Abstract
Incidence of early-onset (< 40 years) type 2 diabetes (T2D) is increasing. While multiple risk factors have been identified, particularly obesity and low socioeconomic status, early-life factors are hypothesised to play a role via fetal programming. We investigated sociodemographic and early-life factors in relation to early-onset T2D using a family-based design that accounts for shared genetic and environmental factors. We included 1,814,062 individuals born in Sweden 1983 to 2002 with follow-up data until 2020, and identified early-onset (age 19–39) T2D cases (n = 3505) through National Diabetes, Patient and Prescribed Drug Registers. Perinatal and sociodemographic factors were retrieved from registers. We used a cohort and sibling design, with multivariable-adjusted Cox proportional hazards regression. Sociodemographic factors associated with early-onset T2D included low parental education, single parenthood, younger parental age and non-Swedish origin. The latter association did not remain after mutual adjustment. Regarding perinatal factors, a higher incidence was noted in relation to lower birth weight (hazard ratio 2.38 [95% confidence interval: 1.98–2.87] and 1.43[1.33–1.54] for < 2500 g and 2500-3500 g, respectively, vs 3500-4500 g), small-for-gestational-age (SGA) (2.24[1.96–2.56]), large-for-gestational-age (LGA) (1.19[1.01–1.39]), and maternal obesity (2.34[2.04–2.69]), diabetes (1.59[1.36–1.85]), smoking (1.59[1.48–1.71]), and infection (1.21[1.03–1.41]) during pregnancy. In the sibling analysis, only low birth weight and SGA remained associated with early-onset T2D. Early-onset T2D is associated with sociodemographic and multiple perinatal factors; only growth restriction likely reflects fetal programming, while other perinatal-related associations might involve confounders. This study highlights the need for early-life, targeted strategies to prevent T2D and reduce health inequities.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10654-025-01261-6.
Keywords: Early-onset type 2 diabetes, Perinatal factors, Birth weight, Preterm birth, Fetal programming, Maternal diabetes
Background
Type 2 diabetes (T2D) has been consistently associated with early-life conditions, particularly low birth weight (LBW, considered an indicator of adverse uterine conditions interacting with fetal growth), preterm birth, and maternal obesity and diabetes during pregnancy [1, 2]. Albeit also criticized, the Barker hypothesis—also referred to as the fetal programming hypothesis—posits that adverse exposures during early life, such as maternal metabolic disorders, trigger fetal adaptive mechanisms that can lead to adverse metabolic outcomes later in life [3]. This hypothesis is supported by the consistent associations observed between perinatal factors and metabolic diseases, including T2D, across different cohorts worldwide, as well as experimental evidence from animal models [1, 2]. However, the proposed pathophysiological mechanisms, including epigenetic modifications, alterations in body composition, and pancreatic beta-cell dysfunction, are diverse and not yet fully elucidated [1].
Although primarily affecting middle-aged or elderly adults, T2D is increasing among young people (< 40 years) [4]. This group, termed “early-onset T2D”, has seen a global increase in prevalence, rising from 2.9% to 3.8% between 2013 and 2021 in the adults aged 20–39 years [5]. This is concerning considering that T2D seems to be a more aggressive disease when diagnosed early, carrying higher risks of complications, comorbidities, and death compared to T2D diagnosed at later ages [5, 6]. Like T2D at higher ages, early-onset T2D is closely linked to obesity, family history of diabetes, low socioeconomic status and certain ethnic backgrounds [7]. It is however conceivable that the pathways to obesity and subsequent T2D are different in early-onset T2D, primarily involving exposures accumulated in early life rather than in adulthood. Fetal programming may thus play an even more significant role for the development of early-onset T2D.
Studies on youth-onset (< 19 years) T2D provide support for the hypothesis of a strong influence of fetal programming, noting high risks associated with intrauterine exposure to maternal diabetes and obesity [8–10]. As an example, the SEARCH study estimated odds ratios of youth-onset T2D at 5.7 for gestational diabetes and 2.8 for maternal obesity [8]. Regarding T2D in young adults, a limited number of studies indicate increased risks related to being first born, LBW, being small-for-gestational-age (SGA) and preterm birth [11–13]. However, a comprehensive analysis of the risk of early-onset T2D in relation to a wide range of early-life factors has not yet been conducted.
Furthermore, it is challenging to separate the effects of fetal programming from those of genetic or environmental factors linked to maternal conditions and the long-term risk of diabetes in the offspring. Family-based designs can alleviate such confounding, e.g. by comparing siblings with different perinatal exposures. Thereby, the influence of fetal exposures on diabetes risk can be assessed while partly accounting for genetic susceptibility and lifestyle factors shared between siblings like infant feeding, childhood diet, physical activity, and family sociodemographic characteristics [14].
This study aimed to investigate the incidence of early-onset T2D in relation to sociodemographic and perinatal factors – including maternal obesity, diabetes, smoking or infection during pregnancy, pre-eclampsia, birth order, gestational age, birth weight, size for gestational age and mode of delivery – in the entire Swedish population born since 1983, using information from a wide range of high-quality registers. To reduce confounding, we complemented a traditional cohort design with a family-based design, comparing the incidence of early-onset T2D among siblings born to the same mother with different perinatal exposures[15]. This quasi-experimental design is made possible by the world’s largest national multigenerational register [16, 17].
Methods
Study population
We used personal identification numbers (PIN) to connect data from several Swedish registers, including the National Medical Birth Register (MBR), the Swedish Multi-Generation Register (MGR), the National Diabetes Register (NDR), the National Patient Register (NPR), the National Prescribed Drug Register (NPDR), the Health Insurance and Labour Market Studies register (LISA), as well as the Swedish emigration and death registers [16, 18–22].
MBR, which has been created in 1973, compiles standardized data from perinatal exams for all births in Sweden, including the international classification of diseases (ICD) codes for certain maternal diagnoses [18]. Our study included all individuals born in Sweden and recorded in the MBR from 1983 to 2002 (1,960,785 live births). These years were selected because some early-life exposures were not documented in the MBR before 1983 (specifically maternal body mass index [BMI], maternal smoking, and family situation at childbirth), and because we set the starting age of follow-up to be 18 years (data availability for identification of diabetes cases ends in 2020). This implies that we could follow people to a maximum of age 37 (people born 1983). Thus, we focused on early-onset T2D (≤ 37 years) and excluded youth-onset cases (< 18 years) to minimize the risk of including T1D cases since previous studies indicated very low rates of youth-onset T2D in Sweden [13, 23].
We excluded pregnancies with multiple births (50,066, 2.6%) and participants without linkages to the MGR or with system-identified duplicated or reused PINs (160, < 1%). Additionally, participants whose mothers were not identified in the MGR or had a duplicated or reused PIN were excluded (1518, < 1%). Participants that could not be linked to their fathers were retained in the study, considering paternal information missing.
The exclusion of participants who emigrated from Sweden or died before reaching 18 years of age (81,136, 4%) and those diagnosed with any type of diabetes before age 18 or without a reliable diagnosis date (13,843, < 1%) resulted in a final cohort of 1,814,062 individuals. For the sibling cohort, we included only sibling groups (born from the same mother) with at least one sibling affected by T2D (n = 6350). The population selection is summarized in Fig. 1. The study was approved by the Swedish Ethics Review Board (registration number: Dnr 2021–02881).
Fig. 1.
Population selection
Information on diabetes
We identified diabetes cases using available data from the NDR and the NPR until 2020. Additionally, we used the NPDR to enhance precision regarding diabetes type and onset date. The NDR, established in 1996, encompasses both primary and secondary diabetes care in Sweden, and was estimated to account for 87% of all individuals living with diabetes in the country in 2019 [24]. The NPR includes inpatient (hospital discharge) and outpatient specialist care data since 1964 and 2001, respectively [20].The NPDR, starting from 2005, records all prescribed medications dispensed in Sweden, categorized according to the Anatomical Therapeutic Chemical (ATC) classification [21].
We defined diabetes cases (n = 21,898) as participants with at least one record in the NDR or at least one visit documented in the NPR with a primary or secondary diagnosis code of “250” (ICD-8/ICD-9 code) or “E10-E14” (ICD-10 codes).
Cases were classified as T2D (n = 4036) if they had no record of another type of diabetes in NDR or NPR (primary diagnosis). To preserve sensitivity, missingness on diabetes type in NDR or E14 coding (“unspecified diabetes mellitus”) in NPR were not considered disqualifying conditions for T2D as these situations can occur when diabetes type is unspecified at early stage after diagnosis and additional diagnostic information is needed, especially in young adults. To ensure specificity, subjects with other types of diabetes formally recorded in NDR or NPR (i.e., E10, E12, E13) and individuals exclusively treated with insulin, for the whole follow-up period, according to data available from NPDR, were censored at the age of diagnosis (n = 17,862). Age of diagnosis was based on the first record in any of the registers.
Familial and sociodemographic factors
We determined parental age (< 20 years, ≥ 20 & < 25 years, ≥ 25& < 30 years and ≥ 30 year) and family situation (two-parent, single-parent, or other situation) at birth using data from the MBR, and the highest parental educational level at birth (categorized as “Primary” for up to compulsory education [≤ 16 years old], “Secondary” for upper secondary education [16–19 years old], and “University” for college/university and further education) from the LISA register [22]. Additionally, the parental countries of birth were identified via the total population register and parents were categorized as Sweden or non-Sweden born, distinctly for the mother and the father. Finally, we identified parental diabetes cases (regardless of type) using the same registers as for study participants. For maternal history of diabetes, we additionally accounted for the information available in the MBR regarding pregestational and gestational diabetes diagnoses using ICD codes (ICD-8: 250; ICD-9: 250, 648A, 648W; ICD-10: O240-44) and self-report. Then, we combined all the information to identify the lifetime maternal and paternal history of diabetes. To examine maternal diabetes as a perinatal risk factor, we separately assess the T2D incidence in relation to maternal diabetes with a diagnosis before the participant’s birth (thus accounting for pregestational and gestational diabetes) from cases with a diagnosis after the participant’s birth.
Pre- and perinatal factors
Using data from the MBR, we examined maternal BMI (< 18.5 kg/m2[underweight], 18.5–25 kg/m2[normal], 25–30 kg/m2 [overweight], and ≥ 30 kg/m2 [obesity]), and maternal smoking (none, 1 to 9 cigarettes per day, and ≥ 10 cigarettes per day) at the time of enrollment in maternal health care (first trimester). Additional factors studied included the mode of delivery (C-section or vaginal), gestational age (categorized as extremely to very preterm [≥ 22 to < 32 weeks], moderate to late preterm [≥ 32 to < 37 weeks], early term [≥ 37 to < 39 weeks], full term [≥ 39 weeks]), size for gestational age (small [SGA] or large [LGA] for gestational age, defined as birth weight below -2 or above + 2 standard deviations for the sex-specific distribution of birth weight in people born at the same gestational age), birth weight (< 2500 g, ≥ 2500 g and < 3500 g, ≥ 3500 g and < 4500 g, ≥ 4500 g), and birth order [25, 26].
Additionally, we identified pre-eclampsia using ICD codes (ICD-8: 637; ICD-9: 642E-G; ICD-10: O14-O15) from the MBR, and maternal infections during pregnancy using infection-related ICD codes from the NPR, as detailed in supplementary Table S1.
Statistical analyses
We described the distribution of exposures and covariates using frequencies and proportions for categorical variables, and means and standard deviations (SD) or median and interquartile range (IQR) for continuous variables. We used Cox proportional hazards (PH) regression models to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of early-onset T2D in relation to early-life exposures. The follow-up period was calculated from the participants’ 18th birthday to the occurrence of diabetes, death, emigration, or April 21, 2020. The models used age as the time scale.
Missing values for categorical variables were treated as a separate category. For continuous covariates, the median value of the distribution was imputed for individuals with missing values, with an additional variable to indicate if the value was imputed or not. Overall, the percentage of missing values was low (ranging from 0 to 6%) except for maternal BMI (29%), which is due to the gradual introduction of the variable in the 1980s, as well as a complete lack of data for two consecutive years caused by an error in the data collection system rather than selective reporting of the mothers [18].
In the full cohort analysis, Cox models were conducted with a cluster-robust standard error to account for familial clustering among siblings born to the same mother. Each association between exposure and early-onset T2D was separately adjusted for sex and year of birth (Model 1). Then, all perinatal and familial factors were mutually adjusted for (Model 2) and finally (Model 3) we added adjustment for parental lifetime history of diabetes.
In the sibling analysis, we performed the Cox model with stratification on sibling groups for suitable exposures (sex, maternal BMI, diabetes, smoking and infection during pregnancy, pre-eclampsia, gestational age, birth weight, birth size, and mode of delivery), excluding exposures that were quasi-systematically concordant (family socio-educational background and parental lifetime history of diabetes) or systematically discordant (year of birth, birth order and parental age at childbirth) within siblings. Each association between exposure and early-onset T2D was separately adjusted for sex (Model 1). Then, all perinatal factors were mutually adjusted for (Model 2). Methods and interpretation of the sibling analysis are further detailed in the supplementary material.
Finally, we tested for an interaction between sex and perinatal exposures associated with early-onset T2D in the full and sibling cohort analyses, as sex-specific susceptibility to the intrauterine environment has previously been described, and sex differences have been observed in attributable risk factors for early-onset T2D [4]. To do so, we compared models with and without an interaction term, using the Likelihood ratio test.
We verified the PH assumption by plotting Schoenfeld residuals and performed stratified analysis if a violation was observed. All analyses were conducted using R, version 4.3.1.
Results
Incidence of early-onset T2D
In the full cohort, we identified 3,505 cases of early-onset T2D over 17,449,085 person-years of follow-up, resulting in an incidence rate of 20.1 per 100 000 person-years, 20.8 in men and 19.3 in women. The median (IQR) duration of follow-up was 9.8 (8.8) years and the median age at diagnosis of T2D was 27.0 (7.4) years. Overall, 89% of the participants were born from a two-parents family, and 39% had at least one parent with a college or university-level of education. Eighty-one percent of the participants had both parents born in Sweden, 11% of fathers had diabetes vs 6% of mothers. Preterm birth accounted for 5% of the cohort and mean (SD) birth weight was 3,542 (553) g. The sibling cohort involved 64,040 person-years and 2,472 cases of early-onset T2D. The characteristics of the participants in the full and sibling cohorts, according to early-onset T2D status, are detailed in Table 1.
Table 1.
Characteristics of participants by early-onset type 2 diabetes (T2D) status in full and sibling cohorts
| Full cohort | Sibling cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| No T2D | T2D | No T2D | T2D | |||||
| n | 1 810 557 | 3 505 | 3878 | 2472 | ||||
| Men n (%) | 929,921 | (51.4) | 1872 | (53.4) | 2016 | (52.0) | 1298 | (52.5) |
| Year of birth n (%) | ||||||||
| 1983 to 1987 | 449,962 | (24.9) | 1832 | (52.3) | 1141 | (29.4) | 1156 | (46.8) |
| 1988 to 1992 | 545,854 | (30.1) | 1176 | (33.6) | 1508 | (38.9) | 940 | (38.0) |
| 1993 to 1997 | 466,730 | (25.8) | 428 | (12.2) | 849 | (21.9) | 333 | (13.5) |
| 1998 to 2002 | 348,011 | (19.2) | 69 | (2.0) | 380 | (9.8) | 43 | (1.7) |
| Family situation n (%) | ||||||||
| Two-parent family | 1,607,780 | (88.8) | 2977 | (84.9) | 3363 | (86.7) | 2107 | (85.2) |
| Single mother or another situation | 89,280 | (4.9) | 307 | (8.8) | 274 | (7.1) | 202 | (8.2) |
| Missing | 113,497 | (6.3) | 221 | (6.3) | 241 | (6.2) | 163 | (6.6) |
| Highest parental degree at the child’s birth n (%) | ||||||||
| ≤ Compulsory education | 134,200 | (7.4) | 571 | (16.3) | 618 | (15.9) | 369 | (14.9) |
| Upper secondary | 971,159 | (53.6) | 2258 | (64.4) | 2567 | (66.2) | 1641 | (66.4) |
| College/university | 704,308 | (38.9) | 675 | (19.3) | 692 | (17.8) | 462 | (18.7) |
| Missing | 890 | (0.0) | 1 | (0.0) | 1 | (0.0) | 0 | (0.0) |
| Parental country of birth n (%) | ||||||||
| Both parents born in Sweden | 1,467,386 | (81.0) | 2723 | (77.7) | 3060 | (78.9) | 1962 | (79.4) |
| Sweden-born mother only | 100,903 | (5.6) | 235 | (6.7) | 245 | (6.3) | 157 | (6.4) |
| Sweden-born father only | 86,267 | (4.8) | 180 | (5.1) | 175 | (4.5) | 114 | (4.6) |
| Both parents born outside Sweden | 146,496 | (8.1) | 331 | (9.4) | 370 | (9.5) | 218 | (8.8) |
| Missing | 9505 | (0.5) | 36 | (1.0) | 28 | (0.7) | 21 | (0.8) |
| Lifetime history of diabetes in the mother n (%) | ||||||||
| History of diabetes | 110,984 | (6.1) | 1089 | (31.1) | 1032 | (26.6) | 705 | (28.5) |
| Lifetime history of diabetes in the father n (%) | ||||||||
| History of diabetes | 188,909 | (10.4) | 1232 | (35.1) | 1268 | (32.7) | 882 | (35.7) |
| Missing data | 9505 | (0.5) | 36 | (1.0) | 28 | (0.7) | 21 | (0.8) |
| Age of the mother n (%) | ||||||||
| < 20 years | 45,128 | (2.5) | 199 | (5.7) | 130 | (3.4) | 158 | (6.4) |
| ≥ 20 to < 25 years | 366,061 | (20.2) | 1007 | (28.7) | 913 | (23.5) | 821 | (33.2) |
| ≥ 25 to < 30 years | 663,730 | (36.7) | 1180 | (33.7) | 1376 | (35.5) | 896 | (36.2) |
| ≥ 30 years | 735,638 | (40.6) | 1119 | (31.9) | 1459 | (37.6) | 597 | (24.2) |
| Age of the mother (mean (SD)) | 29.00 | (5.08) | 27.74 | (5.42) | 28.55 | (5.22) | 26.74 | (4.89) |
| Age of the father n (%) | ||||||||
| < 20 years | 11,217 | (0.6) | 54 | (1.5) | 26 | (0.7) | 35 | (1.4) |
| ≥ 20 to < 25 years | 183,255 | (10.1) | 535 | (15.3) | 496 | (12.8) | 436 | (17.6) |
| ≥ 25 to < 30 years | 542,994 | (30.0) | 1087 | (31.0) | 1072 | (27.6) | 853 | (34.5) |
| ≥ 30 years | 1,063,586 | (58.7) | 1793 | (51.2) | 2256 | (58.2) | 1127 | (45.6) |
| Missing | 9505 | (0.5) | 36 | (1.0) | 28 | (0.7) | 21 | (0.8) |
| Age of the father (mean (SD)) | 31.88 | (6.01) | 31.03 | (6.41) | 31.87 | (6.36) | 30.15 | (5.97) |
| Full cohort | Sibling cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| No type 2 diabetes | Type 2 diabetes | No type 2 diabetes | Type 2 diabetes | |||||
| n | 1 810 557 | 3 505 | 3878 | 2472 | ||||
| BMI of the mother in early pregnancy n (%) | ||||||||
| < 18.5 kg/m2 | 66,109 | (3.7) | 107 | (3.1) | 107 | (2.8) | 85 | (3.4) |
| ≥ 18.5 to < 25 kg/m2 | 911,149 | (50.3) | 1402 | (40.0) | 1328 | (34.2) | 986 | (39.9) |
| ≥ 25 to < 30 kg/m2 | 235,361 | (13.0) | 557 | (15.9) | 715 | (18.4) | 413 | (16.7) |
| ≥ 30 kg/m2 | 74,748 | (4.1) | 276 | (7.9) | 437 | (11.3) | 180 | (7.3) |
| Missing | 523,190 | (28.9) | 1163 | (33.2) | 1291 | (33.3) | 808 | (32.7) |
| BMI of the mother in early pregnancy (mean (SD)) | 22.96 | (3.36) | 23.76 | (3.97) | 24.44 | (4.26) | 23.71 | (3.82) |
| Exposure to maternal diabetes during pregnancy n (%) | 22,876 | (1.3) | 211 | (6.0) | 190 | (4.9) | 119 | (4.8) |
| Smoking during pregnancy n (%) | ||||||||
| No smoking | 1,328,290 | (73.4) | 1813 | (51.7) | 2173 | (56.0) | 1328 | (53.7) |
| 1 to 9 cigarettes per day | 233,893 | (12.9) | 746 | (21.3) | 752 | (19.4) | 500 | (20.2) |
| 10 or more cigarettes per day | 138,043 | (7.6) | 690 | (19.7) | 713 | (18.4) | 465 | (18.8) |
| Missing | 110,331 | (6.1) | 256 | (7.3) | 240 | (6.2) | 179 | (7.2) |
| Infection during pregnancy n (%) | 61,399 | (3.4) | 163 | (4.7) | 167 | (4.3) | 102 | (4.1) |
| Pre-eclampsia or eclampsia n (%) | 50,573 | (2.8) | 163 | (4.7) | 117 | (3.0) | 102 | (4.1) |
| Birth order n (%) | ||||||||
| 1 | 748,863 | (41.4) | 1487 | (42.4) | 748 | (19.3) | 1077 | (43.6) |
| 2 | 651,182 | (36.0) | 1104 | (31.5) | 1321 | (34.1) | 818 | (33.1) |
| ≥ 3 | 410,512 | (22.7) | 914 | (26.1) | 1809 | (46.6) | 577 | (23.3) |
| Birth order (mean (SD)) | 1.92 | (1.03) | 1.97 | (1.11) | 2.62 | (1.33) | 1.92 | (1.08) |
| Gestational age n (%) | ||||||||
| Extremely to very preterm (≥ 22 to < 32 weeks) | 9806 | (0.5) | 51 | (1.5) | 23 | (0.6) | 33 | (1.3) |
| Moderate to late preterm (≥ 32 to < 37 weeks) | 79,866 | (4.4) | 255 | (7.3) | 218 | (5.6) | 162 | (6.6) |
| Early birth term (≥ 37 to < 39 weeks) | 333,863 | (18.4) | 728 | (20.8) | 815 | (21.0) | 511 | (20.7) |
| Full term (≥ 39 weeks) | 1,384,253 | (76.5) | 2457 | (70.1) | 2812 | (72.5) | 1759 | (71.2) |
| Missing | 2769 | (0.2) | 14 | (0.4) | 10 | (0.3) | 7 | (0.3) |
| Birth weight n (%) | ||||||||
| < 2500 g | 57,209 | (3.2) | 264 | (7.5) | 143 | (3.7) | 173 | (7.0) |
| ≥ 2500 g and < 3500 g | 760,840 | (42.0) | 1733 | (49.4) | 1648 | (42.5) | 1232 | (49.8) |
| ≥ 3500 g and < 4500 g | 920,690 | (50.9) | 1367 | (39.0) | 1843 | (47.5) | 966 | (39.1) |
| ≥ 4500 g | 65,657 | (3.6) | 124 | (3.5) | 237 | (6.1) | 91 | (3.7) |
| Missing | 6161 | (0.3) | 17 | (0.5) | 7 | (0.2) | 10 | (0.4) |
| Birth weight (g) (mean (SD)) | 3543 | (552) | 3369 | (654) | 3548 | (611) | 3376 | (644) |
| Size for gestational age n (%) | ||||||||
| Small for gestational age | 45,381 | (2.5) | 245 | (7.0) | 132 | (3.4) | 171 | (6.9) |
| Normal for gestational age | 1,694,157 | (93.6) | 3058 | (87.2) | 3481 | (89.8) | 2162 | (87.5) |
| Large for gestational age | 62,261 | (3.4) | 172 | (4.9) | 248 | (6.4) | 123 | (5.0) |
| Missing | 8758 | (0.5) | 30 | (0.9) | 17 | (0.4) | 16 | (0.6) |
| Mode of delivery n (%) | ||||||||
| Vaginal delivery | 1,603,729 | (88.6) | 3017 | (86.1) | 3467 | (89.4) | 2169 | (87.7) |
| C section | 206,828 | (11.4) | 488 | (13.9) | 411 | (10.6) | 303 | (12.3) |
| Censored during follow-up n (%) | ||||||||
| Other type of diabetes | 4550 | (0.3) | 33 | (0.9) | ||||
| Emigration during follow-up (> 18 years old) | 51,398 | (2.8) | 83 | (2.1) | ||||
| Death during follow-up (> 18 years old) | 66 | (0.0) | 0 | (0.0) | ||||
Sociodemographic factors, parental diabetes and incidence of early-onset T2D
The incidence of early-onset T2D was higher in men than in women (hazard ratio [HR] 1.12, 95% confidence interval [CI]: 1.05–1.20) and increased with year of birth, being 60% higher in those born in 1998–2002 compared with 1983–1987 (Table 2, model 3). Having a mother or a father or both parents born outside Sweden vs. two Sweden-born parents was associated with a higher risk (+ 35%, + 19% and + 58%, respectively) of early-onset T2D (Table 2, model 1). The incidence was also higher in those born to a single mother (1.83[1.62–2.06]), in those whose parents had low education level (3.54[3.16—3.96] for compulsory-only education vs college-to-university education) or were young at childbirth (2.29[1.97–2.67] for mother age < 20 vs ≥ 30, and 2.49[1.90–3.26] for father age < 20 vs ≥ 30) and in those with parental lifetime history of diabetes (5.61[5.21–6.04] when in the mother, 3.75[3.49–4.03] when in the father). These associations were attenuated but remained after being mutually adjusted (Table 2, model 3) except for the association with parental country of birth which was attenuated almost to the null after adjustment for parental educational level and diabetes.
Table 2.
Incidence of early-onset type 2 diabetes and hazard ratio (CI95) according to exposure variables in the full cohort
| Cases | Person-years | Incidence rate* | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Women | 1 633 | 8 457 221 | 19.31 | 1.00 | 1.00 | |
| Men | 1 872 | 8 991 864 | 20.82 | 1.12 (1.04—1.19) | 1.12 (1.05—1.20) | |
| Year of birth | ||||||
| 1983 to 1987 | 1 832 | 7 235 448 | 25.32 | 1.00 | 1.00 | |
| 1988 to 1992 | 1 176 | 6 250 068 | 18.82 | 1.05 (0.96—1.14) | 1.19 (1.09—1.30) | |
| 1993 to 1997 | 428 | 3 228 202 | 13.26 | 1.00 (0.88—1.13) | 1.30 (1.15—1.47) | |
| 1998 to 2002 | 69 | 735 366 | 9.38 | 1.10 (0.85—1.42) | 1.60 (1.23—2.07) | |
| Family situation | ||||||
| Two-parent family | 2 977 | 15 538 042 | 19.16 | 1.00 | 1.00 | 1.00 |
| Single mother or another situation | 307 | 875 154 | 35.08 | 1.83 (1.62—2.06) | 1.24 (1.10—1.41) | 1.21 (1.07—1.37) |
| Highest parental degree at the child’s birth | ||||||
| Up to compulsory education | 571 | 1 459 330 | 39.13 | 3.54 (3.16—3.96) | 2.14 (1.89—2.42) | 1.81 (1.59—2.05) |
| Upper secondary | 2 258 | 9 518 494 | 23.72 | 2.26 (2.07—2.46) | 1.71 (1.56—1.88) | 1.54 (1.41—1.69) |
| College/university | 675 | 6 464 983 | 10.44 | 1.00 | 1.00 | 1.00 |
| Parental country of birth | ||||||
| Both parents born in Sweden | 2 723 | 14 399 265 | 18.91 | 1.00 | 1.00 | 1.00 |
| Sweden-born mother only | 235 | 940 577 | 24.98 | 1.35 (1.18—1.55) | 1.19 (1.04—1.37) | 1.14 (1.00—1.31) |
| Sweden-born father only | 180 | 812 620 | 22.15 | 1.19 (1.02—1.38) | 1.17 (1.00—1.36) | 1.09 (0.94—1.27) |
| Both parents born outside Sweden | 331 | 1 206 038 | 27.45 | 1.58 (1.41—1.78) | 1.33 (1.18—1.50) | 0.99 (0.88—1.12) |
| Lifetime diabetes history in the mother | ||||||
| No diabetes | 2 416 | 16 213 281 | 14.90 | 1.00 | 1.00 | |
| History of diabetes | 1 089 | 1 235 779 | 88.12 | 5.61 (5.21—6.04) | 4.02 (3.68—4.38) | |
| Lifetime diabetes history in the father | ||||||
| No diabetes | 2 237 | 15 242 367 | 14.68 | 1.00 | 1.00 | |
| History of diabetes | 1 232 | 2 116 135 | 58.22 | 3.75 (3.49—4.03) | 3.16 (2.94—3.41) | |
| Age of the mother at the child’s birth | ||||||
| < 20 years | 199 | 482 875 | 41.21 | 2.29 (1.97—2.67) | 1.67 (1.38—2.03) | 1.84 (1.51—2.23) |
| ≥ 20 to < 25 years | 1 007 | 3 883 381 | 25.93 | 1.46 (1.34—1.59) | 1.32 (1.17—1.49) | 1.44 (1.28—1.63) |
| ≥ 25 to < 30 years | 1 180 | 6 468 308 | 18.24 | 1.06 (0.97—1.15) | 1.10 (1.00—1.20) | 1.18 (1.08—1.30) |
| ≥ 30 years | 1 119 | 6 614 521 | 16.92 | 1.00 | 1.00 | 1.00 |
| Age of the father at the child’s birth | ||||||
| < 20 years | 54 | 115 027 | 46.95 | 2.49 (1.90—3.26) | 1.22 (0.90—1.66) | 1.47 (1.09—1.99) |
| ≥ 20 to < 25 years | 535 | 1 958 707 | 27.31 | 1.45 (1.31—1.59) | 0.99 (0.88—1.13) | 1.15 (1.01—1.30) |
| ≥ 25 to < 30 years | 1 087 | 5 426 169 | 20.03 | 1.08 (1.00—1.17) | 0.97 (0.89—1.06) | 1.07 (0.98—1.17) |
| ≥ 30 years | 1 793 | 9 858 598 | 18.19 | 1.00 | 1.00 | 1.00 |
| Maternal BMI in early pregnancy | ||||||
| < 18.5 kg/m2 | 107 | 774 751 | 13.81 | 0.79 (0.65—0.96) | 0.65 (0.53—0.79) | 0.68 (0.56—0.83) |
| ≥ 18.5 and < 25 kg/m2 | 1 402 | 8 746 670 | 16.03 | 1.00 | 1.00 | 1.00 |
| ≥ 25 and < 30 kg/m2 | 557 | 1 813 525 | 30.71 | 2.16 (1.96—2.39) | 1.99 (1.80—2.20) | 1.54 (1.39—1.70) |
| ≥ 30 kg/m2 | 276 | 477 259 | 57.83 | 4.60 (4.04—5.24) | 3.67 (3.21—4.19) | 2.34 (2.04—2.69) |
| Maternal diabetes during pregnancy | ||||||
| No | 3 294 | 17 245 135 | 19.10 | 1.00 | 1.00 | 1.00 |
| Yes | 211 | 203 924 | 103.47 | 5.69 (4.95—6.56) | 4.79 (4.15—5.53) | 1.59 (1.36—1.85) |
| Maternal smoking during pregnancy | ||||||
| No smoking | 1 813 | 12 194 972 | 14.87 | 1.00 | 1.00 | 1.00 |
| Smoking | 1 436 | 4 153 779 | 34.57 | 2.18 (2.03—2.34) | 1.71 (1.58—1.84) | 1.59 (1.48—1.71) |
| 1 to 9 cigarettes per day | 746 | 2 570 517 | 29.02 | 1.84 (1.69—2.01) | 1.51 (1.38—1.65) | 1.43 (1.31—1.56) |
| 10 or more cigarettes per day | 690 | 1 583 261 | 43.58 | 2.73 (2.50—2.99) | 2.01 (1.83—2.21) | 1.83 (1.67—2.02) |
| Infection during pregnancy | ||||||
| No | 3 342 | 16 900 556 | 19.77 | 1.00 | 1.00 | 1.00 |
| Yes | 163 | 548 529 | 29.72 | 1.50 (1.28—1.75) | 1.24 (1.06—1.46) | 1.21 (1.03—1.41) |
| Pre-eclampsia | ||||||
| No | 3 342 | 16 961 609 | 19.70 | 1.00 | 1.00 | 1.00 |
| Yes | 163 | 487 477 | 33.44 | 1.65 (1.41—1.93) | 1.26 (1.07—1.49) | 1.14 (0.96—1.34) |
| Birth order | ||||||
| 1 | 1 487 | 7 162 640 | 20.76 | 1.00 | 1.00 | 1.00 |
| 2 | 1 104 | 6 241 830 | 17.69 | 0.85 (0.79—0.92) | 0.97 (0.89—1.06) | 0.96 (0.88—1.04) |
| ≥ 3 | 914 | 4 044 616 | 22.60 | 1.08 (1.00—1.18) | 1.13 (1.02—1.25) | 1.04 (0.94—1.15) |
| Gestational age | ||||||
| Non-full-term birth (< 39 weeks) | 1 034 | 4 155 016 | 24.89 | 1.32 (1.23—1.42) | 0.96 (0.88—1.05) | 0.94 (0.86—1.02) |
| Extremely to very preterm (≥ 22 to < 32 weeks) | 51 | 91 662 | 55.64 | 3.03 (2.30—4.00) | 1.17 (0.84—1.63) | 1.15 (0.83—1.60) |
| Moderate to late preterm (≥ 32 to < 37 weeks) | 255 | 790 902 | 32.24 | 1.70 (1.50—1.94) | 0.95 (0.81—1.13) | 0.93 (0.79—1.09) |
| Early birth term (≥ 37 to < 39 weeks) | 728 | 3 272 452 | 22.25 | 1.18 (1.09—1.28) | 0.96 (0.88—1.05) | 0.94 (0.86—1.02) |
| Full term (≥ 39 weeks) | 2 457 | 13 264 589 | 18.52 | 1.00 | 1.00 | 1.00 |
| Birth weight | ||||||
| < 2500 g | 264 | 567 773 | 46.50 | 2.95 (2.58—3.37) | 2.40 (1.99—2.90) | 2.38 (1.98—2.87) |
| ≥ 2500 g and < 3500 g | 1 733 | 7 484 730 | 23.15 | 1.48 (1.37—1.59) | 1.42 (1.32—1.53) | 1.43 (1.33—1.54) |
| ≥ 3500 g and < 4500 g | 1 367 | 8 742 745 | 15.64 | 1.00 | 1.00 | 1.00 |
| ≥ 4500 g | 124 | 589 109 | 21.05 | 1.36 (1.13—1.63) | 1.23 (1.02—1.47) | 1.13 (0.94—1.36) |
| Size for gestational age** | ||||||
| Small for gestational age | 245 | 461 832 | 53.05 | 2.78 (2.44—3.17) | 2.31 (2.02—2.64) | 2.24 (1.96—2.56) |
| Normal for gestational age | 3 058 | 16 321 465 | 18.74 | 1.00 | 1.00 | 1.00 |
| Large for gestational age | 172 | 572 920 | 30.02 | 1.62 (1.39—1.90) | 1.32 (1.13—1.55) | 1.19 (1.01—1.39) |
| Mode of delivery | ||||||
| Vaginal delivery | 3 017 | 15 555 373 | 19.40 | 1.00 | 1.00 | 1.00 |
| C section | 488 | 1 893 712 | 25.77 | 1.33 (1.21—1.47) | 1.02 (0.92—1.13) | 0.99 (0.89—1.09) |
*For 100 000 person years
Model 1: each association with early-onset T2D is separately adjusted for sex and year of birth
Model 2: mutual adjustment for sex, year of birth, parental country of birth, parental highest educational level, family situation, parental age at delivery, maternal BMI, diabetes, smoking and infection during pregnancy, pre-eclampsia, birth order, gestational age, birth weight, and mode of delivery
Model 3: Model 2 + adjustment for parental lifetime history of diabetes
**In Model 2 and 3, size for gestational age was adjusted for the same variables except gestational age and birth weight
We detected violations to the PH assumption for some exposures (sex, birth year and parental history of diabetes) and consequently stratified the analyses by attained age (Supplemental Table S2). These analyses indicate a reverse association between sex and early-onset T2D, as the incidence was higher in women compared to men (1.29[1.15–1.44]) before 25 years, and higher in men compared to women (1.40[1.28–1.52]) after 25 years. Additionally, the risk associated with year of birth and parental history of diabetes was higher before 25 years.
Perinatal factors and incidence of early-onset T2D
In the full cohort, we found a dose–response association between gestational age and early-onset T2D, with the highest HR (3.03[2.30–4.00]) for extremely to very preterm birth (< 32 weeks vs ≥ 39 weeks) (Table 2, model 1). We found a U-shaped association between birth weight and early-onset T2D with the lowest incidence observed in participants born with birth weight between 3500 and 4500 g. In comparison, participants born < 2500 g and 2500 to 3500 g had a higher risk of early-onset T2D (2.95[2.58–3.37] and 1.48[1.37–1.59], respectively), as well as those born ≥ 4500 g (1.36[1.13–1.63]). The association with size for gestational age showed the same pattern, with a higher risk for small (2.78 [2.44–3.17]) and large (1.62 [1.39–1.90]) compared to normal for gestational age. After mutual adjustment (Table 2, model 2 and 3), the higher risk observed for LBW and SGA was slightly attenuated, while the other associations were markedly attenuated. These results were consistent with those in the sibling cohort (Table 3), where we observed a higher risk of early-onset T2D only for LBW (2.48[1.65–3.72] and 1.35[1.15–1.58] for < 2500 g and 2500 to 3500 g, respectively, vs 3500 g to 4500 g) and SGA (2.05[1.47–2.86]).
Table 3.
Incidence of early-onset type 2 diabetes and hazard ratios (CI95) according to exposure variables in the sibling cohort
| Cases | Person-years | Incidence rate* | Model 1 | Model 2 | |
|---|---|---|---|---|---|
| Sex | |||||
| Women | 1 174 | 29 757 | 39.45 | 1.00 | |
| Men | 1 298 | 34 283 | 37.86 | 0.90 (0.80—1.02) | |
| BMI of the mother in early pregnancy | |||||
| < 18.5 kg/m2 | 85 | 2 224 | 38.22 | 1.08 (0.72–1.63) | 1.04 (0.69–1.57) |
| ≥ 18.5 and < 25 kg/m2 | 986 | 25 355 | 38.89 | 1.00 | 1.00 |
| ≥ 25 and < 30 kg/m2 | 413 | 10 599 | 38.97 | 0.94 (0.76–1.16) | 0.94 (0.76–1.17) |
| ≥ 30 kg/m2 | 180 | 4 553 | 39.53 | 0.87 (0.63–1.18) | 0.92 (0.67–1.27) |
| Exposure to maternal diabetes during pregnancy | |||||
| No | 2 353 | 61 465 | 38.28 | 1.00 | 1.00 |
| Yes | 119 | 2 575 | 46.21 | 1.18 (0.76–1.85) | 1.25 (0.79–1.96) |
| Smoking during pregnancy | |||||
| No smoking | 1 328 | 34 513 | 38.48 | 1.00 | 1.00 |
| Smoking | 965 | 25 198 | 38.30 | 1.08 (0.84–1.39) | 1.02 (0.79–1.32) |
| 1 to 9 cigarettes per day | 500 | 13 202 | 37.87 | 1.04 (0.80–1.36) | 0.99 (0.75–1.29) |
| 10 or more cigarettes per day | 465 | 11 996 | 38.76 | 1.15 (0.86–1.53) | 1.08 (0.80–1.45) |
| Infection during pregnancy | |||||
| No | 2 370 | 61 417 | 38.59 | 1.00 | 1.00 |
| Yes | 102 | 2 623 | 38.89 | 0.98 (0.72–1.34) | 0.94 (0.68–1.29) |
| Pre-eclampsia | |||||
| No | 2 370 | 61 800 | 38.35 | 1.00 | 1.00 |
| Yes | 102 | 2 240 | 45.53 | 1.18 (0.80–1.73) | 1.08 (0.73–1.61) |
| Gestational age | |||||
| Non-full-term birth (< 39 weeks) | 706 | 17 581 | 40.16 | 1.14 (0.97–1.33) | 0.97 (0.82–1.15) |
| Extremely to very preterm (≥ 22 to < 32 weeks) | 33 | 503 | 65.60 | 4.30 (1.68–10.96) | 2.25 (0.83–6.07) |
| Moderate to late preterm (≥ 32 to < 37 weeks) | 162 | 3 690 | 43.90 | 1.21 (0.90–1.63) | 0.87 (0.63–1.22) |
| Early birth term (≥ 37 to < 39 weeks) | 511 | 13 388 | 38.17 | 1.09 (0.92–1.29) | 0.98 (0.82–1.16) |
| Full term (≥ 39 weeks) | 1 759 | 46 298 | 37.99 | 1.00 | 1.00 |
| Birth weight | |||||
| < 2500 g | 173 | 3 127 | 55.33 | 2.60 (1.83–3.69) | 2.48 (1.65–3.72) |
| ≥ 2500 g and < 3500 g | 1 232 | 30 139 | 40.88 | 1.34 (1.15–1.56) | 1.35 (1.15–1.58) |
| ≥ 3500 g and < 4500 g | 966 | 27 643 | 34.94 | 1.00 | 1.00 |
| ≥ 4500 g | 91 | 2 945 | 30.90 | 0.73 (0.51–1.03) | 0.72 (0.51–1.03) |
| Size for gestational age** | |||||
| Small for gestational age | 171 | 3 048 | 56.10 | 2.11 (1.51–2.93) | 2.05 (1.47–2.86) |
| Normal for gestational age | 2 162 | 57 393 | 37.67 | 1.00 | 1.00 |
| Large for gestational age | 123 | 3 260 | 37.73 | 1.01 (0.74–1.40) | 1.02 (0.73–1.40) |
| Mode of delivery | |||||
| Vaginal delivery | 2 169 | 57 054 | 38.02 | 1.00 | 1.00 |
| C section | 303 | 6 986 | 43.37 | 1.19 (0.89–1.60) | 1.05 (0.77–1.44) |
*For 1 000 person years
Model 1: each association with early-onset T2D is separately adjusted for sex
Model 2: mutual adjustment for sex, maternal BMI, diabetes, smoking and infection during pregnancy, pre-eclampsia, gestational age, birth weight, and mode of delivery
**In Model 2, size for gestational age was adjusted for the same variables except gestational age and birth weight
Regarding other perinatal exposures, we observed a higher incidence (Table 2, model 1) of early-onset T2D in participants exposed to maternal obesity (4.60 [4.04–5.24]), diabetes (5.69 [4.95–6.56]), smoking (2.18 [2.03–2.34]) or infection (1.50 [1.28–1.75]) during pregnancy, as well as pre-eclampsia (1.65 [1.41–1.93]) and C-Sect. (1.33 [1.21–1.47]). These associations were attenuated but remained significant after mutual adjustment (except for pre-eclampsia and C-section) (Table 2). However, all these associations were null in the sibling analysis (Table 3). Notably, exposure to maternal obesity (0.92 [0.67 – 1.27]) and diabetes (1.25 [0.79—1.96]) did not alter the risk of early-onset T2D among siblings born to the same mother.
The PH assumption was respected for all perinatal exposures except for the associations with maternal obesity and diabetes, which showed attenuation during follow-up (Supplementary Table S2 and S3 present results stratified by age at onset in the full cohort and the sibling cohort, respectively).
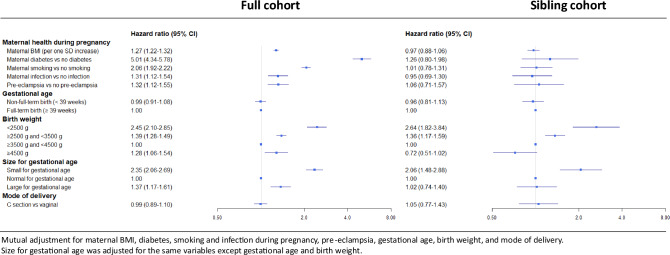
Figure 2 presents a summary of the full cohort and sibling cohort analyses, both based on mutual adjustment for the same set of variables to ensure comparability of estimates between the two cohorts.
Fig. 2.
Forest plot summarizing the associations between early life exposures and early-onset type 2 diabetes in the full cohort and in the sibling cohort. Results are mutually adjusted for all variables presented in the forest plot except for the size for gestational age. Size for gestational age was adjusted for all variables except gestational age and birth weight
Discussion
This study is the first to provide a comprehensive analysis of the incidence of T2D in young adults in relation to early-life exposures based on European nationwide data. In a national cohort involving the whole Swedish population, we observed that low and high birth weight (also when corrected for gestational age), and exposure to maternal obesity, diabetes, smoking, or infection during pregnancy were associated to early-onset T2D. However, in a subset cohort that included T2D cases and their T2D-free siblings as controls (sibling cohort), we could only confirm the higher risk for people born with LBW or SGA. These findings support the hypothesis of a fetal programming of early-onset T2D in the context of growth restriction, whereas associations with other perinatal factors, notably exposure to maternal diabetes and obesity, may reflect genetic susceptibility or the influence of environmental factors shared among family members.
Growth restriction resulting in LBW has been associated with long-term adverse metabolic outcomes, including T2D [1, 12]. Although there are arguments for causality based on animal studies, human data are observational. Therefore, providing a quasi-experimental design through a large sibling analysis raises the level of evidence. In terms of pathophysiology, several mechanisms have been proposed, some of which have been validated in experimental models. One key hypothesis involves rapid postnatal catch-up growth, typically observed following intrauterine growth restriction. It has been linked to unfavorable changes in body composition, including altered fat distribution with increased visceral adiposity [27]. These changes are known to negatively affect insulin sensitivity, further exacerbating the risk of glucose dysregulation. In addition, growth restriction has been associated with impaired pancreatic beta cell development, which can result in a lifelong reduction in insulin secretory capacity [28]. More recently, epigenetic modifications, such as DNA methylation changes in genes involved in metabolic regulation, and alterations in the early-life gut microbiome have also been proposed as contributing factors to long-term health adverse outcomes [29, 30]. In any case, history of fetal growth restriction should have clinical implications in diabetes screening strategy. The American Diabetes Association has previously identified SGA as a risk factor that should prompt diabetes screening in asymptomatic children and adolescents who are overweight or obese [31]. The results of our study are consistent with this statement and even support extending this recommendation to young adults.
On the other hand, our findings challenge the fetal programming hypothesis of early-onset T2D regarding in utero exposure to maternal obesity and diabetes. We observed an association between prenatal exposure to maternal obesity or diabetes and early-onset T2D in participants of the total cohort, aligning with previous findings [8–10]. But the association turned out to be neutral in the sibling cohort. Therefore, the association observed in the total cohort is likely reflecting genetic susceptibility for diabetes, and/or environmental factors shared among family members such as diet and physical inactivity. Indeed, studies that compared T2D within siblings exposed and unexposed to maternal diabetes during pregnancy are scarce and relates to specific populations. Notably, one study investigating T2D in a Native American population (aged 6 to 24 years), showed a significant excess risk of T2D in siblings exposed to maternal diabetes during pregnancy [32]. Thus, it is possible that maternal diabetes affects fetal programming of children and adolescent-onset T2D but not in older ages, or that this effect varies depending on ethnicity. To our knowledge, no similar sibling study has been conducted in a European population with early-T2D as the outcome.
Regarding other perinatal exposures, we found an association between high birth weight, preterm birth (although non-persistent in fully adjusted models) and early-onset T2D in the total cohort, aligning with previous literature [12, 13]. In addition, we found an association with maternal smoking, maternal infection and early-onset T2D. To our knowledge, these last associations have not been identified previously. However, all these associations were null in the sibling cohort, and are, as for the association with maternal diabetes and obesity, likely to result from confounders.
Parental history of diabetes was the strongest risk factor for early-onset T2D in our study. The associations were most pronounced for T2D diagnosed before age 25. This is consistent with the previous observation of an inverse association between the number of T2D-affected family members and the age of diabetes onset [33]. Previous studies have linked early-onset T2D to lower socioeconomic status, parental educational level and younger age at childbirth [7, 11]. In confirmation hereof, we observed a higher risk of early-onset T2D in people born from non-Sweden-born parents, young parents, parents with low level of education, and single-parent families. These associations may reflect more early-life exposure to unhealthy dietary habits and less physical activity, contributing to obesity which in turn, is closely tied to disparities in educational level and socio-economic status as well as the risk of T2D. Consequently, significant public health improvements could likely be achieved by developing targeted prevention strategies for high-risk families, aiming to reduce the risk of obesity and related adverse metabolic outcomes, particularly in young adults with a history of LBW or SGA.
Finally, this study confirms that early-onset T2D should be considered a growing threat to public health as we observed an increasing risk of early-onset T2D along with the year of birth, especially in people younger than 25 years, which is consistent with the increasing incidence of early-onset T2D observed worldwide [4]. Using a strict definition of T2D (excluding people with conflicting diagnoses or treatments), which prioritizes specificity over sensitivity, we have for the first time provided a reliable estimate of the incidence of early-onset T2D in the Swedish population. We also observed a time-dependent association between sex and early-onset T2D, with an excess risk for women before 25 years of age and an excess risk for men thereafter. This pattern has been observed in several cohort studies of early-onset T2D and is thought to be related to sex-specific weight gain and distribution before and after puberty [5]. Indeed, young women are more affected by high BMI, which is the most important attributable risk factor for early-onset T2D [4]. However, BMI at the time of T2D onset was not available in this study, limiting our ability to directly test this hypothesis. Additionally, the possibility of a detection bias cannot be ruled out, as women are more likely to seek healthcare earlier, particularly in relation to reproductive health.
This study has many strengths, including the collection of national data from reliable registries with multiple variables allowing for the consideration of a wide range of exposures and confounders while reducing selection and avoiding recall biases. In particular, the sibling analysis, using the larger national multigenerational registry in the world, allowed us to investigate the fetal programming hypothesis of early-onset T2D in relation to in utero exposure to maternal diabetes, which, to our knowledge, has never been done in a European nationwide cohort [16, 17]. However, this study has several limitations. Because early-onset T2D cases could not be clinically validated, we have excluded individuals younger than 18 years to avoid misclassification with type 1 diabetes, resulting in lack of data on childhood and adolescent-onset T2D. Regarding the incidence of T2D in individuals aged 18–40, it is likely to be underestimated due to undiagnosed T2D cases or management in primary care centers whose data are captured (with high coverage) in NDR but not in NPR. Another limitation relates to the assessment of exposures, as data collection is based on national registries. Thus, some exposures were not considered at all, particularly nutritional habits, since unavailable in national registries. Additionally, some studied exposures may be underestimated, such as minor maternal infections that do not require specialist care. Overall, misclassification on exposures or T2D diagnosis might have biased some associations toward the null, which is further concerning for the sibling cohort that has a limited sample size and for whom more statistical power is necessary to detect weak associations. Finally, the external validity of the study may be limited to European populations. Conducting similar analyses in other populations, particularly those with a high prevalence of early-onset type 2 diabetes, would be of considerable interest.
Conclusions
To conclude, this study highlights the growing incidence of early-onset T2D and emphasizes the urgent need for prevention and screening strategies that go beyond traditional risk factors. Given the strong link between fetal growth restriction and later-life T2D, tailored interventions must incorporate early-life health conditions, particularly in vulnerable populations. Addressing this issue is not only a medical priority but also a socioeconomic imperative, as disadvantaged communities often face higher exposure to T2D risk factors, exacerbating health inequities. Proactive measures can reduce the long-term socio-economic burden of diabetes while improving outcomes for at-risk groups.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- BMI
Body mass index
- ICD
International classification of diseases
- LBW
Low birth weight
- LGA
Large for gestational age
- LISA
Health Insurance and Labour Market Studies register [Longitudinell Integrationsdatabas för Sjukförsäkrings- och Arbetsmarknadsstudier]
- MBR
Medical birth register
- MGR
Multi-Generation Register
- NDR
National diabetes register
- NPDR
National prescribed drugs register
- NPR
National patient resgister
- PIN
Personal identification number
- SGA
Small for gestational age
- T2D
Type 2 diabetes
Author contributions
S.C. conceived and designed the study. Y.W. contributed to methodological issues. C.A. analyzed data. C.A. wrote the first draft of the manuscript. All authors critically revised the manuscript for important intellectual content and made substantial contributions to the interpretation of data. All authors reviewed and approved the final manuscript. C.A. and S.C. are the guarantors of this work and, as such, take responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all the data in the study.
Funding
Open access funding provided by Karolinska Institute. C.A. received scholarship from the French-speaking diabetes society (Société francophone du diabète) and Paris-Saclay University. Y.W. received a scholarship from the China Scholarship Council. The other funding sources include the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, the Strategic Research Area Diabetes at Karolinska Institutet, and the Swedish Diabetes Foundation.
Data availability
Data used for the study is register-based data that is pseudonymised and thus subject to General Data Protection Regulation (GDPR) and cannot be shared openly. Only metadata is published openly. Underlying data from the registers can be made available upon request to the Research Data Office at the Karolinska Institutet via rdo@ki.se after ensuring compliance with relevant legislation and GDPR.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This nationwide cohort study was approved by the Swedish Ethics Review Board (registration number: Dnr 2021–02881).
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoffman DJ, Powell TL, Barrett ES, Hardy DB. Developmental origins of metabolic diseases. Physiol Rev. 2021;101:739–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao L, Deng Y, Zhao D. Association of low birth weight and premature birth with the risk of metabolic syndrome: a meta-analysis. Front Pediatr. 2020;8:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet Lond Engl. 1986;1:1077–81. [DOI] [PubMed] [Google Scholar]
- 4.Xie J, Wang M, Long Z, Ning H, Li J, Cao Y, et al. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: systematic analysis of the Global Burden of Disease Study 2019. BMJ. 2022;379: e072385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra S, Ke C, Srinivasan S, Goyal A, Nyriyenda MJ, Florez JC, et al. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. 2023;11:768–82. [DOI] [PubMed] [Google Scholar]
- 6.Bjornstad P, Chao LC, Cree-Green M, Dart AB, King M, Looker HC, et al. Youth-onset type 2 diabetes mellitus: an urgent challenge. Nat Rev Nephrol. 2023;19:168–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perng W, Conway R, Mayer-Davis E, Dabelea D. Youth-onset type 2 diabetes: the epidemiology of an awakening epidemic. Diabetes Care. 2023;46:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabelea D, Mayer-Davis EJ, Lamichhane AP, D’Agostino RB, Liese AD, Vehik KS, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008;31:1422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millar K, Dean HJ. Developmental origins of type 2 diabetes in aboriginal youth in Canada: it is more than diet and exercise. J Nutr Metab. 2012;2012: 127452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young TK, Martens PJ, Taback SP, Sellers EAC, Dean HJ, Cheang M, et al. Type 2 diabetes mellitus in children: prenatal and early infancy risk factors among native Canadians. Arch Pediatr Adolesc Med. 2002;156:651–5. [DOI] [PubMed] [Google Scholar]
- 11.Lammi N, Moltchanova E, Blomstedt P, Eriksson JG, Taskinen O, Sarti C, et al. The effect of birth order and parental age on the risk of type 1 and 2 diabetes among young adults. Diabetologia. 2007;50:2433–8. [DOI] [PubMed] [Google Scholar]
- 12.Olaiya MT, Wedekind LE, Hanson RL, Sinha M, Kobes S, Nelson RG, et al. Birthweight and early-onset type 2 diabetes in American Indians: differential effects in adolescents and young adults and additive effects of genotype. BMI Maternal Diabetes Diabetologia. 2019;62:1628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia. 2020;63:508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisell T. Invited commentary: sibling-comparison designs, are they worth the eFFORT? Am J Epidemiol. 2021;190:738–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Onofrio BM, Class QA, Rickert ME, Sujan AC, Larsson H, Kuja-Halkola R, et al. Translational epidemiologic approaches to understanding the consequences of early-life exposures. Behav Genet. 2016;46:315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekbom A. The Swedish multi-generation register. Methods Mol Biol Clifton NJ. 2011;675:215–20. [DOI] [PubMed] [Google Scholar]
- 17.Hamm NC, Hamad AF, Wall-Wieler E, Roos LL, Plana-Ripoll O, Lix LM. Multigenerational health research using population-based linked databases: an international review. Int J Popul Data Sci. 2021;6:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cnattingius S, Källén K, Sandström A, Rydberg H, Månsson H, Stephansson O, et al. The Swedish medical birth register during five decades: documentation of the content and quality of the register. Eur J Epidemiol. 2023;38:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nationella Diabetesregistret [Internet]. [cited 2024 Jan 9]. Available from: https://www.ndr.nu/#/english
- 20.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wettermark B, Hammar N, MichaelFored C, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register—Opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–35. [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34:423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zachrisson I, Tibell C, Bang P, Ortqvist E. Prevalence of type 2 diabetes among known cases of diabetes aged 0–18 years in Sweden (Abstract). Diabetologia. 2003;46:A25. [Google Scholar]
- 24.Svensson A-M, Eliasson B, Linder E, Almskog I, Eeg-Olofsson K, Miftaraj M, et al. The Swedish National Diabetes Register (NDR) - Nationwide results 1996–2020 [Internet]. The Swedish National Diabetes Register; 2021 p. 16473344 byte. Available from: https://registercentrum.blob.core.windows.net/refdocs/10.18158/SJeNL4Vwiu.pdf
- 25.ACOG Committee Opinion No 579: Definition of term pregnancy. Obstet Gynecol. 2013;122:1139–40. [DOI] [PubMed]
- 26.Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr Oslo Nor. 1992;1996(85):843–8. [DOI] [PubMed] [Google Scholar]
- 27.Rolfe EDL, Loos RJF, Druet C, Stolk RP, Ekelund U, Griffin SJ, et al. Association between birth weight and visceral fat in adults. Am J Clin Nutr. 2010;92:347–52. [DOI] [PubMed] [Google Scholar]
- 28.Boehmer BH, Limesand SW, Rozance PJ. The impact of IUGR on pancreatic islet development and β-cell function. J Endocrinol. 2017;235:R63-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaiserman A, Lushchak O. Developmental origins of type 2 diabetes: Focus on epigenetics. Ageing Res Rev. 2019;55:100957. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar A, Yoo JY, Valeria Ozorio Dutra S, Morgan KH, Groer M. The association between early-life gut microbiota and long-term health and diseases. J Clin Med. 2021;10:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care. 2023;47:S20–42. [DOI] [PMC free article] [PubMed]
- 32.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–11. [DOI] [PubMed] [Google Scholar]
- 33.Molyneaux L, Constantino M, Yue D. Strong family history predicts a younger age of onset for subjects diagnosed with type 2 diabetes. Diabetes Obes Metab. 2004;6:187–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for the study is register-based data that is pseudonymised and thus subject to General Data Protection Regulation (GDPR) and cannot be shared openly. Only metadata is published openly. Underlying data from the registers can be made available upon request to the Research Data Office at the Karolinska Institutet via rdo@ki.se after ensuring compliance with relevant legislation and GDPR.