Abstract
Background
Low-dose Colchicine therapy has demonstrated clinical benefit as secondary preventive therapy in patients with chronic coronary artery disease. While prior studies have reported its cost-effectiveness in this patient group, this has not yet been studied in post-coronary artery bypass grafting (CABG) patients. Therefore, we evaluated the 5-year potential clinical benefit and lifetime cost-effectiveness of adding low-dose Colchicine to routine secondary preventive therapy among U.S. Veterans post-CABG.
Methods
A discrete time-homogeneous, annual cycle, state-transition Markov Chain model was developed using MACE (myocardial infarction, stroke, coronary reintervention, and all-cause mortality) information from 27,443 U.S. Veterans who underwent CABG. Cost and utility estimates were sourced from peer-reviewed literature. Current secondary preventive therapy was compared with secondary preventive therapy + Colchicine over a 35-year period. Clinical benefit was extrapolated from a nationwide perspective. Cost-effectiveness was modeled by calculating incremental cost-effectiveness ratios (ICERs) across a wide range of willingness-to-pay (WTP) thresholds. Deterministic, probabilistic, and alternative real-world scenarios were modeled to evaluate real-world use.
Results
In 150,000 isolated CABG patients who received surgery in 2019 across the U.S., Colchicine therapy could potentially reduce 8266 myocardial infarctions, 16,674 strokes, and 9472 coronary reinterventions over 5 years. Lifetime Colchicine therapy yielded a QALY gain of 0.74 and an ICER of $26,684 per QALY. Monte Carlo simulation supported these findings (median ICER: $19,598; IQR: $17,995–$20,921). Colchicine remained cost-effective at WTP thresholds >$20,000 per QALY, even with 5-year or mixed adherence.
Conclusion
Adjunct life-long Colchicine therapy may reduce cardiovascular events at an acceptable additional cost post-CABG.
Clinical perspective
What is new?
-
•
This study demonstrated that lifelong colchicine therapy, when added to current secondary prevention strategies for post-CABG patients resulted in a reduced number of adverse cardiovascular events and was also cost-effective. This benefit was also observed with more realistic scenarios such as 5-year therapy or with mixed adherence to Colchicine modeled.
-
•
Prior research had demonstrated that Colchicine use was beneficial in patients with stable coronary artery disease and post-myocardial infarction; this study expanded these results to post-CABG patients, a cohort with multiple co-morbidities and at high risk for recurrent adverse cardiovascular events.
What are the clinical implications?
-
•
Oral low-dose Colchicine should be considered as an adjunct to current secondary preventive therapies in patients after coronary artery bypass grafting.
-
•
Our simulation models demonstrated that the adoption of this strategy nationwide would results in thousands of adverse cardiovascular events avoided in the patient's lifetime.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) and its complications contributes a substantial financial burden to the US healthcare system [1]. Despite numerous medical advancements and interventions, this burden will increase in the coming decade with an expected national expenditure of almost $309 billion in 2035 [2]. Apart from its economic impact, ASCVD also impacts resource utilization in the system, productivity, and quality of life [[3], [4], [5]]. As a result, understanding the pathways to mitigate risk and treatments to improve outcomes at lower costs, has garnered significant attention in cardiovascular research.
The NLRP3 inflammasome is a multi-protein complex that plays a pivotal role in regulating the innate immune system and inflammatory signaling. Upon activation by pathogen or disease associated danger signals, NLRP3 initiates the processing and release of pro-inflammatory cytokines IL-1β. Canakinumab is a first generation monoclonal antibody that targets downstream IL-1β signaling which showed an improved outcome in patients with a history of myocardial infarction and elevated C-reactive protein level [6]. Colchicine is an established treatment medication for inflammatory conditions such as gout and pericarditis, whose mechanism of action involves preventing proteolysis and IL-1”β” maturation through the inhibition of NLRP3 inflammasome [7]. ,Two small sized clinical trials have explored the efficacy colchicine in patients with established CAD [8,9]. Colchicine Cardiovascular Outcomes Trial (COLCOT) showed improved cardiovascular outcomes with 0.5 mg daily colchicine when compared with placebo in patients who were initiated on treatment within 30 days of MI. Low Dose Colchicine in chronic coronary disease (LoDoCo2) Trial also reported improved outcomes with colchicine 0.5 mg daily in patients with chronic coronary disease when compared to placebo [8,9]. Given these favorable outcomes, colchicine has been included in both the European and American guidelines as a Class 2 A and 2B indications respectively. However, the potential implementation of such an approach has implications for additional costs to the healthcare system and may benefit from additional analyses in target patient populations, for whom there is limited data.
Accordingly, the aim of our study was to perform a cost-effectiveness analysis of colchicine when compared to the current standard of care in a subset of patients undergoing CABG who are well-known to be high risk, but also suffer from substantial co-morbidities with competing risks. We used a multi-state explanatory Markov Chain model using annual event estimates for competing events obtained from the VA Quality Initiative Project (VASQIP) registry, which provides real-world event estimates to simulate a lifetime trajectory.
2. Methods
2.1. Data source
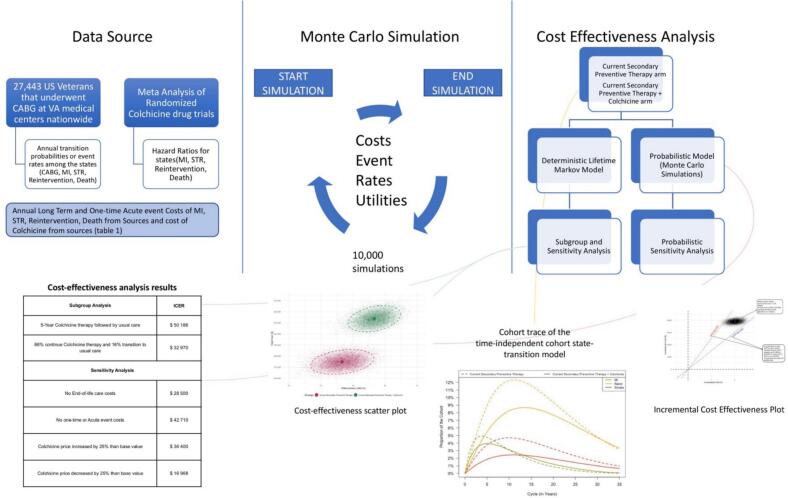
Real world evidence from 27,443 patients that underwent isolated CABG (2010–2019) in VA medical centers nationally was collected to inform the Markov discrete state transition model, Fig. 1 represents the central illustration and complete workflow process. The VA Quality Initiative Project (VASQIP) registry, which is a carefully curated data source maintained by the National Surgery Office of the Veterans Affairs Administration, was the primary data source. Rigorously defined, nurse-adjudicated information on the pre-, intra-, and post-operative period for all patients receiving cardiac surgery at VA medical centers is available in this data repository. This VASQIP data was supplemented with information from the corporate data warehouse (CDW) that contains data regarding their non-index in- and out-patient visits, laboratory test results, and echocardiographic data. The vital status for each patient was obtained by linking our cohort to the National Death Index, the Center for Medicare and Medicaid Services, and the Mortality Data Repository, a curated repository of vital status information maintained by the Department of Veterans Affairs and updated each year quarter. Quarterly. The patients were followed up till May 2020 with a median and maximum follow-up durations for our cohort were 4.9 and 10.4 years, respectively.
Fig. 1.
Title – central illustration: cost-effectiveness simulation framework for evaluating low-dose colchicine in post-CABG patients.
Legend - This schematic summarizes the overall framework used to evaluate the cost-effectiveness of low-dose colchicine therapy in U.S. veterans following coronary artery bypass grafting (CABG). Data sources include a cohort of 27,443 veterans from VA medical centers and a meta-analysis of randomized colchicine trials, providing transition probabilities, hazard ratios, and cost inputs. A Monte Carlo simulation with 10,000 iterations incorporates event rates, costs, and utility values to model outcomes. Cost-effectiveness analysis compares current standard secondary preventive therapy to an intervention arm adding colchicine, using both deterministic and probabilistic lifetime Markov models. Subgroup and sensitivity analyses examine the impact of varying assumptions and inputs. Key outputs include the incremental cost-effectiveness ratio (ICER), cost-effectiveness scatter and plane plots, and cohort state-transition trajectories over time.
2.2. Markov chain model
The cost-effectiveness of adding life-long oral low-dose Colchicine therapy to secondary preventative therapy in patients that underwent isolated CABG was evaluated according to the US healthcare perspective. A first-order discrete-time-homogeneous multi-state Markov Chain model was fitted using event rate parameter estimates obtained from our cohort of post-CABG US Veterans. Given the cohort mean age (65 years), the Markov model was developed over a 35-year time period to simulate a lifetime trajectory with state transition possible at each yearly time interval. All patients started the model in a healthy post-CABG state. The adverse cardiovascular events considered for the Markov model were myocardial infarction (MI), stroke, and coronary reintervention. Correspondingly, the intermediate transition states modeled were the post-MI, post-stroke, and post-coronary reintervention state. All-cause mortality was the final absorbing state. These transition states were selected, as they are important major adverse cardiovascular event (MACE) components in studies related to CABG outcome and have also been implemented in prior cost-effectiveness analyses. The annual deterministic and probabilistic annual transition probabilities used in our analyses are provided in Fig. 2. Table 1 presents the utilities and costs used in our study. As the only important adverse effect observed in low-dose Colchicine trials was a slight increase in pneumonia in the treatment arm, a 3 % annual probability of pneumonia in the low-dose Colchicine arm was modeled.
Fig. 2.
Title – Transition states in the Markov Model.
Legend - This figure illustrates the state transition structure used in the Markov model for evaluating outcomes in patients who start in the Coronary Artery Bypass Graft (CABG) state. All patients enter the model in the CABG state, and the final absorbing state is death (Dead). Transition probabilities between states represent the annual likelihood of moving from one state to another and are shown in the accompanying table with their 95 % confidence intervals. These probabilities form the foundation for simulating long-term outcomes in the study.
The states included are CABG (grey), myocardial infarction (MI, green), reintervention (Reint, orange), stroke (red), and death (black). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
This table presents the values for each parameter included in the Markov model. For probabilistic modeling, both utilities and costs were modeled using a truncated gamma distribution. While the hazard ratios were kept constant, the event transition probabilities were modeled using a beta distribution as reported in Fig. 2.
2.3. Event rate parameters
Event probabilities were obtained by stratifying our time-to-event data into a multi-state modeling framework considering the first MACE event to occur as the endpoint. From this long-format data, separate Cox proportional hazard models were fitted for each reported transition probability. These models were then used to obtain the marginal annual event probability adjusted for sex and age. These annual probabilities were used in the current Markov model. The annual transition probabilities for the Colchicine arm were calculated by multiplying the annual event rates for the routine post-operative care with the hazard ratios (for each selected transition state) obtained from a recent meta-analysis [10] that pooled data from 13 randomized trials using Colchicine (with 13,125 patients included in the study).
2.4. Cost and utility parameters
Various peer-reviewed published sources were used to obtain the cost and utility parameters included in our Markov model. The deterministic estimates, probabilistic distribution, and source are provided in Table 2. Apart from various annual costs, a one-time state transition cost and disutility for each modeled adverse cardiovascular event were also included. Patients also may incur a cost related to end-of-life care in a hospital or hospice facility; this parameter was also included in our primary Markov model.
Table 2.
This table presents the number adverse cardiovascular events that can potentially occur if 150,000 patients entered the Markov model fitted in our study. According to the Society of Thoracic Surgeons Annual report (2019) 157704 patients underwent isolated CABG in that year [22].
| Time | Treatment | MI* | Reintervention* | Stroke* | Deaths |
|---|---|---|---|---|---|
| 5 Years | Current Secondary Prevention Therapy | 32,588 | 42,397 | 18,710 | 33,415 |
| Current secondary preventive therapy + Colchicine | 24,322 | 25,723 | 9238 | 30,058 | |
| Events Prevented | 8266 | 16,674 | 9472 | 3357 | |
| 10 Years | Current Secondary Prevention Therapy | 60,906 | 127,744 | 52,479 | 68,112 |
| Current secondary preventive therapy + Colchicine | 50,812 | 81,118 | 26,403 | 61,773 | |
| Events Prevented | 10,0094 | 46,626 | 26,076 | 6339 |
2.5. Potential clinical benefit
The potential benefit of adding low-dose Colchicine therapy to current secondary preventive therapy was evaluated by applying the cumulative event rates for each adverse cardiovascular event (MI, stroke, coronary reintervention) and all-cause mortality obtained from the Markov Model to the annual number of isolated CABG procedures performed nationally at non-federal institutions in the US [11]. From these data, the number of events that potentially occurred at the 5- (mid-term) and 10-year (long-term) mark for each group were calculated and the difference therefore provided the number of events that could be potentially prevented by supplementing current secondary preventive therapy with low-dose Colchicine over the time period.
2.6. Cost-effectiveness analysis
2.6.1. Primary analysis
The primary analysis consisted of evaluating the lifetime cost-effectiveness of adding oral low-dose Colchicine therapy to the routine secondary preventive therapy. This was performed using a deterministic (base-case) model and a probabilistic model using repeated Monte Carlo simulations. While the deterministic model incorporates base-case parameters, the probabilistic model supports this by introducing and accounting for measurement uncertainty. Separate Markov models were fitted for the routine secondary preventive therapy including with a PCSK9i alone and with Colchicine. Applying the selected costs and utilities, the overall lifetime cost and utility for each arm was obtained and the incremental cost-effectiveness for Colchicine as add-on therapy to routine secondary preventive therapy was calculated. Half-cycle correction was applied for cost and utility calculations. Deterministic and probabilistic models were fitted for the primary analysis. For the probabilistic sensitivity analysis, 10,000 Monte Carlo simulations of our model using the distributions reported in Table 2 were fitted and the median (25th percentile, 75th percentile) of the incremental cost-effectiveness was reported in Table 3. The cost-effectiveness acceptability curve was also graphed across a wide range of ICER values.
Table 3.
This table presents the cost effectiveness results obtained from the primary analysis and other alternative scenarios fitted in our study.
| Outcome | Current secondary preventive therapy | Current secondary preventive therapy + Colchicine |
Difference | ICER (per QALY gained) |
|---|---|---|---|---|
| Primary analysis | ||||
| Deterministic model | $ 26,684 | |||
| QALY per person | 9.34 | 10.08 | 0.74 | |
| Cost per person | $ 139,447 | $ 159,105 | $ 19,658 | |
| Probabilistic model (10,000 Monte Carlo simulations) | $ 19,598 (IQR: $17,995, $20921) |
|||
| QALY per person | 13.33 (IQR: 12.70, 13.97) | 15.34 (14.82, 15.87) | 2.01(1.86, 2.16) | |
| Cost per person | $ 214,678 ($211,118, $218234) | $ 253,580 ($ 249,858, $257452) | $ 38,902 | |
| Subgroup analysis | ||||
| 5-Year Colchicine therapy followed by usual care | $ 50,186 | |||
| QALY per person | 9.34 | 9.73 | 0.39 | |
| Cost per person | $ 139,447 | $ 158,887 | $ 19,440 | |
| 86 % continue Colchicine therapy and 16 % transition to usual care | $ 32,970 | |||
| QALY per person | 9.34 | 9.99 | 0.65 | |
| Cost per person | $ 139,447 | $ 160,607 | $ 21,160 | |
| Type 2 Diabetes Mellitus | $ 24,115 | |||
| QALY per person | 9.63 | 10.63 | 1.00 | |
| Cost per person | $ 121,537 | $ 145,721 | $ 24,184 | |
| Chronic Kidney Disease | $ 23,547 | |||
| QALY per person | 10.13 | 11.28 | 1.14 | |
| Cost per person | $ 116,728 | $ 143,662 | $ 26,935 | |
| Sensitivity analysis | ||||
| No end-of-life care costs | $ 28,500 | |||
| QALY per person | 9.34 | 10.08 | 0.74 | |
| Cost per person | $ 90,544 | $ 111,540 | $ 20,996 | |
| No one-time or Acute event costs | $ 42,710 | |||
| QALY per person | 9.34 | 10.08 | 0.74 | |
| Cost per person | $ 57,629 | $ 89,093 | $ 31,464 | |
| Colchicine price increased by 25 % than base value | $ 36,400 | |||
| QALY per person | 9.34 | 10.08 | 0.74 | |
| Cost per person | $ 139,447 | $ 166,263 | $ 26,816 | |
| Colchicine price decreased by 25 % than base value | $ 16,968 | |||
| QALY per person | 9.34 | 10.08 | 0.74 | |
| Cost per person | $ 139,447 | $ 151,947 | $ 12,500 | |
IQR – interquartile range, QALY – quality adjusted life-year.
CKD is defined as eGFR <60 ml/min/m2.
2.6.2. Alternative analyses
Apart from the base-case analysis, Markov models were also fitted to evaluate the cost-effectiveness for the following pragmatic scenarios: (i) 5-year Colchicine therapy: As the LODOCO2 trial reported event rates for 5 years post-randomization, an alternative analysis was fitted where patients in the Colchicine therapy arm received a combination of low-dose oral Colchicine added to routine secondary preventive therapy for the first 5 years post-CABG, (ii) Mixed treatment: In real life, drug therapy may be stopped due to side-effects or drug-related concerns. A recent meta-analysis reported a Colchicine adherence rate of 86 % during the trial study period [12]. As our Markov model was built on the 1-year time scale, cost effectiveness was modeled for a situation where 86 % patients in the current secondary preventive therapy + Colchicine arm continued to take the drug 1 year after starting the Markov model, while the remaining 14 % stopped Colchicine therapy and received only current secondary preventive therapies.
Additionally, the following sensitivity analyses were also conducted: (i) one-time cost of MI, stroke, and reintervention was excluded from the analysis, (ii) end-of-life cost prior to death was excluded from the model, and (iii) the cost of Colchicine was set at 25 % above and below the cost considered in the deterministic model, and (iv) subgroup analyses considering only patients with baseline type 2 diabetes or chronic kidney disease (defined as an estimated glomerular filtration rate < 60 ml/min/m2).
Statistical analyses were done using R 4.3.1 (The R foundation for Statistical Computing) and the modeling framework provided by the Decision Analysis in R for Technologies in Health (DARTH) study group. Primary packages used for cost effectiveness calculations and Markov modeling were ‘dampack’ and ‘darthtools’ [13,14]. The study was conducted and reported according to the Consolidated Health Economics Evaluation Reporting Standards 2022 (CHEERS-2022) (checklist is provided in the supplemental section).
3. Results
The Markov model was based on 27,443 US Veterans (66 years median age, 1.1 % females, 10 % Black patients) that received CABG at 40 different VA medical centers nationwide.
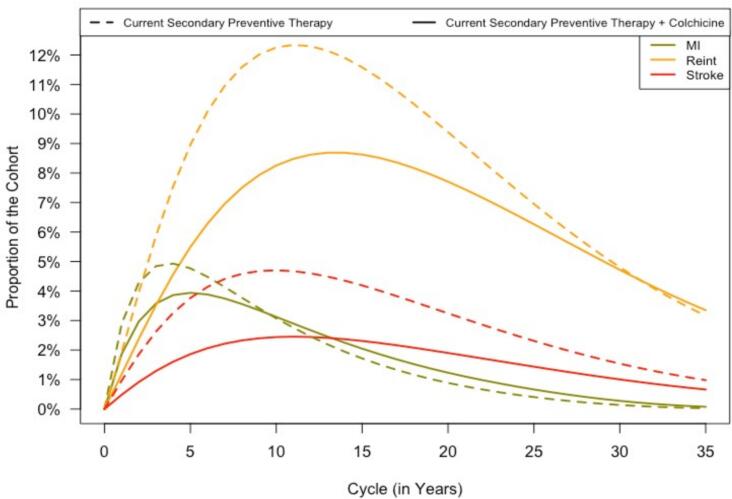
The cohort trace plot (Fig. 3) from the Markov Model shows a significant decrease in the number of events when Colchicine was added to current secondary preventive therapy. At 5 years post-CABG, in 150,000 patients, the addition of Colchicine potentially prevented 16,674 stroke events, 9472 coronary re-intervention and 9472 MI events (Table 2).
Fig. 3.
Title - Cohort trace of the time-independent cohort state- transition model
Legend - This figure illustrates the proportion of a simulated patient cohort experiencing specific health states over time, based on a state-transition model. Two treatment scenarios are compared: Current Secondary Preventive Therapy (dashed lines) and Current Secondary Preventive Therapy with Colchicine (solid lines).
The states analyzed include myocardial infarction (MI, green), reintervention (orange), and stroke (red). The x-axis represents the time in years (cycles), while the y-axis indicates the proportion of the cohort in each state at a given time point.
Adding colchicine results in visible differences in the trajectory of health states, with reductions in the proportions of patients experiencing MI, reintervention, and stroke over time compared to standard therapy alone. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the deterministic model, adding the low-dose Colchicine to current secondary preventive therapy increased quality-adjusted life years (QALYs) from 9.34 to 10.08 at an incremental cost of $19,658 per patient. This resulted in an incremental cost-effectiveness ratio (ICER) of $26,684 per QALY. In the probabilistic model (Fig. 4), even greater benefits were observed, with median QALYs increasing by 2.01 (IQR: 1.86, 2.16) (median QALY of 15.34 vs 13.33 for current secondary preventive therapy + lifetime Colchicine therapy and current secondary preventive therapy respectively) resulting in a median ICER of $19,598 (IQR: $17,995, $20,921) per QALY (Fig. 5).
Fig. 4.
Title – Cost-effectiveness scatter plot.
Legend - This scatter plot illustrates the cost-effectiveness of two strategies: Current Secondary Preventive Therapy (maroon) and Current Secondary Preventive Therapy + Colchicine (green). Each scatter point represents a simulation from a probabilistic sensitivity analysis, displaying the total cost (y-axis) versus effectiveness in quality-adjusted life years (QALYs, x-axis).
The dashed ellipses represent the 95 % confidence regions for each strategy, indicating the spread and uncertainty of the simulated outcomes. The larger, filled points mark the centroid of each strategy, representing the mean total cost and mean effectiveness. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Title - incremental cost effectiveness plot.
Legend - The incremental cost-effectiveness plane shows the relationship between incremental costs and incremental quality-adjusted life years (QALYs) gained for a hypothetical health intervention compared to a baseline. The scatter points represent probabilistic simulations of cost-effectiveness outcomes.
The red dashed line indicates a willingness-to-pay (WTP) threshold of $25,000 per QALY, while the blue dashed line represents a WTP threshold of $15,000 per QALY. The median QALY gain is 2.01 with an incremental cost of $38,902, resulting in an incremental cost-effectiveness ratio (ICER) of $19,598 per QALY.
For a gain of 2.01 QALYs, the total WTP ranges between $30,150 (at $15,000/QALY) and $50,250 (at $25,000/QALY). The results suggest the intervention is cost-effective within these WTP thresholds. The black dotted lines denote the axes origin at zero incremental cost and zero incremental QALYs. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
When Colchicine was added to secondary preventive therapy for a 5-year period post-CABG, the patients in the current secondary preventive therapy +5-year Colchicine therapy group reported a QALY gain of 0.39 compared to the current secondary preventive therapy only group with an ICER of $50,186 per QALY. A mixed treatment scenario where a Colchicine adherence rate of 86 % was modeled reported a QALY gain of 0.65 (current secondary preventive therapy + mixed adherence Colchicine therapy group vs current secondary preventive therapy group) and an ICER of $32,970 per QALY. When end-of-life care costs were excluded from the analysis, the ICER was $28,500 per QALY. Similarly, excluding all one-time acute event costs yielded an ICER of $42,710 per QALY gained. Additionally, analyses limited to those with type 2 diabetes or chronic kidney disease reported greater benefit for low-dose Colchicine (Table 3).
The cost-effectiveness benefit for lifetime Colchicine therapy added to current secondary preventive therapy was present even with a 25 % increase in colchicine price (ICER to $36,400 per QALY) while it was naturally even more cost-effective if the lifetime Colchicine cost were reduced by 25 % (ICER to $16,968 per QALY). Colchicine + current secondary preventive therapy was more cost-effective than current secondary preventive therapy alone when the willingness-to-pay threshold increased beyond US$ 20,000 (Fig. 6).
Fig. 6.
Title - Cost-effectiveness acceptability Curve (CEAC).
Legend - This figure presents the cost-effectiveness acceptability curve (CEAC) for two strategies: Current Secondary Preventive Therapy (red line) and Current Secondary Preventive Therapy + Colchicine (blue line). The x-axis represents the willingness-to-pay (WTP) threshold in thousand dollars per quality-adjusted life year (QALY), while the y-axis shows the probability of each strategy being cost-effective at a given threshold.
The CEAC demonstrates how the probability of cost-effectiveness changes as the WTP threshold increases. At lower thresholds, Current Secondary Preventive Therapy is the preferred strategy, but as the WTP threshold exceeds approximately $15,000 per QALY, Current Secondary Preventive Therapy + Colchicine becomes increasingly favored and dominates at higher thresholds.
The black square markers denote the cost-effectiveness frontier, highlighting the threshold points where each strategy is most likely to be cost-effective. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We used real world data from a nationwide cohort of U.S. Veterans post-CABG to evaluate the potential clinical benefit and cost-effectiveness of adding oral low-dose Colchicine therapy to current secondary preventive therapies over the lifetime horizon. We found that the addition of low-dose Colchicine may result in substantially reduced adverse cardiovascular events after CABG and was likely to be cost effective if the healthcare system was willing to pay $ 20,000 per lifetime QALY gained.
Recent evidence has emerged that inflammation is an important pathway in the progression of atherosclerotic cardiovascular disease including MI, and stroke. Colchicine is an anti-inflammatory drug used to treat gout, pericarditis and juvenile arthritis [15]. However, interest in using this drug as secondary preventive therapy in patients with ASCVD emerged from results of the COLCOT and LoDoCo2 trials. After the initial pilot trial (LoDoCo), the COLCOT trial demonstrated that, in a cohort of post-MI patients, the addition of oral low-dose Colchicine resulted in 23 % less MACE events compared to placebo therapy [16] . Results of the LoDoCo2 trial then further supported the use of low-dose Colchicine even for people with stable coronary artery disease [12] where those receiving Colchicine had 31 % lower events compared to those on a placebo. Two prior studies based on the Canadian healthcare perspective have both reported that the addition of Colchicine therapy to current secondary preventive therapy resulted in improved QALY with a lower overall cost [15,16]. Another study used data from the SMART-REACH registry and reported an average lifetime benefit of 2 years with the addition of Colchicine therapy in patients with stable ASCVD [17], but did not perform a cost-effectiveness analysis. While our study supported observations from all these prior reports, we found that the use of Colchicine also resulted in higher overall costs, both over the 5-year timespan as well over the lifetime horizon. This difference is clearly largely driven by the substantial difference in the cost of Colchicine therapy between Canada (CA $0.26 per pill) and US (US $7.5 per pill). However, even though the current cost of Colchicine is high in the US, the ICER obtained in our models is much lower than that reported for other novel secondary preventive therapies. Recent lipid lowering therapies such as Inclisiran or PSCK9i reported ICER of US$ 51,686 per QALY [18], while that of Alirocumab was reported as US$ 92,200 per QALY [19]. Additionally, both the COLCOT and LoDoCo2 trials reported very low adverse effects of Colchicine therapy. There was a small signal of numerically more non cardiovascular deaths in the Colchicine arm (53 vs 35 events), however, the exact cause of such events was unclear. More importantly, apart from a small increase in gastrointestinal discomfort, and a small increase in pneumonia rates, low-dose Colchicine was well tolerated in the majority of patients that participated in the trial [20]. Furthermore, the therapeutic benefit of colchicine observed in the COLCOT trial was primarily driven by reduced rates of stroke and coronary reintervention. In fact, these results are consistent with colchicine's mechanism of action as an anti-inflammatory agent that inhibits the NLRP3 inflammasome pathway, a critical driver of atherosclerosis-related inflammation. The NLRP3 pathway has been implicated in the progression of plaque instability with subsequent acute thrombotic events. Thus, we would argue that Colchicine use is even more important in a CABG cohort as these patients are at a higher risk for stroke and the long-term benefit of CABG largely depends upon maintaining graft patency. Like prior studies [15], we also have demonstrated the robustness of our primary analyses using different alternative scenarios as well as probabilistic models. A recent trial (CLEAR SYNERGY) [21] reported a statistically non-significant benefit for using low-dose Colchicine after coronary stenting in post-MI patients. However, their observations may in part be due to their study cohort. Multiple prior studies have demonstrated that Colchicine is useful in patients with multi-vessel CAD, hence it would be most useful in post-CABG patients. Furthermore, post-CABG are at risk for long-term plaque deposition in their native coronary arteries as well as grafts, both of which may be reduced or prevented by the anti-inflammatory effect of Colchicine. Additionally, as most of the patients in the CLEAR SYNERGY trial had only critical single-vessel disease, a 5-year time frame may be too small to observe any clinical benefit for low-dose Colchicine. Therefore, despite the disappointing results of this trial, we still believe that low-dose Colchicine is a cost-effective adjunct to current secondary preventive therapy in selected CAD patients.
Our study findings need to be understood on the background of some study limitations. The Markov model was based on real-world event rates from post-CABG US Veterans. Importantly, the cohort was racially/ethnically diverse and contained patients across a wide range of the social determinants of health. However, our cohort was largely males. Hence, our findings need to be evaluated in a female population. Additionally, as US Veterans have been observed to have higher adverse events rates compared to the general population, the ICER values in the general population may be different. Secondly, the treatment effect of Colchicine included in our models was based on information from drug trials which were conducted in patients with MI or stable coronary artery disease. The actual benefit of Colchicine in patients' post-CABG may vary; however, as Colchicine has been found beneficial in reducing atrial fibrillation post-CABG [20], its efficacy in this patient cohort may even be greater than that observe in the clinical trials. Finally, our Markov chains assume that transitions take place only once per observation cycle. In reality, multiple unobserved transitions can take place at any time including between cycle assessments.
In conclusion, the addition of lifetime oral low-dose Colchicine to current secondary preventive therapy post-CABG may potentially result in substantial reductions in adverse cardiovascular events. Additionally, this therapy was observed to be reasonably cost effective in the US.
CRediT authorship contribution statement
Sai Rahul Ponnana: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis. Gauranga Mahalwar: Methodology, Investigation, Formal analysis, Data curation. Joris Holtrop: Writing – review & editing. Steven H.J. Hageman: Writing – review & editing. Tong Zhang: Writing – review & editing. Santosh Kumar Sirasapalli: Writing – review & editing. Skanda Moorthy: Writing – review & editing. Zhuo Chen: Writing – review & editing. Jean-Eudes Dazard: Writing – review & editing. Sadeer Al-Kindi: Writing – review & editing. Yakov Elgudin: Writing – review & editing. Naveed Sattar: Writing – review & editing. Sanjay Rajagopalan: Writing – review & editing, Supervision. Salil V. Deo: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Formal analysis, Conceptualization.
Funding sources
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Keeling W., Binongo J., Wei J., Leshnower B., Farrington W., Halkos M. National trends in emergency coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2023;64 doi: 10.1093/ejcts/ezad352. [DOI] [PubMed] [Google Scholar]
- 2.Raza S., Deo S., Kalra A., et al. Stability after initial decline in coronary revascularization rates in the United States. Ann. Thorac. Surg. 2019;108:1404–1408. doi: 10.1016/j.athoracsur.2019.03.080. [DOI] [PubMed] [Google Scholar]
- 3.Alkhouli M., Alqahtani F., Kalra A., et al. Trends in characteristics and outcomes of patients undergoing coronary revascularization in the United States, 2003-2016. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohri S., Benedetto U., Luthra S., et al. Coronary artery bypass surgery in the UK, trends in activity and outcomes from a 15-year complete national series. Eur. J. Cardiothorac. Surg. 2022;61:449–456. doi: 10.1093/ejcts/ezab391. [DOI] [PubMed] [Google Scholar]
- 5.Pacaric S., Turk T., Eric I., et al. Assessment of the quality of life in patients before and after coronary artery bypass grafting (CABG): a prospective study. Int. J. Environ. Res. Public Health. 2020;17(4):1417 doi: 10.3390/ijerph17041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deo S., Al-Kindi S., Virani S., Fremes S. Novel therapies to achieve the recommended low-density lipoprotein cholesterol concentration (LDL-C) targets for patients after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2024;167 doi: 10.1016/j.jtcvs.2023.05.028. 723-730.e4. [DOI] [PubMed] [Google Scholar]
- 7.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Sabatine M., Giugliano R., Keech A., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz G.G., Steg P.G., Szarek M., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 10.Kofler T., Kurmann R., Lehnick D., et al. Colchicine in patients with coronary artery disease: a systematic review and meta-analysis of randomized trials. J. Am. Heart Assoc. 2021;10(16) doi: 10.1161/JAHA.121.021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez F.G., Shahian D.M., Kormos R., et al. The Society of Thoracic Surgeons National Database 2019 annual report. Ann. Thorac. Surg. 2019;108(6):1625–1632. doi: 10.1016/j.athoracsur.2019.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Nidorf S.M., Fiolet A.T.L., Mosterd A., et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 2020;383(19):1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 13.Alarid-Escudero F., Krijkamp E., Enns E.A., et al. A tutorial on time-dependent cohort state-transition models in R using a cost-effectiveness analysis example. Med. Decis. Mak. 2023;43(1):21–41. doi: 10.1177/0272989X221121747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alarid-Escudero F., Krijkamp E.M., Pechlivanoglou P., et al. A need for change! A coding framework for improving transparency in decision modeling. Pharmacoeconomics. 2019;37(11):1329–1339. doi: 10.1007/s40273-019-00837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boczar K.E., Beanlands R., Wells G., Coyle D. Cost-effectiveness of colchicine for recurrent cardiovascular events. CJC Open. 2023;5(5):348–356. doi: 10.1016/j.cjco.2023.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel M., Tardif J.C., Khairy P., et al. Cost-effectiveness of low-dose colchicine after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT) Eur Heart J Qual Care Clin Outcomes. 2021;7(5):486–495. doi: 10.1093/ehjqcco/qcaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger P.M., Dorresteijn J.A.N., Fiolet A.T.L., et al. Individual lifetime benefit from low-dose colchicine in patients with chronic coronary artery disease. Eur. J. Prev. Cardiol. 2023;30(18):1950–1962. doi: 10.1093/eurjpc/zwad221. [DOI] [PubMed] [Google Scholar]
- 18.Alkeylani A., Miller D.D., Shaw L.J., et al. Influence of race on the prediction of cardiac events with stress technetium-99m sestamibi tomographic imaging in patients with stable angina pectoris. Am. J. Cardiol. 1998;81(3):293–297. doi: 10.1016/s0002-9149(97)00896-5. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt D.L., Briggs A.H., Reed S.D., et al. Cost-effectiveness of Alirocumab in patients with acute coronary syndromes: the ODYSSEY OUTCOMES trial. J. Am. Coll. Cardiol. 2020;75(18):2297–2308. doi: 10.1016/j.jacc.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Deftereos S.G., Beerkens F.J., Shah B., et al. Colchicine in cardiovascular disease: in-depth review. Circulation. 2022;145(1):61–78. doi: 10.1161/CIRCULATIONAHA.121.056171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolly S.S., d’Entremont M.A., Lee S.F., et al. Colchicine in acute myocardial infarction. N. Engl. J. Med. 2024;392(7):633–642. doi: 10.1056/NEJMoa2405922. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez F.G., Shahian D.M., Kormos R., et al. The Society of Thoracic Surgeons National Database 2019 annual report. Ann. Thorac. Surg. 2019;108(6):1625–1632. doi: 10.1016/j.athoracsur.2019.09.034. [DOI] [PubMed] [Google Scholar]