Abstract
Although the existence of a regulatory paracrine feedback system between oocytes and follicular somatic cells has been postulated for some time, there has not yet been any definitive evidence that such a communication system exists. Herein we present a previously undescribed oocyte-granulosa cell (GC) feedback communication system involving an oocyte-derived factor, bone morphogenetic protein-15 (BMP-15) and a GC-derived factor, kit ligand (KL), both of which have been shown to be crucial regulators of female reproduction. We used a coculture system of rat oocytes and GCs and found that BMP-15 stimulates KL expression in GCs, whereas KL inhibits BMP-15 expression in oocytes, thus forming a negative feedback loop. Moreover, KL, like BMP-15, exhibited mitotic activity on GCs in the presence of oocytes. Because c-kit (KL receptor) is expressed in oocytes but not GCs, the oocytes must be involved in mediating the KL-induced GC mitosis. Furthermore, the blockage of c-kit signaling in oocytes by using a c-kit neutralizing antibody markedly suppressed BMP-15-induced GC mitosis, suggesting that the oocyte must play a role in the GC responses to BMP-15. In contrast, the c-kit antibody had no effect on the mitotic activities of two other known GC mitogens, activin-A and BMP-7. Altogether, this study presents direct evidence of a negative feedback system governed by oocyte-derived BMP-15 and GC-derived KL, and demonstrates that the mitotic activities of BMP-15 and KL for GCs depend on this oocyte–GC communication system. We hypothesize that the negative feedback system most likely plays a pivotal role in early folliculogenesis.
There is extensive evidence that growth factors produced locally by the ovary play important roles in regulating ovarian folliculogenesis. Our laboratory has recently reported that bone morphogenetic proteins (BMPs), a subset of the transforming growth factor-β (TGF-β) superfamily, form a functional paracrine regulatory system in the ovary replete with ovarian expression of BMP receptors and ligands as well as important functions of BMP-4, -6, -7, and -15 in regulating ovarian folliculogenesis and ovulation (1–6). In particular, BMP-15 and its close homologue growth differentiation factor-9 (GDF-9) are of interest in female reproduction because of their exclusive expression in oocytes throughout folliculogenesis (2, 7, 8). The importance of GDF-9 was established by the finding that mice with a targeted disruption of the gdf9 gene were infertile because of a block in the primary stage of folliculogenesis (9). This finding was supported by the demonstration that recombinant GDF-9 regulates proliferation, cytodifferentiation, and cumulus expansion of granulosa cells (GCs) in vitro (10, 11). Although BMP-15 knockout female mice exhibit only reduced fertility (12), BMP-15 has recently been shown to be necessary for normal female fertility in sheep based on the finding that naturally occurring mutations in the bmp15 gene in Inverdale (FecXI) ewes cause increased ovulation rates in the heterozygotes but infertility in the homozygotes because of the arrest of follicle development at the primary stage (13). In this regard, we have recently reported two key functions of BMP-15: (i) the inhibition of follicle-stimulating hormone (FSH)-induced GC cytodifferentiation through the inhibition of FSH receptor expression in GCs, and (ii) the stimulation of GC proliferation, which is involved in early follicular growth (2, 3). Therefore, we proposed that, in heterozygous Inverdale ewes, the reduced levels of intact BMP-15 results in higher levels of FSH receptors in GCs, which, in turn, leads to increased numbers of healthy dominant follicles (3). The end result of this sequence of events would lead to an increased ovulation rate. In the homozygotes, however, the total lack of intact BMP-15 mitotic property results in the cessation of GC proliferation at the primary stage, leading to the arrest of follicle development. Thus, the regulation of GC proliferation and cytodifferentiation by the oocyte growth factor, BMP-15, appears to be crucial for female reproduction. This action of BMP-15 is an example of oocyte-to-GC communication (14).
An example of the importance of GC-to-oocyte (as opposed to oocyte-to-GC) communication in mammalian female reproduction was provided by the discovery that naturally occurring mutations at the W (white spotting) and Sl (steel) loci in mice resulted in developmental abnormalities in oogenesis and folliculogenesis leading to infertility (15). Genetic mapping studies have revealed that the W locus encodes a member of the tyrosine kinase receptor family termed c-kit proto-oncogene (16, 17). The Sl locus encodes a ligand for c-kit termed kit ligand (KL; also called Sl factor, stem cell factor, or mast cell growth factor) (18–22). During postnatal ovarian development, c-kit (KL receptor) mRNA and protein are localized to oocytes at all stages of follicular development (23–25). In contrast, the KL mRNA expression in the follicle is localized to GCs (26–28). Thus, the action of KL takes place by virtue of GC-to-oocyte communication. Importantly, these findings suggest that the interaction of GC-derived KL and oocyte c-kit is indispensable for normal fertility.
Because BMP-15 and KL are concomitantly expressed in the early stages of follicular development and appear to be involved in GC mitosis, we have formulated a working hypothesis that oocyte-derived BMP-15 and GC-derived KL may exert their functions in a concerted manner for the oocyte–GC communication-dependent follicular growth. To examine this hypothesis, we have used primary GCs in the presence or absence of oocytes and investigated the BMP-15 and KL systems in regulating GC mitosis. Here we propose that oocyte-derived BMP-15 and GC-derived KL form a negative feedback loop, and that the mitotic properties of these factors are highly controlled by this previously undescribed oocyte–somatic cell communication system.
Materials and Methods
Reagents and Supplies.
Diethylstilbestrol (DES) was purchased from Sigma, and female Sprague–Dawley (SD) rats were purchased from Charles River Laboratories (Wilmington, MA). Recombinant soluble KL (amino-terminal 165-aa chain of mouse KL produced by Escherichia coli), anti-c-kit blocking antibody (IgG fraction of polyclonal antibody from a goat immunized with purified extracellular domain of human c-kit), and control goat antibody were purchased from R&D Systems. Recombinant human BMP-15 tagged with a Flag-epitope (BMP-15) was produced by 293 cells and purified by using anti-Flag monoclonal antibody as reported (2). Recombinant rat activin-A was expressed by Chinese hamster ovary (CHO) cells by using rat inhibin/activin βA cDNA clone, βA30 (29), and purified by using a follistatin affinity column followed by two steps of reverse-phase HPLC. Recombinant human BMP-7 was generously provided by Kuber Sampath (Creative BioMolecules, Hopkinton, MA).
Culture of Rat GC With or Without Oocytes.
Female 23-day-old SD rats were implanted with Silastic capsules containing 10 mg of DES to increase GC number. After 4 days of DES exposure, the ovarian follicles were punctured with a 28-gauge needle, and the isolated mixture of GCs and oocytes was cultured for 48 h in serum-free McCoy's 5a medium supplemented with 2 mM l-glutamine and antibiotics at 37°C in an atmosphere of 5% CO2 in air. For indicated experiments, GCs were separated from oocytes by filtering 5 ml of the oocyte/GC suspension through a nylon mesh (40 μm; BD Falcon, Bedford, MA), which allowed GCs but not oocytes to pass through. The purified GCs were cultured in serum-free McCoy's 5a medium as described above. The animal protocols were approved by the University of California at San Diego Institutional Animal Care and Use Committee.
RNA Extraction and Analysis by Quantitative Competitive Reverse Transcription (RT)-PCR.
Total cellular RNA was extracted by guanidium isothiocyanate/acid/phenol/chloroform methods using Trizol (Life Technologies, Rockville, MD). Oligonucleotides used for RT-PCR were custom-ordered from Life Technologies. The steady-state levels of mRNA encoding KL, BMP-15, and L19 were analyzed by quantitative competitive RT-PCR procedures established in our laboratory (3, 6) using internal control DNAs with the same target-specific primer sequences. PCR primer pairs were selected from different exons of the corresponding genes to discriminate PCR products that might arise from possible chromosome DNA contaminants. Specifically, they were derived from the cDNA clones at the following nucleotide numbers: 186–194 and 706–723 for KL (GenBank accession no. AF071204); 566–583 and 797–814 for BMP-15 (GenBank accession no. AJ132407); and 401–421 and 575–595 for ribosomal protein-L19 (L19; GenBank accession no. J02650). The relative integrated density of each band was digitized by NIH IMAGE 1.62 and the ratios of the densitometric readings of the amplified target cDNA and internal control DNA were analyzed as described (3, 4).
Thymidine Incorporation Assay.
Thymidine incorporation assay was performed as reported (2, 4, 5). Briefly, GCs (2 × 105 viable cells), either alone or with oocytes, were initially precultured for 24 h in a 1.5-ml polypropylene tube containing 200 μl (final volume) of culture medium with no additions. After the 24-h preculture period, 0.5 μCi per tube of [methyl-3H]thymidine ([3H]dT) (Amersham Pharmacia; 1 Ci = 37 GBq) and indicated concentrations of growth factors and/or antibodies (IgGs) were added to the culture medium and the cells were cultured for another 24 h. To stop the culture, cells were washed with PBS, centrifuged (2,000 × g, 30 min), and incubated with ice-cold 10% trichloroacetic acid for 30 min at 4°C. The cell pellet was then solubilized in 0.2 M NaOH, and its radioactivity was counted.
Statistical Analysis.
Results are expressed as the mean ± SEM of at least three separate experiments, with triplicate determinations for each treatment. Differences between groups were analyzed for statistical significance by using ANOVA or unpaired t tests (STATVIEW 5.0 software, Abacus Concepts, Berkeley, CA). P values ≤ 0.05 were accepted as statistically significant.
Results
Previous studies have shown that injection of anti-c-kit antibodies into the ovaries of immature mice causes a disruption of GC proliferation in early antral follicles (30). However, no direct evidence has been provided with regard to whether KL stimulates GCs in vivo or in vitro. To investigate whether the administration of KL causes changes in GC mitosis, it was necessary to use cell cultures that contained both oocytes and GCs because c-kit is expressed only in oocytes, not GCs (23–25). Therefore, cell cultures containing a mixture of oocytes and GCs were used for this study. These cell cultures contained oocytes and GCs at a ratio of ≈1 oocyte to 5,000 GCs.
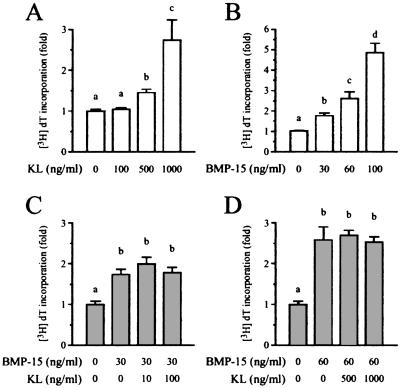
In this culture system, KL stimulated DNA synthesis in a dose-dependent fashion over a concentration range from 0 to 1,000 ng/ml, with 1,000 ng/ml KL causing an ≈2.5-fold increase in basal [3H]dT incorporation (Fig. 1A). When these cells were incubated with BMP-15, the incorporation of [3H]dT increased in a dose-dependent manner, with 100 ng/ml BMP-15 causing a 5-fold stimulation of GC DNA synthesis (Fig. 1B). Thus, in comparison to KL, BMP-15 was much more potent and efficacious in stimulating GC DNA synthesis. We next explored possible interactions between these pathways in regulating GC mitosis. As shown in Fig. 1 C and D, coincubation of KL (10 to 1,000 ng/ml) with submaximal concentrations of BMP-15 (30 or 60 ng/ml) did not lead to further increases in [3H]dT incorporation above that evoked by BMP-15 alone. This finding suggests that BMP-15- and KL-stimulated GC DNA synthesis is mediated through a common pathway, but, under these conditions, the BMP-15 response is stronger and can completely override the effects of KL.
Figure 1.
Effects of KL and BMP-15 on GC mitosis. GCs were cocultured with oocytes in a serum-free medium for 24 h with [3H]dT and increasing doses of KL (0–1,000 ng/ml; A) or BMP-15 (0–100 ng/ml; B). The coculture was also treated with combinations of BMP-15 (30 and 60 ng/ml) and increasing doses of KL (0–1,000 ng/ml) for 24 h (C and D). The [3H]dT level incorporated into the cells was counted. Bars with different letters indicate that group means are significantly different at P ≤ 0.05.
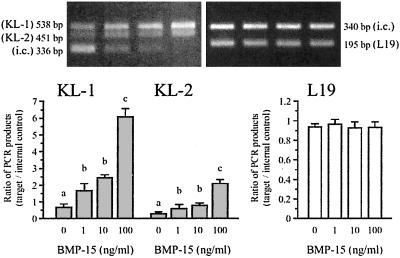
To begin to examine the nature of this oocyte–GC communication, we tested the hypothesis that BMP-15 may regulate KL expression in GCs. Fig. 2 Upper Left shows that GCs express two splicing variants of KL transcripts, KL-1 and KL-2. The consequent proteins translated from the two transcripts are both cell-membrane associated and susceptible to proteolytic cleavage at multiple positions within their extracellular domains (31, 32), which leads to the production of soluble KL predominantly from the KL-1 transcripts (32, 33). Interestingly, stimulation with BMP-15 induced a marked dose-dependent increase in the steady-state levels of KL-1 mRNA, and the increases with the 100 ng/ml dose of BMP-15 were 7.5-fold above basal levels (Fig. 2). KL-2 mRNA also increased after stimulation with BMP-15, but the levels were lower than that of KL-1. There was no effect of BMP-15 on the mRNA levels of the housekeeping gene L19. These data suggest that oocyte-derived BMP-15 is directly involved in stimulating KL expression in GCs. Of interest, this effect of BMP-15 is the opposite of that of GDF-9, which has been shown to be potent in the suppression of KL mRNA expression in GCs (34).
Figure 2.
Effect of BMP-15 on the expression of KL mRNA. GCs were cocultured with oocytes for 48 h in the presence of increasing doses of BMP-15 (0–100 ng/ml), after which total cellular RNA was extracted, and the steady-state levels of mRNA encoding KL and L19 were analyzed by quantitative competitive RT-PCR using the internal control (i.c.) DNAs. Two bands, denoted by KL-1 and KL-2, represent the PCR products from two alternatively spliced mRNA variants. The gel pictures of the PCR products are shown in Upper and the ratios of the PCR products (target/internal control) are graphed in Lower. Bars with different letters indicate that group means are significantly different at P ≤ 0.05.
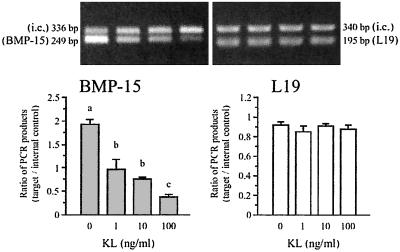
To extend this relationship, we investigated the effects of KL on oocyte BMP-15 mRNA levels. Interestingly, stimulating oocyte-GC cultures with soluble KL-1 protein (0–100 ng/ml) induced a pronounced dose-dependent reduction in the steady-state mRNA levels of oocyte BMP-15 (Fig. 3). After stimulation with 100 ng/ml soluble KL, the levels of BMP-15 mRNA were reduced by ≈75%. The physiological importance of this response is shown by the fact that a significant (P ≤ 0.05) inhibitory effect of KL on BMP-15 mRNA was observed with as little as 1 ng/ml of KL. Thus, KL is more potent (≈500-fold) in exerting effects on oocyte BMP-15 mRNA expression than in exerting effects on DNA synthesis in GCs (compare Figs. 1A and 3).
Figure 3.
Effect of soluble KL on the expression of BMP-15 mRNA. GCs were cocultured with oocytes for 48 h in the presence of increasing doses of soluble KL (0–100 ng/ml), after which total cellular RNA was extracted and the steady-state levels of mRNA encoding BMP-15 and L19 were analyzed by quantitative competitive RT-PCR using the internal control DNAs (i.c.). The PCR products are shown in Upper and the ratios of PCR products (target/internal control) are graphed in Lower. Bars with different letters indicate that group means are significantly different at P ≤ 0.05.
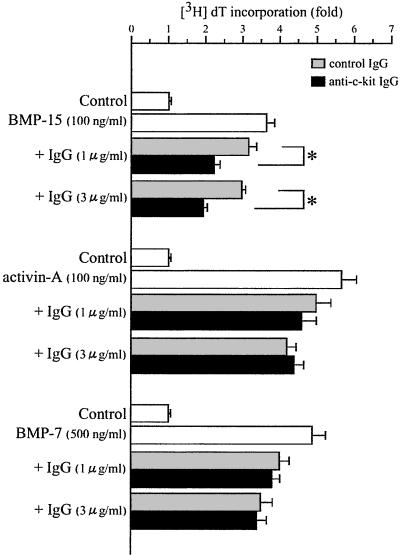
We next attempted to link these two growth factor actions in the regulation of GC DNA synthesis. Here we asked the question whether the BMP-15 stimulation of GC mitosis might involve a stimulation of KL expression in GCs. To test this link we used an anti-c-kit antibody that is capable of blocking KL activities. Interestingly, the stimulation of [3H]dT incorporation induced by 100 ng/ml BMP-15 was significantly suppressed by coincubation with the c-kit blocking antibody compared with the effects of the control antibody (Fig. 4). This finding provides strong support for a role of GC-derived KL in the mitotic effects of oocyte-derived BMP-15 for GCs. To demonstrate specificity, we tested the effect of the c-kit blocking antibody on the stimulation of GC DNA synthesis by two known GC mitogens, activin-A (35) and BMP-7 (5). In contrast to the effects of KL activity on BMP-15-induced GC DNA synthesis, coincubation with anti-c-kit antibody did not change the stimulatory effect of a saturating dose of either activin-A or BMP-7 on [3H]dT incorporation (Fig. 4). A confirmatory study using another anti-c-kit antibody (sc-1494; Santa Cruz Biotechnology), which has been shown to block antrum formation in follicles and aromatase activity in GCs (36), also showed specific inhibition of GC mitosis induced by BMP-15, but not by activin-A or BMP-7 (data not shown).
Figure 4.
Effect of anti-c-kit antibody on GC mitosis induced by BMP-15, activin-A, or BMP-7. GCs were cocultured with oocytes in a serum-free medium for 24 h with [3H]dT and BMP-15 (100 ng/ml) in the presence of increasing doses of either anti-c-kit IgG (1 and 3 μg/ml) or control IgG (1 and 3 μg/ml). The fold changes in [3H]dT incorporation as a comparison with the effect of the control IgG are presented. Bars with an asterisk indicate that group means are significantly different at P ≤ 0.05.
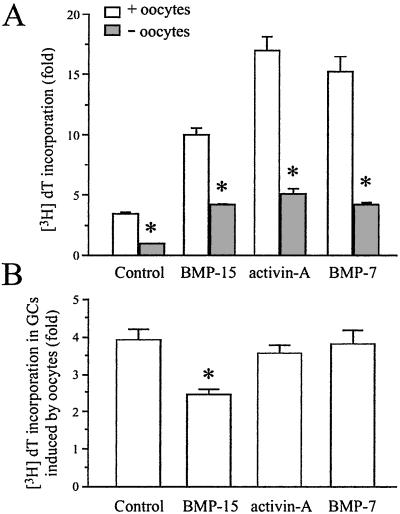
To show the direct importance of the oocyte in GC mitosis induced by BMP-15, we prepared a population of oocyte-free GCs and determined the effects of growth factors on DNA synthesis. The oocytes were removed from the GCs by passing the cell suspension through nylon mesh having a 40-μm pore size (Fig. 5). Removing the oocytes had a great effect on basal DNA synthesis by GCs, reducing [3H]dT incorporation in the GCs by ≈70% (control in Fig. 6A). Treatment with BMP-15 (100 ng/ml) clearly stimulated DNA synthesis by GCs in the oocyte-free culture, but the response was significantly less than that observed in the presence of oocytes. Similar results were obtained with activin-A and BMP-7 (Fig. 6A). Collectively, these data indicate that the effects of BMP-15, activin-A, and BMP-7 on GC DNA synthesis are, at least in part, direct effects in the GCs themselves. However, BMP-15 exhibited significantly lower dependency on the oocytes with regard to DNA synthesis in GCs compared with control, activin-A, and BMP-7 (Fig. 6B).
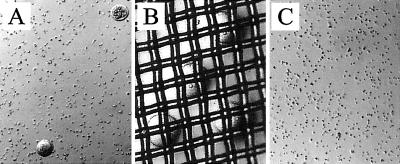
Figure 5.
Elimination of oocytes from GC/oocyte mixture. From the GC/oocyte mixture pool (A), the oocytes ranging from 70 to 80 μm in diameter were separated by using a nylon mesh (40 μm) cell strainer (B). After the cell straining, no oocytes were found in the GC pool (C).
Figure 6.
Effect of oocytes on GC mitosis induced by BMP-15, activin-A or BMP-7. In a serum-free medium, GCs were cultured with or without oocytes (+ or − oocytes) and treated for 24 h with [3H]dT plus BMP-15 (100 ng/ml), activin-A (100 ng/ml), or BMP-7 (500 ng/ml). (A) The [3H]dT level incorporated into the cells was counted. (B) The effect of oocytes on GC incorporation of [3H]dT in each treatment is represented as the ratio of [3H]dT incorporation levels from the treatments with and without oocytes. Bars with asterisk indicate that group means are significantly different from the with oocyte group (A) or among the groups (B) at P ≤ 0.05.
Discussion
There is abundant evidence supporting the importance of KL in the initiation of folliculogenesis by inducing primordial follicle growth (15). However, no evidence has been provided that KL stimulates GC proliferation. In the present study, we first demonstrated that administration of KL to GC/oocyte cocultures simulates GC DNA synthesis. This stimulation of DNA synthesis in the GCs must involve the oocyte because c-kit is present only on the surface of oocytes, not on GCs. This concept implies that an oocyte-derived mitogen might be involved in mediating the mitotic properties of KL. We used the same experimental conditions, and found that BMP-15 exhibits much higher potency in stimulating GC DNA synthesis than does KL. We are unaware, at present, of why such high concentrations (500–1,000 ng/ml) of soluble KL are required to exert its mitotic activity. Given that KL receptor (c-kit) is expressed in oocytes and yet KL stimulates GC DNA synthesis, an unidentified factor secreted from oocytes because of the KL stimulation must mediate the mitotic action. Such a sequential process could be coupled to the requirement of high concentration of KL to exert its mitotic activity for GCs. In contrast, the same soluble KL has exhibited its activity in suppressing BMP-15 mRNA expression in oocytes at much lower concentrations (Fig. 3) because this action of KL is a direct event to oocytes. In this study we have also observed that the addition of increasing doses of KL to a constant dose of BMP-15 fails to enhance GC DNA synthesis above that of BMP-15 alone. Given these findings, we hypothesized that BMP-15 could stimulate the expression of endogenous KL in GCs that could be a mediator, in part, of BMP-15-stimulated GC mitosis.
This hypothesis has been supported by the finding that BMP-15 stimulates both KL-1 and KL-2 mRNA steady-state levels in a dose-dependent manner. In this regard, Joyce and colleagues (37) reported interesting data that the steady-state mRNA level of KL is regulated by oocytes in a developmental stage-dependent manner by using a mouse GC/oocyte coculture system. Specifically, they concluded that the addition of partly grown oocytes increases, KL mRNA expression in GCs, but addition of fully grown oocytes decreases it (37). Based on the present and previous (34) findings that BMP-15 stimulates and that GDF-9 inhibits KL mRNA expression, respectively, it is interesting to hypothesize that the stimulatory factor of KL mRNA expression secreted from the partly grown oocytes may be BMP-15, whereas the inhibitory factor secreted from the fully grown oocytes may be GDF-9. However, because studies by in situ hybridization and immunohistochemistry demonstrated that BMP-15 and GDF-9 mRNA and protein are both produced by oocytes regardless of their growth stages throughout folliculogenesis, how can this be possible? A very important issue in this regard is that currently there is no evidence on the secretion patterns of the biologically active forms of these molecules from the oocyte into the follicular microenvironment. It has been shown in a number of biological systems that the secretion of biologically active forms of TGF-β superfamily members, in particular, is largely determined by posttranslational processing of the precursors (proproteins) (38). A useful method to measure the levels of biologically active forms (as opposed to inactive proprotein) of BMP-15 and GDF-9 in situ during follicular development needs to be developed to prove this intriguing hypothesis. In addition, the presence of binding proteins has also been shown to be of paramount importance in the in situ regulation of the bioavailability of TGF-β superfamily members (39). Our recent finding that follistatin can inhibit BMP-15 functions by forming an inactive complex begins to establish that this inhibitory mechanism also applies to BMP-15 (6). Binding proteins in the follicular microenvironment may, thus, be involved in the regulation of the bioavailability of BMP-15 and possibly GDF-9 throughout folliculogenesis.
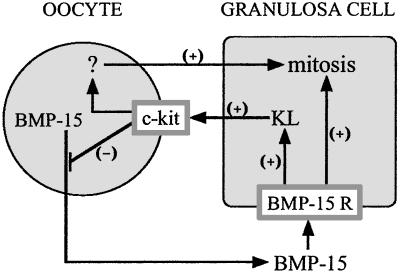
Another important finding in this study is that KL is a very potent inhibitor of BMP-15 mRNA expression. This finding was surprising because we predicted that KL would enhance the expression of BMP-15, which, in turn, stimulates GC proliferation. The inverse relationship between the regulation of BMP-15 expression by KL and the regulation of KL expression by BMP-15 establishes a previously undescribed oocyte-somatic cell paracrine negative feedback loop (Fig. 7). To further investigate the nature of this negative feedback loop, an attempt was made to disrupt the c-kit signaling in oocytes by using a functionally blocking c-kit antibody. Unexpectedly, the GC mitosis induced by BMP-15 was markedly suppressed after incubation with anti-c-kit antibody, whereas that induced by either activin-A or BMP-7 was not significantly altered by the antibody. This finding indicates that c-kit signaling in the oocyte is important in the mechanism of BMP-15 stimulation of GC mitosis. The physiological relevance of our finding is supported by the work of Yoshida et al. (30) showing that the in vivo injection of anti-c-kit antibody into developing mice disturbed the development (but not formation) of primordial follicles. In a different in vivo study, it was shown that female mice with a point mutation in the c-kit gene (tyrosine to phenylalanine at residue 719, which prevents the binding of SH2 domain proteins, including the p85 subunit of phosphatidylinositol 3′-kinase, thereby abolishing subsequent signaling events) exhibit impaired recruitment of primordial follicles leading to a reduced number of developing follicles (40).
Figure 7.
Proposed interaction of BMP-15 and KL in the regulation of GC mitosis. Oocyte-derived BMP-15 acts through the BMP-15 receptor (BMP-15 R) on GCs to stimulate mitosis and KL expression. KL, in turn, acts through c-kit on the surface of the oocyte to inhibit BMP-15 expression, forming a negative feedback loop. KL also causes an increase in GC mitosis, presumably by stimulating the oocyte to secrete a currently unidentified mitogen denoted by “ ? ”.
Is the KL–c-kit signaling system necessary for BMP-15 to exert its mitotic activity on GCs? The experiments using GCs without oocytes revealed that BMP-15 retains its ability to increase the [3H]dT incorporation by GCs, suggesting that BMP-15 promotes GC mitosis by dual pathways, one residing exclusively in GCs and the other through oocytes (Fig. 7). It is intriguing that BMP-15 as well as activin-A and BMP-7 exhibited higher GC mitotic activity in the presence of oocytes than that without oocytes. This finding indicates that all these ligands have a functional dependence on the oocyte for maximum stimulation of GC mitosis; however, it appears that the dependency of BMP-15 on oocytes is significantly lower than that of activin-A and BMP-7 (Fig. 6B). This difference could be explained by the diverse localization of functional receptors for these ligands. Activin-A signal transduction pathway is activated by activin type II receptors (ActRII and ActRIIB) (41, 42), whereas BMP-7 signal transduction can be activated through the ActRII and the BMP type II receptor (BMPRII) (43). In rats, ActRII mRNA is highly expressed in oocytes and the signal in GCs is very weak, whereas the expression of ActRIIB mRNA in both oocytes and GCs is very low (44, 45). In contrast, BMPRII mRNA is intensely expressed in GCs and barely detectable in oocytes (1). Therefore, it is possible that the mitotic activities of BMP-7 and activin-A are mediated predominantly through the oocyte ActRII, whereas that of BMP-15 is mainly through the GC BMPRII. The fact that dependency on oocytes for the activities of activin-A and BMP-7 is higher than that of BMP-15 may support this hypothesis. Our preliminary data, indeed, have demonstrated that the extracellular domain of BMPRII markedly inhibits BMP-15-induced GC DNA synthesis (data not shown). An important current finding concerning the mechanism whereby these three growth factors exert their mitotic activity is that only BMP-15 activity is dependent on the oocyte c-kit signaling.
In total, our present study shows a previously uncharacterized negative feedback system between the oocyte and the GCs in which BMP-15 stimulates KL expression in GCs, and KL-c-kit interaction, in turn, inhibits BMP-15 expression in oocytes. Moreover, the mitotic activities of both BMP-15 and KL depend on this negative feedback system. Because of the importance of these two factors in the local regulation of follicular recruitment and development, this gametic-somatic cell feedback system is most likely crucial in the balance needed for controlled folliculogenesis. A better understanding of this feedback system will provide a considerable contribution to our understanding of ovarian folliculogenesis and pathophysiology.
Acknowledgments
We thank Dr. Gregory F. Erickson and Dr. R. Kelly Moore for helpful discussion and critical reading of the manuscript, and Ms. Mei Wang for excellent technical assistance. This work was supported in part by the National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54HD12303 as part of Specialized Cooperative Centers Program in Reproduction Research, National Institutes of Health Grant R01 HD41494, and the University of California at San Diego Academic Senate (RA 882M). F.O. was supported by a fellowship from The Lalor Foundation.
Abbreviations
- ActRII
activin type II receptor
- BMP
bone morphogenetic protein
- BMPRII
bone morphogenetic protein type II receptor
- DES
diethylstilbestrol
- FSH
follicle-stimulating hormone
- GC
granulosa cell
- GDF-9
growth differentiation factor 9
- [3H]dT
[methyl-3H]thymidine
- KL
kit ligand
- TGF-β
transforming growth factor β
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Shimasaki S, Zachow R J, Li D, Kim H, Iemura S-I, Ueno N, Sampath K, Chang R J, Erickson G F. Proc Natl Acad Sci USA. 1999;96:7282–7287. doi: 10.1073/pnas.96.13.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otsuka F, Yao Z, Lee T H, Yamamoto S, Erickson G F, Shimasaki S. J Biol Chem. 2000;275:39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- 3.Otsuka F, Yamamoto S, Erickson G F, Shimasaki S. J Biol Chem. 2001;276:11387–11392. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- 4.Otsuka F, Moore R K, Shimasaki S. J Biol Chem. 2001;276:32889–32895. doi: 10.1074/jbc.M103212200. [DOI] [PubMed] [Google Scholar]
- 5.Lee W, Otsuka F, Moore R K, Shimasaki S. Biol Reprod. 2001;65:994–999. doi: 10.1095/biolreprod65.4.994. [DOI] [PubMed] [Google Scholar]
- 6.Otsuka F, Moore R K, Iemura S-I, Ueno N, Shimasaki S. Biochem Biophys Res Commun. 2001;289:961–966. doi: 10.1006/bbrc.2001.6103. [DOI] [PubMed] [Google Scholar]
- 7.McGrath S A, Esquela A F, Lee S-J. Mol Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 8.Laitinen M, Vuojolainen K, Jaatinen R, Ketola I, Aaltonen J, Lehtonen E, Heikinheimo M, Ritvos O. Mech Dev. 1998;78:135–140. doi: 10.1016/s0925-4773(98)00161-0. [DOI] [PubMed] [Google Scholar]
- 9.Dong J, Albertini D F, Nishimori K, Kumar T R, Lu N, Matzuk M. Nature (London) 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 10.Vitt U A, Hsueh A J W. Mol Cell Endocrinol. 2001;183:171–177. doi: 10.1016/s0303-7207(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Matzuk M M. Rev Endocr Metab Disord. 2002;3:27–32. doi: 10.1023/a:1012796601311. [DOI] [PubMed] [Google Scholar]
- 12.Yan C, Wang P, DeMayo J, DeMayo F J, Elvin J A, Carino C, Prasad S V, Skinner S S, Dunbar B S, Dube J L, et al. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 13.Galloway S M, McNatty K P, Cambridge L M, Laitinen M P E, Juengel J L, Jokiranta T S, McLaren R J, Luiro K, Dodds K G, Montgomery G W, et al. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 14.Eppig J J. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 15.Driancourt M A, Reynaud K, Cortvrindt R, Smitz J. Rev Reprod. 2000;5:143–152. doi: 10.1530/ror.0.0050143. [DOI] [PubMed] [Google Scholar]
- 16.Geissler E N, Ryan M A, Housman D E. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 17.Chabot B, Stephenson D A, Chapman V M, Besmer P, Bernstein A. Nature (London) 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 18.Copeland N G, Gilbert D J, Cho B C, Donovan P J, Jenkins N A, Cosman D, Anderson D, Lyman S D, Williams D E. Cell. 1990;63:175–183. doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- 19.Flanagan J G, Leder P. Cell. 1990;63:185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- 20.Huang E, Nocka K, Beier D R, Chu T-Y, Buck J, Lahm H-W, Wellner D, Leder P, Besmer P. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 21.Zsebo K M, Williams D A, Geissler E N, Broudy V C, Martin F H, Atkins H L, Hsu R-Y, Birkett N C, Okino K H, Murdock DC, et al. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 22.Anderson D M, Lyman S D, Baird A, Wignall J M, Eisenman J, Rauch C, March C J, Boswell H S, Gimpel S D, Cosman D, et al. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 23.Manova K, Nocka K, Besmer P, Bachvarova R F. Development (Cambridge, UK) 1990;110:1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- 24.Orr-Urtreger A, Avivi A, Zimmer Y, Givol D, Yarden Y, Lonai P. Development (Cambridge, UK) 1990;109:911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- 25.Horie K, Takakura K, Taii S, Narimoto K, Noda Y, Nishikawa S, Nakayama H, Fujita J, Mori T. Biol Reprod. 1991;45:547–552. doi: 10.1095/biolreprod45.4.547. [DOI] [PubMed] [Google Scholar]
- 26.Manova K, Huang E J, Angeles M, De Leon V, Sanchez S, Pronovost S M, Besmer P, Bachvarova R F. Dev Biol. 1993;157:85–99. doi: 10.1006/dbio.1993.1114. [DOI] [PubMed] [Google Scholar]
- 27.Ismail R S, Okawara Y, Fryer J N, Vanderhyden B C. Mol Reprod Dev. 1996;43:458–469. doi: 10.1002/(SICI)1098-2795(199604)43:4<458::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Laitinen M, Rutanen E, Ritvos O. Endocrinology. 1995;136:4407–4414. doi: 10.1210/endo.136.10.7545103. [DOI] [PubMed] [Google Scholar]
- 29.Esch F S, Shimasaki S, Cooksey K, Mercado M, Mason A J, Ying S-Y, Ueno N, Ling N. Mol Endocrinol. 1987;1:388–396. doi: 10.1210/mend-1-5-388. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa S I. Dev Biol. 1997;184:122–137. doi: 10.1006/dbio.1997.8503. [DOI] [PubMed] [Google Scholar]
- 31.Flanagan J G, Chan D C, Leder P. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 32.Huang E J, Nocka K H, Buck J, Besmer P. Mol Biol Cell. 1992;3:349–362. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besmer P, Manova K, Duttlinger R, Huang E J, Packer A, Gyssler C, Bachvarova R F. Dev Suppl. 1993;118:125–137. [PubMed] [Google Scholar]
- 34.Joyce I M, Clark A T, Pendola F L, Eppig J J. Biol Reprod. 2000;63:1669–1675. doi: 10.1095/biolreprod63.6.1669. [DOI] [PubMed] [Google Scholar]
- 35.Li R, Phillips D M, Mather J P. Endocrinology. 1995;136:849–856. doi: 10.1210/endo.136.3.7867593. [DOI] [PubMed] [Google Scholar]
- 36.Reynaud K, Cortvrindt R, Smitz J, Driancourt M-A. Mol Reprod Dev. 2000;56:483–494. doi: 10.1002/1098-2795(200008)56:4<483::AID-MRD6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 37.Joyce I M, Pendola F L, Wigglesworth K, Eppig J J. Dev Biol. 1999;214:342–353. doi: 10.1006/dbio.1999.9437. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H-J, Burgess A W. Mol Cell Biol Res Commun. 2001;4:321–330. doi: 10.1006/mcbr.2001.0301. [DOI] [PubMed] [Google Scholar]
- 39.Bernard D J, Chapman S C, Woodruff T K. Mol Cell Endocrinol. 2001;180:55–62. doi: 10.1016/s0303-7207(01)00500-7. [DOI] [PubMed] [Google Scholar]
- 40.Kissel H, Timokhina I, Hardy M P, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow S R, Manova K, Besmer P. EMBO J. 2000;19:1312–1326. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathews L S, Vale W W. Cell. 1991;65:973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- 42.Attisano L, Wrana J L, Cheifetz S, Massague J. Cell. 1992;68:97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita H, ten Dijke P, Huylebroeck D, Sampath T K, Andries M, Smith J C, Heldin C-H, Miyazono K. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron V A, Nishimura E, Mathews L S, Lewis K A, Sawchenko P E, Vale W W. Endocrinology. 1994;134:799–808. doi: 10.1210/endo.134.2.8299574. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J, Taverne M A M, van der Weijden G C, Bevers M M, van den Hurk R. Biol Reprod. 2001;65:967–977. doi: 10.1095/biolreprod65.3.967. [DOI] [PubMed] [Google Scholar]