Abstract
C-type cytochromes are essential for almost all organisms; they are characterized by the covalent attachment of heme to protein through two thioether bonds to a Cys-Xaa-Xaa-Cys-His peptide motif. Here we show, contrary to opinion of 30 years standing, that a c-type cytochrome can form from heme and apoprotein in vitro under mild conditions and in the absence of any biosynthesis apparatus. This reaction occurs provided formation of a disulfide bond within the Cys-Xaa-Xaa-Cys-His motif is avoided. There are important implications for understanding in vivo cytochrome c assembly.
Archaea, bacteria, fungi, plant thylakoids, and almost all higher organisms use c-type cytochromes (1–5). They are characterized by the attachment of heme (Fe-protoporphyrin IX) to protein through two thioether bonds to a Cys-Xaa-Xaa-Cys-His peptide motif (1–3). Their usual function is electron transfer (e.g., in respiratory chains) (4–6), but they also may be found at the catalytic site of enzymes (7, 8), and in higher cells they are involved in apoptosis (9). Yet despite the essential role of these proteins in biology, it is not clear either how or why the heme becomes attached covalently to the polypeptide (1); it is not bound covalently in most hemoproteins (3). Remarkably, three different c-type cytochrome biogenesis systems have been identified to date; that from many Gram-negative bacteria involves more than 10 gene products (1, 2, 10, 11).
A surprising experimental finding is that heme may be attached covalently to cytochrome c552 from Hydrogenobacter thermophilus, which has a typical cytochrome c fold (12), in the cytoplasm of Escherichia coli (13, 14). It is not clear how this process happens; the usual location of c-type cytochrome formation in bacteria is the periplasm (2, 3). It has been proposed that cytoplasmic assembly occurs because the apoprotein adopts a conformation that can bind heme (15, 16). This proposal is consistent with the observation that replacement of both of the heme-binding cysteine residues by alanines (C11A/C14A) results in formation of a b-type cytochrome, the heme of which is not bound covalently to the polypeptide and can be removed reversibly in vitro (15).
Formation of the c-type cytochrome thioether bonds between cysteine residues and the vinyl substituents of heme is not chemically facile and is understood poorly (1). Side products have been considered to be formed readily (1, 17). Sano and coworkers (18, 19) demonstrated the covalent addition of protoporphyrinogen to bovine apocytochrome c. However, their reaction was conducted under harshly acidic conditions (pH 3.5 or below), the porphyrin they used was reduced by six electrons compared with the physiologically relevant protoporphyrin IX, and it did not contain the iron atom. It is accepted now that cytochrome c biosynthesis requires the covalent attachment of heme to the polypeptide rather than the attachment of porphyrin with subsequent incorporation of iron (4, 20–22). Since Anfinsen and coworkers' seminal observations on the properties of apocytochrome c 30 years ago (23), it has been widely believed that uncatalyzed covalent attachment of heme to apoprotein cannot occur in vitro.
The unusual cytoplasmic formation of H. thermophilus cytochrome c552 in E. coli and the properties of the double alanine mutant suggested that investigation of the reaction between heme and the apo (i.e., lacking heme) wild-type form of the protein may prove instructive. In this paper we report the results of such a study.
Methods
Wild-type H. thermophilus cytochrome c552 and the C11A/C14A, C11A, and C14A variants were expressed and purified as described (15, 24, 25). Apo wild-type H. thermophilus cytochrome c552 was prepared analogously to the method of Fisher et al. (23). After removal of the Ag+ by addition of DTT, the protein was dialyzed extensively in aerated sodium phosphate buffer (pH 7.0, 20 mM) to obtain oxidized apoprotein; reduced apocytochrome was obtained by dialysis in deoxygenated sodium acetate buffer (pH 5.0, 25 mM). During these procedures, a fraction of the protein showed a tendency to oxidize by the addition of oxygen to one or more amino acid residues (presumably methionines other than the heme axial ligand, but including the N-terminal residue) as judged by electrospray mass spectrometry (ES-MS).
Reconstitution of cytochromes was achieved by the addition of apoprotein to heme (or mesoheme) in sodium phosphate buffer (pH 7.0 unless stated, 50 mM) at 25°C. Fe-porphyrins were reduced with disodium dithionite. Apoprotein was kept reduced by the addition of 5 mM DTT. The oxidation states of the heme and apoprotein also could be varied as described in Results and Discussion. The presence of oxygen was avoided by thoroughly sparging all solutions with humidified argon. Reactions were carried out in the dark. The kinetics of cytochrome c formation were monitored at wavelengths characteristic of the b-type cytochrome intermediate leading to cytochrome c552 and of cytochrome c552. Kinetic data were analyzed by using TABLECURVE (Jandel, San Rafael, CA).
SDS/PAGE analysis was carried out by using the buffer system described by Laemmli (26), and heme-activity staining was achieved by the method of Goodhew et al. (27). Ellman's reagent was used according to Riddles et al. (28). Cyanogen bromide protein digestion was performed as described by Means and Feeney (29). The concentration of reduced holo (and reconstituted) cytochrome c552 was determined by using the extinction coefficient 25.4 mM−1⋅cm−1 at 552 nm (O.D., unpublished data) and of apocytochrome c552 by using the extinction coefficient 15.2 mM−1⋅cm−1 (calculated from the protein sequence) at 280 nm. The absorption spectra of reduced hemes in the presence of hydroxide and pyridine are characteristic of the type of Fe-porphyrin present as well as of any modifications to it (e.g., covalent attachment to the polypeptide in a c-type cytochrome); such pyridine hemochrome spectra were obtained according to the method of Bartsch (30). Far UV circular dichroism (CD) spectra were recorded on a Jasco J720 spectropolarimeter. Electrospray-ionization mass spectra were recorded on a Micromass Bio-Q II-ZS triple-quadrupole atmospheric pressure mass spectrometer equipped with an electrospray interface. Samples (10 μl) were introduced into the electrospray source via a loop injector as a solution [20 pmol μl−1 in water/acetonitrile (1:1)/1% formic acid] at a flow rate of 10 μl min−1.
Results and Discussion
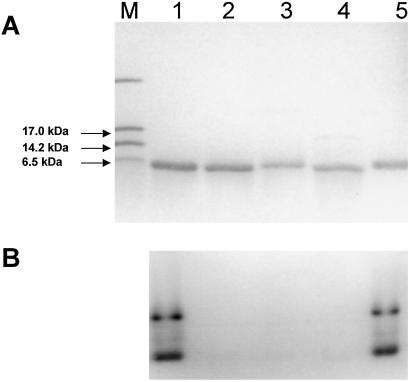
Covalently attached heme was removed from H. thermophilus cytochrome c552 by treatment of the protein with silver sulfate (23). Formation of the apoprotein (and removal of the silver) was demonstrated by the absence of a band in heme-stained (27) SDS/PAGE gels (Fig. 1), ES-MS (calculated mass of apoprotein, 8,700 Da; observed mass, 8,699 Da), and the disappearance of bands characteristic of heme-containing cytochrome c552 from the absorption spectrum (data not shown).
Figure 1.
SDS/17.5% PAGE analysis stained with Coomassie blue (A) or activity stained for covalently bound heme (B). Lanes: M, molecular mass markers; 1, wild-type H. thermophilus cytochrome c552 as purified from E. coli; 2, apocytochrome c552 produced from the holoprotein; 3, the b-type cytochrome analogue formed from apoprotein and Fe-mesoporphyrin; 4, the C11A/C14A mutant of H. thermophilus cytochrome c552, which is a b-type cytochrome (15); 5, reconstituted cytochrome c552 produced by the reaction of wild-type apocytochrome and heme. The higher molecular mass bands in lanes 1 and 5 of the heme-stained gel are caused by polymerized protein; they are not visible by using the less-sensitive Coomassie stain. For Coomassie staining, 200 pmol of protein were applied to each lane of the gel, and for heme staining, 20 pmol were applied.
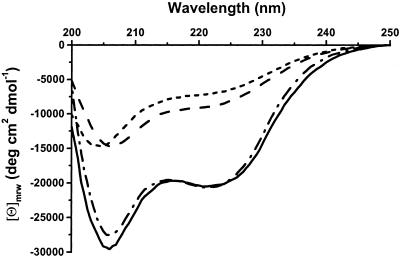
A disulfide bond formed in the apoprotein between the cysteine residues of the Cys-Xaa-Xaa-Cys-His heme-binding motif (these are the only cysteines in the protein; ref. 12), as demonstrated with Ellman's reagent (28), which indicated two free thiols per mole of reduced protein and zero per mole of oxidized protein. Formation of a disulfide bond was confirmed by treatment with cyanogen bromide, which cleaves a peptide specifically after methionine residues (29). In H. thermophilus cytochrome c552, the amino acid sequence of the heme-binding motif is Cys-Met-Ala-Cys-His (12). After digestion of the apoprotein with cyanogen bromide, one of the fragments isolated by HPLC had two N termini present in equimolar amounts; these contained the sequences Asn-Glu-Gln-Leu-Ala-Lys-Gln (the N terminus of the polypeptide; ref. 12) and Ala-Cys-His-Asp-Leu-Lys, showing that the protein had cleaved after the methionine between the two cysteine residues (12). On reduction of this fragment with DTT, two separate peptides each with one of these same two N-terminal sequences were resolved by HPLC, which demonstrates that the oxidized protein contained a disulfide bond that was broken on reduction. Matrix-assisted laser desorption ionization/time-of-flight and ES-MS spectra gave masses of 8,697 and 8,699 Da, respectively, for the undigested oxidized apoprotein, indicating that it was monomeric. Therefore, the disulfide bond between the two cysteines of the Cys-Met-Ala-Cys-His sequence is intramolecular. Air oxidation was sufficient to form the disulfide, which is the first to be observed between the heme-binding cysteines of a c-type cytochrome. CD spectra indicate that formation of this disulfide bond induces secondary structure in the protein relative to the reduced (free thiol) state (Fig. 2). The CD spectrum of reduced apocytochrome c552 is very similar to that reported for the apo C11A/C14A (b-type cytochrome) variant (15).
Figure 2.
CD spectra of H. thermophilus apocytochrome c552 reduced with DTT (- - -), apocytochrome c552 air-oxidized (— — —), holocytochrome c552 as purified from E. coli (⋅ — ⋅ — ⋅), and reconstituted cytochrome c552 formed by adding heme to apoprotein (———). [Θ]mrw is mean molar ellipticity per residue. Spectra were recorded by using 25 μM protein in 20 mM sodium phosphate buffer, pH 7.0.
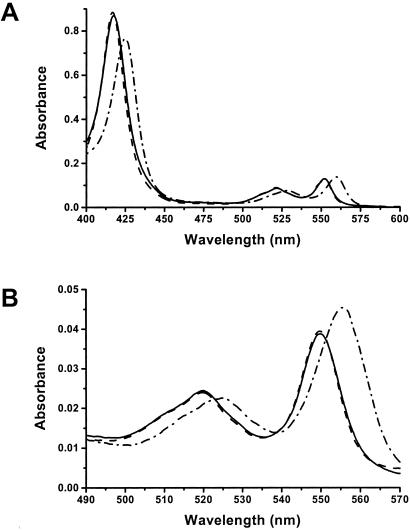
Mixing reduced H. thermophilus apocytochrome c552 (no disulfide bond) with ferrous [Fe(II)] heme in the presence of DTT at pH 7.0 resulted in an increase in absorption around 423 nm relative to that of heme alone; the same trend was observed around 528 and 560 nm, bands characteristic of the presence of a reduced cytochrome (Fig. 3A). The pyridine hemochrome spectrum of this mixture had its α-band at 556 nm (Fig. 3B), indicating that the heme contained two unreacted vinyl groups (30, 31). These results show that the apoprotein and heme initially form a b-type cytochrome, in which the heme is not attached covalently to the peptide but in which its iron atom is coordinated by two amino acid side chains from the protein. With a protein concentration of 10 μM and a stoichiometric quantity of heme, formation of this cytochrome b was complete in ≈20 min at 25°C. We observed a very similar time course on mixing the apo C11A/C14A mutant of H. thermophilus cytochrome c552 with heme under otherwise identical conditions.
Figure 3.
Absorption spectra (A) and pyridine hemochrome spectra (B) of wild-type H. thermophilus cytochrome c552 as purified from E. coli (— — —, largely obscured by the solid line), reconstituted cytochrome c552 (———) recorded 18 h after adding heme to apoprotein, and the b-type cytochrome intermediate (⋅ — ⋅ — ⋅) formed 30 min after adding heme to apoprotein. Absorption spectra were recorded by using 5 μM protein in 50 mM sodium phosphate buffer, pH 7.0; pyridine hemochrome spectra were obtained by using 1.25 μM protein in 19% (vol/vol) pyridine and 0.15 M NaOH. All samples were reduced by using disodium dithionite.
After formation of the b-type cytochrome from the mixture of wild-type H. thermophilus apocytochrome c552 and heme in reducing conditions, the maximum at 560 nm in the protein absorption spectrum shifted to 552 nm (Fig. 3A). After 18 h, the pyridine hemochrome spectrum of the purified reduced protein had a resolved band at 549.7 nm (Fig. 3B), characteristic of saturation of both vinyl groups of the heme (30, 31). These observations decisively indicate the formation of a c-type cytochrome, an interpretation that was substantiated further by demonstration of the absence of free thiol groups (28), heme staining of the proteins on SDS/PAGE gels (Fig. 1), treatment of the heme containing protein with acidified acetone, and ES-MS analysis in denaturing conditions; in the latter three experiments, noncovalently bound heme dissociates from protein, but covalently bound heme does not. The visible absorption spectra of both the ferric [Fe(III)] and ferrous forms of the in vitro-synthesized cytochrome c together with its reduced pyridine hemochrome spectrum were indistinguishable from those of holoprotein prepared from E. coli (Fig. 3 A and B; oxidized spectra not shown); the CD spectra of the oxidized cytochrome c made in vivo or in vitro demonstrated that each must have the same protein fold (Fig. 2). These data show that these two forms of cytochrome c552 are indistinguishable with respect to both the environment of the heme and overall conformation. The ES-MS spectrum of the in vitro product cytochrome c had a peak at the correct mass for H. thermophilus holocytochrome c552.
Formation at pH 7.0 of the covalent bond(s) in the product c-type cytochrome occurred with biphasic kinetics. The first phase, accounting for ≈70% of the total change, had an observed first-order rate constant of 0.4 h−1; this value was independent of the initial heme and apoprotein concentrations, implying that the reaction was intramolecular. The second phase (≈30% of the amplitude) had a rate constant of ≈0.15 h−1. By analogy with many hemoproteins that contain noncovalently bound heme, heme may incorporate into the b-type cytochrome intermediate, leading to cytochrome c552, as a mixture of isomers in which the heme is rotated by 180° about the α,γ-meso axis (32–36). For other heme proteins (32–36), the initial proportions of the two isomers varied, and equilibration was observed after such heme binding, ultimately resulting in >85% of the noncovalently bound heme having a single orientation. At pH 7.0, this reorientation typically occurred on a time scale of hours (32–34), comparable to the rate for the second phase of in vitro holocytochrome c552 formation. Thus, our observations are consistent with a mechanism in which heme can bind covalently the cysteine thiols of apocytochrome c552 in only one orientation, and therefore, heme in the incorrect orientation in the b-type intermediate must reorient before covalent attachment can occur. Hence the portion of the heme that initially binds the apocytochrome c in the correct orientation can form holocytochrome c relatively rapidly (the first observed phase), whereas covalent attachment of the remainder of the heme is rate-limited by its reorientation (the second phase). Support for this idea is found in the structure of H. thermophilus cytochrome c552, produced in the cytoplasm of E. coli; the heme orientation and the stereochemistry of each of the thioether bonds are homogeneous in such protein (P. D. Barker and S.J.F., unpublished observations) and the same as for all cytochromes c made by biogenesis proteins (1).
After 2, 3.25, and 5 h of reaction between apocytochrome c552 and heme, i.e., during the first phase of cytochrome c552 formation, protein was treated with acidified acetone or guanidine hydrochloride to remove any noncovalently bound heme (15, 25). At each of these time points, in the absorption spectrum of the resolubilized protein the α-band maximum was at 552.5 nm, and in the pyridine hemochrome spectrum the corresponding band was at 550 nm, observations indicative of cytochrome c with the heme covalently bound to the polypeptide through two thioether bonds. The equivalent wavelengths for cytochrome c552 with a Cys-Xaa-Xaa-Ala-His or Ala-Xaa-Xaa-Cys-His heme-binding motif are 553 nm for both pyridine hemochrome spectra and 556 and 558 nm for the absorption spectra, respectively (25). Thus, the reaction kinetics for the in vitro cytochrome c formation described in the present work suggest that the faster first phase involves reaction of one of the cysteine thiols with heme as the rate-determining step with subsequent quicker formation of the second thioether bond; an alternative is that both thioether bonds form in a concerted fashion. In principle, such events could be monitored by NMR. However, the apocytochrome c552 is not stable at the millimolar concentrations this technique would require (note that to follow the reaction kinetics early in the heme attachment process, it would be necessary to identify resonances from each of the thioether bonds, some of which are intrinsically broad, in a small percentage of the total protein). In vitro cytochrome c formation from heme and apo C11A and C14A variants of H. thermophilus cytochrome c552, both of which form holocytochromes with a single thioether bond in the cytoplasm of E. coli (25), proceeded similarly to the wild-type (double cysteine) protein. Both mutants showed biphasic kinetics, with the normalized amplitudes and rate constants of both phases at pH 7.0 being approximately equal to those for wild-type cytochrome c552. Thus, there is no evidence that the two thioether bonds can form at substantially different rates, whereas the observation that the slower phase of the reaction is the same for both single and double cysteine proteins supports the idea that heme reorientation is rate-limiting.
The chemistry of thiol attachment to the vinyl groups of heme is understood poorly, and therefore we also have studied the kinetics of in vitro wild-type cytochrome c reconstitution as a function of pH. The rate of first phase of the reaction increased as the pH was lowered (kobs = 0.9, 0.4, and 0.3 h−1 at pH 6.0, 7.0, and 8.0, respectively). Thus, protonation of a titratable group near the vinyl group(s) of the heme that could act as a proton donor, or perhaps protonation of the β-carbon of the vinyl group itself, is presumably mechanistically more important in the physiological pH region than deprotonation of the cysteine thiols. Addition of cysteine to the vinyl group of 5-vinyluracil in acidic conditions has been shown to proceed via a carbonium ion intermediate (37); the particular local environment of the vinyl groups within a c-type cytochrome might facilitate a similar reaction mechanism. The second phase of the covalent heme attachment reaction showed no significant pH dependence in this range.
An analogue of the b-type cytochrome intermediate formed after the addition of reduced Fe-mesoporphyrin to reduced apocytochrome c552. The product had visible absorption maxima at 550, 519, and 415 nm and did not heme-stain on an SDS/PAGE gel (Fig. 1). Mesoporphyrin has ethyl groups in the positions of the vinyl groups of protoporphyrin (31) and therefore cannot form thioether bonds with the polypeptide. There was no evidence for any other type of covalent attachment of mesoporphyrin to apoprotein.
It is commonly believed that reducing conditions are required for the in vivo synthesis of c-type cytochromes (1, 38, 39); reductant may be necessary to ensure that the cysteine residues have free thiol groups and/or to provide ferrous heme. On mixing oxidized (disulfide-bonded) H. thermophilus apocytochrome c552 and ferric [Fe(III)] heme, no reaction was observed. When ferric heme was reacted with reduced apoprotein, products different from H. thermophilus cytochrome c552 were obtained, as shown by ES-MS analysis, absorption, and pyridine hemochrome spectra, which concurs with previous proposals (1, 17) that interaction of reduced apocytochromes c with ferric heme in vivo is far from optimal for generating the correct covalent attachment. When reduced heme was mixed with oxidized apoprotein in the absence of excess reductant, a mixture of products also was obtained. These data show that to effectively form the correct product c-type cytochrome, both the heme iron and apocytochrome disulfide bond must be reduced.
Our data show that a c-type cytochrome, containing heme attached to a polypeptide through two thioether bonds, can form in vitro without the action of any biosynthesis proteins. Initially, the apocytochrome c552 from H. thermophilus bound heme noncovalently, involving coordination of amino acid side chains to the heme iron. It is extremely likely that the highly conserved histidine residue of the Cys-Xaa-Xaa-Cys-His motif, which binds the heme in the holo c-type cytochrome, provided one of these axial iron ligands. Subsequently, thioether bonds formed, presumably because of the proximity of the vinyl groups of the heme and the cysteine residues of the protein. The presence of reduced heme (and reduced apoprotein) avoided formation of side products observed when ferric heme was used. It is probable that heme must be reduced when any c-type cytochrome is made in vivo, as has been deduced already for mitochondrial cytochrome c (38, 39). A requirement for reduced apoprotein is consistent with the roles in bacteria of some of the cytochrome c maturation (Ccm) proteins (2, 3) as disulfide reductases in the periplasm (2, 40); Ccm proteins also may keep heme reduced. The present work shows that a disulfide bond can form in the Cys-Xaa-Xaa-Cys-His heme-binding motif of an apocytochrome. The bacterial periplasm has a very active system (Dsb) for catalyzing the formation of disulfide bonds (2, 40). Such a disulfide may act as an internal protecting group for the reactive cysteine thiols. However, the reversal or avoidance of the propensity of the Cys-Xaa-Xaa-Cys-His motif of an apocytochrome to form a disulfide clearly is an important factor in the biogenesis of cytochromes c.
Very many studies (6, 41, 42) have been made of the refolding of mitochondrial cytochrome c (closely related in structure to the cytochrome c552 studied here) after denaturation in vitro, which leaves the thioether bonds to the heme intact. The relevance of such observations (6, 41, 42) to in vivo formation of mitochondrial cytochrome c is unclear. It is possible that the specific mitochondrial heme lyase enzymes (refs. 2 and 43; one for cytochrome c and one for cytochrome c1) catalyze heme attachment to the unfolded protein, which then would fold as in the in vitro studies. However, the present data suggest that consideration should be given to the notion that the apoprotein adopts a folded conformation with a nascent heme-binding site, as might be deduced also from the observations of Dumont et al. (44). Roles of the heme lyases then could include stabilization of specific conformations of apoproteins (i.e., cytochrome c or cytochrome c1), binding of heme (43) and its subsequent presentation to the apocytochromes c (including control of the stereospecificity of heme attachment), and maintenance of the cysteines as thiols rather than catalyzing formation of the two thioether bonds inside folded proteins. We believe that in this work we have observed the processes that occur during mitochondrial cytochrome c biogenesis but without the acceleration provided by a heme lyase. We undoubtedly have shown that the vinyl groups of heme are more reactive toward cysteine thiols than had been thought previously.
Acknowledgments
We thank Lin Hong, Robin T. Aplin, and Neil Oldham for assistance and advice and R. J. P. Williams for critical reading of the manuscript. This work was supported by Biotechnology and Biological Sciences Research Council Grant C13443 (to S.J.F.) and is a contribution from the Oxford Centre for Molecular Sciences, which is supported by the Biotechnology and Biological Sciences Research Council, Engineering and Physical Sciences Research Council, and Medical Research Council. O.D. is in receipt of a W. R. Miller studentship from St. Edmund Hall (Oxford) and the University of Oxford. S.J.F. and J.W.A.A. are both W. R. Miller Fellows of St. Edmund Hall.
Abbreviations
- ES-MS
electrospray mass spectrometry
- CD
circular dichroism
References
- 1.Barker P D, Ferguson S J. Structure Fold Des. 1999;7:R281–R290. doi: 10.1016/s0969-2126(00)88334-3. [DOI] [PubMed] [Google Scholar]
- 2.Page M D, Sambongi Y, Ferguson S J. Trends Biochem Sci. 1998;23:103–108. doi: 10.1016/s0968-0004(98)01173-6. [DOI] [PubMed] [Google Scholar]
- 3.Thöny-Meyer L. Microbiol Mol Biol Rev. 1997;61:337–376. doi: 10.1128/mmbr.61.3.337-376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettigrew G W, Moore G R. Cytochromes c: Biological Aspects. New York: Springer; 1987. [Google Scholar]
- 5.Moore G R, Pettigrew G W. Cytochromes c: Evolutionary, Structural, and Physicochemical Aspects. New York: Springer; 1990. [Google Scholar]
- 6.Scott R A, Mauk A G. Cytochrome c: A Multidisciplinary Approach. Mill Valley, CA: University Science Books; 1995. [Google Scholar]
- 7.Einsle O, Messerschmidt A, Stach P, Bourenkov G P, Bartunik H D, Huber R, Kroneck P M. Nature (London) 1999;400:476–480. doi: 10.1038/22802. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi N, Moriyama H, Fujiwara T, Fukumori Y, Tanaka N. Nat Struct Biol. 1997;4:276–284. doi: 10.1038/nsb0497-276. [DOI] [PubMed] [Google Scholar]
- 9.Martinou J C, Desagher S, Antonsson B. Nat Cell Biol. 2000;2:E41–E43. doi: 10.1038/35004069. [DOI] [PubMed] [Google Scholar]
- 10.Thöny-Meyer L. Biochim Biophys Acta. 2000;1459:316–324. doi: 10.1016/s0005-2728(00)00167-5. [DOI] [PubMed] [Google Scholar]
- 11.Schulz H, Hennecke H, Thöny-Meyer L. Science. 1998;281:1197–1200. doi: 10.1126/science.281.5380.1197. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa J, Yoshida T, Yamazaki T, Sambongi Y, Yu Y, Igarashi Y, Kodama T, Yamazaki K, Kyogoku Y, Kobayashi Y. Biochemistry. 1998;37:9641–9649. doi: 10.1021/bi9803067. [DOI] [PubMed] [Google Scholar]
- 13.Sambongi Y, Ferguson S J. FEBS Lett. 1994;340:65–70. doi: 10.1016/0014-5793(94)80174-6. [DOI] [PubMed] [Google Scholar]
- 14.Sinha N, Ferguson S J. FEMS Microbiol Lett. 1998;161:1–6. doi: 10.1111/j.1574-6968.1998.tb12921.x. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson E J, Ferguson S J. Proc Natl Acad Sci USA. 2000;97:5156–5160. doi: 10.1073/pnas.090089397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wain R, Pertinhez T A, Tomlinson E J, Hong L, Dobson C M, Ferguson S J, Smith L J. J Biol Chem. 2001;276:45813–45817. doi: 10.1074/jbc.M107572200. [DOI] [PubMed] [Google Scholar]
- 17.Barker P D, Ferrer J C, Mylrajan M, Loehr T M, Feng R, Konishi Y, Funk W D, MacGillivray R T, Mauk A G. Proc Natl Acad Sci USA. 1993;90:6542–6546. doi: 10.1073/pnas.90.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sano S, Granick S. J Biol Chem. 1961;236:1173–1180. [PubMed] [Google Scholar]
- 19.Sano S, Tanaka K. J Biol Chem. 1964;239:PC3109–PC3110. [PubMed] [Google Scholar]
- 20.Colleran E M, Jones O T. Biochem J. 1973;134:89–96. doi: 10.1042/bj1340089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennig B, Neupert W. Eur J Biochem. 1981;121:203–212. doi: 10.1111/j.1432-1033.1981.tb06450.x. [DOI] [PubMed] [Google Scholar]
- 22.Garrard W T. J Biol Chem. 1972;247:5935–5943. [PubMed] [Google Scholar]
- 23.Fisher W R, Taniuchi H, Anfinsen C B. J Biol Chem. 1973;248:3188–3195. [PubMed] [Google Scholar]
- 24.Sanbongi Y, Yang J H, Igarashi Y, Kodama T. Eur J Biochem. 1991;198:7–12. doi: 10.1111/j.1432-1033.1991.tb15979.x. [DOI] [PubMed] [Google Scholar]
- 25.Tomlinson E J, Ferguson S J. J Biol Chem. 2000;275:32530–32534. doi: 10.1074/jbc.M004022200. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Goodhew C F, Brown K R, Pettigrew G W. Biochim Biophys Acta. 1986;852:288–294. [Google Scholar]
- 28.Riddles P W, Blakeley R L, Zerner B. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- 29.Means G E, Feeney R E. Chemical Modification of Proteins. San Francisco: Holden–Day; 1971. [Google Scholar]
- 30.Bartsch R G. Methods Enzymol. 1971;23:344–363. [Google Scholar]
- 31.Falk J E. Porphyrins and Metalloporphyrins; Their General, Physical, and Coordination Chemistry and Laboratory Methods. Amsterdam: Elsevier; 1964. [Google Scholar]
- 32.Yamamoto Y, La Mar G N. Biochemistry. 1986;25:5288–5297. doi: 10.1021/bi00366a045. [DOI] [PubMed] [Google Scholar]
- 33.La Mar G N, Toi H, Krishnamoorthi R. J Am Chem Soc. 1984;106:6395–6401. [Google Scholar]
- 34.McLachlan S J, La Mar G N, Burns P D, Smith K M, Langry K C. Biochim Biophys Acta. 1986;874:274–284. doi: 10.1016/0167-4838(86)90026-9. [DOI] [PubMed] [Google Scholar]
- 35.Banci L, Bertini I, Rosato A, Scacchieri S. Eur J Biochem. 2000;267:755–766. doi: 10.1046/j.1432-1327.2000.01054.x. [DOI] [PubMed] [Google Scholar]
- 36.Wu J Z, La Mar G N, Yu L P, Lee K B, Walker F A, Chiu M L, Sligar S G. Biochemistry. 1991;30:2156–2165. doi: 10.1021/bi00222a020. [DOI] [PubMed] [Google Scholar]
- 37.Jones A S, Slater M J, Walker R T. J Chem Soc Perkin Trans 1. 1987;6:1325–1329. [Google Scholar]
- 38.Nicholson D W, Neupert W. Proc Natl Acad Sci USA. 1989;86:4340–4344. doi: 10.1073/pnas.86.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong J, Margoliash E. J Biol Chem. 1998;273:25695–25702. doi: 10.1074/jbc.273.40.25695. [DOI] [PubMed] [Google Scholar]
- 40.Fabianek R A, Hennecke H, Thöny-Meyer L. FEMS Microbiol Rev. 2000;24:303–316. doi: 10.1111/j.1574-6976.2000.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 41.Englander S W, Sosnick T R, Mayne L C, Shtilerman M, Qi P X, Bai Y. Acc Chem Res. 1998;31:737–744. [Google Scholar]
- 42.Yeh S, Han S, Rousseau D L. Acc Chem Res. 1998;31:727–736. [Google Scholar]
- 43.Steiner H, Kispal G, Zollner A, Haid A, Neupert W, Lill R. J Biol Chem. 1996;271:32605–32611. doi: 10.1074/jbc.271.51.32605. [DOI] [PubMed] [Google Scholar]
- 44.Dumont M E, Corin A F, Campbell G A. Biochemistry. 1994;33:7368–7378. doi: 10.1021/bi00189a043. [DOI] [PubMed] [Google Scholar]