Abstract
With the continuous development of research on natural medicines, quinone compounds have become increasingly important in the research field of chemical constituents of natural treatments. However, there is a lack of in-depth and systematic collation of their types, distribution, pharmacological activities, and potential toxicities. This article comprehensively reviews the structural types, biogenetic pathways, extraction and separation methods, structural identification techniques, pharmacological activities, and toxicities of quinone compounds. It is found that the main difficulties in the research of quinone compounds lie in the cumbersome traditional separation and structural identification processes, as well as the insufficient in-depth studies on the mechanisms of their activities and toxicities. This review aims to provide a reference for research on quinone compounds in natural products and offer ideas and suggestions for subsequent in-depth exploration of the pharmacological activities of quinone compounds, prevention and control of their toxicities, and the realization of rational drug use.
Keywords: quinones, chemical components, synthetic pathway, pharmacological activities, toxicity
1. Introduction
Quinone compounds are an important class of chemical constituents in natural medicines. They refer to natural organic compounds with an unsaturated cyclohexanedione structure within the molecule or are easily transformed into such structures [1]. According to the differences in their structures, quinone compounds are mainly classified into benzoquinones, naphthoquinones, phenanthraquinones, and anthraquinones [2], among which anthraquinones and their derivatives are the most numerous types. Quinone compounds are widely distributed in plants of families such as Polygonaceae Juss., Rubiaceae Juss., Leguminosae Lindl., Rhamnaceae Juss., and Liliaceae Juss., and are also present in the metabolites of some lower plants such as lichens and fungi. They possess various biological activities, including purgative, antibacterial, anti-tumor, diuretic, and hemostatic effects. In recent years, significant breakthroughs have been achieved in the research and development of new drugs derived from natural quinone compounds across multiple fields. For example, sodium tanshinone IIA sulfonate, a drug for treating coronary heart disease [3], and buparvaquone, an antimalarial drug [4]. In recent years, remarkable progress has been made in the research on the pharmacological activities of quinone chemical constituents in traditional Chinese medicines. However, quinone compounds have many adverse reactions, such as hepatotoxicity, nephrotoxicity, and carcinogenicity, which require widespread attention. Traditional Chinese medicines containing anthraquinone components may cause adverse reactions, such as melanosis coli, drug-induced liver injury, and drug-induced kidney injury in clinical practice [5]. Zhou Xujun’s analysis of 130 patients with melanosis coli showed that among 108 patients with constipation, 97 had a history of taking anthraquinone laxatives. Among them, 73 patients were grade III, and the medication duration was 1–4 years [6]. Wang Xiong [7] conducted a retrospective analysis of 12 inpatients with drug-induced liver injury caused by taking Pleuropterus multiflorus (Thunb.) Nakai and its related preparations were admitted to the Department of Hepatology of the First Affiliated Hospital of Hunan University of Chinese Medicine from January 2017 to March 2024. The severity classification was as follows: 8 cases; grade 1, 3 cases; and grade 3, and 1 case was at grade 4. All patients with grade 3 and above liver injury received traditional Chinese medicine prescriptions, and liver injury in those who took proprietary Chinese medicines was mostly mild. After discontinuing the related preparations and receiving symptomatic supportive treatments, such as liver protection and transaminase level reduction, all patients improved and were discharged from the hospital. The occurrence of drug-induced kidney injury may be related to Aloe vera (Haw.) Berg, Senna alexandrina Mill., Astragalus membranaceus (Fisch.) Bunge, Reynoutria japonica Houtt., Senna obtusifolia (L.) H. S. Irwin and Barneby [8]. Zhao Fengbo [9] analyzed 172 patients with renal parenchymal acute kidney injury (AKI). The results showed that 39 cases were caused by the consumption of Chinese herbal medicines. Among the causative herbal medicines, Aloe vera (Haw.) Berg containing anthraquinone components was included.
The dynamic changes in the number of literature, to a certain extent, reflect the academic community’s attention and research progress on quinone compounds. This article conducted searches in the China National Knowledge Infrastructure (CNKI) and Web of Science databases. In CNKI, the advanced search method was adopted, with “quinones (exact)” as the search term; in the Web of Science, the search condition is set as: Topic = “quinone”. The search period was set from 1995 to 2024. After the search, 62,241 literature were obtained, among which 7927 were included in CNKI and 54,314 were included in the Web of Science. The number of references on quinone compounds has generally shown an upward trend. An increasing number of new quinone compounds have been extracted, separated, and identified, and their pharmacological activities and synthesis pathways have been further elucidated. This review elaborates on the chemical constituents, synthesis pathways, pharmacological activities, and toxicities of quinone compounds in traditional Chinese medicine, with the aim of providing scientific references for subsequent research on the pharmacological activities of quinone compounds, toxicity prevention and control, and safety evaluation standards.Figure 1 introduces the number of references based on quinone compounds.
Figure 1.
The number of references based on quinones.
2. Progress in Chemical Composition Research
2.1. Structure Type and Distribution
2.1.1. Benzoquinones
Benzoquinones are structurally divided into two major groups: ortho-benzoquinone and para-benzoquinone, and compounds with pro-benzoquinone structures are unstable; therefore, most naturally occurring benzoquinone compounds are para-benzoquinone derivatives [10]. The substituents of benzoquinone are more varied and are usually classified into small and large groups. Common small groups include hydroxyl, methoxy, carboxyl, and smaller hydrocarbon groups containing less than three carbons, while large groups include saturated or unsaturated chain hydrocarbons containing more than three carbon atoms, benzene rings, and more complex carbon-containing substituents. Figure 2 introduces the classification of the skeletal structures of quinone compounds.
Figure 2.
Classification of the skeletal structures of quinone compounds.
Benzoquinones can be categorized into small-molecule benzoquinones, advanced straight-chain hydrocarbon benzoquinones, isopentenyl benzoquinones, furanobenzoquinones, flavonoid benzoquinones, terpene benzoquinones, and benzoquinones based on the nature of the substituent groups [11]. Small-molecule benzoquinones are common small-molecule substituents, such as hydroxyl, methoxy, and alkyl groups, which are attached to the parent nucleus of the benzoquinone. A total of 14 types of small-molecule benzoquinones have been identified, and examples of small-molecule benzoquinones include 2-methyl-p-quinone, 2, 6-dimethoxy-1, 4-benzoquinone, and others. Advanced straight-chain hydrocarbon benzoquinones have at least one advanced straight-chain aliphatic hydrocarbon attached to the parent nucleus of the benzoquinone, and nine types have been found, such as primin and arnebifuranone. Isopentenyl benzoquinones have a variable number of isopentenyl groups attached to the parent nucleus of the benzoquinone, of which 12 have been found, such as omphalone, 3-bydroxy-2-methyl-5-(3-methyl-2-butenyl)benzo-1,4-quinone. Furobenzoquinones are compounds formed by the fusion of a benzoquinone with a furan ring, of which there are three. An example of a furan-based benzoquinone is cyperaquinone. Flavonoid benzoquinones are structurally characterized by a skeleton similar to that of flavonoids, with the difference that the B ring of this class of compounds is not a benzene ring, but a benzoquinone and its derivatives, of which there are four, such as cyclofissoquinone and bodimoquinone. Terpene quinones are compounds with a terpene skeleton but with a benzoquinone structure in the molecule. There are four kinds, such as 3-acetoxymo-quinone. Biphenylquinone is a dimer consisting of two identical or different benzoquinones linked by a carbon-carbon bond, there are seven kinds. Examples of biphenylquinones include methylvilangin and lanciaquinone.

1,4 Benzoquinone was synthesized using a two-step process. In the first step, compound 1 was reacted with paraformaldehyde in different solvents (37% hydrochloric acid, 47% hydrogen bromide, morpholine, and piperidine) for 2 h at 35 °C to give compounds 2a–2d in high yields. The second step involved the oxidation of compounds 1a–1d with cerium ammonium nitrate (CAN) at room temperature to obtain the desired compounds 2a–2d in good yields. This method is short, high-yield, and easy to post-process [12]. Figure 3 introduces the synthetic pathways of quinone compounds.
Figure 3.
Synthetic pathways of quinone compounds.
Benzoquinones are found in Leguminosae Lindl., Asteraceae L., Comfreyaceae L., Araceae Juss., and some fungi. Among them, four isopentenyl-substituted benzoquinones were isolated from Nephthea chabrolii Audouin, one small-molecule benzoquinone, one high-level straight-chain hydrocarbon benzoquinone, and two isopentenyl-substituted benzoquinones from Arnebia euchroma (Royle) I.M. Johnst., and four small-molecule benzoquinones from Antrodia cinnamomea T. T. Chang & W. N. Chou. Three flavonoid benzoquinones were isolated from Dalbergia odorifera T. Chen. Two biphenoquinones and one advanced straight-chain hydrocarbon benzoquinone were isolated from Myrsine africana L. var. acuminata C. Y. Wu et C. Chen (synonym). Two isopentenyl-substituted benzoquinones were isolated from Atractylodes koreana (Nakai) Kita. Two terpene benzoquinones were isolated from Helianthus annuus L. Two advanced straight-chain hydrocarbon benzoquinones were isolated from Embelia ribes Burm. f. Table 1 presents the names and molecular formulas of benzoquinone compounds.
Table 1.
Names and molecular formulas of the benzoquinone compounds.
| No. | Name | Resource | Molecular | Classification | Ref. |
|---|---|---|---|---|---|
| 1 | 2-methyl-p-quinone | Blaps rynchopetera Fairmaire | C7H6O2 | small molecule benzoquinone | [13] |
| 2 | 2,5-dimethyl-3-methoxy-p-benzoquinone | Fluridobulus penneri | C9H10O3 | small molecule benzoquinone | [14] |
| 3 | 2, 6-dimethoxy-1, 4-benzoquinone | Atractylodes macrocephala Koidz | C8H8O4 | small molecule benzoquinone | [15] |
| 4 | aurantiogliocladin | Arnebia euchroma (Royle) I.M. Johnst. | C10H12O4 | small molecule benzoquinone | [16] |
| 5 | 2-hydroxy-3-methoxy-5-methyl-p-benzoquinone | Antrodia cinnamomea T. T. Chang & W. N. Chou | C8H8O4 | small molecule benzoquinone | [17] |
| 6 | 2-methoxy-6-methyl-p-benzoquinone | Antrodia cinnamomea T. T. Chang & W. N. Chou | C8H8O3 | small molecule benzoquinone | [17] |
| 7 | 2,3-dimethoxy-5-methyl-p-benzoquinone | Antrodia cinnamomea T. T. Chang & W. N. Chou | C9H10O4 | small molecule benzoquinone | [17] |
| 8 | 2-hydroxy-5-methoxy-3-methyl-p-benzoquinone | Antrodia cinnamomea T. T. Chang & W. N. Chou | C8H8O4 | small molecule benzoquinone | [17] |
| 9 | anserinone A | Podospora anserina (Rabenh.) Niessl | C11H12O4 | small molecule benzoquinone | [18] |
| 10 | anserinone B | Podospora anserina (Rabenh.) Niessl | C11H14O4 | small molecule benzoquinone | [18] |
| 11 | 2-hydroxy-3-methyl-5-methoxy-p-benzoquinone | Pterospermum heterophyllum Hance | C8H8O4 | small molecule benzoquinone | [14] |
| 12 | 2.3-dimethyl-5, 6-dimethoxy-p-benzoquinone | Gliocladium penicilloides Corda | C10H12O4 | small molecule benzoquinone | [14] |
| 13 | 2, 5-dimethoxy-3, 6-dimethyl-p-benzoquinone | Neonectria fuckeliana (C. Booth) Castl. & Rossman | C10H12O4 | small molecule benzoquinone | [14] |
| 14 | thymoquinone | Nigella sativa L. | C10H12O2 | small molecule benzoquinone | [19] |
| 15 | primin | Miconia lepidota DC. | C12H16O3 | advanced straight-chain hydrocarbon benzoquinone | [20] |
| 16 | embelin | Embelia ribes Burm. f | C17H26O4 | advanced straight-chain hydrocarbon benzoquinone | [21] |
| 17 | 2,5-dihydroxy-3-tridecyl-1, 4-benzoquinone | Embelia ribes Burm. f. | C19H30O4 | advanced straight-chain hydrocarbon benzoquinone | [21] |
| 18 | myrsinone | Myrsine africana L. var. acuminata C. Y. Wu et C. Chen (synonym) | C17H26O4 | advanced straight-chain hydrocarbon benzoquinone | [14] |
| 19 | idebenone | - | C19H30O5 | advanced straight-chain hydrocarbon benzoquinone | [22] |
| 20 | 2-methoxy-6-nonadecyl-1,4-benzoquinone | Miconia lepidota DC. | C26H44O3 | advanced straight-chain hydrocarbon benzoquinone | [23] |
| 21 | (-)-a-tocospirone | Gynura japonica (Thunb.) Juel | C29H50O4 | advanced straight-chain hydrocarbon benzoquinone | [24] |
| 22 | maesaquinone | Maesa japonica (Thunb.) Moritzi | C26H42O4 | advanced straight-chain hydrocarbon benzoquinone | [25] |
| 23 | paphionone | Paphiopedilum exul (Ridl.) Rolfe | C20H30O5 | advanced straight-chain hydrocarbon benzoquinone | [26] |
| 24 | isopentenyl p-benzoquinone | Phagnalon purpurescens Sch. Bip. | C11H12O2 | isopentenyl benzoquinone | [14] |
| 25 | 3,5,6-trimethoxy-2-isopentene-p-benzoquinone | Dendrobium nobile Lindl. | C14H18O5 | isopentenyl benzoquinone |
[14] |
| 26 | omphalone | Lentinellus micheneri (Berk. & M. A. Curtis) Pegler | C11H8O3 | isopentenyl benzoquinone | [27] |
| 27 | 2(E) -2-geranyl-6-methyl p-benzoquinone | Atractylodes koreana (Nakai) Kita. | C17H22O2 | isopentenyl benzoquinone | [14] |
| 28 | 2-(Z) -2-geranyl-6-methyl p-benzoquinone | Atractylodes koreana (Nakai) Kita. | C17H22O2 | isopentenyl benzoquinone | [14] |
| 29 | amebifuranone | Arnebia euchroma (Royle) I.M. Johnst | C18H20O5 | isopentenyl benzoquinone | [14] |
| 30 | arnebinone | Arnebia euchroma (Royle) I.M. Johnst | C18H22O4 | isopentenyl benzoquinone | [14] |
| 31 | chabrolobenzoquinone E | Nephthea chabrolii Audouin | C27H38O3 | isopentenyl benzoquinone | [28] |
| 32 | chabrolobenzoquinone F | Nephthea chabrolii Audouin | C29H40O4 | isopentenyl benzoquinone | [28] |
| 33 | chabrolobenzoquinone G | Nephthea chabrolii Audouin | C27H38O3 | isopentenyl benzoquinone | [28] |
| 34 | chabrolobenzoquinone H | Nephthea chabrolii Audouin | C29H42O5 | isopentenyl benzoquinone | [28] |
| 35 | atrovirinone | Garcinia atroviridis Griffith ex T. Anderson | C25H28O8 | isopentenyl benzoquinone | [29] |
| 36 | cyperaquinone | Cyperus nipponicus Franch. & Sav. | C14H10O4 | furanobenzoquinone | [30] |
| 37 | albidin | Penicillium albidum Sopp | C10H8O4 | furanobenzoquinone | [14] |
| 38 | graphisquinone | Graphis scripta (L.) Ach. | C11H10O5 | furanobenzoquinone | [14] |
| 39 | chrysoquinane | Euphorbia esula L. | C19H16O9 | flavonoid benzoquinone | [14] |
| 40 | claussequinone | Dalbergia odorifera T.Chen | C16H16O5 | flavonoid benzoquinone | [14] |
| 41 | bowdichione | Dalbergia odorifera T.Chen | C16H10O6 | flavonoid benzoquinone | [14] |
| 42 | donoherbivol-cyclocledoquinone | Dalbergia odorifera T.Chen | C32H28O9 | flavonoid benzoquinone | [14] |
| 43 | 3-Acetoxymo-quinone | Cordia oncocalyx (Allemão) Baill. | C12H14O4 | terpenebenzoquinone | [31] |
| 44 | glanduline A | Helianthus annuus L. | C15H20O2 | terpenebenzoquinone | [14] |
| 45 | glanduline B | Helianthus annuus L. | C15H18O2 | terpenebenzoquinone | [14] |
| 46 | methylvilangin | Myrsine africana L. var. acuminata C. Y. Wu et C. Chen (synonym) | C36H54O8 | biphenylquinone | [25] |
| 47 | methylanhydrovilangin | Myrsine africana L. var. acuminata C. Y. Wu et C. Chen (synonym) | C16H52O7 | biphenylquinone | [25] |
| 48 | lanciaquinone | Ardisia japonica (Thunb.) Bl. | C27H36O7 | biphenylquinone | [32] |
| 49 | neonambiquinone A | Neonothopanus nambi (Speg.) R. H. Petersen & Krisai | C19H14O6 | biphenylquinone | [33] |
| 50 | volucrisporin | Volucrispora aurantiaca Haskins | C18H12O4 | biphenylquinone | [34] |
| 51 | oosporein | Beauveria bassiana (Bals.-Criv.) Vuill. | C14H18O8 | biphenylquinone | [35] |
| 52 | biembelin | Rapanea melanophloeos (L.) Meisn. | C34H50O8 | biphenylquinone | [14] |
| 53 | embenones A | Knema globularia (Lam.) Warb. | C15H18O4 | other | [35] |
| 54 | embenones B | Knema globularia (Lam.) Warb. | C15H20O4 | other | [35] |
| 55 | triaziquone | Artemisia sieberi. J | C12H13N3O2 | other | [36] |
| 56 | aziridyl benzoquinone | - | C16H22N2O6 | other | [37] |
| 57 | erectquione B | Hypericum erectum Sol. ex R.Br. | C29H40O6 | other | [38] |
| 58 | erectquione C | Hypericum erectum Sol. ex R.Br. | C25H34O6 | other | [38] |
| 59 | Atromentin | Ascocoryne sarcoides | C18H12O6 | other | [39] |
| 60 | Erectquione A | Hypericum erectum Sol. ex R.Br. | C21H28O4 | ortho-benzoquinone | [38] |



2.1.2. Naphthoquinones
Naphthoquinones can be structurally divided into three types: α(1,4) naphthoquinone, β(1,2) naphthoquinone, and amphi(2,6) naphthoquinone, of which most naturally occurring naphthoquinones are α-naphthoquinone derivatives [40]. They are mostly orange or orange-red crystals, and a few are purple.

Common naphthoquinone substituents include hydroxyl, methoxy, aliphatic, and aromatic hydrocarbons. Naphthoquinones can be categorized based on the type of substituents as small-molecule-substituted naphthoquinones, benzoisochromanquinones, furanonaphthoquinones, isopentenyl naphthoquinones, etc. [11]. Small-molecule naphthoquinones are common small-molecule substituents, such as hydroxyl, methoxy, and alkyl groups, attached to the parent nucleus of naphthoquinone. Currently, 24 small-molecule-substituted naphthoquinones have been identified, including juglone and plumbagin; 20 benzoisochroman quinones, including davidianone A and mansonin A; 28 furano-naphthoquinones, including arthoniafurone B and cribrarione A; and 23 isopentenyl naphthoquinones, including lapachol and crassiflorone.
There are two mainstream methods for synthesizing 2-methyl-1,4-naphthoquinone. The first method uses 2-methylnaphthalene as the raw material and glacial acetic acid as the solvent, and 2-methyl-1,4-naphthoquinone is obtained via one-step oxidation with chromium trioxide. The main advantage of this method is that 2-methylnaphthalene is inexpensive, and the route is only one step. 2-Methylnaphthalene hydroquinone is obtained by Diels-Alder cycloaddition of butadiene and methylbenzoquinone, followed by oxidation with chromic anhydride to obtain 2-methyl-1,4-naphthoquinone [41].

Naphthoquinones are mainly distributed in plants of the families Ulmaceae Mirb., Persicaceae Raf., and Albiziaceae Raf., in addition to some microorganisms and marine organisms. Among them, 20 naphthoquinones were isolated from Rhinacanthus nasutus (L.) Kurz, containing six benzoisochromanquinones and eight isoprenoid naphthoquinones; 7 naphthoquinones were isolated from Cordia curassavica (Jacq.) Roem. & Schult; Five naphthoquinones, containing one small-molecule naphthoquinone, and three furanoquinones were isolated from Plumbago zeylanica L.; four naphthoquinones were isolated from Chirita eburnea Hance; four benzoisochromanquinones were isolated from Ulmus pumila L.; three small-molecule naphthoquinones were isolated from Diospyros maritima Blume; three small-molecule naphthoquinones and three benzisochromanquinones were isolated from Ulmus davidiana Planch. Table 2 presents the names and molecular formulas of naphthoquinone compounds.
Table 2.
Names and molecular formulas of naphthoquinone compounds.
| No. | Name | Resource | Formula | Classification | Ref. |
|---|---|---|---|---|---|
| 61 | 3-bromoplumbagin | Diospyros maritima Blume | C11H7BrO3 | small molecule naphthoquinones | [42] |
| 62 | 3-(2-hydroxyethyl)plumbagin | Diospyros maritima Blume | C13H12O4 | small molecule naphthoquinones | [42] |
| 63 | 6-(1-ethoxyethyl)plumbagin | Diospyros maritima Blume | C15H16O4 | small molecule naphthoquinones | [43] |
| 64 | juglone | Juglans regia L. | C10H6O3 | small molecule naphthoquinones | [14] |
| 65 | 2-methyl-1, 4-naphthoquinone | Juglans regia L. | C11H8O2 | small molecule naphthoquinones | [14] |
| 66 | lawsone | Lythrum salicaria L. | C10H6O4 | small molecule naphthoquinones | [14] |
| 67 | 2-amino-1.4-naphthoquinone | Laurus nobilis L. | C10H7NO3 | small molecule naphthoquinones | [14] |
| 68 | plumbagin | Plumbago zeylanica L. | C11H8O3 | small molecule naphthoquinones | [14] |
| 69 | isoplumbagin | Impatiens balsamina L. | C11H8O3 | small molecule naphthoquinones | [14] |
| 70 | chimaphilin | Pyrola soldanellifolia Andres | C12H10O3 | small molecule naphthoquinones | [14] |
| 71 | 7-methyl juglone | Diospyros usambarensis Engl. | C11H8O3 | small molecule naphthoquinones | [14] |
| 72 | 2-methoxy-6-acetyl-7-methyljuglone | Pleuropterus multiflorus (Thunb.) Nakai | C13H12O5 | small molecule naphthoquinones | [44] |
| 73 | 2-methoxystypandrone | Rumex japonicus Houtt | C14H12O5 | small molecule naphthoquinones | [45] |
| 74 | 2-butanoyl-3,6,8-trihydroxy-1,4-naphthoquinone 6-O-sulfate |
Oxycomanthus japonicus J. F. W. Mller | C14H11NaO9S | small molecule naphthoquinones | [46] |
| 75 | 2-butanoyl-3,6,8-trihydroxy-1,4-naphthoquinone | Oxycomanthus japonicus J. F. W. Mller | C14H12O6 | small molecule naphthoquinones | [46] |
| 76 | cribrarione B | Cribraria cancellata (Batsch) Nann.-Bremek. | C12H10O6 | small molecule naphthoquinones | [47] |
| 77 | fusarnaphthoquinoe A | Fusarium spp. | C15H18O7 | small molecule naphthoquinones | [48] |
| 78 | 7-carbomethoxy-2,8-dimethoxy-5-hydroxy-l,4-naphthoquinone | Penicillium raistrickii Stolk & Scott | C14H13O7 | small molecule naphthoquinones | [49] |
| 79 | 2,7-dimethoxy-5-hydroxy-1,4-naphthoquinone | Penicillium raistrickii Stolk & Scott | C12H10O5 | small molecule naphthoquinones | [49] |
| 80 | 8-formyl-7-hydroxy-5-isopropyl-2-methoxy-3-methyl-1,4-naphthoquinone | Ceiba pentandra (L.) Gaertn. | C16H16O5 | small molecule naphthoquinones | [50] |
| 81 | 2,7-dihydroxy-8-formyl-5-isopropyl-3-methyl-1.4-naphthoquinone | Ceiba pentandra (L.) Gaertn. | C15H14O5 | small molecule naphthoquinones | [50] |
| 82 | 7-hydroxy-5-isopropyl-2-methoxy-3-methylnaphthoquinone | Bombax malabaricum DC. | C15H16O4 | small molecule naphthoquinones | [51] |
| 83 | lanigerone | Salvia lanigera Poir. (Lamiaceae) | C14H14O3 | small molecule naphthoquinones | [52] |
| 84 | salvigerone | Salvia lanigera Poir. (Lamiaceae) | C21H26O4 | small molecule naphthoquinones | [52] |
| 85 | droserone | Plumbago capensis Thunb | C11H8O4 | small molecule naphthoquinones | [53] |
| 86 | davidianone A | Ulmus davidiana Planch. | C15H12O4 | benzoisochromanquinone | [54] |
| 87 | davidianone B | Ulmus davidiana Planch. | C16H12O5 | benzoisochromanquinone | [54] |
| 88 | davidianone C | Ulmus davidiana Planch. | C17H16O5 | benzoisochromanquinone | [54] |
| 89 | mansonone E | Ulmus pumila L. | C15H14O3 | benzoisochromanquinone | [55] |
| 90 | mansonone F | Ulmus pumila L. | C15H12O3 | benzoisochromanquinone | [55] |
| 91 | mansonone H | Ulmus pumila L. | C15H14O4 | benzoisochromanquinone | [56] |
| 92 | mansonone I | Ulmus pumila L. | C15H14O4 | benzoisochromanquinone | [57] |
| 93 | rhinacanthone | Rhinacanthus nasutus (L.) Kurz | C15H14O3 | benzoisochromanquinone | [58] |
| 94 | rhinacanthin A | Rhinacanthus nasutus (L.) Kurz | C15H14O4 | benzoisochromanquinone | [59] |
| 95 | rhinacanthin O | Rhinacanthus nasutus (L.) Kurz | C24H26O5 | benzoisochromanquinone | [58] |
| 96 | rhinacanthin P | Rhinacanthus nasutus (L.) Kurz | C24H26O5 | benzoisochromanquinone | [58] |
| 97 | rhinacanthin S | Rhinacanthus nasutus (L.) Kurz | C24H24O5 | benzoisochromanquinone | [58] |
| 98 | rhinacanthin T | Rhinacanthus nasutus (L.) Kurz | C24H26O5 | benzoisochromanquinone | [60] |
| 99 | mansonin A | Mansonia altissima A. Chev. | C17H18O5 | benzoisochromanquinone | [60] |
| 100 | mansonin B | Mansonia altissima A. Chev. | C17H18O6 | benzoisochromanquinone | [60] |
| 101 | 5-methoxy-3,4-dehydroxanthomegnin | Paepalanthus latipes Silveira | C16H12O7 | benzoisochromanquinone | [61] |
| 102 | pyranokunthone A | Stereospermum kunthianum Cham. | C20H20O4 | benzoisochromanquinone | [62] |
| 103 | 4-O-methyl erythrostominone | Cordyceps unilateralis (Tul.) Sacc. var. clavata (Y. Kobayasi) | C18H18O8 | benzoisochromanquinone | [63] |
| 104 | halawanone A | Streptomyces Schröter | C23H22O9 | benzoisochromanquinone | [64] |
| 105 | pyranokunthone B | Stereospermum kunthianum Cham. | C20H20O4 | benzoisochromanquinone | [62] |
| 106 | (3a,3′a,4β,β)-3,3′-dimethoxy-cis-[4,4′-bis(3,4,5,10-tetra-hydro-1H-naphtho(2,3-clpyran)]-5.5.10,10-tetraone | Pentas longiflora Oliv. | C28H22O8 | benzoisochromanquinone | [65] |
| 107 | arthoniafurone B | Arthonia cinnabarina Ach. | C14H10O5 | furanonaphthoquinone | [66] |
| 108 | fusarnaphthoquinone B | Fusarium Link | C15H16O5 | furanonaphthoquinone | [48] |
| 109 | arthoniafurone A | Arthonia cinnabarina (DC.) Wallr. | C14H8O5 | furanonaphthoquinone | [66] |
| 110 | cribrarione A | Cribraria purpurea Schwein. | C13H10O7 | furanonaphthoquinone | [67] |
| 111 | 8-hydroxy-1-methylnaphtho[2,3-c]furan-4,9-dione | Bulbine capitata Poelln. | C13H8O4 | furanonaphthoquinone | [68] |
| 112 | 5,8-dihydroxy-1-methylnaphtho[2,3-c]furan-4,9-dione | Aloe ferox Mill. | C13H8O5 | furanonaphthoquinone | [69] |
| 113 | 5,8-dihydroxy-1-hydroxymethylnaphtho[2,3-c]furan-4,9-dione | Aloe ferox Mill. | C13H8O6 | furanonaphthoquinone | [69] |
| 114 | avicequinone A | Avicennia alba Blume | C15H14O5 | furanonaphthoquinone | [70] |
| 115 | avicequinone B | Avicennia alba Blume | C12H6O3 | furanonaphthoquinone | [70] |
| 116 | avicequinone C | Avicennia alba Blume | C15H12O4 | furanonaphthoquinone | [70] |
| 117 | avicequinone D | Avicennia alba Blume | C15H12O5 | furanonaphthoquinone | [70] |
| 118 | avicequinone E | Mendoncia cowanii (S. Moore) Benoist | C15H14O5 | furanonaphthoquinone | [71] |
| 119 | 2-(1′-methylethenyl)naphtho[2,3-b]furan-4,9-dione | Newbouldia laevis (P. Beauv.) Seem. ex Bureau | C15H10O3 | furanonaphthoquinone | [72] |
| 120 | 2-isopropenyl-9-methaxy-1,8-dioxa-dicyclopenta[b,g]naphthal-ene-4,10-dione | Plumbago zeylanica L. | C18H12O5 | furanonaphthoquinone | [73] |
| 121 | 9-hydroxy-2-isopropenyl-1,8-dioxa-dicyclopenta[b,g]naphthal-ene-4,10-dione | Plumbago zeylanica L. | C17H10O5 | furanonaphthoquinone | [74] |
| 122 | 2-(1-hydroxy-l-methyl-ethyl)-9-methoxy-1,8-dioxa-dicyclo-penta[b,g]naphthalene-4,10-dione | Plumbago zeylanica L. | C18H14O6 | furanonaphthoquinone | [73] |
| 123 | (R)-7-hydroxy-a-dunnione | Chirita eburnea Hance | C15H14O4 | furanonaphthoquinone | [74] |
| 124 | (R)-8-hydroxy-a-dunnione | Chirita eburnea Hance | C15H14O4 | furanonaphthoquinone | [74] |
| 125 | (R)-a-7,8-dihydroxy-a-dunnione | Chirita eburnea Hance | C15H14O5 | furanonaphthoquinone | [74] |
| 126 | (R)-7-methoxy-6,8-dihydroxy-a-dunnione | Chirita eburnea Hance | C16H16O6 | furanonaphthoquinone | [74] |
| 127 | 7,8-dimethoxydunnione | Sinningia leucotricha (Hoehne) H. E. Moore | C17H18O5 | furanonaphthoquinone | [75] |
| 128 | dehydro-a-isodunnione | Tectona grandis L. f. | C15H12O3 | furanonaphthoquinone | [76] |
| 129 | 5-hydroxy-7-methoxydehydroiso-a-lapachone | Newbouldia laevis (P. Beauv.) Seemann ex Bureau | C16H14O5 | furanonaphthoquinone | [77] |
| 130 | glycoquinone | Glycosmis pentaphylla (Retz.) Corrêa | C20H24O4 | furanonaphthoquinone | [78] |
| 131 | (2R)-6,8-dihydroxy-a-dunnione | Lysionotus pauciflorus Maxim. | C15H14O5 | furanonaphthoquinone | [79] |
| 132 | balsaminone D | Impatiens balsamina L. | C20H14O7 | furanonaphthoquinone | [80] |
| 133 | (2R)-6-hydroxy-7-methoxy-dehydroiso-α-lapachone | Spermacoce latifolia Aubl. | C15H14O5 | furanonaphthoquinone | [81] |
| 134 | crassiflorone | Diospyros crassiflora Hiern | C21H12O6 | furanonaphthoquinone | [82] |
| 135 | lapachol | Tabebuia avellanedae Lorentz ex Griseb. | C15H14O3 | isopentenyl naphthoquinone | [83] |
| 136 | hydroxysesamone | Sesamum indicum L. | C15H14O5 | isopentenyl naphthoquinone | [84] |
| 137 | 2,3-epoxysesamone | Sesamum indicum L. | C15H14O5 | isopentenyl naphthoquinone | [84] |
| 138 | lantalucratin D | Lantana involucrata L. | C17H18O5 | isopentenyl naphthoquinone | [85] |
| 139 | lantalucratin E | Lantana involucrata L. | C17H18O6 | isopentenyl naphthoquinone | [85] |
| 140 | lantalucratin F | Lantana involucrata L. | C17H18O7 | isopentenyl naphthoquinone | [85] |
| 141 | butylalkannin | Arnebia hispidissima (Sieber ex Lehm.) A.DC. | C20H22O6 | isopentenyl naphthoquinone | [86] |
| 142 | alkannin | Arnebia hispidissima (Sieber ex Lehm.) A.DC. | C6H16O5 | isopentenyl naphthoquinone | [86] |
| 143 | rhinacanthin B | Rhinacanthus nasutus (L.) Kurz | C25H28O5 | isopentenyl naphthoquinone | [59] |
| 144 | rhinacanthin C | Rhinacanthus nasutus (L.) Kurz | C25H30O5 | isopentenyl naphthoquinone | [58] |
| 145 | rhinacanthin G | Rhinacanthus nasutus (L.) Kurz | C25H30O6 | isopentenyl naphthoquinone | [58] |
| 146 | rhinacanthin H | Rhinacanthus nasutus (L.) Kurz | C25H30O6 | isopentenyl naphthoquinone | [58] |
| 147 | rhinacanthin I | Rhinacanthus nasutus (L.) Kurz | C25H30O6 | isopentenyl naphthoquinone | [58] |
| 148 | rhinacanthin J | Rhinacanthus nasutus (L.) Kurz | C25H28O6 | isopentenyl naphthoquinone | [58] |
| 149 | rhinacanthin K | Rhinacanthus nasutus (L.) Kurz | C25H32O7 | isopentenyl naphthoquinone | [58] |
| 150 | rhinacanthin L | Rhinacanthus nasutus (L.) Kurz | C25H32O8 | isopentenyl naphthoquinone | [58] |
| 151 | cordiaquinone A | Cordia curassavica (Jacq.) Roem. & Schult | C21H26O3 | isopentenyl naphthoquinone | [87] |
| 152 | chabrolonaphthoquinone A | Nephthea chabrolii Milne Edwards & Haime | C27H32O4 | isopentenyl naphthoquinone | [88] |
| 153 | chabrolonaphthoquinone B | Nephthea chabrolii Milne Edwards & Haime | C29H38O5 | isopentenyl naphthoquinone | [28] |
| 154 | 6,8-dihydroxy-2,7-dimethoxy-3-(1,1-dimethylprop-2-enyl)-1,4-naphthoquinones | Lysionotus pauciflorus Maxim. | C17H18O6 | isopentenyl naphthoquinone | [79] |
| 155 | 7-hydroxy-2-O-methyldunniol | Sinningia conspicua (Seem.) Focke | C16H15O4 | isopentenyl naphthoquinone | [89] |
| 156 | 7-methoxy-2-O-methyldunniol | Sinningia conspicua (Seem.) Focke | C17H17O4 | isopentenyl naphthoquinone | [89] |
| 157 | 3,5,8-tribydroxy-6-methoxy-2-(5-oxohexa- 1,3-dienyl-1.4-naphthoquinone |
Cordyceps unilateralis (Tul.) Petch | C17H14O7 | isopentenyl naphthoquinone | [63] |
| 158 | rhinacanthin D | Rhinacanthus nasutus (L.) Kurz | C23H20O7 | other | [58] |
| 159 | rhinacanthin M | Rhinacanthus nasutus (L.) Kurz | C22H20O5 | other | [90] |
| 160 | rhinacanthin N | Rhinacanthus nasutus (L.) Kurz | C27H24O7 | other | [58] |
| 161 | rhinacanthin Q | Rhinacanthus nasutus (L.) Kurz | C28H26O7 | other | [58] |
| 162 | rhinacanthin U | Rhinacanthus nasutus (L.) Kurz | C17H18O5 | other | [58] |
| 163 | rhinacanthin V | Rhinacanthus nasutus (L.) Kurz | C25H22O6 | other | [58] |
| 164 | cordiaquinone E | Cordia curassavica (Jacq.) Roemer&Schultes | C21H24O3 | other | [87] |
| 165 | cordiaquinone B | Cordia curassavica (Jacq.) Roemer&Schultes | C21H24O3 | other | [87] |
| 166 | cordiaquinone K | Cordia curassavica (Jacq.) Roemer&Schultes | C21H22O3 | other | [87] |
| 167 | cordiaquinone F | Cordia curassavica (Jacq.) Roemer&Schultes | C26H30O5 | other | [87] |
| 168 | cordiaquinone G | Cordia curassavica (Jacq.) Roemer&Schultes | C21H26O4 | other | [87] |
| 169 | cordiaquinone H | Cordia curassavica (Jacq.) Roemer&Schultes | C21H26O4 | other | [87] |
| 170 | cordiaquinone J | Cordia curassavica (Jacq.) Roemer&Schultes | C21H24O3 | other | [87] |
| 171 | isagarin | Pentas longiflora | C15H12O4 | other | [91] |
| 172 | 3-hydroxy-2-metoxy-8,8,10-trimethyl-8H-antracen-1,4,5-trione | Byrsonima microphylla A.Juss. | C18H16O5 | other | [92] |
| 173 | 3,7-dihydroxy-2-methoxy-8,8,10-trimethyl- 7,8-dihydro-6H-antracen-1,4,5-trione |
Byrsonima microphylla A.Juss. | C18H18O6 | other | [92] |
| 174 | sterekunthal A | Stereospermum kunthianum Cham. | C20H18O5 | other | [62] |
| 175 | stereiqunone C | Stereospermum kunthianum Cham. | C19H16O3 | other | [93] |
| 176 | sterequinone E | Stereospermum personatum (Hassk.) Chatterjee | C19H16O4 | other | [93] |
| 177 | sterekunthal B | Stereospermum personatum (Hassk.) Chatterjee | C20H18O4 | other | [62] |
| 178 | sterequinone B | Stereospermum personatum (Hassk.) Chatterjee | C21H20O5 | other | [93] |
| 179 | 3,8′-biplumbagin | Diospyros maritima Blume | C22H14O6 | other | [43] |
| 180 | isozeylanone | Plumbago zeylanica L. | C22H14O6 | other | [94] |
| 181 | ethylidene-3,3′-biplumbagin | Diospyros maritima Blume | C24H18O6 | other | [43] |
| 182 | ethylidene-3,6′-biplumbagin | Diospyros maritima Blume | C24H18O6 | other | [43] |
| 183 | ethylidene-6,6′-biplumbagin | Diospyros maritima Blume | C24H18O6 | other | [95] |
| 184 | balsaminone E | Impatiens balsamina L. | C22H16O5 | other | [80] |
| 185 | adenophyllone | Heterophragma adenophyllum Seem | C30H22O5 | other | [96] |
| 186 | dilapachone | Heterophragma adenophyllum Seem | C30H26O6 | other | [96] |
| 187 | fusarnaphthoquinone C | Fusarium spp. | C29H26O11 | other | [48] |
| 188 | hygrocin A | Streptomyces hygroscopicus Jensen | C28H31NO8 | other | [97] |
| 189 | hygrocin B | Streptomyces hygroscopicus Jensen | C28H29NO8 | other | [97] |
| 190 | lippisidoquinone | Lippia sidoides Cham. | C30H26O5 | other | [98] |
| 191 | phytonadione | Anethum graveolens L. | C31H46O2 | other | [99] |
| 192 | maritinone | Diospyros anisandra S.F.Blake | C22H14O6 | other | [100] |







2.1.3. Phenanthrenequinones
Phenanthrenequinones are an important class of natural products widely distributed in nature. These compounds are characterized by a tricyclic structure containing three rings and are classified mainly based on variations in the oxygen substitution site of the parent structure. Depending on the oxygen substitution site, phenanthrenequinones can be classified as para-oxygen substituted 1,4 phenanthrenequinone (para-phenanthrenequinone), pro-oxygen substituted 9,10 phenanthrenequinone (o-phenanthrenequinone I), and 3,4 phenanthrenequinone (o-Phenanthrenequinone II) [101].

The “one-pot method has become a powerful example of resource and energy efficiency, as well as environmental sustainability. The ability to perform multiple synthetic transformations in a single reaction vessel. The pot method reduces chemical waste and makes the overall operation more environmentally friendly. Pompy Sarkar discovered the synthesis of 9,10-phenanthrenequinone by the one-pot method. In the initial step, 2-bromobenzaldehyde (1a) was coupled with 2-formylphenylboronic acid (2) under standard Pd(0) conditions. The appearance of 3a was observed under standard Suzuki reaction conditions. The resulting product was then treated with Cu salt and TBHP. This combination leads to the formation of 9,10-phenanthrenequinone [102].
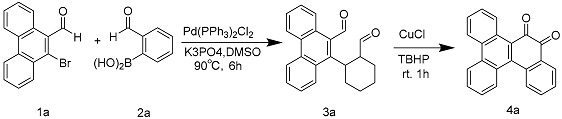
Phenanthrenequinone is mainly found in plants of Labiatae Juss., Orchidaceae Juss., and Senecio L., as well as in Streptomyces Waksman & Henrici. Among them, 11 phenanthrenequinones were isolated from Salvia miltiorrhiza Bunge, comprising one para-phenanthrenequinone and 10 type II o-phenanthrenequinones; six para-phenanthrenequinones were isolated from Dendrobium nobile Lindl.; and three phenanthrenequinones, comprising one para-phenanthrenequinone and two type II o-phenanthrenequinones, were isolated from Salvia trijuga Diels. Table 3 introduces the names and molecular formulas of phenanthraquinone compounds.
Table 3.
Names and molecular formulas of phenanthraquinone compounds.
| No. | Name | Resource | Formula | Classification | Ref. |
|---|---|---|---|---|---|
| 193 | trijuganone A | Salvia trijuga Diels. | C18H14O4 | para-phenanthrenequinone | [103] |
| 194 | bauhinione | Bauhinia variegata L. | C17H16O4 | para-phenanthrenequinone | [104] |
| 195 | ochrone A | Coelogyne ochracea Lindl. | C13H12O4 | para-phenanthrenequinone | [105] |
| 196 | stemanthraquinone | Stemona tuberosa Lour. | C16H14O4 | para-phenanthrenequinone | [106] |
| 197 | dioscoreanone | Dioscorea membranacea Pierre | C16H12O5 | para-phenanthrenequinone | [107] |
| 198 | denbinobin | Dendrobium nobile Lindl. | C16H12O5 | para-phenanthrenequinone | [108] |
| 199 | 7-hydroxy-5,6-dimethoxy-1,4-phenanthrenequinone | Dendrobium moniliforme (L.) Sw. | C16H12O5 | para-phenanthrenequinone | [109] |
| 200 | moniliformin | Fusarium verticillioides (Sacc.) Nirenberg | C16H10O6 | para-phenanthrenequinone | [110] |
| 201 | phenanobiles A | Dendrobium nobile Lindl. | C14H8O5 | para-phenanthrenequinone | [101] |
| 202 | phenanobiles B | Dendrobium nobile Lindl. | C16H13O5 | para-phenanthrenequinone | [101] |
| 203 | phenanobiles C | Dendrobium nobile Lindl. | C14H10O4 | para-phenanthrenequinone | [101] |
| 204 | 6,7-dihydroxy-2-methoxy-1,4-phenanthrenedione | Dioscorea opposita Thunb. | C15H10O5 | para-phenanthrenequinone | [101] |
| 205 | pyranospiranthoquinone | Spiranthes sinensis (Pers.) Ames | C20H18O5 | para-phenanthrenequinone | [14] |
| 206 | ephemeranthoquinone | Flickingeria comata (Bl.) Hawkes. | C15H12O4 | para-phenanthrenequinone | [111] |
| 207 | annoquinone A | Annona montana Macfad. | C15H10O3 | para-phenanthrenequinone | [112] |
| 208 | danshenxinkun C | Salvia miltiorrhiza Bunge | C21H20O4 | para-phenanthrenequinone | [110] |
| 209 | cypripediquinone A | Cypripedium macranthum Sw. | C17H14O5 | o-phenanthrenequinone I | [111] |
| 210 | bulbophyllanthrone | Bulbophyllum odoratissimum (J. E. Sm.) Lindl. | C17H14O6 | o-phenanthrenequinone I | [112] |
| 211 | Sch6 86 31 | Spiromyces sp. | C19H16O4 | o-phenanthrenequinone I | [14] |
| 212 | biruloquinone | Mycosphaerella rubella (Westend.) | C17H10O7 | o-phenanthrenequinone I | [14] |
| 213 | danshenxinkun A | Salvia miltiorrhiza Bunge | C18H16O4 | o-phenanthrenequinone II | [113] |
| 214 | danshenxinkun B | Salvia miltiorrhiza Bunge | C16H12O3 | o-phenanthrenequinone II | [113] |
| 215 | danshenxinkun D | Salvia miltiorrhiza Bunge | C18H16O3 | o-phenanthrenequinone II | [113] |
| 216 | cryptotanshinone | Salvia miltiorrhiza Bunge | C19H20O3 | o-phenanthrenequinone II | [113] |
| 217 | tanshinone I | Salvia miltiorrhiza Bunge | C18H12O3 | o-phenanthrenequinone II | [113] |
| 218 | dihydrotanshinone I | Salvia miltiorrhiza Bunge | C18H14O3 | o-phenanthrenequinone II | [113] |
| 219 | tanshinone IIA | Salvia miltiorrhiza Bunge | C19H18O3 | o-phenanthrenequinone II | [113] |
| 220 | hydroxytanshinone IIA | Salvia miltiorrhiza Bunge | C19H18O4 | o-phenanthrenequinone II | [113] |
| 221 | tanshinone IIB | Salvia miltiorrhiza Bunge | C19H18O4 | o-phenanthrenequinone II | [113] |
| 222 | miltirone | Salvia miltiorrhiza Bunge | C18H17O2 | o-phenanthrenequinone II | [113] |
| 223 | trijuganone B | Salvia trijuga Diels. | C18H16O3 | o-phenanthrenequinone II | [103] |
| 224 | trijuganone C | Salvia trijuga Diels. | C20H20O5 | o-phenanthrenequinone II | [103] |


2.1.4. Anthraquinones
Anthraquinones are the most abundant natural quinones [1]. Anthraquinones include anthraquinone derivatives, their reduction products, oxyanthrone or anthrone, and derivatives of their dimers. In anthraquinones, positions 1, 4, 5, and 8 are referred to as α-positions, positions 2, 3, 6, and 7 are referred to as β-positions, and positions 9 and 10 are referred to as meso-positions. The substituents of anthraquinones include methyl, hydroxymethyl, carboxyl, aldehyde, hydroxyl, and methoxy groups. Compared with benzoquinone and naphthoquinone, anthraquinone substituents contain fewer carbons, generally no more than six carbons, and the complexity and diversity of substituents are not as great as those of benzoquinone and naphthoquinone.
There are two main biosynthetic pathways for anthraquinones in medicinal plants: the polyketide pathway and the mangiferyl/pho-succinyl benzoic acid pathway [114,115,116,117]. The polyketide pathway uses acetyl coenzyme A and malonyl coenzyme A as substrates to generate anthraquinones via polyketide synthase III. The mangiferolic acid/o-succinylbenzoic acid pathway uses isobranchialic acid, α-ketoglutaric acid, and thiamine diphosphate as substrates to synthesize anthraquinones in a series of reactions catalyzed by o-succinylbenzoic acid synthase [118].
Polyketide pathway (top) and mangiferyl/phosuccinobenzoic acid pathway (bottom)

Based on the structure of the parent nucleus, anthraquinones can be categorized into two main groups: monoanthraquinones and dianthraquinones [119]. The vast majority of natural anthraquinones are found in higher plants, fungi, and lichens. Among higher plants, quinones are most abundant in the Rubiaceae Juss., and anthraquinones are more abundant in the Fabaceae Lindl. and Rhamnaceae Juss., Polygonaceae Juss., Zygophyllaceae R. Br., and Liliaceae Juss. Anthraquinones are more abundant in Aspergillus Micheli ex Fries and Penicillium spp. among molds. Twenty-one anthraquinones were found in Pleuropterus multiflorus (Thunb.) Nakai, including four rhodopsin-type anthraquinones, three anthraquinone glycosides, and 14 dianthrone compounds; Seventeen anthraquinones were found in Rheum palmatum L., containing five rhodopsin-anthraquinones, two anthraquinones oxidized, one anthrone, and seven dianthrones; thirteen anthraquinones, including three anthraquinones oxidized and nine anthraquinones, were isolated from the plant Harungana madagascariensis Lam. ex Poir.; ten anthraquinones were isolated and obtained from the plant Galium sinaicum (Delile ex Decne.) Boiss., which contains seven alizarin-type anthraquinones. Nine anthraquinones, including eight anthraquinones (including three anthraquinone glycosides) and one oxidized anthracenol, were identified in the plant Picramnia antidesma Sieber ex Steud.Ten anthraquinones, including three alizarin-type anthraquinones and three anthraquinone oxidizers, were found in Rubia cordifolia L.; Seven anthraquinones, including five rhodopsin-type anthraquinones and two rhodopsin-type anthraquinone glycosides, were found in the Bulbine frutescens (L.) Willd. Seven anthraquinones, including six alizarin-type anthraquinones, were found in the Prismatomeris tetrandra (Roxb.) K. Schum. Six anthraquinones have been found in Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb., and five dianthrones have been found in the Senna alexandrina Milll.
Monoanthraquinones
The vast majority of natural anthraquinones contain hydroxyl groups, and mono-anthracene-nucleated anthraquinones are usually classified into rhodopsin- and chrysophanol-types based on the substitution position of the hydroxyl group [1]. Anthraquinones with hydroxyl groups on both benzene rings belong to the rhodopsin type, such as chrysazin and chrysophorol. Anthraquinones with a hydroxyl group on one benzene ring are of the chrysin type, such as alizarin and digitolutein. Some anthraquinones also exist as glycosides. Table 4 presents the names and molecular formulas of anthraquinone compounds.
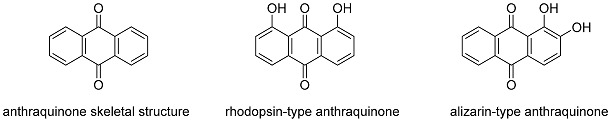
Table 4.
Names and molecular formulas of anthraquinone compounds.
| No. | Name | Resource | Formula | Classification | Ref. |
|---|---|---|---|---|---|
| 225 | chrysazin | Rheum palmatum L. | C14H8O4 | rhodopsin-type anthraquinone | [14] |
| 226 | chrysophanol | Rheum palmatum L. | C15H10O4 | rhodopsin-type anthraquinone | [14] |
| 227 | emodin | Rheum palmatum L. | C15H10O5 | rhodopsin-type anthraquinone | [120] |
| 228 | isochrysophanol | Rheum palmatum L. | C15H12O4 | rhodopsin-type anthraquinone | [14] |
| 229 | Rhein | Rheum palmatum L. | C15H8O6 | rhodopsin-type anthraquinone | [14] |
| 230 | 4-hydroxymethyl chrysazin | Tripterygium wilfordii Hook. f | C15H12O5 | rhodopsin-type anthraquinone | [14] |
| 231 | 1,8-dihydroxy-4-methylanthraquinone | cyanobacterium | C15H10O4 | rhodopsin-type anthraquinone | [121] |
| 232 | monodictyquinone A | Monodictys cerebriformis G. Z. Zhao & T. Y. Zhang | C16H12O5 | rhodopsin-type anthraquinone | [122] |
| 233 | carviolin | Penicillium Link ex Fr. | C16H12O6 | rhodopsin-type anthraquinone | [123] |
| 234 | 1-O-methylemodin | Senna obtusifolia (L.) H. S. Irwin & Barneby. | C16H12O5 | rhodopsin-type anthraquinone | [124] |
| 235 | ω-acetylcarviolin | Zopfiella longicaudata (Ces.) Sacc. | C18H14O7 | rhodopsin-type anthraquinone | [125] |
| 236 | ω-hydroxyemodin | Zopfiella longicaudata (Ces.) Sacc. | C15H10O6 | rhodopsin-type anthraquinone | [46] |
| 237 | lunatin | Curvularia lunata (Wakker) Boedijn | C15H10O6 | rhodopsin-type anthraquinone | [125] |
| 238 | ptilometric acid 6-O-sulfate | Tropiometra afra macrodiscus (Hartlaub) | C18H13NaO10S | rhodopsin-type anthraquinone | [46] |
| 239 | ptilometric acid | Tropiometra afra macrodiscus (Hartlaub) | C18H14O7 | rhodopsin-type anthraquinone | [46] |
| 240 | cassanthraquinone A | Cassia siamea Lam. | C20H14O6 | rhodopsin-type anthraquinone | [126] |
| 241 | ventilanone L | Ventilago denticulata Willd. | C18H14O7 | rhodopsin-type anthraquinone | [127] |
| 242 | ventilanone M | Ventilago denticulata Willd. | C18H16O6 | rhodopsin-type anthraquinone | [127] |
| 243 | 1,8-dihydroxy-3-succinic acid monoethyl ester-6-methylanthraquinone | - | C19H13O8 | rhodopsin-type anthraquinone | [128] |
| 244 | Aloe emodin | Pleuropterus multiflorus (Thunb.) Nakai | C15H10O5 | rhodopsin-type anthraquinone | [44] |
| 245 | emodin methyl ether | Pleuropterus multiflorus (Thunb.) Nakai | C16H12O5 | rhodopsin-type anthraquinone | [44] |
| 246 | ω-hydroxyemodin 8-methyl ether | Pleuropterus multiflorus (Thunb.) Nakai | C16H12O6 | rhodopsin-type anthraquinone | [44] |
| 247 | emodin 8-methyl ether | Pleuropterus multiflorus (Thunb.) Nakai | C16H12O5 | rhodopsin-type anthraquinone | [44] |
| 248 | vismiaquinone C | Vismia martiana Rchb.f. | C21H20O5 | rhodopsin-type anthraquinone | [129] |
| 249 | asparasone A | Aspergillus parasiticus Speare | C18H14O8 | rhodopsin-type anthraquinone | [130] |
| 250 | laurentiquinone A | Vismia laurentii De Wild. | C22H20O7 | rhodopsin-type anthraquinone | [131] |
| 251 | laurenquinone A | Vismia laurentii De Wild. | C22H20O7 | rhodopsin-type anthraquinone | [132] |
| 252 | 3-O-(2-hydroxy-3-methylbut-3-enyl)-emodin | Vismia guineensis (L.) Choisy | C20H18O6 | rhodopsin-type anthraquinone | [133] |
| 253 | 3-O-(2-methoxy-3-methylbut-3-enyl)-emodin | Vismia guineensis (L.) Choisy | C21H20O6 | rhodopsin-type anthraquinone | [133] |
| 254 | 3-O-(E-3-hydroxymethylbut-2-enyl)-emodin | Vismia guineensis (L.) Choisy | C20H18O6 | rhodopsin-type anthraquinone | [133] |
| 255 | 3-O-(3-hydroxymethyl-4-hydroxybut-2-enyl)-emodin | Vismia guineensis (L.) Choisy | C20H18O7 | rhodopsin-type anthraquinone | [133] |
| 256 | pruniflorone J | Cratoxylum formosum (Jack) Dyer | C25H26O6 | rhodopsin-type anthraquinone | [134] |
| 257 | araliorhamnone A | Araliorhamnus vaginata H.Perrier | C18H12O8 | rhodopsin-type anthraquinone | [135] |
| 258 | laurenquinone B | Vismia laurentii De Wild. | C22H18O7 | rhodopsin-type anthraquinone | [132] |
| 259 | laurentiquinone C | Vismia laurentii De Wild. | C24H20O9 | rhodopsin-type anthraquinone | [136] |
| 260 | ploiariquinone A | Ploiarium alternifolium (Szyszył.) Melch. | C25H24O5 | rhodopsin-type anthraquinone | [137] |
| 261 | 4′-demethylknipholone | Bulbine capitata Poelln. | C23H16O8 | rhodopsin-type anthraquinone | [138] |
| 262 | knipholone | Kniphofia foliosa Hochst. | C24H18O8 | rhodopsin-type anthraquinone | [139] |
| 263 | isoknipholone | Kniphofia foliosa Hochst. | C24H18O8 | rhodopsin-type anthraquinone | [140] |
| 264 | knipholone-6-methyl ether | Bulbine capitata Poelln. | C25H20O8 | rhodopsin-type anthraquinone | [68] |
| 265 | gaboroquinone A | Bulbine frutescens (L.) Willd. | C24H18O9 | rhodopsin-type anthraquinone | [141] |
| 266 | gaboroquinone B | Bulbine frutescens (L.) Willd. | C24H18O9 | rhodopsin-type anthraquinone | [141] |
| 267 | sodium ent-knipholone 6′-O-sulfate | Bulbine frutescens (L.) Willd. | C24H17NaO11S | rhodopsin-type anthraquinone | [142] |
| 268 | sodium 4′-O-demethylknipholone 6′-O-sulfate | Bulbine frutescens (L.) Willd. | C23H15NaO11S | rhodopsin-type anthraquinone | [142] |
| 269 | sodium isoknipholone 6-O-sulfate | Bulbine frutescens (L.) Willd. | C24H17NaO11S | rhodopsin-type anthraquinone | [142] |
| 270 | 11-hydroxysulfurmycinone | Streptomyces sp. | C23H20O10 | rhodopsin-type anthraquinone | [143] |
| 271 | blanchaquinone | Streptomyces sp. | C22H20O7 | rhodopsin-type anthraquinone | [143] |
| 272 | brasiliquinone D | Nocardia brasiliensis Lindenberg & Cohn | C28H29NO8 | rhodopsin-type anthraquinone | [144] |
| 273 | cratoxyarborequinone A | Cratoxylum sumatranum (Jack) Blume | C44H46O9 | rhodopsin-type anthraquinone | [144] |
| 274 | cratoxyarborequinone B | Cratoxylum sumatranum(Jack) Blume | C49H54O9 | rhodopsin-type anthraquinone | [145] |
| 275 | floribundone | Senna septemtrionalis (Viv.) H. S. Irwin & Barneby. | C32H22O10 | rhodopsin-type anthraquinone | [146] |
| 276 | phaeosphenone | Phaeosphaeria sp. | C30H26O10 | rhodopsin-type anthraquinone | [147] |
| 277 | R-(-)-skyrin-6-O-β-xylopyranoside | Hypericum perforatum L. | C35H26O14 | rhodopsin-type anthraquinone | [148] |
| 278 | 8-O-β-D-glucopyranosyl-1,1′,8′-trihydroxy- 3,3′-dimethyl-2,7′-bianthraquinone |
Eremurus chinensis O.Fedtsch. | C36H28O13 | rhodopsin-type anthraquinone | [149] |
| 279 | floribundiquinone A | Berchemia polyphylla var. leioclada (Hand.-Mazz.) Hand.-Mazz. | C32H26O10 | rhodopsin-type anthraquinone | [150] |
| 280 | floribundiquinone B | Berchemia polyphylla var. leioclada (Hand.-Mazz.) Hand.-Mazz. | C32H26O10 | rhodopsin-type anthraquinone | [150] |
| 281 | floribundiquinone C | Berchemia polyphylla var. leioclada (Hand.-Mazz.) Hand.-Mazz. | C31H24O9 | rhodopsin-type anthraquinone | [150] |
| 282 | floribundiquinone D | Berchemia polyphylla var. leioclada (Hand.-Mazz.) Hand.-Mazz. | C32H26O10 | rhodopsin-type anthraquinone | [150] |
| 283 | anhydrophlegmacin-9′,10′-quinone | Cassia torosa Cav. | C32H26O10 | rhodopsin-type anthraquinone | [151] |
| 284 | isosengulone | Senna multiglandulosa (Jacq.) H.S.Irwin & Barneby. | C32H22O10 | rhodopsin-type anthraquinone | [152] |
| 285 | icterinoidin A | Dermocybe icterinoides (Peck) Hesler & A.H. Sm. | C30H22O10 | rhodopsin-type anthraquinone | [153] |
| 286 | icterinoidin B | Dermocybe icterinoides (Peck) Hesler & A.H. Sm. | C30H22O10 | rhodopsin-type anthraquinone | [153] |
| 287 | febrifuquinoe | Psorospermum febrifugum Spach. | C40H38O10 | rhodopsin-type anthraquinone | [154] |
| 288 | chaetomanone | Chaetomium globosum Kunze | C31H24O12 | rhodopsin-type anthraquinone | [155] |
| 289 | bulbineloneside A | Bulbinella floribunda (Aiton) T.Durand & Schinz. | C30H28O13 | rhodopsin-type anthraquinone | [156] |
| 290 | bulbineloneside B | Bulbinella floribunda (Aiton) T.Durand & Schinz. | C28H24O12 | rhodopsin-type anthraquinone | [156] |
| 291 | bulbineloneside C | Bulbinella floribunda (Aiton) T.Durand & Schinz. | C28H24O12 | rhodopsin-type anthraquinone | [156] |
| 292 | bulbineloneside D | Bulbinella floribunda (Aiton) T.Durand & Schinz. | C29H26O13 | rhodopsin-type anthraquinone | [156] |
| 293 | alizarin | Rubia cordifolial L. | C14H8O4 | alizarin-type anthraquinone | [14] |
| 294 | alizarin 2-methyl ether | Rubia cordifolia L. | C15H10O4 | alizarin-type anthraquinone | [14] |
| 295 | digitolutein | Ventilago goughii Gamble | C16H14O4 | alizarin-type anthraquinone | [14] |
| 296 | 6-ethylalizarin | Galium spurium L. | C15H12O4 | Alizarin-type anthraquinone | [14] |
| 297 | altersolanol A | Stemphylium botryosum var. lactucum | C16H13O7 | alizarin-type anthraquinone | [14] |
| 298 | rubiawallin A | Rubia wallichiana Decne | C16H12O5 | alizarin-type anthraquinone | [157] |
| 299 | 1,4-dihydroxy-2,3-dimethoxyanthraquinone | Hedyotis herbacea L. | C16H12O6 | alizarin-type anthraquinone | [158] |
| 300 | 2-methoxy-1,3,6-trihydroxyanthraquinone | Morinda citrifolia L. | C15H10O6 | alizarin-type anthraquinone | [159] |
| 301 | 6-methylanthragallol 3-methyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C16H12O5 | alizarin-type anthraquinone | [160] |
| 302 | 7-methylanthragallol 1,3-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C17H14O5 | alizarin-type anthraquinone | [160] |
| 303 | 7-methylanthragallol 2-methyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C16H12O5 | alizarin-type anthraquinone | [160] |
| 304 | 7-formylanthragallol 1,3-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C17H12O6 | alizarin-type anthraquinone | [160] |
| 305 | 8-hydroxy-6,7-dimethoxy-2-methyl-9,10-anthraquinone | Prismatomeris tetrandra (Roxb.) K. Schum. | C17H14O5 | alizarin-type anthraquinone | [161] |
| 306 | 1,3-dihydroxy-5,6-dimethoxy-2-methyl-9,10-anthraquinone | Prismatomeris tetrandra (Roxb.) K. Schum. | C17H14O6 | alizarin-type anthraquinone | [162] |
| 307 | 3-dihydroxy-1,5,6-trimethoxy-2-methyl-9,10-anthraquinone | Prismatomeris tetrandra (Roxb.) K. Schum. | C18H16O6 | alizarin-type anthraquinone | [162] |
| 308 | 6-hydroxy-1, 2, 3-trimethoxy-7-methylanthracene-9, 10-dione | Prismatomeris tetrandra (Roxb.) K. Schum. | C18H16O6 | alizarin-type anthraquinone | [162] |
| 309 | 6-(hydroxymethyl)-1, 2,3-trimethoxyanthracene-9, 10-dione | Prismatomeris tetrandra (Roxb.) K. Schum. | C18H16O6 | alizarin-type anthraquinone | [163] |
| 310 | 7-hydroxy-6-(hydroxymethyl)-1, 2-dimethoxyanthracene-9,10-dione | Prismatomeris tetrandra (Roxb.) K. Schum. | C17H14O6 | alizarin-type anthraquinone | [163] |
| 311 | 8-hydroxyanthragallol 2,3-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C16H12O6 | alizarin-type anthraquinone | [160] |
| 312 | copareolatin 5,7-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C17H14O6 | alizarin-type anthraquinone | [160] |
| 313 | copareolatin 6,7-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C17H14O6 | alizarin-type anthraquinone | [160] |
| 314 | 5,15-dimethylmorindol | Morinda citrifolia L. | C17H14O6 | alizarin-type anthraquinone | [164] |
| 315 | 1,5,15-tri-O-methylmorindol | Morinda citrifolia L. | C18H16O6 | alizarin-type anthraquinone | [165] |
| 316 | (2R)-6-hydroxy-7-methoxy-dehydroiso-α-lapachone | Spermacoce alata Aubl. | C15H10O6 | alizarin-type anthraquinone | [81] |
| 317 | ventilanone N | Ventilago denticulata Willd. | C16H12O6 | alizarin-type anthraquinone | [127] |
| 318 | 3,4,8-trihydroxy-1-methylanthra-9,10-quinone-2-carboxylic acid methyl ester | Eleutherine plicata Herb. | C17H12O7 | alizarin-type anthraquinone | [166] |
| 319 | 4,8-dihydroxy-3-methoxy-1-methylanthra-9,10-quinone-2-carboxylic acid methyl ester | Eleutherine plicata Herb. | C18H14O7 | alizarin-type anthraquinone | [167] |
| 320 | 2-hydroxyemodin 1-methyl ether | Senna tora (L.) Roxb. | C16H12O6 | alizarin-type anthraquinone | [168] |
| 321 | araliorhamnone B | Araliorhamnus vaginata H.Perrier | C19H14O8 | alizarin-type anthraquinone | [135] |
| 322 | bostrycoidin | Fusarium solani (Mart.) Sacc. | C15H11NO5 | alizarin-type anthraquinone | [169] |
| 323 | 6-methoxylucidinω-ethyl ether | Prismatomeris tetrandra (Roxb.) K. Schum. | C18H16O6 | other | [161] |
| 324 | guinizarin | Galium sinaicum (Delile ex Decne.) Boiss. | C14H8O4 | other | [14] |
| 325 | pachybasin | Rheum moorcroftianum Royle | C15H10O3 | other | [14] |
| 326 | 2-hydroxy-3-methyl-anthraquinone | Hedyotis diffusa Willd. | C15H10O3 | other | [14] |
| 327 | tectoquinone | Acatypha india L. | C15H10O2 | other | [14] |
| 328 | 1-hydroxyanthraquinone | Morinda officinalis How | C15H10O2 | other | [14] |
| 329 | 2-methylol anthraquinone | Morinda parvifolia Bartl. ex DC. | C15H10O3 | other | [14] |
| 330 | 5-hydroxy-2-methyl-anthraquinone | Rubia tinctorum Linn. | C15H10O3 | other | [14] |
| 331 | barleriaquinone I | Barleria buxifolia L. | C15H10O3 | other | [14] |
| 332 | barleriaquinone II | Barleria buxifolia L. | C16H10O5 | other | [14] |
| 333 | 2-methylquinizarin | Galium sinaicum (Delile ex Decne.) Boiss. | C15H12O4 | other | [14] |
| 334 | damnacanthol | Damnacanthus major Siebold & Zucc. | C16H14O5 | other | [14] |
| 335 | ziganein | Salvia przewalskii Maxim. | C15H10O4 | other | [14] |
| 336 | 1-amino-2,4-dibromoanthraquinone | - | C14H7Br2NO2 | other | [14] |
| 337 | munjistin methyl ester | Salvia miltiorrhiza Bunge | C16H10O6 | other | [116] |
| 338 | fridamycin E | Spiroplectammina parvula Schwager | C20H20O7 | other | [14] |
| 339 | soranjidiol | Morinda elliptica (Hook.f.) Ridl. | C15H10O4 | other | [14] |
| 340 | ω-hydroxy-phomarin | Digitalis cariensis Boiss. ex Jaub. & Spach | C15H10O5 | other | [14] |
| 341 | rubiawallin C | Rubia wallichiana Decne | C16H10O5 | other | [157] |
| 342 | 2-formyl-1-hydroxyanthraquinone | Morinda elliptica (Hook.f.) Ridl. | C15H8O4 | other | [170] |
| 343 | sterequinone F | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C19H16O3 | other | [170] |
| 344 | sterequinone H | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C19H18O3 | other | [171] |
| 345 | 1-acetoxy-3-methoxy-9,10-anthraquinone | Rubia cordifolia L. | C17H12O5 | other | [172] |
| 346 | ophiohayatone C | Ophiorrhiza hayatana Ohwi | C15H8O5 | other | [173] |
| 347 | munjistin-1-O-methyl ether | Rhynchotechum vestitum Wall. ex Clatke | C16H10O6 | other | [174] |
| 348 | 1,3-dimethoxy-2-methoxymethylanthraquinone | Coussarea macrophylla (Mart.) Müll.Arg. | C18H16O5 | other | [175] |
| 349 | 1-hydroxy-2-hydroxymethyl-3-methoxyanthraquinone | Rubia wallichiana Decne | C16H12O5 | other | [157] |
| 350 | 2-n-butoxymethyl-1,3-dihydroxyanthraquinone | Morinda angustifolia Roxb. | C19H18O5 | other | [176] |
| 351 | 1-methoxy-3-hydroxy-2-carbomethoxy-9,10-anthraquinone | Saprosma scortechinii King & Gamble | C17H12O6 | other | [177] |
| 352 | rubiawallin B | Rubia wallichiana Decne | C16H12O4 | other | [157] |
| 353 | 1,7-dihydroxy-2-hydroxymethyl-9,10-anthraquinone | Hemiboea subcapitata Clarke | C15H10O5 | other | [178] |
| 354 | sterequinone G | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C20H18O4 | other | [171] |
| 355 | anthrakunthone | Stereospermum kunthianum Cham. | C19H16O4 | other | [62] |
| 356 | 3,6-dihydroxy-2-hydroxymethyl-9,10-anthraquinone | Knoxia valerianoides Thorel ex Pitard | C15H10O5 | other | [179] |
| 357 | ophiohayatone A | Ophiorrhiza hayatana Ohwi | C16H12O5 | other | [173] |
| 358 | pustuline | Heterophyllaea pustulata Hook.f. | C16H12O4 | other | [180] |
| 359 | 6-hydroxyxanthopurpurin | Galium sinaicum (Delile ex Decne.) Boiss. | C14H8O5 | other | [160] |
| 360 | 3-methoxycarbonyl-1,5-dihydroxyanthraquinone | Engelhardia roxburghiana Wall. | C16H10O6 | other | [181] |
| 361 | 1,3,6-trihydroxy-2-methoxymethyl-9,10-anthraquinone | Saprosma scortechinii King & Gamble | C16H12O6 | other | [177] |
| 362 | 1-methoxy-3,6-dihydroxy-2-hydroxymethyl-9,10-anthra-quinone | Saprosma scortechinii King & Gamble | C16H12O6 | other | [177] |
| 363 | aloesaponarin I | Aloe camperi Schweinf. | C17H12O6 | other | [182] |
| 364 | aloesaponarin I 3-methyl ether | Aloe camperi Schweinf. | C18H14O6 | other | [183] |
| 365 | alatinone | Cassia alata L. | C15H10O5 | other | [184] |
| 366 | przewalskinone B | Cassia italica Mill. | C16H12O5 | other | [185] |
| 367 | 2-Methyl-1-nitroanthraquinone | - | C15H9NO4 | other | [186] |
| 368 | 3,8-dihydroxy-6-methoxy-1-methylanthra-9,10-quinone-2-carboxylic acid methyl ester | Gladiolus gandavensis Van Houtte | C18H14O7 | other | [187] |
| 369 | ventilanone O | Ventilago denticulata Willd. | C16H12O6 | other | [127] |
| 370 | scorpinone | Amorosia littoralis Mantle & D.Hawksw. B.R. | C16H13NO4 | other | [188] |
| 371 | 1-amino-2-methylanthraquinone | - | C15H11NO2 | other | [189] |
| 372 | dielsiquinone | Guatteria dielsiana R.E.Fr. | C15H11NO4 | other | [190] |
| 373 | marcanine B | Goniothalamus marcanii Craib | C16H13NO4 | other | [129] |
| 374 | marcanine C | Goniothalamus marcanii Craib | C16H13NO5 | other | [123] |
| 375 | marcanine D | Goniothalamus marcanii Craib | C15H11NO5 | other | [129] |
| 376 | marcanine E | Goniothalamus marcanii Craib | C16H13NO5 | other | [129] |
| 377 | araliorhamnone C | Araliorhamnus vaginata H.Perrier | C17H10O7 | other | [135] |
| 378 | laurentiquinone B | Vismia laurentii De Wild. | C22H18O7 | other | [136] |
| 379 | sterequinone I | Stereospermum personatum (Hassk.) Chatterjee | C20H18O4 | other | [171] |
| 380 | sterequinone A | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C19H14O2 | other | [93] |
| 381 | sterequinone D | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C20H16O3 | other | [93] |
| 382 | 2-hydroxymethyl-10-hydroxy-1,4-anthraquinone | Hedyotis herbacea Lour. | C15H10O4 | other | [190] |
| 383 | 2,3-dimethoxy-9-hydroxy-1,4-anthraquinone | Hedyotis herbacea Lour. | C16H12O5 | other | [163] |
| 384 | 9,10-dimethoxy-2-methylanthra-1,4-quinone | - | C17H14O4 | other | [191] |
| 385 | physcion | Rheum palmatum L. | C16H12O5 | other | [192] |
| 386 | 2-aminoanthraquinone | - | C14H9NO2 | other | [193] |
| 387 | kengaquinone | Harungana madagascariensis Lam. ex Poir. | C25H26O5 | other | [194] |
| 388 | newbouldiaquinone | Newbouldia laevis (P.Beauv.) Seem. ex Bureau | C25H14O5 | other | [195] |
| 389 | newbouldiaquinone A | Newbouldia laevis (P.Beauv.) Seem. ex Bureau | C25H14O6 | other | [196] |
| 390 | tectograndone | Tectona grandis L. f. | C30H20O10 | other | [197] |
| 391 | (S)-5,5′-bisoranjidiol | Heterophyllaea pustulata Hook.f. | C30H18O8 | other | [180] |
| 392 | presengulone | Senna sophera (L.) Roxb. | C32H26O10 | other | [198] |
| 393 | scutianthraquinone A | Scutia myrtina (L.) Roxb. | C39H32O13 | other | [199] |
| 394 | scutianthraquinone B | Scutia myrtina (L.) Roxb. | C38H30O13 | other | [199] |
| 395 | scutianthraquinone C | Scutia myrtina (L.) Roxb. | C34H24O12 | other | [199] |
| 396 | scutianthraquinone D | Scutia myrtina (L.) Roxb. | C61H53O20 | other | [199] |
| 397 | mitoxantrone | - | C22H28N4O6 | Other | [200] |
| 398 | sulfemodin 8-O-β-D-glucoside | Rheum palmatum L. | C21H20O13S | anthraquinone glycosides of rhodopsin type | [201] |
| 399 | 1-methyl-8-hydroxyl-9,10-anthraquinone-3-O-β-D-glucopyranoside | Rheum palmatum L. | C22H19O11 | anthraquinone glycosides of rhodopsin type | [202] |
| 400 | 4′-O-demethylknipholone-4′-O-β-D-glucoside | Bulbine frutescens (L.) Willd. | C29H26O13 | anthraquinone glycosides of rhodopsin type | [142] |
| 401 | sodium-4′-O-demethylknipholone-4′-β-D-gluc-opyranoside 6′-O-sulfate | Bulbine frutescens (L.) Willd. | C29H25NaO16S | anthraquinone glycosides of rhodopsin type | [142] |
| 402 | aloin | Aloe vera (L.) Burm.f. | C21H22O9 | anthraquinone glycosides of rhodopsin type | [203] |
| 403 | emodin-1-O-β-gentiobioside | Cassia obtusifolia | C27H30O15 | anthraquinone glycosides of rhodopsin type | [204] |
| 404 | knipholone-8-β-D-gentiobioside | Bulbine narcissifolia | C36H38O18 | anthraquinone glycosides of rhodopsin type | [205] |
| 405 | bulbineloneside E | Bulbinella floribunda | C34H34O17 | anthraquinone glycosides of rhodopsin type | [156] |
| 406 | emodin-8-O-β-D-glucopyranoside | Pleuropterus multiflorus (Thunb.) Nakai | C21H20O10 | anthraquinone glucoside | [44] |
| 407 | emodin methyl ether-8-O-β-D-glucopyranoside | Pleuropterus multiflorus (Thunb.) Nakai | C22H22O10 | anthraquinone glucoside | [44] |
| 408 | polygonum multiflorum ethyl | Pleuropterus multiflorus (Thunb.) Nakai | C21H22O9 | anthraquinone glucoside | [44] |
| 409 | halawanone C | Streptomycete | C21H20O7 | anthraquinone glucoside | [64] |
| 410 | nepalenside A | Rumex nepalensis Spreng. | C21H22O11 | anthraquinone glucoside | [206] |
| 411 | nepalenside B | Rumex nepalensis Spreng. | C21H22O11 | anthraquinone glucoside | [206] |
| 412 | rubiadin-3-O-β-glucoside | Rhynchotechum vestitum Wall. ex C. B. Clarke | C21H20O9 | anthraquinone glucoside | [174] |
| 413 | lucidin-3-O-β-glucoside | Rhynchotechum vestitum Wall. ex C. B. Clarke | C21H20O10 | anthraquinone glucoside | [174] |
| 414 | lasianthuoside A | Lasianthus acuminatissimus Miq. | C22H22O10 | anthraquinone glucoside | [207] |
| 415 | lasianthuoside B | Lasianthus acuminatissimus Miq. | C23H24O10 | anthraquinone glucoside | [207] |
| 416 | lasianthuoside C | Lasianthus acuminatissimus Miq. | C28H32O14 | anthraquinone glucoside | [208] |
| 417 | putorinoside A | Putoria calabrica Pers. | C22H22O12 | anthraquinone glucoside | [209] |
| 418 | putorinoside B | Putoria calabrica Pers. | C22H22O11 | anthraquinone glucoside | [209] |
| 419 | 1,3-dihydroxy-2-carbomethoxy-9,10-anthraquinone3-O-β-primeveroside | Saprosma scortechinii King & Gamble | C27H28O15 | anthraquinone glucoside | [177] |
| 420 | 1.3,6-trihydroxy-2-hydroxymethyl-9,10-anthraquinone 3-O-β-primeveroside |
Saprosma scortechinii King & Gamble |
C26H28O15 | anthraquinone glucoside | [177] |
| 421 | emodin-6-O-β-D-glucopyranoside | Reynoutria japonica Houtt. | C21H20O10 | anthraquinone glucoside | [210] |
Anthraquinones, in a broad sense, include anthraquinone derivatives and their products with different degrees of reduction, such as oxyanthrone and anthrone. The reduction of anthraquinone in an acidic environment produces anthranol and its reciprocal isomer, anthrone. The hydroxyl derivatives of anthranol (or anthrone) often co-exist with the corresponding hydroxyl anthraquinone in plants in either the free or bound state. Table 5 presents the names and molecular formulas of oxanthrol and anthrone compounds.
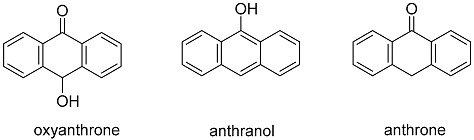
Table 5.
Names and molecular formulas of oxanthrol and anthrone compounds.
| No. | Name | Resource | Formula | Classification | Ref. |
|---|---|---|---|---|---|
| 422 | rubiasin A | Rubia cordifolia L. | C15H16O2 | oxyanthrone | [211] |
| 423 | rubiasin B | Rubia cordifolia L. | C15H16O2 | oxyanthrone | [211] |
| 424 | rubiasin C | Rubia cordifolia L. | C15H16O2 | oxyanthrone | [211] |
| 425 | 1-oxo-4(S),9-dihydroxy-8-methoxy-6-hydroxymethyl-1,2,3,4-tetrahydroanthracene | Eremurus chinensis O.Fedtsch. | C16H16O5 | oxyanthrone | [149] |
| 426 | aloesaponol III-8-methyl ether | Eremurus persicus (Jaub. & Spach) Boiss. | C16H16O4 | oxyanthrone | [212] |
| 427 | kenganthranol A | Harungana madagascariensis Lam. ex Poir. | C30H36O5 | oxyanthrone | [194] |
| 428 | kenganthranol B |
Harungana madagascariensis Lam. ex Poir. |
C25H28O5 | oxyanthrone | [194] |
| 429 | kenganthranol C |
Harungana madagascariensis Lam. ex Poir. |
C26H30O6 | oxyanthrone | [194] |
| 430 | 10-hydroxycascaroside C | Rheum australe D. Don | C27H32O14 | oxyanthrone glycoside | [213] |
| 431 | 10-hydroxycascaroside D | Rheum australe D. Don | C27H32O14 | oxyanthrone glycoside | [213] |
| 432 | mayoside | Mycobacterium microti | C26H24O11 | oxyanthrone glycoside | [214] |
| 433 | mayoside B | Mycobacterium microti | C26H24O11 | oxyanthrone glycoside | [214] |
| 434 | mayoside C | Picramnia teapensis Tul. | C33H34O16 | oxyanthrone glycoside | [215] |
| 435 | mayoside E | Picramnia latifolia Tul. | C27H24O9 | oxyanthrone glycoside | [216] |
| 436 | rubanthrone A | Rubus ulmifolius Schott | C17H14O10 | anthrone | [217] |
| 437 | rubanthrone B | Rubus ulmifolius Schott | C17H16O9 | anthrone | [217] |
| 438 | rubanthrone C | Rubus ulmifolius Schott | C16H12O10 | anthrone | [217] |
| 439 | knipholone anthrone | Kniphofia foliosa Hochst. | C24H20O7 | anthrone | [218] |
| 440 | isoknipholone anthrone | Kniphofia foliosa Hochst. | C24H20O7 | anthrone | [218] |
| 441 | harunganol A | Harungana madagascariensis Lam. ex Poir. | C25H28O4 | anthrone | [219] |
| 442 | harunganol B | Harungana madagascariensis Lam. ex Poir. | C30H36O4 | anthrone | [219] |
| 443 | harungin anthrone | Harungana madagascariensis Lam. ex Poir. | C30H36O4 | anthrone | [194] |
| 444 | bazouanthrone | Harungana madagascariensis Lam. ex Poir. | C30H36O5 | anthrone | [194] |
| 445 | harunmadagascarin A | Harungana madagascariensis Lam. ex Poir. | C30H34O4 | anthrone | [194] |
| 446 | harunmadagascarin B | Harungana madagascariensis Lam. ex Poir. | C35H42O4 | anthrone | [194] |
| 447 | harunmadagascarin C | Harungana madagascariensis Lam. ex Poir. | C30H36O4 | anthrone | [220] |
| 448 | harunmadagascarin D | Harungana madagascariensis Lam. ex Poir. | C30H36O5 | anthrone | [220] |
| 449 | kenganthranol D | Harungana madagascariensis Lam. ex Poir. | C30H32O6 | anthrone | [220] |
| 450 | abyquinone C | Bulbine abyssinica A.Rich. | C30H24O8 | anthrone | [221] |
| 451 | (R)-prechrysophanol | Streptomyces Waksman & Henrici | C15H14O4 | anthrone | [222] |
| 452 | torosachrysone | Dermocybe splendida E. Horak | C16H16O5 | anthrone | [223] |
| 453 | atrochrysone | Aspergillus oryzae (Ahlburg) Cohn | C15H14O5 | anthrone | [224] |
| 454 | aloe barbendol | Aloe vera (L.) Burm. f. | C15H14O4 | anthrone | [225] |
| 455 | acetyltorosachrysone | Psorospermum glaberrimum Hochr. | C18H18O6 | anthrone | [226] |
| 456 | vismione H | Psorospermum glaberrimum Hochr. | C22H24O6 | anthrone | [227] |
| 457 | vismione D | Vismia orientalis (Engl.) Byng & Christenh. | C25H30O5 | anthrone | [228] |
| 458 | vismione L | Psorospermum aurantiacum Engl. | C25H30O5 | anthrone | [229] |
| 459 | vismione M | Psorospermum aurantiacum Engl | C26H32O5 | anthrone | [229] |
| 460 | asperflavin | Microsporum sp. | C21H24O9 | anthrone | [230] |
| 461 | 5-hydroxyaloin A | Aloe nobilis A.Berger | C21H22O10 | anthrone glycoside | [231] |
| 462 | 5-hydroxyaloin A 6′-O-acetate | Aloe nobilis A.Berger | C23H24O11 | anthrone glycoside | [231] |
| 463 | picramnioside A | Picramnia antidesma Sieber ex Steud. | C27H24O10 | anthrone glycoside | [232] |
| 464 | picramnioside B | Picramnia antidesma Sieber ex Steud. | C22H22O10 | anthrone glycoside | [232] |
| 465 | picramnioside C | Picramnia antidesma Sieber ex Steud. | C22H22O10 | anthrone glycoside | [232] |
| 466 | 10-epi-uveoside | Picramnia antidesma Sieber ex Steud. | C27H24O9 | anthrone glycoside | [233] |
| 467 | uveoside | Picramnia antidesma Sieber ex Steud. | C27H24O9 | anthrone glycoside | [233] |
| 468 | microstigmin A | Aloe microstigma Salm-Dyck | C30H28O13 | anthrone glycoside | [234] |
| 469 | microdontin A | Aloe microdonta Salm-Dyck | C30H28O11 | anthrone glycoside | [234] |
| 470 | microdontin B | Aloe microdonta Salm-Dyck | C30H28O13 | anthrone glycoside | [235] |
| 471 | cascaroside E | Rhamnus purshiana DC. | C27H32O14 | anthrone glycoside | [236] |
| 472 | cascaroside F | Rhamnus purshiana DC. | C27H32O14 | anthrone glycoside | [236] |
| 473 | 10R-chrysaloin 1-O-β-D-glucopyranoside | Rheum emodi D. Don | C27H32O13 | anthrone glycoside | [213] |
| 474 | isofoliosone | Bulbine capitata Poelln. | C24H20O8 | anthrone glycoside | [138] |
| 475 | picramnioside D | Picramnia teapensis Tul. | C26H24O10 | anthrone glycoside | [237] |
| 476 | picramnioside E | Picramnia teapensis Tul. | C26H24O10 | anthrone glycoside | [237] |
| 477 | picramnioside F | Picramnia teapensis Tul. | C33H34O15 | anthrone glycoside | [215] |
| 478 | picramniosdie G | Picramnia latifolia Tul. | C27H24O8 | anthrone glycoside | [216] |
| 479 | picramnioside H | Picramnia latifolia Tul. | C27H24O8 | anthrone glycoside | [216] |
| 480 | mayoside D | Picramnia latifolia Tul. | C27H24O9 | anthrone glycoside | [216] |










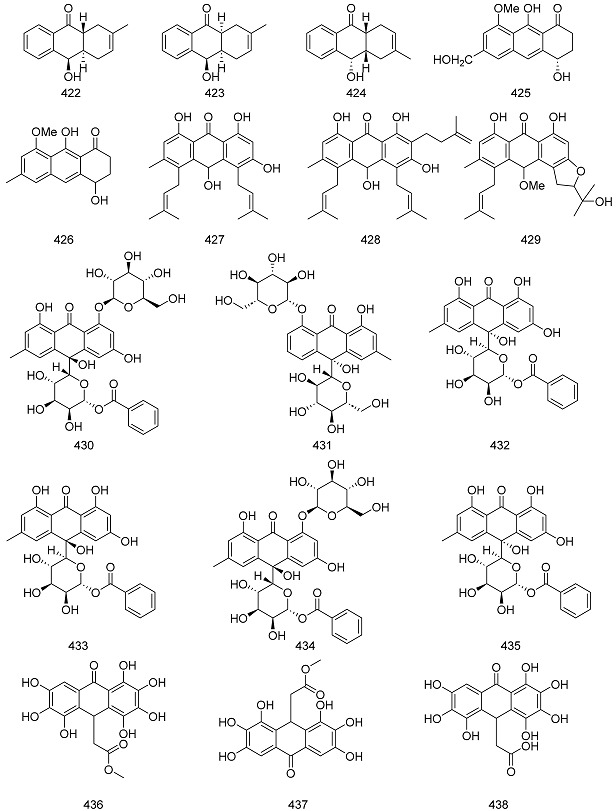
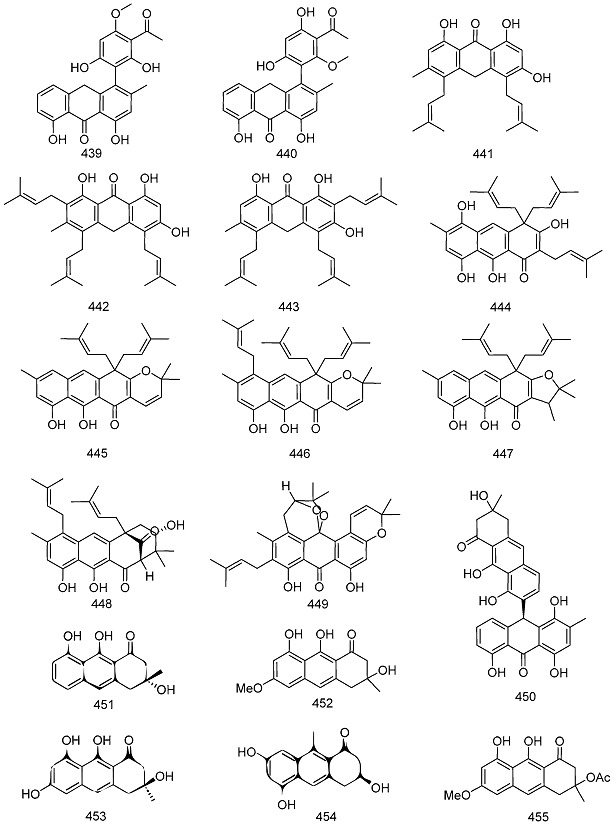
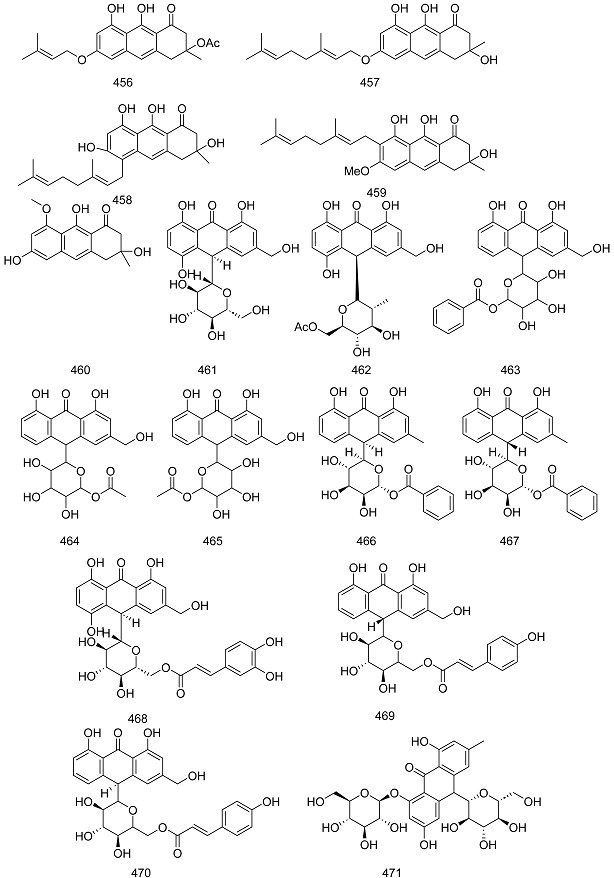
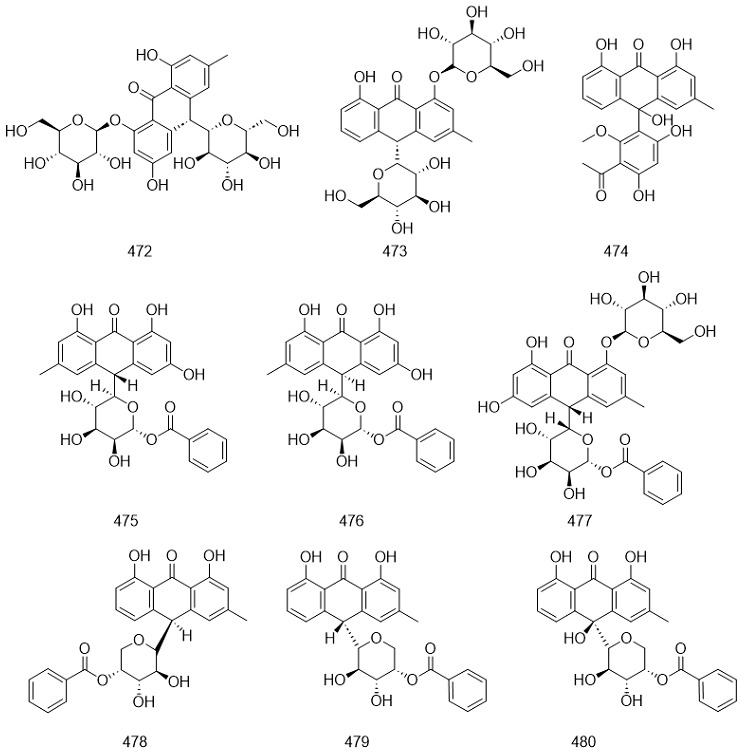
Dithranones
To date, about 63 species of dianthrones have been reported. These dianthrones can be classified into eight types based on their aglycone models. Type I compounds are emodin (C10→C10) emodin linked dianthrones, type II compounds are emodin (C10→C10) physcion linked dianthrones, type III are physcion (C10→C10) physcion linked dianthrones, type IV compounds are aloe-emodin (C10→C10) aloe-emodin linked dianthrones, type V compounds are rhein (C10→C10) rhein linked dianthrones, type VI compounds are rhein (C10→C10) aloe-emodin linked dianthrones, type VII compounds are chrysophanol (C10→C10) chrysophanol linked dianthrones and type VIII compounds are emodin (C10→C10) chrysophanol linked dianthrones. There are different kinds of substituent groups in these dianthrones, such as glycosylation, hydroxyl, isopentene, and malonyl groups. Table 6 introduces the names and molecular formulas of dianthrone compounds.
Table 6.
Names and molecular formulas of dianthrone compounds.
| No. | Name | Resource | Formula | Type | Ref. |
|---|---|---|---|---|---|
| 481 | polygonumnolide C1 | Pleuropterus multiflorus (Thunb.) Nakai | C36H32O13 | type I | [238] |
| 482 | polygonumnolide C2 | Pleuropterus multiflorus (Thunb.) Nakai | C36H32O13 | type I | [238] |
| 483 | polygonumnolide C3 | Pleuropterus multiflorus (Thunb.) Nakai | C36H32O13 | type I | [238] |
| 484 | polygonumnolide C4 | Pleuropterus multiflorus (Thunb.) Nakai | C36H32O13 | type I | [238] |
| 485 | trans-emodin dianthrones | Pleuropterus multiflorus (Thunb.) Nakai | C30H22O8 | type I | [238] |
| 486 | cis-emodin dianthrones | Pleuropterus multiflorus (Thunb.) Nakai | C30H22O8 | type I | [238] |
| 487 | (+)-crinemodin-rhodoptilometrin dianthrone |
Himerometra magnipinna AH Clark | C35H32O8 | type I | [239] |
| 488 | 7,7′-dichlorohypericin | Heterodermia obscurata (Nyl.) Trevis. | C30H14Cl2O8 | type I | [240] |
| 489 | nephrolaevigatin A | Nephroma laevigatum Ach. | C30H20Cl2O8 | type I | [241] |
| 490 | nephrolaevigatin B | Nephroma laevigatum Ach. | C30H20ClO8 | type I | [241] |
| 491 | bioanthrone 1 | Vismia guineensis (L.) Choisy | C50H54O8 | type I | [242] |
| 492 | flavoobscurin B | Heterodermia obscurata (Nyl.) Trevis. | C30H19Cl4O8 | type I | [241] |
| 493 | 8,8′-dihydroxy-1,1′,3,3′-tetramethoxy-6,6′-dimethyl-10,10′-dianthrone | Aspergillus wentii Wehmer | C34H30O8 | type I | [243] |
| 494 | hypericin | Hypericum monogynum L. | C30H16O8 | type I | [244] |
| 495 | pseudohypericin | Hypericum monogynum L. | C30H16O9 | type I | [244] |
| 496 | neobulgarone E | Limonium tubiflorum (Delile) Kuntze | C32H24Cl2O8 | type I | [245] |
| 497 | polygonumnolide A1 | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [246] |
| 498 | polygonumnolide A2 | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [246] |
| 499 | polygonumnolide A3 | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [246] |
| 500 | polygonumnolide A4 | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [246] |
| 501 | polygonumnolide B1 | Pleuropterus multiflorus (Thunb.) Nakai | C43H44O18 | type II | [246] |
| 502 | polygonumnolide B2 | Pleuropterus multiflorus (Thunb.) Nakai | C43H44O18 | type II | [246] |
| 503 | polygonumnolide B3 | Pleuropterus multiflorus (Thunb.) Nakai | C43H44O18 | type II | [246] |
| 504 | polygonumnolide E | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [247] |
| 505 | adamadianthrone | Psorospermum febrifugum Spach | C45H46O8 | type II | [154] |
| 506 | bioanthrone 2 | Vismia guineensis (L.) Choisy | C30H20O11 | type II | [242] |
| 507 | glaberianthrone | Psorospermum glaberrimum Hochr. | C45H46O8 | type II | [248] |
| 508 | prinoidin-emodin dianthrones | Rhamnus napalensis (Wall.) Lawson | C40H37O14 | type II | [249] |
| 509 | (S)-2-hydroxybutyl-4,4′,5,5′,7-pentahydroxy-2′-methoxy-2,7′-dimethyl-10,10′-dioxo-9,9′,10,10′-tetrahydro-[9,9′-bianthracene]-3-carboxylate | Aspergillus wentii Wehmer | C36H32O11 | type II | [249] |
| 510 | (S)-2-hydroxybutyl 4,4′,5,7-tetrahydroxy-5′,7′-dimethoxy-2,2′-dimethyl-10,10′-dioxo-9,9′,10,10′-tetrahydro-[9,9′-bianthracene]-3-carboxylate | Aspergillus wentii Wehmer | C37H34O11 | type II | [249] |
| 511 | 2,4′,5-trihydroxy-4,5′,7′-trimethoxy-2′,7-dimethyl-[9,9′-bianthracene]-10,10′(9H,9′H)-dione | Aspergillus wentii Wehmer | C33H28O8 | type II | [249] |
| 512 | dianthrone A1 | Psorospermum febrifugum Spach | C50H54O8 | type III | [154] |
| 513 | bioanthrone 3 | Vismia guineensis | C30H20O12 | type III | [242] |
| 514 | dianthrone A2a | Psorospermum glaberrimum Hochr. | C45H46O8 | type III | [242] |
| 515 | dianthrone A2b | Psorospermum glaberrimum Hochr. | C40H38O8 | type III | [248] |
| 516 | prinoidin dianthrones rhamnepalins | Rhamnus napalensis (Wall.) M.A.Lawson | C50H51O20 | type III | [249] |
| 517 | 8,8′-dihydroxy-1,1′,3,3′-tetramethoxy-6,6′-dimethyl-10,10′-dianthrone | Aspergillus wentii Wehmer | C34H30O8 | type III | [243] |
| 518 | physcion-10,10′-bianthrone |
Cassia didymobotrya Fresen. |
C32H28O8 | type III | [250] |
| 519 | dianthrone J | Cratoxylum formosum subsp. pruniflorum (Kurz) Gogelein | C42H42O8 | type III | [251] |
| 520 | (−)-trans-2,2′-Digeranyloxy-7,7′-dimethyl-4,4′,5,5′-tetrahydroxy-9,9′-dianthrone | Ochna pulchra Hook. | C50H54O8 | type III | [252] |
| 521 | trans aloe-emodin dianthrone diglucoside | Cassia angustifolia Vahl | C42H42O18 | type IV | [253] |
| 522 | sennoside B | Senna alexandrina Milll. | C42H38O20 | type V | [254] |
| 523 | (−)-ochnadianthrone | Ochna pulchra Hook. | C50H54O8 | type V | [255] |
| 524 | sennidin C | Rheum palmatum L. | C30H20O9 | type VI | [255] |
| 525 | sennoside A | Senna alexandrina Milll. | C42H40O19 | type VI | [254] |
| 526 | sennoside D | Senna alexandrina Milll. | C48H44O25 | type VI | [256] |
| 527 | sennoside E | Senna alexandrina Milll. | C48H44O25 | type VI | [254] |
| 528 | sennoside F | Senna alexandrina Milll. | C48H44O25 | type VI | [254] |
| 529 | chrysophanol dianthrone | Heterodermia obscurata (Nyl.) Trevis. | C30H21O6 | type VII | [240] |
| 530 | chrysophanol-l0,l0′-dianthrone | Cassia didymobotrya Fresen. | C30H22O6 | type VII | [250] |
| 531 | chrysophanol-isophyscion dianthrone | Senna longiracemosa (Vatke) Lock | C31H25O7 | type VII | [257] |
| 532 | isophyscion dianthrone | Senna longiracemosa (Vatke) Lock | C32H28O8 | type VII | [257] |
| 533 | martianine 1 | Senna martiana (Benth.) H. S. Irwin & Barneby | C43H44O16 | type VII | [258] |
| 534 | palmidin B | Rheum palmatum L. | C30H22O7 | type VII | [258] |
| 535 | palmidin C | Rheum palmatum L. | C30H22O7 | type VIII | [259] |
| 536 | neobulgarone G | Limonium tubiflorum (Delile) Kuntze | C32H24Cl2O9 | other | [245] |
| 537 | chrysophanol-physcion-l0,l0′-dianthrone | Cassia didymobotrya Fresen. | C31H25O7 | other | [250] |
| 538 | 1,8,1′,8′-tetrahydroxy-10,10′-dianthrone | Hypericum Tourn. ex L. | C28H18O6 | other | [260] |
| 539 | palmidin A | Rheum palmatum L. | C30H22O8 | other | [259] |
| 540 | rendin A | Rheum palmatum L. | C30H20O9 | other | [255] |
| 541 | rendin B | Rheum palmatum L. | C30H20O8 | other | [255] |
| 542 | rendin C | Rheum palmatum L. | C31H22O9 | other | [255] |
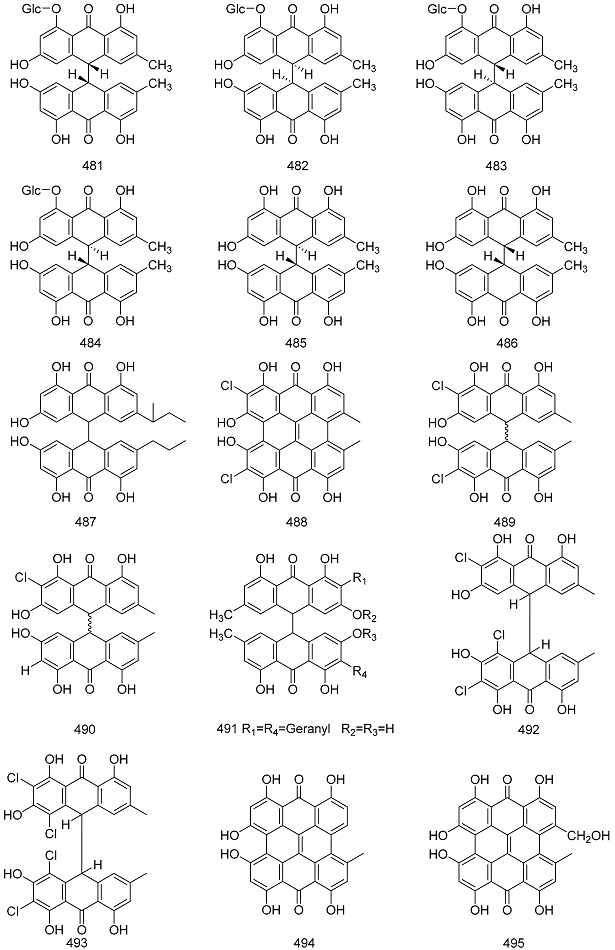

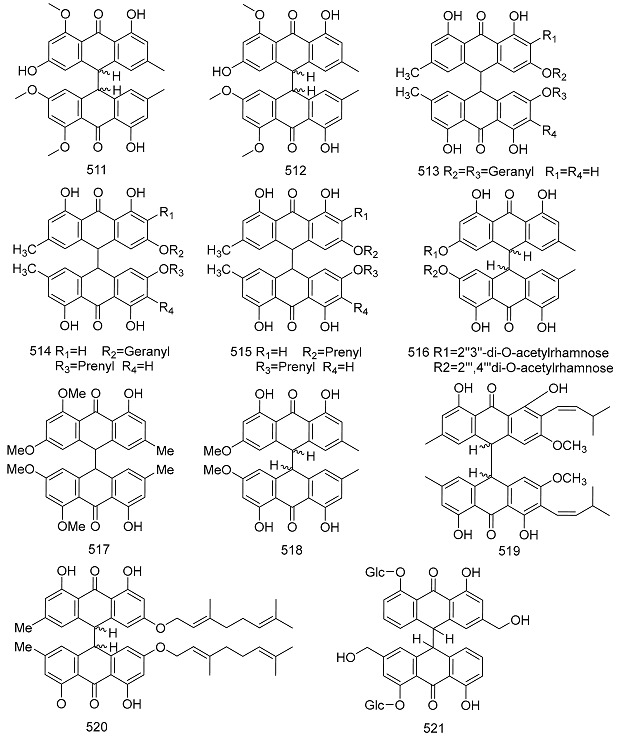


2.2. Extraction and Separation Methods
Quinones are the active chemical components of several traditional Chinese medicines. In nature, quinones exist in two forms: free and glycosylated. The physical and chemical properties of glycosides differ greatly, especially their polarity and solubility; therefore, their extraction and separation methods are different.Figure 4 introduces the extraction and separation methods of quinone compounds.
Figure 4.
Extraction and separation methods for quinone compounds.
2.2.1. Extraction
Chinese medicines often contain both anthraquinones and their glycosides. The first step in the extraction of anthraquinone glycosides is to determine whether they should be extracted simultaneously or separately. Currently, the available extraction methods include alkaline extraction and acid precipitation, organic solvent extraction, physical field-enhanced extraction, water vapor distillation, lead salt method, supercritical fluid extraction, pressurized liquid extraction, and solid-phase extraction [14].
Alkali Extraction and Acid Precipitation Method
The acid precipitation method is applicable to quinone compounds containing acidic groups. In the alkali extraction and acid precipitation methods, the substance to be measured is first dissolved in a suitable solvent to form a solution. Then, an appropriate amount of alkali solution was added dropwise to the solution to neutralize the acidic substance with the alkali. When the hydrogen ions in the acidic substance are completely neutralized, the resulting salt forms ions in the solution that remain dissolved. Quinone compounds with different positions and numbers of free hydroxyl groups have different degrees of acidity; therefore, they can be extracted using different concentrations of alkaline aqueous solutions. Zhang Yuebin [261] extracted cornhusk rutin by alkali extraction and acid precipitation method, and the optimal process determined by response surface method was as follows: material-liquid ratio of 1:17 (g/mL), water bath temperature of 85 °C, and water bath time of 40 min, and the extraction rate of cornhusk rutin was 6.5328%.
Organic Solvent Extraction Methods
The most commonly used method for extracting quinones is organic solvent extraction, and the commonly used solvents include methanol and ethyl acetate. Zhang Liangming [262] extracted the total anthraquinones from cassia seeds, and the optimal process was 70% volume fraction of ethanol, extraction time of 2.0 h, material-liquid ratio of 1:30 (g:mL), and extraction temperature of 85 °C, which resulted in a high extraction rate and a stable process. Under these conditions, the average extraction rate of total anthraquinone from cassia seed was 4.79%.
Physical Field Enhanced Extraction
The addition of a physical field (e.g., microwave or ultrasound) to the traditional solvent can improve the extraction effect and shorten the extraction time. Lili Cao [263] extracted anthraquinones from the rhizomes of Rubia cordifolia by an ultrasonic-assisted method. The optimal extraction conditions were an ultrasonic time of 31.29 min, solvent dosage of 13.47 mL, solvent concentration of 81.15%, and a theoretical prediction of anthraquinone extraction rate in the rhizome of Cynanchum officinale of 7.64%.
Steam Distillation Method
Some compounds with small relative molecular masses are volatile and can be distilled with water vapor. If the compounds are volatile and water-insoluble, they can be extracted using water vapor distillation. The water vapor distillation method is applicable to benzoquinone and naphthoquinone compounds. Du Zexiang [264] used hydrodistillation to extract quinones from the stems of Plumbago zeylanica and determined the content of plumbagin in the compounds. The results showed that the plumbagin content in the fresh and dried stems of Plumbago zeylanica was 0.0423% and 0.0420%, respectively.
Lead Salt Method
Lead salt precipitation is a classical method for separating certain herbal components. Since lead acetate and alkaline lead acetate can form insoluble lead salts or complex salt precipitates with a variety of herbal ingredients in aqueous and alcoholic solutions, this property can be utilized to separate the active ingredients from impurities [265].
Supercritical Fluid Extraction Methods
The CO2-supercritical fluid extraction method utilizes the properties of high density, low viscosity, and large diffusion coefficient of CO2 in the supercritical state to extract the active ingredients, which have the advantages of low extraction temperature, high extraction rate of the active ingredients, and short operation cycle [266]. Zhu K [267] determined the optimal extraction process for the determination of anthraquinone in Rheum officinale by CO2-supercritical fluid method using the orthogonal test method, the optimal extraction conditions were 40 °C maintaining 20 MPa pressure for 2 h, and using 75% ethanol as the entraining agent.
Solid-Phase Extraction Method
Solid-phase extraction (SPE) is a simple and convenient method for the pretreatment of samples that can effectively eliminate the interference of the sample matrix, simplify the elution conditions of liquid chromatography analysis, and shorten the analysis time. Zhao Jiangli [268] established an analytical method for the determination of hydroquinone and phenol in cosmetics by solid-phase extraction and high-performance liquid chromatography, and the detected concentration andquantitative concentration can meet the technical requirements of the «Cosmetic Safety Code», which can be used for the determination of hydroquinone and phenol in cosmetics with complex matrices.
Pressurized Liquid Extraction Method
The pressurized liquid extraction method uses a conventional solvent to extract solid or semi-solid samples under relatively high temperature and pressure [269]. Ong and Soon [270] employed pressurized liquid extraction (PLE) to extract thermally unstable components, such as tanshinone I and tanshinone IIA, from Salvia miltiorrhiza Bunge. PLE was carried out dynamically under the following conditions: a flow rate of 1 mL/min, temperature of 95–140 °C, applied pressure of 10–20 bar, and extraction times of 20 and 40 min. The extraction efficiency of PLE is higher than that of other methods.
2.2.2. Separation
pH Gradient Extraction Method
pH gradient extraction is a traditional method for separating quinones. Quinones contain free hydroxyl groups at different locations and numbers, with different acidic strengths, and different quinones can be selectively extracted using different concentrations of alkaline aqueous solutions [271]. He Ying [272] determined the anthraquinones in the browning products of pomegranate pericarp and used pH gradient extraction for separation and column chromatography purification to obtain four anthraquinones, which were identified as rhubarb phenol, rhubarb, rhubarb acid, and rhubarb methyl ether, and the optimal process conditions were ethanol concentration of 75%, ethanol dosage of 90 mL, extraction time of 25 min, and extraction temperature of 25 °C. The extracts were extracted at 25 °C, and the extracts were extracted at 25 min.Figure 5 introduces the flow chart for the separation of anthraquinone compounds from pomegranate peels.
Figure 5.
Flow chart of the separation of anthraquinone compounds from pomegranate peels.
Chromatographic Methods
The most commonly used method for separating quinones is chromatography, which is particularly effective for separating quinones with free phenolic hydroxyl groups, especially anthraquinones. Conventional chromatographic methods include paper chromatography and column chromatography. An increasing number of new techniques have been applied to the separation of quinone compounds, such as high-performance liquid chromatography, high-performance countercurrent chromatography, droplet countercurrent chromatography, large-pore adsorbent resin, and flash column chromatography. High-performance liquid chromatography (HPLC) is a great complement to traditional chromatography, and with the continuous development of technology, HPLC has been greatly improved, and its operation and data processing are more automated. Chromatographic columns are packed with an ever-increasing variety of materials that can separate substances under normal-phase, reversed-phase, and even chiral conditions.HPLC instruments can be connected to a wide variety of monitors and are increasingly used in the separation of quinones. Jun Huang [273] established a high-performance liquid chromatographic assay for the separation of lawsone, which was sensitive, rapid, and simple, and was corroborated by high-performance liquid chromatography-tandem mass spectrometry to ensure accurate results. High-speed countercurrent chromatography (HSCCC) is a continuous liquid-liquid chromatographic technique that does not require solid-phase carriers. Tian, G [274] used multidimensional high-performance countercurrent chromatography to obtain four major components, tanshinone IIA, tanshinone I, dihydrotanshinone I, and cryptotanshinone II, with purities above 95%. Droplet countercurrent chromatography (DCCC) separates compounds based on differences in partitioning between two immiscible liquid phases. This method requires the system to be separated into two phases in a short period and form droplets efficiently [275].
Macroporous Adsorption Resin Method
Macroporous adsorption resin separation technology is a process of extraction and refinement that uses special adsorbents to selectively adsorb the active ingredients and remove the ineffective ingredients from the compound decoction of traditional Chinese medicine [276]. Zhenkang Lu [277] used macroporous adsorbent resin for the separation and purification of Juglans cyan bark pigment, and the dynamic adsorption and desorption experiments showed that the D-101 macroporous adsorbent resin was the most effective for the separation and purification of Juglans cyan bark pigment. The optimum conditions for adsorption were an initial concentration of 1.5 mg/mL, a flow rate of 0.5 mL/min, a pH of 3, and a volume of 50 mL of sample solution. The optimum conditions for desorption were an elution flow rate of 1.5 mL/min, ethanol concentration of 90% in the eluate, and elution pH of 4.
2.3. Structural Identification Methods
Common methods for the structural identification of benzoquinone include ultraviolet absorption spectroscopy, infrared absorption spectroscopy, nuclear magnetic resonance spectroscopy, and mass spectrometry.
2.3.1. Benzoquinones
Benzoquinones exist in a long conjugated system, and in the UV absorption spectrum, the molecules can show long absorption peaks in both the near-UV and visible regions. The three main absorption bands of benzoquinone are: ca. 240 nm (strong absorption); ca. 285 nm (medium to strong absorption); and ca. 400 nm (weak absorption). Benzoquinones are most characterized in the infrared spectra by the telescopic vibrational absorption peaks of carbonyl, hydroxyl, and double bonds at 1675–1653 cm−1 (νC=O), 3600–3140 cm−1 (νOH), 1640–1200 cm−1 (νC=C). The number of absorption peaks and the wavenumber of the carbonyl group of the benzoquinone compound are closely related to the substituents on benzoquinone. When there is a hydroxyl substitution in the molecule, the hydrogen bonding between the carbonyl group and the hydroxyl group will cause a significant decrease in the wavenumber of the carbonyl absorption peak. If the molecular structure is symmetrical after the substitution of the substituent group, the compound is the same as unsubstituted benzoquinone, and there is only one base absorption peak in the infrared absorption spectrum. In the NMR hydrogen spectrum, the chemical shift of the unsubstituted benzoquinone ring proton is δH 6.72(s); when there is a substitution of an electron-donating group on the ring, it causes the chemical shifts of the other protons to be shifted to the higher field. In the NMR carbon spectra, the chemical shift of the unsubstituted benzoquinone carbonyl carbon is around δC 187, and substitution of the substituents around the benzoquinone carbonyl group induces a shift in the chemical shift of the carbonyl carbon. In the mass spectrum, the chemical shift of the carbonyl carbon is shifted to a higher field when the electron-donating group is substituted. In the mass spectrum, the molecular ion peak of unsubstituted benzoquinone is m/z 108, and cleavage fragments of m/z 82, m/z 80, and m/z 54 appear in its mass spectrum. A fragmentation ion peak (m/z 52) with two consecutive CO removals was present in the mass spectrum of benzoquinone. For substituted benzoquinones, this cleavage pattern provides an important basis for deducing the type of substituent.
2.3.2. Naphthoquinones
The UV absorption of naphthoquinone mainly originates from two parts of the structure: the naphthalene-like and quinone-like structures. The naphthalene structure has three main absorption bands at 245, 251, and 335 nm, while the quinone structure has a main absorption band at 257 nm [1]. When OH−, OCH3−, and other electron-donating groups are substituted in the molecule, the corresponding absorption bands are redshifted. The characteristic absorption peaks in the IR pattern of naphthoquinone remained in the carbonyl stretching vibration absorption peak from 1675 to 1653 cm−1 and the backbone vibration absorption peak between 1635 and 1648 cm−1 of the aromatic ring. In the NMR hydrogen spectra, when there is no substituent on the naphthoquinone (1.4-naphthoquinone) ring, the chemical shift of the ring proton is δH 6.95. In NMR carbon spectra, when there is an electron-donating substituent on the quinone ring, the quinone ring proton is shifted to the high field, and the degree of shift is related to the magnitude of the electron-donating effect [278]. When there is an electron-donating substituent on the quinone ring, such as C3 substituted with -OH or -OR, the chemical shift of C-3 is shifted to the low field by about 20 ppm, and that of C-2 is shifted to the high field by 30 ppm. When the C2 substituent is R, the C-2 signal shifts to the low field by about 10, and the C3 signal shifts to the high field by about 8. The extent of the shift of C2 to the low field increased with increasing R. The C2 substituent is a substituent of the C2 position in quinone rings.
2.3.3. Phenanthrenequinones
Phenanthrenequinone, although structurally classified as a phenanthrenequinone, is biosynthetically classified as a diterpene quinone based on the structures of other coexisting congeners [11]. The vast majority of diterpene quinones have a rosinane or rearranged rosinane-type skeleton and include many pro-quinone types. Most quinone carbonyls in this group are present on the C-ring of the rosinane diterpenes, with 1,4-p-quinone and, in a few cases, also the o-type, usually with an isopropyl unit on the C-ring. The presence of this structural unit can be judged mainly by the chemical shifts of the protons and the shape of the peaks on ′H NMR. Most of the quinone carbonyls in this group are present on the C-ring of the rosinane diterpenes, with 1,4-p-quinone and, in a few cases, also the o-type, usually with an isopropyl unit on the C-ring, and the presence of this structural unit can be judged mainly by the chemical shifts of the protons and the shape of the peaks on 1H NMR. Generally, the chemical shifts of 16-CH3 and 17-CH3 are around 1.10, each appearing as a double peak. The C-15 hypomethyl proton appeared around 3.00 and showed a heptagonal peak due to coupled cleavage with both methyl protons. The 13C NMR chemical shift values of the carbonyl group are mainly derived from the presence or absence of hydroxyl groups in the neighboring environment, and the chemical shift of the carbonyl group with hydrogen bonding is shifted to a lower field. Methyl, hydroxyl, acetyl, and a third carbonyl group are also often present in the diterpene skeleton structure, and the substitution positions of these groups are usually based on two-dimensional mapping. The positions of these substituents are usually determined by a comprehensive analysis of the 1H COSY, HMQC, and HMBC spectra. In addition, the diterpene skeleton is often broken in this type of structure, and the identification of its structure is also mainly based on the analysis of NMR data. Where conditions permitted, confirmation was made by X-ray single-crystal diffraction data analysis [14].
2.3.4. Anthraquinones
In the UV absorption spectrum, anthraquinone has four main absorption bands caused by the benzene-like and quinone-like structures, with four absorption peaks at 252, 325, 272, and 405 nm. Most natural anthraquinones have hydroxyl substitutions, and the UV absorption spectra of hydroxy anthraquinones have five main absorption peaks: the I absorption peak is around 230 nm; the II absorption peak is 240–260 nm (caused by the benzene-like structure); the III absorption peak is 262–295 nm (caused by the quinone-like structure); the IV absorption peak is 305–389 nm (caused by the benzene-like structure); and the V absorption peak is greater than 400 nm (caused by C=O in the quinone-like structure) [1]. The information provided by the UV-Vis spectra of anthraquinones is of some use for structural speculation; however, because of the plethora of exceptions, UV-Vis spectral data are usually used only as circumstantial evidence for structural analysis. The IR absorption spectra of hydroxyanthraquinone are characterized by carbonyl stretching vibrational absorption near 1670 cm−1. Hydroxyl stretching vibrational absorption in the 3600–3150 cm−1 interval and benzene ring backbone vibrational absorption in the 1600–1480 cm−1 interval [279].In 1H NMR, the NMR signals of the aryl hydrogens of the anthraquinone parent nucleus can be divided into two categories: α-aryl hydrogens are in the negatively shielded region of C=O, which are more affected by the carbonyl group, and the resonance occurs in the lower magnetic field region, with the peak centered around δ8.07 [280]; β-aryl hydrogens are less affected by the carbonyl group, and the resonance occurs in the higher magnetic field region, with the peak centered around δ6.67 [271]. 13C NMR plays an important role in the identification of quinones. 13C NMR is important for the identification of quinones. The carbon atoms of the quinone parent nucleus can be classified into four groups, and the chemical shift values of these carbons in unsubstituted anthraquinones are as follows: α-C 126.6, β-C 134.3, carbonyl carbon 182.5, and quaternary carbon 132.9. When there is a hydroxyl substitution at the α-position, the chemical shift of the carbonyl carbon is shifted to the lower field to about 187 [14].
3. Progress in Pharmacological Activity Research
Quinones are abundant in nature, and their pharmacological activities, including immunomodulatory, antitumor, anti-inflammatory, antibacterial, antioxidant, and laxative effects, have received widespread attention.
3.1. Immunomodulatory Effects
Quinones exert multiple regulatory effects on the immune system. At the level of immune cells, it can activate macrophages to enhance phagocytosis, regulate their polarization, affect the differentiation and cytotoxicity of T-lymphocyte subpopulations, and regulate the activation and proliferation of B-lymphocytes and antibody secretion. Shen Jie established an SLE model and tested the parameters of lymph node size, spleen index, kidney index, Th cell subpopulation, and B cell activation index in mice. After Embelin treatment, the Th1/Th2 and Treg/Th17 ratios in the lymph nodes and spleens of SLE mice were significantly elevated. Moreover, the concentrations of dsDNA, ssDNA, and IgG in the serum of mice were significantly decreased. It was concluded that embelin exerts a therapeutic effect on SLE mice by regulating the balance of Th cell subpopulations and inhibiting the activation of Th and B cells, demonstrating that letterbox quinone has immunomodulatory and therapeutic effects on SLE [281].
3.2. Anti-Tumor Activity
Quinones exhibit anti-tumor effects. On the one hand, quinones can induce apoptosis in cancer cells by activating the endogenous apoptotic pathway. On the other hand, quinones can interfere with the cell cycle of tumor cells, causing them to stagnate at a certain stage and inhibiting the proliferation of tumor cells. In addition, quinones can inhibit tumor angiogenesis and reduce nutrient supply to tumors. Moreover, it can enhance the immune function of the body and activate immune cells to recognize and kill cancer cells, thus playing a multi-faceted positive role in the anti-tumor process. Common antitumor components include embelin [282], emodin [283], chrysophanol [284], tanshinone IIA [285], juglone [286], plumbagin [287], aloe-emodin [288], dioscoreanone [289], and denbinobin [290]. Table 7 introduces the anti-proliferative effects of quinone compounds on cells.
Table 7.
Anti-proliferative effects of quinone compounds on cells.
| No. | Name | Cell Line | IC50 |
|---|---|---|---|
| 15 | embelin | PC-3 | 3.7 μmol/L |
| LNCaP | 5.7 μmol/L | ||
| HeLa | 5–7 μmol/L | ||
| 64 | juglone | BxPC-3 | 21.05 μmol/L |
| 68 | plumbagin | HepG2 | (27.08 ± 0.40) μmol/L |
| HL-60 | 0.8 μmol/L | ||
| 197 | dioscoreanone | MCF-7 | 20 μmol/L |
| 198 | denbinobin | K562 | 1.84 μmol/L |
| GSK5182 | 1.6 μmol/L | ||
| 219 | tanshinone IIA | A549 | 42.45 μmol |
| BGC-823 | 61.46 μmol/L | ||
| Hep-2 | 9.6 μmol/L | ||
| 226 | chrysophanol | HepG2 | 30 μmol/L |
| MCF-7 | 25 μmol/L | ||
| A549 | 18 μmol/L | ||
| 227 | emodin | HL-60/ADR | 5.79 μmol/L |
| SMMC-7721 | 21.6 μmol/L | ||
| HL-60 | 20 μmol/L | ||
| L02 | 135 μmol/L | ||
| 521 | aloe-emodin | HeLa | 58.3 μmol/L |
| HepG2 | 10 μmol/L | ||
| HCT116 | 8.7 μmol/L |
Avci, H [286] used MTT to determine the cytotoxic effect of juglone. Treatment of BxPC-3 human pancreatic cancer cells with different concentrations of juglone reduced the expression of MMP-2 and -9 genes in a dose-dependent manner, and VEGF induced a significant reduction in the level of expression of Phactr-1 gene, indicating that huperzine has an anti-metastatic effect on human pancreatic cancer cells. Zhang utilized the thiazolyl blue reduction method (MMT) to detect the antiproliferative effect of Dendrobium officinale phenanthrenequinone on human ovarian cancer cells HO-8910PM, while the Transwell assay was used to detect changes in the metastatic ability of the cells. The expression of apoptosis- and metastasis-related genes and protein levels in HO-8910PM cells was detected using reverse transcription-polymerase chain reaction and protein blotting. The results of the MTT assay showed that the proliferation inhibitory effect of dendrobium phenanthrenequinone at 3 μmol/L and 10 μmol/L on ovarian cancer cells was significant, and dendrobium phenanthrenequinone inhibited the proliferation and metastasis of ovarian cancer cells by upregulating the expression of CASP3, CASP9, and CAV1, and downregulating the expression of SOX2. The experimental results demonstrated that dendrobium phenanthrenequinone has anti-invasive and metastatic therapeutic effects on human ovarian cancer cells [291]. Yang suggested that rhodopsin inhibited SREBP1-dependent and SREBP1-non-dependent cell proliferation and led to caspase-dependent and caspase-non-dependent induction of endogenous apoptosis in HCC [292]. The IC50 value of rhodopsin in L02 cells was 36.69 μg/L [293]. The toxicity of rhodopsin on normal human cells (IC50 values ranging from 92.59 to 185.18 μmol/L) was slightly lower than the IC50 values of rhodopsin on cancer cells (10 to 80 μmol/L).
3.3. Antioxidant Activity
Anthraquinones possess antioxidant effects and play a positive role in protecting the body against oxidative stress damage. Quinones with antioxidant effects include idebenone [294], plumbagin [295], juglone [296], alkannin [297], tanshinone I [298], tanshinone IIA [299], emodin, physcion [300], and aloe-emodin [301]. Idebenone exerts antioxidant effects that are mainly dependent on the benzoquinone ring, which has both reduced (hydroquinone) and oxidized forms [287]. The ketone bond can generate unstable semiquinone through a reduction reaction or further reduction to form dihydroubiquinone, which exhibits strong antioxidant activity. Hao Xu [294] examined the expression of SIRT3 in oxidative stress-injured HT22 cells before and after the use of ibuprofen and found that ibuprofen counteracted oxidative stress-injured neuronal apoptosis by affecting the CD38-SIRT3-P53 pathway. The optimal extraction process of naphthoquinones in water walnut leaves was determined by one-way and orthogonal tests, i.e., 50% v/v ethanol solution as extraction solvent, 1:50 (g/mL), extraction temperature of 60 °C, and extraction time of 5 h. The extraction of naphthoquinones reached 168.14 mg/g under these conditions. Hu Tian determined the optimal extraction process of naphthoquinone components in the leaves of Platycarya strobilacea Siebold & Zucc, through a single-factor and orthogonal experiment. That is, an ethanol solution with a volume fraction of 50% was used as the extraction solvent, with a solid-liquid ratio of 1:50 (g/mL), extraction temperature of 60 °C, and extraction time of 5 h. Under these conditions, the amount of naphthoquinone extract reached 168.14 mg/g. By measuring their reducing power, it was found that the DPPH radical scavenging ability of both the naphthoquinone extract of Narcissus aquifolium Pourr., and VC gradually increased with increasing sample mass concentration. However, the scavenging rate of DPPH radicals by both the naphthoquinone extract of Narcissus aquifolium Pourr., and VC gradually stabilized when the mass concentration of the naphthoquinone extract of Narcissus aquifolium Pourr., and VC was greater than 0.6 mg/mL. The results indicated that the naphthoquinone constituents of water walnut leaves have good antioxidant activity in vitro [302]. Table 8 introduces the antioxidant activity of quinone compounds.
Table 8.
Antioxidant activity of quinone compounds.
| No. | Name | DPPH | ABTS |
|---|---|---|---|
| 68 | plumbagin | IC50 = 50 μmol/L | |
| 64 | juglone | IC50 = 0.498 mg/mL | IC50 = 0.189 mg/mL |
| 142 | alkannin | IC50 = 40 μg/mL | |
| 217 | tanshinone I | IC50 = 0.07 μmol/L | |
| 227 | emodin | EC50 = 147.87 mg/L IC50 = 112.32 mg/mL |
|
| 385 | physcion | IC50 = 56.05 mg/mL | |
| 521 | aloe-emodin | EC50 = 6.03 mg/L |
3.4. Anti-Inflammatory Activity
Anthraquinones have significant anti-inflammatory effects, and their mechanism of action mainly involves the regulation of inflammatory factors and the inhibition of related signaling pathways. Through in vivo experiments in mice, Jie found that alcohol extracts of Rubia cordifolia L. exert anti-inflammatory effects by inhibiting the production of pro-inflammatory factors in serum and promoting the production of anti-inflammatory factors. Rubia cordifolia L. alcohol extract in the middle concentration group and high concentration group had similar therapeutic effects to that of dexamethasone on adjuvant arthritis in mice, resulting in a reduction in inflammatory cell infiltration in the articular cavity of the ankle joint in mice. The MDA and SOP levels in liver homogenates showed that the components in Rubia cordifolia L. inhibit inflammation partly through the elimination of free radicals and reactive oxygen molecules in vivo and partly through the metabolism of glutathione in the liver [303]. Liu Mingxin demonstrated that the naphthoquinone constituents of Arnebia euchroma (Royle) I. M. Johnst. were able to downregulate the expression of inflammatory mediators PGE2, NO, and inflammatory cytokines IL-1β and TNF-α, inhibit xylene-induced mouse auricular swelling, and exert certain anti-inflammatory effects in vitro using a macrophage inflammation model and in vivo in an animal model [304].
3.5. Antimicrobial Activity
Quinones have significant antimicrobial effects and inhibit a wide range of bacteria to varying degrees [305]. Their inhibitory mechanism mainly lies in their ability to inhibit the oxidation and dehydrogenation processes of bacterial sugars and metabolic intermediates, and they can bind to DNA, interfering with its template function, and thus inhibiting the synthesis of proteins and nucleic acids [306]. Zhenkang Lu treated E. coli with juglone at concentrations of 0.0625, 0.125, 0.25, 0.5, 1, 2 mg/mL, and 4 mg/mL, and the relative conductivity of E. coli cell membranes increased which means that juglone resulted in impaired integrity of E. coli membranes, and increased permeability of cell membranes. Fluorescence emission spectroscopy results showed that juglone interacts with membrane proteins, thereby changing the structure of the E. coli cell membrane. The results of crystal violet and bladed azurite staining experiments showed that juglone could weaken the respiration of E. coli by inhibiting the formation of E. coli biofilms and eventually inhibiting its activity. SDS—PAGE and E. coli genome synthesis analysis revealed that juglone inhibited the expression of proteins, DNA, and RNA in E. coli, thereby acting as an antibacterial agent [307].
3.6. Anti-Fibrotic Effect
Quinones have antifibrotic effects. One of these mechanisms involves the inhibition of fibrosis-related cytokine expression, interference with signaling pathways, and reduction of extracellular matrix synthesis. Anthraquinone can inhibit the over-activation of the MAPK pathway in hepatic stellate cells, thereby inhibiting the activation of hepatic stellate cells, reducing their transformation to myofibroblasts, reducing the synthesis of extracellular matrix, and reducing the degree of liver fibrosis. In vitro antifibrotic tests on rat HSC were performed, and it was concluded that 2,3,5-trihydroxy-4,9-dimethoxyphenanthrene, 2,3,5-trihydroxy-4-methoxyphenanthrene, and denbinobin phenanthrenequinone from Dendrobium officinale could all reduce the number of HSC cells. These three phenanthrenes exhibit antifibrotic activity by inducing the selective death of hematopoietic stem cells, providing a new avenue for the prevention and treatment of liver fibrosis [308].
3.7. Laxative Effect
Quinone compounds have purgative effects. The primary action sites of the combined anthraquinones of Rhei Radix or the free anthraquinones of Rheum palmatum L. Radix are the small intestine and stomach, followed by the colon. It can be seen that the anthraquinones of Rheum palmatum L. Radix et Rhizoma can act directly without the need for transformation in the large intestine [309]. Chen Yan-Yan [310] showed that after administration of Da Huang Gan Cao Tang to constipated mice, the time to peak and the area under the drug-time curve of the plasma anthraquinones emodin, aloe emodin, emodin-8-O-β-D-glucoside, conjugated dianthrones senecioside A, and glycyrrhetinic acid were higher than those of control mice. Compared to normal mice, rhubarb-glycyrrhiza glabra soup exhibited a stronger purifying effect in constipated mice, with an increase in fecal excretion and a shorter time to the first detachment.
3.8. Antidepressant Effects
Anthraquinones from medicinal plants, such as chrysin, also have antidepressant activity and are often used in antidepressant therapy. Chrysin has been found to improve depressive symptoms in rats, and high doses of chrysin can activate 5-hydroxytryptamine receptors (5-HT) in the hippocampus of depressed rats, stimulate neurotransmitter transmission, and increase the degree of excitability in rats. This anthraquinone analog reduced the degree of depression in rats [118].
4. Progress in Toxicity Studies
4.1. Digestive System Toxicity
4.1.1. Hepatotoxicity
As exogenous substances, the main chemical components of quinones are oxidized and reduced under the action of the cytochrome P450-based monooxygenase system in the liver and are finally converted into polar compounds for excretion [311]. Hu Xichen conducted three consecutive months of gavage and histopathological examination of the rat liver. At the end of three months, histopathological sections of the liver showed scattered inflammatory cell infiltration, congestion of hepatic sinusoids, active proliferation of Kupffer cells, and phagocytosis of pigment particles under a light microscope. In the transmission electron microscopy of the high-dose group, chromatin was clumped together in the nuclei of some hepatocytes or collected in the subnuclear membranes, the mitochondria were mildly swollen, the structure of capillary bile ducts was not clear in individual specimens, and the number of Kupffer cells was increased. In some specimens, the structure of the capillary bile ducts was unclear, and the number of Kupffer cells was increased. After the recovery period, no obvious pathological changes were observed in the liver pathology section under the microscope in each administered group. The results demonstrated that long-term gavage of prepared Polygonum multiflorum can cause liver inflammatory injury in rats, and the liver can be normalized after stopping the drug [312]. Z.H. Mao investigated the potential cytotoxicity and DNA-breaking effects of rhein, chrysophanol, emodin methyl ether, and aloe emodin on HepaRG in normal human-derived hepatocytes by using high-concentration assay and alkaline comet electrophoresis. The four rhubarb anthraquinones were found to be toxic to hepatocytes to varying degrees. Among them, the effects of emodin methyl ether on elevated reactive oxygen species and mitochondrial damage were more pronounced, and the toxicity of aloe emodin was mainly manifested by the modulation of free Ca2+ levels in hepatocytes. Oxidative stress injury may be an important molecular mechanism responsible for potential hepatocytotoxicity and genotoxicity [313].
4.1.2. Enterotoxicity
Quinones usually exist as glycosides and are not degraded by gastric acid. When anthraquinones is administered orally, it enters the stomach and small intestine through the esophagus, is absorbed into the bloodstream through the small intestinal mucosa, is converted to glucuronide conjugates by phase II enzymes in the liver and intestines [314], and is transported to various tissues and organs throughout the body through the heart to exert a variety of pharmacological effects [315]. Prolonged use of laxatives containing anthraquinones can cause colorectal melanosis (MC). MC is a non-inflammatory, benign, reversible pigmentation characterized by colorectal mucosal lesions [316], which has been found to be due to intestinal mucosal epithelial cellular turnover and deposition of lipofuscin by electron microscopy and histopathology. The presence of anthraquinone-containing laxatives in the colon significantly increases the risk of developing colorectal melanosis. SteerH W Ultrastructural and histochemical staining of colonic tissues from six normal colons and seven patients with melanotic polyps revealed that anthraquinone laxatives increased the number of macrophages in the lamina propria of the colonic mucosa. In addition, they enhance the lysosomal activity of macrophages, Schwann cells, and neuronal cells in the lamina propria of the colonic mucosa, as well as increase the number of lysosomes [317].
Cheng Ying used acridine orange staining and mitochondrial membrane potential staining to detect the effects of rhubarb sap metabolites, rhein, emodin, and aloe emodin on the acidic vesicular organelles and mitochondrial membrane potential in NCM460 and HT29 cells, respectively, and the effects of autophagy and apoptosis-related proteins on the expression levels were detected by western blot. The results showed that rhubarb sap metabolites, rhein, emodin, and aloe emodin, induced autophagy and apoptosis in NCM460 and HT29 cells, suggesting that rhubarb may exert a toxic effect on human colon cells by promoting autophagy and apoptosis [318].
4.2. Urinary Toxicity
Quinones can cause proteinuria, oliguria, anuria, hematuria, and other symptoms, and long-term or large amounts of exposure may lead to acute and chronic nephritis, renal failure, and even uremia and other serious kidney diseases, seriously affecting the normal function of the urinary system and overall health of the body. When emodin is ingested in excess, it interferes with the filtration function of the kidneys and the reabsorption of the renal tubules, resulting in the excretion of protein components that should have been reabsorbed back into the bloodstream through urine, leading to proteinuria. Lan Jie observed the effects of anthraquinone components in Pleuropterus multiflorus (Thunb.) Nakai on human renal cortical proximal tubule epithelial cell line HK-2 cells and detected the changes in mitochondrial membrane potential of HK-2 cells using JC-10. The mitochondrial membrane potential of the five anthraquinone monoconstituents declined with an increase in the treatment concentration and prolongation of the administration time, among which chrysophanin and aloe emodin had the fastest rate of decline, followed by rhodochrospiracol. The apoptosis of HK-2 cells after the administration of the five anthraquinone monomer components were detected by flow assay, and it was found that significant apoptosis was visible only after the administration of Rhein, Aloe emodin greater than or equal to 25 μmol/L for 48 h and and and Rhein 50 and 100 μmol/L for 48 h (p < 0.05). It was concluded that emodin, Aloe emodin, and Rhein can damage HK-2 cells with a potential risk of nephrotoxicity [319].
4.3. Reproductive Toxicity
Quinones may have adverse effects on the uterus and placenta during pregnancy. They cross the placental barrier and exert direct toxic effects on the fetus. Chang determined that emodin induced apoptosis, i.e., embryonic cytotoxicity, in mouse blastocysts by treating them with 25, 50, or 75 μmol/L emodin for 24 h at 37 °C and examining DNA fragmentation using the TUNEL assay. Membrane-associated protein V staining revealed a significantly higher number of membrane-associated protein V-positive/PI-negative (apoptotic) cells in the ICM and TE of emodin-treated blastocysts than in the control group. Emodin significantly inhibited cell proliferation and induced apoptosis in the ICM and TE of mouse blastocysts. Selective inhibition of RAR activity in emodin-treated blastocysts. Therefore, this substance may negatively affect embryonic development by decreasing RARβ expression, which in turn downregulates the RARβ-mediated developmental signaling pathways. Emodin triggers apoptosis in mouse blastocysts, leading to impaired embryonic development via the intrinsic cell death pathway [320].
Quinones can interfere with the normal physiological processes of testicular spermatogenic cells and damage DNA in sperm cells, causing gene mutations or chromosomal aberrations, thereby reducing the quality and quantity of spermatozoa. N-(1,3-Dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) is acutely toxic to organisms. Yao Kezhen exposed C57Bl/6 male mice to 6PPD-Q for 40 days at a dose of 4 mg/kg bw. After 40 days of exposure to C57Bl/6 male mice, exposure to 6PPD-Q not only resulted in decreased testosterone levels but also adversely affected semen quality and in vitro fertilization (IVF) results, thus indicating that 6PPD-Q exposure leads to impaired male fertility [321].
4.4. Carcinogenicity
The International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) classifies carcinogens into five groups, of which quinones are classified as group 2B and group 3 carcinogens. The IARC classifies 1-amino-2,4-dibromoanthraquinone, anthraquinone, dantron (chrysazin; 1,8-dihydroxyanthraquinone), 1-hydroxyanthraquinone, 2-methyl-1-nitroanthraquinone (uncertain purity), and mitoxantrone as Group 2B carcinogens, i.e., possibly carcinogenic to humans, but evidence of carcinogenicity in humans is limited. Limited evidence of carcinogenicity in humans and insufficient evidence of carcinogenicity in experimental animals, or insufficient evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals. 1-amino-2-methylanthraquinone, 2-aminoanthraquinone, and aziridyl benzoquinone are classified as Group 3 carcinogens, i.e., their carcinogenicity to humans is doubtful, and there are insufficient human or animal data. Table 9 introduces carcinogenic quinone compounds and their classifications.
Table 9.
Carcinogenic quinone compounds and their classification.
| No. | Name | Classification |
|---|---|---|
| 225 | dantron(chrysazin;1,8-dihydroxyanthraquinone) | 2B |
| 328 | 1-hydroxyanthraquinone | 2B |
| 336 | 1-amino-2,4-dibromoanthraquinone | 2B |
| 337 | 2-methyl-1-nitroanthraquinone | 2B |
| 397 | mitoxantrone | 2B |
| 59 | tris(aziridinyl)-para-benzoquinone (triaziquone) | 3 |
| 60 | aziridyl benzoquinone | 3 |
| 371 | 1-amino-2-methylanthraquinone | 3 |
| 386 | 2-aminoanthraquinone | 3 |
5. Summary
Summarizing and analyzing the research literature at home and abroad, the current research on quinones focuses on their types, pharmacological activities, and toxicity, and abundant research results have been achieved in these fields. The application and potential risks of quinones in the field of medicine and health have been investigated from the perspectives of classification of chemical structure, verification of biological activity, and exploration of toxicity mechanism, which provides a rich theoretical basis and practical experience for the development of quinones in natural medicine and related products. However, it should be pointed out that this study also has certain limitations. For example, in toxicity research, the molecular mechanism of quinone toxicity remains unclear; in technical terms, the efficiency of extraction and separation techniques is limited, and structural identification is overly dependent on traditional methods. Based on the limitations of the current study, further systematic research can be carried out in the following aspects: firstly, a systematic toxicity evaluation of quinones in traditional Chinese medicine and risk assessment to evaluate the safety of these ingredients; secondly, with the help of a 3D organoid co-culture model, further in-depth investigation into the toxicity mechanism of quinones, clarifying the key links and molecular mechanisms of their toxicity, and synthesizing quinone compounds with high bioactivity and low toxicity. We will synthesize quinone derivatives with high biological activity and low toxicity to expand the application potential of quinone compounds. Finally, we will develop an efficient and accurate online identification technology for the rapid identification of quinone compounds in traditional Chinese medicine.
Acknowledgments
Thanks to Yang Jianbo and Liu Xiaoqiu for their guidance on this study.
Author Contributions
Conceptualization, Z.L., J.Y., X.L., Y.P., P.C., L.Z., J.Y., X.C.,W.J., X.G. and F.W.; investigation, Z.L., R.Y., and H.G.; writing—original draft preparation, Z.L.; writing—review and editing, Z.L., J.Y. and X.L.; supervision, X.L., Y.P., P.C., L.Z., J.Y., X.C., W.J., X.G. and F.W.; project administration, J.Y.; funding acquisition, J.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by Textual Research on the Original Sources of Commonly Used Medicinal Materials in Ethnic Medicines and Study on Reference Medicinal Materials, Research on Key Technologies for Quality Control of Characteristic Ethnic Medicines in Xinjiang and Their Industrial Application and Research on Rapid Identification Methods for Varieties Based on the “Identity Card” Characteristics of Chemical Components, grant numbers “2023B03001-2, 2024B02023-2 and 2023YFC3504105”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Peng Y.H., Liu X.Q., Lv H.Y. Natural Medicinal Chemistry. 2nd ed. Chemical Industry Press; Beijing, China: 2020. [Google Scholar]
- 2.Yang Y.T., Zhang G.Y., Yang D.F., Zhang Y.Q., Zhang L., Wu S., Li X., Zhou Y.Z. Research progress on the regulation of lipid metabolism in the body by active components of traditional Chinese medicine. Chin. Herb. Med. 2024;55:3127–3136. [Google Scholar]
- 3.Wu R., Li K.F., Wang W.F. Efficacy of sodium tanshinone IIA sulfonate combined with metoprolol in the treatment of senile coronary heart disease and its effect on ventricular remodeling. Clin. Med. Res. Pract. 2025;10:53–56. doi: 10.19347/j.cnki.2096-1413.202502014. [DOI] [Google Scholar]
- 4.Yuan H.L., Zhao Y.L., Fan D.S., He W.L., Zhao P.P., Cai Y. Improved synthesis method of buparvaquone. Chem. Bull. 2021;84:952–957. doi: 10.14159/j.cnki.0441-3776.2021.09.012. [DOI] [Google Scholar]
- 5.Tao M.B., Zhang L., Liu F., Chen L., Liu Y.P., Chen H.P. Research progress on the safety of traditional Chinese medicines containing anthraquinone components. Pharmacol. Clin. Chin. Mater. Med. 2016;32:238–243. doi: 10.13412/j.cnki.zyyl.2016.06.069. [DOI] [Google Scholar]
- 6.Zhou X.J. Analysis of clinical characteristics of 130 cases of melanosis coli. Jilin Med. J. 2014;35:3487–3488. [Google Scholar]
- 7.Wang X., Sun K.W., Tian T., Zeng W.T., Yuan W. Clinical analysis of 12 cases of drug-induced liver injury caused by Polygonum multiflorum and its related preparations. Liver. 2024;29:1538–1540. doi: 10.14000/j.cnki.issn.1008-1704.2024.12.025. [DOI] [Google Scholar]
- 8.Duan Y.H. Literature analysis of traditional Chinese medicine varieties causing kidney damage. Strait. Pharm. J. 2014;26:138–139. [Google Scholar]
- 9.Zhao F.B., Li T.J., Zhao H., Guo J.W., Zhang J.G. Clinical analysis of 356 cases of acute kidney injury in inpatients of a nephrology-specialized hospital. J. Intern. Med. Theor. Pract. 2015;10:177–180. doi: 10.16138/j.1673-6087.2015.03.004. [DOI] [Google Scholar]
- 10.Holliday A.E., Walker F.M., Brodie E.D., III, Formica V.A. Differences in defensive volatiles of the forked fungus beetle, Bolitotherus cornutus, living on two species of fungus. J. Chem. Ecol. 2009;35:1302–1308. doi: 10.1007/s10886-009-9712-7. [DOI] [PubMed] [Google Scholar]
- 11.Dong M., Ming X., Xiang T., Feng N., Zhang M., Ye X., He Y., Zhou M., Wu Q. Recent research on the physicochemical properties and biological activities of quinones and their practical applications: A comprehensive review. Food Funct. 2024;15:8973–8997. doi: 10.1039/D4FO02600D. [DOI] [PubMed] [Google Scholar]
- 12.Qiu Y.F., Lu B., Yan Y.Y., Luo W.Y., Gao Z.Q., Wang J. A convenient synthesis of 1,4-benzoquinones. J. Chem. Res. 2019;43:124–126. doi: 10.1177/1747519819841806. [DOI] [Google Scholar]
- 13.Shi Z.M., Wang Q., Lu X.X., Zeng H.P., Xiao H., Zhang C.G., Liu H. Study on the physicochemical properties and druglikeness prediction of methyl-p-benzoquinone. J. Dali. Univ. 2024;9:26–32. [Google Scholar]
- 14.Lu Y. Chemistry of Quinones (Series of Natural Product Chemistry) 2nd ed. Chemical Industry Press; Beijing, China: 2009. [Google Scholar]
- 15.Yang D.Y., Yu H., Wu X.Y., Zhu Y.H., Xiao X.L., Xu W.A., Chen Y.X., Gong Q.F. Research progress on chemical components and biological activities of Atractylodes macrocephala Koidz. Chin. Arch. Tradit. Chin. Med. 2023;41:171–182. doi: 10.13193/j.issn.1673-7717.2023.05.037. [DOI] [Google Scholar]
- 16.Zhang W.Q. Master’s Thesis. Southern Medical University; Guangzhou, China: 2022. Study on the Chemical Components and Anti-Inflammatory Activities of Arnebia euchroma (Royle) Johnst. [Google Scholar]
- 17.Chen C.Y., Wang J.J., Kao C.L., Li H.T., Wu M.D., Cheng M.J. A New Benzoquinone from Antrodia camphorata. Chem. Nat. Compd. 2022;58:614–616. doi: 10.1007/s10600-022-03754-2. [DOI] [Google Scholar]
- 18.Wang H.J., Gloer K.B., Gloer J.B., Scott J.A., Malloch D. Anserinones A and B: New antifungal and antibacterial benzoquinones from the coprophilous fungus Podospora anserina. J. Nat. Prod. 1997;60:629–631. doi: 10.1021/np970071k. [DOI] [PubMed] [Google Scholar]
- 19.Gupta I., Peddha M.S. Anti-adipogenic activity of oleoresin from Nigella sativa L. seeds via modulation of PPAR-γ and C/EBP-α expression in 3T3-L1 adipocytes. Adv. Tradit. Med. 2024 doi: 10.1007/s13596-024-00808-4. [DOI] [Google Scholar]
- 20.Ko J.-H., Lee S.-G., Yang W.M., Um J.-Y., Sethi G., Mishra S., Shanmugam M.K., Ahn K.S. The Application of Embelin for Cancer Prevention and Therapy. Molecules. 2018;23:621. doi: 10.3390/molecules23030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J. Master’s Thesis. Dali Univercity; China: 2018. Study on the Chemical Components and Biological Activities of Embelia ribes Burm.f. and Hypericum spathulatum Hook.f. & Thomson ex Dyer. [Google Scholar]
- 22.De Gaetano F., Mannino D., Celesti C., Bulzomí M., Iraci N., Giofrè S.V., Esposito E., Paterniti I., Ventura C.A. Randomly methylated β-cyclodextrin improves water-solubility, cellular protection and mucosa permeability of idebenone. Int. J. Pharm. 2024;665:124718. doi: 10.1016/j.ijpharm.2024.124718. [DOI] [PubMed] [Google Scholar]
- 23.Gunatilaka A.A., Berger J.M., Evans R., Miller J.S., Wisse J.H., Neddermann K.M., Bursuker I., Kingston D.G. Isolation, synthesis, and structure-activity relationships of bioactive benzoquinones from Miconia lepidota from the Suriname rainforest. J. Nat. Prod. 2001;64:2–5. doi: 10.1021/np000219r. [DOI] [PubMed] [Google Scholar]
- 24.Lin W.Y., Kuo Y.H., Chang Y.L., Teng C.M., Wang E.C., Ishikawa T., Chen I.S. Anti-platelet aggregation and chemical constituents from the rhizome of Gynura japonica. Planta Med. 2003;69:757–764. doi: 10.1055/s-2003-42796. [DOI] [PubMed] [Google Scholar]
- 25.Arot Manguro L.O., Midiwo J.O., Kraus W., Kraus W., Ugi I. Benzoquinone derivatives of Myrsine africana and Maesa lanceolata. Phytochemistry. 2003;64:855–862. doi: 10.1016/S0031-9422(03)00428-X. [DOI] [PubMed] [Google Scholar]
- 26.Lertnitikul N., Teerasukpimol L., Aekanantakul P., Pooreecharurot N., Sukrong S., Boonyong C., Poldorn P., Rungrotmongkol T., Sukandar E.R., Aonbangkhen C., et al. A new benzoquinone and a new stilbenoid from Paphiopedilum exul (Ridl.) Rolfe. Nat. Prod. Res. 2024;22:1–9. doi: 10.1080/14786419.2024.2344196. [DOI] [PubMed] [Google Scholar]
- 27.Zhou R.Y., Li N., Luo W.Y., Wang L.L., Zhang Y.Y., Wang J. A Simple and Convenient Two-step Synthesis of Idebenone. Org. Prep. Proced. Int. 2021;53:397–401. doi: 10.1080/00304948.2021.1917945. [DOI] [Google Scholar]
- 28.Su J.H., Ahmed A.F., Sung P.J., Wu Y.C., Sheu J.H. Meroditerpenoids from a Formosan soft coral Nephthea chabrolii. J. Nat. Prod. 2005;68:1651–1655. doi: 10.1021/np050278a. [DOI] [PubMed] [Google Scholar]
- 29.Permana D., Lajis N.H., Mackeen M.M., Ali A.M., Aimi N., Kitajima M., Takayama H. Isolation and bioactivities of constitutents of the roots of Garcinia atroviridis. J. Nat. Prod. 2001;64:976–979. doi: 10.1021/np000563o. [DOI] [PubMed] [Google Scholar]
- 30.Pirouz M., Abaee M.S., Harris P., Mojtahedi M.M. One-pot synthesis of benzofurans via heteroannulation of benzoquinones. Heterocycl. Commun. 2021;27:24–31. doi: 10.1515/hc-2020-0120. [DOI] [Google Scholar]
- 31.Ferreira P.M.P., De Almeida A.A.C., Conceição M.L.P., Pessoa O.D.L., Marques L.G.A., Capasso R., Pessoa C. Cordia oncocalyx and oncocalyxones: From the phytochemistry to the anticancer action and therapeutic benefits against chronic diseases. Fitoterapia. 2023;169:105624. doi: 10.1016/j.fitote.2023.105624. [DOI] [PubMed] [Google Scholar]
- 32.Guillonneau L., Taddei D., Moody C.J. Synthesis of the reported structure of the bisbenzoquinone lanciaquinone, isolated from Maesa lanceolata. Org. Lett. 2008;10:4505–4508. doi: 10.1021/ol801697g. [DOI] [PubMed] [Google Scholar]
- 33.Sangsopha W., Lekphrom R., Schevenels F.T., Saksirirat W., Bua-Art S., Kanokmedhakul K., Kanokmedhakul S. New p-terphenyl and benzoquinone metabolites from the bioluminescent mushroom Neonothopanus nambi. Nat. Prod. Res. 2020;34:2186–2193. doi: 10.1080/14786419.2019.1578763. [DOI] [PubMed] [Google Scholar]
- 34.Chandra P., Read G., Vining L.C. Studies on the biosynthesis of volucrisporin. II Metabolism of some phenylpropanoid compounds by Volucrispora aurantiaca Haskins. Can. J. Biochem. 1966;44:403–413. doi: 10.1139/o66-050. [DOI] [PubMed] [Google Scholar]
- 35.Truong Nguyen H., Duong T.H., Dang M.K., Pham M.D., Pham N.K., Tri Mai D., Son Dang V., Nguyen N.H., Sichaem J. Two New Benzoquinone Derivatives from Vietnamese Knema globularia Stems. Chem. Biodivers. 2024;21:4. doi: 10.1002/cbdv.202400380. [DOI] [PubMed] [Google Scholar]
- 36.Lin Y.L., Su Y.T., Chen B.H. A study on inhibition mechanism of breast cancer cells by bis-type triaziquone. Eur. J. Pharmacol. 2010;637:1–10. doi: 10.1016/j.ejphar.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Prins B., Koster A.S., Verboom W., Reinhoudt D.N. Microsomal superoxide anion production and NADPH-oxidation in a series of 22 aziridinylbenzoquinones. Biochem. Pharmacol. 1989;38:3753–3757. doi: 10.1016/0006-2952(89)90581-9. [DOI] [PubMed] [Google Scholar]
- 38.An T.-Y., Shan M.-D., Hu L.-H., Liu S.-J., Chen Z.-L. Polyprenylated phloroglucinol derivatives from Hypericum erectum. Phytochemistry. 2002;59:395–398. doi: 10.1016/S0031-9422(01)00408-3. [DOI] [PubMed] [Google Scholar]
- 39.Wieder C., Peres da Silva R., Witts J., Jäger C.M., Geib E., Brock M. Characterisation of ascocorynin biosynthesis in the purple jellydisc fungus Ascocoryne sarcoides. Fungal. Biol. Biotechnol. 2022;9:8. doi: 10.1186/s40694-022-00138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pei J., Hsu C.-C., Wang Y., Yu K.F. Corona discharge-induced reduction of quinones in negative electrospray ionization mass spectrometry. RSC Adv. 2017;7:43540–43545. doi: 10.1039/C7RA08523K. [DOI] [Google Scholar]
- 41.Ding H., Sun B., Jin C. Review on the synthesis methods of 2-methyl-1,4-naphthoquinone. Zhejiang Chem. Ind. 2023;54:23–26. [Google Scholar]
- 42.Higa M., Ogihara K., Yogi S. Bioactive naphthoquinone derivatives from Diospyros maritima BLUME. Chem. Pharm. Bull. 1998;46:1189–1193. doi: 10.1248/cpb.46.1189. [DOI] [PubMed] [Google Scholar]
- 43.Higa M., Noha N., Yokaryo H., Ogihara K., Yogi S. Three new naphthoquinone derivatives from Diospyros maritima Blume. Chem. Pharm. Bull. 2002;50:590–593. doi: 10.1248/cpb.50.590. [DOI] [PubMed] [Google Scholar]
- 44.Yao R., Guo H., Zhang X.S., Wang Y., Guo X.H., Chen J., Li J.H., Xu L., Yang J.B., Jing W.G., et al. Research progress on the processing technology, chemical components and pharmacological activities of processed Polygonum multiflorum. Front. Pharm. 2024;28:523–535. [Google Scholar]
- 45.Nishina A., Kubota K., Osawa T. Antimicrobial components, trachrysone and 2-methoxystypandrone, in Rumex japonicus Houtt. J. Agric. Food Chem. 1993;41:1772–1775. doi: 10.1021/jf00034a047. [DOI] [Google Scholar]
- 46.Takahashi D., Maoka T., Tsushima M., Fujitani K., Kozuka M., Matsuno T., Shingu T. New Quinone Sulfates from the Crinoids Tropiometra afra macrodiscus and Oxycomanthus japonicus. Chem. Pharm. Bull. 2002;50:1609–1612. doi: 10.1248/cpb.50.1609. [DOI] [PubMed] [Google Scholar]
- 47.Iwata D., Ishibashi M., Yamamoto Y., Cribrarione B. A new naphthoquinone pigment from the myxomycete Cribraria cancellata. J. Nat. Prod. 2003;66:1611–1612. doi: 10.1021/np030308e. [DOI] [PubMed] [Google Scholar]
- 48.Trisuwan K., Khamthong N., Rukachaisirikul V., Phongpaichit S., Preedanon S., Sakayaroj J. Anthraquinone, Cyclopentanone, and Naphthoquinone Derivatives from the Sea Fan-Derived Fungi Fusarium spp. PSU-F14 and PSU-F135. J. Nat. Prod. 2010;73:1507–1511. doi: 10.1021/np100282k. [DOI] [PubMed] [Google Scholar]
- 49.Rong X.G., Ma L.Y., Liu W.Z., Liu D.S. A new naphthoquinone compound from Penicillium raistrickii. Chin. J. Mar. Drugs. 2024;43:10–16. doi: 10.13400/j.cnki.cjmd.2024.01.003. [DOI] [Google Scholar]
- 50.Kishore P.H., Reddy M.V., Gunasekar D., Caux C., Bodo B. A new naphthoquinone from Ceiba pentandra. J. Asian Nat. Prod. Res. 2003;5:227–230. doi: 10.1080/1028602031000105812. [DOI] [PubMed] [Google Scholar]
- 51.Sreeramulu K., Rao K.V., Rao C.V., Gunasekar D. A new naphthoquinone from Bombax malabaricum. J. Asian Nat. Prod. Res. 2001;3:261–265. doi: 10.1080/10286020108040365. [DOI] [PubMed] [Google Scholar]
- 52.Lee I.S., Kaneda N., Suttisri R., El-Lakany A.M., Sabri N.N., Kinghorn A.D. New orthoquinones from the roots of Salvia lanigera. Planta Med. 1998;64:632–634. doi: 10.1055/s-2006-957536. [DOI] [PubMed] [Google Scholar]
- 53.Sreelatha T., Hymavathi A., Murthy J.M., Rani P.U., Rao J.M., Babu K.S. Bioactivity-guided isolation of mosquitocidal constituents from the rhizomes of Plumbago capensis Thunb. Bioorg. Med. Chem. Lett. 2010;20:2974–2977. doi: 10.1016/j.bmcl.2010.02.107. [DOI] [PubMed] [Google Scholar]
- 54.Kim J.P., Kim W.G., Koshino H., Jung J., Yoo I.D. Sesquiterpene O-naphthoquinones from the root bark of Ulmus davidiana. Phytochemistry. 1996;43:425–430. doi: 10.1016/0031-9422(96)00279-8. [DOI] [PubMed] [Google Scholar]
- 55.Wang D., Xia M.Y., Cui Z., Tashiro S., Onodera S., Ikejima T. Cytotoxic effects of mansonone E and F isolated from Ulmus pumila. Biol. Pharm. Bull. 2004;27:1025–1030. doi: 10.1248/bpb.27.1025. [DOI] [PubMed] [Google Scholar]
- 56.Changwong N., Sabphon C., Ingkaninan K., Sawasdee P. Acetyl- and Butyryl-cholinesterase Inhibitory Activities of Mansorins and Mansonones. Phytother. Res. 2012;26:392–396. doi: 10.1002/ptr.3576. [DOI] [PubMed] [Google Scholar]
- 57.El-Halawany A.M., Chung M.H., Ma C.-M., Komatsu K., Nishihara T., Hattori M. Anti-estrogenic activity of mansorins and mansonones from the heartwood of Mansonia gagei DRUMM. Chem. Pharm. Bull. 2007;55:1332–1337. doi: 10.1248/cpb.55.1332. [DOI] [PubMed] [Google Scholar]
- 58.Panichayupakaranant P., Charoonratana T., Sirikatitham A. RP-HPLC Analysis of Rhinacanthins in Rhinacanthus nasutus: Validation and Application for the Preparation of Rhinacanthin High-Yielding Extract. J. Chromatogr. Sci. 2009;47:705–708. doi: 10.1093/chromsci/47.8.705. [DOI] [PubMed] [Google Scholar]
- 59.Wu T.-S., Tien H.-J., Yeh M.-Y., Lee K.-H. Isolation and cytotoxicity of rhinacanthin-A and -B, two naphthoquinones, from Rhinacanthus nasutus. Phytochemistry. 1988;27:3787–3788. doi: 10.1016/0031-9422(88)83017-6. [DOI] [Google Scholar]
- 60.Luo J., Wu Z.L., Huang X.J., Zhang X.Q., Fan C.L., Ye W.C., Wang Y. Two new pyranonaphthoquinone compounds from Mansoa alliacea (Lam.) A.H.Gentry. China J. Chin. Mat. Med. 2021;46:3364–3367. doi: 10.19540/j.cnki.cjcmm.20210317.201. [DOI] [PubMed] [Google Scholar]
- 61.Kitagawa R.R., Villegas W., Carlos I.Z., Raddi M.S.G. Antitumor and immunomodulatory effects of the naphthoquinone 5-methoxy-3,4-dehydroxanthomegnin. Rev. Bras. Farmacogn. 2011;21:1084–1088. doi: 10.1590/S0102-695X2011005000136. [DOI] [Google Scholar]
- 62.Onegi B., Kraft C., Köhler I., Freund M., Jenett-Siems K., Siems K., Beyer G., Melzig M.F., Bienzle U., Eich E. Herbal remedies traditionally used against malaria part 6 -: Antiplasmodial activity of napthoquinones and one anthraquinone from Stereospermum kunthianum. Phytochemistry. 2002;60:39–44. doi: 10.1016/S0031-9422(02)00072-9. [DOI] [PubMed] [Google Scholar]
- 63.Kittakoop P., Punya J., Kongsaeree P., Lertwerawat Y., Jintasirikul A., Tanticharoen M., Thebtaranonth Y. Bioactive naphthoquinones from Cordyceps unilateralis. Phytochemistry. 1999;52:453–457. doi: 10.1016/S0031-9422(99)00272-1. [DOI] [Google Scholar]
- 64.Ford P.W., Gadepelli M., Davidson B.S. Halawanones A-D, New polycyclic quinones from a marine-derived streptomycete. J. Nat. Prod. 1998;61:1232–1236. doi: 10.1021/np980126y. [DOI] [PubMed] [Google Scholar]
- 65.El-Hady S., Bukuru J., Kesteleyn B., Van Puyvelde L., Nguyen T.V., De Kimpe N. New pyranonaphthoquinone and pyranonaphthohydroquinone from the roots of Pentas longiflora. J. Nat. Prod. 2002;65:1377–1379. doi: 10.1021/np020110e. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto Y., Kinoshita Y., Thor G.R., Hasumi M., Kinoshita K., Koyama K., Takahashi K., Yoshimura I. Isofuranonaphthoquinone derivatives from cultures of the lichen Arthonia cinnabarina (DC.) Wallr. Phytochemistry. 2002;60:741–745. doi: 10.1016/S0031-9422(02)00128-0. [DOI] [PubMed] [Google Scholar]
- 67.Naoe A., Ishibashi M., Yamamoto Y. Cribrarione A, a new antimicrobial naphthoquinone pigment from a myxomycete Cribraria purpurea. Tetrahedron. 2003;59:3433–3435. doi: 10.1016/S0040-4020(03)00514-3. [DOI] [Google Scholar]
- 68.Bezabih M., Motlhagodi S., Abegaz B.M. Isofuranonaphthoquinones and phenolic and knipholone derivatives from the roots of Bulbine capitata. Phytochemistry. 1997;46:1063–1067. doi: 10.1016/S0031-9422(97)00402-0. [DOI] [Google Scholar]
- 69.Piggott M.J., Wege D. The synthesis of 5-hydroxy-3-methylnaphtho[2,3-c]furan-4,9-dione and 5,8-dihydroxy-1-methylnaphtho[2,3-c]furan-4,9-dione. Aust. J. Chem. 2003;56:691–702. doi: 10.1071/CH02253. [DOI] [Google Scholar]
- 70.Ito C., Katsuno S., Kondo Y., Tan H.T., Furukawa H. Chemical constituents of Avicennia alba. Isolation and structural elucidation of new naphthoquinones and their analogues. Chem. Pharm. Bull. 2000;48:339–343. doi: 10.1248/cpb.48.339. [DOI] [PubMed] [Google Scholar]
- 71.Williams R.B., Norris A., Miller J.S., Razafitsalama L.J., Andriantsiferana R., Rasamison V.E., Kingston D.G.I. Two new cytotoxic naphthoquinones from Mendoncia cowanii from the rainforest of Madagascar. Planta Med. 2006;72:564–566. doi: 10.1055/s-2006-931554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gormann R., Kaloga M., Li X.C., Ferreira D., Bergenthal D., Kolodziej H. Furanonaphthoquinones, atraric acid and a benzofuran from the stem barks of Newbouldia laevis. Phytochemistry. 2003;64:583–587. doi: 10.1016/S0031-9422(03)00277-2. [DOI] [PubMed] [Google Scholar]
- 73.Kishore N., Mishra B.B., Tiwari V.K., Tripathi V. Difuranonaphthoquinones from Plumbago zeylanica roots. Phytochem. Lett. 2010;3:62–65. doi: 10.1016/j.phytol.2009.11.007. [DOI] [Google Scholar]
- 74.Cai X.H., Luo X.D., Zhou J., Hao X.J. Quinones from Chirita eburnea. J. Nat. Prod. 2005;68:797–799. doi: 10.1021/np049632f. [DOI] [PubMed] [Google Scholar]
- 75.Verdán M.H., Koolen H.H.F., Salvador M.J., Barison A., Stefanello M.E.A. A New Naphthoquinone from Sinningia leucotricha (Gesneriaceae) Nat. Prod. Commun. 2015;10:625–626. doi: 10.1177/1934578X1501000423. [DOI] [PubMed] [Google Scholar]
- 76.Gupta P.K., Singh P. A naphthoquinone derivative from Tectona grandis (Linn.) J. Asian Nat. Prod. Res. 2004;6:237–240. doi: 10.1080/10286020310001653192. [DOI] [PubMed] [Google Scholar]
- 77.Gafner S., Wolfender J.-L., Nianga M., Hostettmann K. A naphthoquinone from Newbouldia laevis roots. Phytochemistry. 1998;48:215–216. doi: 10.1016/S0031-9422(97)00502-5. [DOI] [Google Scholar]
- 78.Ito C., Kondo Y., Rao K.S., Tokuda H., Nishino H., Furukawa H. Chemical constituents of Glycosmis pentaphylla: Isolation of a novel naphthoquinone and a new acridone alkaloid. Chem. Pharm. Bull. 1999;47:1579–1581. doi: 10.1248/cpb.47.1579. [DOI] [Google Scholar]
- 79.Zhong Y.J., Wen Q.F., Li C.Y., Su X.H., Yuan Z.P., Li Y.F. Two New Naphthoquinone Derivatives from Lysionotus pauciflorus. Helv. Chim. Acta. 2013;96:1750–1756. doi: 10.1002/hlca.201200627. [DOI] [Google Scholar]
- 80.Li Q., Guo Z.H., Wang K.B., Zhang X.S., Lou Y.T., Zhao Y.Q. Two new 1,4-naphthoquinone derivatives from Impatiens balsamina L. flowers. Phytochem. Lett. 2015;14:8–11. doi: 10.1016/j.phytol.2015.08.011. [DOI] [Google Scholar]
- 81.Luo Y., Shen H.Y., Shen Q.X., Cao Z.H., Zhang M., Long S.Y., Wang Z.B., Tan J.W. A new anthraquinone and a new naphthoquinone from the whole plant of Spermacoce latifolia. J. Asian Nat. Prod. Res. 2017;19:869–876. doi: 10.1080/10286020.2017.1279609. [DOI] [PubMed] [Google Scholar]
- 82.Tangmouo J.G., Meli A.L., Komguem J., Kuete V., Ngounou F.N., Lontsi D., Beng V.P., Choudhary M.I., Sondengam B.L. Crassiflorone, a new naphthoquinone from Diospyros crassiflora (Hien) Tetrahedron Lett. 2006;47:3067–3070. doi: 10.1016/j.tetlet.2006.03.006. [DOI] [Google Scholar]
- 83.Hussain H., Krohn K., Ahmad V.U., Miana G.A., Green I.R. Lapachol: An overview. Arkivoc. 2007;2007:145–171. doi: 10.3998/ark.5550190.0008.204. [DOI] [Google Scholar]
- 84.Hasan A., Furumoto T., Begum S., Fukui H. Hydroxysesamone and 2,3-epoxysesamone from roots of Sesamum indicum. Phytochemistry. 2001;58:1225–1228. doi: 10.1016/S0031-9422(01)00357-0. [DOI] [PubMed] [Google Scholar]
- 85.Hayashi K., Chang F.R., Nakanishi Y., Bastow K.F., Cragg G., McPhail A.T., Nozaki H., Lee K.H. Antitumor agents. 233. Lantalueratins A–F, new cytotoxic naphthoquinones from Lantana involucrata. J. Nat. Prod. 2004;67:990–993. doi: 10.1021/np030450f. [DOI] [PubMed] [Google Scholar]
- 86.Shukla Y.N., Srivastava A., Singh S.C., Kumar S. New naphthoquinones from Arnebia hispidissima roots. Planta Med. 2001;67:575–577. doi: 10.1055/s-2001-16470. [DOI] [PubMed] [Google Scholar]
- 87.Ioset J.R., Marston A., Gupta M.P., Hostettmann K. Antifungal and larvicidal cordiaquinones from the roots of Cordia curassavica. Phytochemistry. 2000;53:613–617. doi: 10.1016/S0031-9422(99)00604-4. [DOI] [PubMed] [Google Scholar]
- 88.Sheu J.H., Su J.H., Sung P.J., Wang G.H., Dai C.F. Novel meroditerpenoid—related metabolites from the formosan soft coral Nephthea chabrolii. J. Nat. Prod. 2004;67:2048–2052. doi: 10.1021/np0401314. [DOI] [PubMed] [Google Scholar]
- 89.Winiewski V., Verdán M.H., Oliveira C.S., Su X.H., Yuan Z.P., Li Y.F. Two new naphthoquinone derivatives from Sinningia conspicua (Gesneriaceae) Nat. Prod. Res. 2023;39:88–93. doi: 10.1080/14786419.2023.2253971. [DOI] [PubMed] [Google Scholar]
- 90.Kongkathip N., Luangkamin S., Kongkathip B., Sangma C., Grigg R., Kongsaeree P., Prabpai S., Pradidphol N., Pitaviriyagul S., Siripong P. Synthesis of novel rhinacanthins and related anticancer naphthoquinone esters. J. Med. Chem. 2004;47:4427–4438. doi: 10.1021/jm030323g. [DOI] [PubMed] [Google Scholar]
- 91.Van Puyvelde L., El Hady S., De Kimpe N., Feneau-Dupont J., Declercq J.P. Isagarin, a new type of tetracyclic naphthoquinone from the roots of Pentas longiflora. J. Nat. Prod. 1998;61:1020–1021. doi: 10.1021/np970589o. [DOI] [PubMed] [Google Scholar]
- 92.Aguiar R.M., David J.P., David J.M. Unusual naphthoquinones, catechin and triterpene from Byrsonima microphylla. Phytochemistry. 2005;66:2388–2392. doi: 10.1016/j.phytochem.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 93.Kumar U.S., Aparna P., Rao R.J., Rao T.P., Rao J.M. 1-methyl anthraquinones and their biogenetic precursors from Stereospermum personatum. Phytochemistry. 2003;63:925–929. doi: 10.1016/S0031-9422(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 94.Sankaram A.V.B., Rao A.S., Shoolery J.N. Zeylanone and Isozeylanone, two novel quinones from Plumbago zeylanica. Tetrahedron. 1979;35:1777–1782. doi: 10.1016/0040-4020(79)88008-4. [DOI] [Google Scholar]
- 95.Higa M. A new binaphthoquinone from Diospyros maritima Blume. Chem. Pharm. Bull. 1988;36:3234. doi: 10.1248/cpb.36.3234. [DOI] [PubMed] [Google Scholar]
- 96.Jassbi A.R., Singh P., Jain S., Tahara S. Novel naphthoquinones from Heterophragma adenophyllum. Helv. Chim. Acta. 2004;87:820–824. doi: 10.1002/hlca.200490080. [DOI] [Google Scholar]
- 97.Cai P., Kong F.M., Ruppen M.E., Glasier G., Carter G.T. Hygrocins A and B, naphthoquinone macrolides from Streptomyces hygroscopicus. J. Nat. Prod. 2005;68:1736–1742. doi: 10.1021/np050272l. [DOI] [PubMed] [Google Scholar]
- 98.Costa S.M.O., Lemos T.L.G., Pessoa O.D.L., Assunção J.C.C., Braz-Filho R. Constituintes químicos de Lippia es (Cham.) Verbenaceae. Rev. Bras. Farmacogn. 2002;12((Suppl. S1)):7. doi: 10.1590/S0102-695X2002000300032. [DOI] [Google Scholar]
- 99.Bryshten I., Paprotny Ł., Olszowy-Tomczyk M., Wianowska D. Quantitative Study of Vitamin K in Plants by Pressurized Liquid Extraction and LC-MS/MS. Molecules. 2024;29:4420. doi: 10.3390/molecules29184420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uc-Cachón A.H., Borges-Argáez R., Said-Fernández S., Vargas-Villarreal J., González-Salazar F., Méndez-González M., Cáceres-Farfán M., Molina-Salinas G.M. Naphthoquinones isolated from Diospyros anisandra exhibit potent activity against pan-resistant first-line drugs Mycobacterium tuberculosis strains. Pulm. Pharmacol. Ther. 2014;27:114–120. doi: 10.1016/j.pupt.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y.H., Lin F.X., Tan Y.F., Yang J.Y., Zhang B., Zhou X.M., Song X.M. Three new phenanthrenequinone compounds from the roots of Dendrobium. Chin. J. Org. Chem. 2021;41:2112–2115. doi: 10.6023/cjoc202012005. [DOI] [Google Scholar]
- 102.Sarkar P., Ahmed A., Ray J.K. Suzuki cross coupling followed by cross dehydrogenative coupling: An efficient one pot synthesis of Phenanthrenequinones and analogues. Tetrahedron Lett. 2020;61:151701. doi: 10.1016/j.tetlet.2020.151701. [DOI] [Google Scholar]
- 103.Xuezhao L., Houwei L., Masatake N. Trijuganone A and B: Two New Phenanthrenequinones from Roots of Salvia trijuga. Planta Med. 1990;56:87–88. doi: 10.1055/s-2006-960892. [DOI] [PubMed] [Google Scholar]
- 104.Zhao Y.Y., Cui C.B., Cai B., Han B., Sun Q.S. A new phenanthraquinone from the stems of Bauhinia variegata L. J. Asian Nat. Prod. Res. 2005;7:835–838. doi: 10.1080/10286020410001721140. [DOI] [PubMed] [Google Scholar]
- 105.Bhaskar M.U., Rao L.J.M., Rao N.S.P., Rao P.R.M. Ochrone A, a novel 9,10-dihydro-1,4-phenanthraquinone from Coelogyne ochracea. J. Nat. Prod. 1991;54:386. doi: 10.1021/np50074a006. [DOI] [Google Scholar]
- 106.Lin L.-G., Yang X.-Z., Tang C.-P., Ke C.Q., Zhang J.B., Ye Y. Antibacterial stilbenoids from the roots of Stemona tuberosa. Phytochemistry. 2007;69:457–463. doi: 10.1016/j.phytochem.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 107.Itharat A., Plubrukarn A., Kongsaeree P., Bui T., Keawpradub N., Houghton P.J. Dioscorealides and dioscoreanone, novel cytotoxic naphthofuranoxepins, and 1,4-phenanthraquinone from Dioscorea membranacea Pierre. Org. Lett. 2003;5:2879–2882. doi: 10.1021/ol034926y. [DOI] [PubMed] [Google Scholar]
- 108.Talapatra B., Mukhopadhyay P., Chaudhury P., Talapatra S.K. Denbinobin, a new phenanthraquinone from Dendrobium nobile Lindl (Orchidaceae) Indian J. Chem. Sect. B. 1982;21:386. [Google Scholar]
- 109.Bae E.Y., Oh H., Oh W.K., Kim M.S., Kim B.S., Kim B.Y., Sohn C.B., Osada H., Ahn J.S. A new VHR dual-specificity protein tyrosine phosphatase inhibitor from Dendrobium moniliforme. Planta Med. 2004;70:869–870. doi: 10.1055/s-2004-827238. [DOI] [PubMed] [Google Scholar]
- 110.Harvey R.B., Edrington T.S., Kubena L.F., Rottinghaus G.E., Turk J.R., Genovese K.J., Ziprin R.L., Nisbet D.J. Toxicity of fumonisin from Fusarium verticillioides culture material and moniliformin from Fusarium fujikuroi culture material when fed singly and in combination to growing barrows. J. Food Prot. 2002;65:373–377. doi: 10.4315/0362-028X-65.2.373. [DOI] [PubMed] [Google Scholar]
- 111.Chen D.N., Wu Y.P., Chen Y.J., Liu W.J., Wang J.X., He F., Jiang L. Two new stilbenoids from aerial parts of Flickingeria fimbriata. J. Asian Nat. Prod. Res. 2019;21:117–122. doi: 10.1080/10286020.2017.1392942. [DOI] [PubMed] [Google Scholar]
- 112.Wu T.S., Jong T.T., Tien H.J., Kuoh C.S., Furukawa H., Lee K.H. Annoquinone-A, an antimicrobial and cytotoxic principle from Annona montana. Phytochemistry. 1987;26:1623–1625. doi: 10.1016/S0031-9422(00)82257-8. [DOI] [Google Scholar]
- 113.Tezuka Y., Kasimu R., Basnet P., Namba T., Kadota S. Aldose reductase inhibitory constituents of the root of Salvia miltiorhiza Bunge. Chem. Pharm. Bull. 1997;45:1306–1311. doi: 10.1248/cpb.45.1306. [DOI] [PubMed] [Google Scholar]
- 114.Ju J.H., Yang J.S., Li J., Xiao P.G. Cypripediquinone A, a new phenanthraquinone from Cypripedium macranthum (Orchidaceae) Chin. Chem. Lett. 2000;11:37–38. [Google Scholar]
- 115.Majumder P.L., Sen R.C. Bulbophyllanthrone, a phenanthraquinone from Bulbophyllum odoratissimum. Phytochemistry. 1991;30:2092. doi: 10.1016/0031-9422(91)85078-E. [DOI] [Google Scholar]
- 116.Zhao L.Y. Master’s Thesis. Hubei University of Science and Technology; China: 2023. Study on the Chemical Constituents of Salvia bowleyana Dunn. [Google Scholar]
- 117.Liang W., Sun J.C., Guo F.X., Zhang X.M., Xu B., Chen Y., Li X. Research progress on the synthesis of anthraquinones based on the shikimic acid/o-succinylbenzoic acid pathway and polyketide pathway. Chin. Herb. Med. 2020;51:1939–1950. [Google Scholar]
- 118.Lu J., Guo Y.S., Shi C. Research progress on the sources and pharmacological activities of anthraquinones in medicinal plants. Guiding J. Tradit. Chin. Med. Pharm. 2024;30:111–116. doi: 10.13862/j.cn43-1446/r.2024.01.023. [DOI] [Google Scholar]
- 119.He X.Q. Master’s Thesis. Zhejiang Chinese Medical University; Hangzhou, China: 2024. Re-Evaluation of the Quality of Rhubarb Chinese Medicine Formula Granules and Comparison of Laxative Effects with Rhubarb Chinese Medicine Decoction Pieces. [DOI] [Google Scholar]
- 120.Dong X., Fu J., Yin X., Cao S.L., Li X.C., Lin L.F., Huyiligeqi N.J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016;30:1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang A., Wu C.-H., Biehl E. Unambiguous synthesis and spectral characterization of 1,8-dihydroxy-4-methylanthraquinone. Arkivoc. 2002;2002:80–84. doi: 10.3998/ark.5550190.0003.113. [DOI] [Google Scholar]
- 122.El-Beih A.A., Kawabata T., Koimaru K., Ohta T., Tsukamoto S. Monodictyquinone A: A new antimicrobial anthraquinone from a sea urchin-derived fungus Monodictys sp. Chem. Pharm. Bull. 2007;55:1097–1098. doi: 10.1248/cpb.55.1097. [DOI] [PubMed] [Google Scholar]
- 123.Hind H.G. A coloring matter of Penicillium carmino-violaceum Biourge—The constitution of carviolin. Biochem. J. 1940;34:577. doi: 10.1042/bj0340577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fujimoto H., Nakamura E., Okuyama E., Ishibashi M. Six immunosuppressive features from an ascomycete, Zopfiella longicaudata, found in a screening study monitored by immunomodulatory activity. Chem. Pharm. Bull. 2004;52:1005–1008. doi: 10.1248/cpb.52.1005. [DOI] [PubMed] [Google Scholar]
- 125.Jadulco R., Brauers G., Edrada R.A., Ebel R., Wray V., Sudarsono, Proksch P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002;65:730–733. doi: 10.1021/np010390i. [DOI] [PubMed] [Google Scholar]
- 126.Wang Y.D., Dong W., Zhou K., Liu G.Y., Li L.M., Lou J., Hu Q.F., Yang H.Y. A new anthraquinone compound from the branches of Cassia siamea Lam., a Dai ethnic medicine. Chin. Herb. Med. 2015;46:1727–1729. [Google Scholar]
- 127.Hangsamai N., Photai K., Mahaamnart T., Kanokmedhakul S., Kanokmedhakul K., Senawong T., Pitchuanchom S., Nontakitticharoen M. Four New Anthraquinones with Histone Deacetylase Inhibitory Activity from Ventilago denticulata Roots. Molecules. 2022;27:1088. doi: 10.3390/molecules27031088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li X., Hu X.L., Pan T.F., Dong L., Ding L.L., Wang Z.Z., Song R., Wang X.Z., Wang N., Zhang Y., et al. Kanglexin, a new anthraquinone compound, attenuates lipid accumulation by activating the AMPK/SREBP-2/PCSK9/LDLR signalling pathway. Biomed. Pharmacother. 2021;133:110802. doi: 10.1016/j.biopha.2020.110802. [DOI] [PubMed] [Google Scholar]
- 129.Soonthornchareonnon N., Suwanborirux K., Bavovada R., Patarapanich C., Cassady J.M. New cytotoxic 1-azaanthraquinones and 3-aminonaphthoquinone from the stem bark of Goniothalamus marcanii. J. Nat. Prod. 1999;62:1390–1394. doi: 10.1021/np990197c. [DOI] [PubMed] [Google Scholar]
- 130.Malysheva S.V., Arroyo-Manzanares N., Cary J.W., Ehrlich K.C., Vanden Bussche J., Vanhaecke L., Bhatnagar D., Di Mavungu J.D., De Saeger S. Identification of novel metabolites from Aspergillus flavus by high resolution and multiple stage mass spectrometry. Food Addit. Contam. Part A. 2014;31:111–120. doi: 10.1080/19440049.2013.859743. [DOI] [PubMed] [Google Scholar]
- 131.Kamnaing P., Free S.N.Y.F., Nkengfack A.E., Folefoc G., Fomum Z.T. An isoflavan—quinone and a flavonol from Millettia laurentii. Phytochemistry. 1999;51:829–832. doi: 10.1016/S0031-9422(99)00043-6. [DOI] [Google Scholar]
- 132.Wabo H.K., Kouam S.F., Krohn K., Hussain H., Tala M.F., Tane P., van Ree T., Hu Q.X., Schulz B. Prenylated anthraquinones and other constituents from the seeds of Vismia laurentii. Chem. Pharm. Bull. 2007;55:1640–1642. doi: 10.1248/cpb.55.1640. [DOI] [PubMed] [Google Scholar]
- 133.Bilia A.R., Yusuf A.W., Bracà A., Keita A., Morelli I. New prenylated anthraquinones and xanthones from Vismia guineensis. J. Nat. Prod. 2000;63:16–21. doi: 10.1021/np990226j. [DOI] [PubMed] [Google Scholar]
- 134.Boonnak N., Karalai C., Chantrapromma S., Ponglimanont C., Fun H.K., Kanjana-Opas A. Bioactive prenylated xanthones and anthraquinones from Cratoxylum formosum ssp. pruniflorum. Tetrahedron. 2006;62:8850–8859. doi: 10.1016/j.tet.2006.06.003. [DOI] [Google Scholar]
- 135.Mammo W., Dagne E., Casser I., Steglich W. Unusual anthraquinone lactones from Araliorhamnus vaginata. Phytochemistry. 1990;29:2637. doi: 10.1016/0031-9422(90)85202-Q. [DOI] [Google Scholar]
- 136.Noungoue D.T., Antheaume C., Chaabi M., Lenta Ndjakou B., Ngouela S., Lobstein A., Tsamo E. Anthraquinones from the fruits of Vismia laurentii. Phytochemistry. 2008;69:1024–1028. doi: 10.1016/j.phytochem.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 137.Tchizhova A.Y., Anufriev V.P., Denisenko V.A., Novikov V.L. Synthesis of (±)-ploiariquinones A and B. J. Nat. Prod. 1995;58:1772. doi: 10.1021/np50125a023. [DOI] [Google Scholar]
- 138.Bezabih M., Abegaz B.M. 4′-Demethylknipholone from aerial parts of Bulbine capitata. Phytochemistry. 1998;48:1071–1073. doi: 10.1016/S0031-9422(97)01053-4. [DOI] [Google Scholar]
- 139.Dagne E., Steglich W. Knipholone: A unique anthraquinone derivative from Kniphofia foliosa. Phytochemistry. 1984;23:1729. doi: 10.1016/S0031-9422(00)83479-2. [DOI] [Google Scholar]
- 140.Yenew A., Dagne E., Müller M., Steglich W. An anthrone, an anthraquinone and two oxanthrones from Kniphofia foliosa. Phytochemistry. 1994;37:525. doi: 10.1016/0031-9422(94)85092-5. [DOI] [Google Scholar]
- 141.Abegaz B.M., Bezabih M., Msuta T., Brun R., Menche D., Muehlbacher J., Bringmann G. Gaboroquinones A and B and 4′-O-Demethylknipholone-4′-O-β-D-glucopyranoside, phenylanthraquinones from the roots of Bulbine frutescens. J. Nat. Prod. 2002;65:1117–1121. doi: 10.1021/np0201218. [DOI] [PubMed] [Google Scholar]
- 142.Mutanyatta J., Bezabih M., Abegaz B.M., Dreyer M., Brun R., Kocher N., Bringmann G. The first 6′-O-sulfated phenylanthraquinones: Isolation from Bulbine frutescens, structural elucidation, enantiomeric purity, and partial synthesis. Tetrahedron. 2005;61:8475–8484. doi: 10.1016/j.tet.2005.06.055. [DOI] [Google Scholar]
- 143.Clark B., Capon R.J., Stewart M., Lacey E., Tennant S., Gill J.H. Blanchaquinone: A new anthraquinone from an Australian Streptomyces sp. J. Nat. Prod. 2004;67:1729–1731. doi: 10.1021/np049826v. [DOI] [PubMed] [Google Scholar]
- 144.Tsuda M., Nemoto A., Komaki H., Tanaka Y., Yazawa K., Mikami Y., Kobayashi J. Nocarasins A-C and Brasiliquinone D, new metabolites from the actinomycete Nocardia brasiliensis. J. Nat. Prod. 1999;62:1640–1642. doi: 10.1021/np990265v. [DOI] [Google Scholar]
- 145.Seo E.-K., Kim N.-C., Wani M.C., Wall M.E., Navarro H.A., Burgess J.P., Kawanishi K., Kardono L.B.S., Riswan S., Rose W.C., et al. Cytotoxic prenylated xanthones and the unusual compounds anthraquinobenzophenones from Cratoxylum sumatranum. J. Nat. Prod. 2002;65:299–305. doi: 10.1021/np010395f. [DOI] [PubMed] [Google Scholar]
- 146.Alemayehu G., Woldeyesus B., Abegaz B.M. (+)-Floribundone 3 from the pods of Senna septentrionalis. Bull. Chem. Soc. Ethiop. 1997;11:25–29. doi: 10.4314/bcse.v11i1.21010. [DOI] [Google Scholar]
- 147.Zhang C., Ondeyka J.G., Zink D.L., Basilio A., Vicente F., Collado J., Platas G., Bills G., Huber J., Dorso K., et al. Isolation, structure, and antibacterial activity of phaeosphenone from a Phaeosphaeria sp. discovered by antisense strategy. J. Nat. Prod. 2008;71:1304–1307. doi: 10.1021/np8001833. [DOI] [PubMed] [Google Scholar]
- 148.Wirz A., Simmen U., Heilmann J., Calis I., Meier B., Sticher O. Bisanthraquinone glycosides of Hypericum perforatum with binding inhibition to CRH-1 receptors. Phytochemistry. 2000;55:941–947. doi: 10.1016/S0031-9422(00)00305-8. [DOI] [PubMed] [Google Scholar]
- 149.Li C., Shi J.-G., Zhang Y.-P., Zhang C.-Z. Constituents of Eremurus chinensis. J. Nat. Prod. 2000;63:653–656. doi: 10.1021/np9904915. [DOI] [PubMed] [Google Scholar]
- 150.Jing Y., Yang J., Wang Y., Yang X.S., Mu S.Z. Anthraquinone-benzisochromanquinone dimers from Berchemia polyphylla var. leioclada. Chin. Pharm. J. 2011;46:661–664. [Google Scholar]
- 151.Takahashi S., Kitanaka S., Takido M., Sankawa U., Shibata S. Phlegmacins and anhydrophlegmacinquinones: Dimeric hydroanthracenes from seedlings of Cassia torosa. Phytochemistry. 1977;16:999–1002. doi: 10.1016/S0031-9422(00)86709-6. [DOI] [Google Scholar]
- 152.Alemayehu G., Abegaz B.M. Bianthraquinones from the seeds of Senna multiglandulosa. Phytochemistry. 1996;41:919. doi: 10.1016/0031-9422(95)00645-1. [DOI] [Google Scholar]
- 153.Gill M., Morgan P.M. Pigments of fungal. Part 73. Absolute stereochemistry of fungal metabolites: Icterinoidins A1 and B1, and atrovirins B1 and B2. Arkivoc. 2004;2004:152–165. doi: 10.3998/ark.5550190.0005.a15. [DOI] [Google Scholar]
- 154.Tsaffack M., Nguemeving J.R., Kuete V., Ndejouong Tchize Ble S., Mkounga P., Penlap Beng V., Hultin P.G., Tsamo E., Nkengfack A.E. Two new antimicrobial dimeric compounds: Febrifuquinone, a vismione-anthraquinone coupled pigment and adamabianthrone, from two Psorospermum species. Chem. Pharm. Bull. 2009;57:1113–1118. doi: 10.1248/cpb.57.1113. [DOI] [PubMed] [Google Scholar]
- 155.Kanokmedhakul S., Kanokmedhakul K., Phonkerd N., Soytong K., Kongsaeree P., Suksamrarn A. Antimycobacterial anthraquinone-chromanone compound and diketopiperazine alkaloid from the fungus Chaetomium globosum KMITL-N0802. Planta Med. 2002;68:834–836. doi: 10.1055/s-2002-34415. [DOI] [PubMed] [Google Scholar]
- 156.Kuroda M., Mimaki Y., Sakagami H., Sashida Y. Bulbinelonesides A-E, phenylanthraquinone glycosides from the roots of Bulbinella floribunda. J. Nat. Prod. 2003;66:894–897. doi: 10.1021/np030061l. [DOI] [PubMed] [Google Scholar]
- 157.Wu T.-S., Lin D.-M., Shi L.-S., Damu A.G., Kuo P.-C., Kuo Y.-H. Cytotoxic anthraquinones from the stems of Rubia wallichiana Decne. Chem. Pharm. Bull. 2003;51:948–950. doi: 10.1248/cpb.51.948. [DOI] [PubMed] [Google Scholar]
- 158.Perman D., Lajis N.H., Othman A.G., Ali A.M., Aimi N., Kitajima M., Takayama H. Anthraquinones from Hedyotis herbacea. J. Nat. Prod. 1999;62:1430–1431. doi: 10.1021/np990101e. [DOI] [PubMed] [Google Scholar]
- 159.Pawlus A.D., Su B.-N., Keller W.J., Kinghorn A.D. An anthraquinone with potent quinone reductase-inducing activity and other constituents of the fruits of Morinda citrifolia (Noni) J. Nat. Prod. 2005;68:1720–1722. doi: 10.1021/np050383k. [DOI] [PubMed] [Google Scholar]
- 160.El-Gamal A.A., Takeya K., Itokawa H., Falim A.F., Amer M.M., Saad H.-E.A., Awad S.A. Anthraquinones from Galium sinaicum. Phytochemistry. 1995;40:245. doi: 10.1016/0031-9422(95)00145-W. [DOI] [PubMed] [Google Scholar]
- 161.Zhang C.L., Guan H., Xi P.Z., Deng T., Gao J.M. Anthraquinones from the roots of Prismatomeris tetrandra. Nat. Prod. Commun. 2010;5:1251–1252. doi: 10.1177/1934578X1000500821. [DOI] [PubMed] [Google Scholar]
- 162.Feng Z.M., Jiang J.S., Wang Y.H., Zhang P.C. Anthraquinones from the roots of Prismatomeris tetrandra. Chem. Pharm. Bull. 2005;53:1330–1332. doi: 10.1248/cpb.53.1330. [DOI] [PubMed] [Google Scholar]
- 163.Chen X.Y., Zhang C., Dong Y.R., Zang Y.D., Chen J.Q., Jin H.T., Zhang D.M. Three new anthraquinoids from Radix Dalbergiae (Huanggen) and their neuroprotective effects on nerve cells. Acta Pharm. Sin. B. 2023;58:3710–3714. doi: 10.16438/j.0513-4870.2023-1104. [DOI] [Google Scholar]
- 164.Deng S., West B.J., Jensen C.J., Basar S., Westendorf J. Development and validation of an RP-HPLC method for the analysis of anthraquinones in noni fruits and leaves. Food Chem. 2009;116:505–508. doi: 10.1016/j.foodchem.2009.02.070. [DOI] [Google Scholar]
- 165.Akihisa T., Matsumoto K., Tokuda H., Yasukawa K., Seino K.-i., Nakamoto K., Kuninaga H., Suzuki T., Kimura Y. Anti-inflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (Noni) J. Nat. Prod. 2007;70:754–757. doi: 10.1021/np068065o. [DOI] [PubMed] [Google Scholar]
- 166.Komura K., Mizukawa Y., Minami H., Qin G.W., Xu R.S. New anthraquinones from Eleutherine americana. Chem. Pharm. Bull. 1983;31:4206–4208. doi: 10.1248/cpb.31.4206. [DOI] [Google Scholar]
- 167.Mahabusarakam W., Hemtasin C., Chakthong S., Voravuthikunchai S.P., Olawumi I.B. Naphthoquinones, anthraquinones and naphthalene derivatives from the bulbs of Eleutherine americana. Planta Med. 2010;76:345–349. doi: 10.1055/s-0029-1186143. [DOI] [PubMed] [Google Scholar]
- 168.Paudel P., Kim D.H., Jeon J., Park S.E., Seong S.H., Jung H.A., Choi J.S. Neuroprotective Effect of Aurantio-Obtusin, a Putative Vasopressin V(1A) Receptor Antagonist, on Transient Forebrain Ischemia Mice Model. Int. J. Mol. Sci. 2021;22:3335. doi: 10.3390/ijms22073335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arsenault G.P. Fungal metabolites II. Structure of bostrycoidin, a β-azaanthraquinone from Fusarium solani D2 purple. Tetrahedron Lett. 1965;6:4033. doi: 10.1016/S0040-4039(01)99610-8. [DOI] [Google Scholar]
- 170.Ismail N.H., Ali A.M., Aimi N., Kitajima M., Takayama H., Lajis N.H. Anthraquinones from Morinda elliptica. Phytochemistry. 1997;45:1723–1725. doi: 10.1016/S0031-9422(97)00252-5. [DOI] [Google Scholar]
- 171.Kumar U.S., Tiwari A.K., Reddy S.V., Aparna P., Rao R.J., Ali A.Z., Rao J.M. Free-radical-scavenging and xanthine oxidase inhibitory constituents from Stereospermum personatum. J. Nat. Prod. 2005;68:1615–1621. doi: 10.1021/np058036y. [DOI] [PubMed] [Google Scholar]
- 172.Son J.K., Jung S.J., Jung J.H., Fang Z., Lee C.S., Seo C.S., Moon D.C., Min B.S., Kim M.R., Woo M.H. Anticancer constituents from the roots of Rubia cordifolia L. Chem. Pharm. Bull. 2008;56:213–216. doi: 10.1248/cpb.56.213. [DOI] [PubMed] [Google Scholar]
- 173.Chan H.-H., Li C.-Y., Damu A.G., Wu T.S. Anthraquinones from Ophiorrhiza hayatana Ohwi. Chem Pharm. Bull. 2005;53:1232–1235. doi: 10.1248/cpb.53.1232. [DOI] [PubMed] [Google Scholar]
- 174.Yang L., Pei-Juan X., Ze-Nai C., Liu G.-M. The anthraquinones of Rhynchotechum vestitum. Phytochemistry. 1998;47:315–317. doi: 10.1016/S0031-9422(97)00561-X. [DOI] [Google Scholar]
- 175.Chiriboga X., Gilardoni G., Magnaghi I., Finzi P.V., Zanoni G., Vidari G. New anthracene derivatives from Coussarea macrophylla. J. Nat. Prod. 2003;66:905–909. doi: 10.1021/np030066i. [DOI] [PubMed] [Google Scholar]
- 176.Xiang W., Song Q.S., Zhang H.J., Guo S.P. Antimicrobial anthraquinones from Morinda angustifolia. Fitoterapia. 2008;79:501–504. doi: 10.1016/j.fitote.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 177.Ling S.-K., Komorita A., Tanaka T., Fujioka T., Mihashi K., Kouno I. Iridoids and anthraquinones from the Malaysian medicinal plant, Saprosma scortechinii (Rubiaceae) Chem. Pharm. Bull. 2002;50:1035–1040. doi: 10.1248/cpb.50.1035. [DOI] [PubMed] [Google Scholar]
- 178.Qiu B.-L., Xu L.-X., Wei X.-Y., Kang M., Lin L.-d. Anthraquinones from Hemiboea subcapitata. Redai Yaredai Zhiwu Xuebao (J. Trop. Subtrop. Bot.) 2014;22:507–510. [Google Scholar]
- 179.Yoo N.H., Jang D.S., Lee Y.M., Jeong I.H., Cho J.-H., Kim J.-H., Kim J.S. Anthraquinones from the roots of Knoxia valerianoides inhibit the formation of advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharmacal. Res. 2010;33:209–214. doi: 10.1007/s12272-010-0204-7. [DOI] [PubMed] [Google Scholar]
- 180.Núñez Montoya S.C., Agnese A.M., Cabrera J.L. Anthraquinone derivatives from Heterophyllaea pustulata. J. Nat. Prod. 2006;69:801–803. doi: 10.1021/np050181o. [DOI] [PubMed] [Google Scholar]
- 181.Lin W.Y., Peng C.F., Tsai I.L., Chen J.J., Cheng M.J., Chen I.S. Antitubercular constituents from the roots of Engelhardia roxburghiana. Planta Med. 2005;71:171–175. doi: 10.1055/s-2005-837786. [DOI] [PubMed] [Google Scholar]
- 182.Nagaoka S.-I., Uno H., Huppert D. Ultrafast excited-state intramolecular proton transfer of aloesaponarin I. J. Phys. Chem. B. 2013;117:4347–4353. doi: 10.1021/jp306870y. [DOI] [PubMed] [Google Scholar]
- 183.Yang Y., Liu Y., Yang D., Li H., Jiang K., Sun J.F. Photoinduced excited state intramolecular proton transfer and spectral behaviors of aloesaponarin 1. Spectrochim. Acta Part A. 2015;151:814–820. doi: 10.1016/j.saa.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 184.Hemlata K., Kalidhar S.B. Alatinone, an anthraquinone from Cassia alata. Phytochemistry. 1993;32:1616. doi: 10.1016/0031-9422(93)85192-T. [DOI] [Google Scholar]
- 185.Kelly T.R., Ma Z., Xu W. Revision of the structure of przewalskinone B. Tetrahedron Lett. 1992;33:7713. doi: 10.1016/0040-4039(93)88024-D. [DOI] [Google Scholar]
- 186.Gruen H., Görner H. Photoreduction of 2-methyl-1-nitro-9,10-anthraquinone in the presence of 1-phenylethanol. Photochem. Photobiol. Sci. 2008;7:1344–1352. doi: 10.1039/b811372f. [DOI] [PubMed] [Google Scholar]
- 187.Chen B., Wang D.Y., Ye Q., Li B.G., Zhang G.L. Anthraquinones from Gladiolus gandavensis. J. Asian Nat. Prod. Res. 2005;7:197–204. doi: 10.1080/10286020310001653336. [DOI] [PubMed] [Google Scholar]
- 188.Van Wagoner R.M., Mantle P.G., Wright J.L.C. Biosynthesis of scorpinone, a 2-azaanthraquinone from Amorosia littoralis, a fungus from marine sediment. J. Nat. Prod. 2008;71:426–430. doi: 10.1021/np070614i. [DOI] [PubMed] [Google Scholar]
- 189.Scoles D.R., Gandelman M., Paul S., Dexheimer T., Dansithong W., Figueroa K.P., Pflieger L.T., Redlin S., Kales S.C., Sun H. A quantitative high-throughput screen identifies compounds that lower expression of the SCA2- and ALS-associated gene ATXN2. J. Biol. Chem. 2022;298:102228. doi: 10.1016/j.jbc.2022.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Goulart M.O.F., Sant’Anna A.E.G., de Oliveira A.B., De Oliveira G.G., Maia J.G.S. Azafluorenones and azaanthraquinone from Guatteria dielsiana. Phytochemistry. 1986;25:1691. doi: 10.1016/S0031-9422(00)81237-6. [DOI] [Google Scholar]
- 191.Mal D., Ray S. First synthesis of 9,10-dimethoxy-2-methyl-1,4-anthraquinone: A naturally occurring unusual anthraquinone. Eur. J. Org. Chem. 2008;2008:3014–3020. doi: 10.1002/ejoc.200800218. [DOI] [Google Scholar]
- 192.Qi F., Zhang W., Xue Y., Geng C., Jin Z.G., Li J.B., Guo Q., Huang X.N., Lu X.F. Microbial production of the plant-derived fungicide physcion. Metab. Eng. 2022;74:130–138. doi: 10.1016/j.ymben.2022.10.007. [DOI] [PubMed] [Google Scholar]
- 193.Sardashti-Birjandi A., Mollashahi E., Maghsoodlou M.T., Yazdani-Elah-Abadi A. Green and catalyst-free synthesis of aminoanthraquinone derivatives in solvent-free conditions. Res. Chem. Intermed. 2021;47:3597–3608. doi: 10.1007/s11164-021-04485-9. [DOI] [Google Scholar]
- 194.Kouam S.F., Khan S.N., Krohn K., Ngadjui B.T., Kapche D.G., Yapna D.B., Zareem S., Moustafa A.M., Choudhary M.I. Alpha-glucosidase inhibitory anthranols, kenganthranols A-C, from the stem bark of Harungana madagascariensis. J. Nat. Prod. 2006;69:229–233. doi: 10.1021/np050407n. [DOI] [PubMed] [Google Scholar]
- 195.Eyong K.O., Krohn K., Hussain H., Folefoc G.N., Nkengfack A.E., Schulz B., Hu Q.X. Newbouldiaquinone and newbouldiamide: A new naphthoquinone-anthraquinone coupled pigment and a new ceramide from Newbouldia laevis. Chem. Pharm. Bull. 2005;53:616–619. doi: 10.1248/cpb.53.616. [DOI] [PubMed] [Google Scholar]
- 196.Eyong K.O., Folefoc G.N., Kuete V., Beng V.P., Krohn K., Hussain H., Nkengfack A.E., Saeftel M., Sarite S.R., Hoerauf A. Newbouldiaquinone A: A naphthoquinone-anthraquinone ether coupled pigment, as a potential antimicrobial and antimalarial agent from Newbouldia laevis. Phytochemistry. 2006;67:605–609. doi: 10.1016/j.phytochem.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 197.Aguinaldo A.M., Ocampo O.P.M., Bowden B.F., Gray A.I., Waterman P.G. Tectograndone, an anthraquinone-naphthoquinone pigment from the leaves of Tectona grandis. Phytochemistry. 1993;33:933. doi: 10.1016/0031-9422(93)85309-F. [DOI] [Google Scholar]
- 198.Alemayehu G., Abegaz B., Kraus W. A 1,4-anthraquinone-dihydroanthracenone dimer from Senna sophera. Phytochemistry. 1998;48:699–702. doi: 10.1016/S0031-9422(98)00031-4. [DOI] [Google Scholar]
- 199.Hou Y.P., Cao S.G., Brodie P.J., Callmander M.W., Ratovoson F., Rakotobe E.A., Rasamison V.E., Ratsimbason M., Alumasa J.N., Roepe P.D., et al. Antiproliferative and antimalarial anthraquinones of Scutia myrtina from the Madagascar forest. Bioorg. Med. Chem. 2009;17:2871–2876. doi: 10.1016/j.bmc.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang L., Hu H., Cai W., Chen S., Sheng P., Fu X. CaCO3-complexed pH-responsive nanoparticles encapsulating mitoxantrone and celastrol enhance tumor chemoimmunotherapy. Pt AInt. J. Pharm. 2024;667:124860. doi: 10.1016/j.ijpharm.2024.124860. [DOI] [PubMed] [Google Scholar]
- 201.Krenn L., Presser A., Pradhan R., Bahr B., Paper D.H., Mayer K.K., Kopp B. Sulfemodin 8-O-β-D-glucoside, a new sulfated anthraquinone glycoside, and antioxidant phenolic compounds from Rheum emodi. J. Nat. Prod. 2003;66:1107–1109. doi: 10.1021/np0301442. [DOI] [PubMed] [Google Scholar]
- 202.Xia Z.X., Tang Z.Y., An R., Chen Y., Zhang Y.Z., Wang X.H. A new anthraquinone glycoside from Rheum officinale. Acta Pharm. Sin. B. 2012;47:1183–1186. doi: 10.16438/j.0513-4870.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 203.Tomasin R., Ferreira I.C., Sawaya A.C.H.F., Mazzafera P., Pascoal A.C.R.F., Salvador M.J., Gomes-Marcondes M.C.C. Honey and Aloe vera Solution Increases Survival and Modulates the Tumor Size In Vivo. Mol. Nutr. Food Res. 2024;68:e2400378. doi: 10.1002/mnfr.202400378. [DOI] [PubMed] [Google Scholar]
- 204.Zhang C., Wang R., Liu B., Tu G. Structure elucidation of a sodium salified anthraquinone from the seeds of Cassia obtusifolia by NMR technique assisted with acid-alkali titration. Magn. Reson. Chem. 2011;49:529–532. doi: 10.1002/mrc.2771. [DOI] [PubMed] [Google Scholar]
- 205.Qhotsokoane-Lusunzi M.E.A., Karuso P. Secondary metabolites from Basotho medicinal plants. I Bulbine narcissifolia. J. Nat. Prod. 2001;64:1368–1372. doi: 10.1021/np010279c. [DOI] [PubMed] [Google Scholar]
- 206.Mei R., Liang H., Wang J., Zeng L.H., Lu Q., Cheng Y.X. New seco-anthraquinone glucosides from Rumex nepalensis. Planta Med. 2009;75:1162–1164. doi: 10.1055/s-0029-1185467. [DOI] [PubMed] [Google Scholar]
- 207.Li B., Zhang D.-M., Luo Y.-M., Chen X.-G. Three new and antitumor anthraquinone glycosides from Lasianthus acuminatissimus Merr. Chem. Pharm. Bull. 2006;54:297–300. doi: 10.1248/cpb.54.297. [DOI] [PubMed] [Google Scholar]
- 208.Huang T., Ming J., Zhong J., Zhong Y., Wu H., Liu H., Li B. Three new anthraquinones, one new benzochromene and one new furfural glycoside from Lasianthus acuminatissimus. Nat. Prod. Res. 2019;33:1916–1923. doi: 10.1080/14786419.2018.1480617. [DOI] [PubMed] [Google Scholar]
- 209.Calis I., Tasdemir D., Ireland C.M., Sticher O. Lucidin type anthraquinone glycosides from Putoria calabrica. Chem. Pharm. Bull. 2002;50:701–702. doi: 10.1248/cpb.50.701. [DOI] [PubMed] [Google Scholar]
- 210.Lee W., Ku S.-K., Kim T.H., Bae J.S. Emodin-6-O-β-D-glucoside inhibits HMGB1-induced inflammatory responses in vitro and in vivo. Food Chem. Toxicol. 2013;52:97–104. doi: 10.1016/j.fct.2012.10.061. [DOI] [PubMed] [Google Scholar]
- 211.Chang L.C., Chávez D., Gills J.J., Fong H.H.S., Pezzuto J.M., Kinghorn A.D. Rubiasins A-C, new anthracene derivatives from the roots and stems of Rubia cordifolia. Tetrahedron Lett. 2000;41:7157–7162. doi: 10.1016/S0040-4039(00)01205-3. [DOI] [Google Scholar]
- 212.Rossi D., Ahmed K.M., Gaggeri R., Della Volpe S., Maggi L., Mazzeo G., Longhi G., Abbate S., Corana F., Martino E., et al. (R)-(−)-Aloesaponol III 8-Methyl Ether from Eremurus persicus: A Novel Compound against Leishmaniosis. Molecules. 2017;22:519. doi: 10.3390/molecules22040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Krenn L., Pradhan R., Presser A., Reznicek G., Kopp B. Anthrone C-glucosides from Rheum emodi. Chem. Pharm. Bull. 2004;52:391–393. doi: 10.1248/cpb.52.391. [DOI] [PubMed] [Google Scholar]
- 214.Thurman P.F., Chai W., Rosankiewicz J.R., Rogers H.J., Lawson A.M., Draper P. Possible intermediates in the biosynthesis of mycoside B by Mycobacterium microti. Eur. J. Biochem. 1993;212:705–711. doi: 10.1111/j.1432-1033.1993.tb17708.x. [DOI] [PubMed] [Google Scholar]
- 215.Rodríguez-Gamboa T., Victor S.R., Fernandes J.B., Fo E.R., Silva M.d.G.d., Vieira P.C., Pagnocca F.C., Bueno O.C., Hebling M.J.A., Castro O.C. Anthrone and oxanthrone C,O-diglycosides from Picramnia teapensis. Phytochemistry. 2000;55:837–841. doi: 10.1016/S0031-9422(00)00323-X. [DOI] [PubMed] [Google Scholar]
- 216.Diaz F., Chai H.B., Mi Q., Su B.-N., Vigo J.S., Graham J.G., Cabieses F., Farnsworth N.R., Cordell G.A., Pezzuto J.M., et al. Anthrone and oxanthrone C-glycosides from Picramnia latifolia collected in Peru. J. Nat. Prod. 2004;67:352–356. doi: 10.1021/np030479j. [DOI] [PubMed] [Google Scholar]
- 217.Flamini G., Catalano S., Caponi C., Panizzi L., Morelli I. Three anthrones from Rubus ulmifolius. Phytochemistry. 2002;59:873–876. doi: 10.1016/S0031-9422(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 218.Habtemariam S. Knipholone anthrone from Kniphofia foliosa induces a rapid onset of necrotic cell death in cancer cells. Fitoterapia. 2010;81:1013–1019. doi: 10.1016/j.fitote.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 219.Ndjakou Lenta B., Ngouela S., Fekam Boyom F., Tantangmo F., Tchouya G.R.F., Tsamo E., Gut J., Rosenthal P.J., Connolly J.D. Anti-plasmodial activity of some constituents of the root bark of Harungana madagascariensis Lam. (Hypericaceae) Chem. Pharm. Bull. 2007;55:464–467. doi: 10.1248/cpb.55.464. [DOI] [PubMed] [Google Scholar]
- 220.Kouam S.F., Yapna D.B., Krohn K., Ngadjui B.T., Ngoupayo J., Choudhary M.I., Schulz B. Antimicrobial prenylated anthracene derivatives from the leaves of Harungana madagascariensis. J. Nat. Prod. 2007;70:600–603. doi: 10.1021/np060556l. [DOI] [PubMed] [Google Scholar]
- 221.Wanjohi J.M., Yenew A., MIDIWO J.O., Heydenreich M., Peter M.G., Dreyer M., Reichert M., Bringmann G. Three dimeric anthracene derivatives from the fruits of Bulbine abyssinica. Tetrahedron. 2005;61:2667–2674. doi: 10.1016/j.tet.2005.01.040. [DOI] [Google Scholar]
- 222.Fiedler H.P., Dieter A., Gulder T.A., Kajahn I., Hamm A., Brown R., Jones A.L., Goodfellow M., Müller W.E., Bringmann G. Genoketides A1 and A2, new octaketides and biosynthetic intermediates of chrysophanol produced by Streptomyces sp. AK 671. J. Antibiot. 2008;61:464–473. doi: 10.1038/ja.2008.63. [DOI] [PubMed] [Google Scholar]
- 223.Elsworth C., Gill M., Saubern S. Biosynthesis of tetrahydroanthraquinones in fungi. Phytochemistry. 2000;55:23–27. doi: 10.1016/S0031-9422(00)00181-3. [DOI] [PubMed] [Google Scholar]
- 224.Kan E., Katsuyama Y., Maruyama J.I., Tamano K., Koyama Y., Ohnishi Y. Efficient heterologous production of atrochrysone carboxylic acid-related polyketides in an Aspergillus oryzae host with enhanced malonyl-coenzyme A supply. J. Gen. Appl. Microbiol. 2020;66:195–199. doi: 10.2323/jgam.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 225.Sánchez M., González-Burgos E., Iglesias I., Gómez-Serranillos M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules. 2020;25:1324. doi: 10.3390/molecules25061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Botta B., Delle Monache F., Delle Monache G., Menichini F. Psorolactones and other metabolites from Psorospermum glaberrimum. Tetrahedron. 1988;44:7193–7198. doi: 10.1016/S0040-4020(01)86089-0. [DOI] [Google Scholar]
- 227.Chevalier Q., Gallé J.B., Wasser N., Mazan V., Villette C., Mutterer J., Elustondo M.M., Girard N., Elhabiri M., Schaller H., et al. Unravelling the puzzle of anthranoid metabolism in living plant cells using spectral imaging coupled to mass spectrometry. Metabolites. 2021;11:571. doi: 10.3390/metabo11090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mbwambo Z.H., Apers S., Moshi M.J., Kapingu M.C., Van Miert S., Claeys M., Brun R., Cos P., Pieters L., Vlietinck A. Anthranoid compounds with antiprotozoal activity from Vismia orientalis. Planta Med. 2004;70:706–710. doi: 10.1055/s-2004-827199. [DOI] [PubMed] [Google Scholar]
- 229.Tiani G.M., Ahmed I., Krohn K., Green I.R., Nkengfack A.E. Kenganthranol F, a new anthranol from Psorospermum aurantiacum. Nat. Prod. Commun. 2013;8:103–104. doi: 10.1177/1934578X1300800123. [DOI] [PubMed] [Google Scholar]
- 230.Li Y., Li X., Lee U., Kang J.S., Choi H.D., Sona B.W. A new radical scavenging anthracene glycoside, asperflavin ribofuranoside, and polyketides from a marine isolate of the fungus Microsporum. Chem. Pharm. Bull. 2006;54:882–883. doi: 10.1248/cpb.54.882. [DOI] [PubMed] [Google Scholar]
- 231.Lv L., Tian X.Y., Fang W.S. Three new antioxidant C-glucosylanthrones from Aloe nobilis. J. Asian Nat. Prod. Res. 2010;12:443–447. doi: 10.1080/10286020.2010.490211. [DOI] [PubMed] [Google Scholar]
- 232.Solis P.N., Ravelo A.G., Gonzalez A.G., Gupta M.P., Phillipson J.D. Bioactive anthraquinone glycosides from Picramnia antidesma spp. fessonia. Phytochemistry. 1995;38:477–480. doi: 10.1016/0031-9422(94)00598-N. [DOI] [PubMed] [Google Scholar]
- 233.Hernández-Medel R., Pereda-Miranda R. Cytotoxic anthraquinone derivatives from Picramnia antidesma. Planta Med. 2002;68:556–558. doi: 10.1055/s-2002-32562. [DOI] [PubMed] [Google Scholar]
- 234.Dagne E., Bisrat D., Van Wyk B.E., Viljoen A., Hellwig V., Steglich W. Anthrones from Aloe microstigma. Phytochemistry. 1997;44:1271–1274. doi: 10.1016/S0031-9422(96)00710-8. [DOI] [Google Scholar]
- 235.Farah M.H., Andersson R., Samuelsson G. Microdontin A and B: Two new aloin derivatives from Aloe microdonta. Planta Med. 1992;58:88–93. doi: 10.1055/s-2006-961397. [DOI] [PubMed] [Google Scholar]
- 236.Rho T., Kil H.W., Seo Y.J., Shin K.J., Wang D., Yoon K.D. Isolation of six anthraquinone diglucosides from cascara sagrada bark by high-performance countercurrent chromatography. J. Sep. Sci. 2020;43:4036–4046. doi: 10.1002/jssc.202000597. [DOI] [PubMed] [Google Scholar]
- 237.Rodríguez-Gamboa T., Fernandes J.B., Fo E.R., da Silva M.F.D.G.F., Vieira P.C., Castro O.C. Two anthrones and one oxanthrone from Picramnia teapensis. Phytochemistry. 1999;51:583–586. doi: 10.1016/S0031-9422(99)00023-0. [DOI] [PubMed] [Google Scholar]
- 238.Yang J.B., Li L., Dai Z., Wu Y., Geng X.C., Li B., Ma S.C., Wang A.G., Su Y.L. Polygonumnolides C1-C4; minor dianthrone glycosides from the roots of Polygonum multiflorum Thunb. J. Asian Nat. Prod. Res. 2016;18:813–822. doi: 10.1080/10286020.2016.1171758. [DOI] [PubMed] [Google Scholar]
- 239.Rideout J., Sutherland I. Pigments of marine animals. XV Bianthrones and related polyketides from Lamprometra palmata gyges and other species of crinoids. Aust. J. Chem. 1985;38:793–808. doi: 10.1071/CH9850793. [DOI] [Google Scholar]
- 240.Overy D.P., Berrue F., Correa H., Hanif N., Hay K., Lanteigne M., Mquilian K., Duffy S., Boland P., Jagannathan R., et al. Sea foam as a source of fungal inoculum for the isolation of biologically active natural products. Mycology. 2014;5:130–144. doi: 10.1080/21501203.2014.931893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cohen P.A., Towers G.H.N. The anthraquinones of Heterodermia obscurata. Phytochemistry. 1995;40:911–915. doi: 10.1016/0031-9422(95)00407-X. [DOI] [Google Scholar]
- 242.Politi M., Sanogo R., Ndjoko K., Guilet D., Wolfender J.L., Hostettmann K., Morelli I. HPLC-UV/PAD and HPLC-MS(n) analyses of leaf and root extracts of Vismia guineensis and ion and identification of two new bianthrones. Phytochem. Anal. 2010;15:364. doi: 10.1002/pca.788. [DOI] [PubMed] [Google Scholar]
- 243.Form I.C., Bonus M., Gohlke H., Lin W., Daletos G., Proksch P. Xanthone, benzophenone and bianthrone derivatives from the hypersaline lake-derived fungus Aspergillus wentii. Bioorg. Med. Chem. 2019;27:115005. doi: 10.1016/j.bmc.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 244.Meirelles G.D.C., Bridi H., Rates S.M.K., Poser G.L.V. Southern Brazilian Hypericum species, promising sources of bioactive metabolites. Stud. Nat. Prod. Chem. 2018;59:491–507. [Google Scholar]
- 245.Aly A.H., Debbab A., Clements C., Edrada-Ebel R.A., Orlikova B., Diederich M., Wray V., Lin W.H., Proksch P. NF-κB inhibitors and antitrypanosomal metabolites from endophytic fungus Penicillium sp. isolated from Limonium tubiflorum. Bioorg. Med. Chem. 2011;19:414–421. doi: 10.1016/j.bmc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 246.Yang J., Yan Z., Ren J., Su Y. Polygonumnolides A1-B3, minor dianthrone derivatives from the roots of Polygonum multiflorum Thunb. Arch. Pharm. Res. 2018;41:617–624. doi: 10.1007/s12272-016-0816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yang J.B., Tian J.Y., Dai Z., Ye F., Ma S.C., Wang A.G. α-Glucosidase inhibitors extracted from the roots of Polygonum multiflorum Thunb. Fitoterapia. 2017;117:65–70. doi: 10.1016/j.fitote.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 248.Lenta B.N., Devkota K.P., Ngouela S., Boyom F.F., Naz Q., Choudhary M.I., Tsamo E., Rosenthal P.J., Sewald N. Anti-plasmodial and cholinesterase inhibiting activities of some constituents of Psorospermum glaberrimum. Chem. Pharm. Bull. 2010;56:222–226. doi: 10.1248/cpb.56.222. [DOI] [PubMed] [Google Scholar]
- 249.Le P., Mai F., Guéritte V., Dumontet M., Van T. Cytotoxicity of rhamnosylanthraquinones and rhamnosylanthrones from Rhamnus nepalensis. J. Nat. Prod. 2001;64:1162–1168. doi: 10.1021/np010030v. [DOI] [PubMed] [Google Scholar]
- 250.Botta B., Dall’Olio G., Ferrari F., Vinciguerra V., Scurria R., Iacomacci P., Ferrari F., Delle Monache G., Misiti D. Cell suspension cultures of Cassia didymobotrya: Mization of growth and secondary metabolite production by application of the orthogonal n method. J. Plant Physiol. 1989;135:290–294. doi: 10.1016/S0176-1617(89)80121-X. [DOI] [Google Scholar]
- 251.Nawong B., Karalai C., Chantrapromma S., Ponglimanont C., Kanjana-Opas A., Chantrapromma K., Fun H.-K. Quinonoids from the barks of Cratoxylum formosum subsp. pruniflorum. Can. J. Chem. 2007;85:341–345. doi: 10.1139/v07-031. [DOI] [Google Scholar]
- 252.Sibanda S., Nyanyira C., Nicoletti M., Galefi C. Ochnabianthrone: A trans-9,9′-bianthrone from Ochna pulchra. Phytochemistry. 1990;29:3974–3976. doi: 10.1016/0031-9422(90)85383-Q. [DOI] [Google Scholar]
- 253.Sasakl K.N.N., Yamauchi K., Kuwano S. Isolation of a new aloe-emodin dianthrone diglucoside from senna and its potentiating effect on the purgative activity of sennoside A in mice. J. Pharm. Pharmacol. 1985;37:703–706. doi: 10.1111/j.2042-7158.1985.tb04946.x. [DOI] [PubMed] [Google Scholar]
- 254.Oshio H., Imai S., Fujioka S., Sugawara T., Miyamoto M., Tsukui M. Investigation of Rhubarbs. III New purgative constituents, sennosides E and F. Chem. Pharm. Bull. 1974;22:823–831. doi: 10.1248/cpb.22.823. [DOI] [Google Scholar]
- 255.Lemli J., Bequeker R., Cuveele J. Sennidin C, reidin B and reidin C, heterodianthrones of the fresh roots of rhubarb. Pharm. Weekbl. 1964;99:613–616. [PubMed] [Google Scholar]
- 256.Gao W., Jin L., Liu C., Zhang N., Zhang R., Bednarikova Z., Gazova Z., Bhunia A., Siebert H.C., Dong H. Inhibition behavior of sennoside A and sennoside C on amyloid fibrillation of human lysozyme and its possible mechanism. Int. J. Biol. Macromol. 2021;178:424–433. doi: 10.1016/j.ijbiomac.2021.02.213. [DOI] [PubMed] [Google Scholar]
- 257.Alemayehu G., Abegaz B., Snatzke G., Duddeck H. Bianthrones from Senna longiracemosa. Phytochemistry. 1993;32:1273–1277. doi: 10.1016/S0031-9422(00)95104-5. [DOI] [Google Scholar]
- 258.Macedo E.M.S.d., Wiggers H.J., Silva M.G.V., Braz-Filho R., Andricopulo A.D., Montanari C.A. A new bianthron glycoside as itor of Trypanosoma cruzi glyceraldehyde 3-phosphate dehydrogenase activity. J. Braz. Chem. 2009;20:947–953. doi: 10.1590/S0103-50532009000500021. [DOI] [Google Scholar]
- 259.Xiang W., Long Z., Zeng J., Zhu X., Yuan M., Wu J., Wu Y., Liu L. Mechanism of Radix Rhei et rhizome intervention in cerebral infarction: A research based on chemoinformatics and systematic pharmacology. Evid.-Based Complement. Alternat. Med. 2021;2021:6789835. doi: 10.1155/2021/6789835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sehgal V.N., Verma P., Khurana A. Anthralin/dithranol in dermatology. Int. J. Dermatol. 2014;53:e449-60. doi: 10.1111/j.1365-4632.2012.05611.x. [DOI] [PubMed] [Google Scholar]
- 261.Zhang Y.B., Wang Y.R., Wang Z.Y., Ma C.K., Shi Y.J. Optimization of the extraction process for rutin from corn silk by alkali extraction and acid precipitation. Chem. Biol. Eng. 2021;38:27–31. [Google Scholar]
- 262.Zhang L.M., Tian B., Mao H., Su Y.Q., Lv D. Optimization of the extraction process for anthraquinones from Cassia obtusifolia. Biochem. Eng. J. 2024;10:93–96+108. [Google Scholar]
- 263.Cao L.L., Ruan C.Q., Gao J.L., Li X., Zhao M., Li Q. Study on the extraction process of anthraquinones from Rubia cordifolia rhizomes. Liaoning Chem. Ind. 2024;53:501–505+529. doi: 10.14029/j.cnki.issn1004-0935.2024.04.003. [DOI] [Google Scholar]
- 264.Du Z.X. Determination of plumbagin content in fresh and dry stems of Plumbago zeylanica. Anhui Agric. Sci. 2009;37:16363–16364. doi: 10.13989/j.cnki.0517-6611.2009.33.159. [DOI] [Google Scholar]
- 265.Li M.J., Deng Q.G., Andong Z.Y. Overview of separation and purification processes for active ingredients in Chinese medicine. J. Qiqihar Univ. 2006;2:7–10. [Google Scholar]
- 266.Dong J.X., Jia A.L., Dong X.L., Qiu Z.D. Study on the extraction process of anthraquinones from Polygonum multiflorum. J. Changchun Univ. Chin. Med. 2012;28:150–151. doi: 10.13463/j.cnki.cczyy.2012.01.069. [DOI] [Google Scholar]
- 267.Zhu K., Yu Y., Dong X.L., Qiu Y., Zhang X.Y., Qiu Z.D. Study on the extraction process of anthraquinones from Rheum officinale by CO2 supercritical fluid extraction. J. Jilin Chin. Med. 2013;33:1261–1263. doi: 10.13463/j.cnki.jlzyy.2013.12.040. [DOI] [Google Scholar]
- 268.Zhao J.L., Kang Y., Sun H., Li X.Q. Determination of hydroquinone and phenol in cosmetics by solid-phase extraction-high performance liquid chromatography. Chin. J. Health Lab. Sci. 2019;29:16–18. [Google Scholar]
- 269.Chen D.M. Ph.D. Thesis. Huazhong Agricultural University; Wuhan, China: 2010. Key Technologies for Quantitative and Confirmatory Analysis of Veterinary Drug Residues in Animal-Derived Foods. [Google Scholar]
- 270.Ong E.S., Len S.M. Evaluation of pressurized liquid extraction and pressurized hot water extraction for tanshinone I and IIA in Salvia miltiorrhiza using LC and LC-ESI-MS. J. Chromatogr. Sci. 2004;42:211–216. doi: 10.1093/chromsci/42.4.211. [DOI] [PubMed] [Google Scholar]
- 271.Wang P. Master’s Thesis. Northwest Agriculture and Forestry University; Xianyang, China: 2013. Isolation and Identification of Polyphenols and Anthraquinones from Smilax scobinicaulis. [DOI] [Google Scholar]
- 272.He Y., Zhang Y.L., Zhang G.R. Extraction, separation and structure identification of browning products from pomegranate peel. Sci. Technol. Food Ind. 2008;4:133–136. doi: 10.13386/j.issn1002-0306.2008.04.037. [DOI] [Google Scholar]
- 273.Huang J., Yu L., Peng X.S., Zheng R., Xu Y., Ni L.J. Determination and mass spectrometric confirmation of lawsone in cosmetics by high performance liquid chromatography. Chin. J. Health Lab. Sci. 2022;32:1182–1186. [Google Scholar]
- 274.Tian G., Zhang T.Y., Zhang Y.B., Ito Y. Separation of tanshinones from Salvia miltiorrhiza Bunge by multidimensional counter-current chromatography. J. Chromatogr. A. 2002;945:281–285. doi: 10.1016/S0021-9673(01)01495-9. [DOI] [PubMed] [Google Scholar]
- 275.Lu C.G. Master’s Thesis. Zhengzhou University; Zhengzhou, China: 2007. Study on the Extraction Process of Effective Components from Aloe by Supercritical CO2. [Google Scholar]
- 276.Yang N., Zhou C.J., Wen R. Research progress in extraction and separation technologies of Chinese herbal medicines. J. Baotou Med. Coll. 2015;31:143–145. doi: 10.16833/j.cnki.jbmc.2015.04.092. [DOI] [Google Scholar]
- 277.Lu Z.K. Master’s Thesis. Shihezi University; Shihezi, China: 2022. Component Analysis and Biological Activity Study of Green Walnut Peel Pigment. [DOI] [Google Scholar]
- 278.Zhang Z.J. Master’s Thesis. Hunan University; Shihezi, China: 2008. Study on the Synthesis Methods of Resveratrol Compounds and Their Derivatives. [Google Scholar]
- 279.Qiu B.L. Master’s Thesis. Fuzhou University; Shihezi, China: 2010. Synthesis and Property Study of Emodin Derivatives. [Google Scholar]
- 280.Wang Y., Chen J.W., Li F., Bian H.T. Acute phototoxicity mechanism and QSAR study of anthraquinones to Daphnia magna; Proceedings of the 5th National Conference on Environmental Chemistry; Dalian, China. 10 May 2009. [Google Scholar]
- 281.Shen J., Zhang M.Y., Guo Z.T., Han S.H., Li H.T., Zhou Z.Y., Peng M.Y. Immunomodulatory and therapeutic effects of embelin on systemic lupus erythematosus mice. J. Army Med. Univ. 2022;44:363–370. doi: 10.16016/j.2097-0927.202109056. [DOI] [Google Scholar]
- 282.Siegelin M.D., Gaiser T., Siegelin Y. The XIAP inhibitor Embelin enhances TRAIL-mediated apoptosis in malignant glioma cells by down-regulation of the short isoform of FLIP. Neurochem. Int. 2009;55:423–430. doi: 10.1016/j.neuint.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 283.Chen Y.Y., Li J., Hu J.D., Zheng J., Zheng Z.H., Zhu L.F., Chen X.J., Lin Z.X. Study on the reversal effect of emodin on multidrug resistance in HL-60/ADR resistant cells. J. Exp. Hematol. 2013;21:1413–1422. doi: 10.7534/j.issn.1009-2137.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 284.Chen S.-C., Chen Q.-W., Ko C.-Y. Chrysophanol induces cell death and inhibits invasiveness through alteration of calcium levels in HepG2 human liver cancer cells. Chin. J. Integr. Med. 2024;31:434–440. doi: 10.1007/s11655-024-3817-2. [DOI] [PubMed] [Google Scholar]
- 285.Kraemer S., Crauwels P., Bohn R., Radzimski C., Szaszak M., Klinger M., Rupp J., van Zandbergen G. AP-1 transcription factor serves as a molecular switch between Chlamydia pneumoniae replication and persistence. Infect. Immun. 2015;83:2651–2660. doi: 10.1128/IAI.03083-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Avci E., Arikoglu H., Kaya D.E. Investigation of juglone effects on metastasis and angiogenesis in pancreatic cancer cells. Gene. 2016;588:74–78. doi: 10.1016/j.gene.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 287.Zhu F., Wu G., He Y.Q., Li Z.Y., Peng G., Ren J.H. Effects of plumbagin on proliferation of hepatoma cell 2 and expression of vascular endothelial growth factor. Chin. Herbal Med. 2010;41:775–778. [Google Scholar]
- 288.Yang J.G., Wang H.Y., Gao X.S., Li X.H. Effects of aloe-emodin on the biological behaviors of cervical cancer HeLa cells. Hebei Med. J. 2018;40:814–818. [Google Scholar]
- 289.Itharat A., Plubrukan A., Kaewpradub N., Chuchom T., Ratanasuwan P., Houghton P.J. Selective cytotoxicity and antioxidant effects of compounds from Dioscorea membranacea rhizomes. Nat. Prod. Commun. 2007;2:643–648. doi: 10.1177/1934578X0700200605. [DOI] [Google Scholar]
- 290.Thangaraj S., Tsao W.-S., Luo Y.-W., Lee Y.-J., Chang C.-F., Lin C.-C., Uang B.-J., Yu C.-C., Guh J.-H., Teng C.-M. Total synthesis of moniliformediquinone and calanquinone A as potent inhibitors for breast cancer. Tetrahedron. 2011;67:6166–6172. doi: 10.1016/j.tet.2011.06.054. [DOI] [Google Scholar]
- 291.Zhang X.W., Cheng M., Wang X.J., Ji Y.L., Zhou C.S. Inhibitory effects of phenanthrenequinone from Dendrobium nobile on the proliferation and metastasis of human ovarian cancer cells. Pharmacol. Clin. Chin. Mat. Med. 2016;32:72–75+19. doi: 10.13412/j.cnki.zyyl.2016.03.019. [DOI] [Google Scholar]
- 292.Yang N., Li C., Li H.L., Liu M., Cai X.J., Cao F.J., Feng Y.B., Li M.L., Wang X.B. Emodin induced SREBP1-dependent and SREBP1-independent apoptosis in hepatocellular carcinoma cells. Front. Pharmacol. 2019;10:709. doi: 10.3389/fphar.2019.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li H., Wang X., Liu Y., Pan D.F., Wang Y., Yang N., Xiang L.C., Cai X.J., Feng Y.B. Hepatoprotection and hepatotoxicity of Polygonum multiflorum, a Chinese medicinal herb: Context of the paradoxical effect. Food Chem. Toxicol. 2017;108:407–418. doi: 10.1016/j.fct.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 294.Xu H. Master’s Thesis. Jilin University; Ürümqi, China: 2023. Idebenone Protects Against Oxidative Stress-Induced Neuronal Cell Apoptosis by Regulating the CD38-SIRT3-P53 Pathway. [Google Scholar]
- 295.Kumar S., Gautam S., Sharma A. Antimutagenic and antioxidant properties of plumbagin and other naphthoquinones. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013;755:30–41. doi: 10.1016/j.mrgentox.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 296.Liu T. Master’s Thesis. Shanxi Normal University; Ürümqi, China: 2018. Study on the Antioxidant and Antibacterial Activities and Antibacterial Mechanism of Juglone. [Google Scholar]
- 297.Hou Y.S. Master’s Thesis. Xinjiang Medical University; Ürümqi, China: 2020. Analysis of Naphthoquinones in Arnebia euchroma and Their Antioxidant Ntitumor Activities In Vitro. [DOI] [Google Scholar]
- 298.Chen J., Yin Z., Yu N., Ou S.S., Wang X., Li H.P., Zhu H.L. Tanshinone alleviates UVA-induced melanogenesis in melanocytes via the Nrf2-regulated antioxidant defense signaling pathway. Curr. Mol. Med. 2024;24:1529–1539. doi: 10.2174/0115665240263196230920161019. [DOI] [PubMed] [Google Scholar]
- 299.Fang M., Huang D.R., Zhang J.W., Liao W.J., Wu F., Liu Y.W. Tanshinone IIA inhibits oxidative stress and exerts anti-hepatocellular carcinoma effects by regulating the PI3K/Akt and Nrf2/HO-1 signaling pathways. China J. Chin. Mater. Med. 2024;49:6724–6734. doi: 10.19540/j.cnki.cjcmm.20240711.401. [DOI] [PubMed] [Google Scholar]
- 300.Mellado M., Madrid A., Peña-Cortés H., López R., Jara C., Espinoza L. Antioxidant activity of anthraquinones isolated from leaves of Muehlenbeckia hastulata (J.E.Sm.) Johnst. (Polygonaceae) J. Chil. Chem. Soc. 2013;58:1767–1770. doi: 10.4067/S0717-97072013000200028. [DOI] [Google Scholar]
- 301.Liu M.Y., Pan X.L., Li X.B., Wu B. Study on the antioxidant activity of metal complexes of aloe-emodin. J. Sichuan Univ. Med. Sci. Ed. 2021;52:241–247. doi: 10.12182/20201160110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hu T., Feng D., Teng L., Zhou G.F., Liu S. Extraction of naphthoquinones from the leaves of Juglans mandshurica and their antioxidant activities in vitro. Food Ind. 2022;43:29–32. [Google Scholar]
- 303.Pan J. Master’s Thesis. Guangzhou University of Chinese Medicine; Guangzhou, China: 2017. Extraction of Total Anthraquinones from Rubia cordifolia and Preliminary Study on Their Antioxidant and Anti-Inflammatory Activities. [DOI] [Google Scholar]
- 304.Liu M.X., Yang S.Q., Xia X.K., Tan W.J., Xiang M.X. Optimization of extraction process and anti-inflammatory study of naphthoquinones from Arnebia euchroma. J. Wuhan Inst. Technol. 2023;45:401–406. doi: 10.19843/j.cnki.CN42-1779/TQ.202303004. [DOI] [Google Scholar]
- 305.Wang Q. Master’s Thesis. Nanjing University of Chinese Medicine; Nanjing, China: 2016. Study on the Necrosis-Targeting Property of Mono-Anthraquinones and Their Application in the Evaluation of Myocardial Activity. [Google Scholar]
- 306.Hu X.H., Meng K., Wang Z., Di M.J. Research progress on the antitumor activity of anthraquinones. Chin. J. Med. Guid. 2023;25:1223–1229. [Google Scholar]
- 307.Lu Z.K., Wu Q.Z., Zhang J., Mao X.Y. Antibacterial effect and mechanism of juglone green walnut peel against Escherichia coli. Food Sci. 2023;44:65–73. [Google Scholar]
- 308.Yang H., Lee P.J., Jeong E.J., Kim H.P., Kim Y.C. Selective apoptosis in hepatic stellate cells ates the antifibrotic effect of phenanthrenes from Dendrobium nobile. Phytother. Res. 2012;26:980. doi: 10.1002/ptr.3632. [DOI] [PubMed] [Google Scholar]
- 309.Tang D.X., Tan Z.H., Liang Y.Y., Cheng L., Huang L. Preliminary study on the cathartic effect and anism of anthraquinones from Rheum officinale. Lishizhen Med. Mater. Med. Res. 2007;6:1314. [Google Scholar]
- 310.Chen Y.Y., Cao Y.J., Tang Y.P., Yue S.J., Duan J.A. Comparative pharmacodynamic, pharmacokinetic and tissue distribution of Dahuang-Gancao decoction in normal and experimental constipation mice. Chin. J. Nat. Med. 2019;17:871–880. doi: 10.1016/S1875-5364(19)30104-9. [DOI] [PubMed] [Google Scholar]
- 311.Zhang Z.Y., Yang L., Huang X.Y., Wang M.X., Ma Z.C., Tang X.L., Wang Y.G., Gao Y. Regulatory effects of THSG and anthraquinones from Polygonum multiflorum on CYP3A4 mediated by human pregnane X receptor. China J. Chin. Mater. Med. 2017;42:4827–4833. doi: 10.19540/j.cnki.cjcmm.20171010.006. [DOI] [PubMed] [Google Scholar]
- 312.Hu X.Q., Geng Z.Y., Li Q.L., Fang H.L., Zhang X.Q. Experimental study on different doses of processed Polygonum multiflorum and the degree of liver injury in rats. Shaanxi J. Tradit. Chin. Med. 2007;10:1420–1421. [Google Scholar]
- 313.Mao Z.H., Liu Q., Wang X., Wen H.R. Study on the hepatotoxicity of anthraquinones from Rheum officinale; Proceedings of the 6th National Annual Conference on Pharmacotoxicology; Chongqing, China. 2016. [Google Scholar]
- 314.Wen H.R., Wang Y.N., Yang Y., Zhao T.T., Ma S.C., Wang Q. Risk assessment of gene mutations induced by emodin-type monoanthrones. Chin. J. Pharmacovigil. 2020;17:455–460. doi: 10.19803/j.1672-8629.2020.08.02. [DOI] [Google Scholar]
- 315.Li C.L., Ma J., Li H.J. Research progress on the absorption and metabolism of anthraquinones. Pharm. Biotechnol. 2012;19:557–560. doi: 10.19526/j.cnki.1005-8915.2012.06.021. [DOI] [Google Scholar]
- 316.Huang H., Li X.Y. Research progress of melanosis coli. Yunnan Med. J. 2008;29:595–597. [Google Scholar]
- 317.Steer H.W., Colin-Jones D.G. Melanosis coli: Studies of the toxic effects of irritant purgatives. J. Pathol. 1975;115:199–205. doi: 10.1002/path.1711150403. [DOI] [PubMed] [Google Scholar]
- 318.Cheng Y. Master’s Thesis. Northwestern University; Xi’an, China: 2021. Study on the Potential Toxic Effects of Metabolic Products and Monomer Components of Anthraquinones from Rheum officinale on Human Colon Cells. [DOI] [Google Scholar]
- 319.Lan J., Wen H.R., Huang Z.Y., Wang Q., Ma S.C. Study on the cytotoxicity of anthraquinone monomer components from Polygonum multiflorum to HK-2 cells. Chin. J. Pharmacovigil. 2023;20:616–622+628. doi: 10.19803/j.1672-8629.20230138. [DOI] [Google Scholar]
- 320.Chang M.H., Huang F.J., Chan W.H. Emodin induces embryonic toxicity in mouse blastocysts through apoptosis. Toxicology. 2012;299:25–32. doi: 10.1016/j.tox.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 321.Yao K.Z., Kang Q.M., Liu W.B., Chen D.N., Wang L.F., Li S. Chronic exposure to tire rubber-derived contaminant 6PPD-quinone impairs sperm quality and induces the damage of reproductive capacity in male mice. J. Hazard. Mater. 2024;470:134165. doi: 10.1016/j.jhazmat.2024.134165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.







