Abstract
Vascular endothelial growth factor (VEGF) plays a key role in the growth and metastasis of solid tumors. We generated a fusion protein containing VEGF121 linked by a flexible G4S tether to the toxin gelonin (rGel) and expressed this as a soluble protein in bacteria. Purified VEGF121/rGel migrated as an 84-kDa homodimer under nonreducing conditions. VEGF121/rGel bound to purified, immobilized Flk-1, and the binding was competed by VEGF121. Both VEGF121/rGel and VEGF121 stimulated cellular kinase insert domain receptor (KDR) phosphorylation. The VEGF121/rGel fusion construct was highly cytotoxic to endothelial cells overexpressing the KDR/Flk-1 receptor. The IC50 of the construct on dividing endothelial cells expressing 105 or more KDR/Flk-1 receptors per cell was 0.5–1 nM, as compared with 300 nM for rGel itself. Dividing endothelial cells overexpressing KDR were approximately 60-fold more sensitive to VEGF121/rGel than were nondividing cells. Endothelial cells overexpressing FLT-1 were not sensitive to the fusion protein. Human melanoma (A-375) or human prostate (PC-3) xenografts treated with the fusion construct demonstrated a reduction in tumor volume to 16% of untreated controls. The fusion construct localized selectively to PC-3 tumor vessels and caused thrombotic damage to tumor vessels with extravasation of red blood cells into the tumor bed. These studies demonstrate the successful use of VEGF121/rGel fusion construct for the targeted destruction of tumor vasculature in vivo.
Vascular endothelial growth factor (VEGF)-A plays a central role in the growth and metastasis of solid tumors (1–10). Through alternative splicing of RNA, human VEGF exists as at least four isoforms of 121, 165, 189, or 206 aa (11–14). The lowest molecular weight isoform, designated VEGF121, is a non-heparan sulfate-binding isoform that exists in solution as a disulfide-linked homodimer.
The angiogenic actions of VEGF are mediated through two related receptor tyrosine kinases, kinase domain receptor (KDR) and FLT-1 in the human, and Flk-1 and Flt-1 in the mouse. Both are largely restricted to vascular endothelial cells (15–18). KDR/Flk-1 and FLT-1 receptors are overexpressed on the endothelium of tumor vasculature (15–24). In contrast, these receptors are almost undetectable in the vascular endothelium of adjacent normal tissues (18). The receptors for VEGF thus seem to be excellent targets for the development of therapeutic agents that inhibit tumor growth and metastatic spread through inhibition of tumor neovascularization. To this end, VEGF121 would be an appropriate carrier to deliver a toxic agent selectively to tumor vascular endothelium.
The recombinant toxin gelonin (rGel) is a single chain N-glycosidase similar in its action to ricin A chain (25–28). Immunotoxins and fusion constructs containing rGel specifically kill tumor cells in vitro and in vivo (ref. 29 and M.G.R., L.C., C. R. Parach, and J.W.M., unpublished work) and have antitumor activity in mice. Gelonin does not seem to generate capillary leak syndrome (31), which limits use of other toxins.
Molecular engineering enabled the synthesis of novel chimeric molecules having therapeutic potential (32, 33). Chimeric fusion constructs targeting the IL-2 receptor, the EGF receptor, and other growth factor/cytokine receptors have been described (34, 35). Studies by Ramakrishnan et al. (36) showed that a chemical conjugate of VEGF and truncated diphtheria toxin has impressive cytotoxic activity on cell lines expressing receptors for VEGF. Further studies with VEGF/DT fusion constructs demonstrated selective toxicity to Caprice's sarcoma cells and dividing endothelial cells in vitro and in vivo (37).
Materials and Methods
The PCR reagents were obtained from Fisher Scientific, and the molecular biology enzymes were purchased from Roche Molecular Biochemicals or New England Biolabs. Bacterial strains, pET bacterial expression plasmids, and recombinant enterokinase were obtained from Novagen. All other chemicals were obtained from Sigma or Fisher Scientific.
Metal affinity resin (Talon) was obtained from CLONTECH. Other chromatography resin and materials were purchased from Amersham Pharmacia. Endothelial cell growth supplement from bovine neural tissue was obtained from Sigma. Murine brain endothelioma bEnd.3 cells were provided by Werner Risau (Max Plank Institute, Munich, Germany). Porcine aortic endothelial cells transfected with either the human FLT-1 receptor (PAE/FLT-1) or the KDR receptor (PAE/KDR) were developed as described (38). Soluble mouse Flk-1 was expressed in Sf9 cells as described by Warren et al. (24). The human melanoma A-375 M cell line was obtained from American Type Culture Collection. Tissue culture reagents were from GIBCO/BRL or Mediatech Cellgro (Herndon, VA). Rabbit anti-gelonin antisera was obtained from the Veterinary Medicine Core Facility at M. D. Anderson Cancer Center. BALB/c nude mice were purchased from The Jackson Laboratory and maintained under sterile pathogen-free conditions according to American Association of Laboratory Animal Care standards.
Construction of VEGF121/rGel.
The cDNA encoding human VEGF121 and recombinant gelonin were fused together by using the splice overlap extension PCR method with VEGF and gelonin DNA as templates. Primers used were: VEGF Nterm, (5′-TGGTCCCAGGCTCATATGGCACCCATGGCAGAA-3′); VEGF Cterm, (5′-TCTAGACCGGAGCCACCGCCACCCCGCCTCGGCTTGTC-3′); Gel Nterm, (5′-GGTGGCGGTGGCTCCGGTCTAGACACCGTGAGC-3′); Gel Cterm, (5′-AAGGCTCGTGTCGACCTCGAGTCATTAAGCTTTAGGATCTTTATC-3′). A G4S linker was incorporated between the VEGF121 and the rGel sequences. Purified PCR products were digested with the restriction enzymes BspHI and XhoI and ligated into the pET-32a. The constructs were transformed into Escherichia coli strain AD494 (DE3) pLys S for expression of the fusion protein.
Protein Expression in E. coli.
Bacterial colonies transformed with the plasmid carrying the VEGF121/rGel insert were cultured in LB growth medium (Sigma) containing 200 μg/ml ampicillin, 70 μg/ml chloramphenicol, and 15 μg/ml kanamycin at 37°C overnight in a shaker bath at 240 rpm. The cultures then were diluted 1:20 with fresh LB medium with antibiotics and grown to early log phase (A600 = 0.6) at 37°C. Thereafter, the cultures were diluted 1:1 with fresh LB medium plus antibiotics; protein synthesis was induced at 23°C by the addition of 0.1 mM isopropyl β-d-thiogalactoside (IPTG) overnight. The cells were collected by centrifugation, resuspended in 10 mM Tris⋅HCl (pH 8.0), and frozen.
Protein Purification.
Frozen bacterial cells were thawed, sonicated (5× for 10 sec each), and then lysed further by the addition of 1 mg/ml lysozyme in 10 mM Tris⋅HCl (pH 8.0) for 30 min at 4°C. The bacterial lysates were ultracentrifuged at 60,000 × g for 45 min at 4°C, adjusted to 40 mM Tris⋅HCl (pH 8.0), filtered (0.22 μm filter), and then loaded at room temperature onto a Talon-containing column. The column was washed with 40 mM Tris⋅HCl (pH 8.0) and 500 mM NaCl containing 5 mM imidazole and eluted with 100 mM imidazole. Protein-containing fractions were dialyzed into 20 mM Tris⋅HCl (pH 7.4) and 50 mM NaCl. The fusion protein was digested for 4 h at room temperature with recombinant enterokinase (20 units/mg of fusion protein) in the presence of 2 mM CaCl2 to remove the hexa-histidine tag and then dialyzed into 20 mM Tris⋅HCl (pH 8.0)/50 mM NaCl. The samples were filter-sterilized before further use and stored at 4°C.
Anti-VEGF and Anti-rGel Western Blot Analysis.
Protein samples were analyzed by SDS/15% PAGE under reducing conditions. The gel was electrophoretically transferred to nitrocellulose overnight at 4°C in transfer buffer (25 mM Tris⋅HCl, pH 7.6/190 mM glycine/20% HPLC-grade methanol). The membranes were blocked by the addition of 5% BSA in Western blocking buffer [Tris-buffered saline (0.05 M Tris, 0.15 M NaCl) (TBS)/0.5% Tween-20] and then incubated for 1 h with rabbit anti-gelonin polyclonal antibody (2 μg/ml in TBS/Tween) or mouse anti-VEGF monoclonal antibody 2C3 (2 μg/ml in TBS/Tween). The membrane then was incubated with goat-anti-rabbit IgG horseradish peroxidase (HRP) or goat-anti-mouse IgG-HRP (1: 5,000 dilution in TBS/Tween). Then, the membrane was developed with the Amersham Pharmacia enhanced chemiluminescence (ECL) detection system and exposed to x-ray film.
Rabbit Reticulocyte Lysate Assay.
The functional activity of rGel and VEGF121/rGel were assayed by using a cell-free protein translation inhibition assay kit from Amersham Pharmacia as described by the manufacturer.
Binding of VEGF121/rGel to Flk-1.
Binding to Flk-1 was tested on microtiter plates coated with soluble mouse Flk-1. Plates were treated with 2 μg/ml of NeutrAvidin (Pierce) for 6 h. Purified, biotinylated Flk-1 (24) was incubated with NeutrAvidin-coated wells for 2 h. VEGF121 or VEGF121/rGel was added to the wells at various concentrations in the presence of PBS containing 2% (vol/vol) BSA. After 2 h of incubation, plates were washed and incubated with nonblocking mouse monoclonal anti-VEGF antibody, 2C3 (39), or rabbit polyclonal anti-gelonin IgG. For competition studies of VEGF121/rGel and VEGF121, binding of the VEGF121/rGel fusion protein was detected by using a rabbit anti-gelonin antibody. Mouse and rabbit IgG were detected by HRP-labeled goat anti-mouse and anti-rabbit antibodies, respectively (Dako). Peroxidase activity was measured by adding O-phenylenediamine (0.5 mg/ml) and hydrogen peroxide (0.03% vol/vol) in citrate-phosphate buffer (pH 5.5). The reaction was stopped by the addition of 100 μl of 0.18 M of H2SO4. The absorbance was read at 490 nM. In competition experiments, a 10-fold molar excess of VEGF121 was premixed with VEGF121/rGel before addition to the plate.
Cytotoxicity of VEGF121/rGel to Adult Bovine Aortic Arch-Derived Endothelial (ABAE) Cells.
Log-phase ABAE cells in DMEM [10% (vol/vol) FBS] were diluted to 4,000 cells per 200 μl. Aliquots (200 μl) were added to 96-well flat-bottomed tissue culture plates and incubated at 37°C for 1–72 h in 5% CO2. Purified VEGF121/rGel or rGel were diluted in culture medium to various concentrations, added to the plate, and the cultures were incubated for 72 h. Remaining adherent cells were stained by the addition of 100 μl of crystal violet [0.5% in 20% (vol/vol) methanol]. Dye-stained cells were solubilized by the addition of 100 μl of Sorenson's buffer [0.1 M sodium citrate, pH 4.2 in 50% (vol/vol) ethanol]. The absorbance was measured at 595 nM.
Cytotoxicity of VEGF121/rGel to Mouse Brain-Derived Endothelial Cells bEnd.3.
Cells were seeded at a density of 50,000 per well in 24-well plates. Twenty-four hours later, VEGF 121/rGel or rGel alone were added at various concentrations. After 5 days of treatment at 37°C, remaining attached cells were trypsinized and counted. The results are presented as total cell number per well. Two identical experiments were performed in duplicate. Standard error in all experiments was less than 5% of the mean.
Cytotoxicity of VEGF121/rGel to PAE/KDR Cells and PAE/FLT-1 Cells.
Log-phase PAE/KDR cells and PAE/FLT-1 cells in F-12 medium [10% (vol/vol) FBS] were diluted to 3,000 cells per 200 μl. Aliquots (200 μl) were added to 96-well flat-bottomed tissue culture plates and incubated at 37°C for 24 h in 5% CO2. Purified VEGF121/rGel or rGel were diluted in culture medium, added to the plate, and incubated for 72 h. Adherent cells were quantified by using the crystal violet staining method described above.
Kinase Activity of KDR in PAE/KDR Cells Exposed to VEGF121/rGel or VEGF121.
PAE/KDR cells were incubated overnight in F-12 culture medium and then incubated at 37°C for 5 min with 100 μM Na3 VO4. VEGF or VEGF121/rGel then were added and, at various times, cells were lysed by the addition of a lysis buffer [50 mM Hepes, pH 7.4/150 mM NaCl/1 mM EGTA/10 mM sodium pyrophosphate/1.5 mM MgCl2/100 mM NaF/10% (vol/vol) glycerol/1% Triton X-100]. Cell lysates were centrifuged (16,000 × g), the supernatants were removed, and their protein concentrations were determined. Lysate supernatants were incubated with 9 μg anti-phosphotyrosine monoclonal antibody (Santa Cruz Biotechnology) for 2 h at 4°C and then precipitated by the addition of Protein A Sepharose beads for 2 h at 4°C. Beads were washed and mixed with SDS sample buffer, heated for 5 min at 100°C, centrifuged, analyzed by SDS/10% PAGE, and then transferred to nitrocellulose filters. The membranes were blocked with 5% nonfat dry milk and incubated with rabbit polyclonal anti-KDR antibody (1:250; Santa Cruz Biotechnology) for 1 h at room temperature. The membranes then were washed, incubated with a peroxidase-linked goat anti-rabbit antibody (1:2,000) for 1 h at room temperature, and then enhanced chemiluminescence reagent (Amersham Pharmacia) was used to visualize the immunoreactive bands.
Immunohistochemical Analysis of VEGF121/rGel in Tumor Xenografts.
Mice (three mice per group) bearing PC-3 tumors were injected intravenously with 50 μg of the fusion protein gelonin. The mean tumor volume per group was 260 mm3. Thirty minutes later, mice were killed, exsanguinated, and all major tissues were snap-frozen. Frozen sections were cut and double stained with pan-endothelial marker MECA-32 (5 μg/ml) followed by detection of the localized fusion protein by using rabbit anti-gelonin antibody (10 μg/ml). MECA-32 rat IgG was visualized with goat anti-rat IgG conjugated to FITC (red fluorescence). Anti-gelonin antibody was detected with goat anti-rabbit IgG conjugated to Cy-3 (green fluorescence). Colocalization of both markers was indicated by a yellow color. Anti-gelonin antibody had no reactivity with tissue sections from mice injected with saline or VEGF121. To determine the percentage of vessels with localized fusion protein, the number of vessels stained with MECA-32 (red), gelonin (green), or both (yellow) were counted at a magnification of ×200 in at least 10 fields per section. Two slides from each mouse were analyzed, and the average percentage of positive vessels was calculated.
In Vivo Therapy in Xenograft Models.
Human melanoma.
Female nu/nu mice were divided into groups of five mice each. Log-phase A-375M human melanoma cells were injected s.c. (5 × 106 cells per mouse) into the right flank. After the tumors had become established (≈50 mm3), the mice were injected with VEGF121/rGel through a tail vein five times over an 11 day period. The total dose of VEGF/rGel was 17 or 25 mg/kg. Other mice received rGel alone at a dose totaling 10 mg/kg. Mice were killed by cervical dislocation after the 40th day of tumor measurement.
Human prostate cancer.
Male nude mice weighing ≈20 g were divided into groups of five mice each. Log-phase PC-3 human prostate tumor cells were injected s.c. (5 × 106 cells per mouse) in the right flank. The mice were injected with VEGF121/rGel through a tail vein every 2–3 days for 11 days. The total dose of VEGF121/rGel was 20 mg/kg. Other mice received rGel alone at a dose totaling 10 mg/kg. Tumor volume was calculated according to the formula: volume = L × W × H, where L = length, W = width, H = height.
A histological study was performed in which groups of three mice bearing PC-3 tumors were given i.v. injections of either saline or VEGF121/rGel (2.5 mg/kg). Forty-eight hours later, the mice were killed, and organs and tumors were removed and fixed in formalin. Paraffin sections were prepared, stained with hematoxylin and eosin, and examined by light microscopy.
Results
Plasmid Construction, Bacterial Expression, and Purification of Fusion Protein.
The cDNA encoding human VEGF121, a G4S flexible tether, and rGel were joined with a PCR-based method (see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). The construct was ligated into the pET-32a vector, transformed into E. coli strain AD494(DE3) plys S, and the fusion protein was expressed and purified from bacterial supernatant. Fig. 7, which is published as supporting information on the PNAS web site, shows the SDS/PAGE analysis of protein expression after induction with IPTG. The lane containing the induced culture shows a new protein at 62 kDa, which is the expected molecular weight for the fusion protein plus the 21 kDa purification tag. This material was purified by binding and elution from immobilized metal affinity (IMAC) resin. Cleavage with recombinant enterokinase removed the tag resulting in a 42-kDa protein under reducing conditions (Fig. 7). The construct migrated as a homodimer at 84 kDa under nonreducing conditions. The fusion construct was immunoreactive with antibodies to both VEGF and rGel (Fig. 7). One liter of induced bacterial culture initially contained ≈2,000 μg of soluble fusion construct. Initial IMAC purification resulted in 750 μg of VEGF121/rGel product (yield 37.5%), and digestion with recombinant enterokinase generated 400 μg of target protein (yield 20%). Subsequent purification yielded 230 μg of VEGF121/rGel final product (11.5% overall yield).
Biological Activity of the rGel Component.
The ability of VEGF121/rGel and rGel to inhibit translation in a cell-free system was determined by using a rabbit reticulocyte translation assay. The purified VEGF121/rGel and rGel had IC50 values of ≈47 and 185 pM, respectively, showing that fusion of rGel and VEGF121 did not reduce the activity of the toxin component.
Binding of VEGF121/rGel to Soluble Flk-1 Receptor.
The fusion protein was tested for its ability to bind to the Flk-1 receptor by ELISA. Fig. 8, which is published as supporting information on the PNAS web site, shows that VEGF121/rGel and native human VEGF121 bind equally well to Flk-1 at all concentrations, indicating that the VEGF component of the fusion protein is fully capable of binding to Flk-1. The specificity of binding of VEGF121/rGel to Flk-1 was confirmed by using a 10-fold molar excess of free VEGF121.
VEGF121/rGel and VEGF121-Induced Phosphorylation of KDR.
PAE/KDR cells overexpressing the KDR were incubated with VEGF121/rGel fusion construct or VEGF121 itself, and Western analysis of phospho-tyrosine content of the receptors was measured at various later time points. As shown in Fig. 9, which is published as supporting information on the PNAS web site, addition of VEGF121/rGel or VEGF121 increased phosphotyrosine content. There were two phases of phosphorylation; an early phase (1–10 min) and a later phase (4–8 h). The time course of induction of KDR phosphorylation was the same for VEGF121/rGel and VEGF121. Phosphorylation of FLT-1 in PAE/FLT-1 cells treated with either VEGF121/rGel or VEGF121 was not observed, as expected from the weaker signaling of FLT-1 compared with KDR observed by others (40).
Cytotoxicity of VEGF121/rGel to Endothelial Cells in Vitro.
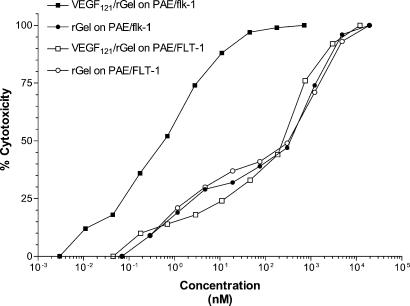
VEGF121/rGel was specifically toxic to KDR/Flk-1 expressing endothelial cells in vitro (Fig. 1 and Table 1). The IC50 values for VEGF121/rGel on log-phase PAE/KDR, ABAE, and bEnd.3 cells, which express 1–3 × 105 KDR/Flk-1 receptors per cell, was 0.06 to 1 nM. Cells expressing FLT-1 and having low endogenous expression of KDR (PAE/FLT-1, HUVEC) were several hundred-fold more resistant to VEGF121/rGel than were the KDR/Flk-1 expressing cells. Thus, FLT-1 appears not to mediate cytotoxicity of VEGF121/rGel, in agreement with earlier reports (41). The ratio of IC50 values of rGel to VEGF121/rGel was calculated for each cell type. This ratio (the targeting index) represents the ability of the VEGF component of the fusion construct to mediate the delivery of the toxin to the endothelial cell surface and into the intracellular ribosomal compartment. As summarized in Table 1, bEnd.3 and ABAE cells were, respectively, 100-fold and 9-fold more sensitive to the fusion construct than they were to free rGel.
Figure 1.
Cytotoxicity of VEGF121/rGel to KDR-expressing PAE-transfected cells. Cells transfected with either the FLT-1 or KDR receptor were treated with various doses of VEGF121/rGel or rGel for 72 h. Cells expressing the FLT-1 receptor were equally insensitive to VEGF121/rGel and rGel (IC50 ≃ 300 nM). In contrast, cells expressing KDR were about 200-fold more sensitive to the fusion construct (IC50 of 0.5 nM) than they were to rGel.
Table 1.
Correlation between number of VEGF receptors per cell and sensitivity to VEGF121/rGel
| Cell type | Number of FLT-1 sites per cell | Number of KDR sites per cell | IC50 for VEGF121/rGel, nM | IC50 for rGel, nM | Targeting index* |
|---|---|---|---|---|---|
| PAE/KDR (log phase) | 0 | 2–3 × 105 (ref. 41) | 0.5 | 300 | 600 |
| PAE/KDR (confluent) | 0 | 2–3 × 105 (ref. 38) | 30 | 5,000 | 167 |
| bEnd3 (log phase) | N.D. | 2 × 105† | 1 | 100 | 100 |
| ABAE (log phase) | 0 | 0.4 × 105 (ref. 56) | 0.059 | 0.524 | 8.9 |
| HUVEC (hypoxia) | N.D. | 0.023 × 105 (ref. 30) | 700 | >1,000 | ≈1 |
| HUVEC (normoxia) | N.D. | 0.017 × 105 (ref. 30) | 800 | >1,000 | ≈1 |
| PAE/FLT-1 (log phase) | 0.5 × 105 (ref. 38) | N.D. | 300 | 300 | 1 |
| PAE/FLT-1 (confluent) | 0.5 × 105 (ref. 38) | N.D. | >5,000 | 10,000 | <2 |
| A-375 (log phase) | N.D. | N.D. | 330 | 109 | 0.3 |
| PC-3 (log phase) | N.D. | N.D. | 225 | 100 | 0.4 |
Numbers in parentheses indicate text reference. N.D., not done.
Targeting index is defined as: (IC50/IC50)(rGel/VEGF121/rGel).
†S. Ran, unpublished data.
Selective Cytotoxicity of VEGF121/rGel for Dividing PAE/KDR Cells.
VEGF121/rGel was 60-fold more toxic to PAE/KDR cells in log-phase growth than it was to PAE/KDR cells that had been grown to confluence and rested (Table 1). This effect was not caused by differences in KDR expression, because the cells expressed the same number of KDR receptors per cell in both phases of growth. The log-phase PAE/KDR cells also were more sensitive to rGel itself than were the confluent cells, suggesting that the quiescence of confluent cells impacts their sensitivity to both targeted and nontargeted rGel. It is possible that the rate or route of entry of both VEGF121/rGel and rGel is different for dividing and nondividing cells.
Inhibition of Tumor Growth in Vivo by VEGF121/rGel.
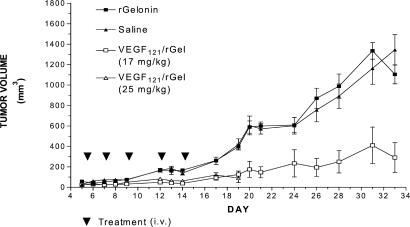
Saline-treated human melanoma (A-375M) tumors showed an increase in tumor volume 24-fold (from 50 mm3 to 1200 mm3) over the 30-day observation period (Fig. 2). Treatment of the mice with VEGF121/rGel strongly retarded tumor growth. At high doses of VEGF121/rGel totaling 25 mg/kg, tumor growth was completely prevented, but all mice died from drug toxicity on day 19. At lower doses totaling 17 mg/kg, all mice survived. Tumor growth was completely prevented throughout the 14-day course of treatment, but thereafter, tumor regrowth slowly recurred. Compared with controls, mice treated with VEGF121/rGel at doses totaling 17 mg/kg showed a 6-fold decrease in tumor volume (1,200 mm3 vs. 200 mm3).
Figure 2.
Inhibition of human melanoma growth in mice by VEGF/rGel. Groups of nude mice bearing A-375M tumors were treated intravenously with saline, rGel, or fusion construct every 2–3 days for 11 days. Administration of rGel did not affect tumor growth. Treatment with VEGF121/rGel at a total dose of either 17 mg/kg or 25 mg/kg significantly suppressed tumor growth. However, treatment at the 25 mg/kg dose level resulted in mortality by day 19. None of the animals dosed at 17 mg/kg showed gross evidence of toxicity.
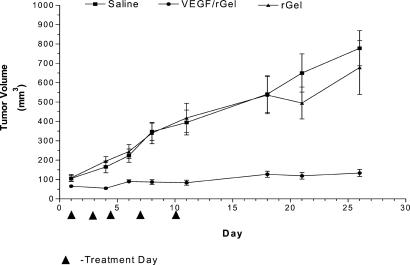
Human prostatic carcinoma (PC-3) tumors increased 12-fold in volume during the 26-day observation period (Fig. 3). Treatment of the mice with five doses of VEGF121/rGel totaling 20 mg/kg virtually abolished tumor growth, even after cessation of treatment. Tumor volume in the treated group only increased from 100 to 200 mm3 over the course of the experiment. Compared with controls, treatment with VEGF121/rGel resulted in a 7-fold decrease in tumor volume (1,400 mm3 vs. 200 mm3).
Figure 3.
Inhibition of human prostate carcinoma growth in mice by VEGF/rGel. Groups of nude mice bearing PC-3 tumors were treated intravenously with saline, rGel, or the VEGF121/rGel fusion construct (20 mg/kg total dose) every 2–3 days for 11 days. Administration of rGel (10 mg/kg) had no effect on tumor growth. In contrast, treatment with the fusion construct completely inhibited tumor growth for 26 days and resulted in a 7-fold reduction in tumor volume compared with saline-treated or rGel-treated controls.
Localization of VEGF121/rGel to Vascular Endothelium in PC-3 Tumor Xenografts.
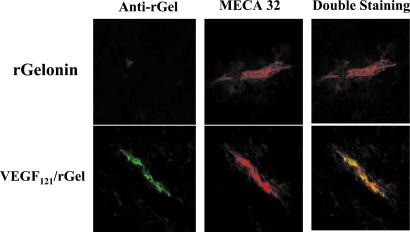
Mice bearing s.c. PC-3 tumors were given one i.v. dose of VEGF121/rGel (2.5 mg/kg) or free gelonin (1 mg/kg) and, 30 min later, the mice were exsanguinated. Frozen sections of tumor and normal organs were examined immunohistochemically. VEGF121/rGel was detected primarily on vascular endothelium of PC-3 tumors (Fig. 4). On average, 62% of vessels positive for MECA 32 also were positive for VEGF121/rGel, as detected by using anti-gelonin antibody. In tumor regions of increased vascularity (“hot spots”), approximately 90% of tumor vessels had bound VEGF121/rGel. Vessels in normal organs were unstained, with the exception of the kidney, where weak and diffuse staining was detected in the glomeruli. Free gelonin did not localize to tumor or normal vessels in any of the mice. These results indicate that VEGF121/rGel localized specifically to tumor vessels after i.v. injection.
Figure 4.
Specific localization of VEGF/rGel to tumor vasculature in PC3 tumors. Nude mice bearing human prostate PC-3 tumors were injected i.v. with VEGF121/rGel or rGel (2.5 mg/kg). Thirty minutes after administration, tissues were removed and snap frozen. Sections were stained with immunofluorescent reagents to detect murine blood vessels (MECA-32, red) and with anti-rGel (green). Vessels stained with both reagents appear yellow. VEGF/rGel localized to tumor vessels, whereas rGel did not. Vessels in all normal organs other than the kidney (glomerulus) were unstained by VEGF/rGel.
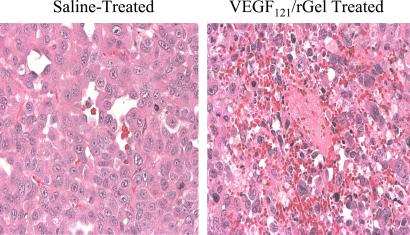
Destruction and Thrombosis of Tumor Vessels by VEGF121/rGel.
Mice bearing s.c. PC-3 tumors were given one i.v. dose of VEGF121/rGel (2.5 mg/kg) or saline. The mice were killed 48 h later, and the tumors and various organs were removed. Paraffin sections were prepared and stained with hematoxylin and eosin. The tumors from VEGF121/rGel recipients (Fig. 5) displayed damaged vascular endothelium, thrombosis of vessels, and extravasation of RBC components into the tumor interstitium. Normal tissues had undamaged vasculature. Treatment of mice with saline had no effect on tumor or normal tissues. As assessed by image analysis, necrotic areas of the tumor increased from ≈4% in saline-treated mice to >12% after treatment with the fusion construct.
Figure 5.
Destruction and thrombosis of tumor blood vessels by VEGF/rGel. Nude mice bearing human prostate PC-3 tumors were treated i.v. with one dose of VEGF121/rGel (2.5 mg/kg). Forty-eight hours after administration, tissues were snap-frozen, sectioned, and stained with hematoxylin and eosin. As shown in this representative image, tumors from mice treated with the fusion construct had damaged vascular endothelium. Clots were visible in the larger vessels of the tumors, and erythrocytes were visible in the tumor interstitium, indicating a loss of vascular integrity. In contrast, histological damage was not visible in any normal organs, including the kidneys, of treated mice.
Discussion
The expression of VEGF and its receptors has been closely linked to tumor vascularity, metastasis, and progression (42–45). Several groups have developed antiangiogenic drugs that block kinase activity of the VEGF receptors (46) or monoclonal antibodies that block VEGF–receptor interactions (47, 48).
The current study demonstrates a chimeric fusion construct containing VEGF and the plant toxin gelonin. VEGF121/rGel was found to be selectively toxic to dividing endothelial cells overexpressing the KDR/Flk-1 receptor. Nondividing (confluent) endothelial cells were almost 60-fold more resistant than were dividing cells to the fusion construct and also were more resistant to free rGel (Table 1). These findings accord with those of previous studies by Wild et al. (32), who showed that conjugates of VEGF and DT were highly toxic to log-phase cells but were not toxic to confluent endothelial cells. The greater sensitivity of dividing endothelial cells to VEGF-toxin constructs may be because of differences in intracellular routing or catabolism of the construct as observed with other targeted therapeutic agents (49).
Cytotoxicity studies demonstrated that expression of the KDR/Flk-1 receptor is needed for VEGF121/rGel to be cytotoxic. Cells overexpressing KDR/Flk-1 (>1 × 105 sites per cell) were highly sensitive to the VEGF121/rGel fusion construct, whereas cells expressing fewer than 0.4 × 105 sites per cell were no more sensitive to the fusion toxin than they were to free rGel. Again, the requirement to surpass a threshold level of KDR/Flk-1 for cytotoxicity may contribute to the safety of VEGF121/rGel. In normal organs, including the kidney glomerulus and pulmonary vascular endothelium (19), the level of KDR/Flk-1 may be below that needed to cause toxicity. The number of receptors for VEGF on endothelial cells in the vasculature of normal organs has been reported by Brown et al. (19, 50) to be significantly lower than on tumor vasculature. Indeed, we could not detect binding of VEGF121/rGel to normal vascular endothelium in organs other than the kidney, where weak binding was observed. Furthermore, no damage to vascular endothelium was observed in normal organs, including the kidney.
Other gelonin-based-targeted therapeutics also have been observed to become toxic to cells only when a certain threshold level of binding is surpassed. In a recent study of immunotoxins directed against the c-erb-2/HER2/neu oncogene product, immunotoxins were not cytotoxic to tumor cells expressing less than about 1 × 106 HER2/neu sites per cell (29). The lack of sensitivity of cells having low levels of receptors is presumably because the cells internalize too little of the toxin or traffic it to compartments that do not permit translocation of the toxin to the ribosomal compartment.
Our study also demonstrates that the presence of FLT-1, even at high levels, does not seem to mediate cellular toxicity of the VEGF121/rGel fusion toxin. Although VEGF binds to the FLT-1 receptor (51), the current study (40, 52) has been unable to demonstrate receptor phosphorylation as a result of ligand binding. Dougher and Terman (53) suggested that receptor phosphorylation may be required for KDR signaling and internalization. If so, the receptor–fusion–toxin complex may not internalize efficiently enough after binding to FLT-1 for the fusion protein to be routed to an intracellular compartment from which the toxin can escape to the cytosol. The relative contributions of the FLT-1 and KDR receptors to the biological effects of VEGF examined by using a monoclonal antibody that blocks the interaction of VEGF with KDR/Flk-1 but not FLT-1 demonstrate that KDR/Flk-1 is the major receptor determining the vascular permeability-inducing and angiogenic effects of VEGF in tumors (48).
Although VEGF121/rGel induces phosphorylation of KDR receptor, we observed no growth-stimulatory effects of the fusion toxin on VEGF receptor-expressing cells. These findings are in keeping with studies of other fusion toxins such as IL-2/DT that initially stimulate target cells in a manner similar to that of IL-2 itself, but ultimately kill the target cells through the actions of the internalized toxin (54).
The antitumor effects of the VEGF121/rGel fusion construct against both melanoma and human prostate carcinoma xenografts was impressive in magnitude and prolonged. A-375M and PC-3 cells in culture were resistant to the fusion construct in vitro, despite the reported presence of KDR on the melanoma (but not on PC-3) cells (55). Therefore, the antitumor effects observed in vivo appear not to be caused by direct cytotoxic effects of VEGF121/rGel on the tumor cells themselves. The antitumor effect seems to be exerted indirectly on the tumor cells through specific damage to tumor vasculature. The VEGF121 fusion toxin localized to tumor blood vessels after i.v. administration. Vascular damage and thrombosis of tumor blood vessels were observed within 48 h of administration of VEGF121/rGel to PC-3 mice, consistent with the primary action of the construct being exerted on tumor vascular endothelium.
In summary, our results indicate that selective destruction of tumor vasculature can be achieved with VEGF121/rGel in mice, giving impressive antitumor effects. Gross morphological toxicity to the normal organs was not visible in animals treated with a therapeutic dose. These studies suggest that VEGF121/rGel has potential as an antitumor agent for treating cancer patients.
Supplementary Material
Acknowledgments
We thank Ms. J. Merchant for her excellent assistance in the preparation of this report. This study was supported by grants from the University of Utrecht and the Dutch Cancer Foundation, Koningin Wilhelmina Fonds, Cancer Center Support Grant 5P30CA16672-26 from the National Cancer Institute, National Institutes of Health Grant ROI CA 7495, and Arcus Therapeutics LLC, Boston. This research was conducted, in part, by The Clayton Foundation for Research.
Abbreviations
- VEGF
vascular endothelial growth factor
- rGel
recombinant toxin gelonin
References
- 1.Klagsbrun M, D'Amore P A. Annu Rev Physiol. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Shing Y. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 3.Folkman J. J Natl Cancer Inst. 1991;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Weidner N, Semple J P, Welch W R, Folkman J. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 5.Leung D W, Cachianes G, Kuang W J, Goeddel D V, Ferrara N. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 6.Senger D R, Galli S J, Dvorak A M, Perruzzi C A, Harvey V S, Dvorak H F. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Henzel W J. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 8.Plouet J, Schilling J, Gospodarowicz D. EMBO J. 1989;8:3801–3806. doi: 10.1002/j.1460-2075.1989.tb08557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poon R T, Fan S T, Wong J. J Clin Oncol. 2001;19:1207–1215. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 10.Price D J, Miralem T, Jiang S, Steinberg R, Avraham H. Cell Growth Differ. 2001;12:129–135. [PubMed] [Google Scholar]
- 11.Cvetkovic D, Movsas B, Dicker A P, Hanlon A L, Greenberg R E, Chapman J D, Hanks G E, Tricoli J V. Urology. 2001;57:821–825. doi: 10.1016/s0090-4295(00)01044-x. [DOI] [PubMed] [Google Scholar]
- 12.Conn G, Bayne M L, Soderman D D, Kwok P W, Sullivan K A, Palisi T M, Hope D A, Thomas K A. Proc Natl Acad Sci USA. 1990;87:2628–2632. doi: 10.1073/pnas.87.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leenders W, van Altena M, Lubsen N, Ruiter D, De Waal R. Int J Cancer. 2001;91:327–333. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1052>3.3.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Houck K A, Ferrara N, Winer J, Cachianes G, Li B, Leung D W. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 15.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 16.Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matushime H, Sato M. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- 17.De Vries C, Escobedo J A, Ueno H, Houck K, Ferrara N, Williams L T. Science. 1992;225:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 18.Peters K G, De Vries C, Williams L T. Proc Natl Acad Sci USA. 1993;90:8915–8919. doi: 10.1073/pnas.90.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown L F, Berse B, Jackman R W, Tognazzi K, Guidi A J, Dvorak H F, Senger D R, Connolly J L, Schnitt S J. Hum Pathol. 1995;26:86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 20.Plate K H, Breier G, Millauer B, Ullrich A, Risau W. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 21.Easty D J, Herlyn M, Bennett D C. Int J Cancer. 1995;60:129–136. doi: 10.1002/ijc.2910600119. [DOI] [PubMed] [Google Scholar]
- 22.Hatva E, Kaipainen A, Mentula P, Jaaskelainen J, Paetau A, Haltia M, Alitalo K. Am J Pathol. 1995;146:368–378. [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi A, Sasaki H, Kim S J, Tobisu K, Kakizoe T, Tsukamoto T, Kumamoto Y, Sugimura T, Terada M. Cancer Res. 1994;54:4233–4237. [PubMed] [Google Scholar]
- 24.Warren R S, Yuan H, Matli M R, Gillett N A, Ferrara N. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stirpe F, Olsnes S, Pihl A. J Biol Chem. 1980;255:6947–6953. [PubMed] [Google Scholar]
- 26.Sivam G, Pearson J W, Bohn W, Oldham R K, Sadoff J C, Morgan A C., Jr Cancer Res. 1987;47:3169–3173. [PubMed] [Google Scholar]
- 27.Sperti S, Brigotti M, Zamboni M, Carnicelli D, Montanaro L. Biochem J. 1991;277:281–284. doi: 10.1042/bj2770281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenblum M G, Kohr W A, Beattie K L, Beattie W G, Marks W, Toman P D, Cheung L. J Interferon Cytokine Res. 1995;15:547–555. doi: 10.1089/jir.1995.15.547. [DOI] [PubMed] [Google Scholar]
- 29.Rosenblum M G, Shawver L K, Marks J W, Brink J, Cheung L, Langton-Webster B. Clin Cancer Res. 1999;5:865–874. [PubMed] [Google Scholar]
- 30.Brogi E, Schatteman G, Wu T, Kim E A, Varticovski L, Keyt B. J Clin Invest. 1996;97:469–476. doi: 10.1172/JCI118437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gawlak S L, Neubauer M, Klei H E, Chang C Y, Einspahr H M, Siegall C B. Biochemistry. 1997;36:3095–3103. doi: 10.1021/bi962474+. [DOI] [PubMed] [Google Scholar]
- 32.Wild R, Dhanabal M, Olson T A, Ramakrishnan S. Br J Cancer. 2000;83:1077–1083. doi: 10.1054/bjoc.2000.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda M, Psarras K, Jinno H, Ikeda T, Enomoto K, Kitajima M, Futami J, Yamada H, Seno M. Breast Cancer. 1997;4:253–255. doi: 10.1007/BF02966516. [DOI] [PubMed] [Google Scholar]
- 34.Dore J M, Gras E, Wijdenes J. FEBS Lett. 1997;402:50–52. doi: 10.1016/s0014-5793(96)01493-7. [DOI] [PubMed] [Google Scholar]
- 35.Sweeney E B, Murphy J R. Essays Biochem. 1995;30:119–131. [PubMed] [Google Scholar]
- 36.Ramakrishnan S, Olson T A, Bautch V L, Mohanraj D. Cancer Res. 1996;56:1324–1330. [PubMed] [Google Scholar]
- 37.Arora N, Masood R, Zheng T, Cai J, Smith D L, Gill P S. Cancer Res. 1999;59:183–188. [PubMed] [Google Scholar]
- 38.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin C H. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 39.Brekken R A, Huang X, King S W, Thorpe P E. Cancer Res. 1998;58:1952–1959. [PubMed] [Google Scholar]
- 40.Esser S, Lampugnani M G, Corada M, Dejana E, Risau W. J Cell Sci. 1998;111:1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 41.Backer M V, Budker V G, Backer JM. J Controlled Release. 2001;74:349–355. doi: 10.1016/s0168-3659(01)00346-7. [DOI] [PubMed] [Google Scholar]
- 42.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 43.Millauer B, Longhi M P, Plate K H, Shawver L K, Risau W, Ullrich A, Strawn L M. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- 44.Takahashi Y, Kitadai Y, Bucana C D, Cleary K R, Ellis L M. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 45.Baker E A, Bergin F G, Leaper D J. Mol Pathol. 2000;53:307–312. doi: 10.1136/mp.53.6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendel D B, Schreck R E, West D C, Li G, Strawn L M, Tanciongco S S, Vasile S, Shawver L K, Cherrington J M. Clin Cancer Res. 2000;6:4848–4858. [PubMed] [Google Scholar]
- 47.Kozin S V, Boucher Y, Hicklin D J, Bohlen P, Jain R K, Suit H D. Cancer Res. 2001;61:39–44. [PubMed] [Google Scholar]
- 48.Brekken R A, Overholser J P, Stastny V A, Waltenberger J, Minna J D, Thorpe P E. Cancer Res. 2000;60:5117–5124. [PubMed] [Google Scholar]
- 49.Walz G, Zanker B, Brand K, Waters C, Genbauffe F, Zeldis J B. Proc Natl Acad Sci USA. 1989;86:9485–9488. doi: 10.1073/pnas.86.23.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown L F, Berse B, Jackman R W, Tognazzi K, Guidi A J, Dvorak H F. Hum Pathol. 1995;26:86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 51.Shibuya M. Int J Biochem Cell Biol. 2001;33:409–420. doi: 10.1016/s1357-2725(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 52.Kanno S, Oda N, Abe M, Terai Y, Ito M, Shitara K, Tabayashi K, Shibuya M, Sato Y. Oncogene. 2000;19:2138–2146. doi: 10.1038/sj.onc.1203533. [DOI] [PubMed] [Google Scholar]
- 53.Dougher M, Terman B I. Oncogene. 1999;18:1619–1627. doi: 10.1038/sj.onc.1202478. [DOI] [PubMed] [Google Scholar]
- 54.St. Croix B, Rago C, Velculescu V, Traverso G, Romans K E, Montgomery E. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 55.Liu B, Earl H M, Baban D, Shoaibi M, Fabra A, Kerr D J, Seymour L W. Biochem Biophys Res Commun. 1995;217:721–727. doi: 10.1006/bbrc.1995.2832. [DOI] [PubMed] [Google Scholar]
- 56.Vaisman N, Gospodarowicz D, Neufeld G. J Biol Chem. 1990;266:19461–19466. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.