Abstract
Purpose
Previous literature suggests a relationship between carpal tunnel syndrome (CTS) and heart failure (HF), indicating that patients with CTS are more likely to experience cardiomyopathy. Amyloid deposition leading to CTS may be prodromal for the development of amyloid cardiomyopathy. We hypothesized that patients undergoing surgery for CTS would have an increased risk of HF compared to those who did not undergo surgery.
Methods
The TriNetX database was queried using the primary ICD-10 code for CTS to identify patients. Patients were included if they had surgical treatment for CTS, reported as endoscopic or open carpal tunnel release, or median nerve release. The primary outcome was HF. Propensity scoring adjusted for demographics and comorbidities. Statistical significance was set at P < .05, and odds ratios were calculated at 95% confidence intervals (CI).
Results
The incidence of HF in patients who underwent carpal tunnel release was 3.49%, 5.98%, 6.947%, and 7.102% at 5, 10, 15, and 20 years after surgery. Compared to nonsurgical patients, the risk difference of 0.399 (95% CI, 0.202–0.596) became statistically significant at five years (P < .0001) with an increased risk ratio (RR) of 1.129 (95% CI, 1.0063–1.198) for surgical patients. The risk difference peaked at 2.007% (95% CI, 1.744–2.27) at the 20-year mark with an RR of 1.369 (95% CI, 1.311–1.456).
Conclusions
This study demonstrates that patients who underwent carpal tunnel release were more likely to experience HF than nonsurgical matched controls. It also showed an increased association with CTS and HF compared to HF progression in the general population In CTS patients with a higher risk of heart failure, hand surgeons can have a crucial role in early identification and facilitating further cardiovascular assessment.
Type of study/level of evidence
Prognostic IV.
Key words: Carpal tunnel syndrome, Database study, Heart failure
Carpal tunnel syndrome (CTS) is the most prevalent nerve compression disorder, leading to considerable functional impairment and discomfort in a considerable portion of the population.1 It arises from compression of the median nerve, resulting in pain, numbness, and weakness that travels to the wrist and hand in the radial most 3.5 digits.1 The global prevalence of CTS is thought to be approximately 4% to 5%, typically presenting at ages 40–60 years, and is relatively more common in women than men.2
Common comorbidities associated with CTS include smoking, increased body mass index, diabetes, and other endocrinopathies.3 Recent studies accordingly have investigated the overlap between atherosclerosis risk factors in patients with CTS.4 Factors such as obesity, high LDL cholesterol and triglyceride levels, hypertension, and cardiac arrhythmias, have been associated with CTS.4 Similarly, the association of cardiac amyloidosis and subsequent heart failure (HF) in patients with CTS recently has been elucidated.5, 6, 7
Given the association between CTS and subsequent HF, the present study aims to characterize the incidence of HF in patients who underwent carpal tunnel release (CTR) compared to patients who have CTS and were treated without surgery. Based on current literature, we expected to see an increased rate of cardiac adverse events and HF in patients who have undergone CTR surgery compared to patients with CTS treated without surgery.
Materials and Methods
No informed consent was administered in this study as all data were retrospective.
This study used the Global Collaborative Network database, which contains the data for >100 million patients within the TriNetX database. TriNetX is a global research database that uses nearly 170 health care organizations and over 400 million patients, with various subsets for different countries and networks. TriNetX is a deidentified database organized by patient diagnosis, procedures, genomics, demographic data, laboratory, and medication data. Health care organizations send their data to TriNetX and undergo an authorized deidentification process authorized by the Health Insurance Portability and Accountability Act.
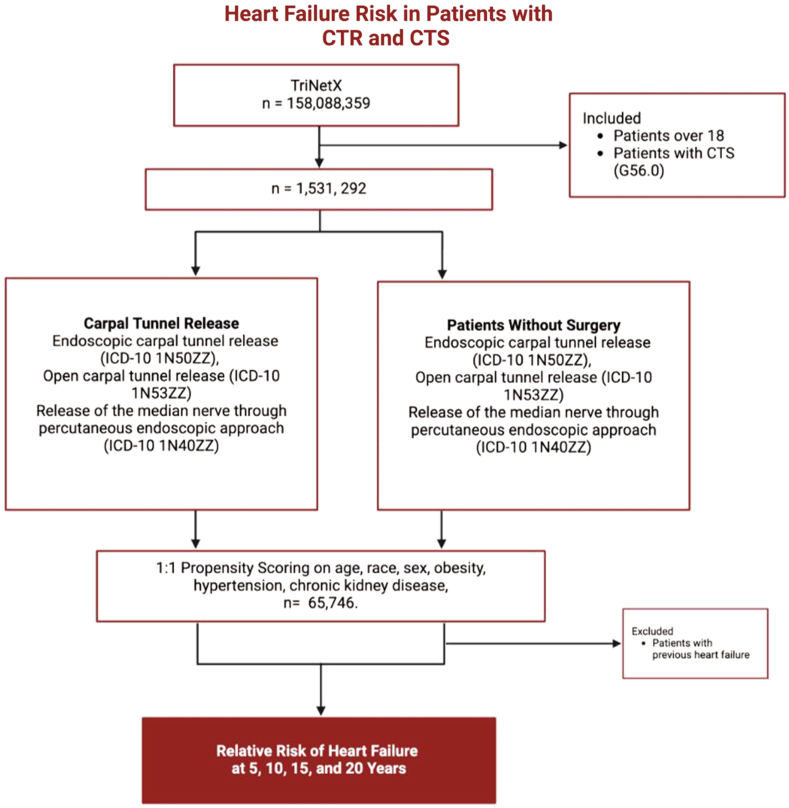
Using the primary ICD-10 code for CTS (G56.0), 1,459,342 patients were identified to have CTS. After identifying patients diagnosed with CTS, we separated the population into two cohorts, one consisting of surgical patients and the other populated with nonsurgical patients. The surgical treatment cohort was identified and analyzed by using ICD-10 Procedure Coding System (ICD-10 PCS) codes for endoscopic CTR (ICD-10 1N50ZZ), open CTR (ICD-10 1N53ZZ), and release of the median nerve through a percutaneous endoscopic approach (ICD-10 1N40ZZ). The study primarily compared the surgical group with a nonsurgical cohort consisting of patients who did not undergo surgery and, therefore, did not have the ICD-10 codes listed previously. The primary outcome of the analysis was HF, defined as ICD-10 code I50 for congestive HF and all subtypes (including left ventricular failure, systolic HF, diastolic HF, and biventricular HF) and I11.0 for hypertensive heart disease. These outcomes were measured at intervals of 5, 10, 15, and 20 years after surgery to determine the rate of progression in each group.
To balance the patient population, we used propensity score matching through TriNetX, which accounts for demographic factors, such as age, race, obesity, diabetes, chronic kidney disease, and hypertension. This process involved creating a nonsurgical control cohort by matching each surgical patient with a nonsurgical counterpart with similar characteristics, thereby reducing the influence of confounding factors. After applying propensity score matching, the final balanced patient population comprised 65,746 individuals (Fig. 1). In a separate analysis, we compared 230,174 patients aged ≥30 years with and 230,174 without a history of CTS or CTR. This control group consisted of patients with a health care encounter coded under ICD-10 code Z00.00 (encounter for general adult medical examination without abnormal findings). These patients then were matched on demographic factors, such as age, race, obesity, diabetes, chronic kidney disease, and hypertension. All statistical analyses were performed through the TriNetX platform, and P values were used to determine statistical significance between cohorts. Any P < .05 was considered significant, and odds ratios were calculated at 95% confidence intervals (CI).
Figure 1.
Study algorithm using TriNetX.
Results
A total of 65,746 patients underwent surgical treatment for CTS. Another 65,746 patients with CTS did not undergo surgery, matched through propensity scoring for age, race, sex, essential hypertension diagnosis, diabetes diagnosis, obesity, and chronic kidney disease diagnosis. Demographic details are in the Table 1.
Table 1.
Baseline Characteristics of Study Population After Matching
| No. (%) | |||
|---|---|---|---|
| Characteristic | CTS with Surgery (n = 65,746) |
CTS Without Surgery (n = 65,746) |
P Value |
| Age (y) at index, mean (SD) | 55.8 ± 14.9 | 55.8 ± 14.8 | 0.943 |
| Sex No.(%) | |||
| Female | 41,514 | 41,520 | 0.972 |
| Male | 23,901 | 23,895 | 0.972 |
| Race | |||
| White | 32,026 | 32,026 | >.99 |
| Black or African American | 5,298 | 5,297 | 0.991 |
| Asian | 2,515 | 2,487 | 0.686 |
| Comorbidities | |||
| Essential | 15,516 | 15,533 | 0.912 |
| Hypertension | |||
| Diabetes mellitus | 7,428 | 7,425 | 0.979 |
| Obesity | 5,235 | 5,246 | 0.910 |
| Chronic kidney disease | 2,031 | 2,020 | .860 |
The results were analyzed at intervals of 5, 10, 15, and 20 years. At the 5-year follow-up, the database identified 63,375 patients who did and 63,077 who did not undergo surgery. Of those in the surgical group, 2,217 experienced HF, resulting in a risk of 3.498%, compared to 1,995 patients in the nonsurgical group, with a risk of 3.099%. The risk ratio, odds ratio, and 95% CIs are detailed in the Table 2.
Table 2.
Heart Failure Risk Difference
| Time Interval (y) | Risk Difference (95% CI) | z score | P Value | Risk Ratio (95% Cl) | Odds Ratio (95% Cl) |
|---|---|---|---|---|---|
| 5 | .399 (0.202–0.596) | 3.97 | < 0.001 | 1.129 (1.0063–1.198) | 1.133 (1.065–1.206) |
| 10 | 1.387 (1.141–1.633) | 11.058 | < 0.001 | 1.302 (1.242–1.365) | 1.3 |
| 15 | 1.91 (1.682–2.20) | 14.355 | < 0.001 | 1.379 (1.32–1.441) | 1.407 (1.343–1.475) |
| 20 | 2.007 (1.744–2.27) | 14.959 | < 0.001 | 1.394 (1.334–1.456) | 1.424 (1.359–1.492) |
At the 10-year follow-up, the database identified 63,811 patients with CTS who had undergone surgery and 63,509 patients with CTS who did not. In the surgical group, 3,816 patients (5.98%) experienced HF, compared to 2,917 patients (4.593%) in the nonsurgical group, with this difference being statistically significant (P < .001).
By the 15-year follow-up, the rate of HF among the surgical group had increased to 6.947%, compared to 5.037% in the nonsurgical group. The risk ratio at 15 years was 1.379 (95% CI, 1.321–1.441), and the odds ratio was 1.407 (95% CI, 1.343–1.475) (Fig. 2A).
Figure 2.
A Rate of CTR patients with heart failure compared to patients with CTS without surgical treatment. The CTR group had a steeper increase in HF risk during the first 10 years, reaching a plateau at approximately 6.5% to 7% after 15 years. In contrast, the nonsurgical cohort shows a slower progression, plateauing at approximately 4.5% after 10 years. B Rate of CTR patients with HF compared to control patients. The CTR group had a consistently higher rate of HF compared to control patients at 5, 10, 15, and 20 years. The rate in the control group plateaued at 15 years at 5.5% whereas the rate in the CTR group plateaued at 15 years at 7.5% risk of HF.
The highest risk ratio was observed at the 20-year follow-up, with the difference in HF risk between the surgical and nonsurgical groups reaching 2.007%. The surgical group had a HF risk of 7.102%, while the nonsurgical group had a risk of 5.095%. The risk differences are summarized in the Table 3.
Table 3.
Risk of Progression
| Time Interval (y) | Incidence Rate in CTS with Surgery | Incidence Rate in CTS Without Surgery |
|---|---|---|
| 5 | 3.498% | 3.099% |
| 10 | 5.98% | 4.593% |
| 15 | 6.947% | 5.037% |
| 20 | 7.102% | 5.095% |
In comparison to the control group, patients with CTR had a higher rate of HF following 5, 10, 15, and 20 years after surgery. All results were significant (P < .001). After 5 years, 4.723% of CTR patients experienced HF compared to 3.754% of controls. This gap widened at the 10-year mark, with rates rising to 6.834% and 5.118%, respectively. By 15 years, the risk had climbed to 7.464% for CTR patients and 5.511% for controls. At 20 years, the progression rates reached 7.576% and 5.541%, indicating that while the increase slowed, CTR patients remained at a greater risk throughout the period. The Figure 2B summarizes the rate of progression to HF in control patients compared to CTR patients.
Discussion
This study aimed to characterize the association between CTS and HF by comparing demographic-matched patients who had CTS surgery to those who did not. The study found that at 5-, 10-, 15-, and 20-years after surgery, patients who had undergone surgery were at higher risk of HF. The results suggest a correlation between CTS severity and adverse cardiovascular outcomes, particularly in patients progressing to CTR. To the best of our knowledge, this is the largest database study to date on the incidence and risk of cardiovascular outcomes in CTS patients. This is the largest database study to date on the incidence and risk difference of cardiovascular outcomes such as HF in patients with CTS.
The finding of increased incidence of HF in patients with CTS who underwent CTR as compared to those managed nonoperatively aligns with the current understanding of the etiology of CTS-associated HF. Previous research has detailed the association between CTS and wild-type transthyretin amyloidosis (ATTRwt), causing the destabilization of transthyretin and deposition of amyloid fibrils.8 In these patients, CTS can be more complicated, often failing nonsurgical management and requiring subsequent carpal tunnel release.8 A previous study by Sperry et al9 found that 10.2% of patients undergoing CTR surgery had a positive biopsy for amyloid, with 2 patients receiving a diagnosis of ATTR and an additional 2 patients having cardiac involvement. Another study conducted in 2021 showed that 75% of a cohort of patients with ATTR cardiac amyloidosis (ATTR-CA) also had bilateral CTS, suggesting that bilateral and, thus, more severe CTS could be a positive predictor for cardiac amyloidosis.10 While previous studies have used bilateral CTS as a marker for CTS severity, reported incidences of bilateral involvement are approximately 50%, indicating that undergoing surgery may be a more appropriate marker of "severe" CTS in lieu of bilaterality.11
The association between HF and CTS helps define the role hand surgeons should take to help prevent the progression of patients to HF. Orthopedic manifestations of amyloidosis, including bilateral CTS, lumbar canal stenosis, atraumatic rupture of the biceps tendon, and rotator cuff injuries, are thought to precede cardiac manifestations, providing orthopedic physicians with the unique ability to catch patients early.12 In older patients with musculoskeletal disease symptoms, concern for amyloidosis should be considered among the constellation of possible diagnoses. Orthopedic surgeons may catch these patients earlier, as patients with ATTR who have cardiac amyloid fibril deposition have poor outcomes because of rapid deterioration, culminating in a survival of 3.5 years.13 An “alarm” symptom of cardiac amyloidosis is CTS, which could trigger the diagnostic work-up for cardiac amyloidosis.
Previous studies have shown that biopsies taken during CTR for idiopathic CTS may reveal amyloid.14, 15, 16, 17, 18, 19, 20, 21 One study showed that of 98 patients, 10 (10.2%) had a positive biopsy.9 Recently, it has been reported that orthopedic surgeons can perform a biopsy of the transverse carpal ligament and tenosynovium during CTR as an alternative for amyloidosis biopsy confirmation, as the procedure is associated with quick recovery and low risk of serious complications.22 A study by Elzinga et al21 found the pathological yield in the biopsy taken during CTS was 100%. Thus, in patients with a higher risk for cardiac amyloidosis, particularly those with a family history of ATTR amyloidosis, patients >50 years old, with a history of multiple myeloma, or recurrent CTS requiring revision, surgeons should consider biopsy testing for cardiac amyloidosis.22 Another recent case report detailed a 71-year-old patient who was diagnosed with cardiac amyloid following trigger finger release surgery and was then placed on disease-modifying therapy.23 This exemplifies the growing role that orthopedic surgeons can have in decreasing the burden of cardiac amyloidosis.23 Early detection of cardiac amyloidosis is crucial because of its severe clinical and financial impact, with high hospitalization costs and mortality rates.13 Cardiac amyloidosis has a high financial and clinical burden on the United States health care system, with average hospital hospitalizations costing over $20,000 per admission, and one-quarter of patients were admitted into the intensive care units.24 The mortality rate also is high, with 9% of patients dying in-hospital and 11% mortality following readmission.23
Patients undergoing CTR have a higher incidence of HF. Given that amyloid deposition is associated with HF and CTS, there may be a connection between all three. However, cardiac amyloidosis is only one possible underlying factor, and other conditions, such as valvular heart disease, chronic inflammatory disorders (rheumatoid arthritis for example), and metabolic syndromes, also may contribute to this association. For patients undergoing CTR, additional risk factors for HF should be taken into account and appropriate counseling or referral should be considered as indicated.
This study has several limitations. First, it relies on billing codes (ICD-10) from TriNetX, which may have inaccuracies or classification errors, potentially affecting risk assessment. The study used ICD-10 for cardiomyopathy rather than a specific code for transthyretin amyloid cardiomyopathy (ATTR-CM). It also used surgical management of CTS as a proxy for CTS severity, which may oversimplify the diagnostic process. Additionally, familial history of ATTR and preoperative cardiac amyloidosis data were not available. Despite using propensity scoring to adjust for demographics and comorbidities, misclassification and confounding biases remain because of the electronic medical record data. The study also is limited by TriNetX's focus on an American population, affecting global generalizability. Lastly, it relies on correlative data from a pre-existing database.
Future studies should follow patients who undergo biopsy to investigate if early diagnosis of cardiac amyloid may subsequently lead to increased survival outcomes. Retrospective reviews of the outcomes of post-biopsy patients also could be used to study long-term follow-up characterization of patients with cardiac amyloid in patients with CTS.
Conflicts of Interest
No benefits in any form have been received or will be received related directly to this article.
References
- 1.Genova A, Dix O, Saefan A, Thakur M, Hassan A. Carpal tunnel syndrome: a review of literature. Cureus. 12(3):e7333. [DOI] [PMC free article] [PubMed]
- 2.Chammas M., Boretto J., Burmann L.M., Ramos R.M., Dos Santos Neto F.C., Silva J.B. Carpal tunnel syndrome - Part I (anatomy, physiology, etiology and diagnosis) Rev Bras Ortop. 2014;49(5):429–436. doi: 10.1016/j.rboe.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geoghegan J.M., Clark D.I., Bainbridge L.C., Smith C., Hubbard R. Risk factors in carpal tunnel syndrome. J Hand Surg Br. 2004;29(4):315–320. doi: 10.1016/j.jhsb.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Shiri R., Heliövaara M., Moilanen L., Viikari J., Liira H., Viikari-Juntura E. Associations of cardiovascular risk factors, carotid intima-media thickness and manifest atherosclerotic vascular disease with carpal tunnel syndrome. BMC Musculoskelet Disord. 2011;12:80. doi: 10.1186/1471-2474-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fosbøl E.L., Rørth R., Leicht B.P., et al. Association of carpal tunnel syndrome with amyloidosis, heart failure, and adverse cardiovascular outcomes. J Am Coll Cardiol. 2019;74(1):15–23. doi: 10.1016/j.jacc.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 6.Luedde M., Schmidt V.J., Gänsbacher-Kunzendorf J., Kostev K. Association between carpal tunnel syndrome and subsequent heart failure among adults in Germany. JAMA Netw Open. 2023;6(7) doi: 10.1001/jamanetworkopen.2023.23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shetty N.S., Pampana A., Patel N., et al. Carpal tunnel syndrome and transthyretin amyloidosis in the All of Us Research Program. Mayo Clin Proc. 2024;99(7):1101–1111. doi: 10.1016/j.mayocp.2023.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitaoka H., Izumi C., Izumiya Y., et al. JCS 2020 Guideline on diagnosis and treatment of cardiac amyloidosis. Circ J. 2020;84(9):1610–1671. doi: 10.1253/circj.CJ-20-0110. [DOI] [PubMed] [Google Scholar]
- 9.Sperry B.W., Reyes B.A., Ikram A., et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018;72(17):2040-–2050. doi: 10.1016/j.jacc.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 10.Cappelli F., Zampieri M., Fumagalli C., et al. Tenosynovial complications identify TTR cardiac amyloidosis among patients with hypertrophic cardiomyopathy phenotype. J Intern Med. 2021;289(6):831–839. doi: 10.1111/joim.13200. [DOI] [PubMed] [Google Scholar]
- 11.Burton C.L., Chesterton L.S., Chen Y., van der Windt D.A. Predicting surgical intervention in patients presenting with carpal tunnel syndrome in primary care. Clin Epidemiol. 2018;10:739–748. doi: 10.2147/CLEP.S154409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruberg F.L., Grogan M., Hanna M., Kelly J.W., Maurer M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strouse C., Briasoulis A., Fonseca R., Jethava Y. Approach to a patient with cardiac amyloidosis. J Geriatr Cardiol. 2019;16(7):567–574. doi: 10.11909/j.issn.1671-5411.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández Fuertes J., Rodríguez Vicente Ó., Sánchez Herráez S., Ramos Pascua L.R. Early diagnosis of systemic amyloidosis by means of a transverse carpal ligament biopsy carried out during carpal tunnel syndrome surgery. Med Clin (Barc) 2017;148(5):211–214. doi: 10.1016/j.medcli.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Kyle R.A., Gertz M.A., Linke R.P. Amyloid localized to tenosynovium at carpal tunnel release. Immunohistochemical identification of amyloid type. Am J Clin Pathol. 1992;97(2):250–253. doi: 10.1093/ajcp/97.2.250. [DOI] [PubMed] [Google Scholar]
- 16.Gioeva Z., Urban P., Meliss R.R., et al. ATTR amyloid in the carpal tunnel ligament is frequently of wild-type transthyretin origin. Amyloid. 2013;20(1):1–6. doi: 10.3109/13506129.2012.750604. [DOI] [PubMed] [Google Scholar]
- 17.Nakamichi K.I., Tachibana S. Amyloid deposition in the synovium and ligament in idiopathic carpal tunnel syndrome. Muscle Nerve. 1996;19(10):1349–1351. doi: 10.1002/(SICI)1097-4598(199610)19:10<1349::AID-MUS16>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 18.Sekijima Y., Uchiyama S., Tojo K., et al. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol. 2011;42(11):1785–1791. doi: 10.1016/j.humpath.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Stein K., Störkel S., Linke R.P., Goebel H.H. Chemical heterogeneity of amyloid in the carpal tunnel syndrome. Virchows Arch A Pathol Anat Histopathol. 1987;412(1):37–45. doi: 10.1007/BF00750729. [DOI] [PubMed] [Google Scholar]
- 20.Sueyoshi T., Ueda M., Jono H., et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Hum Pathol. 2011;42(9):1259–1264. doi: 10.1016/j.humpath.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Elzinga K., Khayambashi S., Hahn C., Mahe E., Fine N.M. Amyloidosis and carpal tunnel syndrome: surgical technique for extended carpal tunnel release with tenosynovium and transverse carpal ligament biopsies. Plast Reconstr Surg Glob Open. 2023;11(1) doi: 10.1097/GOX.0000000000004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon S.D., Adams D., Kristen A., et al. Effects of Patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation. 2019;139(4):431–443. doi: 10.1161/CIRCULATIONAHA.118.035831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson E., Ebong I.A., Darrow M.A., Xiong G., Lipira A.B., Sood R.F. Early diagnosis and treatment of cardiac amyloidosis by screening biopsy during trigger finger release. J Hand Surg Glob Online. 2024;6(6):920–923. doi: 10.1016/j.jhsg.2024.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smiley D.A., Rodriguez C.M., Maurer M.S. Transthyretin cardiac amyloidosis: an evolution in diagnosis and management of an "old" disease. Cardiol Clin. 2022;40(4):541–558. doi: 10.1016/j.ccl.2022.06.008. [DOI] [PubMed] [Google Scholar]