Abstract
Objectives: To explore whether vancomycin (VAN) plus piperacillin-tazobactam (PTZ) was associated with an increased risk of acute kidney injury (AKI) compared with VAN plus other beta-lactams (BLs) or monotherapy in critically ill patients, where the evidence remains controversial. Data sources: PubMed, Cochrane, Web of Science, and Embase were searched from inception to June 2024. Study selection: Studies comparing the risk of AKI with one group receiving VAN+PTZ, and other groups receiving VAN plus other BLs, or monotherapy in critically ill. Data synthesis: This analysis included 20 articles with 28 243 participants. The majority of included studies were retrospective (95%, 19/20) and had moderate risks of bias (80.0%, 16/20). The results indicated VAN+PTZ was associated with a significantly higher risk of AKI compared with VAN plus other BLs (OR = 1.66, 95% CI = 1.42-1.94, P < 0.001). Subgroup analyses showed that compared with adults, children were associated with a higher risk of AKI when receiving VAN+PTZ (OR = 3.16 vs 1.59). Also, VAN+PTZ was associated with a significantly higher risk of severe stage 2 to 3 AKI than VAN plus other BLs (OR = 1.63, 95% CI = 1.28-2.06, P < 0.001). No significant difference was identified in mortality, dialysis, time to AKI, and length of stay between patients receiving VAN plus PTZ and other combinations. Conclusions: In critically ill, VAN plus PTZ was associated with an increased risk of AKI and severe stage 2 to 3 AKI compared with VAN plus other BLs, especially in children. However, more high-quality multicenter, prospective cohort studies, and randomized controlled studies are needed.
Keywords: vancomycin, piperacillin-tazobactam, acute kidney injury, beta-lactams, critically ill
Background
Vancomycin (VAN) in combination with beta-lactams (BLs) (piperacillin-tazobactam [PTZ], cefepime [FEP], meropenem [MEM], etc.) is widely used for the initial management of suspected infections where methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa are suspected in critically ill patients. 1 Substantial observational evidence suggests that VAN + PTZ increases the synergistic risk of acute kidney injury (AKI) by up to 2- to 3-fold compared with VAN + other BLs.2,3 However, the data in general medical and mixed population studies may not be extrapolated to the patients in the ICU, and the evidence has been inconclusive in the critically ill.4,5
Recently, the Antibiotic Choice on Renal Outcomes (ACORN) revealed that there was no association between receipt of FEP or PTZ and the primary outcome of AKI or death by day 14, where >75% of the population received concomitant VAN. 6 This result prompted clinicians to further support the idea that VAN+PTZ does not increase the risk of additional AKI. However, the current definition of AKI for VAN is based on creatinine-defined AKI, which is affected by proximal tubular secretion.2,5,7 Tubular secretion accounts for 10% to 40% of creatinine clearance in healthy adults, and this can rise to 60% in chronic kidney disease (CKD) patients. 7 VAN and PTZ both interact with renal transporters, with vancomycin inhibiting organic anion transporters 1 (OAT1) and OAT3, 8 and piperacillin competing for transporter-mediated secretion.9,10 As a result, the rise in serum creatinine observed with VAN+PTZ may reflect reduced tubular secretion rather than true renal injury, leading to pseudo-toxicity. However, a pseudo-AKI caused by a pseudo-nephrotoxic mechanism may be sufficient to meet the KDIGO criteria for stage 1 AKI but it is unlikely to explain the 2-fold SCr increase observed in severe AKI cases and should not be associated with progression to severe AKI.4,5 Recently, Miano TA et al 7 showed that, in critically ill, VAN+PTZ was associated with creatinine-defined AKI, but not changes in alternative kidney biomarkers (plasma cystatin C, blood urea nitrogen), or clinical outcomes, which further emphasizes the plausibility of the phenomenon of pseudo-nephrotoxicity. However, some evidence suggests that VAN+PTZ realistically leads to an increased risk of AKI in critically ill. A clinical analysis by Kane-Gill SL et al 11 showed critically ill patients, exposed to VAN+PTZ, the VAN +PTZ combination compared with VAN plus other BLs was associated with increased risk of AKI stage 2 to 3, death or dialysis at 9 months. Recently, Chen AY et al 4 published the largest retrospective multicenter cohort study to date in critically ill patients and demonstrated that VAN+PTZ was associated with a higher risk of AKI stage 2 to 3, dialysis, and in-hospital mortality compared with those receiving VAN+FEP or VAN+MEM. Mild kidney injury (eg, AKI stage 1) may be a pseudo-toxicity due to PTZ-induced increases in serum creatinine (SCr) secretion, but the increased risk of severe stage 2 to 3 AKI, dialysis, and mortality may reflect true nephrotoxicity.2,5
Whether VAN+PTZ is associated with a higher risk of AKI in critically ill remains controversial. Given the latest optimal evidence, this meta-analysis was conducted to explore whether VAN+PTZ actually increases AKI compared with VAN plus other BLs (mainly FEP, MEM) or VAN alone in critically ill patients. To get closer to the truth, the primary outcome indicators of AKI and severe stage 2 to 3 AKI were designated for this study.
Methods
The meta-analysis protocol was registered on PROSPERO (CRD42024511084), and designed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The PRISMA Extension Statement was also used as guidance for this analysis (Supplemental Table S1). 12
Study Selection
Databases including PubMed, Cochrane, Web of Science, and Embase were searched to identify the candidate articles published with a search from database inception to June 13, 2024. The search algorithm was: [vancomycin AND (piperacillin OR tazobactam)] AND [(kidney OR renal) OR (nephrotoxicity OR nephritis] AND [critical illness OR critically ill OR intensive care units OR ICU OR septic OR sepsis]. Detailed algorithms for each database are listed in Supplemental Table S2. No language restriction was applied.
All randomized controlled trials and cohort studies (prospective or retrospective) were included. Conference abstracts, case report, case series, and reviews were excluded. Inclusion criteria were: (1) Studies with critically ill patients or septic patients; (2) Studies with one group receiving VAN+PTZ, and other groups receiving VAN plus other BLs, VAN monotherapy, or PTZ monotherapy; (3) Studies reported the incidence of AKI. Exclusion criteria were: (1) AKI occurring within 48 hours of hospital admission; (2) Patients with chronic kidney diseases stage 5 with and without hemodialysis and patients with a prior renal transplant status; (3) Duration of the studied antibiotics less than 48 hours.
Data Extraction
The following information was extracted from the articles: (1) Basic information of the studies: authors, publication year, study design, country, inclusion and exclusion criteria, definition of AKI, sample size; (2) Antibiotics used in the studies: comparison groups, duration of treatment and follow-up; (3) Baseline characteristics of included patients: sex, age, body mass index (BMI), weight, SCr and estimated glomerular filtration rate (eGFR); (4) Outcomes: measures of outcomes (eg, incidence of AKI and severe stage 2 to 3 AKI, dialysis, mortality, length of stay, and time to AKI).
Quality Assessment
The Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was used to assess the quality of the studies. 13 Seven domains of bias were evaluated, and each domain was assigned a rating of low risk, moderate risk, serious risk, critical risk, or no information, followed by an overall assessment. The certainty of evidence for all the outcomes were accessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. 14 Two authors (Ranyi Li and Yanli Li) independently conducted the literature selection, data extraction, and quality assessment. Any disagreements were resolved by consensus among the following authors: Ranyi Li, Yanli Li, Chenqi Xu.
Outcomes
The primary outcomes were AKI and severe stage 2 to 3 AKI. Acute kidney injury was identified according to the 3 commonly used criteria: Kidney Disease: Improving Global Outcomes (KDIGO), 2 Acute Kidney Injury Network (AKIN), 15 or Risk, Injury, Failure, Loss, End-Stage (RIFLE). 16 Acute kidney injury was also defined as an acute increase of 50% in SCr in 7 days in the absence of using the previous 3 AKI criteria. Severe AKI was defined as stage 2 to 3 in KDIGO/AKIN and injury or failure stage in RIFLE.15 -17 Other outcomes included access to dialysis treatment, hospital mortality, hospital length of stay, and time to AKI. Patients on hemodialysis prior to ICU admission were excluded for this research, therefore access to dialysis treatment was defined as the new starts of hemodialysis or CRRT. We defined other BLs as BLs other than PTZ. Beta-lactams other than PTZ, FEP, and MEM were defined as uncommonly used beta-lactam antibiotics (UC-BLs), including aztreonam, ceftazidime, ceftriaxone, imipenem, and imipenem-cilastin.
Statistical Analysis
The odds ratio (OR) was calculated for binary outcomes using the number of events and sample size in each arm. Standardized mean difference (SMD) was calculated for continuous outcomes using the mean, standard deviation, and sample size in each arm. If the studies reported the continuous outcomes using median and range, the conversion was performed to ensure consistency.18,19 The data were pooled and estimated using the random-effects model. Mantel-Haenszel method was used to calculate the ORs. Random-effects model and inverse variance method was used to calculate the SMDs. A 95% confidence interval (CI) was applied to all estimates, and 2-tailed significance tests were used, with P < 0.05 considered statistically significant.
Heterogeneity was assessed using the Higgins’ inconsistency test (I2), defining substantial heterogeneity as I2 greater than 50%. Meta-regression analyses were performed to explore the potential sources of heterogeneity. Subgroup analyses were conducted to explore potential sources of heterogeneity, including different age groups (adults or children), gender, country of study, and year of publication, definition of AKI used (eg, KDIGO, RIFLE, or other criteria), the overall risk of bias, and the type of antibiotics administered (eg, VAN+FEP, VAN+MEM, or VAN+UC-BLs). Sensitivity analyses were conducted by excluding each study to assess the impact of individual studies on the overall pooled estimates. Publication bias was assessed using a funnel plot. Statistical analysis were conducted in Review Manager (RevMan, Version 5.3. Copenhagen) software and R-4.2.1 with the meta package.
Results
This analysis included 20 articles with 28 243 participants. A total of 2230 citations were retrieved through the search. After removing 306 duplicate citations and excluding 1924 citations based on title and abstract, 77 articles qualified for review (Figure 1).
Figure 1.
Flow diagram.
Study characteristics are depicted in Table 1 (details in Supplemental Table S3). The included studies were mainly retrospective (95%, 19/20), with only one prospective study and 0 randomized controlled trials (RCTs). The country distribution of study institutions is mainly from the United States with 70.0% (14/20) and one study from each of the following countries: Saudi Arabia, China, Japan, Korea, Australia, and Oman. Of the included studies, 11 studies were in adult patients, 4 were in children, 1 was divided into adults and children, and the remaining 4 studies did not describe age range.
Table 1.
Characteristics of Included Studies.
| Author | Year | Country | Design | Population | Comparison | Sample size | AKI | Severe AKI |
|---|---|---|---|---|---|---|---|---|
| Pipkin T | 2024 | USA | S, R | / | VAN + PTZ vs VAN + FEP/CAZ/MEM | 44/65 | 50% vs 58.5% | 15.9% vs 6.2% |
| Almutairi MS | 2023 | Saudi Arabia | S, R | Adults | VAN + PTZ vs Other BLs a | 50/29 | 52% vs 52% | / |
| Chen AY | 2023 | USA | M, R | / | VAN + PTZ vs VAN + FEP | 6371/6371 | 26.9% vs 21.8% | 19.7% vs 15.2% |
| / | VAN + PTZ vs VAN + MEM | 1824/1824 | 26.6% vs 21.1% | 18.3% vs 15.0% | ||||
| Buckley MS | 2022 | USA | M, R | Adults | VAN + PTZ vs VAN + FEP/MEM | 758/286 | 21.9% vs 16.8% | 7.3% vs 8.0% |
| Elliott BP | 2022 | USA | S, R | Adults | VAN + PTZ vs VAN + FEP | 127/36 | 14.2% vs 16.7% | / |
| Miano TA | 2022 | USA | S, P | / | VAN + PTZ vs VAN + FEP | 297/442 | 42.1% vs 30.6% | 20.2% vs 15.2% |
| Bartlett JW | 2020 | USA | S, R | Infants | VAN + PTZ vs VAN + FEP | 58/42 | 3.4% vs 4.8% | / |
| Inage S | 2020 | Japan | S, R | Adults | VAN + PTZ vs VAN | 44/143 | 19.8% vs 8.0% | / |
| Molina KC | 2020 | USA | S, R | Adults | VAN + PTZ vs VAN + FEP | 258/136 | 28.7% vs 21.3% | 12.4% vs 8.1% |
| O’Callaghan K | 2020 | Australia | S, R | / | VAN + PTZ vs VAN + FEP/MEM | 153/75 | 25.5% vs 16.0% | / |
| Sheikh S | 2020 | Oman | S, R | Adults | VAN + PTZ vs VAN | 53/60 | 26.4% vs 53.3% | 13.2% vs 41.7% |
| Blevins AM | 2019 | USA | S, R | Adults | VAN + PTZ vs VAN + FEP vs VAN + MEM | 366/1734/392 | 39.3% vs 24.2% vs 23.5% | 21.6% vs 7.6% vs 7.9% |
| Joyce EL | 2019 | USA | S, R | Children | VAN + PTZ vs VAN + FEP | 785/265 | 16.7% vs 10.6% | / |
| Kang S | 2019 | Korea | S, R | Adults | VAN + PTZ vs VAN + MEM/VAN | 74/83/183 | 52.7% vs 25.7% vs 27.7% | 29.7% vs 11.5% vs 12.0% |
| Schreier DJ | 2019 | USA | S, R | Adults | VAN + PTZ vs VAN + FEP vs VAN + MEM | 1540/1373/386 | 39.0% vs 29.0% vs 35.0% | 9.0% vs 8.0% vs 10.1% |
| Buckley MS | 2018 | USA | S, R | Adults | VAN + PTZ vs VAN + FEP | 200/133 | 19.5% vs 17.3% | 15.5% vs 13.5% |
| Holsen MR | 2017 | USA | S, R | Children | VAN + PTZ vs VAN + CRO | 58/35 | 25.9% vs 8.6% | 12.1% vs 0% |
| Hundeshagen G | 2017 | USA | S, R | Adults | VAN + PTZ vs VAN + IPC vs VAN | 81/53/112 | 27.2% vs 0% vs 3.6% | 12.3% vs 0% vs 1.8% |
| Hammond DA | 2016 | USA | S, R | Children | VAN + PTZ vs VAN + FEP | 178/268/26 | 9.0% vs 1.9% vs 15.4% | 3.4% vs 0.4% vs 0% |
| Adults | 49/73 | 32.7% vs 28.8% | / | |||||
| Gao X | 2014 | China | S, R | Children | VAN + PTZ vs VAN + FEP | 125/125 | 48.8% vs 12.0% | / |
Abbreviations: AKI, acute kidney injury; VAN, vancomycin; PTZ, piperacillin-tazobactam; FEP, cefepime; MEM, meropenem; USA, the United States of America; LOS, Length of stay; ATM, aztreonam, CAZ, ceftazidime, CRO, Ceftriaxone, IPC, imipenem-cilastin, IPM, imipenem, Study Design: S, single-center, M, multicenter R, retrospective cohort study P= prospective. Definition of AKI: Acute Kidney Injury Network; RIFLE, Risk, Injury, Failure, Loss, End-Stage; Kidney Disease: Improving Global Outcomes; Serum creatinine increase: acute increase in serum creatinine of 0.3 mg/dL or 50%;
Other BLs including ATM, CAZ, MEM, CRO, IPM, or FEP.
Patient characteristics included were 56.2% male (15 869/28 243), with a mean age of 63.9 years for adults, 6.1 years for children, and 35.9 weeks for infants. The average BMI ranged from 22.6 to 29.0. The included literature was first published in 2014. The mean baseline SCr ranged from 0.35 to 1.88 mg/dL. Four studies were 3-armed and the rest were 2-armed.
Quality Assessment
Three studies (15.0%, 3/20) were classified as having a low risk of bias, and 16 (80.0%) had a moderate risk of bias using the ROBINS-I tool (Supplemental Table S4). The remaining one study was at high risk of bias due to a lack of baseline information and selective reporting of outcomes. Other concerns regarding bias primarily originated from the domain of confounding due to unbalanced patient characteristics and the omission of important confounding factors, such as comorbidities and concomitant nephrotoxic drugs (Supplemental Table S4). The certainty of the evidence for AKI was accessed as moderate mainly due to the serious risk of bias. The summary of findings with GRADE for all the outcomes is presented in Supplemental Table S5.
Incidence of AKI
All included 20 studies reported the incidence of AKI (N = 28 243) (Figure 2).4,7,20 -37 The results showed that the estimated overall incidence of AKI in critically ill was VAN+PTZ [28% (3778/13 493)], VAN+other BLs [22.5% (3314/14 750)], VAN+FEP [22.9% (2461/10 730)], VAN+MEM [23.6% (634/2685)], VAN+UC-BLs [14.4% (117/811)] and VAN [19.4% (102/524)] respectively. The analysis indicated that VAN+PTZ was associated with significantly higher AKI risk compared with VAN plus other BLs (OR = 1.66, 95% CI = 1.42-1.94, P < 0.001). Subgroup analyses showed that VAN+PTZ was associated with a significantly higher risk of AKI compared with VAN+FEP (OR = 1.66, 95% CI = 1.35-2.03, P < 0.001), VAN+MEM (OR = 1.61, 95% CI = 1.20-2.16, P = 0.004), and VAN+UC-BLs (OR = 1.99, 95% CI = 1.15-3.47, P = 0.008) (Supplemental Figure S1). No significant difference in AKI was observed with VAN+PTZ compared with VAN monotherapy (OR = 1.69, 95% CI = 0.53-5.39, P = 0.38) (Supplemental Figure S1).
Figure 2.
Forest plot demonstrating the odds of AKI with VAN+PTZ versus VAN+other BLs or alone. Data are expressed as odds ratio and 95% confidence intervals. In studies (Blevins AM, Hundeshagen G, Kang S and Schreier DJ) including VAN+PTZ, VAN+other BLs, and VAN monotherapy, VAN+PTZ may appear more than once in pairwise comparisons; thus, to avoid duplication in the overall estimate, only one comparison per study was included in the total, combining VAN+other BLs and monotherapy as a single reference group. Chen AY, 2023a represents the comparison groups of VAN+PTZ versus VAN+FEP; Chen AY, 2023b represents the comparison groups of VAN+PTZ versus VAN+MEM; Hundeshagen G, 2017a represents the population of adult; Hundeshagen G, 2017b represents the population of children. BLs, beta-lactams: other BLs was defined as BLs other than PTZ.
Abbreviations: AKI, acute kidney injury; VAN, vancomycin; PTZ, piperacilline-tazobactam.
Note. Chen AY, 2023a and Chen AY, 2023b represent two subgroup comparisons within the same study: VAN+PTZ vs. VAN+FEP (2023a) and VAN+PTZ vs. VAN+MEM (2023b). Hundeshagen G, 2017a and Hundeshagen G, 2017b represent two subgroup comparisons within the same study: population of adult (2017a) and population of children (2017b)
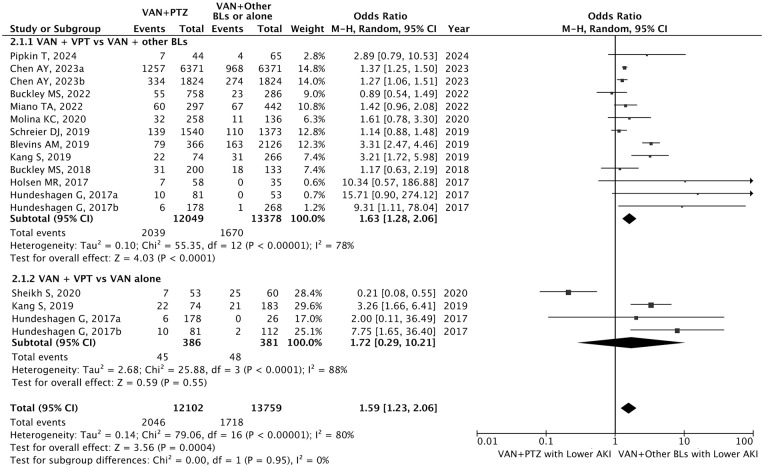
Severe Stage 2 to 3 AKI
Twelve studies (60.0%, 12/20) reported the incidence of AKI at different severity stages (N = 26 064).4,7,23,26,28,29,32,34 -36 Estimated overall incidence of AKI in critically ill was VAN+PTZ [16.9% (2046/12 102)], VAN+other BLs or VAN alone [12.4% (1736/13 962)], VAN+FEP [12.8% (1306/10 189)], VAN+MEM [13.1% (353/2685)], VAN+UC-BLs [4.6% (28/607)] and VAN [12.6% (48/381)] respectively. VAN+PTZ was associated with a significantly higher risk of severe stage 2 to 3 AKI incidence than VAN plus other BLs (OR = 1.63, 95% CI = 1.28-2.06, P < 0.001) (Figure 3), as well as VAN+FEP (OR = 1.57, 95% CI = 1.13-2.16, P < 0.001) (Supplemental Figure S2). No significant difference in stage 2 to 3 AKI incidence was found in the comparison of VAN+PTZ and VAN+MEM (OR = 1.75, 95% CI = 1.00-3.05, P = 0.05), VAN+UC-BLs (OR = 3.51, 95% CI = 0.73-16.94, P = 0.12), and VAN monotherapy (OR = 1.72, 95% CI = 0.29-10.21, P = 0.55) (Supplemental Figure S2).
Figure 3.
Forest plot demonstrating the odds of severe stage 2 to 3 AKI with VAN+PTZ versus VAN plus other BLs or monotherapy. Data are expressed as odds ratio and 95% confidence intervals. In studies (Blevins AM, Hundeshagen G, Kang S and Schreier DJ) including VAN+PTZ, VAN+other BLs, and VAN monotherapy, VAN+PTZ may appear more than once in pairwise comparisons; thus, to avoid duplication in the overall estimate, only one comparison per study was included in the total, combining VAN+other BLs and monotherapy as a single reference group. Chen AY, 2023a represents the comparison groups of VAN+PTZ versus VAN+FEP; Chen AY, 2023b represents the comparison groups of VAN+PTZ versus VAN+MEM; Hundeshagen G, 2017a represents the population of adult; Hundeshagen G, 2017b represents the population of children. BLs = beta-lactams: other BLs was defined as BLs other than PTZ.
Abbreviations: AKI, acute kidney injury; VAN, vancomycin; PTZ, piperacilline-tazobactam.
Note. Chen AY, 2023a and Chen AY, 2023b represent two subgroup comparisons within the same study: VAN+PTZ vs. VAN+FEP (2023a) and VAN+PTZ vs. VAN+MEM (2023b). Hundeshagen G, 2017a and Hundeshagen G, 2017b represent two subgroup comparisons within the same study: population of adult (2017a) and population of children (2017b)
Other Outcomes
In-hospital mortality (10 studies, N = 22 605) (Supplemental Figure S3),4,7,22,23,26,28,29,31,32,34,36 dialysis (10 studies, N = 23 337) (Supplemental Figure S4),4,7,21,23,24,26,29,31,32,34 time to AKI (7 studies, N = 4825)23,24,27,32 -34,36 (Supplemental Figure S5) and length of stay (11 studies, N = 21 893)4,21,23,26,28,29,31,33,34,36 (Supplemental Figure S6) were analyzed. No significant difference was identified in mortality (OR = 1.03, 95% CI = 0.84-1.26, P = 0.81), dialysis (OR = 1.18, 95% CI = 0.90-1.54, P = 0.24), time to AKI (SMD = −0.01, 95% CI = −0.22 to 0.20, P = 0.95) and length of stay (SMD = 0.04, 95% CI = −0.03 to 0.10, P = 0.24) between patients receiving VAN+PTZ and other combinations or monotherapy (Supplemental Figures S3-S6). Subgroup analysis showed that VAN plus PTZ, compared with VAN plus MEM, significantly increased the risk of receiving dialysis (3 studies, 4563 patients, OR = 1.51, 95% CI = 1.19-1.91).
Meta-Analysis and Publication Bias
Meta-regression has been calculated for age, gender, country, year of publication, the definition of AKI, risk of bias, and type of antibiotics. The results showed that age (−0.016, 95% CI = −0.030 to 0.002, P = 0.029) and publish year (−0.151, 95% CI = −0.259 to −0.042, P = 0.009) may be the significant effect size predictor for AKI and the remaining factors showed no significant effect size predictor (Table S6). Thus, we performed subgroup analyses based on age, and the results showed that VAN+PTZ was significantly associated with an increased risk of AKI which varied by age groups. Compared with adults, children were associated with a higher risk of AKI (Children vs Adult, OR = 3.16 vs 1.59) (Figure 4). Sensitivity analyses, performed by sequentially excluding each study, did not change the effect on the various studied endpoints (Supplemental Figure S8). The funnel plots displayed visible gross asymmetry, indicating potential publication bias (Supplemental Figure S7).
Figure 4.
Forest plot demonstrating the odds of AKI with VAN+PTZ versus VAN plus other BLs (Subgroup analysis of studies based on population: adults, children). Data are expressed as odds ratio and 95% confidence intervals. Hundeshagen G, 2017a represents the population of adult; Hundeshagen G, 2017b represents the population of children. BLs = beta-lactams: other BLs was defined as BLs other than PTZ.
Abbreviations: AKI, acute kidney injury; VAN, vancomycin; PTZ, piperacilline-tazobactam.
Note. Hundeshagen G, 2017a and Hundeshagen G, 2017b represent two subgroup comparisons within the same study: population of adult (2017a) and population of children (2017b)
Discussion
This study demonstrates that in critically ill patients, VAN plus PTZ significantly increases the risk of developing AKI and severe stage 2 to 3 AKI compared with VAN plus other BLs. This is the largest evidence-based study to date in critically ill patients that focused on whether VAN+PTZ was associated with additional AKI risk and is the first to use meta-analyze severe AKI as an outcome metric. In the only RCT to date: the ACORN study argued that VAN+PTZ did not increase the risk of additional AKI, but it was only 75% of VAN coadministration in that study. Based on the current evidence, the higher risk associated with VAN+PTZ still needs to be considered.
When considering the risk of AKI, the VAN plus PTZ combination has received the most attention recently. 5 Several meta-analyses in the last several years have shown that VAN+PTZ was associated with a 2- to 3-fold increase in the incidence of AKI compared with VAN plus other BLs.3,38 -41 However, published meta-analyses mainly focus on the general medical population, and the evidence has been inconclusive for critically ill.20 -22 We conducted this meta-analysis trying to answer whether VAN+PTZ was associated with an increased AKI in the critically ill. To date, only one meta-analysis compared the risk of kidney injury with VAN plus PTZ combination or an alternative in critically ill patients. 42 However, approximately 40% of the studies included in the meta-analysis were ICU patients, and the remaining 60% were patients in ICU mixed general wards and therefore this only study is not representative of critically ill patients. The current study provides evidence for clinicians and pharmacists making decisions regarding anti-infective treatment regimens, and the risk of combining VAN with PTZ or with other BLs, especially for patients at high risk of AKI.
VAN+PTZ might cause pseudo-nephrotoxicity due to the effect of VAN and PTZ on serum creatinine secretion.2,43 VAN suppresses the expressions of OAT1 and OAT3. 8 Piperacillin is a strong affinity substrate for OAT, which are associated with tubular processing of creatinine (OAT1 and OAT3),9,10 while MEM was a low-affinity substrate of OAT1/3. 44 Thus, the combined influence of VAN-mediated transporter inhibition and PTZ-mediated inhibition of creatinine secretion seems a plausible mechanism of increased creatinine concentration.2,43 To help discriminate between true nephrotoxicity and pseudo-nephrotoxicity, this study also set severe stage 2 to 3 AKI as the primary outcomes. To the best of our knowledge, this is the first study that evaluated the increased severe AKI risk of VAN plus PTZ in critically ill patients. This study demonstrates that VAN plus PTZ significantly increases the risk of developing severe stage 2 to 3 AKI compared with VAN plus other BLs, which is consistent with the study by Chen AY et al. 4 The study of Chen AY et al may dominate the results. Baseline SCr was estimated using the lowest SCr value recorded within the window of ICU admission in this study, which is confounded by dilution, augmented renal clearance, decreased creatinine clearance, and deconditioning, therefore altering the incidence of AKI and creating ascertainment and information bias. We further excluded the study by Chen et al and the results showed that VAN+PTZ was still associated with a higher risk of severe stage 2 to 3 AKI (Supplemental Figure S10).
Regarding secondary outcomes including mortality, dialysis, and length of stay, VAN+PTZ was associated with an increased risk of severe AKI, but this finding was not statistically different. Subgroup analysis showed that VAN+PTZ was associated with a significantly increased severe risk of AKI compared with VAN+MEM. We agree that based on the available evidence combined, clinicians and pharmacists need to be aware of the additional risk of AKI associated with VAN+PTZ compared with VAN+BLs.
The studies included in this meta-analysis were predominantly retrospective and with moderate risk of bias, thus we further analyzed the sources of study heterogeneity. Four definitions of AKI were included in the study, yet did not add significant heterogeneity, as well as type of antibiotics. Age was the only source of heterogeneity in the primary outcome of AKI. Further subgroup analyses revealed higher AKI risk values for VAN+PTZ in children compared with adults, which is consistent with the findings of a recent meta-analysis of VAN+PTZ associated AKI in children. 45 Children are more susceptible to AKI than adults due to physiological differences, including glomerular filtration rate, and clearance and expression of cytochrome P450 enzymes in the liver and kidney. 45 Interestingly, we find that publication time was another source of heterogeneity of AKI. In the time when the viewpoint that VAN+PTZ is associated with increased nephrotoxicity was proposed, studies showed higher values of increased risk, whereas more recent years have shown a lower additional risk. We speculate that improved research methodologies over time may allow for clearer differentiation between true effects and confounding factors, which could explain why publication time is a source of heterogeneity. In addition, the shift from trough-based to area under the curve (AUC)-based vancomycin dosing strategies may be associated with a decreased risk of AKI, 46 which could partly explain the lower risk observed in more recent studies. In this study, despite differences among the 4 AKI criteria (KDIGO, AKIN, RIFLE, and SCr increase), the meta-regression revealed that varying AKI definitions did not contribute to heterogeneity.
This review has several strengths. First, after a systematic and comprehensive search of databases, this meta-analysis was the largest study to date comparing the risk of AKI with other antibiotic regimens (VAN+FEP, VAN+MEM, VAN+UC-BLs, PTZ alone, VAN alone) in critically ill. Second, for the first time, severe stage 2 to 3 AKI was analyzed as the primary outcome to help discriminate between true nephrotoxicity and pseudo-nephrotoxicity. Third, the source of heterogeneity of VAN + PTZ induced AKI was analyzed in critically ill patients stratified by age and subgroup analysis was performed.
This review has several limitations. First, the studies included in this meta-analysis were mainly retrospective, which could not avoid the interference of confounding factors on the outcome. Second, most of the studies had moderate to severe bias, and heterogeneity was observed in some comparisons. Third, this study did not include urine volume as a criterion for AKI due to the primarily retrospective nature of the included studies, which made accurate data difficult to obtain. Fourth, not all studies reported data on all outcomes, including severe AKI, mortality, and dialysis, which could lead to reporting bias. We believe that more high-quality evidence is needed, including high-quality multicenter, prospective cohort studies and even randomized controlled studies.
Conclusions
In critically ill patients, VAN plus PTZ was associated with increased risk of AKI and severe stage 2 to 3 AKI compared with VAN plus other BLs, especially in children. We agree that, based on the available combined evidence, clinicians and pharmacists need to realize the additional AKI risk associated with VAN+PTZ and consider replacement options, especially for patients at high risk for AKI. However, more high-quality multicenter, prospective cohort studies and randomized controlled studies are needed.
Supplemental Material
Supplemental material, sj-docx-1-pmt-10.1177_87551225251350894 for Incidence of Acute Kidney Injury in Critically Ill Patients Receiving Vancomycin With Concomitant Piperacillin-Tazobactam Versus Other Beta-Lactams: A Systematic Review and Meta-Analysis by Ranyi Li, Yanli Li, Chenqi Xu, Ziyan Shen, Xialian Xu, Xiaoqiang Ding, Xiaoyu Li, Qianzhou Lv and Kunming Pan in Journal of Pharmacy Technology
Footnotes
Author Contributions: K.P., X.L., and Q.L. conceived the study. K.P., X.L., and Q.L. designed the analysis. R.L., Y.L., and C.X. screened the records and extracted the data. R.L., Y.L., and C.X. acquired the data and judged the risk of bias in the studies. K.P., R.L., and Y.L. performed the analysis. K.P., R.L., Z.S., X.X., and X.D. interpreted the data analyses. K.P. and R.L. drafted the manuscript. K.P., X.L., and Q.L. provided critical revisions to the manuscript. R.L., Y.L., and C.X. contributed equally as co-first authors. All the authors approved of the final manuscript and had final responsibility for the decision to submit for publication.
Availability of Data and Material: All data generated or analyzed during this study are included in this published article and its supplementary information files.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No. 82204520), China International Medical Foundation (No. Z-2021-46-2101), the Chinese Pharmaceutical Association (No. CPA-Z05-ZC-2023-003), the Shanghai Municipal Health Commission (No. 202240293), Shanghai Municipal Health Commission (No. 20244Y0101), Shanghai “Rising Stars of Medical Talent” Youth Development Program Youth Medical Talents – Clinical Pharmacist Program (SHWSRS(2023)_106), and Shanghai Hospital Development Center Foundation (SHDC12024632).
ORCID iDs: Qianzhou Lv  https://orcid.org/0000-0002-0866-1546
https://orcid.org/0000-0002-0866-1546
Kunming Pan  https://orcid.org/0000-0002-3907-2939
https://orcid.org/0000-0002-3907-2939
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Goodman KE, Cosgrove SE, Pineles L, et al. Significant regional differences in antibiotic use across 576 US hospitals and 11 701 326 adult admissions, 2016-2017. Clin Infect Dis. 2021;73(2):213-222. doi: 10.1093/cid/ciaa570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Côté JM, Desjardins M, Murray PT. Does vancomycin-piperacillin-tazobactam cause pseudo-AKI, true nephrotoxicity, or both? Chest. 2023;164(2):273-274. doi: 10.1016/j.chest.2023.05.009 [DOI] [PubMed] [Google Scholar]
- 3. Bellos I, Karageorgiou V, Pergialiotis V, Perrea DN. Acute kidney injury following the concurrent administration of antipseudomonal β-lactams and vancomycin: a network meta-analysis. Clin Microbiol Infect. 2020;26(6):696-705. doi: 10.1016/j.cmi.2020.03.019 [DOI] [PubMed] [Google Scholar]
- 4. Chen AY, Deng CY, Calvachi-Prieto P, et al. A large-scale multicenter retrospective study on nephrotoxicity associated with empiric broad-spectrum antibiotics in critically ill patients. Chest. 2023;164(2):355-368. doi: 10.1016/j.chest.2023.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Côté JM, Kane-Gill SL, Murray PT. A ray of hope in the discord: is adding piperacillin-tazobactam to vancomycin truly more nephrotoxic? Intensive Care Med. 2022;48(9):1208-1210. doi: 10.1007/s00134-022-06861-4 [DOI] [PubMed] [Google Scholar]
- 6. Qian ET, Casey JD, Wright A, et al. Cefepime vs piperacillin-tazobactam in adults hospitalized with acute infection: the ACORN randomized clinical trial. JAMA. 2023;330(16):1557-1567. doi: 10.1001/jama.2023.20583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miano TA, Hennessy S, Yang W, et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48(9):1144-1155. doi: 10.1007/s00134-022-06811-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wen S, Wang C, Huo X, et al. JBP485 attenuates vancomycin-induced nephrotoxicity by regulating the expressions of organic anion transporter (Oat) 1, Oat3, organic cation transporter 2 (Oct2), multidrug resistance-associated protein 2 (Mrp2) and P-glycoprotein (P-gp) in rats. Toxicol Lett. 2018;295:195-204. doi: 10.1016/j.toxlet.2018.06.1220 [DOI] [PubMed] [Google Scholar]
- 9. Wen S, Wang C, Duan Y, et al. OAT1 and OAT3 also mediate the drug-drug interaction between piperacillin and tazobactam. Int J Pharm. 2018;537(1-2):172-182. doi: 10.1016/j.ijpharm.2017.12.037 [DOI] [PubMed] [Google Scholar]
- 10. Vallon V, Eraly SA, Rao SR, et al. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am J Physiol Renal Physiol. 2012;302(10):F1293-F1299. doi: 10.1152/ajprenal.00013.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kane-Gill SL, Ostermann M, Shi J, Joyce EL, Kellum JA. Evaluating renal stress using pharmacokinetic urinary biomarker data in critically ill patients receiving vancomycin and/or piperacillin-tazobactam: a secondary analysis of the multicenter sapphire study. Drug Saf. 2019;42(10):1149-1155. doi: 10.1007/s40264-019-00846-x [DOI] [PubMed] [Google Scholar]
- 12. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 13. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed September 12, 2024. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 14. Schünemann HJ, Higgins JPT, Vist GE, et al. Chapter 14: completing “summary of findings” tables and grading the certainty of the evidence. Accessed September 12, 2024. https://training.cochrane.org/handbook/current/chapter-14
- 15. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-R212. doi: 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-c184. doi: 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 18. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785-1805. doi: 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 19. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elliott BP, Tang MM, Madden JA, et al. A retrospective cohort study assessing acute kidney injury and renal recovery among septic patients empirically treated with vancomycin piperacillin-tazobactam versus vancomycin cefepime. Intern Emerg Med. 2022;17(1):91-99. doi: 10.1007/s11739-021-02772-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hammond DA, Smith MN, Painter JT, Meena NK, Lusardi K. Comparative incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin-tazobactam or cefepime: a retrospective cohort study. Pharmacotherapy. 2016;36(5):463-471. doi: 10.1002/phar.1738 [DOI] [PubMed] [Google Scholar]
- 22. O’Callaghan K, Hay K, Lavana J, McNamara JF. Acute kidney injury with combination vancomycin and piperacillin-tazobactam therapy in the ICU: a retrospective cohort study. Int J Antimicrob Agents. 2020;56(1):106010. doi: 10.1016/j.ijantimicag.2020.106010 [DOI] [PubMed] [Google Scholar]
- 23. Buckley MS, Hartsock NC, Berry AJ, et al. Comparison of acute kidney injury risk associated with vancomycin and concomitant piperacillin/tazobactam or cefepime in the intensive care unit. J Crit Care. 2018;48:32-38. doi: 10.1016/j.jcrc.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 24. Buckley MS, Komerdelj IA, D’Alessio PA, et al. Vancomycin with concomitant piperacillin/tazobactam vs. cefepime or meropenem associated acute kidney injury in the critically ill: a multicenter propensity score-matched study. J Crit Care. 2022;67:134-140. doi: 10.1016/j.jcrc.2021.10.018 [DOI] [PubMed] [Google Scholar]
- 25. Almutairi MS, Alnezary FS, Chestnutt J, McAllister M, Almohammed OA, Alhifany AA. Acute kidney injury associated with piperacillin-tazobactam versus other antibiotics combined with vancomycin in critically ill patients: a retrospective cohort study. Saudi Pharm J. 2023;31(12):101844. doi: 10.1016/j.jsps.2023.101844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blevins AM, Lashinsky JN, McCammon C, Kollef M, Micek S, Juang P. Incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin-tazobactam, cefepime, or meropenem. Antimicrob Agents Chemother. 2019;63(5):e02658-18. doi: 10.1128/AAC.02658-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bartlett JW, Gillon J, Hale J, Jimenez-Truque N, Banerjee R. Incidence of acute kidney injury among infants in the neonatal intensive care unit receiving vancomycin with either piperacillin/tazobactam or cefepime. J Pediatr Pharmacol Ther. 2020;25(6):521-527. doi: 10.5863/1551-6776-25.6.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holsen MR, Meaney CJ, Hassinger AB, Fusco NM. Increased risk of acute kidney injury in critically ill children treated with vancomycin and piperacillin/tazobactam. Pediatr Crit Care Med. 2017;18(12):e585-e591. doi: 10.1097/PCC.0000000000001335 [DOI] [PubMed] [Google Scholar]
- 29. Hundeshagen G, Herndon DN, Capek KD, et al. Co-administration of vancomycin and piperacillin-tazobactam is associated with increased renal dysfunction in adult and pediatric burn patients. Crit Care. 2017;21(1):318. doi: 10.1186/s13054-017-1899-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao X, Li J, Li ZP. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized children with or without concomitant piperacillin-tazobactam. Fudan Univ J Med Sci. 2015;42(6):743. doi: 10.3969/j.issn.1672-8467.2015.06.009 [DOI] [Google Scholar]
- 31. Joyce EL, Kane-Gill SL, Priyanka P, Fuhrman DY, Kellum JA. Piperacillin/tazobactam and antibiotic-associated acute kidney injury in critically ill children. J Am Soc Nephrol. 2019;30(11):2243-2251. doi: 10.1681/ASN.2018121223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang S, Park J, Yu YM, Park MS, Han E, Chang MJ. Comparison of acute kidney injury and clinical prognosis of vancomycin monotherapy and combination therapy with beta-lactams in the intensive care unit. PLoS ONE. 2019;14(6):e0217908. doi: 10.1371/journal.pone.0217908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Molina KC, Barletta JF, Hall ST, Yazdani C, Huang V. The risk of acute kidney injury in critically ill patients receiving concomitant vancomycin with piperacillin-tazobactam or cefepime. J Intensive Care Med. 2020;35(12):1434-1438. doi: 10.1177/0885066619828290 [DOI] [PubMed] [Google Scholar]
- 34. Pipkin T, Pope S, Killian A, Green S, Albrecht B, Nugent K. Nephrotoxic risk associated with combination therapy of vancomycin and piperacillin-tazobactam in critically ill patients with chronic kidney disease. J Intensive Care Med. 2024;39(9):860-865. doi: 10.1177/08850666241234577 [DOI] [PubMed] [Google Scholar]
- 35. Schreier DJ, Kashani KB, Sakhuja A, et al. Incidence of acute kidney injury among critically ill patients with brief empiric use of antipseudomonal β-lactams with vancomycin. Clin Infect Dis. 2019;68(9):1456-1462. doi: 10.1093/cid/ciy724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sheikh S, Baig MA, Sharafat MA, et al. Concerning nephrotoxicity of top guns: concomitant piperacillin-tazobactam and vancomycin with vancomycin alone during treatment of critically ill patients. Eurasian J Emerg Med. 2020;19(1):1-5. doi: 10.4274/eajem.galenos.2019.04934 [DOI] [Google Scholar]
- 37. Inage S, Nakamura S, Isoe Y. et al. Acute kidney injury in non-intensive care and intensive care patients treated with vancomycin and piperacillin-tazobactam. J Nippon Med Sch. 2020;87(2):66-72. doi:10.1272/jnms.JNMS.2020_87-203 [DOI] [PubMed] [Google Scholar]
- 38. Alshehri AM, Alzahrani MY, Abujamal MA, et al. Comparative risk of acute kidney injury following concurrent administration of vancomycin with piperacillin/tazobactam or meropenem: a systematic review and meta-analysis of observational studies. Antibiotics (Basel). 2022;11(4):526. doi: 10.3390/antibiotics11040526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalligeros M, Karageorgos SA, Shehadeh F, Zacharioudakis IM, Mylonakis E. The association of acute kidney injury with the concomitant use of vancomycin and piperacillin/tazobactam in children: a systematic review and meta-analysis. Antimicrob Agents Chemother. 2019;63(12):e01572-19. doi: 10.1128/AAC.01572-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med. 2018;46(1):12-20. doi: 10.1097/CCM.0000000000002769 [DOI] [PubMed] [Google Scholar]
- 41. Hammond DA, Smith MN, Li C, Hayes SM, Lusardi K, Bookstaver PB. Systematic review and meta-analysis of acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam. Clin Infect Dis. 2017;64(5):666-674. doi: 10.1093/cid/ciw811 [DOI] [PubMed] [Google Scholar]
- 42. Blears EE, Morris J, Popp D, Lee JO, Norbury WB. Kidney injury in critically ill patients treated with vancomycin and zosyn or an alternative: a systematic review and meta-analysis. Surg Infect (Larchmt). 2022;23(6):516-524. doi: 10.1089/sur.2022.128 [DOI] [PubMed] [Google Scholar]
- 43. Avedissian SN, Pais GM, Liu J, Rhodes NJ, Scheetz MH. Piperacillin-tazobactam added to vancomycin increases risk for acute kidney injury: fact or fiction? Clin Infect Dis. 2020;71(2):426-432. doi: 10.1093/cid/ciz1189 [DOI] [PubMed] [Google Scholar]
- 44. Dong J, Liu Y, Li L, Ding Y, Qian J, Jiao Z. Interactions between meropenem and renal drug transporters. Curr Drug Metab. 2022;23(5):423-431. doi: 10.2174/1389200223666220428081109 [DOI] [PubMed] [Google Scholar]
- 45. Zhang M, Huang L, Zhu Y, et al. Epidemiology of vancomycin in combination with piperacillin/tazobactam-associated acute kidney injury in children: a systematic review and meta-analysis. Ann Pharmacother. 2024;58(10):1034-1044. doi: 10.1177/10600280231220379 [DOI] [PubMed] [Google Scholar]
- 46. Abdelmessih E, Patel N, Vekaria J, et al. Vancomycin area under the curve versus trough only guided dosing and the risk of acute kidney injury: systematic review and meta-analysis. Pharmacotherapy. 2022;42(9):741-753. doi: 10.1002/phar.2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pmt-10.1177_87551225251350894 for Incidence of Acute Kidney Injury in Critically Ill Patients Receiving Vancomycin With Concomitant Piperacillin-Tazobactam Versus Other Beta-Lactams: A Systematic Review and Meta-Analysis by Ranyi Li, Yanli Li, Chenqi Xu, Ziyan Shen, Xialian Xu, Xiaoqiang Ding, Xiaoyu Li, Qianzhou Lv and Kunming Pan in Journal of Pharmacy Technology