Abstract
Objective
To develop a quality consistency evaluation strategy for traditional Chinese medicine (TCM) granules using sugar free Yangwei Granules as a model drug, and demonstrate the effectiveness of the developed method.
Methods
The strategy integrates several methods including, HPLC fingerprint and physical fingerprint methods analyze the similarity in chemical and physical properties of the TCM granule samples. Near-infrared (NIR) spectroscopy with principal components cluster analysis method is used to monitor normal operating conditions (NOC) samples accurately and to identify different types of abnormal operating conditions (AOC) samples, particularly those that deviate from the normal range.
Results
The combined use of HPLC fingerprint and physical fingerprint provides insights into the chemical and physical properties of the samples. NIR spectroscopy, combined with principal components cluster analysis, achieves high accuracy in monitoring NOC samples and identifying AOC samples without misjudgment. The approach proves useful as a complementary method in cases where HPLC fingerprint and physical fingerprint alone lack sufficient resolution.
Conclusion
This study establishes the feasibility and utility of the integrated approach for assessing the quality consistency of TCM granules. The strategy shows a high degree of generalization and holds significant importance for enhancing the quality control processes of TCM granules.
Keywords: chemometrics, multivariate analysis, near-infrared spectroscopy, quality consistency evaluation strategy, sugar-free Yangwei Granules, traditional Chinese medicine granules
1. Introduction
Traditional Chinese medicine (TCM) is playing an increasingly important role in disease prevention and treatment around the world (Li et al., 2020, Li et al., 2020, Wang et al., 2021). TCM preparations are complex systems compared to chemical medicines (Li, Yang, Pang & Sun, 2021). Granules, being a significant form of medicinal preparation of TCM, have advantages such as easy consumption, portability, storage, and transportation. Conducting studies on evaluating and controlling the quality of TCM granules can contribute to enhancing their overall quality. Besides their complex and multi-component chemical composition, granules also have important physical properties such as homogeneity, stackability and flowability. To evaluate and control the quality of TCM granules, it is necessary to consider not only their chemical properties but also their physical indicators. Quality consistency is a fundamental attribute of medication and a prerequisite for consistent efficacy (Li, Zhang & Han, 2022). Therefore, the development of a scientific and standardized method for evaluating and controlling the quality of TCM granules is essential for the future advancement of TCM.
TCM granules are commonly produced by fluid-bed granulation (FBG) in pharmaceutical factories. FBG is a wet granulation technique that combines mixing, granulation, and drying in one unit, and is known for its excellent heat and mass transfer, making it more efficient than traditional granulation methods (Tian et al., 2020, Zhao et al., 2019). However, FBG is a complicated process that involves nucleation, coalescence, breakage and layering rate processes, and it can be the challenge for its application due to various technique difficulties. If the FBG process is not properly monitored and controlled, it may result in products with poor quality consistency (Tian et al., 2018, Zhao et al., 2020). Therefore, it is crucial to analyze the changes in granules quality caused by operating conditions and material properties. Developing an evaluation method or procedure to analyze and quantify the differences in granule quality consistency between batches is necessary in the development and production of granulation processes.
At present, fingerprint analysis is a common method of quality consistency evaluation for TCM in the pharmaceutical industry, and it is included in Center for Drug Evaluation as a quality control standard for TCM (Yan et al., 2019, Yang et al., 2016, Zhong et al., 2022). However, the HPLC fingerprints of drugs mainly provide information on their chemical property, such as chemical composition, relative content, and material basis (Wei et al., 2020). Many improved methods for quality consistency evaluation were proposed (Li et al., 2021, Liang et al., 2019, Wang et al., 2020). For example, combining pharmacokinetics with metabolomics can offer a comprehensive perspective on the consistency evaluation of Chaihuang Granules (Zhang et al., 2021). Integrating biological activity determination with spectroscopy can be used to quickly detect the quality consistency of Glycyrrhiza formula granules (Zhou et al., 2023). Additionally, biological characteristics integrates chemical can evaluate the quality consistency between dispensing granule and traditional decoction from Coptidis Rhizoma (Zhang, Li, Li, Jiang & Li, 2020). However, implementing these methods can be time-consuming and labor-intensive, and it proves to be difficult to implement them in products that undergo industrial manufacturing.
Considering the complexity of TCM granule preparation, the comprehensive quality consistency evaluation method should be rapid, eco-friendly, and able to incorporate maximum information from the samples. Near-infrared (NIR) spectroscopy has shown to be effective in terms of economy, practicality, and operability. It can provide both physical and chemical information in a rapid, noninvasive, and environmentally friendly way, making it a promising method for quality consistency evaluation of TCM granule. Therefore, the utilization of NIR spectroscopy technologies, combined with new chemometrics, can be employed to evaluate quality consistency for samples (Liu et al., 2018).
Sugar-free Yangwei Granules are a typical TCM granules preparation used to treat chronic gastritis and chronic atrophic gastritis (Wang et al., 2011, Teng et al., 2022). It composes of eight Chinese herbs: namely Astragali Radix (Huangqi in Chinese), Codonopsis Radix (Dangshen in Chinese), Citri Reticulatae Pericarpium (Chenpi in Chinese), Cyperi Rhizoma (Xiangfu in Chinese), Paeoniae Radix Alba (Baishao in Chinese), Dioscoreae Rhizoma (Shanyao in Chinese), Mume Fructus (Wumei in Chinese), and Nardostachyos Radix et Rhizoma (Gancao in Chinese). This preparation has been listed in the Chinese Pharmacopoeia (2020, Vol 1) and has been used in clinics for many years (Zhao, Tian, Qiu & Qu, 2021). The main effective pharmaceutical ingredients contained in Yangwei Granules include albiflorin, paeoniflorin and benzoylpaeoniflorin. Research has shown that paeoniflorin is a potential therapeutic agent for treating ulcerative colitis (Zhou, Gong, Zhang & Peng, 2020). There is a literature report on the pharmacokinetics and tissue distribution of albiflorin as the main active ingredient in normal and chronic gastritis rats (Liu, Nong, Qu & Li, 2023). While benzoylpaeoniflorin itself has the ability to effectively alleviate various types of pain, and the recent research has shown that benzoylpaeoniflorin mainly contribute to the anti-anaphylactic activity of Paeonia lactiflora Pall., and P. lactiflora plays an important role in preventing intestinal diseases (Zhong et al., 2021). This study aimed to develop a quality consistency evaluation method for TCM granules with high generalization ability, using sugar-free Yangwei Granules produced by FBG as an example. To ensure the models' identification ability, samples produced by different production staff were collected for the calibration set, in order to incorporate sufficient variation (Xie et al., 2008), and improve the model's identification ability.
In this study, HPLC fingerprint, physical fingerprint, and NIR spectroscopy coupled with chemometric techniques were used as a quality consistency evaluation methods to evaluate the quality consistency of sugar-free Yangwei Granules. A quality evaluation strategy for TCM granules was developed. In conclusion, the work demonstrates how to establish a strategy with high generalization ability for evaluating the quality consistency of the commercialized sugar-free Yangwei Granules, so as to improve the quality of TCM granules.
2. Materials and methods
2.1. Materials
The final products and the intermediates of sugar-free Yangwei Granules (Batch number of samples were in the supplementary file) were obtained from Chiatai Qingchunbao Pharmaceutical Co., Ltd. (Hangzhou, China), and produced by the commercial-scale FBG (Chongqing Eagle Pharmaceutical Machinery Co., Ltd., Chongqing, China). The standard substances of albiflorin (> 98%, Lot. 180516), paeoniflorin (> 98%, Lot. 180420) and benzoylpaenoniflorin (> 98%, Lot. 180531), were purchased from Shanghai RongHe Pharmaceutical Science and Technology Development Co., Ltd. (Shanghai, China).
2.2. Sample preparation
In the manufacturing process of sugar-free Yangwei Granules, multiple FBGs were operated simultaneously to produce the intermediates. Each machine was operated by a designated individual. When enough intermediates are produced by these FBGs for a single batch, they were mixed together and sieved to create a final batch of products with a commercial batch number. To ensure that the collected samples contain sufficient variation, intermediates produced by five different individuals (labeled as No. A–E) were gathered. Specifically, six intermediates produced by individual A were collected and labeled A-1 to A-6. Similarly, intermediates produced by individuals B, C, D, and E were also collected and labeled as B-1 to B-5, C-1 to C-5, D-1 to D-4, and E-1 to E-5, respectively. At the same time, a total of 152 final product batches, each with a commercial batch number, were collected for modeling. Additionally, 66 batches were selected as the validation set to assess the models’ performance. In the validation set, batches 153–162 were produced under normal operating conditions (NOC), and samples from batches 163–219 were collected under abnormal operating conditions (AOC). The details of the sample information are presented in Table 1. Samples 163–172, 173–182, and 183–192 were affected by dampness, having been exposed to the laboratory environment for 1 d, 3 d, and 7 d, respectively. Samples 193–197, 198–202, and 203–207 were expired products, exceeding their expiration dates by 0.5, 1, and 1.5 years in relation to the date of the experiment, respectively. Samples 208–219 were abandoned because their particle size distribution (PSD) did not meet the required qualifications. Considering that the samples were in commercial production, we collected prepared samples without displaying the specific operating parameters as commercial contract.
Table 1.
Details of sample information.
| Data set | Classification | Sample number | Sample information |
|---|---|---|---|
| Calibration set | NOC samples | 1–152 | − |
| Validation set | NOC samples | 153–162 | − |
| Damped samples | 163–172 | Exposed 1 d | |
| 173–182 | Exposed 3 d | ||
| 183–192 | Exposed 7 d | ||
| Expired samples | 193–197 | Expired 0.5 year | |
| 198–202 | Expired 1 year | ||
| 203–207 | Expired 1.5 year | ||
| Abandoned samples | 208–219 | PSD is unqualified |
2.3. Methods for quality consistency evaluation
2.3.1. HPLC fingerprint
A pre-development HPLC method (Wang, Shao, Qu, Cheng, & Wu, 2006) was used to construct the HPLC fingerprint for sugar-free Yangwei Granules. The analysis of active pharmaceutical ingredients (API) content was carried out using an Agilent 1100 HPLC system (Agilent Technologies, USA). The system was equipped with a quaternary pump, an online vacuum degasser, an autosampler, a thermostated column compartment, and an ultraviolet detector. Prior to the test, the methodology validation results showed that the specificity, instrument precision, intra-day precision, inter-day precision, and accuracy of each API were acceptable (Zhao, Tian, Qiu & Qu, 2021). The spectra were then compared with the “Similarity Evaluation System for Chromatographic Fingerprint of TCM” software (2012 A edition).
2.3.2. Physical fingerprint
The physical fingerprint can comprehensively characterize the granule properties, which is a useful tool for evaluating the quality consistency of samples through the similarity evaluation method (Luo et al., 2017). In this study, we comprehensively characterized the physical properties of stackability, compressibility, flowability, stability, and homogeneity, including bulk density (ρb), tap density (ρt), porosity (ε), Carr's index (IC), Hausner ratio (HR), angle of repose (α), moisture content (MC), d10, d50, d90, span, and relative size distribution width (RW). The measurement methods for physical properties and the construction of physical fingerprints can be found in our previous work (Zhao, Qu, Tian & Wei, 2020).
2.3.3. Principal components cluster analysis of NIR spectra
The NIR spectra were measured by an FT-NIR spectrometer (Thermo Fisher Scientific Inc., Massachusetts, USA) equipped with the integrating sphere module. A background spectrum was obtained before each measurement, and the spectrum for each sample was obtained by averaging 32 scans within the wavenumber of 10 000 and 4 000 cm−1, with a resolution was 8.0 cm−1.
Principal components cluster analysis was used to assess the quality consistency of samples from different individuals. The principal component analysis (PCA) method extracted a set of latent variables from the spectral data to reduce the multivariate data dimension, eliminating the overlapping information, and then constructed a new set of independent comprehensive latent variables to reflect the information contained in the original variables as much as possible. The model was established and analyzed by SIMCA-P version 13.0 software (Umetrics, Sweden).
2.4. Quality consistency evaluation for sugar-free Yangwei granules
2.4.1. Data preparation and preprocessing
To reduce the influence of outliers on the model, outlier samples were discriminated by the Mahalanobis distance based on Chauvenet rule (Ping et al., 2022). According to the Chauvenet rule, the probability of random error of a single sample will not be higher than 1/2n when the sample is measured many times (n is the number of samples). When the sample deviation, i.e., the difference between the measured value and its arithmetic mean value, exceeds this range, it means that the measured value is suspicious and should be discarded. Besides the outlier samples distinguished by the Mahalanobis distance based on Chauvenet rule, there are certain outlier samples that are caused by the comprehensive fluctuation of multiple variables. These can be detected in the score plot or Hotelling's T2 control chart of the sample set, and can be directly removed before establishing the model.
2.4.2. Quality consistency evaluation strategy
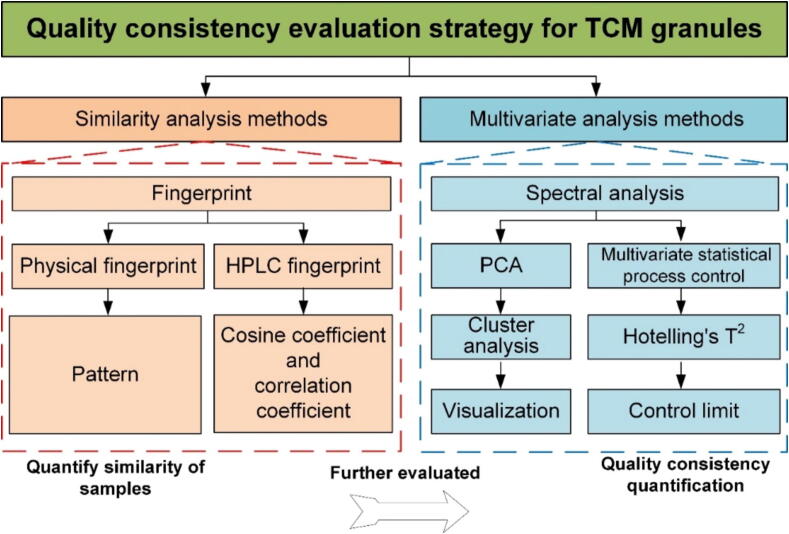
A strategy for evaluating the quality consistency of TCM granules has been proposed in Fig. 1. The strategy integrates current qualitative and quantitative analysis methods. First, physical fingerprint and HPLC fingerprint similarity evaluations are used to assess the differences in physical and chemical properties of the samples. Second, the quality consistency of the samples is evaluated by conducting a principal components cluster analysis of their NIR spectra. Finally, the degree and cause of quantitative differences are further analyzed by multivariate analysis. The combination of qualitative and quantitative methods constitutes the quality consistency evaluation method applicable to TCM granules. In this strategy, the PCA model was established based on the NIR spectra, which treated with outlier sample discrimination. The number of PCs was determined by cross-validation method. The 95% Hotelling's T2 statistic was used as the control limit to evaluate the quality consistency between different batches of the final production of sugar-free Yangwei Granules.
Fig. 1.
Quality consistency evaluation strategy for TCM granules.
3. Results and discussion
3.1. Quality consistency evaluation method development
3.1.1. Construction of HPLC fingerprint for sugar free Yangwei granules
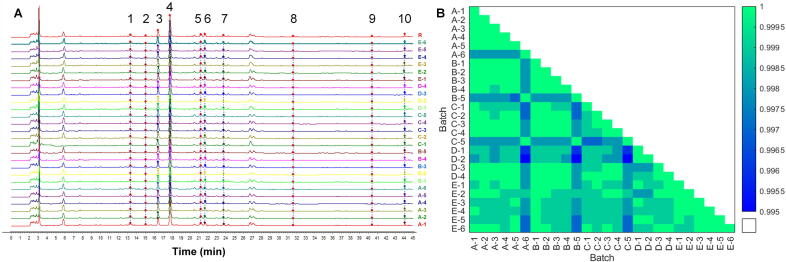
The HPLC fingerprints of 26 samples were established to analyze the similarities between intermediates produced by different individuals. Fig. 2A showed the raw HPLC fingerprints at 230 nm. Ten common peaks were detected, with peak 3 identified as albiflorin, peak 4 identified as paeoniflorin, and peak 9 identified as benzoylpaenoniflorin. Sample A-1 was chosen as the reference to calculate the similarities, and the values were shown by heatmap in Fig. 2B. The 26 samples exhibited high similarity with a minimum value of 0.995. The results indicate that the samples are identical in both composition and content of chemical components, suggesting that the quality of these samples produced by different individuals with high quality consistency.
Fig. 2.
HPLC fingerprint (A) and cosine coefficients (B) of 26 samples.
3.1.2. Construction physical fingerprint for sugar free Yangwei granules
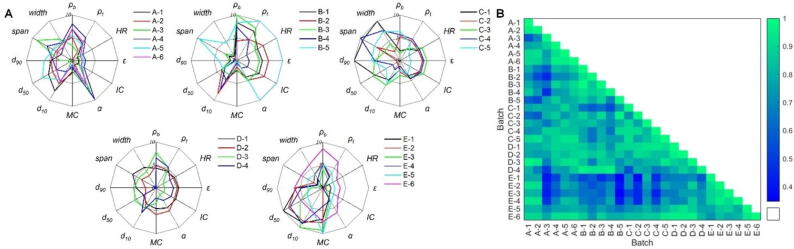
The physical properties of 26 samples have been characterized and normalized using a linear normalization algorithm. Based on the results of the powder properties, a physical fingerprint was constructed (Fig. 3A). The fingerprint showed distinct differences between samples obtained from different individuals. The similarity among the samples was quantified using cosine coefficients, and the corresponding heatmap was presented in Fig. 3B. The results indicated that the cosine coefficients were greater than 0.5 when comparing the intermediates produced by individuals A, B, C, and D, except for samples A-3 and B-4. This suggested that the physical properties of the intermediates produced by A, B, C, and D were highly similar. It was observed that the main difference in physical properties between samples A-3 and B-4 was due to the particle size distribution. Among the samples from A, B, and C, there were significant differences compared to the samples from E. The cosine coefficients of A-3, B-3, B-5, C-2, and C-4 were less than 0.5 when compared with E. On the other hand, all the samples from D and E exhibited high similarity as the cosine coefficients exceed 0.5. The results have demonstrated that physical fingerprints can distinguish the samples that are significantly different from others. However, the classification effect is not significant.
Fig. 3.
Physical fingerprint (A) and cosine coefficients (B) of 26 samples.
3.1.3. Principal components cluster analysis of NIR spectra for sugar free Yangwei granules
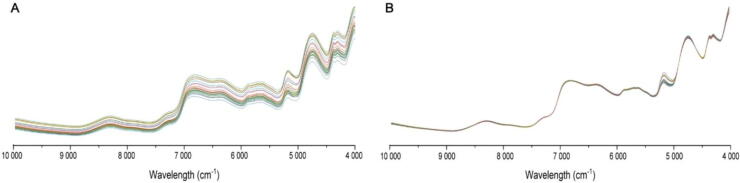
The PCA analysis was conducted by performing a singular value decomposition of the data array. It intuitively provides insights into the differences among granules and helps with making a pre-judgment to evaluate the quality consistency of granules. Prior to modeling, the spectra data was preprocessed using a Savitzky-Golay (SG) filter (Fransson & Folestad, 2006) with a 15-point window and multiplicative scatter correction (MSC) method (Liu et al., 2017). This preprocessing helped reduce the inclusion of invalid information, enhance the effective information, and improve the accuracy of the model. The original NIR spectra and the preprocessed NIR spectra can be seen in Fig. 4.
Fig. 4.
Original (A) and preprocessed (B) NIR spectra.
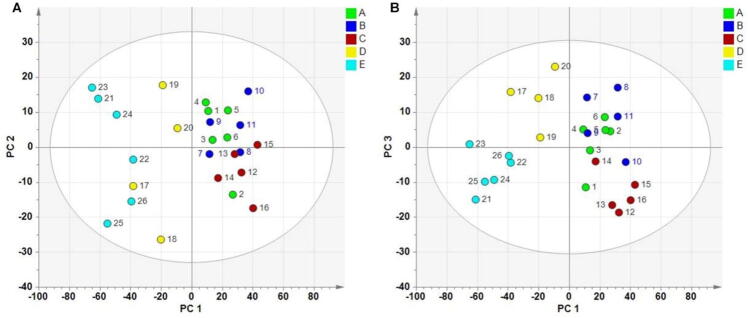
A PCA model containing three PCs was established, and the PCs accounted for a total of 96.5% of the data variance (PC1: 78.1%; PC2: 9.9%; and PC3: 8.5%). The score plot was presented in Fig. 5. The PCA results provided a visual determination of the similarity among the samples. It can be observed that all the PC scores were within the 95% Hotelling's T2 control limit. The results indicated differences among the batches produced by different individuals. The whole samples from A, B, C clustered together, suggesting their similarity. The resulting PC1-PC2 and PC1-PC3 score plot made a clear distinction between the E and the other samples. Samples from D and E exhibited noticeable separation from the other samples, indicating significant differences between them.
Fig. 5.
Score plot of 26 samples for sugar free Yangwei Granules form A–E. A, PC1-PC2; B, PC1-PC3.
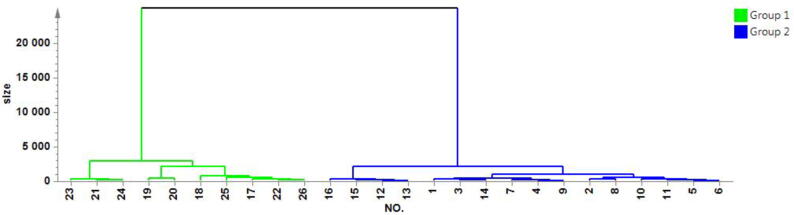
Based on the PCA results of the NIR spectrum, hierarchical cluster analysis (HCA) was performed to classify the samples into two groups. Group 1 included samples 23, 21, 24, 19, 20, 18, 25, 17, 22, and 26, while Group 2 consisted of samples 16, 15, 12, 13, 1, 3, 14, 7, 4, 9, 2, 8, 10, 11, 5, and 6. The samples from individuals A, B, and C were classified in Group 1 due to their similar properties, whereas individuals D and E were classified in Group 2 for their similar properties. The dendrograms in Fig. 6 depict the classification results.
Fig. 6.
Classification results of 26 batches of Sugar-free Yangwei Granules by principal components cluster analysis.
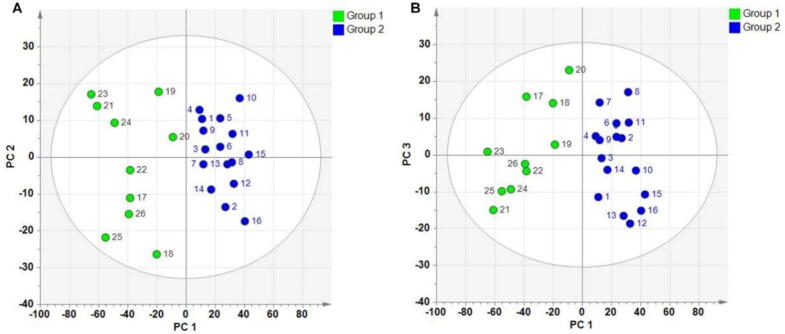
The PCA method applied to the NIR spectrum is ineffective in distinguishing between the differences in samples (Fig. 7). However, when performing principal components cluster analysis on the NIR spectra, the classification effect of samples with minor differences in quality is remarkably accurate. These findings are consistent with the outcome of the physical fingerprint examination.
Fig. 7.
Classification results of PCA cluster analysis. A, PC1-PC2; B, PC1-PC3.
3.1.4. Quality consistency evaluation strategy for TCM granules
Among the different methods used to evaluate the quality consistency of sugar-free Yangwei granules produced by FBG, the results of the HPLC fingerprint similarity analysis showed that different samples made by different individuals of Yangwei Granules exhibited high similarity in terms of chemical characteristics, with minimal changes in overall component content. Thus, it is challenging to distinguish differences other than chemical property by HPLC fingerprint. Physical quality attributes are crucial for TCM granule quality and cannot be ignored. Physical fingerprint can identify samples that significantly different from others, but its classification effect is not significant. Using NIR spectroscopy in combination with multivariate data analysis can effectively distinguish between different samples, providing satisfactory results for classification. The proposed strategy provides a comprehensive evaluation method of the quality consistency of sugar-free Yangwei Granules, and can serve as a useful tool for evaluating quality consistency in TCM granules.
3.2. Quality consistency evaluation for sugar-free Yangwei granules
3.2.1. Overview of primitive data
The raw NIR spectra and preprocessed data for the 219 collected samples, as shown in Table 1, were depicted in Fig. 8.
Fig. 8.
Raw (A) and preprocessed (B) NIR spectra data for 219 collected samples.
3.2.2. Outlier sample discriminating
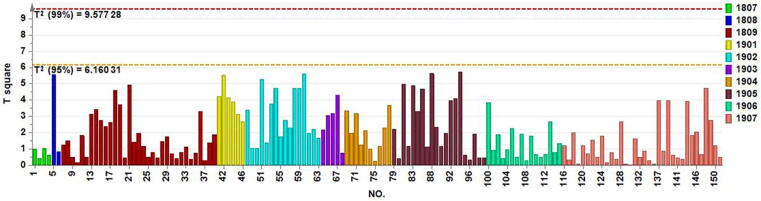
The Mahalanobis distance was calculated for the original NIR spectra, and the values were sorted. According to the Chauvenet rule, sample 84 was identified as an outlier. After removing the outlier sample (sample 84), PCA modeling was performed on the NIR spectra of 151 batches. The NIR spectra were preprocessed using SG filter (15 points) combined with the MSC method. Through cross-validation, two PCs were selected, which accounted for 83.8% (PC1: 62.0%, PC2: 21.8%) of the variance in the spectral variables. Fig. 9A showed the score chart of the two PCs. The ellipse displayed in Fig. 9B represents the 95% Hotelling's T2 control limit. The sample points were color-coded based on their manufacturing dates (Fig. 9, Fig. 10), specifically, the 4-digit number on the label represented the production year with the first two digits, and the production month with the last two digits. It can be seen that samples 41, 47, 136, 144, and 152 had fallen outside the 95% Hotelling's T2 control limit and were considered as outlier samples. This could be due to the comprehensive fluctuations of multiple variables.
Fig. 9.
PCA score of 151 batches after eliminating abnormal samples by Mahalanobis distance method based on Chauvenet rule (A), and score chart for 146 samples after outlier samples removed (B). The sample points were color-coded based on their manufacturing dates.
Fig. 10.
Hotelling’s T2 control chart of 146 samples. The sample points were color-coded based on their manufacturing dates.
Overall, six outlier samples have been identified. Sample 84 was identified by the Mahalanobis distance method based on Chauvenet rule, and samples 41, 47, 136, 144, and 152 were identified by the PCA model.
3.2.3. Quality consistency evaluation model construction
PCA modeling was carried out for 146 samples of their NIR spectra after removing outlier samples in section “3.2.2”. The NIR spectra were preprocessed using an SG filter (15 points) and MSC method. Two PCs were selected through cross validation, accounting for 82.6% (PC1: 62. 4%; PC2: 22.2%) of the variance of variables. Hotelling's T2 statistical value and its 95% control limit were calculated as the quality consistency evaluation index for sugar free Yangwei Granules (Fig. 10).
3.2.4. Quality consistency evaluation for sugar free Yangwei granules
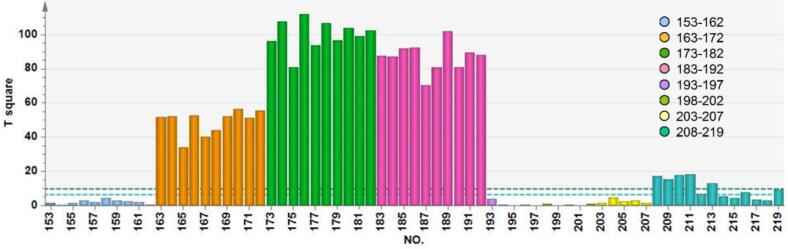
The quality consistency evaluation model, which was constructed in section 3.2.3, was used to assess the quality consistency of the commercialized sugar-free Yangwei Granules. The validation set comprised NOC samples 153–162, damped samples 163–192, expired products 193–207, and unqualified samples 208–219, as illustrated in Table 1. These samples were processed in the same manner as the calibration set, and then mapped onto the above PCA model. The score chart of the validation set was shown in Fig. 11, and the Hotelling's T2 control chart was shown in Fig. 12.
Fig. 11.
Score chart of validation set.
Fig. 12.
Hotelling's T2 control chart of validation set.
The scores of the test samples 153–162 of sugar-free Yangwei Granules fell within the ellipses on the score chart, as well as within the control limit on the Hotelling's T2 control chart. Samples 153–162 were qualified products that met the quality requirement, they showed quality consistency with the modeling samples, and exhibited negligible differences in quality among themselves. Furthermore, the results indicated that the model developed for quality consistency evaluation could effectively evaluate the newly produced NOC samples (Fig. 11, Fig. 12).
Sample 163–172, 173–182, and 183–192 were damped samples that were exposed to laboratory environment for 1 d, 3 d, and 7 d, respectively, in order to achieve abnormal samples. It can be observed that samples with the same expiration days were scattered in approximately the same region and deviated significantly from the normal range in both the score control chart and Hotelling's T2 control chart. Sugar-free Yangwei Granules, as a TCM granule preparation, have strong hygroscopicity. When exposed to an environment with a relative humidity that exceeds its critical relative humidity, it rapidly absorbs moisture and negatively affects the product quality. The applied quality consistency evaluation model showed deviations from the normal range for the monitored samples.
Samples 193–197, 198–202, and 203–207 were expired products, with expiration dates of 0.5 year, 1 year, and 1.5 year from the experimental date, respectively. However, the model was unable to identify these NOC samples. The expired samples fell within the normal range in both the score control chart and the Hotelling's T2 control chart. These samples had been stored appropriate in a drying cabinet and were packaged in the polyester/aluminum/polyethylene composite film for drug packaging. In accordance with the distinct properties of TCM, it is not always the case that the quality of certain drugs deteriorates after their expiration dates. Therefore, although the established quality consistency evaluation models may have overlooked the abnormality of expired samples, this result can still be deemed reasonable. Nonetheless, further research is warranted to fully comprehend the effects of expired TCM drugs and their potential deviation from conventional medicines.
Samples 208–219 are not qualified due to their PSD not meeting the requirement. These samples can be used to evaluate the adaptability of the established model. Most samples fall outside the Hotelling's T2 control limit, with only a small number within the limit. This indicates that the established models can identify abnormal samples that deviate significantly from the expected quality. Furthermore, it demonstrates good generalization ability of the established models.
4. Conclusion
A proposed quality consistency evaluation strategy for TCM granules has been proven to be feasible. In this study, three different models were used to evaluate the quality consistency of sugar-free Yangwei Granules. These models included HPLC fingerprint, physical fingerprint, and NIR spectroscopy coupled with multivariate data analysis. Through the HPLC fingerprint and physical fingerprint, both the chemical and physical properties of the samples were evaluated for consistency. Additionally, NIR combined with multivariate analysis proved to be an effective method for evaluating quality consistency. This approach helps upplement the issue of insufficient resolution in HPLC and physical fingerprint for products with similar properties. The results demonstrate that the established multivariate data analysis methods can accurately monitor NOC samples without any misjudgment. The approach can identify different AOC types of samples, particularly those that deviate from the normal range, such as damped samples or PSD unqualified samples. However, the methods were not sensitive to expired samples, which may be attributed to the unique properties of TCM. The proposed strategy comprehensively evaluates the quality consistency of commercialized sugar-free Yangwei Granules produced by FBG. It could be employed in the future to improve the quality while saving both material and time. This strategy is significant for the quality control of TCM granules and can serve as a certain reference for the industry.
CRediT authorship contribution statement
Jie Zhao: Visualization, Writing – original draft. Geng Tian: Writing – review & editing. Haibin Qu: Supervision, Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2023YFC3504501), the China Postdoctoral Science Foundation (No. 2024M752839), the National Natural Science Foundation of China (No. 82404868), and Postdoctoral Fellowship Program of CPSF (No. GZC20232358).
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.chmed.2024.11.001.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
References
- Fransson M., Folestad S. Real-time alignment of batch process data using COW for on-line process monitoring. Chemometrics & Intelligent Laboratory Systems. 2006;84(1):56–61. [Google Scholar]
- Li L.F., Zhang Q.W., Han Q.B. Recent advances in qualitative and quantitative analysis of polysaccharides in natural medicines: A critical review. Journal of Pharmaceutical and Biomedical Analysis. 2022;220 doi: 10.1016/j.jpba.2022.115016. [DOI] [PubMed] [Google Scholar]
- Li X., Yang H., Pang X., Sun G. Entirely control the quality consistency of Rong'e Yishen oral liquid by both quantified profiling and quantitative analysis of multi-components by single marker method. Journal of Pharmaceutical Biomedical Analysis. 2021;193 doi: 10.1016/j.jpba.2020.113719. [DOI] [PubMed] [Google Scholar]
- Li Y.X., Li J., Zhong D.L., Zhang Y., Zhang Y.G., Guo Y., et al. Clinical practice guidelines and experts’ consensuses of traditional Chinese herbal medicine for novel coronavirus (Covid-19): Protocol of a systematic review. Systematic Reviews. 2020;9(1):170. doi: 10.1186/s13643-020-01432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.X., Liu X.B., Guo L.X., Li J., Jin R.J. Traditional Chinese herbal medicine for treating novel coronavirus (COVID-19) pneumonia: Protocol for a systematic review and meta-analysis. Systematic Reviews. 2020;9(1):75. doi: 10.1186/s13643-020-01343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D.Y., Miao Y.H., Zheng L.Y., Gao X., Chen W., et al. Integrating chemical similarity and bioequivalence: a pilot study on quality consistency evaluation of dispensing granule and traditional decoction of Scutellariae Radix by a totality-of-the-evidence approach. Journal of Pharmaceutical and Biomedical Analysis. 2019;169:1–10. doi: 10.1016/j.jpba.2019.02.030. [DOI] [PubMed] [Google Scholar]
- Liu F., Nong X.J., Qu W.H., Li X.B. Pharmacokinetics and tissue distribution of 12 major active components in normal and chronic gastritis rats after oral administration of Weikangling capsules. Journal of Ethnopharmacology. 2023;316 doi: 10.1016/j.jep.2023.116722. [DOI] [PubMed] [Google Scholar]
- Liu R.H., Sun Q.F., Hu T., Li L., Nie L., Wang J.Y., et al. Multi-parameters monitoring during traditional Chinese medicine concentration process with near infrared spectroscopy and chemometrics. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2018;192:75–81. doi: 10.1016/j.saa.2017.10.068. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., André S., Cristau L., Lagresle S., Hannas Z., Calvosa É., et al. Multivariate statistical process control (MSPC) using Raman spectroscopy for in-line culture cell monitoring considering time-varying batches synchronized with correlation optimized warping (COW) Analytica Chimica Acta. 2017;952:9–17. doi: 10.1016/j.aca.2016.11.064. [DOI] [PubMed] [Google Scholar]
- Luo G., Xu B., Zhang Y., Cui X.L., Li J.Y., Shi X.Y., et al. Scale-up of a high shear wet granulation process using a nucleation regime map approach. Particuology. 2017;31:87–94. [Google Scholar]
- Ping X., Yang F.B., Zhang H.G., Xing C.D., Yao B.F., Wang Y. An outlier removal and feature dimensionality reduction framework with unsupervised learning and information theory intervention for organic Rankine cycle (ORC) Energy. 2022;254 [Google Scholar]
- Teng K.X., Fu H., Wang Z.C., Shen Y.Q., Xie X.Y., Zhao J., et al. Quality consistency evaluation method for Yangwei Granule extracts based on physical fingerprint. Chinese Traditional and Herbal Drugs. 2022;53(3):712–719. [Google Scholar]
- Tian G., Wei Y.D., Zhao J., Li W.L., Qu H.B. Application of near-infrared spectroscopy combined with design of experiments for process development of the pulsed spray fluid bed granulation process. Powder Technology. 2018;339:521–533. [Google Scholar]
- Tian G., Wei Y.D., Zhao J., Li W.L., Qu H.B. Application of pulsed spray and moisture content control strategies on quality consistency control in fluidized bed granulation: A comparative study. Powder Technology. 2020;363:232–244. [Google Scholar]
- Wang H., Chen M.L., Li J., Chen N., Chang Y.X., Dou Z.Y., et al. Quality consistency evaluation of Kudiezi Injection based on multivariate statistical analysis of the multidimensional chromatographic fingerprint. Journal of Pharmaceutical and Biomedical Analysis. 2020;177 doi: 10.1016/j.jpba.2019.112868. [DOI] [PubMed] [Google Scholar]
- Wang J., Shao Q., Qu H.B., Cheng Y.Y., Wu X.Z. HPLC determination of four active components in Sugar-free YanWei Granules. Chinese Journal of Pharmaceutical Analysis. 2006;26(12):1804–1806. [Google Scholar]
- Wang S.F., Fang H.Y., Qu H.B. Optimization of micellar electrokinetic capillary chromatography method using central composite design for the analysis of components in Yangwei granule. Journal of Zhejiang University-Science B. 2011;12(3):193–200. doi: 10.1631/jzus.B1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lu C., Li H., Qi W.S., Ruan L.G., Bian Y.J., et al. Effcacy and safety assessment of severe COVID-19 patients with Chinese medicine: A retrospective case series study at early stage of the COVID-19 epidemic in Wuhan, China. Journal of Ethnopharmacology. 2021;277 doi: 10.1016/j.jep.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X.C., Cao B., Luo C.H., Huang H.Z., Tan P., Xu X.R., et al. Recent advances of novel technologies for quality consistency assessment of natural herbal medicines and preparations. Chinese Medicine. 2020;15(1):56. doi: 10.1186/s13020-020-00335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B.G., Gong T., Tang M.H., Mi D.F., Zhang X., Liu J., et al. An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Liuwei Dihuang Pills produced by different manufacturers. Journal of Pharmaceutical and Biomedical Analysis. 2008;48(4):1261–1266. doi: 10.1016/j.jpba.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Yan H., Sun G.X., Chi H.X., Zhang J., Sun W.Y., Hou Z.F., et al. Quality consistency evaluation of Fufanggancao tablets based on the control mode of standard preparation and quantitative fingerprint. Chinese Journal of Chromatography. 2019;37(11):1200–1208. doi: 10.3724/SP.J.1123.2019.06010. [DOI] [PubMed] [Google Scholar]
- Yang L., Yang L.P., Sun G.X., Guo Y., Hou Z.F., Chen S. Holistic evaluation of quality consistency of Ixeris sonchifolia (Bunge) Hance Injectables by quantitative fingerprinting in combination with antioxidant activity and chemometric methods. PLos One. 2016;11(2) doi: 10.1371/journal.pone.0148878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.Y., Li X.X., Li P., Jiang Y., Li H.J. Consistency evaluation between dispensing granule and traditional decoction from Coptidis Rhizoma by using an integrated quality-based strategy. Phytochemical Analysis. 2021;32(2):153–164. doi: 10.1002/pca.2905. [DOI] [PubMed] [Google Scholar]
- Zhang Q.Q., Cao C.R., Guo Y.D., Hu Y.C., Lv J., Zhu C.Y., et al. Consistency evaluation of Chaihuang granules based on pharmacokinetics and metabolomics. Journal of Pharmaceutical and Biomedical Analysis. 2021;202 doi: 10.1016/j.jpba.2021.114170. [DOI] [PubMed] [Google Scholar]
- Zhao J., Li W.L., Qu H.B., Tian G., Wei Y.D. Application of definitive screening design to quantify the effects of process parameters on key granule characteristics and optimize operating parameters in pulsed-spray fluid-bed granulation. Particuology. 2019;43:56–65. [Google Scholar]
- Zhao J., Li W.L., Qu H.B., Tian G., Wei Y.D. Real-time monitoring and fault detection of pulsed-spray fluid-bed granulation using near-infrared spectroscopy and multivariate process trajectories. Particuology. 2020;53:112–123. [Google Scholar]
- Zhao J., Qu H.B., Tian G., Wei Y.D. Quality consistency evaluation method for granules in fluidized bed granulation based on powder properties. Journal of Zhejiang University (Engineering Science) 2020;54(2):374–380. [Google Scholar]
- Zhao J., Tian G., Qiu Y.Y., Qu H.B. Rapid quantification of active pharmaceutical ingredient for sugar-free Yangwei granules in commercial production using FT-NIR spectroscopy based on machine learning techniques. Spectrochimica Acta Part A-Molecular and Biomolecular Spectroscopy. 2021;245 doi: 10.1016/j.saa.2020.118878. [DOI] [PubMed] [Google Scholar]
- Zhong W.B., Pang Y., Lan L.L., Zhang X.T., Li X.F., Li Q., et al. Evaluating quality consistency of cigarette by 3 kinds of quantum fingerprints. Spectrochim Acta A: Molecular and Biomolecular Spectroscopy. 2022;282 doi: 10.1016/j.saa.2022.121678. [DOI] [PubMed] [Google Scholar]
- Zhong W.C., Li E.C., Hao R.R., Zhang J.F., Jin H.T., Lin S. Anti-anaphylactic potential of benzoylpaeoniflorin through inhibiting HDC and MAPKs from Paeonia lactiflora. Chinese Journal of Natural Medicines. 2021;19(11):825–835. doi: 10.1016/S1875-5364(21)60086-9. [DOI] [PubMed] [Google Scholar]
- Zhou Y.F., Wang J.N., Tan P., Wu Q.H., Chen J., Liu J.X., et al. A novel method for quality consistency evaluation of Glycyrrhiza formula granules based on a spectrum-effect study. Journal of Separation Science. 2023;46 doi: 10.1002/jssc.202200433. [DOI] [PubMed] [Google Scholar]
- Zhou Y.X., Gong X.H., Zhang H., Peng C. A review on the pharmacokinetics of paeoniflorin and its anti-inflammatory and immunomodulatory effects. Biomedicine & Pharmacotherapy. 2020;130 doi: 10.1016/j.biopha.2020.110505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.