Graphical abstract
Keywords: Polysaccharide iron, Multiomics, Spermatogenesis, Steroid hormone
Highlights
-
•
LRPF enhances spermatogenesis through regulates the homeostasis of intestinal flora, and synergistically improves serum steroid and fatty acid-related metabolites.
-
•
LRPF increases the expression of PPARγ protein to promote steroid hormone synthesis.
-
•
Iron deficiency mainly increases Ethyl chrysanthemumate content and inhibits PPARγ protein expression in the testis, thereby inhibiting STAR and CYP17A1 protein expression.
Abstract
Iron deficiency is a common nutritional issue that seriously affects male reproductive health. Lotus root polysaccharide iron (LRPF), a novel nutritional supplement, may ameliorate the damage caused by iron deficiency, however, the mechanism is unclear. In this study, we comprehensively determined the benefits of LRPF on reproduction in iron-deficient mice by integrating transcriptomics, microbiomics and serum metabolomics. Microbiomics showed that LRPF could restore changes to the intestinal microbiota caused by iron deficiency. Metabolomics results showed that LRPF stabilised steroid hormone and fatty acid metabolism in iron-deficient mice, reduced the content of ethyl chrysanthemumate (EC) and ameliorated the reproductive impairment. The transcriptomic analysis showed that LRPF regulated steroid hormone synthesis and the peroxisome proliferator-activated receptor (PPAR) signalling pathway in iron-deficient mice. In vitro experiments showed that LRPF could promote steroid hormone synthesis in Leydig cells by activating PPARγ. In conclusion, this study highlights the advantage of LRPF to improve testicular development.
Introduction
Iron deficiency exerts negative impacts on different systems in the body, including the reproductive, immune, and nervous systems [1], [2], [3], [4]. Iron is a key element in the synthesis of haemoglobin and other important biomolecules. In terms of reproductive system, it is essential for maintaining normal reproductive system function. Iron deficiency can adversely affect spermatogenesis, resulting in decreased sperm count and quality, thus affecting fertility [5], [6]. Lotus root polysaccharide iron (LRPF), a new type of iron supplement, has several unique advantages: it improves iron absorption, reduces gastrointestinal reactions, helps regulate the intestinal microbial balance and improves blood metabolite levels, all of which make it an effective treatment for iron deficiency [7]. LRPF can replenish sufficient iron, which can effectively combat iron deficiency and ensure the smooth progress of sperm production [8]. Moreover, the other active ingredients in LRPF can improve the overall health of the ingestor [9], [10], [11].
The intricate connections among intratesticular steroid hormones underscore the critical role of hormonal regulation in maintaining reproductive function [12]. Testosterone, the primary male sex hormone, exerts a significant influence at every stage of spermatogenesis, from the proliferation of spermatogonia to the maturation of spermatozoa [13]. Disruptions in steroid hormone synthesis, stemming from conditions such as hypogonadism or testicular disorders, can lead to significant impairments in spermatogenesis and fertility [14], [15]. Interestingly, the previous studies observed that the intestinal microbiota ferments dietary fiber to produce short-chain fatty acids (SCFAs), which can affect gut and systemic hormone levels, including steroid hormones [16], [17]. Disturbances in fatty acid metabolism in men with infertility may be associated with dysregulated expression of genes that encode rate-limiting enzymes and genes involved in fatty acid metabolism in testicular tissue [18], [19].
Peroxisome proliferator-activated receptor (PPAR) acts as an important transcription factor and regulates the expression of genes associated with fatty acid metabolism [20]. Notably, lipid homeostasis is crucial for the functioning of endocrine reproductive organs, with PPARγ playing a pivotal role in regulating the expression of genes involved in fatty acid metabolism in the testes of men with impaired spermatogenesis [21]. Cholesterol is an important component of testosterone biosynthesis and plays a vital role in maintaining and regulating male fertility [22].
Multi-omics analysis can synthesize data from different omics levels to provide a more comprehensive and systematic perspective on testicular development and spermatogenesis. Multiomics studies are becoming an important tool for understanding testicular development and spermatogenesis. For example, In their 2022 study, Zhang et al. highlighted the important role of vitamin A metabolism in the gut-testicular axis by analyzing spermatogenesis disorders in a model of metabolic syndrome [23]. In their 2021 study, Zhang et al. demonstrated the positive effects of alginate oligosaccharides on sperm quality and spermatogenesis through fecal microbiota transplantation [24]. These studies show that multi-omics studies not only help to identify the key factors influencing sperm quality, but also open up the possibility of developing new therapeutic strategies that may improve spermatogenesis by modulating the gut microbiota and metabolic pathways.
In this study, the multi-omics integrated analysis was used to evaluate the potential benefits of LRPF to counteract the effects of iron deficiency on male mice reproductive health. In this paper, we elucidated the relationship between LRPF in improving iron deficiency-induced intestinal microbiota imbalance, metabolic disorders and abnormal spermatogenesis, and explored the mechanism of PPARγ regulating steroid hormone synthesis in Leydig cells.
Materials and methods
Ethics statement
The study was approved by the Animal Protection and Ethics Committee of Qingdao Agricultural University (No. 2021-021) and the animal experiments were conducted in accordance with the Guidelines for the Management and Use of Laboratory Animals of the National Institutes of Health of China.
Animals and treatment
Male ICR mice were housed in the vivarium of the School of Veterinary Medicine, Qingdao Agricultural University. The room was well ventilated with a controlled temperature (24 ± 2°C) and relative humidity (60 %–80 %) and a 12 h photoperiod. Water and food were provided ad libitum.
For the iron deficiency and supplementation experiments, 3-week-old male ICR mice were acclimatised for 7 days and then divided into the control (CON) group (n = 6), the iron deficiency (FeD) group (n = 9) and the LRPF group (n = 10). The CON group was fed a normal diet. For the other two groups, an iron deficiency model was developed by feeding the mice a low-iron diet based on the AIN93G standard with an iron content of 12 ppm for 4 weeks. The FeD group continued to be fed with this diet for 4 weeks, while the LRPF group was fed with this diet as well as 20 mg/kg LRPF for 4 weeks. We have previously shown that this approach generates an iron-deficiency anaemia mouse model, and LRPF supplementation significantly improves various blood parameters in these mice [5], [7].
A separate group of 7-week ICR male mice were acclimatised for 7 days and then divided into three groups: the control (CON) group (n = 7), the ethyl chrysanthemumate (EC) group (n = 6) and the N-phenylacetylglutamine (N-PAGln) group (n = 6). The EC group received 200 mg/kg EC (Aladdin, E103553, Shanghai, China) via oral gavage for 4 weeks. The N-PAGln group received 100 mg/kg N-PAGln (Leyan, 1067675, Shanghai, China) via oral gavage for 4 weeks. After the last treatment, the mice were fasted for 12 h, the blood was collected to centrifuge the serum, the mice were euthanized to collect the testes, the contents of the small intestine were scraped with sterile instruments, and all samples were stored in liquid nitrogen for the first time.
Cultivation and treatment of mouse a Leydig cell line
We use in vitro to understand molecular mechanisms and pathological processes using mouse Leydig cell line (TM3), which are responsible for secreting multiple growth factors, hormones, and other substances to regulate the development and maturation of germ cells within the seminiferous tubules [25]. TM3 was cultured with Dulbecco’s Modified Eagle’s Medium (DMEM) (Pricella, PM150210, Wuhan, China) supplemented with 10 % foetal bovine serum (FBS (Procell, 164210, Wuhan, China) and 1 % penicillin–streptomycin (Gibco, 15140163, NY, USA) at 37 °C in a cell incubator with 5 % CO2. To explore the effect of EC on steroid hormone synthesis in vitro, 10 μM EC was used to treat TM3 cells for 24 h. To explore the role of FABP5 and PPARγ in steroid hormone synthesis, TM3 cells were treated with 50 μM FABP5 inhibitor SBFI-26 (GLPBIO, GC39236, Shanghai, China) and 30 μM PPARγ antagonists GW9662 (MedChemExpress, HY-16578, Shanghai, China) for 24 h. To further explore the effect of LRPF on TM3 cells in an iron-deficient environment, we cultured TM3 in serum-free DMEM medium with the addition of LRPF. In addition, 10 μM PPARγ agonist GW1929 (MedChemExpress, HY-15655, Shanghai, China) is added under serum-free culture conditions. To further prove that LRPF promotes steroid hormone synthesis through the PPARγ, after adding LRPF to TM3 cells cultured in serum-free medium, 30 μM GW9662 were added respectively to treat the cells for 24 h.
MTT assay
Using a MTT detection kit (Solarbio, M1020), according to the instructions, 10 μg/mL, 50 μg/mL and 100 μg/mL, 250 μg/mL, 500 μg/mL and 1000 μg/mL concentrations of LRPF were prepared in the control group. After incubation for 24 h, MTT was incubated for 4 h and then formazan solution was added. After shaking and mixing, the absorbance of each well was measured at 490 nm using an ultrasensitive multifunction microplate detector.
Evaluation of sperm parameters
The step was consistent with previous studies [5]. The tail of the epididymis was removed from and placed in a 3-cm Petri dish with 200 μL of preservation solution. The Petri dish was placed on a heating table set at 37 ℃, and the epididymis was cut with a scalpel. The sample was incubated for 5 min to completely release sperm from the caudal epididymis. A pipette gun was used to transfer 20 μL of sperm storage solution to 200 μL of sperm storage solution. A pipette gun was used to transfer 4 μL of diluted sperm storage solution onto a sperm counting plate, and the sperm parameters were evaluated under a light microscope with the help of a computer-assisted sperm assay (CASA) system.
Western blotting
The step was consistent with previous studies [5]. Total protein was obtained from TM3 cells (n ≥ 3 per group) or mouse testes (n ≥ 3 per group) using radioimmunoprecipitation assay (RIPA) lysis buffer. Some of the protein was subjected to high-temperature (100 °C) denaturation and then, proteins were resolved using sodium dodecyl sulphate (SDS)–polyacrylamide gel electrophoresis on a 10 % gel. After separation, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes and incubated with primary antibodies (Table S1). Finally, the proteins were visualised using the Sparkjade ECL Super Kit and then photographed. ImageJ software (version 1.53e; National Institutes of Health, Bethesda, MD, USA) was used to quantify protein expression levels.
Haematoxylin and eosin (HE) and immunofluorescence staining
Testes and epididymides were fixed for 12 h in 4 % paraformaldehyde, rinsed in running water, dehydrated, embedded in paraffin and cut into 5-μm sections. Then, the sections were deparaffinised, rehydrated, blocked and incubated with antibodies or stained with HE [26]. Finally, fluorescence signals were detected using a fluorescence microscope (Olympus, Tokyo, Japan) and quantified using ImageJ software (version 1.53e).
GeneCards target prediction and RNA sequencing of testes
In the Gene Cards database, the keywords “oligospermia” and “iron deficiency” were entered to screen and identify the target genes. RNA sequencing (RNA-seq) further revealed the mechanism of action of LRPF on testicular genes in iron-deficient mice. mRNA-seq was performed with an Illumina Hiseq 4000 platform and PE150 model by Beijing Novogene Technology Co., Ltd. (Beijing, China). Low-quality reads were removed by using Fastp [27]. High-quality (clean) reads were compared with the mouse reference genome using STAR; FeatureCounts was used to calculate the gene expression levels [28], [29]. Differentially expressed genes (DEGs) had a p-value < 0.05. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of DEGs were performed using clusterProfiler in R (version 4.2.2) [30]. Protein–protein interaction (PPI) networks of DEGs were generated in the STRING database and visualised using the Cytoscape software (version 3.9.1) [31]. KEGG analysis was performed by analysing combinations of consensus DEGs by Cytoscape software and ClueGO plug-in [32].
16S ribosomal RNA (rRNA) sequencing of the intestinal microbiota
The small intestinal microbiota was analysed with 16 rRNA sequencing. Briefly, the small intestinal contents of mice were obtained using sterilised instruments at a clean bench and frozen in liquid nitrogen [26]. The total DNA was obtained using the EZNAR Stool DNA Kit (Omega Bio-tek Inc., Norcross, GA, USA). Libraries were constructed from the V3–V4 region and then sequenced. The raw data were spliced and filtered to obtain clean data, which was imported into qiime2 [33]. Amplicon sequence variants (ASVs) were obtained by filtering sequences with an abundance of < 5 using DADA2 [34]. The abundance and alpha and beta diversity of the ASVs were determined to assess species richness [33]. The community structure differences among the groups were assessed with random forest, linear discriminant analysis effect size (LEfSe) and ternaryplot were used to evaluate significant differences in species composition and community structure. The PICRUSt2 software was used for functional prediction analysis of microbial communities in ecological samples [35]. The R software was used to visualise the results.
Untargeted serum metabolomics
Serum samples from mice were collected, frozen in liquid nitrogen, and sent to Novogene (Beijing, China) for testing. Serum metabolites were detected using liquid chromatography-mass spectrometer (LC/MS), specifically ACQUITY UPLC and AB Sciex Triple TOF 5600 equipment. Univariate analysis of variables and variable importance of projection (VIP) analysis were used to identify differential metabolites. These metabolites were annotated using the KEGG database to understand their functional characteristics and classification. MetOrigin traceability analysis of metabolite sources were also performed (https://metorigin.met-bioinformatics.cn).
Detection of reproduction-related biochemical indexes by enzyme linked immunosorbent assays (ELISAs)
Blood was collected from mice and used to isolate serum. The serum levels of reproductive factors were determined using ELISA kits (Solarbio, Shanghai, China). The serum testosterone (JM-02852 M2, JingMei, Jiangsu, China), luteinising hormone (LH) (JM-11607 M2, JingMei) and follicle-stimulating hormone (FSH) (JM-11681 M2, JingMei). In summary, the standard was added to the microplate with serum, reacted at 37 °C for 90 min, added biotin antibody working solution after washing, reacted at 37 °C for 60 min, washed again and added chromogenic working solution, and reacted at 37 °C protected from light. The microplate reader was used to detect the OD value at 450 nm.
Statistical analysis
The data are presented as the mean ± standard error of the mean (SEM) of at least three biological replicates for each experiment [5]. Student’s t-test was used to determine significant differences (GraphPad Prism 8.0, GraphPad Software, SanDiego, CA, USA). Sperm concentration, western blotting, immunofluorescence, and metabolites were used Student’s t-test. A p-value < 0.05 was considered significant, and p-value < 0.01 was considered very significant. Correlation analysis was done using the R package corrplot, which was visualized in R.
Results
Screening gene targets of oligospermia caused by iron deficiency
To evaluate the effect of LRPF on testicular development in iron-deficient mice, we established an iron-deficient mouse model and a rescue model supplemented with LRPF, and performed multi-omics analyses on them, including whole transcriptomics, microbiomes, and metabolomics (Fig. 1A). With the GeneCards database, we identified 349 genes that are highly associated with iron deficiency leading to oligospermia (Fig. 1B). Enrichment analysis using the GOGeNET database revealed that these target genes are associated with male infertility and oligospermia (Fig. 1C). Pathway and process enrichment analysis revealed that these genes are also associated with response to hormones, response to steroid hormones, reproductive structure development, gamete generation and regulation of the mitotic cell cycle, suggesting that iron deficiency may induce the development of oligospermia by interfering with steroid synthesis (Fig. 1D). In the PPI network, we have identified several key proteins involved in hormone synthesis (Fig. 1E).
Fig. 1.
Screening of targets for oligospermia caused by iron deficiency. (A) Schematic representation of the experiment to investigate the effect of LRPF on mouse reproduction following iron deficiency. (B) The Venn diagram shows the iron deficiency and oligospermia target genes from GeneCards. (C) Enrichment analysis based on the GOGeNET database. (D) Enrichment analysis of common target genes based on the online tools in Metascape. (E) The PPI networks for common target genes.
LRPF effectively mitigates male reproductive dysfunction caused by iron deficiency
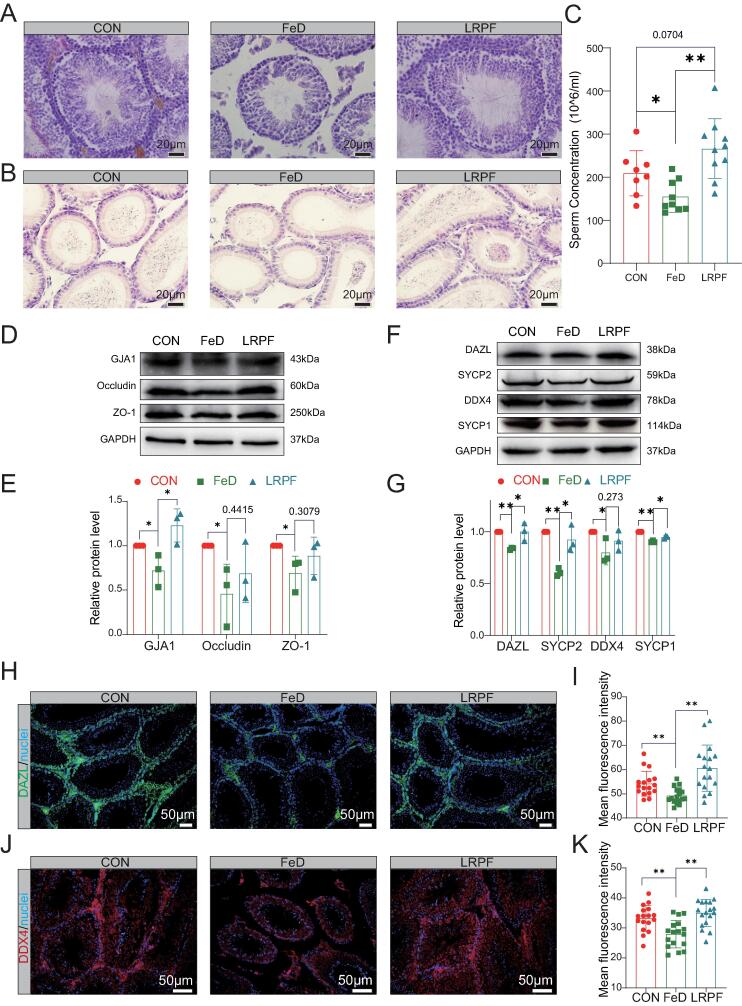
In the FeD group, the Leydig cells exhibited a looser structure, the seminiferous tubule walls became increasingly thin and the number of germ cells in the lumen was noticeably reduced. However, following LRPF supplementation, the testicular structure appeared more compact, the interstitium appeared fuller, the seminiferous tubule walls appeared to recover, the number of germ cells increased (Fig. 2A). HE results showed that LRPF increased the number of spermatozoa in the lumen of the epididymis in iron-deficient mice (Fig. 2B). After LRPF supplementation, the sperm concentration increased significantly compared with the FeD group (P < 0.01), and was not significant difference compared with the CON group (Fig. 2C). Tight junction proteins are a crucial component of the blood–testis barrier. The FeD group showed a significant reduction in the expression of Gap junction alpha-1 protein (GJA1), Occludin, and Zonula occludens-1 (ZO-1) in the testes. However, LRPF supplementation effectively counteracted this change, suggesting it has a potential role in maintaining the integrity of the blood–testis barrier (Fig. 2D, E). Meiosis is a pivotal stage in spermatogenesis and plays a fundamental role in ensuring the stability and transmission of genetic material, species diversity and reproductive health. WB results showed decreased expression of Deleted In Azoospermia Like (DAZL), DEAD-box helicase 4 (DDX4), Synaptonemal Complex Protein 1 (SYCP1) and Synaptonemal Complex Protein 2 (SYCP2) proteins in FeD testes. However, LRPF increases the expression of these proteins, suggesting that it has the potential to enhance meiosis (Fig. 2F, G). Immunohistochemistry further confirmed that LRPF could increase the expression of DAZL and DDX4 proteins in testes of iron-deficient mice (Fig. 2H-K).
Fig. 2.
LRPF promotes testis development and spermatogenesis in iron-deficient mice. (A) Testicular H&E staining: CON for control group, FeD for iron deficiency exposure treatment group, LRPF represents the LRPF perfusion group. Bar = 20 μm. (B) Epididymis H&E staining, Bar = 20 μm. (C) The histogram shows the change in sperm concentration (n ≥ 3). The data are expressed as the mean of ± SEM. * p < 0.05 and ** p < 0.01. (D) The characteristic bands of the testicular structure–related proteins GJA1, occludin and ZO-1. (E) The statistics data for protein expression level. The data are expressed as the mean of ± SEM (n = 3). * p < 0.05 and ** p < 0.01. (F) The characteristic bands of the meiosis-related proteins DAZL, SYCP2, DDX4 and SYCP1. (G) The statistics data for protein expression level. The data are expressed as the mean of ± SEM (n = 3). * p < 0.05 and ** p < 0.01. (H) Immunostaining of DAZL in the testis. (I) The fluorescence density statistics of DAZL relative expression. The data are expressed as the mean of ± SEM (n ≥ 3). ** p < 0.01.(J) Immunostaining of DDX4 in the testis. (K) The fluorescence density statistics of DDX4 relative expression. The data are expressed as the mean of ± SEM (n ≥ 3). ** p < 0.01.
LRPF effectively mitigates intestinal microbial imbalance caused by iron deficiency
We analysed the relative abundance of the intestinal microbiota at various taxonomic levels for the three groups to identify specific changes in the intestinal microbiota composition. The intestinal microbiota was dominated by Firmicutes, Proteobacteria and Cyanobacteria at the phylum level. Proteobacteria and Actinobacteriota had higher relative abundances, while Proteobacteria, Bacteroidota and Cyanobacteria had lower relative abundances in the FeD group compared with CON group. Compared with the FeD group, LRPF significantly reversed the changes in Firmicutes, Proteobacteria, Cyanobacteria, Actinobacteriota and Bacteroidota. The Firmicutes/Bacteroidota ratio decreased in the LRPF group compared with the FeD group (Fig. 3A). At the genus level, LRPF significantly reversed the changes in Pseudomonas, Lactobacillus, Staphylococcus, Enterococcus and Chloroplast in the FeD group (Fig. 3B). Venn diagrams of the species abundance tables showed that LRPF caused significant changes in the intestinal microbiota of the FeD group (Fig. 3C).
Fig. 3.
LRPF effectively mitigates the intestinal microbial imbalance caused by iron deficiency. (A) Stacked histograms show the differences at the phylum level. (B) Stacked histograms show the differences at the genus level. (C) A Venn diagram of the ASVs for each group. (D) Cloud and rain maps of the Chao1 and Shannon indices for α diversity for each group. (E) NMDS analysis shows differences in the CON, FeD and LRPF intestinal microbiota. (F) The ternary phase diagram shows the differences in the dominant species among the groups. (G) LEfSe analysis shows significant differences in biomarkers of the intestinal microbiota among the groups. (H) Random forest analysis shows the classification of horizontal intestinal flora of each genus. (I) The heatmap shows the Spearman correlation analysis between the top 10 enriched microbes (based on horizontal richness) and the sperm concentration. Green indicates a positive correlation and purple indicates a negative correlation.
Alpha diversity describes the abundance and diversity of microbial communities; the Chao and Shannon indices reflect the abundance and diversity of microbiota, respectively. LRPF showed a certain improvement trend on the diversity of intestinal microbes in the state of iron deficiency, which showed that the Shannon index increased, but the chao1 index did not change significantly (Fig. 3D). Non-metric multidimensional scaling (NMDS) showed that the stress value was 0.12, and the results were reliable (Fig. 3E). The ternary plot showed differences in dominant species among the three groups at different taxonomic levels. Staphylococcus was the dominant genus in the FeD group and Enterococcus was the dominant genus in the LRPF group (Fig. 3F).
We performed LEfSe to identify biomarkers with significant differences among the groups. The cladogram showed the taxonomic hierarchical distribution of marker species in each group, and the histograms of the linear discriminant analysis (LDA) value distribution of significantly different species showed significantly enriched species within each group and their degree of importance (Fig. 3G). We used the random forest algorithm subordinate level to classify the intestinal microbial community efficiently (Fig. 3H). Based on these two algorithms, we found Pseudomonas, Lactobacillus, Enterococcus and Achromobacter as the peugeot organism (Fig. S1A). We performed Pearson correlation analysis to find linear correlations between taxa (p < 0.05 and r > 0.6) to construct a correlation network for genus-level microbes across the three groups (Fig. S1B). We performed Spearman correlation analysis between the top 10 enriched genera and the sperm concentration and found that polymicrobial changes correlated with the sperm concentration, suggesting an interconnection between the intestinal microbiota and the sperm concentration (Fig. 3I). Iron deficiency reduces the metabolic function of intestinal microorganisms in mice, such as steroid biosynthesis. After LRPF supplementation, the function of intestinal microorganisms is restored (Fig. S2A, B).
LRPF improves serum metabolic alterations caused by iron deficiency
We performed principal component analysis for each group and then used orthogonal partial least squares discriminant analysis (OPLS-DA) to predict and validate the sample classes. OPLS-DA analysis of all metabolites showed that the overall serum distribution trend of mice in the LRPF and FeD groups was significantly separated (Fig. 4A). We conducted a KEGG enrichment analysis of differential metabolites between the FeD and LRPF groups and observed significant changes in steroid hormone biosynthesis, fatty acid degradation, ovarian steroidogenesis and steroid biosynthesis (Fig. 4B). Through cluster heat map analysis, we observed that LRPF effectively alleviated steroid and fatty acid metabolism disorders caused by iron deficiency (Fig. S3A, B). The microbiome and its metabolites are closely related to human health and disease, so we analysed the differential metabolites with MetOrigin to identify which bacteria participate in certain metabolic reactions. We first characterised the origin of metabolites (Fig. S3D). At the family level, we identified 177 microorganisms from the 16S rRNA sequencing and co-metabolism analysis that may play a role in the metabolic processes of steroids and fatty acids (Fig. S3E). We performed Spearman correlation analysis of microorganisms and the top 10 enriched metabolites and found that Pseudomonadaceae was negatively correlated with PC and LPC, and positively correlated with artemisinin, lysoPS and cholecalciferol (Fig. S3F).
Fig. 4.
LRPF counteracts the serum metabolic alterations caused by iron deficiency. (A) PLS-DA analysis shows differences in serum metabolites between the CON and FeD groups, and between the FeD and LRPF groups. (B) A matrix dot map of KEGG pathway enrichment of differential metabolites. (C) A Venn diagram of serum metabolites shows differences between the CON and FeD groups, and between the FeD and LRPF groups. (D) A heatmap of the differential metabolites for the CON, FeD and LRPF groups. (E) Spearman correlation between serum metabolites and the sperm concentration. Purple indicates a positive correlation and green indicates a negative correlation. * p < 0.05, ** p < 0.01 and *** p < 0.001. (F) Spearman linear correlation analysis of Ethyl chrysanthemumate and sperm concentration in serum. (G) Histogram of Ethyl chrysanthemumate content in serum of each group. * p < 0.05 and ** p < 0.01. (H) Spearman linear correlation analysis of N-Phenylacetylglutamine and sperm concentration in serum. (I) Histogram of N-Phenylacetylglutamine content in serum of each group. * p < 0.05. (J) Testicular H&E staining: CON for control group, EC for Ethyl chrysanthemumate exposure treatment group, N-PAGln for N-Phenylacetylglutamine exposure treatment group. Bar = 20 μm. (K) The histogram shows the change in sperm concentration (n ≥ 3). The data are expressed as the mean of ± SEM. * p < 0.05. (L) Immunostaining of DAZL in the testis. (M) The fluorescence density statistics of DAZL relative expression. The data are expressed as the mean of ± SEM (n ≥ 3). * p < 0.05 and ** p < 0.01.
There was a total of 20 differential metabolites for the FeD and LRPF groups, and cluster expression heatmaps show their expression (Fig. 4C, D). Spearman correlation analysis of the differential metabolites and the sperm concentration revealed a significant positive correlation between the sperm concentration and N-PAGln, as well as a significant negative correlation between the sperm concentration and EC and cis-2-decenoic acid (Fig. 4E). We used linear correlation analysis to explore the relationship between each group and the sperm concentration, as well as the EC and N-PAGln levels. There was a negative correlation between EC and the sperm concentration, with increased levels in the FeD group and a significant decrease in the LRPF group (Fig. 4F, G). Conversely, N-PAGln showed a positive correlation with the sperm concentration, decreasing in the FeD group but increasing in the LRPF group. These findings suggest that LRPF may regulate the levels of both metabolites, potentially benefiting male reproduction (Fig. 4H, I). After intragastric administration of the above substances in mice, we observed that the EC significantly reduced sperm concentration (p < 0.05), while N-PAGln had no significant effect on sperm concentration (Fig. 4K). Histological analysis revealed that EC had severe negative effects on the internal structure of the testis (Fig. 4J). After administration of EC, DAZL and DDX4 protein expression in the testis decreased, suggesting that EC may interfere with testicular meiosis and negatively affect male reproduction. After administration of N-PAGln, DAZL protein expression in the testis increased, but DDX4 expression did not change significantly (Fig. S3C). Testicular immunofluorescence revealed that EC decreased DAZL protein expression and N-PAGln increased DAZL protein expression (Fig. 4L, M). These findings suggests that LRPF can ameliorate male reproductive damage caused by iron deficiency by regulating metabolism.
Transcriptomic effects of LRPF on testicular development in mice with iron deficiency
The FeD group showed a total of 564 DEGs, including 314 upregulated genes and 250 downregulated genes (Fig. 5A). The heatmap in Fig. 5B shows gene expression patterns and clustering. GO analysis for biological processes of DEGs in testes of the FeD group revealed enrichment of metabolic process, steroid cellular process, metabolic hormone process, steroid hormone metabolic process, steroid hormone biosynthetic process and hormone process (Fig. 5C). KEGG enrichment analysis also revealed an association with ovarian steroidogenesis and steroid biosynthesis (Fig. 5D). The above enrichment results were similar to the common target genes we found by searching GeneCards with the keywords ‘iron deficiency’ and ‘oligospermia’, suggesting that our mouse model of iron deficiency–induced oligospermia is feasible. The LRPF group showed 1672 upregulated genes and 2352 downregulated genes in testis (Fig. 5E). The heatmap in Fig. 5F shows the expression pattern and clustering of DEGs after LRPF supplementation. GO enrichment analysis for biological processes of the DEGs for the LRPF group revealed an association with steroid metabolic process. These results suggest that LRPF may ameliorate reproductive damage caused by iron deficiency by regulating steroid metabolic metabolism (Fig. 5G). The results of the KEGG enrichment analysis indicate that the differential genes are significantly enriched in fatty acid metabolism, suggesting that fatty acid metabolism may play an important role in the biological process of interest in this study (Fig. 5H).
Fig. 5.
Transcriptome effects of LRPF on testicular development in mice with iron deficiency. (A) The volcano plot shows the changes in gene expression in the testis after iron deficiency. (B) The cluster heatmap shows the distribution of DEGs in the testis after iron deficiency. (C) GO enrichment for biological processes of DEGs. (D) KEGG pathway enrichment analysis of DEGs. (E) The volcano plot shows the changes in gene expression in the testes of the FeD and LRPF groups. (F) The cluster heatmap shows the distribution of DEGS in the testes of the FeD and LRPF groups. (G) GO enrichment for biological processes of DEGs. (H) KEGG pathway enrichment of DEGs.
LRPF promotes steroid hormone expression in testes of iron-deficient mice
CompareCluster analysis revealed that steroid biosynthetic process, steroid metabolic process and steroid biosynthesis were enriched in the FeD and LRPF groups (Fig. 6A, B). We used ClueGO to construct a network of related genes in relation to their corresponding pathways (Fig. 6C). We generated a Venn diagram to identify the key target genes of LRPF in the treatment of iron deficiency and oligospermia. This approach yielded seven consensus-related genes. A PPI network validated Cytochrome P450 family 11 subfamily A member 1 (CYP11A1) and Cytochrome P450 family 17 subfamily A member 1(CYP17A1) as essential (Fig. 6D). We examined the expression of steroid anabolism–related proteins in the testis and found that LRPF significantly restored the Steroidogenic acute regulatory protein (StAR), hydroxysteroid 17-beta dehydrogenase 6 (HSD178B6), CYP11A1, CYP17A1 and Luteinizing Hormone/Choriogonadotropin Receptor (LHCGR) protein levels that were reduced by iron deficiency (Fig. 6E, F). Immunohistochemistry further demonstrated that LRPF promoted the expression of StAR and CYP17A1 proteins in the testes of iron-deficient mice (Fig. 6G, H). LRPF could significantly increase the serum testosterone, LH and FSH levels (Fig. 6I). In addition, StAR and CYP17A1 protein expression was significantly decreased in the testes of EC-treated mice, but there were no changes in the testes of N-PAGln-treated mice (Fig. 6J, K). Immunohistochemistry further demonstrated that EC decreased CYP17A1 expression in the testes (Fig. 6L, M). To further validate the interference of EC on steroid synthesis, we treated TM3 cells with EC and found decreased expression of StAR, HSD17B6, CYP17A1 and LHCGR (Fig. S4A). To explore and visualise the interactive metabolites and genes between functional correlates, we constructed a gene–metabolite interaction network by using MetaboAnalyst to display the enriched steroid-related metabolites and genes (Fig. S4B).
Fig. 6.
LRPF promotes steroid hormone expression in the testes of mice with iron deficiency. (A) GO enrichment for biological processes of DEGs in the testis of FeD and LRPF mice. (B) KEGG pathway enrichment of DEGs. (C) The pathway enrichment network based on ClueGO. (D) A Venn diagram of the DEGs, the common oligospermia and iron deficiency target genes and the core target genes of the PPI network for the FeD and LRPF groups. (E) A representative western blot and StAR, HSD17B6, CYP11A1, CYP17A1 and LHCGR protein expression levels in the testes of the FeD and LRPF groups. (F) The statistics data for protein expression level. The data are expressed as the mean of ± SEM (n = 3). * p < 0.05 and ** p < 0.01. (G) Immunostaining of StAR and CYP17A1 in the testis. (H) The fluorescence density statistics of StAR and CYP17A1 relative expression. The data are expressed as the mean of ± SEM (n ≥ 3). * p < 0.05 and ** p < 0.01. (I) Serum testosterone (n = 3), LH (n = 3) and FSH (n = 3) in the FeD and LRPF groups. The data are expressed as the mean ± SEM. * p < 0.05 and ** p < 0.01. (J) A representative western blot and StAR, CYP17A1 and PARPγ protein expression levels in the testes of the EC and N-PAGln-treated mice. (K) The statistics data for protein expression level. The data are expressed as the mean of ± SEM (n = 3). * p < 0.05 and ** p < 0.01. (L) Immunostaining of CYP17A1 in the testis. (M) The fluorescence density statistics of CYP17A1 relative expression. The data are expressed as the mean of ± SEM (n ≥ 3). * p < 0.05.
LRPF activates PPARγ in mouse testis to promote steroids synthesis
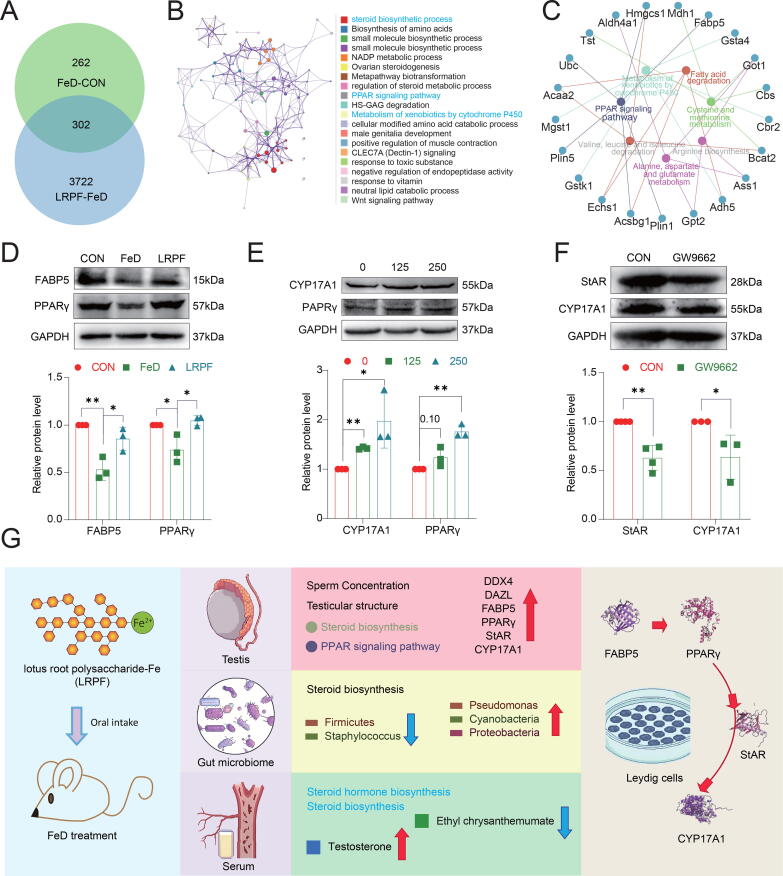
We next investigated the regulatory mechanism by which LRPF ameliorates male reproductive damage caused by iron deficiency. We found a total of 302 DEGs in LRPF testes versus FeD testes (Fig. 7A). Functional enrichment analysis of the DEGs revealed an association with the biosynthetic steroid process, metabolism of xenobiotics by cytochrome P450 and the PPAR signalling pathway (Fig. 7B). We used ClueGO to analyse the relationship between the core genes and the PPAR signalling pathway (Fig. 7C). There was decreased testicular FABP5 and PPARγ expression in the FeD group, which was significantly improved after LRPF supplementation (Fig. 7D). In the following experiments, we further validated the unique mechanism of action of LRPF in TM3 hormone synthesis. Different concentrations of LRPF were added to serum-free cultured TM3 to verify the effect of LRPF on TM3 steroid hormone synthesis in an iron-free environment. MTT results showed that LRPF promoted the proliferation of TM3 cells at the release concentration, and the proliferation was significantly promoted in the concentration range of 100 μg/mL to 500 μg/mL (Fig. S5A). We selected 125 μg/mL and 250 μg/mL concentrations of the optimal LRPF concentration range to promote TM3 cell proliferation to treat TM3 cells for validation of LRPF in vitro. The results of cell immunofluorescence showed that LRPF could significantly promote the expression of StAR and FABP5 proteins in TM3 cells (Fig. S5B). In addition, WB results showed that 250 μg/ml LRPF could promote the expression of CYP17A1 and PPARγ in TM3 cultured in iron deficiency state (Fig. 7E). To further investigate the link between testicular PPAR signalling and steroid synthesis, we treated TM3 cells with 30 μM GW9662, a PPARγ inhibitor, and found that it significantly decreased PPARγ and FABP5 expression (Fig. S4C). In addition, StAR and CYP17A1 protein expression was also decreased in TM3 cells after decreasing PPARγ (Fig. 7F). To simulate the iron deficiency environment in vitro, we added 10 μM GW1929 to TM3 cultured in serum-free DMEM medium to explore the effect of activation of PPARγ on steroid hormone synthesis in TM3 cells under iron deficiency environment. GW1929 can promote the expression of StAR, CYP17A1 and PPARγ proteins in TM3 (Fig. S4D). We treated TM3 cells with 50 μM of the FABP5 inhibitor SBFI-26, and WB results showed reduced StAR CYP17A1 protein expression (Fig. S4E). To further demonstrate the role of PPARγ in LRPF in promoting steroid hormone synthesis in TM3 cells, we added 30 μM GW9662 to 250 μg/mL LRPF-exposed TM3. Cell immunofluorescence results showed that GW9662 could significantly reduce the expression of StAR and FABP5 proteins in LRPF-treated TM3 cells (Fig. S5C). WB results also showed that the expression of CYP17A1 in TM3 under LRPF exposure decreased after GW9662 treatment (Fig. S5D). These results demonstrate that LRPF can promote steroid hormone synthesis in the testis by activating the PPARγ in Leydig cells (Fig. S4F). Notably, in vivo we found that EC significantly decreased PPARγ protein expression in the testes while N-PAGln had no significant effect on PPARγ expression (Fig. 6J, K). An additional in vitro experiment in TM3 cells showed that EC could significantly reduce FABP5 and PPARγ protein expression (Fig. S4A).
Fig. 7.
LRPF promotes the synthesis of TM3 steroid hormone by activating PPARy. (A) The Venn diagram shows the DEGs between the LRPF and FeD groups. (B) There are common functional enrichment networks of DEGs. (C) DEGs associated with the PPAR signalling pathway. (D) A representative western blot and the FABP5 and PPARγ protein expression levels in the testes of the FeD and LRPF groups (n = 3). The data are expressed as the mean ± SEM. * p < 0.05. (E) A representative western blot and the CYP17A1 and PPARγ protein expression levels of TM3 cells treated with LRPF (n ≥ 3). The data are expressed as the mean ± SEM. * p < 0.05 and ** p < 0.01. (F) A representative western blot and the StAR and CYP17A1 protein expression levels of TM3 cells treated with GW9662 (n ≥ 3). The data are expressed as the mean ± SEM. * p < 0.05 and ** p < 0.01. (F).
Discussion
Iron is essential for male reproductive function, but traditional ferric sulfate supplements suffer from poor absorption, many side effects, and low bioavailability. In contrast, LRPF, as a new nutritional supplement, not only prevents iron deficiency anemia, but also significantly improves the impact of iron deficiency by regulating intestinal microbes, showing broad prospects in the field of food and medicine.Iron plays an important role in male reproductive function [5], [7], [36]. In this study, we used a multi-omics approach to comprehensively investigate how LRPF counteracts iron deficiency–mediated male reproductive damage.
Testicular meiosis is of great significance in maintaining the normal function of the reproductive system and maintaining the genetic diversity of species [37]. It mainly occurs in the seminiferous tubules of the testis, and abnormal meiosis can lead to male infertility [38], [39]. We found that LRPF could effectively resolve the abnormal testicular meiosis caused by iron deficiency and then restore normal reproductive function. In mammals, DAZL is mainly expressed in the testis and is involved in some critical steps during spermatogenesis. Moreover, it is crucial for the normal function of Sertoli cells and the normal development of spermatozoa [40]. DDX4, SYCP1 and SYCP2 are involved in the regulation of sperm production, development and function [41].
There is a consensus that hormones participate in regulating meiosis [42], [43]. Iron deficiency not only alters the expression of genes involved in steroid synthesis, but also affects the function of the gut microbiota, which plays a key role in steroid synthesis. Certain metabolites produced by the gut microbiota, including short-chain fatty acids, lactic acid, and other organic acids, have the ability to acidify the intestinal microenvironment, facilitate the conversion of iron to ferrous iron, and promote iron absorption [44]. A previous study reported that the gut microbiota is a major regulator of androgen metabolism in intestinal contents [45]. Li et al. further investigated the relationship between androgens and the gut microbiota, suggesting a possible microbiota-gut-testicular axis [46]. Currently, Hsiao et al. found that androgens in the gut of male mice break down intestinal bacteria to regulate circulating androgen levels [46]. Above findings highlight the importance of the gut microbiota in regulating androgen levels. The presence of the gut microbiota-testicular axis was further confirmed by Hao et al. in a 2022 study, where they found that the negative effects of a high-fat diet on male fertility can be mitigated by fecal microbiota transplantation (FMT), which is achieved by improving the metabolome of the whole body and testes [47]. The mechanisms by which intestinal microbes regulate serum metabolites are complex and diverse and may involve multiple aspects. First, intestinal microbes can produce metabolites by directly metabolising substrates that affect the host’s systemic metabolism after entering the blood circulation [63]. Second, intestinal microbes can affect the permeability of the host’s intestinal mucosal barrier, thereby regulating the absorption and transport of metabolites [64]. In addition, intestinal microbes can indirectly influence the levels of serum metabolites by regulating the host immune system and affecting gene expression [65]. We identified Pseudomonadaceae as involved in steroid hormone synthesis and fatty acid metabolism, further reflecting the effects of iron deficiency and LRPF treatment on the intestinal microbiota composition and function as well as the serum metabolite levels. Future studies can explore the mechanisms underlying the interactions between intestinal microbes and serum metabolites and provide new ideas and methods for the prevention and treatment of metabolic dysfunction caused by iron deficiency. LRPF can regulate intestinal microbial homeostasis, serum metabolite changes and intestinal microbial balance to improve serum steroid hormone metabolism.
LRPF can also counter the testicular steroid hormone synthesis deficiency caused by iron deficiency and increase the expression of StAR, CYP17A1 and other proteins in the testis. Cholesterol is a substrate for the synthesis of steroid hormones such as testosterone, while the StAR protein, which is mainly expressed in the interstitial cells of the testis, is involved in the transport of cholesterol from the cytoplasm to the mitochondrial matrix [48], [49], [50]. CYP17A1 is mainly expressed in Sertoli and interstitial cells, and it is involved in the synthesis of steroid hormones such as testosterone [51]. Tsao et al. showed that FeSO4 supplementation could significantly increase the expression of StAR in the testes of iron-deficient rats, but had no significant effect on CYP17A1[6]. Our in vitro studies have demonstrated that LRPF can promote the expression of StAR and CYP17A1 proteins in TM3 cells in serum-free culture. These results suggest that LRPF can be more effective in improving the reduction of testosterone synthesis caused by iron deficiency. RNA sequencing revealed shared DEGs that were enriched in the PPAR signalling pathway in testes of the FeD and LRPF groups. The PPAR signalling pathway affects the synthesis and metabolism of steroid hormones as well as sperm production and function [52], [53]. We found that LRPF could promote FABP5, PAPRγ protein expression in the testes of iron-deficient mice and in serum-free cultured TM3 cells in vitro. The sperm PPARγ protein levels showed a higher trend in normal animal spermatozotic men than in men with soft spermia, while the PPARγ agonist pioglitazone could protect testicular tissue and sperm in hypothyroid rats [54], [55]. PPARγ is expressed in Leydig cells and is involved in testosterone synthesis and secretion [56]. By activating PPARγ, it can promote esterification of cholesterol and increase testosterone synthesis in interstitial cells. The PPAR signalling pathway is also involved in energy metabolism and metabolite transport during spermatogenesis and maturation [52], [57], [58]. FABP5 is required for PPARγ activation, and FABP5 antagonist SBFI26 has been found to inhibit the expression of PPARγ protein in prostate cancer cells, and we have found that inhibiting the expression of PPARγ in TM3 also inhibits FABP5 expression in reverse [59], [60]. The above conclusions indicate that FABP5 and PPARγ are positively moderated. We found that LRPF could restore the iron deficiency–induced abnormality in the FABP5–PPARγ pathway. Our in vitro experiments showed that after inhibiting the expression of FABP5, PPARγ in TM3 cells, the expression of StAR and CYP17A1, key components of steroid synthesis, decreased, while the expression of activated PAPRγ promoted the synthesis of steroid hormones. Kotula-Balak et al. showed that the expression of PAPRγ in human stromal stromal tumors was lower than that of normal mesenchymal cells, and the expression of StAR was also reduced [61]. Veerasamy et al.'s study of hamster testes and Leydig cells found that Tributyltins simultaneously reduced the expression of StAR, CYP17A1, and PPARγ proteins [62]. Thus, PPARγ positively regulates steroid hormone synthesis in Leydig cells. In vitro serum-free cultureTM3 cells were cultured with LRPF and then 30 μM of GW9662 was added, and we found a decrease in StAR CYP17A1 protein expression. These findings suggest that LRPF may benefit spermatogenesis by activating PPARγ to promote steroid hormone synthesis in the testes of iron-deficient mice.
We discovered some interesting commonalities between the CON, FeD and LRPF groups. By comparing the FeD and CON groups and the LRPF and FeD groups, we found that some common metabolites; these metabolites may play an important role in the processes associated with iron deficiency and iron supplementation. We found that EC was significantly negatively correlated with sperm concentration, suggesting that EC negatively affects male reproduction. The serum EC level increased significantly after iron deficiency. Researchers have shown the negative effects of pyrethroids on sperm count, sperm motility, sperm morphology, testis weight, epididymis weight and serum testosterone in men [66]. We also found a positive correlation between N-PAGln and the sperm concentration, and serum metabolomics showed that LRPF could significantly restore the iron deficiency–mediated reduction in N-PAGln. However, our in vivo experiments revealed that N-PAGln supplementation alone did not significantly promote spermatogenesis. It is possible we did not use an appropriate concentration of N-PAGln, or this metabolite may only significantly benefit spermatogenesis in certain injury contexts. Ethyl pyrethroate is an important pyrethroid. Pyrethroids have endocrine disrupting effects on the hypothalamic-pituitary–gonadal (HPG) axis [67]. We found that EC could significantly reduce the sperm concentration and interfere with testicular meiosis in mice. Steroid hormone synthesis was also disrupted, and StAR and CYP17A1 were decreased in the testes of the EC-treated mice. In line with our findings, a cross-sectional association analysis of 1235 adults aged ≥ 20 years in China by Xu et al. showed that environmental pyrethroid exposure was associated with adult sex hormone changes and negatively correlated with total testosterone [68]. We further demonstrated the negative effects of EC on steroid hormone synthesis with our in vitro experiments. Specifically, treating TM3 cells with 10 µM EC for 24 h reduced the expression of key proteins involved in steroid synthesis, including StAR, CYP17A1 and LHCGR. We also found that EC inhibited the PPARγ in vitro and in vivo. Thus, EC interfered with male steroid hormone synthesis by inhibiting the PPARγ. However, we confirmed that LRPF could significantly ameliorate the increased EC serum level caused by iron deficiency. So, LRPF can restore the ability of testosterone hormone synthesis by regulating the EC level in the body.
Conclusion
This study revealed multiple mechanisms of action of LRPF in addressing iron deficiency-induced reproductive dysfunction in mice (Fig. 7G). Specifically, LRPF significantly increased sperm concentration, improved testicular structure, restored the integrity of the seminiferous tubule wall, and increased the number of sperm within the epididymis. At the same time, LRPF effectively maintained the integrity of the blood-testicular barrier by increasing the expression of tight junction proteins GJA1, Occludin and ZO-1 in the testis. During meiosis, a critical stage of spermatogenesis, LRPF restores the expression of key proteins such as DAZL, DDX4, SYCP1, and SYCP2, thereby promoting spermatogenesis. In addition, LRPF significantly ameliorates the gut microbial imbalance caused by iron deficiency and restores gut health by regulating the composition and diversity of gut microbes. Most critically, LRPF promotes the synthesis of steroid hormones in the testes by activating PPARγ in mouse Leydig cells, and restores the expression of steroid synthesis-related proteins such as StAR and CYP17A1 that are reduced due to iron deficiency. This mechanism of action further enhances the potential benefits of LRPF for male reproductive health by modulating the levels of specific metabolites EC. These findings together constitute a comprehensive mechanism of LRPF in improving the effect of iron deficiency on reproductive function in mice, and provide a solid experimental basis for the application of LRPF in the field of male reproductive health.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by Horizontal Topic Research “Screening, Evaluation and Functional Product Development of Natural Active Ingredients” (20240102), High level talents research fund project of Qingdao Agricultural University in China to Xi-Feng Zhang (1120043), Science & Technology Fund Planning Projects of Qingdao City in China (24-1-8-xdny-5-nsh).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2024.09.022.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Muto Y., Nishiyama M., Nita A., Moroishi T., Nakayama K.I. Essential role of FBXL5-mediated cellular iron homeostasis in maintenance of hematopoietic stem cells. Nat Commun. 2017;8:16114. doi: 10.1038/ncomms16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassan T.H., Badr M.A., Karam N.A., Zkaria M., El S.H., Abdel R.D., et al. Impact of iron deficiency anemia on the function of the immune system in children. Medicine. 2016;95:e5395. doi: 10.1097/MD.0000000000005395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokusoglu M., Nevruz O., Baysan O., Uzun M., Demirkol S., Avcu F., et al. The altered autonomic nervous system activity in iron deficiency anemia. Tohoku J Exp Med. 2007;212:397–402. doi: 10.1620/tjem.212.397. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Duan X., Tan D., Zhang B., Xu A., Qiu N., et al. Iron deficiency and overload in men and woman of reproductive age, and pregnant women. Reprod Toxicol. 2023;118 doi: 10.1016/j.reprotox.2023.108381. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F.L., Yuan S., Dong P.Y., Ma H.H., De Felici M., Shen W., et al. Multi-omics analysis reveals that iron deficiency impairs spermatogenesis by gut-hormone synthesis axis. Ecotox Environ Safe. 2022;248 doi: 10.1016/j.ecoenv.2022.114344. [DOI] [PubMed] [Google Scholar]
- 6.Tsao C.W., Liao Y.R., Chang T.C., Liew Y.F., Liu C.Y. Effects of iron supplementation on testicular function and spermatogenesis of iron-deficient rats. Nutrients. 2022;14 doi: 10.3390/nu14102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan S., Dong P.Y., Ma H.H., Liang S.L., Li L., Zhang X.F. Antioxidant and biological activities of the lotus root polysaccharide-iron (III) complex. Molecules. 2022;27 doi: 10.3390/molecules27207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tvrda E., Peer R., Sikka S.C., Agarwal A. Iron and copper in male reproduction: a double-edged sword. J Assist Reprod Gen. 2015;32:3–16. doi: 10.1007/s10815-014-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang D., Wang Q., Ke L., Jiang J., Ying T. Antioxidant activities of various extracts of lotus (Nelumbo nuficera Gaertn) rhizome. Asia Pac J Clin Nutr. 2007;16(Suppl 1):158–163. [PubMed] [Google Scholar]
- 10.Yi Y., Huang X.Y., Zhong Z.T., Huang F., Li S.Y., Wang L.M., et al. Structural and biological properties of polysaccharides from lotus root. Int J Biol Macromol. 2019;130:454–461. doi: 10.1016/j.ijbiomac.2019.02.146. [DOI] [PubMed] [Google Scholar]
- 11.Han X., Liang Q., Rashid A., Qayum A., Rehman A., Zhong M., et al. The effects of different hydrocolloids on lotus root starch gelatinization and gels properties. Int J Biol Macromol. 2024;257 doi: 10.1016/j.ijbiomac.2023.128562. [DOI] [PubMed] [Google Scholar]
- 12.Matsuyama S., DeFalco T. Steroid hormone signaling: multifaceted support of testicular function. Front Cell Dev Biol. 2023;11:1339385. doi: 10.3389/fcell.2023.1339385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen A.H., Priskorn L., Hansen L.S., Carlsen E., Joensen U.N., Jacobsen F.M., et al. Testicular torsion and subsequent testicular function in young men from the general population. Hum Reprod. 2023;38:216–224. doi: 10.1093/humrep/deac271. [DOI] [PubMed] [Google Scholar]
- 14.Kathrins M., Niederberger C. Diagnosis and treatment of infertility-related male hormonal dysfunction. Nat Rev Urol. 2016;13:309–323. doi: 10.1038/nrurol.2016.62. [DOI] [PubMed] [Google Scholar]
- 15.Tournaye H., Krausz C., Oates R.D. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endo. 2017;5:544–553. doi: 10.1016/S2213-8587(16)30040-7. [DOI] [PubMed] [Google Scholar]
- 16.Shin J.H., Park Y.H., Sim M., Kim S.A., Joung H., Shin D.M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170:192–201. doi: 10.1016/j.resmic.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Cong J., Zhou P., Zhang R. Intestinal microbiota-derived short chain fatty acids in host health and disease. Nutrients. 2022;14 doi: 10.3390/nu14091977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y., Chen G., Wang L., Kong J., Pan J., Xi Y., et al. Triptolide-induced mitochondrial damage dysregulates fatty acid metabolism in mouse sertoli cells. Toxicol Lett. 2018;292:136–150. doi: 10.1016/j.toxlet.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Varma S., Molangiri A., Kona S.R., Ibrahim A., Duttaroy A.K., Basak S. Fetal exposure to endocrine disrupting-bisphenol A (BPA) alters testicular fatty acid metabolism in the adult offspring: relevance to sperm maturation and quality. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24043769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke S.D., Thuillier P., Baillie R.A., Sha X. Peroxisome proliferator-activated receptors: a family of lipid-activated transcription factors. Am J Clin Nutr. 1999;70:566–571. doi: 10.1093/ajcn/70.4.566. [DOI] [PubMed] [Google Scholar]
- 21.Olia B.F., Alizadeh A., Sadighi G.M., Shahhoseini M. Role of peroxisome proliferator-activated receptor gamma (PPARgamma) in the regulation of fatty acid metabolism related gene expressions in testis of men with impaired spermatogenesis. Reprod Biol. 2021;21 doi: 10.1016/j.repbio.2021.100543. [DOI] [PubMed] [Google Scholar]
- 22.Wang L., Lu H., Wang S., Liu H., Guo M., Bai H., et al. Vitamin D Receptor affects male mouse fertility via regulation of lipid metabolism and testosterone biosynthesis in testis. Gene. 2022;834 doi: 10.1016/j.gene.2022.146589. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T., Sun P., Geng Q., Fan H., Gong Y., Hu Y., et al. Disrupted spermatogenesis in a metabolic syndrome model: the role of vitamin A metabolism in the gut - testis axis. Gut. 2022;71:78–87. doi: 10.1136/gutjnl-2020-323347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P., Feng Y., Li L., Ge W., Yu S., Hao Y., et al. Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut. 2021;70:222–225. doi: 10.1136/gutjnl-2020-320992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou R., Wu J., Liu B., Jiang Y., Chen W., Li J., et al. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol Life Sci. 2019;76:2681–2695. doi: 10.1007/s00018-019-03101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S., Zhou Y., Chen Y., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 29.Wu T., Hu E., Xu S., Chen M., Guo P., Dai Z., et al. Yu, clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb) 2021;2 doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szklarczyk D., Kirsch R., Koutrouli M., Nastou K., Mehryary F., Hachilif R., et al. protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51(2023):D638–D646. doi: 10.1093/nar/gkac1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J., Holmes S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang C., Mai J., Cao X., Burberry A., Cominelli F., Zhang L. ggpicrust2: an R package for PICRUSt2 predicted functional profile analysis and visualization. Bioinformatics. 2023;39 doi: 10.1093/bioinformatics/btad470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soliman A., Yassin M., De Sanctis V. Intravenous iron replacement therapy in eugonadal males with iron-deficiency anemia: Effects on pituitary gonadal axis and sperm parameters. A pilot study. Indian J Endocrinol Metab. 2014;18:310–316. doi: 10.4103/2230-8210.131158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin T., Ji D., Su X., Zhou X., Wang X., He S., et al. Using Bayesian and weighted regression to evaluate the association of idiopathic oligoastenoteratozoospermia with seminal plasma metal mixtures. Chemosphere. 2024;351 doi: 10.1016/j.chemosphere.2024.141202. [DOI] [PubMed] [Google Scholar]
- 37.Griswold M.D. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salian S., Doshi T., Vanage G. Perinatal exposure of rats to Bisphenol A affects fertility of male offspring–an overview. Reprod Toxicol. 2011;31:359–362. doi: 10.1016/j.reprotox.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Eisenberg M.L., Esteves S.C., Lamb D.J., Hotaling J.M., Giwercman A., Hwang K., et al. Male infertility. Nat Rev Dis Primers. 2023;9:49. doi: 10.1038/s41572-023-00459-w. [DOI] [PubMed] [Google Scholar]
- 40.Mikedis M.M., Fan Y., Nicholls P.K., Endo T., Jackson E.K., Cobb S.A., et al. DAZL mediates a broad translational program regulating expansion and differentiation of spermatogonial progenitors. Elife. 2020;9 doi: 10.7554/eLife.56523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medrano J.V., Pera R.A., Simon C. Germ cell differentiation from pluripotent cells. Semin Reprod Med. 2013;31:14–23. doi: 10.1055/s-0032-1331793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Gendt K., Swinnen J.V., Saunders P.T., Schoonjans L., Dewerchin M., Devos A., et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larose H., Kent T., Ma Q., Shami A.N., Harerimana N., Li J.Z., et al. Regulation of meiotic progression by Sertoli-cell androgen signaling. Mol Biol Cell. 2020;31:2841–2862. doi: 10.1091/mbc.E20-05-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Y., Zhang W., Wang Y., Yi C., Yu B., Pang X., et al. Crosstalk between intestinal flora and human iron metabolism: the role in metabolic syndrome-related comorbidities and its potential clinical application. Microbiol Res. 2024;282 doi: 10.1016/j.micres.2024.127667. [DOI] [PubMed] [Google Scholar]
- 45.Collden H., Landin A., Wallenius V., Elebring E., Fandriks L., Nilsson M.E., et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am J Physiol-Endoc M. 2019;317:E1182–E1192. doi: 10.1152/ajpendo.00338.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Cheng W., Shang H., Wei H., Deng C. The interplay between androgen and gut microbiota: is there a microbiota - gut -testis axis. Reprod Sci. 2022;29:1674–1684. doi: 10.1007/s43032-021-00624-0. [DOI] [PubMed] [Google Scholar]
- 47.Hao Y., Feng Y., Yan X., Chen L., Ma X., Tang X., et al. Gut microbiota-testis axis: FMT mitigates high-fat diet-diminished male fertility via improving systemic and testicular metabolome. Microbiol Spectr. 2022;10:e0002822. doi: 10.1128/spectrum.00028-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stocco D.M. Intramitochondrial cholesterol transfer. Biochim Biophys Acta. 2000;1486:184–197. doi: 10.1016/s1388-1981(00)00056-1. [DOI] [PubMed] [Google Scholar]
- 49.Jefcoate C.R., Lee J. Cholesterol signaling in single cells: lessons from STAR and sm-FISH. J Mol Endocrinol. 2018;60:R213–R235. doi: 10.1530/JME-17-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musicki B., Zhang Y., Chen H., Brown T.R., Zirkin B.R., Burnett A.L. Mechanism of testosterone deficiency in the transgenic sickle cell mouse. PLoS One. 2015;10:e0128694. doi: 10.1371/journal.pone.0128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge R.S., Li X., Wang Y. Leydig cell and spermatogenesis. Adv Exp Med Biol. 2021;1288:111–129. doi: 10.1007/978-3-030-77779-1_6. [DOI] [PubMed] [Google Scholar]
- 52.Liu L.L., Xian H., Cao J.C., Zhang C., Zhang Y.H., Chen M.M., et al. Peroxisome proliferator-activated receptor gamma signaling in human sperm physiology. Asian J Androl. 2015;17:942–947. doi: 10.4103/1008-682X.150253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crisostomo L., Alves M.G., Gorga A., Sousa M., Riera M.F., Galardo M.N., et al. Molecular mechanisms and signaling pathways involved in the nutritional support of spermatogenesis by sertoli cells. Methods Mol Biol. 2018;1748:129–155. doi: 10.1007/978-1-4939-7698-0_11. [DOI] [PubMed] [Google Scholar]
- 54.Mousavi M.S., Shahverdi A., Drevet J., Akbarinejad V., Esmaeili V., Sayahpour F.A., et al. Peroxisome proliferator-activated receptors (PPARs) levels in spermatozoa of normozoospermic and asthenozoospermic men. Syst Biol Reprod Med. 2019;65:409–419. doi: 10.1080/19396368.2019.1677801. [DOI] [PubMed] [Google Scholar]
- 55.Narjes Jalilvand M.H.F.B., Ebrahimzadeh-Bideskan A. Protective effect of PPARγ agonist pioglitazone, on testicular tissue and sperm parameters in hypothyroid rats. Toxin Rev. 2021;40:267–276. [Google Scholar]
- 56.Badr G., Abdel-Tawab H.S., Ramadan N.K., Ahmed S.F., Mahmoud M.H. Protective effects of camel whey protein against scrotal heat-mediated damage and infertility in the mouse testis through YAP/Nrf2 and PPAR-gamma signaling pathways. Mol Reprod Dev. 2018;85:505–518. doi: 10.1002/mrd.22987. [DOI] [PubMed] [Google Scholar]
- 57.Santoro M., Guido C., De Amicis F., Sisci D., Vizza D., Gervasi S., et al. Sperm metabolism in pigs: a role for peroxisome proliferator-activated receptor gamma (PPARgamma) J Exp Biol. 2013;216:1085–1092. doi: 10.1242/jeb.079327. [DOI] [PubMed] [Google Scholar]
- 58.Santoro M., De Amicis F., Aquila S., Bonofiglio D. Peroxisome proliferator-activated receptor gamma expression along the male genital system and its role in male fertility. Hum Reprod. 2020;35:2072–2085. doi: 10.1093/humrep/deaa153. [DOI] [PubMed] [Google Scholar]
- 59.Al-Jameel W., Gou X., Forootan S.S., Fayi M.S.A., Rudland P.S., Forootan F.S., et al. Inhibitor SBFI26 suppresses the malignant progression of castration-resistant PC3-M cells by competitively binding to oncogenic FABP5. Oncotarget. 2017;8:31041–31056. doi: 10.18632/oncotarget.16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El K.M., Aviszus K., Sasse S.K., Zhao X., Serban K.A., Majka S.M., et al. Macrophage programming is regulated by a cooperative interaction between fatty acid binding protein 5 and peroxisome proliferator-activated receptor gamma. FASEB J. 2022;36:e22300. doi: 10.1096/fj.202200128R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotula-Balak M., Gorowska-Wojtowicz E., Milon A., Pawlicki P., Tworzydlo W., Płachno B.J., et al. Towards understanding leydigioma: do G protein-coupled estrogen receptor and peroxisome proliferator–activated receptor regulate lipid metabolism and steroidogenesis in Leydig cell tumors? Protoplasma. 2020;257:1149–1163. doi: 10.1007/s00709-020-01488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanimozhi V., Palanivel K., Akbarsha M.A., Kadalmani B. Molecular mechanisms of tributyltin-induced alterations in cholesterol homeostasis and steroidogenesis in hamster testis: in vivo and in vitro studies. J Cell Biochem. 2018;119:4021–4037. doi: 10.1002/jcb.26564. [DOI] [PubMed] [Google Scholar]
- 63.Ma J., Piao X., Mahfuz S., Long S., Wang J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim Nutr. 2022;9:159–174. doi: 10.1016/j.aninu.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh S., Whitley C.S., Haribabu B., Jala V.R. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenter. 2021;11:1463–1482. doi: 10.1016/j.jcmgh.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X., Zhang T., Ren X., Chen X., Wang S., Qin C. Pyrethroids toxicity to male reproductive system and offspring as a function of oxidative stress induction: rodent studies. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.656106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye X., Liu J. Effects of pyrethroid insecticides on hypothalamic-pituitary-gonadal axis: a reproductive health perspective. Environ Pollut. 2019;245:590–599. doi: 10.1016/j.envpol.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 68.Xu H., Bo Y. Associations between pyrethroid exposure and serum sex steroid hormones in adults: findings from a nationally representative sample. Chemosphere. 2022;300 doi: 10.1016/j.chemosphere.2022.134591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.