Abstract
For a long time, the sense of smell was considered the neglected stepbrother of human sensory abilities, and the loss of smell has received little attention. This perception changed dramatically with the COVID-19 pandemic, which led to millions of people losing their sense of smell, and some never recovering. COVID-19 not only increased general awareness of olfactory disorders but also accelerated research into the role of smell in nonverbal communication and mental health. This review aims to summarize the literature on the impact of olfactory disorders on quality of life. Starting from the functions of olfaction in healthy individuals, we will briefly describe the most common olfactory disorders and their effect on an individual’s life, including nutrition and eating behaviors, social and psychological well-being, and exposure to environmental hazards. Consequences of olfactory loss permeate many spheres of daily life. On average, dysosmia has a moderate impact on quality of life, though for some patients the effects can be severe.
Keywords: olfaction, olfactory loss, anosmia, mental health, coping strategies
Introduction
The lay view on olfaction is that our sense of smell is not essential. This assumption was potentially based on older research indicating that humans are “microsmic” (Loos et al. 2023; McGann 2017) and surveys reporting that people declare they would rather give up their sense of smell than any of their other four main senses (Schifferstein 2006), their smartphones (McCann Worldgroup 2011), their little toe, or hearing in one ear (Wrzesniewski 1999). However, the scientific view on the capacity and functions of olfaction for humans changed dramatically in the last decades (McGann 2017; Loos et al. 2023), and general awareness increased due to the COVID-19-related smell loss that affected millions of people (Desiato et al. 2021).
Olfaction—one of the three chemical senses
Olfaction is one of the sensory systems serving to detect and decode chemical stimuli in the environment. In olfaction, volatile chemical compounds in the air are inhaled through the nose, bind to olfactory receptors located in the olfactory epithelium, and generate signals that are integrated by the olfactory bulb before being transmitted to the primary and secondary olfactory cortices for interpretation. Gustation allows detection and perception of the chemical compounds dissolved in saliva to give the sensations of salty, sweet, sour, bitter, or umami tastes, possibly also fat and water. Chemesthesis allows detection of agents that have reached the mucosa (for instance of the oral and nasal cavity) by activating receptors involved in other sensory systems, to raise sensations such as burning, stinging, pain, stringency, or cooling. All three senses interact in aroma perception during eating.
Functions of olfaction
As comprehensively delineated by Stevenson (2010), the main functions of olfaction include guidance toward objects with positive connotations, such as delicious foods or beautifully smelling flowers, cueing social interactions, and warning about environmental hazards like spoiled food or leaking gas. The individual hedonic valence of an odor hence determines the behavioral response of approaching or avoiding the source of a smell (Arshamian et al. 2017).
Olfaction is a key modality for regulating appetite, food intake, and appreciation of a meal. Pleasantly perceived food odors indicate edibility, increase appetite, and aid food localization. During chewing, odorous molecules are pumped into the retronasal passage and reach the olfactory mucosa, evoking olfactory perception of food aroma (Boesveldt and de Graaf 2017). Thus, both orthonasal and retronasal routes bear a regulatory role in eating behaviors.
Olfaction is also a cue in social communication. Body odors transport a variety of information guiding nonverbal communication, such as familiarity, nutrition, hormonal status, emotionality, or inflammation (Loos et al. 2023). As a result, body odors impact mother–child bonding (Schäfer and Croy 2023) and mating (Mahmut and Croy 2019).
Concerning the olfactory warning function, olfaction is a rather slow processing near-distance sense. Human olfaction appears to be tuned to detect gaseous hazards at very low concentrations, such as smoke and fire but also pathogens or metabolic products of pathogens, as they evolve in inflamed organisms or decay, for instance. Those odors evoke a typical disgusted expression (Saluja et al. 2024).
Situations in which we use the sense of smell are almost always multimodal processes involving multiple sensory modalities, such as tasting, smelling, and chemesthesis for eating, or seeing, hearing, and smelling for person perception. Olfaction facilitates spatial navigation and the formation of cognitive maps (Raithel and Gottfried 2021; Schwarz et al. 2024). It also supports some motor functions like mobility, balance, fine motor function, manual dexterity (Tian et al. 2016), respiratory function (Gorodisky et al. 2024), and swallowing (Ryan and Hummel 2013; Jestrović et al. 2015; Loret 2015; Yamamura et al. 2016).
Types of olfactory impairment and prevalence
Olfactory loss etiologies
Many people gradually lose their sense of smell, with aging being the main cause of olfactory deterioration (Doty and Kamath 2014). Other causes for gradual olfactory loss include allergic rhinitis, chronic rhinosinusitis, or neurodegenerative disorders (Whitcroft et al. 2023). Idiopathic olfactory loss (without a clear underlying cause) is associated with an increased risk of developing neurodegenerative diseases such as Parkinson’s disease (Doty et al. 1988; Ponsen et al. 2004, 2009; Haehner et al. 2009, 2011), Alzheimer’s disease (Thomann et al. 2009; Wilson et al. 2009), or dementia (McLaughlin and Westervelt 2008; Stanciu et al. 2014). However, some people are congenitally anosmic, meaning they were born without a sense of smell. Congenital anosmia is relatively rare (Karstensen and Tommerup 2012; Schriever and Hummel 2020). It often has a genetic basis and can be an associated symptom of syndromes, such as Kallman syndrome, CHARGE syndrome, the Bardet-Biedl syndrome, and SCN9A-associated insensitivity to pain (Deller et al. 2022).
Quantitative olfactory impairment
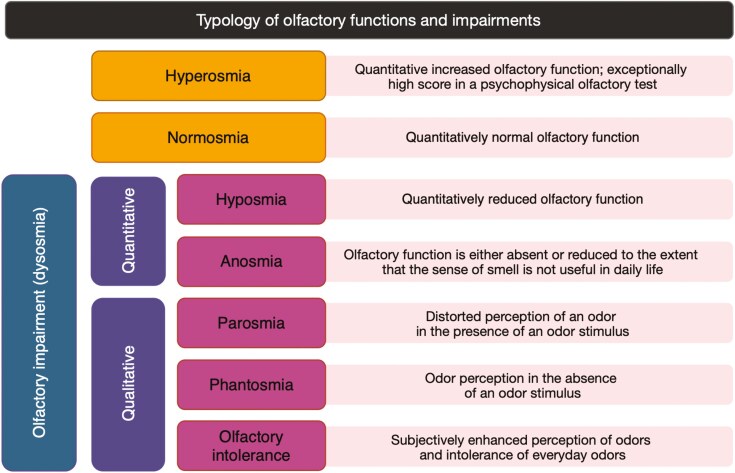
Screening tests such as the “University of Pennsylvania Smell Identification Test” (UPSIT) (Doty et al. 1984) or the “Sniffin’ Sticks” (Hummel et al. 1997) can reliably distinguish olfactory impairment from normal olfactory function based on an individual numerical score referred to normative values in the population. Individual scores can be categorized as normosmic (normal olfactory function), hyposmic (impaired olfactory function), or anosmic (including a residual ability to perceive odors with limited usefulness in daily life or no olfactory function). Exceptionally high scores are termed hyperosmia (Hernandez et al. 2023).
Qualitative olfactory impairment
Patients may also experience qualitative olfactory disorders. Parosmia is a distorted olfactory sensation in the presence of an odor source (e.g. sensing coffee aroma as a rotten smell). Phantosmia is an olfactory sensation without the presence of an odor source, i.e. olfactory hallucination (Frasnelli et al. 2004). Qualitative olfactory dysfunction typically manifests with unpleasant olfactory perceptions. Patients with parosmia complain that coffee, meat, onion, or toothpaste begin to smell repulsive and disgusting. Some patients also complain about triggered, identifiable, and usually unpleasant olfactory percepts that persist, sometimes for days, in the absence of an ongoing stimulus, what has been called “odor-induced phantosmias.” Qualitative olfactory disorders are often symptoms accompanying recovery from a viral infection, possibly due to a miswiring of newly proliferating olfactory receptor neurons in the olfactory bulb but have also been reported by patients with sinonasal and post-traumatic olfactory dysfunction (Pellegrino et al. 2021).
A distinctive category of qualitative olfactory impairment is complaints about the subjectively enhanced olfactory perception that is termed “chemical odor intolerance.” Odor intolerance manifests with the experience of feeling ill (e.g. nausea, headache, breathing difficulties) in the presence of ambient everyday odors (Hernandez et al. 2023; Nordin Millqvist, et al. 2003; Ryan et al. 2006). The types of olfactory functions and impairments are summarized in Fig. 1.
Fig. 1.
Causes and types of olfactory impairment.
Olfactory loss prevalence
A meta-analysis summarizing results from 25 epidemiological studies, including a population of 175,073 participants aged between 18 and 101 years (mean age 63 years, 56% men), indicates the overall prevalence of olfactory disorders to be 22.2% (95% CI 14.8 to 30.6%). Based on the results of psychophysical tests, the prevalence is 28.8%, and based on the self-reported olfactory disorders, it is 9.5% (Desiato et al. 2021). Other studies suggest that anosmia concerns 3.6% to 5.8% of the general population, while hyposmia is estimated to be present in 13% to 18% of people (Hummel et al. 2017). Parosmia occurs in 4.8% of the general population (Olofsson et al. 2022), and phantosmia in 4.2% (Wehling et al. 2021). Qualitative olfactory disorders are often (19% to 34%) comorbid with quantitative olfactory dysfunction, i.e. hyposmia or anosmia (Reden et al. 2006; Pellegrino et al. 2021).
The gap between the self-reported olfactory loss and the results of psychophysical diagnostic tests (Desiato et al. 2021) indicates that many people have a distorted sense of smell but still consider their olfaction normal. Studies on the quality of life in patients with olfactory loss are often biased as the participants of these studies reported themselves to the ENT clinics. They decided to seek medical help because olfactory loss has become bothersome, and they want to eliminate it from their lives. But, since olfaction is the least valued of the senses, some people likely do not realize they are missing out on odors. Indeed, empirical evidence shows that there are people in the general population who rate their sense of smell as normal, while psychophysical tests suggest anosmia or hyposmia (Oleszkiewicz and Hummel, 2019), and for whom the lack of odor sensation is not distressing (Oleszkiewicz et al. 2019).
Epidemiological estimates for olfactory dysfunctions vary as a function of sample demographics, the definition of impairment, and measurement methods. Olfactory deficits are more frequent among men (Sorokowski et al. 2019) and the elderly (Attems et al. 2015; Olofsson et al. 2021). Previous COVID-19 infection also increases the odds of olfactory dysfunction, as 5% to 10% of the patients with COVID-19-associated olfactory loss do not fully recover (Kim et al. 2024).
Consequences of olfactory loss in daily life
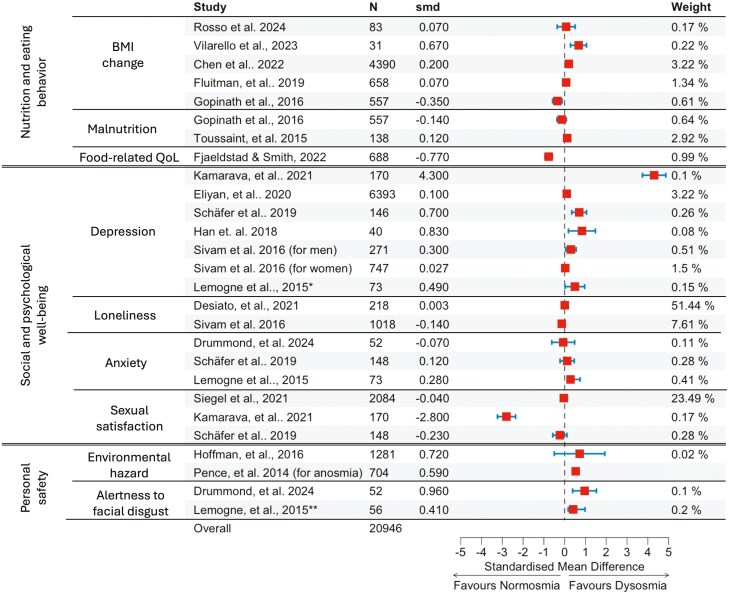
Patients with olfactory loss report complaints in the areas that mirror the functions of olfaction, i.e. nutrition, social and psychological functioning, and exposure to environmental hazards. We elaborate on these difficulties below and summarize the effect sizes for these complaints reported by the respective studies in Fig. 2. According to the effect sizes mentioned in the papers published in the last decade, the consequences of olfactory loss permeate many spheres of daily life, but this impact is not debilitating.
Fig. 2.
Forest plot of the effects of olfactory loss on the aspects of quality of life. The selection of studies for Fig. 2 was based on the following criteria: (1) published after 2014, (2) presenting a cross-sectional comparison of dysosmic (anosmic, hyposmic, or both) and healthy controls in the respected area impacted by the smell loss or longitudinal data for dysosmia, and (3) available descriptive data to calculate Cohen’s d effect size. Thus, the presented selection is not a systematic review or meta-analysis. Despite Kamarava (2021) study being an outlier, we decided not to exclude it from the Figure. Smd—Standardized mean difference (Cohen’s d), N—total number of participants for the study, QoL—quality of life. Horizontal blue lines depict 95% confidence interval for the effect sizes marked with red squares. Study weight is proportional to study precision, specifically the span of the standard error of the estimate of a study. For Pence et al. 2014 no confidence interval data is reported.
Consequences for nutrition and eating behavior
Anosmia and hyposmia
Food odors trigger appetite and drive eating behaviors (Zoon et al. 2014; Morquecho-Campos et al. 2020). In patients with olfactory loss, retronasal olfaction has been found to contribute more to the quality of life than orthonasal olfaction, pinpointing the critical role of olfaction in food appreciation (Oleszkiewicz et al. 2019). Smell loss often has negative consequences on food intake and energy balance, leading to non-uniform weight changes. When food becomes flavorless, some patients suffering from smell loss lose interest in eating and reduce food consumption. Some are afraid of consuming rotten food because they are unable to assess food freshness accurately (BurgesWatson et al. 2021). These changes in eating habits may cause weight loss (Purdy et al. 2020) and malnutrition, the latter being prevalent in the geriatric population (Gopinath et al. 2016; Fluitman et al. 2021). Other patients with smell loss may gain weight by increasing food intake and consuming more sugary and fatty products to compensate for the lack of aroma perception (Gaillet-Torrent et al. 2014; Ramaekers et al. 2015; Proserpio et al. 2017; Van Regemorter et al. 2020; Chen et al. 2022; Vilarello et al. 2023; Rosso et al. 2025). Some studies yield no significant results on the change of weight in relation to olfactory loss (Fluitman et al. 2019), likely because the two mechanisms cancel each other out.
Eating is a social act, e.g. family and friends gathering, preparing meals together, and going out to restaurants, cafes, or bars. Olfactory loss deprives patients of active participation in these activities (Boesveldt and Parma, 2021), resulting in distress and the feeling of longing and depression (Burges Watson et al. 2018, 2021). With olfactory loss, food-related quality of life decreases. Eating becomes sustenance stripped of its joy. Patients with anosmia report avoiding cooking. Their decisions related to food choices become more complicated, confidence in their own cooking skills weakens, and the results of cooking become less predictable. Consequently, patients begin to see cooking as not a fulfilling activity and point to the inability to prepare new meals successfully (Fjaeldstad and Smith, 2022).
Coping strategies to appreciate food and drinks include a shift toward texture-rich foods to increase oral sensations (Burges Watson et al. 2021). The use of capsaicin, an active component of chilli peppers that produces a sensation of burning, may help patients with olfactory loss to increase the perception (and decrease the use) of salt (Hunter et al. 2023). An important, already progressing initiative is culinary education tailored for individuals suffering from olfactory disorders. People suffering from smell loss are instructed on how to make their food more interesting and stimulating while keeping it healthy and nutritious. The course-related cookbook presents recipes for texture-rich meals at varying temperatures (Fjaeldstad, 2024). Patients with anosmia are also known to develop compensatory mechanisms to aid in the lack of food-related chemosensory perceptions. It has been demonstrated that they better hear the level of liquid carbonization (providing more rich, trigeminal sensation) as compared to individuals with a normal sense of smell (Oleszkiewicz et al. 2023).
Parosmia and phantosmia
Parosmia and phantosmia have rather uniform consequences on eating behaviors, as the patients most often experience negative distortions of aroma perceptions. They describe food-related odorous and mouth sensations as “disgusting to eat”, “awful,” and “metallic”, resulting in food avoidance (Fjaeldstad and Smith, 2022). Some patients with parosmia mention leaving the house while the meal is being prepared to avoid repulsive olfactory sensations (Burges Watson et al. 2021; Pellegrino et al. 2021), often arising when roasting or heating (Fjaeldstad and Smith, 2022).
Consequences for social and psychological well-being
Anosmia and hyposmia
Olfactory disorders are accompanied by depressive symptoms in 36% to 76% of the patients, depending on the etiology of the olfactory loss (Deems et al. 1991; Temmel et al. 2002a; Jung et al. 2014; Smith and Alt 2020; Kamrava et al. 2021; Sabiniewicz et al. 2022), in a dose-dependent way (Sharma et al. 2022a). Patients with olfactory loss present reduced central processing of emotional stimuli (Han et al. 2019). In congenital anosmia, 29% of the cases are associated with mild or severe depression (Croy et al. 2012a). In patients whose sense of smell improved during so-called “olfactory training”—a 12-week long, regular, intermittent exposure to a set of four odors (rose, eucalyptus, lemon, and cloves) (Hummel et al. 2009; Pieniak et al. 2022), relief of depressive symptoms has been observed (Sabiniewicz et al. 2022 but see: Pabel et al. 2020).
Two mutually non-exclusive pathways explain the mechanisms linking olfactory loss and depression (Croy et al. 2014). First, olfactory loss is associated with a loss of a source of pleasant sensations (Naudin et al. 2014; Parker et al. 2022), such as the appreciation of food. The enhanced social insecurity and loss of joy in the social aspects of eating may furthermore lead to social withdrawal, potentially accelerated by worries about own profession. This detriment in olfaction-related quality of life can increase depressive symptoms (Liu, Prem, Sharma, et al. 2022a). Second, altered brain functioning after olfactory loss may affect emotion processing. Animal studies, for instance, showed that olfactory loss reduces projections toward the amygdala (Carlsen et al. 1982; Mucignat-Caretta et al. 2004)—one of the major emotion-processing brain areas. In line, patients with olfactory loss assess emotional pictures as less salient and show diminished brain responses when viewing them (Han et al. 2019). Interestingly, the size of the olfactory bulb relates inversely to depression (Negoias et al. 2010) and even predicts the success of psychotherapy (Negoias et al. 2016). In some aetiologies of olfactory disorder, such as CRS, proinflammatory cytokines IL-6 and TNF-α may cross the blood-brain barrier to affect the amygdala and promote emotional instability (Soler et al. 2015; Yuan and Slotnick, 2014). In line, respiratory diseases moderate the relationship between depression and olfaction (Pabel et al. 2018). Also, other mental disorders, such as schizophrenia, are related to olfactory disorders (Moberg et al. 2014).
Olfactory loss affects quality of life less than blindness or deafness (Fischer et al. 2009) but is somewhat related to psychological distress (Bochicchio et al. 2023), depression (Croy and Hummel, 2017; Eliyan et al. 2021; Kamrava et al. 2021), and loneliness (Sivam et al. 2016). This relation is mediated by the temporal decline of olfaction, and patients with sudden olfactory loss—as due to COVID-19—are on higher risk for psychological distress (Kim et al. 2024). Altered behaviors in the areas affected by the olfactory disorders, along with the feeling of being lost and surprised by the sudden smell disorder, often result in the feeling of not being fully understood by close family members and friends (Burges Watson et al. 2021). Without the sense of smell, friends and romantic relationships are more difficult to initiate and maintain (Mahmut and Croy, 2019; Blomkvist and Hofer, 2021). Anosmia is related to a less satisfying sex life, especially for men (Schäfer et al. 2019; Deng et al. 2020; Siegel et al. 2021) and in people in stable romantic relationships (Hofer et al. 2025).
Olfactory loss imposes anxiety (Lemogne et al. 2015; Schäfer et al. 2019; Drummond et al. 2024). One of the spheres reflecting elevated anxiety is social chemical communication. The inability to smell hinders control over one’s body odor and raises concerns about the perception by others (Blomqvist et al. 2004; Nordin et al. 2011; Boesveldt and Parma, 2021). For the smell-disordered parents, these worries extrapolate to children (Croy et al. 2014; Lee et al. 2024). Consequently, patients suffering from smell loss may exaggerate personal hygiene routines by washing themselves several times a day or overdosing on scented cosmetics to mask the potential occurrence of unpleasant body odor (Miwa et al. 2001; Temmel et al. 2002b). Some of them withdraw from social events to avoid being singled out by body odor (Pellegrino et al. 2021).
Professional life may be negatively impacted by the loss of the sense of smell. 3-8% of the patients complain about their work performance after losing the sense of smell (Blomqvist et al. 2004; Nordin et al. 2011). Occupations particularly affected by this disorder are care professions, such as nurses and nursery teachers but also firefighters, chefs, perfumers, and sommeliers. Up to 60% of patients report the need to adjust their professional position to their condition, while 5% report the need to shift careers (Haxel et al. 2012).
Parosmia and phantosmia
Patients with qualitative olfactory disorders admit they get disgusted by their partner’s smell and avoid sharing this struggle with their partner so as not to hurt them (Burges Watson et al. 2021). In extreme cases, patients report for instance “quitting dating due to the lack of control over their body odor and the inability to imagine how a potential partner smells” or “altered feelings of intimacy due to the inability to smell the body odor of a partner or the partner’s body odor becoming disturbing” (Burges Watson et al. 2021). In their statements, patients with qualitative olfactory disorders often point out that they cannot share these feelings with their partner, as admitting that they are disgusted by their partner’s smell would hurt them.
Consequences for personal safety and exposure to hazardous events
Anosmia and hyposmia
Olfactory loss exposes individuals to the risk of inhaling toxins and not detecting smoke or gas leaks. Dangerous events of potential poisoning happen to patients with anosmia 2 to 3 times more often than to people without olfactory impairment (Santos et al. 2004; Croy et al. 2012b; Pence et al. 2014; Coelho et al. 2021). Consequently, patients with smell disorders are anxious about staying home alone (Pellegrino et al. 2021; Lee et al. 2024). To cope with the difficulties in detecting environmental threats, patients with smell loss develop certain protective behaviors like specific attention not to leave the iron alone or to consult other household members about the freshness of the food products (Croy et al. 2012c; Elkholi et al. 2021). Increased alertness to facial expressions of fear, anger, and disgust has also been observed in anosmic individuals, concluding that they may be more vigilant to the social and visual cues for disgusting odors in the environment (Lemogne et al. 2015; Drummond et al. 2024).
Measuring quality of life in olfactory disorders
Many studies concerning olfactory disorders concentrate on the general quality of life, which may sometimes be hard to examine from the perspective of olfactory disorders, as the questions do not tackle activities directly engaging the nose (Neuland et al. 2011). Several questionnaires dedicated to quality of life in olfactory disorders are available for different groups of patients (Han et al. 2020). An important development in understanding what aspects of the quality of life are affected by olfactory disorders is the involvement of the patients and the public. A summary of questionnaires on the quality of life associated with olfactory loss is presented in Table 1.
Table 1.
Summary of available methods to measure general and olfaction-specific quality of life. Referenced methods appear in chronological order.
| Reference | Questionnaire | Comment |
|---|---|---|
| (Ware and Sherbourne, 1992) | Short Form-36 Health Survey | General health survey |
| (Anderson et al. 1999) | Sinonasal Outcome Test (SNOT-16) | For patients with chronic rhinosinusitis (CRS), directly assesses olfactory dysfunction |
| (de Jong et al. 1999) | Appetite, hunger, subjective taste and smell questionnaire | Describes sensory impressions and feelings of appetite and hunger |
| (Miwa et al. 2001) | Questionnaire on the impact of olfactory impairment on quality of life and disability | Describes impairment in 15 olfactory-related daily life activities and general enjoyment of life |
| (Nordin et al. 2003) | Multi-Clinic Smell and Taste Questionnaire | Assessment of the consequences of olfactory dysfunction |
| (Frasnelli and Hummel 2005) | Questionnaire of Olfactory Dysfunction | Assessment of daily life problems associated with olfactory loss |
| (McDowell, 2006) | General Well-Being Schedule | Contains positive and negative questions across six dimensions: well-being, self-control, vitality, depression, anxiety, and general health |
| (Croy et al. 2010) | Individual significance of olfaction (ISoO) | Addresses associations, applications, and consequences of olfaction in daily life |
| (Pusswald et al. 2012) | Brief Self-Report Inventory to Measure Olfactory Dysfunction and Quality of Life | Assessment of the subjective general and odor-specific olfactory function and olfaction-related quality of life |
| (Mattos et al. 2018) | Questionnaire of Olfactory Dysfunction—Negative Statements (QOD-NS) | QOD-NS describes the consequences of olfactory loss. Subscale of Positive Statements (QOD-PS) is considered a measure of how well a patient is coping with the olfactory disorder (Liu et al. 2022b) |
| (Lee et al. 2022) | Olfactory Dysfunction Outcomes Rating (ODOR) | Includes questions about the consequences of olfactory dysfunction |
Dysosmia-related quality of life across the lifespan
Children and adolescents
Our understanding of the prevalence of olfactory disorders in the pediatric population and their consequences for the children is still far from complete. Despite the increasing evidence for high olfactory abilities in children and functionality of the olfactory system since birth (Schaal 1988, 2000; Soussignan and Schaal, 1996; Stevenson et al. 2007; Gellrich et al. 2017; Oleszkiewicz et al. 2022; Ustun et al. 2022), we poorly understand the impact odors have on children and whether olfactory loss has implications on their quality of life. Since children and adolescents are a minority of patients with olfactory loss referred to ENT clinics (2% to 4% of all patients), it can be expected that olfactory loss is less prevalent in the pediatric population—but may also go unnoticed (Gellrich et al. 2025). It is plausible that children adjust to the smell disorder quickly and do not report problems with olfaction to their parents or healthcare professionals. To our knowledge, this assumption has not been tested empirically. More studies involving the perspective of children and adolescents suffering from olfactory loss are needed to understand the consequences of olfactory disorders in their daily lives, including family bonding, relationships with peers, and effects on emotional and cognitive functioning.
Adults
Olfactory disorders are most salient for the subgroup of people who are used to very good olfactory function—young and healthy individuals—and for the subgroup who value their sense of smell highly—younger women (Murr et al. 2018). Indeed, younger patients with olfactory loss and women report lower olfaction-related quality of life (Zou et al. 2021). Sudden loss of the ability to perceive odors is harder to adapt to in comparison to congenital anosmia (Romanowicz et al. 2022). This is particularly evident among adults who have suddenly lost their sense of smell due to COVID-19 (Coelho et al. 2021; Elkholi et al. 2021; Bochicchio et al. 2023; Hofer et al. 2025).
In contrast, some people practically cannot smell, but they still consider their olfaction normal and do not notice the deficit. Unawareness of olfactory loss is mostly driven by age and often goes unnoticed under the cover of other emerging health conditions (Oleszkiewicz and Hummel, 2019). People with undetected olfactory loss, who have not been recruited in the ENT clinic, report similar quality of life, and depressive symptoms as individuals with normosmia, but exhibit slightly lower cognitive capacities possibly related to the association between olfactory loss and cognitive dysfunction (Oleszkiewicz et al. 2019).
Older adults
Older individuals typically report that their vision and audition declined considerably as compared to when they were young—however, they also report an unaffected sense of smell (Cavazzana et al. 2018). This is surprising as age is the number one cause for quantitative olfactory disorders. This result exemplifies how little attention slowly evolving olfactory disorders receive. In line, people with sudden olfactory loss, as in viral or traumatic cases, report higher impairment (Zou et al. 2021). Due to the gradual development, olfactory deficits may go unnoticed, exposing elderly people to environmental hazards and household accidents related to respiratory intoxication or food poisoning (Stevenson, 2010; Croy et al. 2014). Among people older than 70 years, 20% to 31% cannot detect odors of smoke and natural gas (Hoffman et al. 2016). Olfactory disorders emerging with aging may contribute to poor diet (Toussaint et al. 2015), especially in women presenting moderate/severe olfactory impairment (Gopinath et al. 2016). In the geriatric population, olfactory loss has also been linked to lesser variability of chosen foods (Rolls and McDermott, 1991; Kremer et al. 2014). Consequences of olfactory loss of social functioning, and depression are also amplified with aging (Eliyan et al. 2021). Olfactory loss in aging individuals comes with a smaller social network (Zou et al. 2016; Boesveldt et al. 2017), and loneliness (Sivam et al. 2016; Desiato et al. 2021). Olfactory loss is being considered a mortality risk marker in older individuals due to its relationship with increased frailty, neurodegeneration, poor nutrition, and inflated risk of being exposed to life-threatening situations (Pinto et al. 2017; Van Regemorter et al. 2020; Ruane et al. 2025).
Treatment strategies and coping with the olfactory disorders
As we already concluded in previous reviews about QoL in olfactory disorders and found confirmed with updated literature from the last decade, loss of the sense of smell negatively impacts quality of life, exposes patients with anosmia or hyposmia to environmental hazards, devoid them of eating pleasures, and hinders social interactions (Hummel and Nordin 2005; Croy et al. 2014).
Olfactory disorders are not irreversible; multiple treatment options exist (Whitcroft et al. 2023). Olfactory training has been demonstrated to be an efficient treatment method in multiple olfactory loss etiologies, including post-traumatic and post-infectious olfactory loss (Hummel et al. 2017; Whitcroft et al. 2023). Olfactory training can be recommended to individuals with lower baseline scores, but who have some degree of olfactory function (to sense the odorants they sniff bidaily). Individuals who began regaining olfactory function and manifesting parosmia are also likely to benefit from olfactory training (Liu et al. 2021). Besides the sense of smell, olfactory training may benefit cognitive and emotional functions (Pieniak et al. 2024), especially in the geriatric population (Wegener et al. 2018; Oleszkiewicz et al. 2021; Oleszkiewicz et al. 2021; Woo et al. 2023; Vance et al. 2024) which ultimately should improve quality of life. Still, many patients remain unaware of such treatment possibility (Li et al. 2024). Patients who reported the greatest reduction in the quality of life because of olfactory disorders are most motivated to perform an olfactory training regimen, but the lack of noticeable improvement within a short period of time is the main reason patients drop the procedure after approximately one month (Li et al. 2024). Thus, medical recommendations for olfactory training should be accompanied by a clear message that the method is only effective when performed regularly over at least 12 weeks.
Pharmacological treatment is also available to patients suffering from olfactory loss. While awaiting rigorous examination in appropriate double-blind, multicentric investigations (Patel et al. 2022), preliminary evidence shows therapeutic effects in olfactory dysfunction due to various causes, e.g. sodium citrate (Whitcroft et al. 2017, 2021), topical vitamin A (Reden et al. 2012; Hummel et al. 2017), zinc (Lyckholm et al. 2012; Jiang et al. 2015), or acupuncture (Drews et al. 2021). In addition, intranasal and systemic corticosteroids, surgery, or monoclonal antibodies are recommended for patients with olfactory loss resulting from sinunasal disease (Whitcroft and Hummel, 2019).
In addition to these forms of treatment, which have been used for years, sometimes with limited effectiveness (Patel et al. 2022), several new therapeutic options are currently being investigated (Mainland et al. 2020). They include injections of platelet-rich plasma into the olfactory cleft (Yan et al. 2023) or the topical administration of theophylline. Other work is currently underway on the effects of transcutaneous electrical stimulation to augment smell training (Maharjan et al. 2018). More futuristic aspects of therapy (Gunder et al. 2023) include work on olfactory implants (Lipp et al. 2025)—in analogy to cochlear implants—or transplantation of olfactory mucosa (Kurtenbach et al. 2019).
Patient survey data, however, showed that most frequently used treatment options—nasal and oral steroids and smell training—are perceived as only slightly or not effective at all by the majority of participants in the survey. Younger age thereby seemed the main predictor of treatment success (Murphy et al. 2024). Treatment options should hence be tailored to the patient’s age and also to the cause of olfactory loss to ensure maximal efficiency, and more research is needed to better target mechanisms for chemosensory impairment.
Most individuals adapt to the chemosensory deficit, and their quality of life improves again with the duration of the dysfunction (Auinger et al. 2021; Liu et al. 2022), while the importance of olfaction decreases (Liu et al. 2020). Coping strategies can potentially be guided during psychotherapy, which has been proven to be a successful intervention to reduce anxiety and depression in individuals suffering from sensory impairments in visual and auditory domains (Trott et al. 2025). Empirical evidence regarding olfactory dysfunctions is needed.
Support groups may also turn out helpful when adjusting to olfactory loss. There are non-governmental organizations (NGOs) dedicated to olfactory disorders (e.g. Chrissi Kelly on Smell [CKOS], Smell and Taste Association of North America [STANA], Fifth Sense, or Reuksmaakstoornis), or a recently opened NIH National Smell and Taste Center that combines research and treatment of olfactory disorders. These organizations create forums for patients to seek peer support, promote the inclusion of patient voices in the treatment process and research on olfactory dysfunction, advocate for funding aimed at better understanding olfactory disorders and treating them, and build networks connecting patients, healthcare professionals, and institutions to educate about olfactory health and disease. Patients and public involvement appear critical for the development of research, social strategies, clinical approaches, and science aimed at understanding olfactory disorders (Gane et al. 2020; Parker et al. 2021a, 2021b, 2022; Smith et al. 2021).
Contributor Information
Anna Oleszkiewicz, Interdisciplinary Center Smell & Taste, Department of Otorhinolaryngology, Faculty of Medicine Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany; Institute of Psychology, University of Wroclaw, Wrocław, Poland.
Ilona Croy, Department of Clinical Psychology, Institute of Psychology, Friedrich-Schiller-University Jena, Jena, 07743 , Germany; Department of Psychotherapy and Psychosomatic Medicine, Faculty of Medicine and University Hospital Carl Gustav Carus, TUD Dresden University of Technology, Dresden, 01307, Germany; German Centre for Mental Health (DZPG), site Halle-Jena-Magdeburg, Halle-Jena-Magdeburg, Germany.
Thomas Hummel, Interdisciplinary Center Smell & Taste, Department of Otorhinolaryngology, Faculty of Medicine Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
Funding
This work was supported by the National Science Center (Poland) Grant Number: OPUS 20 #2020/39/B/HS6/01533 awarded to AO. IC and TH were supported by the European Innovation Council, Smellodi project (agreement ID: 101046369)
Conflict of interest
The authors declare no competing interests.
Data availability
No new data were generated or analysed in support of this research.
References
- Anderson ER, Murphy MP, Weymuller EA.. Clinimetric evaluation of the Sinonasal Outcome Test‐16. Otolaryngology–Head and Neck Surgery 1999:121(6):702–707. doi: https://doi.org/ 10.1053/hn.1999.v121.a100114 [DOI] [PubMed] [Google Scholar]
- Arshamian A, Laska M, Gordon AR, Norberg M, Lahger C, Porada DK, Jelvez Serra N, Johansson E, Schaefer M, Amundin M, et al. A mammalian blood odor component serves as an approach-avoidance cue across phylum border - from flies to humans. Sci Rep. 2017:7(1):13635. doi: https://doi.org/ 10.1038/s41598-017-13361-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Walker L, Jellinger KA.. Olfaction and aging: a mini-review. Gerontology. 2015:61(6):485–490. doi: https://doi.org/ 10.1159/000381619 [DOI] [PubMed] [Google Scholar]
- Auinger AB, Besser G, Liu DT, Renner B, Mueller CA.. Long-term impact of olfactory dysfunction on daily life. Wien Klin Wochenschr. 2021:133(19-20):1004–1011. doi: https://doi.org/ 10.1007/s00508-020-01751-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomkvist A, Hofer M.. Olfactory impairment and close social relationships. A narrative review. Chem Senses. 2021:46:1–10. doi: https://doi.org/ 10.1093/chemse/bjab037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist EH, Brämerson A, Stjärne P, Nordin S.. Consequences of olfactory loss and adopted coping strategies. Rhinology. 2004:42(4):189–194. doi: https://doi.org/ 10.4193/Rhin [DOI] [PubMed] [Google Scholar]
- Bochicchio V, Mezzalira S, Maldonato NM, Cantone E, Scandurra C.. Olfactory-related quality of life impacts psychological distress in people with COVID-19: the affective implications of olfactory dysfunctions. J Affect Disord. 2023:323:741–747. doi: https://doi.org/ 10.1016/j.jad.2022.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesveldt S, de Graaf K.. The differential role of smell and taste for eating behavior. Perception. 2017:46(3-4):307–319. doi: https://doi.org/ 10.1177/0301006616685576 [DOI] [PubMed] [Google Scholar]
- Boesveldt S, Parma V.. The importance of the olfactory system in human well-being, through nutrition and social behavior. Cell Tissue Res. 2021:383(1):559–567. doi: https://doi.org/ 10.1007/s00441-020-03367-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesveldt S, Yee JR, McClintock MK, Lundström JN.. Olfactory function and the social lives of older adults: a matter of sex. Sci Rep. 2017:7(1):45118. doi: https://doi.org/ 10.1038/srep45118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burges Watson DL, Campbell M, Hopkins C, Smith B, Kelly C, Deary V.. Altered smell and taste: anosmia, parosmia and the impact of long Covid-19. PLoS One. 2021:16(9):e0256998. doi: https://doi.org/ 10.1371/journal.pone.0256998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burges Watson DL, Lewis S, Bryant V, Patterson J, Kelly C, Edwards-Stuart R, Murtagh MJ, Deary V.. Altered eating: a definition and framework for assessment and intervention. BMC Nutrition 2018:4:14. doi: https://doi.org/ 10.1186/s40795-018-0221-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen J, de Olmos J, Heimer L.. Tracing of two‐neuron pathways in the olfactory system by the aid of transneuronal degeneration: projections to the amygdaloid body and hippocampal formation. J Comp Neurol. 1982:208(2):196–208. doi: https://doi.org/ 10.1002/cne.902080208 [DOI] [PubMed] [Google Scholar]
- Cavazzana A, Röhrborn A, Garthus-Niegel S, Larsson M, Hummel T, Croy I.. Sensory-specific impairment among older people. An investigation using both sensory thresholds and subjective measures across the five senses. PLoS One. 2018:13(8):e0202969. doi: https://doi.org/ 10.1371/journal.pone.0202969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Masala C, Oleszkiewicz A, Englmaier V, Gunder N, Menzel S, Haehner A, Hummel T.. Nonlinear association between chemosensory dysfunction and body mass index. J Sens Stud. 2022:37(1). doi: https://doi.org/ 10.1111/joss.12715 [DOI] [Google Scholar]
- Coelho DH, Reiter ER, Budd SG, Shin Y, Kons ZA, Costanzo RM.. Quality of life and safety impact of COVID-19 associated smell and taste disturbances. Am J Otolaryngol. 2021:42(4):103001. doi: https://doi.org/ 10.1016/j.amjoto.2021.103001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Buschhüter D, Seo H-S, Negoias S, Hummel T.. Individual significance of olfaction: development of a questionnaire. Eur Arch oto-rhino-laryngology 2010:267(1):67–71. doi: https://doi.org/ 10.1007/s00405-009-1054-0 [DOI] [PubMed] [Google Scholar]
- Croy I, Hummel T.. Olfaction as a marker for depression. J Neurol. 2017:264(4):631–638. doi: https://doi.org/ 10.1007/s00415-016-8227-8 [DOI] [PubMed] [Google Scholar]
- Croy I, Negoias S, Novakova L, Landis BN, Hummel T.. Learning about the functions of the olfactory system from people without a sense of smell. PLoS One. 2012a:7(3):e33365. doi: https://doi.org/ 10.1371/journal.pone.0033365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Negoias S, Novakova L, Landis BN, Hummel T.. Learning about the functions of the olfactory system from people without a sense of smell. PLoS One. 2012b:7(3):e33365. doi: https://doi.org/ 10.1371/journal.pone.0033365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Negoias S, Novakova L, Landis BN, Hummel T.. Learning about the functions of the olfactory system from people without a sense of smell. PLoS One. 2012c:7(3):e33365. doi: https://doi.org/ 10.1371/journal.pone.0033365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Nordin S, Hummel T.. Olfactory disorders and quality of life-an updated review. Chem Senses. 2014:39(3):185–194. doi: https://doi.org/ 10.1093/chemse/bjt072 [DOI] [PubMed] [Google Scholar]
- de Jong N, Mulder I, de Graaf C, van Staveren WA.. Impaired sensory functioning in elders: the relation with its potential determinants and nutritional intake. J Gerontol A Biol Sci Med Sci. 1999:54(8):B324–B331. doi: https://doi.org/ 10.1093/gerona/54.8.b324 [DOI] [PubMed] [Google Scholar]
- Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB.. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch otolaryngology--Head & Neck Surgery 1991:117(5):519–528. doi: https://doi.org/ 10.1001/archotol.1991.01870170065015 [DOI] [PubMed] [Google Scholar]
- Deller M, Gellrich J, Lohrer EC, Schriever VA.. Genetics of congenital olfactory dysfunction: a systematic review of the literature. Chem Senses. 2022:47:bjac028. doi: https://doi.org/ 10.1093/chemse/bjac028 [DOI] [Google Scholar]
- Deng HY, Feng JR, Zhou WH, Kong WF, Ma GC, Hu TF, Luo SG, Xi Y, Zhang Y, Yang QT.. Olfactory sensitivity is related to erectile function in adult males. Front Cell Dev Biol. 2020:8:501405. doi: https://doi.org/ 10.3389/fcell.2020.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, Schlosser RJ.. The prevalence of olfactory dysfunction in the general population: a systematic review and meta-analysis. Am J Rhinol Allergy 2021:35(2):195–205. doi: https://doi.org/ 10.1177/1945892420946254 [DOI] [PubMed] [Google Scholar]
- Desiato VM, Soler ZM, Nguyen SA, Salvador C, Hill JB, Lamira J, Rowan NR, Yoo F, Little RE, Matthews LJ, et al. Evaluating the relationship between olfactory function and loneliness in community-dwelling individuals: a cross-sectional study. Am J Rhinol Allergy 2021:35(3):334–340. doi: https://doi.org/ 10.1177/1945892420958365 [DOI] [PubMed] [Google Scholar]
- Doty R, Shaman P, Dann M.. Development of the University of Pennsylvania smell identification test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984:32(3):489–502. doi: https://doi.org/ 10.1016/0031-9384(84)90269-5 [DOI] [PubMed] [Google Scholar]
- Doty RL, Deems DA, Stellar S.. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988:38(8):1237–1244. doi: https://doi.org/ 10.1212/wnl.38.8.1237 [DOI] [PubMed] [Google Scholar]
- Doty RL, Kamath V.. The influences of age on olfaction: a review. Front Psychol. 2014:5:20. doi: https://doi.org/ 10.3389/fpsyg.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews T, Hummel T, Rochlitzer B, Hauswald B, Hähner A.. Acupuncture is associated with a positive effect on odour discrimination in patients with postinfectious smell loss—a controlled prospective study. Eur Arch Otorhinolaryngol. 2021:279(3):1329–1334. doi: https://doi.org/ 10.1007/s00405-021-06872-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond J, Makdani A, Pawling R, Walker SC.. Congenital anosmia and facial emotion recognition. Physiol. Behav. 2024:278:114519. doi: https://doi.org/ 10.1016/j.physbeh.2024.114519 [DOI] [PubMed] [Google Scholar]
- Eliyan Y, Wroblewski KE, McClintock MK, Pinto JM.. Olfactory dysfunction predicts the development of depression in older US adults. Chem Senses. 2021:46:bjaa075. doi: https://doi.org/ 10.1093/chemse/bjaa075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkholi SMA, Abdelwahab MK, Abdelhafeez M.. Impact of the smell loss on the quality of life and adopted coping strategies in COVID-19 patients. Eur Arch Oto-Rhino-Laryngology 2021:278(9):3307–3314. doi: https://doi.org/ 10.1007/s00405-020-06575-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Klein BEK, Klein R, Schubert CR, Wiley TL.. Multiple sensory impairment and quality of life. Ophthalmic Epidemiol. 2009:16(6):346–353. doi: https://doi.org/ 10.3109/09286580903312236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjaeldstad AW. Using cooking schools to improve the pleasure of food and cooking in patients experiencing smell loss. Foods 2024:13(12):1821. doi: https://doi.org/ 10.3390/foods13121821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjaeldstad AW, Smith B.. The effects of olfactory loss and parosmia on food and cooking habits, sensory awareness, and quality of life—a possible avenue for regaining enjoyment of food. Foods 2022:11(12):1686. doi: https://doi.org/ 10.3390/foods11121686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluitman KS, Hesp AC, Kaihatu RF, Nieuwdorp M, Keijser BJF, IJzerman RG, Visser M.. Poor taste and smell are associated with poor appetite, macronutrient intake, and dietary quality but not with undernutrition in older adults. J Nutr. 2021:151(3):605–614. doi: https://doi.org/ 10.1093/jn/nxaa400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluitman KS, Nadar HJ, Roos DS, Berendse HW, Keijser BJF, Nieuwdorp M, Ijzerman RG, Visser M.. The association of olfactory function with BMI, appetite, and prospective weight change in dutch community-dwelling older adults. J Nutr Health Aging. 2019:23(8):746–752. doi: https://doi.org/ 10.1007/s12603-019-1241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasnelli J, Hummel T.. Olfactory dysfunction and daily life. Eur Arch oto-rhino-laryngology 2005:262(3):231–235. doi: https://doi.org/ 10.1007/s00405-004-0796-y [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Landis BN, Heilmann S, Hauswald B, Hüttenbrink KB, Lacroix JS, Leopold DA, Hummel T.. Clinical presentation of qualitative olfactory dysfunction. Eur Arch Otorhinolaryngol. 2004:261(7):411–415. doi: https://doi.org/ 10.1007/s00405-003-0703-y [DOI] [PubMed] [Google Scholar]
- Gaillet-Torrent M, Sulmont-Rossé C, Issanchou S, Chabanet C, Chambaron S.. Impact of a non-attentively perceived odour on subsequent food choices. Appetite. 2014:76:17–22. doi: https://doi.org/ 10.1016/j.appet.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Gane SB, Kelly C, Hopkins C.. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020:58(3):299–301. doi: https://doi.org/ 10.4193/Rhin20.114 [DOI] [PubMed] [Google Scholar]
- Gellrich J, Lohrer EC, Hummel T, Schriever VA.. Olfactory dysfunction in children and adolescents—a diagnostic pathway. Neuropediatrics. 2025:56(4):215–220. doi: https://doi.org/ 10.1055/a-2509-8547 [DOI] [PubMed] [Google Scholar]
- Gellrich J, Stetzler C, Oleszkiewicz A, Hummel T, Schriever VA.. Olfactory threshold and odor discrimination ability in children-evaluation of a modified “sniffin’’ Sticks” test.”. Sci Rep. 2017:7(1):1928. doi: https://doi.org/ 10.1038/s41598-017-01465-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath B, Russell J, Sue CM, Flood VM, Burlutsky G, Mitchell P.. Olfactory impairment in older adults is associated with poorer diet quality over 5 years. Eur J Nutr. 2016:55(3):1081–1087. doi: https://doi.org/ 10.1007/s00394-015-0921-2 [DOI] [PubMed] [Google Scholar]
- Gorodisky L, Honigstein D, Weissbrod A, Weissgross R, Soroka T, Shushan S, Sobel N.. Humans without a sense of smell breathe differently. Nat Commun. 2024:15(1):8809. doi: https://doi.org/ 10.1038/s41467-024-52650-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunder N, Dörig P, Witt M, Welge-Lüssen A, Menzel S, Hummel T.. Future therapeutic strategies for olfactory disorders: electrical stimulation, stem cell therapy, and transplantation of olfactory epithelium-an overview. HNO. 2023:71(Suppl 1):35–43. doi: https://doi.org/ 10.1007/s00106-022-01249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehner A, Hummel T, Reichmann H.. Olfactory dysfunction as a diagnostic marker for Parkinson’s disease. Expert Rev Neurother. 2009:9(12):1773–1779. doi: https://doi.org/ 10.1586/ern.09.115 [DOI] [PubMed] [Google Scholar]
- Haehner A, Hummel T, Reichmann H.. Olfactory loss in Parkinson’s disease. Parkinson’s Disease 2011:2011:450939. doi: https://doi.org/ 10.4061/2011/450939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Hummel T, Raue C, Croy I.. Olfactory loss is associated with reduced hippocampal activation in response to emotional pictures. Neuroimage. 2019:188:84–91. doi: https://doi.org/ 10.1016/j.neuroimage.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Han P, Su T, Qin M, Chen H, Hummel T.. A systematic review of olfactory related questionnaires and scales. Rhinology Journal 2020:0(0):0–0. doi: https://doi.org/ 10.4193/rhin20.291 [DOI] [PubMed] [Google Scholar]
- Haxel BR, Nisius A, Fruth K, Mann WJ, Muttray A.. Defizite der ärztlichen Beratung bei Riechstörungen. HNO. 2012:60(5):432–438. doi: https://doi.org/ 10.1007/s00106-011-2448-z [DOI] [PubMed] [Google Scholar]
- Hernandez AK, Landis BN, Altundag A, Fjaeldstad AW, Gane S, Holbrook EH, Huart C, Konstantinidis I, Lechner M, Macchi A, et al. Olfactory nomenclature: an orchestrated effort to clarify terms and definitions of dysosmia, anosmia, hyposmia, normosmia, hyperosmia, olfactory intolerance, parosmia, and phantosmia/olfactory hallucination. ORL: Journal for Otorhinolaryngol Relat Spec 2023:85(6):312–320. doi: https://doi.org/ 10.1159/000530211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MK, Blume L, Turner BJ, Schäfer L, Croy I, Hummel T.. The impact of COVID-19-related smell dysfunction on sexual and mental wellbeing: data from a longitudinal sample. Biol Psychol. 2025:195:109002. doi: https://doi.org/ 10.1016/j.biopsycho.2025.109002 [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Rawal S, Li C-M, Duffy VB.. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 2016:17(2):221–240. doi: https://doi.org/ 10.1007/s11154-016-9364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Nordin S.. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005:125(2):116–121. doi: https://doi.org/ 10.1080/00016480410022787 [DOI] [PubMed] [Google Scholar]
- Hummel T, Rissom K, Reden J, Hähner A, Weidenbecher M, Hüttenbrink K-B.. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009:119(3):496–499. doi: https://doi.org/ 10.1002/lary.20101 [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G.. ‘Sniffin’ Sticks’: olfactory performance assessed by the combined testing of odour identification, odor discrimination and olfactory threshold. Chem Senses. 1997:22(1):39–52. doi: https://doi.org/ 10.1093/chemse/22.1.39 [DOI] [PubMed] [Google Scholar]
- Hummel T, Whitcroft K, Andrews P, Altundag A, Cinghi C, Costanzo RM, Damm M, Frasnelli J, Gudziol H, Gupta N, et al. Position paper on olfactory dysfunction. Rhinology. 2017:54(Supplement 26):1–30. doi: https://doi.org/ 10.4193/Rhin16.248 [DOI] [PubMed] [Google Scholar]
- Hummel T, Whitcroft KL, Rueter G, Haehner A.. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur arch oto-rhino-laryngology 2017:274(7):2819–2825. doi: https://doi.org/ 10.1007/s00405-017-4576-x [DOI] [PubMed] [Google Scholar]
- Hunter SR, Beatty C, Dalton PH.. More spice, less salt: How capsaicin affects liking for and perceived saltiness of foods in people with smell loss. Appetite. 2023:190:107032. doi: https://doi.org/ 10.1016/j.appet.2023.107032 [DOI] [PubMed] [Google Scholar]
- Jestrović I, Coyle JL, Sejdić E.. Decoding human swallowing via electroencephalography: a state-of-the-art review. J Neural Eng. 2015:12(5):051001. doi: https://doi.org/ 10.1088/1741-2560/12/5/051001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Twu C, Liang K.. Medical treatment of traumatic anosmia. Otolaryngology--Head and Neck Surgery 2015:152(5):954–958. doi: https://doi.org/ 10.1177/0194599815571272 [DOI] [PubMed] [Google Scholar]
- Jung YG, Lee J, Park GC.. Does post‐infectious olfactory loss affect mood more severely than chronic sinusitis with olfactory loss? Laryngoscope 2014:124(11):2456–2460. doi: https://doi.org/ 10.1002/lary.24691 [DOI] [PubMed] [Google Scholar]
- Kamrava SK, Tavakol Z, Talebi A, Farhadi M, Jalessi M, Hosseini SF, Amini E, Chen B, Hummel T, Alizadeh R.. A study of depression, partnership and sexual satisfaction in patients with post-traumatic olfactory disorders. Sci Rep. 2021:11(1):20218. doi: https://doi.org/ 10.1038/s41598-021-99627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karstensen H, Tommerup N.. Isolated and syndromic forms of congenital anosmia. Clin Genet. 2012:81(3):210–215. doi: https://doi.org/ 10.1111/j.1399-0004.2011.01776.x [DOI] [PubMed] [Google Scholar]
- Kim S, Finlay JB, Ko T, Goldstein BJ.. Long‐term olfactory loss post‐COVID‐19: pathobiology and potential therapeutic strategies. World J Otorhinolaryngol - Head and Neck Surgery 2024:10(2):148–155. doi: https://doi.org/ 10.1002/wjo2.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer S, Holthuysen N, Boesveldt S.. The influence of olfactory impairment in vital, independently living older persons on their eating behaviour and food liking. Food Qual Preference. 2014:38:30–39. doi: https://doi.org/ 10.1016/j.foodqual.2014.05.012 [DOI] [Google Scholar]
- Kurtenbach S, Goss GM, Goncalves S, Choi R, Hare JM, Chaudhari N, Goldstein BJ.. Cell-based therapy restores olfactory function in an inducible model of hyposmia. Stem Cell Rep. 2019:12(6):1354–1365. doi: https://doi.org/ 10.1016/j.stemcr.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Mahadev A, Kallogjeri D, Peterson AM, Gupta S, Khan AM, Jiramongkolchai P, Schneider JS, Piccirillo JF.. Development and psychometric validation of the olfactory dysfunction outcomes rating. JAMA Otolaryngology-- Head & Neck Surgery 2022:148(12):1132–1139. doi: https://doi.org/ 10.1001/jamaoto.2022.3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Luke L, Boak D, Philpott C.. Impact of olfactory disorders on personal safety and well-being: a cross-sectional observational study. Eur Arch Otorhinolaryngol. 2024:281(7):3639–3647. doi: https://doi.org/ 10.1007/s00405-024-08529-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Smadja J, Zerdazi E-H, Soudry Y, Robin M, Berthoz S, Limosin F, Consoli SM, Bonfils P.. Congenital anosmia and emotion recognition: a case-control study. Neuropsychologia. 2015:72:52–58. doi: https://doi.org/ 10.1016/j.neuropsychologia.2015.04.028 [DOI] [PubMed] [Google Scholar]
- Li Z, Pellegrino R, Kelly C, Hummel T.. Olfactory training: perspective from people who were disturbed by their smell problems. Eur Arch Otorhinolaryngol. 2024:281(12):6423–6430. doi: https://doi.org/ 10.1007/s00405-024-08911-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp C, Laamari L, Bertsch A, Podlesek D, Bensafi M, Hummel T, Brugger J.. Devices for the electrical stimulation of the olfactory system: a review. Biosens. Bioelectron 2025:271:117063. doi: https://doi.org/ 10.1016/j.bios.2024.117063 [DOI] [PubMed] [Google Scholar]
- Liu DT, Besser G, Prem B, Speth MM, Sedaghat AR, Mueller CA.. Individual importance of olfaction decreases with duration of smell loss. J Rhinol 2020:0(0):0–0. doi: https://doi.org/ 10.4193/rhin20.196 [DOI] [PubMed] [Google Scholar]
- Liu DT, Prem B, Besser G, Renner B, Mueller CA.. Olfactory-related quality of life adjustments in smell loss during the coronavirus-19 pandemic. Am J Rhinol Allergy 2022:36(2):253–260. doi: https://doi.org/ 10.1177/19458924211053118 [DOI] [PubMed] [Google Scholar]
- Liu DT, Prem B, Sharma G, Kaiser J, Besser G, Mueller CA.. Depression symptoms and olfactory‐related quality of life. Laryngoscope 2022a:132(9):1829–1834. doi: https://doi.org/ 10.1002/lary.30122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DT, Prem B, Sharma G, Kaiser J, Besser G, Mueller CA.. Depression symptoms and olfactory‐related quality of life. Laryngoscope 2022b:132(9):1829–1834. doi: https://doi.org/ 10.1002/lary.30122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DT, Sabha M, Damm M, Philpott C, Oleszkiewicz A, Hähner A, Hummel T.. Parosmia is associated with relevant olfactory recovery after olfactory training. Laryngoscope. 2021:131(3):618–623. doi: https://doi.org/ 10.1002/lary.29277 [DOI] [PubMed] [Google Scholar]
- Loos HM, Schaal B, Pause BM, Smeets MAM, Ferdenzi C, Roberts SC, de Groot J, Lübke KT, Croy I, Freiherr J, et al. Past, present, and future of human chemical communication research. Perspect Psychol Sci.. 2023:20(1):20–44. doi: https://doi.org/ 10.1177/17456916231188147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loret C. Using sensory properties of food to trigger swallowing: a review. Crit Rev Food Sci Nutr. 2015:55(1):140–145. doi: https://doi.org/ 10.1080/10408398.2011.649810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyckholm L, Heddinger SP, Parker G, Coyne PJ, Ramakrishnan V, Smith TJ, Henkin RI.. A randomized, placebo controlled trial of oral zinc for chemotherapy-related taste and smell disorders. J Pain Palliat Care Pharmacother 2012:26(2):111–114. doi: https://doi.org/ 10.3109/15360288.2012.676618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharjan A, Wang E, Peng M, Cakmak YO.. Improvement of olfactory function with high frequency non-invasive auricular electrostimulation in healthy humans. Front Neurosci. 2018:12(APR). doi: https://doi.org/ 10.3389/fnins.2018.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmut MK, Croy I.. The role of body odors and olfactory ability in the initiation, maintenance and breakdown of romantic relationships – A review. Physiology & Behavior 2019:207:179–184. doi: https://doi.org/ 10.1016/j.physbeh.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Mainland JD, Barlow LA, Munger SD, Millar SE, Vergara MN, Jiang P, Schwob JE, Goldstein BJ, Boye SE, Martens JR, et al. Identifying treatments for taste and smell disorders: gaps and opportunities. Chem Senses. 2020:45(7):493–502. doi: https://doi.org/ 10.1093/chemse/bjaa038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos JL, Schlosser RJ, DeConde AS, Hyer M, Mace JC, Smith TL, Soler ZM.. Factor analysis of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2018:8(7):777–782. doi: https://doi.org/ 10.1002/alr.22112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann Worldgroup. The Truth About Youth. http://micco.se/wp-content/uploads/2011/06/McCann-Worldgroup-Truth-About-Youth.pdf [Google Scholar]
- McDowell, I. Measuring Health. Oxford University Press. 2006. doi: https://doi.org/ 10.1093/acprof:oso/9780195165678.001.0001 [DOI] [Google Scholar]
- McGann JP. Poor human olfaction is a 19th-century myth. Science (New York, N.Y.) 2017:356(6338):eaam7263. doi: https://doi.org/ 10.1126/science.aam7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin NCR, Westervelt HJ.. Odor identification deficits in frontotemporal dementia: A preliminary study. Arch Clin Neuropsychol. 2008:23(1):119–123. doi: https://doi.org/ 10.1016/j.acn.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Miwa T, Furukawa M, Tsukatani T, Costanzo RM, DiNardo LJ, Reiter ER.. Impact of olfactory impairment on quality of life and disability. Archives of Otolaryngology--Head & Neck Surgery 2001:127(5):497–503. doi: https://doi.org/ 10.1001/archotol.127.5.497 [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn C-G, Borgmann-Winter KE, Kohler CG, Gur RE, Turetsky BI.. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull. 2014:40(1):50–59. doi: https://doi.org/ 10.1093/schbul/sbt049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquecho-Campos P, de Graaf K, Boesveldt S.. Smelling our appetite? The influence of food odors on congruent appetite, food preferences and intake. Food Qual Preference. 2020:85:103959. doi: https://doi.org/ 10.1016/j.foodqual.2020.103959 [DOI] [Google Scholar]
- Mucignat-Caretta C, Bondi’ M, Caretta A.. Animal models of depression: olfactory lesions affect amygdala, subventricular zone, and aggression. Neurobiol Dis. 2004:16(2):386–395. doi: https://doi.org/ 10.1016/j.nbd.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Murphy C, Dalton P, Boateng K, Hunter S, Silberman P, Trachtman J, Schrandt S, Naimi B, Garvey E, Joseph PV, et al. Integrating the patient’s voice into the research agenda for treatment of chemosensory disorders. Chem Senses. 2024:49:20. doi: https://doi.org/ 10.1093/chemse/bjae020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr J, Hummel T, Ritschel G, Croy I.. Individual significance of olfaction: a comparison between normosmic and dysosmic people. Psychosomatics. 2018:59(3):283–292. doi: https://doi.org/ 10.1016/j.psym.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Naudin M, Carl T, Surguladze S, Guillen C, Gaillard P, Belzung C, El-Hage W, Atanasova B.. Perceptive biases in major depressive episode. PLoS One. 2014:9(2):e86832. doi: https://doi.org/ 10.1371/journal.pone.0086832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T.. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 2010:169(1):415–421. doi: https://doi.org/ 10.1016/j.neuroscience.2010.05.012 [DOI] [PubMed] [Google Scholar]
- Negoias S, Hummel T, Symmank A, Schellong J, Joraschky P, Croy I.. Olfactory bulb volume predicts therapeutic outcome in major depression disorder. Brain Imaging and Behavior 2016:10(2):367–372. doi: https://doi.org/ 10.1007/s11682-015-9400-x [DOI] [PubMed] [Google Scholar]
- Neuland C, Bitter T, Marschner H, Gudziol H, Guntinas-Lichius O.. Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. Laryngoscope 2011:121(4):867–872. doi: https://doi.org/ 10.1002/lary.21387 [DOI] [PubMed] [Google Scholar]
- Nordin S, Brämerson A, Murphy C, Bende M.. A Scandinavian adaptation of the multi-clinic smell and taste questionnaire: evaluation of questions about olfaction. Acta Otolaryngol. 2003:123(4):536–542. doi: https://doi.org/ 10.1080/00016480310001411 [DOI] [PubMed] [Google Scholar]
- Nordin S, Hedén Blomqvist E, Olsson P, Stjärne P, Ehnhage A; for the NAF2S2 Study Group. Effects of smell loss on daily life and adopted coping strategies in patients with nasal polyposis with asthma. Acta Otolaryngol. 2011:131(8):826–832. doi: https://doi.org/ 10.3109/00016489.2010.539625 [DOI] [PubMed] [Google Scholar]
- Nordin S, Millqvist E, Löwhagen O, Bende M.. The Chemical Sensitivity Scale: psychometric properties and comparison with the noise sensitivity scale. J. Environ. Psychol 2003:23(4):359–367. doi: https://doi.org/ 10.1016/s0272-4944(03)00002-1 [DOI] [Google Scholar]
- Oleszkiewicz A, Abriat A, Doelz G, Azema E, Hummel T.. Beyond olfaction: beneficial effects of olfactory training extend to aging-related cognitive decline. Behav Neurosci. 2021:135(6):732–740. doi: https://doi.org/ 10.1037/bne0000478 [DOI] [PubMed] [Google Scholar]
- Oleszkiewicz A, Behl O, Grahl T, Hummel T.. Odor discrimination in children aged 4–12 years. Chem Senses. 2022:47:bjac005. doi: https://doi.org/ 10.1093/chemse/bjac005 [DOI] [PubMed] [Google Scholar]
- Oleszkiewicz A, Bottesi L, Pieniak M, Fujita S, Krasteva N, Nelles G, Hummel T.. Olfactory training with Aromastics: olfactory and cognitive effects. Eur Arch Otorhinolaryngol. 2021:279(1):225–232. doi: https://doi.org/ 10.1007/s00405-021-06810-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszkiewicz A, Hummel T.. Whose nose does not know? Demographical characterization of people unaware of anosmia. Eur Arch Otorhinolaryngol. 2019:276(6):1849–1852. doi: https://doi.org/ 10.1007/s00405-019-05414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszkiewicz A, Kunkel F, Larsson M, Hummel T.. Consequences of undetected olfactory loss for human chemosensory communication and well-being. Philos Trans R Soc Lond B Biol Sci 2019:375(1800):20190265. doi: https://doi.org/ 10.1098/rstb.2019.0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszkiewicz A, Park D, Resler K, Draf J, Schulze A, Zang Y, Hähner A, Hummel T.. Quality of life in patients with olfactory loss is better predicted by flavor identification than by orthonasal olfactory function. Chem Senses. 2019:44(6):371–377. doi: https://doi.org/ 10.1093/chemse/bjz027 [DOI] [PubMed] [Google Scholar]
- Oleszkiewicz A, Schmidt P, Smith B, Spence C, Hummel T.. Effects of blindness and anosmia on auditory discrimination of temperature and carbonation of liquids. Food Qual Preference. 2023:107:104852. doi: https://doi.org/ 10.1016/j.foodqual.2023.104852 [DOI] [Google Scholar]
- Olofsson JK, Ekesten F, Nordin S.. Olfactory distortions in the general population. Sci Rep. 2022:12(1):9776. doi: https://doi.org/ 10.1038/s41598-022-13201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Ekström I, Larsson M, Nordin S.. Olfaction and aging: a review of the current state of research and future directions. I-Perception. 2021:12(3):20416695211020331. doi: https://doi.org/ 10.1177/20416695211020331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabel LD, Hummel T, Weidner K, Croy I.. The impact of severity, course and duration of depression on olfactory function. J Affect Disord. 2018:238:194–203. doi: https://doi.org/ 10.1016/j.jad.2018.05.033 [DOI] [PubMed] [Google Scholar]
- Pabel LD, Murr J, Weidner K, Hummel T, Croy I.. Null effect of olfactory training with patients suffering from depressive disorders—An exploratory randomized controlled clinical trial. Front Psychiatry. 2020:11:593. doi: https://doi.org/ 10.3389/fpsyt.2020.00593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JK, Kelly CE, Gane SB.. Insights into the molecular triggers of parosmia based on gas chromatography olfactometry. Communications Medicine 2022:2(1):58. doi: https://doi.org/ 10.1038/s43856-022-00112-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JK, Kelly CE, Smith BC, Kirkwood AF, Hopkins C, Gane S.. Patients’ perspectives on qualitative olfactory dysfunction: thematic analysis of social media posts. JMIR Formative Research 2021a:5(12):e29086. doi: https://doi.org/ 10.2196/29086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JK, Kelly CE, Smith BC, Kirkwood AF, Hopkins C, Gane S.. Patients’ perspectives on qualitative olfactory dysfunction: thematic analysis of social media posts. JMIR Formative Research 2021b:5(12):e29086. doi: https://doi.org/ 10.2196/29086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel ZM, Holbrook EH, Turner JH, Adappa ND, Albers MW, Altundag A, Appenzeller S, Costanzo RM, Croy I, Davis GE, et al. International consensus statement on allergy and rhinology: Olfaction. Int Forum Allergy Rhinol 2022:12(4):327–680. doi: https://doi.org/ 10.1002/alr.22929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino R, Mainland JD, Kelly CE, Parker JK, Hummel T.. Prevalence and correlates of parosmia and phantosmia among smell disorders. Chem Senses. 2021:46. doi: https://doi.org/ 10.1093/chemse/bjab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence TS, Reiter ER, DiNardo LJ, Costanzo RM.. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol Head Neck Surg 2014:140(10):951–955. doi: https://doi.org/ 10.1001/jamaoto.2014.1675 [DOI] [PubMed] [Google Scholar]
- Pieniak M, Oleszkiewicz A, Avaro V, Calegari F, Hummel T.. Olfactory training – Thirteen years of research reviewed. Neurosci Biobehav Rev. 2022:141:104853. doi: https://doi.org/ 10.1016/j.neubiorev.2022.104853 [DOI] [PubMed] [Google Scholar]
- Pieniak M, Rokosz M, Ivcevic Z, Reichert A, Żyżelewicz B, Nawrocka P, Lebuda I, Oleszkiewicz A.. Olfactory training improves emotion matching ability in 6–9 years old children — preliminary evidence. J Sens Stud. 2024:39(2 https://doi.org/ 10.1111/joss.12912 [DOI] [Google Scholar]
- Pinto JM, Wroblewski KE, Huisingh-Scheetz M, Correia C, Lopez KJ, Chen RC, Kern DW, Schumm PL, Dale W, McClintock MK.. Global sensory impairment predicts morbidity and mortality in older U.S. adults. J Am Geriatr Soc. 2017:65(12):2587–2595. doi: https://doi.org/ 10.1111/jgs.15031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsen MM, Stoffers D, Booij J, van Eck-Smit BLF, Wolters EC, Berendse HW.. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. 2004:56(2):173–181. doi: https://doi.org/ 10.1002/ana.20160 [DOI] [PubMed] [Google Scholar]
- Ponsen MM, Stoffers D, Twisk JWR, Wolters EC, Berendse HW.. Hyposmia and executive dysfunction as predictors of future Parkinson’s disease: a prospective study. Mov Disord. 2009:24(7):1060–1065. doi: https://doi.org/ 10.1002/mds.22534 [DOI] [PubMed] [Google Scholar]
- Proserpio C, de Graaf C, Laureati M, Pagliarini E, Boesveldt S.. Impact of ambient odors on food intake, saliva production and appetite ratings. Physiol Behav. 2017:174:35–41. doi: https://doi.org/ 10.1016/j.physbeh.2017.02.042 [DOI] [PubMed] [Google Scholar]
- Purdy F, Luo Z, Gardiner JC, Pinto JM, Shiroma EJ, Simonsick EM, Harris TB, Chen H.. Olfaction and changes in body composition in a large cohort of older U.S. adults. J Gerontol A Biol Sci Med Sci. 2020:75(12):2434–2440. doi: https://doi.org/ 10.1093/gerona/glaa085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusswald G, Auff E, Lehrner J.. Development of a brief self-report inventory to measure olfactory dysfunction and quality of life in patients with problems with the sense of smell. Chemosens Percept. 2012:5(3-4):292–299. doi: https://doi.org/ 10.1007/s12078-012-9127-7 [DOI] [Google Scholar]
- Raithel CU, Gottfried JA.. Using your nose to find your way: ethological comparisons between human and non-human species. Neurosci Biobehav Rev 2021:128:766–779. doi: https://doi.org/ 10.1016/j.neubiorev.2021.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers MG, Verhoef A, Gort G, Luning PA, Boesveldt S.. Metabolic and sensory influences on odor sensitivity in humans. Chem Senses. 2015:41(2):bjv068. doi: https://doi.org/ 10.1093/chemse/bjv068 [DOI] [PubMed] [Google Scholar]
- Reden J, Lill K, Zahnert T, Haehner A, Hummel T.. Olfactory function in patients with postinfectious and posttraumatic smell disorders before and after treatment with vitamin A: a double-blind, placebo-controlled, randomized clinical trial. Laryngoscope 2012:122(9):1906–1909. doi: https://doi.org/ 10.1002/lary.23405 [DOI] [PubMed] [Google Scholar]
- Reden J, Maroldt H, Fritz A, Zahnert T, Hummel T.. A study on the prognostic significance of qualitative olfactory dysfunction. Eur Arch Otorhinolaryngol. 2006:264(2):139–144. doi: https://doi.org/ 10.1007/s00405-006-0157-0 [DOI] [PubMed] [Google Scholar]
- Rolls B, McDermott T.. Effects of age on sensory-specific satiety. Am J Clin Nutr. 1991:54(6):988–996. doi: https://doi.org/ 10.1093/ajcn/54.6.988 [DOI] [PubMed] [Google Scholar]
- Romanowicz A, Kwaśniewski K, Brzoznowski W, Tretiakow D, Plichta L, Skorek A.. Multi-level comparison of congenital and acquired anosmia. Polski Przegląd Otorynolaryngologiczny 2022:11(2):8–13. doi: https://doi.org/ 10.5604/01.3001.0015.8948 [DOI] [Google Scholar]
- Rosso C, De Corso E, Urbanelli A, Fadda G, Saibene AM, Ferella F, Spanu C, Pipolo C.. Changes in weight secondary to improved odor perception in chronic rhinosinusitis with nasal polyps’ patients treated with Dupilumab. Eur Arch Otorhinolaryngol. 2025:282(1):251–256. doi: https://doi.org/ 10.1007/s00405-024-09021-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruane R, Lampert O, Larsson M, Vetrano DL, Laukka EJ, Ekström I.. Olfactory deficits and mortality in older adults. JAMA Otolaryngol Head Neck Surg. 2025:151(6):558. doi: https://doi.org/ 10.1001/jamaoto.2025.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C, Hummel T.. Gustation, Olfaction, and Deglutition. In Principles of Deglutition. NY: Springer; 2013. pp. 19–24. doi: https://doi.org/ 10.1007/978-1-4614-3794-9_2 [DOI] [Google Scholar]
- Ryan CM, Morrow LA, Hodgson M.. Cacosmia and neurobehavioral dysfunction associated with occupational exposure to mixtures of organic solvents. Am J Psychiatry 2006:145(11):1442–1445. doi: https://doi.org/ 10.1176/ajp.145.11.1442 [DOI] [PubMed] [Google Scholar]
- Sabiniewicz A, Hoffmann L, Haehner A, Hummel T.. Symptoms of depression change with olfactory function. Sci Rep. 2022:12(1):5656. doi: https://doi.org/ 10.1038/s41598-022-09650-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja S, Croy I, Gruhl A, Croy A, Kanbaty M, Hellmann A, Stevenson RJ.. Facial disgust in response to touches, smells, and tastes. Emotion 2024:24(1):2–14. doi: https://doi.org/ 10.1037/emo0001257 [DOI] [PubMed] [Google Scholar]
- Santos DV, Reiter ER, DiNardo LJ, Costanzo RM.. Hazardous events associated with impaired olfactory function. Arch Otol Neck Surg 2004:130(3):317–319. doi: https://doi.org/ 10.1001/archotol.130.3.317 [DOI] [PubMed] [Google Scholar]
- Schaal B. Olfaction in infants and children: developmental and functional perspectives. Chem Senses. 1988:13(2):145–190. doi: https://doi.org/ 10.1093/chemse/13.2.145 [DOI] [Google Scholar]
- Schaal B, Marlier L, Soussignan R.. Human foetuses learn odours from their pregnant mother’s diet. Chem Senses. 2000:25(6):729–737. doi: https://doi.org/ 10.1093/chemse/25.6.729 [DOI] [PubMed] [Google Scholar]
- Schäfer L, Croy I.. An integrative review: human chemosensory communication in the parent-child relationship. Neurosci Biobehav Rev. 2023:153:105336. doi: https://doi.org/ 10.1016/j.neubiorev.2023.105336 [DOI] [PubMed] [Google Scholar]
- Schäfer L, Mehler L, Hähner A, Walliczek U, Hummel T, Croy I.. Sexual desire after olfactory loss: quantitative and qualitative reports of patients with smell disorders. Physiol. Behav. 2019:201:64–69. doi: https://doi.org/ 10.1016/j.physbeh.2018.12.020 [DOI] [PubMed] [Google Scholar]
- Schifferstein HNJ. The perceived importance of sensory modalities in product usage: A study of self-reports. Acta Psychol (Amst). 2006:121(1):41–64. doi: https://doi.org/ 10.1016/j.actpsy.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Schriever VA, Hummel T.. Etiologies of olfactory dysfunction in a pediatric population: based on a retrospective analysis of data from an outpatient clinic. Eur Arch Otorhinolaryngol. 2020:277(11):3213–3216. doi: https://doi.org/ 10.1007/s00405-020-06087-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Yang AL, Hamburger K.. The human sense of smell in spatial orientation: A state-of-the-art review. Psychol of Conscious. 2024:12(2):169–185. doi: https://doi.org/ 10.1037/cns0000395 [DOI] [Google Scholar]
- Siegel JK, Kung SY, Wroblewski KE, Kern DW, McClintock MK, Pinto JM.. Olfaction is associated with sexual motivation and satisfaction in older men and women. J Sex Med. 2021:18(2):295–302. doi: https://doi.org/ 10.1016/j.jsxm.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivam A, Wroblewski KE, Alkorta-Aranburu G, Barnes LL, Wilson RS, Bennett DA, Pinto JM.. Olfactory dysfunction in older adults is associated with feelings of depression and loneliness. Chem Senses. 2016:41(4):293–299. doi: https://doi.org/ 10.1093/chemse/bjv088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, B., Hopkins, C., Whitcroft, K., Kelly, C., Burges Watson, D. L., and Deary. Vincent. (2021). Covid and Society: Accessing healthcare before, during and after the pandemic. The British Academy, School of Advanced Studies, University of London. https://www.thebritishacademy.ac.uk/publications/covid-decade-accessing-healthcare-before-during-after-pandemic/ [Google Scholar]
- Smith KA, Alt JA.. The relationship of chronic rhinosinusitis and depression. Current Opinion in Otolaryngology & Head & Neck Surgery 2020:28(1):1–5. doi: https://doi.org/ 10.1097/MOO.0000000000000595 [DOI] [PubMed] [Google Scholar]
- Soler ZM, Eckert MA, Storck K, Schlosser RJ.. Cognitive function in chronic rhinosinusitis: a controlled clinical study. Int Forum Allergy Rhinol 2015:5(11):1010–1017. doi: https://doi.org/ 10.1002/alr.21581 [DOI] [PubMed] [Google Scholar]
- Sorokowski P, Karwowski M, Misiak M, Marczak MK, Dziekan M, Hummel T, Sorokowska A.. Sex differences in human olfaction: a meta-analysis. Front Psychol. 2019:10:242. doi: https://doi.org/ 10.3389/fpsyg.2019.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussignan R, Schaal B.. Children’s facial responsiveness to odors: influences of hedonic valence of odor, gender, age, and social presence. Dev Psychol. 1996:32(2):367–379. doi: https://doi.org/ 10.1037/0012-1649.32.2.367 [DOI] [Google Scholar]
- Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson L-G, Olofsson JK.. Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc. 2014:20(2):209–217. doi: https://doi.org/ 10.1017/S1355617713001409 [DOI] [PubMed] [Google Scholar]
- Stevenson RJ. An Initial Evaluation of the Functions of Human Olfaction. Chem Senses. 2010:35(1):3–20. doi: https://doi.org/ 10.1093/chemse/bjp083 [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Mahmut M, Sundqvist N.. Age-related changes in odor discrimination. Dev Psychol. 2007:43(1):253–260. doi: https://doi.org/ 10.1037/0012-1649.43.1.253 [DOI] [PubMed] [Google Scholar]
- Temmel AFP, Quint C, Schickinger-Fischer B, Klimek L, Stoller E, Hummel T.. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg 2002a:128(6):635. doi: https://doi.org/ 10.1001/archotol.128.6.635 [DOI] [PubMed] [Google Scholar]
- Temmel AFP, Quint C, Schickinger-Fischer B, Klimek L, Stoller E, Hummel T.. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg 2002b:128(6):635. doi: https://doi.org/ 10.1001/archotol.128.6.635 [DOI] [PubMed] [Google Scholar]
- Thomann PA, Dos Santos V, Toro P, Schönknecht P, Essig M, Schröder J.. Reduced olfactory bulb and tract volume in early Alzheimer’s disease—A MRI study. Neurobiol Aging. 2009:30(5):838–841. doi: https://doi.org/ 10.1016/j.neurobiolaging.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Tian Q, Resnick SM, Studenski SA.. Olfaction is related to motor function in older adults. J Gerontol A Biol Sci Med Sci. 2016:glw222:glw222. doi: https://doi.org/ 10.1093/gerona/glw222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint N, de Roon M, van Campen JPCM, Kremer S, Boesveldt S.. Loss of olfactory function and nutritional status in vital older adults and geriatric patients. Chem Senses. 2015:40(3):197–203. doi: https://doi.org/ 10.1093/chemse/bju113 [DOI] [PubMed] [Google Scholar]
- Trott M, Koblitz A, Pardhan S.. Cognitive behavioural therapy versus other interventions on mental health in people with sensory impairments: a systematic review and meta-analysis. J Psychosom Res. 2025:189:111998. doi: https://doi.org/ 10.1016/j.jpsychores.2024.111998 [DOI] [PubMed] [Google Scholar]
- Ustun B, Reissland N, Covey J, Schaal B, Blissett J.. Flavor sensing in utero and emerging discriminative behaviors in the human fetus. Psychol Sci. 2022:33(10):1651–1663. doi: https://doi.org/ 10.1177/09567976221105460 [DOI] [PubMed] [Google Scholar]
- Van Regemorter V, Hummel T, Rosenzweig F, Mouraux A, Rombaux P, Huart C.. Mechanisms linking olfactory impairment and risk of mortality. Front Neurosci 2020: 14: 140. doi: https://doi.org/ 10.3389/fnins.2020.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Del Bene VA, Kamath V, Frank JS, Billings R, Cho D-Y, Byun JY, Jacob A, Anderson JN, Visscher K, et al. Does olfactory training improve brain function and cognition? A systematic review. Neuropsychol Rev. 2024:34(1):155–191. doi: https://doi.org/ 10.1007/s11065-022-09573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarello BJ, Jacobson PT, Tervo JP, Gallagher LW, Caruana FF, Gary JB, Saak TM, Gudis DA, Joseph PV, Goldberg TE, et al. BMI increases in individuals with COVID-19-associated olfactory dysfunction. Nutrients 2023:15(21):4538. doi: https://doi.org/ 10.3390/nu15214538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD.. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection on JSTOR. Med Care. 1992:30(6):473–483. https://www.jstor.org/stable/3765916?seq=1#metadata_info_tab_contents [PubMed] [Google Scholar]
- Wegener B-A, Croy I, Hähner A, Hummel T.. Olfactory training with older people. Int J Geriatr Psychiatry. 2018:33(1):212–220. doi: https://doi.org/ 10.1002/gps.4725 [DOI] [PubMed] [Google Scholar]
- Wehling E, Bless JJ, Hirnstein M, Kråkvik B, Vedul-Kjelsås E, Hugdahl K, Kalhovde AM, Larøi F.. Olfactory hallucinations in a population-based sample. Psychiatry Res. 2021:304:114117. doi: https://doi.org/ 10.1016/j.psychres.2021.114117 [DOI] [PubMed] [Google Scholar]
- Whitcroft KL, Altundag A, Balungwe P, Boscolo-Rizzo P, Douglas R, Enecilla MLB, Fjaeldstad AW, Fornazieri MA, Frasnelli J, Gane S, et al. Position paper on olfactory dysfunction:2023. Rhinology. 2023:61(33):1–108. doi: https://doi.org/ 10.4193/Rhin22.483 [DOI] [PubMed] [Google Scholar]
- Whitcroft KL, Ezzat M, Cuevas M, Andrews P, Hummel T.. The effect of intranasal sodium citrate on olfaction in post-infectious loss: results from a prospective, placebo-controlled trial in 49 patients. Clin. Otolaryngol 2017:42(3):557–563. doi: https://doi.org/ 10.1111/coa.12789 [DOI] [PubMed] [Google Scholar]
- Whitcroft KL, Gunder N, Cuevas M, Andrews P, Menzel S, Haehner A, Hummel T.. Intranasal sodium citrate in quantitative and qualitative olfactory dysfunction: results from a prospective, controlled trial of prolonged use in 60 patients. Eur Arch Otorhinolaryngol. 2021:278(8):2891–2897. doi: https://doi.org/ 10.1007/s00405-020-06567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcroft KL, Hummel T.. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngology-- Head & Neck Surgery. 2019:145(9):846. doi: https://doi.org/ 10.1001/jamaoto.2019.1728 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA.. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann N Y Acad Sci. 2009:1170:730–735. doi: https://doi.org/ 10.1111/j.1749-6632.2009.04013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CC, Miranda B, Sathishkumar M, Dehkordi-Vakil F, Yassa MA, Leon M.. Overnight olfactory enrichment using an odorant diffuser improves memory and modifies the uncinate fasciculus in older adults. Front Neurosci. 2023:17:1200448. doi: https://doi.org/ 10.3389/fnins.2023.1200448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzesniewski A, McCauley C, Rozin P.. Odor and affect: individual differences in the impact of odor on liking for places, things and people. Chem Senses. 1999:24(6):713–721. doi: https://doi.org/ 10.1093/chemse/24.6.713 [DOI] [PubMed] [Google Scholar]
- Yamamura K, Kurose M, Okamoto K.. Chemical sensing regulates mastication/swallowing. Curr Pharm Des. 2016:22(15):2279–2284. doi: https://doi.org/ 10.2174/1381612822666160216151150 [DOI] [PubMed] [Google Scholar]
- Yan CH, Jang SS, Lin HFC, Ma Y, Khanwalkar AR, Thai A, Patel ZM.. Use of platelet-rich plasma for COVID-19-related olfactory loss: a randomized controlled trial. Int. Forum Allergy Rhinol 2023:13(6):989–997. doi: https://doi.org/ 10.1002/ALR.23116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T-F, Slotnick BM.. Roles of olfactory system dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2014:54:26–30. doi: https://doi.org/ 10.1016/j.pnpbp.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Zoon HFA, He W, de Wijk RA, de Graaf C, Boesveldt S.. Food preference and intake in response to ambient odours in overweight and normal-weight females. Physiol Behav 2014:133:190–196. doi: https://doi.org/ 10.1016/j.physbeh.2014.05.026 [DOI] [PubMed] [Google Scholar]
- Zou L, Hummel T, Otte MS, Bitter T, Besser G, Mueller CA, Welge-Lüssen A, Bulut OC, Göktas O, Negoias S, et al. Association between olfactory function and quality of life in patients with olfactory disorders: a multicenter study in over 760 participants. Rhinol J. 2021:0(0):0–0. doi: https://doi.org/ 10.4193/rhin20.403 [DOI] [PubMed] [Google Scholar]
- Zou L, Yang Z, Wang Y, Lui SSY, Chen A, Cheung EFC, Chan RCK.. What does the nose know? Olfactory function predicts social network size in human. Sci Rep. 2016:6(1):25026. doi: https://doi.org/ 10.1038/srep25026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.