SUMMARY
T helper 1 (Th1) cell initiation pathways are well characterized; however, those regulating their contraction are less understood. Here, we define a CD4+ T cell-autonomous pathway in which complement C5 orchestrated a shift from prostaglandin E2 (PGE2) dominance to enhanced prostacyclin (PGI2) production via activation of C5a receptor 2 (C5aR2). This pivot in lipid mediators induced autocrine signaling through the PGI2 receptor and expression of the interleukin-1 (IL-1) decoy IL-1 receptor type 2 (IL-1R2), which sequestered Th1 cell-driving intrinsic IL-1β, facilitating Th1 cell contraction. Disruption of this C5aR2-PGI2-R axis was a hallmark of pathologically persistent Th1 cell activity in inflammatory conditions, including cryopyrin-associated periodic syndromes (CAPS), Crohn’s disease, and rheumatoid arthritis. Rebalancing this axis through selective PGE2 synthase inhibition rectified the hyperactive Th1 cell phenotype in vitro in T cells from individuals with CAPS. Therefore, complement is a key controller of prostanoid metabolism, and the latter is an intrinsic—and potentially druggable—checkpoint for the cessation of Th1 cell effector responses.
In brief
The pathways regulating Th1 cell contraction are not well understood. Rahman et al. demonstrate that complement receptor C5aR2 controls T cell-intrinsic prostanoid metabolism as an integral component of Th1 cell contraction. Faulty C5aR2-prostanoid rewiring marks autoimmunity-associated hyper-Th1 cell responses in arthritis and Crohn’s disease. Therefore, prostanoid metabolism is a potentially druggable checkpoint for the cessation of Th1 cell effector responses.
Graphical Abstract
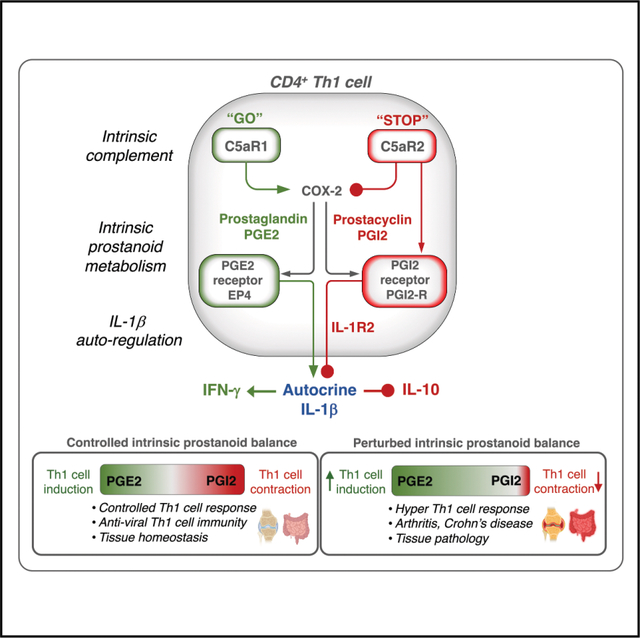
INTRODUCTION
Timely contraction of successful T helper 1 (Th1) cell responses, characterized by the downregulation of interferon (IFN)-γ production and co-expression of the anti-inflammatory cytokine interleukin-10 (IL-10), is essential in limiting Th1 cell-driven tissue pathologies1 and preventing autoimmune disease.2 T cell-intrinsically operating complement system components are central orchestrators of Th1 cell induction and contraction.3 Human CD4+ T cells require cell-autonomous and temporally controlled engagement of the human-specific complement regulator and receptor CD46 during T cell receptor (TCR) stimulation to trigger the cell metabolic changes underlying IFN-γ production and Th1 cell lineage initiation and IL-10 co-expression and Th1 contraction.4–6
The magnitude of Th1 cell responses is further controlled by intracellular C5 activation and stimulation of the G protein-coupled receptor (GPCR) C5a receptor 1 (C5aR1) expressed by mitochondria (mtC5aR1). MtC5aR1 triggers reactive oxygen species (ROS) and Nod-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome-mediated secretion of mature IL-1β by Th1 cells, which augments IFN-γ production and restrains IL-10 co-expression.7 The cell-surface-expressed alternative GPCR, C5a receptor 2 (C5aR2), engaged by T cell-secreted C5a (or C5a-desArg, the desarginized form of C5a), fosters IL-10 co-secretion and Th1 cell contraction through an unknown mechanism.7 Uncontrolled IL-1β production by CD4+ T cells has been pinpointed as a driver of the hyperactive Th1 cell response in individuals suffering from cryopyrin-associated periodic syndromes (CAPS),7 of sustained tissue inflammation in rheumatoid arthritis (RA),8 and of CD4+ T cell depletion during human immunodeficiency virus-1 (HIV-1) infection.9 However, the mechanism by which CD4+ T cell-autonomous IL-1β production is regulated remains elusive.
In this work, we report that Th1 cells expressed and engaged all arms of the prostanoid production and signaling machinery and that a C5aR2-controlled shift toward intrinsic prostacyclin (prostaglandin I2 [PGI2]) production generated IL-1β-neutralizing IL-1 receptor type 2 (IL-1R2 or IL-1R II) in a protein kinase A (PKA)-dependent fashion, which allowed for transition into IL-10 production demarcating Th1 cell contraction. Perturbations in the regulative C5aR2-prostacyclin axis were a prominent feature of hyper-Th1 cell responses in CAPS, Crohn’s disease, and RA. Thus, we provided insights on the intrinsic metabolic programs governing Th1 cell responses, identified complement as a key controller of prostanoid metabolism, and defined a cell-autonomous T cell signaling axis enabling IL-1β autoregulation. Further, successful in vitro normalization of augmented Th1 cell activity in CD4+ T cells from individuals with CAPS through pharmacological inhibition of prostaglandin E2 (PGE2) synthase suggests that expanding currently limited investigations into the effects of nonsteroidal anti-inflammatory drugs (NSAIDs) on T cell response modulation may be warranted.
RESULTS
C5aR2 triggers CD4+ T cell-intrinsic prostacyclin generation and Th1 cell contraction
To determine how T cell surface-expressed C5aR2 controls the magnitude of the Th1 cell response (Figure 1A), we activated human CD4+ T cells with anti-CD3 and anti-CD46 antibodies in the presence or absence of a cell non-permeable C5aR antagonist (C5aRA) and performed RNA sequencing (RNA-seq) (GEO: GSE69090).7 Along with an enrichment of classical inflammatory pathways such as “IFN signaling” and “nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) signaling,” gene set enrichment analysis highlighted “prostaglandin signaling” among the top biological pathways induced by C5aR2 antagonism (Figure 1B). In agreement, the principal component analysis showed that the top 10 loadings from PC3—separating groups by C5aR2 antagonist treatment (Figure S1A)—included the apex enzyme controlling prostaglandin synthesis, PTGS2 (encoding COX-2; Figures 1C and S1B; Table S1), with IFNG and IL1B transcription also augmented (Figure S1B).
Figure 1. C5aR2 triggers CD4+ T cell-intrinsic prostacyclin generation and Th1 cell contraction.

(A) Schematic of CD46- and C5aR1/2-controlled human Th1 cell induction (IFN-γ) and contraction (IL-10 co-induction) via modulation of CD4+ T cell-intrinsic IL-1β secretion.
(B) Pathway analysis using differentially-expressed genes (DEGs) of human CD4+ T cells activated with CD3 + CD46 (6 h) with or without addition of a C5aR2 antagonist (C5aRA) (n = 3 donors, one experiment), and prostaglandin pathway is indicated in red and insert.
(C) Heatmap depicting the top 10 DEGs derived from (B).
(D) IFN-γ and IL-10 production and IFN-γ:IL-10 production ratio of CD3 + CD46 activated (36 h) T cells with or without concurrent incomplete COX-2 inhibition (5 μM) (n = 8, three independent experiments).
(E) Schematic of major prostanoid synthesis enzymes and products downstream of COX-2-generated PGH2, including relevant prostanoid receptors.
(F) Kinetics of PTGES2, TBXAS1, and PTGIS gene transcription in CD3 + CD46-activated CD4+ T cells (n = 4, two independent experiments).
(G) IFN-γ and IL-10 production and IFN-γ:IL-10 production ratio of CD3 + CD46 activated (36 h) T cells with or without concurrent addition of either PGE2 (left) or prostacyclin (PGI2, right) at 12 h post activation (n = 4–6, four independent experiments).
(H) Intrinsic PGE2 and PGI2 production by non-activated (NA) or subsorted IFN-γ+, IFN-γ+IL-10+, or IL-10+ T cells after CD3 + CD46 stimulation for either 2 or 36 h (n = 3–4, two independent experiments). 2 h stimulation generates IFN-γ+ cells only; thus, IFN-γ+ IL-10+ or IL-10+ cells could not be assessed (na) for prostanoid production at this time point.
(I) IFN-γ and IL-10 production by CD3 + CD46 activated (36 h) T cells in which PTGES2, PTGIS, or PTGIR expression had been inhibited by siRNA treatment as indicated or which had been activated in the presence or absence of an EP4 inhibitor (20 μM) (n = 3, three independent experiments).
(J) Effects of 15-PGDH inhibition with or without concurrent EP4 or PGI2-R antagonism on Th1 cell IFN-γ and/or IL-10 (co)producing cells at 36 h post CD3 + CD46 activation. Representative fluorescence-activated cell sorting (FACS) plots shown on the left (statistical analyses are summarized in Figure S1P) (n = 3–4, three independent experiments).
(K) Schematic of C5aR2-controlled intrinsic PGE2 vs. PGI2 generation balance and subsequent effects on IFN-γ and IL-10 production by activated Th1 cells.
Groups were compared using one-way ANOVA test or paired Student’s t test and error bars in graphs represent mean ± SD. *p < 0.05, **p < 0.01. See also Figure S1.
We therefore next assessed COX-2 protein expression in CD4+ T cells during the CD3 + CD46 stimulation-controlled Th1 cell induction (IFN-γ secretion; from ~2 h on) and contraction (IL-10 co-production; from ~18 h on) phases at 0, 2, 18, and 36 h (IL-10 peak production) post activation.4 We had previously shown that the co-induction of IL-10 in T-box expressed in T cells (T-bet)-positive Th1 cells serves as a suitable surrogate marker for monitoring the transition kinetics of productive infection-fighting Th1 cell induction into its contraction and resolution phase.10 Resting T cells expressed low basal amounts of COX-2, which increased rapidly at 2 h of CD3 + CD46 activation and gradually returned to basal amounts at 36 h post activation (Figure S1C). The addition of high doses (>5 μM) of a COX-2 inhibitor abrogated T cell proliferation upon CD3 + CD46 stimulation (and CD3 or CD3 + CD28 activation, not shown) without inducing cell death (Figures S1D and S1E), indicating that optimal COX-2 activity in CD4+ T cells was required for normal proliferation upon stimulation. However, more tempered reduction of COX-2 activity through the application of an inhibitor dose that left T cell proliferation unaffected significantly increased IL-10 production (and reduced the IFN-γ:IL-10 ratio) by CD3 + CD46-stimulated CD4+ T cells without consistent impact on IFN-γ (Figure 1D), IL-17, tumor necrosis factor (TNF), or IL-6 production (Figure S1F). This suggested that T cell-intrinsic COX-2 activity may control the Th1 cell shutdown program.
COX-2-generated PGH2 can be further converted into distinct prostanoids by three enzymes: PGE2 synthase (PTGES2), generating PGE2; thromboxane A synthase 1 (TBXAS1), generating thromboxane (TXA2); and the PGI2 synthase (also known as prostacyclin synthase [PTGIS]), generating PGI2/prostacyclin (Figure 1E). Unexpectedly, we observed the presence of all three enzymes operating downstream of COX-2 in CD4+ T cells, albeit with different expression kinetics (Figures 1F, S1G, and S1H): while TBXAS1 and PTGES2 gene (and protein) expression were evident at the steady-state state, PTGIS transcription was not detectable in resting T cells. Conversely, upon T cell activation, TBXAS1 and PTGES2 transcription (and protein) expression were predominantly downregulated or absent at 18–36 h, while PTGIS transcription and protein generation were induced and sustained at 18–36 h post activation (Figures 1F, S1G, and S1H), overall implying that TBXAS1 and PTGES2 may be involved in Th1 cell induction, whereas PTGIS may participate in Th1 cell contraction. Although activated CD4+ T cells generated TXA2, the inhibition of TXBAS1 activity reduced only TNF secretion and did not affect cell survival, proliferation, or Th1, Th2, or Th17 cell cytokine production upon T cell stimulation (Figure S1I). We therefore focused on cell-intrinsic activities of PTGES2 and PTGIS. We detected only minimal expression of the PGE2 receptor EP2 on resting and activated T cells, and CD46 engagement, as previously described,11 further reduced EP2 protein expression (Figure S1H). Also aligning with published data,12 resting CD4+ T cells expressed intermediate basal amounts of the PGE2 receptor EP4 constitutively and increased EP4 expression upon activation rapidly (Figure S1H). Measurable expression of the PGI2/prostacyclin receptor PGI2 receptor (PGI2-R), however, required T cell stimulation, was most strongly expressed late during activation, and coincided with PTGIS protein expression (Figure S1H). Further, addition of PGE2 to T cell cultures during CD3 + CD46 activation reduced IL-10 production by T-bet+ CD4+ T cells, whereas provision of PGI2 (prostacyclin) facilitated transition into T-bet+ IL-10 co-producing cells (Figures 1G and S1J), supporting the notion that the balance of PGE2 vs. prostacyclin sensed by Th1 cells may control IL-10 co-induction and contraction. Retinoic orphan receptor-γ t (RORγt)+ Th17 cells or Foxp3+ regulatory T cells (Tregs) represented a negligible source of IL-10-producing cells in cultures (Figures S1J and S1K).
Prostanoids impacting on T cell effector responses are largely thought to be derived from exogenous sources.12,13 However, the presence of the full prostanoid production and response machinery in Th1 cells suggested that they generate and engage PGE2 and PGI2 in a cell-autonomous fashion. Indeed, mass spectrometry measurement of cell-intrinsic PGE2 and prostacyclin amounts produced by Th1 cells during their CD3 + CD46-mediated induction and contraction phases (IFN-γ+, IFN-γ/IL-10+, and IL-10+; Figure 1H) in lipid-free media revealed only PGE2 in resting or very early activated T cells (2 h, which triggers limited IFN-γ+ and no IL-10+ T cells). Conversely, only prostacyclin was detected in T cells that had been activated for 18–36 h, with the largest amounts noted in IL-10-secreting cells (Figure 1H). Mirroring their respective prostanoid ligand generation, expression of EP4 was highest on IFN-γ-producing cells, and PGI2-R expression was most prominent on IL-10-secreting cells (Figure S1L). Next, we assessed the effect of intrinsic PTGES2 vs. PTGIS enzyme balance modulation on Th1 cell responses in vitro. Inhibition of PTGES2 activity in CD4+ T cells abrogated normal cell division by stimulated T cells (Figure S1M) and, in consequence, cytokine secretion overall (Figures 1I and S1N). The addition of PGE2 to cultures partially rescued IFN-γ, but not IL-10, production by T cells with impeded PTGES2 activity (Figure S1O). Preventing EP4 signaling mimicked the effects of PTGES2 inhibition (Figures 1I, S1M, and S1N), suggesting that PTGES2-mediated generation of PGE2 downstream of COX-2 and engagement of EP4 by PGE2 are required for human CD4+ T cell proliferation upon stimulation. Inhibition of EP2 during T cell activation had no effect on cell proliferation and only moderately reduced IFN-γ production. However, EP2 inhibition modestly potentiated the effect of EP4 antagonism and further reduced the generation of IFN-γ+ cells (Figures S1M and S1N), implying that cell-intrinsic EP2 stimulation by PGE2 may play a (redundant) role during Th1 cell induction, as has recently been shown for anti-tumor CD8+ T cells.14 The reduction of PTGIS or PGI2-R expression by small interfering RNA (siRNA) technique, on the other hand, led to a specific loss in IL-10 secretion by Th1 cells (Figure 1I), which was partially restored by provision of PGI2 into culture media (Figure S1O). We used an additional method to change the intrinsic balance of PGE2 vs. prostacyclin amounts during T cell activation, namely inhibition of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) function, which blocks the degradation of PGE2 into 15-ketoPGE2 and thus increases cellular PGE2 amounts artificially (Figure 1E). Treatment of T cells with the 15-PGDH inhibitor significantly increased the number of IFN-γ vs. IL-10-producing T cells (Figures 1J and S1P). Tempered inhibition of the EP4 receptor (with an inhibitor concentration preserving T cell proliferation; Figure S1M) after 15-PGDH inhibition restored IL-10 induction, while additional PGI2-R inhibition led to a complete absence of IL-10+ T cells in cultures (Figures 1J and S1P). Of note, PTGIS generates prostacyclin but also the peroxisome proliferator-activated receptor (PPAR)-γ agonist 15-deoxydelta prostaglandin J2 (15d-PGJ2), which is critical for normal T cell survival and control of T cell lineage differentiation15 and particularly Th2 cell induction.16,17 However, neither knockdown of PTGIS nor inhibition of PPAR-γ affected the amounts of the Th2 cell cytokines IL-5 and IL-13 detected in cultures (Figure S1Q), suggesting that PTGIS activity did not bolster Th1 cell-restraining Th2 cell responses in our in vitro system. Addition of PGE2 to activated innate lymphoid cells such as natural killer (NK) cells or natural killer T (NKT) cell cultures reduced, whereas PTGES2 inhibition increased their IFN-γ production (Figure S1R). This agrees with previous observations showing that PGE2 suppresses NK cell-derived IFN-γ production and cell function.18,19 Thus, the IFN-γ-supporting effect of intrinsic PGE2 generation may be associated specifically with adaptive Th1 T cells. This notion, however, should be further explored in the future.
The C5aR2 (which was expressed most prominently by IL-10-producing Th1 cells; Figure S1S) orchestrated IL-10 co-induction in Th1 cells by tempering PTGS2 gene transcription (Figures 1C and S1B) after T cell activation in a β-arrestin-dependent fashion (Figure S1T). This was distinct from classic GPCRs that signal through an associated G protein20 but aligns with the previous finding that CD4+ T cell-specific deficiency of β-arrestin in mice increases in vivo Th1 cell responses and exacerbates experimental autoimmune encephalomyelitis (EAE)21—although IL-10 production by Th1 cells was not explored in this work. C5aR2-mediated signals also increased PTGIS and PGI2-R protein expression (Figure S1U) to further support engagement of the CD4+ T cell PTGIS-PGI2-R axis.
Overall, these data indicate that human CD4+ T cells generate PGE2 and prostacyclin (PGI2), respond to T cell-intrinsic changes in prostaglandin species production chiefly via EP4 and PGI2-R, respectively, and that a timely transition from initial PGE2 production to predominantly prostacyclin generation is required for normal Th1 cell contraction (Figure 1K).
The CD4+ T cell-intrinsic prostacyclin receptor restrains in vivo Th1 cell responses
We next addressed the effect of ablating incoming prostacyclin receptor signals on the co-induction of IL-10 by Th1 cells in vivo. As Ptgir−/− mice or tissues were not available to us, we generated a Ptgir−/− mouse strain (Figure S2A; the mouse Ptgir gene encodes the mouse prostacyclin receptor). CD4+ T cells isolated from Ptgir−/− animals proliferated normally ex vivo (Figure S2B), albeit with a trend toward reduced viability when compared with wild-type (WT) CD4+ T cells (Figure S2C). Ptgir−/− T cells also produced TNF and IL-6 in amounts comparable to those from WT control mice when activated with CD3 + CD28 ex vivo under non-polarizing conditions, with a trend toward reduced IL-17 production (Figure S2D). However, like human T cells in which intrinsic PGI2 signaling is pharmacologically or genetically reduced during activation, ex vivo stimulated Ptgir-deficient mouse T cells secreted increased amounts of IFN-γ and reduced amounts of IL-10, with a significantly augmented IFN-γ:IL-10 ratio compared with T cells from WT mice (Figure S2E). Prostacyclin signaling can support the suppressive activity of natural and induced Tregs.22 We therefore employed a Th1 cell-dependent T cell-transfer colitis model based on the injection of sorted naive CD4+CD25− T cells into Rag2−/− mice to assess the impact of Ptgir deficiency on effector Th1 cells during intestinal inflammation. Mice that received Ptgir-deficient T cells showed a trend toward increased weight loss (Figure 2A), consistent with the observation that weight loss is a suitable readout for dextran sulfate sodium (DSS)-induced colitis but less so for T cell-transfer models.23 The mice receiving Ptgir−/− naive CD4+ T cells, however, had a reduction in colon length, a dependable measure of more severe intestinal inflammation, when compared with animals that were injected with WT CD4+ T cells (Figure 2B). Further, histological examination of colonic tissue showed that mice injected with Ptgir−/− T cells had a higher pathology score with increased lamina propria perturbances and cryptitis and elevated goblet cell loss (Figures 2C and S2F). Aligning with these observations, Ptgir-deficient T cells re-isolated from the culled colitogenic mice produced augmented IFN-γ and reduced IL-10 amounts when compared with WT T cells (Figure 2D). IL-10 was produced predominantly by T-bet+ Th1 cells, with only negligible numbers of RORγt+ Th17 cells generating IL-10 in both WT and Ptgir-deficient colitogenic CD4+ T cells (Figures S2G and S2H). Further, numbers of pathogenic IFN-γ-co-producing Th17 cells24 between animal groups remained unchanged (Figure S2G). In line with the capacity of IFN-γ to suppress Th17 cell induction25 and our previous observation that Th1 cells negatively control Th17 cell responses in a T cell-transfer colitis model,7 we noted a significant reduction in IL-17 generation by in vivo elicited Ptgir−/− T cells (Figure S2G). We were able to source spleens and femurs from Ptgis−/− animals.26 Utilizing naive Foxp3−CD4+ T cells from Ptgis−/− mice in the T cell-transfer colitis model demonstrated that Ptgis-deficient T cells largely phenocopied the effects of Ptgir−/− T cells. Ptgis−/− T cells proliferated normally in vivo (Figure S2I) and induced weight loss (Figure 2E) and a significant reduction in colon length in recipient animals (Figure 2F). There was a trend (which did not reach statistical significance, probably because of the smaller number of animals we could inject due to limited materials) toward increased intestinal pathology in mice that received Ptgis-deficient T cells compared with those injected with WT T cells (Figure 2G). Further, cultures of Ptgis−/− CD4+ T cells re-isolated from the colons of colitogenic mice contained substantially fewer IL-10-producing T cells and had an increased ratio of IFN-γ:IL-10 production when compared with WT T cells (Figure 2H). The numbers of IL-17+IFN-γ+ T cells between animals were also unchanged, however, and in contrast to the experiments using Ptgir−/− T cells, Th17 cells were not reduced in numbers in mice injected with Ptgis−/− T cells (Figure S2I).
Figure 2. The CD4+ T cell-intrinsic prostacyclin receptor restrains in vivo Th1 cell responses.

(A) Body weight change of Rag2−/− mice injected with sorted naive CD4+ T cells isolated from wild-type (WT) or Ptgir−/− mice (n = 6–8, two independent experiments).
(B) Colon length at the study endpoint (6 weeks) of mice as treated under (A).
(C) Representative hematoxylin and eosin (H&E) colon tissue histology staining of mice injected with either WT or Ptgir−/− naive CD4+ T cells at 6 weeks post injection (left) with pathology scores across three colon sections/mouse (right) at 6 weeks post injection (n = 6–8, two independent experiments).
(D) Percentages of IFN-γ- and IL-10-producing colonic CD4+ T cells re-isolated at the study endpoint (A–C) after phorbol myristate acetate (PMA) + ionomycin stimulation (5 h) (n = 6–8, two independent experiments).
(E) Body weight change of Rag2−/− mice injected with sorted naive CD4+ T cells isolated from WT or Ptgis−/− mice (n = 4, one experiment).
(F) Colon length at the study endpoint (6 weeks) of mice as treated under (E).
(G) Representative H&E colon tissue histology staining of mice injected with either WT or Ptgis−/− naive CD4+ T cells at 6 weeks post injection (left) with pathology scores across three colon sections/mouse (right) at 6 weeks post injection (n = 4, one experiment).
(H) Percentages of IFN-γ- and IL-10-producing colonic CD4+ T cells re-isolated at the study endpoint (E–G) after PMA + ionomycin stimulation (5 h) (n = 4, one experiment).
(I) Schematic of influenza infection model used (left) with a representative FACS plot of IFN-γ and/or IL-10-producing lung, bone marrow (BM)-derived dendritic cell-restimulated CD4+ T cells (middle), and the summary of data from four animals used (right) (n = 4, one experiment).
Groups were compared using paired or non-paired Student’s t test where appropriate, and error bars in graphs represent mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.005. See also Figure S2.
Utilizing the bone marrow (BM) of Ptgis−/− mice, we explored an in vivo role for T cell-intrinsic PTGIS activity for IL-10 co-induction in Th1 cells also in an infection model: we generated bone marrow (BM) chimeric mice by reconstituting irradiated mice with equal parts BM isolated from WT or Ptgis−/− mice and infected these mice with Puerto Rico/8/1934 H1N1 (PR8) influenza A virus (IVA) (Figure 2I). Here, the CD4+ T cell response is Th1 cell dominated and encompasses both the IFN-γ-heavy Th1 induction and the IL-10-producing contraction phases.27 Indeed, Ptgis−/− Th1 cells isolated from the lungs of infected mice showed, similar to what we observed in the colitis model, a defect in IL-10 co-induction (Figure 2I).
Together, these data indicate that intrinsic PGI2-R signaling induced by CD4+ T cell-autonomous prostacyclin production is required for IL-10 co-induction and initiation of the contraction program by Th1 cells in vivo and restrains Th1 cell immunity during intestinal inflammation and viral infection.
The PGI2-R limits intrinsic Th1 cell-sustaining IL-1β activity via IL-1R2 induction
To identify how PGI2-R controls Th1 cell contraction, we performed RNA-seq analyses of ex vivo CD3 + CD28-activated CD4+ T cells from Ptgir−/− and WT animals (Figure 3A). Among the highest-ranking genes showing expression reduction in Ptgir−/− T cells, we noted, as expected, the prostacyclin receptor but also the Il1r2 gene encoding the IL-1R2 (Figure 3B; Table S2). Accordingly, CD4+ T cells from both Ptgir−/− and C5ar2−/− mice showed a significant impairment of IL-1R2 protein upregulation after CD3 + CD28 stimulation (Figure 3C). IL-1R2 is expressed by a range of immune and non-immune cells, is considered an IL-1 decoy receptor and negative regulator of IL-1 signaling,28,29 and can modulate Th17 and Treg cell responses.30,31 Given the central role of complement-triggered T cell-intrinsic IL-1β production as a driver of Th1 cell induction and restrainer of Th1 cell contraction (Figure 1A),7 we focused on a potential role for IL-1R2 in enabling co-expression of IL-10 by Th1 cells. We infected WT and Il1r2−/− BM chimeric mice with PR8 IVA (Figure 3D). Nine days after virus infection, the numbers of influenza-specific NP311–325 tetramer-, CD11a-, and CD49d-positive CD4+ T cells in the lung producing IFN-γ were comparable among all animals (Figures 3D and S3A), indicating that Il1r2−/− cells induce anti-viral Th1 cell responses normally. There was also no significant difference in the in vivo proliferative capacity of WT and Il1r2-deficient Th1 cells (Figure S3B). However, Il1r2-deficient Th1 cells showed a marked defect in IL-10 co-induction when (re)activated with PR8-IVA-infected BM-derived dendritic cells ex vivo (Figure 3D), demonstrating that CD4+ T cell-intrinsic expression of IL-1R2 is required for normal IL-10 co-induction in infection-fighting Th1 cells.
Figure 3. PGI2-R limits intrinsic Th1 cell-sustaining IL-1β activity via IL-1R2 induction.

(A) Volcano plot depicting differentially expressed genes (DEGs) between in vitro CD3 + CD28 stimulated (24 h) CD4+ T cells isolated from wild-type (WT) or Ptgir−/− mice (knockout [KO]) (n = 4, one experiment).
(B) Heatmap showing the top DEGs derived from (A) (n = 4).
(C) Percentage of surface IL-1R2-expressing CD3 + CD28-activated (72 h) mouse CD4+ T cells (natural Treg cell-depleted) isolated from WT, Ptgir−/−, or C5ar2−/− animals (n = 3–4, one experiment).
(D) Schematic of influenza infection model used (left) with a representative FACS plot of IFN-γ and/or IL-10-producing lung, bone marrow (BM)-derived dendritic cell-restimulated CD4+ T cells (middle), and the summary of data from four animals used (right) (n = 4, one experiment).
(E) Percentage of surface IL-1R2-expressing human CD4+ T cells (nTreg cell-depleted) after CD3 + CD28 or CD3 + CD46 activation (36 h) (n = 5, two independent experiments).
(F) Impact of PGI2-R antagonism on IL-1R2 expression induction by CD3 + CD46 stimulation of CD4+ T cells (n = 3, two independent experiments).
(G) IFN-γ and/or IL-10 production by IL-1R2negative/low, IL-1R2intermediate, and IL-2R2high T cells after CD3 + CD46 activation (36 h). Shown is a representative FACS plot (statistical analysis in Figure S3E, n = 3, three independent experiments).
(H) IFN-γ+, IL-10+, and IFN-γ:IL-10+ T cell ratio of CD3 + CD46 activated (36 h) T cells with or without concurrent addition of a neutralizing antibody to IL-1R2 (n = 5, three independent experiments; −, cells treated with an isotype control).
(I) Activated IL-1β protein amounts detected in culture media in experiments performed as described under (H) (n = 3).
(J) Suggested model of T cell-intrinsic C5aR2/PGI2-R-induced IL-1R2 expression, which negatively controls cell-autonomous IL-1β production and enables timely Th1 cell IL-10 switching.
Groups were compared using one-way or two-way ANOVA or the paired Student’s t test and error bars in graphs represent mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.005. See also Figure S3.
This mechanism seems to extend to human Th1 cells. CD3 + CD46 activation (which is upstream of the C5 axis in human T cells; Figure 1A) of purified and natural Treg (nTreg)-depleted human CD4+ T cells induced significantly increased expression of IL-1R2 when compared with CD3 + CD28 stimulated T cells at 18 h in culture (Figure 3E). Antagonizing PGI2-R or C5aR2 activity strongly reduced induction of IL-1R2 expression during activation (Figures 3F and S3C). Furthermore, IL-10-producing Th1 cells expressed elevated amounts of IL-1R2 compared with IL-10-non-producing Th1 cells, with the highest amounts of IL-1R2 protein present on IFN-γ−IL-10+ T cells (Figure S3D). Similarly, assessment of IFN-γ and IL-10 production by IL-1R2negative/low, IL-1R2intermediate, and IL-1R2high CD4+ T cells in culture after 36 h of CD3 + CD46 activation demonstrated that amounts of IL-1R2 expression correlated positively with IL-10 co-production and that IL-1R2high CD4+ T cells were the predominant IL-10-producing cells (Figures 3G and S3E). Curtailing the IL-1β inhibitory activity of IL-1R2 through the addition of a function-blocking antibody during CD3 + CD46 activation had no impact on IFN-γ responses but reduced specifically the number of IL-10+ T cells and the amounts of IL-10 secreted into the cell supernatant and sustained an increased IFN-γ:IL-10 ratio when compared with T cells activated in the presence of an isotype control antibody (Figures 3H and S3F). As expected, IL-1β amounts detectable in the cell supernatant by CD3 + CD46-induced Th1 cells also increased during IL-1R2 blockage (Figure 3I), while IL-17 production and cell viability remained unaffected during in vitro activation (Figures S3G and S3H).
Overall, these data indicate that PGI2-R engagement by Th1 cells downstream of C5aR2 induces IL-1R2 expression that neutralizes CD4+ T cell-intrinsic IL-1β production and enables IL-10 co-induction and Th1 cell contraction (Figure 3J).
PGI2-R mediates Th1 cell contraction through PKA activity
The four PGE2 receptors, EP1 to 4, and the prostacyclin PGI2-R are major inducers of cyclic adenosine monophosphate (cAMP),32,33 and a key activity of cAMP in T cells is the activation of protein kinase A (PKA).34 Active PKA, in turn, restrains TCR signaling and controls T cell activation overall but also induces T cell gene expression by phosphorylation of the transcription factors cAMP responsive element (CRE)-binding protein (CREB) specifically during Th1 cell induction.34,35 “cAMP-mediated signaling” was the most downregulated signaling pathway in Ptgir-deficient mouse CD4+ T cells, while “CD28 signaling in T helper cells” and “TCR signaling” were among the top three up-regulated pathways (Figure 4A). This suggested to us that cell-intrinsic PGI2 production may control Th1 cell IL-10 co-induction through modulation of PKA activity. Indeed, we observed stark differences in the amounts of activated PKA between subsorted human IFN-γ+IL-10−, IFN-γ+IL-10+, and IFN-γ−IL-10+ CD4+ T cells, with significantly increased active PKA when Th1 cells initiated IL-10 co-production (Figure 4B). CD3 + CD46 activation moderately augmented PKA activation in bulk human CD4+ T cell cultures (Figures 4C and S4A). However, the addition of PGE2 during stimulation reduced activation of PKA below the amounts induced by CD3 + CD46 activation (Figures 4C and S4A). PGI2 supplementation, on the other hand, increased PKA activation in stimulated T cells (Figures 4C and S4A). Changes in PKA-mediated phosphorylation of CREB in IL-10+ T cells mimicked those observed for PKA activation in the presence of PGE2 or PGI2 (Figure S4B). We next assessed the effect of PGE2 or PGI2 provision on Th1 cell induction or contraction during CD3 + CD46 stimulation in the presence or absence of PKA inhibition. Abrogation of PKA activity had no consistent effect on Th1 cell IFN-γ production but hampered their ability to transition into IL-10 co-generation (Figures 4D and S4C) and to increase IL-2R2 expression (Figure 4E). Inhibition of PKA also impeded the PGI2-driven increase in CREB phosphorylation (Figure S4B), suggesting that PGI2-R signaling enables Th1 cell IL-10 switching through the PKA-CREB pathway.
Figure 4. PGI2-R mediates Th1 cell contraction through PKA activity.

(A) Top increased (orange) and reduced (blue) biological pathways based on differentially expressed genes (DEGs) derived from in vitro CD3 + CD28 stimulated CD4+ T cells isolated from wild-type (WT) or Ptgir−/− (KO) mice (n = 4, one experiment).
(B) Quantification of activated PKA (phosphorylated [pPKA], normalized to total PKA [tPKA]) in IFN-γ+IL-10−, IFN-γ+IL-10+, and IFN-γ−IL-10+ CD4+ T cells generated by CD3 + CD46 activation (36 h) (n = 3, three independent experiments).
(C) Amounts of pPKA in CD3 + CD46-activated T cells (18 h) (left) with or without addition of either PGE2 or PGI2 (right) (n = 5, three independent experiments).
(D) Impact of PGE2 vs. PGI2 provision in the presence or absence of a PKA inhibitor (inh.) on IFN-γ and IL-10 production in CD3 + CD46-activated T cells (36 h). Representative FACS plots from one of three donors (left) with statistical analysis of cumulative data (right, n = 3, three independent experiments).
(E) Effect of PKA inhibition on IL-1R2 expression by CD3 + CD46-activated T cells (36 h). Representative FACS plots from one of three donors (left) and statistical analysis of cumulative data (right, n = 3, two independent experiments).
(F) Amounts of mRNAs encoding PTGES2, IL1R2, or IL10 in CD3 + CD46-activated T cells (8 h) with or without PGI2 addition to media assessed by quantitative PCR (n = 3, one experiment).
(G) Effect of PGI2-R expression reduction by PTGIR siRNA treatment in CD3 + CD46-activated T cells (see also Figure S4E) on IL-10 expression and PKA and CREB activation (n = 3, three independent experiments).
(H) Effect of PGI2-R expression reduction by PTGIR siRNA treatment in CD3 + CD46-activated T cells (48 h) on PTGES2, IL1R2, or IL10 transcription (n = 4–5, four independent experiments).
(I) Schematic of the effects of PGE2-EP4 vs. PGI2-PGI2-R activity on CD4+ T cell PKA activation and transcription of indicated genes. The effect of PGI2-R engagement on IL10 transcription vs. translation is not clear (denoted by “?”).
Groups were compared using one-way ANOVA or the Student’s t test. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001. See also Figure S4.
The addition of PGI2 to CD3 + CD46-activated bulk CD4+ T cells after 8 h of stimulation failed to change transcription of the PTGES2 gene but substantially augmented IL1R2 transcription, which was partially reversed by PKA inhibition (Figures 4F and S4D). Unexpectedly, we observed a significant decrease in IL10 transcript amounts upon PGI2 provision at this time point (Figure 4F). The expression reduction of PGI2-R via PTGIR siRNA treatment of CD4+ T cells reduced the numbers of IL-10-, activated PKA-, and pCREB-positive T cells in CD3 + CD46-activated cultures at 48 h post activation (Figures 4G, S4E, and S4F). Furthermore, CD3 + CD46-activated T cells with an engineered reduction in PTGIR expression displayed higher PTGES2 and lower IL1R2 gene transcription but no changes in IL10 transcription (Figure 4H). The discrepancy between PGI2-R-mediated effects on IL10 gene transcription and IL-10 protein generation may reflect more complex kinetics of IL10 gene expression during the Th1 cell life cycle or potentially the involvement of PGI2 in translational control of IL-10.36
These data collectively indicate that PGI2-R (and not EP4)-mediated activation of PKA is a prerequisite for IL-10 co-expression in Th1 cells and involves the direct modulation of PTGES2 and IL1R2 transcription (Figure 4J).
Unbalanced intrinsic prostanoid metabolism limits Th1 cell contraction in individuals with CAPS
CD4+ T cells from rare individuals with gain-of-function (GoF) mutations in the NLRP3 gene and that suffer from CAPS have, in addition to increased production of IL-1β by innate immune cells,37 increased Th1 cell induction due to their uncontrolled NLRP3-driven T cell-autologous IL-1β generation that sustains IFN-γ secretion (Figure S5A).7 Whether T cell-intrinsic prostanoid metabolism and the IL-10 co-induction program may be perturbed in Th1 cells from individuals with CAPS is unexplored. We therefore activated purified CD4+ T cells from nine individuals with CAPS (Table S3) in vitro with CD3 + CD46 and assessed their C5aR2-controlled prostanoid generation and response machinery in comparison with CD4+ T cells isolated from sex- and age-matched healthy control individuals. Due to the limited blood volume available from individuals with CAPS, select experiments were performed with each sample. As previously observed,7 CD4+ T cells from individuals with CAPS secreted significantly higher amounts of IL-1β and IFN-γ (Figures 5A and 5B). This was associated with substantially reduced co-induction of IL-10 (Figures 5A and 5B) and viability (Figure S5B) when compared with healthy control T cells. Further, in vitro activated CAPS T cells showed a significant shift toward the enzyme axis driving PGE2 production with concurrent blunting of the prostacyclin “arm” mediating Th1 cell contraction: COX-2 and PTGES2 enzyme expression was augmented, while PTGIS induction was reduced, and the expression of C5aR2, PGI2-R, and IL1-R2 proteins was decreased (EP4 expression could not be assessed due to limited material) in T cells from individuals with CAPS when compared with T cells from healthy controls (Figures 5C, 5D, S5C, and S5D). In agreement with our finding that intrinsic PGI2-R stimulation drives PKA activation and CREB phosphorylation upstream of IL-10 induction (Figure 4) and that this axis is muted in T cells isolated from individuals with CAPS, we noted that amounts of active PKA (and possibly pCREB) were reduced in the two CAPS T cell samples we could assess (Figure S5D).
Figure 5. Unbalanced intrinsic prostanoid metabolism limits Th1 cell contraction in individuals with CAPS.

(A) IL-1β, IFN-γ, and IL-10 secreted by CD4+ T cells isolated from the indicated individuals with cryopyrin-associated periodic syndrome (CAPS) or sex- and age-matched healthy control donors (HDs) measured at 12–24 h post CD3 + CD46 activation (n = 5, five independent experiments).
(B) Representative FACS plot of cell staining for IFN-γ and IL-10 production by HD3 and CAPS3 CD4+ T cells upon CD3 + CD46 activation (24 h) (of a total of n = 3, three independent experiments).
(C) Expression of COX-2, PTGES2, and PTGIS in CD4+ T cells isolated from the indicated individuals with CAPS or HDs measured at 36 h post CD3 + CD46 activation (n = 3–4, three to four independent experiments).
(D) Protein expression of C5aR2, PTGI2-R, and IL-1R2 in CD4+ T cells isolated from the indicated individuals with CAPS or HDs measured at 36 h post CD3 + CD46 activation (n = 3–4, three to four independent experiments).
(E) IL-10 production by T cells isolated from indicated individuals with CAPS after CD3 + CD46 activation with or without either a specific PTGES2 inhibitor (MF63, 1 μM) or soluble IL-1R2 (5 μg/mL) at 12 h post activation (n = 6–7, six independent experiments).
(F) Schematic detailing the shifted balance of the intrinsic prostanoid generation and response machinery toward PGE2 production underlying faulty IL-10 production in CAPS T cells, which could be rescued by PTGES2 inhibition or IL-1R2 provision.
Groups were compared using the paired or non-paired Student’s t test, error bars in graphs represent mean ± SD. *p < 0.05, **p < 0.01. See also Figure S5.
We next attempted to restore Th1 cell contraction in T cells from individuals with CAPS in vitro. Neither the addition of a specific C5aR2 (P32)38 nor PGI2-R agonist during CD3 + CD46 activation normalized IL-10 production by the individuals’ T cells (Figure S5E), likely due to reduced C5aR2 and PGI2-R expression by CAPS Th1 cells. However, both the provision of soluble IL-1R2 during T cell activation and the inhibition of PTGES2 with the specific inhibitor MF6339 caused a substantial increase in IL-10-producing Th1 cells (Figures 5E and S5F). Inhibition of PTGES2 activity in T cells from individuals with CAPS also reinstated the defective expression of IL-1R2 (Figure S5G), aligning with our cumulative data showing that PGE2 production by T cells restrains engagement of the prostacyclin/IL-1R2 shutdown axis.
In summary, CD4+ T cells from individuals with CAPS are, in addition to mutated NLRP3-driven hyper-IL-1β production, characterized by a pathologically imbalanced intrinsic PGE2 vs. prostacyclin induction axis (Figure 5F), which limits their normal IL-10 co-induction and Th1 cell contraction program. Th1 cell contraction can be restored ex vivo by normalizing hyperactive PGE2 generation through PTGES2 inhibition or by provision of soluble IL-1R2, which neutralizes IL-1β.
C5aR2-PTGIS-PGI2-R axis perturbations demarcate autoimmunity-driving CD4+ T cells
We next addressed whether CD4+ T cells associated with Th1 cell-driven autoimmunity show signatures associated with prostacyclin dysregulation. We first performed RNA-seq upon either PTGIS or PTGIR knockdown in human nTreg-depleted CD4+ T cells after anti-CD3 and anti-CD46 stimulation for 48 h (Figure 6A). PTGIS knockdown in in vitro activated T cells caused substantial cell death (Figure S6) and hence reduced generation of IFN-γ+IL-10− cells, meaning cell numbers were too few for RNA-seq. Nonetheless, for other comparisons, we saw substantial transcriptional changes, particularly in IFN-γ−IL-10− cell populations, including, as expected, a large overlap in differentially expressed genes (DEGs) between PTGIR and PTGIS knockdown conditions (Figures 6B and 6C; Table S4). We next used these DEGs to define a CD4+ T cell-specific bespoke gene signature representing a perturbed intrinsic PTGIS-PGI2-R axis (n = 224 genes; Figure 6C). To understand if this prostacyclin-perturbed signature is present in Th1 cell-driven inflammatory disease, we utilized three public datasets: one consisting of intraepithelial lymphocytes in healthy vs. Crohn’s intestinal tissue and two consisting of immune cells from matched blood and synovial fluid samples of individuals with psoriatic arthritis or juvenile idiopathic arthritis to identify perturbed pathways upon migration of CD4+ T cells into an inflammatory environment in the same individual (Figures 6D and 6E; STAR Methods). After cell clustering (Figure 6E), we performed pathway analysis with single-cell pathway analysis (SCPA)40 for each CD4+ T cell cluster across conditions and for each dataset: i.e., T effector memory (Tem) cells in healthy vs. Tem cells in Crohn’s disease, then T resident memory (Trm) cells in healthy vs. Trm cells in Crohn’s disease, and so on. This allowed an understanding of pathways that are perturbed in each CD4+ T cell population across disease conditions. In addition to the expected “IFNG response” that usually demarcates hyper-Th1 cell activity, our gene signatures representing perturbed CD4+ T cell-intrinsic C5AR2 and prostacyclin (PTGIR/PTGIS) activity were among the topmost dysregulated pathways across all diseases (Figures 6F and 6G). This suggested that CD4+ T cells contributing to human inflammatory diseases share similar transcriptomic differences to those observed upon PTGIR or PTGIS deficiency.
Figure 6. C5aR2-PTGIS-PGI2-R axis perturbations demarcate autoimmunity-driving CD4+ T cells.

(A) Outline for RNA-seq experiment where Foxp3− human CD4+ T cells were CD3 + CD46 stimulated (48 h) in the presence of either a scrambled control (scr) or PTGIR or PTGIS targeting siRNA and subsequent bulk RNA-seq of sorted IFN-γ−IL-10− and IFN-γ+ cells (n = 3, one experiment).
(B) Volcano plots showing logFC and false discovery rate (FDR) values from differential expression testing, and PTGIR and PTGIS expression is highlighted in each plot. Significant DEGs are highlighted in blue/red, based on FDR < 0.05 and absolute logFC > 0.6 (n = 3, one experiment).
(C) Venn diagram depicting the strategy employed to generate a PTGIS-PTGIR signature for downstream analyses.
(D) Tissue sources of scRNA-seq datasets used for pathway analysis with intestinal datasets from healthy vs. individuals with Crohn’s samples and psoriatic arthritis (PSA) and juvenile idiopathic arthritis (JIA) datasets of matched blood and joint samples, respectively.
(E) Uniform manifold approximation and projection (UMAP) representations of the three datasets used, showing cell clustering (upper UMAPs) and tissue status or origin (lower UMAPs) for each.
(F) Heatmap of pathway analysis results from single-cell pathway analysis (SCPA). Each row represents a comparison of the specified cell type across conditions, e.g., the first row shows pathway changes in the Tem cell population in the blood of JIA vs. the joint, and so on.
(G) Rank plot from SCPA focusing on the JIA dataset, highlighting the rank of the IFNG response (derived from an IFN signature indiscriminate of type I and II responses), PTGIR-PTGIS, and C5AR2 signatures across cell types.
See also Figure S6.
These data indicate that the CD4+ T cell-intrinsic prostacyclin machinery shows significant alterations in arthritis and Crohn’s disease and may contribute to the hyper-Th1 cell responses underlying these common autoimmune pathologies.
DISCUSSION
Here, we discovered that Th1 cells harbor and engage an intrinsic C5-controlled prostanoid production and response machinery and that perturbations in the temporally controlled balance of cell-autonomous prostanoid metabolism are associated with hyper-Th1 cell responses in inflammatory diseases. A generally held belief is that T cells are solely responsive to environmental lipid mediators and that the effects of PGE2 on T cell responses are largely activating. However, these two perceptions have recently been challenged, as CD4+ T effector cells can produce PGE2, and, further, such T cell-generated PGE2 dictates T cell function in an intrinsic fashion.41,42 Moreover, PGE2 exerts pleotropic and context-dependent effects on - T cell priming and effector functions,43 which include both inhibition and augmentation of Th1, Th17, and nTreg cell responses.41,42,44,45 An elegant concept developed by the Crofford laboratory suggests that different concentrations of PGE2 collectively provided by tissue CD4+ T and non-T cells shape the pro- and anti-inflammatory T cell response at mucosal interfaces.41 Our data here suggest that this concept extends to prostacyclin (PGI2).
The impact of exogenous PGI2 on effector T cell activity is less explored but seems to be largely immunosuppressive46 and operates partially through licensing of nTreg cell function.22 T cell-intrinsic generation and function of prostacyclin have not been described, and its effects on Th1 cell biology remain unexplored.47 Moreover, the effects of distinct prostanoid lipid mediator activities on T cells have been studied in isolation. The integration here of cell-autonomous, somewhat opposing, effects of PGE2 and prostacyclin into the distinct Th1 cell life cycle phases aligns with the concept of an intrinsic “checks and balances” system that Th1 cells employ to prevent hyper-Th1 cell-mediated tissue pathology.48 Our suggestion that changes in the PGE2/PGI2 balance not only contribute to uncontrolled Th1 cell responses seen in CAPS but also to those underlying RA and Crohn’s disease aligns with our previous work where we defined the induction of arachidonic acid (the substrate for COX-1/2 enzymes) metabolism as a prerequisite for IFN-γ production by CD4+ T cells40 and with a recent study that demonstrated increased arachidonic acid responsiveness in RA-driving T cells.49 Furthermore, PTGIS emerged as one of the top genes reduced through promotor hypermethylation in the tissue of colons from individuals with Crohn’s disease when compared with healthy donors, although the cell type associated with PTGIS reduction was not identified in this study.50 The presence of an unexpected sliding balance between PGE2 and PGI2 generation within a Th1 cell response population may also explain the divergent results that COX-1/2 inhibitors can exert on acute and/or chronic Th1 cell activity51,52: timing and dosing will impose distinct effects on the normal COX-1/2 downstream PGE2 vs. PGI2 production balance. It will likely also be important to better dissect the roles of intrinsic EP2 and EP4 receptor signaling and their specific effects on general T cell activation and proliferation vs. IFN-γ production. Thus, as has been shown for PGE2, we expect the net outcome of intrinsic and environmental prostacyclin-driven PGI2-R engagement to be complex and context-dependent.
The identification of the T cell-autonomous C5 system as apex controller of specific prostanoid lipid mediator generation is in agreement with the emerging central role of cell-intrinsic complement (the complosome) as key regulator of the metabolic pathways directing immune cell responses, which include glycolysis, oxidative phosphorylation, and fatty acid synthesis.7,53,54 Given the expansive biological roles of prostanoids in, for example, pain perception, endothelial and epithelial cell responsiveness, platelet activation and thrombosis,55 and the ubiquitous presence of cell-autonomous complement across immune and non-immune cells,56 we expect the functional C5-prostanoid connection discovered here in Th1 cells to extend to other cells and activities and, thus, be of broader significance. For example, we explored here mostly effector Th1 cell-prone experimental systems; however, C5aR signaling impacts on cells underlying type 2 and 3 immunity,57,58 which also integrate prostanoid-derived (and specifically prostacyclin-mediated) signals for functional control.59–62 Also, cell-autonomous C5aR signaling engages during stimulation of CD8+ cytotoxic T cells,63 cells on which PGI2-R signaling controls exhaustion.64 Thus, exploring these cell populations may be a suitable starting point to understand the biological reach of the C5-prostanoid crosstalk.
C5’s integration into both induction (C5aR1-driven PGE2 generation and activated IL-1β secretion) and contraction (C5aR2-induced prostacyclin production and IL-1R2 expression) of Th1 cell activity fuels the field’s departure from the long-standing concept that C5, and complement in general, is solely pro-inflammatory toward the understanding that it is also a critical driver of the resolution of tissue inflammation.65,66 The identification of C5aR2 as inducer of IL-1β-neutralizing IL-1R2 is particularly exciting, as IL-1β is a central trigger and perpetuator of inflammation, while comparatively less is known about the endogenous pathways that rein it in.67 It may be beneficial to explore whether the inhibitory activity of C5aR2 on other immune cells, for example, macrophages, is also mediated through tempering of intrinsic IL-1β production via IL-1R2 provision.8,53,68
The circuitous functional relationship between IL-1β and prostaglandins has been recognized,69,70 and both players are considered valuable therapeutic targets for the treatment of inflammatory conditions.71,72 Complement C5 emerges as an important upstream controller of this circuit and may provide an additional means to modulate prostanoid and IL-1β generation at will. Of note, C5aR1-mediated signals emerged as strong upstream inducers of NLRP3 activation,53,73 which suggests that targeting C5aR signaling could potentially impact another central component of the IL-1β induction pathway. Successful therapies will, however, need to embrace the understanding that immune- and non-immune cell-derived complement critical to protective and aberrant tissue immunity74–76 operates largely independent from the classic serum-derived complement system.5,77 This is reflected by the fact that currently approved complement therapeutics target circulating liver-derived complement only and are effective in complement-driven blood disorders (paroxysmal nocturnal hemoglobinuria, hemolytic uremic syndrome) and vasculopathies56,78 but failed in clinical trials for tissue inflammatory conditions such as RA or multiple sclerosis.56 Thus, a better understanding of when and how to inhibit or activate the complement system for a desired outcome needs to go hand-in-hand with increasing tissue penetrance (and cell permeability) of complement drugs.
In summary, we provide fundamental insights into the metabolic rewiring underlying the induction and contraction of Th1 cell effector responses and identify complement C5 as a key controller of T cell-intrinsic prostanoid generation and IL-1β “self-control.”
Limitations of the study
We have currently not integrated the role of C5aR1-mediated NLRP3 activation,7,53 which is an important component of IL-1β maturation, into the cell-autonomous C5aR-prostanoid axis discovered here. Similarly, the functional significance of TXA2 intrinsically generated by activated CD4+ T cells warrants further exploration. We have also not studied the effects of the cell-intrinsic C5-prostanoid crosstalk on other aspects of Th1 cell biology, for example, memory responses and exhaustion.79 Here, we focused largely on the regulative or contractive phase of Th1 cell immunity and its importance in the prevention of tissue pathology. However, both prostacyclin and IL-1R2 also impact nTreg cells, an independent key population mediating immune control and tolerance.29,62,80 Although we have removed nTregs in our in vitro and in vivo experiments performed here, it will nonetheless be beneficial to explore in the future whether the C5-prostanoid axis may also impact the biology of nTreg cells. Finally, although our data indicate that perturbations in the Th1 cell C5aR2-PGI2-R axis may extend beyond CAPS to RA and Crohn’s disease, this remains to be proven formally.
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Claudia Kemper (claudia.kemper@nih.gov).
Materials availability
No unique materials or reagents were generated in this study.
Data and code availability
RNA-seq data for Figure 1 (C5aR2 antagonist-treated human CD4+ T cells), RNA-seq data for Figure 3 (mouse WT vs. Ptgir−/− CD4+ T cells), and RNA-seq data for Figure 6 (human PTGIS- and PTGIR-deficient CD4+ T cells) are deposited to the GEO database. The accession numbers are listed in the key resources table. The data are publicly available from the date of publication.
This paper analyzes existing, publicly available data, related to Figure 6.81–83 The pertinent references are listed in the key resources table.
This paper does not report original code. Code to replicate all microarray, bulk RNA-seq and single-cell RNA-seq (scRNA-seq) analysis in the paper can be found at https://github.com/jackbibby1/Rahman-J-2024 (Zenodo https://doi.org/10.5281/zenodo.15222862).
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| PE-anti-human T-bet (QA18A24) | Biolegend | Cat# 365904; RRID: AB_3675491 |
| Pacific Blue-mouse IgG1κ, isotype ctrl. (MOPC-21) | Biolegend | Cat#400151; RRID: AB_3675489 |
| Pacific Blue-anti-human/mouse T-bet (4B10) | Biolegend | Cat# 644808; RRID: AB_1595479 |
| FITC-mouse IgG1κ, isotype ctrl. (MOPC-21) | Biolegend | Cat# 400138; RRID: AB_3675490) |
| FITC-anti-human CD3 (HIT3a) | Biolegend | Cat# 300306; RRID: AB_314041) |
| Brilliant Violet 421-anti-mouse CD4 (GK1.5) | Biolegend | Cat# 100437; RRID: AB_10900241 |
| Brilliant Violet 421-anti-human FOXP3 (206D) | Biolegend | Cat# 320124; RRID: AB_2565972 |
| Alexa Fluor 647-anti-mouse IL-10 (JES5-16E3) | Biolegend | Cat# 505014; RRID: AB_493511 |
| PE-anti-mouse CD11a (I21/7) | Biolegend | Cat# 153104; RRID: AB_2716034 |
| Alexa Fluor-647 anti-human IL-10 (JES3-9D7) | Biolegend | Cat# 501412, RRID: AB_493318 |
| Brilliant Violet 711-anti-human IFN-γ(4S.B3) | Biolegend | Cat# 502540; RRID: AB_2563506 |
| PE-anti-mouse CD25 (A18246A) | Biolegend | Cat# 113703; RRID: AB_2927943 |
| APC-anti-mouse CD25 (PC61) | Biolegend | Cat# 102012; RRID: AB_312861 |
| FITC-anti-mouse CD45RB (C363-16A) | Biolegend | Cat# 103305: RRID: AB_313012 |
| PE-Cyanine7-anti-mouse CD49d (R1-2) | Biolegend | Cat# 103618; RRID: AB_2563700 |
| PerCp/Cyanine5.5-anti-mouse CD45.1 (A20) | Biolegend | Cat# 110728; RRID: AB_893346 |
| APC-Cyanine7-anti-mouse CD45.1 (A20) | Biolegend | Cat# 110716; RRID: AB_313505 |
| APC-anti-human CD56 (5.1H11) | Biolegend | Cat# 362504; RRID: AB_2563912 |
| FITC-anti-mouse CD45.2 (104) | Biolegend | Cat# 109806; RRID: AB_313442 |
| Brilliant Violet 510-anti-mouse CD90.2 (Thy1.2) (53-2.1) | Biolegend | Cat# 140319; RRID: AB_2561395 |
| Alexa Fluor 488-anti-mouse CD107a (1D4B) | Biolegend | Cat# 121608; RRID: AB_571983 |
| Alexa Fluor 488-anti-mouse CD107b (M3/84) | Biolegend | Cat# 108510; RRID: AB_493308 |
| Brilliant Violet 605-anti-mouse CD4 (GK1.5) | Biolegend | Cat# 100451; RRID: AB_2564591 |
| PE-anti-mouse FOXP3 (MF-14) | Biolegend | Cat# 126404; RRID: AB_1098117 |
| Rabbit anti-human HSP90 (C45G5) | Cell Signaling Technology | Cat# 4877; RRID: AB_2233307 |
| Rabbit anti-human PTGIS | LS bio | Cat# LS-C372587; RRID: AB_3675478 |
| Rabbit anti-human PTGIR | LS bio | Cat# LS-B11456-50; RRID: AB_3675477 |
| PE-anti-mouse IL-10 (JESS-16E3) | Invitrogen | Cat# 12-710182; RRID: AB_466176 |
| APC-anti-mouse IL-17A (TC11-1810.1) | Biolegend | Cat# 11-7311-82; RRID: AB_465412 |
| Rabbit anti-human β-Actin | LS bio | Cat# LS-B1040-50; RRID: AB_968470 |
| Alexa Fluor 488-anti-mouse IFN-γ (XMG1.2) | Biolegend | Cat# 505813; RRID: AB_493312 |
| PE-anti-mouse IL-17A (TC11-18H10.1) | Biolegend | Cat# 506904; RRID: AB_315464 |
| Anti-mouse IL-1R2 (AF563) | R & D Systems | Cat# AF563; RRID: AB_2125055 |
| Brilliant Violet 711-anti-mouse/human Ki67 (B56) | BD Biosciences | Cat# 563755; RRID: AB_2738406 |
| PE-anti-human IL-1RII Antibody (34141) | R & D Systems | Cat# FAB663P; RRID: AB_1964613 |
| Brilliant Violet 510-anti-mouse TCRβ (H57-597) | Biolegend | Cat# 109234; RRID: AB_2562350 |
| PE-anti-mouse IL-17A (TC11-18H10.1) | Biolegend | Cat# 506904; RRID: AB_315464 |
| Goat anti-human IL-1RII Antibody | R & D Systems | Cat# AF-263-NA; RRID: AB_354431 |
| Rabbit anti-human PTGER4 | Thermo Fisher Scientific | Cat# PA5-18476; RRID: AB_10981059 |
| Anti-human COX-2 (495222) | R & D Systems | Cat # 612596; RRID: AB_399879 |
| APC-anti-goat IgG | R & D Systems | Cat# F0108; RRID: AB_573124 |
| Mouse monoclonal IgG (20116) | R & D Systems | Cat# MAB004; RRID: AB_357346 |
| APC-anti-human IL-10 | Biolegend | Cat# 506807; RRID: AB_315457 |
| PE-Violet 770-anti-human IFN-γ (45-15) | Miltenyi Biotec | Cat# 130-113-499; RRID: AB_2726168 |
| APC-anti-human CD4 (OKT4) | Biolegend | Cat# 317416; RRID: AB_571945 |
| APC-anti-human CD69 (FN50) | Biolegend | Cat# 310910; RRID: AB_314845 |
| APC-anti-human C5aR2 (1D9-M12) | Biolegend | 342406; RRID: AB_2564331 |
| Alexa Fluor 488-anti-human RORγt (Q21-559) | BD Biosciences | Cat# 563621; RRID: AB_2738325 |
| Pacific Blue-anti-mouse IFN-γ (XMG1.2) | Biolegend | Cat# 505818; RRID: AB_528922 |
| Alexa Fluor 647-anti-mouse IL-10 (JES5-16E3) | Biolegend | Cat# 505818; RIDD: AB_2125096 |
| PE-Cyanine7-anti-human IL-17A (eBio64DEC17) | Thermo Fisher Scientific | Cat# 25-7179-42; RRID: AB_11063994 |
| Anti-human PTGER2 (ARC1393) | Thermo Fisher Scientific | Cat# MA5-35750; RRID: AB_2849650 |
| Alexa Fluor 488-anti-human IL-17A | Biolegend | Cat# 512308; RRID: AB_961386 |
| Anti-mouse CD3 (145-2C11) | BioXcell | Cat# BE0001-1; RRID: AB_1107634 |
| Anti-mouse CD28 (37.51) | BioXcell | Cat # BE0015-1; RRID: AB_1107624 |
| Anti-human CD3 (OKT3) | BioXcell | Cat# BE0001-2; RRID: AB_1107632 |
| Anti-human CD28 (CD28.2) | BD Biosciences | Cat # 555726; RRID: AB_396069 |
| Anti-human CD46 (TRA2-10) | Wash-U Hybridoma Center (WUHC) | Clone TRA-2-10; RRID: AB_10895912 |
| Rabbit anti-human PTGES2 | Thermo Fisher Scientific | Cat# PA5-22213; RRID: AB_11154153 |
| PE-Cy7-anti-Mouse RORγt (Q31-378) | BD Biosciences | Cat# 567305; RRID: AB_2916546 |
| TruStain FcX PLUS anti-mouse CD16/32 (S17011E) | Biolegend | Cat# 156604; RRID: AB_2783137 |
|
| ||
| Bacterial and virus strains | ||
|
| ||
| Influenza PR8-33 (H1N1) | Mueller et al.84 | N/A |
|
| ||
| Biological samples | ||
|
| ||
| Healthy donor blood buffy coats | NIH blood Bank | N/A |
| Blood samples from individuals with CAPS | NIH, IRB#17-I-0016, Table S3 | N/A |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Brilliant Violet-conjugated NP311-325 | NIH Tetramer Core | N/A |
| tetramer | ||
| Human IL-1R2 protein, recombinant | R & D Systems | Cat# 263-2R-050/CF |
| C5aR2 agonist (P32, Ac-RHYPYWR-OH) | Li et al.68 | N/A |
| Human IL-2 protein, recombinant | Peprotech | Cat# 200-02 |
| PGI2 (prostacyclin) | Cayman Chemicals | Cat# 14010 |
| PGI2-R agonist (epoprostenol) | TOCRIS | Cat# 2989 |
| PGI2-R antagonist | TOCRIS | Cat# 4268 |
| PGE2 (prostaglandin E2) | Cayman Chemicals | Cat# 14010 |
| EP4 receptor antagonist (L-161,982) | Sigma | Cat# SML0690 |
| EP2 antagonist (C52) | Sigma | Cat# SML3643 |
| LIVE/DEAD™ Fixable Near-IR | Invitrogen | Cat# L10119 |
| LIVE/DEAD™ Fixable Aqua | Invitrogen | Cat# L34965 |
| Cell trace violet | Invitrogen | Cat# C34557 |
| COX-2 inhibitor | Cayman Chemicals | Cat# 70210 |
| mPGES-1 inhibitor (MF63) | Cayman Chemicals | Cat# 13217 |
| 15-hydroxy PGDH inhibitor | Cayman Chemicals | Cat# 18040 |
| TBXAS1 inhibitor | Cayman Chemicals | Cat# 70510 |
| PPARγ antagonist | Cayman Chemicals | Cat# 10026 |
| Cell Stimulation Cocktail (plus protein transport inhibitors) (500x) | Thermo Fisher Scientific | Cat# 00-4975-93 |
| Barbadin | MedChem Express | Cat# HY-119706 |
| RIPA Lysis and Extraction Buffer | Thermo Fisher Scientific | Cat# 89900 |
| Halt™ Protease Inhibitor Cocktail (100x) | Thermo Fisher Scientific | Cat# 78430 |
| Halt Phosphatase Inhibitor Cocktail | Thermo Fisher Scientific | Cat# 78420 |
| PKA (protein kinase A) inhibitor KT 5720 | R & D Systems | Cat# 1288 |
| 4x Laemmli Sample Buffer | BioRad | Cat# 1610747 |
| Trans-Blot Turbo Midi Nitrocellulose Transfer Packs | BioRad | Cat# 1704159EDU |
| eBioscience™ Protein Transport Inhibitor Cocktail (500x) | Thermo Fisher Scientific | Cat# 00498003 |
| Mouse GM-CSF (granulocyte-macrophage colony-stimulating factor), recombinant | Biolegend | Cat# 576304 |
| 1x PBS (phospahe-buffered saline) with 1% Casein | BioRad | Cat# 1610783 |
| 2-Mercaptoethanol | Thermo Fisher Scientific | Cat# 21985-023 |
| GolgiStop | BD Biosciences | Cat# 554724 |
| BD GolgiPlug™ | BD Biosciences | Cat# 555029 |
| Collagenase | Sigma-Aldrich | Cat# C7657 |
| DNAse I | Sigma-Aldrich | Cat# DN25 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Accell Human PTGES2 siRNA | HORIZON | Cat# E-021342-00-0005 |
| Accell Human PTGIS siRNA | HORIZON | Cat# E-004691-00-0005 |
| Accell Human PTGIR siRNA | HORIZON | Cat# E-005716-00-0005 |
| Accell Non-targeting Control Pool | HORIZON | Cat# D-001910-10-05 |
| Human TaqMan probe RPL7 | Thermo Fisher Scientific | CAT# Hs02596927_g1 |
| Human TaqMan probe PTGES2 | Thermo Fisher Scientific | CAT# Hs00228159_m1 |
| Human TaqMan probe PTGIR | Thermo Fisher Scientific | CAT# #Hs01125026_m1 |
| Human TaqMan probe PTGIS | Thermo Fisher Scientific | CAT# Hs00919949_m1 |
| Human TaqMan probe TBXAS1 | Thermo Fisher Scientific | CAT# Hs01022706_m1 |
| Human TaqMan probe IL1R2 | Thermo Fisher Scientific | CAT# Hs00174759_m1 |
| Human TaqMan probe IL10 | Thermo Fisher Scientific | CAT# Hs00961622_m1 |
| Mouse TaqMan probe Hprt | Thermo Fisher Scientific | CAT# Mm01545399_m1 |
| Mouse TaqMan probe Ptges2 | Thermo Fisher Scientific | CAT# Mm00460181_m1 |
| Mouse TaqMan probe Ptgir | Thermo Fisher Scientific | CAT# Mm00801939_m1 |
| Mouse TaqMan probe Ptgis | Thermo Fisher Scientific | CAT# Mm00447271_m1 |
| PKA Assay Kit | Abcam | Cat# 139435 |
| Ebioscience Foxp3/Transcription Factor Staining Buffer Set | Invitrogen | Cat# 00-5521-00 |
| Cytofix/Cytoperm Fixation/Permeabilization Solution Kit | BD Biosciences | Cat# BDB554714 |
| Mouse CD4 T cell isolation kit | Miltenyi Biotech | Cat# 130-104-454 |
| Mouse CD4 T cell isolation kit | Stem Cell Technologies | Cat# 19852 |
| Human CD4 T cell isolation kit | Miltenyi Biotech | Cat# 130-091-155 |
| Human CD4 T cell isolation kit | Stem Cell Technologies | Cat# 17952 |
| EasySep Human CD4+ CD127low CD25+ Regulatory T Cell Isolation Kit | Stem Cell Technologies | Cat# 18063 |
| IFN-γ Secretion Assay | Miltenyi Biotec | Cat# 130-054-202 |
| IL-10 Secretion Assay | Miltenyi Biotec | Cat# 130-090-761 |
| LEGENDplex Human T helper cytokine panel | Biolegend | Cat# 741028 |
| LEGENDplex Human Inflammation panel | Biolegend | Cat# 740809 |
| LEGENDplex Mouse Inflammation panel | Biolegend | Cat# 740446 |
| Human IL-1β/IL-1F2 DuoSet Kit | R & D Systems | Cat# DY201 |
| RNAqueous Micro Kit | Invitrogen | Cat# AM1931 |
| NEBNext Ultra II RNA Library Prep Kit for Illumina | NEB | Cat# E7770 |
| TURBO DNA-free Kit | Invitrogen | Cat# AM1907 |
| High-Capacity cDNA Reverse Transcription Kit | Invitrogen | Cat# 4387406 |
| TaqMan Universal Master Mix II, no UNG | Invitrogen | Cat# 4440043 |
| NEBNext Poly(A) mRNA Magnetic Isolation Module | NEB | Cat# E7490 |
|
| ||
| Deposited data | ||
|
| ||
| RNA-seq of in vitro activated human CD4+ T cells | This paper, Figure 3 | GEO: GSE288921 |
| RNA-seq of in vitro activated mouse CD4+ T cells | This paper, Figure 6 | GEO: GSE288969 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| T cells, bone marrow (BM) cells isolated from C57BL/6 littermate control mice to KO lines used | The Jackson Laboratory | Strain# 000664 |
| T cells, BM cells isolated from Ptgis-/- mice | Ge et al.26 | N/A |
| T cells isolated from Ptgir-/- mice | NIH, Transgenic Core | N/A |
| T cells, BM cells isolated from Il1r2-/- mice | Pinteaux et al.85 | N/A |
| Mouse strain B6 CD45.1 | The Jackson Laboratory | IMSR_JAX:002014 |
| Mouse strain C57BL/10 Rag2-/- | Taconic | Line# 103 |
|
| ||
| Software and algorithms | ||
|
| ||
| R (v4.2.2) | Carvalho et al.86 | N/A |
| FlowJo 10 software | Treestar | https://www.flowjo.com/ |
| FastQC (v0.11.9) | Andrews87 | N/A |
| MultiQC (v1.9) | Ewels et al.88 | N/A |
| edgeR (v3.40.2) | Robinson et al.89 | https://bioconductor.org |
| GraphPad PRISM 9.4.1 | Graphpad software | https://www.graphpad.com |
| Limma | Ritchie et al.90 | https://bioconductor.org |
| STAR (v2.7.10b) | Liao et al.91 | N/A |
| Subread v2.0.3) | Liao et al.91 | N/A |
| ggplot2 (v3.4) | Wickham92 | N/A |
| ComplexHeatmap (v2.14) | Gu et al.93 | N/A |
| Harmony (v1.1) | Korsunsky et al.94 | N/A |
| SCPA (v1.5.4) | Bibby et al.40 | N/A |
|
| ||
| Others | ||
|
| ||
| PBS | Corning | Cat# 21-040-CV |
| RPMI | Thermo Fisher Scientific | Cat# 21870-076 |
| IMDM | Thermo Fisher Scientific | Cat# 12440-053 |
| Lymphoprep separation medium | Corning | Cat# 25-072-CI |
| HBSS | Thermo Fisher Scientific | Cat# 14170-112 |
| Percoll Cytvia | Sigma-Aldrich | Cat# GE17-0891-01 |
| FBS | Sigma-Aldrich | Cat# F0926 |
| HEPES | Corning | Cat# 25-060-CI |
| Penicillin-Streptomycin | Thermo Fisher Scientific | Cat# 15070-63 |
| Glutamine | Thermo Fisher Scientific | Cat# 25030-149 |
STAR★METHODS
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Human participants
Blood samples were obtained from healthy donors and individuals with CAPS and processed with appropriate ethical and institutional approvals from the National Institutes of Health review board (IRB 17-I-0016). All healthy donors and the individuals with CAPS gave informed consent prior to sample collection. CD4+ T cells from adult healthy volunteers (and that were sex- and age-matched to individuals with CAPS in pertinent experiments) were purified from freshly drawn blood or from buffy coats (both NIH Blood Bank, Bethesda, USA) and activated as described in method details. Detailed information on the nine individuals with CAPS and the healthy donors is provided in Table S3.
Mice
Il1r2flox/flox mice were generated by homologous recombination of a targeting vector with F3 and FRT flanked Neo and Puro selection and LoxP sites flanking exon 3 of Il1r2 into a C57BL/6N Tac ES cells, followed by standard generation of chimaeras and Flp removal of selection cassettes (Taconic). Il1r2flox/flox mice on a C57BL/6J background were crossed to CMV-Cre (#6054; Jax) to generate Il1r2+/− and Il1r2−/− littermates, which were born at expected frequencies with no gross phenotype.85 Spleens and femurs were obtained from 5–8 week old female Il1r2−/− mice and appropriate litter-mate controls that were housed in accordance with UK Home Office guildines and with access to food at libidum. Spleens and femurs from Ptgis−/− mice on a C57BL/6N background were obtained from View Solid Biotechnology (Beijing, China)26 and maintained at the Laboratory Animal Center of Shantou University Medical College in line with University animal welfare regulations and with free access to food. Tissues from female 4–7 week old mice were collected and shipped at 4 °C to the National Heart Lung and Blood Institute. The Ptgir−/− mouse strain was generated by the National Heart Lung and Blood Institute’s Transgenic Core using the CRISPR/Cas9 method.95 Briefly, two single guide RNAs (sgRNA), one targeting the first exon (GGGTCCAGGATGTCCATCGC) and the other one targeting the last exon (CCCTTTCCAGACCTGCATCG) of the mouse Ptgir gene, were generated using ThermoFisher’s in vitro transcription service. These two sgRNAs (20 μg/ml each) were co-microinjected with Cas9 mRNA (50 μg/ml, TriLink BioTechnologies) into the cytoplasm of zygotes collected from C57Bl/6N mice (Charles River Laboratories). Injected embryos were cultured overnight in KSOM medium (Millipore Sigma) in a 37 °C incubator with 6% CO2. The next day, embryos that had reached the 2-cell stage of development were implanted into the oviducts of pseudo-pregnant surrogate mothers (CD1 strain from Charles River Laboratory). Offspring born to the foster mothers were genotyped using PCR followed by Sanger sequencing. Founder mice with deletion of the entire ~2Kb between the first and last exons were backcrossed with C57BL/6J mice (Bar Harbor, ME, USA; strain #: 000664) for eight generations. The C57BL/10 female Rag2−ol mice were obtained from Taconic (Hudson, NY, USA), used at an age between 6–14 weeks, and in all experiments appropriate litter mate mice were used as controls. All animals housed at the NIH were maintained in American Association for Accreditation of Laboratory Animal Care (AAALAC)-accredited Biological Safety Level 2 (BSL2) or BSL3 facilities at the NIH, and experiments were conducted in accordance with the ethical review process at NIH and performed in compliance with an animal study proposal approved by the National Heart Lung and Blood Institute Animal Care and Use Committee.
METHOD DETAILS
Antibodies, agonists/antagonists, inhibitors, and prostaglandins
Monoclonal antibodies used to activate human CD4+ T cells were bought from BD Biosciences, San Diego, CA (anti-hCD28, clone CD28.2, #555726), purified from a specific hybridoma (anti-hCD3; OKT-3) or generated in-house (anti-CD46; TRA-2–10) and used at the concentrations (2μg/ml) described under the T cell stimulation protocol. Additional antibodies used for flow cytometric analyses, and/or Western blotting, or inhibition experiments included anti-human CD4 APC (clone OKT4, #317416), anti-human CD69 APC (clone FN50, # 310910), anti-human C5aR2 APC (clone 1D9-M12, # 342406), Brilliant Violet 711™ anti-human IFN-γ (clone 4S.B3, #502540), Alexa Fluor(R) 647 anti-human IL-10 (clone JES3–9D7, Cat# 501412), Brilliant Violet 421™ anti-human FOXP3 (clone 206D,# 320124), Pacific Blue™ anti-T-bet (clone 4B10, # 644808), Pacific Blue™ Mouse IgG1κ Isotype Ctrl (clone MOPC-21, Cat# 400151), FITC Mouse IgG1κ Isotype Ctrl (ICFC) (clone MOPC-21, Cat#400138), PE anti-human T-bet (clone QA18A24,# 365904), Alexa Fluor® 488 anti-mouse IFN-γ (clone XMG1.2, #505813), PE anti-mouse IL-17A (clone TC11–18H10.1, Cat# 506904), APC anti-human CD56 (clone 5.1H11, # 362504), and FITC anti-human CD3 Antibody (HIT3a, #300306) were all bought from BioLegend (San Diego, CA, USA). Anti-mouse IL-10 (Invitrogen, Waltham, MA, USA; clone JESS-16E3, #12–7101-82), anti-mouse IL-17A (BioLegend; clone TC11–18H10.1, #506916), and anti-mouse IFN-γ (ThermoFisher Scientific, Waltham, MA, USA; clone XMG1.2, #11–7311-82), BD Pharmingen™ Alexa Fluor® 488 mouse anti-human RORγt (clone Q21–559, # 563621), BD Pharmingen™ PE-Cy7 mouse anti-mouse RORγt (clone Q31–378, # 567305) and Thermo Fisher PE-Cyanine7 anti-human IL-17A antibodies (clone eBio64DEC17, # 25–7179) were used for intracellular cytokine staining.
For mouse cell sorting and ex vivo staining, anti-mouse CD45RB FITC (clone C36316A, #103305), anti-mouse CD25 PE (clone A18245A, #113703) and anti-mouse CD4 BV421 (clone GK1.5, #100437), PE anti-mouse CD11a (I21/7, # 153104), APC anti-mouse CD25 (PC61, #102012), PE-Cyanine7 anti-mouse CD49d (R1–2, # 103618), PerCp/Cyanine5.5 anti-mouse CD45.1 (A20, #110728), APC-Cyanine7 anti-mouse CD45.1 (clone A20, # 110716), FITC anti-mouse CD45.2 (clone 104,# 109806), Brilliant Violet 510™ anti-mouse CD90.2 (Thy1.2, clone 53–2.1, #140319), Brilliant Violet 711™ anti-mouse/human Ki67 (clone B56,# 563755), Brilliant Violet 510™ anti-mouse TCRβ (clone H57–597, #109234), Pacific Blue™ anti-mouse IFN-γ (clone XMG1.2, #505818), Alexa Fluor® 647 anti-mouse IL-10 (clone JES5–16E3, # 505014), Brilliant Violet 605™ anti-mouse CD4 (clone GK1.5,# 100451), PE anti-mouse FOXP3 (clone MF-14, #126404), and TruStain FcX™ PLUS (anti-mouse CD16/32, clone S17011E, # 156604) antibodies were all purchased from BioLegend.
Anti-human IFN-γ PE-Vio® 770 was bought from Miltenyi Biotec (Bergisch Gladbach, Germany; clone 45–15, #130–113-499). Anti-human IL-10 APC (clone JES3–19F1, #506806) and anti-human IL-17 Alexa Fluor 488 (clone BL168, #512308) were purchased from BioLegend. Alexa Fluor 488 anti mouse CD107a (1D4B; #121608) and anti-mouse CD107b (M3/84; #108510) were from BioLegend. From R&D Systems (Minneapolis, MI, USA) the following antibodies were sourced: anti-human IL-1R2 PE-conjugated (clone 34141, #FAB663P), polyclonal anti-human IL-1R2 antibody (#AF263NA), polyclonal anti-mouse IL-1R2 (#AF563), anti-human COX-2 (clone 495222, #MAB4198), mouse monoclonal IgG (clone 20116, #MAB004), and anti-human PTGER2 (EP2) (clone ARC1393, #MA5–35750) ). Polyclonal anti-human PTGES2 (#PA522213) and anti-human PTGER4 (EP4) (#PA518476) were both purchased from Invitrogen. Polyclonal anti-human PTGIR (#B11456), anti-human PTGIS (#C372587), and anti-beta-ACTIN (#LSC19587) where obtained from LSBio (Lynnwood, WA, USA) and anti-human HSP90 was bought from Cell Signaling Technology (Danvers, MA, USA; #4877s). Recombinant human IL-1R2 was purchased from R&D Systems (#263–2R050/CF). The C5aR2 agonist (P32, Ac-RHYPYWR-OH, used at 10 μM) was generated and provided by Trent Woodruff (University of Queensland, Brisbane, Australia).68 Prostaglandin E2 (PGE2) was obtained from Cayman Chemical (Ann Arbor Michigan, MI, USA; #14010, used at 1 μM) as was Prostaglandin I2 (prostacyclin #18220, used at 1 μM). Prostaglandin I2 (PGI2-R-agonist) has a short half-life, is soluble under alkaline conditions, and is highly light sensitive. The compound was therefore stored and used under appropriate precautions and carrier-controls. The PGI2-R/PTGIR (IP receptor) agonist (#2989, used at 1 μM) and the PGI2-R antagonist (#4268, used at 10 μM) were obtained from TOCRIS (Minneapolis, MN, USA). The EP4 receptor antagonist (L-161,982; #147776–06-5, used at various conc. 1–100 μM) and EP2 antagonist C52 (# SML3643) were purchased from Sigma Aldrich (Saint Louis, MO, USA). The specific COX-2 inhibitor, (#70210, used at 5μM), mPGES-1 inhibitor (MF63, #13217, used at 1μM), 15-hydroxy prostaglandin dehydrogenase (15-hydroxy PGDH) inhibitor (#18040, used at 10 μM), and Thromboxane A2 (TXA2) synthase inhibitor (#70510, used at 1 – 10μM) and PPARγ antagonist (#10026, used at 1–5 μM) were bought from Cayman Chemicals. The β-arrestin inhibitor Barbadin (#HY-119706) was purchased from MedChemExpress (Monmouth Junction, NJ, USA), whilst the protein kinase A (PKA) inhibitor KT 5720 (#1288) was obtained from R&D systems.
CD4+ T cell isolation and activation
Human
CD4+ T cells were isolated from human PBMC or buffy coats after centrifugation using Lymphoprep separation medium (Corning, Vienna, VA, USA) and a negative selection kit according to the manufacturer’s protocol (either #130–091-155 from Miltenyi Biotec) or #17952 from Stem Cell Technologies (Vancouver, Canada)). Purity of isolated cells was typically >95%, and cells were resuspended in complete RPMI medium (10% FCS, 2mM glutamine (#25030–149, Gibco, Billings, MT, USA), 50U/ml Penicillin + 50μg/ml streptomycin (#15070–063, Gibco)). Purified CD4+ T cells were activated for the indicated time points in 96- or 48 well culture plates (Greiner, Monroe, NC, USA) pre-coated with antibodies to CD3 (clone OKT3, generated in house) and CD46 (clone TRA2.10, generated in house)5 at 1.5–2 ×105 cells/well (96-well) or 3.5–5×105/well (48-well) in media containing 50 U/ml recombinant human IL-2 (PeproTech, Cranbury, NJ, USA) followed by adding cell stimulation cocktail to the wells (Thermo Fisher, 500x, #00–4975-03) where indicated (last four hours of activation) in an incubator at 37°C and 5% CO2. Note: PGE2 or PGI2 was added 8 hrs post initial activation and cells then further incubated and harvested and assayed at the indicated timepoints.
Mouse
CD4+ T cells were isolated from mouse spleens using a negative selection kit according to the manufacturer’s protocol (either #130–104-454 from Miltenyi Biotec or #19852 from Stem Cell Technologies). Purity of isolated cells was typically >95%, and cells were resuspended in complete RPMI medium (10% FCS, 2mM glutamine (#25030–149, Gibco), 50U/ml Penicillin + 50μg/ml streptomycin (#15070–063, Gibco) and 2-betamercaptoethanol (21985–023, Gibco). Cells were activated in plates pre-coated with 2ug/ml of anti-CD3 (clone 145–2C11, BioXcell, West Lebanon, NH, USA) at 2×105 per well of a 96 well plate and 5×105 per well of a 48 well plate in the presence of 1ug/ml soluble anti-CD28 (clone 37.51, BioXcell). After 3 days, the cells were harvest, split into half and seeded into a new plate with 25U/ml Human IL-2 (PeproTech). Cells were harvested and assayed at the indicated timepoints.
CD4+ T cell proliferation assessment
Proliferation of in vitro activated human or mouse CD4+ T cells was assessed using cell trace violet. Isolated human or mouse CD4+ T cells were labeled with 2μM cell trace violet (CTV, #C34557, Thermo Fisher Scientific) according to the manufacturer’s protocol. For human T cells, cells were harvested at day-5 post-activation with immobilized antibodies to CD3 and CD46. For mouse T cells, cells were harvested at day-3 post-activation with immobilized antibodies to CD3 in the presence of soluble anti-CD28 antibody. Cells were stained with LIVE/DEAD Fixable Far Red Dead Cell Stain (Invitrogen, #L10120) solution and CTV dilution and division index was assessed using a BD Canto (BD Biosciences, Franklin Lakes, NJ, USA) and FlowJo™ v10 software (BD Biosciences).
Protein kinase A (PKA) activation measurement
Amounts of active (phosphorylated) PKA protein was measured in cell pellets of human, activated and sorted CD4+ T cells using the Abcam PKA Assay Kit (#139435) according to the manufacturer’s protocol. Human T cells were activated, and IFN-γ and/or IL-10-producing cells sorted. Equal numbers of cells were sonicated in assay buffer and the resulting lysates used to assess amounts of active PKA.
Cytokine measurements
Cytokine secretion into cell supernatants by human CD4+T cells was assessed using the human Th1/Th2/Th17 Cytometric Bead Array (BD Biosciences, #560484) LEGENDplex™ Human T helper cytokine panel (Biolegend, # 741028), LEGENDplex™ Human Inflammation panel (Biolegend, #740809) or the IFN-γ Secretion Assay (#130–054-202) in combination with the IL-10 Secretion Assay-Detection Kit (APC, #130–090-761) both from Miltenyi Biotec). Secreted active human IL-1β was measured using the Human IL-1β/IL-1F2 DuoSet Kit (R&D Systems, #DY201). For intracellular cytokine detection assay, activated CD4+ T cells (anti-CD3+anti-CD46) were stimulated with Cell Stimulation Cocktail (plus protein transport inhibitors) (500x) (ThermoFisher Scientific; # 00–4975-93) for the last 4 hrs. Cells were fixed and permeabilized for intracellular IFN-γ, IL-17A and IL-10 staining using eBioscience™ Intracellular (Foxp3) Fixation & Permeabilization Buffer Set (#00–5521-00).
Cytokine production by mouse CD4+T cells was quantified in cell culture supernatants using the mouse Th cytokine or Inflammation Legendplex kit (BioLegend, #740446) according to the manufacturer’s protocol, or by intracellular cytokine staining and flow cytometry after addition of cell Stimulation Cocktail (plus protein transport inhibitors) (500x) (ThermoFisher Scientific; # 00–4975-93) for the last 4 hrs. Cells were then harvested, stained for live/dead and CD4 and then fixed and permeabilized (#554714, Cytofix/Cytoperm Solution Kit; BD Biosciences) and stained intracellularly for cytokines (IFN-γ, IL-17, and/or IL-10) and assessed by flow cytometry on a BD FACS CANTO (BD Biosciences).
Western Blotting
Where indicated, activated or nonactivated (control) human or mouse CD4+ T cells were lysed with RIPA Lysis and Extraction Buffer (ThermoFisher Scientific, #89900) in the presence of Halt Protease Inhibitor Cocktail (ThermoFisher Scientific, #78430) and Halt Phosphatase Inhibitor Cocktail (ThermoFisher Scientific, #78420) proteinase inhibitor at concentrations between 5–10×106 cells/ml. Samples were prepared for gel electrophoresis with 4x Laemmli Sample Buffer (BioRad, Hercules, CA, USA; #1610747) supplemented with 10% 2-Mercaptoethanol, denatured at 95 °C for 5 min, and separated using a kD Criterion TGX gel system (BioRad). The Trans-Blot® Turbo™ Transfer System was used to transfer proteins using a Trans-Blot® Turbo™ Midi Nitrocellulose Transfer Pack (Cat#1704159EDU) as followed by manufacturer’s instructions (BioRad). Membranes were then incubated in blocking buffer (1 x PBS supplemented with 1% casein, BioRad; #1610783) for 1 h at room temperature prior to the addition of primary antibodies for overnight incubation at 4°C. After washing 3 x in wash buffer (1x PBS with 0.1 % Tween-20), the membrane was incubated with the desired secondary antibody diluted in blocking buffer for 1 h at room temperature. Following 3 x wash, protein bands were visualized using the ChemiDoc MP Imaging System (BioRad). Relative intensity of protein bands was determined with Image Lab software (BioRad) and data analysis or graphs were generated in Prism 10 (GraphPad, La Jolla, CA, USA).
Eicosanoid mass spectrometry
Sorted, IFN-γ and/or IL-10-producing human CD4+ T cells (induced by anti-CD3 and anti-CD46 activation) were pelleted and flash frozen on dry ice. Samples were submitted and processed at the Vanderbilt University Eicosanoid Core Laboratory and processed as previously described.96 Briefly, samples were homogenized and extracted in ice-cold methanol with indomethacin and butylated hydroxytoluene (BHT). Samples were injected for liquid chromatography - mass spectrometry (LC-MS). Eicosanoids were identified and quantified based on the mass and amounts of known standards as previously described.
CD4+ T cell transfer colitis model
Splenic CD4+ T cells were isolated from either Ptgir−/− or Ptgis−/− (KO) mice and wild type (WT) control mice using a negative selection CD4 T cell isolation kit (#19852 from Stem Cell Technologies). Cells were stained with anti-CD45RB FITC, anti-CD25 PE and anti-CD4 BV421 and sorted on a SH800S cell sorter (Sony Biotechnology Inc., San Jose, Ca, USA) for CD4+ CD25−CD45RBhi (brightest 35%) cells. 2.3 ×105 of KO or WT cells were injected i.p. into age and sex matched C57BL/10 Rag2−/− mice (Taconic, Line # 103). The mice were monitored for developing colitis symptoms, such as weight loss and skin dermatitis and sacrificed 6 weeks post adoptive transfer. Intestinal-draining lymph nodes, spleens, and colons were removed, spleens and colons weighted (colons were weighted after flushing out the feces) and colons measured for length.
Ex vivo T cell stimulation
Spleen, draining lymph node, colon intraepithelial CD4+ T cells were isolated and stimulated overnight with 1 ug/ml of soluble CD3 (clone 145–2C11, #BE00001; BioXcell, Lebanon, NH, USA) and CD28 (clone 37.51, #BE0015–1; BioXcell) antibodies. The following morning, GolgiPlug and GolgiStop (BD Biosciences, #BDB555029 and #BDB554724) were added to the cultures for 5 hrs. The cells were then stained with CD4 and a live/dead fixable near IR (Thermo Fisher Scientific; #L10119), fixed and permeabilized (Cytofix/Cytoperm Fixation/Permeabilization Solution Kit, #BDB554714; BD Biosciences) and stained intracellularly for IFN-γ, IL-17A and IL-10. IL-10 staining was performed on cells upon additional activation with Cell Stimulation Cocktail (plus protein transport inhibitors) (500x) (ThermoFisher Scientific; # 00–4975-93) for the last 4 hrs.
Pathology scoring
After flushing the colons of feces, proximal, mid-, and distal colon samples were excised and placed into 3.7% formaldehyde solution, and then paraffin embedded. Cross-sectional sections were cut and stained with hematoxylin and eosin (H&E). Colon pathology scores were based on severity of mononuclear cell inflammation, intestinal wall thickening, including infiltration to the muscularis, and epithelial damage, including edema, degeneration, and necrosis on a graded scale where 0 = normal, 0.5 = very mild, 1 = mild, 2 = moderate, 3 = severe. Samples were blindly scored by a pathologist from the NIH Pathology Core.
Generation of mixed bone marrow chimeras
CD45.1.1+ or CD45.1.2+ C57BL/6 mice were lethally irradiated (950 rad) and reconstituted with a total of 0.5–1×106 donor BM cells from mixed WT C57BL/6 and KO BM cells. To achieve this, BM from CD45.1/1+ or CD45.1/2+ C57BL/6 wild-type mice were mixed in equal parts with BM cells from CD45.2/2+ C57BL/6 mice deficient in Il1r2 or ptgis. Mice were allowed to reconstitute for at least 8 weeks before infection with PR8 influenza A.
Influenza infection model
Bone marrow chimeric mice were infected intranasally (i.n.) with 104 EID50 of recombinant PR8 influenza virus (expressing the LCMV gp33–41 eptiope (KAVYNFATM) inserted into the NA of A/PR/8/34 (H1N1) (PR8–33) (kindly provided by Dr. Rafi Ahmed, Emory University).84
Lung cell isolation
Lymphocytes were isolated from the lungs as previously described. Briefly, the mice were perfused with PBS, lungs excised, minced and incubated 1mg/ml Collagenase (#C7657) and 1mg/ml DNAse (#DN25) both from Sigma-Aldrich) in 3ml of RPMI for 45 min at 37 degrees. Single cell suspensions were obtained by pushing the digested lung pieces through a 40 uM mesh filter (BD Biosciences). Lung lymphocytes were then purified by a 44/67% percoll gradient (Sigma-Aldrich, #GE17–0891-01) and centrifugation at 2000 rpm for 20 min at room temperature.
Influenza cytokine assay
Femurs of B6 CD45.1 mice were placed in 70% ethanol for 2 min and then flushed with cold 1xPBS plus 2% FCS. The cells were pipetted up and down to disperse clumps, washed with PBS, lysed with ACK lysis buffer, washed two times with complete RPMI media and counted. 2×106 bone marrow cells were seeded per 100mM petri dish in 10 ml of complete RPMI media containing 20 ng/ml of recombinant mouse Granulocyte-macrophage colony-stimulating factor (rmGM-CSF, Biolegend, # 576304) and incubated at 37° C for 3 days. On day 3, 10 ml of fresh complete RPMI media containing 20 ng/ml rmGM-CSF was added to the plates. On day 7 the bone marrow-derived dendritic cells (BMDCs) were harvested, washed with complete media, counted and washed in 1xPBS containing 0.1% BSA. 4×106 BMDCs in 500ul of 1xPBS containing 0.1% BSA were seeded per well into a 6 well plate and 500ul of PR8 influenza virus diluted in PBS containing 0.1% BSA was added per well. The cells were then infected by incubating them for 5 hrs at 37 °C. After 5 hrs the cells were gently scrapped off the plates with cell scrapers, washed 2 times and then kept on ice until co-culture with the lung lymphocytes. 7.5×105 PR8-infected BMDCs were mixed with 5.0×105 lung lymphocytes (purified as described above) per well of a 96-well plate in complete RPMI media plus 40U/ml human IL-2 and protein transport inhibitor (ThermoFisher, # 00498003) plus anti-CD107a and CD107b antibodies (or isotypes) for 6 hrs at 37 °C. After 6 hrs, the cells were harvested and stained with live/dead and CD4, CD8, CD90.2 to gate on live lymphocytes and CD45.1 was used to gate out BMDCs. They were then washed, fixed and permeabilized and stained intracellularly for IFN-γ, IL-10 and IL-17A and assessed by flow cytometry.
Human PTGIR and PTGIS RNA silencing
Knock down of mRNAs encoding human PTGIS or PTGIR was achieved by silencing RNA technique. SiRNAs targeting PTGES2 (#E-021342–00-0005), PTGIS (#E-004691–00-0005) or PTGIR (#E-005716–00-0005), Accell Non-targeting Control Pool (#D-001910–10-05) were purchased from HORIZON (Cambridge, MA, USA) as single ACCELLSMARTpools which contain 3 or 4 individual siRNAs targeting a single gene. Delivery of siRNAs into CD4+ T cells that had been depleted of natural regulatory T cells (Tregs) by using CD4+ CD25+ Regulatory T cell Isolation Kit (STEMCELL #18063) was followed the protocol provided by the vendor. Briefly, the siRNA stock was prepared at the recommended concentration using 1x siRNA Buffer (#B-002000-UB-100) which was then mixed with primary human CD4+ T cells resuspended in Accell Delivery Media to archive a final siRNA concentration of 1 μM in the cell culture. Cells were either kept non-activated or activated with immobilized antibodies to CD3 (2 μg/ml) and CD46 (2 μg/ml) for 48 hrs and then harvested for cytokine production assessment and processing for RNA-sequencing.
RNA extraction and quantitative RT-PCR
RNA was extracted from human or mouse CD4+ T cell using the RNAqueous Micro Kit (Invitrogen, #AM1931,) followed by DNase digestion (TURBO DNA-free™ Kit, Invitrogen, # AM1907). RNA concentration (A260) and purity (A260/A280 ≈ 2.0) was monitored using the spectrophotometer NanoDrop 1000 (ThermoFisher Scientific) and the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific, #4387406) used to generate cDNA. Quantitative PCR analysis for genes of interest was performed using desired VIC or FAM-labeled TaqMan probes in conjunction with the TaqMan™ Universal Master Mix II, no UNG (ThermoFisher Scientific, #4440043). Their respective sequences are proprietary and are hence not publicly available. Data were acquired on the CFX96 Touch Real-Time PCR Detection System (BioRad).
Human TaqMan probes
RPL7 (ribosomal protein L7), #Hs02596927_g1; PTGES2 (prostaglandin E synthase 2), #Hs00228159_m1; PTGIR (prostaglandin I2 (prostacyclin) receptor (IP)), #Hs01125026_m1; PTGIS (prostaglandin I2 (prostacyclin) synthase), # Hs00919949_m1; TBXAS1 (thromboxane A synthase 1), #Hs01022706_m1. IL1R2 (interleukin 1 receptor type 2), #Hs00174759_m1. IL10 (interleukin 10), #Hs00961622_m1.
Mouse TaqMan probes
Hprt (hypoxanthine guanine phosphoribosyl transferase), #Mm01545399_m1; Ptges2 (prostaglandin E synthase 2), #Mm0046 0181_m1; Ptgir (prostaglandin I receptor (IP)), #Mm00801939_m1; Ptgis (prostaglandin I2 (prostacyclin) synthase), #Mm004 47271_m1.
Microarray and RNA-sequencing data
For impact of C5aR2 T cell surface inhibition on Th1 cell induction, previously published microarray data (Affymetrix) on transcriptomic profiling of human CD4+ T cells that were activated with anti-CD3 and anti-CD46 for 2 hrs in the presence or absence of a cell non-permeable C5aR1/2 double inhibitor (human CD4+ T cells express only the C5aR2 on the surface, whereas the C5aR1 is expressed solely intracellularly) were mined.7 Transcriptome profiling was previously performed by the KCL Genomic Centre (London) using human exon 1.0 ST arrays (Affymetrix). For RNA sequencing of human CD4+ T cells, Treg-depleted CD4+ T cells were subjected to treatment with siRNAs targeting either PTGIR or PTGIS or a control scrambled siRNA, IFN-γ− and IFN-γ+ cells sorted from these three conditions (knockdown of PTGIR or PTGIS reduced IL-10 production by Th1 cells), and total RNA extracted from sorted cells as described above. For RNA-sequencing of Ptgir−/− mouse CD4+ T cells, purified CD4+ T cells from Ptgir−/− or WT mice were CD3+CD28 activated for 4 hrs, and total RNA extracted from bulk cells. Human and mouse RNA was quantified and applied to the 4200 TapeStation System (Agilent Technologies, Savage, MD, USA) for quality assessment. RNA samples were subjected to library preparation using poly(A) mRNA capture (New England Biolabs, Ipswich, MA. USA; # E7770 and #E7490) according to the manufacturer’s protocol. Libraries were sequenced on a Novaseq 6000 System (Illumina, San Diego, CA, USA). Analysis of microarray data was performed in R (v4.2.2) using the oligo (v1.64.1)86 and limma (v3.56.3)90 packages. Data were normalized using the Robust Multichip Average algorithm, and genes with negligible expression were filtered out. Subsequent statistical analysis was performed using lmFit and eBayes. Principal component analysis was performed on the normalized expression values, and the top 10 PC3 loadings were used in the heatmap visualization.
RNA-sequencing analysis
Sequencing quality control was performed using FastQC (v0.11.9)87 and MultiQC (v1.9).88 Sequences were then trimmed using Trim Galore!97 and alignment was performed using STAR (v2.7.10b).98 Finally, quantification was performed using featureCounts (Subread v2.0.3).91 Downstream analysis was performed in R (v4.3.1) with data filtering, normalization and statistical analysis performed in edgeR (v3.40.2).89 For PTGIR−/−- samples, edgeR processing involved filtering genes based on expression using filterByExpr, Trimmed Mean of M-values library size adjustment with counts per million normalization using cpm, and statistical comparison using exactTest(). For analysis of PTGIR and PTGIS siRNA experiments, the same data filtering and normalization through edgeR was applied, although, due to the presence of donor batch effects, statistical analysis was performed using a model.matrix(~0 + donor_batch + group) > estimateDisp() > glmQLFit() > glmQLFTest() pipeline. Visualization was then performed using ggplot2 (v3.4)92 and ComplexHeatmap (v2.14).93
Single cell RNA-sequencing analysis
Data for Figure 6 were taken from Jaeger et al.,81 Penkava et al.,82 and Maschmeyer et al.83 and processed independently broadly following a standard Seurat (v4) pipeline. For each dataset, cells were excluded that had >10% genes mapping to mitochondrial reads, variable features classified using FindVariableFeatures(), scaled using ScaleData(), underwent principal component analysis with RunPCA, integrated across conditions using Harmony (v1.1),94 and downstream clustering using RunUMAP > FindNeighbors (dims = 1:20) > FindClusters(). Differential expression testing was then perfomed using FindAllMarkers(), and pathway analysis was performed using SCPA (v1.5.4).40
Pathway analysis
For microarray data, Gene Set Enrichment Analysis was performed using normalized expression values with parameters: 1000 permutations, gene-set permutation, weighted enrichment statistic, Signal2Noise ranking metric, and min/max genes at 15 and 500 respectively. For scRNA-seq pathway analysis, differentially expressed genes from the above microarray data were grouped to form a C5AR signature, and differentially expressed genes from the PTGIS and PTGIR siRNA knockdown experiments were pooled to form a PTGIR/PTGIS signature (Table S1). Pathway analysis was then performed in SCPA (v1.5.4)40 using the C5AR and PTGIS/R signatures, along with Hallmark, KEGG, and wikipathways from MSigDB, retrieved with msigdbr (v7.5.1). Q-values from SCPA were then used to rank and visualize pathway perturbations.
QUANTIFICATION AND STATISTICAL ANALYSIS
Comparison between two (independent) groups were performed using the paired or non-paired Student’s t-test. Multiple group comparison was performed using one-way or two-way analysis of variance with Bonferroni correction where appropriate (GraphPad Prism software, La Jolla, CA, USA). Data were expressed as mean ± standard deviation and statistical significance was attributed to p values < 0.05.
Supplementary Material
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.immuni.2025.05.003.
Highlights.
T cell-autonomous prostanoid metabolism controls Th1 cell induction and contraction
C5aR2 shifts intrinsic, Th1 cell-inducing PGE2 toward Th1 cell-restraining PGI2
PGI2-R-induced IL-1R2 sequesters intrinsic IL-1β, enabling Th1 cell autoregulation
C5aR2-PGI2-R axis perturbations demarcate hyper-Th1 cells driving autoimmunity
ACKNOWLEDGMENTS
We thank the individuals with CAPS and the healthy donors that provided samples for this work. We thank the NHLBI Sequencing Core for their support and services in generating the sequencing data and the NHLBI Transgenic Core for the generation of the Ptgir−/− mouse strain and the NIH Tetramer Core for provision of the major histocompatibility complex (MHC) II tetramer to influenza PR8 (NP311–325). We thank Emmanuel Pinteaux (University of Manchester) for providing the Il1r2−/− mice. Work in this study was supported by the following funding bodies and grants: the National Institutes of Health (NIH) grant ZIA/hl1006223 (C.K.); NIH grant ZIA/DK07549 (B.A.); NIH grant R01 DK122147–01 (C.H.S.); and the British Heart Foundation grants FS/13/3/30038, FS/18/19/33371, RG/16/8/32388, and SP/F/22/150038 (M.C.H.C.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Jankovic D, Kugler DG, and Sher A (2010). IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 3, 239–246. 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan U, and Ghazanfar H (2018). T Lymphocytes and Autoimmunity. Int. Rev. Cell Mol. Biol. 341, 125–168. 10.1016/bs.ircmb.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 3.West EE, Kolev M, and Kemper C (2018). Complement and the Regulation of T Cell Responses. Annu. Rev. Immunol. 36, 309–338. 10.1146/annurev-immunol-042617-053245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, Suddason T, Lord G, Atkinson JP, Cope A, et al. (2010). Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat. Immunol. 11, 862–871. 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, Subias M, Pickering MC, Drouet C, Meri S, et al. (2013). Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39, 1143–1157. 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolev M, Dimeloe S, Le Friec G, Navarini A, Arbore G, Povoleri GA, Fischer M, Belle R, Loeliger J, Develioglu L, et al. (2015). Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity 42, 1033–1047. 10.1016/j.immuni.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbore G, West EE, Spolski R, Robertson AAB, Klos A, Rheinheimer C, Dutow P, Woodruff TM, Yu ZX, O’Neill LA, et al. (2016). T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science 352, aad1210. 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Shen Y, Jin K, Wen Z, Cao W, Wu B, Wen R, Tian L, Berry GJ, Goronzy JJ, et al. (2019). The DNA Repair Nuclease MRE11A Functions as a Mitochondrial Protector and Prevents T Cell Pyroptosis and Tissue Inflammation. Cell Metab. 30, 477–492.e6. 10.1016/j.cmet.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doitsh G, Galloway NLK, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Muñoz-Arias I, et al. (2014). Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514. 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West EE, Merle NS, Kamiński MM, Palacios G, Kumar D, Wang L, Bibby JA, Overdahl K, Jarmusch AK, Freeley S, et al. (2023). Loss of CD4(+) T cell-intrinsic arginase 1 accelerates Th1 response kinetics and reduces lung pathology during influenza infection. Immunity 56, 2036–2053.e12. 10.1016/j.immuni.2023.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kickler K, Maltby K, Ni Choileain S, Stephen J, Wright S, Hafler DA, Jabbour HN, and Astier AL (2012). Prostaglandin E2 affects T cell responses through modulation of CD46 expression. J. Immunol. 188, 5303–5310. 10.4049/jimmunol.1103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, and Narumiya S (2009). Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat. Med. 15, 633–640. 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 13.Okano M, Sugata Y, Fujiwara T, Matsumoto R, Nishibori M, Shimizu K, Maeda M, Kimura Y, Kariya S, Hattori H, et al. (2006). E prostanoid 2 (EP2)/EP4-mediated suppression of antigen-specific human T-cell responses by prostaglandin E2. Immunology 118, 343–352. 10.1111/j.1365-2567.2006.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francica BJ, Holtz A, Lopez J, Freund D, Chen A, Wang D, Powell D, Kipper F, Panigrahy D, Dubois RN, et al. (2023). Dual Blockade of EP2 and EP4 Signaling is Required for Optimal Immune Activation and Antitumor Activity Against Prostaglandin-Expressing Tumors. Cancer Res Commun. 3, 1486–1500. 10.1158/2767-9764.CRC-23-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JM, and Bothwell ALM (2012). The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol. Cells 33, 217–222. 10.1007/s10059-012-2297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T, Tibbitt CA, Feng X, Stark JM, Rohrbeck L, Rausch L, Sedimbi SK, Karlsson MCI, Lambrecht BN, Karlsson Hedestam GB, et al. (2017). PPAR-gamma promotes type 2 immune responses in allergy and nematode infection. Sci. Immunol. 2, eaal5196. 10.1126/sciimmunol.aal5196. [DOI] [PubMed] [Google Scholar]
- 17.Nobs SP, Natali S, Pohlmeier L, Okreglicka K, Schneider C, Kurrer M, Sallusto F, and Kopf M (2017). PPARgamma in dendritic cells and T cells drives pathogenic type-2 effector responses in lung inflammation. J. Exp. Med. 214, 3015–3035. 10.1084/jem.20162069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt D, Ma X, Kundu N, and Fulton A (2011). Prostaglandin E(2) (PGE (2)) suppresses natural killer cell function primarily through the PGE(2) receptor EP4. Cancer Immunol. Immunother. 60, 1577–1586. 10.1007/s00262-011-1064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi PC, Zhou X, Cuchens M, and Jones Q (2001). Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. J. Immunol. 166, 885–891. 10.4049/jimmunol.166.2.885. [DOI] [PubMed] [Google Scholar]
- 20.Hilger D, Masureel M, and Kobilka BK (2018). Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 25, 4–12. 10.1038/s41594-017-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Liu C, Wei B, and Pei G (2013). Loss of beta-arrestin 2 exacerbates experimental autoimmune encephalomyelitis with reduced number of Foxp3+ CD4+ regulatory T cells. Immunology 140, 430–440. 10.1111/imm.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norlander AE, Bloodworth MH, Toki S, Zhang J, Zhou W, Boyd K, Polosukhin VV, Cephus JY, Ceneviva ZJ, Gandhi VD, et al. (2021). Prostaglandin I2 signaling licenses Treg suppressive function and prevents pathogenic reprogramming. J. Clin. Invest. 131, e140690. 10.1172/JCI140690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, and Grisham MB (2009). T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G135–G146. 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, and Weaver CT (2009). Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107. 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiner SL (2007). Development in motion: helper T cells at work. Cell 129, 33–36. 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Ge J, Zhou Y, Li H, Zeng R, Xie K, Leng J, Chen X, Yu G, Shi X, Xu Y, et al. (2024). Prostacyclin Synthase Deficiency Leads to Exacerbation or Occurrence of Endothelium-Dependent Contraction and Causes Cardiovascular Disorders Mainly via the Non-TxA(2) Prostanoids/TP Axis. Circ. Res. 135, e133–e149. 10.1161/CIRCRESAHA.124.324924. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Madan R, Karp CL, and Braciale TJ (2009). Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 15, 277–284. 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters VA, Joesting JJ, and Freund GG (2013). IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 32, 1–8. 10.1016/j.bbi.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Supino D, Minute L, Mariancini A, Riva F, Magrini E, and Garlanda C (2022). Negative Regulation of the IL-1 System by IL-1R2 and IL-1R8: Relevance in Pathophysiology and Disease. Front. Immunol. 13, 804641. 10.3389/fimmu.2022.804641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DH, Kim HY, Cho S, Yoo SJ, Kim WJ, Yeon HR, Choi K, Choi JM, Kang SW, and Lee WW (2021). Induction of the IL-1RII decoy receptor by NFAT/FOXP3 blocks IL-1beta-dependent response of Th17 cells. eLife 10, e61841. 10.7554/eLife.61841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolouli E, Elfaki Y, Herppich S, Schelmbauer C, Delacher M, Falk C, Mufazalov IA, Waisman A, Feuerer M, and Huehn J (2021). Recirculating IL-1R2(+) Tregs fine-tune intrathymic Treg development under inflammatory conditions. Cell. Mol. Immunol. 18, 182–193. 10.1038/s41423-019-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai MK, Hsieh CC, Kuo HF, Yang SN, Kuo CH, Huang MY, Tsai YM, Lee MS, and Hung CH (2014). Effect of prostaglandin I2 analogs on macrophage inflammatory protein 1alpha in human monocytes via I prostanoid receptor and cyclic adenosine monophosphate. J. Investig. Med. 62, 332–339. 10.2310/JIM.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Wei X, Ji C, Yu W, Song C, and Wang C (2022). PGE2 inhibits neutrophil phagocytosis through the EP2R-cAMP-PTEN pathway. Immun. Inflamm. Dis. 10, e662. 10.1002/iid3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bielenberg M, Kurelic R, Frantz S, and Nikolaev VO (2024). A mini-review: phosphodiesterases in charge to balance intracellular cAMP during T-cell activation. Front. Immunol. 15, 1365484. 10.3389/fimmu.2024.1365484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao C, Hirata T, Soontrapa K, Ma X, Takemori H, and Narumiya S (2013). Prostaglandin E(2) promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat. Commun. 4, 1685. 10.1038/ncomms2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.William M, Leroux LP, Chaparro V, Lorent J, Graber TE, M’Boutchou MN, Charpentier T, Fabié A, Dozois CM, Stäger S, et al. (2018). eIF4E-Binding Proteins 1 and 2 Limit Macrophage Anti-Inflammatory Responses through Translational Repression of IL-10 and Cyclooxygenase-2. J. Immunol. 200, 4102–4116. 10.4049/jimmunol.1701670. [DOI] [PubMed] [Google Scholar]
- 37.Almeida de Jesus A, and Goldbach-Mansky R (2013). Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin. Immunol. 147, 155–174. 10.1016/j.clim.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Croker DE, Monk PN, Halai R, Kaeslin G, Schofield Z, Wu MC, Clark RJ, Blaskovich MA, Morikis D, Floudas CA, et al. (2016). Discovery of functionally selective C5aR2 ligands: novel modulators of C5a signalling. Immunol. Cell Biol. 94, 787–795. 10.1038/icb.2016.43. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YY, Yao YD, Luo JF, Liu ZQ, Huang YM, Wu FC, Sun QH, Liu JX, and Zhou H (2022). Microsomal prostaglandin E(2) synthase-1 and its inhibitors: Molecular mechanisms and therapeutic significance. Pharmacol. Res. 175, 105977. 10.1016/j.phrs.2021.105977. [DOI] [PubMed] [Google Scholar]
- 40.Bibby JA, Agarwal D, Freiwald T, Kunz N, Merle NS, West EE, Singh P, Larochelle A, Chinian F, Mukherjee S, et al. (2022). Systematic single-cell pathway analysis to characterize early T cell activation. Cell Rep. 41, 111697. 10.1016/j.celrep.2022.111697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maseda D, Johnson EM, Nyhoff LE, Baron B, Kojima F, Wilhelm AJ, Ward MR, Woodward JG, Brand DD, and Crofford LJ (2018). mPGES1-Dependent Prostaglandin E(2) (PGE(2)) Controls Antigen-Specific Th17 and Th1 Responses by Regulating T Autocrine and Paracrine PGE(2) Production. J. Immunol. 200, 725–736. 10.4049/jimmunol.1601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, Aoki T, Thumkeo D, Siriwach R, Yao C, and Narumiya S (2019). T cell-intrinsic prostaglandin E(2)-EP2/EP4 signaling is critical in pathogenic T(H)17 cell-driven inflammation. J. Allergy Clin. Immunol. 143, 631–643. 10.1016/j.jaci.2018.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sreeramkumar V, Hons M, Punzón C, Stein JV, Sancho D, Fresno M, and Cuesta N (2016). Efficient T-cell priming and activation requires signaling through prostaglandin E2 (EP) receptors. Immunol. Cell Biol. 94, 39–51. 10.1038/icb.2015.62. [DOI] [PubMed] [Google Scholar]
- 44.Fabricius D, Neubauer M, Mandel B, Schütz C, Viardot A, Vollmer A, Jahrsdörfer B, and Debatin KM (2010). Prostaglandin E2 inhibits IFN-alpha secretion and Th1 costimulation by human plasmacytoid dendritic cells via E-prostanoid 2 and E-prostanoid 4 receptor engagement. J. Immunol. 184, 677–684. 10.4049/jimmunol.0902028. [DOI] [PubMed] [Google Scholar]
- 45.Bloom D, Jabrane-Ferrat N, Zeng L, Wu A, Li L, Lo D, Turck CW, An S, and Goetzl EJ (1999). Prostaglandin E2 enhancement of interferon-gamma production by antigen-stimulated type 1 helper T cells. Cell. Immunol. 194, 21–27. 10.1006/cimm.1999.1479. [DOI] [PubMed] [Google Scholar]
- 46.Zhou W, Zhang J, Goleniewska K, Dulek DE, Toki S, Newcomb DC, Cephus JY, Collins RD, Wu P, Boothby MR, et al. (2016). Prostaglandin I2 Suppresses Proinflammatory Chemokine Expression, CD4 T Cell Activation, and STAT6-Independent Allergic Lung Inflammation. J. Immunol. 197, 1577–1586. 10.4049/jimmunol.1501063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel K, and Peebles RS Jr. (2022). Prostacyclin Regulation of Allergic Inflammation. Biomedicines 10, 2862. 10.3390/biomedicines10112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trinchieri G (2007). Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med. 204, 239–243. 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye Z, Shen Y, Jin K, Qiu J, Hu B, Jadhav RR, Sheth K, Weyand CM, and Goronzy JJ (2021). Arachidonic acid-regulated calcium signaling in T cells from patients with rheumatoid arthritis promotes synovial inflammation. Nat. Commun. 12, 907. 10.1038/s41467-021-21242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadler T, Bhasin JM, Xu Y, Barnholz-Sloan J, Chen Y, Ting AH, and Stylianou E (2016). Genome-wide analysis of DNA methylation and gene expression defines molecular characteristics of Crohn’s disease-associated fibrosis. Clin. Epigenet. 8, 30. 10.1186/s13148-016-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilaplana C, Marzo E, Tapia G, Diaz J, Garcia V, and Cardona PJ (2013). Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J. Infect. Dis. 208, 199–202. 10.1093/infdis/jit152. [DOI] [PubMed] [Google Scholar]
- 52.Mortensen R, Clemmensen HS, Woodworth JS, Therkelsen ML, Mustafa T, Tonby K, Jenum S, Agger EM, Dyrhol-Riise AM, and Andersen P (2019). Cyclooxygenase inhibitors impair CD4 T cell immunity and exacerbate Mycobacterium tuberculosis infection in aerosol-challenged mice. Commun. Biol. 2, 288. 10.1038/s42003-019-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niyonzima N, Rahman J, Kunz N, West EE, Freiwald T, Desai JV, Merle NS, Gidon A, Sporsheim B, Lionakis MS, et al. (2021). Mitochondrial C5aR1 activity in macrophages controls IL-1beta production underlying sterile inflammation. Sci. Immunol. 6, eabf2489. 10.1126/sciimmunol.abf2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hess C, and Kemper C (2016). Complement-Mediated Regulation of Metabolism and Basic Cellular Processes. Immunity 45, 240–254. 10.1016/j.immuni.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmid T, and Brüne B (2021). Prostanoids and Resolution of Inflammation - Beyond the Lipid-Mediator Class Switch. Front. Immunol. 12, 714042. 10.3389/fimmu.2021.714042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West EE, Woodruff T, Fremeaux-Bacchi V, and Kemper C (2024). Complement in human disease: approved and up-and-coming therapeutics. Lancet 403, 392–405. 10.1016/S0140-6736(23)01524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grailer JJ, Bosmann M, and Ward PA (2012). Regulatory effects of C5a on IL-17A, IL-17F, and IL-23. Front. Immunol. 3, 387. 10.3389/fimmu.2012.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmudde I, Ströver HA, Vollbrandt T, König P, Karsten CM, Laumonnier Y, and Köhl J (2013). C5a receptor signalling in dendritic cells controls the development of maladaptive Th2 and Th17 immunity in experimental allergic asthma. Mucosal Immunol. 6, 807–825. 10.1038/mi.2012.119. [DOI] [PubMed] [Google Scholar]
- 59.Bao YS, Zhang P, Xie RJ, Wang M, Wang ZY, Zhou Z, Zhai WJ, Feng SZ, and Han MZ (2011). The regulation of CD4+ T cell immune responses toward Th2 cell development by prostaglandin E2. Int. Immunopharmacol. 11, 1599–1605. 10.1016/j.intimp.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Maric J, Ravindran A, Mazzurana L, Björklund ÅK, Van Acker A, Rao A, Friberg D, Dahlén SE, Heinemann A, Konya V, et al. (2018). Prostaglandin E(2) suppresses human group 2 innate lymphoid cell function. J. Allergy Clin. Immunol. 141, 1761–1773.e6. 10.1016/j.jaci.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duffin R, O’Connor RA, Crittenden S, Forster T, Yu C, Zheng X, Smyth D, Robb CT, Rossi F, Skouras C, et al. (2016). Prostaglandin E(2) constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science 351, 1333–1338. 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou W, Dowell DR, Huckabee MM, Newcomb DC, Boswell MG, Goleniewska K, Lotz MT, Toki S, Yin H, Yao S, et al. (2012). Prostaglandin I2 signaling drives Th17 differentiation and exacerbates experimental autoimmune encephalomyelitis. PLoS One 7, e33518. 10.1371/journal.pone.0033518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arbore G, West EE, Rahman J, Le Friec G, Niyonzima N, Pirooznia M, Tunc I, Pavlidis P, Powell N, Li Y, et al. (2018). Complement receptor CD46 co-stimulates optimal human CD8(+) T cell effector function via fatty acid metabolism. Nat. Commun. 9, 4186. 10.1038/s41467-018-06706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dahabieh MS, DeCamp LM, Oswald BM, Kitchen-Goosen SM, Fu Z, Vos M, Compton SE, Longo J, Williams KS, Ellis AE, et al. (2024). NRF2-dependent regulation of the prostacyclin receptor PTGIR drives CD8 T cell exhaustion. Preprint at bioRxiv. 10.1101/2024.06.23.600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ricklin D, Hajishengallis G, Yang K, and Lambris JD (2010). Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797. 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiss MG, Papac-Miličević N, Porsch F, Tsiantoulas D, Hendrikx T, Takaoka M, Dinh HQ, Narzt MS, Göderle L, Ozsvár-Kozma M, et al. (2023). Cell-autonomous regulation of complement C3 by factor H limits macrophage efferocytosis and exacerbates atherosclerosis. Immunity 56, 1809–1824.e10. 10.1016/j.immuni.2023.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Q, and Kanneganti TD (2017). Cutting Edge: Distinct Regulatory Mechanisms Control Proinflammatory Cytokines IL-18 and IL-1beta. J. Immunol. 198, 4210–4215. 10.4049/jimmunol.1700352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li XX, Clark RJ, and Woodruff TM (2020). C5aR2 Activation Broadly Modulates the Signaling and Function of Primary Human Macrophages. J. Immunol. 205, 1102–1112. 10.4049/jimmunol.2000407. [DOI] [PubMed] [Google Scholar]
- 69.Bakker R, Pierce S, and Myers D (2017). The role of prostaglandins E1 and E2, dinoprostone, and misoprostol in cervical ripening and the induction of labor: a mechanistic approach. Arch. Gynecol. Obstet. 296, 167–179. 10.1007/s00404-017-4418-5. [DOI] [PubMed] [Google Scholar]
- 70.Mayer-Barber KD, and Yan B (2017). Clash of the Cytokine Titans: counter-regulation of interleukin-1 and type I interferon-mediated inflammatory responses. Cell. Mol. Immunol. 14, 22–35. 10.1038/cmi.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng C, Liu J, Zheng X, Hu X, and He Y (2023). Prostaglandin and prostaglandin receptors: present and future promising therapeutic targets for pulmonary arterial hypertension. Respir. Res. 24, 263. 10.1186/s12931-023-02559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 377, 1119–1131. 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 73.Arbore G, and Kemper C (2016). A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur. J. Immunol. 46, 1563–1573. 10.1002/eji.201546131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kolev M, West EE, Kunz N, Chauss D, Moseman EA, Rahman J, Freiwald T, Balmer ML, Lötscher J, Dimeloe S, et al. (2020). Diapedesis-Induced Integrin Signaling via LFA-1 Facilitates Tissue Immunity by Inducing Intrinsic Complement C3 Expression in Immune Cells. Immunity 52, 513–527.e8. 10.1016/j.immuni.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friščić J, Böttcher M, Reinwald C, Bruns H, Wirth B, Popp SJ, Walker KI, Ackermann JA, Chen X, Turner J, et al. (2021). The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity 54, 1002–1021.e10. 10.1016/j.immuni.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 76.West EE, and Kemper C (2023). Complosome - the intracellular complement system. Nat. Rev. Nephrol. 19, 426–439. 10.1038/s41581-023-00704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghannam A, Fauquert JL, Thomas C, Kemper C, and Drouet C (2014). Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Mol. Immunol. 58, 98–107. 10.1016/j.molimm.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 78.Ricklin D, Mastellos DC, Reis ES, and Lambris JD (2018). The renaissance of complement therapeutics. Nat. Rev. Nephrol. 14, 26–47. 10.1038/nrneph.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu VH, Yung BS, Faraji F, Saddawi-Konefka R, Wang Z, Wenzel AT, Song MJ, Pagadala MS, Clubb LM, Chiou J, et al. (2023). The GPCR-Galpha(s)-PKA signaling axis promotes T cell dysfunction and cancer immunotherapy failure. Nat. Immunol. 24, 1318–1330. 10.1038/s41590-023-01529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu W, Li H, Zhang X, Wen D, Yu F, Yang S, Jia X, Cong B, and Ma C (2013). Prostaglandin I2-IP signalling regulates human Th17 and Treg cell differentiation. Prostaglandins Leukot. Essent. Fatty Acids 89, 335–344. 10.1016/j.plefa.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 81.Jaeger N, Gamini R, Cella M, Schettini JL, Bugatti M, Zhao S, Rosadini CV, Esaulova E, Di Luccia B, Kinnett B, et al. (2021). Single-cell analyses of Crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat. Commun. 12, 1921. 10.1038/s41467-021-22164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Penkava F, Velasco-Herrera MDC, Young MD, Yager N, Nwosu LN, Pratt AG, Lara AL, Guzzo C, Maroof A, Mamanova L, et al. (2020). Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat. Commun. 11, 4767. 10.1038/s41467-020-18513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maschmeyer P, Heinz GA, Skopnik CM, Lutter L, Mazzoni A, Heinrich F, von Stuckrad SL, Wirth LE, Tran CL, Riedel R, et al. (2021). Antigen-driven PD-1(+) TOX(+) BHLHE40(+) and PD-1(+) TOX(+) EOMES(+) T lymphocytes regulate juvenile idiopathic arthritis in situ. Eur. J. Immunol. 51, 915–929. 10.1002/eji.202048797. [DOI] [PubMed] [Google Scholar]
- 84.Mueller SN, Langley WA, Li G, García-Sastre A, Webby RJ, and Ahmed R (2010). Qualitatively different memory CD8+ T cells are generated after lymphocytic choriomeningitis virus and influenza virus infections. J. Immunol. 185, 2182–2190. 10.4049/jimmunol.1001142. [DOI] [PubMed] [Google Scholar]
- 85.Pinteaux E, Abdulaal WH, Mufazalov IA, Humphreys NE, Simonsen-Jackson M, Francis S, Müller W, and Waisman A (2020). Cell-specific conditional deletion of interleukin-1 (IL-1) ligands and its receptors: a new toolbox to study the role of IL-1 in health and disease. J. Mol. Med. (Berl.) 98, 923–930. 10.1007/s00109-020-01928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carvalho BS, and Irizarry RA (2010). A framework for oligonucleotide microarray preprocessing. Bioinformatics 26, 2363–2367. 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andrews S (2010). FastQC: A Quality Control Tool for High Throughput Sequence. http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- 88.Ewels P, Magnusson M, Lundin S, and Käller M (2016). MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048. 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47. 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liao Y, Smyth GK, and Shi W (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 92.Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis. Use R!, Second Edition (Springer International Publishing; ). [Google Scholar]
- 93.Gu Z, Eils R, and Schlesner M (2016). Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849. 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 94.Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, and Raychaudhuri S (2019). Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296. 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, and Jaenisch R (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918. 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brandt SL, Wang S, Dejani NN, Klopfenstein N, Winfree S, Filgueiras L, McCarthy BP, Territo PR, and Serezani CH (2018). Excessive localized leukotriene B4 levels dictate poor skin host defense in diabetic mice. JCI Insight 3, e120220. 10.1172/jci.insight.120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martin M (2011). Cutadapt Removes Adapter Sequences From High-Throughput Sequencing Reads. EMBnet.journal 17, 10–12. 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 98.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data for Figure 1 (C5aR2 antagonist-treated human CD4+ T cells), RNA-seq data for Figure 3 (mouse WT vs. Ptgir−/− CD4+ T cells), and RNA-seq data for Figure 6 (human PTGIS- and PTGIR-deficient CD4+ T cells) are deposited to the GEO database. The accession numbers are listed in the key resources table. The data are publicly available from the date of publication.
This paper analyzes existing, publicly available data, related to Figure 6.81–83 The pertinent references are listed in the key resources table.
This paper does not report original code. Code to replicate all microarray, bulk RNA-seq and single-cell RNA-seq (scRNA-seq) analysis in the paper can be found at https://github.com/jackbibby1/Rahman-J-2024 (Zenodo https://doi.org/10.5281/zenodo.15222862).
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| PE-anti-human T-bet (QA18A24) | Biolegend | Cat# 365904; RRID: AB_3675491 |
| Pacific Blue-mouse IgG1κ, isotype ctrl. (MOPC-21) | Biolegend | Cat#400151; RRID: AB_3675489 |
| Pacific Blue-anti-human/mouse T-bet (4B10) | Biolegend | Cat# 644808; RRID: AB_1595479 |
| FITC-mouse IgG1κ, isotype ctrl. (MOPC-21) | Biolegend | Cat# 400138; RRID: AB_3675490) |
| FITC-anti-human CD3 (HIT3a) | Biolegend | Cat# 300306; RRID: AB_314041) |
| Brilliant Violet 421-anti-mouse CD4 (GK1.5) | Biolegend | Cat# 100437; RRID: AB_10900241 |
| Brilliant Violet 421-anti-human FOXP3 (206D) | Biolegend | Cat# 320124; RRID: AB_2565972 |
| Alexa Fluor 647-anti-mouse IL-10 (JES5-16E3) | Biolegend | Cat# 505014; RRID: AB_493511 |
| PE-anti-mouse CD11a (I21/7) | Biolegend | Cat# 153104; RRID: AB_2716034 |
| Alexa Fluor-647 anti-human IL-10 (JES3-9D7) | Biolegend | Cat# 501412, RRID: AB_493318 |
| Brilliant Violet 711-anti-human IFN-γ(4S.B3) | Biolegend | Cat# 502540; RRID: AB_2563506 |
| PE-anti-mouse CD25 (A18246A) | Biolegend | Cat# 113703; RRID: AB_2927943 |
| APC-anti-mouse CD25 (PC61) | Biolegend | Cat# 102012; RRID: AB_312861 |
| FITC-anti-mouse CD45RB (C363-16A) | Biolegend | Cat# 103305: RRID: AB_313012 |
| PE-Cyanine7-anti-mouse CD49d (R1-2) | Biolegend | Cat# 103618; RRID: AB_2563700 |
| PerCp/Cyanine5.5-anti-mouse CD45.1 (A20) | Biolegend | Cat# 110728; RRID: AB_893346 |
| APC-Cyanine7-anti-mouse CD45.1 (A20) | Biolegend | Cat# 110716; RRID: AB_313505 |
| APC-anti-human CD56 (5.1H11) | Biolegend | Cat# 362504; RRID: AB_2563912 |
| FITC-anti-mouse CD45.2 (104) | Biolegend | Cat# 109806; RRID: AB_313442 |
| Brilliant Violet 510-anti-mouse CD90.2 (Thy1.2) (53-2.1) | Biolegend | Cat# 140319; RRID: AB_2561395 |
| Alexa Fluor 488-anti-mouse CD107a (1D4B) | Biolegend | Cat# 121608; RRID: AB_571983 |
| Alexa Fluor 488-anti-mouse CD107b (M3/84) | Biolegend | Cat# 108510; RRID: AB_493308 |
| Brilliant Violet 605-anti-mouse CD4 (GK1.5) | Biolegend | Cat# 100451; RRID: AB_2564591 |
| PE-anti-mouse FOXP3 (MF-14) | Biolegend | Cat# 126404; RRID: AB_1098117 |
| Rabbit anti-human HSP90 (C45G5) | Cell Signaling Technology | Cat# 4877; RRID: AB_2233307 |
| Rabbit anti-human PTGIS | LS bio | Cat# LS-C372587; RRID: AB_3675478 |
| Rabbit anti-human PTGIR | LS bio | Cat# LS-B11456-50; RRID: AB_3675477 |
| PE-anti-mouse IL-10 (JESS-16E3) | Invitrogen | Cat# 12-710182; RRID: AB_466176 |
| APC-anti-mouse IL-17A (TC11-1810.1) | Biolegend | Cat# 11-7311-82; RRID: AB_465412 |
| Rabbit anti-human β-Actin | LS bio | Cat# LS-B1040-50; RRID: AB_968470 |
| Alexa Fluor 488-anti-mouse IFN-γ (XMG1.2) | Biolegend | Cat# 505813; RRID: AB_493312 |
| PE-anti-mouse IL-17A (TC11-18H10.1) | Biolegend | Cat# 506904; RRID: AB_315464 |
| Anti-mouse IL-1R2 (AF563) | R & D Systems | Cat# AF563; RRID: AB_2125055 |
| Brilliant Violet 711-anti-mouse/human Ki67 (B56) | BD Biosciences | Cat# 563755; RRID: AB_2738406 |
| PE-anti-human IL-1RII Antibody (34141) | R & D Systems | Cat# FAB663P; RRID: AB_1964613 |
| Brilliant Violet 510-anti-mouse TCRβ (H57-597) | Biolegend | Cat# 109234; RRID: AB_2562350 |
| PE-anti-mouse IL-17A (TC11-18H10.1) | Biolegend | Cat# 506904; RRID: AB_315464 |
| Goat anti-human IL-1RII Antibody | R & D Systems | Cat# AF-263-NA; RRID: AB_354431 |
| Rabbit anti-human PTGER4 | Thermo Fisher Scientific | Cat# PA5-18476; RRID: AB_10981059 |
| Anti-human COX-2 (495222) | R & D Systems | Cat # 612596; RRID: AB_399879 |
| APC-anti-goat IgG | R & D Systems | Cat# F0108; RRID: AB_573124 |
| Mouse monoclonal IgG (20116) | R & D Systems | Cat# MAB004; RRID: AB_357346 |
| APC-anti-human IL-10 | Biolegend | Cat# 506807; RRID: AB_315457 |
| PE-Violet 770-anti-human IFN-γ (45-15) | Miltenyi Biotec | Cat# 130-113-499; RRID: AB_2726168 |
| APC-anti-human CD4 (OKT4) | Biolegend | Cat# 317416; RRID: AB_571945 |
| APC-anti-human CD69 (FN50) | Biolegend | Cat# 310910; RRID: AB_314845 |
| APC-anti-human C5aR2 (1D9-M12) | Biolegend | 342406; RRID: AB_2564331 |
| Alexa Fluor 488-anti-human RORγt (Q21-559) | BD Biosciences | Cat# 563621; RRID: AB_2738325 |
| Pacific Blue-anti-mouse IFN-γ (XMG1.2) | Biolegend | Cat# 505818; RRID: AB_528922 |
| Alexa Fluor 647-anti-mouse IL-10 (JES5-16E3) | Biolegend | Cat# 505818; RIDD: AB_2125096 |
| PE-Cyanine7-anti-human IL-17A (eBio64DEC17) | Thermo Fisher Scientific | Cat# 25-7179-42; RRID: AB_11063994 |
| Anti-human PTGER2 (ARC1393) | Thermo Fisher Scientific | Cat# MA5-35750; RRID: AB_2849650 |
| Alexa Fluor 488-anti-human IL-17A | Biolegend | Cat# 512308; RRID: AB_961386 |
| Anti-mouse CD3 (145-2C11) | BioXcell | Cat# BE0001-1; RRID: AB_1107634 |
| Anti-mouse CD28 (37.51) | BioXcell | Cat # BE0015-1; RRID: AB_1107624 |
| Anti-human CD3 (OKT3) | BioXcell | Cat# BE0001-2; RRID: AB_1107632 |
| Anti-human CD28 (CD28.2) | BD Biosciences | Cat # 555726; RRID: AB_396069 |
| Anti-human CD46 (TRA2-10) | Wash-U Hybridoma Center (WUHC) | Clone TRA-2-10; RRID: AB_10895912 |
| Rabbit anti-human PTGES2 | Thermo Fisher Scientific | Cat# PA5-22213; RRID: AB_11154153 |
| PE-Cy7-anti-Mouse RORγt (Q31-378) | BD Biosciences | Cat# 567305; RRID: AB_2916546 |
| TruStain FcX PLUS anti-mouse CD16/32 (S17011E) | Biolegend | Cat# 156604; RRID: AB_2783137 |
|
| ||
| Bacterial and virus strains | ||
|
| ||
| Influenza PR8-33 (H1N1) | Mueller et al.84 | N/A |
|
| ||
| Biological samples | ||
|
| ||
| Healthy donor blood buffy coats | NIH blood Bank | N/A |
| Blood samples from individuals with CAPS | NIH, IRB#17-I-0016, Table S3 | N/A |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Brilliant Violet-conjugated NP311-325 | NIH Tetramer Core | N/A |
| tetramer | ||
| Human IL-1R2 protein, recombinant | R & D Systems | Cat# 263-2R-050/CF |
| C5aR2 agonist (P32, Ac-RHYPYWR-OH) | Li et al.68 | N/A |
| Human IL-2 protein, recombinant | Peprotech | Cat# 200-02 |
| PGI2 (prostacyclin) | Cayman Chemicals | Cat# 14010 |
| PGI2-R agonist (epoprostenol) | TOCRIS | Cat# 2989 |
| PGI2-R antagonist | TOCRIS | Cat# 4268 |
| PGE2 (prostaglandin E2) | Cayman Chemicals | Cat# 14010 |
| EP4 receptor antagonist (L-161,982) | Sigma | Cat# SML0690 |
| EP2 antagonist (C52) | Sigma | Cat# SML3643 |
| LIVE/DEAD™ Fixable Near-IR | Invitrogen | Cat# L10119 |
| LIVE/DEAD™ Fixable Aqua | Invitrogen | Cat# L34965 |
| Cell trace violet | Invitrogen | Cat# C34557 |
| COX-2 inhibitor | Cayman Chemicals | Cat# 70210 |
| mPGES-1 inhibitor (MF63) | Cayman Chemicals | Cat# 13217 |
| 15-hydroxy PGDH inhibitor | Cayman Chemicals | Cat# 18040 |
| TBXAS1 inhibitor | Cayman Chemicals | Cat# 70510 |
| PPARγ antagonist | Cayman Chemicals | Cat# 10026 |
| Cell Stimulation Cocktail (plus protein transport inhibitors) (500x) | Thermo Fisher Scientific | Cat# 00-4975-93 |
| Barbadin | MedChem Express | Cat# HY-119706 |
| RIPA Lysis and Extraction Buffer | Thermo Fisher Scientific | Cat# 89900 |
| Halt™ Protease Inhibitor Cocktail (100x) | Thermo Fisher Scientific | Cat# 78430 |
| Halt Phosphatase Inhibitor Cocktail | Thermo Fisher Scientific | Cat# 78420 |
| PKA (protein kinase A) inhibitor KT 5720 | R & D Systems | Cat# 1288 |
| 4x Laemmli Sample Buffer | BioRad | Cat# 1610747 |
| Trans-Blot Turbo Midi Nitrocellulose Transfer Packs | BioRad | Cat# 1704159EDU |
| eBioscience™ Protein Transport Inhibitor Cocktail (500x) | Thermo Fisher Scientific | Cat# 00498003 |
| Mouse GM-CSF (granulocyte-macrophage colony-stimulating factor), recombinant | Biolegend | Cat# 576304 |
| 1x PBS (phospahe-buffered saline) with 1% Casein | BioRad | Cat# 1610783 |
| 2-Mercaptoethanol | Thermo Fisher Scientific | Cat# 21985-023 |
| GolgiStop | BD Biosciences | Cat# 554724 |
| BD GolgiPlug™ | BD Biosciences | Cat# 555029 |
| Collagenase | Sigma-Aldrich | Cat# C7657 |
| DNAse I | Sigma-Aldrich | Cat# DN25 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Accell Human PTGES2 siRNA | HORIZON | Cat# E-021342-00-0005 |
| Accell Human PTGIS siRNA | HORIZON | Cat# E-004691-00-0005 |
| Accell Human PTGIR siRNA | HORIZON | Cat# E-005716-00-0005 |
| Accell Non-targeting Control Pool | HORIZON | Cat# D-001910-10-05 |
| Human TaqMan probe RPL7 | Thermo Fisher Scientific | CAT# Hs02596927_g1 |
| Human TaqMan probe PTGES2 | Thermo Fisher Scientific | CAT# Hs00228159_m1 |
| Human TaqMan probe PTGIR | Thermo Fisher Scientific | CAT# #Hs01125026_m1 |
| Human TaqMan probe PTGIS | Thermo Fisher Scientific | CAT# Hs00919949_m1 |
| Human TaqMan probe TBXAS1 | Thermo Fisher Scientific | CAT# Hs01022706_m1 |
| Human TaqMan probe IL1R2 | Thermo Fisher Scientific | CAT# Hs00174759_m1 |
| Human TaqMan probe IL10 | Thermo Fisher Scientific | CAT# Hs00961622_m1 |
| Mouse TaqMan probe Hprt | Thermo Fisher Scientific | CAT# Mm01545399_m1 |
| Mouse TaqMan probe Ptges2 | Thermo Fisher Scientific | CAT# Mm00460181_m1 |
| Mouse TaqMan probe Ptgir | Thermo Fisher Scientific | CAT# Mm00801939_m1 |
| Mouse TaqMan probe Ptgis | Thermo Fisher Scientific | CAT# Mm00447271_m1 |
| PKA Assay Kit | Abcam | Cat# 139435 |
| Ebioscience Foxp3/Transcription Factor Staining Buffer Set | Invitrogen | Cat# 00-5521-00 |
| Cytofix/Cytoperm Fixation/Permeabilization Solution Kit | BD Biosciences | Cat# BDB554714 |
| Mouse CD4 T cell isolation kit | Miltenyi Biotech | Cat# 130-104-454 |
| Mouse CD4 T cell isolation kit | Stem Cell Technologies | Cat# 19852 |
| Human CD4 T cell isolation kit | Miltenyi Biotech | Cat# 130-091-155 |
| Human CD4 T cell isolation kit | Stem Cell Technologies | Cat# 17952 |
| EasySep Human CD4+ CD127low CD25+ Regulatory T Cell Isolation Kit | Stem Cell Technologies | Cat# 18063 |
| IFN-γ Secretion Assay | Miltenyi Biotec | Cat# 130-054-202 |
| IL-10 Secretion Assay | Miltenyi Biotec | Cat# 130-090-761 |
| LEGENDplex Human T helper cytokine panel | Biolegend | Cat# 741028 |
| LEGENDplex Human Inflammation panel | Biolegend | Cat# 740809 |
| LEGENDplex Mouse Inflammation panel | Biolegend | Cat# 740446 |
| Human IL-1β/IL-1F2 DuoSet Kit | R & D Systems | Cat# DY201 |
| RNAqueous Micro Kit | Invitrogen | Cat# AM1931 |
| NEBNext Ultra II RNA Library Prep Kit for Illumina | NEB | Cat# E7770 |
| TURBO DNA-free Kit | Invitrogen | Cat# AM1907 |
| High-Capacity cDNA Reverse Transcription Kit | Invitrogen | Cat# 4387406 |
| TaqMan Universal Master Mix II, no UNG | Invitrogen | Cat# 4440043 |
| NEBNext Poly(A) mRNA Magnetic Isolation Module | NEB | Cat# E7490 |
|
| ||
| Deposited data | ||
|
| ||
| RNA-seq of in vitro activated human CD4+ T cells | This paper, Figure 3 | GEO: GSE288921 |
| RNA-seq of in vitro activated mouse CD4+ T cells | This paper, Figure 6 | GEO: GSE288969 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| T cells, bone marrow (BM) cells isolated from C57BL/6 littermate control mice to KO lines used | The Jackson Laboratory | Strain# 000664 |
| T cells, BM cells isolated from Ptgis-/- mice | Ge et al.26 | N/A |
| T cells isolated from Ptgir-/- mice | NIH, Transgenic Core | N/A |
| T cells, BM cells isolated from Il1r2-/- mice | Pinteaux et al.85 | N/A |
| Mouse strain B6 CD45.1 | The Jackson Laboratory | IMSR_JAX:002014 |
| Mouse strain C57BL/10 Rag2-/- | Taconic | Line# 103 |
|
| ||
| Software and algorithms | ||
|
| ||
| R (v4.2.2) | Carvalho et al.86 | N/A |
| FlowJo 10 software | Treestar | https://www.flowjo.com/ |
| FastQC (v0.11.9) | Andrews87 | N/A |
| MultiQC (v1.9) | Ewels et al.88 | N/A |
| edgeR (v3.40.2) | Robinson et al.89 | https://bioconductor.org |
| GraphPad PRISM 9.4.1 | Graphpad software | https://www.graphpad.com |
| Limma | Ritchie et al.90 | https://bioconductor.org |
| STAR (v2.7.10b) | Liao et al.91 | N/A |
| Subread v2.0.3) | Liao et al.91 | N/A |
| ggplot2 (v3.4) | Wickham92 | N/A |
| ComplexHeatmap (v2.14) | Gu et al.93 | N/A |
| Harmony (v1.1) | Korsunsky et al.94 | N/A |
| SCPA (v1.5.4) | Bibby et al.40 | N/A |
|
| ||
| Others | ||
|
| ||
| PBS | Corning | Cat# 21-040-CV |
| RPMI | Thermo Fisher Scientific | Cat# 21870-076 |
| IMDM | Thermo Fisher Scientific | Cat# 12440-053 |
| Lymphoprep separation medium | Corning | Cat# 25-072-CI |
| HBSS | Thermo Fisher Scientific | Cat# 14170-112 |
| Percoll Cytvia | Sigma-Aldrich | Cat# GE17-0891-01 |
| FBS | Sigma-Aldrich | Cat# F0926 |
| HEPES | Corning | Cat# 25-060-CI |
| Penicillin-Streptomycin | Thermo Fisher Scientific | Cat# 15070-63 |
| Glutamine | Thermo Fisher Scientific | Cat# 25030-149 |


