Summary
Morphogens choreograph the generation of remarkable cellular diversity in the developing nervous system. Differentiation of stem cells in vitro often relies upon combinatorial modulation of these signaling pathways. However, the lack of a systematic approach to understand morphogen-directed differentiation has precluded the generation of many neural cell populations and the general principles of regional specification and maturation remain incomplete. Here, we developed an arrayed screen of 14 morphogen modulators in human neural organoids cultured for over 70 days. Deconvolution of single cell-multiplexed RNA sequencing data revealed design principles of brain region specification. We tuned neural subtype diversity to generate a recently described TAC3-expressing striatal interneuron type within assembloids. To circumvent limitations of in vitro neuronal maturation, we used a neonatal rat transplantation strategy that enabled human Purkinje neurons to develop their hallmark complex dendritic branching. This comprehensive platform yields insights into the factors influencing stem cell-derived neural diversification and maturation.
Introduction
Human stem cell biology for neuroscience offers the promise to generate a diversity of neural cell types of interest to study human development 1–3, identify the pathophysiology of neuropsychiatric disorders 4–7, and develop cell-based therapies 8–10. By mimicking in vivo morphogen signaling events in vitro, human induced pluripotent stem (hiPS) cells can be guided to differentiate into specific neuronal and glial populations. Decades of research have revealed that morphogen gradients are established by neurodevelopmental organizers in vivo that induce the specification of discrete cell fates in the neural tube 11–14. The subsequent replication of these signaling pathways in vitro using stem cells have further elucidated major rules of how concentration gradients, combinatorial signaling, and timing of morphogen action coordinate cell type specification of the nervous system 15–17. For example, the application of sonic hedgehog (SHH), which is secreted by floor plate cells located in the ventral neural tube, guides stem cells to ventral neural cell fates 14,18–22. In fact, protocols combining SHH with WNT and FGF8 agonists yield human stem cell-derived midbrain dopaminergic neurons that have enabled recent transplantation clinical trials for Parkinson’s disease 23,24. Alternatively, combining SHH with WNT and retinoic acid (RA) generates human spinal motor neurons that have been used to model motor neuron disease 25.
Generally, these protocols have been developed by taking knowledge of spatiotemporal morphogen expression patterns and iteratively modulating morphogen pathways in vitro by trial and error to generate a neural population of interest. Unfortunately, our understanding of human morphogen signaling is incomplete, and it is still not clear how a limited set of signaling pathways give rise to the immense cell diversity in the nervous system. Furthermore, approaches to differentiate hiPS cells have not been comprehensive, and even with the advent of guided and unguided organoid differentiation approaches 26, we have yet to generate many neural cells in vitro.
Towards this goal, we built an arrayed morphogen screening platform that leverages regionalization of human neural organoids, multiplexed single cell RNA sequencing and transcriptomic mapping onto reference atlases of the human developing nervous system. Using this approach, we found that discrete molecular conditions generated highly regionalized neural organoid cultures that collectively cover cell diversity across the neural axis. We developed and applied bioinformatic analyses to deconvolute morphogen-cell type relationships. This enabled us to extract principles of morphogenesis including discrete timing windows for patterning and protocols to generate Cajal-Retzius (CR) cells and a TAC3+ human interneuron subtype recently described in primates that we characterized within assembloids using spatial transcriptomics 27–29. Moreover, our functional characterization of neural populations revealed an in vitro limitation in stem cell-derived human Purkinje neuron morphological maturation, highlighting the need for additional interventions to faithfully recapitulate hallmark neural characteristics. Towards addressing this, we transplanted cerebellar organoids into the neonatal rat which enabled human Purkinje neurons to develop complex dendritic branches, characteristic of the late gestation human cerebellum. Ultimately, the promise of this scalable platform is to build a comprehensive morphogen atlas of human neural cell fate specification and functional maturation to accelerate human research into neurobiology, cellular pathophysiology, and cell-based therapies across nervous system disorders.
Results
Development of multiplexed morphogen organoid screen
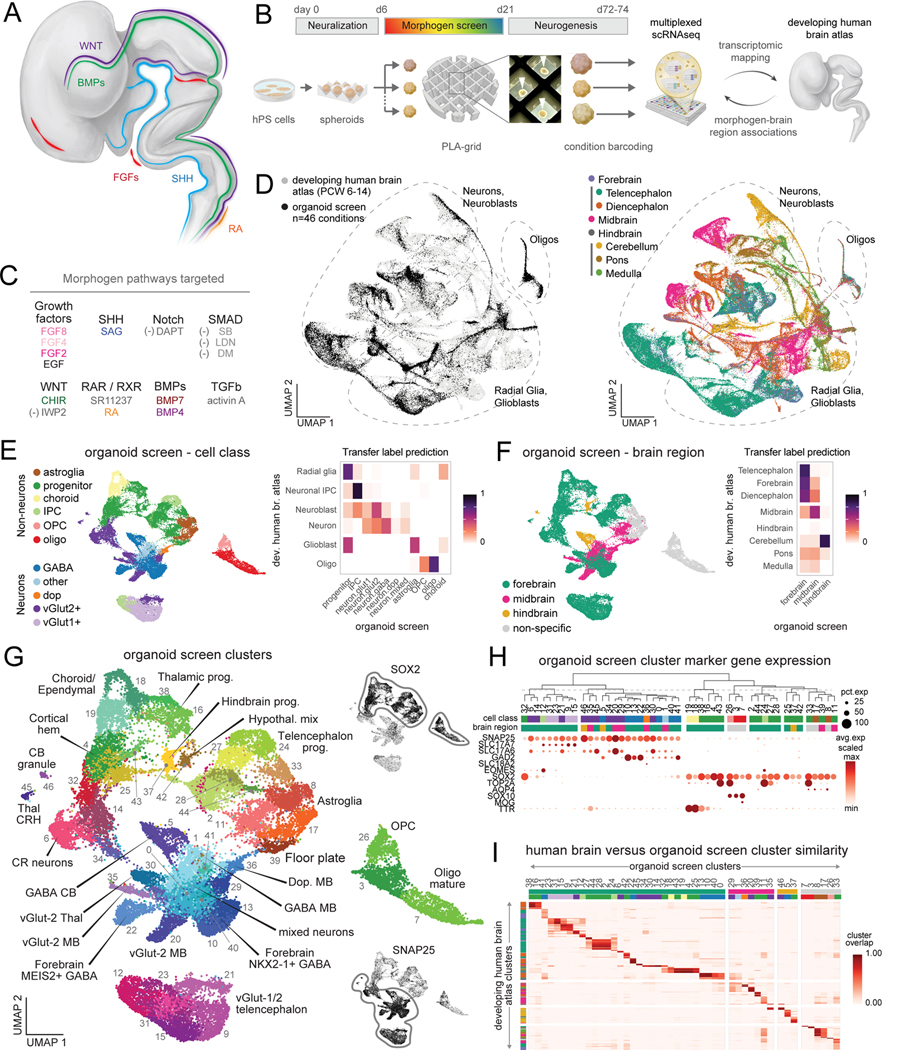
Cell fate specification is influenced by the identity, timing, duration, concentration, and combination of specific morphogens (Fig. 1A)14,30. To understand the principles of morphogen action upon human neural differentiation, we designed a screen in hiPS cell-derived organoids (Fig. 1B). We first generated spheroids from hiPS cells in microwells and neuralized them with dual SMAD inhibitors, as previously described 17,31,32. We screened modulators of 8 morphogen pathways using 14 different molecules in 46 unique combinations, timings, concentrations, and durations in an arrayed format (Fig. 1C, Table S1). To generate the greatest diversity of neural cell populations, we modulated these parameters by applying morphogens individually and in combination. We further modulated SHH (with smoothened agonist, SAG) and WNT (positively with CHIR and negatively with IWP2), varying their relative timings and concentrations. We included previously validated protocols known to generate specific central nervous system regions (such as the spinal cord or choroid plexus) (Fig. S1A) 33–35. Concentration ranges were chosen based upon previous use in stem cell cultures (see Methods section). To facilitate the standardized application of morphogens, we designed and fabricated polylactic acid (PLA) polymer grids with 3D printing that separated 30 organoids per condition in 6-well plates, allowing us to grow a panel of nearly 1500 individually spaced organoids (Fig. S1B). We tracked organoid size over time, and at days 72–74 of differentiation, we used tissue clearing methods to examine organoid morphological features and dissociated organoids for multiplexed single cell RNA sequencing (scRNA-seq) using split-pool combinatorial barcoding 36.
Figure 1. Generation of human neural diversity with a multiplexed morphogen screen in organoids.
(A) Schematic of morphogen expression in the developing human fetal nervous system (adapted from 30).
(B) Diagram of the arrayed screening platform. White arrowheads indicate human brain organoids within the polylactic acid (PLA) grid.
(C) Molecules used to modulate morphogen pathways. “(−)” denotes pathway inhibitor. SAG, Smoothened agonist; RA, retinoic acid; DM, dorsomorphin; SB, SB-431542; LDN, LDN-193189; SR11, SR11237; FGF, fibroblast growth factor; EGF, epidermal growth factor; SHH, sonic hedgehog; TGF, transforming growth factor; BMP, bone morphogenic protein; CHIR, CHIR99021.
(D) UMAP of integrated cellular profiles from organoid morphogen screen and human fetal brain atlas 37 colored by dataset (left) and fetal brain region (right).
(E) Left: UMAP visualization of major cell class annotations. Right: overlap of label transfer predictions from primary human fetal reference scRNA-seq data 37.Overlap values are normalized across rows.
(F) Left: UMAP visualization of brain regions annotations. Right: overlap of label transfer prediction from primary human fetal reference scRNA-seq data 37.Overlap values are normalized across rows.
(G) Annotated clusters of neural cells from all organoid screen conditions. Insets show gene expression of indicated markers.
(H) Dot plot of cell class gene expression in single cell clusters from all organoid conditions. Top: hierarchical clustering based on top 20 cluster marker genes.
(I) Heatmap of cluster overlap between integrated primary and organoid cell clusters. Cluster overlap score of 1 indicates perfect cluster overlap in the integrated dataset. Primary fetal clusters with cluster overlap < 0.25 to organoid clusters are not shown.
After filtering, we successfully captured 36,265 high quality cells from all organoid conditions (average of 788 ± 57 (standard error of the mean) cells per condition, Fig. S1C-D). To map cellular diversity, we integrated cells from all conditions with a comprehensive, high resolution transcriptomic atlas of the pre-natal human brain from developmental time points corresponding to the approximate stage of our organoids (Fig. S1E)37. Cells from the organoid screen spanned neuronal, astroglial, and oligodendrocyte cell types from the telencephalon to the hindbrain (Fig. 1D). We next examined scRNA-seq clusters to investigate cell type transcriptional identity in the arrayed multiplexed data. Transfer labeling from the reference primary human fetal annotations identified corresponding cell labels and regional identities of clustered organoid cells (Fig. 1E,F, Fig. S1F, Table S2). Regional identities were further confirmed using VoxHunt to map cell clusters to developing mouse brain in situ hybridization data, which identified organoid clusters corresponding to telencephalon, diencephalon, midbrain, and cerebellar regions (Fig. S1G)38. We integrated the results from each of these analyses to annotate the cell populations generated in the arrayed screen which were corroborated by examining the expression of specific marker genes (Fig. 1G, H, Fig. S1H-I). This revealed clusters of forebrain glutamatergic neurons, GABAergic neurons, and TP73+/RELN+ Cajal-Retzius (CR) cells, as well as cerebellar Purkinje neurons and granule cells, and thalamic VGLUT2+ and corticotropin releasing hormone (CRH) expressing neurons. We also generated diverse populations of non-neuronal cell types such as TTR+/CLIC2+ choroid plexus cells, MOG+ maturing oligodendrocytes, and cortical hem cells. Finally, we examined the transcriptional similarity of single cell clusters from primary fetal brain data with corresponding clusters from the organoid screen (Fig. 1I, Fig. S2A). Overall, organoid clusters mapped to 358 out of 550 central nervous system-derived fetal clusters (65.1%) with at least 25% cluster overlap (see Methods), indicating we captured a large proportion of brain regional diversity found in vivo.
Associations between morphogen conditions and neural cell type generation
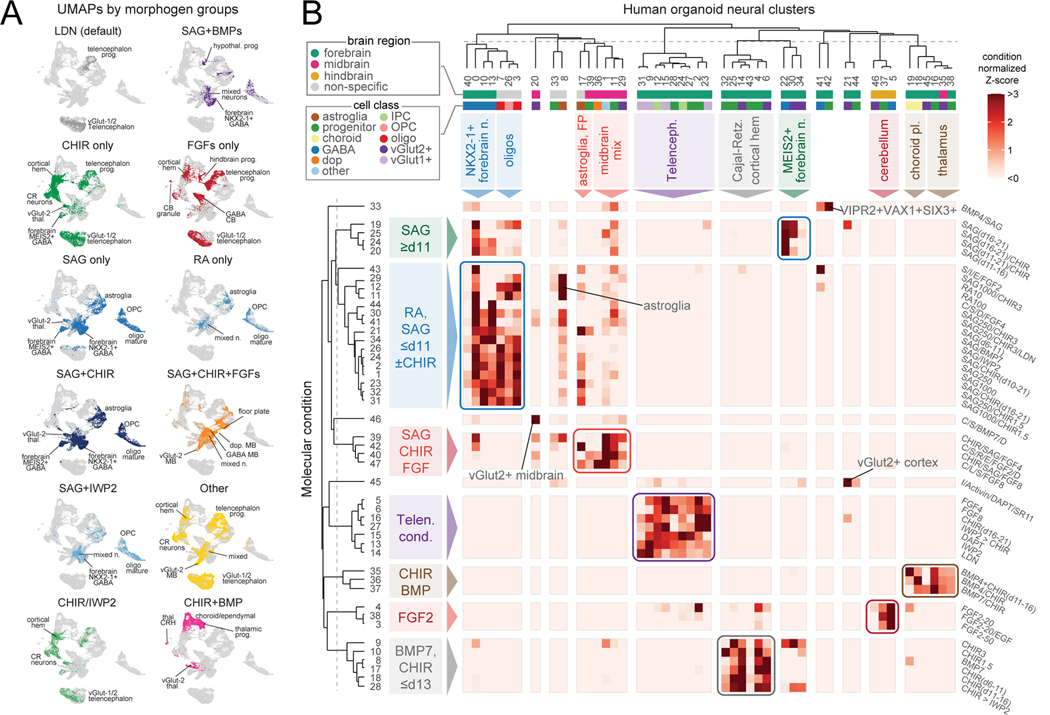
To examine associations between morphogen conditions and neural patterning at higher resolution, we quantified the contribution of cells from each condition to single cell clusters to identify condition-to-cell type associations (Fig. S2B-D, Table S3). On average, each condition contributed at least 10% of cells to 3.37 (± 0.16, standard error) neural clusters, reflecting the specificity of cell type generation within organoids from a single condition. Furthermore, most single cell clusters were populated with cells originating from more than one condition. By calculating a similarity matrix of conditions based upon contribution to clusters, we identified molecular conditions that generated organoids with similar cellular composition, allowing us to examine shared features of molecule identity, timing, concentration, and combination that led to neural cell formation (Fig. S2E). Exposure to continued SMAD inhibition with LDN was used as a default condition for comparison and generated human cortical organoids (hCO) containing telencephalic cortical progenitors and neurons39.
Interestingly, we found groups of conditions that generated very similar neural populations including interneuron subtypes, midbrain, cerebellum, cortical hem, Cajal-Retzius cells, thalamus, and choroid plexus (Fig. 2A-B). We next inspected cell diversity generated from each molecular condition. Conditions exposed to high or low concentrations of the same molecule generated cells that overlapped in UMAP space (Fig. S2F). Sequencing additional fixed and cryopreserved cells from two conditions with lower cell numbers in the original screen confirmed the robustness of our identification of cell populations (Fig. S2G). Conditions exposed to IWP2 or DAPT, inhibitors of the WNT and Notch pathways, respectively, almost exclusively generated telencephalic progenitors and glutamatergic neurons, while activation of SHH signaling resulted in forebrain GABAergic neurons, astrocytes, and oligodendrocytes (Fig. 2A-B). We quantified the relative generation of unique cell populations by aggregate molecular condition and found that certain populations such as dopaminergic neurons and choroid plexus were only generated when organoids were exposed to specific combinations of molecules, SAG+CHIR+FGF8 and BMP+CHIR, respectively (as expected from prior studies 24,35,40–42) (Fig. S2D, H). Progenitors, VGLUT1+ neurons, VGLUT2+ neurons, and GABAergic neurons were present more broadly across conditions with shared molecular features (Fig. 2B).
Figure 2. Deconvoluting morphogen modulators driving cellular composition.
(A) UMAP visualization of cells from aggregated molecular conditions sharing the indicated molecule(s).
(B) Heatmap of cell composition (normalized by total number of cells in each condition) by molecular condition and scRNA-seq cluster. Groups of conditions that similarly contribute to sets of single cell clusters are color-coded and annotated. Dendrograms based on hierarchical clustering.
Combinatorics of morphogen signaling pathways
We examined the influence of morphogen combinatorics on neural cell identity, finding examples of morphogen dominance and synergistic interactions. We observed that SAG alone or when combined with either CHIR and BMP7 generated GABAergic interneurons and oligodendrocytes, reflecting the dominance of SHH signaling over WNT and BMP signaling pathways to induce cell identity (Fig. S3A,B). Interestingly, the combination of some molecules enhanced the generation of specific brain regions and cell types. For example, combining SAG+CHIR with either FGF4 or FGF8 greatly increased the proportion of midbrain cellular identities which comprised only a minority of cells in the SAG+CHIR condition and were absent when FGF4 or FGF8 were added alone (Fig. S3C). Finally, we assessed more complex combinatorics of conditions that contained SAG and another molecule from days 6 to 21. To visualize cellular diversity, we performed hierarchical clustering and principal components analysis (PCA) on the relative cellular contribution of each condition to each neural cluster (Fig. S3D-F). When SAG was combined with IWP2, BMP7 or CHIR, the efficient generation of NKX2–1+ GABAergic neurons was not affected. In contrast, addition of BMP4 yielded hypothalamic cell populations not observed with SAG alone, suggesting morphogen pathway synergy.
Neuropsychiatric disease gene enrichment across organoid conditions
As stem cell-derived organoids are used to investigate the cellular phenotypes of genetic mutations associated with neuropsychiatric conditions 43,44, we examined the expression of genes with coding variation associated with neurodevelopmental disorders (NDD)45, autism spectrum disorder (ASD)45, and schizophrenia (SCZ)46 in cells generated in the organoid screen (Fig. S3G). Disease genes were broadly expressed and enriched across clusters with on average 81% (n= 373 genes), 84% (n= 184 genes), and 60% (n= 244 genes) of disease genes expressed per cluster from NDD, ASD, and SCZ, respectively. We used a specificity index to visualize disease-associated genes with restricted expression across clusters (Fig. S3H-I). While many neuropsychiatric genes are ubiquitously expressed across neuronal and non-neuronal cells, others are found in only a subset of clusters that correspond to specific molecular conditions (Fig. S3J). For example, EBF3, which is associated with a NDD syndrome characterized by autistic features and cerebellar hypoplasia among other symptoms47, is expressed in organoid cerebellum Purkinje, glutamatergic midbrain, and Cajal-Retzius cells (Fig. S3K). Similarly, GATA3, which is associated with NDD, and NR4A2, which is associated with SCZ, demonstrated restricted expression in organoid midbrain GABAergic and dopaminergic neuron clusters, respectively (Fig. S3K). Thus, we generated a diversity of human neural cell populations that exhibit selective expression of disease related genes and can be used to investigate gene-phenotype relationships in the relevant cell type.
Associations between morphogen conditions and organoid morphology
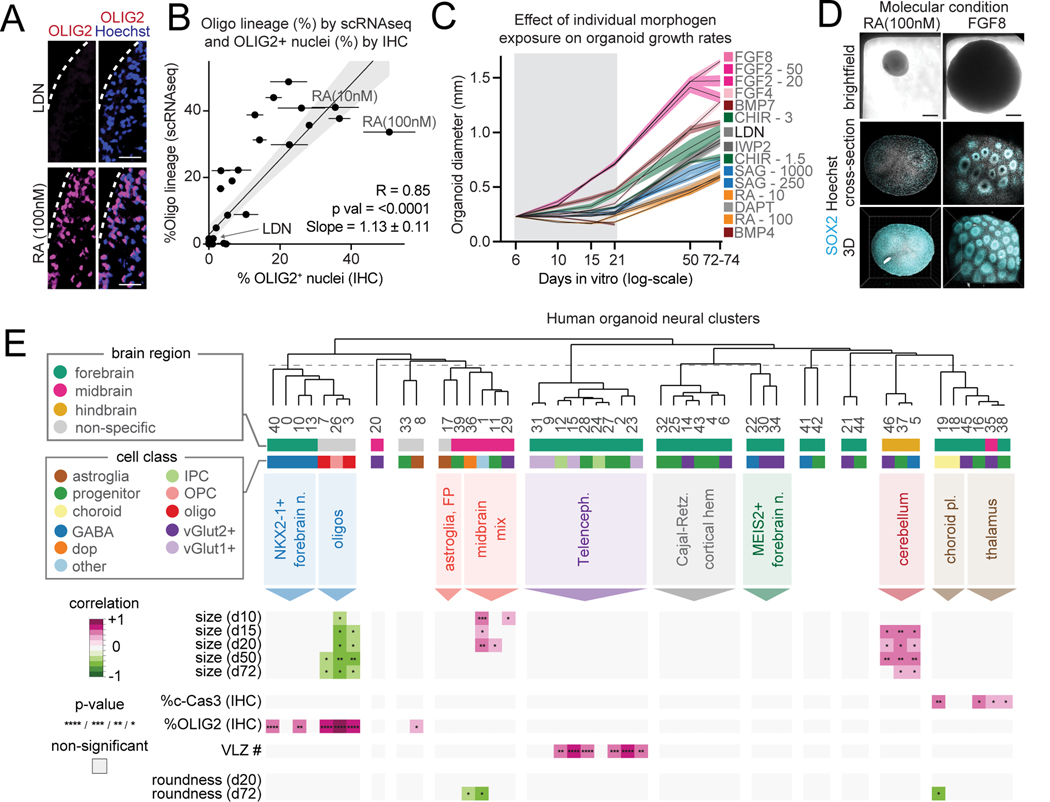
We applied a series of cytoarchitectural and immunostaining analyses in organoids from every condition to validate our single cell transcriptomic data and to provide additional insights into the influence of morphogens (Fig. S3L-O). The fraction of OLIG2+ nuclei in cryo-sectioned organoids from the same condition were highly consistent, and OLIG2 percentage by IHC and the percentage of oligodendrocyte lineage cells determined by scRNA-seq were correlated across conditions (r = 0.85, P< 0.0001, Fig. 3A, B, Fig. S4A). Conditions that contained RA and SAG generated the highest numbers of oligodendrocyte lineage cells compared to conditions without these molecules (Fig. 3A, B, Table S3), consistent with prior reports in organoids and 2D stem cell cultures 48–50. These results demonstrate that this platform can make quantitative assessments of morphogen effects on organoid cell composition.
Figure 3. Cytoarchitectural features associated with morphogen application and cell composition.
(A) Immunohistochemistry of OLIG2 expression in d72 organoids treated with LDN or 100nM retinoic acid (RA). (25 nm, scale bar)
(B) Scatter plot of the percentage of OLIG2+ nuclei by IHC versus percentage of oligodendrocytes by scRNA-seq in organoids from each condition. (mean, standard error, linear regression, 95% confidence interval)
(C) Mean organoid diameter from conditions exposed to a single molecule (standard error) over time. One condition treated with BMP4 alone decreased in size and disintegrated.
(D) Brightfield and CUBIC-cleared organoids from FGF8 and 100nM RA-treated d72 organoids. (scale bar, 0.2mm)
(E) Multiple correlation of quantitative organoid cytoarchitectural and immunohistochemical features with the relative contribution to organoid neural clusters across conditions. Only associations that pass BH-corrected P-value cut-offs are shown *<0.05, **<0.01, ***<0.001, ****<0.0001 Pearson correlation.
In addition to affecting cell type composition, molecular signaling in organoids may influence cytoarchitecture and proliferation. Thus, we used imaging to extract organoid silhouette features over time and quantified organoid growth rate and roundness by morphogen exposure (Fig. 3C, Fig. S4B, Table S4). Among conditions containing a single morphogen modulator, application of FGF8 (100 ng/mL) resulted in organoids with an average diameter that was 2.82 times (P<0.0001) larger than those exposed to RA (100 nM). To assess organoid morphology differences, we also performed organoid clearing with CUBIC and whole mount SOX2 staining (Fig. 3D, Fig. S4C)51. Strikingly, organoids exposed to FGF8 contained numerous ventricular-like zones (VLZs) compared with organoids exposed to other molecules such as RA (Fig. 3D, Fig. S4D, E). In organoids lacking VLZs, SOX2 and KI67 immunostaining revealed a distributed configuration of proliferative cells (Fig. S4F-H). By staining organoids from each condition with cleaved Caspase 3 (cCas3), we examined if smaller organoids had higher rates of programmed cell death but found no association between size and percentage of cCas3+ nuclei (Fig. S4I-J).
We integrated these analyses with our single cell transcriptomic data by performing a multiple correlation analysis to identify statistically significant associations between morphogen exposure, relative proportions of cellular identities by neural cluster, and organoid cytoarchitectural features (Fig. 3E, Fig. S4K, L). The percentage of OLIG2+ nuclei and percentage of cells mapping to our oligodendrocyte lineage clusters were negatively correlated with organoid size. We interestingly found that telencephalic neural identity within organoids is positively correlated with the presence of more VLZs but not with increased organoid size (Fig. 3E). In contrast, cerebellar identity was positively correlated with organoid size but not with the presence of ventricular like zones. Thus, the development of certain cytoarchitectural features in organoids is associated with the underlying brain regional identity rather than proliferative rates. In sum, these results demonstrate that a comprehensive screening approach can extract physical and cell composition features associated with morphogen signaling in organoids.
Morphological maturation of human Purkinje neurons after rat transplantation
Our association analysis identified three conditions that yielded high percentages of cerebellar neurons, and we investigated these cell clusters more closely by performing transcriptomic mapping onto a recent dataset from the human fetal cerebellum (Fig. 4A) 52. Three primary cerebellar clusters mapped with high specificity onto cells from the screen, including cerebellar Purkinje neurons expressing SKOR2/CA8/TFAP2A, PAX2+ cerebellar interneurons, and granule cells expressing RBFOX3, CBLN1, RELN, and PAX6 (Fig. S5A). By deconvoluting morphogen identity with brain region generation, we found that FGF2 in the presence of N2 supplement was the dominant feature of molecular conditions that yielded these cerebellar populations, similar to a prior report (Fig. 4B)53. Indeed, cerebellar progenitors and neurons were generated with high efficiency in three conditions containing FGF2 alone or in combination with EGF, but not in conditions with FGF4 or FGF8 (Fig. 4C). This contrasts with typical hCO protocols which use FGF2 but without N2 supplement31. By immunostaining, we confirmed SKOR2+/TFAP2A+ nuclei in organoids from these conditions in addition to populations of PAX6+ cells (Fig. 4D-E, Fig. S5B). We performed new FGF2 (50ng/mL, day 6 to 21) differentiations generating human cerebellar organoids (hCbO) in three hiPS cell lines and performed scRNA-seq (13,040 high-quality cells) which identified the generation of diverse hindbrain populations: PAX2+ interneurons, Purkinje neurons (SKOR2+/CB8+/CALB1+), LMX1A+/EOMES+ unipolar brush cells (UBCs), ATOH1+/BARHL1+ rhombic lip progenitors, PAX6-high/BARHL1+ granule cells, cerebellar nuclei (CN), choroid plexus, and astrocytes (Fig 4F-H, Fig. S5C-E, Table S5).
Figure 4. Generation of human cerebellar organoids.
(A) Label transfer prediction scores of primary cerebellar populations 52 to scRNA-seq data identifies Purkinje neurons, PAX2+ interneurons, and granule cells.
(B) UMAP highlighting cells originating from three conditions exposed to FGF2 generating high numbers of cerebellar cells.
(C) Quantification of cell proportions by scRNA-seq in the indicated conditions.
(D) Quantification of Purkinje neuron proportions by immunohistochemistry in the indicated conditions. (mean ± SEM, unpaired ANOVA, ***p < 0.0001).
(E) Immunohistochemistry for Purkinje neuron transcription factor markers SKOR2 and TFAP2A at day 72. Scale bar, 25 nm.
(F) UMAP visualization of clustered and integrated hCbO cells from new differentiations (n= 13,040 cells; n=3 hiPS cell lines, day 80). Insets show cells from each hiPS cell line.
(G) Dot plot of cluster marker gene expression in indicated clusters. Cyc_prog: cycling progenitors; Prog.: progenitors; RL: rhombic lip; GCP: granule cell progenitors; GC: granule cells; PAX2-IN: PAX2+ interneurons; CN: cerebellar nuclei; UBC: unipolar brush cells; Exc_hindb.: excitatory hindbrain neurons; BG: Bergmann glia.
(H) Cell type proportions across hCbO hiPS cell lines colored by clusters.
(I) Gene Ontology (GO) term enrichment analysis of genes significantly upregulated in primary developing human Purkinje neurons compared with hCbO Purkinje neurons (adjusted P < 0.05, fold change > 2, expressed in at least 10% of cells).
(J) Immunohistochemical identification of Purkinje neuron morphology at 8 months of differentiation. Scale bar, 9 nm.
Comparison of Purkinje neuron transcriptomes from in vitro hCbO and in vivo PCW9–17 human fetal cerebellum revealed an in vivo enrichment of genes related to dendritic development gene ontology (GO) terms (Fig. 4I, Fig. S5F-G, Table S6). CALB1 and SKOR2 immunostaining and L7::GFP viral labeling of human Purkinje neurons in hCbO at day 185 of differentiation revealed simple fusiform morphology comparable to the PCW12–16 fetal cerebellum (Fig. 4J, Fig S5H-I)54. The absence of complex dendritic branching – a hallmark of Purkinje neuron morphology – in hCbO even after 6 months of culture suggested limitations in stem cell-derived neuronal maturation in vitro55. In fact, prior reports described stem cell-derived human Purkinje neuron dendritic morphology characteristic of early or mid-gestation, but not late gestation, when co-cultured with dissociated rodent cerebellar cells for trophic support 56.
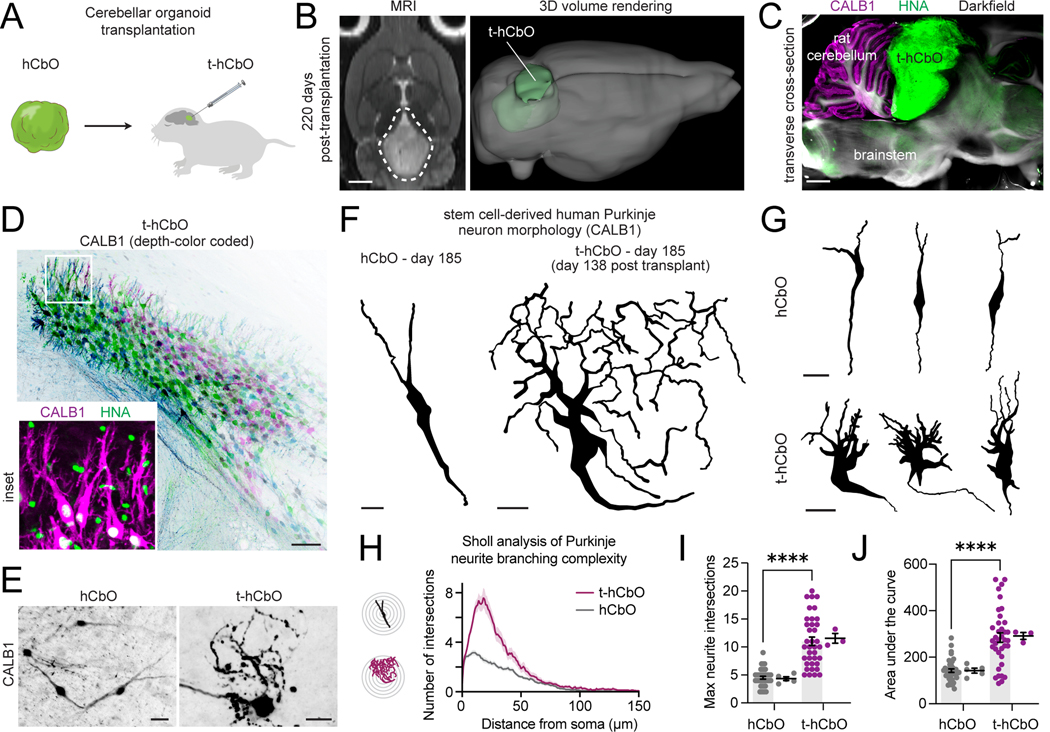
We previously reported that stem cell-derived human cortical neurons grow larger after intact organoid transplantation into the cortex of immunodeficient developing rats57. To investigate the ability of the in vivo cellular context to overcome the in vitro barrier in Purkinje morphological maturation, we developed a stereotactic hCbO transplantation protocol into the cerebellar cortex of early postnatal athymic rats (postnatal days 3–7) (Fig. 5A, Fig. S5J). We tracked the location and growth of transplanted hCbO (t-hCbO) in living animals over several months using T2-weighted MRI reconstructions (Fig. 5B, Fig. S5K-L). We assessed t-hCbO positioning by dissecting and vibratome sectioning post-mortem brain tissues, performing tissue clearing, and immunostaining for human nuclear antigen (HNA)-positive cells (Fig. S5M-N); Fig. 5C). We confirmed human cerebellar cellular diversity in t-hCbO by identifying TFAP2A+ progenitors, BARHLH1+ granule cell lineage cells, and HNA+CALB1+SKOR2+ Purkinje neurons (Fig. 5D, Fig. S5O-P). We found that 8 out of 9 transplanted rats exhibited positive CALB1, SKOR2, and HNA immunostaining in t-hCbO. We next performed single neuron tracing of isolated CALB1+ Purkinje neuron morphology in cleared in vitro hCbO and in vivo t-hCbO samples that originated from the same stem cell differentiation at 185 days (138 days post-transplantation for t-hCbO) (Fig. 5E-G, Supplemental Video S1-2). Purkinje neurons in hCbO exhibited fusiform morphology while those in t-hCbO exhibited more mature complex dendritic branching, quantified by greater neurite complexity using a Sholl analysis of max neurite intersections and area under the curve (p<0.0001) (Fig. 5H-J). In summary, we found that the morphological maturation of stem cell-derived Purkinje neurons in hCbO stalls at the fusiform stage, roughly corresponding to the late first trimester, even after 6 months of in vitro culture. In contrast, t-hCbO Purkinje neuron morphology resembles the complex dendritic patterns previously observed in the late gestation human fetal cerebellum at PCW3254.
Figure 5. Complex dendritic morphology of stem cell-derived human Purkinje neurons after rat transplantation.
(A) Schematic of the experimental design. hCbO generated from hiPS cells are transplanted at days 41–48 of differentiation into the cerebellar cortex of newborn athymic rats.
(B) Left: horizontal view T2-weighted MRI images showing t-hCbO in the cerebellum at 220 days post-transplantation. Scale bar, 2 mm. Right: 3D MRI volume reconstruction.
(C) Vibratome-sectioned rat brain after tissue clearing and immunostaining for HNA and CALB1. Scale bar, 2 mm.
(D) Cluster of HNA+ human Purkinje neurons within t-hCbO immunostained for CALB1 (depth-color coded). Scale bar, 50nm.
(E) Representative z-projection of stem cell-derived human Purkinje neurons. Scale bar, 30nm.
(F) Representative reconstructions of CALB1 neurite tracing of human Purkinje neurons at day 185 of differentiation (day 138 post-transplantation for t-hCbO) Scale bar, 15nm.
(G) Additional representative reconstructions shown.
(H) Sholl analysis of human Purkinje neurite branching complexity. Data are presented as mean ± s.e.m. (n = 39, 38 neurons traced from hCbO and t-hCbO; n = 5 hCbO organoids, 3 t-hCbO (transplanted rats)).
(I) Maximum neurite intersections by neuron replicates (left) and by organoid replicates (right) (****P < 0.0001).
(J) Area under the curve of Sholl analysis by neuron replicates (left) and by organoid replicates (right) (****P < 0.0001).
Generation of human medial pallium organoids containing Cajal-Retzius cells
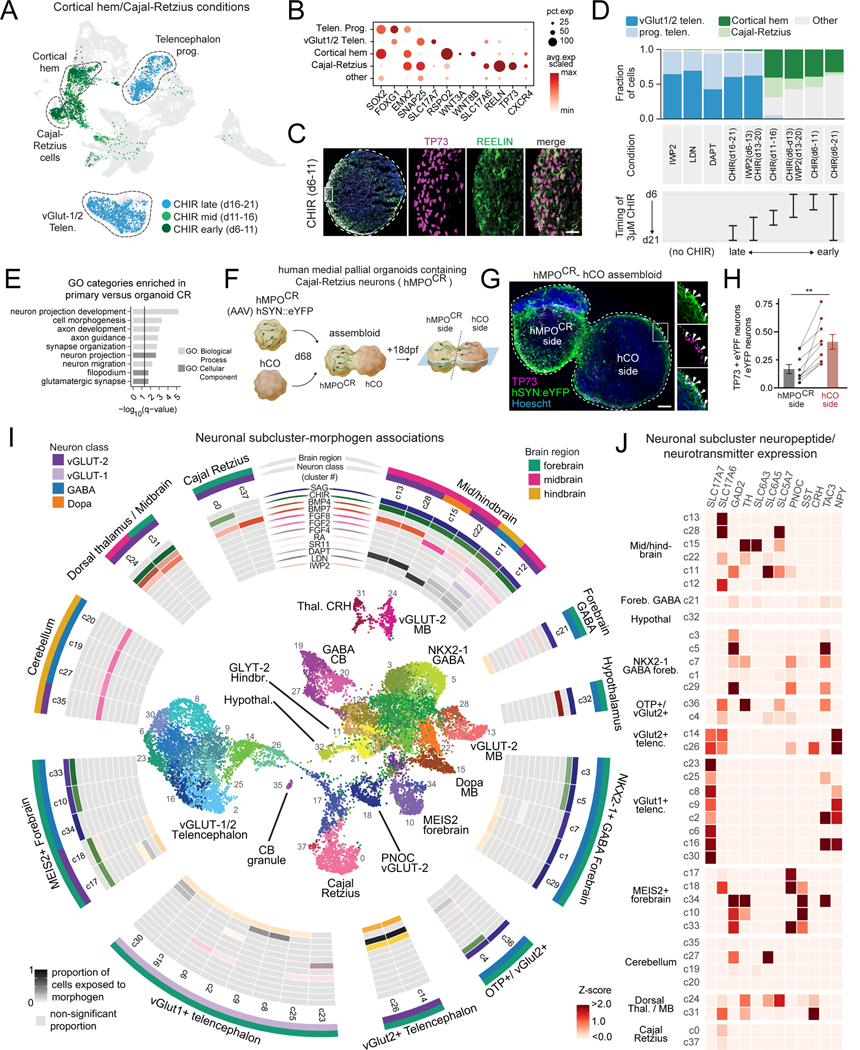
When we inspected conditions that were exposed to the WNT activator CHIR, we observed that some conditions generated high percentages of glutamatergic cortical neurons, while others generated medial pallium-derived cortical hem (CH) and Cajal-Retzius (CR) cells, from which they principally originate (Fig. 6A-C, Fig. S6A)58,59. A closer examination of these molecular conditions revealed that CH/CR fates were specified when CHIR was applied starting early during differentiation, before day 11 (Fig. 6D). WNT activation from day 6 to 13 followed by WNT inhibition from day 13 to 21 still resulted in CR/CH cells, indicating that sustained WNT activation is not critical for this switch in cell fate. Interestingly, CHIR application starting after day 13 yielded glutamatergic cortical neurons, similar to conditions in which IWP2, LDN, or DAPT were applied.
Figure 6. Deconvolution of morphogen features generating migratory Cajal-Retzius cells and human neural diversity.
(A) UMAP highlighting cells from three conditions exposed to the WNT activator CHIR for 5 days at sequential time windows.
(B) Dot plot of marker gene expression in select clusters.
(C) Immunohistochemistry of CR cell transcription factor marker TP73 and cytoplasmic marker REELIN in organoid cryosection. Scale bar, 30 nm.
(D) Quantification of cell composition from scRNAseq data, arranged by timing of CHIR application.
(E) Gene Ontology (GO) term enrichment analysis of genes significantly upregulated in primary human versus organoid Cajal-Retzius cells. (adjusted P < 0.05, fold change > 2, expressed in at least 10% of cells).
(F) Schematic of the experimental design. Virally labeled hMPOCR cells and unlabeled hCO to make assembloids (day 86, 18 days post fusion (dpf)).
(G) Immunohistochemistry of representative hMPOCR-hCO assembloid. Insets show TP73+eYFP+ CR cells originated from hMPOCR that migrated into hCO. Scale bar, 50 nm.
(H) Fraction of TP73+eYFP+ neurons of all eYFP+ neurons per each side of assembloid (n = 9 assembloids, s.e.m. shown, Wilcoxon matched-pairs signed rank test, ** P = 0.0039).
(I) Inside: UMAP visualization of neuron-subset clusters. Outside: Neuronal scRNA-seq subclusters visualized in a circular heatmap of statistically significant associations of proportion of cells exposed to each morphogen, based on permutation testing (see Methods).
(J) Z-score normalized expression of neurotransmitter-associated genes and neuropeptides in each single neuronal subcluster.
To validate the robustness of our protocol (CHIR, day 6 to11) to generate human medial pallium organoids containing Cajal-Retzius cells (denoted hMPOCR), we quantified TP73+ nuclei by immunostaining (n = 4 hiPS cell lines) and performed scRNA-seq (n = 2 hiPS cell lines) in new differentiations at day 80. After quality filtering, we integrated scRNA-seq data (n= 12,500 high-quality cells) and found reproducible cell clusters that contained markers of CR cells (TP73+RELN+) and cortical hem (WNT3A/RSPO2) across lines (Fig. S6B-G, Table S7). Comparing the transcriptomes of hiPS cell-derived CR cells with stage-matched human fetal CR cells revealed lower expression of gene sets associated with neuron migration and cell morphogenesis in stem cell-derived CR cells (Fig. 6E, Fig. S6H, Table S8). CR cells are known to be highly migratory in the developing cortex and we next asked if hMPOCR integrated with hCOs in assembloids would enable assessment of the ability of CR cells to migrate 60. We virally labelled neurons within hMPOCR with an AAV expressing hSYN1::eYFP prior to assembly with hCO (Fig. 6F). Immunostainings of assembloids identified CR cells (TP73+eYFP+) that migrated within hCOs (day 68, 18 days after fusion) (Fig. 6G). The proportion of CR cells (TP73+eYFP+ of all eYFP+ neurons) was higher on the hCO side of the assembloid, demonstrating CR cells were much more likely to migrate to hCO than other hSYN1:eYFP+ neurons present in the organoid (Fig. 6H). Thus, this arrayed screen identified a surprisingly narrow critical timing window in which WNT activation can generate migratory CR cells .
Neuronal cell type diversity
To examine neuronal cell type diversity in greater detail, we subclustered neuronal cells for further investigation. Using a permutation analysis, we found molecules statistically associated with the generation of each neuronal population, yielding a morphogen map of neuronal diversity (Fig. 6I, Table S9). For example, TCF7L2+ thalamic neurons were generated from conditions in which CHIR with or without BMP7 or BMP4 were added (Fig. 6J, Fig. S6I-K). Furthermore, we identified a condition with the ‘dorsalizing’ and ‘ventralizing’ morphogens BMP4 and SAG that generated a population of cells enriched for VIPR2, VAX1, FZD5, and SIX3 that are representative of the ventral hypothalamus (Fig. S6L). We performed new differentiations of four stem cell lines to day 133 and observed expression of these and other hypothalamic markers by qPCR (Fig. S6M). Next, we identified the expression of a diverse array of brain regional, neurotransmitter, and neuropeptide-associated genes that mark distinct populations (Fig. 6J, Fig. S7A-C). Interestingly, many genes are expressed in clusters with high specificity. For example, CRH expression was found in thalamic neurons in conditions applied with CHIR and BMP, and acetylcholinesterase (ACHE) expression was found in ISL1+/PHOX2B+ motor neurons from a condition in which SAG, CHIR and RA were applied (Fig. S7C). Thus, this arrayed screening platform generates great neuronal diversity, including some highly specialized neuronal populations.
Generation of human TAC3 GABAergic neurons
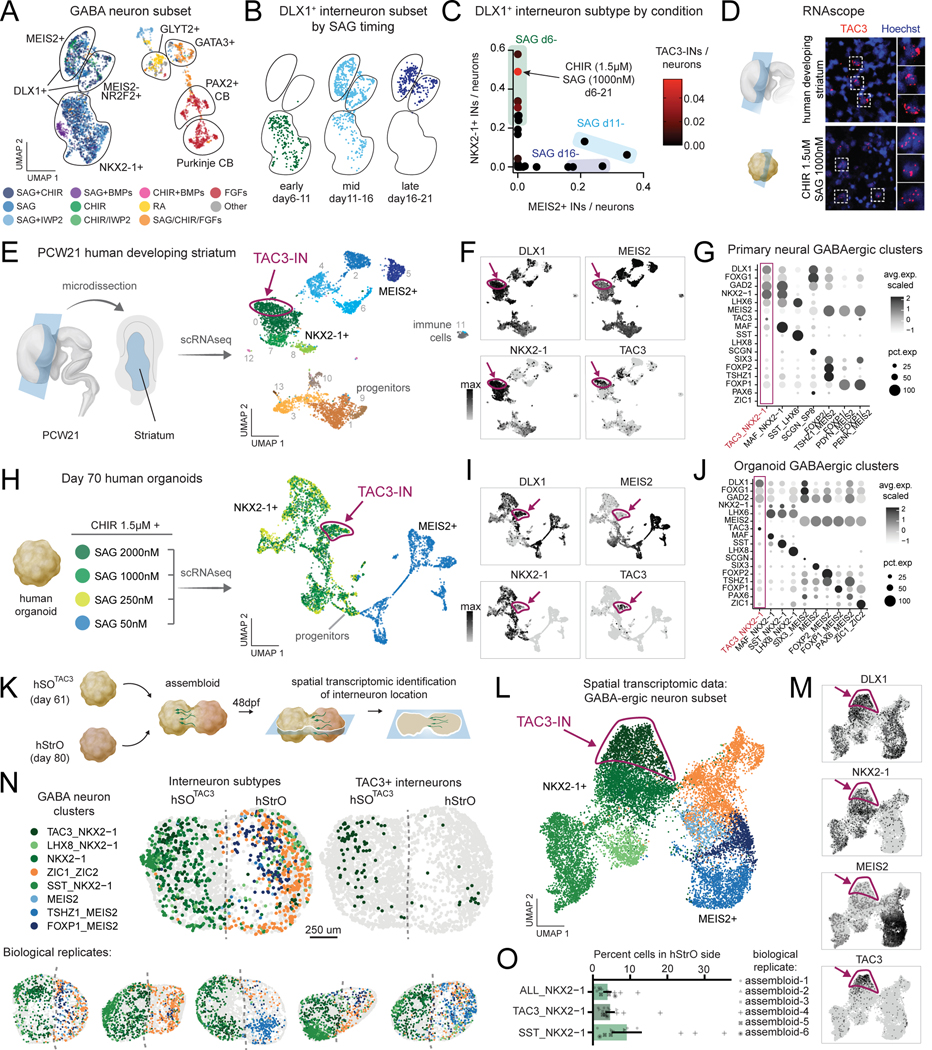
We observed that our screen generated a large proportion of neurons expressing the GABA synthesizing enzyme and we subclustered these cells for closer examination (Fig. 7A, Fig. S7D). Conditions with SAG with or without CHIR generated forebrain DLX1+ interneurons with diverse neurotransmitter identities that segregated by MEIS2+ and NKX2–1+ transcription factor expression (Fig. S7E). Early application of 250 nM SAG preferentially generated NKX2–1+ interneurons which are located more ventrally in vivo, while late application of 250 nM SAG generated more MEIS2+ interneurons, located more dorsally in vivo (Fig. 7B), confirming previous observations61. When SAG was applied at the intermediate time point day 11, a mixture of both subtypes was observed, reflecting a graded effect of SAG timing on interneuron subtype generation (Fig. 7B, Fig. S7F-H).
Figure 7. Conditions generating hiPS cell-derived TAC3+ striatal interneurons.
(A) UMAP of GABAergic neurons, color coded by aggregate molecular conditions and annotated by gene markers.
(B) UMAP of DLX1+ forebrain interneuron clusters annotated by the timing of 250 nM SAG.
(C) Scatter plot of percentage of MEIS2+ and NKX2–1+ interneurons generated in each condition. Conditions exposed to SAG early generate high percentages of NKX2–1+ interneurons and low percentage of MEIS2+ interneurons. Conditions are color coded by the percentage of TAC3-INs generated.
(D) RNAscope of TAC3 in human primary striatal and organoid cells.
(E) Left: diagram of PCW21 micro-dissected human striatum. Right: UMAP of scRNA-seq clusters highlights progenitors, GABAergic interneurons including TAC3-INs, and a small population of immune cells. Cells are color coded by cluster.
(F) Interneuron marker gene expression (UMAP) in primary human cells with TAC3-INs highlighted.
(G) Dot plot gene expression of previously identified TAC3 and striatal cell type marker genes in subpopulations of primary human fetal cells.
(H) Left: diagram of organoid conditions exposed to 1.5 μM CHIR and 4 different concentrations of SAG. Right: UMAP visualization of day 70 scRNA-seq organoid conditions. Cells are color coded by condition.
(I) Interneuron marker gene expression (UMAP) in human organoids with TAC3-INs highlighted.
(J) Dot plot gene expression of previously identified TAC3 and striatal cell type marker genes in subpopulations of organoid cells.
(K) Schematic of the experimental design. hSOTAC3 (day 61) and hStrO (day 80) were integrated to make assembloids. Sections were collected at day 48 post fusion for spatial transcriptomics (MERFISH).
(L) UMAP visualization of GABAergic cell clusters from MERFISH of hSOTAC3-hStrO assembloids (n = 14 cryosections, 6 assembloids, n=47,022 cells).
(M) GABAergic marker gene expression (UMAP) in assembloids with TAC3-INs highlighted.
(N) Representative MERFISH sections annotated by GABAergic cell clusters.
(O) Quantification of percent cells of indicated GABAergic cell clusters located in hStrO-side (n=14 cryosections from 6 assembloids). Data are presented as mean ± s.e.m.
When we examined neuropeptide diversity, we found tachykinin 3 (TAC3) was expressed in a subset of NKX2–1+/DLX1+ interneurons (Fig. 6J, Fig. 7C, Fig. S7E). Interestingly, a population of striatal-enriched TAC3+/DLX1+/NKX2–1+ forebrain interneurons (TAC3-INs) was recently identified in macaque and marmoset brains by single cell profiling 27–29. TAC3-INs were not found in striatum from mouse or ferret. Of all conditions from the organoid screen, CHIR-1.5 μM and SAG 1000nM generated the greatest fraction of human TAC3-INs (Fig. 7C). We next prepared coronal sections of the developing human striatum from post conception week (PCW) 21 and performed RNAscope to confirm expression of TAC3 in primary and organoid cells (Fig. 7D). To further explore the transcriptomic identity of human TAC3-INs, we performed scRNA-seq on micro-dissected human primary striatum (Fig. 7E, Table S10). We identified a cluster of human TAC3+/DLX1+/NKX2-1+ neurons with gene expression patterns resembling those found in developing macaque and adult marmoset (Fig. 7F, G).
To further refine TAC3-IN generation from human stem cells, we performed new organoid differentiations in which we applied CHIR 1.5 μM and varied the concentration of SAG (Fig. 7H, Fig. S8A, Table S11). Low SAG (50 nM) generated MEIS2+ forebrain interneurons and higher SAG concentrations generated NKX2–1+ forebrain interneurons (Fig. 7I, Fig. S8B). The greatest fraction of TAC3-INs was observed with the highest concentration of SAG (2000 nM) (Fig. S8C), which we refer to as human subpallial TAC3 organoids (hSOTAC3). Previously reported marker genes for TAC3-IN from non-human primates showed similar expression patterns observed in TAC3-INs from organoids although differential expression analysis highlighted differences between in vitro and in vivo populations (Fig. 7J, Fig. S8D-E, Table S12).
TAC3-IN are enriched in the striatum and we next examined whether TAC3-INs could integrate with human striatal cells by assembling day 61 hSOTAC3 with d80 human striatal organoids (hStrO) to generate hSOTAC3 – hStrO assembloids (Fig. 7K, Fig. S8F-G) 27,29,62. Identification of TAC3-IN cellular identity and position was performed using spatial transcriptomics (Table S13). We processed, clustered, and annotated n=47,022 segmented cells across biological assembloid replicates 48 days after fusion (Fig. S8H). We identified TAC3-INs located on the hStrO side of assembloids, demonstrating the utility of spatial transcriptomic validation of TAC3-IN derived from our screen (n = 14 cryosections from 6 assembloids) (Fig. 7N-M, Fig S8I-K, Table S14). Thus, we demonstrate that this organoid screening approach generated a reproducible protocol to further investigate a subtype of striatal human interneurons.
Discussion
Advancing a mechanistic understanding of human cellular neurobiology and the pathophysiology of brain disorders relies upon building neuronal models that capture the cellular diversity of the human brain. Furthermore, development of cell therapies for conditions ranging from epilepsy to neurodegeneration requires detailed knowledge of how key brain cell types are generated from stem cells. To these ends, we bring together human brain organoid technology, multiplexed scRNA-seq, and transcriptomic mapping in an arrayed screen of morphogen modulators. This platform generated highly regionalized neural organoid cultures that collectively account for a large diversity of neuronal and non-neuronal brain cell types. We identified critical factors driving cerebellar differentiation and differential switch-like versus graded effects of morphogen timing on the generation of CR cells and GABAergic interneurons, respectively. Demonstrating the utility of this platform to identify and generate rare brain cell types, we characterized a protocol yielding a recently described forebrain interneuron subtype, TAC3-IN, that can be used to study an evolutionary innovation in striatal circuits. Beyond transcriptomics, we characterize the maturation of key cell populations using organoid transplantation, integration into assembloids, and spatial transcriptomics. These studies reveal the importance of in vitro assembloid models to reveal neural migration and an in vivo cellular context for stem cell-derived human Purkinje neurons to develop their hallmark complex dendritic arborizations.
We anticipate that technology developments, automation, and decreases in the cost of multiplexed sequencing over time will enable rapid scaling of this approach. Combining morphogens and transcription factor overexpression strategies may further accelerate efforts to generate cell states in vitro 63,64. Building larger datasets, mapping cells to reference atlases and applying advanced machine learning methods may ultimately lead to conditions that generate precise in vitro cell types in unexplored regions of the nervous system.
Limitations of the Study
There are multiple pathways controlling cell fate specification and the combinatorial possibilities to modulate them are large. With the number of conditions included in this effort, we were able to generate a fraction of regional brain diversity. Although we were able to detect and reproduce the generation of neural cell types, further efforts could utilize even greater numbers of cells in order to detect even more rare cell populations. To build a comprehensive morphogen map of cellular differentiation for the human nervous system and other organ systems, future efforts will require more conditions screened. Furthermore, many in vivo cues for neural development are missing in vitro, including activity-dependent processes that shape cell fate specification and maturation. In fact, we find that transplantation is required for Purkinje neurons to reach maturation stages not observed in vitro. Additionally, the effect of many morphogens will depend on pluripotency states and future studies should explore their impact across cell lines. Finally, comprehensive and well-annotated cellular atlases across human neurodevelopment will be needed to map cell types with higher precision.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Sergiu P. Pasca (spasca@stanford.edu).
Materials Availability
All the reagents in this study were included in the key resources table.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Reelin (CR-50) | MBL International | D223–3 |
| P73 (EP436Y) | Abcam | ab40658 |
| Olig2 | EMD Millipore | AB9610 |
| SOX2 | Santa Cruz | sc-365823 |
| Cleaved Caspase-3 Antibody (Asp175) | Cell Signaling Technology | 9661S |
| SKOR2 | Novus Biologicals | NBP2–14565 |
| TFAP2A | Santa Cruz | sc-12726 |
| Calbindin D28k (CALB1) | Synaptic Systems | 214 005 |
| HNA | Abcam | ab191181 |
| Anti-GFP | GeneTex | GTX13970 |
| MAP2 | Sigma-Aldrich | M4403–50UL |
| FOXP2 | Santa Cruz | sc-517261 |
| CLIC2 | Abcam | ab175230 |
| KI67 | BioLegend | 350504 |
| PAX6 | Sigma-Aldrich | AB2237 |
| BARHL1 | Sigma-Aldrich | HPA004809 |
| TCF7L2 | Cell Signaling Technology | 2569S |
| Bacterial and virus strains | ||
| AAV-DJ-hSYN1::eYFP | Stanford Gene Vector and Virus Core | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| CHIR 99021 | Selleckchem | S1263 |
| SAG | Millipore | 566660 |
| BMP4 | PeproTech | 120–05ET |
| BMP7 | PeproTech | 120–03P |
| FGF2 | R&D Systems | 233-FB |
| FGF4 | PeproTech | 100–31 |
| FGF8 | PeproTech | 100–25 |
| Retinoic Acid | Sigma-Aldrich | R2625 |
| IWP2 | STEMCELL Technologies | 72124 |
| LDN-193189 | Selleckchem | S7507 |
| Dorsomorphin | Sigma-Aldrich | P5499 |
| SB | Tocris | 3748 |
| Y-27632 | Selleck chem | S1049 |
| EGF | R&D Systems | 236-EG |
| DAPT | STEMCELL Technologies | 72082 |
| Vitronectin | Thermo Fisher Scientific | A14700 |
| E8 medium | Life Technologies | A1151700 |
| Human recombinant BDNF | Peprotech | 450–02 |
| Human recombinant NT3 | Peprotech | 450–03 |
| L-Ascorbic Acid 2-phosphate Trisodium Salt | Wako | 323–44822 |
| N6, 2’-O-Dibutyryladenosine 3’, 5’ -cyclic monophosphate sodium salt (cAMP) | Millipore Sigma | D0627 |
| N-2 supplement | Life Technologies | 17502048 |
| Essential 6 medium | Life Technologies | A1516401 |
| Neurobasal A | Life Technologies | 10888 |
| B-27 supplement without Vit A | Life Technologies | 12587 |
| Glutamax | Thermo Fisher Scientific | 35050079 |
| Penicillin and streptomycin | Thermo Fisher Scientific | 15140122 |
| Accutase | Innovate Cell Technologies | AT–104 |
| Activin A | PeproTech | 120–14P |
| SR11237 | Tocris | 3411 |
| Critical commercial assays | ||
| Chromium Single cell 3′ GEM, Library & Gel Bead Kit v3 | 10x Genomics | PN: 1000075 |
| Evercode Whole Transcriptome Kit | Parse Biosciences | ECW02030 |
| Parse Biosciences Cell Fixation | Parse Biosciences | ECF2001 |
| Deposited data | ||
| Spatial transcriptomics data | This paper | Zenodo: https://doi.org/10.5281/zenodo.13835782 |
| Single cell RNA sequencing data | This paper | GEO: GSE233574 https://cells-test.gi.ucsc.edu/?ds=morphogen-screen |
| Human fetal brain scRNA-seq dataset | Braun et al. 37 | https://github.com/linnarsson-lab/developing-human-brain |
| Human fetal cerebellum scRNA-seq dataset | Aldinger et al.52 | https://cells.ucsc.edu/?ds=cbl-dev |
| Primate TAC3-IN scRNA-seq dataset | Schmitz et al.28 | https://dev-inhibitory-neurons.cells.ucsc.edu/ |
| Human fetal striatum scRNA-seq dataset | Bhaduri et al.65 | https://data.nemoarchive.org/biccn/grant/u01_devhu/kriegstein/transcriptome/scell/10x_v2/human/processed/counts/ |
| Experimental models: Cell lines | ||
| Human iPSC line 2242–1 | Pasca Lab | N/A |
| Human iPSC line Q-0306–1 | Pasca Lab | N/A |
| Human iPSC line Q-0294–2 | Pasca Lab | N/A |
| Human iPSC line NIH2788 | Pasca Lab | N/A |
| Experimental models: Organisms/strains | ||
| FOXN1−/− rats | Charles River Laboratories | Crl:NIH-Foxn1rnu |
| Software and algorithms | ||
| ImageJ (Fiji) | Schindelin et al., 2012 66 | https://imagej.net/Fiji |
| Cell Ranger v6.1.0, v7.1.0 | 10x Genomics | https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger |
| Seurat v4.3.0 | Satija Lab; Hao et al., 2021 67 | https://satijalab.org/seurat/ |
| MERlin spatial transcriptomics processing pipeline | Emanuel et al., 2020 68 | https://github.com/emanuega/merlin |
| Cellpose segmentation algorithm | Stringer et al., 2021 69 | https://www.cellpose.org/ |
| Other | ||
| 10 cm ultra-low attachment plates | Corning | 3262 |
| 6-well ultra-low attachment plates | Corning | 3471 |
| White PLA filament | Prusa | PRM-PLA-175-WHT |
| Original Prusa i3 MK3S+ 3D Printer | Prusa | PRI-MK3S-COM-ORG-PEI |
| FLOWMI Cell Strainer (40, 70uM) | SP Bel Art | 136800070 |
| MERSCOPE | Vizgen | https://vizgen.com/ |
| AggreWell 800 plates | STEMCELL Technologies | 34815 |
Data and Code Availability
Single-cell RNA-sequencing data have been deposited at GEO as GSE233574 and are available as of the date of the publication. Spatial transcriptomics data have been deposited at Zenodo (https://doi.org/10.5281/zenodo.13835782) and are available as of the date of the publication.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental Model and Subject Details
Characterization and maintenance of hiPS cells
hiPS cell lines used were validated using methods as previously described. Genome-wide SNP genotyping was performed using Illumina genome-wide GSAMD-24v2–0 SNP microarray at the Children’s Hospital of Philadelphia (CHOP). Cultures were tested for and maintained Mycoplasma free. A total of 4 control hiPS cell lines derived from fibroblasts collected from 4 healthy subjects were used for experiments. Approval for this study was obtained from the Stanford IRB panel and informed consent was obtained from all subjects.
Human primary tissue
Human brain samples were obtained under a protocol approved by the Research Compliance Office at Stanford University. The PCW21 tissue was dissected prior to dissociation for the scRNAseq and RNA scope experiments.
Method details
Organoid screen culture
Prior to neural differentiation, hiPS cells were cultured on vitronectin-coated plates (5 μg ml–1, Thermo Fisher Scientific, A14700) in Essential 8 medium with supplement (Thermo Fisher Scientific, A1517001). Cells were dissociated every 5 to 6 days with UltraPure™ 0.5 mM EDTA, pH 8.0 (Thermo Fisher Scientific, 15575) and passaged onto new plates. For the generation of 3D neural spheroids (day –1), hiPS cells were washed with PBS and incubated with Accutase (Innovative Cell Technologies, AT104) at 37°C for 5–7 min. Cells were manually triturated with a P1000 into a single cell suspension and were passed through a 40 μM filter (FloMi). To generate spheroids, 3 × 106 hiPS cells were added to each AggreWell-800 well in Essential 8 medium supplemented with the ROCK inhibitor Y27632 (10 μM, Selleckchem, S1049), centrifuged at 100 g for 1 min, and then incubated at 37°C in 5 % CO2. After 24 hours, spheroids of approximately 10,000 cells were formed. Spheroids were lifted from each microwell by pipetting medium in the well up and down with a cut P1000 pipet tip and were placed in Essential 6 medium (Thermo Fisher Scientific, A1516401) with the SMAD pathway inhibitors dorsomorphin (2.5 μM, Sigma-Aldrich, P5499) and SB-431542 (10 μM, R&D Systems, 1614).
Approximately 200–300 spheroids collected from one AggreWell well were spread across 6 wells of an AggreWell plate, such that each well contained approximately 30 spheroids. Due to the microwell patterned bottom surface of the AggreWell, spheres within the same well did not come in contact. Between day 15 and day 21, growing organoids from a single AggreWell well were transferred to an ultra-low attachment 6-well plate (Corning, 3471) with a custom 3D fabricated polylactic acid grid inserted and adhered with inert silicone adhesive. Each organoid was placed within a single chamber of the grid. In brief, printing template was designed with TinkerCad (Autodesk 2023) and exported in a stereolithographic (stl) file. The printing parameters were as follows: layer height = 0.2 mm, minimum shell thickness (perimeter) = 0.6 mm (top) 0.7mm (bottom), nozzle temperature = 210C, bed temperature = 60C, and print speed = 75%. Grids were printed on a 3D printer (Prusa i3 MK3S+) using polylactic acid filament (Prusament PLA White, PRM-PLA-JET-1000). After printing, grids were sterilized by soaking in 70% ethyl alcohol (Sigma-Aldrich, E7023) for one hour. Grids were then placed in a 2% solution of poly(2-hydroxyethyl methacrylate) (Sigma-Aldrich, P3932–10G) in ethanol to create a hydrophobic coating. Grids were allowed to dry in a sterile laminar flow hood and were UV treated for 30 minutes on each side. Grids were then inserted into each well of an ultra-low attachment 6-well plate (Corning, 3471) and a small amount (~1g) of silicone adhesive (RS Hughes Inc RTV108, NC0380109) was applied around all sides to secure it to the plastic well. The silicone was allowed to cure for 24 hours. Wells with the 3D printed grids were washed 3 times in sterile PBS (Thermo Fisher Scientific, 14190250) prior to use.
Molecules in the screen were added to the following basal media: from day 0 to day 6, spheres were cultured in Essential 6 medium and media was exchanged daily. From day 6 to day 21, spheres were cultured in neural medium consisting of Neurobasal™-A Medium (Thermo Fisher Scientific, 10888022), B-27™ Supplement, minus vitamin A (Thermo Fisher Scientific, 12587010), GlutaMAX™ Supplement (1:100, Thermo Fisher Scientific, 35050079), and Penicillin-Streptomycin (1:100, Thermo Fisher Scientific, 15070063) and media was exchanged daily. From day 21 onwards, spheres were cultured in Neurobasal™-A Medium (Thermo Fisher Scientific, 10888022), B-27™ Supplement, minus vitamin A (Thermo Fisher Scientific, 12587010), GlutaMAX™ Supplement (1:100, Thermo Fisher Scientific, 35050079), N-2 supplement (Life Technologies, 17502048) and Penicillin-Streptomycin (1:100, Thermo Fisher Scientific, 15070063) with BDNF (20 ng/ml; Peprotech), NT3 (20 ng/ml; Peprotech), cAMP (50 μM, Millipore Sigma, D0627), L-Ascorbic Acid 2-phosphate Trisodium Salt (AA; 200 μM, Wako, 323–44822) and media was exchanged every 2–3 days.
Molecules used: EGF (R&D Systems, 236-EG), FGF2 (R&D Systems, 233-FB), IWP-2 (STEMCELL Technologies, 72124), RA (Sigma, R2625), 2.5 μM DAPT (STEMCELL Technologies, 72082), brain-derived neurotrophic factor (BDNF; 20ng ml-1, PeproTech, 450–02), NT3 (20 ng ml-1, PeproTech, 450–03), FGF4 (PeproTech, 100–31), FGF8 (PeproTech, 100–25), SAG (Millipore, 5666660), CHIR 099021 (Selleckchem, S1263), BMP4 (PeproTech, 120–05ET), BMP7 (PeproTech, 120–03P), LDN-193189 (S7507, Selleckchem), Activin A (50 ng/mL, PeproTech, 120–14P), SR11237 (100 nM, Tocris, 3411), dorsomorphin (DM, Sigma Aldrich P5499), SB (Tocris, 3748), Y-27643 (Selleckchem), L-ascorbic acid (FUJIFILM Wako Chemical Corporation, 344–44822), cAMP (Millipore Sigma, D0627).
Selection of morphogen conditions
We varied a total of 14 morphogens across our conditions. They were chosen based upon their reported importance in the patterning of neural tissues (as reviewed in 70). We chose to apply SB and DM for 6 days to ensure organoid neuroectodermal patterning, as we have previously shown 32. We chose the application of molecules from day 6 (d6) to d21 (15 total days) based upon known developmental timing windows of cell fate specification and our experience with generating various regionalized neural organoids.
In 14 conditions (#1–14), a single morphogen modulator was applied from d6 to d21 with 4 conditions having different concentrations of the same molecule (SAG, FGF2, CHIR, RA). In one additional condition (#15), DAPT was added in a shorter time window to replicate its prior pulsed usage as a potentiator of neurogenesis 33. In an additional 6 conditions (#16–21), either CHIR or SAG were applied alone for the same 5 day-duration, but in varying time windows (d6 to d11, d11 to d16, and d16 to d21). In 4 conditions (#22–25), we applied CHIR and SAG together but varied the initiation day of one or the other molecule to model the effects of sequential signaling pathway activation. In 1 condition (#26), we applied SAG with the WNT inhibitor IWP2, to examine the effects of SHH activation in both settings of WNT activation and inhibition. In two conditions (#27–28), we applied WNT activation with CHIR and WNT inhibition with IWP2 in either sequence to examine the impact of oppositional signaling switches. In 4 conditions (#29–32), we applied SAG and CHIR together with the same timing and duration but varied their relative concentrations. In 5 conditions (#33–37), we applied either BMP4 or BMP7 in combination with other morphogen modulators SAG and CHIR. In two of these conditions (#35–36), we applied both CHIR and BMP4 together but changed the duration of application based upon a published report that yielded choroid plexus 35. In 5 conditions (#38–41), we applied multiple morphogens in combination to study the impact of concurrent combinatoric signaling. In the remaining 6 conditions (#42–47), we applied morphogen protocols developed in our lab, including those previously published to generate spinal cord/hindbrain organoids (#42) 33. Condition #7 in which BMP4 was applied alone was observed to reduce organoid size, consistent with differentiation into a neural crest lineage that is highly migratory and less likely to maintain a stable 3D culture 71. All other conditions yielded organoids and no conditions were excluded from single cell sequencing or other analyses.
Cryoprotection and immunocytochemistry
Organoids were fixed in 4% paraformaldehyde (PFA)/phosphate buffered saline (PBS) overnight at 4°C. Organoids were then washed in PBS and transferred to 30% sucrose/PBS overnight. Organoids were rinsed in optimal cutting temperature (OCT) compound (Tissue-Tek OCT Compound 4583, Sakura Finetek) and frozen using dry ice. For immunofluorescence staining, 25 μm-thick sections were cut using a Leica Cryostat (Leica, CM1860). Cryosections were washed with PBS and blocked in 1% BSA, 0.1% Triton-X, sodium azide diluted in PBS for 1 h at room temperature. The sections were then incubated overnight at 4°C with primary antibodies diluted in 1% BSA, 0.1% Triton-X, sodium azide. Cryosections were washed three times and were incubated with secondary antibodies in 1% BSA, 0.1% Triton-X, sodium azide. Aquamount (Polysciences, 18606) was used to mount coverglass slides. Imaging was performed on a Leica TCS SP8 confocal microscope or Zeiss upright fluorescent microscope. Images were processed and analyzed using Fiji (NIH) and IMARIS (Oxford Instruments). To quantify percentage of oligodendrocyte precursor cells, images of Hoechst and OLIG2+ nuclei were taken. Hoechst images were converted into 8-bit images, performed automatic image threshold (Default, Dark Background) into a binary image, converted to Mask, performed Watershed (FiJi, NIH). OLIG2 nuclei images were manually thresholded due to inconsistency of background signals by a researcher blinded to molecular condition. Nuclei from Hoechst and OLIG2 images were quantified using Analyze Particles with pixel size 30–1000, and the number of OLIG2 nuclei counts were divided by Hoechst nuclei counts to obtain a percentage. Ventricular-like regions were quantified by examining cross sections of organoids stained with Hoechst and counting clearly identifiable ventricle-like structures.
Antibodies used: anti-reelin CR-50 (MBL International, D223–3), anti-P73 (EP436Y, Abcam, ab40658), Olig2 (AB9610, EMD Millipore), SKOR2 (NBP2–14565, Novus), SOX2 (D6D9, Cell Signaling Technologies), PAX6 (Invitrogen, MA1–109 (13B10–1A10)), MAP2 (Sigma, M4403–50uL), TFAP2A (sc-12726, Santa Cruz Biotechnologies), CLIC2 (EPR6469, ab175230, Abcam), FOXP2 (mouse, 1:500; sc-517261, Santa Cruz Biotechnology), GFP (chicken IgY, 1:250; GTX13970, GeneTex), CALB1 (guinea pig, 1:500; #214 005, Synaptic Systems), Ki-67 (mouse, BD Pharmingen, #550609), cleaved Caspase 3 (rabbit, 1:100; #9661, Cell Signaling Technology), TCF7L2 (rabbit, 1:200; C48H11, Cell Signaling Technology), BARHL1 (rabbit, 1:150; HPA004809, Sigma), HNA (mouse, 1:200; ab191181, Abcam). To detect single mRNA molecules, RNAscope was performed using the Muliplex Fluorescent Reagent Kit v2 (Advanced Cell Diagnostics, 323100) following manufacturer’s instructions. RNAscope human probe used: TAC3 (Advanced Cell Diagnostics, 507301)
Clearing and 3D staining of organoids
We applied the hydrophilic chemical cocktail-based CUBIC protocol. Organoids at day 72 were fixed with a 4% PFA/PBS solution at 4°C overnight, washed twice with PBS, and incubated in Tissue-Clearing Reagent CUBIC-L (TCI, T3740) at 37°C for 2 days. After washing three times in PBS, nuclei were stained with Hoechst. Samples were washed twice with PBS, and once with solution containing 10 mM HEPES, 10% TritonX-100, 200 mM NaCl and 0.5% BSA (HEPES-TSB) at 37°C for 2 hours, and then stained with anti-SOX2 (rabbit, Cell Signaling Technology, #3579, 1:100 dilution) antibody in HEPES-TSB solution at 37°C for 2 days. Samples were washed twice with 10% Triton X-100 in PBS and once with HEPES-TSB solution for 2 h each and then incubated with a donkey anti-rabbit IgG (H&L) highly cross-adsorbed secondary antibody, Alexa Fluor 488 (Thermo Fisher Scientific, A-21206, 1:300 dilution) in HEPES-TSB solution at 37°C for 2 days. Samples were washed twice with 10% Triton X-100 in PBS for 30 min and once with PBS for 1 hour. After washing with PBS, samples were incubated with Tissue-Clearing Reagent CUBIC-R+ (TCI, T3741) at room temperature for 2 d for refractive index matching. CUBIC-cleared organoids were then transferred into a well of a Corning 96-well microplate (Corning, 4580) in 150 μL of CUBIC-R+ solution and imaged using a 10x objective on a Leica TCS SP8 confocal microscope.
Organoid morphology quantification
Brightfield images of individual organoids were taken at each time point from each condition. ROIs for all spheres were created manually using the polygon tool in ImageJ version 2.3.0/1.53f. For each resulting ROI, morphology features were analyzed using ImageJ. The resulting spreadsheets were merged into final data files with Python 3.8 using packages Pandas 1.5.2 and NumPy 1.23.5, from which figures were plotted in GraphPad Prism 9. Pearson correlations were performed with R studio package “corrplot”. Dendrograms were generated with Morpheus (Broad Institute, https://software.broadinstitute.org/orpheus), Sankey plots were generated with Flourish (Kiln Enterprises Ltd, UK).
Transplantation of hCbO
All animal procedures followed animal care guidelines approved by Stanford University’s Administrative Panel on Laboratory Animal Care (APLAC). Pregnant RNU euthymic (rnu/+) rats were purchased (Charles River Laboratories). Animals were maintained under a 12-h light–dark cycle and provided food and water ad libitum. Three-to-seven-day-old athymic (FOXN1–/–) rat pups were identified by immature whisker growth before culling. Pups (male and female) were anaesthetized with 2–3% isoflurane and mounted on a stereotaxic frame. A craniotomy, about 2–3 mm in diameter, was performed 2.5mm posterior to the skull landmark lambda, 0mm medial/lateral, and DV 1.4mm, preserving the dura intact. Next, the dura mater was punctured using a 30-G needle (approximately 0.3 mm) close to the anterior side of the craniotomy. A hCbO was next moved onto a 3 × 3-cm parafilm and excess medium was removed. Using a Hamilton syringe connected to a 22 G, 45° needle, the hCbO was gently pulled into the most distal tip of the needle. The syringe was next mounted on a syringe pump connected to the stereotaxic device. The sharp tip of the needle was next positioned above the 0.3-mm-wide pre-made puncture in the dura mater (z = 0 mm), the syringe was reduced 1–2 mm (z = approximately –1.5 mm), and until a tight seal between the needle and the dura mater was formed. Next, the syringe was elevated to the center of the cortical surface at z = –0.5 mm, and the hCbO was ejected at a speed of 5 μl per minute. After injection of hCbO was completed, the needle was retracted at a rate of 0.2–0.5 mm per minute, the skin was closed, and the pups were immediately placed on a warmed heat pad until complete recovery.
MRI of transplanted rats
All MR studies were performed at 7T Bruker BioSpec system (Bruker Corp.) equipped with BGA-12S gradient insert (660 mT/m, 4570 T/m/s) and interfaced to ParaVision 360 V. 3.5. A 2×2 receive-only rat brain array coil and 86mm volume coil were used for MR imaging. Animals were anesthetized with 3% isoflurane mixed in O2 and maintained with 1–1.5% isoflurane during the imaging procedure. Core body temperature was maintained at 37°C using a warm water circulator pad and a temperature controller (ThermoScientific). Animal’s respiration was also monitored via pneumatic pad placed under the animal (SA instruments, NY). T2w 2D Turbo RARE sequence was used to obtain transverse structural MRI (TR/TE = 5500/49.48 ms, FOV = 30 × 30 mm2, matrix = 200 × 200 yielding 150 mm in-plane resolution, slice thickness = 0.5 mm with 52 slices covering whole brain, RARE Factor = 8, NEX = 2, scan time = 4 min 24 sec). T2 weighed MRI volume was quantified using Slicer 3D software.
Human Purkinje neurite tracing
Immunocytochemistry and immunohistochemistry of free-floating organoids.
For immunocytochemistry free-floating hCbO and vibratome sectioned (0.5mm) rat brains containing t-hCbO were blocked overnight at 4°C (1% BSA, 0.3% Triton X-100 diluted in PBS). Samples were subsequently incubated overnight at 4°C with primary antibodies in blocking solution. The next day, cryosections were washed with blocking solution and then incubated with Alexa Fluor secondary antibodies (1:1000 dilution in blocking solution) overnight at 4°C. The following antibodies were used for free-floating immunostaining: anti-SKOR2 antibody (1:100 dilution, rabbit, Novus Biologicals), anti-HNA antibody (1:100 dilution, mouse, Abcam), anti-CALB1 antibody (1:100 dilution, guinea pig, Synaptic Systems).Nuclei were visualized with RedDot2 (Biotium).
Tissue clearing of in vitro and in vivo organoids.
After immunostaining hCbO and vibratome sectioned (0.5mm) rat brains containing t-hCbO, samples were dehydrated in increasing concentrations of methanol (20%, 50%, 80%, 100%) for 5 min incubation periods and incubated in 100% methanol overnight at 4°C on a shaker. Dehydrated samples were then transferred to vacuum grease wells on a microscope slide. Residual methanol was aspirated and 1:2 benzyl alcohol/benzyl benzoate (BABB) was added and sealed with a glass coverslip for clearing.
Image acquisition and neural morphology analysis.
Images were acquired on an inverted confocal microscope (Leica). Human Purkinje neurons were identified based on immunolabeling of CALB1, SKOR2, and HNA. Z-stacks from individual neurons were acquired and neural morphology was semi-manually traced in neuTube72. Sholl analysis was then carried out on z-projected traces of individual neurons using Simple Neurite Tracer plugin (https://imagej.net/plugins/snt/) in FIJI (ver. 2.14.0).
Real-time qPCR
mRNA from organoids were isolated using the RNeasy Mini kit (Qiagen, 74106) with Dnase I, Amplification Grade (Thermo Fisher Scientific, 18068–015). Template cDNA was prepared by reverse transcription using the SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR (Thermo Fisher Scientific, 11752250). qPCR was performed using the SYBR™ Green PCR Master Mix (Thermo Fisher Scientific, 4312704) on a QuantStudio™ 6 Flex Real-Time PCR System (Thermo Fisher Scientific, 4485689).
Single cell RNA-seq library preparation and data analysis
Three organoids from each condition were pooled at day 72–74, incubated in 30 U/mL papain enzyme solution (Worthington Biochemical, LS003126) and 0.4% DNase (12,500 U/mL, Worthington Biochemical, LS2007) at 37 °C for 45 minutes. Organoids were triturated with a P1000 pipette and were resuspended in FBS followed by trituration with a P200 pipette tip. Cells were resuspended in PBS with 0.4% DNase and filtered through a 70 μm Flowmi Cell Strainer (Bel-Art, H13680–0070). The samples were centrifuged at 200 x g for 3 minutes and were resuspended in Parse Cell Buffer containing BSA. Cells were then fixed using the Parse Biosciences Cell Fixation (Parse Biosciences, ECF2001) kit and stored at −80°C until the start of barcoding and library prep with the Evercode Whole Transcriptome kit (Parse Biosciences, ECW02030). Samples were prepared with v1 kits, with the exception of SAG concentration series which was prepared with v2 kits. Libraries from different samples were pooled and sequenced by Admera Health on a NovaSeq S4 (Illumina).
To obtain single cell suspension from micro-dissected developing striatum, the tissue was incubated in 30 U/mL papain enzyme solution (Worthington Biochemical, LS003126) and 0.4% DNase (12,500 U/mL, Worthington Biochemical, LS2007) at 37 °C for 45 minutes. Then, tissue was washed with a solution including protease inhibitor after enzymatic dissociation, and gently triturated to obtain a single-cell suspension. Single cells were resuspended in 0.04% BSA/PBS (Millipore-Sigma, B6917–25MG) and filtered through a 70 μm Flowmi Cell Strainer (Bel-Art, H13680–0070), and the cell number was counted. To target 7,000 cells after recovery, approximately 11,600 cells were loaded per lane on a Chromium Single Cell 3′chip (Chromium Next GEM Chip G Single Cell Kit, 10x Genomics, PN-1000127) and cDNA libraries were generated with the Chromium Next GEM Single Cell 3ʹ GEM, Library & Gel Bead Kit v3.1 (10x Genomics, PN-1000128), according to the manufacturer’s instructions. The library was sequenced using the Illumina NovaSeq S4 2 × 150 bp at Admera Health.
Singe cell expression analysis
Preprocessing and quality filtering
For the multiplex organoid scRNAseq screen, gene expression levels were quantified for each putative cell using the Parse Biosciences analysis software suite (split-pipe command, version 0.9.3p). Specifically, reads were mapped to a human (GRCh38, Ensemble release 93) reference genome created using the mkref mode and quantified using default parameters. All subsequent analyses were performed on the filtered count matrices using the R (version 4.1.2) package Seurat (version 4.3.0)67.
To ensure only high-quality cells were included for downstream analyses, an iterative filtering process was implemented. First, cells with less than the 5th percentile within the dataset of unique genes detected (785 genes) and with mitochondrial counts greater than 20% of the total counts were identified and removed. Subsequently, raw gene count matrices were normalized by regularized negative binomial regression using the sctransform function (vst.flavor=”v2”), which also identified the top 3,000 highly variable genes using default parameters. Dimensionality reduction using principal component analysis (PCA) on the top variable genes was performed and clusters of cells were identified in PCA space by shared nearest-neighbor graph construction and modularity detection implemented by the FindNeighbors and FindClusters functions using a dataset dimension of 50 (dims=50 chosen based on visual inspection of elbow plot) with default parameters. We subsequently performed iterative rounds of clustering (resolution = 2) to identify and remove clusters of putative low-quality cells based on outlier low gene counts (median below the 10th percentile), outlier high fraction mitochondrial genes (median above the 95th percentile), outlier high fraction ribosomal genes (median above the 95th percentile), and/or high proportions of putative doublets identified by the DoubletFinder package73 (median DoubletFinder score above the 95th percentile). We iteratively removed low-quality clusters until no further clusters met the above thresholds. Following low quality cell removal, an additional round of filtering of putative stressed cells was performed using the Gruffi R package74 in addition to manual inspection and removal of clusters expressing markers associated with unfolded protein response (e.g. DDIT3, EIF2AK3).
Analogous preprocessing and filtering were performed on the primary human fetal striatum, the secondary GABAergic neuron organoid screen, and cerebellar organoid scRNAseq datasets, with the following exceptions. Filtered count matrices were obtained using the CellRanger analysis software suite (version 6.1.2) for the human fetal striatum dataset and version 7.1.0 for the cerebellar and Cajal-Retzius organoid datasets. Filtered count matrices were obtained using updated Parse Biosciences software for the GABAergic secondary screen (split-pipe command, 1.0.4p). Dataset dimension of 30 was chosen with cluster resolution of 1 for the GABAergic organoid and primary fetal datasets and resolution of 2 for the cerebellar organoid dataset.
Cell clustering and annotation
Following low quality cell removal, datasets were clustered (FindClusters function; resolution = 2 for primary screen; resolution = 1 for primary fetal striatum and secondary GABAergic organoid screen) and embedded for visualization purposes with Uniform Manifold Approximation and Projection (UMAP)75. Marker genes for each cluster were determined using the FindMarkers function with default parameters (Wilcoxon Rank Sum test), calculated on the normalized gene expression data. We identified and categorized major cell classes through a combination of marker gene expression and annotation via integration with a comprehensive reference human fetal brain datasets37,52. Specifically, progenitor clusters displayed expression of mitotic transcripts (e.g., MKI67 and TOP2A) and/or SOX2, and had high cluster overlap to radial glial clusters annotated in fetal brain. Intermediate progenitor cells (IPC) expressed EOMES and mapped to fetal IPCs. We used the term astroglia to encompass multiple states of astrocyte differentiation and clusters expressed high levels of SLC1A3 and AQP4 and demonstrated mapping to fetal glioblasts. Oligodendrocyte progenitor cells (OPCs) expressed PDGFRA and SOX10, while oligodendrocytes expressed markers of myelination (MOG, MYRF). Glutamatergic neurons were identified by the presence of neuronal transcripts (STMN2, SNAP25) and presence of either VGLUT1 (SLC17A7) or VGLUT2 (SLC17A6). GABAergic neurons expressed GABA processing enzymes (GAD1, GAD2) and dopaminergic neurons expressed the dopamine transporter SLC6A3. Choroid-like cells were defined by expression of TTR. Similarly broad developmental region annotations were performed using a combination of integration/label transfer with the human fetal brain dataset and expression of known marker gene expression. Forebrain clusters were identified by expression of FOXG1 (telencephalon), EMX2, DLX1, and/or TCF7L2 (diencephalon) in addition to mapping to fetal forebrain, telencephalon, and diencephalon clusters. Midbrain clusters expressed EN1, OTX2, GATA3, and/or markers of dopaminergic neurons in addition to mapping to fetal midbrain clusters. Hindbrain clusters primarily mapped to fetal hindbrain and cerebellum and contained markers of cerebellar cell populations.
To map our organoid clusters to annotated cell clusters from reference primary fetal single scRNAseq data, we performed a pairwise dataset integration approach. Given the complex batch structure described in the Braun et al. fetal reference publication, we focused only on samples acquired using the 10x v3 chemistry. We further removed cell clusters annotated to non-central nervous system derived populations (erythrocyte, fibroblast, immune, neural crest, and placode annotated clusters) and removed cells from the earliest developmental stages which did not contain detailed regional information (samples from 6 PCW or younger). We used Seurat’s LogNormalize method (as implemented with the NormalizeData function) and integrated (IntegrateData function) using each donor as a batch (using dataset dimension of 50 and cluster resolution=1). The reference dataset was randomly subsetted to have a maximum of 500 cells per original cluster to improve computational efficiency. Using an analogous approach described previously 76, cluster overlap was defined as the proportion of cells in each integrated cluster that overlapped with the reference cluster labels. For the fetal cerebellum reference dataset, we downloaded processed data from UCSC cell browser, which was processed in the original study using similar workflow in Seurat. To further classify organoid cells, we utilized Seurat’s TransferData workflow to map reference regional annotation or fetal cerebellum clusters to our organoid cells.
For the primary fetal and organoid GABAergic secondary screen, annotation of clusters was based on marker genes and cell annotations used in a recent fetal macaque inhibitory neuron reference atlas 28.
Cell condition associations
To determine conditions associated with cell populations across the primary organoid screen clusters, we normalized the contribution of each condition to each cluster by the total number of cells present in each condition. To visualize positive associations, we scaled (to achieve mean = 0, standard deviation= 1) condition contributions to each cluster for each condition (visualized in Fig. 2F). For neuronal subclusters, we performed a permutation approach to calculate the statistical association of each applied molecule to a given neuronal subcluster. Specifically, for each cluster we calculated the proportion of cells that were exposed to a given molecule. To determine statistical associations for each molecule, we randomly shuffled molecular annotations (n= 1000 permutations) and determined p-values by calculating the number of permutated molecular proportions greater than the observed value and dividing by the number of permutations.
Disease gene enrichment
Neurodevelopmental disorder (NDD), autism spectrum disorder (ASD), and schizophrenia (SCZ) associated gene lists were compiled from recent rare coding variant association studies 45,46. Using cutoffs defined by Fu et al., we focused on larger disease gene lists with the following criteria for each gene set: NDD (genes associated at FDR ≤ 0.001, 373 genes), ASD (genes associated at FDR ≤ 0.05, 185 genes), SCZ (genes associated at P < 0.01, 244 genes). To calculate enrichment of disease genes across organoid clusters, a one-sided Fisher’s exact test was performed for each set of cluster marker genes (adjusted P-value < 0.05) and disease gene sets. Focusing on genes with at least some minimal expression in our organoid data (defined as non-zero expression in at least 10% of cells in at least one organoid cluster) we determined which genes displayed the most specific expression across organoid clusters by calculating a Tau score. Tau varies from 0 to 1, where 0 means broadly expressed, and 1 is specific.
Differential gene expression analysis
Differential gene expression analysis between primary fetal and organoid cell clusters was performed using a pseudobulk approach across biological replicates, implemented with the Libra R package (version 1.0.0) 77. Specifically, the edgeR (version 4.0.15, R package) log-likelihood ratio test was performed between groups on gene counts summed across cells for a given cell class for each sample replicate. For heat map visualizations, counts per million (CPM) normalized expression values were calculated using edgeR (cpm() function) and scaled (to achieve mean = 0, standard deviation = 1). Gene Ontology (GO) enrichment analyses among significantly altered genes (Benjamini–Hochberg-adjusted P values less than 0.05, expressed in at least 10% of cells, and fold change increase of at least 2) were performed using the ToppGene Suite (https://toppgene.cchmc.org/) 78. We used the ToppFun application with default parameters and reported Benjamini–Hochberg-adjusted P values calculated from hypergeometric tests from GO annotations.
MERFISH
Gene panel selection
Multiplexed error-robust fluorescence in situ hybridization (MERFISH) was performed using Vizgen MERSCOPE platform using manufacturer’s instructions and outlined here. We cross-referenced prior published human and mouse brain MERFISH gene panels, literature, and utilized published bulk developing human brain RNA-sequencing to generate a 500 gene panel for MERFISH. Specifically, we incorporated the 140 human gene panel designed by Allen Institute, ~100 shared genes present on both Vizgen’s 500 PanNeuro mouse gene panel and a recently designed whole brain 500 mouse gene panel 79. The remainder of the gene panel was selected by manual annotation to maximize annotation of neural cell types and region-restricted developmental gene signatures. We incorporated bulk RNA-sequencing reads per kilobase per million mapped reads (RPKM) expression values obtained from published developing human frontal cortex, to finalize the gene selection, to ensure that the summed RPKM values of all genes in the panel was less than 10,000 to minimize optical crowding (per Vizgen recommendations) 80. Note that this panel was also designed to be compatible with organoid transplantation experiments in rats and thus contains 11 genes targeting the rat transcriptome, which were not included in analyses below.
Sample Preparation and Cryosectioning
Organoids and assembloids were fixed in 4% PFA overnight at 4°C and subsequently dehydrated in 30% sucrose for 48 hours at 4°C, or until the samples sank to the bottom of the well. Samples were then embedded in OCT (Tissue-Tek OCT Compound 4583, Sakura Finetek) and frozen using dry ice. Using cryostat (Leica, CM1860), 10 μm sections were collected and adhered to a MERSCOPE Slide (Vizgen, 20400001) and placed in a 6 cm petri dish. The slide was left in the cryostat at −20°C for 25–30 minutes to allow the section to fully adhere to the slide. The slide was then washed with 5 mL of 1× PBS three times for 5 min each and allowed to dry at room temperature for 1 hour. The slide was stored in 5 mL of 70% ethanol at 4°C for a minimum of 24 hours and a maximum of 1 month before proceeding with sample processing.
Sample Processing
After sections were allowed to permeabilize in 70% ethanol for at least 24 hours, the slide was washed once with 5 mL of Sample Prep Wash Buffer (Vizgen, 20300001) and incubated at 37°C for 30 minutes with 5 mL of Formamide Wash Buffer (Vizgen, 20300002). Formamide Wash Buffer was then carefully aspirated from the slide, and 50 μL of MERSCOPE Gene Panel Mix was added directly to the center of the section. The section was then covered with parafilm and allowed to incubate with the Gene Panel Mix for 36–48 hours at 37°C in a humidified incubator.
After probe hybridization, the parafilm was carefully removed and the slide was washed with 5 mL of Formamide Wash Buffer two times for 30 minutes each at 47°C. The slide was then incubated with 5 mL of Sample Prep Wash Buffer for 2 minutes at room temperature before embedding the section in a 4% polyacrylamide gel. The Gel Embedding Solution was prepared by combining 5 mL of Gel Embedding Premix (Vizgen, 20300004) with 25 mL of 10% w/v ammonium persulfate solution (Millipore-Sigma, 09913–100G) and 2.5 μL of N,N,N’,N’-Tetramethylethylenediamine (Millipore-Sigma, T7024–25ML). After incubating with Sample Prep Wash Buffer for 2 minutes at room temperature, 100 μL of Gel Embedding Solution was retained and the sample was incubated with 4.9 mL of Gel Embedding Solution for 1 minute at room temperature before transferring the majority of the solution to a waste tube to monitor gel formation. 50 μL of the retained Gel Embedding Solution was then added to the center of the section and covered with a 20 mm glass coverslip coated with 100 μL of Gel Slick Solution (VWR, 12001–812). The gel was allowed to solidify for 90 minutes at room temperature before the coverslip was removed and the Clearing Solution was added. The Clearing Solution was prepared by combining 5 mL Clearing Premix (Vizgen, 20300003) pre-warmed to 37°C for 30 minutes with 50 μL Proteinase K (New England Biolabs, P8107S). Clearing Solution was added to the slide and the sample was incubated at 47°C for 24 hours.
After the sample was allowed to incubate in Clearing Solution at 47°C for 24 hours in a humidified incubator, the slide was washed with Sample Prep Wash Buffer two times for 5 minutes each at room temperature. The slide was then incubated with 3 mL DAPI and PolyT Staining Reagent (Vizgen, 20300021) for 15 minutes at room temperature on a rocker while protected from light. The slide was then incubated with 5 mL Formamide Wash Buffer for 10 minutes at room temperature and subsequently moved to 5 mL Sample Prep Wash Buffer.
Imaging
Prior to imaging, a MERSCOPE Imaging Cartridge (Vizgen, 20300019) was allowed to thaw at 37°C in a water bath for 1 hour. Once completely thawed, the cartridge was activated by adding 100 μL of RNase Inhibitor (New England Biolabs, M0314L) to 250 μL Imaging Buffer Activator (Vizgen, 20300022) and adding 350 μL of this Imaging Activation Mix to the cartridge via the Cartridge Activation Port. Finally, 15 mL of Mineral Oil (Millipore-Sigma M5904–6X500ML) was carefully layered on top of the liquid in the MERSCOPE Imaging Cartridge via the Cartridge Activation Port, and the activated imaging cartridge was placed into the MERSCOPE Instrument. The MERSCOPE Flow Chamber (Vizgen, 10300102) was assembled with the slide according to the manufacturer’s instructions and connected to the fluidic lines in the MERSCOPE Instrument. Images were acquired using both 10× objective and 63x immersion oil objectives per manufacturer’s instructions.
Data processing
Raw imaging data were processed using the Vizgen analysis pipeline (based on the MERlin python package68), which included cell segmentation with Cellpose based on the DAPI and PolyT staining 69. The outputted cell-by-gene count matrix was filtered to include cells with at least 50 mRNA molecules to remove putative low-quality cells. Data was preprocessed using the Seurat (version 5.0.1) R package workflow. Specifically, we performed sctransform-based normalization, with modified clipping parameters (−10,10) to account for the effect of outliers, per Seurat author suggestions for smFISH experiments. Dimensionality reduction using PCA on human genes was performed and clusters of cells were identified in PCA space by shared nearest neighbor graph construction and modularity detection implemented by the FindNeighbors and FindClusters (resolution=2) functions using a dataset dimension of 30 with default parameters. Marker genes for each cluster were determined using the FindAllMarkers function with default parameters. GABAergic clusters were identified by expression of GAD1/GAD2 and annotated by marker genes.
Quantifications and statistical analyses
Data are presented as mean ± s.e.m. Raw data were tested for normality of distribution, and statistical analyses were performed by paired and unpaired t-test (two-tailed), Mann-Whitney test, Wilcoxon test, one-way ANOVA, or Kruskal-Wallis test with multiple comparison tests depend on the dataset. Sample sizes were estimated empirically. GraphPad Prism Version 9.3.1 or MATLAB (version R2018a, 9.4.0, MathWorks) were used for statistical analyses.
Supplementary Material
Table S1, related to Figure 1. Molecular protocols for all conditions. Details of protocols and molecules applied at each time point.
Table S2, related to Figure 1. Organoid screen scRNA-seq cluster annotation and marker genes. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S3, related to Figure 2. Organoid screen molecular condition cell numbers for each scRNAseq cluster.
Table S4, related to Figure 3. Morphological and cell composition features by condition.
Table S5. Cerebellar organoid scRNA-seq cluster annotation and marker genes, related to Figure 4. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S6. Primary fetal versus organoid cerebellum cell differential gene expression and Purkinje neuron gene ontology (GO) enrichment, related to Figure 4. (A) Pseudobulk differential gene expression analysis (EdgeR log likelihood ratio test with Benjamini-Hochberg adjustment). Shown are significant differentially regulated genes (defined as adjusted P value < 0.05, expressed in at least 10% of cells, and absolute fold change of 2). GO term enrichment for significant differentially regulated genes increased in primary (B) or organoid (C) Purkinje neurons.
Table S7. Cajal-Retzius organoid scRNA-seq cluster annotation and marker genes, related to Figure 6. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S8. Primary fetal versus organoid Cajal-Retzius cell differential gene expression and gene ontology (GO) enrichment, related to Figure 6. (A) Pseudobulk differential gene expression analysis (EdgeR log likelihood ratio test with Benjamini-Hochberg adjustment). Shown are significant differentially regulated genes (defined as adjusted P value < 0.05, expressed in at least 10% of cells, and absolute fold change of 2). (B) GO term enrichment for significant differentially regulated genes increased in primary CR cells.
Table S9. Neuronal organoid scRNA-seq subcluster annotation and marker genes, related to Figure 6. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S10. Primary fetal striatum scRNA-seq cluster annotation and marker genes, related to Figure 7. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S11. Secondary GABAergic organoid screen scRNA-seq cluster annotation and marker genes, related to Figure 7. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S12. Primary fetal versus organoid TAC3 GABAergic neuron differential gene expression and gene ontology (GO) enrichment, related to Figure 7. (A) Pseudobulk differential gene expression analysis (EdgeR log likelihood ratio test with Benjamini-Hochberg adjustment). Shown are significant differentially regulated genes (defined as adjusted P value < 0.05, expressed in at least 10% of cells, and absolute fold change of 2). GO term enrichment for significant differentially regulated genes increased in primary (B) or organoid (C) TAC3 neurons.
Table S13. MERFISH gene panel, related to Figure 7.
Table S14. Subpallial TAC3 organoid MERFISH cluster annotation and marker genes, related to Figure 7. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Video S1, related to Figure 5. Confocal z-projection of CALB1 immunostaining in cleared hCbO identifying Purkinje neuron complex dendritic branching at day 185
Video S2, related to Figure 5. Confocal z-projection of CALB1 immunostaining in cleared t-hCbO identifying Purkinje neuron complex dendritic branching at day 185 (138 days post transplantation).
Acknowledgements
This work was supported by Stanford Brain Organogenesis Big Idea Grant from the Wu Tsai Neurosciences Institute (to S.P.P.), the NYSCF Robertson Stem Cell Investigator Award (to S.P.P.), the Kwan Research Fund (to S.P.P.), the Coates Foundation (to S.P.P.), the Senkut Research Funds (to S.P.P.), The Ludwig Foundation (to S.P.P.), the Chan Zuckerberg Initiative Ben Barres Investigator Award (to S.P.P.), CZ BioHub (to S.P.P.), NINDS K08-NS123544-01 (to N.D.A), Brain and Behavior Research Foundation (to N.D.A), Foundations of the National Institutes of Health Deeda Blair Research Initiative (to N.D.A.), NIMH T32MH019938 (to K.W.K), Stanford Maternal & Child Health Research Institute (MCHRI) Postdoctoral Fellowships (to Y.M.).
Footnotes
Declaration of interests
G.N. was an employee of Daiichi-Sankyo Co., Ltd, when performing the experiments for this study, however, the company did not have any input and interpretation on the design of experiments and the data. Stanford University holds patents that cover the generation of regionalized neural organoids. All other authors declare no competing interests.
References
- 1.Kelley KW, and Pasca SP (2022). Human brain organogenesis: Toward a cellular understanding of development and disease. Cell 185, 42–61. 10.1016/j.cell.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Lodato S, and Arlotta P. (2015). Generating neuronal diversity in the mammalian cerebral cortex. Annu Rev Cell Dev Biol 31, 699–720. 10.1146/annurev-cellbio-100814-125353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansour AA, Schafer ST, and Gage FH (2021). Cellular complexity in brain organoids: Current progress and unsolved issues. Semin Cell Dev Biol 111, 32–39. 10.1016/j.semcdb.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Amin ND, and Pasca SP (2018). Building Models of Brain Disorders with Three-Dimensional Organoids. Neuron 100, 389–405. 10.1016/j.neuron.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Di Lullo E, and Kriegstein AR (2017). The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 18, 573–584. 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancaster MA, and Knoblich JA (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125. 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 7.Qian X, Song H, and Ming GL (2019). Brain organoids: advances, applications and challenges. Development 146. 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacomelli E, Vahsen BF, Calder EL, Xu Y, Scaber J, Gray E, Dafinca R, Talbot K, and Studer L. (2022). Human stem cell models of neurodegeneration: From basic science of amyotrophic lateral sclerosis to clinical translation. Cell Stem Cell 29, 11–35. 10.1016/j.stem.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabar V, and Studer L. (2014). Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet 15, 82–92. 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Temple S. (2023). Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell 30, 512–529. 10.1016/j.stem.2023.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driever W, Thoma G, and Nusslein-Volhard C. (1989). Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature 340, 363–367. 10.1038/340363a0. [DOI] [PubMed] [Google Scholar]
- 12.Basson MA, Echevarria D, Ahn CP, Sudarov A, Joyner AL, Mason IJ, Martinez S, and Martin GR (2008). Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development 135, 889–898. 10.1242/dev.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada T, Placzek M, Tanaka H, Dodd J, and Jessell TM (1991). Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell 64, 635–647. 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- 14.Briscoe J, and Small S. (2015). Morphogen rules: design principles of gradient-mediated embryo patterning. Development 142, 3996–4009. 10.1242/dev.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wichterle H, Lieberam I, Porter JA, and Jessell TM (2002). Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397. 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang SC, Wernig M, Duncan ID, Brustle O, and Thomson JA (2001). In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19, 1129–1133. 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 17.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, and Studer L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27, 275–280. 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagner A, and Briscoe J. (2017). Morphogen interpretation: concentration, time, competence, and signaling dynamics. Wiley Interdiscip Rev Dev Biol 6. 10.1002/wdev.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dessaud E, McMahon AP, and Briscoe J. (2008). Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489–2503. 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- 20.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, et al. (2017). Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59. 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, et al. (2013). Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 12, 559–572. 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, et al. (2013). Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 12, 573–586. 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim TW, Piao J, Koo SY, Kriks S, Chung SY, Betel D, Socci ND, Choi SJ, Zabierowski S, Dubose BN, et al. (2021). Biphasic Activation of WNT Signaling Facilitates the Derivation of Midbrain Dopamine Neurons from hESCs for Translational Use. Cell Stem Cell 28, 343–355 e345. 10.1016/j.stem.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piao J, Zabierowski S, Dubose BN, Hill EJ, Navare M, Claros N, Rosen S, Ramnarine K, Horn C, Fredrickson C, et al. (2021). Preclinical Efficacy and Safety of a Human Embryonic Stem Cell-Derived Midbrain Dopamine Progenitor Product, MSK-DA01. Cell Stem Cell 28, 217–229 e217. 10.1016/j.stem.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. (2008). Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221. 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 26.Pasca SP, Arlotta P, Bateup HS, Camp JG, Cappello S, Gage FH, Knoblich JA, Kriegstein AR, Lancaster MA, Ming GL, et al. (2022). A nomenclature consensus for nervous system organoids and assembloids. Nature 609, 907–910. 10.1038/s41586-022-05219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krienen FM, Goldman M, Zhang Q, R CHDR, Florio M, Machold R, Saunders A, Levandowski K, Zaniewski H, Schuman B, et al. (2020). Innovations present in the primate interneuron repertoire. Nature 586, 262–269. 10.1038/s41586-020-2781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitz MT, Sandoval K, Chen CP, Mostajo-Radji MA, Seeley WW, Nowakowski TJ, Ye CJ, Paredes MF, and Pollen AA (2022). The development and evolution of inhibitory neurons in primate cerebrum. Nature 603, 871–877. 10.1038/s41586-022-04510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corrigan Emily K., M.D., Poddar Aunoy, García Miguel Turrero, Schmitz Matthew T., Harwell Corey C., Paredes Mercedes F., Krienen Fenna M., Pollen Alex A.. (2024). Conservation, alteration, and redistribution of mammalian striatal interneurons. bioRxiv. [Google Scholar]
- 30.Wurst W, and Bally-Cuif L. (2001). Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci 2, 99–108. 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- 31.Yoon SJ, Elahi LS, Pasca AM, Marton RM, Gordon A, Revah O, Miura Y, Walczak EM, Holdgate GM, Fan HC, et al. (2019). Reliability of human cortical organoid generation. Nat Methods 16, 75–78. 10.1038/s41592-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O’Rourke NA, Nguyen KD, et al. (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 12, 671–678. 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, Singh M, Chen X, Thete MV, Walczak EM, et al. (2020). Generation of Functional Human 3D Cortico-Motor Assembloids. Cell 183, 1913–1929 e1926. 10.1016/j.cell.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, and Studer L. (2004). Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A 101, 12543–12548. 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellegrini L, Bonfio C, Chadwick J, Begum F, Skehel M, and Lancaster MA (2020). Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369. 10.1126/science.aaz5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, Graybuck LT, Peeler DJ, Mukherjee S, Chen W, et al. (2018). Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182. 10.1126/science.aam8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun E, Danan-Gotthold M, Borm LE, Lee KW, Vinsland E, Lonnerberg P, Hu L, Li X, He X, Andrusivova Z, et al. (2023). Comprehensive cell atlas of the first-trimester developing human brain. Science 382, eadf1226. 10.1126/science.adf1226. [DOI] [PubMed] [Google Scholar]
- 38.Fleck JS, Sanchis-Calleja F, He Z, Santel M, Boyle MJ, Camp JG, and Treutlein B. (2021). Resolving organoid brain region identities by mapping single-cell genomic data to reference atlases. Cell Stem Cell 28, 1148–1159 e1148. 10.1016/j.stem.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 39.van den Ameele J, Tiberi L, Vanderhaeghen P, and Espuny-Camacho I. (2014). Thinking out of the dish: what to learn about cortical development using pluripotent stem cells. Trends Neurosci 37, 334–342. 10.1016/j.tins.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Goke J, Tan ZY, Saw TY, Tan CP, Lokman H, et al. (2016). Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 19, 248–257. 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, and Sasai Y. (2015). Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun 6, 8896. 10.1038/ncomms9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe M, Kang YJ, Davies LM, Meghpara S, Lau K, Chung CY, Kathiriya J, Hadjantonakis AK, and Monuki ES (2012). BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors. J Neurosci 32, 15934–15945. 10.1523/JNEUROSCI.3227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon A, Yoon SJ, Tran SS, Makinson CD, Park JY, Andersen J, Valencia AM, Horvath S, Xiao X, Huguenard JR, et al. (2021). Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat Neurosci 24, 331–342. 10.1038/s41593-021-00802-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amiri A, Coppola G, Scuderi S, Wu F, Roychowdhury T, Liu F, Pochareddy S, Shin Y, Safi A, Song L, et al. (2018). Transcriptome and epigenome landscape of human cortical development modeled in organoids. Science 362. 10.1126/science.aat6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu JM, Satterstrom FK, Peng M, Brand H, Collins RL, Dong S, Wamsley B, Klei L, Wang L, Hao SP, et al. (2022). Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat Genet 54, 1320–1331. 10.1038/s41588-022-01104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh T, Poterba T, Curtis D, Akil H, Al Eissa M, Barchas JD, Bass N, Bigdeli TB, Breen G, Bromet EJ, et al. (2022). Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 604, 509–516. 10.1038/s41586-022-04556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deisseroth CA, Lerma VC, Magyar CL, Pfliger JM, Nayak A, Bliss ND, LeMaire AW, Narayanan V, Balak C, Zanni G, et al. (2022). An Integrated Phenotypic and Genotypic Approach Reveals a High-Risk Subtype Association for EBF3 Missense Variants Affecting the Zinc Finger Domain. Ann Neurol 92, 138–153. 10.1002/ana.26359. [DOI] [PubMed] [Google Scholar]
- 48.Marton RM, and Pasca SP (2020). Organoid and Assembloid Technologies for Investigating Cellular Crosstalk in Human Brain Development and Disease. Trends Cell Biol 30, 133–143. 10.1016/j.tcb.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Douvaras P, and Fossati V. (2015). Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nat Protoc 10, 1143–1154. 10.1038/nprot.2015.075. [DOI] [PubMed] [Google Scholar]
- 50.Hu BY, Du ZW, and Zhang SC (2009). Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc 4, 1614–1622. 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Susaki EA, Shimizu C, Kuno A, Tainaka K, Li X, Nishi K, Morishima K, Ono H, Ode KL, Saeki Y, et al. (2020). Versatile whole-organ/body staining and imaging based on electrolyte-gel properties of biological tissues. Nat Commun 11, 1982. 10.1038/s41467-020-15906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aldinger KA, Thomson Z, Phelps IG, Haldipur P, Deng M, Timms AE, Hirano M, Santpere G, Roco C, Rosenberg AB, et al. (2021). Spatial and cell type transcriptional landscape of human cerebellar development. Nat Neurosci 24, 1163–1175. 10.1038/s41593-021-00872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, and Sasai Y. (2015). Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep 10, 537–550. 10.1016/j.celrep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 54.Zecevic N, and Rakic P. (1976). Differentiation of Purkinje cells and their relationship to other components of developing cerebellar cortex in man. J Comp Neurol 167, 27–47. 10.1002/cne.901670103. [DOI] [PubMed] [Google Scholar]
- 55.Atamian A, Birtele M, Hosseini N, Nguyen T, Seth A, Del Dosso A, Paul S, Tedeschi N, Taylor R, Coba MP, et al. (2024). Human cerebellar organoids with functional Purkinje cells. Cell Stem Cell 31, 39–51 e36. 10.1016/j.stem.2023.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchholz DE, Carroll TS, Kocabas A, Zhu X, Behesti H, Faust PL, Stalbow L, Fang Y, and Hatten ME (2020). Novel genetic features of human and mouse Purkinje cell differentiation defined by comparative transcriptomics. Proc Natl Acad Sci U S A 117, 15085–15095. 10.1073/pnas.2000102117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, Li MY, Birey F, Yang X, Saw NL, et al. (2022). Maturation and circuit integration of transplanted human cortical organoids. Nature 610, 319–326. 10.1038/s41586-022-05277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, and Pierani A. (2005). Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci 8, 1002–1012. 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- 59.Moore SA, and Iulianella A. (2021). Development of the mammalian cortical hem and its derivatives: the choroid plexus, Cajal-Retzius cells and hippocampus. Open Biol 11, 210042. 10.1098/rsob.210042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Causeret F, Moreau MX, Pierani A, and Blanquie O. (2021). The multiple facets of Cajal-Retzius neurons. Development 148. 10.1242/dev.199409. [DOI] [PubMed] [Google Scholar]
- 61.Danjo T, Eiraku M, Muguruma K, Watanabe K, Kawada M, Yanagawa Y, Rubenstein JL, and Sasai Y. (2011). Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci 31, 1919–1933. 10.1523/JNEUROSCI.5128-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krienen FM, Levandowski KM, Zaniewski H, Del Rosario RCH, Schroeder ME, Goldman M, Wienisch M, Lutservitz A, Beja-Glasser VF, Chen C, et al. (2023). A marmoset brain cell census reveals regional specialization of cellular identities. Sci Adv 9, eadk3986. 10.1126/sciadv.adk3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng AHM, Khoshakhlagh P, Rojo Arias JE, Pasquini G, Wang K, Swiersy A, Shipman SL, Appleton E, Kiaee K, Kohman RE, et al. (2021). A comprehensive library of human transcription factors for cell fate engineering. Nat Biotechnol 39, 510–519. 10.1038/s41587-020-0742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joung J, Ma S, Tay T, Geiger-Schuller KR, Kirchgatterer PC, Verdine VK, Guo B, Arias-Garcia MA, Allen WE, Singh A, et al. (2023). A transcription factor atlas of directed differentiation. Cell 186, 209–229 e226. 10.1016/j.cell.2022.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhaduri A, Sandoval-Espinosa C, Otero-Garcia M, Oh I, Yin R, Eze UC, Nowakowski TJ, and Kriegstein AR (2021). An atlas of cortical arealization identifies dynamic molecular signatures. Nature 598, 200–204. 10.1038/s41586-021-03910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, et al. (2021). Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 e3529. 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emanuel G, Eichhorn SW & Zhuang X. (2020). MERlin—scalable and extensible MERFISH analysis software, v0.1.6. Zenodo. 10.5281/zenodo.3758540 [DOI] [Google Scholar]
- 69.Stringer C, Wang T, Michaelos M, and Pachitariu M. (2021). Cellpose: a generalist algorithm for cellular segmentation. Nat Methods 18, 100–106. 10.1038/s41592-020-01018-x. [DOI] [PubMed] [Google Scholar]
- 70.Tao Y, and Zhang SC (2016). Neural Subtype Specification from Human Pluripotent Stem Cells. Cell Stem Cell 19, 573–586. 10.1016/j.stem.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mizuseki K, Sakamoto T, Watanabe K, Muguruma K, Ikeya M, Nishiyama A, Arakawa A, Suemori H, Nakatsuji N, Kawasaki H, et al. (2003). Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc Natl Acad Sci U S A 100, 5828–5833. 10.1073/pnas.1037282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng L, Zhao T, and Kim J. (2015). neuTube 1.0: A New Design for Efficient Neuron Reconstruction Software Based on the SWC Format. eNeuro 2. 10.1523/ENEURO.0049-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGinnis CS, Murrow LM, and Gartner ZJ (2019). DoubletFinder: Doublet Detection in Single-Cell RNA Sequencing Data Using Artificial Nearest Neighbors. Cell Syst 8, 329–337 e324. 10.1016/j.cels.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vertesy A, Eichmuller OL, Naas J, Novatchkova M, Esk C, Balmana M, Ladstaetter S, Bock C, von Haeseler A, and Knoblich JA (2022). Gruffi: an algorithm for computational removal of stressed cells from brain organoid transcriptomic datasets. EMBO J 41, e111118. 10.15252/embj.2022111118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, and Newell EW (2018). Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- 76.Bakken TE, Jorstad NL, Hu Q, Lake BB, Tian W, Kalmbach BE, Crow M, Hodge RD, Krienen FM, Sorensen SA, et al. (2021). Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature 598, 111–119. 10.1038/s41586-021-03465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Squair JW, Gautier M, Kathe C, Anderson MA, James ND, Hutson TH, Hudelle R, Qaiser T, Matson KJE, Barraud Q, et al. (2021). Confronting false discoveries in single-cell differential expression. Nat Commun 12, 5692. 10.1038/s41467-021-25960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Bardes EE, Aronow BJ, and Jegga AG (2009). ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 37, W305–311. 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao Z, van Velthoven CTJ, Kunst M, Zhang M, McMillen D, Lee C, Jung W, Goldy J, Abdelhak A, Aitken M, et al. (2023). A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature 624, 317–332. 10.1038/s41586-023-06812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li M, Santpere G, Imamura Kawasawa Y, Evgrafov OV, Gulden FO, Pochareddy S, Sunkin SM, Li Z, Shin Y, Zhu Y, et al. (2018). Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 362. 10.1126/science.aat7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhong W, Barde S, Mitsios N, Adori C, Oksvold P, Feilitzen KV, O’Leary L, Csiba L, Hortobagyi T, Szocsics P, et al. (2022). The neuropeptide landscape of human prefrontal cortex. Proc Natl Acad Sci U S A 119, e2123146119. 10.1073/pnas.2123146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1, related to Figure 1. Molecular protocols for all conditions. Details of protocols and molecules applied at each time point.
Table S2, related to Figure 1. Organoid screen scRNA-seq cluster annotation and marker genes. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S3, related to Figure 2. Organoid screen molecular condition cell numbers for each scRNAseq cluster.
Table S4, related to Figure 3. Morphological and cell composition features by condition.
Table S5. Cerebellar organoid scRNA-seq cluster annotation and marker genes, related to Figure 4. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S6. Primary fetal versus organoid cerebellum cell differential gene expression and Purkinje neuron gene ontology (GO) enrichment, related to Figure 4. (A) Pseudobulk differential gene expression analysis (EdgeR log likelihood ratio test with Benjamini-Hochberg adjustment). Shown are significant differentially regulated genes (defined as adjusted P value < 0.05, expressed in at least 10% of cells, and absolute fold change of 2). GO term enrichment for significant differentially regulated genes increased in primary (B) or organoid (C) Purkinje neurons.
Table S7. Cajal-Retzius organoid scRNA-seq cluster annotation and marker genes, related to Figure 6. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S8. Primary fetal versus organoid Cajal-Retzius cell differential gene expression and gene ontology (GO) enrichment, related to Figure 6. (A) Pseudobulk differential gene expression analysis (EdgeR log likelihood ratio test with Benjamini-Hochberg adjustment). Shown are significant differentially regulated genes (defined as adjusted P value < 0.05, expressed in at least 10% of cells, and absolute fold change of 2). (B) GO term enrichment for significant differentially regulated genes increased in primary CR cells.
Table S9. Neuronal organoid scRNA-seq subcluster annotation and marker genes, related to Figure 6. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S10. Primary fetal striatum scRNA-seq cluster annotation and marker genes, related to Figure 7. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S11. Secondary GABAergic organoid screen scRNA-seq cluster annotation and marker genes, related to Figure 7. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Table S12. Primary fetal versus organoid TAC3 GABAergic neuron differential gene expression and gene ontology (GO) enrichment, related to Figure 7. (A) Pseudobulk differential gene expression analysis (EdgeR log likelihood ratio test with Benjamini-Hochberg adjustment). Shown are significant differentially regulated genes (defined as adjusted P value < 0.05, expressed in at least 10% of cells, and absolute fold change of 2). GO term enrichment for significant differentially regulated genes increased in primary (B) or organoid (C) TAC3 neurons.
Table S13. MERFISH gene panel, related to Figure 7.
Table S14. Subpallial TAC3 organoid MERFISH cluster annotation and marker genes, related to Figure 7. Marker genes for each annotated cell cluster as determined using the Seurat FindMarkers function with default parameters (two-sided Wilcoxon Rank Sum test with Bonferroni correction).
Video S1, related to Figure 5. Confocal z-projection of CALB1 immunostaining in cleared hCbO identifying Purkinje neuron complex dendritic branching at day 185
Video S2, related to Figure 5. Confocal z-projection of CALB1 immunostaining in cleared t-hCbO identifying Purkinje neuron complex dendritic branching at day 185 (138 days post transplantation).
Data Availability Statement
Single-cell RNA-sequencing data have been deposited at GEO as GSE233574 and are available as of the date of the publication. Spatial transcriptomics data have been deposited at Zenodo (https://doi.org/10.5281/zenodo.13835782) and are available as of the date of the publication.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.