Abstract
Background
Lamotrigine is a preferred antiepileptic drug for women with epilepsy planning or undergoing pregnancy, owing to its relatively low teratogenic risk. However, serious adverse reactions, including life-threatening conditions such as Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), can occur.
Case presentation
We report the case of a 33-year-old woman of childbearing age with epilepsy who developed TEN following the initiation of lamotrigine as part of a preconception antiepileptic regimen. The patient presented with high fever, a rapidly spreading erythematous rash, bullae formation, and extensive epidermal detachment. Prompt and comprehensive multidisciplinary treatment led to significant clinical improvement and eventual full recovery. The therapeutic approach included intravenous dexamethasone sodium phosphate, fluid resuscitation, intravenous immunoglobulin (IVIG), oral antihistamines, traditional Chinese medicine (TCM), and meticulous care of the skin and mucosal surfaces.
Conclusion
While antiepileptic drugs (AEDs) such as lamotrigine are crucial for managing epilepsy, they may induce severe skin toxicity, potentially leading to fatal outcomes. Healthcare providers must remain vigilant for early symptoms and intervene promptly to mitigate adverse effects. This case report, alongside a literature review, aims to underscore the importance of comprehensive care throughout the preconception, pregnancy, and postpartum phases for women with epilepsy.
Keywords: Lamotrigine, Epilepsy, Planning pregnancy, Stevens-Johnson syndrome, Toxic epidermal necrolysis, Case report, Literature review
Background
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are rare, life-threatening mucocutaneous diseases considered different manifestations of drug-induced delayed hypersensitivity reactions, characterized by erythema, bullae, and epidermal detachment [1]. Due to their rarity, large-scale epidemiological studies are limited, and the pathogenesis remains incompletely understood, posing a challenging and difficult-to-treat condition. Women of childbearing age are particularly at high risk, with TEN mortality reaching up to 30% [2].
Epilepsy is a significant concern for reproductive safety in women of childbearing age, as antiepileptic drugs (AEDs) therapy can cause severe adverse reactions. Commonly prescribed AEDs include carbamazepine, oxcarbazepine, phenobarbital, and phenytoin sodium. Additionally, newer-generation AEDs such as lamotrigine and levetiracetam are widely used due to their improved safety profiles. Among them, lamotrigine, an aromatic antiepileptic drug, is considered a first-line treatment owing to its low teratogenicity and minimal impact on infant neurodevelopment and cognitive function [3–5].
This study reports the first case in our institution of a woman with epilepsy planning pregnancy who developed SJS progressing to TEN after lamotrigine treatment. By detailing the progression and outcomes of this case, we aim to emphasize the importance of patient safety, mental health, and rights protection. This report calls for increased attention to the physical and mental health rights of women of childbearing age and provides recommendations for clinicians to protect the health of women with epilepsy during preconception, pregnancy, and postpartum periods.
Case report
A 33-year-old Chinese woman (BMI: 19.10 kg/m²) presented with a persistent high fever lasting nine days (maximum temperature: 39.8 °C), a widespread erythematous rash, oral mucosal erosions with dysphagia, and limb pain. The rash initially appeared on the trunk and rapidly disseminated over 48 h. She had a 20-year history of epilepsy but had been seizure-free since 2013, maintained on long-term Oxcarbazepine (150 mg/day) combined with Topiramate (100 mg/day). Eighteen days before symptom onset, her antiepileptic regimen was modified in preparation for pregnancy, initiating Lamotrigine (25 mg/day) with a gradual reduction in Oxcarbazepine (current dose: 75 mg/day). The patient had no history of pemphigus, autoimmune diseases, or pregnancy.
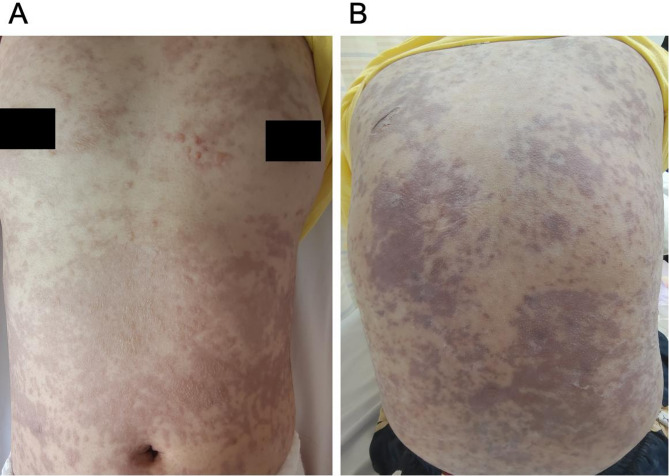
Upon admission on the first day, she exhibited extensive purple-red macules, papules, and vesicles with notable skin swelling, predominantly on the trunk. Partial skin flaking and a positive Nikolsky sign were also observed (Fig. 1). Her vital signs included a temperature of 39.8 °C, a heart rate of 134 bpm, a respiratory rate of 21 breaths per minute, and blood pressure of 92/76 mmHg. Significant erosion of lips and oral cavity was observed, accompanied by bilateral submandibular lymphadenopathy. Laboratory test results indicated decreases in white blood cells, red blood cells (WBC, RBC), platelet count (PLT), hemoglobin (HGB), and lymphocyte percentage (LY%). Biochemical indicators such as total protein (TP) and albumin (ALB) were also reduced, while electrolyte and coagulation abnormalities were noted. Conversely, aspartate aminotransferase (AST), beta 2 Microglobulin (β2M), lactate dehydrogenase (LAD), neutrophil ratio (NEUT%), and high-sensitivity C-reactive protein (hs-CRP) were elevated (Table 1). Chest CT (Computed Tomography) scans was unremarkable, Serum-specific IgE testing did not detect any allergen sensitization, indicating no evidence of an immediate hypersensitivity reaction. But she refused to undergo skin biopsy. Based on the extensive mucosal involvement, a positive Nikolsky sign, the temporal association with lamotrigine use, and a systemic inflammatory response (elevated high sensitive C-Reactive Protein and neutrophilia) [7, 8], pemphigus and other autoimmune blistering diseases were effectively excluded.
Fig. 1.
Presentation of the patient’s skin lesions upon admission: (A) chest and (B) back, showig widespread purplish-red patches and papules, accompanied by blister formation. The skin exhibits swelling, and certain areas show partial epidermal detachment, though the affected skin area remains below 10%
Table 1.
Laboratory indexes at admission of the patient
| Test indexes | Values | Reference ranges |
|---|---|---|
| Blood routine | ||
| WBC | 3.02 × 109/L | 3.5–9.5 × 109/L |
| RBC | 3.34 × 1012/L | 3.8–5.1 × 1012/L |
| PLT | 116 g/L | 125–350 g/L |
| HGB | 105 g/L | 115–150 g/L |
| NEUT% | 85.5% | 40–75% |
| LY% | 10.6% | 20–50% |
| Biochemical function | ||
| ALT | 30.4 U/L | 7–40 U/L |
| AST | 45.2 U/L | 13–35 U/L |
| TP | 63.3 g/L | 65–85 g/L |
| ALB | 37.7 g/L | 40–55 g/L |
| BUN | 3.57 mmol/L | 2.9–7.1 mmol/L |
| β2M | 5.8 mg/L | 1–3 mg/L |
| LAD | 586 U/L | 120–250 U/L |
| GLU | 4.95 mmol/L | 3.9–6.1 mmol/L |
| hs-CRP | 67.3 mg/L | 0–3 mg/L |
| Coagulation function | ||
| prothrombin concentration | 69.4% | 70–130% |
| serum fibrinogen assay | 4.691 g/L | 2–4 g/L |
| D-Dimer | 2.01 mg/L | 0-0.55 mg/L |
| FDP | 5.73 ug/L | 0–5 ug/L |
| Electrolyte | ||
| K+ | 3.32 mmol/L | 3.5–5.3 mmol/L |
| Na+ | 136 mmol/L | 137–147 mmol/L |
| Ca2+ | mol/L | 2.11–2.65 mol/L |
WBC, white blood cell count; RBC, red blood cell count; PLT, platelet count; HGB, haemoglobin; NEUT%, neutrophils ratio; LY%, lymphocyte percentage; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; BUN, blood urea nitrogen; β2M, beta 2 Microglobulin; LAD, lactic dehydrogenase; GLU, blood glucose; hs-CRP, high sensitive C-Reactive Protein; FDP, fibrinogen degradation product; K+, potassium; Na+, sodium; Ca2+, calcium
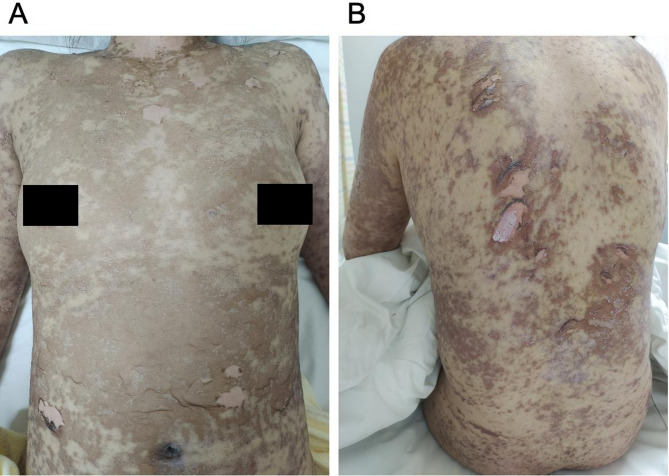
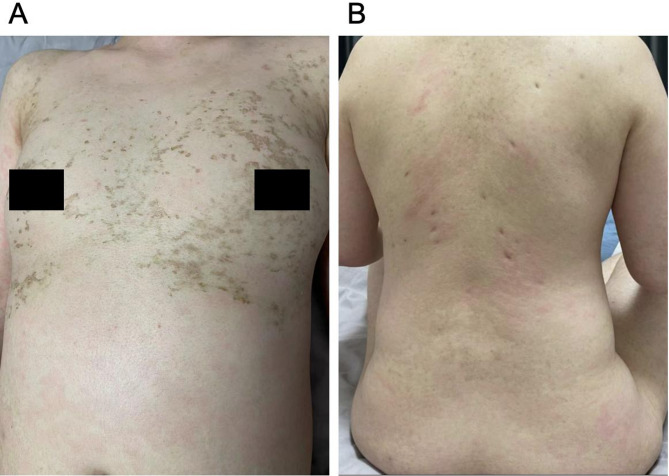
Initially, as the affected BSA was below 10%, she was tentatively diagnosed with SJS induced by lamotrigine, with a SCORTEN score of 1 (Table 2) [9]. Lamotrigine was immediately discontinued, and treatments initiated included antihistamines (Loratadine 10 mg, taken orally once every morning, and cetirizine hydrochloride 10 mg, taken orally once every evening), daily fluid supplementation, and intravenous dexamethasone phosphate at 10 mg/day. By the fourth day of hospitalization, the situation escalated as diffuse brownish swollen bullae and epidermal detachment extended beyond 36% of BSA (Fig. 2), prompting a diagnosis revision to TEN. So the patient’s treatment involved coordinated efforts from gynecologists, neurologists, ophthalmologists, dermatologists, hematologists, and nutritionists, to optimize clinical outcomes. The progression to TEN can be attributed to several critical factors, including the drug residual effect, dysregulated immune response, and compromised skin barrier. The treatment regimen was augmented with intravenous immunoglobulin (IVIG) at 10 g/day for one week and enhanced care for skin and mucosa (Eyes: Tobramycin-dexamethasone eye drops, twice daily; Skin and Mucous Membranes: 20% Calamine lotion 200 ml + 0.1% Halcinonide 20 ml, twice daily. Later, mupirocin ointment was applied to ulcerated areas, and halometasone propionate cream was applied to the entire body, twice daily, for a total duration of 14 days), topical corticosteroids (20% Calamine lotion 200 ml + 0.1% ciclesonide 20 ml. twice a day), and anti-inflammatory therapies (10 mg of dexamethasone phosphate injection + 100 ml of 5% glucose, administered intravenously once daily for 14 days) Traditional Chinese medicine decoction (Rehmanniae Radix (20 g), Paeoniae Radix Rubra (15 g), Moutan Cortex (15 g), Sophorae Flavescentis Radix (8 g), Imperatae Rhizoma (10 g), and Glycyrrhizae Radix Et Rhizoma (10 g) etc. ) aimed at clearing heat, detoxifying, cooling blood, and eliminating dampness were also administered. After 16 days of comprehensive treatment, the patient’s condition markedly improved; her rash receded, and all health indicators normalized, including hs-CRP (Fig. 3). Her SCORTEN score was adjusted to 0 (Table 2), evidencing significant symptomatic relief and subsequent recovery, allowing for discharge. A follow-up one month post-discharge showed no signs of recurrence (Fig. 4).
Table 2.
SCORTEN during the patient’s hospitalization
| Risk factors | Score | On admission 12 − 09 |
Exacerbation 12–13 |
Recovery 12–25 |
|---|---|---|---|---|
| Age above 40 years | 1 | 0 | 0 | 0 |
| Presence of malignancy | 1 | 0 | 0 | 0 |
| Heart rate > 120 beats per minute | 1 | 1 | 1 | 0 |
|
Initial epidermal detachment > 10% |
1 | 0 | 1 | 0 |
| Serum urea > 10 mmol/L | 1 | 0 | 0 | 0 |
| Serum glucose > 14 mmol/L | 1 | 0 | 0 | 0 |
| Serum bicarbonate < 20 mmol/L | 1 | 0 | 0 | 0 |
| Total | 7 | 1 | 2 | 0 |
Fig. 2.
Presentation of the patient’s skin lesions on the fourth day of admission: (A) chest and (B) back, showing worsened conditions with diffuse brownish swollen bullae and extensive skin detachment, covering up to 36% of BSA
Fig. 3.
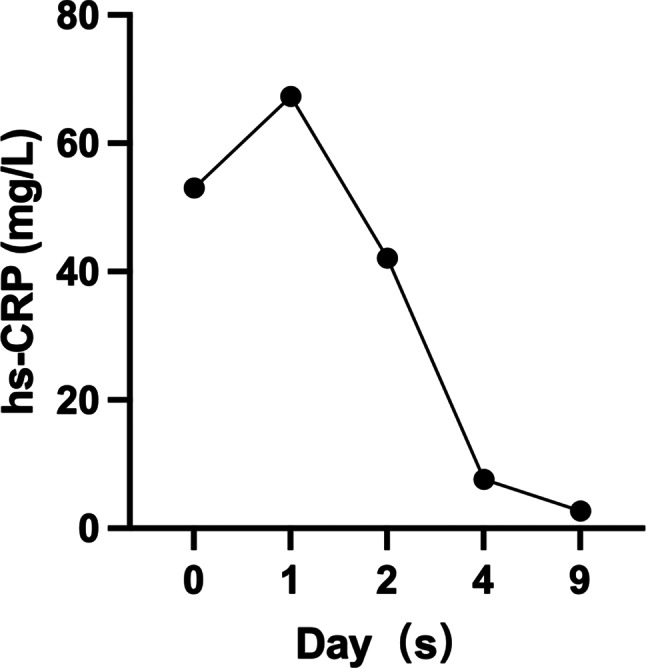
Trend chart of hs-CRP levels. The x-axis represents the days of hospitalization, and the y-axis represents the hs-CRP values
Fig. 4.
Presentation of the patient’s skin lesions during follow-up: (A) chest and (B) back, showing brown scabs, pigmentation, and depressed scars
To evaluate the likelihood of drug-induced TEN, we applied the Naranjo Adverse Drug Reaction Probability Scale [10], a widely recognized and validated tool for assessing adverse drug reactions (Table 3). Based on the scoring system, Lamotrigine received a score of 5/10 (probable causal relationship), whereas Oxcarbazepine received a score of 0.5/10 (possible causal relationship). Based on the extensive mucosal involvement, a positive Nikolsky sign, the temporal association with lamotrigine use, and a systemic inflammatory response (elevated hs-CRP and neutrophilia) [1, 2], pemphigus and other autoimmune blistering diseases were effectively excluded.
Table 3.
Naranjo score for drug-induced TEN
| Assessment Criteria | Lamotrigine | Oxcarbazepine & Topiramate |
|---|---|---|
| Temporal Association | Symptoms appeared 9 days after initiation (+ 2) | Long-term use, no recent dose modification (0) |
| Dechallenge Outcome | Symptom improvement after discontinuation (+ 1) | Not discontinued (0) |
| Rechallenge Outcome | Not re-exposed (N/A) | N/A |
| Established Risk Profile | Strongly associated with SJS/TEN (+ 1) | Low risk of rash (+ 0.5) |
| Exclusion of Alternative Causes | Negative infectious/autoimmune workup (+ 1) | Long-term tolerance (+ 0) |
| Total Score | Probable (5 points) | Possible (0.5 points) |
Patient perspective
Clinical recovery was satisfactory. The skin rash and systemic symptoms completely resolved, with only residual pigmentation and no sequelae observed. Based on the neurologist’s recommendation, lamotrigine was discontinued and replaced with oxcarbazepine (150 mg/day) and levetiracetam (500 mg/day) as the new antiepileptic regimen. If the patient plans to conceive in the future, the selection of antiepileptic drugs should balance seizure control efficacy and fetal safety. We recommend adjusting the medication regimen prior to pregnancy planning.
Discussion
Lamotrigine-induced adverse reactions in childbearing-age women with epilepsy
Lamotrigine-induced SJS and TEN pose significant threats to the life and health of women of childbearing age [3, 11, 12], while epilepsy also significantly increases the complexity of pregnancy in this population. Lamotrigine falls under the Type B category(Table 4), a large-scale European studies indicate that TEN is more prevalent in women of reproductive age, with a female-to-male incidence ratio of approximately 3:1 [13–16]. A study by Viale et al. demonstrated that the mortality rate among pregnant women with epilepsy is ten times higher than that of non-epileptic women, significantly impacting pregnancy outcomes [4]. Although lamotrigine is favored for its reduced risk of fetal malformations [6, 17], the potential for severe cutaneous reactions remains a critical concern. Lamotrigine-induced adverse reactions, including SJS and TEN, may be associated with dose-related risks, suggesting that cautious dose escalation could help mitigate these risks [18]. Additionally, metabolic changes leading to the accumulation of toxic intermediates may affect lamotrigine clearance [19–21], further complicating safe medication use during pregnancy.
Table 4.
Classification and characteristics of AED-associated ADRs
| ADR Type | Characteristics | Representative Drugs | Typical ADRs | Management Strategy | Remarks |
|---|---|---|---|---|---|
| Type A | Dose-dependent, predictable, related to drug action | Phenytoin, Carbamazepine | Drowsiness, ataxia, tremors, GI symptoms | Dose adjustment, slow titration, symptomatic management | Most common ADR type (~ 30%) [22] |
| Type B | Idiosyncratic, immune/genetic-related, unpredictable | Lamotrigine, Valproic acid | SJS/TEN, hepatotoxicity, aplastic anemia | Immediate drug discontinuation, antihistamines, supportive care | Genetic screening recommended (e.g., HLA-B*1502 for lamotrigine-related SJS) [23, 24] |
| Type C | Chronic cumulative effects, related to long-term use | Phenobarbital, Phenytoin | Osteoporosis, gingival hyperplasia, cognitive impairment | Long-term monitoring (bone density, cognition), calcium/Vitamin D supplementation | Higher risk in elderly patients [25] |
| Type D | Delayed toxicity (months to years), genetic or developmental impact | Valproic acid, Phenytoin | Teratogenicity, carcinogenicity, neurodevelopmental abnormalities | Avoid in pregnancy; enhanced fetal monitoring | Valproic acid poses a teratogenic risk of up to 10% [26] |
ADR: Adverse Drug Reaction
The unpredictability of epileptic seizures exacerbates the challenges to mental health and life safety for women considering pregnancy. Balancing effective seizure control with minimal drug exposure requires a delicate approach. This case underscores the importance of rigorous medication management for women with epilepsy during preconception and pregnancy, including thorough risk assessment, continuous monitoring, and careful weighing of therapeutic benefits against potential risks. Implementing comprehensive preventive and treatment strategies is crucial for reducing the likelihood of adverse reactions.
A systematic literature search was conducted on PubMed, Google Scholar, and Scopus using the following search strategy: (“lamotrigine[MeSH]” OR “lamictal”) AND (“toxic epidermal necrolysis[MeSH]” OR “TEN” OR “Stevens-Johnson syndrome” OR “SJS” OR “drug-induced skin reaction”). The search covered the period from 1990 (the year lamotrigine was introduced to the market) to 2024, and key findings were summarized in Tables 5 and 6 [27–29].
Table 5.
Reported TEN cases associated with lamotrigine
| Study Type | Number of Cases | Key Findings | References |
|---|---|---|---|
| Case Reports | ≥ 50 cases | TEN typically occurs within 2–8 weeks of drug initiation, often linked to rapid dose escalation. | [15, 27–35] |
| Multicenter Cohort Study | 12 cases | Lamotrigine accounts for 5.3% of all drug-induced TEN cases, with a mortality rate of approximately 30% (SCORTEN-dependent). | [31, 33, 36] |
| Asian Population Studies | 23 cases | HLA-B*15:02 carriers have an 80-fold increased risk, underscoring the need for genetic screening before lamotrigine initiation. | [33, 36] |
| Meta-analysis | 7 studies | Risk of lamotrigine-induced TEN estimated at 1/1000 to 1/5000, significantly higher than that of other new-generation AEDs. | [16, 23, 35] |
Table 6.
Clinical characteristics of previously reported cases of lamotrigine-induced TEN
| Study | Age/sex | Lamotrigine Dosage | Onset (Days) | Mucosal Involvement |
Treatment | Outcome |
|---|---|---|---|---|---|---|
|
Sullivan JR et al. [37], 1996 |
29/F |
25 mg alternate nights |
9 | Oral, genital | Cyclosporine (4.5 mg/kg/d) | Cured |
|
Fernández CalvoC et al. [38], 2000 |
44/F | N/A | 42 | N/A | GC | Cured |
|
Tristani-FirouziP et al. [39], 2002 |
14/F | N/A | N/A | N/A | IVIG | Cured |
|
Sladden M et al. [40], 2004 |
26/F |
25 mg alternate days |
14 | Oral, genital | Supportive therapy | Cured |
|
ChangCC et al. [41], 2005 |
32/F | 12.5 mg/d | 14 | N/A | MP | Cured |
|
Rodríguez-BlancoI et al. [42], 2005 |
37/M | 50 mg/d | 3 | N/A | PD | Cured |
|
VargheeSP et al. [43], 2006 |
14/F | 25 mg/d | 21 | N/A | MP | Cured |
| 28/F | 25 mg/d | 18 | N/A | MP | Cured | |
| 14/F | 50 mg/d | 21 | N/A | MP | Cured | |
|
DuparA et al. [44], 2010 |
16/F | N/A | 15 | oral | N/A | Cured |
|
Fayaz A et al. [45], 2011 |
20/F | 200 mg/d | 18 | Oral | Intravenous dexamethasone for 3 days | Cured |
|
Kazeem et al. [26], 2009 |
37/F |
12.5 mg/d With valproic acid1050 mg |
21 | Oral, ocular | steroid hormone 1000 mg/d | Died |
|
Kazee et al. [26], 2009 |
19/F | 100 mg/d | 14 | Oral, ocular |
0.5 mg/kg/d of IgM-IVIG |
Cured |
|
HaoxiangX et al. [46], 2009 |
72/F | 75 mg/d | N/A | N/A | IVIG + MP | Cured |
|
HaoxiangX et al. [46], 2009 |
35/F | 50 mg/d | 10 | N/A | Corticosteroids | Cured |
|
HuangHT et al. [47], 2010 |
30/M | 30 mg/d | 19 | N/A | Antibiotics | Cured |
|
BarvaliyaMJ et al. [48], 2012 |
12/M | 50 mg/d | 28 | N/A | Steroids | Died |
|
KaurSKad DograA [49], 2013 |
47/F | 100 mg/d | 12 | N/A | PD | Cured |
| 26/F | N/A | 28 | N/A | PD | Uneventful Recovery | |
|
Russomanno et al. [50], 2021 |
22/F | N/A | 14 | Oral, ocular | Cyclosporine×5d | Cured |
|
Lindsey J et al. [51], 2022 |
29/F | Lamotrigine (L) and TMP-SMX | 17 |
Oral, ocular, esophagel, genital |
Cyclosporine×14 d | Cured |
| Current case | 33/F | Lamotrigine 25 mg/d, oxcarbazepine 75 mg/d and topiramate 100 mg/d | 9 | Oral, ocular | dexamethasone combined with IVIG etc. | Cured |
Abbreviations: TEN: Toxic Epidermal Necrolysis; F: female; M: male; d: day; GC: glucocorticoid; IVIG: intravenous immunoglobulin; NOS: not otherwise specified; N/A: not applicable; TMP-SMX: trimethoprim-sulfamethoxazole; PD: prednisone; MP: methylprednisolone; Onset: Time from Drug Exposure to Rash Onset
The unpredictability of epileptic seizures adds to the mental health and life safety challenges for women contemplating pregnancy. Managing medication to minimize exposure while ensuring effective seizure control requires a delicate balance. This narrative detailed a case where a woman with a lengthy epilepsy history encountered severe skin reactions following a switch to lamotrigine, necessitating emergency interventions like drug discontinuation and supportive treatments, which eventually led to recovery. This instance highlights the critical need for vigilant medication management in women with epilepsy during the pre-pregnancy and pregnancy phases, prioritizing rigorous risk assessments, continuous monitoring, and the careful weighing of therapeutic advantages against potential dangers. Adopting comprehensive preventive and therapeutic strategies is vital to diminish the likelihood of adverse reactions.
Medication literature review: providing medication reference for pre-pregnancy, pregnancy, and postpartum breastfeeding
Clinical medication management emphasizes epilepsy control and mitigates the teratogenic risks of AEDs during pre-pregnancy, aiming for seizure control with the lowest effective single-drug dose. Notably, sodium valproate, while effective for seizure control, carries a high teratogenic risk. Topiramate is associated with a notable rate of congenital malformations, contrasting with the lower fetal malformation risks of lamotrigine and levetiracetam [52, 53]. Lamotrigine outperforms levetiracetam and zonisamide in therapeutic efficacy and cost-effectiveness [54]. Early pregnancy administration of a lamotrigine-levetiracetam combination shows promise for epileptic patients with reproductive potential [55], minimizing neurodevelopmental disorders in fetuses and slashing the risk of congenital malformations by 60% [56], without showing inferiority to sodium valproate [54]. Research by Chadwick D [57] suggests considering AED discontinuation for seizure-free individuals with a normal EEG (Electroencephalogram) for at least two years, recommending early folic acid supplementation to decrease teratogenic risk. Thus, pre-pregnancy medication necessitates a balanced consideration of benefits and risks to select a suitable AED regimen for women with reproductive potential [58].
During pregnancy, the focus shifts to monitoring clinical symptoms and the pharmacokinetics effects of physiological changes on serum drug concentrations. Clark, C. T’s [59] findings indicate a decrease in lamotrigine drug concentration during pregnancy, with carbamazepine minimally affecting drug concentration, and rectal administration of phenobarbital, carbamazepine, and lamotrigine ensuring adequate bioavailability [60]. Pregnancy could heighten the risk of epileptic seizures; barring phenytoin, which permits plasma concentration monitoring, dose adjustments for other drugs should be symptom-based [61]. Enzyme-inducing AEDs like carbamazepine, phenobarbital, primidone, and phenytoin heighten the bleeding risk in newborns [62], making vitamin K supplementation advisable [63].
For breastfeeding, it’s imperative to monitor the infant’s serum drug concentration when on AEDs. Studies by Davanzo R [64] recommend older AEDs like carbamazepine, phenytoin, phenobarbital, and primidone during breastfeeding due to their low infant plasma concentrations, suggesting carbamazepine’s safety [65]. However, lamotrigine may raise drug levels in infant plasma and is thus not advised during breastfeeding [58]. Therefore, diligent monitoring of the infant’s blood drug concentration is vital to reduce teratogenesis risk, warranting cautious selection of AEDs based on individual cases.
Screening and record-keeping to improve clinical management before, during, and after pregnancy
This case underscores the critical need for comprehensive screening and meticulous record-keeping across all stages for epilepsy patients, particularly for women of childbearing age contemplating pregnancy. Studies have shown that antiepileptic drug-induced SJS and TEN are strongly associated with specific leukocyte allele genes [66, 67]. Notably, the incidence of SJS/TEN induced by aromatic AEDs like lamotrigine, phenytoin, and carbamazepine is significantly linked to the HLA-B1502 allele in Chinese Han and Southeast Asian populations. Furthermore, the HLA-DRB101 [68] allele, prevalent in the Iranian populace, is connected to SJS/TEN induced by these drugs, while in European demographics, HLA-B*38:01 poses a risk for lamotrigine-induced SJS/TEN [69]. These insights highlight the necessity for genetic screening when prescribing aromatic AEDs to mitigate adverse drug reactions.
In the pre-pregnancy phase, thorough genetic screening and establishing comprehensive health records are advised to tailor medication treatment plans, ensuring the wellbeing of both the patient and the fetus. During pregnancy, the emphasis should be on controlling epileptic seizures, dynamically monitoring serum drug levels, and adjusting medication doses in response to the physiological changes of pregnancy and their impact on pharmacokinetics. Postpartum management should aim at safeguarding the health of both mother and infant, with continuous condition monitoring post-birth and providing necessary psychological and emotional support to affected patients, fostering the overall well-being of both the patient and their family.
We acknowledge the limitations of this study. Firstly, this report describes a unique case but is limited to a single patient. Due to the absence of data from multiple cases or cohort studies, it is not possible to generalize the safety or pathogenic risk of lamotrigine in a larger sample. Secondly, while this study emphasizes the importance of balancing efficacy and maternal-fetal safety when selectin AEDs during pregnancy, there is still a lack of unified consensus on medication use during pregnancy in existing guidelines. Standardized processes such as dynamic monitoring of blood drug concentrations, individualized dose adjustments, and multidisciplinary collaboration (e.g., between neurology and obstetrics) have not been established in clinical practice. There is an urgent need for evidence-based standardized protocols to optimize outcomes for both mother and child. Lastly, this study mentions a strong association between genetic factors and TEN induced by aromatic AEDs. However, the widespread implementation of genetic screening is constrained by ethnic specificity and regional disparities in medical resources (for instance, limited access to testing technologies in low-income countries). Additionally, cost-benefit analyses, such as comparing universal screening with targeted screening of high-risk populations, remain unclear, potentially impeding clinical adoption. Future efforts should explore cost-effective screening strategies and incorporate data from more diverse populations to enhance risk stratification.
Conclusion
This study reports a case of a severe skin reaction in a woman of reproductive age, possibly associated with lamotrigine use, which rapidly progressed to TEN. Furthermore, we reviewed relevant literature to explore the potential risks of using AEDs among epileptic women of reproductive age. Our analysis underscores that while the teratogenic potential of AEDs is an important concern, the health of pregnant women should not be overlooked.
Due to the uniqueness of this patient’s condition and treatment plan, we have documented the patient’s treatment process and regimen in detail. Our aim is to provide clinicians with recommendations and references when dealing with similar TEN cases. Through this case, we advocate for increased awareness of medication safety among women who are planning to become pregnant, are already pregnant, or are breastfeeding. We recommend strengthening genetic screening, maintaining detailed medication records, and regularly monitoring drug levels to optimize clinical management strategies before and during pregnancy, thereby ensuring the health and well-being of both mother and child.
Acknowledgements
The author is grateful to the patient who was allowed to report her clinical manifestations in this report for her valuable contribution.
Abbreviations
- β2M
Beta 2 Microglobulin
- ADR
Adverse Drug Reaction
- AEDs
Antiepileptic drugs
- ALB
Albumin
- AST
Aspartate aminotransferase
- BSA
Body surface area
- CT
Computed Tomography
- d
Day
- EEG
Electroencephalogram
- F
Female
- GC
Glucocorticoid
- HGB
Haemoglobin
- hs-CRP
High sensitive C-Reactive Protein
- IVIG
Intravenous immunoglobulin
- LAD
Lactic dehydrogenase
- LY%
Lymphocyte percentage
- M
Male
- MP
Methylprednisolone
- NEUT%
Neutrophil ratio
- NOS
Not otherwise specified
- Onset
Time from Drug Exposure to Rash Onset
- PD
Prednisone
- PLT
Platelet count
- RBC
Red blood cell count
- SJS
Stevens-Johnson Syndrome
- TEN
Toxic Epidermal Necrolysis
- TMP-SMX
Trimethoprim-sulfamethoxazole
- TP
Total protein
- WBC
White blood cell count
Authors’ contributions
Zhanxue Sun conceptualized the manuscript. Lili Zhang and Pingping Yang drafted the initial and final manuscript. Ying Zhu and Kexin Liu analyze data.
Funding
This work was supported by the National Administration of Traditional Chinese Medicine National Clinical Excellent Talents Advanced Study and Training Program (Letter to National Traditional Chinese Medicine Educators [2022] No. 1), Beijing University of Chinese Medicine “Qihuang Elite Talent Famous Doctor Cultivation Program” (Y2023A05), and Construction Project of Primary-level Inheritance Studios for TCM Characteristic Specialized Diseases (Symptoms) in Chaoyang District, Beijing (No. Chaoweitong [2024] 201).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. It has been reviewed and approved by the Research Ethics Committee of the Beijing University of Chinese Medicine Third Affiliated Hospital (BZYSY-2024YJSKTPJ-13).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lili Zhang and Pingping Yang contributed equally to this work.
References
- 1.Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. 2018;54(1):147–76. [DOI] [PubMed] [Google Scholar]
- 2.Viale L, Allotey J, Cheong-See F, Arroyo-Manzano D, McCorry D, Bagary M, Mignini L, Khan KS, Zamora J, Thangaratinam S, et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386(10006):1845–52. [DOI] [PubMed] [Google Scholar]
- 3.Wang ML, Tao YY, Sun XY, Guo Y, Wang ZY, Cao YF, Zhao L. Estrogen profile- and pharmacogenetics-based lamotrigine dosing regimen optimization: recommendations for pregnant women with epilepsy. Pharmacol Res. 2021;169:105610. [DOI] [PubMed] [Google Scholar]
- 4.Nucera B, Brigo F, Trinka E, Kalss G. Treatment and care of women with epilepsy before, during, and after pregnancy: a practical guide. Ther Adv Neurol Disord. 2022;15:17562864221101687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, Sabers A, Thomas SV, Vajda F. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530–8. [DOI] [PubMed] [Google Scholar]
- 6.Diederich S, Hemmeter U, Paulmann M, Mockenhaupt M. Effects of dosage in new users of lamotrigine inducing epidermal necrolysis: results of the German registry of severe skin reactions. Epilepsia. 2023;64(5):1259–65. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Fierro ML, Garza-Veloz I, Zorrilla-Alfaro SM, Campuzano-Garcia AE, Rodriguez-Borroel M. Peripheral blood mononuclear cells cytokine profile in a patient with toxic epidermal necrolysis triggered by lamotrigine and COVID-19: a case study. Int J Mol Sci. 2025;26(3). [DOI] [PMC free article] [PubMed]
- 8.Deng E, Craig TJ, Nguyen DV, Al-Shaikhly T. COVID-19 and severe cutaneous allergic reactions to sulfonamides. Allergy Asthma Proc. 2024;45(6):e93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastuji-Garins. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129. [PubMed]
- 10.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45. [DOI] [PubMed] [Google Scholar]
- 11.Marianne L, Carlo M, Benedetta TB-P, Thomas H. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. 2017;54(1). [DOI] [PubMed]
- 12.Viale L, Allotey J, Cheong-See F, Arroyo-Manzano D, McCorry D, Bagary M, Mignini L, Khan KS, Zamora J, Thangaratinam S. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386(10006):1845–52. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Yang J, Liu R. A case of toxic epidermal necrolysis caused by lamotrigine combined with valproic acid and literature review. Cureus. 2023;15(9):e45334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lofton AL, Klein-Schwartz W. Evaluation of lamotrigine toxicity reported to poison centers. Annals Pharmacotherapy. 2004;38(11):1811–5. [DOI] [PubMed] [Google Scholar]
- 15.Ordonez L, Salgueiro E, Jimeno FJ, Manso G. Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs. Eur Rev Med Pharmacol Sci. 2015;19(14):2732–7. [PubMed] [Google Scholar]
- 16.Glahn JZ, Almeida MN, Kochen A, Noel O, Stogner V, Hsia HC, Savetamal A. Lamotrigine emerging as a driver of Stevens-Johnson syndrome and toxic epidermal necrolysis: an 8-year retrospective study. Burns: J Int Soc Burn Injuries. 2024;50(8):2114–23. [DOI] [PubMed] [Google Scholar]
- 17.Farooq O, Abbas A, Ahmad M, Manzoor AB. Lamotrigine-associated toxic epidermal necrolysis. Pakistan J Med Sci. 2023;39(6):1883–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pa B, G SS, Thomas G, Kp A. Dosage optimization of lamotrigine in pregnancy: a pharmacometric approach using modeling and simulation. J Clin Pharmacol. 2022;62(12):1557–65. [DOI] [PubMed] [Google Scholar]
- 19.Roberti R, De Caro C, Iannone LF, Zaccara G, Lattanzi S, Russo E. Pharmacology of cenobamate: mechanism of action, pharmacokinetics, drug-drug interactions and tolerability. CNS Drugs. 2021;35(6):609–18. [DOI] [PubMed] [Google Scholar]
- 20.Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. 2016;136(7):1387–97. [DOI] [PubMed] [Google Scholar]
- 21.Wadelius M, Karlsson T, Wadelius C, Rane A. Lamotrigine and toxic epidermal necrolysis. Lancet. 1996;348(9033):1041. [DOI] [PubMed] [Google Scholar]
- 22.Perucca E, Brodie MJ, Kwan P, Tomson T. 30 years of second-generation antiseizure medications: impact and future perspectives. Lancet Neurol. 2020;19(6):544–56. [DOI] [PubMed] [Google Scholar]
- 23.Sabourirad S, Mortezaee R, Mojarad M, Eslahi A, Shahrokhi Y, Kiafar B, Jarahi L, Afkhami Ardakani S, Farrokhi S. Investigating the association of lamotrigine and phenytoin-induced Stevens-Johnson syndrome/toxic epidermal necrolysis with HLA-B*1502 in Iranian population. Exp Dermatol. 2021;30(2):284–7. [DOI] [PubMed] [Google Scholar]
- 24.Perucca P, Gilliam FG. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):792–802. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22(9):739–60. [DOI] [PubMed] [Google Scholar]
- 26.Kazeem GR, Cox C, Aponte J, Messenheimer J, Brazell C, Nelsen AC, Nelson MR, Foot E. High-resolution HLA genotyping and severe cutaneous adverse reactions in lamotrigine-treated patients. Pharmacogenet Genomics. 2009;19(9):661–5. [DOI] [PubMed] [Google Scholar]
- 27.Kc KK, Limbu T, Maskay SS, Bhasima A, Acharya SP. Lamotrigine induced toxic epidermal necrolysis: a case report. Annals Med Surg (2012). 2020;60:468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenei Kluch L, Erdei I, Remenyik E, Suranyi E, Bodnar F, Emri G, Szegedi A. Does the dose of the culprit drug matter in hypersensitivity reactions? Orv Hetil. 2020;161(46):1959–65. [DOI] [PubMed] [Google Scholar]
- 29.Sukasem C, Sririttha S, Chaichan C, Nakkrut T, Satapornpong P, Jaruthamsophon K, Jantararoungtong T, Koomdee N, Medhasi S, Oo-Puthinan S, et al. Spectrum of cutaneous adverse reactions to aromatic antiepileptic drugs and human leukocyte antigen genotypes in Thai patients and meta-analysis. Pharmacogenomics J. 2021;21(6):682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Y, Li S, Zhang L, Jin H, Zou X. Association between HLA alleles and lamotrigine-induced cutaneous adverse drug reactions in Asian populations: a meta-analysis. Seizure. 2018;60:163–71. [DOI] [PubMed] [Google Scholar]
- 31.Rashid M, Rajan AK, Chhabra M, Kashyap A, Chandran VP, Venkataraman R, Nair S, Thunga G. Role of human leukocyte antigen in anti-epileptic drugs-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: a meta-analysis. Seizure. 2022;102:36–50. [DOI] [PubMed] [Google Scholar]
- 32.An DM, Wu XT, Hu FY, Yan B, Stefan H, Zhou D. Association study of lamotrigine-induced cutaneous adverse reactions and HLA-B*1502 in a Han Chinese population. Epilepsy Res. 2010;92(2–3):226–30. [DOI] [PubMed] [Google Scholar]
- 33.Diphoorn J, Cazzaniga S, Gamba C, Schroeder J, Citterio A, Rivolta AL, Vighi GD, Naldi L. Incidence, causative factors and mortality rates of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in Northern Italy: data from the REACT registry. Pharmacoepidemiol Drug Saf. 2016;25(2):196–203. [DOI] [PubMed] [Google Scholar]
- 34.Bloom R, Amber KT. Identifying the incidence of rash, Stevens-Johnson syndrome and toxic epidermal necrolysis in patients taking lamotrigine: a systematic review of 122 randomized controlled trials. An Bras Dermatol. 2017;92(1):139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukasawa T, Takahashi H, Takahashi K, Tanemura N, Amagai M, Urushihara H. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with anticonvulsants in a Japanese population: matched case-control and cohort studies. Allergology International: Official J Japanese Soc Allergology. 2021;70(3):335–42. [DOI] [PubMed] [Google Scholar]
- 36.Park HJ, Kim SR, Leem DW, Moon IJ, Koh BS, Park KH, Park JW, Lee JH. Clinical features of and genetic predisposition to drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a single Korean tertiary institution patients-investigating the relation between the HLA -B*4403 allele and lamotrigine. Eur J Clin Pharmacol. 2015;71(1):35–41. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan JR, Watson A. Lamotrigine-induced toxic epidermal necrolysis treated with intravenous cyclosporin: a discussion of pathogenesis and immunosuppressive management. Australas J Dermatol. 1996;37(4):208–12. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Calvo C, Olascoaga J, Resano A, Urcola-Echevarria J, Turneu A, Zubizarreta J. Lyell’s syndrome associated with lamotrigine. RN. 2000;31(12):1162–4. [PubMed] [Google Scholar]
- 39.Payam T-F, Marta JP, Jeffrey RS, Stephen EM, John JZ. Treatment of toxic epidermal necrolysis with intravenous immunoglobulin in children. J Am Acad Dermatol. 2002;47(4). [DOI] [PubMed]
- 40.Sladden M, Mortimer N, Chave T. Toxic epidermal caused by lamotrigine. Aus Fam Physician. 2004;33(10):829–30. [PubMed] [Google Scholar]
- 41.Chuan-Chia C, I-Shin S, Hsin-An C, San-Yuan H. Toxic epidermal necrolysis with combination lamotrigine and valproate in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2005;30(1). [DOI] [PubMed]
- 42.Isabel R-B, Dolores S-A, Jaime T. Toxic epidermal necrolysis caused by lamotrigine. Actas Dermosifiliogr. 2006;96(2).
- 43.Susan PV, Linwood RH, Mary Lou P, Robert EG, Bruce HA. Lamotrigine-induced toxic epidermal necrolysis in three patients treated for bipolar disorder. Pharmacotherapy. 2006;26(5). [DOI] [PubMed]
- 44.Duparc A, Lasek A, Gros C, Delaporte E, Van der Linden T, Modiano P. Nécrolyse épidermique toxique à La Lamotrigine par erreur de délivrance. Ann De Dermatologie Et De Vénéréologie. 2010;137(11):736–8. [DOI] [PubMed] [Google Scholar]
- 45.Sofi FA, Koul PA, Mufti SA, Dhobi GN. Lamotrigine-induced toxic epidermal necrolysis in a young epileptic. BMJ case reports. 2011;2011. [DOI] [PMC free article] [PubMed]
- 46.Wang XQ, Lv B, Wang HF, Zhang X, Yu SY, Huang XS, Zhang JT, Tian CL, Lang SY. Lamotrigine-induced severe cutaneous adverse reaction: update data from 1999–2014. J Clin Neuroscience: Official J Neurosurgical Soc Australasia. 2015;22(6):1005–11. [DOI] [PubMed] [Google Scholar]
- 47.Hsien-Te H, Chen-Lin C, Dong-Sheng T. Toxic epidermal necrolysis after sun-exposure probably due to lamotrigine and chlorpromazine. Asian J Psychiatr. 2010;3(4). [DOI] [PubMed]
- 48.Manish JB, Mahendra KP, Tejas KP, CBT. Toxic epidermal necrolysis due to lamotrigine in a pediatric patient. J Pharmacol Pharmacother. 2013;3(4). [DOI] [PMC free article] [PubMed]
- 49.Kaur S, Dogra A. Toxic epidermal necrolysis due to concomitant use of lamotrigine and valproic acid. Indian J Dermatology. 2013;58(5):406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russomanno K, DiLorenzo A, Horeczko J, Deng M, Cardis M, Petronic-Rosic V, Johnson LS, Pasieka HB. Photodistributed toxic epidermal necrolysis in association with lamotrigine and tanning bed exposure. JAAD Case Rep. 2021;14:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaghan LJ, Coates MM, Crouse LN, Miedema J, Mervak JE, Ziemer CM. Photodistributed toxic epidermal necrolysis. JAMA Dermatology. 2022;158(7). [DOI] [PMC free article] [PubMed]
- 52.Koch S, Lösche G, Jager-Romän E, Jakob S, Rating D, Deichl A, Helge H. Major and minor birth malformations and antiepileptic drugs. Neurology. 1992;42(4 Suppl 5):83–8. [PubMed] [Google Scholar]
- 53.Rosenow F, Winter Y, Leunikava I, Brunnert M, Joeres L, Sutphin J, Boeri M, Smith J, Villani F, Brandt C. Relative importance of clinical outcomes and safety risks of antiseizure medication monotherapy for patients and physicians: discrete choice experiment eliciting preferences in real-world study VOTE. Epilepsia. 2022;63(2):451–62. [DOI] [PubMed] [Google Scholar]
- 54.Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G, Tudur-Smith C, Plumpton C, Hughes DA, Williamson P, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet. 2021;397(10282):1375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bjørk MH, Zoega H, Leinonen MK, Cohen JM, Dreier JW, Furu K, Gilhus NE, Gissler M, Hálfdánarson Ó, Igland J, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022;79(7):672–81. [DOI] [PubMed] [Google Scholar]
- 56.Cohen JM, Alvestad S, Cesta CE, Bjørk MH, Leinonen MK, Nørgaard M, Einarsdóttir K, Engeland A, Gissler M, Karlstad Ø, et al. Comparative safety of antiseizure medication monotherapy for major malformations. Ann Neurol. 2023;93(3):551–62. [DOI] [PubMed] [Google Scholar]
- 57.Chadwick D. The withdrawal of antiepileptic drugs. 1995.
- 58.Delgado-Escueta AV, Janz D. Consensus guidelines: preconception counseling, management, and care of the pregnant woman with epilepsy. Neurology. 1992;42(4 Suppl 5):149–60. [PubMed] [Google Scholar]
- 59.Clark CT. Psychotropic drug use in perinatal women with bipolar disorder. Semin Perinatol. 2020;44(3):151230. [DOI] [PubMed] [Google Scholar]
- 60.Kaplan YC, Demir O. Use of phenytoin, phenobarbital carbamazepine, levetiracetam lamotrigine and valproate in pregnancy and breastfeeding: risk of major malformations, dose-dependency, monotherapy vs polytherapy, pharmacokinetics and clinical implications. Curr Neuropharmacol. 2021;19(11):1805–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arfman IJ, Wammes-van der Heijden EA, Ter Horst PGJ, Lambrechts DA, Wegner I, Touw DJ. Therapeutic drug monitoring of antiepileptic drugs in women with epilepsy before, during, and after pregnancy. Clin Pharmacokinet. 2020;59(4):427–45. [DOI] [PubMed] [Google Scholar]
- 62.Fishel Bartal M, Sibai BM. Eclampsia in the 21st century. Am J Obstet Gynecol. 2022;226(2s):S1237–53. [DOI] [PubMed] [Google Scholar]
- 63.McAuley JW, Anderson GD. Treatment of epilepsy in women of reproductive age: pharmacokinetic considerations. Clin Pharmacokinet. 2002;41(8):559–79. [DOI] [PubMed] [Google Scholar]
- 64.Davanzo R, Dal Bo S, Bua J, Copertino M, Zanelli E, Matarazzo L. Antiepileptic drugs and breastfeeding. Ital J Pediatr. 2013;39:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomson T, Battino D, Bromley R, Kochen S, Meador KJ, Pennell PB, Thomas SV. Breastfeeding while on treatment with antiseizure medications: a systematic review from the ILAE women task force. Epileptic Disorders: Int Epilepsy J Videotape. 2022;24(6):1020–32. [DOI] [PubMed] [Google Scholar]
- 66.Cheung YK, Cheng SH, Chan EJ, Lo SV, Ng MH, Kwan P. HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia. 2013;54(7):1307–14. [DOI] [PubMed] [Google Scholar]
- 67.Hung SI, Chung WH, Liu ZS, Chen CH, Hsih MS, Hui RC, Chu CY, Chen YT. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010;11(3):349–56. [DOI] [PubMed] [Google Scholar]
- 68.Dastgheib L, Rostami F, Gharesi-Fard B, Asadi-Pooya AA, Namjoo S, Tahmasebi F, Hadibarhaghtalab M. Association of human leukocyte antigen alleles with carbamazepine- or lamotrigine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in an Iranian population: a case-control study. Iran J Med Sci. 2023;48(1):70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramírez E, Bellón T, Tong HY, Borobia AM, de Abajo FJ, Lerma V, Moreno Hidalgo MA, Castañer JL, Cabañas R, Fiandor A, et al. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol Res. 2017;115:168–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.