Abstract
Infected cell protein 0 (ICP0) of herpes simplex virus-1 is a 775-aa residue multifunctional protein that acts as a promiscuous transactivator linked to the degradation of several proteins. ICP0 is the only protein known which encodes two physically separated E3 ubiquitin (Ub) ligase domains, one, designated herpes virus Ub ligase 1 (HUL-1) located in a domain encoded in exon 3 and one designated herpes virus Ub ligase 2 (HUL-2) associated with the really interesting new gene (RING) finger domain encoded by exon 2. We report the following: (i) ICP0 residues 543–680 are sufficient for HUL-1 E3 activity and necessary determinants are encoded between residues 616 and 680. (ii) In substrate independent in vitro ubiquitylation reactions, a chimeric protein containing the HUL-1 domain promotes the ubiquitylation of itself and the ubiquitin conjugating enzyme (E2) cdc34 and interacts with cdc34. (iii) The mechanism of HUL-1 E3 function does not involve formation of a thioester between the HUL-1 domain and Ub. (iv) Residues 621–625 are essential for in vitro HUL-1 E3 activity and interaction between the HUL-1 domain and cdc34, suggesting that this interaction is required for HUL-1 E3 function.
Infected cell protein 0 (ICP0) of herpes simplex virus-1 is a multifunctional protein translated from a spliced mRNA containing three exons encoding 19, 222, and 534 codons (reviewed in ref. 1). Substitution of aspartate-199 in ICP0 with alanine (D199A) abolishes the ability of ICP0 to interact with and stabilize cyclin D3, attenuates viral growth in quiescent primary fibroblasts, and impairs neuroinvasiveness (2–4). In addition, ICP0 also stabilizes cyclin D1 even though a physical interaction between ICP0 and cyclin D1 has not been detected (3, 4).
Multiple lines of evidence indicate that ICP0 functionally and physically interacts with the ubiquitin (Ub)-proteasomal degradation pathway (reviewed in ref. 5). ICP0 interacts with the Ub-specific protease (USP)7 (6–8) and dynamically associates with the 26S proteasome and remains bound to proteasomes in the presence of the proteasome inhibitor MG132 (9, 10). Furthermore, a zinc-binding really interesting new gene (RING) finger motif (amino acids 106–149) characteristic of a class of ubiquitin ligase (E3) enzymes is encoded by ICP0 exon 2 (5, 11).
Two different regions of ICP0 have been shown to possess E3 Ub ligase activity in conjunction with different E2 Ub-conjugating enzymes in substrate-independent in vitro ubiquitylation reactions (9, 12, 13). The one reported first and designated herpesvirus Ub ligase 1 (HUL-1) was initially mapped to the C terminus of exon 3 (amino acids 543–768) and functions in conjunction with the E2 enzyme cdc34 (UbcH3) but not with UbcH5a, UbcH6, or UbcH7 (9, 12). As the RING finger domain is not required for HUL-1 E3 function and no homology has been detected between the HUL-1 domain and other known classes of E3 enzymes, it appears to represent a novel class of E3 enzymes (12). Cdc34 was shown to interact with the domain encoded by ICP0 exon 2 (amino acids 20–241), and this interaction was attenuated but not ablated by the D199A mutation (9, 12). The second E3 site designated here as HUL-2 was mapped to exon 2 containing the RING finger and in in vitro ubiquitylation reactions functionally interacts with UbcH6 and UbcH5a but not with UbcH7 or cdc34 (9, 12, 13). These results led to the unprecedented conclusion that ICP0 encodes two physically separate E3 ligase domains that belong to different classes of E3 enzymes and have different E2 specificities (12).
In vivo, ICP0 promotes the proteasome-dependent degradation of multiple cellular proteins in a RING finger-dependent manner, indicating they are substrates of the HUL-2 RING finger E3 ligase (reviewed in refs. 1 and 11). In addition, aspartate-199 is required for ICP0 to efficiently promote the interaction of ubiquitylated cdc34 with proteasomes in the presence of MG132 and the proteasome-dependent degradation of cdc34 in infected cells (9, 12).
On the basis of these observations, we proposed a model where the HUL-1 E3 ligase promotes the ubiquitylation of the E3 cdc34 whereas the HUL-2 E3 ligase promotes degradation of other cellular proteins (12). Thus HUL-2 may target proteins whose proteasome-dependent turnover is regulated by UbcH5a and UbcH6 whereas HUL-1 targets cdc34 for degradation to preclude the degradation of proteins whose proteasome-dependent degradation is regulated by that E2 enzyme. This model is consistent with and provides an explanation of the mechanism by which ICP0 stabilizes cyclin D1 in a D199-dependent manner without binding it (12). Cdc34 is the major E2 that interacts with the Skp1-cdc53-F-box (SCF) E3 complex (5), and SCF complexes containing the F-box protein Skp2 promote the ubiquitylation and degradation of cyclin D1 (14, 15). The fact that the D199A substitution impairs the interaction of cdc34 with ICP0 as well as the ability of ICP0 to promote the interaction of ubiquitylated cdc34 with and its degradation by the 26S proteasome suggests that cdc34 must be firmly tethered to ICP0 to allow its ubiquitylation, but the requirements for ICP0 interaction with cdc34 must be less stringent for ICP0 to promote transfer of Ub from cdc34 and Ub-protein conjugation in vitro as the cdc34-binding site encoded by exon 2 is not required for E3 activity in vitro (9, 12).
In this report, we show that critical determinants for HUL-1 E3 activity map to residues 616–680 and that this region also contains a sequence that interacts with the E2 cdc34. Residues 621–625 are essential for both E3 activity and interaction of the HUL-1 domain with cdc34, suggesting that interaction with the E2 is required for this atypic E3 enzyme to promote Ub-protein ligation.
Materials and Methods
Plasmids and Fusion Proteins.
pRB4995 encoding herpes simplex virus-1 strain F ICP0 codons 543–768 inserted into vector pGEX 4T-1 in frame with glutathione S-transferase (GST) was described elsewhere (16). Pm1I/SalI, BsiWI/SalI, and SfiI/SalI fragments of pRB4995 containing the pGEX vector sequences and a portion of ICP0 fused in frame with GST were incubated with DNA polymerase I klenow fragment to fill in sticky ends, purified, and ligated together to generate pRB5842, pRB5843, and pRB5844, respectively. pRB5842, pRB5843, and pRB5844 encode ICP0 residues 543–714, 543–680, and 543–615 fused in frame with GST, respectively. Site-directed mutagenesis where complimentary oligonucleotides containing specific mutations in ICP0 were annealed to pRB4995 DNA was performed by using the QuikChange XL site-directed mutagenesis kit (Stratagene) to individually generate single amino acid substitutions C621A, C666A, K624I, and K660I and the deletion of ICP0 codons 621–625 in the ICP0 fragment contained in pRB4995. The resulting plasmids were designated pRB5845, pRB5846, pRB5847, pRB5848, and pRB5849, respectively, and sequenced at The University of Chicago Cancer Research Center DNA Sequencing Facility to confirm that the ICP0 fragments they encoded contained the appropriate mutation. GST fusion proteins encoded by pRB4994, pRB4995, pRB5842, pRB5843, pRB5844, pRB5845, pRB5846, pRB5847, pRB5848, and pRB5849 were designated GST-exon 2, GST-exon 3, GST-714, GST-680, GST-615, GST-C621A, GST-C666A, GST-K624I, GST-K660I, and GST-Δ621–625, respectively. GST fusion proteins were expressed in Escherichia coli BL21 cells and purified as described (16).
N-Ethylmaleimide (NEM) Treatment of GST Fusion Proteins.
NEM treatment of GST-exon 3 was performed as described (17). Glutathione Sepharose 4B beads (Amersham Pharmacia Biotech) bound to GST-exon 3 were incubated in the presence of 5 mM NEM in PBS at 24°C for 10 min at which point DTT was added to a concentration of 10 mM, and incubation at 24°C was continued for another 10 min. As a control, beads, to which 10 mM DTT was added just before the 5 mM NEM, were incubated at 24°C for 10 min. Beads were then washed for 30 min three times at 4°C. GST fusion proteins were eluted from the beads in 50 mM Tris, pH 8, containing 100 mM glutathione (16) and dialyzed into PBS at 4°C.
Substrate-Independent in Vitro Ubiquitylation Reactions.
Reactions were performed as described (9) in 30 μl of ubiquitylation buffer (50 mM Tris, pH 7.5/2.5 mM MgCl2/0.5 mM DTT) containing 40 ng of recombinant rabbit Ub-activating enzyme E1 (Calbiochem), 40 ng of [His-6]-UbcH3 (Affiniti Research Products, Mamhead, Exeter, UK), 1 μg of biotinylated Ub (Affiniti, catalog no. UW8705), 0.2 mM ATP, an ATP-regenerating system consisting of 1 mM creatine phosphate and 15 units porcine heart creatine phosphokinase (Calbiochem), and 5 μg of GST or the indicated purified GST fusion protein. The reactions were thoroughly mixed and incubated at 37°C for 90 min. The reaction was then stopped by the addition of one part 4× disruption buffer (6.85% SDS/24% glycerol/3.3% β-mercaptoethanol/233 mM Tris, pH 6.8/0.008% bromophenol blue) to three parts reaction mixture or preparation for affinity capture as described below.
Affinity Capture Experiments.
Affinity capture of GST fusion proteins was performed as described (12) with glutathione Sepharose 4B resin (Amersham Pharmacia Biotech). For each sample, 15 μl from the in vitro ubiquitylation reactions described above was added to 485 μl of cold PBS on ice. Diluted reaction mixtures were mixed with 20 μl of resin and reacted overnight at 4°C. Sepharose pellets were collected by centrifugation and rinsed for 30 min, three times, at 4°C before addition of disruption buffer.
Abs and Immunoblotting.
Anti-GST rabbit polyclonal Ab was made against the chimeric protein GST-ORF P (18) and used at a dilution of 1:2,000 for immunoblots. Rabbit polyclonal Ab against cdc34 (Neomarkers, Fremont, CA) was used at a dilution of 1:500. Biotinylated Ub was detected with streptavidin-horseradish peroxidase (Bio-Rad) at a dilution of 1:1,000. Electrophoretic separations of total or precipitated proteins from in vitro ubiquitylation reactions, immunoblotting, and development of immunoblots by enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech) or 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT, Sigma) were performed as described (9).
Results
Characterization of in Vitro Ubiquitylation Mediated by the HUL-1 E3 Ligase.
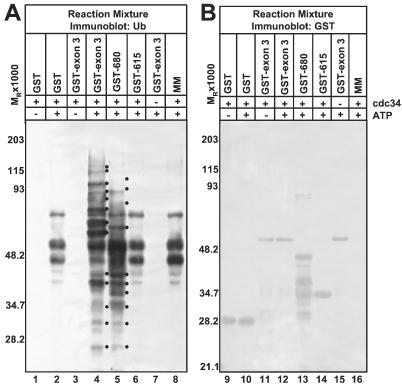
In substrate-independent in vitro ubiquitylation reactions, the ability of a protein to promote Ub-protein ligation is indicative of E3 activity (5, 9). Addition of an E3 enzyme to the reaction would be expected to effect the accumulation higher Mr Ub-protein conjugates, additional Ub-protein conjugates, and/or increased levels of Ub-protein conjugates as compared to reactions to which an E3 enzyme was not added. It is important to note that a basal level of Ub-protein ligation is mediated by the E2 in the absence of an E3. As activation of Ub by E1 is ATP-dependent (19), Ub-protein ligation mediated by the ubiquitylation machinery included in such in vitro reactions requires ATP. We have reported that ICP0 chimeric protein GST-exon 3 (ICP0 amino acids 543–768) promotes Ub-protein ligation in an ATP-dependent manner in the substrate-independent in vitro ubiquitylation system described in Materials and Methods (9). Reaffirming this result, streptavidin-horseradish peroxidase reacted with higher Mr protein conjugates with biotinylated Ub and increased levels and numbers of ubiquitylated products from electrophoretically separated reaction mixtures containing GST-exon 3 than from reactions containing GST or no GST fusion protein (Fig. 1A, compare lane 4 to lanes 2 and 8). As expected, both basal Ub-protein ligation and enhanced levels Ub-protein ligation mediated by GST-exon 3 were ATP-dependent (Fig. 1A, compare lane 1 to 2 and lane 3 to 4). Furthermore, both basal and GST-exon 3-promoted Ub-protein conjugation require the E2 cdc34 (Fig. 1A, compare lanes 4 and 7). Fig. 1B shows that the mixtures contain comparable amounts of GST and chimeric proteins, indicating that differences in Ub-protein ligation observed in the reactions are not the results of differential loading. Therefore, GST-exon 3 is a bona fide E3 in that it stimulates Ub-protein ligation mediated by the E1 and E2 components of the ubiquitylation machinery.
Figure 1.
Analysis of HUL-1 E3 ligase function. (A) GST (lanes 1 and 2), GST-exon 3 (lanes 3, 4, and 7), GST-680. (lane 5), GST-615 (lane 6), or no additional protein were added to the in vitro ubiquitylation reaction master mix (MM) containing recombinant E1, biotinylated Ub, and ubiquitylation buffer in the presence and absence of recombinant cdc34 and ATP and the ATP-regenerating system as indicated. Reaction mixtures were electrophoretically separated in a denaturing polyacrylamide gel and probed with streptavidin. Dots to the right of bands indicate polyubiquitylated species that are specifically present or augmented in reactions containing GST-exon 3 or GST-680. (B) Electrophoretically separated reaction mixtures containing GST (lanes 9 and 10), the indicated ICP0 chimeric protein (lanes 11–15) in addition to the master mix, or the master mix alone (lane 16) along with the indicated reaction components were probed with an Ab against GST. Reactive bands were detected with BCIP/NBT. All procedures in this and other figure legends were as described in Materials and Methods.
Mapping of the HUL-1 E3 Ligase Domain.
To define the ICP0 residues required for HUL-1 E3 ligase activity, we constructed terminal deletions of GST-exon 3: GST-714 (residues 543–714), GST-680 (residues 543–680), and GST-615 (residues 543–615). GST-680 promoted Ub-protein ligation above basal levels (Fig. 1A, compare lane 5 to lanes 2 and 8) whereas GST-615 did not (Fig. 1A, compare lane 6 to lanes 2 and 8). Solubility problems were encountered with GST-714, but it also promoted Ub-protein ligation (data not shown). Fig. 1B shows that GST and chimeric proteins were of the expected size and present in reactions in comparable amounts. From these results, we conclude that determinants essential for HUL-1 E3 activity map between codons 616 and 680 and that residues 543–680 are sufficient for HUL-1 E3 activity.
HUL-1 E3 Activity Promotes the Ubiquitylation of HUL-1 Chimeric Proteins and the E2 cdc34 in Vitro.
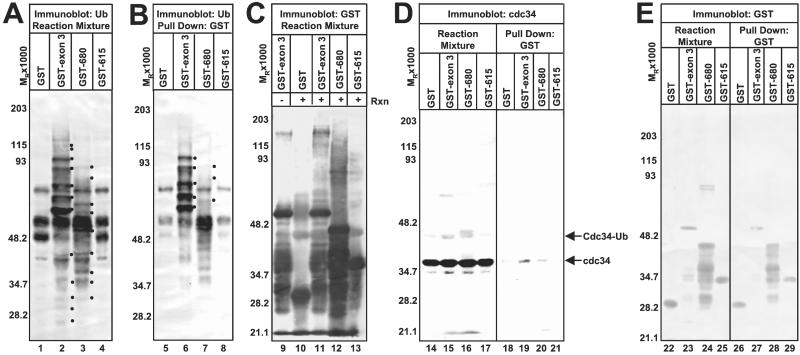
That GST-exon 3, GST-680, and GST-714 differ in size and produce ladders where some Ub-protein conjugates (especially those of high Mr) are of different sizes (Fig. 1A, lanes 4 and 5 and data not shown). We examined the possibility that the HUL-1 promotes its own uibquitylation. We precipitated GST and GST fusion proteins by using glutathione Sepharose beads and reacted electrophoretically separated precipitates with streptavidin-horseradish peroxidase (Fig. 2B). Ub conjugates of GST-exon 3 and GST-680 are readily apparent (Fig. 2B, lanes 6 and 7) and correspond to prominent Ub-protein conjugates in the total reaction mixture (Fig. 2A), which are unique to either the GST-exon 3 or GST-680 ladders (compare Fig. 2 A lanes 2 and 3 to B lanes 6 and 7). As expected from their sizes, the ladder of ubiquitylated GST-680 is shifted downward compared to that of ubiquitylated GST-exon 3 (Fig. 2B, lanes 6 and 7). Basal Ub-protein ligation activity of the E2 promotes low-level ubiquitylation of catalytically inactive proteins GST and GST-615 (Fig. 2B, lanes 5 and 8). GST and ICP0 chimeric proteins are of the proper size and present in comparable amounts in both total reaction mixtures and GST precipitates (Fig. 2E). Ubiquitylated proteins in GST precipitates that are of lower Mr than the chimeric proteins (Fig. 2B, lanes 6 and 7) are likely degradation products of the chimeric proteins. In a complementary approach, we reacted electrophoretically separated reaction mixtures with Ab to GST and visualized GST with the highly sensitive ECL detection procedure (Fig. 2C) to detect low abundance Ub conjugates that may not be detected by the alkaline phosphatase method. A ladder of proteins reacting with the anti-GST Ab with Mr higher than GST-exon 3 or GST-680 is observed (Fig. 2C, lanes 11 and 12). Because these high Mr bands are not present in purified GST-exon 3 preparations (Fig. 2C, compare lanes 9 and 11), the high Mr protein species represent ubiquitylated isoforms of GST-exon 3 and GST-680 formed as a result of the E3 activity of these chimeric proteins during the reaction. Ub conjugates to GST and GST-615 generated by basal Ub-protein ligation activity are present is significantly lower amounts than those present in reactions containing the active HUL-1 E3 ligase (GST-exon 3 or GST-680) (Fig. 2C, compare lanes 10 and 13 to lanes 11 and 12).
Figure 2.
The HUL-1 E3 encoded by ICP0 exon 3 promotes its own ubiquitylation as well as that of cdc34 in vitro. (A) In vitro ubiquitylation reactions containing GST or the indicated GST fusion protein, the master mix, recombinant cdc34, ATP, and the ATP-regenerating system were separated by SDS/PAGE and probed with streptavidin. Dots to the right of bands indicate polyubiquitylated species that are specifically present or augmented in reactions containing GST-exon 3 or GST-680. (B) GST or GST-fusion proteins were precipitated from reaction mixtures with glutathione Sepharose beads. Precipitates were separated as above and probed with streptavidin. Dots to the right of bands indicate ubiquitylated GST-exon 3 or GST-680. (C) Purified GST-exon 3 (lane 9) or reaction mixtures containing GST or the indicated GST fusion protein (lanes 10–13) were electophoretically separated and probed with Ab against GST. Reactive bands were detected by ECL. (D) GST and GST-fusion proteins were precipitated from reactions as above. Precipitates (lanes 18–21) and total reaction mixtures (lanes 14–17) were separated as above and probed with Ab against cdc34. Cdc34 was visualized by ECL. (E) GST and GST-fusion proteins were precipitated as above. Precipitates (lanes 26–29) and total reaction mixtures (lanes 22–25) were separated and probed with Ab against GST. Bands reacting with the Ab were detected with BCIP/NBT.
To reaffirm the previous result that HUL-1 E3 activity promotes autoubiquitylation of cdc34 (9), we reacted electrophoretically separated reaction mixtures with Ab to cdc34 (Fig. 2D, lanes 14–17). Although unmodified cdc34 is present in comparable amounts in all reactions, a band of a Mr consistent with monoubiquitylated cdc34 is prevalent in reactions containing GST-exon 3 and GST-680 but is faint in reactions containing GST and GST-615 (Fig. 2D, compare lanes 14 and 17 to lanes 15 and 16). Additional high Mr cdc34 isoforms are also present in reactions containing GST-exon 3 and GST-678 (Fig. 2D, compare lanes 14 and 17 to lanes 15 and 16). Although more of the ubiquitylation mediated by the HUL-1 E3 is directed against the HUL-1 chimeric protein, the HUL-1 E3 ligase also promotes the ubiquitylation of cdc34.
ICP0 Encodes a Second Interaction Site for the E2 cdc34 in the HUL-1 Domain in Exon 3.
As interactions between E3 enzymes and their cognate E2 can often be detected (5), we examined the possibility that the HUL-1 domain interacted with cdc34 by reacting electrophoretically separated GST and GST fusion proteins from in vitro ubiquitylation reactions with Ab directed against cdc34 and detecting precipitated cdc34 by ECL (Fig. 2D, lanes 18–21). Cdc34 precipitated with GST-exon 3 and GST-680 (Fig. 2D, lanes 19 and 20) but not with GST or GST-615. Thus, we conclude that cdc34 specifically interacts with the HUL-1 domain and that critical determinants for this interaction are encoded in the region between residues 616 and 680. In a previous report (9), we were unable to detect precipitation of cdc34 by GST-exon 3. However, the alkaline phosphatase detection method used in that study is much less sensitive than the ECL method used here. As a very small fraction of the total cdc34 in the reaction is precipitated by the HUL-1 domain (Fig. 2D, compare lanes 19 and 20 to lanes 15 and 16), it is likely that the interaction between cdc34 and the HUL-1 domain is weak or transient in nature and was below level of detection of the previous study.
The Catalytic Mechanism of the HUL-1 E3 Does Not Involve Formation of Thioester Bonds with Ub.
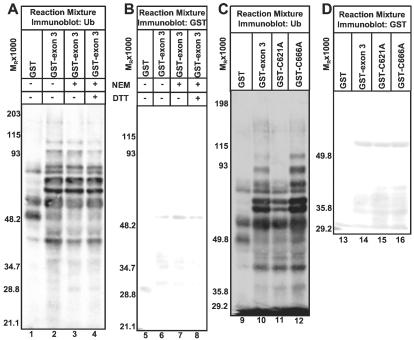
HECT (homologous to E6-AP C terminus domain family E3 enzymes accept Ub from E2 enzymes via a thioester with a catalytic cysteine and directly catalyze Ub transfer to the target protein and are inactivated by sulfhydryl-modifying agents (17, 20). To address whether the HUL-1 E3 acts by a mechanism similar to HECT E3s, we preincubated GST-exon 3 with the sulfhydryl-modifying agent NEM and quenched further modification of cysteine residues with DTT before use in in vitro ubiquitylation reactions. NEM treatment had no effect on HUL-1 E3 activity as GST-exon 3 treated with NEM promoted levels of Ub-protein ligation equivalent to untreated GST-exon 3 or GST-exon 3 treated with NEM inactivated by DTT (Fig. 3A, lanes 2–4). In a complimentary approach, we also mutated the two cysteine residues in the region required for HUL-1 E3 activity (residues 616–680) at positions 621 and 666 to alanine (GST-C621A and GST-C666A) as mutation of the catalytic cysteine of a HECT domain E3 completely destroys its function (20). Whereas the C621A mutation impairs the ability of GST-exon 3 to promote Ub-protein ligation (Fig. 3C, compare lanes 2 and 3), GST-C621A promotes Ub-protein ligation above basal levels as greater numbers and levels of Ub-protein conjugates are present in reactions containing GST-621A than those containing GST (Fig. 3C, compare lanes 1 and 3). Because mutating C621 only has a partial effect on E3 activity, C621 is not analogous to HECT domain catalytic cysteine. The C666A mutation had no effect on HUL-1 E3 function (Fig. 3C, lane 4), and GST and chimeric proteins were included in reactions in comparable amounts in these studies (Fig. 3 B and D). We conclude that the catalytic mechanism of the HUL-1 E3 does not involve thioester formation between Ub and the HUL-1 domain.
Figure 3.
HUL-1 E3 Ub ligase activity does not require thioester formation between ICP0 and ubqiutin. (A) GST, GST-exon 3, or GST-exon 3 treated with the indicated chemicals was added to in vitro ubiquitylation reactions performed as in Fig. 2. Reaction mixtures were electophoretically separated in a denaturing polyacrylamide gel and probed with streptavidin. (B) Electrophoretically separated reactions as in A were probed with Ab against GST. GST was detected with BCIP/NBT. (C) In vitro ubiquitylation reactions containing GST or the indicated GST-fusion protein were electrophoretically separated as in Fig. 2 and reacted with streptavidin. (D) Electrophoretically separated reaction mixtures containing GST or the indicated GST fusion protein were probed with Ab against GST, and reactive bands were detected with BCIP/NBT.
ICP0 Residues 621–625 Are Necessary for HUL-1 Domain E3 Function and Interaction with cdc34.
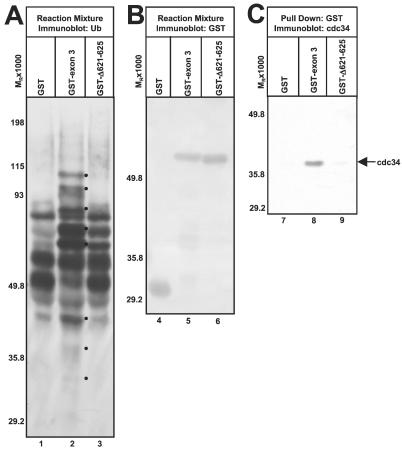
As previous results have shown that mutating lysine-620 to isoleucine has no effect on the E3 activity of GST-exon 3 (12), we hypothesized that C621 and the region slightly downstream of it might define a critical determinant for HUL-1 E3 activity. Thus, we deleted residues 621–625 from GST-exon 3 (GST-Δ621–625). GST-Δ621–625 does not promote the appearance of Ub-protein conjugates, which are not present in reactions containing GST (Fig. 4A, lanes 1 and 3). Also, most ubiquitylated bands present in reactions containing GST-Δ621–625 and GST are of comparable intensity (Fig. 4A, lanes 1 and 3). Notably absent from reactions containing GST-Δ621–625 are high and low Mr Ub-protein conjugates present in reactions containing GST-exon 3 (Fig. 4A, compare lanes 2 and 3). Therefore, GST-Δ621–625 does not significantly promote Ub-protein ligation above basal levels and is not a functional E3 ligase. The mutation of lysine-624 to isoleucine partially impairs HUL-1 E3 activity although this effect is less severe than that of the C621A mutation whereas a K660I mutation has no effect (data not shown). As the Δ621–625 blocked E3 function, we asked whether it also disrupted the interaction of cdc34 with the HUL-1 domain (Fig. 4C). Although cdc34 precipitated with GST-exon 3 (Fig. 4C, lane 8), significant amounts of cdc34 did not precipitate with GST-Δ621–625 (Fig. 4C, lane 9). GST and GST fusion proteins were of the expected size and present in the reaction mixtures in comparable amounts (Fig. 4B). From these data, we conclude that residues 621–625 are necessary for HUL-1 E3 activity and interaction of the HUL-1 domain with cdc34. Thus, interaction with the E2 cdc34 appears to be required for HUL-1 E3 activity.
Figure 4.
ICP0 residues 621–625 are essential for HUL-1 E3 activity and interaction with cdc34. (A) In vitro ubiquitylation reactions containing GST, GST-exon 3, or GST-Δ621–625 performed as in Fig. 2 were separated in a denaturing polyacrylamide gel and probed with streptavidin. Dots to the right of bands indicated polyubiquitylated species specifically present or augmented in reactions containing GST-exon 3. (B) Reactions were separated as in A and reacted with Ab against GST. Reactive bands were detected with BCIP/NBT. (C). GST and GST fusion proteins were precipitated from reactions containing GST, GST-exon 3, or GST-Δ621–625 as in Fig. 2. Precipitates were separated by SDS/PAGE and reacted with Ab against cdc34. Cdc34 was visualized by ECL.
Discussion
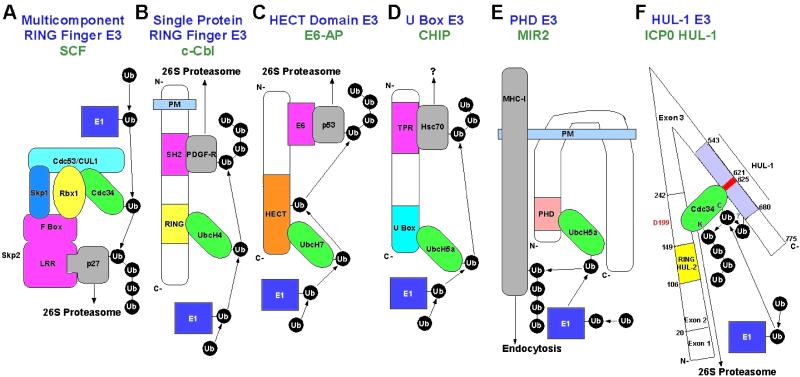
ICP0 is unique in that it is the only protein hitherto described to encode two discrete E3 Ub ligase functions. The mouse E3 enzyme KIAA0860 encodes both U box and RING finger domains, but only the U-box is responsible for E3 activity (21). Sequence comparisons failed to show any significant homology between the HUL-1 domain and any members of the existing five classes of E3 ligases (Fig. 5 A–E). The HUL-1 domain also did not contain any known conserved domains. The HUL-1 domain appears to be a novel, sixth class of E3 enzymes (Fig. 5F). Recently, two new domains with E3 activity, the U box (21, 22) and PHD (plant homeodomain) (23), were identified. Unlike these domains (21, 23), the HUL-1 does not show any similarity to a RING finger domain. However, sequences encoding determinants required for HUL-1 E3 function (residues 616–680) show strong sequence homology to corresponding regions of ICP0 proteins encoded by herpes simplex virus 2 and macrodipid herpesvirus 1, a pathogen of wallabies. Thus, it is conceivable that these ICP0 proteins also encode a HUL-1 E3 activity.
Figure 5.
Known classes of E3 enzymes. (A) Multicomponent E3 complex modeled after SCF (adapted from ref. 29). p27 is recruited to the complex by the F-box protein Skp2 via its leucine-rich repeat (LRR) domain. Other F-box proteins recruit different substrates (reviewed in ref. 5). The F-box protein interacts with SCF components Skp1 and the RING finger protein Rbx1. The cullin (Cdc53/CUL1) is a backbone, which binds Skp1, Rbx1, and the E2 cdc34, which also interacts with the Rbx1 RING finger. (B) Single protein RING finger E3 modeled after c-Cbl (adapted from ref. 5). The RING finger interacts with and allosterically activates the E2 UbcH4 (30). A SH2 (src homology 2) domain binds substrates phosphorylated at tyrosine such as platelet-derived growth factor-Rβ. (C) HECT domain E3 modeled after E6-AP (adapted from ref. 5). The E2 UbcH7 binds the HECT domain, and Ub is transferred from the E2 to form a thioester with a conserved cysteine in the HECT domain. Ub is then subsequently transferred to the substrate p53. p53 is recruited to E6-AP by the adaptor E6. (D) U box E3 modeled after CHIP. The U box of CHIP interacts with and activates members of the UbcH5 E2 family and TPR (tetratricopeptide repeat) domains near the N terminus interact with the substrate Hsc70 (22). (E) PHD (plant homeodomain) E3 modeled after MIR2. Human herpesvirus 8 MIR2 recruits UbcH5a to the membrane and activates it via its PHD domain (24). MIR2 (modulator of immune recognition) transmembrane (TM) domains mediate recognition of the transmembrane region(s) in the plane of the membrane of target molecules such as MHC class I causing the cytoplasmic domain of the target to be brought into close proximity with the E2 (24). (F). HUL-1 E3 domain. The HUL-1 domain of ICP0 has E3 activity in conjunction with cdc34 and interaction of the HUL-1 domain with cdc34 is required for E3 activity as evidenced by the fact that residues 621–625 are required for both functions. Cdc34 also interacts with ICP0 in the region encoded by exon 2 such that aspartate-199 is required for optimal interaction. The model proposes that the interaction site encoded by ICP0 exon 2 tethers cdc34 to the HUL-1 domain so its E2 activity can be efficiently stimulated by HUL-1 E3 activity via the interaction between cdc34 and the HUL-1 domain leading to the efficient autouibquitylation of cdc34. It also is possible there are other substrates for the HUL-1 E3.
Another unique aspect of ICP0 is that it encodes two distinct binding sites for the E2 cdc34. In an earlier report (12), we surmised that cdc34 must be firmly tethered to the interaction domain in exon 2 to enable efficient polyubiquitination of the protein by the HUL-1 E3 ligase as replacement of a single amino acid in exon 2 impairs cdc34 degradation. However, this interaction is not required for the HUL-1 domain to stimulate transfer of Ub from cdc34. In this report, we have identified a second interaction site for cdc34 in the HUL-1 domain. Because the interaction between cdc34 and the HUL-1 domain is required for HUL-1 E3 activity in vitro and appears to be relatively weak, it is likely that this interaction is catalytic in nature and is required for the HUL-1 E3 to promote transfer of Ub from cdc34. Thus, we propose a model where both interactions are required for the HUL-1 domain to promote cdc34 autoubiquitylation in vivo. In addition to being charged with Ub at cysteine for its E2 function, yeast cdc34 is ubqiuitiylated at multiple lysines in vitro and in vivo (17, 24, 25), Furthermore, a portion of yeast cdc34 not required for E2 activity is necessary for its ubiquitylation in vivo (25). Although this region contains a major ubiquitylation site, cdc34 is also ubiquitylated elsewhere (24). Thus, this region might mediate the tethering of cdc34 to E3 enzymes such that they might promote cdc34 autoubiquitylation. Ubiquitylated cdc34 products formed in reactions containing the HUL-1 E3 are ubiquitylated conjugates at lysine because reducing agents in the disruption buffer would destroy the thioester linkage at cysteine (26). Thus, cdc34 is a bona fide substrate for HUL-1 E3 activity in vitro. We also hypothesized that a larger GST fusion containing the HUL-1 domain and the cdc34 tethering domain in exon 2 would promote more efficient cdc34 ubiquitylation (9).
Although the E3 is often ubiquitylated in substrate-independent in vitro experiments (5), our results are not instructive as to whether ICP0 is ubiquitylated in vivo. An ICP0 isoform (ICP0R) in which only intron 1 is spliced out is stably ubiquitylated in infected cells (27). This isoform contains only a small portion of the sequences encoded in exon 3 and lacks the HUL-1 site. We have been unable to detect ubiquitylated ICP0 in infected cells, and ICP0 is not degraded during infection (9),
The region encoded by ICP0 residues 621–625 that is essential for HUL-1 E3 activity and cdc34 binding also appears to be critical for USP7 binding. Mutation of arginine-623 and lysine-624 together abrogates USP7 binding (28). However, HUL-1 E3 and USP7 binding determinants are not identical as mutation of lysine-620 to isoleucine ablates USP7 binding (28) but has no effect on HUL-1 E3 activity (12). Thus, it is possible that USP7 and cdc34 compete for binding to the same region of ICP0, that USP7 regulates HUL-1 E3 activity by cleaving Ub from HUL-1 substrates as it is in the vicinity of the E3 domain or that ICP0 further enhances the ubiquitylation of substrates by sequestering USP7. Therefore, this region encoded by ICP0 exon 3 plays a seminal role in the interaction of ICP0 with the Ub-proteasomal pathway.
Acknowledgments
We thank Charles Van Sant and Guoying Zhou for invaluable advice and Pascal Lopez for some of the purified GST-exon 3 used in this study. These studies were aided by grants from the National Cancer Institute of the U.S. Public Health Service (CA78766, CA71933, CA83939, CA87661, and CA88860). R.H. is a Howard Hughes Medical Institute Predoctoral Fellow.
Abbreviations
- GST
glutathione S-transferase
- ICP0
infected cell protein 0
- HUL-1 and 2
herpesvirus ubiquitin ligase 1 and 2
- Ub
ubiquitin
- NEM
N-ethtylmaleimide
- ECL
enhanced chemiluminescence
- USP
Ub-specific protease
- BCIP/NBT
5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium
- SCF
Skp1-cdc53-F-box
- HECT
homologous to E6-AP C terminus
- E2
ubiquitin conjuating enzyme
- E3
ubiquitin ligase
References
- 1.Roizman B, Knipe D M. In: Fields Virology. 4th Ed. Knipe D M, Howley P, Griffin D E, Lamb R A, Martin M A, Roizman B, Straus S E, editors. Philiadelphia: Lippincott; 2001. pp. 2399–2459. [Google Scholar]
- 2.Kawaguchi Y, Van Sant C, Roizman B. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Sant C, Kawaguchi Y, Roizman B. Proc Natl Acad Sci USA. 1999;96:8184–8189. doi: 10.1073/pnas.96.14.8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Sant C, Lopez P, Advani S J, Roizman B. J Virol. 2001;75:1888–1898. doi: 10.1128/JVI.75.4.1888-1898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson P K, Eldridge A G, Freed E, Furstenthal L, Hsu J Y, Kaiser B K, Reimann J D R. Trends Cell Biol. 2000;10:429–439. doi: 10.1016/s0962-8924(00)01834-1. [DOI] [PubMed] [Google Scholar]
- 6.Everett R D, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. EMBO J. 1997;16:1519–1530. doi: 10.1093/emboj/16.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meredith M, Orr A, Everett R D. Virology. 1994;200:457–469. doi: 10.1006/viro.1994.1209. [DOI] [PubMed] [Google Scholar]
- 8.Meredith M, Orr A, Elliott M, Everett R D. Virology. 1995;209:174–187. doi: 10.1006/viro.1995.1241. [DOI] [PubMed] [Google Scholar]
- 9.Van Sant C, Hagglund R, Lopez P, Roizman B. Proc Natl Acad Sci USA. 2001;98:8815–8820. doi: 10.1073/pnas.161283098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez P, Van Sant C, Roizman B. J Virol. 2001;75:3832–3840. doi: 10.1128/JVI.75.8.3832-3840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett R D. BioEssays. 2000;22:761–770. doi: 10.1002/1521-1878(200008)22:8<761::AID-BIES10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Hagglund R, Van Sant C, Lopez P, Roizman B. Proc Natl Acad Sci USA. 2002;99:631–636. doi: 10.1073/pnas.022531599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutell C, Sadis S, Everett R D. J Virol. 2002;76:841–850. doi: 10.1128/JVI.76.2.841-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Z-K, Gervais J L M, Zhang H. Proc Natl Acad Sci USA. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganiatsas S, Dow R, Thompson A, Schulman B, Germain D. Oncogene. 2001;20:3641–3650. doi: 10.1038/sj.onc.1204501. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Bruni R, Roizman B. J Virol. 1997;71:1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seol J H, Feldman R M R, Zachariae W, Shevchenko A, Correll C C, Lyapina S, Chi Y, Galova M, Claypool J, Sandmeyer S, et al. Genes Dev. 1999;13:1614–1626. doi: 10.1101/gad.13.12.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagunoff M, Randall G, Roizman B. J Virol. 1996;70:1810–1817. doi: 10.1128/jvi.70.3.1810-1817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciechanover A, Heller H, Kats-Etzion R, Hershko A. Proc Natl Acad Sci USA. 1981;78:761–765. doi: 10.1073/pnas.78.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheffner M, Nuber U, Huibregtse J M. Nature (London) 1995;373:81–83. doi: 10.1038/373081a0. [DOI] [PubMed] [Google Scholar]
- 21.Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama K-I. J Biol Chem. 2001;276:33111–33120. doi: 10.1074/jbc.M102755200. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Ballinger C A, Wu Y, Dai Q, Cyr D M, Höhfeld J, Patterson C. J Biol Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- 23.Coscoy L, Sanchez D J, Ganem D. J Cell Biol. 2001;155:1265–1273. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee A, Gregori L, Xu Y, Chau V. J Biol Chem. 1993;268:5668–5675. [PubMed] [Google Scholar]
- 25.Goebl M G, Goetsch L, Byers B. Mol Cell Biol. 1994;14:3022–3029. doi: 10.1128/mcb.14.5.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huibregtse J M, Scheffner M, Beaudenon S, Howley P M. Proc Natl Acad Sci USA. 1995;92:2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber P C, Spatz S J, Nordby E C. Virology. 1999;253:288–298. doi: 10.1006/viro.1998.9502. [DOI] [PubMed] [Google Scholar]
- 28.Everett R D, Meredith M, Orr A. J Virol. 1999;73:417–426. doi: 10.1128/jvi.73.1.417-426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyers M, Willems A R. Science. 1999;284:603–604. doi: 10.1126/science.284.5414.601. [DOI] [PubMed] [Google Scholar]
- 30.Joaziero C A P, Wing S S, Huang H, Leverson J D, Hunter T, Liu Y-C. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]