Abstract
FtsH is a membrane-bound, ATP-dependent metalloprotease in Escherichia coli that degrades some integral membrane proteins and cytoplasmic proteins. In this study, we show that FtsH-dependent degradation of both membrane-bound and soluble proteins is retarded when cells are treated with carbonyl cyanide-3-chlorophenylhydrazone or 2,4-dinitrophenol uncouplers, which dissipate the proton-motive force. In vitro casein degradation by membrane-integrated FtsH was stimulated by succinate, a respiratory substrate; this stimulation was counteracted by cyanide-3-chlorophenylhydrazone. Potassium thiocyanate, which specifically collapses Δψ, partially canceled the effect of succinate, but ammonium sulfate, which collapses ΔpH, showed little effect. These results indicate that the proton-motive force, in particular the Δψ component, plays a role in efficient degradation of substrates by FtsH in its native state. FtsH variants with altered transmembrane regions did not receive proton-motive force stimulation, suggesting that the proton-motive force activates FtsH, directly or indirectly, through the transmembrane region.
ATP is used as a universal currency of energy in a wide variety of cellular activities. ATP-dependent proteases play pivotal roles in the regulation of various cellular events, including the rapid removal of abnormal proteins. Prokaryotic cells are equipped with several ATP-dependent proteases (1, 2). In Escherichia coli, five enzymes catalyze ATP-dependent proteolysis: Lon, ClpAP, ClpXP, HslUV, and FtsH. FtsH, the only membrane protein among these five enzymes, is a metalloprotease. FtsH has two transmembrane segments in the N-terminal region that are followed by cytoplasmic AAA ATPase and protease domains (see Fig. 1 for a schematic diagram) (3). FtsH has a homooligomeric quaternary structure (4) that is crucial for its ATPase and proteolytic activities (5–7). The N-terminal region of FtsH has dual functions in membrane localization and homooligomerization (4, 5). FtsH forms a complex with another membrane protein complex, HflK/HflC (HflKC), which has been suggested to have a regulatory role in the proteolytic function of FtsH (8–10). The short periplasmic domain between the transmembrane segments of FtsH is essential for the interaction with the periplasmically exposed HflKC complex (11).
Figure 1.
Schematic representations of FtsH-His6-Myc, FtsH(Δperi)-His6-Myc, LacY-FtsH-His6-Myc, and Zip-FtsH(ΔTM)-His6-Myc used in this study. All of the proteins carried the C-terminal His6-Myc tag. Hatched regions are derived from FtsH. TM1 and TM2 indicate the first and the second transmembrane segments of either FtsH or LacY. The leucine-zipper sequence at the N terminus of Zip-FtsH(ΔTM)-His6-Myc mediates the multimerization of the cytoplasmic domain of FtsH. The positions of the AAA ATPase homologous region and the metalloprotease zinc-binding motif (HEXXH) are indicated.
A notable feature of FtsH is its involvement in membrane protein degradation. Substrates of FtsH include several multispanning membrane proteins, such as the SecY subunit of protein translocase (12, 13), subunit a of the proton ATPase (Foa) (14), and the YccA protein of unknown function (10). SecY and the Foa subunit are degraded rapidly when they are not associated with their partner proteins. We previously proposed that FtsH catalyzes processive degradation of membrane proteins, in which the substrate protein is presumably dislocated to the cytoplasm where the enzymatic active sites of FtsH reside (15).
Many membrane-related reactions are driven or promoted by another type of energy source in the cell, the proton-motive force (PMF). PMF is involved in the import and export of various substances, ranging from small ions to macromolecules, across the membrane. Some reactions use both ATP and PMF as energy sources. For example, the translocation of secretory proteins is driven by ATP and PMF (16, 17). In this case, the SecA ATPase drives the stepwise movement of preproteins at the expense of ATP (18, 19), but the mechanism by which PMF promotes translocation is not fully understood. The role of PMF in cellular proteolytic reactions has not yet been elucidated.
In this study, we show that the proteolytic activity of FtsH, an ATP-dependent protease, is significantly stimulated by PMF. Our results shed light on the energy-dependent proteolysis that occurs at the membrane.
Materials and Methods
Bacterial Strains, Plasmids, and Media.
All bacterial strains used in this study were derivatives of E. coli K12. MC4100 [araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR] (20) and CSH26 (Δpro-lac thi) (21) have been described. AD1377 [CSH26, Δ(uncB-uncC) sfhC21 zad-220∷Tn10/F′lacIq] was constructed as follows: first, Δ(uncB-uncC) marker was transferred from DK8 (22) into AD16 (CSH26/F′ lacIq) (12) by P1 transduction by using a nearby ilv∷Tn10 for selection; subsequently, ilv∷Tn10 was eliminated by selecting Ilv+ transductants by using P1 phages that had been grown on MC4100; finally, sfhC21 was introduced with zad-220∷Tn10 from AR3289 (23) by P1 transduction. AD1400 (AD1377, ftsH3∷kan) was constructed by P1-mediated introduction of ftsH3∷kan into AD1377 from AR423 (24). AD1434 [CU141, Δ(uncB-uncC) sfhC21 zad-220∷Tn10 ftsH3∷kan] was similarly constructed by introducing the markers above into CU141 (MC4100/F′lacIq) (24).
pSTD113 (ftsH-his6-myc) (4), pSTD181 (atpB) (14), pSTD243 [ftsH(Δperi)-his6-myc] (11), pSTD348 (lacY-ftsH-his6-myc) (5), pSTD423 [yccA-(P3)-phoA(SSSS)-his6-myc] (5), and pSTD430 [zip-ftsH(ΔTM)-his6-myc] (5) have been described previously. pTYE007B (5) was a derivative of vector pTYE007 (4), and carried a frame shift in the lacZα gene. pSTD466 (yccA-his6-myc) was constructed by cloning a 0.8-kb HindIII–SacI blunt-ended fragment of pKH330 (10) into a SmaI site of pBAD33 (25).
L medium (26) and M9 medium (21) were used. Ampicillin (50 μg/ml) and/or chloramphenicol (20 or 100 μg/ml) were added for growing plasmid-bearing strains.
Pulse–Chase Experiments.
Pulse–chase experiments were performed essentially as described (4). Cells were grown in M9 medium supplemented with 0.4% glucose, 20 μg/ml 18 aa (other than Met and Cys), and appropriate antibiotics at 28°C or 37°C, induced with either 0.4% arabinose or 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) plus 5 mM cAMP and pulse-labeled with [35S]methionine. Chase was initiated by adding 1/100 volume of 20 μg/ml unlabeled methionine followed by either 3/1000 volume of 10 mM carbonyl cyanide-3-chlorophenylhydrazone (CCCP) (dissolved in DMSO) or 1/68 volume of 0.34 M 2,4-dinitrophenol (DNP) (in methanol). Control samples received the same volume of either DMSO or methanol. Immunoprecipitation by using anti-Myc, anti-Foa, anti-σ32, and anti-PhoA was performed as described previously (4). Proteins were separated by 10% polyacrylamide gel electrophoresis (27) or 16.1% acrylamide/0.12% N,N′-methylenebisacrylamide gel electrophoresis (28) and were visualized and quantified with a Fuji BAS1800 imaging analyzer.
In Vitro Proteolytic Activity Assay.
Inverted inner membrane vesicles were prepared from cells of AD1434/pSTD113 and AD1434/pTY007B that had been grown at 37°C for 3 h in L medium containing ampicillin (50 μg/ml), IPTG (1 mM), and cAMP (1 mM) essentially as described (29). Inverted membrane vesicles containing 6.1 μg of protein (except FtsH; the FtsH-overproduced vesicles contained an additional 2.1 μg of FtsH-His6-Myc) were incubated with resorufin-labeled casein (200 μg/ml) at 37°C in buffer containing 50 mM Tris⋅HCl, pH 8.1, 50 mM KCl, 5 mM MgCl2, 25 μM zinc acetate, 10 mM 2-mercaptoethanol. Added as indicated in Fig. 4 were: 5 mM ATP, 4.5 mM succinate, 10 μM CCCP (or DMSO), 12.5 mM potassium thiocyanate, 20 mM ammonium sulfate, and 0.5% Nonidet P-40. A portion of each sample was withdrawn at intervals and mixed with an equal volume of 7% trichloroacetic acid. Photometric quantitation of degraded resorufin-casein in the supernatant was performed as described (6).
Figure 4.
Effects of PMF on in vitro degradation of resorufin-casein by FtsH. Inverted membrane vesicles prepared from AD1434/pSTD113 [V(F)] or from AD1434/pTYE007B [V(Δ)] were incubated with resorufin-casein at 37°C in the presence or absence of 5 mM ATP, 4.5 mM succinate (Suc), 10 μM CCCP (plus 0.25% DMSO), 0.25% DMSO, 20 mM ammonium sulfate (AS), 12.5 mM potassium thiocyanate (KSCN), and 0.5% Nonidet P-40, as indicated. A portion of the samples was withdrawn at the indicated time points, mixed with trichloroacetic acid, and centrifuged. The absorbance of the supernatant at 574 nm was measured.
Measurement of ΔpH and Δψ.
Inverted membrane vesicles (122 μg protein except FtsH-His6-Myc) were incubated at room temperature in the reaction mixture with the same buffer conditions as the resorufin-casein degradation assay, except that resorufin-casein was omitted. The generation of ΔpH and Δψ by succinate (4.5 mM) and the dissipation of ΔpH and Δψ by CCCP (10 μM), potassium thiocyanate (12.5 mM), or ammonium sulfate (20 mM) was monitored by fluorescence quenching of quinacrine (1 μM) and oxonol V (1 μM) included in the mixture above, essentially as described (30, 31).
Results
CCCP Inhibits the FtsH-Catalyzed Degradation of Membrane and Cytoplasmic Proteins in Vivo.
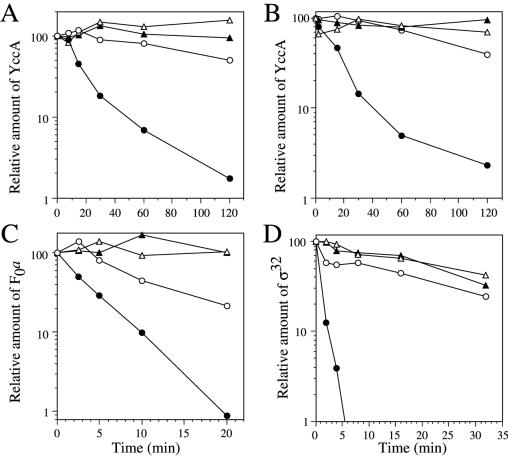
We examined the effects of CCCP and DNP, uncouplers that dissipate PMF, on in vivo degradation of membrane-bound and soluble substrates of FtsH (Fig. 2). YccA is a membrane protein with seven transmembrane segments (15). Although the cellular function of YccA is unknown, it is a substrate of FtsH (10). YccA with a C-terminal His6-Myc tag was expressed from a plasmid and its stability was examined by pulse–chase experiments. In wild-type cells, pulse-labeled YccA was degraded on chase at 28°C (Fig. 2 A and B) and 37°C (data not shown) with a half-life of about 10–13 min. Little degradation of YccA was observed in the FtsH-deleted cells, even after 120 min of chase, indicating that YccA degradation is catalyzed almost exclusively by FtsH. When CCCP (Fig. 2A) was added at the onset of the chase with unlabeled methionine, degradation of YccA was significantly retarded. The stabilization of YccA by CCCP was more remarkable at 28°C (t1/2 was about 120 min; Fig. 2A) than at 37°C (t1/2 was about 45 min; data not shown). A similar YccA-stabilizing effect was observed for another uncoupler, DNP (Fig. 2B).
Figure 2.
Effects of CCCP and DNP on FtsH-dependent protein degradation in vivo. Cells of AD1377 (ftsH+ Δunc; circles) and AD1400 (ΔftsH Δunc; triangles), carrying pSTD466 (yccA; A and B), pSTD181 (atpB; C), or no plasmid (D), were grown in M9 medium at 28°C, pulse-labeled with [35S]methionine from 2 min, and chased in the presence (open symbols) or absence (closed symbols) of either 30 μM CCCP (A, C, and D) or 5 mM DNP (B). For expression of YccA-His6-Myc and Foa, cells were induced for 10 min with 0.4% arabinose, and 1 mM IPTG plus 5 mM cAMP, respectively, before pulse-labeling. Portions of the samples were withdrawn at the indicated time points and subjected to immunoprecipitation either with anti-Myc, anti-Foa, or anti-σ32.
We have shown that a PhoA domain inserted into the third periplasmic region of YccA was degraded in an FtsH-dependent manner when the PhoA domain is unfolded (5, 15). This degradation presumably is accompanied by the dislocation of the periplasmic PhoA domain into the cytoplasm (5, 15). To determine whether PMF affects the dislocation of the PhoA moiety, we examined degradation of YccA-(P3)-PhoA(SSSS), whose PhoA domain is unfolded because of the lack of intramolecular disulfide bonds (5) (data not shown). If PMF is required for FtsH to degrade the periplasmic region of the substrate, even the unfolded PhoA domain might remain undigested under the PMF-dissipated condition. The pulse–chase experiments showed that degradation of YccA-(P3)-PhoA(SSSS) depended on FtsH and significantly retarded by CCCP (data not shown). However, no PhoA-containing degradation intermediate was detected even in the presence of CCCP (data not shown).
We also examined the effects of CCCP on the degradation of Foa, another membrane-bound substrate of FtsH (ref. 14; Fig. 2C). Foa was degraded with a half-life of about 2.5 min at 28°C in wild-type cells. The half-life of Foa was extended ≈3- to 4-fold by the addition of CCCP. These results indicate that PMF accelerates the FtsH-dependent degradation of membrane proteins, but not specifically the dislocation process.
Substrates of FtsH include not only membrane proteins but also some cytoplasmic proteins including a heat-shock sigma factor, σ32. FtsH is the major protease responsible for in vivo degradation of σ32 (32, 33), although other cytoplasmic ATP-dependent proteases (ClpXP, ClpAP, and HslUV) also contribute to the degradation (34). σ32 is degraded very rapidly both at 28°C (Fig. 2D) and 37°C (data not shown). We found that CCCP markedly stabilized σ32, almost to the same extent as in the FtsH-deleted cells. Dissipation of PMF inhibits translocation of secreted proteins (35), leading to the accumulation of their precursors in the cytoplasm. Accumulation of abnormal proteins could cause stabilization of σ32 (36, 37; also see Fig. 5C). However, the σ32-stabilizing effect of CCCP was observed immediately after its addition, suggesting that PMF directly affects the degradation of σ32. In the FtsH-deleted cells, σ32 was still degraded slowly presumably by ClpXP, ClpAP and/or HslUV. The FtsH-independent σ32 degradation was not affected by CCCP. Thus, the CCCP effect is specific for FtsH among the ATP-dependent proteases.
Figure 5.
FtsH variants with an altered membrane region are insensitive to CCCP. (A and B) Plasmids pSTD113 (ftsH-his6-myc; circles in A), pTYE007B (vector; triangles in A), pSTD348 (lacY-ftsH-his6-myc; squares in B) and pSTD243 (ftsH(Δperi)-his6-myc; diamonds in B) were introduced into cells of AD1400 carrying pSTD423 [yccA-(P3)-phoA(SSSS)]. (C) Plasmids pSTD113 (ftsH-his6-myc, circles), pSTD430 (zip-ftsH(ΔTM)-his6-myc, downward triangles) and pUC119 (vector; upward triangles) were introduced into cells of AD1400. Stability of YccA-(P3)-PhoA(SSSS)-His6-Myc (A and B) or σ32 (C) was examined at 28°C by pulse–chase experiments in the presence (open symbols) or absence (closed symbols) of CCCP, essentially as described in the legend to Fig. 2. For expression of the plasmid-encoded proteins, cells were induced for 2 h with 1 mM IPTG plus 5 mM cAMP.
In Vitro Effects of PMF on Casein Degradation by Membrane-Integrated FtsH.
Although an in vitro assay has been established for FtsH-catalyzed proteolysis in the presence of a nonionic detergent (13, 33), the protease activity for membrane-integrated FtsH has not been measured. In this study, we have now developed an in vitro system in which proteolytic activities of FtsH in inverted membrane vesicles can be measured. Inverted membrane vesicles were prepared from FtsH-overproduced and ΔFtsH cells (lacking F1F0-ATPase) (Fig. 3A) and examined for generation of PMF. Δψ and ΔpH were generated by the inverted membrane vesicles with overproduced FtsH on addition of succinate, a respiratory substrate that generates PMF, and both Δψ and ΔpH were collapsed by CCCP (Fig. 3B Top). As reported (31), ΔpH was specifically dissipated by ammonium sulfate (AS; Fig. 3B Middle) and Δψ was specifically dissipated by potassium thiocyanate (KSCN; Fig. 3B Bottom).
Figure 3.
Δψ and ΔpH generation in the FtsH-overproduced inverted membrane vesicles and their dissipation by CCCP, ammonium sulfate, and potassium thiocyanate. (A) Protein profiles of inverted membranes from FtsH-overproduced strain AD1434 (ΔftsH Δunc)/pSTD113 (lane 1) and FtsH-deleted strain AD1434/pTYE007B (lane 2). Open and filled arrow heads indicate intact and C-terminally self-processed forms (40) of FtsH-His6-Myc, respectively. (B) Generation and collapse of Δψ and ΔpH. Generation of Δψ and ΔpH in the inverted membrane vesicles prepared from AD1434/pSTD113 was monitored by fluorescence quenching of oxonol V and quinacrine, respectively, as described in Materials and Methods. Succinate (4.5 mM) was added at the point indicated by filled arrows and CCCP (10 μM), ammonium sulfate (AS; 20 mM), or potassium thiocyanate (KSCN; 12.5 mM) was added at the point indicated by open arrows.
Inverted membrane vesicles were incubated with resorufin-casein (Fig. 4). Resorufin-casein was degraded with time in an ATP-dependent manner (Fig. 4A). The addition of succinate markedly enhanced the degradation of resorufin-casein (Fig. 4A). No degradation was observed with the ΔFtsH inverted membrane vesicles, even in the presence of succinate (Fig. 4A). Thus, the succinate-induced enhancement of casein degradation was not caused by the activation of other proteases in the inverted membrane vesicles. When the vesicles were solubilized with a nonionic detergent, Nonidet P-40, succinate no longer stimulated the degradation of resorufin-casein (Fig. 4D). Solubilized FtsH showed an approximately 3-fold higher proteolytic activity than the PMF-stimulated membrane-integrated form. Although the exact reason for these findings is unclear, solubilization may allow FtsH to have more efficient access to substrates.
The requirement for intact membrane vesicles is consistent with the notion that succinate stimulation was caused by the generation of PMF. Indeed, the stimulatory effect of succinate on the proteolytic activity of FtsH was canceled by addition of CCCP (Fig. 4B) that dissipates PMF (Fig. 3B). CCCP itself did not diminish degradation of resorufin-labeled casein by FtsH (Fig. 4B). To evaluate the contributions of the Δψ and the ΔpH components of PMF in this stimulation of casein degradation, the effects of ammonium sulfate and potassium thiocyanate, which collapse these components individually (Fig. 3B), were examined. As shown in Fig. 4C, ammonium sulfate exerted little effect on succinate-stimulated degradation of casein, whereas potassium thiocyanate partially canceled the effect of succinate. Coaddition of ammonium sulfate with potassium thiocyanate did not cause further reduction in casein degradation. Neither CCCP nor potassium thiocyanate affected the casein degradation in the presence of Nonidet P-40 (Fig. 4D). The observed partial effect of potassium thiocyanate may be due to incomplete collapse of Δψ at the concentration used in these experiments (12.5 mM) (Fig. 3B). Its effects at higher concentrations could not be investigated because the higher concentrations of potassium thiocyanate nonspecifically inhibited the proteolytic activity of FtsH (data not shown). These results, together with the in vivo observations, support the notion that PMF, especially the Δψ component, has a stimulatory effect on the proteolytic activity of FtsH in the membrane.
The Transmembrane Region of FtsH Is Important for the PMF Effect.
The N-terminal transmembrane region of FtsH not only serves as a membrane anchor but also mediates homooligomeric (FtsH–FtsH) and heterooligomeric (FtsH–HflKC) protein interactions (4, 11). To investigate the possible roles of the transmembrane region in PMF-dependent activation of FtsH, we used FtsH derivatives, LacY-FtsH and FtsH(Δperi). In LacY-FtsH, the N-terminal transmembrane region of FtsH was replaced by an N-terminal segment of the LacY protein that includes the first and the second transmembrane segments (5). In FtsH(Δperi), the periplasmic region is almost entirely deleted (11). Both of these FtsH variants are localized in the cytoplasmic membrane and are capable of degrading SecY (5, 11). As shown in Fig. 5, YccA-(P3)-PhoA(SSSS) was degraded in the ΔftsH strain when FtsH+, LacY-FtsH, or FtsH(Δperi) was coexpressed (Fig. 5 A and B). The FtsH+-catalyzed degradation of YccA-(P3)-PhoA(SSSS) was slowed down by CCCP (Fig. 5A). In this case, the effect of CCCP was less pronounced than that observed in the wild-type (FtsH+) strain (Fig. 2), possibly because of overproduction of FtsH. In contrast to the wild-type FtsH, LacY-FtsH and FtsH(Δperi) were little affected by CCCP in degradation of YccA-(P3)-PhoA (Fig. 5B). We believe that YccA-(P3)-PhoA(SSSS) degradation by LacY-FtsH and FtsH(Δperi) was at the PMF-nonstimulated level. We also examined the effects of CCCP on σ32 degradation by Zip-FtsH(ΔTM) (5), a soluble derivative of FtsH that can degrade soluble, but not membrane-embedded substrates (Fig. 5C). CCCP markedly stabilized σ32 in the FtsH+-overproducing cells; the stabilization became appreciable within 2 min. (Substantial degradation of labeled σ32 during the initial 2 min of the chase period was possibly caused by overproduction of FtsH.) On the other hand, σ32 degradation by Zip-FtsH(ΔTM) was little affected by CCCP up to about 8 min of chase. It seems that the stabilization of σ32 in the CCCP-treated cells during the later chase period was at least partly caused by the accumulation of secretory protein precursors. These results strongly suggest that the N-terminal transmembrane region is important for FtsH to be activated by PMF.
Discussion
FtsH is the unique membrane-bound, ATP-dependent protease in E. coli (2). We have shown that the membrane integration of FtsH is related to its ability to degrade membrane proteins (5, 6). Although the monomeric cytoplasmic domain of FtsH was virtually inactive, leucine-zipper-mediated oligomerization makes it proteolytically active against soluble substrates. However, homooligomerization is not sufficient for the degradation of membrane-embedded substrates, which are only degraded by FtsH that contains a transmembrane region. The fact that transmembrane regions from unrelated membrane proteins (LacY and EnvZ) can substitute for the original transmembrane region without severely compromising the activity against membrane-integrated substrates suggests that the membrane association enables FtsH to catalyze degradation of membrane proteins.
In this study, we discovered another link between membrane localization and the proteolytic activities of FtsH. CCCP or DNP treatment significantly retarded degradation of the substrate proteins in vivo. The CCCP effects were appreciable almost immediately after its addition, suggesting that PMF is directly involved in the degradation processes. The effect was specific in that CCCP did not stimulate LacY-FtsH, FtsH(Δperi), and Zip-FtsH(ΔTM), nor did it stimulate the residual degradation of σ32 in ΔftsH cells. We reproduced PMF stimulation of FtsH activity in vitro. These results collectively showed that the proteolytic function of FtsH is stimulated by PMF. The results using the reagents that specifically collapse Δψ or ΔpH in inverted membrane vesicles suggest that Δψ is especially important.
In E. coli, PMF is widely used for transport of various molecules across the membrane in both directions. Thus, PMF may have promoted dislocation of extracytoplasmic and intramembrane regions of membrane proteins to the cytoplasmic side in the FtsH-catalyzed protein degradation. However, our analysis of the degradation of YccA-(P3)-PhoA(SSSS) did not support this possibility. Moreover, PMF stimulated the degradation of soluble proteins σ32 in vivo and resorufin-casein in vitro. Thus, PMF does not seem to affect a process that is specific to the degradation of membrane proteins. Rather, PMF may affect a more fundamental process (or processes) in the proteolytic functions of FtsH. It is conceivable, for instance, that PMF affects substrate binding, substrate unfolding, or the release of degradation products. ATP hydrolysis does not seem to be a target of the PMF activation (unpublished results). We obtained a preliminary result that in vivo degradation of the λ CII protein, another cytoplasmic substrate of FtsH (9, 38), was apparently little affected by the CCCP treatment of cells. Although the involvement of multiple proteases (unpublished results) and regulatory factors (9, 39) in the degradation of CII makes interpretation of these results difficult, some FtsH substrates may have a decreased dependence on PMF for their efficient degradation.
The proteolytic activities of the FtsH variants with altered membrane-associated regions [LacY-FtsH, FtsH(Δperi) and Zip-FtsH(ΔTM)] were virtually unaffected by CCCP. The N-terminal transmembrane region of FtsH is important for both homooligomeric (FtsH–FtsH) and heterooligomeric (FtsH–HflKC) subunit interactions (4, 11). PMF may affect the proteolytic functions of FtsH by modulating subunit interactions in the FtsH complex. However, HflKC is not directly involved because stabilization of YccA and σ32 by CCCP was still observed in HflKC-deleted cells (unpublished results). It is also possible that some other factors associate with FtsH by way of the transmembrane region and activate it in a PMF-dependent manner.
Acknowledgments
We thank K. Ito for discussion, encouragement, and critical reading and editing of the manuscript, H. Mori and K. Inaba for discussion, and M. Yamada, M. Sano, and K. Mochizuki for technical assistance. This work was supported by grants from the Ministry of Education, Science, Sports and Culture, Japan.
Abbreviations
- CCCP
carbonyl cyanide-3-chlorophenylhydrazone
- DNP
2,4-dinitrophenol
- Foa
subunit a of the proton ATPase
- IPTG
isopropyl-β-d-thiogalactopyranoside
- PMF
proton-motive force
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gottesman S. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman S. Curr Opin Microbiol. 1999;2:142–147. doi: 10.1016/S1369-5274(99)80025-3. [DOI] [PubMed] [Google Scholar]
- 3.Tomoyasu T, Yuki T, Morimura S, Mori H, Yamanaka K, Niki H, Hiraga S, Ogura T. J Bacteriol. 1993;175:1344–1351. doi: 10.1128/jb.175.5.1344-1351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama Y, Yoshihisa T, Ito K. J Biol Chem. 1995;270:23485–23490. doi: 10.1074/jbc.270.40.23485. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama Y, Ito K. EMBO J. 2000;19:3888–3895. doi: 10.1093/emboj/19.15.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama Y, Ito K. Biochemistry. 2001;40:7687–7693. doi: 10.1021/bi010039w. [DOI] [PubMed] [Google Scholar]
- 7.Karata K, Inagawa T, Wilkinson A J, Tatsuta T, Ogura T. J Biol Chem. 1999;274:26225–26232. doi: 10.1074/jbc.274.37.26225. [DOI] [PubMed] [Google Scholar]
- 8.Kihara A, Akiyama Y, Ito K. EMBO J. 1996;15:6122–6131. [PMC free article] [PubMed] [Google Scholar]
- 9.Kihara A, Akiyama Y, Ito K. Proc Natl Acad Sci USA. 1997;94:5544–5549. doi: 10.1073/pnas.94.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kihara A, Akiyama Y, Ito K. J Mol Biol. 1998;279:175–188. doi: 10.1006/jmbi.1998.1781. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama Y, Kihara A, Mori H, Ogura T, Ito K. J Biol Chem. 1998;273:22326–22333. doi: 10.1074/jbc.273.35.22326. [DOI] [PubMed] [Google Scholar]
- 12.Kihara A, Akiyama Y, Ito K. Proc Natl Acad Sci USA. 1995;92:4532–4536. doi: 10.1073/pnas.92.10.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama Y, Kihara A, Tokuda H, Ito K. J Biol Chem. 1996;271:31196–31201. doi: 10.1074/jbc.271.49.31196. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama Y, Kihara A, Ito K. FEBS Lett. 1996;399:26–28. doi: 10.1016/s0014-5793(96)01283-5. [DOI] [PubMed] [Google Scholar]
- 15.Kihara A, Akiyama Y, Ito K. EMBO J. 1999;18:2970–2981. doi: 10.1093/emboj/18.11.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Economou A. FEBS Lett. 2000;476:18–21. doi: 10.1016/s0014-5793(00)01662-8. [DOI] [PubMed] [Google Scholar]
- 17.Mori H, Ito K. Trends Microbiol. 2001;9:494–500. doi: 10.1016/s0966-842x(01)02174-6. [DOI] [PubMed] [Google Scholar]
- 18.Schiebel E, Driessen A J M, Hartl F-U, Wickner W. Cell. 1991;64:927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- 19.van der Wolk J P W, de Wit J G, Driessen A J M. EMBO J. 1997;16:7297–7304. doi: 10.1093/emboj/16.24.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casadaban M. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 22.Geller B L, Green H M. J Biol Chem. 1989;264:16465–16469. [PubMed] [Google Scholar]
- 23.Tatsuta T, Tomoyasu T, Bukau B, Kitagawa M, Mori H, Karata K, Ogura T. Mol Microbiol. 1998;30:583–594. doi: 10.1046/j.1365-2958.1998.01091.x. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama Y, Ogura T, Ito K. J Biol Chem. 1994;269:5218–5224. [PubMed] [Google Scholar]
- 25.Guzman L-M, Belin D, Carson M J, Beckwith J. J Bactriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis R W, Botstein D, Roth J R. Advanced Bacterial Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1980. [Google Scholar]
- 27.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama Y, Ito K. EMBO J. 1985;4:3351–3356. doi: 10.1002/j.1460-2075.1985.tb04088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshihisa T, Ito K. J Biol Chem. 1996;271:9429–9436. doi: 10.1074/jbc.271.16.9429. [DOI] [PubMed] [Google Scholar]
- 30.Yamada H, Tokuda H, Mizushima S. J Biol Chem. 1989;264:1723–1728. [PubMed] [Google Scholar]
- 31.Shiozuka K, Tani K, Mizushima S, Tokuda H. J Biol Chem. 1990;31:18843–18847. [PubMed] [Google Scholar]
- 32.Herman C, Thévenet D, D'Ari R, Bouloc P. Proc Natl Acad Sci USA. 1995;92:3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomoyasu T, Gamer J, Bukau J, Kanemori M, Mori H, Rutman A J, Oppenheim A B, Yura T, Yamanaka K, Niki H, et al. EMBO J. 1995;14:2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanemori M, Nishihara K, Yanagi H, Yura T. J Bacteriol. 1997;179:7219–7225. doi: 10.1128/jb.179.23.7219-7225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakker E P, Randall L L. EMBO J. 1984;3:895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goff S A, Goldberg A L. Cell. 1985;41:587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Akiyama Y, Yura T, Shiba K. J Bacteriol. 1986;167:201–204. doi: 10.1128/jb.167.1.201-204.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shotland Y, Koby S, Teff D, Mansur N, Oren D A, Tatematsu K, Tomoyasu T, Kessel M, Bukau B, Ogura T, Oppenheim A B. Mol Microbiol. 1997;24:1303–1310. doi: 10.1046/j.1365-2958.1997.4231796.x. [DOI] [PubMed] [Google Scholar]
- 39.Kihara A, Akiyama Y, Ito K. J Biol Chem. 2001;276:13695–13700. doi: 10.1074/jbc.M011699200. [DOI] [PubMed] [Google Scholar]
- 40.Akiyama Y. Biochemistry. 1999;38:11693–11699. doi: 10.1021/bi991177c. [DOI] [PubMed] [Google Scholar]