Graphical abstract

Keywords: Single-cell analysis, Lung cancer, Tumor heterogeneity, Immunotherapy
Highlights
-
•
Summary of latest research on cancer single cell sequencing and cancer immunotherapy.
-
•
Discussion of the key open issues, prospects and challenges of current single cell analysis.
-
•
Correlation between single cell sequencing and cancer immunotherapy.
-
•
Overview of single-cell sequencing technologies assisted in cancer immunotherapy.
Abstract
Background
Lung cancer is a prevalent form of cancer worldwide, presenting a substantial risk to human well-being. Lung cancer is classified into two main types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). The advancement of tumor immunotherapy, specifically immune checkpoint inhibitors and adaptive T-cell therapy, has encountered substantial obstacles due to the rapid progression of SCLC and the metastasis, recurrence, and drug resistance of NSCLC. These challenges are believed to stem from the tumor heterogeneity of lung cancer within the tumor microenvironment.
Aim of review
This review aims to comprehensively explore recent strides in single-cell analysis, a robust sequencing technology, concerning its application in the realm of tumor immunotherapy for lung cancer. It has been effectively integrated with transcriptomics, epigenomics, genomics, and proteomics for various applications. Specifically, these techniques have proven valuable in mapping the transcriptional activity of tumor-infiltrating lymphocytes in patients with NSCLC, identifying circulating tumor cells, and elucidating the heterogeneity of the tumor microenvironment.
Key scientific concepts of review
The review emphasizes the paramount significance of single-cell analysis in mapping the immune cells within NSCLC patients, unveiling circulating tumor cells, and elucidating the tumor microenvironment heterogeneity. Notably, these advancements highlight the potential of single-cell analysis to revolutionize lung cancer immunotherapy by characterizing immune cell fates, improving therapeutic strategies, and identifying promising targets or prognostic biomarkers. It is potential to unravel the complexities within the tumor microenvironment and enhance treatment strategies marks a significant step towards more effective therapies and improved patient outcomes.
Introduction
Lung cancer is a highly widespread global public health issue, with increasing rates of both incidence and mortality [1], [2]. Scientists have extensively researched and analyzed cancer cells in order to enhance the survival rate and well-being of individuals with lung cancer, employing a range of therapeutic approaches. Even so, lung cancer is a highly perilous and detrimental disease that poses a significant threat to human safety and well-being, and its mechanisms of metastasis, recurrence and immune resistance are still unclear. Throughout history, clinicians and researchers have struggled with how to cure cancer. Surgical resection is the gold standard for patients with stage I and stage II non-small cell lung cancer [3]. For advanced lung cancer, the cure rates of different treatment methods are not ideal. Early metastasis, rapid recurrence and immune resistance are the main causes of death in these patients [3]. Metastasis of lung cancer leads to a shortened survival period for patients. The recurrence of cancer is considered a difficult obstacle to curing advanced cancer and a reason why surgery cannot be performed. The treatment of advanced lung cancer mainly depends on chemotherapy [4]. Due to the lack of effective treatments, it is difficult to cure advanced lung cancer.
Immunotherapy has long been used as a postoperative adjuvant or palliative treatment and offers a new therapeutic direction for patients with non-small cell lung cancer [5]. Immunotherapies have different mechanisms of action, and the types include immune checkpoint inhibitors, adoptive cellular immunotherapy, and active immune vaccines. With the progress of research on molecular immune mechanisms, great progress has also been made in the research of immune checkpoint inhibitors (ICIs), cytokine-induced killer cells (CIKs), chimeric antigen receptor T cells (CAR-T cells), targeted cancer vaccines and gene therapy. The combination of several immunotherapies has been clinically reported as a first-line treatment that improves the safety and specificity of treatment and reduces the incidence of treatment-related serious adverse effects [6]. In the past decade, immunotherapy has created new opportunities for improving the survival of patients with early-stage non-small cell lung cancer [7].
The utilization of single-cell analysis has extensive implications in the realm of cancer research and the development of anticancer immunotherapy. Single-cell analysis entails the sequencing of the genome or transcriptome of an individual cell to acquire multiomics data, such as genomic and transcriptomic information. This approach allows for the identification of variations in population and evolutionary connections among cells. This technique can be employed to examine genetic material and uncover disparities in RNA or protein profiles among cells with exceptional precision, thereby enhancing our comprehension of the microenvironment of an individual cell [8]. The configuration of immune cells within the tumor microenvironment of lung cancer has been documented as a potentially significant biomarker for assessing the effectiveness of immunotherapy [9]. This finding significantly facilitates immunotherapy for lung cancer and supports the development and transformation of immunotherapy.
Although immunotherapy for lung cancer has been used clinically, it is not yet used as a first-line treatment and is usually used as adjuvant therapy. This may be because its efficacy is minimal or even nonexistent for some patients. Fortunately, single-cell sequencing can be used as a new tool to facilitate immunotherapy development in lung cancer, and the analysis of the tumor microenvironment can be used to find new targets or explore the mechanism of immune escape to improve efficiency. This review below will address recent studies related to lung cancer immunotherapy and single-cell analysis.
Single-cell analysis
Conventional sequencing techniques are limited to obtaining the average characteristics of a large number of cells and cannot effectively evaluate a small number of cells, resulting in the loss of information regarding cellular heterogeneity [10]. Traditional sequencing is now called bulk sequencing to distinguish it from single-cell sequencing technology. Single-cell sequencing technique enables the evaluation of the genome, DNA-metHYLome, or transcriptome of individual cells within a population [11]. Different from traditional sequencing methods, recent single-cell analysis techniques are being developed and improved, providing new opportunities for better understanding cancer with decreased analysis costs. Diverse technologies can be employed to examine the diversity within cellular populations at the human transcriptome, genomic, epigenomic, and proteomic levels in order to obtain comprehensive data sets [12]. These integrated data sets can help us identify new physiological mechanisms and pathways with therapeutic potential.
Single-cell RNA sequencing
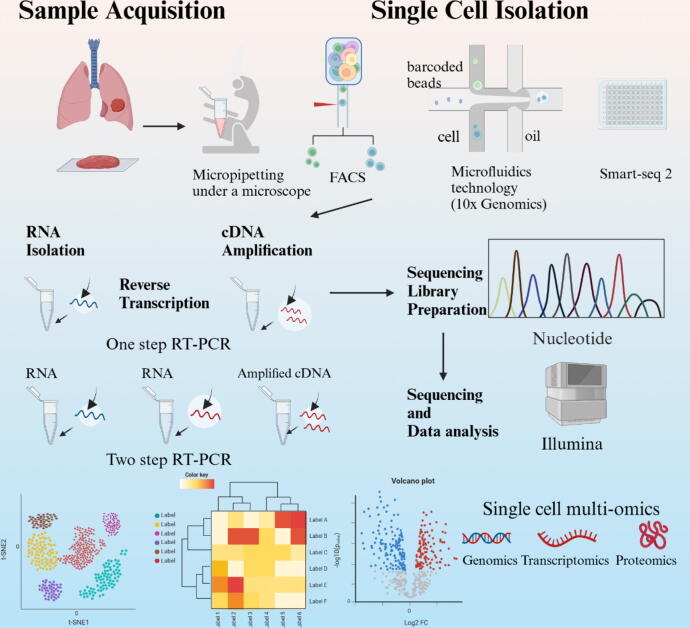
Sample acquisition is the very first step of single-cell sequencing analysis (Fig. 1). Tissues are not usually homogeneous but consist of hundreds of cell types, which are integrated together and exhibit great differences in abundance. The samples can be resected tissues, biopsy tissues, fresh tissues and rapidly frozen tissues [13]. Single-cell isolation is essential after obtaining the sample. There are many sorting and isolation methods for single cells. The conventional methods include limited dilution, micropipetting under a microscope, fluorescence-activated cell sorting and laser capture microdissection. High-throughput sorting methods can be roughly divided into but not limited to microdrop-wrapped single cells based on microfluidics technology (Drop Seq, 10x Genomics, etc.), single-cell separation technology based on micropore arrays (BD Rhapsody, Singleron, etc.). Single cells can be extracted from these tissues by either unbiased or biased sampling methods. Unbiased sampling is ideal for accurately representing the tissue's composition, whereas targeted sampling may be required to separate uncommon cell types [14]. For the analysis of the genome in individual cells, reverse transcription and cDNA amplification are necessary because there are very small amounts of RNA in a single cell [15]. In order to do DNA sequencing, the amplified DNA must first be incorporated into a sequencing library. A sequencing library refers to a compilation of individual DNA fragments, which are single-stranded, either from a group of cells or, in the case of single-cell sequencing, from a particular cell [16]. After sequencing library preparation, cDNA sequencing should be initiated. Smart-seq has been developed for full-length cDNA amplification as a whole-transcriptome amplification [17], [18]. Quartz-Seq [19], mcSCRB-seq [20], CEL-seq [21], BRB-Seq [22], DRUG-Seq [23] and PLATE-Seq [24] have also been developed to stably and sensitively analyze RNAs from a single cell and have obtained convincing sequencing data. Then, in the final step, the sequencing data are subjected to a specific bioinformatics workflow, such as transcriptome analysis or genome analysis, depending on the selected computational application. During the last decade, there has been a substantial increase in the availability of computational tools for analyzing RNA sequencing data. These tools have the capability to conduct quality control on sequencing data in order to obtain clean downstream data, including common tools such as FASTP [25], NGS-QC [26], GIANT [27], START [28]. FASTP efficiently pre-processes FASTQ data using multiple threads, NGS-QC is specifically designed for the processing of NGS data, GIANT incorporates Galaxy-based tools for additional differential analysis, and START provides a wide range of visual data analysis functions. The choice of these tools should be based on the purpose of application and the accuracy needed [25]. In summary, the process of scRNA-seq can be divided into five stages, namely, single-cell isolation, reverse transcription and amplification, RNA sequencing, sequencing library preparation, data analysis and data set integration [26].
Fig. 1.
The workflow of single cell RNA sequencing. The main steps of single cell RNA sequencing include tissue sample processing, single cell isolation and extraction, reverse transcription amplification, generation of cDNA libraries, sequencing, and bioinformatics data analysis. Created with BioRender.com.
Single-cell DNA sequencing
Notably, single-cell DNA sequencing can clarify the complexity of cellular mutation profiles at the single-cell level [27]. The DNA content of a single cell is very low, and it is far from reaching the demand of sequencing database construction. It is essential to amplify single cell DNA. To achieve this, it is necessary to overcome these difficulties, such as how to amplify uniformly and efficiently and how to cover the whole genome. At present, the main methods of single-cell DNA sequencing are DOP-PCR, MDA and MALBAC [28]. MALBAC utilizes Phi 29 polymerase to ensure amplification efficiency and high genome wide coverage, and utilizes special primer in chain hybridization to ensure linear amplification [29]. This solves the problem of low genome coverage caused by amplification bias, while also reducing the template requirement for genome sequencing to the single-cell level [30]. Unlike the above low-throughput single-cell DNA sequencing, Mission Bio uses microfluidic technology similar to scRNA-seq to achieve single-cell separation, and then amplifies specific DNA sequences through multiple amplification primer sets to achieve high-throughput single-cell DNA sequence sequencing. It is mainly applied in revealing tumor subtypes and drug resistance mechanisms [31]. However, the technology is not perfect yet, and there are also issues of low capture rate and amplification bias. At present, single-cell DNA sequencing has not fully opened the door of single-cell genome sequencing.
Single-cell multiomics analysis
Single-cell analysis has been extensively utilized and advanced over time, often in conjunction with other technologies such as transcriptomics, epigenetics, and proteomics [32]. These technologies have developed rapidly, resulting in a variety of single-cell multiomics sequencing technologies, all of which are used to reveal multiple characteristics, such as DNA, RNA, and protein profiles, for the same individual cell (Fig. 2, Table 1). Lin et al. developed scNanoCOOL-seq based on nanopore sequencing platform and conducted multi omics analysis in the same individual cell [33]. Utilizing long reads, scNanoCOOL-seq can jointly analyze transcriptomics and epigenetic features such as DNA methylome and chromatin state, including genome such as copy number variations (Table 1). Single-cell multiomics analysis has proven to be helpful for a comprehensive understanding of cellular events [34].
Fig. 2.
The milestones and findings of multiomics research and sequencing technology. The figure briefly lists the milestones in the development of sequencing technology and multiomics research, and demonstrates what can be achieved by combining single-cell sequencing with multiomics research analysis. Depending on the research objective, multiple aspects of tumor tissues, such as the epigenome, genome, transcriptome, and proteome, can be analyzed. Created with BioRender.com.
Table 1.
Summary of single-cell sequencing technologies.
| Single cell technologies | Omics | Characteristic | Applications | Cell isolation |
|---|---|---|---|---|
| Digital-scRRBS | Epigenomic | Improved scRRBS with high coverage, high positioning, and cost-effectivenessv[116], [117] | DNA methylation | Microfluidics |
| scCHIC-seq | Epigenomic | Detecting specific histone signals in different cell types[118] | Evaluating chromosomal states and whole genome histone modifications | Microfluidics |
| scNanoCOOL-seq | Epigenomic | Joint analysis of genome, DNA methylation, chromatin accessibility, and transcriptome within the same cell[33] | DNA methylation and chromatin accessibility | Nanopore |
| scATACseq | Epigenomic | Easy to exhibit cellular epigenomic heterogeneity, but with low recovery and flux[119] | Epigenomic cellular status | Chromium |
| scWGBS | Genomics | Revealing intercellular heterogeneity under low sequencing coverage, but DNA is susceptible to damage[120] | Epigenomic cellular status | Microfluidics |
| MALBAC | Genomics | Whole genome high coverage and sensitivity[29] | Detecting whole genome mutation and SNV | |
| SCI-seq | Genomics | Simultaneously producing thousands of low throughput single-cell libraries[121] | Detecting copy number variation | xSDS |
| Strand-seq | Genomics | High resolution sister chromatid exchanges localization[122] | Identifying genomic instability | FACS |
| Cel-seq | Transcriptomic | Highly sensitive but low flux and efficiency | Heterogeneity | Micropipette |
| Smart-seq3 | Transcriptomic | High sensitivity but limited efficiency[123] | RNA sequencing | FACS |
| Drop-seq | Transcriptomic | High throughput, low cost[124] | Heterogeneity | Microfluidics |
| scRna-seq | Transcriptomic | High cell capture efficiency, fast cycle time, high cell suitability, and reproducibility | RNA sequencing | Chromium |
| scMS | Proteomic | There are challenges such as low flux, slow speed, and low protein content, and the mass spectrometer is prone to generating noise[43], [125] | Cellular metabolites and peptides[125] | NanoPOTS |
| CITE-seq | Proteomic | Using antibody oligonucleotide complexes to capture cell surface proteins, but antibody cross reactivity is a problem[45] | Combining cell surface proteins and transcriptome information | Chromium |
Transcriptomics
While single-cell data is applicable for single-cell transcriptomics, the process of single-cell separation may result in the loss of spatial information. With the innovation of combined single-cell analysis and spatial transcriptome analysis, the resolution and accuracy of transcriptome profiling have increased [35]. Multi-regional sequencing can analyze the transcriptomic differences in different spaces of tumors and link them to the Intratumor heterogeneity. By analyzing multi-regional sequencing data in different spaces, Mitra et al. reconstructed a significant relationship between genome and immune heterogeneity [36]. The integration of spatial transcriptome and single-cell analysis enables us to obtain more precise insights into the characteristics of individual cells across various spatial regions (Table 1). The spatial characteristics of cells within a tissue are indicative of their function, and the precise cellular positioning plays a crucial role in elucidating the variations in cellular differentiation and cell state [37].
Epigenetics
Tumor cells continually undergo significant dynamic changes, with great intercellular variation, and despite the fact that tumor cells originate from the same cell, lung cancer tissue becomes heterogeneous after tumorigenesis and differentiation [38]. Cellular heterogeneity is largely dependent on epigenetic heterogeneity. Therefore, it is of great necessity to relate the modification, association and conformation of genomic sequences to epigenetic cell fates and tissue-specific functions. At this point, bulk sequencing can easily obscure cellular-level epigenetic alterations in tissues [39]. However, single-cell sequencing analysis is able to reveal differences in single-cell solution, which can be used to detect chromatin accessibility, histone modifications, chromosome conformation and noncoding RNA modifications [39]. These techniques are rapidly becoming powerful tools for studying epigenetic changes in early-stage lung cancer and are extremely valuable for exploring immunotherapy in lung cancer. A variety of new single-cell sequencing technologies have been developed to detect epigenetic levels, such as scATAC-seq (Table 1). Studies on single-cell sequencing technologies have shown that the heterogeneity between cells at the epitope level is correlated with and much greater than that at the transcriptional level, but the associated regulatory mechanisms are not fully understood [40].
Proteomics
Given the various functions that proteins perform in the human body, it is essential to expedite the progress of single-cell proteomics research in order to enhance our comprehension of the proteins that are implicated in both well-being and illness. Single-cell proteome analysis poses a significant problem mostly because to the limited protein content within individual cells, which cannot be amplified akin to genes [41]. Recent advancements in microchip, mass spectrometry, and reiterative staining-based technologies have made it possible to conduct a thorough analysis of proteins in individual cells [41], [42]. Mass spectrometry, as one of the most fundamental and popular tools for studying proteomics, has attracted attention with the joining forces of single-cell analysis. One would like to see more proteins and post-translational modifications analyzed by single-cell mass spectrometry [43]. However, due to the limited ion transmission efficiency and separation rate of mass spectrometry instruments, the throughput and proteome coverage of scMS are also limited [44]. As for noteworthy cell surface proteins, CITE-seq can reflect more detailed cell phenotype characteristics by using oligonucleotide labeled antibodies [45]. In addition, single-cell analysis at the proteome level has been used to find targeted cells that mediate the mechanism of tumor resistance [46], identify important biomarkers that predict the prognosis of patients [47], and evaluate the necessary molecules that activate anticancer therapies [48].
Lung cancer immunotherapy
Lung cancer, being the leading cause of cancer mortality, results in millions of fatalities globally annually. According to the 2021 WHO classification of lung tumors, lung cancers can be divided into non-small cell lung cancers (NSCLC) and small-cell lung cancer (SCLC). NSCLC can be further divided into subgroups based on morphology, immunohistochemistry and molecular techniques [49]. The main causes of lung cancer-related death are early metastasis, rapid recurrence and immunotherapy resistance [1], [2], [49], [50], [51], [52]. Lung cancer commonly spreads to the bone, brain, lung, and liver, which reduces the overall survival rate. The predominant sites of metastasis for lung cancer include the neurological system, skeletal system, liver, respiratory system, and adrenal gland [50], [53], [54]. Liver metastasis and nervous system metastasis are common in patients with small cell lung cancer metastasis, while bone metastasis and respiratory system metastasis are common in adenocarcinoma [50], [55]. Women and young patients have a higher incidence of nervous system metastases [50]. Cancer recurrence in the same lung or at the bronchial stump is referred to as local recurrence; in contrast, recurrence at any other site is referred to as distant recurrence [52]. Recurrent terminal lung cancer is considered difficult to cure since surgery cannot be performed.
The latest TRACERx study (Tracking Non Small Cell Lung Cancer Evolution Through Therapy) offers a comprehensive and prospective characterization of NSCLC, encompassing various aspects of lung cancer and delving into tumor heterogeneity and the underlying molecular mechanisms [56], [57], [58], [59], [60], [61]. Researchers can effectively distinguish mutations in multi-regional tissues from TRACERx, and point out that subclonal whole genome doubling was associated with clinical outcome [57]. In addition, the researchers compared metastatic tumors with primary NCSLC tumors and revealed the presence of selection of the oncogenes within metastatic tumors [58]. By integrating transcriptomic and genomic data, researchers identified that ITH leads to therapeutic resistance and immune evasion underscoring the crucial role of the genome in regulating the development of lung cancer [60]. The tumor microenvironment (TME) is widely appreciated to be composed of cancer cell populations and cancer stem cells as well as stromal cells [62]. There are heterogeneous and interactive cellular components and complex recruited noncellular components in the TME [63]. Lung cancer tissue is usually composed of a variety of cells, which have different genetic characteristics and form a unique TME. The attributes of immune cells and tumor cells within the tumor microenvironment (TME) are intricately linked to their susceptibility to anticancer treatment [64]. Within a diverse system like this, many components or signals that either promote or inhibit tumor growth can influence the advancement of tumors and impact the efficacy of the immune response against them [65]. The development in tumor immune research indicates that not only cancer cells but also the TME play an indispensable role in tumor occurrence and malignant progression and can facilitate precision medicine [62], [66]. The immune system is the body's natural barrier. The role of the immune system is to attack non-self antigens such as viral proteins or tumor antigens and to tolerate the body's own normal antigens, and T cells are the main component of the immune response. Immunotherapy stimulates the immune system to target and attack cancer cells by many methods, including therapeutic vaccines, monoclonal antibodies, ICIs, and T-cell therapy. [67] (Fig. 3). In recent years, immune cell therapy and ICIs such as the cytotoxic T-lymphocyte-associated protein 4 receptor (CTLA-4) and programmed cell death 1 (PD-1) antibodies have become the first choice for immunotherapy of NSCLC (Fig. 3). Today, immunotherapy for NSCLC is available as combination therapy in first-line settings and can be applied earlier than ever before in patients [68].
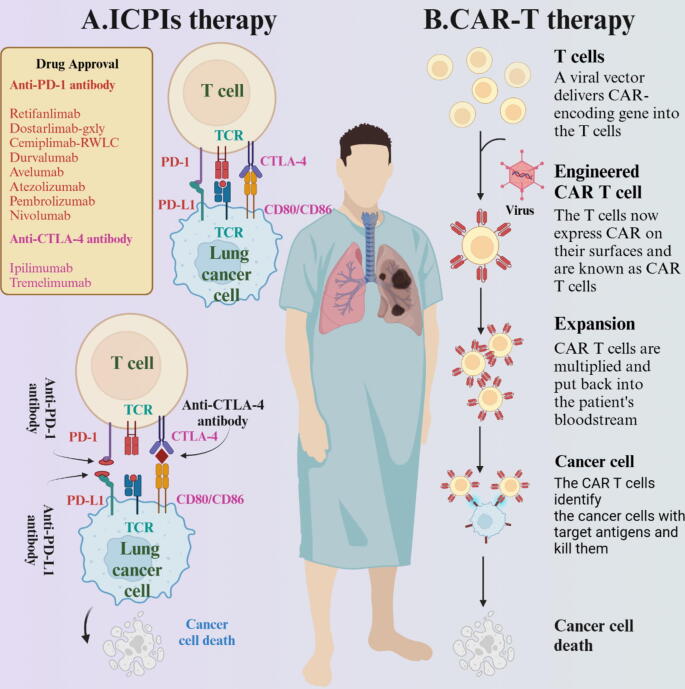
Fig. 3.
ICPIs and immune cell therapy for lung cancer. A. The schematic diagram illustrating the mechanism of immune checkpoint inhibitor therapy shows that the target sites of immune checkpoint therapy mainly include PD-1 and CTLA-4. When the immune checkpoint receptor binds to the ligand, T cells become stationary and inhibit immune cells from attacking tumor cells. The inhibitor maintains the vitality of immune system by blocking this binding. B. Insights to the basic concept of CAR-T cell therapy, which utilizes the artificial addition of specific antibodies to enhance the killing power of human T cells against tumor cells. Created with BioRender.com.
Immune cell therapy
Immune cell therapy is a rapidly evolving class of therapies with a progressively expanding range of applications that have been shown to be effective in cancer. Immune cell therapies can be divided into nonengineered immune cell therapies and engineered immune cell therapies, depending on whether the immune cells are genetically modified [69]. Nonengineered immune cell therapy isolates tumor-infiltrating lymphocytes (TILs) from tumors and expands them back into the body for the purpose of treating the disease. The immune system's T cells primarily identify cancer cells as abnormal, produce a group of cytotoxic T lymphocytes that can move and penetrate the malignancy regardless of its location, and selectively attach to and eliminate cancer cells [70], [71]. Examples of engineered immune cell therapies are CAR-T [72], CAR-NK, T cells engineered with T-cell receptors (TCR) [73] and adoptive T-cell therapies [74]. These therapies rely on enhancing the specificity of immune cells or improving immune safety to enhance the efficacy of immune cell therapies. Immune cell therapies have shown impressive results in certain B-cell malignancies, but there may be some challenges in treating solid tumors [75]. Cytokine-induced killer cells (CIKs) are commonly used to treat solid tumors and have the powerful antitumor activity of T lymphocytes and the non-major histocompatibility antigen (MHC)-restricted tumor killing ability of NK cells (natural killer cells) [76], [77]. Analysis of the immunosuppressive landscape of the tumor immune microenvironment (TIME) is expected to lead to optimization of the therapeutic effect of CAR-T cells in cancer patients [78]. In summary, immune cell therapies have multiple mechanisms involved in tumor immunotherapy; these therapies can directly kill tumor cells while activating and enhancing the immune response of the immune system of lung cancer patients and regulating the balance of the immune system, thus exerting good therapeutic effects. It is hoped that these therapies will have an increasingly important role in the treatment of lung cancer in the coming years.
Immune checkpoint inhibitors
Immunological checkpoints are a group of immunological molecules that have the ability to control the strength and scope of immune responses, thereby preventing harm and destruction to healthy tissues [79]. During cancer and development, immunological checkpoints emerge as significant contributors to immune tolerance. Under normal physiological conditions, T cells rarely respond to autoimmunity, and immune function remains at a moderate intensity because of the balance of immune checkpoint molecules. Tumor cells escape immune surveillance and progression through the immune checkpoint inhibitor pathway [80]. ICI therapy is a treatment method that aims to eliminate cancer cells by manipulating T-cell function through the use of coinhibition or costimulation of several pathways. The programmed cell death-1 (PD-1) or programmed cell death ligand-1 (PD-L1) pathway is the primary mechanism by which tumors suppress the activity of lymphocytes in the TME [81]. ICIs are more potent than immune cell therapies for solid organ and hematologic malignancies, and they are currently the standard of care for the treatment of many types of cancer. ICIs targeting the PD-1 and PD-L1 axis have greatly advanced the treatment of NSCLC [82] and changed the treatment landscape. PD-1 expression is widely detectable in different tumor types, and this has emerged as a marker for the response to PD-1 PD-1/PD-L1 inhibitors [83]. Immunotherapy has characteristics such as durability and low toxicity compared to traditional chemotherapy. PD-1 inhibitors have long-lasting efficacy, and because the immune system has a memory function, unlike chemotherapy and targeted therapy, for which resistance eventually develops, PD-1 inhibitors can induce long-term remission and clinical cure even in a small subset of patients with metastasis [84]. Nevertheless, the utilization of immune checkpoint inhibitor medication is linked to a variety of side effects referred to as immune-related adverse events (irAEs). These irAEs may occur in various organs throughout the body but are mainly concentrated in the GI tract, skin, lungs, and liver [85]. Compared to immune checkpoint inhibitors that target CTLA-4, those that target PD-1 and PD-L1 have a lower incidence of irAEs. The efficacy of ICIs is inconsistent among different patients, which may result from heterogeneity of the TME. The understanding of the genetic or epigenetic factors that contribute to the diversity of lung cancer, the interplay between various cell types, and the connection between this diversity and medication resistance is still limited. The composition of the TME has been shown to influence the reaction to ICIs [86]. Exploring the potential mechanism of the TME in immunotherapy can solve the current dilemma [87].
Single-cell analysis of immunotherapy sensitivity in lung cancer
The advent of single-cell analysis has provided a new avenue for cancer research. Single-cell analysis has been extensively employed to investigate human lung cancer at the cellular, genetic, and molecular levels. Single-cell technologies enable the extraction of high-throughput sequencing data from individual cells within tumor tissues, allowing for the acquisition of detailed information at a single-cell level. In recent years, single-cell analysis has been used to detect the anti-immune regulatory mechanism in the TME with single-cell resolution [88]. Through single-cell analysis, the molecular characteristics of cell populations or rare cells can be observed in a cancer tissue sample. Single cell analysis is an efficient and reliable method for lung cancer research. It is usually used to identify the diversity within tumors, understand the TME, and study the response of tumors to clinical treatment [89] (Fig. 4).
Fig. 4.
Single cell sequencing can be used in multiple fields of cancer research. A. The application of single cell sequencing in depicting tumor microenvironment. Single cell sequencing can detect key genes that change during tumor progression in the tumor microenvironment, identify rare subpopulations, cell states, and perform multi omics analysis. B. Single cell sequencing explores tumor heterogeneity at different levels, including intra patient heterogeneity, inter patient heterogeneity, and intra tumor heterogeneity levels. C. Schematic diagram of the role of CTC cluster in cancer metastasis, as well as the detection and analysis of CTC using single cell sequencing technology. D. Single cell sequencing designs new targeted therapies and signaling pathways for cancer patients, and detects patient resistance and individualized treatment. Created with BioRender.com.
Exploring the tumor microenvironment
The TME is a complex dynamical system with a direct effect on tumor immunity. Zilionis et al. used scRNA-seq to analyze the expression programs of tumor-infiltrating myeloid cells in NSCLC patients and mouse lung adenocarcinoma (LA) models [90]. In this study, TIM populations with similar expression programs were identified in human and mouse models, which provides confidence in linking laboratory research with clinical treatment. Tian et al. revealed different immune phenotypes by analyzing the immune cells infiltration in TME from different SCLC patients [91]. They speculate that the clinical immunotherapy is mediated by these immunological phenotypes. They concluded that SCLC patients with a lower degree of immune cell infiltration had benefited more from ICI therapy [91]. In order to explore the spatial and TME of early LUAD, multi-regional single-cell sequencing analysis of early LUAD patients revealed the cell populations, states, and phenotypes in the spatial and ecological evolution of LUAD, which have the potential to become targets for early interception [92]. There are still many potential mechanisms for how cancer cells resist drugs, although many publications indicate different pathways. Maynard et al. performed single-cell analysis in advanced-stage NSCLC samples from patients during targeted treatment, and the scRNA-seq data showed that cancer cells surviving therapy were present as residual disease, suggesting a therapy-induced primitive cell-state transition, whereas other cells continued to expand during therapy, indicating progressive disease, which was accompanied by upregulation of protumor pathways [93]. This study shows how therapy-induced immunosuppressive adaptation is induced in the TME and provides a potential drug resistance mechanism to be targeted for treatment.
Tumor heterogeneity
Tumor heterogeneity is the occurrence of many divisions and proliferation during the growth of a tumor. Consequently, the daughter cells exhibit alterations in molecular biology or genetics, resulting in modifications in the pace of tumor growth, invasion capability, immune response, and prognosis [94]. Here, the main reference is to intratumor heterogeneity (ITH), which is represented by differences in gene expression and phenotypes even between different cells of the same tumor [95]. Tumor heterogeneity may be the main cause of immune resistance and tumor recurrence [96]. To more effectively research ITH and prevent erroneous interpretation of tumor, multi-region sampling is essential. Tian et al. performed multi-regional sequencing on matched malignant and non-malignant cells from 11 SCLC patients, and found that ITH mainly occurred in differential expression of key factors of SCLC and programs including but not limited to cell cycle, immune and hypoxia [91]. Xin et al. performed multi-regional sequencing of 116 lung nodules to draw a genomic map of lung cancer progression from preneoplasia to invasive lung adenocarcinoma. They analyzed heterogeneity in the same patient because different lesions are driven by mutations in different oncogenes [97]. Due to the variability of ITH in different tumors, studies have shown that accurate methods for studying the ITH of lung cancer include but are not limited to increasing sequencing depth, expanding sampling areas, and adopting reasonable analysis algorithms [98]. Single-cell sequencing can be applied to study the internal heterogeneity of NSCLC at the individual cell level, and related data can be used to map the clonal development and evolution of lung tumor cells [99], [100], as well as to study the invasive spread and metastasis of early lung cancer and predict the evolution of drug resistance during treatment [101].
Liquid biopsy
Circulating tumor cells (CTCs) refer to tumor cells that enter the blood from the primary or metastatic tumor, travel through the peripheral blood circulation, and have the risk of implantation in other organs [102]. Understanding CTCs at single-cell resolution and analyzing the heterogeneity of CTCs often reveals unique information that can be used to study immunotherapy-related issues that are often obscured by bulk/combined analysis of samples [103]. In liquid biopsy, CTCs, as major targets, become important biomarkers in tumor prognosis monitoring. Research on CTCs in NSCLC patients based on single-cell sequencing is worthy of attention. It can not only find new CTC biomarkers but also reveal the mechanism of tumor cell metastasis at the molecular level. Barbirou et al. used the immunofluorescence technique to identify CTCs from the peripheral blood of 20 patients with NSCLC and 11 smokers without tumors. The CTC counts of NSCLC patients are significantly higher than those of other smokers. Even in this case, for studies involving single-CTC analysis, the detection rate of CTCs in the peripheral blood is typically unsatisfactory because of the quantity of CTCs [104]. However, based on data from Barbirou et al., the highest number of mutations detected emutation rate could be detected in CTCs. Single-CTC analysis has potential value in identifying new treatments for NSCLC despite the possibility that CTC counts may be low in blood samples.
Identification of new immunotherapy biomarkers
Single-cell analysis can help to find new biomarkers and observe prognosis after immunotherapy. Many articles have contributed to the precise treatment of tumors through single-cell analysis. Zhou et al. found KAT2B molecules in the RNA transcriptome database of NSCLC clinical patients using bioinformatics technology [105]. Additionally, KAT2B expression correlated positively with the levels of multiple infiltrating immune cells and mRNA expression levels of immune checkpoint genes in NSCLC. The low expression of this molecule is related to the unsatisfactory effect of ICIs and a poor prognosis in lung adenocarcinoma patients. Through single-cell analysis, they found that the KAT2B molecule mainly regulates signaling pathways mediated by immune cells and interferon-γ. This article indicates that KAT2B may serve as a novel biomarker for predicting prognosis and the response to ICIs in NSCLC. Zoledronic acid (ZA) is an antitumor drug that can enhance endocrine therapy, chemotherapy and targeted therapy [106]. To explore the influence of ZA on the clinical efficiency of ICIs in treating NSCLC and its possible mechanism, Zheng et al. detected and analyzed the immune cell population and cytokines in the TME through single-cell analysis technology [107]. They found more CD8 + IFN-gamma + T cells and gamma delta T cells and fewer CD11b cells in the TME of the ZA-treated group than the control-treated group. Antitumor cytokines INF-gamma and IL-18 were elevated in the sera. The above experimental results show that ZA can improve the therapeutic effect of ICIs. Zhang et al. used single-cell low-coverage whole-genome sequencing (scWGS) to detect the plasma-free DNA spectrum of 25 patients with PD-L1-negative advanced NSCLC before ICI treatment to provide information on cell-free copy number variation [108].
Identification of targeted pathways
Moreover, single-cell analysis provides an advanced method to define new tumor molecules, identify new cancer-driving genes, and then further explore the pathological mechanism of lung cancer. It is known that the abundance of T-cell subtypes in the tumor microenvironment is closely related to the response to ICIs. Recent studies have focused on T-cell recognition of mutation-associated neoantigens (MANA), which are important targets of PD-1 blocking the induction of antitumor immunity. It is not known whether resistance factors initially exist or are produced during cancer progression; however, these factors in the TME inhibit MANA-specific TIL responses. To improve response rates to ICIs, Caushi et al. used single-cell analysis to compare patients who do not respond to ICIs and those who do respond to treatment. The results support the hypothesis that the low activity of tumor-specific T cells is related to the poor affinity of TCRs for their cognate peptide MHC [109]. They also analyzed the expression of interleukin-7 receptor (IL-7R) in MANA-specific T cells and found that it is much lower than that in influenza-specific tissue-resident memory cells. However, MANA-specific T cells remain responsive to supraphysiological levels of IL-7. Targeting the IL-7 pathway enhances the ICB response, which is essential for T-cell homeostasis and long-term memory.
Conclusions
Lung cancer exhibits a diverse array of cell types, phases, and interactions. Single-cell RNA sequencing (scRNA-seq) enables a complete analysis of gene expression at the cellular level, facilitating a more profound comprehension of the diverse organs and cell types implicated. This novel approach can be employed to analyze the cellular makeup of tissues, identify rare cell types or conditions, track the dynamic changes in gene expression within cells over time, pinpoint genes that exhibit distinct expression patterns in specific cell types under various conditions (such as treatment or disease), and detect alterations in gene expression across different cell types while incorporating spatial and protein expression data [110], [111], [112]. Without a doubt, the utilization of single cell analysis in the realm of lung cancer research has been referenced in numerous scholarly investigations. Despite the effectiveness and informativeness of scRNA-Seq, it is crucial to acknowledge and tackle the numerous challenges and sources of variability to prevent complications or restrictions in data interpretation. First, single-cell analysis has been combined with multiomics analysis and has greatly improved the universality of single-cell analysis and addressed some issues, such as missing spatial information in single-cell transcriptome analyses [32]. However, there are still some technical issues, and further development of reliable amplification and detection techniques is needed. Moreover, single-cell proteomics is still a technology in need of improvement, and detection of the entire proteome based on antibodies conjugated to targeted DNA barcodes is challenging [37]. Moreover, for single-cell proteomics, it is not only difficult to accurately quantify the single-cell protein level but also impossible to amplify proteins [41].
Sensitivity, contamination, and noise of scRNA sequencing are also issues we need to address. The instability of reporters in techniques such as fluorophore sequencing is a current issue. At present, there is an upper limit of detection (an indicator of sensitivity) in single-cell analysis, which is primarily caused by single-cell burden [46]. The contamination of samples in scRNA sequencing mainly occurs in the cell isolation step. Obtaining pure single-cell samples from tumor tissues for scRNA-seq analysis can be challenging. With this high-resolution transcriptomic approach, technical noise can mask information on low-expression and low-abundance molecules, which is a crucial problem in scRNA sequencing. Although single-cell analysis has advantages in accuracy and resolution, it cannot fully meet the demands of bulk patient analysis and multicenter studies [113].
Additionally, single-cell sequencing is expensive. Sample capture is costly, single-cell sequencing library preparation is more expensive, analysis is more complex, and results are difficult to interpret [114]. At present, single-cell analysis cannot be widely carried out in the laboratory. As a state-of-the-art technology in recent years, instruments, operators, and reagents for single-cell analysis require some time to be popularized, which results in the high cost of single-cell RNA sequencing, and correspondingly, single-cell sequencing of proteins is more expensive [115]. Therefore, most researchers do not choose single-cell RNA-seq unless it is necessary for the experimental question, and they usually use simpler and less costly bulk sequencing to solve problems.
In general, single-cell analysis has been predicted to become an indispensable tool in the field of oncology and lung cancer immunotherapy. Given the heterogeneity of lung cancer, single-cell analysis can be applied for individual patients to identify novel targets and adopt appropriate treatment strategies based on predicted responses to therapies [48]. This is personalized therapy—a goal to strive toward. Single-cell analysis has been routinely performed in the clinic for lung cancer research to improve antitumor treatments by characterizing the heterogeneous TME or CTC population. Of course, this cannot be done without the development of new experimental platform tools or applications that automate the computation of sequencing data. In the future, the identification of new or known cells or cell populations will continue to be an important goal of scRNA-seq experiments. We look forward to seeing how this technology will continue to promote the development of lung cancer treatment in the next decade.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
CRediT authorship contribution statement
Nan Xiao: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Hongyang Liu: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. Chenxing Zhang: Supervision, Writing – review & editing. Huanxiang Chen: Supervision, Writing – review & editing. Yang Li: Resources, Writing – review & editing. Ying Yang: Resources, Writing – review & editing. Hongchun Liu: Formal analysis, Project administration. Junhu Wan: Formal analysis, Project administration.
Funding
This study was supported by grants from the National Natural Science Foundation of China grants (Grant No. 82173018, 82003193), the Medical Science and Technology Provincial and Ministerial Co-construction Project of Henan province (Grant No. SBGJ202102133), the Young and Middle-aged Health Science and Technology Innovation Talents Project of Henan province (Grant No. YXKC2021036), the Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (Grant No. QNCXTD2023005), and was sponsored by Natural Science Foundation of Henan Province (Grant No.232300421054, 232300421286), Zhengzhou University Education and Teaching Reform Research and Practice Project (Grant No. 2022ZZUJG298).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Biographies

Nan Xiao, a graduate student at the First Affiliated College of Zhengzhou University. In June 2018, I completed my undergraduate studies at Zhengzhou University. In September 2018, I was admitted to the First Affiliated College of Zhengzhou University. During my master's degree, I mainly conducted molecular biology experiments related to tumors. And have a certain interest in single-cell sequencing technology.

Hongyang Liu, the attending physician, engaged in clinical, teaching, and research work in obstetrics and gynecology. I have studied at the Obstetrics and Gynecology Hospital affiliated with Fudan University. Proficient in the diagnosis and treatment of diseases such as endometriosis, gynecological tumors, and cervical diseases. The research direction focuses on the molecular mechanisms and clinical diagnosis and treatment of gynecological tumors.

Chenxing Zhang, a master's student in Zhengzhou University, my tutor is Wan Junhu. In June 2022, I completed my undergraduate studies in Zhengzhou University. I was admitted as a graduate student in Clinical Laboratory Diagnostics at the College of Medical Sciences of Zhengzhou University in September 2022. Currently, I am mainly engaged in research related to tumor epigenetics and therapy.

Huanxiang Chen, a master's student in Zhengzhou University, my supervisor is Qiaozhen Kang and Junhu Wan. In June 2022, I completed my undergraduate studies in Jinan University, and in September 2022, I was admitted as a graduate student to Zhengzhou University, Henan Province. At present, I am mainly engaged in the research on the pathogenesis and treatment of cancer.

Yang Li, a PhD student at Zhengzhou University, supervised by Junhu Wan. I completed my undergraduate studies at Kunming Medical University in June 2020, and my master's degree in Biochemistry and Molecular Biology at the Department of Laboratory Medicine of the Third Affiliated Hospital of Kunming Medical University in June 2023.I was accepted into the PhD program in Clinical Laboratory Diagnostics at the College of Medical Sciences of Zhengzhou University in September 2023. Currently, I am mainly engaged in research related to tumor epigenetics and therapy.

Ying Yang, My name is Ying Yang and I am a graduate student at Zhengzhou University. I completed my undergraduate studies at China Medical University. In September 2021, I was admitted as a graduate student in Clinical Laboratory Diagnostics at Zhengzhou University. Currently, I am mainly engaged in lung cancer related research. In March 2023, I published an article entitled “Liquid biopsy on the horizon in immunotherapy of non-small cell lung cancer: current status, challenges, and perspectives,” which reviewed the progress of liquid biopsy in the field of immunotherapy for lung cancer.

Junhu Wan, Associate Professor, Deputy Chief Technician, Doctoral Supervisor, Ph.D. from Peking University, Outstanding Youth in Health Science and Technology Innovation in Henan Province, Top Ten Outstanding Youth of Zhengzhou University First Affiliated Hospital, and Young Member of the Laboratory Branch of Henan Medical Association. Won the first prize of Henan Medical Science and Technology Award. Hosted the National Natural Science Foundation General Program and Youth Fund; Hosted 4 excellent youth projects funded by the Natural Science Foundation of Henan Province and provincial-level scientific research projects. My main research focus is on the molecular mechanisms of epigenetic regulation of tumors, including the role of protein post-translational modifications, RNA modifications, and non coding RNA in tumor regulation. I have published more than 10 research papers as the first and corresponding author in journals such as Cell Research and Nuclear Acids Research.

Hongchun Liu, Professor, Chief Technician, and Master's Supervisor. I have studied in the United States as a visiting scholar for six months. From 2009 to 2013, he was continuously hired by the Provincial Department of Health as a senior judge of the Henan Provincial Health System Senior Professional Title Evaluation Committee. I have led and undertaken 7 scientific and technological research projects, including the Provincial Science and Technology Research Project, the Provincial Department of Education, and the Provincial Department of Health. I have also undertaken 1 horizontal joint project and published over 40 academic papers in SCI, the Chinese series, and core journals.
Contributor Information
Hongchun Liu, Email: xingyunerliu@163.com.
Junhu Wan, Email: wanjh@zzu.edu.cn.
References
- 1.Bade B.C., Dela Cruz C.S. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41:1–24. doi: 10.1016/j.ccm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger D., Wood D., Aisner D., Akerley W., Bauman J., Bharat A., Bruno D., Chang J., Chirieac L., D'Amico T., et al. NCCN guidelines insights: non-small cell lung cancer version 2. Journal of the National Comprehensive Cancer Network : JNCCN. 2021;19:254–266. doi: 10.6004/jnccn.2021.0013. [DOI] [PubMed] [Google Scholar]
- 4.Gubens M.A., Davies M. NCCN guidelines updates: new immunotherapy strategies for improving outcomes in non-small cell lung cancer. J Natl Compr Canc Netw. 2019;17:574–578. doi: 10.6004/jnccn.2019.5005. [DOI] [PubMed] [Google Scholar]
- 5.Kanwal B., Biswas S., Seminara R.S., Jeet C. Immunotherapy in advanced non-small cell lung cancer patients: ushering chemotherapy through the checkpoint inhibitors? Cureus. 2018;10:e3254. doi: 10.7759/cureus.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad M.M., Govindan R., Balogh K.N., Spigel D.R., Garon E.B., Bushway M.E., et al. Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell. 2022;40:1010–1026.e1011. doi: 10.1016/j.ccell.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Thai A.A., Solomon B.J., Sequist L.V., Gainor J.F., Heist R.S. Lung cancer. Lancet. 2021;398:535–554. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 8.Fan X.X., Wu Q. Decoding lung cancer at single-cell level. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.883758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leader A.M., Grout J.A., Maier B.B., Nabet B.Y., Park M.D., Tabachnikova A., et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell. 2021;39:1594–1609. doi: 10.1016/j.ccell.2021.10.009. e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang X., Huang Y., Lei J., Luo H., Zhu X. The single-cell sequencing: new developments and medical applications. Cell Biosci. 2019;9:53. doi: 10.1186/s13578-019-0314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegenhain C., Vieth B., Parekh S., Reinius B., Guillaumet-Adkins A., Smets M., et al. Comparative analysis of single-cell RNA sequencing methods. Mol Cell. 2017;65:631–643. doi: 10.1016/j.molcel.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Mustachio L.M., Roszik J. Single-cell sequencing: current applications in precision onco-genomics and cancer therapeutics. Cancers (Basel) 2022;14 doi: 10.3390/cancers14030657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Meng D., Guo H., Sun C., Chen P., Jiang M., et al. Single-cell sequencing, an advanced technology in lung cancer research. Onco Targets Ther. 2021;14:1895–1909. doi: 10.2147/OTT.S295102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro E., Biezuner T., Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet. 2013;14:618–630. doi: 10.1038/nrg3542. [DOI] [PubMed] [Google Scholar]
- 15.Kashima Y., Sakamoto Y., Kaneko K., Seki M., Suzuki Y., Suzuki A. Single-cell sequencing techniques from individual to multiomics analyses. Exp Mol Med. 2020;52:1419–1427. doi: 10.1038/s12276-020-00499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arsenio J. Single-cell transcriptomics of immune cells: cell isolation and cDNA library generation for scRNA-Seq. Methods Mol Biol. 2020;2184:1–18. doi: 10.1007/978-1-0716-0802-9_1. [DOI] [PubMed] [Google Scholar]
- 17.Goetz J.J., Trimarchi J.M. Transcriptome sequencing of single cells with Smart-Seq. Nat Biotechnol. 2012;30:763–765. doi: 10.1038/nbt.2325. [DOI] [PubMed] [Google Scholar]
- 18.Picelli S., Bjorklund A.K., Faridani O.R., Sagasser S., Winberg G., Sandberg R. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat Methods. 2013;10:1096–1098. doi: 10.1038/nmeth.2639. [DOI] [PubMed] [Google Scholar]
- 19.Sasagawa Y., Danno H., Takada H., Ebisawa M., Tanaka K., Hayashi T., et al. Quartz-Seq2: a high-throughput single-cell RNA-sequencing method that effectively uses limited sequence reads. Genome Biol. 2018;19:29. doi: 10.1186/s13059-018-1407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagnoli J.W., Ziegenhain C., Janjic A., Wange L.E., Vieth B., Parekh S., et al. Sensitive and powerful single-cell RNA sequencing using mcSCRB-seq. Nat Commun. 2018;9:2937. doi: 10.1038/s41467-018-05347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimshony T., Wagner F., Sher N., Yanai I. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2012;2:666–673. doi: 10.1016/j.celrep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Alpern D., Gardeux V., Russeil J., Mangeat B., Meireles-Filho A.C.A., Breysse R., et al. BRB-seq: ultra-affordable high-throughput transcriptomics enabled by bulk RNA barcoding and sequencing. Genome Biol. 2019;20:71. doi: 10.1186/s13059-019-1671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye C., Ho D.J., Neri M., Yang C., Kulkarni T., Randhawa R., et al. DRUG-seq for miniaturized high-throughput transcriptome profiling in drug discovery. Nat Commun. 2018;9:4307. doi: 10.1038/s41467-018-06500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veeranagouda Y., Zachayus J.L., Guillemot J.C., Venier O., Didier M. High-throughput cellular RNA sequencing (HiCAR-Seq): cost-effective, high-throughput 3' mRNA-Seq method enabling individual sample quality control. Curr Protoc Mol Biol. 2020;132:e123. doi: 10.1002/cpmb.123. [DOI] [PubMed] [Google Scholar]
- 25.Hong M., Tao S., Zhang L., Diao L.T., Huang X., Huang S., et al. RNA sequencing: new technologies and applications in cancer research. J Hematol Oncol. 2020;13:166. doi: 10.1186/s13045-020-01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong Z.X., Ho W.Y., Yeap S.K., Wang M.L., Chien Y., Verusingam N.D., et al. Single-cell RNA sequencing in human lung cancer: Applications, challenges, and pathway towards personalized therapy. J Chin Med Assoc. 2021;84:563–576. doi: 10.1097/JCMA.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 27.VanOudenhove J., Halene S., Mendez L. Is it the time to integrate novel sequencing technologies into clinical practice? Curr Opin Hematol. 2023;30:70–77. doi: 10.1097/MOH.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 28.Huang L., Ma F., Chapman A., Lu S., Xie X.S. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu Rev Genomics Hum Genet. 2015;16:79–102. doi: 10.1146/annurev-genom-090413-025352. [DOI] [PubMed] [Google Scholar]
- 29.Zong C., Lu S., Chapman A.R., Xie X.S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science. 2012;338:1622–1626. doi: 10.1126/science.1229164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu S., Zong C., Fan W., Yang M., Li J., Chapman A.R., et al. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Science. 2012;338:1627–1630. doi: 10.1126/science.1229112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waarts M.R., Stonestrom A.J., Park Y.C. Levine RL: Targeting mutations in cancer. J Clin Invest. 2022:132. doi: 10.1172/JCI154943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morf J., Wingett S.W., Farabella I., Cairns J., Furlan-Magaril M., Jimenez-Garcia L.F., et al. RNA proximity sequencing reveals the spatial organization of the transcriptome in the nucleus. Nat Biotechnol. 2019;37:793–802. doi: 10.1038/s41587-019-0166-3. [DOI] [PubMed] [Google Scholar]
- 33.Lin J., Xue X., Wang Y., Zhou Y., Wu J., Xie H., et al. scNanoCOOL-seq: a long-read single-cell sequencing method for multi-omics profiling within individual cells. Cell Res. 2023;33:879–882. doi: 10.1038/s41422-023-00873-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J., Hyeon D.Y., Hwang D. Single-cell multiomics: technologies and data analysis methods. Exp Mol Med. 2020;52:1428–1442. doi: 10.1038/s12276-020-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R., Ferdinand J.R., Loudon K.W., Bowyer G.S., Laidlaw S., Muyas F., et al. Mapping single-cell transcriptomes in the intra-tumoral and associated territories of kidney cancer. Cancer Cell. 2022;40:1583–1599.e1510. doi: 10.1016/j.ccell.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitra A., Andrews M.C., Roh W., De Macedo M.P., Hudgens C.W., Carapeto F., et al. Spatially resolved analyses link genomic and immune diversity and reveal unfavorable neutrophil activation in melanoma. Nat Commun. 1839;2020:11. doi: 10.1038/s41467-020-15538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philpott M., Cribbs A.P., Brown T., Jr., Brown T., Sr., Oppermann U. Advances and challenges in epigenomic single-cell sequencing applications. Curr Opin Chem Biol. 2020;57:17–26. doi: 10.1016/j.cbpa.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Alderton G.K. Epigenetic and genetic heterogeneity in metastasis. Nat Rev Cancer. 2017;17:141. doi: 10.1038/nrc.2017.11. [DOI] [PubMed] [Google Scholar]
- 39.Casado-Pelaez M., Bueno-Costa A., Esteller M. Single cell cancer epigenetics. Trends in Cancer. 2022;8:820–838. doi: 10.1016/j.trecan.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Carter B., Zhao K. The epigenetic basis of cellular heterogeneity. Nat Rev Genet. 2020;22:235–250. doi: 10.1038/s41576-020-00300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao X., Wang X., Guan S., Lin H., Yan G., Gao M., et al. Integrated Proteome Analysis Device for Fast Single-Cell Protein Profiling. Anal Chem. 2018;90:14003–14010. doi: 10.1021/acs.analchem.8b03692. [DOI] [PubMed] [Google Scholar]
- 42.Pham T., Tyagi A., Wang Y.S., Guo J. Single-cell proteomic analysis. WIREs Mech Dis. 2021;13:e1503. doi: 10.1002/wsbm.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennett H.M., Stephenson W., Rose C.M., Darmanis S. Single-cell proteomics enabled by next-generation sequencing or mass spectrometry. Nat Methods. 2023;20:363–374. doi: 10.1038/s41592-023-01791-5. [DOI] [PubMed] [Google Scholar]
- 44.Kelly R.T. Single-cell proteomics: progress and prospects. Mol Cell Proteomics. 2020;19:1739–1748. doi: 10.1074/mcp.R120.002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoeckius M., Hafemeister C., Stephenson W., Houck-Loomis B., Chattopadhyay P.K., Swerdlow H., et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017;14:865–868. doi: 10.1038/nmeth.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mistry A.M., Greenplate A.R., Ihrie R.A., Irish J.M. Beyond the message: advantages of snapshot proteomics with single-cell mass cytometry in solid tumors. FEBS J. 2019;286:1523–1539. doi: 10.1111/febs.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Luo J., Jiang H., Xie T., Zheng J., Tian Y., et al. The tumor suppressor role of zinc finger protein 671 (ZNF671) in multiple tumors based on cancer single-cell sequencing. Front Oncol. 2019;9:1214. doi: 10.3389/fonc.2019.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerzeli I.K., Lord M., Doroszko M., Elgendy R., Chourlia A., Stepanek I., et al. Single-cell RNAseq and longitudinal proteomic analysis of a novel semi-spontaneous urothelial cancer model reveals tumor cell heterogeneity and pretumoral urine protein alterations. PLoS One. 2021;16:e0253178. doi: 10.1371/journal.pone.0253178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholson A.G., Tsao M.S., Beasley M.B., Borczuk A.C., Brambilla E., Cooper W.A., et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17:362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Riihimaki M., Hemminki A., Fallah M., Thomsen H., Sundquist K., Sundquist J., et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 51.Bernstein E., Bade B.C., Akgün K.M., Rose M.G., Cain H.C. Barriers and facilitators to lung cancer screening and follow-up. Semin Oncol. 2022;49:213–219. doi: 10.1053/j.seminoncol.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Schegoleva A.A., Khozyainova A.A., Fedorov A.A., Gerashchenko T.S., Rodionov E.O., Topolnitsky E.B., et al. Prognosis of different types of non-small cell lung cancer progression: current state and perspectives. Cell Physiol Biochem. 2021;55:29–48. doi: 10.33594/000000340. [DOI] [PubMed] [Google Scholar]
- 53.Nishi W., Hayashi K. Lung metastases from pancreatic cancer initially suspected to be primary lung cancer due to lepidic tumor growth. Kyobu Geka. 2021;74:1091–1094. [PubMed] [Google Scholar]
- 54.Zens P., Bello C., Scherz A., Koenigsdorf J., Pollinger A., Schmid R.A., et al. A prognostic score for non-small cell lung cancer resected after neoadjuvant therapy in comparison with the tumor-node-metastases classification and major pathological response. Mod Pathol. 2021;34:1333–1344. doi: 10.1038/s41379-021-00777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yavropoulou M.P., Poulios C., Foroulis C., Tournis S., Hytiroglou P., Kotsa K., et al. Distant lung metastases caused by a histologically benign phosphaturic mesenchymal tumor. Endocrinol Diabetes Metab Case Rep. 2018;2018 doi: 10.1530/EDM-18-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ng K.W., Boumelha J., Enfield K.S.S., Almagro J., Cha H., Pich O., Karasaki T., Moore D.A., Salgado R., Sivakumar M., et al. Antibodies against endogenous retroviruses promote lung cancer immunotherapy. Nature. 2023;616:563–573. doi: 10.1038/s41586-023-05771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frankell A.M., Dietzen M., Al Bakir M., Lim E.L., Karasaki T., Ward S., et al. The evolution of lung cancer and impact of subclonal selection in TRACERx. Nature. 2023;616:525–533. doi: 10.1038/s41586-023-05783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al Bakir M., Huebner A., Martinez-Ruiz C., Grigoriadis K., Watkins T.B.K., Pich O., et al. The evolution of non-small cell lung cancer metastases in TRACERx. Nature. 2023;616:534–542. doi: 10.1038/s41586-023-05729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karasaki T., Moore D.A., Veeriah S., Naceur-Lombardelli C., Toncheva A., Magno N., et al. Evolutionary characterization of lung adenocarcinoma morphology in TRACERx. Nat Med. 2023;29:833–845. doi: 10.1038/s41591-023-02230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez-Ruiz C., Black J.R.M., Puttick C., Hill M.S., Demeulemeester J., Larose Cadieux E., et al. Genomic-transcriptomic evolution in lung cancer and metastasis. Nature. 2023;616:543–552. doi: 10.1038/s41586-023-05706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbosh C., Frankell A.M., Harrison T., Kisistok J., Garnett A., Johnson L., et al. Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature. 2023;616:553–562. doi: 10.1038/s41586-023-05776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 63.He D., Wang D., Lu P., Yang N., Xue Z., Zhu X., et al. Single-cell RNA sequencing reveals heterogeneous tumor and immune cell populations in early-stage lung adenocarcinomas harboring EGFR mutations. Oncogene. 2021;40:355–368. doi: 10.1038/s41388-020-01528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho D.W., Tsui Y.M., Chan L.K., Sze K.M., Zhang X., Cheu J.W., et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun. 2021;12:3684. doi: 10.1038/s41467-021-24010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jia Q., Chu H., Jin Z., Long H., Zhu B. High-throughput single-small es, Cyrillicell sequencing in cancer research. Signal Transduct Target Ther. 2022;7:145. doi: 10.1038/s41392-022-00990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bischoff P., Trinks A., Obermayer B., Pett J.P., Wiederspahn J., Uhlitz F., et al. Single-cell RNA sequencing reveals distinct tumor microenvironmental patterns in lung adenocarcinoma. Oncogene. 2021;40:6748–6758. doi: 10.1038/s41388-021-02054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xue T., Zhao X., Zhao K., Lu Y., Yao J., Ji X. Immunotherapy for lung cancer: Focusing on chimeric antigen receptor (CAR)-T cell therapy. Curr Probl Cancer. 2022;46 doi: 10.1016/j.currproblcancer.2021.100791. [DOI] [PubMed] [Google Scholar]
- 68.Reck M., Remon J., Hellmann M.D. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 2022;40:586–597. doi: 10.1200/JCO.21.01497. [DOI] [PubMed] [Google Scholar]
- 69.Weber E.W., Maus M.V., Mackall C.L. The emerging landscape of immune cell therapies. Cell. 2020;181:46–62. doi: 10.1016/j.cell.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He J., Hu Y., Hu M., Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep. 2015;5:13110. doi: 10.1038/srep13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Darvin P., Toor S.M., Sasidharan Nair V., Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esensten J.H., Bluestone J.A., Lim W.A. Engineering therapeutic T cells: from synthetic biology to clinical trials. Annu Rev Pathol. 2017;12:305–330. doi: 10.1146/annurev-pathol-052016-100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He J., Xiong X., Yang H., Li D., Liu X., Li S., et al. Defined tumor antigen-specific T cells potentiate personalized TCR-T cell therapy and prediction of immunotherapy response. Cell Res. 2022;32:530–542. doi: 10.1038/s41422-022-00627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rath J.A., Arber C. Engineering strategies to enhance TCR-based adoptive T Cell therapy. Cells. 2020;9:1485. doi: 10.3390/cells9061485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mizukoshi E., Kaneko S. Immune cell therapy for hepatocellular carcinoma. J Hematol Oncol. 2019;12 doi: 10.1186/s13045-019-0742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee D.A. Cellular therapy: Adoptive immunotherapy with expanded natural killer cells. Immunol Rev. 2019;290:85–99. doi: 10.1111/imr.12793. [DOI] [PubMed] [Google Scholar]
- 77.Liu S., Meng Y., Liu L., Lv Y., Yu W., Liu T., et al. CD4+ T cells are required to improve the efficacy of CIK therapy in non-small cell lung cancer. Cell Death Dis. 2022;13 doi: 10.1038/s41419-022-04882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Z., Zhou Z., Dang Q., Xu H., Lv J., Li H., et al. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T cell therapy. Theranostics. 2022;12:6273–6290. doi: 10.7150/thno.76854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 80.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Velcheti V., Schalper K.A., Carvajal D.E., Anagnostou V.K., Syrigos K.N., Sznol M., et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agustoni F., Hirsch F.R. PACIFIC trial: new perspectives for immunotherapy in lung cancer. Transl Lung Cancer Res. 2018;7:S19–S24. doi: 10.21037/tlcr.2017.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhong R., Zhang Y., Chen D., Cao S., Han B., Zhong H. Single-cell RNA sequencing reveals cellular and molecular immune profile in a Pembrolizumab-responsive PD-L1-negative lung cancer patient. Cancer Immunol Immunother. 2021;70:2261–2274. doi: 10.1007/s00262-021-02848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen X., Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018:k3529. doi: 10.1136/bmj.k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schneider B.J., Naidoo J., Santomasso B.D., Lacchetti C., Adkins S., Anadkat M., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 86.Petitprez F., Meylan M., de Reynies A., Sautes-Fridman C., Fridman W.H. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front Immunol. 2020;11:784. doi: 10.3389/fimmu.2020.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou L., Liang H., Ge Y., Ding W., Chen Q., Zhang T., et al. Precisely targeted nano-controller of PD-L1 level for non-small cell lung cancer spinal metastasis immunotherapy. Adv Healthc Mater. 2022;11:e2200938. doi: 10.1002/adhm.202200938. [DOI] [PubMed] [Google Scholar]
- 88.Zhou J., Jiang Y., Huang Y., Wang Q., Kaifi J.T., Kimchi E.T., et al. Single-cell RNA sequencing to characterize the response of pancreatic cancer to anti-PD-1 immunotherapy. Transl Oncol. 2022;15 doi: 10.1016/j.tranon.2021.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou M., Ma Y., Chianga C.C., Rock E.C., Luker K.E., Luker G.D., et al. High-throughput cellular heterogeneity analysis in cell migration at the single-cell level. Small. 2022:e2206754. doi: 10.1002/smll.202206754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zilionis R., Engblom C., Pfirschke C., Savova V., Zemmour D., Saatcioglu H.D., et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–1334. doi: 10.1016/j.immuni.2019.03.009. e1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian Y., Li Q., Yang Z., Zhang S., Xu J., Wang Z., et al. Single-cell transcriptomic profiling reveals the tumor heterogeneity of small-cell lung cancer. Signal Transduct Target Ther. 2022;7:346. doi: 10.1038/s41392-022-01150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sinjab A., Han G., Treekitkarnmongkol W., Hara K., Brennan P.M., Dang M., et al. Resolving the spatial and cellular architecture of lung adenocarcinoma by multiregion single-cell sequencing. Cancer Discov. 2021;11:2506–2523. doi: 10.1158/2159-8290.CD-20-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maynard A., McCoach C.E., Rotow J.K., Harris L., Haderk F., Kerr D.L., et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell. 2020;182:1232–1251. doi: 10.1016/j.cell.2020.07.017. e1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McGranahan N., Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168:613–628. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 95.Lim Z.-F., Ma P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12 doi: 10.1186/s13045-019-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L., Chen L., Li S.C., Wang M., Li C., Song T., et al. Heterogeneity in lung cancers by single-cell DNA sequencing. Clin Transl Med. 2023;13:e1388. doi: 10.1002/ctm2.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu X., Fujimoto J., Ying L., Fukuoka J., Ashizawa K., Sun W., et al. Multi-region exome sequencing reveals genomic evolution from preneoplasia to lung adenocarcinoma. Nat Commun. 2019;10:2978. doi: 10.1038/s41467-019-10877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang J., Fujimoto J., Zhang J., Wedge D.C., Song X., Zhang J., et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–259. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu F., Fan J., He Y., Xiong A., Yu J., Li Y., et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat Commun. 2021;12:2540. doi: 10.1038/s41467-021-22801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jamal-Hanjani M., Wilson G.A., McGranahan N., Birkbak N.J., Watkins T.B.K., Veeriah S., et al. Tracking the evolution of non–small-cell lung cancer. N Engl J Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 101.Kim K.-T., Lee H.W., Lee H.-O., Kim S.C., Seo Y.J., Chung W., et al. Single-cell mRNA sequencing identifies subclonal heterogeneity in anti-cancer drug responses of lung adenocarcinoma cells. Genome Biol. 2015;16 doi: 10.1186/s13059-015-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adachi H., Ito H., Sawabata N. Circulating tumor cells and the non-touch isolation technique in surgery for non-small-cell lung cancer. Cancers (Basel) 2022;14 doi: 10.3390/cancers14061448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keller L., Pantel K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer. 2019;19:553–567. doi: 10.1038/s41568-019-0180-2. [DOI] [PubMed] [Google Scholar]
- 104.Barbirou M., Miller A., Manjunath Y., Ramirez A.B., Ericson N.G., Staveley-O'Carroll K.F., et al. Single circulating-tumor-cell-targeted sequencing to identify somatic variants in liquid biopsies in non-small-cell lung cancer patients. Curr Issues Mol Biol. 2022;44:750–763. doi: 10.3390/cimb44020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou X., Wang N., Zhang Y., Yu H., Wu Q. KAT2B is an immune infiltration-associated biomarker predicting prognosis and response to immunotherapy in non-small cell lung cancer. Invest New Drugs. 2022;40:43–57. doi: 10.1007/s10637-021-01159-6. [DOI] [PubMed] [Google Scholar]
- 106.Coleman R.E., Collinson M., Gregory W., Marshall H., Bell R., Dodwell D., et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04) J Bone Oncol. 2018;13:123–135. doi: 10.1016/j.jbo.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng Y., Wang P.P., Fu Y., Chen Y.Y., Ding Z.Y. Zoledronic acid enhances the efficacy of immunotherapy in non-small cell lung cancer. Int Immunopharmacol. 2022;110 doi: 10.1016/j.intimp.2022.109030. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X., Xu Q., Yu X., Huang M., Li S., Sheng L., et al. What Is long-term survival and which first-line immunotherapy brings long-term survival for advanced wild-type non-small cell lung cancer: a network meta-analysis based on integrated analysis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.764643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caushi J.X., Zhang J., Ji Z., Vaghasia A., Zhang B., Hsiue E.H., et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. 2021;596:126–132. doi: 10.1038/s41586-021-03752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suvà M.L., Tirosh I. Single-cell RNA sequencing in cancer: lessons learned and emerging challenges. Mol Cell. 2019;75:7–12. doi: 10.1016/j.molcel.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 111.Jovic D., Liang X., Zeng H., Lin L., Xu F., Luo Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin Transl Med. 2022;12 doi: 10.1002/ctm2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kolodziejczyk Aleksandra A., Kim J.K., Svensson V., Marioni John C., Teichmann Sarah A. The technology and biology of single-cell RNA sequencing. Mol Cell. 2015;58:610–620. doi: 10.1016/j.molcel.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 113.Dong M., Thennavan A., Urrutia E., Li Y., Perou C.M., Zou F., et al. SCDC: bulk gene expression deconvolution by multiple single-cell RNA sequencing references. Brief Bioinform. 2020;22:416–427. doi: 10.1093/bib/bbz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dal Molin A., Di Camillo B. How to design a single-cell RNA-sequencing experiment: pitfalls, challenges and perspectives. Brief Bioinform. 2018;20:1384–1394. doi: 10.1093/bib/bby007. [DOI] [PubMed] [Google Scholar]
- 115.Denyer T., Timmermans M.C.P. Crafting a blueprint for single-cell RNA sequencing. Trends Plant Sci. 2022;27:92–103. doi: 10.1016/j.tplants.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 116.Zeng X., Guo X., Jiang S., Yang X., Zhong Z., Liu S., et al. Digital-scRRBS: a cost-effective, highly sensitive, and automated single-cell methylome analysis platform via digital microfluidics. Anal Chem. 2023;95:13313–13321. doi: 10.1021/acs.analchem.3c02484. [DOI] [PubMed] [Google Scholar]
- 117.Guo H., Zhu P., Guo F., Li X., Wu X., Fan X., et al. Profiling DNA methylome landscapes of mammalian cells with single-cell reduced-representation bisulfite sequencing. Nat Protoc. 2015;10:645–659. doi: 10.1038/nprot.2015.039. [DOI] [PubMed] [Google Scholar]
- 118.Ku W.L., Nakamura K., Gao W., Cui K., Hu G., Tang Q., et al. Single-cell chromatin immunocleavage sequencing (scChIC-seq) to profile histone modification. Nat Methods. 2019;16:323–325. doi: 10.1038/s41592-019-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Buenrostro J.D., Wu B., Litzenburger U.M., Ruff D., Gonzales M.L., Snyder M.P., et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523:486–490. doi: 10.1038/nature14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Farlik M., Sheffield N.C., Nuzzo A., Datlinger P., Schönegger A., Klughammer J., et al. Single-cell DNA methylome sequencing and bioinformatic inference of epigenomic cell-state dynamics. Cell Rep. 2015;10:1386–1397. doi: 10.1016/j.celrep.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vitak S.A., Torkenczy K.A., Rosenkrantz J.L., Fields A.J., Christiansen L., Wong M.H., et al. Sequencing thousands of single-cell genomes with combinatorial indexing. Nat Methods. 2017;14:302–308. doi: 10.1038/nmeth.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Falconer E., Hills M., Naumann U., Poon S.S., Chavez E.A., Sanders A.D., et al. DNA template strand sequencing of single-cells maps genomic rearrangements at high resolution. Nat Methods. 2012;9:1107–1112. doi: 10.1038/nmeth.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hagemann-Jensen M., Ziegenhain C., Sandberg R. Scalable single-cell RNA sequencing from full transcripts with Smart-seq3xpress. Nat Biotechnol. 2022;40:1452–1457. doi: 10.1038/s41587-022-01311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Macosko E.Z., Basu A., Satija R., Nemesh J., Shekhar K., Goldman M., et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rubakhin S.S., Romanova E.V., Nemes P., Sweedler J.V. Profiling metabolites and peptides in single cells. Nat Methods. 2011;8:S20–S29. doi: 10.1038/nmeth.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]