Abstract
A novel Fe/Ca-modified biochar (BC) derived from corn stalk was prepared in this study for the simultaneous adsorption and immobilization of arsenic, cadmium and lead in aqueous solution and soil, respectively. The adsorption experiment in aqueous solution indicated that 1Ca-Fe@BC exhibited excellent removal efficiency (R, %) towards arsenic (46.3%), cadmium (76.3%) and lead (93.6%). The presence of cadmium and lead could enhance the adsorption efficiency of arsenic by facilitating the formation of ternary complexes with it. Moreover, both cooperative and competitive effects occurred between cadmium and lead adsorption. When the distribution coefficient of cadmium and lead was equimolar, cooperative adsorption prevailed. However, when the distribution coefficient deviated from this balance, competitive adsorption dominated with a higher affinity for lead. The soil culture experiment demonstrated that the utilization of 1Ca-Fe@BC could enhance the stability of arsenic, cadmium and lead in soil by enhancing soil cation exchange capacity and pH while reducing dissolved organic carbon. The competitive and cooperative effects of arsenic, cadmium and lead in soil were consistent with those found in aqueous solution. The results showed that 1Ca-Fe@BC exhibited an excellent remediation efficiency for both arsenic, cadmium and lead-contaminated aqueous solution and soil.
Keywords: Multi-contamination, biochar, Fe/Ca modified, simultaneous remediation, heavy metal
Graphical Abstract.
This is a visual representation of the abstract.
Highlights
Ca-Fe@BC showed excellent adsorption performance for As, Cd and Pb in solution.
Cooperative adsorption of As, Cd and Pb was mainly due to surface complexation (Fe–Pb–As/Fe–Cd–As).
Competition was mainly between Cd and Pb, and Pb was more competitive than Cd.
The stability of As, Cd and Pb in soil was significantly affected by soil properties.
Introduction
The global alarm over arsenic (As), cadmium (Cd) and lead (Pb) pollution, notorious for their toxic nature and tendency to accumulate in the environment, is undeniable (Kumar et al., 2021; Ramlan et al., 2022; Wang et al., 2021). These pollutants, which insidiously lurk in soil, unleash a spectrum of health hazards – hypertension, fatigue, sleep disruption and cognitive decline, each posing a significant threat to human well-being (Jiang et al., 2012; Qu et al., 2013; Timofeev et al., 2018). Human activities, including mining, smelting, fertilization and sewage irrigation, had exacerbated this issue by intertwining these toxic elements in both water and soil (Fu et al., 2013; Lu et al., 2015; Yang et al., 2018). In this ecological quandary, Cd(II) and Pb(II) is presented as cations, whereas As(V) predominantly existed as anions such as H2AsO4−, HAsO42− and AsO43− (Park et al., 2016; Yao et al., 2021). The combined effect of these metals resulted in heightened environmental toxicity, surpassing the impact of any single metal, thereby complicating the quest for remediation. Addressing this required the selection of an appropriate repair method, which is a task of urgent and critical importance (Fan et al., 2021; Zhang et al., 2020a).
In the complex arena of environmental remediation, researchers have extensively studied the intricate interactions among diverse materials and their combined effectiveness in eliminating arsenic, cadmium and lead from both aqueous solution and soil (Diagboya et al., 2015; Mokwenye et al., 2016; Yao et al., 2021). Regarding the investigation of aqueous solution, Arancibia-Miranda et al. (2020) embarked on an exploration, utilizing magnetic nanocomposites to extract copper, cadmium and arsenic from water. Their research unveiled a notable dynamic: the coexistence of copper and cadmium hindered the adsorption capacity. However, interestingly, specific arsenic species seemed to facilitate the removal of cadmium. Say et al. (2003) investigated the competitive adsorption behaviours of cadmium, lead and arsenic. Their findings highlighted the preferential affinity of lead over other metal ions, providing a deeper understanding of the complex interplay among metal ions (Say et al., 2003). Regarding the investigation of soil, Rocco et al. (2018) investigated the impact of both inorganic and organic amendments on soils co-contaminated with arsenic and cadmium. Their study revealed a complex scenario: alkaline soil conditions reduced the bioavailability of cadmium while increasing that of arsenic. This outcome was attributed to an enhancement in cadmium’s precipitation and ion exchange processes induced by pH, consequently reducing its mobility in soil. Additionally, the release of dissolved organic carbon (DOC) from organic compounds competed for adsorption sites with arsenic, significantly increasing solubility and mobility of arsenic (Rocco et al., 2018). Although many studies had been devoted to the removal mechanism of arsenic, cadmium and lead in solution and soil, their synergies and competition mechanism remained unclear, necessitating further research.
Biochar (BC) is easily produced and highly economically efficient (Igalavithana et al., 2019). Its high porosity, large specific surface area and diverse surface functional groups enable it to effectively adsorb arsenic, cadmium and lead (El-Naggar et al., 2021). However, the adsorption capacity (q, mg g−1) of unmodified biochar for arsenic, cadmium and lead is constrained (Beesley and Marmiroli, 2011). Recent advancements have highlighted the role of iron (Fe) modification in significantly enhancing biochar’s capacity to adsorb these contaminants (Zhou et al., 2017). Lin et al. (2017) discovered that iron impregnation significantly enhanced the q of biochar for As(V) by modifying its surface functional groups. Moradi and Karimi (2021) and Ni et al. (2019) discovered that Fe-modified biochar’s unique physical and chemical properties fostered mechanisms such as ion exchange, complexation, electrostatic attraction and precipitation, effectively stabilizing cadmium and lead. The adsorption mechanism for Cd–Pb and As–Cd by Fe-modified biochar have been partially investigated. Alghamdi and Alasmary (2023) investigated the competitive adsorption mechanism of coexisting cadmium and lead, revealing lead’s higher potential to be adsorbed, possibly due to Pb(II)’s larger ionic radius. Zhang et al. (2019) showed that Fe-modified biochar exhibited remarkable efficacy in removing both arsenic and cadmium from bimetallic solutions, potentially because As(V) provided negatively charged sites for cadmium adsorption, forming Fe–As–Cd complexes on the biochar surface. The addition of calcium to Fe-modified biochar has been shown to increase the q for Cd and Pb. Chen et al. (2020) and Wu et al. (2020) investigated the simultaneous adsorption mechanism of Fe/Ca-modified biochar in aqueous solution for As–Pb and in soil for As–Cd, respectively. However, the simultaneous adsorption mechanism of three heavy metals, that is, arsenic, cadmium and lead, in both aqueous solution and soil by Fe/Ca-modified biochar remained an uncharted territory, beckoning further exploration.
The objectives of this study were (1) to determine an Fe/Ca-modified biochar with the optimal Fe-to-Ca ratio, in order to maximize its remediation efficacy towards arsenic, cadmium and lead, (2) to reveal the cooperative and competitive mechanism of simultaneous adsorption of arsenic, cadmium and lead in aqueous solution by Fe/Ca-modified biochar, adsorption experiments arsenic, cadmium and lead were conducted along with characterization analysis and (3) to investigate the stabilization mechanism of As, Cd and Pb by Fe/Ca-modified biochar in soil. The findings will establish a theoretical foundation for the simultaneous remediation of arsenic, cadmium and lead in both aqueous solution and soil through Fe/Ca-modified biochar.
Materials and methods
Reagents
Ferric sulphate heptahydrate (FeSO4·7H2O) and ferric chloride hexahydrate (FeCl3·6H2O), were obtained from Sinopharm Co., Ltd (Beijing, China). Calcium oxide (CaO) and Lead nitrate (Pb(NO3)2) were obtained from Xi Long Science Co., Ltd (Shanghai, China). Cadmium nitrate tetrahydrate (Cd(NO3)2·4H2O) was obtained from Aladdin Co., Ltd (Beijing, China). Sodium arsenate heptahydrate (HAsNa2O4·7H2O) was obtained from SIGMA Co., Ltd (Hong Kong, China). The experimental solution was prepared using deionized water (see details in Supplemental Text S1).
Soil preparation
The samples were burrowed from the topsoil (0–20 cm) of the Botanical Garden of Nanjing Normal University. Firstly, the soil sample was pretreated by impurities removal, air drying, crushing and screening to obtain the original soil sample with particle size less than 10 mesh. Secondly, a 240-mL mixed solution containing 4.4 mmol L−1 Na2HAsO4·7H2O, 0.7 mmol L−1 Cd(NO3)2·4H2O and 32.2 mmol L−1 Pb(NO3)2 was uniformly sprayed onto soil samples weighing 1600 g. Thirdly, the heavy metal-pretreated soil sample was moved into a constant temperature humidity chamber and aged for 30 days, and the moisture content of soil was controlled at 70%. Finally, the aged soil was crushed and sieved to particle size less than 10 mesh. The physicochemical properties and heavy metals’ concentration of test and aged soil sample were listed in Supplemental Table S1.
Biochar preparation
Corn stalk (CS) was collected from Lianyungang, China, underwent pyrolysis in a fixed-bed reactor under N2 atmosphere at 450°C for 2 hours. The pyrolysed CS, called biochar (BC), was crushed into particle less than 10 mesh and stored in a sealed container. Two modified biochars involving Fe and Fe/Ca-modified biochar were prepared based on the as-prepared BC.
As for Fe-modified biochar (Fe@BC), firstly, weighed 20 g of as-prepared biochar and put into a 1 L beaker. Secondly, weighed a certain amount of FeCl3·6H2O and FeSO4·7H2O (the molar ratio of Fe3+/Fe2+ = 2, their detailed dosages seen in Supplemental Table S2) and dissolved into 200 mL of deionized water, then transferred the solution into the beaker and stirred with a glass rod to moisten biochar thoroughly. Thirdly, adjusted its pH to 11–12 with 5 mol L−1 NaOH, and then immersed in a water bath set at 60°C for 2 hours. Finally, moved the biochar into a ventilated drying oven to dry at 105°C for 24 hours, then grinded and refined to the particle size smaller than 100 mesh. The detailed Fe-modified biochars were called 3Fe@BC, 5Fe@BC, 10Fe@BC and 20Fe@BC, where the values 3–20 represents for the mass ratio of total Fe (TFe) to biochar of 3%, 5%, 10% and 20%, respectively.
As for Fe/Ca-modified biochar (Ca-Fe@BC), the preparation procedure was the same as preparation of Fe-modified biochar. However, there were two differences, the first one was to fix the mass ratio of TFe/BC of 1:10, and the second one was to add a certain of CaO (also seen in Supplemental Table S2) at the second step of 10Fe@BC preparation. Fe/Ca-modified biochars were called 0.1Ca-Fe@BC, 0.5Ca-Fe@BC, 1Ca-Fe@BC, 2Ca-Fe@BC and 3Ca-Fe@BC, respectively, in which, 0.1–3 represents the mass ratio of CaO to biochar of 0.1%, 0.5%, 1%, 2% and 3%, respectively (see details in Supplemental Text S2).
Experiment procedures
Adsorption experiment
Single adsorption of arsenic, cadmium and lead
As the q of lead by biochar exceeded that of cadmium, arsenic and cadmium were chosen as representatives in the study of q and adsorption efficiency. Weighed 10, 20, 40, 100 and 200 mg of as-prepared biochars and placed them into a series of 100 mL plastic centrifuge tubes, then added 20 mL solution with concentrations of arsenic at 20 mg·L−1, cadmium at 50 mg·L−1 and lead at 100 mg·L−1 into the respective centrifuge tubes. The choice of solution with concentrations of arsenic at 20 mg·L−1 was determined by Fan et al.’s (2018) research, cadmium at 50 mg·L−1 was determined by Ren et al.’s (2021) research, and lead at 100 mg·L−1 was determined by Luo et al.’s (2020) research. The levels of As, Cd and Pb in the solution was determined after 24 hours of constant temperature oscillation. The impact of the biochar type and quantity on the adsorption of As, Cd and Pb in solution was studied. For adsorption kinetics, A 20 mL solution containing As, Cd and Pb was treated with 40 mg 1Ca-Fe@BC and sampled at different times (60–720 minutes). For the adsorption isotherm experiment of single solute system, 40 mg 1Ca-Fe@BC was added to 20 mL of As at concentrations ranging from 0.5 to 20 mg L−1, Cd at concentrations ranging from 20 to 100 mg L−1, and Pb at concentration ranging from 25 to 200 mg L−1, respectively.
Simultaneous adsorption of arsenic, cadmium and lead
Firstly, to study the q values of different modified biochar in a ternary cation adsorption system of As, Cd and Pb, 40 mg of biochars and 20 mL of heavy metal solution containing As, Cd and Pb were added into 100 mL centrifuge tube. To analyse the competitive adsorption mechanisms of As, Cd and Pb, their adsorption performance was studied both in single-metal and multi-metal contaminated solutions across a range of concentrations. The concentrations of Pb were controlled to be 220, 240, 260, 280 and 300 mg L−1, respectively. The concentrations of Cd were controlled to be 20, 40, 60, 80 and 100 mg L−1, respectively.
For all tests, the pH of the solution was controlled at 5 by pH metre. When the solution was configured, it was then shaken at a speed of 205 rpm in the oscillator. The shaking time of single and simultaneous adsorption experiments was set to be 24 hours. The isothermal adsorption model and the composite system experiment required 12 hours of oscillation. For the adsorption kinetics experiment, the shaking time was controlled to be 1, 2, 4, 8 and 12 hours, respectively. After centrifugation, the sample was passed through a 0.45-μm nylon filter membrane, yielding the filtrate. The filtrate was analysed for heavy metal content using inductively coupled plasma (ICP) emission spectrometry. Detailed experiment procedure were given in Supplemental Text S3.
Adsorption amount (qt, mg g−1) and removal efficiency (R, %) were calculated based on the following equations:
| (1) |
| (2) |
where qt is the adsorption amount at adsorption time t (minutes), mg g−1; C0 is the original concentration of the adsorption solution, mg·L−1; Ct is the instantaneous concentration at adsorption time t, mg L−1; V is the solution volume, L; m is the mass of 1Ca-Fe@BC, g; R is the removal efficiency, %.
Soil culture experiment
The culture experiment first weighed 400 g of soil and 400 g 5% (w/w) of different species of biochar (20 g). The soil samples were placed on an overturning oscillator to oscillate continuously at a rotation frequency of 180 rpm for 12 hours. Then 150 of the above biochar-doped soil samples were weighed and laid in 120 (ID) × 23 (H) mm Petri dishes. Based on different biochars, 10 control experiments were carried out and named as soil, 3Fe@BC, 5Fe@BC, 10Fe@BC, 20Fe@BC, 0.1Ca-Fe@BC, 0.5Ca-Fe@BC, 1Ca-Fe@BC, 2Ca-Fe@BC and 3Ca-Fe@BC, respectively. Finally, the Petri dish filled with samples was subjected to a controlled and stable temperature environment cultured at 25°C for 105 days. During culture, the Petri dishes were taken out every 3 days to weigh and replenish water so that the moisture content of soil samples maintained 40%. About 20 g soil samples were collected at 15, 45, 60 and 105 days, respectively. After drying, grinding and sifting through 100 mesh, they were stored in sealed bags for later use. The pH of soil was measured using a pH metre. The soil’s cation exchange capacity (CEC, Cmol+ kg−1) was determined by hexamine cobalt(III) solution spectrophotometric. The DOC in soil (g kg−1) was extracted in water at a 1:2 soil:water ratio and quantified using a TOC analyser (Multi N/C 3100; Analytik Jena, Germany). Each experiment was repeated twice.
The contents of total arsenic, cadmium and lead in soil were determined by ICP after microwave digestion. The modified continuous extraction method of European Community Bureau of Reference (BCR) was used to extract arsenic, cadmium and lead from soil (Rauret et al., 1999). The experiment was repeated only once. The extraction sequence is as follows: (1) acid extractable state, extracting with 0.11 mol L−1 CH3COOH solution for 16 hours; (2) 0.5 mol L−1 NH2OH·HCl induced reduction; (3) extracting the oxidizable state under the conditions of 8.8 mol L−1 H2O2 and 1 mol L−1 NH4OAc and (4) subtracting the aforementioned three steps from the total could derive the residual state content. In this article, the content of acid extractable state and residue state was mainly used to characterize the availability of heavy metals in soil (see details in Supplemental Text S4). The percentage of acid extractable state and residue state was calculated by the formula:
| (3) |
where η is the percentage of acid extractable (or residue) arsenic, cadmium and lead in soil, %; v is the level of arsenic, cadmium and lead in acid extractable (or residue) extract, μg mL−1; V0 is the volume of acid extractable (or residue) extract, mL, which is 25 mL in this article; m is the quality of soil sample for the experiment, g, which is 1.0 g in this article and CHM is the total content of arsenic, cadmium and lead per gram of soil, μg g−1.
Characterization
The samples underwent proximate analysis based on GB/T 28731-2012 as shown in Supplemental Table S3. The ultimate analysis of carbon, hydrogen, nitrogen and sulphur in the raw material and biochars was conducted using an elemental analyser (Vario EL III; Elementar, Langenselbold, Germany), with the corresponding results presented in Supplemental Table S4. The specific surface area and pore structure parameters of biochars were determined by N2 adsorption/desorption analyser (Autosorb-IQ; Quantachrome Company, Boynton Beach, Florida, USA). The micromorphology and microelement types of biochars were tested by scanning electron microscope (JSM-5610LV, Tokyo, Japan). The surface functional groups of biochars were determined by Fourier to transform infrared spectroscopy (FTIR; NEXUS670, Madison, Wisconsin, USA). X-ray diffraction (XRD; D/max2500/PC, Tokyo, Japan) was used for the crystal structure analysis of biochars (see details in Supplemental Text S5).
Results and discussion
Characterization
The specific surface area and pore structure parameters of corn stalk and biochars are shown in Supplemental Table S5. The specific surface area of 10Fe@BC exhibited a significant increase when compared to CS and BC, about 14.53 m2 g−1, fivefold increase compared to the original biochar. The micropore volume and the total pore volume were also increased to approximately four times that of the original biochar. The pore structure of biochar was improved by iron modification. After adding Ca, the specific surface area and micropore volume of Ca-Fe@BC increased first and then decreased with the increasing Ca content. When the mass ratio of CaO to biochar was 1%, its specific surface area of 1Ca-Fe@BC was at its highest, about 21.64 m2 g−1, eight times that of the original biochar. Its micropore volume was also at its highest, about 0.0079 cm3 g−1, 11 times that of the original biochar. Thus, it could be inferred that adding a certain amount of calcium could further improve the pore structure of BC; however, too much calcium may cause the blockage its pore structure. Moreover, the pore size of Fe@BC and Ca-Fe@BC belong to the micropore range (<2 nm), which performs strong adsorption. Thus, it could be inferred that Fe- and Fe/Ca-modified biochar exhibits superior adsorption properties towards heavy metals, and 1Ca-Fe@BC is a better adsorption material.
The micro-morphology photos and Energy Dispersive Spectrometer (EDS) spectrum of CS and BC samples are shown in Supplemental Figure S1. It could be found that the pore structure of biochars were obviously improved after Fe and Fe/Ca modification, which is consistent with the conclusion based on the specific surface area and pore structure analysis. It could be observed from Supplemental Figure S1(e) and (h) that the Fe content in 10Fe@BC and the Ca content in 1Ca-Fe@BC was significantly higher than that in BC, indicating that iron and calcium element was effectively loaded on the appearance of biochar.
The XRD patterns of 1Ca-Fe@BC prior to and following the adsorption of arsenic, cadmium and lead, and their mixture are presented in Figure 1(a). CaCO3 shows a peak around 29.4°, while Fe3O4 exhibits peaks at 35.4° and 62.4°. It could be found that the successful loading of iron and calcium onto biochar was achieved in the form of Fe3O4 and CaCO3, respectively. CdCO3 appears at around 23.2°, which was observed after Cd(II) adsorption, indicating that CaCO3 on 1Ca-Fe@BC precipitated with Cd(II). PbSO4 has peaks at 26.0° and 31.8°. PbSO4 and Pb4(SO4)(CO3)2(OH)2 were formed after lead adsorption, indicating that lead would react with sulphate on the surface of biochar and a more complex precipitate would be formed under alkaline conditions. As16Ca11.44Si9.04 at 34.2° was observed after As(V) adsorption, indicating that arsenate may react with SiO2 and CaCO3. Moreover, in the arsenic, cadmium and lead simultaneous adsorption system, Cd2AsCl2 was observed except for As16Ca11.44Si9.04 and PbSO4, which showed that As(V)/Cd(II) form complexes with each other in simultaneous adsorption system. Based on the EDS spectra analysis in Supplemental Figure S1, C, S, Cl and Si are originally present on the surface of biochar materials and may react with other elements to form the above substances.
Figure 1.

(a) XRD patterns and (b) FTIR of 1Ca-Fe@BC.
FTIR: Fourier to transform infrared spectroscopy; XRD: X-ray diffraction.
The FTIR spectra of 1Ca-Fe@BC Pre- and post-adsorption of arsenic, cadmium and lead, and their mixture are given in Figure 1b. The peaks at 3423, 2924, 1595, 1376 and 1106 are attributed to the stretching vibration of –OH, C–H, COOH, C–H and C–O–C, respectively (Mohubedu et al., 2019). The oxygen-containing functional groups could form complexes with heavy metals (Diagboya and Dikio., 2018). The peak at approximately 569 cm−1 is related to the Fe–O vibration, providing evidence that the surface of biochar was successfully modified with loaded Fe3O4. An obvious absorption peak at 879 cm−1 could be observed, which is caused by CO32− stretching vibration. It further confirmed that CaCO3 was produced after modification, which is consistent with the findings obtained from the preceding XRD analysis.
Single adsorption of arsenic, cadmium and lead in aqueous system
Effect of Fe/Ca modification and biochar dosage
Based on previous experimental results, the adsorption of Pb(II) by the biochars prepared in this article was significantly higher than that of arsenic and cadmium. However, this article focused on the simultaneous adsorption of arsenic, cadmium and lead. Therefore, the single adsorption characteristics of arsenic and cadmium by biochar were further inverstigated in this article to improve the adsorption for arsenic and cadmium. Based on Figure 2(a), the removal efficiency and adsorption of As(V) by biochar improved after Fe/Ca modification. The biochars used in this investigation can be ranked based on their adsorption of arsenic as follows: 1Ca-Fe@BC > 0.5Ca-Fe@BC > 10Fe@BC > BC. As shown in Figure 2(b), the adsorption for cadmium by different biochars was roughly the same. Moreover, it was observed that the removal efficiency of arsenic and cadmium increased with the increasing biochar dosage, while the adsorption amount gradually decreased. By combining the data from Figure 2(a) and (b) with Supplemental Figure S2 and Supplemental Table S6, it can be concluded that the optimum biochar dosage for the simultaneous adsorption of arsenic and cadmium was approximately 2 g L−1 considering (Okoli et al., 2014).
Figure 2.
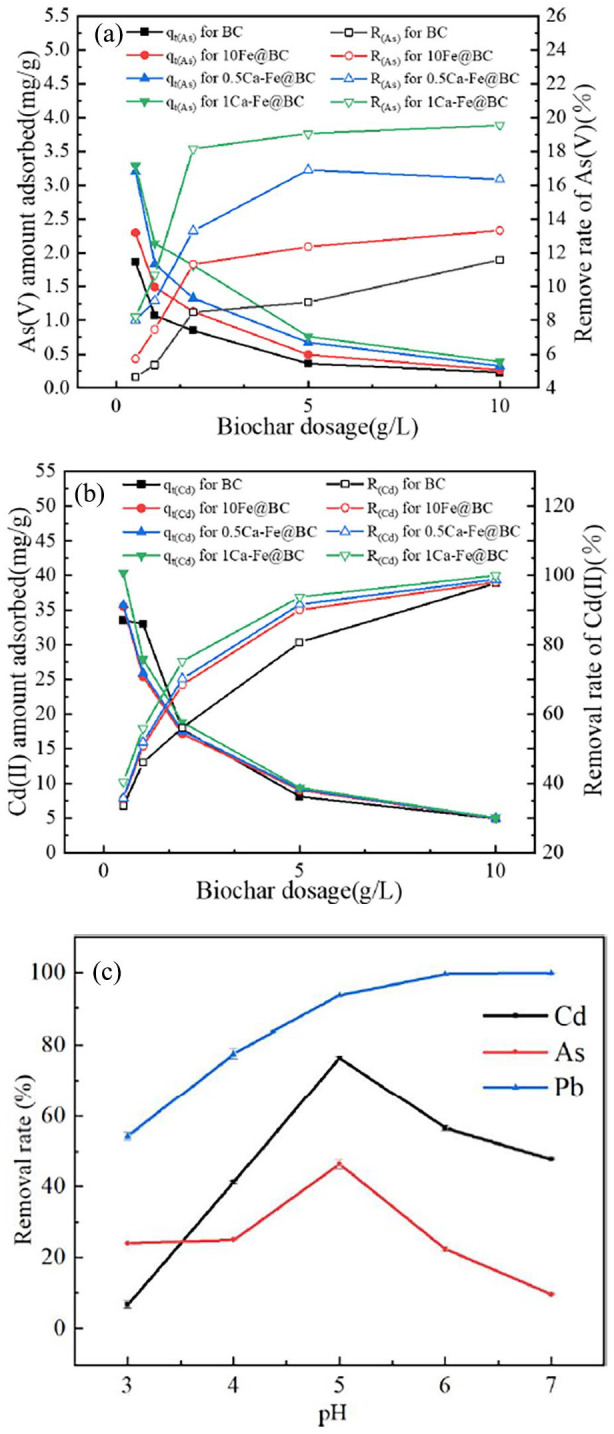
(a) Effect of adsorbent dosage on adsorption and removal efficiency of As; (b) effect of adsorbent dosage on adsorption and removal efficiency of Cd; (c) effect of pH on the adsorption characteristics of arsenic, cadmium and lead.
Effect of pH
Generally, the pH level can significantly affect the heavy metal adsorption performance of biochar. In this study, 1Ca-Fe@BC was used to investigate the impact of pH due to its superior adsorption performance for arsenic, cadmium and lead. The data presented in Figure 2(c) indicate that the removal efficiency of lead by 1Ca-Fe@BC was the highest, followed by cadmium and arsenic. As the initial pH of solution increased, the adsorption performance for lead continuously improved and eventually stabilized. However, for cadmium and arsenic, their removal efficiency initially increased with the rise in pH, reached a peak at pH 5, and then decreased with further pH increases.
For arsenic adsorption, the diminished removal efficiency at low pH was primarily attributed to the partial dissolution of iron oxides on the biochar surface under acidic conditions. As the pH increased, the dissolution of Fe and Mn oxides was inhibited, resulting in an increased removal efficiency of arsenic at pH 5. However, when the pH exceeded 5, the removal efficiency of As(V) declined due to enhanced deprotonation of functional groups on the biochar surface caused by decreased acidity (Wu et al., 2020). For cadmium and lead adsorption, the removal efficiency was constrained at low pH by the high concentration of H+ ions in the solution, which caused electrostatic repulsion with cadmium and lead (Lu et al., 2012). As the primal pH increased, more adsorption sites were generated due to the deprotonation of surface functional groups on the biochar, leading to continuous improvement in its ability to adsorb cadmium and lead. However, the adsorption of cadmium by biochar may be affected by precipitation formation when the pH exceeds 5 (Chen et al., 2015). The maximum removal efficiency of arsenic, cadmium and lead were 46.3%, 76.3% and 93.6%, respectively.
Adsorption kinetics and adsorption isotherm
The utilization of adsorption kinetics model enabled the investigation of biochar’s adsorption process and mechanism. The quasi-first-order and quasi-second order kinetic models and the intraparticle diffusion kinetic model were used to study the adsorption process and mechanism of arsenic, cadmium and lead by 1Ca-Fe@BC (Diagboya and Dikio, 2018; Mohubedu et al., 2019):
| (4) |
| (5) |
where q and qt represent the heavy metal adsorption capacity (mg g−1) at adsorption equilibrium and adsorption time t, k1 and k2 are pseudo-first-order and pseudo-second-order kinetic constants. As illustrated in Supplemental Figure S3(a) to (f) and Supplemental Table S7, quasi-second-order kinetic models showed good linear regression coefficients. The equilibrium adsorption capacities of arsenic, cadmium and lead obtained by quasi-second-order kinetic fitting were 2.4, 20.1 and 50.2 mg g−1. The findings suggested that chemical adsorption plays a predominant role in the adsorption of arsenic, cadmium and lead adsorption by 1Ca-Fe@BC, and the adsorption process involves the formation of chemical bonds (Xu et al., 2021). Moreover, based on the intraparticle diffusion kinetic model in Supplemental Figure S3(a) to (f), the adsorption process of 1Ca-Fe@BC primarily occurred during the initial stage, exhibiting a rapid rate of adsorption. The adsorption rate tended to be stable in the second stage. It could be inferred that the adsorption of 1Ca-Fe@BC mainly exists at the superficial level. Moreover, the linear fitting curves of the two stages did not pass through the origin. This indicates that the adsorption process was not solely governed by intra-particle diffusion but also involved extra-particle diffusion. The adsorption of different Fe-modified biochars for arsenic, cadmium and lead were shown in Supplemental Table S8. Although the amount of iron added to 1Ca-Fe@BC in this experiment was relatively small, it could be observed that its adsorption effect on arsenic and cadmium was comparable to other biochars with high iron content, while exhibiting significant adsorption effect on Pb(II).
Simultaneous adsorption of arsenic, cadmium and lead in aqueous system
Effect of Fe/Ca modification
The adsorption of arsenic, cadmium and lead by different modified biochars in the arsenic–cadmium–lead ternary composite adsorption system is given in Figure 3(a). As shown, the adsorption of arsenic, cadmium and lead initially increased with the rising Fe content in the biochar, followed by a subsequent decrease. The highest adsorption amount was achieved when 10Fe@BC was used. Moreover, with the elevation of calcium content in biochar, the adsorption of arsenic, cadmium and lead also exhibited an increasing trend followed by a decrease, reaching their maximum values when 1Ca-Fe@BC was used. However, the adsorption of Cd by 1Ca-Fe@BC was not as effective, likely due to the strong competitive relationship between the ions (Arcibar-Orozco et al., 2015; Ji et al., 2022). The incorporation of iron and calcium in appropriate amounts enhanced the complexation, electrostatic interactions and ion exchange capacity of biochar towards arsenic, cadmium and lead (Wang et al., 2017; Zhou et al., 2014). However, when the concentration of iron and calcium exceeded a certain threshold, that is, Fe/BC>10% and CaO/BC>1% as shown in Supplemental Table S2, it led to pore blockage within the biochar structure, reducing its specific surface area and ultimately decreasing the adsorption efficiency for arsenic, cadmium and lead (Amen et al., 2020). Therefore, the composite adsorption effect and mechanism of biochar on arsenic, cadmium and lead in solution were investigated using 1Ca-Fe@BC as the model system.
Figure 3.
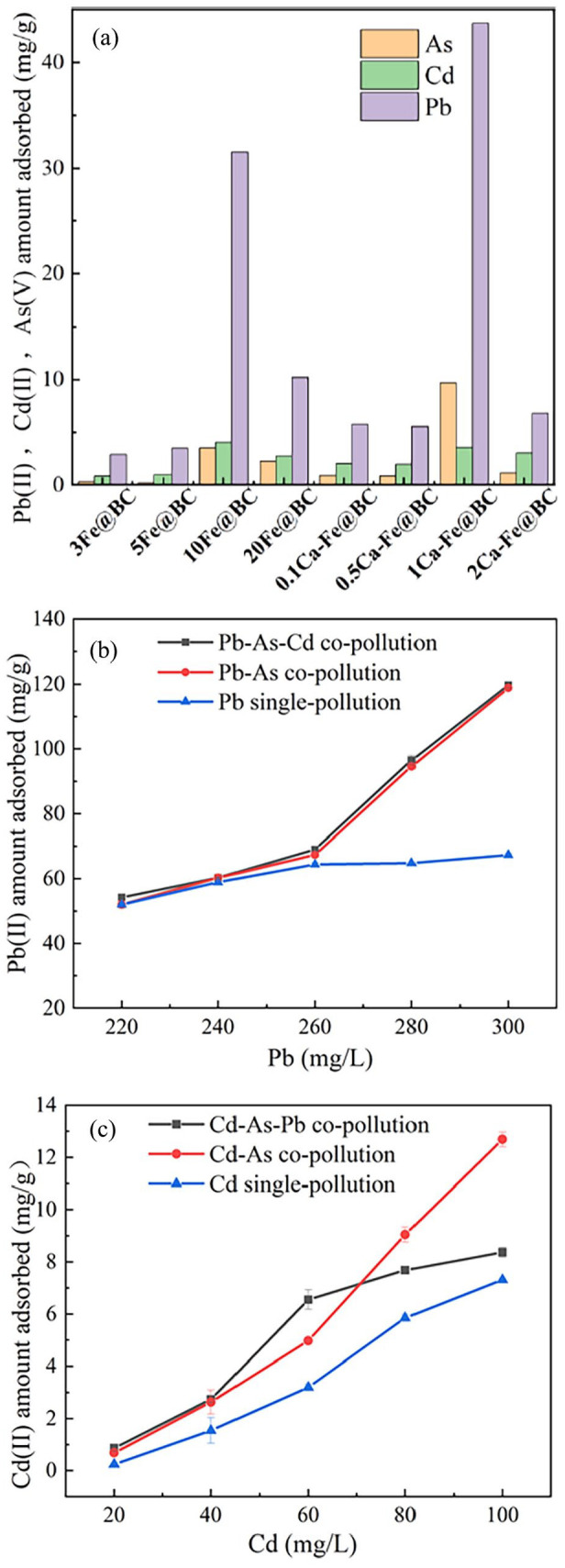
(a) The adsorption of arsenic, cadmium and lead by different modified biochars in arsenic–cadmium–lead ternary composite adsorption system; (b) the impact of lead concentration on the adsorption of lead by 1Ca-Fe@BC in lead, lead–arsenic and lead–arsenic–cadmium systems; (c) the impact of cadmium concentration on the adsorption of cadmium by 1Ca-Fe@BC in cadmium, cadmium–arsenic and cadmium–arsenic–lead systems.
Impact of lead concentration
The impact of lead concentration on its adsorption by 1Ca-Fe@BC in lead, lead–arsenic and lead–arsenic–cadmium systems was illustrated in Figure 3(b). With the increase of initial lead concentration, it was evident that the addition of arsenic to the lead–arsenic system enhances the adsorption for lead (Wu et al., 2018). This was due to the generation of electrostatic attraction between lead cations and AsO43− anions with negative charges. Moreover, cooperative adsorption occurred via complex formation: where one ‘Pb’ is adsorbed, it enhances the adsorption of the other ‘As’ because Pb is a cation while As(V) is an anion (Ren et al., 2012; Wu et al., 2020). In lead–arsenic–cadmium system, the addition of cadmium did not significantly alter the adsorption of Pb(II) in comparison to the lead–arsenic system, which indicated that the adsorption of lead and cadmium by biochar exhibited both synergistic and antagonistic effects, the aforementioned finding was in line with the research outcomes of Hong et al. (2022).
Impact of cadmium concentration
The impact of cadmium concentration on the adsorption of cadmium by 1Ca-Fe@BC in cadmium-only, cadmium–arsenic and cadmium–arsenic–lead systems is shown in Figure 3(c). In cadmium–arsenic system, when the cadmium concentration was below 70 mg L−1, the adsorption of cadmium in the cadmium–arsenic–lead system was more effective than in the cadmium–arsenic system, indicating a synergistic effect between lead and cadmium. However, when the cadmium concentration exceeded 70 mg L−1, the adsorption of cadmium in the cadmium–arsenic system was slightly better than that in the cadmium–arsenic–lead system. This suggests that at higher cadmium concentrations, the presence of lead inhibits the adsorption of cadmium. This was due to the greater stability of complex formation formed by lead and oxygen compared to those formed by cadmium and oxygen, making them more favourable for lead adsorption in competitive adsorption processes on biochar (Ding et al., 2016), so the affinity of 1Ca-Fe@BC for lead was greater than that of cadmium (Ni et al., 2019). Furthermore, in Figure 3(b), the distribution coefficient of lead and cadmium remained approximately constant regardless of changes in lead dosage, and the presence of cadmium promoted the adsorption of lead. In Figure 3(c), the distribution coefficient of lead and cadmium was equimolar when the concentration of cadmium was around 54 mg g−1, and the addition of lead at this point promoted the adsorption of cadmium. When the concentration of cadmium exceeded 70 mg g−1, the distribution coefficient of lead and cadmium differed greatly, and the addition of lead inhibited the adsorption of cadmium. Therefore, it was found that the adsorption of biochar for cadmium and lead was most maximum when their distribution coefficient was equimolar (Chakraborty and Chakrabarti, 2008).
Simultaneous adsorption mechanism of arsenic, cadmium and lead in aqueous system
Simultaneous adsorption mechanism of arsenic, cadmium and lead in aqueous system was illustrated in Figure 4. According to the intraparticle diffusion kinetic and isothermal adsorption analysis, the remediation of arsenic, cadmium and lead by 1Ca-Fe@BC mainly existed on the surface (Diagboya and Dikio, 2018; Mohubedu et al., 2019). Arsenic, cadmium and lead could be effectively adsorbed by 1Ca-Fe@BC through physical adsorption, ion exchange, precipitation, electrostatic interaction and surface complexation.
Figure 4.
Simultaneous adsorption mechanism of arsenic, cadmium and lead in aqueous system.
For physical adsorption, competition occurred during the physical adsorption process of arsenic, cadmium and lead by biochar due to the limited adsorption sites. For ion exchange and precipitation, cadmium and lead were immobilized onto the biochar surface through ion exchange with Mg2+ and Na+ on the surface of biochar (Wang et al., 2017; Zhou et al., 2014). This phenomenon was intensified by the addition of CaO. Cadmium and lead can form precipitates of Pb(OH)2, PbSO4 and CdCO3 when combined with anions such as OH−, SO42− and CO32− (Moradi and Karimi, 2021; Ni et al., 2019). Cadmium and lead engaged in fierce competition with one another during the ion exchange and precipitation processes on the biochar surface.
For electrostatic interaction, pyrolytic biochar itself was negatively charged, enabling it to attract cadmium and lead through electrostatic attraction. Moreover, electrostatic interaction can also generated between H2AsO4−, HAsO42−, AsO43− anions and cadmium, lead cations (Wang et al., 2017; Zhou et al., 2014). For surface complexation, loading iron on biochar could increase the surface functional groups and enhance the complexation of three heavy metals (Moradi and Karimi, 2021; Ni et al., 2019). The hydroxyl groups on the surface of 1Ca-Fe@BC competitively complexed with arsenic, cadmium and lead to form binary complexes, namely Fe–O–As, Fe–O–Pb and Fe–O–Cd, and ternary complexes such as Fe–Pb–As and Fe–Cd–As. As(V) exhibited a synergistic effect on the formation of ternary complexes with cadmium and lead, and a competitive effect on the formation of binary complexes. Cadmium and lead exhibited competitive interactions in the formation of both ternary and binary complexes. The complexation formed by Pb(II) and oxygen exhibited greater stability, thereby rendering Pb(II) more competitive in the formation of complexes. Biochar demonstrates superior adsorption capabilities for both cadmium and lead when their distribution coefficient was equimolar.
Remediation of arsenic, cadmium and lead co-contaminant in soil
Change in soil properties
The consequence of various forms of modified biochar on the change in soil pH, CEC and DOC were referred in Figure 5. During 15–45 days, the overall soil pH decreased, as evidenced by Figure 5(a), which may be attributed to the consumption of alkaline substances in biochar (Xu et al., 2022). The Ca, Mg and K elements in biochars were converted into hydroxides or hydrides during carbonization. After added in soils, hydroxyl groups of these substances in biochar were released into the soil solution, leading to a subsequent increase in soil pH during 45–105 days (Penido et al., 2019). Compared with the original soil, the soil pH after adding biochar was higher, and the soil pH with the addition of Fe/Ca-modified biochar generally exhibited higher values compared to those with Fe-modified biochar. This was because biochar itself was alkaline, and CaO added in the preparation of Fe/Ca-modified biochar was also alkaline (Li et al., 2017). At high pH, the negative charge on soil particles’ surface was enhanced, thereby facilitating electrostatic attraction and precipitation of cadmium and lead. However, as depicted in Figure 5(a), the biochar species had little effect on soil pH after 105 days of cultivation. Therefore, it could be inferred that pH may not be the primary parameter influencing heavy metal immobilization by biochar (Zhang et al., 2020b).
Figure 5.

Change in soil properties: (a) pH, (b) CEC and (c) DOC.
CEC: cation exchange capacity; DOC: dissolved organic carbon.
Based on Figure 5(b), with the increasing culture time, the soil CEC exhibited an initial decrease, followed by a subsequent increase and ultimately a final decline. Upon the incorporation of biochar into the soil, the soil pH increased and heavy metals such as cadmium and lead were converted to precipitate hydroxide, thereby reducing CEC. The following increase and decrease of CEC were attributed to the dissolution and precipitation of hydroxides, which occurred concomitantly with the increase and decrease of pH (Zhang et al., 2020b). After 105 days of cultivation, the soil CEC yielded the most significant improvements when adding 10Fe@BC and 1Ca-Fe@BC. The introduction of cations Fe2+, Fe3+ and Ca2+ into the soil through 10Fe@BC and 1Ca-Fe@BC addition led an increase in CEC. With the further increase of Fe and Ca, soil organic matter content decreased and CEC thus decreased correspondingly (Ouyang et al., 2018). Correlation analysis of BET-specific surface area of biochar and CEC of soil was conducted by using Statistical Product Service Solutions (SPSS). The Pearson correlation coefficient was 0.859, and the significance p = 0.014 < 0.05, so there was a significant positive correlation. That was the larger the specific surface area of biochar, the higher the CEC applied into the soil. The enhancement of soil CEC could facilitate the ion exchange between biochar and cationic heavy metals, thereby reinforcing the immobilization of Cd(II) and Pb(II) (Xu et al., 2022).
Based on Figure 5(c), the trend of soil DOC exhibited a decrease followed by an increase over time. The incorporation of biochar into soil enhanced the microbial decomposition in soil, leading to a reduction in DOC. Meanwhile, after biochar was applied, DOC was difficult to release from the soil immediately, so as time goes on, a small amount of DOC would be released again, and its content would rise slightly. After the addition of various types of biochar, 1Ca-Fe@BC exhibited the lowest soil DOC compared to other biochar. As DOC could compete with heavy metals for soil and biochar adsorption sites, when DOC was reduced, the q of soil and biochar for arsenic, cadmium and lead was enhanced, thereby mitigating their toxicity and migration (Rocco et al., 2018).
Speciation of arsenic, cadmium and lead
Impact of biochar species
The impact of biochar species on the speciation changes of arsenic, cadmium and lead was given in Figure 6. After 105 days of cultivation, the biochar-amended soil exhibited a decrease in acid-soluble arsenic, cadmium and lead content compared to the original soil, while the residual content increased. When 10Fe@BC and 1Ca-Fe@BC were added, the acid-soluble arsenic, cadmium and lead content of soil was minimized while the residual content was maximized, indicating that 10Fe@BC and 1Ca-Fe@BC had the most effective remediation effect on arsenic, cadmium and lead.
Figure 6.
BCR sequential extraction fractions for (a and b) arsenic, (c and d) cadmium and (e and f) lead in soil after 15 and 105 days.
BCR: Community Reference Bureau.
The 10Fe@BC and 1Ca-Fe@BC proved to be the most effective biochars for cadmium and lead remediation, owing to their higher CEC values. The ion exchange capacity of biochar for cadmium and lead was enhanced, ultimately leading to effective immobilization of cadmium and lead. Although the soil pH remained high after biochar addition, which was not conducive to As(V) adsorption, it could be observed that As(V) fixation was still effective due to the synergistic effect of cadmium, lead and arsenic adsorption. After loading cadmium and lead, biochar exhibited a higher positive charge. This enhanced the electrostatic effect on As(V), thereby achieving effective immobilization of As(V). The immobilization efficiency of different Fe-modified biochar on arsenic, cadmium and lead in soil was shown in Supplemental Table S9. It could be found that the Fe/Ca-modified biochar prepared in this study exhibited excellent immobilization efficiency towards arsenic, cadmium and lead in soil, reaching 56.5%, 67.6% and 40.4%, respectively.
Impact of culture time
The influence of culture time on the acid-soluble and residual contents of arsenic, cadmium and lead in soil was shown in Supplemental Figure S4. The results presented in Supplemental Figure S4(a) and (b) demonstrated a decrease in the acid-soluble As(V) content from 15 to 105 days, while the residual content increased. The correlation analysis of the residual content of arsenic, cadmium and lead and soil physical and chemical properties including pH, CEC and DOC was listed in Supplemental Table S10. A significant negative correlation could be observed between the residual content of As(V) and DOC. The residual content of As(V) was also affected by pH and CEC. During 15–60 days, the residual content of As(V) increased due to a continued decline in DOC. The residual content of As(V) between 60 and 105 days showed a slight increase. This was due to the increase in pH and CEC, which enhanced biochar’s adsorption for cadmium and lead. This resulted in an increased amount of positive charges on the surface of biochar, enabling it to absorb As(V) through electrostatic action, thereby reducing its mobility.
As depicted in Supplemental Figure S4(c) and (d), the acid-soluble Cd(II) content exhibited an initial increase from 15 to 45 days, followed by a subsequent decrease from 45 to 105 days. The trend of the residual content was inversely proportional to that of the acid-soluble Cd(II) content. As shown in Supplemental Table S10, the residual content of cadmium was most influenced by CEC. During 15–45 days, the residual content of Cd(II) decreased due to a decrease in CEC, while from day 45 to 60, the increase persisted due to a concurrent rise in CEC levels. During the period of 60–105 days, the increase of the residual content Cd(II) was because Cd(II) was also influenced by DOC, and the decrease of DOC in 60–105 days led to the increase of the residual content Cd(II).
As depicted in Supplemental Figure S4(e) and (f), the acid-soluble Pb(II) content exhibited an initial increase, followed by a decrease, and ultimately another increase. Conversely, the trend in the residual content was opposite to that observed for the acid-soluble Pb(II) content. According to Supplemental Table S10, it could be found that the residual content of Pb(II) had a very significant positive correlation with CEC. The consistency of the residual content of Pb(II) and CEC variation was maintained over the 15–105 day period. Simultaneous adsorption mechanism of arsenic, cadmium and lead in soil system was presented in Supplemental Figure S5.
Conclusions
The Fe/Ca-modified biochar derived from corn stalks was prepared using pyrolysis and impregnation techniques. The impact of Fe/Ca-modified biochar on the adsorption of arsenic, cadmium and lead was investigated in both aqueous and soil systems. The results indicated that under optimal conditions (1Ca-Fe@BC, pH = 5), the removal efficiency of arsenic, cadmium and lead in solution within 24 hours reached 46.3%, 76.3% and 93.6%, respectively. After adding Fe/Ca-modified biochar, the immobilization efficiencies of arsenic, cadmium and lead in soil reached 56.5%, 67.6% and 40.4%, respectively. Compared to biochar prepared in other studies, the Fe/Ca-modified biochar produced in this study exhibited higher immobilization efficiency with lower iron content. Based on simultaneous adsorption experiments and characterization analysis, the simultaneous adsorption mechanism of arsenic, cadmium and lead were analysed. Cooperative effects between the adsorption of arsenic and cadmium–lead by Fe/Ca-modified biochar were observed, attributed to the formation of surface complexation (Fe–Pb–As and Fe–Cd–As) and electrostatic interactions. The primary competition was between cadmium and lead.
Supplemental Material
Supplemental material, sj-docx-1-wmr-10.1177_0734242X241307521 for Simultaneous remediation of As(V), Cd(II) and Pb(II) in aqueous solution and soil using Fe/Ca-modified biochar by Yan Zhou, Wenhui Liu, Dandan Chen, Bangwei Liu, Ping Lu and Yiwei Zhang in Waste Management & Research
Acknowledgments
This work was supported by funds of the National Natural Science Foundation of China (52106253), the Youth project of the Natural Science Foundation of Jiangsu Province (BK20190708) and the Natural Science Foundation of Universities of Jiangsu Province (18KJB470015).
Footnotes
Authors contributions: Yan Zhou, Investigation, Methodology, Data analysis, Writing – original draft. Wenhui Liu, Investigation, Data analysis. Dandan Chen, Conceptualization, Methodology, Validation, Supervision, Writing – Review & editing, Funding acquisition. Bangwei Liu, Investigation. Ping Lu, Supervision, Validation, Project administration. Yiwei Zhang, Data analysis.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The National Natural Science Foundation of China, grant/award number: 52106253; the Youth project of the Natural Science Foundation of Jiangsu Province, grant/award number: BK20190708 and the Natural Science Foundation of Universities of Jiangsu Province, grant/award number: 18KJB470015.
Ethical approval: This study does not involve human and/or animal subjects.
ORCID iD: Dandan Chen  https://orcid.org/0000-0002-5936-6176
https://orcid.org/0000-0002-5936-6176
Data availability: The data and materials that support the findings of this study were available on request from the corresponding author (dandanchen@njnu.edu.cn).
Supplemental material: Supplemental material for this article is available online.
References
- Alghamdi AG, Alasmary Z. (2023) Efficient remediation of cadmium- and lead-contaminated water by using Fe-modified date palm waste biochar-based adsorbents. International Journal of Environmental Research and Public Health 20: 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen R, Bashir H, Bibi I, et al. (2020) A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chemical Engineering Journal 396: 125195. [Google Scholar]
- Arancibia-Miranda N, Manquian-Cerda K, Pizarro C, et al. (2020) Mechanistic insights into simultaneous removal of copper, cadmium and arsenic from water by iron oxide-functionalized magnetic imogolite nanocomposites. Journal of Hazardous Materials 398: 122940. [DOI] [PubMed] [Google Scholar]
- Arcibar-Orozco JA, Rangel-Mendez JR, Diaz-Flores PE. (2015) Simultaneous adsorption of Pb(II)-Cd(II), Pb(II)-phenol, and Cd(II)-phenol by activated carbon cloth in aqueous solution. Water Air and Soil Pollution 226: 2197. [Google Scholar]
- Beesley L, Marmiroli M. (2011) The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environmental Pollution 159: 474–480. [DOI] [PubMed] [Google Scholar]
- Chakraborty P, Chakrabarti CL. (2008) Competition from Cu(II), Zn(II) and Cd(II) in Pb(II) binding to Suwannee River Fulvic Acid. Water Air and Soil Pollution 195: 63–71. [Google Scholar]
- Chen T, Quan XC, Ji ZH, et al. (2020) Synthesis and characterization of a novel magnetic calcium-rich nanocomposite and its remediation behaviour for As(III) and Pb(II) co-contamination in aqueous systems. Science of the Total Environment 706: 135122. [DOI] [PubMed] [Google Scholar]
- Chen T, Zhou Z, Han R, et al. (2015) Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 134: 286–293. [DOI] [PubMed] [Google Scholar]
- Diagboya PN, Dikio ED. (2018) Scavenging of aqueous toxic organic and inorganic cations using novel facile magneto-carbon black-clay composite adsorbent. Journal of Cleaner Production 180: 71–80. [Google Scholar]
- Diagboya PN, Olu-Owolabi BI, Adebowale KO. (2015) Effects of time, soil organic matter, and iron oxides on the relative retention and redistribution of lead, cadmium, and copper on soils. Environmental Science and Pollution Research 22: 10331–10339. [DOI] [PubMed] [Google Scholar]
- Ding Y, Liu Y, Liu S, et al. (2016) Competitive removal of Cd(II) and Pb(II) by biochars produced from water hyacinths: performance and mechanism. RSC Advances 6: 5223–5232. [Google Scholar]
- El-Naggar A, Chang SX, Cai YJ, et al. (2021) Mechanistic insights into the (im)mobilization of arsenic, cadmium, lead, and zinc in a multi-contaminated soil treated with different biochars. Environment International 156: 106638. [DOI] [PubMed] [Google Scholar]
- Fan D, Sun J, Liu C, et al. (2021) Measurement and modeling of hormesis in soil bacteria and fungi under single and combined treatments of Cd and Pb. Science of the Total Environment 783: 147494. [DOI] [PubMed] [Google Scholar]
- Fan J, Xu X, Ni Q, et al. (2018) Enhanced As (V) removal from aqueous solution by biochar prepared from iron-impregnated corn straw. Journal of Chemistry 2018: 1–8. [Google Scholar]
- Fu J, Zhang A, Wang T, et al. (2013) Influence of E-waste dismantling and its regulations: temporal trend, spatial distribution of heavy metals in rice grains, and its potential health risk. Environmental Science & Technology 47: 7437–7445. [DOI] [PubMed] [Google Scholar]
- Hong C, Dong Z, Zhang J, et al. (2022) Effectiveness and mechanism for the simultaneous adsorption of Pb(II), Cd (II) and As(III) by animal-derived biochar/ferrihydrite composite. Chemosphere 293: 133583. [DOI] [PubMed] [Google Scholar]
- Igalavithana AD, Kim K-H, Jung J-M, et al. (2019) Effect of biochars pyrolyzed in N2 and CO2, and feedstock on microbial community in metal(loid)s contaminated soils. Environment International 126: 791–801. [DOI] [PubMed] [Google Scholar]
- Ji Y, Zheng N, An Q, et al. (2022) The effect of carbonization temperature on the capacity and mechanisms of Cd(II)-Pb(II) mix-ions adsorption by wood ear mushroom sticks derived biochar. Ecotoxicology and Environmental Safety 239: 113646. [DOI] [PubMed] [Google Scholar]
- Jiang J, Xu R-K, Jiang T-Y, et al. (2012) Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. Journal of Hazardous Materials 229: 145–150. [DOI] [PubMed] [Google Scholar]
- Kumar A, Subrahmanyam G, Mondal R, et al. (2021) Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 268: 128855. [DOI] [PubMed] [Google Scholar]
- Li H, Dong X, da Silva EB, et al. (2017) Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 178: 466–478. [DOI] [PubMed] [Google Scholar]
- Lin LN, Qiu WW, Wang D, et al. (2017) Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: Characterization and mechanism. Ecotoxicology and Environmental Safety 144: 514–521. [DOI] [PubMed] [Google Scholar]
- Lu H, Zhang W, Yang Y, et al. (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Research 46: 854–862. [DOI] [PubMed] [Google Scholar]
- Lu Y, Song S, Wang R, et al. (2015) Impacts of soil and water pollution on food safety and health risks in China. Environment International 77: 5–15. [DOI] [PubMed] [Google Scholar]
- Luo X, Shen M, Huang Z, et al. (2020) Efficient removal of Pb(II) through recycled biochar-mineral composite from the coagulation sludge of swine wastewater. Environmental Research 190: 110014. [DOI] [PubMed] [Google Scholar]
- Mohubedu RP, Diagboya PNE, Abasi CY, et al. (2019) Magnetic valorization of biomass and biochar of a typical plant nuisance for toxic metals contaminated water treatment. Journal of Cleaner Production 209: 1016–1024. [Google Scholar]
- Mokwenye II, Diagboya PNE, Olu-owolabi BI, et al. (2016) Immobilization of toxic metal cations on goethite-amended soils: a remediation strategy. Journal of Applied Sciences and Environmental Management 20: 436–443. [Google Scholar]
- Moradi N, Karimi A. (2021) Fe-modified common reed biochar reduced cadmium (Cd) mobility and enhanced microbial activity in a contaminated calcareous soil. Journal of Soil Science and Plant Nutrition 21: 329–340. [Google Scholar]
- Ni B-J, Huang Q-S, Wang C, et al. (2019) Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 219: 351–357. [DOI] [PubMed] [Google Scholar]
- Okoli CP, Adewuyi GO, Zhang Q, et al. (2014) Mechanism of dialkyl phthalates removal from aqueous solution using γ-cyclodextrin and starch based polyurethane polymer adsorbents. Carbohydrate Polymers 114: 440–449. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Wu Y, Hao Z, et al. (2018) Combined impacts of land use and soil property changes on soil erosion in a mollisol area under long-term agricultural development. Science of the Total Environment 613: 798–809. [DOI] [PubMed] [Google Scholar]
- Park J-H, Ok YS, Kim S-H, et al. (2016) Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142: 77–83. [DOI] [PubMed] [Google Scholar]
- Penido ES, Martins GC, Matos Mendes TB, et al. (2019) Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotoxicology and Environmental Safety 172: 326–333. [DOI] [PubMed] [Google Scholar]
- Qu X, Alvarez PJJ, Li Q. (2013) Applications of nanotechnology in water and wastewater treatment. Water Research 47: 3931–3946. [DOI] [PubMed] [Google Scholar]
- Ramlan Basir-Cyio M, Napitupulu M, et al. (2022) Pollution and contamination level of Cu, Cd, and Hg heavy metals in soil and food crop. International Journal of Environmental Science and Technology 19: 1153–1164. [Google Scholar]
- Rauret G, Lopez-Sanchez JF, Sahuquillo A, et al. (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. Journal of Environmental Monitoring 1: 57–61. [DOI] [PubMed] [Google Scholar]
- Ren J, Wang C, Zhang F, et al. (2021) Adsorption of Cd~(2+) from aqueous solution by modified rice husk-derived biochars. Journal of Ecology and Rural Environment 37: 73–79. [Google Scholar]
- Ren X, Yang S, Tan X, et al. (2012) Mutual effects of copper and phosphate on their interaction with gamma-Al2O3: Combined batch macroscopic experiments with DFT calculations. Journal of Hazardous Materials 237: 199–208. [DOI] [PubMed] [Google Scholar]
- Rocco C, Seshadri B, Adamo P, et al. (2018) Impact of waste-derived organic and inorganic amendments on the mobility and bioavailability of arsenic and cadmium in alkaline and acid soils. Environmental Science and Pollution Research 25: 25896–25905. [DOI] [PubMed] [Google Scholar]
- Say R, Yilmaz N, Denizli A. (2003) Biosorption of cadmium, lead, mercury, and arsenic ions by the fungus Penicillium purpurogenum. Separation Science and Technology 38: 2039–2053. [Google Scholar]
- Timofeev I, Kosheleva N, Kasimov N. (2018) Contamination of soils by potentially toxic elements in the impact zone of tungsten molybdenum ore mine in the Baikal region: A survey and risk assessment. Science of the Total Environment 642: 63–76. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang H, Tang H, et al. (2021) Heavy metal pollution characteristics and health risk evaluation of soil around a tungsten-molybdenum mine in Luoyang, China. Environmental Earth Sciences 80: 293. [Google Scholar]
- Wang S, Gao B, Li Y, et al. (2017) Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests. Journal of Hazardous Materials 322: 172–181. [DOI] [PubMed] [Google Scholar]
- Wu J, Huang D, Liu X, et al. (2018) Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. Journal of Hazardous Materials 348: 10–19. [DOI] [PubMed] [Google Scholar]
- Wu J, Li Z, Huang D, et al. (2020) A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. Journal of Hazardous Materials 387: 122010. [DOI] [PubMed] [Google Scholar]
- Xu H, Hu X, Chen Y, et al. (2021) Cd(II) and Pb(II) absorbed on humic acid-iron-pillared bentonite: Kinetics, thermodynamics and mechanism of adsorption. Colloids and Surfaces A-Physicochemical and Engineering Aspects 612: 126005. [Google Scholar]
- Xu M, Dai W, Zhao Z, et al. (2022) Effect of rice straw biochar on three different levels of Cd-contaminated soils: Cd availability, soil properties, and microbial communities. Chemosphere 301: 134551. [DOI] [PubMed] [Google Scholar]
- Yang Q, Li Z, Lu X, et al. (2018) A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Science of the Total Environment 642: 690–700. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zhou H, Yan X-L, et al. (2021) The Fe3O4- modified biochar reduces arsenic availability in soil and arsenic accumulation in indica rice (Oryza sativa L.). Environmental Science and Pollution Research 28: 18050–18061. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yuan X, Xiong T, et al. (2020. a) Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. Chemical Engineering Journal 398: 125657. [Google Scholar]
- Zhang W, Tan X, Gu Y, et al. (2020. b) Rice waste biochars produced at different pyrolysis temperatures for arsenic and cadmium abatement and detoxification in sediment. Chemosphere 250: 126268. [DOI] [PubMed] [Google Scholar]
- Zhang YC, Fan JJ, Fu ML, et al. (2019) Adsorption antagonism and synergy of arsenate(V) and cadmium(II) onto Fe-modified rice straw biochars. Environmental Geochemistry and Health 41: 1755–1766. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Gao B, Zimmerman AR, et al. (2014) Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresource Technology 152: 538–542. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu Y-G, Liu S-B, et al. (2017) Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chemical Engineering Journal 314: 223–231. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-wmr-10.1177_0734242X241307521 for Simultaneous remediation of As(V), Cd(II) and Pb(II) in aqueous solution and soil using Fe/Ca-modified biochar by Yan Zhou, Wenhui Liu, Dandan Chen, Bangwei Liu, Ping Lu and Yiwei Zhang in Waste Management & Research





