Abstract
The importance of algal and detrital food supplies to the planktonic food web of a highly disturbed, estuarine ecosystem was evaluated in response to declining zooplankton and fish populations. We assessed organic matter bioavailability among a diversity of habitats and hydrologic inputs over 2 years in San Francisco Estuary's Sacramento–San Joaquin River Delta. Results show that bioavailable dissolved organic carbon from external riverine sources supports a large component of ecosystem metabolism. However, bioavailable particulate organic carbon derived primarily from internal phytoplankton production is the dominant food supply to the planktonic food web. The relative importance of phytoplankton as a food source is surprising because phytoplankton production is a small component of the ecosystem's organic-matter mass balance. Our results indicate that management plans aimed at modifying the supply of organic matter to riverine, estuarine, and coastal food webs need to incorporate the potentially wide nutritional range represented by different organic matter sources.
Ecologists have long recognized the potential importance of terrestrially derived organic matter in supporting secondary productivity within aquatic ecosystems (1–3). Terrestrial–aquatic linkages in organic matter supply have been well documented in many headwater streams draining forested catchments (4–7) and are becoming increasingly documented in large lakes (8), large rivers (9), estuaries (10), and the open ocean (11). These latter findings suggest that aquatic community respiration routinely depends on external detrital inputs even in aquatic ecosystems in which respiration and secondary production were previously thought to be tightly coupled to internal phytoplankton production (12). Although terrestrial–aquatic linkages in organic matter supply and respiration have been documented in increasingly larger aquatic ecosystems, the corresponding strength of these external detrital pathways to higher trophic levels in planktonic food webs remains unclear (13–17). We studied the role of detrital inputs in supporting the food web of a large, highly disturbed estuary. We evaluated the amount, composition, sources, and bioavailability of organic matter among a diversity of habitats and hydrologic inputs for 2 years in San Francisco Estuary's Sacramento-San Joaquin River Delta. Results from this comparative study and a system-wide organic matter budget were used to quantify the potential supply of detrital and algal organic matter to the metazoan food web in this large estuarine ecosystem.
Background
The Sacramento–San Joaquin River Delta is a mosaic of tidal freshwater habitats connecting a 1.6 × 107 ha watershed to the San Francisco Bay (18). The Delta waterways and habitats provide the northern San Francisco Bay with the majority of its river inflow and organic matter supply (18). The Delta waterways provide many important ecosystem services including conveyance of drinking water for over 20 million people, supply of irrigation water for nearly 10 million ha of farmland, and 26,000 ha of open-water habitat for waterfowl and 130 species of fish (19). The Delta ecosystem has experienced 150 years of intense, human-induced disturbance and has one of the highest incidences of invasive species in the world (20). Recent declines in the abundances of many species of fishes, however, have stimulated large-scale ecosystem restoration efforts (19). Although multiple factors may have interacted to depress fish stocks and diversity, a decline in food resources has been hypothesized as being an important factor in explaining declines of juvenile fish (21). Specifically, there have been significant declines in phytoplankton (22) and numerous species of native zooplankton over the past 30 years (23). Declines in phytoplankton have been attributed to the widespread invasion of the suspension-feeding clam Potamocorbula amurensis (22). Phytoplankton biomass accrual has remained low because of continued benthic grazing pressure (24) and low growth rates that are a function of light limitation as opposed to nutrient limitation (25). Long-term declines in Delta phytoplankton, native zooplankton, and native fish suggest a potential trophic linkage (21), but additional complementary approaches are required to determine the strength of this linkage. Ecosystem science requires multiple approaches to provide strong inference (26). Our study was designed to provide an ecosystem-wide assessment of food resources that support the production of lower-trophic-level invertebrates that provide forage food for juvenile fish.
A long historical data set was used to construct a Delta-wide organic matter mass balance (18). On an annual basis, external river inputs accounted for 69% of the organic matter supply to the Delta, whereas primary producers within the system accounted for <15%. External river inputs were dominated by the Sacramento River, which provides 84% of the Delta's freshwater. Internal primary production was dominated by phytoplankton, as opposed to macrophytes and benthic algae, but this production is small compared with other estuaries (27) and has declined 43% since 1975 (22). In a complementary study, we measured the isotopic composition (13C:12C and 15N:14N ratios) of seston, sediments, and living plants collected throughout the estuary. Results suggested that the organic-matter pools within the Delta do not have large components of recently produced plant biomass or detritus, except during phytoplankton blooms (28).
Methods
This study was designed to assess the bioavailability and realized importance of the Delta's potential sources of organic matter. Our experimental design aimed to maximize potential spatial and temporal variation in organic matter bioavailability. We compared 12 habitats and hydrologic inputs during a 2-year period. Sampling sites were selected to span the Delta and extend into Suisun Bay in the northern San Francisco Bay, an area in which phytoplankton and zooplankton declines have been documented (Fig. 1). Sampling dates were selected to span hydrologic conditions and seasons. We partitioned organic matter into ecologically significant size classes (dissolved and particulate) and pools of quality (bioavailable and recalcitrant). Particulate organic matter can be directly consumed by zooplankton, whereas dissolved organic matter usually must be converted to bacterial or protozoan biomass before it is available to zooplankton (29, 30). We operationally defined bioavailable organic matter as the fraction that can be respired or incorporated by bacteria during a 21-day bioassay, which corresponds to the Delta's mean hydraulic-residence time.
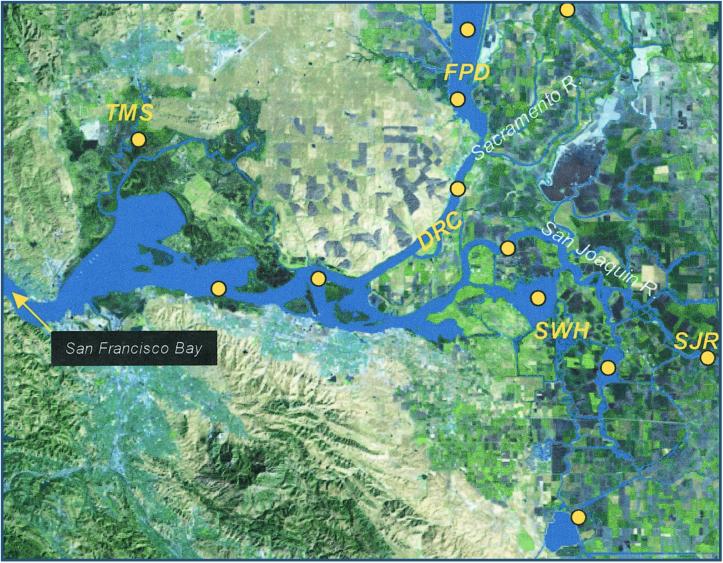
Figure 1.
Enhanced satellite image of the San Francisco Estuary's Sacramento and San Joaquin River Delta. Yellow circles show sites sampled during the 2-year study. These sites were classified as five diverse habitats: (i) deep-river channel (DRC), (ii) tidal-marsh sloughs (TMS), (iii) floodplain drainage (FPD), (iv) shallow-water habitat (SWH), and (v) San Joaquin River (SJR).
Water samples were collected between October 1998 and July 2000 by peristaltic pump and screened through a 210-μm mesh. The demarcation between dissolved organic carbon (DOC) and particulate organic carbon (POC) was 1.0 μm. DOC and POC were measured with high temperature combustion following separate filtration and acidification procedures. Bioavailable DOC (BDOC) and bioavailable POC (BPOC) were operationally defined as the metabolized fraction during independent 21-day incubations in the dark at room temperature. Organic carbon loss was measured directly in both sets of assays and supported with additional assays that measured 21-day biological oxygen demand and the conversion of POC to DOC. Flocculation was not observed during BDOC assays. Chlorophyll a and phaeophytin were determined with standard fluorometric methods.
We categorized our sampling sites into five functionally different habitat types: (i) deep-river channels, (ii) tidal-marsh sloughs, (iii) floodplain drainage, (iv) shallow-water habitats, and (v) San Joaquin River inputs (Fig. 1). Deep-river channel habitat includes three sites along the Sacramento River and two sites in the central Delta that receive water from the Sacramento River (mean depth = 9.7 m). Tidal-marsh slough represents shallow sloughs draining Suisun Marsh, a remnant tule (Scirpus) marsh. Floodplain drainage characterizes water that has traveled through the Yolo Bypass, a large agricultural floodplain that is inundated for flood protection during high flow. Shallow-water habitats represent Franks Tract and Mildred Island, two large lake-like environments in the central Delta. San Joaquin River inputs represent sampling on the San Joaquin River at Mossdale, upstream from the river's entrance into the Delta at Stockton.
In addition to measuring bioavailable pools of organic matter, we estimated the potential contributions of phytoplankton and bacterioplankton to the Delta's metazoan food web. We estimated the potential yield of protozoan biomass by assuming efficient transfers of BDOC to bacteria (growth efficiency = 0.25) and bacteria to protozoans (growth efficiency = 0.25). These estimates represent theoretical maximal yields and were used in comparisons of various pools of organic matter. We aimed to maximize the potential pool of protozoan biomass to highlight the relative importance of algal biomass. Suspension feeders that can filter bacteria directly (e.g., many cladocerans) account for a smaller percentage of the Delta's zooplankton assemblage relative to more selective feeders that rely on larger particles (e.g., some copepods and rotifers).
Rates of phytoplankton and bacterial production were calculated with additional assays that were unrelated to the previously described bioavailability assays. Phytoplankton gross primary production was calculated as a function of chlorophyll a and mean water-column irradiance using an empirical model built from 51 direct measurements of primary productivity at sites within the Delta with a standard NaH14CO3 uptake method (22). Phytoplankton respiration was calculated as a function of biomass and growth rate by using an additional empirical model based on 145 published measurements of algal cultures (31). Bacterial abundance was measured by using direct microscopic counts and converted into bacterial biomass estimates assuming bacterial cell:C = 20 fg per cell (32). Bacterial respiration was calculated as whole-community respiration subtracted by algal respiration. Whole-community respiration was measured as oxygen consumption during 24-h in situ dark incubations. Bacterial productivity was estimated from bacterial respiration, assuming bacterial growth efficiency = 0.25 (this value is similar to the global mean; ref. 33).
Results and Discussion
Our results revealed that a small fraction of the total pool of organic matter was bioavailable (Table 1), suggesting that only a small fraction of the Delta's chemical energy can support secondary productivity. The concentration of DOC was routinely greater than POC, but the bioavailable DOC and bioavailable POC concentrations were more comparable (Table 1). This discrepancy results from a larger percentage of the particulate organic matter being bioavailable (Table 1). This finding is ecologically important because POC enters the metazoan food web at a much greater efficiency than DOC. POC is frequently ingested directly, whereas DOC must be routed through the microbial loop, resulting in a large respiratory loss of carbon (30). Thus zooplankton secondary production is likely to be more strongly linked to bioavailable POC as opposed to bioavailable DOC.
Table 1.
Bioavailability of dissolved and particulate organic carbon
| Habitat type | Dissolved organic carbon
|
Particulate organic carbon
|
||||
|---|---|---|---|---|---|---|
| DOC, mg liter−1 | BDOC, mg liter−1 | BDOC, % | POC, mg liter−1 | BPOC, mg liter−1 | BPOC, % | |
| DRC | 2.4 ± 0.11 | 0.3 ± 0.02 | 12 | 1.0 ± 0.09 | 0.2 ± 0.02 | 23 |
| TMS | 10.5 ± 0.59 | 1.2 ± 0.09 | 11 | 2.8 ± 0.21 | 0.5 ± 0.10 | 21 |
| FPD | 3.0 ± 0.14 | 0.5 ± 0.04 | 15 | 1.6 ± 0.10 | 0.4 ± 0.08 | 22 |
| SWH | 2.7 ± 0.09 | 0.3 ± 0.03 | 13 | 0.6 ± 0.04 | 0.2 ± 0.05 | 27 |
| SJR | 3.2 ± 0.21 | 0.4 ± 0.04 | 11 | 1.8 ± 0.20 | 0.9 ± 0.20 | 33 |
Bioavailability of dissolved (DOC) and particulate organic carbon (POC) among habitat type. Bioavailable dissolved organic carbon (BDOC) and bioavailable particulate organic carbon (BPOC) were operationally defined as the amount of DOC and POC that were metabolized by bacteria during 21-day incubations. Percent of BDOC and BPOC are shown as the median of all samples. Habitat types include deep-river channels (DRC), tidal-marsh sloughs (TMS), floodplain drainage (FPD), shallow-water habitats (SWH), and San Joaquin River inputs (SJR). The data are given as the mean of independent samples ± 1 SE. Sample size varies among habitat type: DRC = 93, TMS = 23, FPD = 39, SWH = 32, and SJR = 23. BPOC was measured on a sub-set of these samples: DRC = 45, TMS = 15, FPD = 24, SWH = 18, and SJR = 12.
Phytoplankton-derived organic matter constituted a small fraction of the total and particulate organic matter found among habitats. For example, phytoplankton biomass typically represented only 5% of the total organic matter (i.e., DOC + POC) and 28% of the POC in the deep-river channel habitat (overall medians; Table 2). However, phytoplankton biomass was a large and important component of the bioavailable POC. Phytoplankton biomass routinely equaled or exceeded bioavailable POC in all habitats (Table 2). Further, phytoplankton biomass and bioavailable POC strongly correlated in all habitats (overall Spearman's coefficient of rank (r) = 0.66, and r > 0.40 regardless of the specific habitat). These findings were surprising because of the small contribution of algal biomass to the Delta's organic-matter mass balance (18). Conversely, detrital-derived POC constituted the majority of the total POC in most habitats (overall median ratio = 0.72), but represented a much smaller component of the bioavailable POC. This finding was surprising because detrital organic matter is the energetic basis of many stream and river ecosystems (5–7). This finding contributes to an emergent general pattern of carbon cycling in large rivers and estuaries. Riverine organic matter may be much older and recalcitrant than previously thought (17). Our findings provide strong evidence that the Delta's planktonic food web may be highly reliant on phytoplankton production although this organic-matter source represents a small amount of the ecosystem's potential energy to higher trophic levels.
Table 2.
Total phytoplankton biomass (PhytoOC) relative to other pools of organic carbon
| Habitat type | PhytoOC: DOC + POC | PhytoOC: POC | PhytoOC: BPOC | ProtOC: PhytoOC | Phyto <10 μm: Total phyto |
|---|---|---|---|---|---|
| DRC | 0.05 | 0.28 | 1.18 | 0.11 | 0.64 |
| TMS | 0.04 | 0.22 | 0.94 | 0.15 | 0.64 |
| FPD | 0.07 | 0.24 | 0.80 | 0.09 | 0.72 |
| SWH | 0.05 | 0.42 | 1.87 | 0.12 | 0.82 |
| SJR | 0.18 | 0.61 | 1.80 | 0.04 | 0.32 |
Phytoplankton biomass was calculated as the sum of chlorophyll a + phaeophytin assuming C:pigment of 35:1. Protozoan biomass (ProtOC) was calculated assuming an efficient transfer of BDOC to protozoan biomass via the microbial loop. Specifically, bacterial growth efficiency during metabolism of BDOC was assumed to equal 0.25 and the assimilation efficiency of protozoans was assumed to equal 0.25 (see Methods for details). The data are given as the median ratio of independent samples. Sample size varies among habitat type: DRC = 93, TMS = 23, FPD = 39, SWH = 32, and SJR = 23. Phytoplankton:BPOC was determined for a sub-set of these samples: DRC = 45, TMS = 15, FPD = 24, SWH = 18, and SJR = 12.
Zooplankton growth experiments conducted in conjunction with the research presented here supported this hypothesized trophic linkage (34). Laboratory growth assays with the cladoceran Daphnia magna showed that zooplankton growth rate was strongly related to phytoplankton biomass and unrelated to the amount of detrital organic matter. Previous research examining clearance rates and assimilation efficiencies in Potamocorbula amurensis, a benthic suspension-feeding clam that is abundant in Suisun Bay, reached similar conclusions (24), but the specific results were not transferable to Delta-wide planktonic-filter feeders. The strength of the relationship between zooplankton growth rate and phytoplankton biomass was most pronounced at chlorophyll a concentrations ranging from 0–10 μg liter−1. This finding suggests that nutritional components associated with phytoplankton, rather than with detritus, regulate zooplankton growth and that Delta zooplankton may be food-limited when chlorophyll a concentration is <10 μg liter−1. Our measurements revealed that chlorophyll a concentrations rarely exceeded 10 μg liter−1 at most Delta sites (San Joaquin River is an exception). For example, size-fractionated phytoplankton (i.e., phytoplankton <10 μm and potentially having high nutritional value) only exceeded 10 μg liter−1 in 4 of 207 samples. These findings are in agreement with a long-term historical analysis of Delta phytoplankton biomass (22). These findings are ecologically important because they suggest that the Delta's zooplankton may be routinely food limited. Our results also suggest that the Delta's phytoplankton, although routinely low in biomass, is generally of high nutritional quality. For example, a large percentage of Delta chlorophyll a is contained in cells smaller than 10 μm (overall median ratio = 0.67; Table 2) and generally consists of a high proportion of diatoms and cryptophytes.
What are the relative strengths of the algal and detrital linkages to Delta zooplankton? The Sacramento River contributes the majority of the Delta's freshwater inputs and a large component of external organic matter inputs (18). Our habitat classification of deep-river channel contains sites that are greatly influenced by Sacramento River discharge. Deep-river channel habitat represents a large percentage of the Delta's volume (52% of the Delta's volume is >3 m in depth). Deep-river channel had the lowest algal biomass among habitats (annual mean = 191 μg of C liter−1; Table 3), but algal biomass represented a large proportion of bioavailable POC (median ratio = 1.18; Table 2). However, could bioavailable DOC augment the contribution of particulate detritus? Even if we assume a direct (i.e., only two trophic steps) and efficient (i.e., high-growth efficiency of 0.25) transformation of DOC to protozoan biomass the annual mean contribution would be 19 μg of C liter−1 (by using the annual mean BDOC concentration in Table 1). Even this upper-limit estimate would only augment the bioavailable POC by 9% (and only 12% when Delta-wide means are used). Alternatively, the ratio of potential protozoan biomass to phytoplankton biomass also shows the relatively small contribution of DOC-derived detritus, regardless of the habitat (Table 2). These estimates of the relative contribution of potential protozoan biomass are almost certainly overestimates because they are based on BDOC pools generated after 21-day incubations and estimated using optimal energy transfers; thus, the conversion of bioavailable DOC into protozoan biomass seems unlikely to substantially augment the Delta's bioavailable POC pool.
Table 3.
Phytoplankton and bacterioplankton activities among habitat type
| Habitat type | Phytoplankton
|
Bacteria
|
||||
|---|---|---|---|---|---|---|
| chl a (μg liter−1) | Biomass (μg C liter−1) | NPP (μg C l−1d−1) | Biomass (μg C liter−1) | Respiration (μg C l−1d−1) | BP (μg C l−1d−1) | |
| DRC | 3.1 ± 0.4 | 191 ± 18 | 10 ± 1 | 53 ± 3 | 36 ± 4 | 9 ± 1 |
| TMS | 7.7 ± 0.8 | 451 ± 44 | 104 ± 18 | 143 ± 10 | 62 ± 17 | 16 ± 4 |
| FPD | 5.4 ± 0.6 | 300 ± 28 | 45 ± 9 | 58 ± 3 | 55 ± 8 | 14 ± 2 |
| SWH | 3.9 ± 0.8 | 240 ± 36 | 58 ± 10 | 78 ± 7 | 70 ± 16 | 17 ± 4 |
| SJR | 26.8 ± 7.2 | 1215 ± 298 | 295 ± 96 | 90 ± 8 | 92 ± 13 | 23 ± 3 |
Phytoplankton biomass was calculated as the sum of chlorophyll a (chl a) + phaeophytin using a C:pigment ratio of 35:1. Net primary production (NPP) was calculated from phytoplankton biomass and light availability (see Methods). Bacterioplankton biomass was calculated using bacterial abundance estimates and a C:cell ratio of 20 fg:cell. Bacterioplankton respiration and production (BP) were derived from measurements of whole-community respiration (see Methods). The data are given as the mean of independent samples ± 1 SE. Phytoplankton sample size varies among habitat type: DRC = 93, TMS = 23, FPD = 39, SWH = 32, and SJR = 23. Bacterioplankton was characterized on a sub-set of these samples: DRC = 45, TMS = 15, FPD = 24, SWH = 18, and SJR = 12.
An alternate approach for assessing the relative strengths of the algal and detrital linkages to Delta zooplankton is to compare the standing stocks and productivity of phytoplankton and bacterioplankton (Table 3). For reasons discussed above, we highlight results from the deep-river channel, where annual mean phytoplankton biomass was ≈4-fold higher than bacterioplankton biomass. Net primary productivity was much lower in the deep-river channel compared with all other habitats (Table 3) because of large respiratory losses in the deep aphotic zone. We estimated bacterial productivity from in situ respiration assuming a bacterial growth efficiency of 0.25. Assuming all bacteria are converted into protozoan biomass with similarly high growth efficiency, we generate <25% of the particulate organic carbon produced by net primary production. All other Delta habitats had much higher phytoplankton biomass and net primary production compared with the deep-river channel habitat (Table 3). Conversely, bacterial biomass and productivity were only slightly higher (generally, a <2-fold increase) in other habitats (Table 3). Thus, the relative importance of phytoplankton-derived organic matter was much greater in the other habitats. Our reported rates of Delta-wide phytoplankton and bacterioplankton production are similar to previously reported estimates. For example, our estimates of bacterioplankton production compared with those of Hollibaugh and Wong (32) for northern San Francisco Bay have similar ranges and only 2-fold differences in mean rates although different methods were used.
The important role of phytoplankton biomass in the Delta's pool of bioavailable POC was supported with three independent approaches: (i) comparison of pools of organic matter and relationships among pools using direct measures and bioavailability bioassays; (ii) comparison of phytoplankton and bacterioplankton activities; and (iii) zooplankton growth and fecundity bioassays. The combined findings suggest that detrital linkages to the planktonic food web are relatively weak even under the best-case scenarios for detrital importance.
Conclusions
Ecologists have long debated the relative importance of algal vs. detrital food supplies to aquatic food webs (1, 2, 7, 13, 14, 16). Experimental manipulations of entire streams, rivers, lakes, watersheds, and masses of marine water have greatly advanced our understanding of energy flow in aquatic ecosystems, but such experiments are rare (35). Our collective findings from long-term, comparative, and experimental approaches provide strong evidence in support of the previously hypothesized food-chain linkage between phytoplankton and the pelagic food web in the San Francisco Estuary's Delta. Our findings were surprising because phytoplankton constitutes a small fraction of the ecosystem's organic matter supply. Our results have important implications for management actions aimed at stabilizing or amplifying populations of valued pelagic species. Specifically, our work documents the link between phytoplankton (a limiting resource) and the pelagic food web and suggests that successful management actions may need to incorporate strategies for enhancing phytoplankton biomass in the Delta ecosystem. Such a management strategy contrasts with strategies in other disturbed ecosystems (e.g., Chesapeake Bay) that aim to decrease phytoplankton production and biomass. In addition, our findings provide valuable information for predicting food-web responses to future manipulations of organic matter supply, both planned and unplanned. Planning and decision-making can be improved by such forecasts of ecosystem state (25, 36).
Our results indicate that management actions aimed at modifying the supply of organic matter to riverine, estuarine, and coastal food webs need to consider the potentially wide nutritional range represented by different organic matter sources. Specifically, the largest organic matter pools may have the weakest nutritional impact, whereas the smallest pools may represent the most important source of energy for the food web. Planktonic food webs in low-productivity estuarine and riverine ecosystems may be especially sensitive to changes in phytoplankton production, although phytoplankton biomass represents a small fraction of the flux of organic matter through many rivers and estuaries. Our results are important because many large estuaries have experienced massive human-induced modifications of external and internal organic matter supply (27). Human actions have resulted in worldwide loss and degradation of tidal marshes and riparian forests, thus altering the supply of external organic matter inputs to estuaries (15, 37). Human actions also have resulted in worldwide manipulation of the hydrologic, chemical, and biological factors that regulate phytoplankton production within estuaries (27, 38, 39). Projected trends in worldwide land use suggest that the world's estuaries will become increasingly reliant on bioavailable organic matter produced within the ecosystem relative to that delivered from external sources (40).
Acknowledgments
We thank E. Canuel, S. Conard, M. Cox, J. Edmonds, K. Forshey, C. Lopez, L. Lucas, N. Monsen, F. Parchaso, B. Richards, L. Schemel, R. Stewart, J. Thompson, B. Topping, and V. Pilon for contributing to this study. Special thanks are extended to A. Arnsberg, B. Cole, and T. Schraga for their dedicated field and laboratory work. J. Kuwabara, L. Lucas, M. Marvin-DiPasquale, L. Schemel, G. Trussell, and three anonymous reviewers provided thoughtful comments on the manuscript. This research was funded by the CALFED Bay-Delta Ecosystem Restoration Program and the U.S. Geological Survey.
Abbreviations
- DOC
dissolved organic carbon
- POC
particulate organic carbon
- BDOC
bioavailable DOC
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lindeman R L. Ecology. 1942;23:399–418. [Google Scholar]
- 2.Teal J M. Ecology. 1962;43:614–624. [Google Scholar]
- 3.Wetzel R G. Limnology: Lake and River Ecosystems. San Diego: Academic; 2001. pp. 731–783. [Google Scholar]
- 4.Fisher S G, Likens G E. Ecol Monogr. 1973;43:421–439. [Google Scholar]
- 5.Vannote R L, Minshall G W, Cumming K W, Sedell J R, Cushing C E. Can J Fish Aquat Sci. 1980;37:130–137. [Google Scholar]
- 6.Webster J R, Meyer J L. J N Am Benthol Soc. 1997;16:5–17. [Google Scholar]
- 7.Wallace J B, Eggert S L, Meyer J L, Webster J R. Science. 1997;277:102–104. [Google Scholar]
- 8.Cole J J, Caraco N F, Kling G W, Kratz T K. Science. 1994;265:1568–1570. doi: 10.1126/science.265.5178.1568. [DOI] [PubMed] [Google Scholar]
- 9.Raymond P A, Caraco N F, Cole J J. Estuaries. 1997;20:381–390. [Google Scholar]
- 10.Smith S V, Hollibaugh J T. Ecol Monogr. 1997;67:509–533. [Google Scholar]
- 11.del Giorgio P A, Cole J J, Cimbleris A. Nature (London) 1997;385:148–151. [Google Scholar]
- 12.Cole J J, Findlay S, Pace M L. Mar Ecol Prog Ser. 1988;43:1–10. [Google Scholar]
- 13.Peterson B J, Howarth R W, Garritt R H. Science. 1985;227:1361–1363. doi: 10.1126/science.227.4692.1361. [DOI] [PubMed] [Google Scholar]
- 14.Deegan L A, Garritt R H. Mar Ecol Prog Ser. 1997;147:31–47. [Google Scholar]
- 15.Zedler J B, Fellows M Q, Trnka S. In: Successes, Limitations, and Frontiers in Ecosystem Science. Pace M L, Groffman P M, editors. New York: Springer; 1998. pp. 69–112. [Google Scholar]
- 16.Lewis W M, Jr, Hamilton S K, Rodriguez M A, Saunders J F, III, Lasi M A. J N Am Benthol Soc. 2001;20:241–254. [Google Scholar]
- 17.Raymond P A, Bauer J E. Nature (London) 2001;409:497–499. doi: 10.1038/35054034. [DOI] [PubMed] [Google Scholar]
- 18.Jassby A D, Cloern J E. Aquatic Conservation: Mar Fresh Ecosyst. 2000;10:323–352. [Google Scholar]
- 19. Lucas, L. V., Cloern, J. E., Thompson, J. K. & Monsen, N. E. (2002) Ecol. Appl., in press.
- 20.Cohen A N, Carlton J T. Science. 1998;279:555–558. doi: 10.1126/science.279.5350.555. [DOI] [PubMed] [Google Scholar]
- 21.Bennett W A, Moyle P B. In: San Francisco Bay: The Ecosystem. Hollibaugh J T, editor. San Francisco: Am. Assoc. Adv. Science; 1996. pp. 519–542. [Google Scholar]
- 22.Jassby A D, Cloern J E, Cole B E. Limnol Oceanogr. 2002;47:698–712. [Google Scholar]
- 23.Kimmerer W J, Orsi J J. In: San Francisco Bay: The Ecosystem. Hollibaugh J T, editor. San Francisco: Am. Assoc. Adv. Science; 1996. pp. 403–424. [Google Scholar]
- 24.Werner I, Hollibaugh J T. Limnol Oceanogr. 1993;38:949–964. [Google Scholar]
- 25.Cloern J E. Aquatic Ecology. 1999;33:3–16. [Google Scholar]
- 26.Carpenter S R. In: Successes, Limitations, and Frontiers in Ecosystem Science. Pace M L, Groffman P M, editors. New York: Springer; 1998. pp. 287–313. [Google Scholar]
- 27.Cloern J E. Mar Ecol Prog Ser. 2001;210:223–253. [Google Scholar]
- 28.Cloern J E, Canuel E A, Harris D. Limnol Oceanogr. 2002;47:713–729. [Google Scholar]
- 29.Pomeroy L R. BioScience. 1974;24:499–504. [Google Scholar]
- 30.Ducklow H W, Purdie D A, Williams P J, Davies J M. Science. 1986;232:865–867. doi: 10.1126/science.232.4752.865. [DOI] [PubMed] [Google Scholar]
- 31.Cloern J E, Grenz C, Lucas L V. Limnol Oceanogr. 1995;40:1313–1321. [Google Scholar]
- 32.Hollibaugh J T, Wong P S. In: San Francisco Bay: The Ecosystem. Hollibaugh J T, editor. San Francisco: Am. Assoc. Adv. Science; 1996. pp. 263–288. [Google Scholar]
- 33.del Giorgio P A, Cole J J. Annu Rev Ecol Syst. 1998;29:503–541. [Google Scholar]
- 34. Müller-Solger, A. B., Jassby, A. D. & Müller-Navarra, D. C. (2002) Limnol. Oceanogr., in press.
- 35.Carpenter S R, Chisholm S W, Krebs C J, Schindler D W, Wright R F. Science. 1995;269:324–327. doi: 10.1126/science.269.5222.324. [DOI] [PubMed] [Google Scholar]
- 36.Clark J S, Carpenter S R, Barber M, Collins S, Dobson A, Foley J A, Lodge D M, Pascual M, Pielke R, Jr, Pizer W, et al. Science. 2001;293:657–660. doi: 10.1126/science.293.5530.657. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson C, Berggren K. BioScience. 1997;50:783–792. [Google Scholar]
- 38.National Resource Council. Clean Coastal Waters: Understanding and Reducing the Effects of Nutrient Pollution. Washington, DC: Natl. Acad. Press; 2000. [Google Scholar]
- 39.Strayer D L, Caraco N F, Cole J J, Findlay S, Pace M L. BioScience. 1999;49:19–27. [Google Scholar]
- 40.Tilman D, Fargione J, Wolff B, D'Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger W H, Simberloff D, Swackhamer D. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]