Highlights
The combination of A. catechu and P. betle methanolic extracts demonstrated significant anthelminthic properties against Haemonchus spp.
The scanning electron microscopy images originally revealed distinct cuticular damage resulting from the exposure to the plant combined extract that likely contributed to worm mortality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-025-04951-1.
Keywords: Haemonchus spp., Areca catechu, Piper betle, Methanolic extract, Anthelmintic effect, Scanning electron microscopy, Ultrastructural alteration
Abstract
Background
This study aimed to evaluate the in vitro anthelmintic effects of methanolic extracts from Areca catechu seeds and Piper betle leaves and their combined mixture against adult Haemonchus spp. Phytochemical analysis of the extracts was performed to identify the constituent compounds. Adult worm motility was evaluated along with inhibition percentages at varying concentrations and time points and subsequently compared to controls. The ultrastructural changes were examined via scanning electron microscopy (SEM). Statistical significance was determined at p < 0.05, and the median effective concentration (EC50) values were determined.
Results
The combined methanolic extracts of A. catechu and P. betle at 5% concentration significantly inhibited the motility of adult female and male Haemonchus within 15 min in vitro. The analysis revealed 58 phytochemicals, including hydroxychavicol (45.66%), eugenol (13.66%), allylpyrocatechol diacetate (8.13%), acetyleugenol (6.16%), isoeugenyl acetate (5.45%), γ-muurolene (3.03%), and arecoline (2.73%) as the major components in combined extract. These herbal extracts affect the anthelmintic vitality, evidently through inducing the cuticular damage and leakage of biological substances through cuticular fissures by SEM.
Conclusions
The study results indicate that the methanolic extracts possess significant ability to inhibit the nematode motility and the morphological destruction caused the leakage of internal substances from circular furrows between the cuticular annuli. The cuticular damage along the entire body after the plant exposure, likely contributed to worm mortality. Taken together, these herbal effects warrant further investigation for controlling gastrointestinal nematode infections in livestock.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-025-04951-1.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-025-04951-1.
Background
Goat farming is a significant agricultural practice worldwide, contribution to milk, meat, and fiber industries. However, it is often challenged by parasitic infections, particularly those caused by helminths, such as Haemonchus spp. Haemonchus spp. are major gastrointestinal nematodes causing weight loss, edema, anemia, and death in small ruminants worldwide [1, 2]. These parasites can significantly affect goat health, productivity, and economic viability, leading to reduced growth rates, decreased milk production, increased mortality, and impaired immune function [3–7]. Moreover, goat farmers, especially smallholder farmers transitioning to commercial production systems in developing regions, face significant economic losses because of these infections [8]. Furthermore, several studies have documented the development of drug resistance in various anthelmintic classes, including benzimidazoles, macrocyclic lactones, and imidazothiazoles [9–11]. Effective control strategies and farm management practices are crucial to mitigate these impacts and sustain goat farming productivity [12].
Anthelmintic resistance poses a significant challenge for controlling gastrointestinal nematodes, including Haemonchus spp., in small ruminants. To address this issue, the use of phytochemical compounds has been explored as an alternative or complementary approach to combat these parasites [13]. Medicinal plants, such as Terminalia glaucescens and Combretum molle, show significant anthelmintic potential. Methanolic extracts of these plants are effective in inhibiting Haemonchus egg hatchability and larval motility [14, 15]. Methanol extraction is a widely recognized method for maximizing the yield of bioactive phytochemicals [16]. These methanol-extracted phytochemicals, particularly tannins, disrupt the Haemonchus cuticle and interfere with its metabolic processes, ultimately leading to larval death [17]. Moreover, crude methanolic extracts from plants, such as Croton macrostachyus, Nicotiana tabacum, and Zingiber officinale, that are rich in tannins, flavonoids, steroids, and terpenoids have been found to impair Haemonchus survival [18]. Areca catechu L. and Piper betle L. are indigenous plants from tropical regions that contain tannins and flavonoids [19, 20].
A. catechu L., the slim monoecious palm is belonging to the Arecaceae family. A. catechu L. seeds, commonly known as betel nuts, are a rich source of bioactive compounds including alkaloids tannins, flavonoids, triterpenes, steroids, and fatty acids. These compounds exhibit a wide range of pharmacological properties, including anthelmintic, anti-inflammatory, anti-tumor, antioxidant, antibacterial, and antiviral activities [21, 22]. To assess the anthelmintic activities of A. catechu seed extracts, Badar et al. [23] conducted a series of in vitro and in vivo studies against Haemonchus contortus, including adult motility assays, egg hatch assays, and fecal egg count reduction tests using crude aqueous-methanol seed extracts. Several studies have explored the anthelmintic potential of areca nut and pumpkin seeds. Combinations of these two agents have been investigated in vivo against Taenia spp [24]. and Heterophyes heterophyes [25]. Moreover, the anthelmintic effect of areca nuts on Fasciola spp. has been studied in vitro [26].
Piper betle L., commonly known as betel leaf, is a perennial climbing vine belonging to the Piperaceae family. This plant has a long history of use in traditional medicine systems across South and Southeast Asia. Recent scientific studies have highlighted the potential anthelmintic properties of P. betle, making it a promising natural alternative to synthetic anthelmintics [27]. Rich in phenolic compounds, P. betle leaves exhibit notable anthelmintic activity [28]. Moreover, ethanol extracts from Thai P. betle leaves have demonstrated antiparasitic effects against Toxoplasma gondii and Neospora caninum both in vitro and in vivo [29, 30]. Furthermore, the essential oil of P. betle exhibits cysticidal activity and inhibits sporulation in Eimeria tenella oocysts [31].
This study aimed to investigate the in vitro effects of methanolic extracts of A. catechu (AC) seeds and P. betle (PB) leaves, as well as their combination (ACPB), on the motility and cuticular surface of adult male and female Haemonchus spp. These indigenous plants may offer promising natural alternatives for mitigating gastrointestinal nematodes in small ruminants.
Methods
Ethics approval and consent to participate
The animal protocol for the use of goat abomasal contents in this study was approved by the Ethical Committee (Approval No. MUVS-2019-12-54) and the Biosafety Committee (Approval No. IBC/MUVS-B-001/2563) of the Faculty of Veterinary Science, Mahidol University, Thailand. Goats were not sacrificed specifically for the study; the parasites were collected post-mortem from animals at a private slaughterhouse, located in Nakhon Pathom province, licensed by the Department of Livestock Development.
Plant extract Preparation
The Areca catechu L. seeds (Fig. 1a) and Piper betle L. leaves (Fig. 1b) were collected from the Rhi Khing subdistrict, Sam Pharn district, Nakhon Pathom province, Thailand. Plant identification was confirmed, and voucher specimens were deposited at the Herbarium of Pharmaceutical Botany, Mahidol University (PBM) under accession numbers 005546–005547 (AC) and 005510–005511 (PB). Collection complied with all relevant institutional and national regulations.
Fig. 1.
The fresh fruit of Areca catechu L. seeds (a) composed of Areca seeds and Piper betle L. leaves (b)
Fresh herbal samples, weighing 1,000 g each, were cut into small pieces measuring approximately 2–3 cm. These pieces were then dried in a hot air oven at 60 °C for 48–72 h. The dried plant materials, 100 g each, were then ground into a fine powder using an herbal grinding machine and stored in a desiccator at room temperature (25 °C ± 2 °C) until further extraction.
The herbal samples were incubated in 95% methanol at a ratio of 100 g of plant material per 500 mL of methanol. This extraction process was conducted in a dark cabinet at room temperature for a period of 72 h. The extracts were filtered through Whatman® filter paper No. 4, evaporated using a rotary evaporator (Rotavapor R-200/205, BÜCHI, Flawil, Switzerland), and then lyophilized in a freeze-dry vacuum chamber (Labconco, Kansas City, MO, USA). The methanolic extracts were stored at − 20 °C until further use, and the yield was calculated based on the initial weight of the dried plant material.
Quantification of the herbal extracts
Gas chromatography–mass spectrometry analysis
The chemical profile of ACPB was determined using gas chromatography-mass spectrometry (GC-MS). Specifically, an Agilent 7890 A gas chromatograph coupled with a 5975 C mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) was employed. Separation of the components was achieved using a DB-5HT capillary column (30 m × 250 μm × 0.1 μm; Agilent Technologies, USA). The sample was introduced via a split injector (1:10 split ratio), with helium serving as the carrier gas at a consistent flow rate of 1 mL/min. The injector temperature was maintained at 250 °C. The oven temperature program commenced with an isothermal hold at 40 °C for 5 min, followed by a linear ramp to 250 °C at a rate of 10 °C/min, and concluded with a final isothermal hold at 250 °C for 5 min.
The mass spectrometer was operated in the electron ionization mode, with an ion source temperature of 250 °C and an ionization energy of 70 eV. Mass spectra were acquired across a mass-to-charge ratio (m/z) of 30–550. Compound identification was accomplished by comparing the acquired mass spectra with those contained in the Wiley 10th edition and NIST 2014 mass spectral libraries (W10N14.L).
Determination of total phenolic content
The total phenolic content of the plant extract was quantified using a modified Folin-Ciocalteu assay, adapting established protocols [32, 33]. Briefly, 20 µL of the stock plant extract solution (1,000 µg/mL) was combined with 100 µL of Folin-Ciocalteu reagent and vortexed for homogeneity. After a 1-min incubation period, 80 µL of a 7.5% (w/v) sodium carbonate (Na2CO3) solution was added, and the mixture was thoroughly vortexed again. The reaction was allowed to proceed for 30 min at ambient temperature. Absorbance was measured at 760 nm using a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). Gallic acid was utilized as the standard, and the total phenolic content was expressed as milligrams of gallic acid equivalents per gram of crude extract (mg GAE/g).
Determination of flavonoid content
Based on the protocol described by Chang et al. [34], the total flavonoid content of the plant extract was quantified using a modified aluminum chloride (AlCl3) colorimetric assay. Briefly, 25 µL of the stock plant extract solution (1,000 µg/mL) was combined with 75 µL of 95% ethanol. Subsequently, 5 µL of 10% AlCl3, 5 µL of 1 M potassium acetate, and 140 µL of ultrapure water were sequentially added to the mixture, with thorough vortexing after each addition. The reaction was allowed to proceed for 30 min at room temperature. Absorbance was immediately measured at 415 nm using a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). Quercetin was utilized as the standard, and the total flavonoid content was expressed as milligrams of quercetin equivalents per gram of sample (mg QE/g).
Determination of total hydrolyzed tannin content
The total hydrolysable tannin content of the plant extract was quantified using a modified Folin-Ciocalteu colorimetric assay, adapting the Singleton and Rossi method [33]. Briefly, 20 µL of the stock plant extract solution (1,000 µg/mL) was combined with 100 µL of Folin-Ciocalteu reagent. After a 1-min incubation period, 80 µL of a 7.5% (w/v) Na2CO3 solution was added, and the mixture was thoroughly vortexed again. The reaction was allowed to proceed for 30 min at room temperature. Absorbance was measured at 760 nm using a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). Gallotannin was utilized as the standard, and the total hydrolysable tannin content was expressed as milligrams of gallotannin equivalents per gram of sample (mg GE/g).
Determination of total condensed tannin content
The total condensed tannin content of the plant extract was quantified using the vanillin-hydrochloric acid assay, modified from the Singleton and Rossi method [30]. Briefly, 20 µL of the stock plant extract solution (1,000 µg/mL) was combined with 50 µL of 1% (w/v) vanillin solution and 50 µL of 25% (v/v) sulfuric acid. The mixture was incubated in a dark cabinet at room temperature for 15 min. Absorbance was measured at 500 nm using a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). Catechin was utilized as the standard, and the total condensed tannin content was expressed as milligrams of catechin equivalents per gram of sample (mg Catechin equivalent (CE)/g).
Parasite collection
Natural Haemonchus-infected goat abomasa were sourced from a standard Nakhon Pathom slaughterhouse, which supplies animals from the province and neighboring regions of Ratchaburi and Kanchanaburi. These abomasa were transported to the Faculty of Veterinary Science, Mahidol University within 40 min. Upon arrival, the abomasa were surgically opened and washed with 0.9% sodium chloride (NaCl) solution. Subsequently, Haemonchus parasites were isolated from the abomasal mucosa and contents. The parasites initially collected from the abomasum by naked eye were subsequently identified at the genus level using a stereomicroscope (Nikon SMZ745; Nikon Corp., Tokyo, Japan). The isolated parasites were then placed in a Petri dish containing 0.9% NaCl solution. The collected worms were then washed three times with RPMI-1640 medium (Thermo Fisher Scientific Inc., Waltham, MA, USA), separated by sex, and preserved in RPMI-1640 containing 100 U/mL penicillin, 100 µg/mL streptomycin (Gibco, NY, USA), 2.5 µg/mL amphotericin B (Gibco, PA4 SRF, UK), and 10 mM N-[2-hydroxyethylpiperazine-N-[4-butanesulfonic acid] (HEPES) buffer (Gibco, NY, USA) [35].
Adult worm motility assay
In total, 252 female and 168 male adults were divided into each triplicated treatment. The study was performed in a sterile 24-well plate, and each well contained no more than 3 worms. All treatments and control groups were maintained in an incubated medium consisting of RPMI-1640 supplemented with 10 U/mL penicillin, 10 µg/mL streptomycin, 0.25 µg/mL amphotericin B, and 10 mmol HEPES. Subsequently, 1 mL of AC, PB, or an ACPB mixture (0.05, 0.1, 0.5, 1.0, 2.5, and 5.0 mg/mL) was added and incubated at various times in a CO2 incubator at 37 °C with 5% CO2. Positive controls consisted of 32 mg/mL (3.2% w/v) albendazole and 1 mg/mL (0.1% w/v) ivermectin (Sigma-Aldrich, Inc., Natick, MA, USA). Worm motility was assessed at 5 min, 15 min, 30 min, 3 h, 12 h, and 24 h by observing the worms for 5 s under a stereomicroscope. Worms were classified as either motile or nonmotile [36, 37]. The percentage of motility inhibition was calculated for each treatment. The death of parasites was confirmed using a blunt sterile needle 26 gauge after 24 h of treatment. Each treatment and control were replicated three times [38, 39].
Scanning electron microscopy
Adult male and female Haemonchus worms, selected from the group treated with 5% ACPB, were used for scanning electron microscopy (SEM). The worms were fixed in 2.5% glutaraldehyde for 24 h, washed with phosphate-buffered saline, and then coated with copper using an auto fine coater for 15 min (JEOL model JEC-3000FC, Tokyo, Japan). The specimens were subsequently examined using a scanning electron microscope (JEOL JSM-IT500, Tokyo, Japan) in SED mode at 10 kV.
Nested and allele-specific PCR
To investigate the association between the F200Y polymorphism in the isotype 1 β-tubulin gene and benzimidazole resistance, albendazole-treated samples of adult Haemonchus spp. were analyzed. These included triplicate male samples (representing 33.33% of the male population at each location) and female samples (representing 16.67% of the female population at each location). Genomic DNA was extracted from these worms using the PureLink™ Genomic DNA Mini Kit (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s protocol. The extracted DNA was subsequently stored at − 20 °C for future analysis.
Nested PCR was performed in a total volume of 20 µL using the AllTaq Master Mix (Qiagen, Hilden, Germany), 10 pmol of each primer, and 2 µL of DNA template (50–100 ng/µL). The primers Pn1 (5′-GGCAAATATGTCCCACGTGC-3′) and Pn2 (5′-GATCAGCATTCAGCTGTCCA-3′), were used in the first-round of nested PCR, while Pn3 (5′-GGAACAATGGACTCTGTTCG-3′) and Pn4 (5′-GGGAATCGAAGGCAGGTCGT-3′) were used in the second round [40] with minor modifications. The first-round PCR conditions were as follows: initial denaturation at 94 °C for 5 min; 1 cycle of denaturation at 94 °C for 2 min, annealing at 52 °C for 55 s, and extension at 72 °C for 55 s; 20 cycles of denaturation at 94 °C for 2 min, annealing at 52 °C for 55 s, and extension at 72 °C for 55 s and a final extension at 72 °C for 10 min. The second-round PCR used 2 µL of the first-round PCR product as the template and followed the same cycling profile for 33 cycles.
Allele-specific PCR (AS-PCR) for Haemonchus spp. was performed in two separate reactions, following the protocol described by Humbert and Elard (1997) with minor modification. Each 20 µL reaction contained AllTaq Master Mix (Qiagen, Hilden, Germany), 10 pmol of the non-specific forward primer Ph1 (5′-GGAACGATGGACTCCTTTCG-3′) and the non-specific reverse primer Ph2 (5′-GATCAGCATTCAGCTGTCCA-3′), 25 pmol of either the resistant allele-specific primer Ph3 (5′-CTGGTAGAGAACACCGATGAAACATA-3′) or the susceptible allele-specific primer Ph4 (5′-ATACAGAGCTTCGTTGTCAATACAGA-3′), and 2.0 µL of DNA template from the second round of nested PCR. The cycling conditions were as follows: initial denaturation at 94 °C for 5 min; 40 cycles of 94 °C for 55 s (denaturation), 53 °C for 55 s (annealing), and 72 °C for 55 s (extension); and a final extension at 72 °C for 10 min [41].
Following AS-PCR amplification, allele-specific products were separated by electrophoresis on a 2.0% agarose gel stained with GelRed™ (Biotium, Hayward, CA, USA) and visualized using a c300 UV transilluminator (Azure Biosystems, Dublin, CA, USA). Bands corresponding to the resistant allele, susceptible allele, and internal control were observed at approximately 250, 550, and 750 bp, respectively. The adult Haemonchus spp. genotypes were classified as homozygous resistant (RR), homozygous susceptible (SS), or heterozygous (SR).
Statistical analysis
Quantitative data are presented as the mean ± standard deviation. Statistical comparisons between the control group and the herbal treatment group were conducted using SPSS software (version 22.0). A p-value of less than 0.05 (p < 0.05) was considered statistically significant.
Results
Qualification of the methanolic extracts from Areca catechu L. seeds and Piper betle L. leaves
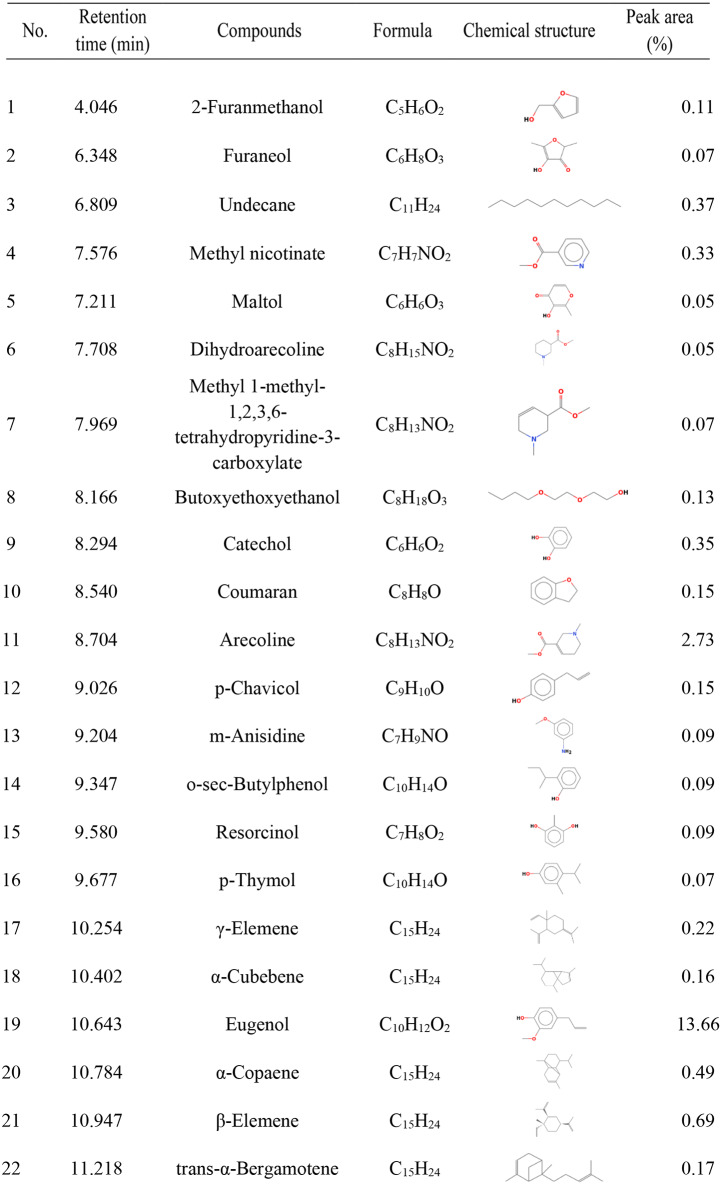
The methanolic extracts from AC seeds were in the pinkish red solid form with a yield of dry AC seeds was 8.95%. The semisolid dark green extract of PB leaves had a 6.71% yield of dry plant. Phytochemical analysis revealed significant variations in the total phenolic content, total flavonoid content, total hydrolyzed tannin content, and total condensed tannin content among the herbal extracts. The ACPB mixture exhibited the highest total phenolic content (4,353.0 ± 52.3 mg GAE/g), followed by the AC (3,929.9 ± 31.5 mg GAE/g) and PB extracts (3,624.3 ± 67.3 mg GAE/g). Conversely, the PB extract demonstrated the highest total flavonoid content (238.9 ± 7.2 mg QE/g), while the AC extract exhibited the lowest. Notably, the ACPB mixture exhibited the highest content levels of both hydrolysable tannins (4,711.6 ± 94.2 mg GE/g) and condensed tannins (21,976.4 ± 253.4 mg CE/g) (Table 1). Then, the AC and PB extracts at a 1:1 ratio was blended into the ACPB mixture before further determining the chemical components by GC-MS analysis (Table 2). From 58 identified compounds, accounting for 97.05% of the total substance, the major constituents of the combined extract were hydroxychavicol (45.66%), eugenol (13.66%), allylpyrocatechol diacetate (8.13%), acetyleugenol (6.16%), isoeugenyl acetate (5.45%), γ-muurolene (3.03%), and arecoline (2.73%). A chromatogram of the major components of the ACPB mixture is shown in Fig. 2.
Table 1.
The quantitative values of the secondary metabolites present in the two plants
| Plant extracts | Total phenolic content (mg GAE/g sample) |
Total flavonoid content (mg QE/g sample) |
Total hydrolyzed tannin content (mg GE/g sample) |
Total condensed tannin content (mg CE/g sample) |
|---|---|---|---|---|
| AC | 3,929.9 ± 31.5 | 5.2 ± 0.3 | 3,918.2 ± 22.1 | 21,632.0 ± 240.0 |
| PB | 3,624.3 ± 67.3 | 238.9 ± 7.2 | 3,322.7 ± 72.5 | 124.0 ± 32.9 |
| ACPB | 4,353.0 ± 52.3 | 9.9 ± 0.5 | 4,711.6 ± 94.2 | 21,976.4 ± 253.4 |
AC = Areca catechu, PB = Piper betle, ACPB = A. catechu and P. betle mixture
Table 2.
The constituents of the Areca catechu and Piper betle (ACPB) mixture determined via gas chromatography–mass spectrometry
Fig. 2.
Chromatogram of the main components of the Areca catechu and Piper betle (ACPB) mixture determined via gas chromatography–mass spectrometry
Benzimidazole resistance gene detection
Despite both male and female worms exhibiting the heterozygous resistant (SR) genotype (F200Y) at codon 200 of the β-tubulin isotype 1 gene, as determined by AS-PCR (Fig. 3), our study demonstrated a distinct benzimidazole-resistant phenotype in the female Haemonchus samples compared to their adult male counterparts.
Fig. 3.
Allele-specific PCR (AS-PCR) used to amplify the F200Y single nucleotide polymorphism in the isotype 1 β-tubulin gene. PCR products were visualized on a 2.0% agarose gel. Haemonchus spp. male and female samples included M1 and F1 (Nakhon Pathom), M2 and F2 (Ratchaburi), and M3 and F3 (Kanchanaburi). Lane M contained the 100 bp DNA ladder. Lanes 1 and 2 represent AS-PCR reactions 1 and 2 for each sample, respectively
The inhibitory effects of AC and PB extracts on the motility of the adult worm
Table 3 represents the significant antimotility effects of AC and PB extracts, as well as ACPB mixture on adult female worms at various times points compared with the untreated control group. The female worms exhibited resistance characteristics following albendazole and ivermectin treatment, with mortality rates of 0 and 33.33% ± 27.22%, respectively. Among the test concentration, only the 5% AC extracts demonstrated a significant inhibitory effect on female worm motility during treatment (24 h post treatment). However, 0.5% and 1%, PB extracts reduced female worm motility by more than 70% within 15 min, showing significant inhibition by 3 h. The 2.5% and 5% PB extracts elicited rapid effects, significantly reducing female worm motility within 15 min after treatment. At 1% and 2.5% ACPB mixture demonstrated the highest antimotility effect at 3 h, resulting in completely mortality of female worms. The 5% ACPB mixture exhibited 66.67% ± 47.14% inhibition at 5 min and significantly induced immobilization by 15 min. Significant female worm mortality was observed at 24 h following the final incubation with 0.05–5% PB and 0.5–5% of ACPB treatments.
Table 3.
The antimotility effects of Areca catechu (AC) seed extracts, Piper betle (PB) leaf extracts, and A. catechu and P. betle (ACPB) mixtures against female Haemonchus spp. At various time points
| Plant extracts | Conc. (% w/v) |
Percentage of immobilized female worm (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Incubation times | 24 h post treatment | ||||||||
| 5 min | 15 min | 30 min | 3 h | 12 h | 24 h | ||||
| RPMI | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| Albendazole | 3.2 | 5.56 ± 7.86 | 5.56 ± 7.86 | 5.56 ± 7.86 | 5.56 ± 7.86 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Ivermectin | 0.1 | 72.22 ± 20.79 | 77.78 ± 15.71 | 83.33 ± 16.67 | 83.33 ± 16.67 | 55.56 ± 15.71 | 55.56 ± 15.71 | 33.33 ± 27.22 | |
| AC | 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 33.33 ± 0.00 | 38.89 ± 7.86 | |
| 0.1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 22.22 ± 15.71 | 16.67 ± 23.57 | ||
| 0.5 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 22.22 ± 15.71 | 22.22 ± 15.71 | 55.56 ± 15.71 | ||
| 1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.56 ± 7.86 | 5.56 ± 7.86 | 27.78 ± 7.86 | 61.11 ± 28.33 | 72.22 ± 20.79 | ||
| 2.5 | 0.00 ± 0.00 | 5.56 ± 7.86 | 11.11 ± 15.71 | 16.67 ± 23.57 | 50.00 ± 13.61 | 77.78 ± 15.71 | 83.33 ± 13.61 | ||
| 5 | 0.00 ± 0.00 | 5.56 ± 7.86 | 11.11 ± 15.71 | 22.22 ± 31.43 | 55.56 ± 15.71 | 88.89 ± 15.71 | 94.44 ± 7.86* | ||
| PB | 0.05 | 0.00 ± 0.00 | 44.44 ± 15.71 | 55.56 ± 15.71 | 88.89 ± 15.71 | 88.89 ± 15.71 | 83.33 ± 23.57 | 94.44 ± 7.86* | |
| 0.1 | 22.22 ± 31.43 | 44.44 ± 15.71 | 55.56 ± 15.71 | 88.89 ± 15.71 | 88.89 ± 15.71 | 88.89 ± 15.71 | 100.00 ± 0.00* | ||
| 0.5 | 22.22 ± 31.43 | 72.22 ± 20.79 | 88.89 ± 15.71 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 1 | 55.56 ± 41.57 | 88.89 ± 15.71 | 88.89 ± 15.71 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 2.5 | 66.67 ± 47.14 | 94.44 ± 7.86* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 5 | 83.33 ± 23.57 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| ACPB | 0.05 | 0.00 ± 0.00 | 22.22 ± 15.71 | 22.22 ± 15.71 | 5.56 ± 7.86 | 27.78 ± 7.86 | 50.00 ± 23.57 | 50.00 ± 23.57 | |
| 0.1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.56 ± 7.86 | 27.78 ± 7.86 | 88.89 ± 15.71 | 72.22 ± 39.28 | 77.78 ± 31.43 | ||
| 0.5 | 22.22 ± 15.71 | 27.78 ± 7.86 | 27.78 ± 7.86 | 72.22 ± 39.28 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 1 | 11.11 ± 15.71 | 27.78 ± 7.86 | 50.00 ± 23.57 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 2.5 | 55.56 ± 41.57 | 83.33 ± 23.57 | 83.33 ± 23.57 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 5 | 66.67 ± 47.14 | 94.44 ± 7.86* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
Conc. = concentration. * p-value ≤ 0.05 compared with RPMI treatment
In male worms, albendazole and ivermectin inhibited motility at 12 h and 15 min, respectively. The 0.5–5% AC extracts became effective at 24 h of incubation, resulting in complete mortality of all the worm. At 15 min, the 0.05–2.5% PB extracts and 2.5–5% ACPB mixture exhibited significant inhibition of male motility. Complete mortality of male worms was recorded at 24 h following treatment with 0.05–5% PB extracts and 0.5–5% ACPB mixture (Table 4). Immobilization effects of the herbal extracts on both the female and male worms were also recorded at 30 min and 9 h, although these were not significantly different from the results at 60 min and 3 h, respectively (data not shown).
Table 4.
The antimotility effects of Areca catechu (AC) seed extracts, Piper betle (PB) leaf extracts, and A. catechu and P. betle (ACPB) mixtures against male Haemonchus spp. At various time points
| Plant extracts | Conc. (% w/v) |
Percentage of immobilized male worm (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Incubation times | 24 h post treatment | ||||||||
| 5 min | 15 min | 30 min | 3 h | 12 h | 24 h | ||||
| RPMI | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| Albendazole | 3.2 | 5.56 ± 7.86 | 72.22 ± 39.28 | 72.22 ± 39.28 | 72.22 ± 39.28 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | |
| Ivermectin | 0.1 | 66.67 ± 47.14 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | |
| AC | 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | 16.67 ± 23.57 | 16.67 ± 23.57 | 88.89 ± 15.71 | 94.44 ± 7.86* | 100.00 ± 0.00* | |
| 0.1 | 0.00 ± 0.00 | 33.33 ± 47.14 | 16.67 ± 23.57 | 16.67 ± 23.57 | 50.00 ± 40.82 | 66.67 ± 47.14 | 100.00 ± 0.00* | ||
| 0.5 | 0.00 ± 0.00 | 33.33 ± 47.14 | 11.11 ± 15.71 | 16.67 ± 23.57 | 50.00 ± 40.82 | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.11 ± 15.71 | 22.22 ± 31.43 | 55.56 ± 41.57 | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 2.5 | 0.00 ± 0.00 | 16.67 ± 23.57 | 22.22 ± 31.43 | 22.22 ± 31.43 | 66.67 ± 47.14 | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 5 | 0.00 ± 0.00 | 16.67 ± 23.57 | 22.22 ± 31.43 | 22.22 ± 31.43 | 66.67 ± 47.14 | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| PB | 0.05 | 66.67 ± 47.14 | 83.33 ± 23.57* | 94.44 ± 7.86* | 94.44 ± 7.86* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | |
| 0.1 | 66.67 ± 47.14 | 83.33 ± 23.57* | 94.44 ± 7.86* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 0.5 | 66.67 ± 47.14 | 88.89 ± 15.71* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 1 | 66.67 ± 47.14 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 2.5 | 66.67 ± 47.14 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 5 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| ACPB | 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 33.33 ± 47.14 | 72.22 ± 39.28 | 94.44 ± 7.86* | |
| 0.1 | 66.67 ± 47.14 | 72.22 ± 39.28 | 72.22 ± 39.28 | 72.22 ± 39.28 | 72.22 ± 39.28 | 83.33 ± 23.57* | 94.44 ± 7.86* | ||
| 0.5 | 66.67 ± 47.14 | 72.22 ± 39.28 | 72.22 ± 39.28 | 77.78 ± 31.43 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 1 | 66.67 ± 47.14 | 72.22 ± 39.28 | 77.78 ± 31.43 | 94.44 ± 7.86 | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 2.5 | 66.67 ± 47.14 | 88.89 ± 15.71* | 88.89 ± 15.71* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
| 5 | 66.67 ± 47.14 | 94.44 ± 7.86* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | 100.00 ± 0.00* | ||
Conc. = concentration. * p-value ≤ 0.05 compared with RPMI treatment
The median effective concentration (EC50) of AC, PB, and ACPB treatments against female and male Haemonchus spp. at 12 h and 24 h are presented in Tables 5 and 6. At 12 h, the AC extract demonstrated EC50 values of 2.73 mg/mL for female worms and 0.19 mg/mL for male worms. However, the EC50 values of PB could not be determined, as even the lowest concentration (0.05 mg/mL) of PB extract caused complete mortality in both female and male worms at both 12 h and 24 h. The EC50 values for the ACPB mixture were similar for female (0.06 mg/mL) and male (0.07 mg/mL) worm at 12 h. The AC extract exhibited higher EC50 values for inhibiting female worm motility compared to male worm motility at both 12 h and 24 h.
Table 5.
The median effective concentration (EC50; mg/mL) of Areca catechu (AC) seed extracts, Piper betle (PB) leave extracts, and A. catechu and P. betle (ACPB) mixtures against female Haemonchus spp. At 12 h and 24 h
| Plant extracts | Incubation times | |||||
|---|---|---|---|---|---|---|
| 12 h | 24 h | |||||
| EC50 | 95% Fiducial CI | EC50 | 95% Fiducial CI | |||
| Lower | Upper | Lower | Upper | |||
| AC | 2.73 | 1.26 | 5.95 | 0.46 | 0.19 | 1.09 |
| PB | ND | ND | ND | ND | ND | ND |
| ACPB | 0.06 | 0.05 | 0.09 | 0.06 | 0.03 | 0.09 |
ND = not determined, 95% Fiducial CI; The 95% Fiducial confidence intervals
Table 6.
The median effective concentration (EC50; mg/mL) of Areca catechu (AC) seed extracts, Piper betle (PB) leaf extracts, and A. catechu and P. betle (ACPB) mixtures against the male Haemonchus spp. At 12 h and 24 h
| Plant extracts | Incubation times | |||||
|---|---|---|---|---|---|---|
| 12 h | 24 h | |||||
| EC50 | 95% Fiducial CI | EC50 | 95% Fiducial CI | |||
| Lower | Upper | Lower | Upper | |||
| AC | 0.19 | 0.01 | 2.90 | 0.13 | 0.09 | 0.19 |
| PB | ND | ND | ND | ND | ND | ND |
| ACPB | 0.07 | 0.04 | 0.10 | 0.02 | 0.01 | 0.05 |
ND = not determined, 95% Fiducial CI; The 95% Fiducial confidence intervals
The effects of herbal extracts on the Haemonchus spp. Morphology by scanning electron microscopy
The SEM images of adult female and male worms originally revealed distinct morphological alterations following treatment with ACPB mixture (5 mg/mL) for 24 h (Figs. 4 and 5). Cuticular damage, characterized by several irregular surfaced, round-shaped appearances, was evident on the surface of the buccal region (Fig. 4b), along the entire body (Fig. 4c) and around the vulva of the female worm (Fig. 4d). Figure 4f illustrates wrinkling of the buccal capsule and the lancet structure in the male worm. The effects of the ACPB mixture, observed at various magnifications, prominently affected the surface of the worm’s body. Leakage of internal biological substances was observed from circular furrows between the cuticular annuli (Fig. 5b, c and e, and 5f). These destructive morphological changes resulting from the exposure to the plant extract likely contributed to worm mortality.
Fig. 4.
The morphological image of the Haemonchus spp. buccal area and body obtained by scanning electron microscopy after Areca catechu and Piper betle (ACPB) mixture treatment at 5 mg/mL for 24 h. Images were captured at various magnifications: (a) control female buccal area (scale bar 5 μm, 3,000×), (b) treated female buccal area (scale bar 10 μm, 2,000×), (c) treated female body (scale bar 50 μm, 370×), (d) treated female vulva (scale bar 50 μm, 370×), (e) control male buccal area (scale bar 5 μm, 3,000×), and (f) treated male buccal area (scale bar 10 μm, 2,000×)
Fig. 5.
The morphological image of the Haemonchus spp. body obtained by scanning electron microscopy after Areca catechu and Piper betle (ACPB) mixture treatment at 5 mg/mL for 24 h. Images were captured at various magnifications: (a) control female cuticle (scale bar 10 μm, 1,000×), (b) and (c) treated female cuticle (scale bar 10 μm, 2,000× and scale bar 2 μm, 7,000×), (d) control male cuticle (scale bar 10 μm, 1,000×), (e) and (f) treated male cuticle (scale bar 10 μm, 1,000× and 2,000×)
Discussion
The increasing resistance of gastrointestinal nematodes to traditional anthelmintics has prompted a search for new methods for controlling parasitic infections, particularly those using plant-based compounds. Several studies have explored the in vitro effects of medicinal plant extracts, such as methanol, ethanol, and essential oils, on parasitic nematodes. These extracts often contain bioactive molecules such as alkaloids, flavonoids, tannins, and terpenes, which can disrupt nematode metabolism and reproduction, resulting in antiparasitic activity [42, 43]. Methanol extraction is frequently employed to isolate these valuable compounds. Its effectiveness lies in its ability to extract a wide range of substances [44]. Our current study revealed substantial levels of phenolics, flavonoids, hydrolyzed tannins, and condensed tannins in methanolic extracts. Our findings are consistent with the results reported by Busari et al. [14], who found that methanolic extracts from Terminalia glaucescens leaves exhibited greater in vitro anthelmintic activity against Haemonchus eggs. Other studies have also highlighted the potential of methanolic extracts. For example, Alowanou et al. [45] investigated the effects of methanolic extracts from Bridelia ferruginea, Combretum glutinosum, and Mitragyna inermis on Haemonchus contortus egg hatching, larval migration, and adult worm motility. Furthermore, Nwosu et al. [46] demonstrated the ovicidal and larvicidal activity of a methanolic extract from Dennettia tripetala fruits against H. contortus.
A previous study evaluated the anthelmintic efficacy of a crude aqueous methanolic extract of AC seeds using in vitro egg hatch and adult motility assays, as well as in vivo fecal egg count reduction tests [23]. In that study, adult motility inhibition of 9.00% ± 0.58% was observed at 50 mg/mL after 10 h, compared to 10.00% ± 0.00% with levamisole. However, our study employed a different outcome, 0.5% (w/v) AC extract achieved 100% motility inhibition in isolated adult male Haemonchus parasites within 24 h. A 5.0% (w/v) concentration demonstrated substantial inhibition, at 94.44% ± 7.86%, in adult female Haemonchus parasites 24 h post-treatment. These findings collectively indicate the AC extract’s capacity to inhibit the motility of adult Haemonchus parasites.
The anthelmintic activity of a methanolic extract of PB leaves has been previously demonstrated in earthworms (Pheritima posthuma) at a concentration range of 10–80 mg/mL, where both paralysis and mortality were observed [28]. In the present study, we evaluated the effects of PB extract on both adult male and female Haemonchus. Specifically, 0.1% (w/v) PB extract exhibited 100% motility inhibition in adult males within 3 h exposure. In adult females, the same concentration induced motility inhibition over a 24 h exposure period. However, 0.5% (w/v) PB extract achieved inhibition within 3 h of exposure. These findings indicate that, under the tested conditions, PB extract effectively inhibits motility in both adult male and female Haemonchus parasites.
Although the combined ACPB methanolic extract exhibited no synergistic anthelmintic effect in adult male Haemonchus parasites, it exhibited enhanced efficacy in adult females compared to AC extract alone. This sex-specific difference warrants further investigation. Notably, the combined use of ACPB has shown therapeutic potential in other biological contexts. For instance, Zhang et al. [47] demonstrated that the combination of areca nut and betel leaf creates a unique metabolomic profile with implications for traditional medicine. However, Sari et al. [48] reported cellular damage in oral keratinocytes and fibroblasts following prolonged exposure to areca nut and betel leaf extracts. In contrast, Nadig et al. [49] found that an aqueous extract of PB and AC protected against seizures and improved cognitive function in zebrafish. These contrasting findings highlight the complex interplay of factors, including extraction methods, dosage, exposure duration, and target organisms, that can influence the biological effects of these plant extracts. Further research is essential to fully elucidate the mechanisms of action and safety profiles of AC and PB extracts, particularly in the context of anthelmintic applications.
The albendazole- and ivermectin-resistant phenotypes observed in the adult female positive controls suggest the presence of anthelmintic-resistant Haemonchus spp. strains within our study population. Consistent with this, resistance to major anthelmintic classes, including benzimidazoles and macrocyclic lactones, has been frequently reported in Thailand [50–53]. Despite the benzimidazole AS-PCR indicating heterozygous resistance (SR) in both male and female positive controls, the adult males demonstrated a susceptible phenotype in the motility assay. This inconsistency may be explained by sex-specific variations in Haemonchus spp. RNA expression [54]. Consequently, both intrinsic biological factors and gene expression likely contribute to the broader range of observed outcomes.
Our findings suggest that AC, PB, or their combined mixture ACPB could serve as alternative anthelmintics in livestock farming, particularly in areas where benzimidazole- and ivermectin-resistant Haemonchus populations are prevalent. Although the median lethal doses of AC and PB extracts have been previously reported [55, 56], further research is necessary to determine the appropriate lethal doses of these methanolic extracts for use in livestock.
Hydroxychavicol, identified as the major phenolic component (45.66%) of the ACPB mixture, exhibits various documented antimicrobial activities. Although its antibacterial [57] and antifungal effects [58, 59] are well-established, the mechanism of action against helminths remains unknown. Previous studies have elucidated the distinct mechanisms of the antimicrobial effects of hydroxychavicol. Specifically, chloroform-extracted hydroxychavicol from PB leaves disrupts cell membrane integrity and increases membrane permeability in Candida albicans [58], whereas methanol-extracted hydroxychavicol induces oxidative stress, leading to membrane and DNA damage in Escherichia coli [57]. Given these established mechanisms of membrane disruption and oxidative stress in other organisms, we hypothesize that the observed cuticular damage and leakage in Haemonchus following ACPB mixture treatment may be attributed to hydroxychavicol. However, the precise mechanism of action of this active compound against helminths warrants further investigation.
Although eugenol, a phenolic compound comprising 13.66% of the ACPB mixture in this study, along with its related compounds acetyleugenol (6.16%) and isoeugenyl acetate (5.45%), has shown anthelmintic potential in other studies, its specific efficacy against adult Haemonchus has not been established. Notably, previous research has highlighted the anthelmintic potential of eugenol. For instance [60], have reported 100% egg hatching inhibition of Haemonchus at eugenol concentrations of 0.5–1.0%, utilizing the essential oil of Ocimum gratissimum, which contains 43.70% eugenol. Furthermore, Boyko and Brygadyrenko [61] demonstrated the larvicidal effects of eugenol, reporting a lethal concentration 50% (LC50) value of 0.0513% ± 0.0049% for the inhibition of third-stage H. contortus larvae at a 1% concentration. This disparity between the well-documented efficacy of eugenol against larvae and eggs and the absence of data on adult Haemonchus underscores the importance of the present investigation. Moreover, eugenol exhibits lethal effects on the adult Trichinella spiralis cuticle, resulting in complete smoothing of the cuticle annulations, widened openings in the bacillary band glands, and regions displaying multiple blebs and fissures [62]. In contrast, our SEM analysis revealed morphological alterations distinct from those described for eugenol-treated Trichinella spiralis. These differences likely arise from variations in the test compounds (ACPB extract against pure eugenol), eugenol concentration, and the parasite species investigated.
Allylpyrocatechol diacetate, a phenolic compound comprising 8.13% of the ACPB mixture in our study, has been reported to exhibit anti-malarial activity in in vivo experiments using methanolic extracts of PB [63]. Furthermore, 4-allylpyrocatechol, extracted from PB leaves, has demonstrated antimicrobial effects against Streptococcus intermedius and Streptococcus mutans by permeabilizing the bacterial membrane, leading to cellular leakage. Moreover, this compound inhibits Candida albicans budding and induces DNA damage via superoxide generation [64].
γ-Muurolene, a sesquiterpene present at 3.03% in the ACPB mixture used in our study, has been shown to inhibit E. coli growth [65]. Moreover, essential oil from O. gratissimum leaves, containing 11.60% γ-muurolene, has demonstrated membrane-disrupting activity against Staphylococcus aureus, E. coli, Salmonella Typhimurium, and Shigella flexneri [66].
Arecoline, an alkaloid comprising 2.73% of the ACPB mixture in our study, has been investigated for its effects on Caenorhabditis elegans. Exposure to 0.2–0.4 mM arecoline induces neurotoxicity, developmental toxicity, and reproductive toxicity in C. elegans [67]. Furthermore, arecoline is known to inhibit acetylcholine receptors, which can lead to paralysis in gastrointestinal nematodes [68].
Our study revealed that the ACPB mixture induced cuticle alterations in Haemonchus spp. This effect might be characterized by a high level of condensed tannins, aligns with observations from previous research. The SEM analysis further showed aggregation around the buccal capsule, alongside transverse and longitudinal thickening and wrinkling of the cuticular ridges following tannin exposure [69]. These findings are supported by recent studies on C. elegans, where condensed tannins were found to induce cuticle rigidity, as evidenced by SEM and atomic force microscopy [70].
While this study provides compelling preliminary in vitro evidence, the absence of in vivo validation represents a clear avenue for future research. The promising initial findings warrant further investigation to fully characterize the pharmacokinetic and pharmacodynamic profiles of the herbal compounds. Subsequent studies should prioritize the assessment of absorption, distribution, metabolism, and excretion to establish clinically relevant dosages and evaluate potential in vivo toxicities. Moreover, the individual analyses of the active compounds within the AC and PB methanolic extracts for understanding will contribute to the observed effects. These investigations will build upon the current findings and provide a more comprehensive understanding of the therapeutic potential of these herbal extracts.
Conclusion
Our study conclusively demonstrates the promising in vitro anthelmintic activity of methanolic extracts from AC and PB, both individually and in combination, against adult Haemonchus parasites of both sexes. The combined extracts induced the parasitic death caused by the distinguished cuticular damage and the separated circular furrows between the cuticular annuli. This plant-based approach presents a novel and potentially sustainable alternative to conventional synthetic anthelmintics, capitalizing on the synergistic phytochemical properties and diverse mechanisms of action inherent in these two plants. These findings strongly support further exploration and integration of phytotherapeutic strategies into comprehensive parasite control programs, particularly in livestock.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by the Faculty of Veterinary Science, Mahidol University. The authors would like to express their sincere gratitude to the Nakhon Pathom goat slaughterhouse for their valuable support. We also thank Mr. Chawalit Takoon and Mr. Pornphawit Mo-mai from the Mahidol University Frontier Research Facility (MU-FRF) for their cooperation and assistance with scanning electron microscopy. Additionally, we extend our thanks to the Monitoring and Surveillance Center for Zoonotic Diseases in Wildlife and Exotic Animals at the Faculty of Veterinary Science, Mahidol University, for providing the necessary laboratory facilities.
Abbreviations
- AC
Areca catechu
- AS-PCR
Allele-specific PCR
- GC-MS
Gas chromatography-mass spectrometry
- HEPES
N-[2-hydroxyethylpiperazine-N-[4-butanesulfonic acid
- RR
Homozygous resistant
- SS
Homozygous susceptible
- SR
Heterozygous
- PB
Piper betle
Author contributions
SS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CC: Investigation, Methodology. CN: Investigation, Methodology. SB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. All authors read and approved the final manuscript.
Funding
This research was funded by the Faculty of Veterinary Science, Mahidol University.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethical approval
The animal protocol was approved by the ethical committee (MUVS-2019-12-54) and biosafety committee (IBC/MUVS-B-001/2563) of the Faculty of Veterinary Science at Mahidol University, Thailand.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abosse JS, Terefe G, Teshale BM. Comparative study on pathological changes in sheep and goats experimentally infected with Haemonchus contortus. Surg Exp Pathol. 2022. 10.1186/s42047-022-00116-8. [Google Scholar]
- 2.Besier RB, Kahn LP, Sargison ND, Van Wyk JA. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv Parasitol. 2016. 10.1016/bs.apar.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Charlier J, Rinaldi L, Musella V, Ploeger HW, Chartier C, Vineer HR, et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev Vet Med. 2020. 10.1016/j.prevetmed.2020.105103. [DOI] [PubMed] [Google Scholar]
- 4.Fthenakis GC, Papadopoulos E. Impact of parasitism in goat production. Small Rumin Res. 2018. 10.1016/j.smallrumres.2017.04.001. [Google Scholar]
- 5.Perry BD, Randolph TF. Improving the assessment of the economic impact of parasitic diseases and of their control in production animals. Vet Parasitol. 1999. 10.1016/S0304-4017(99)00040-0. [DOI] [PubMed] [Google Scholar]
- 6.Pilarczyk B, Tomza-Marciniak A, Pilarczyk R, Bombik E, Seremak B, Udała J, et al. A comparison of the prevalence of the parasites of the digestive tract in goats from organic and conventional farms. Anim (Basel). 2021. 10.3390/ani11092581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zvinorova PI, Halimani TE, Muchadeyi FC, Matika O, Riggio V, Dzama K. Prevalence and risk factors of Gastrointestinal parasitic infections in goats in low-input low-output farming systems in Zimbabwe. Small Rumin Res. 2016. 10.1016/j.smallrumres.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Windsor PA, Nampanya S, Putthana V, Keonam K, Johnson K, Bush RD, et al. The endoparasitism challenge in developing countries as goat Raising develops from smallholder to commercial production systems: A study from Laos. Vet Parasitol. 2018. 10.1016/j.vetpar.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Arsenopoulos KV, Fthenakis GC, Katsarou EI, Papadopoulos E. Haemonchosis: A challenging parasitic infection of sheep and goats. Anim (Basel). 2021. 10.3390/ani11020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotze AC, Prichard RK. Anthelmintic resistance in Haemonchus contortus: history, mechanisms and diagnosis. Adv Parasitol. 2016. 10.1016/bs.apar.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Zajac AM, Garza J. Biology, epidemiology, and control of Gastrointestinal nematodes of small ruminants. Vet Clin North Am Food Anim Pract. 2020. 10.1016/j.cvfa.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 12.van der Voort M, Charlier J, Lauwers L, Vercruysse J, Van Huylenbroeck G, Van Meensel J. Conceptual framework for analysing farm-specific economic effects of helminth infections in ruminants and control strategies. Prev Vet Med. 2013. 10.1016/j.prevetmed.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Kamaraj C, Rahuman AA, Elango G, Bagavan A, Zahir AA. Anthelmintic activity of botanical extracts against sheep Gastrointestinal nematodes, Haemonchus contortus. Parasitol Res. 2011. 10.1007/s00436-010-2218-y. [DOI] [PubMed] [Google Scholar]
- 14.Busari IO, Soetan KO, Aiyelaagbe OO, Babayemi OJ. Phytochemical screening and in vitro anthelmintic activity of methanolic extract of Terminalia glaucescens leaf on Haemonchus contortus eggs. Acta Trop. 2021. 10.1016/j.actatropica.2021.106091. [DOI] [PubMed] [Google Scholar]
- 15.Simon MK, Ajanusi OJ, Abubakar MS, Idris AL, Suleiman MM. The anthelmintic effect of aqueous methanol extract of Combretum Molle (R. Br. X. G. Don) (Combretaceae) in lambs experimentally infected with Haemonchus contortus. Vet Parasitol. 2012. 10.1016/j.vetpar.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (Basel). 2017. 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eguale T, Tadesse D, Giday M. In vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus. J Ethnopharmacol. 2011. 10.1016/j.jep.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 18.Mumed HS, Nigussie DR, Musa KS, Demissie AA. In vitro anthelmintic activity and phytochemical screening of crude extracts of three medicinal plants against Haemonchus contortus in sheep at Haramaya municipal abattoir, Eastern Hararghe. J Parasitol Res. 2022. 10.1155/2022/6331740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavan YV, Singhal RS. Separation of polyphenols and Arecoline from areca nut (Areca catechu L.) by solvent extraction, its antioxidant activity, and identification of polyphenols. J Sci Food Agric. 2013. 10.1002/jsfa.6081 [DOI] [PubMed] [Google Scholar]
- 20.Murugesan S, Ravichandran D, Lakshmanan DK, Ravichandran G, Arumugam V, Raju K, et al. Evaluation of anti rheumatic activity of Piper betle L. (Betelvine) extract using in silico, in vitro and in vivo approaches. Bioorg Chem. 2020. 10.1016/j.bioorg.2020.104227. [DOI] [PubMed] [Google Scholar]
- 21.Sun H, Yu W, Li H, Hu X, Wang X. Bioactive components of Areca nut: an overview of their positive impacts targeting different organs. Nutrients. 2024. 10.3390/nu16050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salehi B, Konovalov DA, Fru P, Kapewangolo P, Peron G, Ksenija MS, Cardoso SM, Pereira OR, Nigam M, Nicola S, Pignata G, Rapposelli S, Sestito S, Anil Kumar NV. Luz Cádiz-Gurrea M, Segura-Carretero A, P Mishra A, Sharifi-Rad M, Cho WC, Taheri Y, Setzer WN, Sharifi-Rad J. Areca catechu-From farm to food and biomedical applications. Phytother Res. 2020;34(9):2140–2158. 10.1002/ptr.6665 [DOI] [PubMed]
- 23.Badar SN, Iqbal Z, Sajid MS, Rizwan HM, Shareef M, Malik MA et al. Comparative anthelmintic efficacy of Arundo donax, Areca catechu, and Ferula assa-foetida against Haemonchus contortus. Braz J Vet Parasitol. 2021; 10.1590/S1984-29612021028 [DOI] [PubMed]
- 24.Qian MB, Xiao N, Li SZ, Abela-Ridder B, Carabin H, Fahrion AS, et al. Control of taeniasis and cysticercosis in China. Adv Parasitol. 2020. 10.1016/bs.apar.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoud LH, Basiouny SO, Dawoud HA. Treatment of experimental heterophyiasis with two plant extracts, areca nut and pumpkin seed. J Egypt Soc Parasitol. 2002;32:501–6. [PubMed] [Google Scholar]
- 26.Yamson EC, Tubalinal GASP, Viloria VV, Mingala CN. Anthelmintic effect of betel nut (Areca catechu) and Neem (Azadirachta indica) extract against liver fluke (Fasciola spp). J Adv Vet Anim Res. 2019. 10.5455/javar.2019.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas P, Anand U, Saha SC, Kant N, Mishra T, Masih H, et al. Betelvine (Piper betle L.): A comprehensive insight into its ethnopharmacology, phytochemistry, and pharmacological, biomedical and therapeutic attributes. J Cell Mol Med. 2022. 10.1111/jcmm.17323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akter KN, Karmakar P, Das A, Anonna SN, Shoma SA, Sattar MM. Evaluation of antibacterial and anthelmintic activities with total phenolic contents of Piper betel leaves. Avicenna J Phytomed. 2014;4:320–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Leesombun A, Boonmasawai S, Shimoda N, Nishikawa Y. Effects of extracts from Thai Piperaceae plants against infection with Toxoplasma gondii. PLoS ONE. 2016. 10.1371/journal.pone.0156116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leesombun A, Boonmasawai S, Nishikawa Y. Effects of Thai piperaceae plant extracts on Neospora Caninum infection. Parasitol Int. 2017. 10.1016/j.parint.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Ristanti R, Hamid PH, Nugroho HA, Nuringtyas TR, Wibowo S, Ferdian PR, et al. Anticoccidial activities of Piper betle L essential oil on Eimeria Tenella oocysts. Sci Rep. 2024. 10.1038/s41598-024-76754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamuela-Raventós RM. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity (eds R. Apak, E. Capanoglu and F. Shahidi). 2018; 10.1002/9781119135388.ch6
- 33.Singleton VL, Rossi JA. Colorimetry of total phenolics with Phosphomolybdic-Phosphotungstic acid reagents. American journal of enology and viticulture. Am J Enol Vitic. 1965. 10.5344/ajev.1965.16.3.144. [Google Scholar]
- 34.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. JFDA. 2002. 10.38212/2224-6614.2748. [Google Scholar]
- 35.Barone CD, Zajac AM, Manzi-Smith LA, Howell AB, Reed JD, Krueger CG, et al. Anthelmintic efficacy of cranberry vine extracts on ovine Haemonchus contortus. Vet Parasitol. 2018. 10.1016/j.vetpar.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katiki LM, Ferreira JF, Gonzalez JM, Zajac AM, Lindsay DS, Chagas AC, et al. Anthelmintic effect of plant extracts containing condensed and hydrolyzable tannins on Caenorhabditis elegans, and their antioxidant capacity. Vet Parasitol. 2013. 10.1016/j.vetpar.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Skantar AM, Agama K, Meyer SL, Carta LK, Vinyard BT. Effects of geldanamycin on hatching and juvenile motility in Caenorhabditis elegans and Heterodera glycines. J Chem Ecol. 2005. 10.1007/s10886-005-7114-z. [DOI] [PubMed] [Google Scholar]
- 38.Barbosa MLF, Ribeiro WLC, de Araújo Filho JV, de Cássia Alves Pereira R, André WPP, Melo ACFL et al. In vitro anthelmintic activity of Lippia alba essential oil chemotypes against Haemonchus contortus. Exp Parasitol. 2023; 10.1016/j.exppara.2022.108439 [DOI] [PubMed]
- 39.Sambodo P, Prastowo J, Kurniasih K, Indarjulianto S. In vitro potential anthelmintic activity of Biophytum Petersianum on Haemonchus contortus. Vet World. 2018. 10.14202/vetworld.2018.1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvestre A, Humbert JF. A molecular tool for species identification and benzimidazole resistance diagnosis in larval communities of small ruminant parasites. Exp Parasitol. 2000. 10.1006/expr.2000.4542. [DOI] [PubMed] [Google Scholar]
- 41.Humbert JF, Elard L. A simple PCR method for rapidly detecting defined point mutations. Tech Tips Online. 1997. 10.1016/S1366-2120(08)70030-8. [Google Scholar]
- 42.Davuluri T, Chennuru S, Pathipati M, Krovvidi S, Rao GS. Vitro anthelmintic activity of three tropical plant extracts on Haemonchus contortus. Acta Parasitol. 2020. 10.2478/s11686-019-00116-x. [DOI] [PubMed] [Google Scholar]
- 43.Mukherjee N, Mukherjee S, Saini P, Roy P, Babu SP. Phenolics and terpenoids; the promising new search for anthelmintics: A critical review. Mini Rev Med Chem. 2016. 10.2174/1389557516666151120121036. [DOI] [PubMed] [Google Scholar]
- 44.Váradyová Z, Pisarčíková J, Babják M, Hodges A, Mravčáková D, Kišidayová S, et al. (Ovicidal and larvicidal activity of extracts from medicinal-plants against Haemonchus contortus. Exp Parasitol. 2018. 10.1016/j.exppara.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Alowanou GG, Olounladé PA, Akouèdegni GC, Faihun AML, Koudandé DO, Hounzangbé-Adoté S. In vitro anthelmintic effects of Bridelia ferruginea, Combretum glutinosum, and Mitragyna inermis leaf extracts on Haemonchus contortus, an abomasal nematode of small ruminants. Parasitol Res. 2019. 10.1007/s00436-019-06262-5. [DOI] [PubMed]
- 46.Nwosu RA, Suleiman MM, Makun HJ, Ameh MP, Shetshak MA, Akefe IO. In vitro anthelmintic activity of Dennettia tripetala G. Baker (Annonaceae) fruits against Haemonchus contortus. J Parasit Dis. 2022. 10.1007/s12639-021-01438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang P, Sari EF, McCullough MJ, Cirillo N. Metabolomic Profile Indonesian Betel Quids Biomolecules. 2022. 10.3390/biom12101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sari EF, Mohammed AI, Celentano A, McCullough MJ, Cirillo N. Cytotoxic effects of Indonesian betel quid components on oral keratinocytes and fibroblasts. BioChem. 2023. 10.3390/biochem3040011. [Google Scholar]
- 49.Nadig APR, Suman Sahyadri M, Mehdi S, Krishna KL. Aqueous extract of Piper betle L. leaf and Areca catechu L. nut protects against pentylenetetrazole-induced seizures and positively modulates cognitive function in adult zebrafish. Adv Tradit Med. 2023. 10.1007/s13596-022-00664-0. [Google Scholar]
- 50.Chan AHE, Kaenkaew C, Pakdee W, Sungpradit S, Thaenkham U. Emergence of dual drug-resistant strongylids in goats: first phenotypic and genotypic evidence from Ratchaburi province, central Thailand. BMC Vet Res. 2025. 10.1186/s12917-025-04700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitaksakulrat O, Chaiyasaeng M, Artchayasawat A, Eamudomkarn C, Thongsahuan S, Boonmars T. The first molecular identification of benzimidazole resistance in Haemonchus contortus from goats in Thailand. Vet World. 2021. 10.14202/vetworld.2021.764-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ratanapob N, Thuamsuwan N, Thongyuan S. Anthelmintic resistance status of goat Gastrointestinal nematodes in Sing Buri province, Thailand. Vet World. 2022. 10.14202/vetworld.2022.83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rerkyusuke S, Lamul P, Thipphayathon C, Kanawan K, Porntrakulpipat S. Caprine roundworm nematode resistance to macrocyclic lactones in Northeastern Thailand. Vet Integr Sci. 2023. 10.12982/VIS.2023.044. [Google Scholar]
- 54.Kellerová P, Matoušková P, Lamka J, Vokřál I, Szotáková B, Zajíčková M, et al. Ivermectin-induced changes in the expression of cytochromes P450 and efflux transporters in Haemonchus contortus female and male adults. Vet Parasitol. 2019. 10.1016/j.vetpar.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Amran AA, Mahmud R. Antimalarial activity of methanolic leaf extract of Piper betle L. Molecules. 2010. 10.3390/molecules16010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng W, Liu YJ, Wu N, Sun T, He XY, Gao YX, et al. Areca catechu L. (Arecaceae): a review of its traditional uses, botany, phytochemistry, Pharmacology and toxicology. J Ethnopharmacol. 2015. 10.1016/j.jep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 57.Singh D, Majumdar AG, Gamre S, Subramanian M. Membrane damage precedes DNA damage in hydroxychavicol treated E. coli cells and facilitates cooperativity with hydrophobic antibiotics. Biochimie. 2021. 10.1016/j.biochi.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Ali I, Khan FG, Suri KA, Gupta BD, Satti NK, Dutt P, et al. In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Ann Clin Microbiol Antimicrobe. 2010. 10.1186/1476-0711-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali I, Satti NK, Dutt P, Prasad R, Khan IA. Hydroxychavicol: A phytochemical targeting cutaneous fungal infections. Sci Rep. 2016. 10.1038/srep37867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pessoa LM, Morais SM, Bevilaqua CM, Luciano JH. Anthelmintic activity of essential oil of Ocimum gratissimum linn. And Eugenol against Haemonchus contortus. Vet Parasitol. 2002. 10.1016/s0304-4017(02)00253-4. [DOI] [PubMed] [Google Scholar]
- 61.Boyko O, Brygadyrenko V. Survival of nematode larvae after treatment with eugenol, isoeugenol, thymol, and carvacrol. Front Biosci (Elite Ed). 2023. 10.31083/j.fbe1504025. [DOI] [PubMed] [Google Scholar]
- 62.ElGhannam M, Dar Y, ElMehlawy MH, Mokhtar FA, Bakr L. Eugenol; effective anthelmintic compound against foodborne parasite Trichinella spiralis muscle larvae and adult. Pathogens. 2023. 10.3390/pathogens12010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamura S, Miyoshi A, Kawano T, Horii T, Itagaki S, Murakami N. Structure-Activity relationship of Anti-malarial Allylpyrocatechol isolated from Piper betle. Chem Pharm Bull (Tokyo). 2020. 10.1248/cpb.c20-00294. [DOI] [PubMed] [Google Scholar]
- 64.Phumat P, Khongkhunthian S, Wanachantararak P, Okonogi S. Comparative inhibitory effects of 4-allylpyrocatechol isolated from Piper betle on Streptococcus intermedius, Streptococcus mutans, and Candida albicans. Arch Oral Biol. 2020. 10.1016/j.archoralbio.2020.104690. [DOI] [PubMed] [Google Scholar]
- 65.Perigo CV, Torres RB, Bernacci LC, Guimarães EF, Haber LL, Facanali R, et al. The chemical composition and antibacterial activity of eleven Piper species from distinct rainforest areas in southeastern Brazil. Ind Crops Prod. 2016. 10.1016/j.indcrop.2016.09.028. [Google Scholar]
- 66.Chimnoi N, Reuk-Ngam N, Chuysinuan P, Khlaychan P, Khunnawutmanotham N, Chokchaichamnankit D, et al. Characterization of essential oil from Ocimum gratissimum leaves: antibacterial and mode of action against selected gastroenteritis pathogens. Microb Pathog. 2018. 10.1016/j.micpath.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 67.Xiang K, Wang B, Wang L, Zhang Y, Li H, Luo Y. Oxidative stress, oxidative damage, and cell apoptosis: toxicity induced by Arecoline in Caenorhabditis elegans and screening of mitigating agents. Toxins (Basel). 2024. 10.3390/toxins16080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zajíčková M, Nguyen LT, Skálová L, Raisová Stuchlíková L, Matoušková P. Anthelmintics in the future: current trends in the discovery and development of new drugs against Gastrointestinal nematodes. Drug Discov Today. 2020. 10.1016/j.drudis.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Martínez-Ortíz-de-Montellano C, Arroyo-López C, Fourquaux I, Torres-Acosta JF, Sandoval-Castro CA, Hoste H. Scanning electron microscopy of Haemonchus contortus exposed to tannin-rich plants under in vivo and in vitro conditions. Exp Parasitol. 2013. 10.1016/j.exppara.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 70.Greiffer L, Liebau E, Herrmann FC, Spiegler V. Condensed tannins act as anthelmintics by increasing the rigidity of the nematode cuticle. Sci Rep. 2022. 10.1038/s41598-022-23566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.