Abstract
Background
Dengue fever (DF) is caused by dengue virus (DENV) and is primarily spread through bites of infected Aedes mosquitoes in tropical and subtropical regions, including Ethiopia. In the Afar region, DF is often misdiagnosed as malaria due to limited diagnostic capabilities. This study assessed the seroprevalence of DENV infection among febrile patients in healthcare facilities in the Afar region.
Methods
A cross-sectional study was conducted from September 2022 to March 2023 in Amibara, Harunka and Awash Sebat city administration districts, involving febrile patients suspected of having malaria. Sociodemographic data, clinical features and blood samples were collected from participants after obtaining informed consent or assent. Following blood film examination for Plasmodium infection, sera were tested for anti-DENV IgM and IgG antibodies using enzyme-linked immunosorbent assay (ELISA). Data were entered into Epi Data 3.1 and analyzed with Stata/SE 14.2. A p-value < 0.05 was considered significant.
Results
A total of 411 febrile patients (55.5% female, aged 5–80 years, with a mean age of 27.3 ± 13.8 years) participated in the study. Among those tested for anti-DENV antibodies, 101/410 (24.6%) were positive for anti-DENV IgM, indicating an acute DENV infection, and 142/367 (38.7%) were positive for anti-DENV IgG, revealing previous exposure. Only 44/411 (10.7%) were positive for Plasmodium infection, while 18/410 (4.4%) were co-infected with malaria and acute DENV infection and 18/367 (4.9%) were positive for both malaria and previous exposure to DENV. Those diagnosed with malaria were nearly 2.5 times more likely to test positive for acute DENV infection than those without malaria (AOR = 2.37; 95% CI 1.14–4.92). The odds of positivity for previous exposure were about two times higher in females than in males (AOR = 2.24; 95% CI 1.32–3.26) and in government and private sector employees than in pastoralists and agro-pastoralists (AOR = 1.90; 95% CI 1.03–8.15), while patients aged 11–20 years had lower odds of previous exposure than those aged 5–10 years (AOR = 0.35; 95% CI 0.14–0.88). Experiencing muscle pain was associated with twice the odds of previous exposure (AOR = 1.90; 95% CI 1.04–3.49).
Conclusion
The study reveals a high seroprevalence of DENV infection among febrile patients in the study area, highlighting the need for routine diagnosis and management of DF in this setting. Improving vector control, disease surveillance and public awareness would be crucial for combating DF and other mosquito-borne diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-11406-3.
Keywords: Seroprevalence, Dengue fever, Febrile illness, Afar region, Ethiopia
Introduction
Dengue fever (DF) is an arboviral disease caused by dengue virus (DENV) infection. This virus comprises four closely related but distinct serotypes (DENV1-4), transmitted to humans through the bites of infected female Aedes aegypti and Ae. albopictus mosquitoes [1, 2]. The incidence of DF has emerged as a significant public health problem in tropical and subtropical regions, including Ethiopia [3, 4]. The rising prevalence of DF in endemic regions, alongside its expansion into new areas, can be attributed to a variety of factors, including globalization, urbanization, increased travel, climate change, and inadequate mosquito control measures. These elements collectively contribute to the proliferation and spread of both the vectors and the virus [1, 5]. Presently, approximately half of the global population is at risk of contracting DENV infection, with over 390 million people infected annually, approximately 100 million of whom exhibit symptoms of DF, resulting in thousands of deaths from severe DF each year [1, 6].
DF presents a wide clinical spectrum, ranging from mild flu-like symptoms to severe febrile illness characterized by symptoms such as high fever, muscle and joint pain, severe headache, pain behind the eyes, nausea, rash, and vomiting. Severe cases may show warning signs, including abdominal pain, persistent vomiting, respiratory distress, and organ impairment [7, 8]. The severity can increase during secondary infections with different serotypes due to the production of weakly neutralizing antibodies from the initial infection, leading to antibody-dependent enhancement (ADE) [9]. Diagnosis typically involves detecting DENV-specific antibodies, non-structural 1 (NS1) protein, or viral RNA, each with its limitations [10]. Anti-DENV IgM antibodies appear 3–5 days after symptom onset and persist for months, while IgG antibodies develop after a week and can persist for life during both primary and secondary infection. In the acute phase of secondary DENV infections, high levels of cross-reactive IgG antibodies are detected before or simultaneously with the IgM response [11–13].
The World Health Organization (WHO) has also established targets to reduce the incidence of DF by 25%, achieve zero case-fatality rates, and enhance outbreak detection and response capabilities by 75% by 2030 [8]. Despite these targets, DF continues to be a significant public health challenge globally, particularly in Africa, where countries such as Ethiopia face recurrent outbreaks [14]. A major challenge in managing this disease is the limited availability of accurate and accessible diagnostic tools, coupled with a shortage of trained healthcare professionals [15]. These factors compromise effective patient care and hinder the accurate assessment of the disease burden in endemic regions. Moreover, DF shares overlapping symptoms such as fever, headache, and muscle pain with malaria, a disease prevalent in tropical and subtropical regions similar to DF [11, 16, 17]. This overlapping clinical presentation can lead to misdiagnosis and inappropriate management of cases.
Ethiopia has been hit by frequent DF outbreaks all year round, particularly in the eastern regions such as Afar, Somali, and Dire Dawa city since the first confirmed case report from non-malarial febrile illness in Dire Dawa city in 2013 [18–22]. The serotypes responsible for these frequent outbreaks have been identified as DENV1-3 serotypes [23–25]. Serological studies conducted in different localities across the country have reported seroprevalence rates ranging from 7.5 to 19% for current or acute infections, and 13–21% for past infections among acute febrile patients in northwestern Ethiopia [18, 25]. In southern Ethiopia, rates of 22.9% IgG and 7.9% IgM against DENV were reported [26]. However, there exists a significant risk of co-existence with malaria and other febrile illnesses, which poses challenges for accurate diagnosis of diseases due to the clinical similarities. Accurately differentiating DF from malaria and other endemic febrile diseases is essential to mitigate the increased morbidity and mortality associated with delayed diagnosis and treatment [27]. Despite these pressing concerns, DF has not been included in the public health priority in Ethiopia, resulting in a neglected burden of the disease. This situation is further compounded by a weak healthcare system, limited experience among healthcare providers, and economic constraints [28, 29] which complicates the diagnostic process for febrile illnesses presenting with overlapping symptoms, often leading to undetermined cases [16, 30]. This study aimed to assess the seroprevalence of DENV infection among malaria-suspected febrile patients in healthcare facilities in selected districts of the Afar region of Northeast Ethiopia, highlighting the need for the implementation of public health interventions. To our knowledge, this is the first study to report seroprevalence of DENV infection, particularly DENV-malarial co-infection and IgG seroprevalence in febrile patients in the Afar Region.
Materials and methods
Study area
This study was conducted in the administration of zone 3 of the Afar region, Northeast Ethiopia. Zone 3 is one of the five zones in the region and bordered by the Oromia region (south), the Amhara region (southwest), Argobba special district and the administration of zone 5(west), the administration of zone 1 (north) and the Somali region(east). This zone is divided into seven districts and two city administrations, each with one or two public health centers (HCs) and various health posts. Additionally, there is one general referral hospital in the zone.
Generally, the Afar region is a pastoralist region, with few practicing agro-pastoralists, and the Awash River is the only water source for the community members and their livestock. The region has an arid climate with an experience of seasonal flooding of the Awash River. It is a known hotspot region for vector-borne diseases, including malaria and arboviral diseases such as DF and chikungunya [31, 32].
Study sites and population
The study was conducted among febrile patients visited public HCs located in three districts of Zone 3, namely Amibara, Harunka, and Awash Sebat city administration districts, with Harunka was in Amibara and Awash Sebat city administration in Awash Fentale districts (Fig. 1). These districts were purposively selected from the zone based on previous outbreaks of arbovirus infection-like illnesses in the areas [32] and their proximity. Four HCs were sampled from the districts. Namely, Andido HC, located in Harunka district, serves a community that reflects the demographics of the Afar population. Awash Sebat HC, situated within Awash Sebat city administration, is located at a major transport hub linking Dire Dewa, Djibouti and Addis Ababa, as well as Harar and Addis Ababa. The city administration represents relatively urbanized community. From Amibara district, Awash Arba and Sidafage HCs were considered. Awash Arba HC, which provides services for urban and rural pastoralists communities residing near the principal road toward Djibouti, while Sidafage HC, which is located away from the main road, provides care to communities that represent semi-pastoralist and pastoralist communities in more rural settings [33].
Fig. 1.
Map of the study sites in the Afar Region, Northeast Ethiopia
Study design and period
A health facility-based cross-sectional study was conducted among febrile patients suspected of having malaria between September 2022 and March 2023.
Study participants and eligibility criteria
The study participants were patients with acute febrile illness presenting to the selected HCs’ adult outpatient department during the study period.
Inclusion criteria: History of fever within the last seven days with measured axillary temperature ≥ 37.5 °C and suspected of having malaria by clinicians in the selected HCs.
Exclusion criteria: patients unwilling to provide informed consent/assent, apparently anemic, pregnant and under five years children were excluded.
Sample size estimation and sampling techniques
The sample size was determined using a single proportion formula based on a previous study in other health institutes in Ethiopia [18] that found the overall seroprevalence of IgM antibodies against DENV infection to be 19% among febrile patients. Therefore, the minimum estimated sample size was 237 at 95% confidence interval and 5% level of precision. Based on the availability of laboratory resources for conducting serological tests for DENV infection, the sample size was increased to 411 patients (on average, 103 per HC) to estimate the prevalence of DENV infections. Using non-probability consecutive sampling technique, all eligible febrile patients visiting the selected HCs during the study period were recruited until the designated sample size was achieved. Due to variability in patient flow, some centers recruited more rapidly while others slowly than initially estimated, but the total sample size across all sites was achieved.
Data collection
Trained local healthcare workers collected data. A structured questionnaire was used to collect sociodemographic and clinical features. The questionnaire was developed through a thorough review of existing literature pertaining to febrile patients with arboviral diseases, including DF, and consulting with experts as detailed in the supplementary file (Additional file). The clinicians measured axial temperature and collected socio-demographic and clinical features from eligible patients after obtaining informed consent/assent. Laboratory technicians collected 5 ml of venous blood samples under aseptic conditions using a vacutainer tube.
Laboratory analysis and interpretation
Blood films were prepared and stained with Giemsa stain to examine hemo-parasites using microscopy at the study sites. The remaining blood was centrifuged, and serum was separated and transported in a cold chain to the laboratory at Aklilu Lemma Institute of Health Research (ALIHR), formerly known as Aklilu Lema Institute of Pathobiology (ALIPB), where it was stored at −80 °C until analyses.
Serum samples were analyzed in the laboratory to detect antibodies against DENV infection using the Serion ELISA Classic IgM (Cat. No. ESR114M, Germany) and IgG (Cat. No. ESR114G, Germany) following the manufacturer’s instructions. The anti-DENV IgM ELISA, with a sensitivity of 91.7% and a specificity of over 99%, was used to identify recent or acute DENV infection. The anti-DENV IgG ELISA, with a sensitivity and specificity of over 99%, was used to detect previous DENV infections. Both tests employed similar procedures, except that the serum was treated with a rheumatoid factor (RF) absorbent during the IgM test to reduce false positives from non-specific RF binding. This RF treatment minimizes interference from IgM autoantibodies, which can affect IgM ELISA results but not the IgG assay. Patient sera or RF-treated sera with RF were diluted in buffer at a ratio of 1:101. The prepared samples, along with ready-to-use negative and positive controls, were transferred to microplates coated with DENV antigen. If antibodies are present in the patient´s serum, they bind to the fixed antigen during incubation at 37 °C for 60 min. The ELISA procedures followed the protocol, which included using alkaline phosphatase-conjugated anti-human IgM/IgG as the conjugate. A secondary antibody conjugated with the enzyme alkaline phosphatase detects and binds to the immune complex. The colorless substrate p-nitrophenylphosphate (pNPP) is then converted into the colored product p-nitrophenol. The incubations of the plate were carried out at 37° C for 30 min for both steps, with a wash four times between steps with Sodium chloride solution with Tween 20 and 30 mM Tris/HCl, diluted in distilled water. After adding 0.1 N sodium hydroxide as the stop solution, the absorbance intensity of the reaction product, which was proportional to the concentration of the analyte in the sample, was measured photometrically.
The absorbance was measured at 405 nm using a Thermo Scientific Multiskan FC Microplate Photometer (Model: 51119000; Thermo Fisher Scientific, Shanghai, China), with a 96-well plate format, following the ELISA kit instructions. The absorbance optical density (OD) values were interpreted according to the cut-off ranges specified in the quality control certificate accompanying each kit lot. The lower and upper OD thresholds were derived from the mean OD of standard sera (STD) tested in duplicate on each plate, covering the entire standard validity range. Based on the measured mean OD of the STDs, the appropriate column from the quality control certificate was selected, which contains information on the upper and lower OD cut-offs for evaluating patient samples. OD values below the lower cut-off were considered negative, those above the upper cut-off were positive and values in between were deemed borderline (equivocal) for each kit, as specified in the manufacturer’s quality control certificate. For results to be valid, the following criteria had to be met. (1) The OD of the substrate blank must be subtracted from the OD of STDs and patient sera before interpretation. (2) The substrate blank’s OD must be less than 0.25. (3) The negative control must be negative, while the mean OD of the STD serum, after blank subtraction, must meet standards outlined in the lot-specific quality control certificate. (4) The variation in OD values between STD sera must be within 20% [34]. Failure to meet these specified criteria invalidates the result; the test would be repeated.
Data entry and analysis
Data were entered into Epi Data v.3.1 and exported to Stata/SE 14.2 for analysis. The analysis involved summarizing socio-demographic and clinical features using frequencies and percentages. The prevalence of anti-DENV antibodies was determined by calculating the ratio of participants with positive test results to the total number of participants in the study. Separate univariable and multivariable logistic regression models were developed for both anti-DENV IgM (recent infection) and anti-DENV IgG (past exposure) to identify risk factors associated with the positivity of these antibodies. This analysis involved categorizing borderline and negative results as a comparative group, while excluding participants with missing variable values.
Univariable logistic regression analysis was conducted to identify associations between the prevalence of anti-DENV IgM and IgG antibodies and demographic characteristics and clinical features. Likewise, multivariable logistic regression analysis was executed to explore the relationship between those antibodies and demographic and clinical features, assessing the impact of these factors on seropositives while controlling for other variables. The model fit for the multivariable logistic regression was evaluated through the Hosmer-Lemeshow goodness-of-fit test, which indicated a satisfactory model fit (p = 0.059 for IgM and p = 0.162 for IgG). Odds ratios (OR) with 95% confidence intervals (CI) were used to assess the strength of the association between seropositivity and variables, with statistical significance established at p-value of < 0.05.
Ethical consideration
Ethical approval for this study was obtained from the former Institutional Review Board of ALIPB, now under the ALIHR, Addis Ababa University (ALIPB IRERC/88/2014/22). The study adheres to the principles and guidelines outlined in the Declaration of Helsinki. Before data collection, permission was obtained from health offices of Amibara, Harunka, and Awash Sebat city administration districts. Informed written consent was obtained from adults and from parents or guardians for children under 12, while informed assent was obtained from children between 12 and 18 years old. Malaria-positive participants were treated in accordance with established malaria treatment protocols in the study stites, while participants without malaria received alternative antibiotics regimens based on the clinician’s differential diagnosis.
Results
Sociodemographic information of the study participants
A total of 411 febrile patients (female 55.5%, age ranged from 5 to 80 years old, median of age 25, mean age of 27.3 years ± 13.8) participated in the study. The majority of the participants were married (60.6%), and resided in the Amibara district (59.4%) (Table 1).
Table 1.
Sociodemographic characteristics of the study participants (N = 411)
| General characteristics | Number (%) | |
|---|---|---|
| Sex | Male | 183 (44.5) |
| Female | 228 (55.5) | |
| Age (years) | 5–10 | 40 (9.7) |
| 11–20 | 106 (25.8) | |
| 21–30 | 148 (36.0) | |
| 31–40 | 57 (13.9) | |
| 41–50 | 34 (8.3) | |
| 51 and above | 26 (6.3) | |
| Education status | No formal education | 191 (46.5) |
| Grade 1-8th | 127 (30.9) | |
| Grade 9-12th | 70 (17.0) | |
| College diploma or above | 23 (5.6) | |
| Resident | Urban | 207 (50.4) |
| Rural | 204 (49.6) | |
| Occupation | Agro/pastoralist | 41 (10.0) |
| Government/private worker | 148 (36.0) | |
| Housewife | 114 (27.7) | |
| Students | 108 (26.3) | |
| Marital status | Married | 249 (60.6) |
| Non-married | 162 (39.4) | |
| Religion | Muslim | 251 (61.1) |
| Christians | 160 (38.9) | |
| Ethnicity | Afar | 199 (48.4) |
| Others | 212 (51.6) | |
| Districts | Amibara | 244 (59.4) |
| Awash Sebat | 126 (30.7) | |
| Harunka | 41 (10.0) | |
| Health institutes | Awash Arba | 200 (48.7) |
| Awash Sebat | 126 (30.7) | |
| Sidafage | 44 (10.7) | |
| Andido | 41 (10.0) | |
Clinical features reported by the study participants
The participants/parents reported that the duration of their febrile illness ranged from 1 to 8 days, with an average of 2.9 ± 1.4 days before they sought medical attention, while the measured axial temperature ranged from 37.5 to 40.0 °C, with an average of 38.1 ± 0.5 °C. Majority of the participants (93.9%) experienced the illness for 5 days or less. In addition to fever, participants reported various other clinical symptoms. The most common symptoms included headache (90.5%), joint pain (71.5%), and back pain (49.9%)(Table 2). Notably, none of the respondents mentioned any additional clinical symptoms typically associated with severe DF, such as mucosal bleeding, bloody vomit or stool, bleeding gums or nose, or petechiae.
Table 2.
Clinical features reported by febrile patients (N = 411)
| Number (%) | |
|---|---|
| Duration of the illness | |
| ≤ 5 days | 386 (93.9) |
| >5 days | 25 (6.1) |
| Headache | 372 (90.5) |
| Joint pain | 294 (71.5) |
| Back pain | 205 (49.9) |
| Chills | 182 (44.3) |
| Nausea | 145 (35.3) |
| Diarrhea | 54 (13.1) |
| Malaise | 91 (22.1) |
| Muscle pain | 86 (20.9) |
| Sore throat | 46 (11.2) |
| Abdominal pain/tenderness | 83 (20.2) |
| Persisting vomits | 52 (12.6) |
| Calf pain | 18 (4.4) |
| Rash | 6 (1.5) |
| Cough | 81 (19.7) |
| Difficulty in breathing | 10 (2.4) |
| Pain behind the eyes | 6 (1.5) |
Seroprevalence of anti-dengue virus antibodies
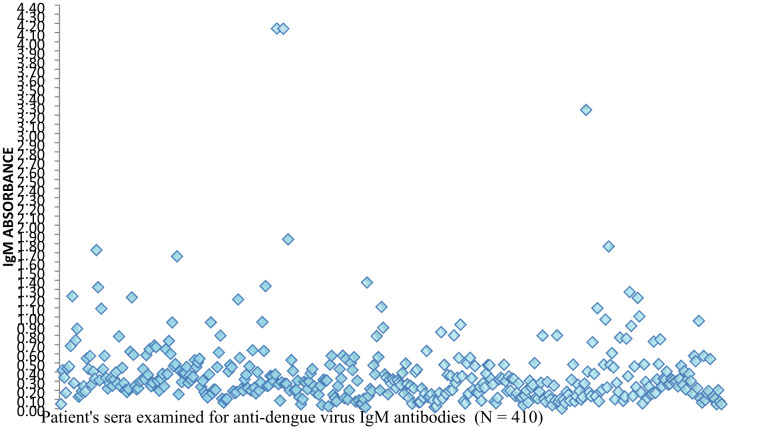
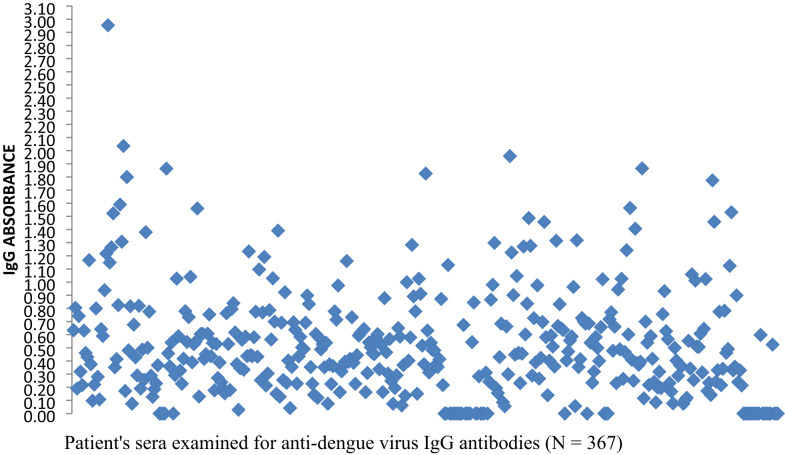
In this study, a total of 410 patient sera were examined for anti-DENV IgM antibodies, with one serum was excluded due to an unsuitable for serological test. Additionally, 367 of these sera were tested for anti-DENV IgG antibodies due to shortage of reagents for the ELISA IgG assays. To visually represent the distribution of ELISA absorbance values, scatter plots were generated for both anti-DENV IgM and IgG assays. These scatter plots display individual OD readings substrate blank subtraction, assisting the visual assessment of serological result patterns. The average upper and lower OD cut-off values for IgM kits were approximately 0.517 and 0.357, respectively, while upper and lower cut-off values for the IgG kits were 0.550 and 0.395, respectively. These thresholds were employed to categorize results into positive, borderline and negative (Figs. 2 and 3).
Fig. 2.
Scatter plot of anti-DENV IgM ELISA absorbance values after subtraction of substrate blank
Fig. 3.
Scatter plot of anti-DENV IgG ELISA absorbance values after subtraction of substrate blank
Out of the 410 sera, 101(24.6%) tested positive, indicating a recent or acute DENV infection. Additionally, 86(21.0%) serum was classified as borderline for IgM antibodies, while the remaining 223(54.4%) serum demonstrated negative results for IgM antibodies. Among 367 patient sera tested for IgG antibodies, 142 (38.7%) tested positive, suggesting previous exposure to DENV, while 75(20.4%) serum samples demonstrated borderline results. Among the 367 patient sera tested for both anti-DENV IgM and IgG antibodies, 196(53.4%) sera exhibited positivity for one or both antibodies, including 38 samples that were positive for both, while a quarter (25.1%) of samples were classified as borderline for either or both antibodies(Table 3).
Table 3.
Seroprevalence of anti-DENV antibodies, malaria parasites and their co-infections
| Diagnoses | Results | Number (%) |
|---|---|---|
| Anti-dengue virus IgM antibodies (N = 410) | Positive | 101 (24.6) |
| Borderline | 86 (21.0) | |
| Negative | 223 (54.4) | |
| Anti-dengue virus IgG antibodies (N = 367) | Positive | 142 (38.7) |
| Borderline | 75 (20.2) | |
| Negative | 150 (40.8) | |
| Microscopy for hemo-parasites (N = 411) | Positive for falciparum | 37 (9.0) |
| Positive for P. vivax | 5 (1.2) | |
| Positive for mixed of both infections | 2 (0.5) | |
| Total malaria positive | 44 (10.7) | |
| Dengue IgM-malaria Co-infection (N = 410) | Positive for falciparum and anti-dengue IgM | 14 (3.4) |
| Positive for vivax and anti-dengue IgM | 3 (0.7) | |
| Positive for Mixed malaria-dengue IgM | 1 (0.2) | |
| Total positive for malaria and anti-dengue IgM | 18 (4.4) | |
| Dengue IgG-malaria Co-infection (N = 367) | Positive for falciparum and anti-dengue IgG | 16 (4.4) |
| Positive for vivax and anti-dengue IgG | 2 (0.5) | |
| Total positive for malaria and anti-dengue IgG | 18 (4.9) |
The seropositivity rate of anti-DENV IgM antibodies was comparable between males and females (25.1% vs. 24.2%) (p = 0.832), while more patients of those diagnosed with malaria parasites were tested positive for anti-DENV IgM antibodies than those patients tested negative for malaria (40.9% vs. 22.7%)(p = 0.008). On the other hand, the seropositivity rate of anti-DENV IgG antibodies were higher in females than in males (45.0 vs. 32.3%) (p = 0.013)(Table 4).
Table 4.
Seropositivity rates of anti-dengue virus infections according to sociodemographic features
| Anti-Dengue IgM (N = 410) | Anti-Dengue IgG (N = 367) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N° tested | N° +ve (%) | X2; P-value |
N° tested | N° +ve (%) | X2; p-value |
|
| Sex | Male | 183 | 46(25.1) | 0.045; 0.832 | 167 | 54(32.3) | 6.123; 0.013 |
| Female | 227 | 55(24.2) | 200 | 90(45.0) | |||
|
Age (years) |
5–10 | 40 | 5(12.5) | 4.950; 0.422 | 37 | 21(56.8) | 10.045; 0.074 |
| 11–20 | 105 | 28(26.7) | 99 | 35(35.4) | |||
| 21–30 | 148 | 36(24.3) | 129 | 42(32.6) | |||
| 31–40 | 57 | 18(31.6) | 48 | 23(47.9) | |||
| 41–50 | 34 | 8(23.5) | 30 | 14(46.7) | |||
| 51 and above | 26 | 6(23.1) | 24 | 9(37.5) | |||
| Education status | No formal education | 191 | 46(24.1) | 3.484; 0.323 | 175 | 67(38.3) | 0.336; 0.953 |
| Grade 1-8th | 126 | 32(25.4) | 109 | 45(41.3) | |||
| Grade 9-12th | 70 | 14(20.0) | 66 | 25(37.9) | |||
| College diploma or above | 23 | 9(39.1) | 17 | 7(41.2) | |||
| Resident | Urban | 206 | 49(23.8) | 0.160; 0.689 | 192 | 82(42.7) | 2.035; 0.154 |
| Rural | 204 | 52(25.5) | 175 | 62(35.4) | |||
| Occupational status | Agro/pastoralist | 41 | 11(26.8) | 1.048; 0.790 | 33 | 8(24.4) | 5.147; 0.161 |
| Government/private worker | 148 | 40(27.0) | 134 | 59(44.0) | |||
| Housewife | 114 | 26(22.8) | 101 | 36(35.6) | |||
| Students | 107 | 24(22.4) | 99 | 41(41.4) | |||
| Marital status | Married | 248 | 66(26.6) | 1.324; 0.250 | 218 | 80(36.7) | 1.452; 0.228 |
| Non-married | 162 | 35(21.6) | 149 | 64(42.9) | |||
| Religion | Muslim | 251 | 54(21.5) | 3.394; 0.065 | 222 | 85(38.3) | 0.212; 0.645 |
| Christians | 159 | 47(29.6) | 145 | 59(40.7) | |||
| Ethnicity | Afar | 199 | 44(22.1) | 1.326; 0.249 | 170 | 61(35.9) | 1.495; 0.221 |
| Others | 211 | 57(27.0) | 197 | 83(42.1) | |||
| Districts | Amibara | 244 | 64(26.2) | 2.545; 0.280 | 225 | 85(37.8) | 1.217; 0.544 |
| Awash Sebat | 125 | 31(24.8) | 121 | 52(43.0) | |||
| Harunka | 41 | 6(14.6) | 21 | 7(33.3) | |||
| Health institutes | Awash Arba | 200 | 58(29.0) | 7.130; 0.068 | 200 | 75(37.5) | 1.275; 0.735 |
| Awash Sebat | 125 | 31(24.8) | 121 | 52(42.9) | |||
| Sidafage | 44 | 6(13.6) | 25 | 10(40.0) | |||
| Andido | 41 | 6(14.6) | 21 | 7(33.3) | |||
| Malaria parasite | Negative | 366 | 83(22.7) | 7.032; 0.008 | 326 | 126(38.6) | 0.421; 0.516 |
| Positive | 44 | 18(40.9) | 41 | 18(43.9) | |||
Prevalence of plasmodium infections and co-positivity with anti-dengue virus antibodies
Among the 411 febrile patients diagnosed microscopically for hemo-parasites, 44(10.7%) were found to be infected with Plasmodium parasites (9.0% identified as P. falciparum, 1.2% as P. vivax and 0.5% presenting with mixed infections). Notably, 18 (4.4%) and 18(4.9%) patients were co-positive for both Plasmodium parasites and anti-DENV IgM and IgG antibodies, respectively (Table 3).
Risk factors associated with dengue virus infection
Univariable and multivariable logistic regression revealed important insights into the risk factors associated with DENV infection (Table 5). There is no significant difference between female and male participants in the positivity rate of recent DENV infection. Age plays a significant role in risk for recent DENV infection. Participants aged between 31 and 40 years exhibited more than three times positivity compared to the reference category of children aged 5–10 years (COR = 3.23, 95% CI: 1.08–9.62), though it became insignificant during multivariable analysis. Additionally, patients diagnosed with malaria parasites had a nearly two and a half times risk for recent DENV infection than those who diagnosed negative in both univariable (COR = 2.36, 95% CI: 1.23–4.52) and multivariable (AOR = 2.37, 95% CI: 1.14–4.92) analyses.
Table 5.
Univariable and multivariable logistic regression analyses of sociodemographic and clinical features with anti-DENV antibodies
| Variable | Anti-DENV IgM | Anti-DENV IgG | |||
|---|---|---|---|---|---|
| COR (95%CI) | AOR (95%CI) | COR (95%CI) | AOR (95%CI) | ||
| Sex | Male | 1 | 1 | 1 | 1 |
| Female | 0.95(0.61–1.50) | 1.14(0.66–1.99) | 1.71(1.12–2.62) | 2.24(1.32–3.26) | |
|
Age (years) |
5–10 | 1 | 1 | 1 | 1 |
| 11–20 | 2.54(0.91–7.15 | 2.56(0.82–7.99) | 0.42(0.19–0.90) | 0.35(0.14–0.88) | |
| 21–30 | 2.25(0.82–6.17) | 1.88(0.53–6.64) | 0.37(0.17–0.78) | 0.36(0.13–1.02) | |
| 31–40 | 3.23(1.08–9.62) | 2.25(0.54–9.48) | 0.70(0.29–1.66) | 0.78(0.22–2.78) | |
| 41–50 | 2.15(0.63–7.35) | 1.48(0.31–7.06) | 0.67(0.25–1.75) | 0.47(0.12–1.85) | |
| 51 and above | 2.10(0.57–7.76) | 1.42(0.27–7.45) | 0.46(0.16–1.31) | 0.51(0.12–2.20) | |
| Education status | No formal education | 1 | 1 | 1 | 1 |
| Grade 1-8th | 1.07(0.64–1.81) | 1.26(0.62–2.58) | 1.13(0.70–1.85) | 0.95(0.46–1.94) | |
| Grade 9-12th | 0.79(0.40–1.54) | 0.62(0.26–1.47) | 0.98(0.55–1.76) | 1.01(0.45–2.25) | |
| College diploma or above | 2.03(0.82–4.99) | 1.87(0.65–5.38) | 1.13(0.41–3.11) | 0.88(0.27–2.83) | |
| Resident | Urban | 1 | 1 | 1 | 1 |
| Rural | 1.10(0.70–1.72) | 1.53(0.82–2.84) | 0.74(0.48–1.12) | 0.82(0.45–1.53) | |
| Occupational status | Agro/pastoralist | 1 | 1 | 1 | 1 |
| Government/private worker | 1.01(0.46–2.20) | 1.12(0.45–2.78) | 2.46(1.03–5.84) | 1.90(1.03–8.15) | |
| Housewife | 0.81(0.35–1.82) | 0.73(0.28–1.94) | 1.73(0.71–4.23) | 1.65(0.55–4.91) | |
| Students | 0.79(0.34–1.80) | 1.17(0.36–3.79) | 2.21(0.91–5.38) | 2.13(0.62–7.29) | |
| Marital status | Married | 1 | 1 | 1 | 1 |
| Non-married | 0.76(0.47–1.21) | 0.63(0.29–1.38) | 1.30(0.85–1.99) | 1.29(0.62–2.70) | |
| Religion | Muslim | 1 | 1 | 1 | 1 |
| Christians | 1.53(0.97–2.41) | 1.59(0.77–3.28) | 1.11(0.72–1.70) | 0.85(0.43–1.66) | |
| Ethnicity | Afar | 1 | 1 | 1 | 1 |
| Others | 1.30 (0.83–2.50) | 0.94(0.45–1.96) | 1.30(0.85–1.98) | 1.07(0.53–2.15) | |
| Districts | Amibara | 1 | 1 | 1 | |
| Awash Sebat | 0.93(0.56–1.20) | 0.93(0. 56-1.52) | 1.24(0.79–1.95) | 0.97(0.52–1.80) | |
| Harunka | 0.48(0.19–3.986) | 048(0.19–1.20) | 0.82(0.31–2.12) | 0.60(0.19–1.91) | |
| Illness duration | ≤ 5 days | 1 | 1 | 1 | 1 |
| > 5 days | 1.20(0.48–2.97) | 1.36(0.49–3.79) | 1.76(0.73–4.26) | 2.10(0.76–5.79) | |
| Joint pain | No | 1 | 1 | 1 | 1 |
| Yes | 1.18(0.71–1.97) | 1.24(0.69–2.22) | 0.90(0.57–1.43) | 0.78(0.46–1.33) | |
| Headache | No | 1 | 1 | 1 | 1 |
| Yes | 0.81(0.39–1.70) | 0.89(0.39–2.03) | 0.78(0.39–1.53) | 0.76(0.35–1.68) | |
| Back pain | No | 1 | 1 | 1 | 1 |
| Yes | 0.92(0.59–1.45) | 0.84(0.50–1.41) | 0.89(0.58–1.35) | 1.04(0.63–1.71) | |
| Chills | No | 1 | 1 | 1 | 1 |
| Yes | 0.91(0.58–1.43) | 1.16(0.65–2.06) | 0.95(0.62–1.45) | 0.68(0.40–1.16) | |
| Nausea | No | 1 | 1 | 1 | 1 |
| Yes | 0.85(0.53–1.37) | 0.77(0.45–1.33) | 1.42(0.92–2.19) | 1.51(0.92–2.45) | |
| Malaise | No | 1 | 1 | 1 | 1 |
| Yes | 0.99(0.57–1.70) | 0.85(0.46–1.57) | 0.61(0.36–1.04) | 0.55(0.30–1.03) | |
| Muscle pain | No | 1 | 1 | 1 | 1 |
| Yes | 1.24(0.73–2.12) | 1.29(0.69–2.43) | 1.57(0.94–2.62) | 1.90(1.04–3.49) | |
| Rash | No | 1 | 1 | 1 | 1 |
| Yes | 3.12(0.62–15.72) | 2.73(0.45–16.51) | 1.56(0.31–7.84) | 2.46(0.34–17.53) | |
| Malaria parasite | Negative | 1 | 1 | 1 | 1 |
| Positive | 2.36(1.23–4.52) | 2.37(1.14–4.92) | 1.24(0.64–2.39) | 1.32(0.62–2.82) | |
Sex and occupational status were significantly associated with previous DENV exposure. The odds of previous DENV exposure was found to be nearly twice as high in females compared to males, with crude OR of 1.71 (95% CI: 1.12–2.62) and adjusted OR of 2.24 (95% CI: 1.32–3.26). Government and private employees had higher odds of previous exposure compared to pastoralist and agro-pastoralists (AOR = 1.90; 95% CI: 1.03–8.15). Patents aged 11–20 had lower odds of previous exposure compared to patients aged from 5 to 10 years (AOR = 0.35; 95% CI: 0.14–0.88). Symptoms also associated with previous exposure. Patients experiencing muscle pain had AOR of 1.90 (95% CI: 1.04–3.49).
Discussion
This study examined the seroprevalence of DENV infection among acute febrile patients in three districts located in zone 3 of the Afar region in northeast Ethiopia, an area known to be malaria-endemic. The ELISA testing showed that more than half (53.4%) of the patient sera were seropositive for anti-DENV IgM and/or IgG antibodies among the febrile patient sera examined for both antibodies. The finding revealed that DF was under-recognized and under-reported in the study area, in alignment with the previous report in Africa [35]. This significant burden of DENV infection supports the idea that DENV is emerging as a major public health challenge in the tropics and sub-tropics, especially in sub-Saharan Africa [36, 37]. The high seroprevalence of DF in this area may be due to several factors, including climate change and local environmental conditions that favor Aedes mosquito breeding, which facilitates the virus transmission; frequent outbreaks that increase the chances of detection both IgM and IgG even during non-outbreak periods; increased urbanization and mobility that may help spread the virus [26]. Additionally, this is due to the disease is often under-recognized in national public health priorities of Ethiopia, resulting in gaps in early detection and response efforts [29, 32]. The finding highlights the need for a comprehensive understanding of the global and local prevalence of DF and its incidence as a neglected disease.
In particular, the seroprevalence of anti-DENV IgM and IgG antibodies was 24.6% and 38.7%, respectively, indicating an active DENV infection and previous exposure to the virus. The rates are higher than those reported in similar studies conducted in Ethiopia and other parts of Africa. For instance, rates of 7.9% for anti-DENV IgM and 22.9% for IgG antibodies were reported among febrile patients visiting health facilities in Borena, Southern Ethiopia [26], while rates of 19% for IgM and 21% for IgG antibodies were reported among febrile patients presented in Metema and Humera hospitals in Northwest Ethiopia [18]. Similarly, pooled seroprevalence estimated 9% for IgM and 21% for IgG against DENV infection in Ethiopia [38], and 24.8% for IgG and 10.8% for IgM among febrile populations in Africa [39]. These differences may be due to variation in the distribution of risk factors, climatic conditions in the study area, and the diversity of studied populations. Additionally, differences in diagnostic methodologies could also account for variability in seroprevalence rates.
In this study, though only 10.7% of patients with febrile symptoms suspected of malaria were diagnosed with malaria parasites, a significant finding was the rate of co-infection with DENV and malaria, which was 4.4% among febrile patients examined for both infections. The rate of co-infection is lower than the rates reported in neighboring Sudan (6.6%) [27], French Guiana (7.1%) [40], India (7.4%) [41] and Brazil (8.3%) [42]. Conversely, the rate is higher than 2.2% reported in Burkina Faso [43]. The variation in co-infection rate may be attributed to local endemicity and differences in socio-demographic characteristics. Furthermore, patients diagnosed with malaria were approximately 2.5 times more likely to test positive for anti-DENV IgM, indicating concurrent recent DENV infection. This poses substantial diagnostic challenges due to overlapping clinical features, which can result in misdiagnosis, and consequently lead to delayed treatment and adverse clinical outcomes characterized by increased morbidity and mortality [44]. These findings underscore the need for heightened attention from healthcare workers, researchers, and health policy makers [39]. To enhance the integration of DENV diagnostic testing into routine evaluation of febrile patients in endemic settings [45], educational intervention campaigns are crucial for healthcare professionals to raise awareness about symptom differentiation and prevention methods [46]. Healthcare workers need to receive trainings, which enable them to differentiate DF, malaria and other febrile illnesses, ensuring timely and accurate diagnoses. Community-based interventions could also play vital roles in mitigating risks by targeting the prevention of both malaria and DENV vectors through eliminating stagnant water sources, improving waste management and promoting the use of bed nets.
The seropositivity for anti-DENV IgM antibodies, indicating recent DENV infection, was found to be relatively low. This may be due to a delay in the production of detectable anti-DENV IgM antibodies, which is usually detected after the first 5–7 days of infection, where the possibility of IgM antibodies remaining negative for the first few days [12, 47]. Moreover, the seropositivity of IgM antibodies did not significantly differ by sociodemographic features and clinical symptoms of the participants. It aligned with a study conducted in Burkina Faso, which also found no symptoms and signs associated with participants who tested positive for anti-DENV IgM antibodies [43]. However, it contrasted the WHO reports, which indicated fever, body pain, headache, nausea, and rash as common symptoms of recent or acute DF [1] and Lima et al. report that identified rash and retro-orbital pain were associated with DF during outbreak period, while rash and nausea/vomiting during non-outbreak period in febrile participants [48]. On the other hand, patients visited Sidafage HC in this study showed lower seropositivity for anti-DENV IgM antibodies. This suggests that residents near Sidafage HC, which is the only HC found in the city located away from the international road crossing to ward Djibouti, may be less likely to be a potential hotspot for DENV transmission. However, longitudinal and entomological studies would be necessary to identify the real picture on the ground to aid in implementing vector control measures.
The seroprevalence of anti-DENV IgG was significantly higher among female patients. This finding disagrees with the studies conducted elsewhere in Ethiopia, where male participants were disproportionately influenced by the rate of anti-DENV IgG exposure [18, 26]. Similarly, in this study, those individuals who were working as government and private employees were more affected compared to pastoralists and agro-pastoralists with previous DENV infections, while patents within the 11–20 years age groups were found less frequently positive for previous DENV infections compared to those age group below 10 years old. This may be due to the working community members may face increased exposure to Aedes mosquitoes due to outdoor work during daylight hours. The observed differences regarding symptoms and demographics are likely influenced by a combination of immunological mechanisms, host factors, epidemiological context and variations in study design and methodology. In addition, the time of data collection, whether during an outbreak or a non-outbreak period, may significantly affect symptom presentation [48].
In this study, the absence of severe DF symptoms, such as mucosal bleeding or hemorrhagic manifestations, persistent vomiting, respiratory distress, and organ impairment, may indicate a lower prevalence of secondary infections. The severity of DF can be aggravated by secondary infection with different serotypes, as the initial infection elicits the production of weakly neutralizing antibodies., which enhances the severity of subsequent infection through a mechanism known as antibody-dependent enhancement (ADE) [9]. Children under five years and pregnant women were excluded higher due to their clinical risk and the potential need for immediate treatment intervention. Additionally, the technical and ethical challenges associated with venous blood collection in pediatric populations and the overlaps of febrile illness with pregnancy-related complications were factors for excluding. The absence of these demographic factors may potentially contribute to under-detection of severe cases. Because, the community members, including neonate or young child, pregnant female, exhibiting high body mass index, and possessing genetic polymorphisms are recognized risk factors for increased DF severity [7, 8]. Moreover, the lack of hematological data, such as platelet counts or hematocrit levels and reliance on self-reported symptoms limits the ability to confirm or rule out severe cases.
Furthermore, this study identified that a quarter (25.1%) were classified as borderline for anti-DENV IgM and/or IgG antibodies among sera examined for both antibodies, with 21.2% borderline for anti-DENV IgM and 20.2% borderline for anti-DENV IgG antibodies of participants, indicating early-stage infections, low-level antibody responses, or cross-reactivity with other flaviviruses. The presence highlights diagnostic uncertainty and the potential underestimation of true exposure. It underscores the critical need for continuous surveillance and molecular testing to accurately assess the burden of DF in the study area and the country as a whole.
Despite the valuable insights of this study’s findings, the study has several limitations. First, the study relied on ELISA-based serological testing, which limits the ability to distinguish between primary and secondary DF, detect the virus serotypes and confirm the borderline results. Secondly, vector and environmental surveillance data were not included, which are crucial for a comprehensive understanding of the dynamics of DENV transmission. Third, the study was facility-based and relied on febrile patients presenting to selected health care facilities, who may not represent the general community or those with asymptomatic or mild dengue infection. Purposive selection of the districts may also limit the generalizability of the findings to other regions in the Afar or even Ethiopia. Fourth, the cross-sectional design restricts to establishment of temporal relationships between risk factors and the infection. Lastly, reliance on self-reported symptoms that may have had recall bias, and no children under five and pregnant women and hematological data may have contributed to underreporting severe DF cases.
Conclusion
This study indicates that DENV is circulating in the Afar region, with a prevalence of 24.6% for anti-DENV IgM and 39.2% for anti-DENV IgG antibodies among febrile patients. This underscores the significant impact of DF in febrile patients in the study area. Additionally, the co-circulation of malaria and DF poses significant diagnostic challenges for healthcare systems, particularly in settings lacking robust diagnostic capabilities. There is an urgent need to establish routine diagnostics for DF in the local health facilities and to enhance epidemiological surveillance systems to accurately assess the disease burden in endemic regions like the Afar region. Longitudinal, molecular studies, such as RT-PCR, and hematological assessments like platelet count and hematocrit tests, could better establish causality and identify circulating DENV serotypes and the risk of severe DF and secondary infections. To effectively reduce morbidity and mortality associated with DF and other emerging arboviral-related diseases, it would be essential to elevate public awareness to recognize and manage diseases. This includes engaging communities in proactive prevention efforts, and providing comprehensive training for healthcare workers at-risk populations like in the Afar region. Furthermore, it is crucial to strengthen vector control measures targeting Aedes mosquito, which is the primary vector of DENV and other arboviruses. Equally important is the management of Anopheles species that transmit malaria, tailoring to their specific behavioral patterns.
Supplementary Information
Acknowledgements
We are grateful to study participants, data collectors and Health Bureau of Amibara district, Harunka district and Awash Sebat city administration and their respective health centers staff members. We are also grateful to Addis Ababa University of Ethiopia and LaRuke Development Inc. of USA for their financial and material supports.
Author contributions
Conceptualization and Design: BZ, GM, and ML. Formal analysis and Writing– original draft: BZ, TK, JWL, and ML. Writing-review and editing: BZ, TK, GM, and ML. All authors reviewed the manuscript.
Funding
The study obtained financial and material supports from Addis Ababa University of Ethiopia and LaRuke Development Inc. of USA.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the former Institutional Review Board of ALIPB, now under the ALIHR, Addis Ababa University (ALIPB IRERC/88/2014/22). The study adheres to the principles and guidelines outlined in the Declaration of Helsinki. Before data collection, permission was obtained from health offices of Amibara, Harunka and Awash Sebat city administration districts. Informed written consent was obtained from adults and from parents or guardians for children under 12, while informed assent was obtained from children between 12 and 18 years old. Malaria-positive participants were treated in accordance with established malaria treatment protocols in the study sites, while participants without malaria received alternative antibiotics regimens based on the clinician’s differential diagnosis.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Dengue and severe dengue. Fact Sheet.World Health Organization. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- 2.Gloria-Soria A, Ayala D, Bheecarry A, Calderon-Arguedas O, Chadee DD, Chiappero M, et al. Global genetic diversity of Aedes aegypti. Mol Ecol. 2016;25(21):5377–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. The Lancet Infectious Diseases. 2017 Mar [;17(3):e101–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473309916305187 [DOI] [PubMed]
- 5.Kajeguka DC, Kaaya RD, Mwakalinga S, Ndossi R, Ndaro A, Chilongola JO et al. Prevalence of dengue and chikungunya virus infections in north-eastern Tanzania: a cross sectional study among participants presenting with malaria-like symptoms. BMC Infect Dis. 2016;16(1):183. Available from: http://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-016-1511-5 [DOI] [PMC free article] [PubMed]
- 6.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global Spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res. 2012;136(3):373–90. [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030.World Health Organization. 2020. Available from: www.eliminateschisto.org/WHO_Revised-Draft-NTD-Roadmap2021-2030-23Apr2020
- 9.Wahala WMPB, de Silva AM. The human antibody response to dengue virus infection. Viruses. 2011;3(12):2374–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raafat N, Blacksell SD, Maude RJ. A review of dengue diagnostics and implications for surveillance and control. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2019;113(11):653–60. Available from: https://academic.oup.com/trstmh/article/113/11/653/5542180 [DOI] [PMC free article] [PubMed]
- 11.WHO. Dengue guidelines for diagnosis, treatment, prevention and control: new edition.World Health Organization. 2009. Available from: https://apps.who.int/iris/handle/10665/44188 [PubMed]
- 12.Chanama S, Anantapreecha S, A-nuegoonpipat A, Sa-gnasang A, Kurane I, Sawanpanyalert P. Analysis of specific IgM responses in secondary dengue virus infections: levels and positive rates in comparison with primary infections. J Clin Virol. 2004;31(3):185–9. [DOI] [PubMed] [Google Scholar]
- 13.CDC. Dengue; prevention.Centres for Disease Control and Prevention. 2021. Available from: https://www.cdc.gov/dengue/prevention/dengue-vaccine.html
- 14.Mwanyika GO, Mboera LEG, Rugarabamu S, Ngingo B, Sindato C, Lutwama JJ, et al. Dengue virus infection and associated risk factors in africa: A systematic review and Meta-Analysis. Viruses. 2021;13(4):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaenisch T, Junghanss T, Wills B, Brady OJ, Eckerle I, Farlow A et al. Dengue Expansion in Africa—Not Recognized or Not Happening? Emerg Infect Dis. 2014;20(10). Available from: http://wwwnc.cdc.gov/eid/article/20/10/14-0487_article.htm [DOI] [PMC free article] [PubMed]
- 16.Eshetu D, Shimelis T, Nigussie E, Shumie G, Chali W, Yeshitela B, et al. Seropositivity to dengue and associated risk factors among non-malarias acute febrile patients in Arba minch districts, Southern Ethiopia. BMC Infect Dis. 2020;20(1):639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Animut A, Mekonnen Y, Shimelis D, Ephraim E. Febrile illnesses of different etiology among outpatients in four health centers in Northwestern Ethiopia. Jpn J Infect Dis. 2009;62(2):107–10. [PubMed] [Google Scholar]
- 18.Ferede G, Tiruneh M, Abate E, Wondimeneh Y, Damtie D, Gadisa E, et al. A serologic study of dengue in Northwest ethiopia: suggesting preventive and control measures. PLoS Negl Trop Dis. 2018;12(5):e0006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AFRO. Ethiopia Steps Up Actions for Dengue Prevention and Control.World Health organization - African Region. 2014. Available from: https://www.afro.who.int/news/ethiopia-steps-actions-dengue-prevention-and-control
- 20.Degife LH, Worku Y, Belay D, Bekele A, Hailemariam Z. October,. Factors associated with dengue fever outbreak in Dire Dawa administration city, 2015, Ethiopia - case control study. BMC Public Health. 2019;19(1):650. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-019-7015-7 [DOI] [PMC free article] [PubMed]
- 21.Gutu MA, Bekele A, Seid Y, Mohammed Y, Gemechu F, Woyessa AB, et al. Another dengue fever outbreak in Eastern Ethiopia-An emerging public health threat. PLoS Negl Trop Dis. 2021;15(1):e0008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf AM, Ibrahim NA. Knowledge, attitude and practice towards dengue fever prevention and associated factors among public health sector health-care professionals: in dire dawa, Eastern Ethiopia. Risk Manage Healthc Policy. 2019;12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woyessa AB, Mengesha M, Kassa W, Kifle E, Wondabeku M, Girmay A, et al. The first acute febrile illness investigation associated with dengue fever in Ethiopia, 2013: a descriptive analysis. Ethiop J Health Dev. 2014;28(3):96. [Google Scholar]
- 24.Sisay C, Waldetensai A, Seyoum M, Tayachew A, Wossen M, Keneni D, et al. Detection of serotype 1-Dengue fever outbreak in dire Dawa city, Eastern Ethiopia. Ethiop J Public Health Nutr. 2022;5(1):49–54. [Google Scholar]
- 25.Akelew Y, Pareyn M, Lemma M, Negash M, Bewket G, Derbew A et al. Aetiologies of acute undifferentiated febrile illness at the emergency ward of the University of Gondar Hospital, Ethiopia. Tropical Med Int Health. 2022;27(3):271–9. Available from: https://onlinelibrary.wiley.com/doi/10.1111/tmi.13721 [DOI] [PubMed]
- 26.Geleta EN. Serological evidence of dengue fever and its associated factors in health facilities in the borena zone, South Ethiopia. Res Rep Trop Med. 2019;10:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsedig K, Eldigail MH, Elduma AH, Elaagip A, Altahir O, Siam HA et al. Prevalence of malaria and dengue co-infections among febrile patients during dengue transmission season in Kassala, eastern Sudan. Viennet E, editor. PLoS Negl Trop Dis. 2023;17(10):e0011660. Available from: 10.1371/journal.pntd.0011660 [DOI] [PMC free article] [PubMed]
- 28.Mohan A, Fakhor H, Nimavat N, Wara UU, Lal PM, Costa ACDS, et al. Dengue and COVID-19: A risk of coepidemic in Ethiopia. J Med Virol. 2021;93(10):5680–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zerfu B, Kassa T, Legesse M. Epidemiology, biology, pathogenesis, clinical manifestations, and diagnosis of dengue virus infection, and its trend in Ethiopia: a comprehensive literature review. Trop Med Health. 2023;51(1):11. Available from: https://tropmedhealth.biomedcentral.com/articles/10.1186/s41182-023-00504-0 [DOI] [PMC free article] [PubMed]
- 30.Endale A, Michlmayr D, Abegaz WE, Asebe G, Larrick JW, Medhin G et al. Community-based sero-prevalence of chikungunya and yellow fever in the South Omo Valley of Southern Ethiopia. Reiner RC, editor. PLoS Negl Trop Dis. 2020;14(9):e0008549. Available from: 10.1371/journal.pntd.0008549 [DOI] [PMC free article] [PubMed]
- 31.Mehari S, Zerfu B, Desta K. Prevalence and risk factors of human brucellosis and malaria among patients with fever in malaria-endemic areas, attending health institutes in Awra and Gulina district, Afar region, Ethiopia. BMC Infect Dis. 2021;21(1):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.EPHI. Arboviral Disease Vectors Surveillance and Control Guideline. Public Health Entomology Research Team.Ethiopia Public Health Institute, Addis Ababa Ethiopia. 2021. Available from: https://ephi.gov.et/wp-content/uploads/2021/12/Arboviral-Disease-Vectors-Surveillance-and-Control-Guideline.pdf
- 33.Hagaman A, Gonzalez Rodriguez H, Egger E, Bitewulign B, Case H, Alemayehu AK et al. Navigating and manipulating childbirth services in Afar, Ethiopia: A qualitative study of cultural safety in the birthing room. Social Science & Medicine. 2023;331:116073. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0277953623004306 [DOI] [PMC free article] [PubMed]
- 34.Dengue ELISA. Instructions - dengue virus igg/igm, dengue virus superior igm. Enzyme-immunoassay for determination of human antibodies for in vitro diagnostic use. Engl (Version V 114.5). 2024.
- 35.Amarasinghe A. Dengue Virus Infection in Africa. Emerg Infect Dis. 2011; Available from: http://wwwnc.cdc.gov/eid/article/17/8/10-1515_article.htm [DOI] [PMC free article] [PubMed]
- 36.Goswami L, Runumi C, Rasul ES. Seroprevalence of Dengue Infection in a Tertiary Care Hospital in Assam. IJMDS. 2018;7(1):1582. Available from: https://informaticsjournals.co.in/index.php/ijmds/article/view/18905
- 37.Aniakwaa-Bonsu E, Amoako-Sakyi D, Dankwa K, Prah JK, Nuvor SV. Seroprevalence of Dengue Viral Infection among Adults Attending the University of Cape Coast Hospital. AID. 2021;11(01):60–72. Available from: https://www.scirp.org/journal/doi.aspx?doi=10.4236/aid.2021.111008
- 38.Nigussie E, Atlaw D, Negash G, Gezahegn H, Baressa G, Tasew A et al. A dengue virus infection in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2024;24(1):297. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-024-09142-1 [DOI] [PMC free article] [PubMed]
- 39.Simo FBN, Bigna JJ, Kenmoe S, Ndangang MS, Temfack E, Moundipa PF et al. Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies. Sci Rep. 2019;9(1):13626. Available from: https://www.nature.com/articles/s41598-019-50135-x [DOI] [PMC free article] [PubMed]
- 40.Epelboin L, Hanf M, Dussart P, Ouar-Epelboin S, Djossou F, Nacher M et al. Is dengue and malaria co-infection more severe than single infections? A retrospective matched-pair study in French Guiana. Malar J. 2012;11(1):142. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/1475-2875-11-142 [DOI] [PMC free article] [PubMed]
- 41.Mohapatra MK, Patra P, Agrawala R. Manifestation and outcome of concurrent malaria and dengue infection. J Vector Borne Dis. 2012;49(4):262–5. [PubMed] [Google Scholar]
- 42.Magalhães BML, Alexandre MAA, Siqueira AM, Melo GC, Gimaque JBL, Bastos MS et al. Clinical Profile of Concurrent Dengue Fever and Plasmodium vivax Malaria in the Brazilian Amazon: Case Series of 11 Hospitalized Patients. Am J Trop Med Hyg. 2012;87(6):1119–24. Available from: https://www.ajtmh.org/view/journals/tpmd/87/6/article-p1119.xml [DOI] [PMC free article] [PubMed]
- 43.Ouédraogo JCRP, Ilboudo S, Compaoré TR, Bado P, Nitiéma M, Ouédraogo WT, et al. Determinants and prevalence of symptomatic dengue fever among adults in the central region of Burkina faso: a hospital-based cross-sectional study. BMC Infect Dis. 2024;24(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward DI. A case of fatal plasmodium falciparum malaria complicated by acute dengue fever in East Timor. Am J Trop Med Hyg. 2006;75(1):182–5. [PubMed] [Google Scholar]
- 45.Gebremariam TT, Schallig HDFH, Kurmane ZM, Danquah JB. Increasing prevalence of malaria and acute dengue virus coinfection in Africa: a meta-analysis and meta-regression of cross-sectional studies. Malar J. 2023;22(1):300. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-023-04723-y [DOI] [PMC free article] [PubMed]
- 46.Socha W, Kwasnik M, Larska M, Rola J, Rozek W. Vector-Borne Viral Diseases as a Current Threat for Human and Animal Health—One Health Perspective. JCM. 2022;11(11):3026. Available from: https://www.mdpi.com/2077-0383/11/11/3026 [DOI] [PMC free article] [PubMed]
- 47.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8(12 Suppl):S7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim JK, Seydou Y, Carabali M, Barro A, Dahourou DL, Lee KS et al. (2019) Clinical and epidemiologic characteristics associated with dengue during and outside the 2016 outbreak identified in health facility-based surveillance in Ouagadougou, Burkina Faso. Forshey BM, editor. PLoS Negl Trop Dis. 13(12):e882. 10.1371/journal.pntd.0007882 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.