Abstract
Background
Patients without diabetes, composed of those with normal glucose regulation or prediabetes, experience a higher complication rate than patients with diabetes in response to surgical stress. It is unknown if excess complications occur equally among all patients without diabetes and if hyperglycemia or inflammatory indices are the best markers of postoperative complications.
Methods
In this retrospective review of medical records, postoperative infections, acute kidney injury (AKI), and composite in-hospital complications were measured after colorectal surgery in patients with varying states of preoperative glucose metabolism. The effects that postoperative hyperglycemia (glucose > 180 mg/dl) and inflammation (systemic inflammatory response index (SIRI) had on the complications within each group were examined.
Results
Hyperglycemic patients without diabetes experienced excess infections (P = 0.028), AKI (P = 0.023), and in-hospital complications (P = 0.009) in comparison with patients with diabetes. However, excess infections occurred exclusively in patients with normal glucose regulation (P = 0.039), not those with prediabetes (P = 0.166), and were associated with inflammation (P = 0.020) and not severe hyperglycemia on multivariable analysis. In contrast, exaggerated AKI was found only in patients with prediabetes (P = 0.005), not with normal glucose regulation (P = 0.296), and was independently associated with elevated morning glucose values (P = 0.000).
Conclusions
Excess complications among patients without diabetes are concentrated in patients with normal glucose regulation or prediabetes. Inflammatory markers are a better predictor of infections, while early hyperglycemia reflects a process injurious to kidney function.
Keywords: Diabetic paradox, Postoperative hyperglycemia, Inflammation, Complications
Background
Perioperative hyperglycemia in patients without diabetes is associated with more significant complications than a similar magnitude of hyperglycemia in patients with diabetes [1]. Multiple surgical specialties have evidence of this paradox [2–7]. However, patients without diabetes are a heterogeneous population, and it is not known if overall or individual complications occur with equal frequency among patients with normal glucose regulation or prediabetes. Since patients with prediabetes are intermediate between those with normal glucose regulation or diabetes, inclusion with either group could affect any analysis of postoperative results and prevention or treatment strategies.
Perioperative hyperglycemia is a marker for complications in patients without diabetes, as evidenced by the diabetic paradox, but it is not known if hyperglycemia is the best marker for individual complications such as infections and AKI. This retrospective, exploratory review of medical records was undertaken for two reasons: first, to determine if all patients without diabetes are equally affected by postoperative hyperglycemia; second, to examine whether a cellular-based inflammatory marker (systemic inflammatory response index SIRI) is a better predictor of complications among patients without diabetes. If inflammation is a strong predictor of complications, then anti-inflammatory measures might be added to anti-hyperglycemic treatment to improve outcomes.
Methods
To address these questions, we updated and retrospectively reviewed the colorectal database of Bayhealth Medical Center (Dover, DE), which was previously described [8, 9], to include 1418 consecutive patients with major colorectal surgery from 8/01/2016 through 12/31/2023. Operations included colon resections and ostomy procedures, including elective or emergent cases, and employing either an open or minimally invasive approach. Septic patients, defined as patients with positive cultures on the day of the procedure, were excluded. The Bayhealth Institutional Review Board (BH 25) approved the study. The Bayhealth IRB waived the requirement for individual consents, and individual consents were not obtained for this study.
In January 2018, Bayhealth initiated an enhanced glucose management program for colorectal surgery. Patients were defined as having diabetes, prediabetes, or normal glucose regulation, using predominantly a preoperative fasting glucose, a HbA1C, if available, or a clinical history of diabetes like previously described [10]. If a fasting glucose was unavailable, a casual glucose was used. Patients with diabetes were identified with a fasting glucose ≥ 126 mg/dl, a casual glucose ≥ 200 mg/dl, a HbA1C ≥ 6.5, or a history of diabetes. Almost all patients with diabetes had confirmatory tests.
Patients without diabetes were classified as recommended by the ADA based on serum glucose levels and HbA1C, if available [11]. Patients with prediabetes were recognized with a fasting glucose ≥ 100 mg/dl and < 126 mg/dl or a HbA1C ≥ 5.7 and ≤ 6.4. Casual glucose values between 140 mg/dl and 199 mg/dl indicated prediabetes. If both a glucose value and HbA1C were available, any test indicating prediabetes was used to classify the patient as having prediabetes. Almost all patients with prediabetes derived from a casual glucose were confirmed with a Hg A1C. Patients with normal glucose regulation had all tests within the normal range (fasting glucose < 100 mg/dl, casual glucose < 140 mg/dl, HbA1C < 5.7).
In the immediate 48 h after surgery, patients were considered to have severe postoperative hyperglycemia with any single glucose value ≥ 180 mg/dl [12].
Patients were part of a glucose management team conducted by a clinical pharmacist, as previously described [10]. The basal/bolus insulin approach was used postoperatively to treat patients with diabetes [13], whether or not they were treated preoperatively with insulin or oral agents. Oral diabetes medications were initially withheld, except for DPP-4 inhibitors. Patients without diabetes with severe hyperglycemia were treated with the basal/bolus technique when feasible but most often treated with correctional insulin.
Derivatives of the complete blood count were used as markers for inflammation on the first day after surgery (SIRI 1), like what is described by other cellular-based inflammatory markers [14, 15].
Postoperative complications
Postoperative complications included infections, acute kidney injury, non-infectious complications (DVT/PE, acute myocardial infarction or stroke, or cardiopulmonary arrest or failure, as defined by software from Conduent-MIDAS Health Solutions), as previously described [8], and 30-day readmissions. In-hospital complications included infections, acute kidney injury, and non-infectious complications. Total complications included in-hospital complications and 30-day readmissions.
A postoperative infection was defined as a patient with any positive pathological culture from the first postoperative day through day 30. Cultures with normal skin flora or mixed urogenital flora were not considered positive. Acute kidney injury was determined with the KDIGO criteria as an increase in creatinine of ≥ 0.3 mg/dl within the first 48 h or an increase of ≥ 1.5 times the baseline creatinine from days 3 through 7. Urine output was not used to define AKI. Postoperative infections, defined by culture criteria, and AKI, defined with increased creatinine, were chosen as suitable parameters to assess postoperative outcomes because they were laboratory data not subject to clinical interpretation.
Predictors of outcomes
Univariate predictors of outcomes were selected from demographics, preoperative and postoperative (within 48 h of surgery) laboratories, and procedure-related features. The outcomes of the regression analyses were postoperative infection, AKI, in-hospital complications, and total complications. All significant univariate predictors were evaluated in a multivariable model with backward elimination to determine the independent predictors of the outcomes. In addition, SIRI 1 was also treated as an outcome so that the predictors of inflammation on the first day after surgery could be assessed.
Continuous data was expressed as means ± standard deviations. Categorical data was expressed as percentages. Two sample t-tests and tests for two proportions were used to compare groups. Regression analyses were run with XLSTAT software (Lumivero [2024]). XLSTAT statistical and data analysis solution. (New York, USA.), with significant (P < 0.05) univariate predictors used for multivariable analysis.
Results
Patient population
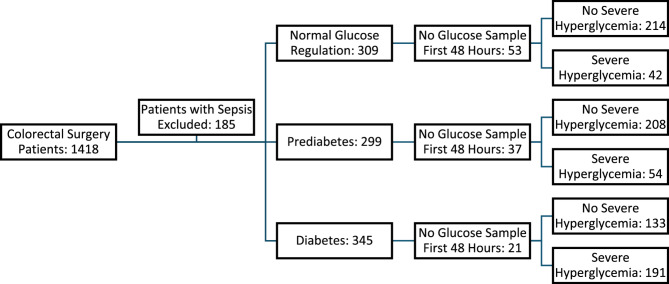
Of the 1418 patients who underwent colorectal surgery, 185 had sepsis on the day of surgery, leaving 1233 patients for further study. Two hundred eighty patients did not have adequate preoperative testing to define their preoperative metabolic status. Of the remaining 953 non-septic patients whose metabolic status was determined preoperatively, 111 (11.7%) did not undergo glucose sampling within the first 48 h postoperatively. At least one glucose sample was taken within the first 48 h postoperatively in 842 (88.3%) metabolically defined patients, or 68.3% of the total population studied. A flow diagram of the patients available for analysis is shown in Fig. 1.
Fig. 1.
Flow Diagram of Patients Available for Study
The demographics of the 953 patients in the three metabolic groups are presented in Table 1 and are compared with ANOVA. There were significant differences in age, American Society of Anesthesiologists (ASA) score, and BMI among the three groups, with nonsignificant differences in the percentage of males and emergent cases. Patients with diabetes were older and had a higher ASA score and BMI. The demographics did not change in the subpopulations of patients with glucose samples in the first 48 h postoperatively (data not shown).
Table 1.
Demographics of patients with normal glucose regulation, prediabetes, and diabetes
| Metabolic Status | Patients | Age (yrs) | ASA | BMI (kg/m2) | % Male | Emergent |
|---|---|---|---|---|---|---|
| Normal | 309 | 64.2 ± 14.8 | 2.9 ± 0.7 | 28.3 ± 6.6 | 137 (44.3%) | 42 (13.6%) |
| Pre Diabetes | 299 | 66.2 ± 13.4 | 2.9 ± 0.7 | 29.1 ± 7.1 | 161 (53.8%) | 37 (12.4%) |
| Diabetes | 345 | 67.2 ± 11.9 | 3.1 ± 0.6 | 32.1 ± 10.2 | 187 (54.2%) | 56 (16.2%) |
| P Value | P = 0.016 | P < 0.0001 | P < 0.0001 | P = 0.928 | P = 0.165 |
The three metabolic groups’ preoperative laboratory values and procedure-related characteristics are presented in Tables 2 and 3. The patients with normal glucose regulation had some favorable (lower creatinine, HbA1C) and unfavorable (lower Hg, albumin) characteristics compared to the prediabetic or diabetic groups. However, the three groups’ procedure-related characteristics of duration, operative approach, and transfusion requirements were similar. The categorization of patients into those with or without severe hyperglycemia did not change the similarity of the demographic characteristics, as seen in Table 4.
Table 2.
Preoperative laboratories
| Metabolic Status | Patients | Hg (g/dl) | Creatinine (mg/dl) | HbA1C | Albumin (g/dl) | WBC (103 cells/µL) |
|---|---|---|---|---|---|---|
| Normal | 309 | 11.9 ± 2.2 | 1.0 ± 0.5 | 5.3 ± 0.3 | 3.1 ± 0.7 | 8.2 ± 4.3 |
| Pre Diabetes | 299 | 12.4 ± 2.0 | 1.0 ± 0.8 | 5.8 ± 0.4 | 3.2 ± 0.7 | 8.8 ± 5.4 |
| Diabetes | 345 | 11.9 ± 2.4 | 1.2 ± 0.8 | 6.8 ± 1.4 | 3.1 ± 0.7 | 9.1 ± 5.1 |
| P Value | P = 0.008 | P = 0.003 | P < 0.0001 | P = 0.002 | P = 0.064 |
Table 3.
Procedure-related characteristics
| Metabolic Status | Patients | Procedure Duration (min) | Transfusion (ml) | Laparoscopic |
|---|---|---|---|---|
| Normal | 309 | 259 ± 122 | 322 ± 915 | 141 (45.6%) |
| Pre Diabetes | 299 | 268 ± 106 | 320 ± 2955 | 147 (49.2%) |
| Diabetes | 345 | 264 ± 109 | 257 ± 872 | 179 (51.9%) |
| P Value | P = 0.483 | P = 0.869 | P = 0.491 |
Table 4.
Demographics of metabolic groups stratified by severe hyperglycemia
| Metabolic Status | Patients | Age | ASA | BMI | Male | Emergent | |
|---|---|---|---|---|---|---|---|
| Normal | No Hyperglycemia | 214 | 64.7 ± 15.0 | 2.9 ± 0.7 | 28.4 ± 6.5 | 98 (45.8%) | 27 (12.6%) |
| Hyperglycemia | 42 | 62.2 ± 13.1 | 3.1 ± 0.8 | 28.3 ± 6.0 | 17 (40.5%) | 4 (9.5%) | |
| P Value | 0.317 | 0.116 | 0.928 | 0.639 | 0.742 | ||
| Pre Diabetes | No Hyperglycemia | 208 | 65.8 ± 13.3 | 2.9 ± 0.7 | 29.2 ± 6.7 | 116 (55.8%) | 25 (12.0%) |
| Hyperglycemia | 54 | 66.9 ± 12.9 | 2.8 ± 0.7 | 29.0 ± 8.2 | 27 (50.0%) | 6 (11.1%) | |
| P Value | 0.590 | 0.760 | 0.825 | 0.546 | 1.000 | ||
| Diabetes | No Hyperglycemia | 133 | 67.5 ± 11.4 | 3.1 ± 0.7 | 31.4 ± 7.0 | 68 (51.1%) | 22 (16.5%) |
| Hyperglycemia | 191 | 67.2 ± 12.1 | 3.1 ± 0.6 | 32.5 ± 11.9 | 107 (56.0%) | 32 (16.8%) | |
| P Value | 0.804 | 0.530 | 0.332 | 0.450 | 1.000 |
We also examined whether the baseline and procedure-related characteristics differed between patients with prediabetes or normal glucose regulation. There was no difference in age (P = 0.079), BMI (P = 0.125), ASA score (P = 0.550), or creatinine (P = 0.208) between the two groups without diabetes. There was a difference in Hg (P = 0.007) and albumin (P = 0.005), with the prediabetes group having more favorable levels.
Perioperative glucose measurements
Perioperative glucose levels for all patients are shown in Table 5. Patients with diabetes had significantly higher glucose values in the perioperative period than the other two groups. Of interest, preoperatively, patients with normal glucose regulation or prediabetes had significant differences in preoperative glucose levels; however, their average glucose levels converged and were similar during the first 48 h after surgery. Severe postoperative hyperglycemia developed in 16.9% of patients with normal glucose metabolism, 20.2% of patients with prediabetes, and 59% of patients with diabetes.
Table 5.
Perioperative glucose values
| Metabolic Status | Patients | Preoperative Glucose | POD 1 Morning Glucose | Glucose Average 48 h |
|---|---|---|---|---|
| Normal | 309 | 97 ± 17 | 121 ± 29 | 126 ± 25 |
| Pre Diabetes | 299 | 110 ± 18 | 124 ± 24 | 129 ± 21 |
| Diabetes | 345 | 151 ± 57 | 164 ± 56 | 155 ± 40 |
| P Value Among Groups | < 0.0001 | < 0.0001 | < 0.0001 | |
| P Value Between Patients with Normal Glucose Regulation and Prediabetes | 0.000 | 0.419 | 0.230 |
Postoperative complications
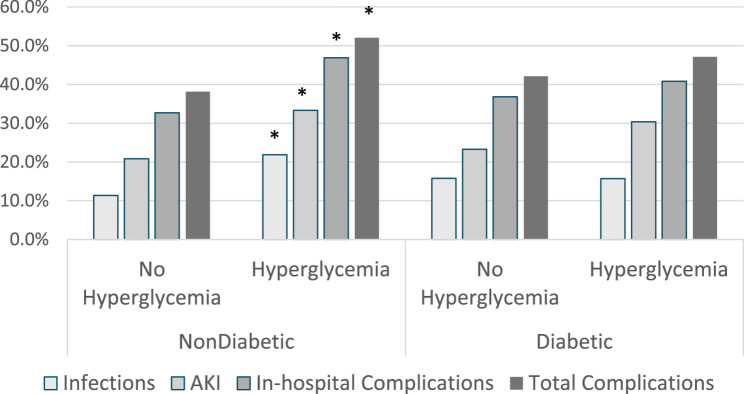
The complications of infection, AKI, in-hospital, and total complications are shown in Fig. 2. In the absence of hyperglycemia, patients without diabetes tend towards a lower complication rate than patients with diabetes. However, the graph demonstrates a diabetic paradox in that severe hyperglycemia was associated with a significant increase in complications in patients without diabetes for infection, AKI, and the composites of in-hospital or total complications (P = 0.028, P = 0.023, P = 0.009, P = 0.018); hyperglycemia was not associated with an increase in complications for patients with diabetes (P = 1.00, P = 0.2195, P = 0.541, P = 0.435, respectively).
Fig. 2.
Patients Without Diabetes Have Increased Complications with Postoperative Hyperglycemia
However, there are distinctions in the relationship between severe hyperglycemia and complications among patients without diabetes. Figure 3 examines infections after separating patients without diabetes into those with normal glucose regulation or prediabetes. Surprisingly, infections significantly increased only in patients with normal glucose regulation, not those with prediabetes.
Fig. 3.
Postoperative Infections Are Increased Only in Patients with Normal Glucose Regulation in the Presence of Severe Hyperglycemia. P Values refer to the difference in infection rates between patients with and without hyperglycemia for the three metabolic groups
Figure 4 examines AKI when patients were grouped similarly. A significant increase in AKI occurred only in patients with prediabetes, not in patients with normal glucose regulation. Thus, excess complications associated with postoperative hyperglycemia in patients without diabetes are dependent upon the specific complication assessed and the subgroup of patients without diabetes.
Fig. 4.
AKI Is Increased Only in Patients with Prediabetes in the Presence of Severe Hyperglycemia. P Values refer to the difference in AKI rates between patients with and without hyperglycemia for the three metabolic groups
The roles of glucose and inflammation as predictors of infections and AKI
Multivariable regressions were performed in patients with normal glucose regulation and prediabetes to assess the relative importance of hyperglycemia and inflammation as predictors of complications. Inflammation (SIRI 1, P = 0.020), albumin (P = 0.003), and a minimally invasive operative approach (P = 0.007) were the only independent predictors of infection in patients with normal glucose regulation, with an AUC of 0.875. Severe hyperglycemia was not an independent predictor of infections.
In contrast, the first postoperative day morning glucose (POD # 1, P = 0.000) was a significant predictor of AKI in patients with prediabetes, along with procedure duration (P = 0.003) and the absence of normal saline boluses (P = 0.018), with an AUC of 0.758. Since duration correlates with inflammation, we consider that hyperglycemia and inflammation affect AKI in patients with prediabetes [8]. The morning glucose was a more sensitive predictor of AKI than severe hyperglycemia, so morning glucose was used in the multivariable analysis.
Cellular inflammatory markers, hyperglycemia, and outcomes
Since SIRI 1 has not often been used as an early postoperative measure of inflammation, we examined the predictors and postoperative complications associated with SIRI 1 elevation. The independent predictors of SIRI 1 are listed in Table 6.
Table 6.
Independent predictors of SIRI 1
| Predictor | t Statistic | P Value | Lower bound (95%) | Upper bound (95%) |
|---|---|---|---|---|
| Procedure Duration | 3.887 | 0.000 | 0.095 | 0.290 |
| ASA | 2.723 | 0.007 | 0.037 | 0.229 |
| Elective | −5.146 | < 0.0001 | −0.345 | −0.154 |
| Diabetes Group-Normal | 2.524 | 0.012 | 0.030 | 0.245 |
| No Severe Hyperglycemia | −2.938 | 0.003 | −0.255 | −0.050 |
SIRI 1 was elevated early after surgery, correlated with the intensity of surgery (procedure duration and emergent status), and was higher in males. Patients with normal glucose regulation, when controlled with other significant predictors on multivariable analysis, had a significantly higher inflammatory response to surgery, as reflected in SIRI 1 (P = 0.012).
SIRI 1 was predictive of postoperative complications, including infections (P = 0.002), AKI (P = < 0.0001), non-infectious complications (P = 0.002), in-hospital complications (P = < 0.0001), and total complications (P = 0.000).
Figure 5 shows that elevations of inflammation (SIRI 1) coincided with severe postoperative hyperglycemia. In comparison with patients without severe hyperglycemia, patients with diabetes or prediabetes show significantly increased levels of inflammation, with a similar trend for patients with normal glucose regulation. Thus, two markers of surgical stress, severe hyperglycemia and SIRI 1, are elevated early after surgery, with the inflammatory marker more critical in predicting infections. In contrast, hyperglycemia appears equally important as inflammation in predicting kidney injury.
Fig. 5.
Inflammation Is Increased in Patients with Severe Hyperglycemia SIRI 1 are the systemic inflammatory response indices on the first day after surgery
Sensitivity analysis
To confirm the consistency of the effects of hyperglycemia on the subgroups of patients without diabetes, we then examined patients with elective surgery only. A similar relationship was found: patients with normal glucose regulation had higher infection rates (P = 0.010), and patients with prediabetes experienced a significantly higher incidence of AKI (P = 0.007) in the presence of severe hyperglycemia. The analysis was comparable when septic patients were added to the elective cases (P = 0.041 for excess infections from hyperglycemia in patients with normal glucose regulation; P = 0.011 for excess AKI from hyperglycemia in patients with prediabetes).
We occasionally used casual glucose to define patients without diabetes [16]. Casual glucose alone was used to define 2.3% of patients with prediabetes. It was used more often in patients with normal glucose regulation: 16.1% of patients were identified as normal glucose regulation with casual glucose alone, with 9.4% having a glucose between 100 mg/dl and 139 mg/dl.
The effect on outcomes of using casual blood glucose levels to define patients without diabetes was evaluated. Patients classified with casual glucose alone were excluded from the analysis. Severe postoperative hyperglycemia was still associated with excess infections in patients with normal glucose regulation (P = 0.010) with no effect on the incidence of AKI (P = 0.610). A similar result occurred in patients with prediabetes: severe hyperglycemia was associated with an increase in AKI (P = 0.007), with no difference in infections (P = 0.770).
Discussion
Despite similar levels of postoperative hyperglycemia, complications are higher in patients in the absence rather than the presence of diabetes. This diabetic paradox has been most commonly observed with postoperative infections but has also been noted for composite complications and mortality [17–19]. In this study, we confirm the existence of this conundrum but think that the specific complications, the subclassification of patients without diabetes, and the independent predictors of the complications help define the anomaly further. Excess infections occur in patients with normal glucose regulation; inflammation, not postoperative hyperglycemia, is an independent predictor. In contrast, in patients with prediabetes, an excess incidence of AKI is associated with elevated postoperative glucose levels but also with systemic inflammation. Because excess infections or AKI occurred in either patients with prediabetes or normal glucose regulation, depending on the complication, the factors that influenced excess complications within each subgroup were examined.
Hyperglycemia may be a less potent cause of postoperative immunosuppression than surgical trauma
In our study, hyperglycemia is less critical than inflammation as a cause of perioperative infectious complications. Others have reported that surgical trauma is a potent cause of immunosuppression. In non-septic patients, surgical trauma releases fragments of dead or injured cells (DAMPS), which are recognized by pattern recognition receptors on multiple cell types [20]. The result is an initial nonspecific activation of leukocyte and monocyte cells of the innate immune system, which initially causes a generalized inflammatory state. Monocytes will travel to the injury sites and release interleukin-6, among other cytokines. However, the initial inflammation is followed by compensatory immunosuppression, with a decrease in antigen presentation to T cells by monocytes, as seen in the downregulation of the cell surface protein HLA-DR [21].
After surgery, postoperative hyperglycemia can potentially add to the immunosuppression, increasing infection rates [22, 23]. Initially, hyperglycemia depresses leukocyte and mononuclear cell migration and phagocytic activity [22, 24]. Later, hyperglycemia decreases the HLA-DR expression in antigen-presenting cells [23]. The question is, to what degree does hyperglycemia aggravate an already potent immunosuppression that occurs as a response to the trauma of surgery? Some insight is presented in patients after a total gastrectomy. Intensive insulin therapy in patients on hyperalimentation partially restored HLA-DR expression towards the baseline, but a substantial decrease in HLA-DR expression remained, likely due to surgical trauma [25]. Thus, severe postoperative hyperglycemia may not be the only or even predominant cause of postoperative immunosuppression, and glucose control may have limits in preventing infection.
Insulin treatment and postoperative complications
If severe hyperglycemia itself is a principal cause of the excess complications that occur in patients without diabetes, then correction with insulin should resolve the problem. The preponderance of evidence suggests that insulin decreases complications, but incompletely. Kwon noted that complications were lower in hyperglycemic patients without diabetes if given adequate insulin treatment [26]. Early studies on strict glucose control demonstrated a striking improvement in patient outcomes [27]. A recent prospective, non-randomized study of general surgical patients without diabetes showed that treatment with intravenous insulin mitigated hyperglycemia-induced complications [1]. Further, meta-analyses of randomized trials of strict glucose control have generally demonstrated decreased surgical site and sepsis rates, particularly in patients without diabetes, including some after abdominal surgery [28, 29]. However, individual studies and meta-analyses have not consistently demonstrated infection control [30, 31] with insulin protocols. Our study suggests that there may be a limitation in improvement in postoperative infections in patients with normal glucose regulation with insulin protocols since hyperglycemia was not an independent cause of postoperative infections.
Postoperative inflammation and hyperglycemia in patients with normal glucose regulation
In a previous report, peak postoperative inflammatory levels were similar for patients with or without diabetes, suggesting that inflammation did not cause excess complications in patients without diabetes [32]. Our results may differ because we measured different markers of inflammation but also because we analyzed patients without diabetes as two distinct groups. The cellular inflammatory marker (SIRI 1) was significantly higher in patients with normal glucose regulation (P < 0.012, Table 6) than in patients with diabetes or prediabetes. In addition, SIRI 1, not severe hyperglycemia, was an independent predictor of infections. Thus, excess inflammation might contribute to infections in patients with normal glucose regulation to a greater extent than hyperglycemia.
Excess AKI in patients with prediabetes
The most important predictor of AKI in patients with prediabetes is the glucose level on the morning after surgery. The reasons for the considerable sensitivity of patients with prediabetes to hyperglycemia-induced AKI are uncertain. Patients with diabetes are thought to be partially protected from hyperglycemia through the mechanism of downregulation of the GLUT proteins, which facilitate the entrance of glucose into renal cells. This mechanism does not appear to offer protection for patients with prediabetes since these patients had the highest incidence of AKI in the presence of hyperglycemia.
The relationship between elevated glucose levels on the morning after surgery and AKI suggests that insulin therapy could significantly benefit this group. Animal studies show that euglycemia reduces AKI, endothelial dysfunction, and systemic and intra-renal inflammation [33–35]. The strongest indicator of the etiologic role of hyperglycemia in AKI would be its prevention with anti-hyperglycemia therapy [36]. Some early clinical studies have shown that strict glucose control can reduce the incidence of AKI [27, 37]. Other later studies did not confirm these findings [38, 39]. Overall, the clinical evidence is mixed on the effectiveness of insulin in preventing AKI: one meta-analysis found a reduced AKI with tight glucose control, while another did not [28, 29]. Less strict insulin protocols are now favored because of the side effects of insulin treatment [38, 39].
Should patients with prediabetes be identified preoperatively??
Patients in our study were defined as having prediabetes with the American Diabetes Association (ADA) guidelines, which are more sensitive than the guidelines of the World Health Organization [11, 16]. It has not been determined which guidelines are more appropriate for surgical patients.
Prediabetes has not been considered a distinct co-morbidity in surgical patients. Some studies in non-surgical patients have shown that patients with prediabetes have an intermediate or increased risk of complications [40, 41]. To our knowledge, there has been little examination of the effects that prediabetes may have on surgical complications. Our study suggests that patients with prediabetes are susceptible to hyperglycemia-induced AKI. The best preventive or treatment strategy is unclear.
Inflammation and hyperglycemia
In clinical studies, there appears to be a complex interaction between inflammation and hyperglycemia. In postoperative cardiac surgery patients, strict glucose control with insulin did not reduce inflammatory markers or postoperative complications [42]. In another study, anti-inflammatory strategies reduced postoperative inflammatory markers, but the effect on outcomes was uncertain [43]. In contrast with these two studies, a marked decrease in inflammatory markers and complications was noted after pancreaticoduodenectomy in patients on hyperalimentation, where insulin was delivered with an artificial pancreas, and substantial insulin doses were administered [44]. Whether a more intensive glucose control would lower inflammatory levels after colorectal surgery is uncertain.
Limitations
This study is retrospective and exploratory, and causation cannot be inferred. The metabolic status of patients without diabetes was defined with the available information and sometimes made with a single parameter, such as a preoperative fasting glucose or HbA1C level, using the ADA criteria. Random glucose levels were generally confirmed with a HbA1C for diagnosing patients with prediabetes; only 2.3% were based on casual glucose alone. The rate of severe postoperative hyperglycemia in patients with normal glucose regulation was low, and the relationship between hyperglycemia and its consequences would be strengthened with a larger sample size. No direct measurements of postoperative inflammation, such as cytokines, were available, so inflammation was inferred from blood count measure
Conclusions
Complications occurring after colorectal surgery associated with the diabetic paradox are dependent on the preoperative glucose regulatory state of the patient. Excess complications occur in patients without diabetes but, depending on the complication, are concentrated in either patients with normal glucose regulation or prediabetes. Higher levels of inflammation may lead to higher infection rates in patients with normal glucose regulation, whereas hyperglycemia is a principal cause of excess AKI in patients with prediabetes. This study suggests that prediabetes may be a risk factor for hyperglycemia-associated AKI. The study also raises the possibility that anti-inflammatory strategies have the potential to reduce excess infections associated with the diabetic paradox.
Acknowledgements
The authors would like to thank Tom Sharkey for his help with data extraction from Epic and data preparation with Excel.
Abbreviations
- AKI
Acute Kidney Injury
- ASA
American Society of Anesthesiologists
- SIRI 1
Systemic inflammatory response index on POD # 1
- DVT/PE
Deep vein thrombosis, pulmonary embolus
- DAMPS
Molecules derived from damaged cells that activate the immune system
- HLA-DR
Cell surface receptor aiding the immune system
- ADA
American Diabetes Association
- WHO
World Health Association
Authors’ contributions
J.M.: conceptualization, design of the study, data curation, formal analysis of data, including statistical analysis, original draft preparation, and revisions; A.R.: conceptualization, resources, review and editing, supervision; A.F.: methodology, review and editing, investigation; M.M.: review and editing, investigation; K.G.: methodology, investigation, review and editing; G.S.: supervision, resources, review and editing.
Funding
There is no external funding for this study.
Data availability
Restrictions apply to the availability of some, or all of the data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conclusions under which access to some data may be provided.
Declarations
Ethics approval and consent to participate
The Bayhealth Institutional Review Board (BH 25) approved the study. The Bayhealth IRB waived the requirement for individual consents, and individual consents were not obtained for this study. The authors conducted this study adhering to the guidelines of the Declaration of Helsinki.
Consent for publication
NA.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen JY, Nassereldine H, Cook SB, Thornblade LW, Dellinger EP, Flum DR. Paradoxical association of hyperglycemia and surgical complications among patients with and without diabetes. JAMA Surg. 2022;157:765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohan S, Kaoutzanis C, Welch KB, Vandewarker JF, Winter S, Krapohl G, et al. Postoperative hyperglycemia and adverse outcomes in patients undergoing colorectal surgery: results from the Michigan surgical quality collaborative database. Int J Colorectal Dis. 2015;30:1515–23. [DOI] [PubMed] [Google Scholar]
- 3.Mraovic B, Suh D, Jacovides C, Parvizi J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiffermiller J, Anderson M, Thompson R. Postoperative length of stay in patients with stress hyperglycemia compared to patients with diabetic hyperglycemia: a retrospective cohort study. J Diabetes Sci Technol. 2024;18:556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Székely A, Levin J, Miao Y, Tudor IC, Vuylsteke A, Ofner P, et al. Impact of hyperglycemia on perioperative mortality after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2011;142:430-7.e1. [DOI] [PubMed] [Google Scholar]
- 6.Tsai YC, Wu SC, Hsieh TM, Liu HT, Huang CY, Chou SE, et al. Association of stress-induced hyperglycemia and diabetic hyperglycemia with mortality in patients with traumatic brain injury: analysis of a propensity score-matched population. Int J Environ Res Public Health. 2020;17:4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakhry SM, Morse JL, Wilson NY, Waswick WA, Garland JM, Chipko JM, et al. Hyperglycemia in nondiabetic adult trauma patients is associated with worse outcomes than diabetic patients: an analysis of 95,764 patients. J Trauma Acute Care Surg. 2022;93:316–22. [DOI] [PubMed] [Google Scholar]
- 8.Mannion JD, Rather A, Fisher A, Gardner K, Ghanem N, Dirocco S, et al. Systemic inflammation and acute kidney injury after colorectal surgery. BMC Nephrol. 2024;25:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannion JD, Rather A, Gardner K, McEvilly M, Fisher A, Harper L, et al. Treatment of severe hyperglycemia in patients without diabetes after colorectal surgery. Surg Infect (Larchmt). 2023;24:344–50. [DOI] [PubMed] [Google Scholar]
- 10.Mannion JD, Rather A, Manifold S, Gardner K, McEvilly M, Yaeger J, et al. Postoperative hyperglycemia in patients with and without diabetes after major joint replacement: the impact of an enhanced glucose management program. JB JS Open Access. 2021;6:e20.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42:59–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis G, Fayfman M, Reyes-Umpierrez D, Hafeez S, Pasquel FJ, Vellanki P, et al. Stress hyperglycemia in general surgery: why should we care? J Diabetes Complications. 2018;32:305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gameiro J, Fonseca JA, Dias JM, Milho J, Rosa R, Jorge S, et al. Neutrophil, lymphocyte and platelet ratio as a predictor of postoperative acute kidney injury in major abdominal surgery. BMC Nephrol. 2018;19:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Zou Z, Zhang Y, Zhu B, Ning Y, Shen B, et al. Dynamics in perioperative neutrophil-to-lymphocyte*platelet ratio as a predictor of early acute kidney injury following cardiovascular surgery. Ren Fail. 2021;43:1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alvarez S, Coffey R, Mathias PM, Algotar AM. Prediabetes. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC. 2025. [PubMed]
- 17.Chen YH, Chou CH, Su HH, Tsai YT, Chiang MH, Kuo YJ, et al. Correlation between neutrophil-to-lymphocyte ratio and postoperative mortality in elderly patients with hip fracture: a meta-analysis. J Orthop Surg Res. 2021;16:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33:1783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alazawi W, Pirmadjid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;264:73–80. [DOI] [PubMed] [Google Scholar]
- 22.Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23:9–15. [DOI] [PubMed] [Google Scholar]
- 23.Lachmann G, Von Haefen C, Wollersheim T, Spies C. Severe perioperative hyperglycemia attenuates postoperative monocytic function, basophil count and T cell activation. Minerva Anestesiol. 2017;83:921–9. [DOI] [PubMed] [Google Scholar]
- 24.Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci. 2016;351:201–11. [DOI] [PubMed] [Google Scholar]
- 25.Cao S, Zhou Y, Chen D, Niu Z, Wang D, Lv L, et al. Intensive versus conventional insulin therapy in nondiabetic patients receiving parenteral nutrition after D2 gastrectomy for gastric cancer: a randomized controlled trial. J Gastrointest Surg. 2011;15:1961–8. [DOI] [PubMed] [Google Scholar]
- 26.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the surgical care and outcomes assessment program. Ann Surg. 2013;257:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003;31:359–66. [DOI] [PubMed] [Google Scholar]
- 28.Kang ZQ, Huo JL, Zhai XJ. Effects of perioperative tight glycemic control on postoperative outcomes: a meta-analysis. Endocr Connect. 2018;7:R316-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YY, Hu SF, Ying HM, Chen L, Li HL, Tian F, et al. Postoperative tight glycemic control significantly reduces postoperative infection rates in patients undergoing surgery: a meta-analysis. BMC Endocr Disord. 2018;18:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agus MS, Steil GM, Wypij D, Costello JM, Laussen PC, Langer M, et al. Tight glycemic control versus standard care after pediatric cardiac surgery. N Engl J Med. 2012;367:1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU: a systematic review and metaanalysis. Chest. 2010;137:544–51. [DOI] [PubMed] [Google Scholar]
- 32.Thompson R, Khor S, Thornblade LW, Flum DR, Sobel M. The paradox of hyperglycemia and surgical outcomes in patients with and without diabetes. Surg Infect (Larchmt). 2019;20:338–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellger B, Debaveye Y, Vanhorebeek I, Langouche L, Giulietti A, Van Etten E, et al. Survival benefits of intensive insulin therapy in critical illness: impact of maintaining normoglycemia versus glycemia-independent actions of insulin. Diabetes. 2006;55:1096–105. [DOI] [PubMed] [Google Scholar]
- 34.Hirose R, Xu F, Dang K, Liu T, Behrends M, Brakeman PR, et al. Transient hyperglycemia affects the extent of ischemia-reperfusion-induced renal injury in rats. Anesthesiology. 2008;108:402–14. [DOI] [PubMed] [Google Scholar]
- 35.Vanhorebeek I, Gunst J, Ellger B, Boussemaere M, Lerut E, Debaveye Y, et al. Hyperglycemic kidney damage in an animal model of prolonged critical illness. Kidney Int. 2009;76:512–20. [DOI] [PubMed] [Google Scholar]
- 36.Mendez CE, Der Mesropian PJ, Mathew RO, Slawski B. Hyperglycemia and acute kidney injury during the perioperative period. Curr Diab Rep. 2016;16:10. [DOI] [PubMed] [Google Scholar]
- 37.Thomas G, Rojas MC, Epstein SK, Balk EM, Liangos O, Jaber BL. Insulin therapy and acute kidney injury in critically ill patients a systematic review. Nephrol Dial Transplant. 2007;22:2849–55. [DOI] [PubMed] [Google Scholar]
- 38.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97. [DOI] [PubMed] [Google Scholar]
- 39.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–44. [DOI] [PubMed] [Google Scholar]
- 40.Ahsan MJ, Latif A, Ahmad S, Willman C, Lateef N, Shabbir MA, et al. Outcomes of prediabetes compared with normoglycaemia and diabetes mellitus in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Heart Int. 2023;17:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan Y, Chen W, Wang Y. Prediabetes and outcome of ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2019;28:683–92. [DOI] [PubMed] [Google Scholar]
- 42.Reyes-Umpierrez D, Davis G, Cardona S, Pasquel FJ, Peng L, Jacobs S, et al. Inflammation and oxidative stress in cardiac surgery patients treated to intensive versus conservative glucose targets. J Clin Endocrinol Metab. 2017;102:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan T, Jiang CY, Zhang H, Han XK, Zhang HT, Jiang XY, et al. The low-dose colchicine in patients after non-CABG cardiac surgery: a randomized controlled trial. Crit Care. 2023;27:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akabori H, Tani M, Kitamura N, Maehira H, Imashuku Y, Tsujita Y, et al. Perioperative tight glycemic control using artificial pancreas decreases infectious complications via suppression of inflammatory cytokines in patients who underwent pancreaticoduodenectomy: a prospective, non-randomized clinical trial. Am J Surg. 2020;220:365–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some, or all of the data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conclusions under which access to some data may be provided.