. D. & Chi, N. C. Nuclear mechanosignaling in striated muscle diseases. Front Physiol.14, 1126111 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Battey, E., Stroud, M. J. & Ochala, J. Using nuclear envelope mutations to explore age-related skeletal muscle weakness. Clin. Sci. (Lond.)134, 2177–2187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zylicz, J. J. & Heard, E. Molecular mechanisms of facultative heterochromatin formation: an X-chromosome perspective. Annu Rev. Biochem89, 255–282 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Brockdorff, N. Polycomb complexes in X chromosome inactivation. Philos Trans R Soc Lond B Biol Sci.372 (2017). [DOI] [PMC free article] [PubMed]
- 65.Weinberg, D. N. et al. The histone mark H3K36me2 recruits DNMT3A and shapes the intergenic DNA methylation landscape. Nature573, 281–286 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dhayalan, A. et al. The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. J. Biol. Chem.285, 26114–26120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berman, B. P. et al. Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nat. Genet.44, 40–46 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou, W. et al. DNA methylation loss in late-replicating domains is linked to mitotic cell division. Nat. Genet.50, 591–602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nora, E. P. et al. Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell169, 930–944.e22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim, H. et al. The gene-silencing protein MORC-1 iopologically entraps DNA and forms multimeric assemblies to cause DNA compaction. Mol. Cell75, 700–710.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biferali, B. et al. Prdm16-mediated H3K9 methylation controls fibro-adipogenic progenitors identity during skeletal muscle repair. Sci Adv7 (2021). [DOI] [PMC free article] [PubMed]
- 72.Marshall, O. J., Southall, T. D., Cheetham, S. W. & Brand, A. H. Cell-type-specific profiling of protein-DNA interactions without cell isolation using targeted DamID with next-generation sequencing. Nat. Protoc.11, 1586–1598 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogel, M. J. et al. Human heterochromatin proteins form large domains containing KRAB-ZNF genes. Genome Res.16, 1493–1504 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong, T. et al. TET2 modulates spatial relocalization of heterochromatin in aged hematopoietic stem and progenitor cells. Nat. Aging3, 1387–1400 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akgol Oksuz, B. et al. Systematic evaluation of chromosome conformation capture assays. Nat. Methods18, 1046–1055 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui, W. et al. Deficiency of the Polycomb protein RYBP and TET methylcytosine oxidases promotes extensive CpG island hypermethylation and malignant transformation. Cancer Res.83, 2480–2495 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krueger, F. & Andrews, S. R. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics27, 1571–1572 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korthauer, K., Chakraborty, S., Benjamini, Y. & Irizarry, R. A. Detection and accurate false discovery rate control of differentially methylated regions from whole genome bisulfite sequencing. Biostatistics20, 367–383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu, T. Use model-based Analysis of ChIP-Seq (MACS) to analyze short reads generated by sequencing protein-DNA interactions in embryonic stem cells. Methods Mol. Biol.1150, 81–95 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res.44, W160–W165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akalin, A., Franke, V., Vlahovicek, K., Mason, C. E. & Schubeler, D. Genomation: a toolkit to summarize, annotate and visualize genomic intervals. Bioinformatics31, 1127–1129 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Durand, N. C. et al. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst.3, 95–98 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knight, P. A. & Ruiz, D. A fast algorithm for matrix balancing. IMA J. Numer. Anal.33, 1029–1047 (2012). [Google Scholar]
- 85.Stansfield, J. C., Cresswell, K. G. & Dozmorov, M. G. multiHiCcompare: joint normalization and comparative analysis of complex Hi-C experiments. Bioinformatics35, 2916–2923 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kruse, K., Hug, C. B. & Vaquerizas, J. M. FAN-C: a feature-rich framework for the analysis and visualisation of chromosome conformation capture data. Genome Biol.21, 303 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao, S. S. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell159, 1665–1680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Weide, R. H. et al. Hi-C analyses with GENOVA: a case study with cohesin variants. NAR Genom. Bioinform3, lqab040 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stansfield, J. C., Cresswell, K. G., Vladimirov, V. I. & Dozmorov, M. G. HiCcompare: an R-package for joint normalization and comparison of HI-C datasets. BMC Bioinforma.19, 279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cresswell, K. G. & Dozmorov, M. G. TADCompare: An R Package for Differential and Temporal Analysis of Topologically Associated Domains. Front Genet.11, 158 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marshall, O. J. & Brand, A. H. damidseq_pipeline: an automated pipeline for processing DamID sequencing datasets. Bioinformatics31, 3371–3373 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci.30, 70–82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stirling, D. R. et al. CellProfiler 4: improvements in speed, utility and usability. BMC Bioinforma.22, 433 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data are available in the main text or the Supplementary Material. Source Data are provided with this paper. Raw sequencing files and processed data files generated in this study are deposited and freely available from Gene Expression Omnibus (GEO: GSE251747- GSE251751). All data is provided at GEO GSE251747 (ChIP-seq), GSE251748 (Dam-ID-seq), GSE251749 (HiC), GSE251750 (RNA-seq), and GSE251751 (WGBS). Source data are provided with this paper.
Both the CellProfiler pipeline and custom ImageJ macro script, are available here: (https://github.com/vaioic/Published_Scripts/tree/main/Pfeifer_Lab/Plot_Profile).