Abstract
Because many survivors of mass extinctions do not participate in postrecovery diversifications, and therefore fall into a pattern that can be termed “Dead Clade Walking” (DCW), the effects of mass extinctions extend beyond the losses observed during the event itself. Analyses at two taxonomic levels provide a first-order test of the prevalence of DCWs by using simple and very conservative operational criteria. For four of the Big Five mass extinctions of the Phanerozoic, the marine genera that survived the extinction suffered ≈10–20% attrition in the immediately following geologic stage that was significantly greater than the losses sustained in preextinction stages. The stages immediately following the three Paleozoic mass extinctions also account for 17% of all order-level losses in marine invertebrates over that interval, which is, again, significantly greater than that seen for the other stratigraphic stages (no orders are lost immediately after the end-Triassic or end-Cretaceous mass extinctions). DCWs are not evenly distributed among four regional molluscan time-series following the end-Cretaceous extinction, demonstrating the importance of spatial patterns in recovery dynamics. Although biotic interactions have been invoked to explain the differential postextinction success of clades, such hypotheses must be tested against alternatives that include stochastic processes in low-diversity lineages—which is evidently not a general explanation for the ordinal DCW patterns, because postextinction fates are not related to the size of extinction bottlenecks in Paleozoic orders—and ongoing physical environmental changes.
Paleontologists have long noted that certain clades (monophyletic evolutionary lineages) survive mass extinctions only to remain marginal or decline in the aftermath, whereas other groups diversify. The aim of this paper is to test the generality of this pattern of survival without recovery, which I term “Dead Clade Walking” (DCW) in homage to an award-winning film based on ref. 1, by moving beyond anecdotal accounts to global and regional analyses of marine invertebrates. Here, I show that more taxa are lost at both the genus and ordinal levels shortly after extinction events than expected from preextinction intensities, that the ordinal pattern cannot be explained simply in terms of the stochastic consequences of the losses suffered in the mass extinction itself, and that DCW molluscan genera are not evenly distributed geographically after the end-Cretaceous (K-T) extinction. Taken together, these analyses provide further evidence that the evolutionary and ecological roles of mass extinctions are larger than indicated simply by tallies of taxa lost at the crisis horizons or intervals (2–4), and that more attention should be paid to taxa that do not fit the end-member cases of complete extinction or unbridled diversification.
Phanerozoic Genera
Methods.
One approach to quantifying the frequency and role of DCWs is to test whether the assemblage of taxa surviving a mass extinction is subject to more severe losses in the immediately ensuing stratigraphic stage than might be expected from the losses suffered at other stage boundaries. Because of the secular decline in extinction rates (e.g., refs 5–7), comparisons are not readily made across an entire Phanerozoic time-series, and so, each of the Big Five mass extinctions was treated separately. To reduce variance in the duration of time bins, longer stages were split into substages, and a few short stages were combined, following Sepkoski (ref. 7, p. 37).
For each of the three stratigraphic stages preceding, and the two stages following a mass extinction, survivorship was calculated at the upper boundary for the set of genera that had crossed the lower boundary. That is, how many taxa that entered the time bin survived beyond it? (This calculation omits taxa originating within the focal stage, so numbers differ from previous analyses of this database.) Survivorship across the upper boundary of the postextinction stage then was compared with that of taxa crossing stage boundaries immediately preceding the extinction boundary.
Results.
For four of the Big Five mass extinctions, the genera that survived the extinction itself had significantly lower survivorship in the immediately following stage or stages than was seen for taxa at preextinction stage boundaries (Fig. 1). Thus, the stages immediately following mass extinctions saw significant attrition among the survivors. This statement is a very conservative estimate of DCW frequencies, of course, because some survivors dwindle for millions of years beyond the test interval before disappearing.
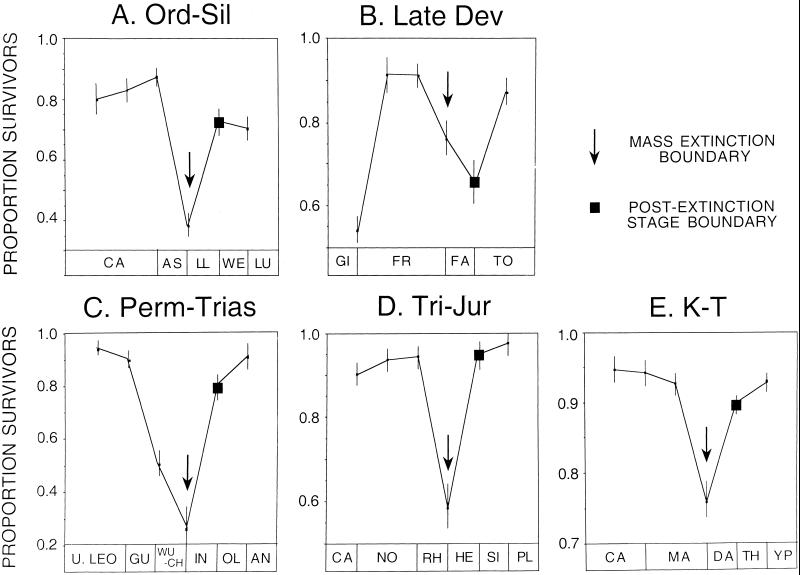
Figure 1.
Genus-level patterns associated with the Big Five mass extinctions, showing percent survivorship across a stage boundary only for those taxa that had crossed the preceding stage boundary. For four of the extinctions (A–C, E), a significantly greater proportion of genera that survived the extinction failed to persist beyond the immediately postextinction stage, relative to preextinction stages. Arrow, extinction boundary; square, survivorship proportion at the postextinction boundary; vertical bars = 95% CI, following Raup (63). Abbreviations at bottom of plots are first two letters of stratigraphic stage codes in ref. 13.
The one exception to this pattern is the end-Triassic extinction, where survivorship was indistinguishable from that preceding the extinction event; this is consistent with previous observations (8). Also consistent with previous work (9, 10) is the low survivorship at the top of the Guadalupian stage, which probably combines a genuine extinction pulse with backwards-smearing from the end-Permian (P-T) extinction event. Losses among the survivors of Late Devonian (Frasnian-Fammenian) extinction actually exceeded those seen at the mass extinction itself. However, in the full dataset, originations within the Fammenian offset the drop in survivorship to produce the conventional portrait of the end-Frasnian as the larger extinction pulse; backwards-smearing of an end-Fammenian event is another possibility, and indeed an extinction pulse also is recorded at that time (7).
Phanerozoic Orders
Methods.
Orders differ in their evolutionary dynamics from genera and families (e.g., 11, 12), so that the DCW hypothesis should also be tested at this level. Extinctions of marine invertebrate orders were tabulated from Benton (13), which gives a more thorough updating at this taxonomic level than the Sepkoski database (see Table 1, which is published as supporting information on the PNAS website, www.pnas.org, for further corrections of stratigraphic ranges; these data show that new and reexamined data tend to sharpen rather than blur the extinction events).
The data analyzed here are entirely postCambrian. Ordinal classification of Cambrian taxa remains unstable, stratigraphic ranges are heavily influenced by exceptional deposits such as the Chengjiang Formation and the Burgess Shale, and Cambrian turnover rates are extraordinarily high and include one or more extinction events of still-uncertain magnitude (4, 14). The reliability of the remaining 142 postCambrian ordinal extinctions is still very uneven, as they range from sturdy articulate brachiopods to weakly skeletonized holothurioids (sea cucumbers). Accordingly, a subset of taxa with relatively high preservation potential was chosen for analysis (see ref. 12 on the analytical utility of separating well preserved and poorly preserved taxa), namely the extinct orders of sponges, coelenterates, arthropods (trilobites and ostracodes only; see ref. 15 on preservational variation among arthropod groups), mollusks, brachiopods, bryozoans, echinoderms (excluding the easily disarticulated ophiuroids and holothurioids), and graptolites. Following Foote (16), the four orders confined to a single stage were also omitted, leaving a total of 115 ordinal extinctions.
Results.
The comparison between ordinal losses in stages immediately after mass extinctions and those lost in the other nonextinction-stages is suggestive but should be interpreted cautiously (Fig. 2). Clearly, the mass extinctions take a significant toll at the ordinal level, even though the Big Five extinctions account for <5% of the total species extinction of the Phanerozoic (e.g., 17). An impressive 39 ± 9% [with 95% confidence interval (CI)] of the extinct orders are lost during the Big Five mass extinctions. This finding yields a median loss of eight orders per stage, very significantly different from the median value of zero for nonextinction stages when the stages immediately after the extinction events (i.e., the potential DCW stages) are excluded (P < 0.0001, Mann–Whitney U test corrected for ties). Whether these ordinal losses are attributable to the removal of dwindling orders by stochastic species loss or to phylogenetic clumping of extinction (i.e., concentration of losses in particular clades) will require comprehensive analysis of the diversity dynamics of these clades. A number of ordinal extinctions involve the final removal of DCWs, e.g., the end-Ordovician paterinate brachiopods, the end-Permian trilobites, and the end-Cretaceous pygasteroid echinoids (see ref. 18, and references to Table 1).
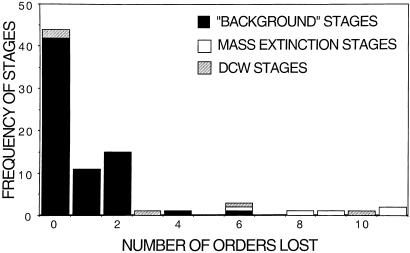
Figure 2.
Frequency distribution of order-level extinction among Phanerozoic stages. Black bars, ordinal losses of “normal stages”; 45 of the “normal stages” have no ordinal extinction. Hatched bars, ordinal losses for the “Big Five” mass extinctions. Stippled bars, ordinal losses in the stages immediately following mass extinctions.
Regarding DCWs, the immediate postextinction stages (which account for an additional 17 ± 7% of Phanerozoic ordinal extinction) differ as a group only marginally from the rest of the nonextinction stages (P = 0.056); the median is three extinctions per DCW stage, but stages following the two postPaleozoic extinctions see no ordinal losses. Ordinal extinction rates tend to be higher throughout the Paleozoic, as expected, given the generally higher extinction rates observed at the genus and family levels (e.g., ref. 7). Taking the Paleozoic extinctions alone, distributions are significantly different when the immediate postextinction intervals are compared with the other nonextinction stages, despite the lower totals (P = 0.005). The immediate postextinction biotas in the Paleozoic included a pool of more volatile taxa (6), and this may have rendered the short-term sorting of DCWs more detectable at the ordinal level. However, the fact that Paleozoic taxa tended to be shorter ranging does not explain their significant concentration in the three postextinction stages relative to the rest of the Paleozoic. Heightened extinction of mass-extinction survivors, therefore, seems to occur in the immediate aftermath of major Paleozoic events, and the lack of such extinctions at the ordinal level following the two postPaleozoic events, despite significant genus-level losses, remains an unresolved problem.
K-T Molluscan Genera
Methods.
Many aspects of diversification, extinction, and recovery seem to have a spatial component (e.g., 19). A preliminary test for geographic concentrations of DCWs among K-T molluscan genera can be performed by using the four regional Paleocene time-series studied in detail by Jablonski (20): the Gulf Coast of North America, northern Europe, northern Africa, and India/Pakistan.
Results.
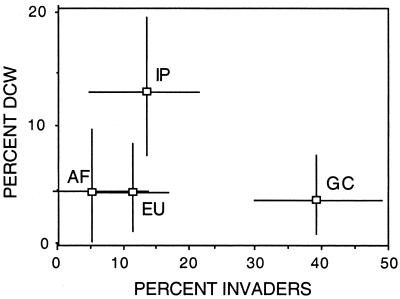
Of the four regions, India/Pakistan contained significantly more DCWs than the others, despite the unexceptional intensity of postextinction invasions reported for this region by Jablonski (ref. 20; Fig. 3). This result is surprising, because damped or failed recoveries are often explained in terms of biotic interactions (see below), so that the region with the greatest influx of potential competitors and predators might be expected to have the greatest proportion of postextinction losses among native clades (e.g., refs. 21 and 22). The higher frequency of DCWs in the India/Pakistan Paleocene is unlikely to be a sampling artifact, because both the latest Cretaceous and the Eocene of this and the other three regions have been more thoroughly sampled than the Paleocene (see references in 20), so that taxon ranges should tend either to stop at the K-T boundary or extend into the Eocene (16). These results demonstrate a biogeographic component to the occurrence of DCWs, suggesting that postextinction losses can be influenced by spatial distribution per se and not only by the kinds of organismal adaptations, such as competitive ability or predation-resistance, often invoked to explain differential survivorship.
Figure 3.
The frequency of bivalve and gastropod DCWs at the genus level is unrelated to invasion intensity suffered by different regions after the end-Cretaceous mass extinction. IP, India-Pakistan area; GC, Gulf Coast of North America; EU, northern Europe; AF, northern Africa. Bars are 95% CIs.
Potential Mechanisms
Artifact.
DCW records need to be assessed critically. Some may be taxonomic errors promoted, for example, by convergence on newly extinct taxa by survivors reoccupying modes of life vacated by related clades (see 23, 24). Other putative DCWs may be specimens reworked from preextinction strata. Such processes commonly leave taphonomic or sedimentary indicators for macrofossils, but this has been a serious concern for microfossils, especially at the K-T boundary (e.g., refs. 25 and 26).
A fully resolved phylogenetic analysis to the species level is not available for fossil marine invertebrates, and so the data analyzed here probably include paraphyletic taxa (i.e., groups that include some but not all descendants of a common ancestor, so that extinction of the taxon does not exterminate the entire genealogical lineage). However, paraphyly is unlikely in itself to have artificially produced the results reported here. Regarding the genus-level analyses, numerical simulations have shown that paraphyly is unlikely to mask or generate major patterns of taxonomic origination and extinction, especially given the large number of taxa involved (27–32). Order-level systematics have been increasingly cast in cladistic terms [e.g., echinoderms (33), trilobites (34), and brachiopods (35)]. Extinct orders that have not been revised from this standpoint probably represent true termination of constituent lineages in most instances, either owing to strict monophyly or because paraclades were split significantly before the last appearance of the ordinal taxon, so that the loss of that taxon is meaningful in terms of biodiversity (27–29). Extensive “pseudoextinction” of orders at or immediately following extinction boundaries is thus unlikely.
DCWs also are unlikely to result from sampling biases related to the sea-level changes often associated with extinction boundaries (reviewed by ref. 36). Although certain combinations of sampling/preservation, origination and extinction could yield patterns resembling those shown here (see ref. 16 for general discussion), evidence seems to be lacking for the requisite parameter combinations near the best studied extinction boundaries. Whenever sampling is poorer immediately after an extinction boundary than in the times preceding it or well after it, as seen for the Ordovician-Silurian, P-T, and K-T events, observed frequencies of DCWs are probably underestimates of the true values, because artificial range terminations are most likely to occur before, but not within, poorly sampled intervals, and originations concentrated after those intervals (e.g., 16).
Bottleneck Effects.
If a taxon loses most of its constituent species, geographic extent, or ecological variety during a mass extinction, stochastic factors may account for its failure to rediversify after this bottleneck. The probability that chance events will drive a clade into extinction within a given time window depends on the size of the bottleneck (i.e., the number of constituent taxa at the extinction boundary), the duration of the low-diversity interval, and the clade-specific extinction and origination rates (e.g., 18, 37, 38). Examples are probably legion at the genus level, as suggested by many short-lived postextinction occurrences of formerly diverse and widespread taxa [e.g., the bivalve genera Permophorus in the early Triassic of the Western Interior Seaway (39) and Linearia in the Paleocene of California (40)]. Lags between extinction and rediversification differ significantly among events (e.g., refs. 41 and 42), so that stochastic loss of clades following the extinction bottleneck should vary predictably among events.
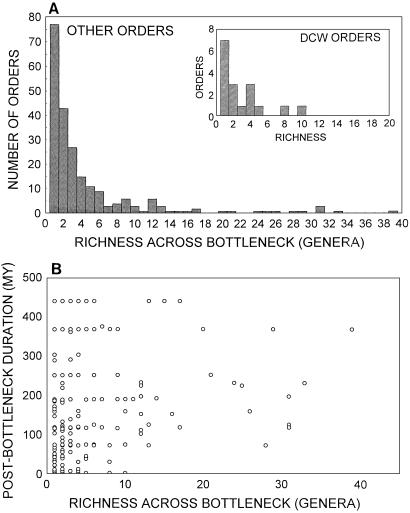
Although a complete analysis requires improved data on turnover rates within clades, the demise of ordinal DCWs probably cannot be attributed to stochastic effects related to the bottleneck imposed by the immediately preceding mass extinction. There is no significant difference in the bottleneck sizes (number of genera crossing an extinction boundary) of Paleozoic DCW orders and the more persistent Paleozoic orders (modes for both groups, one genus; medians for both groups, two genera; Mann–Whitney U test corrected for ties, P = 0.47; Fig. 4A). This result is not simply owing to the simple binary comparison (DCWs vs. all others regardless of subsequent fate) or to the relatively small numbers of DCW orders. As shown in a more detailed analysis (Fig. 4B), there is also no significant relation between bottleneck size and postextinction duration for all of the Paleozoic orders (Pearson product–moment correlation, r = 0.05, P = 0.47; Spearman rank–order correlation, R = 0.02, P = 0.73). These analyses were necessarily confined to the Paleozoic because of the lack of postPaleozoic DCW orders, and the preponderance of orders that persist from the Mesozoic mass extinctions to the present day and, thereby, skew postextinction durations, but the overall result is unlikely to change with the addition of the ≈30 well preserved orders that originate after the Paleozoic (12).
Figure 4.
Survivorship of Paleozoic orders after mass extinctions is unrelated to the size of the extinction bottleneck. (A) The frequency distribution of genera surviving a mass extinction does not differ significantly for DCW orders (which became extinct by the end of the stage immediately following a mass extinction) and the other well preserved, contemporaneous orders. (B) The geologic durations of Paleozoic orders following the end-Ordovician, late Devonian, and end-Permian extinctions are unrelated to the number of genera within those orders that survived those events. Total number of orders = 138; total number of order × mass extinction encounters = 242; only Paleozoic orders were analyzed because postPaleozoic extinction lack ordinal DCWs, and extant orders dominate the postPaleozoic dataset, undermining comparisons of postextinction durations. When no genera within an order were recorded crossing a given extinction event, genealogical continuity was assumed, and a value of one surviving genus was assigned.
The histories of DCWs subjected to more prolonged bottlenecks can be useful in themselves. For example, the contraction of pygasteroid echinoids, nerineacean gastropods, and several families of rudist bivalves near the Cenomanian-Turonian (C-T) boundary, with the final demise of one or a few genera apiece at the K-T boundary, may support the reality of an extinction within the C-T interval despite shorter term sampling artifacts (see ref. 43).
Ongoing Physical Environmental Changes and the Spatial Dimension.
Even after the amelioration of the proximate cause of the extinction episode (which can last on the order of 105–106 yrs for some events; see refs. 41 and 42 for reviews), longer term environmental changes such as global trends in climate or ocean circulation may unfold that drive survivors into extinction. Such changes can affect even those taxa that crossed the extinction boundaries unscathed, but should take the largest toll on clades that have already suffered taxonomic and/or spatial bottlenecks. Temporal coincidence does not equal causation, but causality can be tested by partitioning environmental perturbations and biotic changes spatially (19, 44): if regionally restricted environmental changes prevent the postextinction recovery of taxa, then a significant subset of DCWs should be endemic to environmentally perturbed regions.
Regional environmental change may in fact underlie the concentration of K-T DCWs in the India/Pakistan region (Fig. 3), which underwent significant changes in sedimentary environments in the late Paleocene-early Eocene as the Indian Plate collided with Asia (e.g., refs. 45 and 46). When endemic genera are omitted from this fauna, the proportion of DCWs (6 ± 6%, with binomial 95% CI) no longer differs significantly from the other three regions. A similar mechanism might account for the loss of DCWs in northwestern Europe, where the final Paleocene demise of the Chalk facies removed a number of K-T survivors from the mollusks, echinoids, and bryozoans. Extinction intensities for early Paleocene echinoids (25%) are only moderately lower than those seen at the K-T boundary (34%), for example (47).
On much shorter timescales, Looy et al. (48) apply an “extinction debt” concept to the end-Permian plant extinction, proposing an extinction wave that removed increasingly competitive taxa immediately following the collapse of the Permian ecosystem. However, such short-term processes do not seem to dominate the DCW patterns. For example, of the 325 marine genera that survive a mass extinction only to be lost in the following stage and whose stratigraphic ranges are resolved to the substage level in the Sepkoski database, at least 68 ± 5% (with binomial 95% CI) range through the lower substage and become extinct in the middle or upper substage, long after an initial “extinction debt” would be expected (and even the extinctions in the lower substage may encompass considerable geological time). Thus, the other hypotheses discussed here are more likely to be responsible for DCW losses over evolutionary timescales.
Biotic Interactions.
Low diversification rates and/or geographic restriction in the face of regional change cannot account for all DCWs. For example, losses in the aftermath of the P-T extinction include the rapidly evolving cephalopod order Prolecanitida, which was widely distributed in the early Triassic (49). Adverse interactions with other cephalopod clades, or with diversifying predators, have been invoked to explain this pattern. Similarly, among vertebrates, temnospondyls (“labyrinthodonts”) were thought to have become extinct in the end-Triassic extinction, but rare and geographically scattered specimens have been recorded through the Jurassic and into the Aptian (mid-Cretaceous); their failure to re-diversify, and their final demise, has been attributed to competition or predation by crocodilians and other carnivorous vertebrates (50).
Such biotic interactions are the traditional explanation for the dwindling or long-term suppression of clades after, or even independent of, mass extinctions (e.g., 51). These hypotheses are among the most difficult to test rigorously from clade dynamics alone (52). Tests require not only the rejection of nonbiotic alternatives, but clade-by-clade analyses using additional information such as the life habits, biogeography, and relative abundances of both the DCW and the taxa hypothesized to be damping or reversing its diversification. Individual examples have been corroborated on these grounds, but their pervasiveness remains unclear (see refs. 19, 44, 53, and 54).
Conclusions
Simply surviving a mass extinction is no guarantee of success in the aftermath. The present analyses take a narrow view of the DCW pattern for operational purposes: only taxa lost in the stage immediately following one of the Big Five Mass extinctions were considered to fall into the DCW category. This simplified protocol may explain the lack of order-level DCWs after the earliest Triassic. If extinction rates of genera and families are lower in the postPaleozoic than in earlier times, then attritional loss of their constituent taxa could easily take longer than a single stratigraphic stage to eliminate a DCW clade. The most extinction-resistant taxa can persist at low diversity for protracted periods without succumbing to final extinction, particularly if they find refuges from physical or biotic perturbation (51); those surviving to the present day are often called living fossils.
The analyses presented here demonstrate elevated extinction rates among the survivors of mass extinctions in the postextinction interval. Although these extinction intensities are smaller than the mass extinctions, they are imposed on impoverished and/or undersampled biotas, and DCW effects are defined very narrowly for operational reasons. The results given must, therefore, be underestimates, and events in the “survival” or “recovery” intervals may well prove to be nearly as important in shaping the postextinction biota as the bottleneck imposed by the extinction event itself. Several alternatives must be considered as mechanisms for the marginalization or prolonged demise of once-prominent taxa, and decisive tests will almost always require additional data. Categorizing clades according to postextinction trajectories and testing hypotheses on the factors governing their fates will be an important step in evaluating the role of extinctions and recoveries in shaping the history of life.
Finally, such insights also may be useful to conservation biology, if the goal is to preserve or recover not only the functioning of ecosystems, but the evolutionary potential of clades (55, 56). The fossil record clearly shows that survival alone is not the key to evolutionary success, even with the amelioration of extinction mechanisms. Thus clade-based conservation strategies aimed at “picking winners” over evolutionary timescales (e.g., ref. 57) must be formulated cautiously and should incorporate or substitute factors ranging from phylogenetic distinctness (e.g., ref. 58) to multiclade biodiversity hotspots (e.g., refs. 59–61; see also ref. 62 on the difficulties of estimating area-specific persistence probabilities). With its multitude of natural experiments, the fossil record can provide insights into which clades and regions are most vulnerable to failure during the recovery phase.
Supplementary Material
Acknowledgments
I thank J. Day, M. L. Droser, D. H. Erwin, K. W. Flessa, M. Foote, S. M. Kidwell, C. G. Maples, C. Moritz, A. Nützel, W. A. Oliver, Jr., D. M. Raup, P. D. Taylor, J. W. Valentine, P. J. Wagner, and the late J. J. Sepkoski, Jr., for valuable comments, generous assistance and advice. This work was supported by National Science Foundation Grant EAR 9903030 and by the John Simon Guggenheim Memorial Foundation.
Abbreviations
- DCW
Dead Clade Walking
- K-T
end-Cretaceous
References
- 1.Prejean H. Dead Man Walking. New York: Random House; 1994. [Google Scholar]
- 2.Jablonski D. In: Patterns and Processes in the History of Life. Raup D M, Jablonski D, editors. Berlin: Springer; 1986. pp. 313–329. [Google Scholar]
- 3.Raup D M. Proc Natl Acad Sci USA. 1994;91:6758–6763. doi: 10.1073/pnas.91.15.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jablonski D. Paleobiology. 2000;26, Suppl. to No. 4:15–52. [Google Scholar]
- 5.Raup D M, Sepkoski J J., Jr Science. 1982;215:1501–1503. doi: 10.1126/science.215.4539.1501. [DOI] [PubMed] [Google Scholar]
- 6.Gilinsky N L. Paleobiology. 1994;20:445–458. [Google Scholar]
- 7.Sepkoski J J., Jr . In: Global Events and Event Stratigraphy in the Phanerozoic. Walliser O H, editor. Berlin: Springer; 1996. pp. 35–51. [Google Scholar]
- 8.Hallam A, Wignall P B. Mass Extinctions and Their Aftermath. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 9.Stanley S M, Yang X. Science. 1994;266:1340–1344. doi: 10.1126/science.266.5189.1340. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y G, Wang Y, Wang W, Shang Q H, Cao C Q, Erwin D H. Science. 2000;289:432–436. doi: 10.1126/science.289.5478.432. [DOI] [PubMed] [Google Scholar]
- 11.Erwin D H, Valentine J W, Sepkoski J J., Jr Evolution (Lawrence, Kans) 1987;41:1177–1186. [PubMed] [Google Scholar]
- 12.Jablonski D, Bottjer D J. Science. 1991;252:1831–1833. doi: 10.1126/science.252.5014.1831. [DOI] [PubMed] [Google Scholar]
- 13.Benton M J, editor. The Fossil Record 2. London: Chapman & Hall; 1993. [Google Scholar]
- 14.Zhuravlev A Y, Wood R. Geology. 1996;24:311–314. [Google Scholar]
- 15.Sepkoski J J., Jr Contrib Zool. 2000;69:213–222. [Google Scholar]
- 16.Foote M. Paleobiology. 2000;26, Suppl. to No. 4:74–102. [Google Scholar]
- 17.Raup D M. Paleobiology. 1991;17:37–48. doi: 10.1017/s0094837300010332. [DOI] [PubMed] [Google Scholar]
- 18.Raup D M. Acta Geol Hispanica. 1981;16:25–33. [Google Scholar]
- 19.Miller A I. Science. 1998;281:1157–1160. doi: 10.1126/science.281.5380.1157. [DOI] [PubMed] [Google Scholar]
- 20.Jablonski D. Science. 1998;279:1327–1330. doi: 10.1126/science.279.5355.1327. [DOI] [PubMed] [Google Scholar]
- 21.Baarli B G, Harper D A T. Norges Geologisk Tidsskrift. 1986;66:87–98. [Google Scholar]
- 22.Williamson M. Ecography. 1999;22:5–12. [Google Scholar]
- 23.Erwin D H, Droser M L. Palaios. 1993;8:623–624. [Google Scholar]
- 24.Lethiers F, Casier J G. Geobios. 1999;32:727–731. [Google Scholar]
- 25.MacLeod K G, Huber B T. Geology. 1996;24:463–466. [Google Scholar]
- 26.Pospichal J J. Geology. 1994;22:99–102. [Google Scholar]
- 27.Sepkoski J J, Jr, Kendrick D C. Paleobiology. 1993;19:168–184. doi: 10.1017/s0094837300015852. [DOI] [PubMed] [Google Scholar]
- 28.Wagner P J. Paleobiology. 1995;21:410–439. [Google Scholar]
- 29.Wagner P J. Paleobiology. 2000;26, Suppl. to No. 4:341–371. [Google Scholar]
- 30.Foote M. Evolution (Lawrence, Kans) 1996;50:1–11. doi: 10.1111/j.1558-5646.1996.tb04467.x. [DOI] [PubMed] [Google Scholar]
- 31.Adrain J M, Westrop S R. Science. 2000;289:110–112. doi: 10.1126/science.289.5476.110. [DOI] [PubMed] [Google Scholar]
- 32.Robeck H E, Maley C C, Donoghue M J. Paleobiology. 2000;26:171–187. [Google Scholar]
- 33.Ausich W I, Kammer T W. J Paleontol. 2001;75:1161–1173. [Google Scholar]
- 34.Fortey R A. J Paleontol. 2001;75:1141–1151. [Google Scholar]
- 35.Carlson S J. J Paleontol. 2001;75:1109–1118. [Google Scholar]
- 36.Smith A B. Philos Trans R Soc London B. 2001;356:351–367. doi: 10.1098/rstb.2000.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raup D M. Paleobiology. 1985;11:42–52. [Google Scholar]
- 38.McKinney M L. Annu Rev Ecol Syst. 1997;28:495–516. [Google Scholar]
- 39.Newell N D, Boyd D W. Am Mus Novit. 1999;3263:1–5. [Google Scholar]
- 40.Squires R L, Goedert J L. Veliger. 1994;37:253–266. [Google Scholar]
- 41.Erwin D H. Trends Ecol Evol. 1998;13:344–349. doi: 10.1016/s0169-5347(98)01436-0. [DOI] [PubMed] [Google Scholar]
- 42.Erwin D H. Proc Natl Acad Sci USA. 2001;98:5399–5403. doi: 10.1073/pnas.091092698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith A B, Gale A S, Monks N E A. Paleobiology. 2001;27:241–253. [Google Scholar]
- 44.Roy K. Paleobiology. 1994;20:274–296. [Google Scholar]
- 45.Rowley D B. Earth Planet Sci Lett. 1996;145:1–13. [Google Scholar]
- 46.Najman Y, Garzanti E. Geol Soc Am Bull. 2000;112:435–449. [Google Scholar]
- 47.Smith A B, Jeffrey C H. In: Biotic Response to Global Change: The Last 145 Million Years. Culver S J, Rawson P F, editors. Cambridge: Cambridge Univ. Press; 2000. pp. 181–194. [Google Scholar]
- 48.Looy C V, Twitchett R J, Dilcher D L, Van Konijnenburg-Van Cittert J H A, Visscher H. Proc Natl Acad Sci USA. 2001;98:7879–7883. doi: 10.1073/pnas.131218098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Page K N. In: Ammonoid Paleobiology. Landman N H, Tanabe K, Davis R A, editors. New York: Plenum; 1996. pp. 755–794. [Google Scholar]
- 50.Warren A, Rich T H, Vickers-Rich P. Palaeontographica. 1997;A247:1–24. [Google Scholar]
- 51.Vermeij G J. Escalation in Evolution. Princeton: Princeton Univ. Press; 1987. [Google Scholar]
- 52.Sepkoski J J., Jr . In: Evolutionary Paleobiology. Jablonski D, Erwin D H, Lipps J H, editors. Chicago: Univ. of Chicago Press; 1996. pp. 211–255. [Google Scholar]
- 53.Roy K. Paleobiology. 1996;22:436–452. [Google Scholar]
- 54.Sepkoski J J, Jr, McKinney F K, Lidgard S. Paleobiology. 2000;26:7–18. doi: 10.1666/0094-8373(2000)026<0007:cdappc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Myers N, Knoll A H. Proc Natl Acad Sci USA. 2001;98:5389–5392. doi: 10.1073/pnas.091092498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cowling R M, Pressey R L. Proc Natl Acad Sci USA. 2001;98:5452–5457. doi: 10.1073/pnas.101093498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erwin T L. Science. 1991;253:750–752. doi: 10.1126/science.253.5021.750. [DOI] [PubMed] [Google Scholar]
- 58.Crozier R H. Annu Rev Ecol Syst. 1997;28:243–268. [Google Scholar]
- 59.Roberts C M, McClean C J, Veron J E N, Hawkins J P, Allen G R, McAllister D E, Mittermeier C G, Schueler F W, Spalding M, Wells F, et al. Science. 2002;295:1280–1284. doi: 10.1126/science.1067728. [DOI] [PubMed] [Google Scholar]
- 60.Moritz C, Richardson K S, Ferrier S, Monteith G B, Stanisic J, Williams S E, Whiffin T. Proc R Soc London Ser B. 2001;268:1875–1881. doi: 10.1098/rspb.2001.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myers N, Mittermeier R A, Mittermeier C G, da Fonseca G A B, Kent J. Nature (London) 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 62.Araújo M B, Williams P H. Biol Cons. 2000;96:331–345. [Google Scholar]
- 63.Raup D M. In: Analytical Paleobiology. Gilinsky N L, Signor P W, editors. Lawrence, KS: Paleontological Soc.; 1991. pp. 207–216. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.